Synaptic vesicle endocytosis: fast and slow modes of membrane retrieval (original) (raw)
. Author manuscript; available in PMC: 2013 Apr 15.
Published in final edited form as: Trends Neurosci. 2008 Sep 24;31(11):559–568. doi: 10.1016/j.tins.2008.08.005
Abstract
Several modes of synaptic vesicle release, retrieval and recycling have been identified. In a well-established mode of exocytosis, termed ‘full-collapse fusion’, vesicles empty their neurotransmitter content fully into the synaptic cleft by flattening out and becoming part of the presynaptic membrane. The fused vesicle membrane is then reinternalized via a slow and clathrin-dependent mode of compensatory endocytosis that takes several seconds. A more fleeting mode of vesicle fusion, termed ‘kiss-and-run’ exocytosis or ‘flicker-fusion’, indicates that during synaptic transmission some vesicles are only briefly connected to the presynaptic membrane by a transient fusion pore. Finally, a mode that retrieves a large amount of membrane, equivalent to that of several fused vesicles, termed ‘bulk endocytosis’, has been found after prolonged exocytosis. We are of the opinion that both fast and slow modes of endocytosis co-exist at central nervous system nerve terminals and that one mode can predominate depending on stimulus strength, temperature and synaptic maturation.
Introduction
Neurotransmitter release from nerve terminals underlies synaptic communication in the brain. Repetitive synaptic activity will cause depression if the vesicle pool is depleted at a rate faster than the pool-replenishment process. Endocytosis of fused vesicle membrane is a key step to refilling the vesicle pool and facilitating continuous release. Membrane retrieval is, thus, potentially a crucial bottleneck for vesicle recycling and a likely target for modulating synaptic plasticity.
The classical model for vesicle formation involves invagination of vesicles from clathrin-coated membrane pits (clathrin-mediated endocytosis) or budding from an endosomal structure formed after bulk endocytosis [1] (Figure 1). This process is thought to occur slowly with a time constant of tens of seconds to minutes [2–4]. Although the balance between exocytosis and endocytosis is vital to sustain synaptic transmission and maintain nerve terminal size, it is unlikely that classical endocytosis is fast enough to account for the rapid and continuous rates of transmission observed at many synapses in the nervous system. Crucially, more rapid rates of endocytosis have been demonstrated in nerve terminals (time constant τ = 1–2 s) [5] (Figures 2 and 3) and might explain how vesicle pool depletion is avoided at times of high activity. Kiss-and-run vesicle turnover has been proposed as a mechanism by which more rapid endocytosis could occur [4,6]. However, estimates of the contribution of kiss-and-run to exo-endocytosis at small bouton-type nerve terminals vary from 0 to ~80% of all fusion events. In contrast to clathrin-mediated endocytosis and bulk endocytosis, in kiss-and-run endocytosis a single vesicle is endocytosed rapidly before full collapse has occurred, preventing loss of vesicle identity (Figure 1). Although there is unequivocal evidence for multiple kinetic modes of endocytosis [7] and, in particular, that kiss-and-run endocytosis exists in many neuroendocrine cells, it remains controversial as to whether it has an important role in synaptic vesicle turnover in nerve terminals under physiological conditions [4,8].
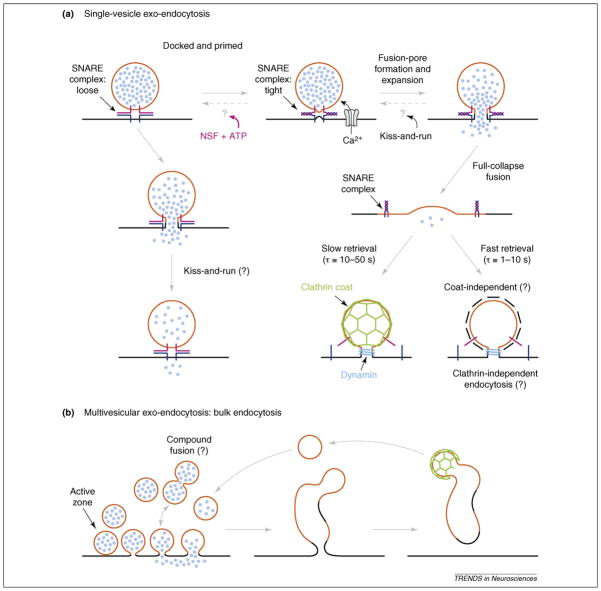
Figure 1.
SNARE complexes and exo-endocytosis. Classical (clathrin-mediated) and alternative routes for vesicular membrane retrieval. (a) The diagram shows that vesicle docking and fusion is mediated by the formation of SNARE (soluble _N_-ethylmaleimide sensitive factor (NSF) attachment protein receptor) complexes. A t-SNARE (target-SNARE or plasma membrane SNARE; dark blue) binds loosely or tightly to a v-SNARE molecule (vesicular-SNARE; purple) to form a SNARE complex [31,72]. Once a synaptic vesicle is docked and primed for exocytosis it proceeds with fusion via tight SNARE complex formation (coiled SNAREs are tightly bound and in a stable low energy state; right hand pathway [81]) or via loose SNARE complex formation which can also lead to fusion [31,32] (left hand pathway). The chaperone protein NSF (plus ATP) can uncoil and reverse the tight SNARE complex after fusion. It is, thus, required to disassemble used tight SNARE complexes and, thus, recycle SNARE proteins. NSF might also act as an ATPase before the fusion event [73]. An influx of Ca2+ ions triggers vesicle fusion via the formation and expansion of a fusion pore. Neurotransmitters are then released in <100 μs by the rapid expansion of the fusion pore in full-collapse fusion. In kiss-and-run exocytosis the fusion pore expands rapidly to a size sufficient for rapid transmitter release (e.g. a pore conductance of 300 pS) and then closes within approximately a second (or a few 100 μs). This mode of endocytosis is, thus, thought to be ‘fast.’ After full-collapse fusion the slow retrieval mode of endocytosis (time constant: τ = 10–50 s) is hypothesized to be mediated by clathrin-coated-pit formation and dynamin binding to the fission pore as a collar that constricts the pore neck via GTP hydrolysis. The fast mode of endocytosis (τ = 1–10 s) is thought to be clathrin-independent and is not well understood at a molecular level. Question marks indicate hypothesized pathways. (b) Multivesicular exo-endocytosis. Strong and prolonged stimulation of synapses might lead to copious exocytosis. It might also lead to compound exocytosis in which vesicles fuse with each other before they fuse to the plasma membrane. The large amount of vesicle membrane might be subsequently endocytosed as one large membrane invagination (bulk endocytosis). Parts of the plasma membrane might also be reinternalized via this mode of endocytosis, which could lead to a fast step-like rate of endocytosis because large amounts of membrane are fissioned at once from the plasma membrane. There is good evidence for this mode of endocytosis after strong stimulation in synaptosomes, ribbon-type synapses and at the NMJ [64,65]. Clathrin-mediated budding of vesicles from the endosome formed after bulk endocytosis can then produce synaptic vesicles that enter the reserve and/or recycling pool of vesicles.
