The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development (original) (raw)
The thymic medulla and an intact mTEC compartment are needed for the development of nTreg cells and negative selection of conventional T cells but not their further maturation.
Abstract
A key role of the thymic medulla is to negatively select autoreactive CD4+ and CD8+ thymocytes, a process important for T cell tolerance induction. However, the involvement of the thymic medulla in other aspects of αβ T cell development, including the generation of Foxp3+ natural regulatory T cells (nTreg cells) and the continued maturation of positively selected conventional αβ T cells, is unclear. We show that newly generated conventional CD69+Qa2− CD4 single-positive thymocytes mature to the late CD69−Qa2+ stage in the absence of RelB-dependent medullary thymic epithelial cells (mTECs). Furthermore, an increasing ability to continue maturation extrathymically is observed within the CD69+CCR7−/loCCR9+ subset of conventional SP4 thymocytes, providing evidence for an independence from medullary support by the earliest stages after positive selection. In contrast, Foxp3+ nTreg cell development is medullary dependent, with mTECs fostering the generation of Foxp3−CD25+ nTreg cell precursors at the CD69+CCR7+CCR9− stage. Our results demonstrate a differential requirement for the thymic medulla in relation to CD4 conventional and Foxp3+ thymocyte lineages, in which an intact mTEC compartment is a prerequisite for Foxp3+ nTreg cell development through the generation of Foxp3−CD25+ nTreg cell precursors.
In the thymus, positive selection of CD4+8+ thymocytes recognizing self-peptide/MHC on cortical thymic epithelial cells (TECs) triggers the entry of CD4/CD8 single-positive (SP) T cells into the thymic medulla, a process essential for tolerance induction (Kurobe et al., 2006). Additionally, the medulla is also considered a key site of differentiation that supports thymocyte maturation after positive selection, including stages defined by loss of CD24/CD69 and acquisition of CD62L/Qa2 (McCaughtry et al., 2007; Li et al., 2007).
Although the medulla also contains SP4 Foxp3+ natural regulatory T cells (nTreg cells; Liston et al., 2008), its role in nTreg cell generation remains unclear, with both medullary TECs (mTECs) and DCs being implicated (Aschenbrenner et al., 2007; Proietto et al., 2008; Spence and Green, 2008; Wirnsberger et al., 2009; Hinterberger et al., 2010). Importantly, nTreg cell development is a multistage process, with TCR–MHC (Lio and Hsieh, 2008) and CD28–CD80/86 interactions (Lio et al., 2010; Vang et al., 2010; Hinterberger et al., 2011) driving the generation of Foxp3−CD25+ nTreg cell precursors that give rise to Foxp3+CD25+ nTreg cells (Lio and Hsieh, 2008). However, the role of mTECs during Foxp3−CD25+ nTreg cell precursor generation is unknown.
Here, we define steps in both conventional and nTreg SP4 thymocyte maturation, mapping their requirements for a RelB-dependent mTEC compartment (Burkly et al., 1995; Weih et al., 1995; Heino et al., 2000). We show that conventional SP4 thymocytes can complete their maturation in the absence of RelB-dependent mTECs, with evidence of thymic independence occurring by the CD69+CCR7−/loCCR9+ SP4 thymocyte stage. In contrast, Foxp3+ nTreg cells require an intact thymic medulla, with a requirement for RelB-dependent mTEC mapping to the generation of Foxp3−CD25+ nTreg cell precursors at the CD69+CCR7+CCR9− stage. Collectively, our data reveal the differential importance of the thymic medulla during SP4 thymocyte development and highlight a specific role for mTECs in Foxp3−CD25+ precursor generation.
