Concise Review: Prospects of Stem Cell Therapy for Temporal Lobe Epilepsy (original) (raw)
. Author manuscript; available in PMC: 2013 Mar 31.
Published in final edited form as: Stem Cells. 2007 Jun 28;25(10):2396–2407. doi: 10.1634/stemcells.2007-0313
Abstract
Certain regions of the adult brain have the ability for partial self-repair after injury through production of new neurons via activation of neural stem/progenitor cells (NSCs). Nonetheless, there is no evidence yet for pervasive spontaneous replacement of dead neurons by newly formed neurons leading to functional recovery in the injured brain. Consequently, there is enormous interest for stimulating endogenous NSCs in the brain to produce new neurons or for grafting of NSCs isolated and expanded from different brain regions or embryonic stem cells into the injured brain. Temporal lobe epilepsy (TLE), characterized by hyperexcitability in the hippocampus and spontaneous seizures, is a possible clinical target for stem cell-based therapies. This is because these approaches have the potential to curb epileptogenesis and prevent chronic epilepsy development and learning and memory dysfunction after hippocampal damage related to status epilepticus or head injury. Grafting of NSCs may also be useful for restraining seizures during chronic epilepsy. The aim of this review is to evaluate current knowledge and outlook pertaining to stem cell-based therapies for TLE. The first section discusses the behavior of endogenous hippocampal NSCs in human TLE and animal models of TLE and evaluates the role of hippocampal neurogenesis in the pathophysiology and treatment of TLE. The second segment considers the prospects for preventing or suppressing seizures in TLE using exogenously applied stem cells. The final part analyzes problems that remain to be resolved before initiating clinical application of stem cell-based therapies for TLE.
Keywords: Temporal lobe epilepsy, Adult neurogenesis, Dentate gyrus, Epilepsy, Hippocampal stem cells Hippocampal progenitors, Neural stem cells, Stem cell grafts
Introduction
Hippocampal lesions inflicted by acute seizures or head injury initially lead to epileptogenic structural changes and then progress into hippocampal dysfunction exemplified by chronic epilepsy, learning and memory impairments, and dramatically declined dentate neurogenesis. Approximately 50 million people suffer from epilepsy and approximately 40% of patients have temporal lobe epilepsy (TLE). TLE is typified by the progressive expansion of spontaneous recurrent motor seizures (SRMS; typically described as complex partial seizures in humans) stemming from the limbic system regions, especially the hippocampus [1, 2]. The TLE with hippocampal sclerosis, one of the most prevailing types of partial seizure disorders [3–5], is often allied with an initial precipitating event such as febrile convulsions, trauma, status epilepticus (SE), or encephalitis [6–8]. The hippocampal sclerosis is characterized by widespread hippocampal neuronal loss and aberrant mossy fiber sprouting [9–11]. Thirty-five percent of the people with TLE have chronic seizures that are resistant to antiepileptic drugs [12–14], and most TLE patients have learning and memory impairments and depression [15–18]. Moreover, antiepileptic drugs merely provide symptomatic treatment without influencing the course of the disease. Thus, there is a pressing need to develop alternative therapeutic approaches that suppress the evolution of both chronic epilepsy and learning and memory dysfunction after the initial precipitating injury (IPI).
The onset of chronic epilepsy following the IPI usually occurs after a latent period [2, 19–21]. Hence, testing the efficacy of promising intervention strategies applied shortly after the injury has immense value. Particularly, grafting of neural stem/progenitor cells (NSCs) expanded from different brain regions has significance, as this strategy may curb epileptogenesis and prevent the development of chronic epilepsy and learning and memory dysfunction after an IPI. Furthermore, grafting of NSCs into the hippocampus and/or application of strategies that activate endogenous NSCs to produce new neurons in the hippocampus during chronic epilepsy may be useful for easing both chronic seizures and learning and memory impairments. In this review, we will discuss the current knowledge and prospects pertaining to stem cell-based therapies for TLE.
Behavior of Endogenous Hippocampal Stem Cells in TLE
The concept of neurogenesis in the adult brain is now widely accepted, particularly in the two neurogenic regions, the subventricular zone of the forebrain and the subgranular zone (SGZ) of the dentate gyrus (DG). Neurogenesis in the DG of the hippocampus occurs in a wide variety of species, which includes rodents [22, 23], tree shrews [24, 25], monkeys [25, 26], and humans [27]. Interestingly, a vast majority of proliferating cells in the hippocampus differentiate into neurons [23, 26, 28–30]. It is believed that a subset of glial-fibrillary acidic protein-expressing cells located in the SGZ and granule cell layer (GCL) are likely NSCs in this region [31–33]. Slow proliferation of these NSCs produces a pool of transit amplifying cells, which proliferate further and give rise to new neurons and glia. Newly differentiated granule cells migrate into the GCL, express the mature neuronal marker neuron-specific nuclear antigen (NeuN), grow dendrites into the molecular layer, and send axons into the CA3 region [32, 34, 35]. During this stage, immature neurons likely receive _γ_-amino butyric acid (GABA) mediated excitatory synaptic inputs [36–38]. However, major glutamatergic synaptic activation from perforant path afferents does not occur until new neurons are 2 or more weeks old [36, 37], which coincides with appearance of spines on dendrites of newly born neurons [39]. Thus, incorporation of newly born neurons in the DG to the functional hippocampal circuitry takes over 2 weeks after their birth. Over the last decade, there has been a lot of interest in identifying the contribution of dentate neurogenesis to the pathophysiology of TLE.
Extent of Dentate Neurogenesis During the Early Phases of TLE
Blumcke and colleagues [40], by evaluating the expression of nestin in the DG of young (<2 years old) TLE patients, reported more nestin+ cells in the DG. An increased Ki-67 proliferation index and clusters of supragranular nestin+ cells within the molecular layer of the DG were also observed. Furthermore, confocal studies revealed colocalization of nestin with _β_-III tubulin, suggesting a neuronal fate for some of these nestin+ cells. Thus, early onset of TLE in pediatric patients is likely associated with increased neurogenesis in the DG, which is consistent with a number of studies in animal models of TLE where SE was found to increase NSC proliferation and neurogenesis in the SGZ of the DG ([41–46]; Figure 1). Hippocampal injury inflicted by excitotoxins such as kainic acid also increases neurogenesis in the DG [47]. Hippocampal injury or SE induces an initial, transitory proliferative surge in the SGZ with the number of new neurons increasing several folds during the first few weeks after injury [44, 45, 48]. This may be due to the release of mitogenic factors from dying neurons, deafferented granule cells, and reactive glia, as several neurotrophic factors are upregulated in the hippocampus after seizures or excitotoxic injury [49–51]. It may also be due to increased levels of neuropeptide Y (NPY), because acute seizures or SE increase NPY expression, and NSCs responsive to NPY exist in the hippocampus [52–56]. Thus, acute seizures or any other IPIs in the hippocampus considerably increase dentate neurogenesis likely through a surge in proliferation of NSCs. However, some of the increase may also be due to an enhanced survival of newly formed granule cells.
Figure 1.
Changes in dentate neurogenesis following kainic acid-induced status epilepticus. Newly born neurons in the DG of a naïve adult rat (A1) and an adult rat that underwent status epilepticus 12 days prior to euthanasia (B1) were visualized with immunostaining for doublecortin, a marker of newly born neurons. (A2) and (B2) show magnified views of regions of dentate gyrus from (A1) and (B1), respectively. Note that, in comparison with the dentate gyrus of a control rat (A1, A2), a rat that underwent status epilepticus (B1, B2) exhibits considerably increased density of doublecortin+ new neurons and abnormal migration of newly born neurons into the dentate hilus (indicated by arrowheads in [B1]). (C1) is a magnified view of a region from (B1) showing aberrantly migrated newly born neurons in the dentate hilus. Scale bar (A1, B1) = 200 _μ_m; (A2, B2, C1) = 50 _μ_m. Abbreviations: DG, dentate gyrus; DH, dentate hilus; GCL, granule cell layer; SGZ, subgranular zone.