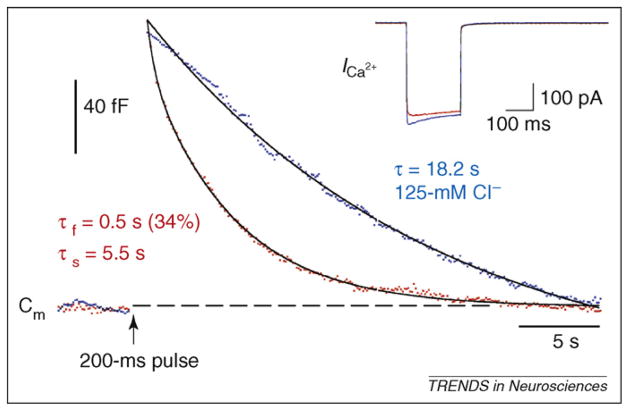
Figure 2.
The kinetics of endocytosis at a ribbon-type synapse. Capacitance (Cm) measurements from an isolated goldfish bipolar cell synaptic terminal embedded in a retinal slice. The Cm jump is triggered by a depolarization from −60 mV to 0 mV for 200 ms (arrow), which elicits the Ca current (_I_Ca) shown in the inset. The Cm decay has both fast and slow components with normal 15-mM Cl− in the patch pipette (red data points; double exponential fit with τf = 0.5 s [fast component; 34% contribution to overall fit] and τs = 5.5 s [slow component]). However, with 125-mM internal Cl−, endocytosis has no fast component (blue data points; Cm decay fit by a single exponential with τ = 18.2 s). High internal Cl−, thus, blocks fast endocytosis selectively, indicating that it is due to a molecular mechanism distinct from slow endocytosis. The internal Ca buffer was 5-mM ethylene glycol tetraacetic acid (EGTA); so high EGTA does not slow the rate of endocytosis per se, as long as the Ca currents are of large amplitude [18]. Reproduced, with permission, from Ref. [18].
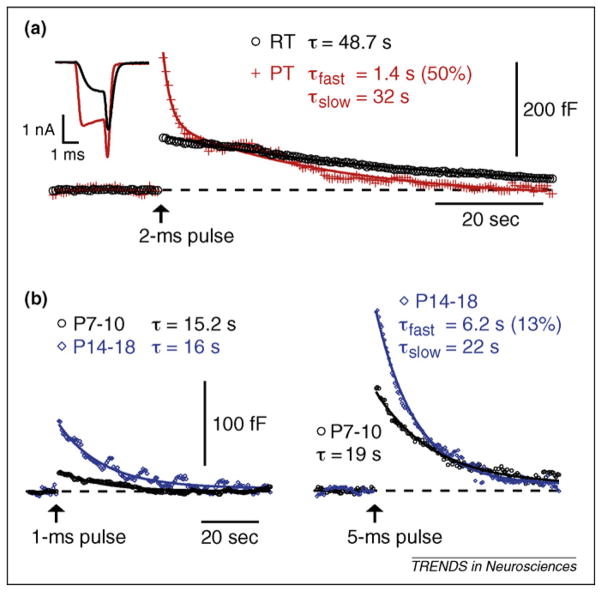
Figure 3.
Endocytosis at a calyx nerve terminal: effects of temperature and synaptic maturation. Membrane capacitance at the calyx of Held nerve terminal was monitored before and after a short depolarization to 0 mV (arrows). This depolarization activated a Ca2+ current (inset in A) and synaptic vesicle exocytosis, causing a capacitance (Cm) jump. Compensatory endocytosis is marked by a decrease in membrane capacitance as vesicle membrane fissions from the cell surface. (a) Increasing temperature accelerates endocytosis. Recording from a calyceal terminal (postnatal day [P] 10) at room temperature (RT) and then at physiological temperature (PT) reveals the addition of a kinetically distinct form of endocytosis at PT. A short depolarization at RT (2 ms, black circles) increased membrane capacitance by 110 fF, followed by a monoexponential return to baseline. At PT, this same calyceal terminal responded to the same depolarization with a larger Cm jump (270 fF, red crosses), and endocytosis was fit by a double exponential composed of a fast and slow component. Shown are the averages of 5 sweeps at RT and 4 sweeps at PT. Ca2+ currents from the step depolarizations are shown in the inset. Ca2+ influx increases at PT owing to the faster activation kinetics for the voltage sensitive Ca2+ channels. (b) The capacity for fast endocytosis increases with synaptic maturation. Short depolarizations (1 ms and 5 ms to 0 mV, arrows) in calyx terminals from young (P7–10, black circles) and more mature (P14–18, blue diamonds) animals at RT result in very different exo-endocytosis profiles. Young terminals show small capacitance jumps (23 fF for 1-ms depolarizing pulses, which is equivalent to the fusion of ~300 synaptic vesicles or that elicited by 1 or 2 action potentials; 120 fF for 5 ms) and slow endocytosis (τ = 15 s). Exocytosis in more mature terminals is greatly increased (77 fF at 1 ms; 211 fF at 5 ms) whereas the endocytotic rate is augmented slightly for large depolarizations, resulting in a similar time course for membrane recovery. Because more fused vesicle membrane is retrieved at similar or faster rates, the capacity for endocytosis is apparently increased at mature nerve terminals, perhaps because they have a larger, or more functional, pool of endocytotic proteins available to promote vesicle membrane fission. Reproduced, with permission, from Ref. [21].