RESULTS AND DISCUSSION
Emergence of conventional and nTreg cell precursors in CD4 thymocytes
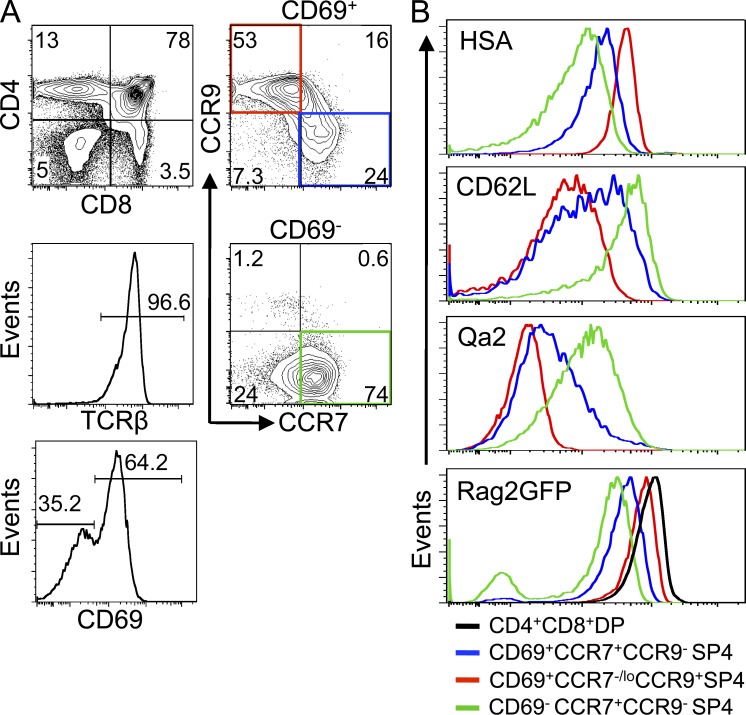
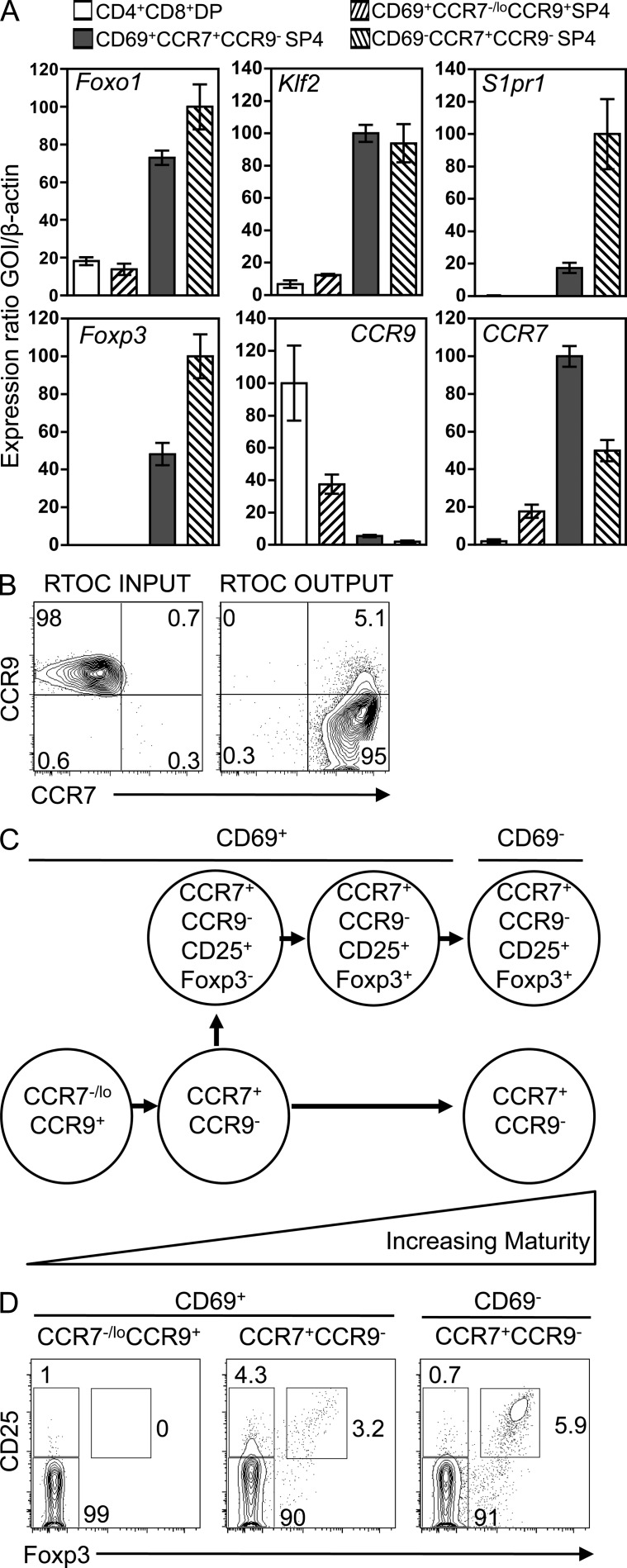
As positive selection involves changes in the chemokine receptors CCR7 and CCR9 (Choi et al., 2008), we used these and other (HSA/CD69/CD62L/Qa2) markers to define distinct subsets of SP4 thymocytes in Rag2GFP reporter mice, in which GFP levels are a measure of maturation status (Boursalian et al., 2004; McCaughtry et al., 2007). Fig. 1 A shows that CD69+αβ TCRhi SP4 thymocytes contain CCR7+CCR9− and CCR7−/loCCR9+ subsets, whereas more mature CD69− cells are CCR7+CCR9−. Analysis of CD62L/Qa2 showed the CD69+CCR7−/loCCR9+ subset to be CD62LloQa2lo and to express the highest levels of Rag2GFP among SP4 thymocytes, whereas CD69−CCR7+CCR9− cells were CD62LhiQa2hi with the lowest levels of Rag2GFP (Fig. 1 B). CD69+CCR7+CCR9− and CD69−CCR7+CCR9− but not CD69+CCR7−/loCCR9+ SP4 thymocytes (Fig. 2 A) expressed mRNA for Foxo1 and Klf2, known regulators of thymocyte egress (Carlson et al., 2006; Gubbels Bupp et al., 2009), whereas mRNA encoding S1pr1, another regulator of thymocyte emigration (Matloubian et al., 2004), was limited to the CD69−CCR7+CCR9− subset (Fig. 2 A). Importantly, when reaggregate thymus organ cultures (Rossi et al., 2007) were initiated with purified CD69+CCR7−/loCCR9+ SP4 thymocytes, their progeny had up-regulated CCR7 and down-regulated both CCR9 (Fig. 2 B) and CD69 (not depicted), providing evidence for a maturation sequence in which the CD69+CCR7−/loCCR9+ subset represents newly generated SP4 thymocytes after positive selection, followed by CD69+CCR7+CCR9− and then CD69−CCR7+CCR9− cells (Fig. 2 C).
Figure 1.
CCR7 and CCR9 define distinct subsets of SP4 thymocytes. (A) CD69+ and CD69− subsets of SP4 thymocytes from Rag2GFP mice analyzed for CCR7/CCR9 expression. Data are typical of four experiments. (B) Levels of HSA, CD62L, Qa2, and Rag2GFP in the following SP4 subsets: CD69+CCR7−/loCCR9+ (red), CD69+CCR7+CCR9− (blue), and CD69−CCR7+CCR9− (green). For comparison, Rag2GFP expression by CD69−CD4+8+ thymocytes is shown (black). Data are typical of three separate experiments.
Figure 2.
Developmental emergence of conventional and Foxp3+ Treg SP4 cells. (A) qPCR in indicated thymocyte subsets. Error bars indicate SEM. mRNA levels were normalized to β-actin. Data are from at least two independently sorted biological samples, with each gene analyzed two times. (B) Phenotype of purified CD69+CCR7−/loCCR9+ SP4 thymocytes before (left) and after (right) incorporation into reaggregate thymus organ cultures (RTOCs). Data are typical of four experiments. (C) Developmental sequence of SP4/nTreg thymocyte maturation. (D) CCR7/CCR9 subsets of adult WT αβ TCRhi SP4 thymocytes for CD69, CD25, and Foxp3 expression. Data represent at least three separate experiments.