Aberrant Migration of Newly Born Granule Cells After Status Epilepticus
In normal conditions, a vast majority of newly born neurons in the SGZ migrates into the GCL. However, in conditions such as SE, a large number of newly born neurons (i.e., granule cells) migrate away from the GCL into the dentate hilus ([45, 48, 57–63]; Figure 1). An elegant study by Parent and associates [58] reports appearance of chain-like progenitor cell formations extending into the hilus and molecular layer after the SE, suggesting that seizures alter migratory behavior of dentate granule cell precursors. Likewise, ectopic dentate granule cells were also found in the hilus and molecular layer of epileptic human DG [58]. Interestingly, ectopic granule cells in the dentate hilus exhibit several features of normal granule cells in the GCL, which comprise outgrowth of mossy fiber axons and incorporation of these axons into the pre-existing hippocampal circuitry [57, 60, 64] and standard granule cell membrane properties and firing behavior [60]. Nevertheless, extensive studies by Scharfman and colleagues have shown that ectopic granule cells exhibit some features that are inconsistent with normal granule cells in the GCL. These include an increased proportion of somatic and dendritic asymmetric (presumably excitatory) synapses, enhanced mossy fiber innervation, a distinct pattern of activation during spontaneous seizures, and the occurrence of spontaneous epileptiform bursts [57, 60, 64–66]. Recently, McCloskey and colleagues [65], by quantifying the population of ectopic granule cells at different times after pilocarpine-induced SE using immunostaining for Prox-1 (a marker of dentate granule cells [67]), demonstrated that the size of the hilar ectopic granule cell population after SE is substantial and stable over time. Additionally, correlation was found between the size of ectopic granule cell population and the frequency of behavioral seizures.
Thus, it appears that newly born granule cells that migrate into the hilus after the SE contribute to the development of chronic epilepsy. From this perspective, blocking the generation of ectopic granule cells following SE might be useful for thwarting the progression of SE into chronic epilepsy. Indeed, a study has tried to suppress the SE induced NSC proliferation in the DG with the antimitotic agent cytosine-_β_-d-arabinofuranoside (Ara-C) and evaluated the frequency of SRMS [68]. In this study, rats received continuous intracerebroventricular infusions of Ara-C or vehicle for 14 days from one day before the onset of SE. Rats were video monitored for ~12 hours per day from day 28 to day 34 after the SE. During this period, SRMS were observed in a majority of both vehicle-treated and Ara-C-treated rats; however, there was ~70% reduction in the frequency of SRMS and ~34% decrease in the duration of individual seizures in the Ara-C group. Interestingly, milder chronic epilepsy in Ara-C treated rats was associated with reduced density of ectopic granule cells in the hilus; however, the aberrant mossy fiber sprouting was unaffected [68]. These results support the idea that newly born granule cells that migrate into the hilus shortly after the SE contribute to the development of chronic epilepsy. However, there are some concerns. First, as analyses of SRMS were done very early (28 –34 days) after the SE, it is unknown whether beneficial effects of Ara-C would persist at later time points after the SE. Because in animal models of epilepsy SRMS are generally very robust at 2– 4 months post-SE [69], it is necessary to analyze the effects of Ara-C exposure during the early post-SE period on SRMS occurring at extended time points after the SE in future studies. Second, examining seizure frequency or duration in the pilocarpine model as an endpoint is difficult because these animals show rather diffuse damage and the SRMS may not arise from the hippocampus. Third, it is not clear whether the positive effects of Ara-C exposure are a result of decreased number of ectopic granule cells or decreased proliferation of glia after the SE. Fourth, it is unknown whether Ara-C treatment would block other epileptogenic changes that promote the development of chronic seizures. Fifth, Ara-C treatment may also suppress the compensatory neurogenesis in the GCL that might otherwise improve inhibition [70]. Thus, it remains to be seen whether complete elimination of aberrant neurogenesis after the SE would prevent the evolution of SE into chronic epilepsy.
Status of Dentate Neurogenesis During Chronic Epilepsy
In contrast to the increased neurogenesis observed in DG following SE or hippocampal injury, in conditions such as chronic epilepsy, DG neurogenesis declines considerably. This was evidenced by the following studies. First, Mathern and associates [71] showed that surgically resected hippocampi from children with frequent seizures exhibit decreased density of cells positive for polysialic acid neural cell adhesion molecule (a marker of newborn neurons) in the DG in comparison with age-matched autopsy cases. This suggested that severe seizures during early childhood are associated with decreased dentate neurogenesis. Second, Pirttila and colleagues [72, 73] report diminished dentate neurogenesis in a group of TLE patients with frequent seizures in comparison with control brains. In addition to seizures, decreased neurogenesis in these patients appeared to be associated with severe hippocampal damage, as also seen in an earlier study [74]. Third, a recent study by Fahrner et al. [75] suggests decreased dentate neurogenesis in the hippocampus of TLE patients based on decreased synthesis of mRNA for doublecortin (a marker of newly born neurons) and absence of cells positive for Ki-67 (a marker of proliferating cells).
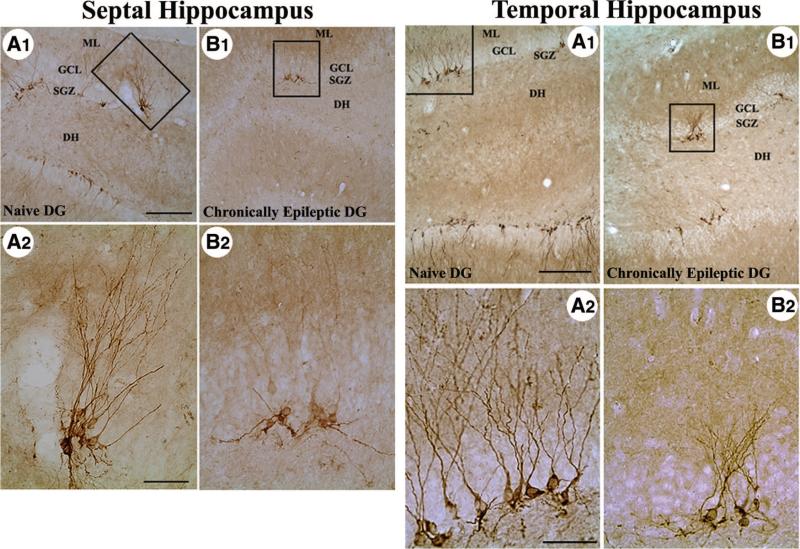
Thus, chronic TLE in humans is associated with decreased DG neurogenesis. These results are supported by studies in kainate models of TLE [48]. By analyzing neurogenesis in two different kainate models of rat TLE at both early and delayed time points after SE and/or hippocampal injury, Hattiangady and colleagues [48] demonstrate that chronic TLE is associated with severely diminished addition of new neurons to the adult DG (Fig. 2). The overall reductions in the addition of new neurons to the chronically epileptic hippocampus ranged from 64% to 81% in comparison with the age-matched intact hippocampus. Interestingly, the overall decrease in neurogenesis was considerably greater in rats exhibiting more SRMS, suggesting that greater frequency of SRMS during chronic epilepsy is more detrimental to DG neurogenesis.
Figure 2.