Synaptic fidelity, short-term depression and endocytosis
The elementary or quantal event in synaptic transmission, the fusion of a single synaptic vesicle with the plasma membrane, was first observed in the 1950 s by using postsynaptic receptors as biosensors for the release of a neurotransmitter [9]. Since then, several techniques and preparations have been developed to further study this event in real time. In a majority of synapses, increasing stimulation strength or frequency results in short-term synaptic depression, which is recoverable after a short period of quiescence. At least part of this depression is due to presynaptic depletion of the pool of release-competent vesicles, although other factors including sodium and calcium channel inactivation, receptor desensitization, feedback via autoreceptors and changes in cleft H+ and Ca2+ concentrations have also been implicated [10–13].
The calyx of Held is a well-studied central nervous system (CNS) nerve terminal that can sustain synaptic transmission at high stimulation frequencies (>600 Hz) [14]. A functional synaptic unit containing 1200–2000 releasable vesicles spread over ~500 release sites has been defined morphologically and functionally [15]. Synaptic depression is mostly due to depletion of the readily releasable pool at stimulation frequencies >100 Hz. Because fast endocytosis has a time constant of 1 to 10 s at the mature calyx of Held (Figure 3), it might interact synergistically with the vesicle pool size and release properties to influence the degree of synaptic depression under different conditions. Substantial synaptic depression is also typical at smaller nerve terminals, such as those found in the nucleus of the solitary tract, where the functional synaptic unit is comprised of a single axon and ~20 release sites each with a release probability of ~0.9 [16]. At this synapse, frequency-dependent depression is even more marked: five stimuli at 50 Hz decreases transmitter release by ~80%.
Although these two synapses vary considerably in size and number of release sites, they both show synaptic depression reflecting depletion of the releasable vesicle pool over the course of a few hundred milliseconds, which is too fast for replenishment by endocytosis or via vesicle recruitment from a reserve pool. Clearly, the impact of a presynaptic action potential on transmission is dependent on the number of release sites in the functional synaptic unit, the release probability and previous synaptic activity. Additionally, the rate of vesicle pool replenishment, and thus the rate of endocytosis, must be taken into account when considering the transfer properties of synaptic terminals.
Fast and slow modes of endocytosis are dissociable: evidence from ribbon and calyx synapses
The first studies to directly measure the rate of endocytosis in CNS nerve terminals employed large terminals and capacitance measurements. Cell surface area is proportional to electrical membrane capacitance (Cm). Consequently, Cm measurements, performed via the patch-clamp technique, permit measurement of net changes in cell surface area over time. Fusion of secretory granules or vesicles with the plasma membrane results in a fast increase in cell surface area as the membranes become contiguous and, thus, causes a ‘Cm jump,’ reflecting exocytosis. The subsequent retrieval of vesicular membrane (endocytosis) results in a decay of Cm as fission occurs between the cell surface and vesicular membranes. The fusion of secretory vesicles can, thus, be directly detected by Cm jumps, whereas membrane retrieval can be monitored in real time and in living cells via the decay of Cm (Figures 2 and 3).
Two kinetically distinct modes of endocytosis were first observed in retinal bipolar cell nerve terminals using Cm measurements: fast (τ = 1–2 s) and slow (τ = 10–30 s) [5] (Figure 2). The fast mode has been reported to be sensitive to Ca levels [5,17] and is selectively inhibited by high chloride concentration [18], indicating that it is probably mediated by a molecularly distinct mechanism from the slow mode (Figure 2). Slow endocytosis is mediated by the elaborate clathrin-coat- and dynamin-dependent mode of endocytosis [19] (Figure 1) and is very sensitive to membrane tension (or patch pipette internal pressure) [20]. This pathway involves invagination of membranous clathrin-coated pits (orchestrated by multiple proteins) and vesicle fission (via dynamin GTPases). Subsequent uncoating, trafficking, re-acidification and recharging with transmitter ready the vesicle for reuse. By contrast, the mechanism for fast endocytosis remains unclear.
Bipolar cell terminals, unlike the majority of bouton-type CNS nerve terminals, contain specialized active zones (synaptic ribbons) and release vesicles in response to graded membrane potential changes. Consequently, it was important to examine endocytosis in nerve terminals that have conventional active zones and fire Na+-based action potentials, such as the calyx of Held. Direct measurements of endocytosis in this large CNS nerve terminal gave rates of capacitance decay that are fast for short depolarizing pulses and slower for longer depolarizations [4,21,28]. Furthermore, Cm measurements at the calyx of Held revealed distinct fast and slow rates of endocytosis at 35°C and at more mature postnatal ages [21] (Figure 3), indicating that both modes of membrane retrieval are likely to be present physiologically. Remarkably, both short and long depolarizing pulses elicit similar rates of capacitance decay (or endocytosis) from more mature nerve terminals, indicating that the capacity for endocytosis increases with synaptic maturation.
Kiss-and-run in large nerve terminals
After kiss-and-run endocytosis, a vesicle can quickly undergo re-release and vesicle turnover will not be limited by slow enzymatic clathrin-cage formation and clathrin uncoating. Presumably, the fewer number of steps required to produce a new vesicle implies a potentially more rapid form of vesicle recycling that could be important for maintaining fast sustained signaling.
Direct data for the existence of kiss-and-run endocytosis first came from capacitance measurements in mast cells, which secrete via large dense-core granules [22]. Amperometry was then used to detect single vesicle fusion, demonstrating that a fusion pore is sometimes transiently formed between the inside of the granule and the extracellular surface of the cell [23]. Recently, the release of dopamine from midbrain neurons via the fusion of small clear core vesicles has been detected by amperometry. Vesicle fusion usually elicited a single monophasic current, but up to 20% of the events were multiphasic with 2–5 peaks of decreasing amplitude, consistent with repetitive release from a flickering fusion pore [24]. However, in leech neurons, serotonin release from small clear-core vesicles was found to be all-or-none and more consistent with full-collapse fusion [25].