In relation to nTreg cell emergence, Foxp3 mRNA was detectable in CD69+CCR7+CCR9− SP4 thymocytes, with higher levels noted in the most mature CD69−CCR7+CCR9− subset (Fig. 2 A), indicating that Foxp3+ nTreg cell development is first detectable within the CD69+ stage of SP4 thymocyte maturation but after induction of CCR7 and loss of CCR9. To further relate changes in CCR7/CCR9 to distinct stages in nTreg cell development, we analyzed CD25 and Foxp3 by flow cytometry. Consistent with quantitative PCR (qPCR) data, the earliest CD69+CCR7−/loCCR9+ cells were Foxp3−, and Foxp3−CD25+ nTreg cell precursors were barely detectable in this population (Fig. 2 D). In contrast, CD69+CCR7+CCR9− SP4 thymocytes contained Foxp3−CD25+ nTreg cell precursors as well as their more mature CD25+Foxp3+ progeny (Fig. 2 D), whereas the most mature CD69−CCR7+CCR9− SP4 thymocytes contained Foxp3+ nTreg cells but lacked Foxp3−CD25+ precursors (Fig. 2 D). Thus, Foxp3−CD25+ nTreg cell precursor appearance maps to the transition between CCR7−/loCCR9+ and CCR7+CCR9− stages in the CD69+ phase of SP4 thymocyte development (Fig. 2 C).
Conventional SP4 thymocyte development occurs independently of RelB-dependent mTECs
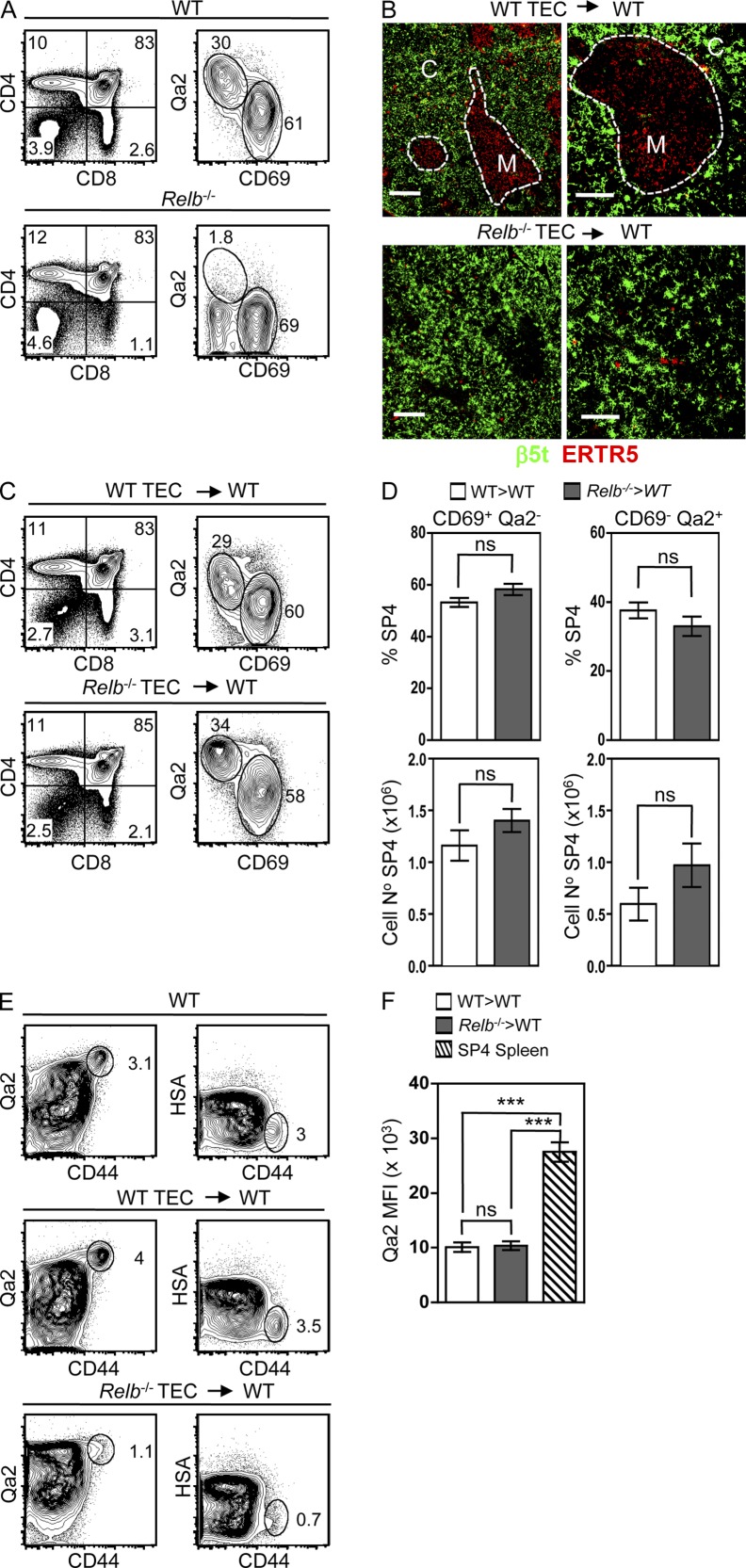
A recent study has suggested a correlation between the thymic medulla and SP4 thymocyte development, most notably the absence of CD69−Qa2+ SP4 thymocytes in Relb−/− mice displaying a severe block in mTEC development (Fig. 3 A; Li et al., 2007). However, detailed analysis of the role of mTECs in Relb−/− mice is confounded by their complex phenotype that includes DC deficiencies and multiorgan autoimmunity (Weih et al., 1995; Wu et al., 1998). We therefore investigated the mTEC requirements of both conventional and nTreg SP4 thymocytes by grafting alymphoid 2-deoxyguanosine (dGuo)–treated fetal thymus organ cultures (Rossi et al., 2007), from either Relb−/− or WT embryos, into unmanipulated WT mice. Importantly, any absence of Qa2+ cells in our experiments is not caused by the genetic background of the Relb−/− mice used here, as Relb−/− fetal liver chimaeras with WT hosts generated Qa2+ SP4 thymocytes (not depicted). Confocal analysis of WT and Relb−/− TEC grafts confirmed an mTEC defect in the latter (Fig. 3 B), consistent with a cell-intrinsic role for RelB in mTEC development. Importantly, although grafting of Relb−/− TECs into nude hosts resulted in autoimmunity (Fig. 4, A and B), WT hosts grafted with Relb−/− TECs showed no signs of disease (not depicted), presumably as a result of peripheral tolerance mechanisms involving Treg cells generated in the host WT thymus. Thus, grafting of unmanipulated WT hosts with thymuses harboring a cell-intrinsic RelB-dependent mTEC deficiency provides a model to study the role of mTECs in SP4 thymocyte development in the absence of autoimmunity.