Distribution of doublecortin-immunopositive (newly formed) neurons in the septal and temporal regions of the chronically epileptic hippocampus. (A1): Dentate gyrus of a naïve, age-matched control rat. (B1): Dentate gyrus of a chronically epileptic rat at 5 months post-kainic acid administration. Left panel, septal hippocampus; right panel, temporal hippocampus. (A2) and (B2) are magnified views of boxed regions in (A1) and (B1). Note that the number of newly formed neurons is much less in the chronically epileptic dentate gyrus in comparison with the age-matched naïve dentate gyrus. In addition, unlike the newly formed neurons with extensive vertically oriented dendrites in the naïve dentate gyrus, newly formed neurons in the chronically epileptic dentate gyrus predominantly exhibit immature horizontally oriented or basal dendrites and less extensive vertical dendrites. Scale bar (A1, B1) = 200 _μ_m; (A2, B2) = 50 _μ_m. Figure reproduced from Hattiangady et al. [48]. Abbreviations: DG, dentate gyrus; DH, dentate hilus; GCL, granule cell layer; ML, molecular layer; SGZ, subgranular zone.
Potential Mechanisms of Decreased Neurogenesis During Chronic Epilepsy
It is generally perceived that increases in neurogenesis after SE or acute seizures are a result of neuronal activity-related enhancements in NSC proliferation [44, 45]. Consistent with this idea, a study by Deisseroth et al. [76] shows that increased excitatory stimuli act directly on hippocampal NSCs to favor neuron production. Likewise, a mild reduction in net hippocampal excitatory activity via diazepam administration reduces neuronal fate-choice of newly born cells, suggesting that excitation-neurogenesis coupling exists in adult hippocampal NSCs [76]. However, the above mechanism is inconsistent with the decreased neurogenesis observed during chronic epilepsy. Since some studies link decreased neurogenesis in certain hippocampal injury models to inflammation [77, 78], one may presume that decreased dentate neurogenesis is linked to the increased number of activated microglia in the chronically epileptic hippocampus. Evaluation of microglia, however, reveals a minimal number of activated microglia during chronic epilepsy, underscoring that dramatically decreased neurogenesis during chronic epilepsy is not due to chronic inflammation [48]. Considering this, decreased neurogenesis during chronic epilepsy may be due to the following. First, it is likely that DG milieu during chronic epilepsy is not conducive for increased NSC proliferation. Indeed, the concentration of several NSC proliferation factors such as fibroblast growth factor (FGF)-2, insulin-like growth factor-1, and brain-derived neurotrophic factor (BDNF) [79, 80] decreases during chronic epilepsy [48, 51]. Second, it is possible that chronic epilepsy depletes the number of NSCs. However, a human study reports an increased expression of NSC marker Musashi-1 in adult epileptic hippocampal tissues obtained from TLE patients [81], suggesting that NSCs persist during chronic epilepsy; however, it is unknown whether these cells exhibit significant proliferation or progeny of these cells differentiate into mature neuronal phenotypes. Detailed studies in future on proliferation of NSCs, neuronal differentiation of newly born cells, and long-term survival of newly differentiated neurons during chronic epilepsy may address these questions.
Is There a Link Between Decreased Neurogenesis and the Pathophysiology of TLE?
Diminished dentate neurogenesis during chronic epilepsy might contribute to the persistence of seizures and learning and memory impairments. This is because one previous study suggests that ~14% of newborn neurons in the DG of young rats differentiate into inhibitory GABA-ergic basket cells [82]. If this finding were valid for new neurons born throughout adulthood, dramatically decreased neurogenesis during chronic epilepsy would result in minimal addition of new GABA-ergic basket cells to the DG circuitry, which may contribute to persistence of reduced inhibitory neurotransmission. This idea is consistent with the observations in animal models of TLE that chronic epilepsy is associated with both reduced inhibition of granule cells and reduced number of GABA-ergic basket cells in the DG [83–87]. In addition, a recent report by Jakubs and coworkers [70] reports that adult-generated hippocampal granule cells in a rat model of epilepsy display reduced excitatory synaptic input and decreased excitability, implying that their functional integration was adjusted to the prevailing functional state in the hippocampal network [88]. From this viewpoint, it appears that reduced neurogenesis during chronic epilepsy contributes to maintenance of DG hyperexcitability. Furthermore, as newly added granule cells get incorporated into circuits supporting spatial memory as they mature, it has been suggested that newly born neurons make a unique contribution to memory processing in the dentate gyrus and formation of the temporal clusters of long-term episodic memories [89–95]. In addition, animal models of depression evince that dentate neurogenesis responds to factors modulating depression such as stress or antidepressant treatment [96, 97], implying some rapport between extent of neurogenesis and mood. Therefore, it is possible that hippocampal-dependent cognitive deficits and depression observed during chronic epilepsy [98, 99] are linked at least partially to diminished dentate neurogenesis.
Potential of Endogenous Hippocampal Stem Cells for Treating TLE
Increased neurogenesis following SE leads to abnormal migration of a substantial number of newly born granule cells into the dentate hilus, where they exhibit spontaneous epileptiform bursts and a distinct pattern of activation during SRMS. There is also evidence that a greater number of ectopic granule cells in the hilus is associated with increased frequency of SRMS, and blocking the ectopic migration of granule cells shortly after the SE results in much milder chronic epilepsy. However, rigorous and long-term studies are needed to ascertain whether complete elimination of aberrant neurogenesis after the SE would prevent the evolution of SE into chronic epilepsy. If successful, a new therapy for prevention of chronic epilepsy that ensues after SE or brain injury may be developed. In contrast to the scenario at early post-SE, chronic TLE is associated with dramatically declined production of new neurons in the adult DG. Because decreased neurogenesis may contribute to the persistence of seizures and impairments in learning and memory during chronic epilepsy, development of strategies that enhance the production of new neurons in the chronically epileptic hippocampus may be useful for treating chronic TLE. Nevertheless, it should be noted that chronic epilepsy is associated with persistent loss of significant fractions of several hippocampal cell types in addition to decreased neurogenesis, which may also affect learning ability. Additionally, a dramatic increase in the production of new neurons in conditions such as chronic epilepsy may be detrimental, as substantially increased neurogenesis observed after the initial SE (i.e., during the acute injury phase) has been found to be pathological and to promote abnormal hyperexcitability [60, 100–102]. Rigorous studies on animal models are needed to comprehend these issues and to develop potential neurogenesis-related therapies for chronic TLE.
Potential of Stem Cell Grafts for Treating TLE
Although the effects of fetal cell grafts have been studied extensively in animal models of TLE [85, 103–109], studies on the efficacy of stem cell grafts for treating TLE are very few hitherto. In the last few years, adult stem cell-, NSC-, and embryonic stem cell-based therapies have received considerable interest for treating a variety of neurodegenerative diseases [110–115]. Both NSC- and ESC-based therapies may be useful for treating chronic epilepsy because of their potential ability to replace neuronal populations that are lost during the course of the disease and to repair the disrupted circuitry. Alternatively, if these cells produce a large number of GABA-ergic inhibitory interneurons, they may be efficient for inhibiting seizure activity. Stem cell grafting may also be useful for cell-based delivery of antiepileptic or neuroprotective factors.
Prospective Donor Stem Cells for Grafting into the Hippocampus in TLE
Neural Stem/Progenitor Cells
Multipotent NSCs present in the developing and adult central nervous system (CNS) can be expanded in culture using mitogens of NSCs such as FGF-2 and epidermal growth factor (EGF). These cells can be maintained in an undifferentiated state or can be induced to differentiate into neurons, astrocytes, and oligodendrocytes [116–122]. Studies on FGF-2- and EGF-responsive NSCs have shown that these cells satisfy the criteria of regional NSCs [123, 124]. Thus, NSCs from different areas of the human CNS could potentially be harvested in culture for prolonged periods and used as a source of donor tissue for grafting in neurodegenerative diseases [125–128]. NSCs may also serve as donor cells for grafting in TLE. However, the functional recovery after grafting of NSCs into the injured hippocampus will depend on the behavior of grafted NSCs. It will be helpful if they differentiate into a large number of neurons specific to the lesioned site of grafting and facilitate the reconstruction of the disrupted circuitry. Alternatively, it may also be useful if they differentiate into GABA-ergic interneurons and establish connectivity with host hippocampal neurons exhibiting hyperexcitability or simply synthesize and secrete antiepileptic factors on a long-term basis.