Single-fusion-event measurements from nerve terminals became possible with the cell-attached Cm technique, where small patches of membrane directly under a pipette tip are monitored [26]. Remarkably low noise levels were obtained (<2 aF), permitting resolution of single microvesicle (≈50 nm in diameter; Box 1) fusion and fission events at pituitary nerve terminals [26] and at the release face of the calyx of Held [27] (Figure 4a,b). At both terminal types, the opening of variable-sized fusion pores resulted in increases in capacitance of variable size, rapidly (τ ≤ 1 s) followed by size-matched decreases in capacitance as the pore closed (Figure 4a,b). This work provided direct evidence for a rapid form of endocytosis in presynaptic nerve terminals. However, there are two concerns about the interpretation of these flicker-fusion events. First, only 5–16% of all exocytotic events were reversible ‘kiss-and-run’ events, indicating a minor role. Second, distortion of the presynaptic membrane at the patch pipette tip during seal formation might alter the fusion properties of vesicles in the cell-attached mode, because normal membrane tension is probably disrupted and it is difficult to predict how this impacts kiss-and-run vesicle cycling. For example, increased hydrostatic pressure is known to inhibit endocytosis in whole-cell Cm recordings [20].
Box 1. Why are synaptic vesicles so small?
Synaptic vesicles are the smallest eukaryotic organelle. Their average outer lipid diameter varies from 32 nm in _C. elegans_[74] to 42 nm in the mammalian CNS [75]. The narrow Gaussian distribution of vesicle diameters has a coefficient of variation (CV) of only 0.1 to 0.2. This uniformity of size minimizes quantal excitatory postsynaptic current variation (CV = 0.3 in the frog NMJ). Indeed, disruption in Drosophila of endocytotic proteins such as AP-180 increased the size and variability of vesicles leading to increased quantal amplitude and variation [76]. What are the reasons for this small and uniform vesicle size? We speculate that the regulation of exocytosis and endocytosis at nerve terminals is likely to place functional constraints on this physical dimension. The effective surface tension of lipid bilayer vesicles is proportional to 1/Rv2, where Rv is the vesicle radius, and increases in surface tension decrease the free energy barrier for vesicle fusion [77,78]. So, in principle, smaller vesicles require less energy to fuse than larger vesicles. This expectation is borne out by mathematical models of curved lipid fusion (i.e. synaptic vesicles) with planar lipids (representing the cell membrane) [79]. A high degree of lipid curvature, promoted perhaps by synaptotagmin interactions with SNAREs, might thus enhance synaptic vesicle fusion [80]. The endocytosis of small patches of membrane via clathrin cages might also consume less ATP overall than the endocytosis of larger membrane patches. Finally, the need to recycle vesicles locally and quickly within a small synaptic bouton (<1 μm) could also place functional constraints on the size of synaptic vesicles (e.g. large dense-core granules need to return to the Golgi if they lose their central core protein). Thus, the need for fast, local and continuous recycling of vesicle membrane, coupled with rapid refilling with neurotransmitter for efficient signaling, might have placed severe functional constraints on synaptic vesicle size.
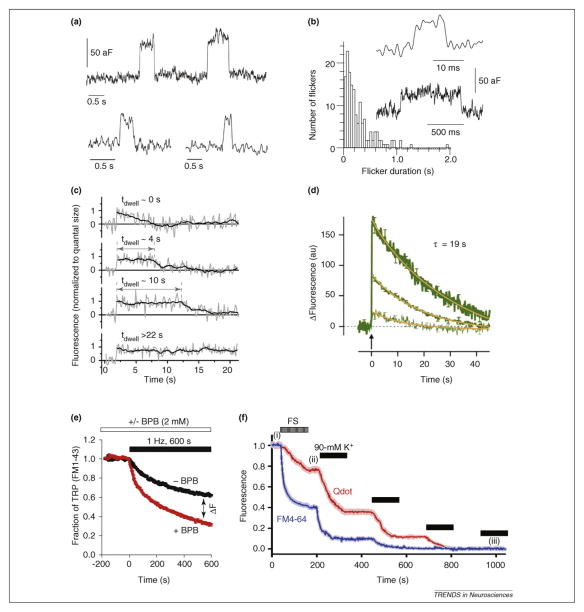
Figure 4.
Methodological summary of recent investigations supports the existence of both classical and kiss-and-run endocytosis. (a) Cell-attached capacitance measurements follow fusion pore opening and closing in real-time. This technique supports kiss-and-run exo-endocytosis. Capacitance flickers in a cell-attached recording from posterior pituitary nerve terminals correspond to kiss-and-run exocytosis of microvesicles (50-nm in diameter [26]). (b) The majority of the paired flicker durations for capacitance up and down steps in cell-attached recording from the release face of the calyx of Held are <1 s. The inset shows two examples (46 nm diameter synaptic vesicles [27]). (c,d) Fluorescently tagged proteins enable observation of single synaptic vesicle exo-endocytosis events. (c) Labeling of the vesicular transporter showed single vesicle dwell time varied for different fusion events as shown in these four example traces indicating different modes of endocytosis. The 0-s dwell-time (tdwell ~ 0 s) event is consistent with kiss-and-run exocytosis and fast endocytosis. (d) Average synaptophysin–GFP signal from ~3000 synapses (middle trace) stimulated by a single field stimulus (at the arrow) decayed with τ = 19 s. This is consistent with a single mode of endocytosis. The synapses with highest and lowest fluorescence decayed with similar rates (upper and lower traces, respectively). (e) The total releasable pool (TRP) as retained FM1-43 dye after exocytosis is quenched by bromophenol blue (red trace), consistent with kiss-and-run. (f) 15-nm quantum dots (Qdot; red trace) escape from vesicles more slowly than FM4-64 signal (blue trace), using either field stimulation (FS) (i) or four rounds of high K+ (90 mM K+), starting at (ii) and ending at (iii), consistent with Qdots reporting only full-collapse fusion and FM reporting both full-collapse and kiss-and-run exo-endocytosis. Part (c) modified from [50], (d) modified from [49], (e) modified from [51] and (f) modified from [52], with permission.