Figure 3.
RelB-dependent mTECs are dispensable for conventional SP4 thymocyte development. (A) Qa2/CD69 expression in WT (top) and Relb−/− (bottom) thymocytes after gating on αβ TCRhi SP4 thymocytes. Data are representative of three experimental replicates. (B) Immunofluorescent staining of WT and Relb−/− graft sections for ERTR5 and β5t. C denotes cortex, and M denotes medulla. Bars: (left) 200 µm; (right) 100 µm. (C) Thymocytes from WT (top) and Relb−/− (bottom) thymus grafts, with Qa2/CD69 levels shown for αβ TCRhi SP4 cells. (D) Frequencies of Qa2/CD69 SP4 thymocytes subsets recovered from WT and Relb−/− grafts. (E) Qa2/HSA/CD44 expression in SP4 thymocytes recovered from WT thymus (top), WT TEC grafts (middle), and Relb−/− TEC grafts (bottom). (F) Mean fluorescence intensity (MFI) of Qa2 expression in CD69− SP4 T cells from WT spleen or CD69− SP4 thymocytes from WT or Relb−/− TEC grafts. Error bars represent SEM; data in C–F are from at least three independent experiments, with a minimum of five of each graft type per experiment. In an unpaired Student’s two-tailed t test, ns denotes a nonsignificant difference where P > 0.1; ***, P < 0.001.
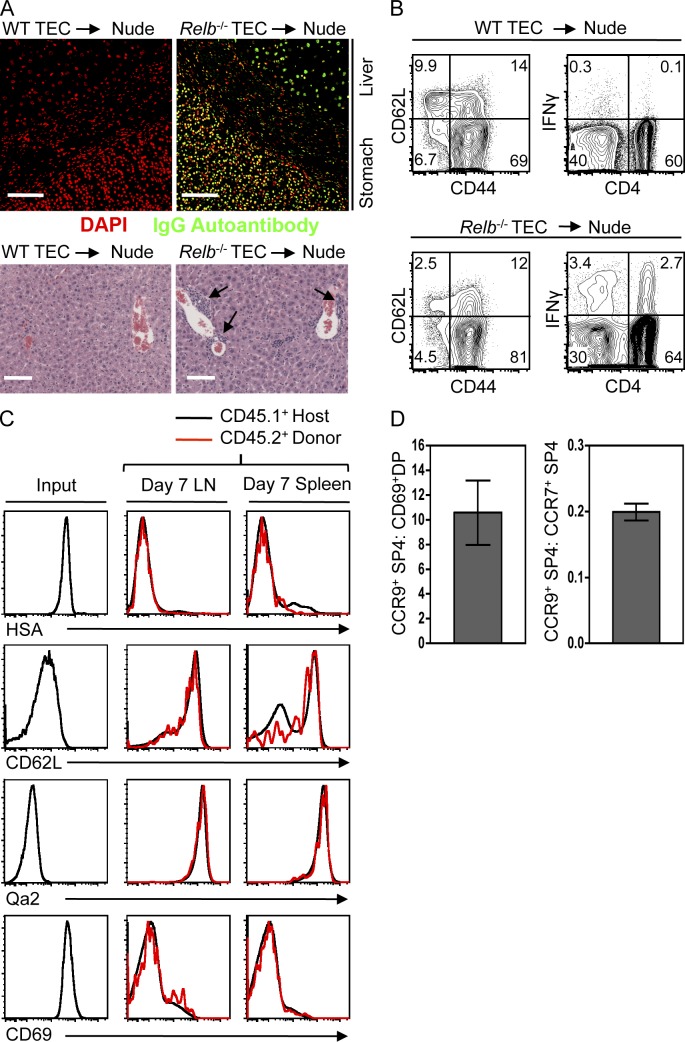
Figure 4.
Extrathymic development of CCR7−/loCCR9+CD69+ SP4 thymocytes. (A) Autoantibodies in serum from nude mice receiving WT or Relb−/− TEC grafts (top) and histological analysis (bottom) of lymphocytic infiltrates (arrows) in liver of the same mice. Bars, 100 µm. (B) CD44/CD62L expression in SP4 LN T cells from nude mice receiving either WT or Relb−/− TEC grafts. Right panels show intracellular IFN-γ in CD3+ LN T cells of the same mice. Data in A and B are typical of three experimental replicates. (C) HSA/CD62L/Qa2/CD69 expression in CD69+CCR7−/loCCR9+ adult αβ TCRhi SP4 thymocytes before transfer (input) and their SP4 T cell progeny (CD45.2+ cells) recovered from both spleen and LN after 7 d (red lines). Black lines show host CD45.1+ SP4 LN and splenic T cells for comparison. (D) Ratio of recovered SP4 progeny in spleen 7 d after coinjection of equal numbers of either CD69+CCR7−/loCCR9+ SP4 thymocytes and CD4+8+69+ thymocytes (left) or CD69+CCR7−/loCCR9+ SP4 thymocytes and CD69−CCR7+ SP4 thymocytes (right). Error bars represent SEM, and data in C and D are typical of four separate experiments.
Analysis of SP4 thymocytes in Relb−/− TEC grafts within WT hosts revealed both early CD69+Qa2− and late CD69−Qa2+ subsets at proportions and numbers comparable with WT grafts (Fig. 3, C and D). Importantly, SP4 thymocytes in WT and Relb−/− grafts were predominantly CD44loHSAint (Fig. 3 E) and expressed lower levels of Qa2 compared with peripheral CD4 T cells (Fig. 3 F), indicating that they were not peripheral T cells circulating back to the thymus (McCaughtry et al., 2007; Hale and Fink, 2009). Our findings do not support previous suggestions from experiments involving in vitro TEC lines (Li et al., 2007), that mTECs provide essential support for SP4 thymocyte maturation. The reason for this difference is unknown, although it may relate to whether in vitro systems reflect the functional capacities of mTECs in vivo. Instead, our data show that conventional SP4 thymocyte development in vivo occurs in the absence of RelB-dependent mTECs. Interestingly, absence of Qa2+CD69− SP4 thymocytes was also reported in Aire−/− mice (Li et al., 2007). Given that Aire−/− mice show an increased mTEC compartment (Anderson et al., 2002), the impact made by Aire on thymocyte development is unclear.