In vitro proliferation and differentiation of NSCs from embryonic day 19 (E19) and postnatal hippocampi have been analyzed [120, 121]. Hippocampal NSCs proliferate in the presence of EGF and/or FGF-2 and form neurospheres in vitro. Proliferating cells within neurospheres are negative for markers of neurons and glia but express various markers of NSCs. Furthermore, some cells within neurospheres have self-renewal ability. NSCs from hippocampus are multipotent, as they can give rise to neurons, astrocytes, and oligodendrocytes. Moreover, the progeny of hippocampal NSCs give rise to larger pyramidal-shaped neurons [120, 121], some of which resemble hippocampal CA3 pyramidal neurons. Only a fraction (~20%) of neurons in neurosphere cultures of E19 hippocampus are GABA+, and CA3 pyramidal-like neurons lack GABA [120] (Fig. 3). Thus, NSCs from E19 hippocampus have the ability to give rise to both hippocampal pyramidal-like neurons and interneurons and hence appear ideal for grafting into the hippocampus in conditions such as TLE. On the other hand, analyses of NSCs isolated from anterior subventricular zone (aSVZ) reveal their preference for differentiating into GABA-ergic neurons (Fig. 3). Grafting of NSCs from aSVZ may also be useful for treating TLE, as depletion of GABA-ergic interneurons appears to be one of the reasons for hippocampal hyperexcitability in TLE, and replenishment of GABA-ergic interneurons through grafting likely strengthens hippocampal inhibition [83–87, 129]. However, considering the ineffectiveness of GABA mimetic drugs for controlling seizures in pharmacoresistant epilepsy [130], it remains to be seen whether furnishing GABA through addition of new inhibitory neurons to the hippocampal circuitry would be useful for restraining seizures on a long-term basis.
Figure 3.
NSCs isolated from embryonic day 19 hippocampus differentiate into both larger pyramidal shaped cells that are immunopositive for TuJ-1 but lack GABA (presumably hippocampal pyramidal neurons) and interneuron-like cells that are immunopositive for both TuJ-1 and GABA (presumably GABA-ergic interneurons). (A1) shows a larger multipolar GABA immunopositive neuron (yellowish green, indicated by arrow), whereas (A2) and (A3) show smaller GABA immunopositive neurons (arrows). Note that larger neurons resembling CA3 pyramidal neurons are immunopositive for TuJ1 (red) but negative for GABA. In contrast, GABA-ergic neurons are positive for both GABA and TuJ1 and hence exhibit yellowish green color in double exposure photography. Figure reproduced from Shetty [120]. Middle panel: (B1) shows differentiation of NSCs isolated from the anterior subventricular zone into TuJ-1 positive neurons (red) and astrocytes (green). Lower panel: (C1–C3) illustrate differentiation of neurons derived from anterior subventricular zone NSCs into GABA-ergic cells. Scale bar = 25 _μ_m. Abbreviations: E19, embryonic day 19; GABA, _γ_-amino butyric acid; GFAP, glial-fibrillary acidic protein; NSCs, neural stem/progenitor cells.
Embryonic Stem Cells
Unlike neural precursors and NSCs, embryonic stem cells are very promising in terms of providing an infinite supply of donor cells for grafting in neurodegenerative diseases. ESCs are derived from the inner cell mass of the developing blastocyst and hence are capable of generating every cell type present in the body. ESCs exhibit high levels of telomerase activity [131] and a short G1 cell cycle checkpoint [132] and therefore exhibit proliferation ceaselessly in culture without losing their pluripotency [133]. However, ESCs may not be ideal for direct transplantation in an undifferentiated state into the brain because they are prone to uncontrolled proliferation and teratoma formation [134]. Therefore, it is necessary to generate cell lines that are committed to specific neural lineages from ESCs in culture prior to grafting. In this way, it is possible to tailor cell therapy from ESCs for specific neurodegenerative disorders. Indeed, recent in vitro studies have reported success in pushing the progeny of ESCs to differentiate into dopaminergic neurons [135–138], spinal motor neurons [139, 140], and oligodendrocytes [141]. Thus, ESCs are very promising as a source of initial donor tissue for generating a variety of neural precursors in culture for grafting in neurodegenerative conditions. However, it remains to be validated whether different types of hippocampal neurons could be generated from ESCs.
Effects of Grafting Human NSCs in a Pilocarpine Model of TLE
Chu and colleagues [142] (Table 1) examined the effects of grafting NSCs on SRMS in rats that underwent SE. Donor cells were _β_-galactosidase (_β_-gal) encoded human NSCs, and the grafting was performed just a day after the induction of SE via i.v. injection. Between 28 and 35 days after the SE, 87% of animals receiving no NSCs (i.e., epilepsy-only group) showed SRMS. In contrast, only 13% of animals receiving NSCs displayed SRMS. Furthermore, in comparison with the epilepsy-only group, animals receiving NSCs exhibited milder SRMS and smaller field excitatory postsynaptic potential amplitudes in the CA1 region following stimulation of Schaffer collaterals. Grafted cells positive for _β_-gal were observed in multiple regions of the brain, including the hippocampus. Investigation of phenotypes revealed that only a few cells derived from NSCs expressed markers of mature neurons. Even the cells that expressed these markers did not morphologically resemble any of the major hippocampal neuronal types. However, a fraction of _β_-gal+ grafted cells coexpressed markers of interneurons such as GABA and parvalbumin (PV) in the hippocampus and the piriform cortex, suggesting that cells derived from NSCs differentiate into GABA-synthesizing cells.
Table 1.
Stem cell transplantation studies in epilepsy models
| Study | Cell type and source | Animal model | Timing of grafting | Route/site of grafting | Therapeutic outcome | Survival and differentiation |
|---|---|---|---|---|---|---|
| Chu et al. [142] | NSCs from VZ of 15 weeks gestation human brain | Pilocarpine model of rat TLE | 1 day after SE | Intravenous injection of NSCs into tail vein | Reduced frequency of SRMS at 28-35 days post-SE | Extent of survival unknownSome cells differentiated into GABA-ergic neurons in the hippocampus, amygdala, and piriform cortex |
| Ruschenschmidt et al. [143] | Mouse embryonic stem cell-derived neuronal precursorsa | Pilocarpine model of rat TLE | 1 month after SE | Intracerebral grafting into hippocampi | Not analyzed | Survival not assessed quantitativelyFunctional properties of grafted cells were similar to grafted cells placed into the hippocampus of sham-control rats |
| Shetty and Hattiangady [144] | Hippocampal NSCs from rat pups | ICV KA model of rat TLE | 4 days after KA lesion | Intracerebral grafting into hippocampi | Not analyzed | Survival excellentDifferentiation into neurons and astrocytes observed. Some grafted cells differentiated into GABA-ergic neurons |
| Acharya et al. [145] | Hippocampal NSCs from rat pups | IP KA model of rat TLE | 4 months after KA induced SE | Intracerebral grafting into hippocampi | ~50% reduction in the frequency of spontaneous seizures | Survival excellentDifferentiation into neurons rarely observed. Majority of grafted cells differentiated into astrocytes |
Thus, suppression of chronic SRMS in animals receiving NSCs is not due to NSC-mediated replenishment of hippocampal cell layers. However, the results suggest that NSCs have the ability to differentiate into GABA-synthesizing cells following engrafting into the injured hippocampus. It is possible that introduction of new GABA-synthesizing cells decreases neuronal excitability in the injured hippocampus and thereby suppresses SRMS. Nevertheless, several issues are obscure. First, it is unclear whether beneficial effects mediated by NSCs at 28–35 days post SE would persist at extended time points after the SE. This is important because, in animal models of epilepsy, SRMS are generally very robust at 2–4 months after the SE [69]. Hence, to fully ascertain the efficacy of NSC administration for blocking the evolution of TLE after the SE, it is necessary to analyze the effects of NSC administration on a long-term basis using both behavioral measures as well as extensive electroencephalographic recordings. Second, it is imperative to examine whether NSC-derived cells expressing GABA and PV are indeed interneurons and whether these cells establish synaptic connectivity with hippocampal neurons exhibiting hyperexcitability. Additionally, it is uncertain whether cells coexpressing _β_-gal and markers of GABA are newly differentiated interneurons or represent existing host neurons that are fused with NSC-derived _β_-gal+ cells. Thus, detailed long-term experiments are critical prior to the clinical application of NSC therapy for TLE.