Caveats notwithstanding, these data provide strong evidence that kiss-and-run endocytosis can exist in nerve terminals; however, the question of the importance of this mechanism at various presynaptic terminals, and under different stimulation protocols, remains open. For instance, single vesicle fusion has been shown to be more consistent with full vesicle collapse into the plasma membrane at large bipolar cell terminals [4]. Conversely, the view that full-collapse, dynamin-dependent vesicle turnover is the only mechanism of vesicle retrieval at nerve terminals [28] has been partially undermined by the demonstration that disruption of the dynamin pathway only transiently blocks endocytosis at the calyx of Held after massive exocytosis triggered by strong stimulation [29]. The physiologically important question remains: is kiss-and-run endocytosis, observed at some larger nerve terminals, also present and abundant at small terminals that form the vast majority of CNS synapses?
SNAREs and kiss-and-run exocytosis
Vesicle fusion is mediated partly by SNARE (soluble _N_-ethylmaleimide sensitive factor [NSF] attachment protein receptor) protein interactions [31]. In brief, vesicular (v)-SNAREs on the vesicle membrane bind to target (t)-SNAREs on the plasma membrane and form tight–SNARE complexes that bring the two bilayers into close apposition (Figure 1). The tight–SNARE complex is an extremely stable, low energy state and, thus, needs the action of the ATPase NSF to be dissociated so that v- and t-SNARE proteins can be recycled after fusion. At nerve terminals, Ca2+ influx followed by Ca2+ binding to synaptotagmin triggers the fusion of synaptic vesicles. The formation of a fusion pore is, thus, usually followed by full collapse of the vesicle membrane onto the plasma membrane. However, the fusion pore can sometimes transiently close, so the process of pore opening is reversible, as clearly evidenced by capacitance measurements in endocrine cells [30]. We speculate that a loose–SNARE complex, which can also lead to fusion [31,32], is more likely to be reversible than the tight–SNARE complex and, thus, provides a possible substrate for kiss-and-run exocytosis (Figure 1). However, the conformational changes in SNARE proteins (and SNARE-interacting proteins) that might favor transient fusion pore openings are still poorly understood [31,33] and the number of SNARE complexes that form the collar around the fusion pore neck has not been determined.
Vesicle pools and signals at small nerve terminals
The cortical bouton-type nerve terminal is an amazing feat of bio-nanotechnology. Its diameter is typically <1 μm and it contains only ~200 synaptic vesicles [34]. A small number of these vesicles (2–10) are docked and ready for fusion with the plasma membrane at the active zone. The small size of this readily releasable pool makes conventional terminals prone to vesicle pool depletion and synaptic depression. Fast endocytosis and vesicle recycling could, therefore, be necessary for their proper function [35]. However, in vivo studies have shown that cortical neurons fire sparsely and in bursts that typically have average rates of below 1 Hz. For example, hippocampal place-field cells of adult rats (awake and freely moving) typically fire action potentials in bursts and have an average overall firing rate of 0.47 Hz [36]. Bursts occur at a rate of one to two per minute and consist of ~10 spikes fired at 2 to 6 Hz. Thus, with these firing patterns, small terminals have substantial time to recover from vesicle pool depletion, in spite of small vesicle numbers, and might not require a fast mode of endocytosis. By contrast, medium-sized nerve terminals in the CNS such as the mossy fiber terminals in the hippocampus and cerebellum often operate in vivo at high spontaneous rates (typically between 4 and 100 Hz) [37,38]. Sensory stimuli can then increase evoked spike rates to 700 Hz. Accordingly, these terminals have a large readily releasable pool of 1000–2000 vesicles [39]. Because synaptic terminals in distinct CNS areas clearly have structural and functional differences, it is possible that endocytosis rates are not identical at all nerve terminals.
Controversies at conventional nerve terminals: fast endocytosis and kiss-and-run
At small nerve terminals the evidence for a vesicle-recycling pathway in which the vesicle does not collapse is indirect, but still compelling. Measurement of vesicle retrieval at small nerve terminals using membrane capacitance has not been feasible so far, although patch-clamp recordings from synaptosomes have recently been performed [11]. Instead, recycling vesicles have been monitored with optical methods. In most of these studies the rates presented reflect internalization of the same membrane that was previously part of a released vesicle. However, it has also been shown that endocytosis does not always immediately retrieve the most recently exocytosed membrane [2,3] (Figure 1).
Functional and structural experiments in a range of preparations indicate at least two distinct pathways for endocytosis. Slow rates for vesicle endocytosis were reported using styryl (FM) dyes (τ recycling and endocytosis = 30–60 s) [41], presumably reflecting vesicle regeneration via an endosomal intermediate. In contrast to this, evidence that vesicles could be recycled without passing through endosomal structures came from the finding that lipid-bound fluorophore staining of vesicles was not diluted after endocytosis in hippocampal nerve terminals [42]. Kinetic and modeling studies of these synapses indicated rapid reuse of vesicles consistent with a recycling pathway that did not include endosomes [43]. Using patch clamp and FM dyes it has been proposed that this fast endocytic pathway might have an important role during both low and high rates of synaptic transmission [44–46]. However, these experiments did not show whether this mode of vesicle recycling was clathrin independent, underlining the need for more direct evidence for kiss-and-run cycling.
Building on earlier data indicating that endocytosis at hippocampal nerve terminals is rapid (τendo = 1–6 s) [47], a sensitive camera was used to resolve the signal from single vesicles stained with lipophilic FM1-43 dye [40]. A mild stimulus stained ≤2 vesicles within each terminal. Subsequent stimulation led to destaining in subquantal steps, indicating that the vesicle did not fully collapse after exocytosis [40]. Kiss-and-run vesicle cycling is consistent with rapid turnover; however, it also predicts that vesicles fuse repeatedly without loss of identity. Aravanis and colleagues [40] argued that the second decrement in fluorescence was due to loss of signal from the same vesicle. A single vesicle might, thus, be reused multiple times simply by staying docked and repetitively opening its fusion pore, akin to chromaffin granules, giving rise to the term kiss-and-stay exocytosis.