We next analyzed the requirements for thymic support after positive selection by investigating the ability of CD69+CCR7−/loCCR9+ SP4 thymocytes to mature extrathymically. Although recent thymic emigrants undergo maturation outside the thymus (Boursalian et al., 2004), it is unclear how far back in development this window of thymic independence extends, particularly in relation to the post–positive selection stages described here. Initially, CD69+CCR7−/loCCR9+ SP4 thymocytes from adult CD45.2+ WT mice, with an HSAhiCD62LloQa2−CD69+ phenotype (Fig. 4 C), were i.v. injected into congenic CD45.1+ WT mice. Analysis after 7 (Fig. 4 C) and 14 d (not depicted) showed that injected cells had acquired an HSAloCD62LhiQa2hiCD69lo phenotype comparable with host naive CD4+ T cells. To provide a comparative analysis of extrathymic maturation, CD69+CCR7−/loCCR9+ thymocytes from CD45.2+ mice were coinjected into CD45.1+CD45.2+ hosts at a 1:1 ratio with either less mature CD45.1+CD4+8+69+ or more mature CD45.1+CD69−CCR7+CCR9− SP4 thymocytes. Analysis of spleen 7 d later revealed a ratio of 10:1 after coinjection of CD69+CCR7−/loCCR9+ SP4 and less mature CD4+8+69+ thymocytes and a 0.2:1 ratio after coinjection of CD69+CCR7−/loCCR9+ SP4 and more mature CD69−CCR7+CCR9− SP4 thymocytes (Fig. 4 D). Thus, CD69+CCR7−/loCCR9+ SP4 thymocytes show an emerging capacity for thymic independence, strengthening the notion that conventional SP4 thymocyte development can occur in the absence of thymic medullary support from the earliest SP4 thymocyte stages. Although mice receiving CD69+CCR7−/loCCR9+ SP4 thymocytes showed no signs of autoimmunity (not depicted), it is unclear whether this reflects the timing of negative selection in relation to this subset or the control of autoreactivity by Treg cells generated in the WT host thymus.
RelB-dependent mTECs control Foxp3−CD25+ nTreg cell precursor generation
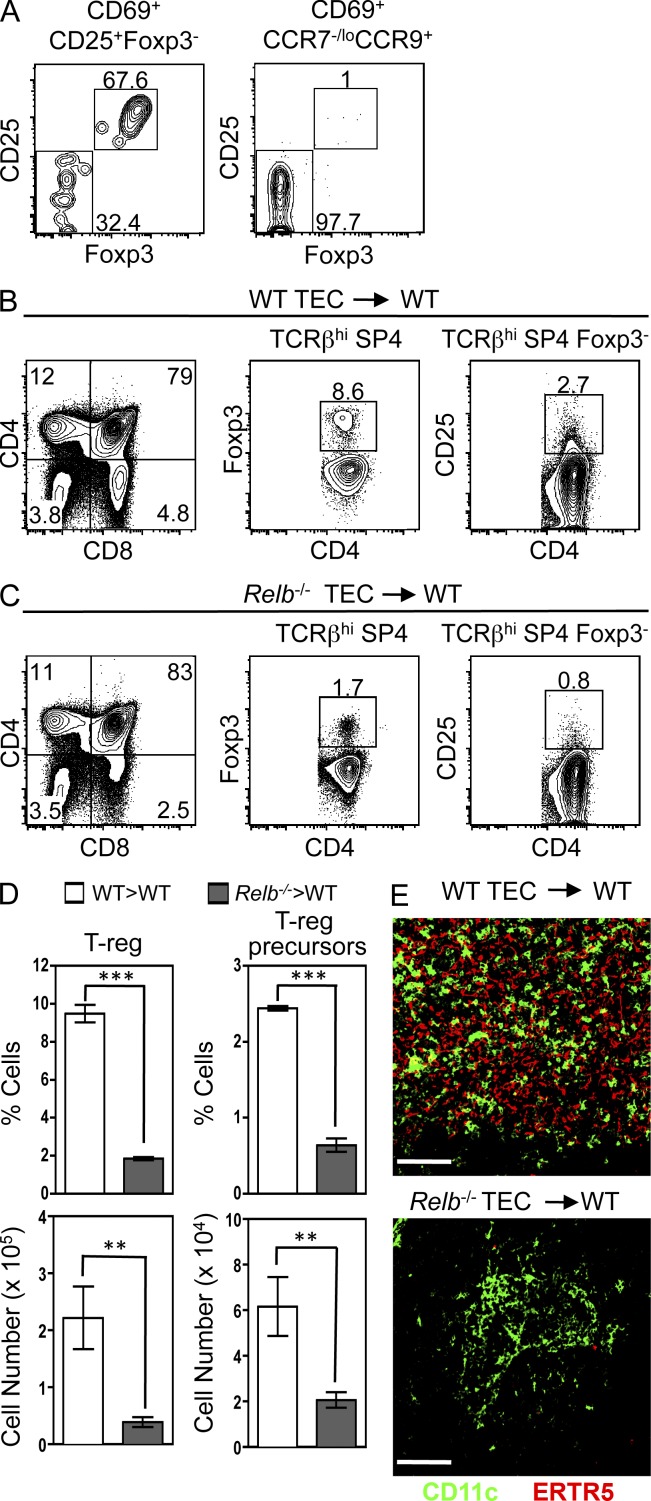
We next examined the role of RelB-dependent mTECs in the development of Foxp3+ nTreg cells and Foxp3−CD25+ precursors. CD69+Foxp3−CD25+ and CD69+CCR7−/loCCR9+ subsets of SP4 thymocytes, the former reflecting nTreg cell precursors (Lio and Hsieh, 2008) and the latter capable of extrathymic generation of conventional CD4 T cells (Fig. 4), were purified from CD45.2+ Foxp3GFP reporter mice and transferred i.v. into WT CD45.1+ hosts. CD69+Foxp3−CD25+ nTreg cell precursors, shown here to be at a CD69+CCR7+CCR9− intermediate stage (Fig. 2 D), generated Foxp3+CD25+ nTreg cells (Fig. 5 A). In contrast to their capacity to generate conventional CD4 T cells extrathymically (Fig. 4), CD69+CCR7−/loCCR9+ SP4 thymocytes failed to give rise to Foxp3+CD25+ nTreg cells outside the thymus (Fig. 5 A). Thus, the CD69+CCR7−/loCCR9+ stage after positive selection marks a point where continued maturation of conventional but not Foxp3+ regulatory SP4 thymocytes can occur independently of thymic support.