Properties of Embryonic Stem Cell Grafts in the Epileptic Hippocampus
Recently, Ruschenschmidt and colleagues [143] (Table 1) examined characteristics of mouse embryonic stem cell-derived neural precursors (ESNs) transplanted into the hippocampi of chronically epileptic and sham-control rats. Most ESNs were found in clusters at the transplant site, although individual ESNs exhibited short-distance migration into the host tissue. However, grafted ESNs extended processes into the surrounding host brain tissue. Electrophysiological analyses of ESNs revealed their ability to generate action potentials and express voltage-gated Na+ and K+ currents, as well as hyperpolarization-activated currents. Furthermore, most ESNs received non-_N_-methyl-d-aspartate and GABAA receptor-mediated synaptic input. Interestingly, no obvious differences were found in the functional properties of ESNs between sham-control and pilocarpine-treated rats. Thus, after transplantation into the hippocampus of sham-control and chronically epileptic rats, ESNs display synaptic properties characteristic of neurons. These results are promising in terms of achieving appropriate survival of grafted ESNs in the chronically epileptic hippocampus. Striking invasion of host tissue by a dense plexus of graft-derived fibers appears useful for widespread delivery of inhibitory mediators to the host brain through grafts of ESNs. However, several issues remain to be addressed in future studies. These include whether or not the grafted ESNs have the ability (a) to survive for prolonged periods in the epileptic hippocampus, (b) to differentiate into functional principal hippocampal neurons and/or GABA-ergic interneurons, and (c) to suppress SRMS and learning and memory deficits observed during chronic epilepsy.
Survival and Differentiation of Hippocampal NSCs in Rat Models of TLE
Grafting studies were conducted to determine whether hippocampal NSCs could survive grafting into the lesioned hippocampus and give rise to multiple cell types [144] (Table 1). Proliferating NSCs in neurospheres were labeled with 5′-bromodeoxyuridine (BrdU), treated with BDNF (20 g/ml), and grafted stereotaxically into the lesioned CA3 region of the hippocampus at 4 days after kainic acid-induced injury, a model of TLE. Grafts were analyzed with immunostaining for BrdU, NeuN, GABA (a marker of inhibitory neurons), and S-100_β_ (a marker of mature astrocytes). All grafts showed widespread dispersion of BrdU-labeled cells away from the transplant core (Fig. 4). Although most of the grafted cells were restricted to the lesioned CA3 region, some cells migrated into the dentate SGZ and GCL and differentiated predominantly into neurons (Fig. 4). Although a fraction of grafted cells differentiated into neurons in the graft core (Fig. 4), a vast majority of graft-derived cells differentiated into S-100_β_+ astrocytes (Fig. 5). Some of the graft-derived neurons expressed the inhibitory neurotransmitter GABA (Fig. 5). Thus, hippocampal NSCs placed into the hippocampus shortly after injury survive grafting and give rise to both neurons (including GABA-ergic neurons) and astrocytes. However, detailed long-term studies are necessary to assess their ability to prevent chronic epilepsy development after SE or brain injury. Recently, the efficacy of hippocampal NSCs for suppressing seizures was examined by grafting these cells into the hippocampi of rats exhibiting chronic TLE [145]. The results suggest that hippocampal NSC grafting is efficacious for reducing both frequency and intensity of SRMS in rats exhibiting chronic TLE (Table 1).
Figure 4.
Dispersion and differentiation of BrdU-labeled NSCs isolated from the embryonic day 19 hippocampus following grafting into the lesioned hippocampal CA3 region of a kainic acid-treated rat. Note that cells derived from NSC grafts disperse extensively in the hippocampus (A1), and the graft core contains a significant number of NeuN immunopositive neurons (B1). (A2) and (B2) are magnified views of graft regions from (A1) and (B1). Panels (C1–C3) illustrate differentiation of BrdU+ NSCs into NeuN immunopositive neurons in the graft core (arrowheads), whereas panels (D1–D3) demonstrate neuronal differentiation of BrdU+ NSCs that migrated into the dentate granule cell layer (arrowheads). Scale bar (A1, B1) = 200 _μ_m; (A2, B2) = 30 _μ_m; (C1–D3) = 20 _μ_m. Abbreviations: BrdU, 5′-bromodeoxyuridine; NeuN, neuron-specific nuclear antigen; NSCs, neural stem/progenitor cells; T, transplant.
Figure 5.
Differentiation of grafted BrdU-labeled hippocampal neural stem/progenitor cells into GABA+ neurons and S-100_β_+ mature astrocytes following grafting into the lesioned hippocampal CA3 region of a kainic acid-treated rat. (A1–A3) demonstrate GABA+ neurons derived from grafted NSCs and (B1–B3) show S-100_β_+ astrocytes derived from grafted NSCs. Scale bar = 20 _μ_m. Abbreviations: BrdU, 5′-bromodeoxyuridine; GABA, _γ_-amino butyric acid; NSCs, neural stem/progenitor cells.
Overall Conclusions
There is no conclusive evidence in support of using stem cells for treating TLE hitherto. However, it is important to acknowledge that this field is still in infancy, and the initial studies are promising. Usefulness of four distinct stem cell-based approaches needs to be assessed rigorously in future using animal models of TLE for further advances in this field. The first approach should involve development of methods for testing whether inhibiting increased proliferation of hippocampal NSCs during the first few weeks following the SE would prevent the development of chronic epilepsy. Addressing this issue is important in light of studies suggesting that epileptic seizures such as SE not only increase dentate neurogenesis but also lead to abnormal migration of newly born granule cells into the dentate hilus, where they exhibit spontaneous epileptiform bursts and may contribute to the development of chronic epilepsy [57, 58, 60, 64, 65, 146]. However, it should be recognized that although the above approach is interesting to pursue, clinical application of this antiepileptogenic therapy for preventing TLE after IPI is complicated. This is because many patients with TLE do not seem to have any IPI, and many patients with IPIs (e.g., prolonged febrile seizures) do not progress into TLE [147–149]. Thus, predicting those that will benefit from early therapeutic interventions will be difficult.
The second approach should focus on developing strategies that activate endogenous NSCs in the chronically epileptic hippocampus to produce a large number of new neurons including GABA-ergic interneurons. This approach has significance because studies in both TLE and animals models of TLE suggest that chronic TLE is associated with dramatically declined production of new neurons in the adult DG. Decreased neurogenesis during chronic epilepsy may contribute to the persistence of seizures possibly due to decreased addition of new GABA-ergic interneurons. Likewise, impairments in learning and memory during chronic epilepsy may be due to participation of minimal or no new neurons in learning processes. Considering this, development of strategies that enhance the production of new neurons and facilitate the differentiation of new neurons into GABA-ergic neurons in the chronically epileptic hippocampus may be useful for treating seizures as well as learning and memory deficits in chronic TLE.