Gandhi and Stevens [47] imaged vesicle turnover by transfecting hippocampal cultures with synaptopHluorin [48], a pH-sensitive green fluorescent protein (GFP) tagged to the vesicle luminal domain of vesicle-associated membrane protein 2 (VAMP2; also known as synaptobrevin). At rest, the vesicle lumen pH, and thus fluorescence, is low. After fusion the vesicle pH and fluorescence increase. Both then decrease after endocytosis and reacidification of the synaptic vesicle. Single vesicle fusion was resolved by photobleaching and, hence, decreasing the background fluorescence. The kinetics of the fluorescence decrease (vesicle endocytosis and reacidification) of averaged traces had three phases: a rapid phase (τ = 0.9 s), a slower compensatory phase (τ = 10.7 s) and a very slow or stranded mode (τ > 45 s). The rapid component was consistent with kiss-and-run endocytosis (Figure 4c), and the differential quenching effects of different sized pH buffers supported the existence of a narrow fusion pore.
GFP-tagged VAMP2 was associated with substantial background fluorescence owing to surface expression of the protein [47,49]. Accordingly, Granseth and colleagues [49] monitored vesicle fusion using synaptopHluorin and a higher signal-noise indicator, GFP-tagged synaptophysin. When the authors examined the distribution of events quantitatively they did not resolve three individual kinetic phases of endocytosis, but instead demonstrated that the fluorescence decreased in a monoexponential manner (τ = 20 s), consistent with a single form of endocytosis (Figure 4d). After correction for vesicle acidification, τen-do = 15 s. A collateral set of experiments using acid quenching confirmed this rate of endocytosis. To further sharpen the vesicle turnover signal, Balaji and Ryan [50] used a GFP–vesicular-glutamate-transporter fusion protein with even lower surface expression than the synaptophysin–GFP indicator and were able to resolve single vesicle events. Quantal increases in fluorescence that decreased after endocytosis and acidification were observed. Membrane dwell-time for ~150 endocytic events was described by three categories (Figure 4c). However, quantification of the dwell-time distribution revealed a monoexponential fit (τ ~13 s) consistent with a single mechanism of endocytosis [49] (Figure 4c,d).
Two other studies also used optical probes to determine if synaptic vesicle ‘kissing’ occurs. Harata and colleagues [51] used the small hydrophilic molecule, bromphenol blue (BPB) to test whether after vesicle fusion there was incomplete collapse of the vesicle. The authors proposed that the more lipophilic FM1-43 might be retained in the setting of rapid endocytosis or the presence of a transient and/or small-diameter fusion pore. They argued that BPB would rapidly enter vesicles via a fusion pore and quench fluorescence from FM1-43 or GFP-tagged VAMP. Indeed, destaining occurred more rapidly with BPB, consistent with it entering vesicles via a fusion pore to quench retained fluorophore (Figure 4e). Up to 80% of releasable vesicles showed this type of fusion, consistent with kiss-and-run, and this proportion decreased as stimulation rates increased. Using the same principle, Zhang and colleagues [52] argued that use of a larger fluorescent marker would delay destaining in the setting of kiss-and-run vesicle cycling. After loading vesicles with 15-nm phospholuminescent ‘quantum dots’ and FM4-64, they were able to monitor the two signals independently and demonstrated that fluorescence loss from the larger quantum dots was much slower than that for the FM signal (Figure 4f). These data are, thus, also consistent with the formation of a transient fusion pore, before full fusion, and might reflect reversible protein–lipid interactions at room temperature [53]. However, stimulation at higher frequencies might shift exocytosis into a full-collapse mode, as illustrated recently using FM1-43 dye imaging of single vesicle fusion [54]. It also remains to be seen whether these same effects are observed in similar proportion at physiological temperatures.
In fact, a majority of experiments examining synaptic vesicle turnover in conventional bouton synapses have been performed at room temperature (~24°C), despite the high temperature sensitivity of mammalian exo-endocytosis (Figure 3a). Indeed, Fernandez-Alfonso and Ryan [55] have observed an accelerated rate of endocytosis at 35°C. Recently, amperometric recordings from PC12 cells showed that kiss-and-run endocytosis was responsible for ~35% of the vesicles that fuse at a lower temperature (12°C), but this percentage was decreased by 50% at 28°C, indicating that the studies discussed here might have overestimated the prevalence of kiss and run [56].
The importance of clathrin-mediated endocytosis at cultured hippocampal synaptic terminals was confirmed by small interfering (si)RNA experiments that completely blocked endocytosis-linked fluorescence decreases for 2 min after stimulated exocytosis, when expression of clathrin heavy chain was inhibited [49]. The absence of a fast, clathrin-independent mechanism for endocytosis, thus, indicate that kiss-and-run endocytosis is not prevalent at small bouton-type terminals; however, it was perhaps surprising that there were still pools of vesicles available for release in these cultures, given the degree of siRNA-mediated inhibition and the level of spontaneous turnover. The presence of a residual pool of synaptic vesicles in the absence of clathrin-mediated endocytosis circumstantially points to at least one other form of vesicle retrieval at these terminals. An acute role for clathrin has also been recently demonstrated at Drosophila synapses, where photoinactivation rapidly inhibited vesicle formation and quickly diminished transmission in an activity-dependent manner [57].
In support of clathrin-independent recycling, Deak and colleagues [58] determined that fast, but not slow, endocytosis was impaired in synaptoptobrevin-2-deficient cultured hippocampal neurons. Specifically, the faster rate of destaining for FM2-10 compared with FM1-43, consistent with kiss-and-run endocytosis in wild-type neurons, was absent in the synaptobrevin-2-null mutants. Additionally, deletion of dynamin-1, an important and abundant mediator of vesicle-neck constriction and vesicle fission, impaired endocytosis increasing t0.5 from ~40 s to 70 s at stimulation rates of 10 Hz, confirming an important endocytic role for dynamin-1 at high stimulus frequencies [59]. However, endocytosis was unaffected in the dynamin-1-null mutant after stimulation for 30 s at rates of 2 and 5 Hz. Consistent with these results, kiss-and-run endocytosis has been suggested to predominate at lower stimulation frequencies [51].