Figure 5.
RelB-dependent mTECs control Foxp3−CD25+ nTreg cell precursor generation. (A) Expression of Foxp3GFP/CD25 in the LN progeny of i.v. injected CD69+Foxp3−CD25+ (left) and CD69+CCR7−/loCCR9+ (right) T cells after gating on CD45.2+ injected cells. Data are typical of two experimental replicates. (B and C) WT (B) and Relb−/− (C) thymus grafts analyzed for Foxp3−CD25+ nTreg cell precursors and Foxp3+ nTreg cells within the SP4 subset. (D) Quantitation of these populations; error bars represent SEM. An unpaired Student’s two-tailed t test was performed: ***, P < 0.001; **, P < 0.01. Data in B–D represent four separate experiments. (E) Confocal analysis of WT and Relb−/− grafts. Bars, 100 µm.
Importantly, and unlike conventional SP4 thymocyte development, Relb−/− TEC grafts showed a significant reduction in Foxp3+ SP4 thymocytes compared with WT grafts (Fig. 5, B–D). Furthermore, nTreg cell precursors were also significantly reduced in mTEC-deficient Relb−/− TEC grafts (Fig. 5, B–D). Although the few nTreg cells in Relb−/− grafts do not rule out inefficient generation via residual RelB-independent mTECs, the large majority of nTreg cell development appears to be controlled by RelB-dependent mTECs. Moreover, although the presence of host-derived CD11c+ DCs in both WT and Relb−/− grafts (Fig. 5 E) argues against the defect in nTreg cell production being solely caused by the absence of DCs, we cannot exclude impaired DC function in the absence of RelB-dependent mTECs.
In conclusion, we show that conventional and Foxp3+ nTreg CD4 T cells demonstrate a differential requirement for RelB-dependent medullary thymic microenvironments during their development. In particular, mTECs support the generation of Foxp3−CD25+ nTreg cell precursors and their Foxp3+CD25+ nTreg cell progeny from within the pool of medullary CCR7+CCR9− SP4 thymocytes. The dependency of Foxp3−CD25+ nTreg cell precursor generation on both CD28–CD80/CD86 and TCR–MHC interactions (Lio and Hsieh, 2008; Lio et al., 2010; Vang et al., 2010; Hinterberger et al., 2011) fits well with the expression of these co-stimulatory molecules and MHC class I/II by mTECs (Rossi et al., 2007) and suggests that provision of these molecules by mTECs is linked to their ability to support nTreg cell generation as shown here. That mTECs provide TCR ligands for nTreg cell development fits well with the generation of antigen-specific TCR transgenic Treg cells after the targeting of model antigens to mTECs (Aschenbrenner et al., 2007; Hinterberger et al., 2010) and the normal numbers of nTreg cells generated when hematopoietic cells are selectively MHC class II deficient (Aschenbrenner et al., 2007; Liston et al., 2008). In contrast, the ability of conventional SP4 thymocytes to continue their maturation, either in the absence of RelB-dependent mTECs or extrathymically, reveals differences in the maturational requirements for nTreg cells and conventional T cells and warrants a rethinking of the role of the medulla in T cell development. Thus, rather than representing a microenvironment fostering late-stage αβ T cell development per se, the primary role of the medulla is in the generation of self-tolerance via negative selection and the generation of Foxp3+ CD4 nTreg cells, a scenario compatible with the T cell–mediated autoimmunity in Ccr7−/− mice that occurs after inefficient thymocyte access to the medulla (Kurobe et al., 2006).
MATERIALS AND METHODS
Mice.
WT CD45.2+ C57BL/6, congenic CD45.1+ C57BL/6 (BoyJ), CD45.1+CD45.2+ C57BL/6, nude C57BL/6, C57BL/6 Relb−/− (Weih et al., 1995) mice, FVB/N RAG2GFP (Yu et al., 1999), and C57BL/6 Foxp3GFP reporter mice (gift from T. Strom, Beth Israel Deaconess Medical Center, Boston, MA; Bettelli et al., 2006) were bred at the University of Birmingham in accordance with Home Office regulations. For timed matings, the day of vaginal plug detection was designated as day 0. All animal experiments were performed in accordance with University of Birmingham (Local Ethical Review Panel) and national UK Home Office regulations.
Antibodies, flow cytometry, and cell sorting.
Thymocyte, splenocyte, and LN suspensions were stained with the following antibodies: PECy7/Alexa Fluor 700/PE anti-CD4 (clone GK1.5; eBioscience) or PerCP-Cy5.5/APC eFluor 780/V500 anti-CD4 (clone RM4-5; eBioscience/BD), eFluor 450/FITC/V500 anti-CD8 (clone 53-6.7; eBioscience/BD) or biotinylated anti-CD8 clone (YTS156.7.7; BioLegend), APC eFluor 780 anti-TCRβ (clone H57-597; eBioscience), FITC/APC/PerCP-Cy5.5 anti-CD69 (clone H1.2F3; eBioscience), APC anti-CD62L (clone MEL-14; BioLegend), FITC/Alexa Fluor 700 anti-CD44 (clone IM7; eBioscience), PE anti-CD3ε (clone 145-2C11; eBioscience), APC eFluor 780/PE anti-HSA/CD24 (clone M1/69; BD/eBioscience), biotinylated/FITC anti-Qa2 (clone 695H1-9.9; BioLegend/eBioscience), eFluor 780/eFluor 450 anti-CD45.1 (clone A20; eBioscience), PE/Alexa Fluor 700 anti-CD45.2 (clone 104; eBioscience), PE anti-CCR9 (clone eBio CW-1.2; eBioscience), APC/PE anti-CD25 (clone PC61/PC61.5; BioLegend/eBioscience), and APC anti–IFN-γ (clone XMG1.2; BD). For surface CCR7 expression, thymocytes were incubated in recombinant CCL19-Ig (eBioscience), followed by biotinylated goat anti–human Ig (eBioscience). All biotinylated antibodies were picked up with PECy7-conjugated streptavidin (eBioscience). For intracellular staining of Foxp3, cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s protocol and stained with PE anti-Foxp3 (clone FJK-16s; eBioscience). Flow cytometry was performed on a Fortessa analyzer using FACSDiva6.2 software (BD), with data subsequently analyzed with FlowJo 8.7 software (Tree Star). Purified populations of thymocytes were sorted from adult thymus suspensions using a MoFlo XDP cell sorter (Beckman Coulter).