The third strategy should comprise rigorous analyses of the efficacy of grafts of NSCs or ESNs placed into the hippocampus after the onset of chronic epilepsy for suppressing seizures and learning and memory deficits. This is because the initial results of stem cell grafting studies in TLE models reported so far [142–145] are promising in terms of their short term survival and their effectiveness for reducing the frequency of seizures. However, it is important to critically examine whether grafting of NSCs or ESNs into the hippocampus during chronic epilepsy would lead to long-term survival of graft-derived cells, differentiation of graft-derived cells into functional principal hippocampal neurons and/or GABA-ergic interneurons, long-term suppression of SRMS, and improvements in learning and memory deficits. In view of findings that delivery of anticonvulsant compounds such as NPY, glial-derived neurotrophic factor, and adenosine is efficacious for reducing seizures in animal models of TLE [150–152], a fourth approach would be to try a combination therapy comprising NSC/ESC cell transplants and cell or recombinant viral vector-based delivery of anticonvulsant compounds into the hippocampus during chronic epilepsy. This strategy might turn out to be very effective, as seizure control would likely be mediated by both GABA-ergic interneurons derived from NSC/ESC transplants and anti-convulsant compounds released by genetically engineered cells or host cells infected with viral vectors encoding these compounds.
Acknowledgments
We gratefully acknowledge the support from National Institute of Neurological Disorders and Stroke (NS 54780 and NS 43507 to A.K.S), National Institute for Aging (AG 20924 to A.K.S.), and Department of Veterans Affairs (VA Merit Awards to A.K.S.) for our research group's contributions to the area of research discussed in this review.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Engel J, Jr., Wilson C, Bragin A. Advances in understanding the process of epileptogenesis based on patient material: What can the patient tell us? Epilepsia. 2003;44(suppl 12):60–71. doi: 10.1111/j.0013-9580.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- 2.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 3.Engel J., Jr Etiology as a risk factor for medically refractory epilepsy: A case for early surgical intervention. Neurology. 1998;51:1243–1244. doi: 10.1212/wnl.51.5.1243. [DOI] [PubMed] [Google Scholar]
- 4.Mathern GW, Adelson PD, Cahan LD, et al. Hippocampal neuron damage in human epilepsy: Meyer's hypothesis revisited. Prog Brain Res. 2002;135:237–251. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- 5.Mathern GW, Babb TL, Mischel PS, et al. Childhood generalized and mesial temporal epilepsies demonstrate different amounts and patterns of hippocampal neuron loss and mossy fibre synaptic reorganization. Brain. 1996;119:965–987. doi: 10.1093/brain/119.3.965. [DOI] [PubMed] [Google Scholar]
- 6.Cendes F. Febrile seizures and mesial temporal sclerosis. Curr Opin Neurol. 2004;17:161–164. doi: 10.1097/00019052-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Fisher PD, Sperber EF, Moshe SL. Hippocampal sclerosis revisited. Brain Dev. 1998;20:563–573. doi: 10.1016/s0387-7604(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 8.Harvey AS, Berkovic SF, Wrennall JA, et al. Temporal lobe epilepsy in childhood: Clinical, EEG, and neuroimaging findings and syndrome classification in a cohort with new-onset seizures. Neurology. 1997;49:960–968. doi: 10.1212/wnl.49.4.960. [DOI] [PubMed] [Google Scholar]
- 9.Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalby NO, Mody I. The process of epileptogenesis: A pathophysiological approach. Curr Opin Neurol. 2001;14:187–192. doi: 10.1097/00019052-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Sutula T, Cascino G, Cavazos J, et al. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 12.Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 13.Litt B, Esteller R, Echauz J, et al. Epileptic seizures may begin hours in advance of clinical onset: A report of five patients. Neuron. 2001;30:51–64. doi: 10.1016/s0896-6273(01)00262-8. [DOI] [PubMed] [Google Scholar]
- 14.McKeown MJ, McNamara JO. When do epileptic seizures really begin? Neuron. 2001;30:1–3. doi: 10.1016/s0896-6273(01)00253-7. [DOI] [PubMed] [Google Scholar]
- 15.Detour J, Schroeder H, Desor D, et al. A 5-month period of epilepsy impairs spatial memory, decreases anxiety, but spares object recognition in the lithium-pilocarpine model in adult rats. Epilepsia. 2005;46:499–508. doi: 10.1111/j.0013-9580.2005.38704.x. [DOI] [PubMed] [Google Scholar]
- 16.Devinsky O. Therapy for neurobehavioral disorders in epilepsy. Epilepsia. 2004;45(suppl 2):34–40. doi: 10.1111/j.0013-9580.2004.452003.x. [DOI] [PubMed] [Google Scholar]
- 17.Helmstaedter C, Brosch T, Kurthen M, et al. The impact of sex and language dominance on material-specific memory before and after left temporal lobe surgery. Brain. 2004;127:1518–1525. doi: 10.1093/brain/awh174. [DOI] [PubMed] [Google Scholar]
- 18.Mazza M, Orsucci F, De Risio S, et al. Epilepsy and depression: Risk factors for suicide? Clin Ter. 2004;155:425–427. [PubMed] [Google Scholar]
- 19.Mathern GW, Babb TL, Pretorius JK, et al. The pathophysiologic relationships between lesion pathology, intracranial ictal EEG onsets, and hippocampal neuron losses in temporal lobe epilepsy. Epilepsy Res. 1995;21:133–147. doi: 10.1016/0920-1211(95)00014-2. [DOI] [PubMed] [Google Scholar]
- 20.Mathern GW, Pretorius JK, Babb TL. Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J Neurosurg. 1995;82:220–227. doi: 10.3171/jns.1995.82.2.0220. [DOI] [PubMed] [Google Scholar]
- 21.Spencer DD, Spencer SS. Hippocampal resections and the use of human tissue in defining temporal lobe epilepsy syndromes. Hippocampus. 1994;4:243–249. doi: 10.1002/hipo.450040303. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 24.Gould E, McEwen BS, Tanapat P, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould E, Reeves AJ, Fallah M, et al. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 28.Cameron HA, Woolley CS, McEwen BS, et al. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 29.Rao MS, Hattiangady B, Abdel-Rahman A, et al. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- 30.Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- 31.Hattiangady B, Shetty AK. Aging does not alter the number or pheno-type of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.09.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempermann G, Jessberger S, Steiner B, et al. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Seri B, Garcia-Verdugo JM, McEwen BS, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt MD, Jessberger S, Steiner B, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 35.Emsley JG, Mitchell BD, Kempermann G, et al. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Esposito MS, Piatti VC, Laplagne DA, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge S, Goh EL, Sailor KA, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao C, Teng EM, Summers RG, Jr, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumcke I, Schewe JC, Normann S, et al. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001;11:311–321. doi: 10.1002/hipo.1045. [DOI] [PubMed] [Google Scholar]
- 41.Bengzon J, Kokaia Z, Elmer E, et al. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekdahl CT, Mohapel P, Elmer E, et al. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2001;14:937–945. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- 43.Madsen TM, Treschow A, Bengzon J, et al. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa E, Aimi Y, Yasuhara O, et al. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41:10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 45.Parent JM, Yu TW, Leibowitz RT, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- 47.Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 48.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Lowenstein DH, Seren MS, Longo FM. Prolonged increases in neurotrophic activity associated with kainate-induced hippocampal synaptic reorganization. Neuroscience. 1993;56:597–604. doi: 10.1016/0306-4522(93)90359-n. [DOI] [PubMed] [Google Scholar]