Bulk endocytosis: new views on an old retrieval mechanism
The presence of bulk endocytosis has been noted since the first models for synaptic vesicle retrieval were introduced, when large cisternae were observed resulting from moderate to heavy stimulation protocols [1]. It has been repeatedly hypothesized that synaptic vesicles can be reformed by internal budding from these endosomal structures and that this mode of recycling underlies the slower endocytosis rates [60]. However, until recently the presence and dynamics of this type of retrieval have been difficult to visualize and test experimentally. At the Drosophila neuromuscular junction (NMJ), clathrin is essential for reformation of synaptic vesicles, either via direct retrieval from the plasma membrane or, more likely, as a result of controlled budding from activity-dependent formation of endosomes [57]. Additionally, clathrin might have a role in controlling the extent of bulk endocytosis, as terminals lacking clathrin activity showed abnormal endosomal topology. Tomographic analysis of tonically active inhibitory neurons from dynamin-1-knockout mice showed a prevalence of clathrin-coated pits, indicating that clathrin mediates endocytosis [61]. However, ultrastructural analysis of activity-dependent endocytosis in excitatory cells showed profiles that were largely composed of endosomes. Thus, the level of activity of the terminal might determine whether bulk endocytosis occurs. At the mouse NMJ, the activation of L-type voltage-activated Ca2+ channels transformed recently endocytosed vesicles into a release-competent state bypassing a reserve pool and endosomal intermediates [62]. These studies reveal that the endosomal compartment might be transient, specific to nerve terminal type and circumvented by high synaptic demand. Notably, under heavy stimulation, endosomes can even be forced to fuse directly with the plasma membrane [63]. This type of exocytosis could underlie the persistence of capacitance increases seen at the calyx of Held in the absence of GTP hydrolysis [29].
Future directions
Recent studies of small and large nerve terminals indicate the existence of both slow clathrin-mediated and fast clathrin-independent modes of endocytosis, in addition to a bulk endocytosis mode [64,65]. These studies prompt several questions. What are the identities of the synaptic proteins that control the switch between the different modes of endocytosis? What signal directs fused vesicle membrane to a particular mode of endocytosis? What molecular mechanisms set the kinetics of membrane retrieval?
Vesicle trafficking, recruitment and endocytosis are highly temperature sensitive, yet the majority of studies of endocytosis are performed at room temperature and in immature synapses or in cultured synapses. The different pathways of vesicle endocytosis might also have very different temperature sensitivities [66], begging the question ‘what are the relative contributions of the kiss-and-run and full-fusion pathways at small nerve terminals in physiological conditions?’ Future studies, thus, need to be performed with mature nerve terminals and closer to the in vivo condition.
Conclusion
Several studies with small and large nerve terminals have demonstrated the existence of rapid (τ ~1 s) and slow forms of endocytosis at CNS synapses. Likewise, at the NMJ, vesicle endocytosis has also been shown to involve two endocytotic pathways distinguishable structurally and kinetically [67,68]. Some synaptic vesicles seem to retain their identity throughout the endocytic cycle, supporting the kiss-and-run mechanism of vesicle cycling, and this pathway might be an important contributor to fast endocytosis at small nerve terminals [40,42,51] (although, recently, full-collapse vesicle fusion has been observed with FM dyes at bouton-type synapses [54]). This contrasts with endocytosis at large nerve terminals, such as the retinal bipolar cell terminal, where fast endocytosis has been observed but kiss-and-run has not been detected [69,70]. Recent studies, thus, provide evidence indicating important functional differences between large and small nerve terminals, which could reflect different release requirements. However, other studies show that the rate of endocytosis at small bouton-type nerve terminals (τ ~13–15 s; Figure 4c–d) is very similar to that found in large nerve terminals, such as the calyx of Held (τ ~15 s; Figure 3b), pointing to a fundamentally conserved process vital to proper synaptic function at phasic synapses [71,72]. Although new inroads have recently been made to understanding the dynamic nature of endocytosis, in addition to the presence and utility of intermediate states between retrieved vesicle membrane and the reconstitution of release-competent vesicles, more molecular and functional studies with new probes are clearly needed to resolve these long-standing controversies and provide explanations that might underlie some of the apparent functional differences in modes of endocytosis at different synapses.
Acknowledgments
Our work was supported by grants from: the National Institute of Neurological Disorders and Stroke; the National Heart, Lung, and Blood Institute; the National Institute on Deafness and Other Communication Disorders; the National Eye Institute; and the Human Frontier Science Program.