Fetal thymus organ culture and thymus grafting.
Embryonic day (E) 15 thymus lobes, cultured for 7 d in 1.35 mM dGuo were transplanted under the kidney capsule of 8-wk-old recipient mice as described previously (Rossi et al., 2007), with grafts in WT mice and nude mice harvested after 8–12 wk and 6 wk, respectively.
i.v. cell transfer.
CD69+CCR7−/loCCR9+Foxp3− and CD69+Foxp3−CD25+ subsets of SP4 αβ TCRhi thymocytes were MoFlo sorted from CD45.2+ FoxP3GFP adult reporter mice and transferred i.v. in a 300-µl volume of PBS into CD45.1+ BoyJ mice. LN and spleen were harvested after 7 d and analyzed by flow cytometry for CD45.1/CD45.2 together with maturation markers to study the persistence of donor-derived cells. For coinjection experiments, purified CD69+CCR7−/loCCR9+ SP4 from CD45.2+ C57BL/6 mice and either CD4+8+69+ or CD69−CCR7+CCR9− SP4 from CD45.1+ BoyJ mice were mixed at a 1:1 ratio and injected i.v. into CD45.1+CD45.2+ hosts as described above. After 7 d, injected cells were identified in LN and spleen preparations on the basis of either a CD45.1+CD45.2− or CD45.1−CD45.2+ phenotype by flow cytometry and expressed as a ratio.
Immunohistochemistry.
Thymus grafts were recovered from host mice, and 7-µm sections were cut, then fixed in acetone, and stained with the following antibodies: the mTEC marker ERTR5 (gift of W. van Ewijk, Riken Yokohama Institute, Yokohama City, Kanagawa, 230-0045), detected with Alexa Fluor 594 goat anti–rat IgM (Invitrogen), the cortical TEC marker rabbit anti-β5t (MBL), detected with donkey anti–rabbit 488 (Invitrogen), FITC-conjugated anti-CD11c (clone N418; eBioscience) followed by rabbit anti-FITC (Invitrogen), and donkey anti–rabbit 488 (Invitrogen). Images were obtained using an LSM 780 microscope and analyzed using LSM software (Carl Zeiss).
Analysis of autoimmunity.
C57BL/6 nude mice, grafted with either WT or Relb−/− dGuo-treated thymus lobes, were sacrificed 6 wk after transplantation, and organs and sera were collected. LN cells from the indicated grafted mice were analyzed for intracellular IFN-γ cytokine production and CD44/CD62L expression as described previously (Gaspal et al., 2011). To detect autoantibodies, sera (1:40 dilution) was added to sections of composite tissue blocks containing the indicated tissues and detected with goat anti–mouse IgG FITC (SouthernBiotech), counterstained with DAPI. Lymphocytic infiltrates were detected in paraffin wax–embedded sections of liver as described previously (Rossi et al., 2007). Images were acquired with a microscope (DM6000; Leica) using a 20× objective.
qPCR.
qPCR analysis of freshly sorted thymocyte populations was performed exactly as described previously (Roberts et al., 2012). Primer used are as follows: β-actin QuantiTect Mm Actb 1SG Primer Assay (QIAGEN QT00095242); Foxo1 forward, 5′-TGTCAGGCTAAGAGTTAGTGAGCA-3′; and reverse, 5′-GGGTGAAGGGCATCTTTG-3′; Klf2 forward, 5′-CTCAGCGAGCCTATCTTGCC-3′; and reverse, 5′-CACGTTGTTTAGGTCCTCATCC-3′; S1pr1 forward, 5′-AAATGCCCCAACGGAGACT-3′; and reverse, 5′-CTGATTTGCTGCGGCTAAATTC-3′; Foxp3 forward, 5′-CCCAGGAAAGACAGCAACCTT-3′; and reverse, 5′-TTCTCACAACCAGGCCACTTG-3′; Ccr9 forward, 5′-ACCATGATGCCCACAGAACT-3′; and reverse, 5′-GGGAAGAGTGGCAAGAAAGA-3′; and Ccr7 forward, 5′-CTAGCTGGAGAGAGACAAGA-3′; and reverse, 5′-TATCCGTCATGGTCTTGAGC-3′.
Acknowledgments
We thank Dr. Terry Strom for Foxp3GFP mice, provided by Dr. Nick Jones.
This study was supported by a Medical Research Council (MRC) Programme Grant. J.E. Cowan is supported by a PhD Studentship from the MRC Centre for Immune Regulation.
The authors have no competing financial interests.