- 50.Shetty AK, Rao MS, Hattiangady B, et al. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- 51.Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87:147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- 52.Howell OW, Doyle K, Goodman JH, et al. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- 53.Howell OW, Scharfman HE, Herzog H, et al. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 54.Howell OW, Silva S, Scharfman HE, et al. Neuropeptide Y is important for basal and seizure-induced precursor cell proliferation in the hippocampus. Neurobiol Dis. 2007;26:174–188. doi: 10.1016/j.nbd.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Madsen TM, Greisen MH, Nielsen SM, et al. Electroconvulsive stimuli enhance both neuropeptide Y receptor Y1 and Y2 messenger RNA expression and levels of binding in the rat hippocampus. Neuroscience. 2000;98:33–39. doi: 10.1016/s0306-4522(00)00078-6. [DOI] [PubMed] [Google Scholar]
- 56.Scharfman HE, Gray WP. Plasticity of neuropeptide Y in the dentate gyrus after seizures, and its relevance to seizure-induced neurogenesis. EXS. 2006;95:193–211. doi: 10.1007/3-7643-7417-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dashtipour K, Tran PH, Okazaki MM, et al. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res. 2001;890:261–271. doi: 10.1016/s0006-8993(00)03119-x. [DOI] [PubMed] [Google Scholar]
- 58.Parent JM, Elliott RC, Pleasure SJ, et al. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 59.Ribak CE, Dashtipour K. Neuroplasticity in the damaged dentate gyrus of the epileptic brain. Prog Brain Res. 2002;136:319–328. doi: 10.1016/s0079-6123(02)36027-8. [DOI] [PubMed] [Google Scholar]
- 60.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: Functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shapiro LA, Korn MJ, Shan Z, et al. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res. 2005;1040:81–91. doi: 10.1016/j.brainres.2005.01.098. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro LA, Ribak CE. Integration of newly born dentate granule cells into adult brains: Hypotheses based on normal and epileptic rodents. Brain Res Rev. 2005;48:43–56. doi: 10.1016/j.brainresrev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Shapiro LA, Ribak CE. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69:53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Pierce JP, Melton J, Punsoni M, et al. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196:316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCloskey DP, Hintz TM, Pierce JP, et al. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24:2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- 67.Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000;20:6095–6105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung KH, Chu K, Kim M, et al. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004;19:3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- 69.Rao MS, Hattiangady B, Reddy DS, et al. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006;83:1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- 70.Jakubs K, Nanobashvili A, Bonde S, et al. Environment matters: Synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Mathern GW, Leiphart JL, De Vera A, et al. Seizures decrease postnatal neurogenesis and granule cell development in the human fascia dentata. Epilepsia. 2002;43(suppl 5):68–73. doi: 10.1046/j.1528-1157.43.s.5.28.x. [DOI] [PubMed] [Google Scholar]
- 72.Pirttila TJ, Lukasiuk K, Hakansson K, et al. Cystatin C modulates neurodegeneration and neurogenesis following status epilepticus in mouse. Neurobiol Dis. 2005;20:241–253. doi: 10.1016/j.nbd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Pirttila TJ, Manninen A, Jutila L, et al. Cystatin C expression is associated with granule cell dispersion in epilepsy. Ann Neurol. 2005;58:211–223. doi: 10.1002/ana.20545. [DOI] [PubMed] [Google Scholar]
- 74.Mikkonen M, Soininen H, Kalvianen R, et al. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44:923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- 75.Fahrner A, Kann G, Flubacher A, et al. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol. 2007;203:320–332. doi: 10.1016/j.expneurol.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 76.Deisseroth K, Singla S, Toda H, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 77.Ekdahl CT, Claasen JH, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 79.Hattiangady B, Rao MS, Shetty GA, et al. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 80.Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- 81.Crespel A, Rigau V, Coubes P, et al. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis. 2005;19:436–450. doi: 10.1016/j.nbd.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 82.Liu S, Wang J, Zhu D, et al. Generation of functional inhibitory neurons in the adult rat hippocampus. J Neurosci. 2003;23:732–736. doi: 10.1523/JNEUROSCI.23-03-00732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashimoto Y, Miyakawa H, Kudo Y, et al. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) increases GABAA receptor-mediated spontaneous postsynaptic currents in the dentate granule cells of rat hippocampal slices. Neurosci Lett. 2004;358:33–36. doi: 10.1016/j.neulet.2003.12.083. [DOI] [PubMed] [Google Scholar]
- 84.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci. 2000;20:8788–8801. doi: 10.1523/JNEUROSCI.20-23-08788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shetty AK, Turner DA. Glutamic acid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons. Exp Neurol. 2001;169:276–297. doi: 10.1006/exnr.2001.7668. [DOI] [PubMed] [Google Scholar]
- 87.Shetty AK, Turner DA. Intracerebroventricular kainic acid administration in adult rat alters hippocampal calbindin and non-phosphorylated neurofilament expression. J Comp Neurol. 1995;363:581–599. doi: 10.1002/cne.903630406. [DOI] [PubMed] [Google Scholar]
- 88.Kempermann G. They are not too excited: The possible role of adult-born neurons in epilepsy. Neuron. 2006;52:935–937. doi: 10.1016/j.neuron.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 90.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 91.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 92.Shors TJ, Townsend DA, Zhao M, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 95.Kee N, Teixeira CM, Wang AH, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 96.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 97.Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 2007;6:205–218. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- 98.Alessio A, Damasceno BP, Camargo CH, et al. Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav. 2004;5:22–27. doi: 10.1016/j.yebeh.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 99.Brown-Croyts LM, Caton PW, Radecki DT, et al. Phenobarbital pre-treatment prevents kainic acid-induced impairments in acquisition learning. Life Sci. 2000;67:643–650. doi: 10.1016/s0024-3205(00)00658-5. [DOI] [PubMed] [Google Scholar]
- 100.Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- 101.Parent JM. The role of seizure-induced neurogenesis in epileptogenesis and brain repair. Epilepsy Res. 2002;50:179–189. doi: 10.1016/s0920-1211(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 102.Scharfman HE, Sollas AL, Smith KL, et al. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J Comp Neurol. 2002;454:424–439. doi: 10.1002/cne.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hattiangady B, Rao MS, Zaman V, et al. Incorporation of embryonic CA3 cell grafts into the adult hippocampus at 4-months after injury: Effects of combined neurotrophic supplementation and caspase inhibition. Neuroscience. 2006;139:1369–1383. doi: 10.1016/j.neuroscience.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 104.Shetty AK, Turner DA. Development of fetal hippocampal grafts in intact and lesioned hippocampus. Prog Neurobiol. 1996;50:597–653. doi: 10.1016/s0301-0082(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 105.Shetty AK, Turner DA. Development of long-distance efferent projections from fetal hippocampal grafts depends upon pathway specificity and graft location in kainate-lesioned adult hippocampus. Neuroscience. 1997;76:1205–1219. doi: 10.1016/s0306-4522(96)00413-7. [DOI] [PubMed] [Google Scholar]
- 106.Shetty AK, Turner DA. Fetal hippocampal cells grafted to kainate-lesioned CA3 region of adult hippocampus suppress aberrant supragranular sprouting of host mossy fibers. Exp Neurol. 1997;143:231–245. doi: 10.1006/exnr.1996.6363. [DOI] [PubMed] [Google Scholar]