Footnotes
Abbreviations used:
dGuo
2-deoxyguanosine
mTEC
medullary TEC
nTreg cell
natural regulatory T cell
qPCR
quantitative PCR
TEC
thymic epithelial cell
References
- Anderson M.S., Venanzi E.S., Klein L., Chen Z., Berzins S.P., Turley S.J., von Boehmer H., Bronson R., Dierich A., Benoist C., Mathis D. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401 10.1126/science.1075958 [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K., D’Cruz L.M., Vollmann E.H., Hinterberger M., Emmerich J., Swee L.K., Rolink A., Klein L. 2007. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8:351–358 10.1038/ni1444 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Boursalian T.E., Golob J., Soper D.M., Cooper C.J., Fink P.J. 2004. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5:418–425 10.1038/ni1049 [DOI] [PubMed] [Google Scholar]
- Burkly L., Hession C., Ogata L., Reilly C., Marconi L.A., Olson D., Tizard R., Cate R., Lo D. 1995. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 373:531–536 10.1038/373531a0 [DOI] [PubMed] [Google Scholar]
- Carlson C.M., Endrizzi B.T., Wu J., Ding X., Weinreich M.A., Walsh E.R., Wani M.A., Lingrel J.B., Hogquist K.A., Jameson S.C. 2006. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 442:299–302 10.1038/nature04882 [DOI] [PubMed] [Google Scholar]
- Choi Y.I., Duke-Cohan J.S., Ahmed W.B., Handley M.A., Mann F., Epstein J.A., Clayton L.K., Reinherz E.L. 2008. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 29:888–898 10.1016/j.immuni.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspal F.M., Withers D., Saini M., Bekiaris V., McConnell F.M., White A., Khan M., Yagita H., Walker L.S., Anderson G., Lane P.J. 2011. Abrogation of CD30 and OX40 signals prevents autoimmune disease in FoxP3-deficient mice. J. Exp. Med. 208:1579–1584 10.1084/jem.20101484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels Bupp M.R., Edwards B., Guo C., Wei D., Chen G., Wong B., Masteller E., Peng S.L. 2009. T cells require Foxo1 to populate the peripheral lymphoid organs. Eur. J. Immunol. 39:2991–2999 10.1002/eji.200939427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale J.S., Fink P.J. 2009. Back to the thymus: peripheral T cells come home. Immunol. Cell Biol. 87:58–64 10.1038/icb.2008.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino M., Peterson P., Sillanpää N., Guérin S., Wu L., Anderson G., Scott H.S., Antonarakis S.E., Kudoh J., Shimizu N., et al. 2000. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur. J. Immunol. 30:1884–1893 [DOI] [PubMed] [Google Scholar]
- Hinterberger M., Aichinger M., Prazeres da Costa O., Voehringer D., Hoffmann R., Klein L. 2010. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat. Immunol. 11:512–519 10.1038/ni.1874 [DOI] [PubMed] [Google Scholar]
- Hinterberger M., Wirnsberger G., Klein L. 2011. B7/CD28 in central tolerance: costimulation promotes maturation of regulatory T cell precursors and prevents their clonal deletion. Front. Immunol. 2:30 10.3389/fimmu.2011.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurobe H., Liu C., Ueno T., Saito F., Ohigashi I., Seach N., Arakaki R., Hayashi Y., Kitagawa T., Lipp M., et al. 2006. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 24:165–177 10.1016/j.immuni.2005.12.011 [DOI] [PubMed] [Google Scholar]
- Li J., Li Y., Yao J.Y., Jin R., Zhu M.Z., Qian X.P., Zhang J., Fu Y.X., Wu L., Zhang Y., Chen W.F. 2007. Developmental pathway of CD4+CD8- medullary thymocytes during mouse ontogeny and its defect in _Aire_−/− mice. Proc. Natl. Acad. Sci. USA. 104:18175–18180 10.1073/pnas.0708884104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio C.W., Hsieh C.S. 2008. A two-step process for thymic regulatory T cell development. Immunity. 28:100–111 10.1016/j.immuni.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio C.W., Dodson L.F., Deppong C.M., Hsieh C.S., Green J.M. 2010. CD28 facilitates the generation of Foxp3(-) cytokine responsive regulatory T cell precursors. J. Immunol. 184:6007–6013 10.4049/jimmunol.1000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A., Nutsch K.M., Farr A.G., Lund J.M., Rasmussen J.P., Koni P.A., Rudensky A.Y. 2008. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc. Natl. Acad. Sci. USA. 105:11903–11908 10.1073/pnas.0801506105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- McCaughtry T.M., Wilken M.S., Hogquist K.A. 2007. Thymic emigration revisited. J. Exp. Med. 204:2513–2520 10.1084/jem.20070601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietto A.I., van Dommelen S., Zhou P., Rizzitelli A., D’Amico A., Steptoe R.J., Naik S.H., Lahoud M.H., Liu Y., Zheng P., et al. 2008. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA. 105:19869–19874 10.1073/pnas.0810268105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N.A., White A.J., Jenkinson W.E., Turchinovich G., Nakamura K., Withers D.R., McConnell F.M., Desanti G.E., Benezech C., Parnell S.M., et al. 2012. Rank signaling links the development of invariant γδ T cell progenitors and Aire(+) medullary epithelium. Immunity. 36:427–437 10.1016/j.immuni.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S.W., Kim M.Y., Leibbrandt A., Parnell S.M., Jenkinson W.E., Glanville S.H., McConnell F.M., Scott H.S., Penninger J.M., Jenkinson E.J., et al. 2007. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 204:1267–1272 10.1084/jem.20062497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence P.J., Green E.A. 2008. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc. Natl. Acad. Sci. USA. 105:973–978 10.1073/pnas.0709071105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang K.B., Yang J., Pagán A.J., Li L.X., Wang J., Green J.M., Beg A.A., Farrar M.A. 2010. Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J. Immunol. 184:4074–4077 10.4049/jimmunol.0903933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih F., Carrasco D., Durham S.K., Barton D.S., Rizzo C.A., Ryseck R.P., Lira S.A., Bravo R. 1995. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 80:331–340 10.1016/0092-8674(95)90416-6 [DOI] [PubMed] [Google Scholar]
- Wirnsberger G., Mair F., Klein L. 2009. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc. Natl. Acad. Sci. USA. 106:10278–10283 10.1073/pnas.0901877106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., D’Amico A., Winkel K.D., Suter M., Lo D., Shortman K. 1998. RelB is essential for the development of myeloid-related CD8alpha- dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity. 9:839–847 10.1016/S1074-7613(00)80649-4 [DOI] [PubMed] [Google Scholar]
- Yu W., Nagaoka H., Jankovic M., Misulovin Z., Suh H., Rolink A., Melchers F., Meffre E., Nussenzweig M.C. 1999. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 400:682–687 10.1038/23287 [DOI] [PubMed] [Google Scholar]