- 107.Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shetty AK, Zaman V, Turner DA. Pattern of long-distance projections from fetal hippocampal field CA3 and CA1 cell grafts in lesioned CA3 of adult hippocampus follows intrinsic character of respective donor cells. Neuroscience. 2000;99:243–255. doi: 10.1016/s0306-4522(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 109.Turner DA, Shetty AK. Clinical prospects for neural grafting therapy for hippocampal lesions and epilepsy. Neurosurgery. 2003;52:632–644. doi: 10.1227/01.neu.0000047825.91205.e6. [DOI] [PubMed] [Google Scholar]
- 110.Borlongan CV, Yu G, Matsukawa N, et al. Cell transplantation: Stem cells in the spotlight. Cell Transplant. 2005;14:519–526. [PubMed] [Google Scholar]
- 111.Lee JP, Jeyakumar M, Gonzalez R, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 112.Singec I, Jandial R, Crain A, et al. The leading edge of stem cell therapeutics. Annu Rev Med. 2007;58:313–328. doi: 10.1146/annurev.med.58.070605.115252. [DOI] [PubMed] [Google Scholar]
- 113.Yasuhara T, Matsukawa N, Hara K, et al. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci. 2006;26:12497–12511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yasuhara T, Matsukawa N, Yu G, et al. Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxicischemic injury. Cell Transplant. 2006;15:231–238. doi: 10.3727/000000006783982034. [DOI] [PubMed] [Google Scholar]
- 115.Yurek DM, Fletcher-Turner A. Comparison of embryonic stem cell-derived dopamine neuron grafts and fetal ventral mesencephalic tissue grafts: Morphology and function. Cell Transplant. 2004;13:295–206. doi: 10.3727/000000004783983954. [DOI] [PubMed] [Google Scholar]
- 116.Goldman JE, Zerlin M, Newman S, et al. Fate determination and migration of progenitors in the postnatal mammalian CNS. Dev Neurosci. 1997;19:42–48. doi: 10.1159/000111184. [DOI] [PubMed] [Google Scholar]
- 117.McKay R. Stem cells and the cellular organization of the brain. J Neurosci Res. 2000;59:298–300. doi: 10.1002/(sici)1097-4547(20000201)59:3<298::aid-jnr2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 118.Palmer TD, Markakis EA, Willhoite AR, et al. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 120.Shetty AK. Progenitor cells from the CA3 region of the embryonic day 19 rat hippocampus generate region-specific neuronal phenotypes in vitro. Hippocampus. 2004;14:595–614. doi: 10.1002/hipo.10206. [DOI] [PubMed] [Google Scholar]
- 121.Shetty AK, Turner DA. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: Inducing effects of brain-derived neurotrophic factor. J Neurobiol. 1998;35:395–425. doi: 10.1002/(sici)1097-4695(19980615)35:4<395::aid-neu7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 122.Shetty AK, Turner DA. Neurite outgrowth from progeny of epidermal growth factor-responsive hippocampal stem cells is significantly less robust than from fetal hippocampal cells following grafting onto organotypic hippocampal slice cultures: Effect of brain-derived neurotrophic factor. J Neurobiol. 1999;38:391–413. doi: 10.1002/(sici)1097-4695(19990215)38:3<391::aid-neu8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 123.Ciccolini F, Svendsen CN. Fibroblast growth factor 2 (FGF-2) promotes acquisition of epidermal growth factor (EGF) responsiveness in mouse striatal precursor cells: Identification of neural precursors responding to both EGF and FGF-2. J Neurosci. 1998;18:7869–7880. doi: 10.1523/JNEUROSCI.18-19-07869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 125.Cao QL, Howard RM, Dennison JB, et al. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- 126.Ginis I, Rao MS. Toward cell replacement therapy: Promises and caveats. Exp Neurol. 2003;184:61–77. doi: 10.1016/s0014-4886(03)00256-5. [DOI] [PubMed] [Google Scholar]
- 127.Park KI, Lachyankar M, Nissim S, et al. Neural stem cells for CNS repair: State of the art and future directions. Adv Exp Med Biol. 2002;506:1291–1296. doi: 10.1007/978-1-4615-0717-8_188. [DOI] [PubMed] [Google Scholar]
- 128.Peterson DA. Stem cells in brain plasticity and repair. Curr Opin Pharmacol. 2002;2:34–42. doi: 10.1016/s1471-4892(01)00118-7. [DOI] [PubMed] [Google Scholar]
- 129.Alvarez-Dolado M, Calcagnotto ME, Karkar KM, et al. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006;26:7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006;129:18–35. doi: 10.1093/brain/awh682. [DOI] [PubMed] [Google Scholar]
- 131.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 132.Savatier P, Lapillonne H, van Grunsven LA, et al. Withdrawal of differentiation inhibitory activity/leukemia inhibitory factor up-regulates D-type cyclins and cyclin-dependent kinase inhibitors in mouse embryonic stem cells. Oncogene. 1996;12:309–322. [PubMed] [Google Scholar]
- 133.Suda Y, Suzuki M, Ikawa Y, et al. Mouse embryonic stem cells exhibit indefinite proliferative potential. J Cell Physiol. 1987;133:197–201. doi: 10.1002/jcp.1041330127. [DOI] [PubMed] [Google Scholar]
- 134.Draper JS, Moore HD, Ruban LN, et al. Culture and characterization of human embryonic stem cells. Stem Cells Dev. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- 135.Chung S, Shin BS, Hwang M, et al. Neural precursors derived from embryonic stem cells, but not those from fetal ventral mesencephalon, maintain the potential to differentiate into dopaminergic neurons after expansion in vitro. Stem Cells. 2006;24:1583–1593. doi: 10.1634/stemcells.2005-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chung S, Sonntag KC, Andersson T, et al. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci. 2002;16:1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morizane A, Takahashi J, Shinoyama M, et al. Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J Neurosci Res. 2006;83:1015–1027. doi: 10.1002/jnr.20799. [DOI] [PubMed] [Google Scholar]
- 139.Singh Roy N, Nakano T, Xuing L, et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 140.Wichterle H, Lieberam I, Porter JA, et al. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 141.Glaser T, Perez-Bouza A, Klein K, et al. Generation of purified oligodendrocyte progenitors from embryonic stem cells. FASEB J. 2005;19:112–114. doi: 10.1096/fj.04-1931fje. [DOI] [PubMed] [Google Scholar]
- 142.Chu K, Kim M, Jung KH, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023:213–221. doi: 10.1016/j.brainres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 143.Ruschenschmidt C, Koch PG, Brustle O, et al. Functional properties of ES cell-derived neurons engrafted into the hippocampus of adult normal and chronically epileptic rats. Epilepsia. 2005;46(suppl 5):174–183. doi: 10.1111/j.1528-1167.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- 144.Shetty AK, Hattiangady B. Survival and differentiation of stem/progenitor cells from the postnatal hippocampus following grafting into the intact or injured young adult and aged hippocampus.. Society for Neuroscience Abstracts; Atlanta. October 16, 2006. [Google Scholar]
- 145.Acharya MM, Rai KS, Hattiangady B, et al. Potential of hippocampal stem/progenitor cell grafts for restraining spontaneous motor seizures during chronic temporal lobe epilepsy.. Society for Neuroscience Abstracts; San Diego. November 3–7, 2007. [Google Scholar]
- 146.Gong C, Wang TW, Huang HS, et al. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mathern GW, Babb TL, Leite JP. The pathogenic and progressive features of chronic human hippocampal epilepsy. Epilepsy Res. 1996;26:151–161. doi: 10.1016/s0920-1211(96)00052-6. et a. [DOI] [PubMed] [Google Scholar]
- 148.Bender RA, Dube C, Baram TZ. Febrile seizures and mechanisms of epileptogenesis: Insights from an animal model. Adv Exp Med Biol. 2004;548:213–225. doi: 10.1007/978-1-4757-6376-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lewis DV. Losing neurons: Selective vulnerability and mesial temporal sclerosis. Epilepsia. 2005;46(suppl 7):39–44. doi: 10.1111/j.1528-1167.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 150.Noe’ F, Nissinen J, Pitkanen A, et al. Gene therapy in epilepsy: The focus on NPY. Peptides. 2007;28:377–383. doi: 10.1016/j.peptides.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 151.Kanter-Schlifke I, Georgievska B, Kirik D, et al. Seizure suppression by GDNF gene therapy in animal models of epilepsy. Mol Ther. 2007;15:1106–1113. doi: 10.1038/sj.mt.6300148. [DOI] [PubMed] [Google Scholar]
- 152.Li T, Steinbeck JA, Lusardi T, et al. Suppression of kindling epilepto-genesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]