References
- 1.Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller VJ, et al. Monitoring clathrin-mediated endocytosis during synaptic activity. J Neurosci. 2004;24:2004–2012. doi: 10.1523/JNEUROSCI.4080-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voglmaier SM, Edwards RH. Do different endocytic pathways make different synaptic vesicles? Curr Opin Neurobiol. 2007;17:374–380. doi: 10.1016/j.conb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Wu LG, et al. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci. 2007;27:11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- 6.Ceccarelli B, et al. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. J Cell Biol. 1997;139:885–894. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harata NC, et al. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J Neurochem. 2006;97:1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- 9.del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushmerick C, et al. Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment. J Neurosci. 2006;26:1366–1377. doi: 10.1523/JNEUROSCI.3889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SM, et al. Recordings from single neocortical nerve terminals reveal a nonselective cation channel activated by decreases in extracellular calcium. Neuron. 2004;41:243–256. doi: 10.1016/s0896-6273(03)00837-7. [DOI] [PubMed] [Google Scholar]
- 12.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 13.Palmer MJ, et al. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sätzler K, et al. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey TW, et al. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci. 2006;26:6131–6142. doi: 10.1523/JNEUROSCI.5176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neves G, et al. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc Natl Acad Sci U S A. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hull C, von Gersdorff H. Fast endocytosis is inhibited by GABA-mediated chloride influx at a presynaptic terminal. Neuron. 2004;44:469–482. doi: 10.1016/j.neuron.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jockusch WJ, et al. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron. 2005;46:869–878. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Heidelberger R, et al. Multiple components of membrane retrieval in synaptic terminals revealed by changes in hydrostatic pressure. J Neurophysiol. 2002;88:2509–2517. doi: 10.1152/jn.00267.2002. [DOI] [PubMed] [Google Scholar]
- 21.Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol. 2007;98:3349–3359. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- 22.Spruce AE, et al. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990;4:643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez de Toledo G, et al. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 24.Staal RG, et al. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- 25.Bruns D, et al. Quantal release of serotonin. Neuron. 2000;28:205–220. doi: 10.1016/s0896-6273(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 26.Klyachko VA, Jackson MB. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- 27.He L, et al. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T, et al. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, et al. GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- 30.Vardjan N, et al. Elementary properties of spontaneous fusion of peptidergic vesicles: fusion pore gating. J Physiol. 2007;585:655–661. doi: 10.1113/jphysiol.2007.136135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han X, Jackson MB. Structural transitions in the synaptic SNARE complex during Ca2+-triggered exocytosis. J Cell Biol. 2006;172:281–293. doi: 10.1083/jcb.200510012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu T, et al. Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- 33.Vardjan N, et al. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pyle JL, et al. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 36.Dobrunz LE, Stevens CF. Response of hippocampal synapses to natural stimulation patterns. Neuron. 1999;22:157–166. doi: 10.1016/s0896-6273(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 37.Rancz EA, et al. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature. 2007;450:1245–1248. doi: 10.1038/nature05995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saviane C, Silver RA. Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature. 2006;439:983–987. doi: 10.1038/nature04509. [DOI] [PubMed] [Google Scholar]
- 39.Hallermann S, et al. A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc Natl Acad Sci U S A. 2003;100:8975–8980. doi: 10.1073/pnas.1432836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aravanis AM, et al. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- 41.Ryan TA, et al. The timing of synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 1996;93:5567–5571. doi: 10.1073/pnas.93.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- 43.Klingauf J, et al. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- 44.Sara Y, et al. Fast vesicle recycling supports neurotransmission during sustained stimulation at hippocampal synapses. J Neurosci. 2002;22:1608–1617. doi: 10.1523/JNEUROSCI.22-05-01608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards DA, et al. Two modes of exocytosis at hippocampal synapses revealed by rate of FM1-43 efflux from individual vesicles. J Cell Biol. 2005;168:929–939. doi: 10.1083/jcb.200407148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens CF, Williams JH. “Kiss and run” exocytosis at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:12828–12833. doi: 10.1073/pnas.230438697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- 48.Miesenbock G, et al. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 49.Granseth B, et al. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 50.Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harata NC, et al. Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron. 2006;49:243–256. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Q, et al. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proc Natl Acad Sci U S A. 2007;104:17843–17848. doi: 10.1073/pnas.0706906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson MB, Chapman ER. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu Rev Biophys Biomol Struct. 2006;35:135–160. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, et al. Release of the styryl dyes from single synaptic vesicles in hippocampal neurons. J Neurosci. 2008;28:1894–1903. doi: 10.1523/JNEUROSCI.4518-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez-Alfonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron. 2004;41:943–953. doi: 10.1016/s0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Jackson MB. Temperature dependence of fusion kinetics and fusion pores in Ca2+-triggered exocytosis from PC12 cells. J Gen Physiol. 2008;131:117–124. doi: 10.1085/jgp.200709891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heerssen H, et al. Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr Biol. 2008;18:401–409. doi: 10.1016/j.cub.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deak F, et al. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson SM, et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 60.Danglot L, Galli T. What is the function of neuronal AP-3? Biol Cell. 2007;99:349–361. doi: 10.1042/BC20070029. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi M, et al. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci U S A. 2008;105:2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perissinotti PP, et al. L-type calcium channels are involved in fast endocytosis at the mouse neuromuscular junction. Eur J Neurosci. 2008;27:1333–1344. doi: 10.1111/j.1460-9568.2008.06113.x. [DOI] [PubMed] [Google Scholar]
- 63.Coggins MR, et al. Stimulated exocytosis of endosomes in goldfish retinal bipolar neurons. J Physiol. 2007;584:853–865. doi: 10.1113/jphysiol.2007.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clayton EL, et al. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci. 2008;28:6627–6632. doi: 10.1523/JNEUROSCI.1445-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paillart C, et al. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23:4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teng H, Wilkinson RS. “Delayed” endocytosis is regulated by extracellular Ca2+ in snake motor boutons. J Physiol. 2003;551:103–114. doi: 10.1113/jphysiol.2003.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards DA, et al. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 68.Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LoGiudice L, Matthews G. Endocytosis at ribbon synapses. Traffic. 2007;8:1123–1128. doi: 10.1111/j.1600-0854.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 70.Llobet A, et al. Real-time measurement of exocytosis and endocytosis using interference of light. Neuron. 2003;40:1075–1086. doi: 10.1016/s0896-6273(03)00765-7. [DOI] [PubMed] [Google Scholar]
- 71.de Lange RP, et al. Two modes of vesicle recycling in the rat calyx of Held. J Neurosci. 2003;23:10164–10173. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schweizer FE, Ryan TA. The synaptic vesicle: cycle of exocytosis and endocytosis. Curr Opin Neurobiol. 2006;16:298–304. doi: 10.1016/j.conb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 73.Kuner T, et al. Photolysis of a caged peptide reveals rapid action of N-ethylmaleimide sensitive factor before neurotransmitter release. Proc Natl Acad Sci U S A. 2008;105:347–352. doi: 10.1073/pnas.0707197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rostaing P, et al. Preservation of immunoreactivity and fine structure of adult C. elegans tissues using high-pressure freezing. J Histochem Cytochem. 2004;52:1–12. doi: 10.1177/002215540405200101. [DOI] [PubMed] [Google Scholar]
- 75.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 76.Zhang B, et al. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 77.Lee JY, Schick M. Calculation of free energy barriers to the fusion of small vesicles. Biophys J. 2008;94:1699–1706. doi: 10.1529/biophysj.107.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zimmerberg J. Membrane biophysics. Curr Biol. 2006;16:R272–R276. doi: 10.1016/j.cub.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 79.Malinin VS, et al. Osmotic and curvature stress affect PEG-induced fusion of lipid vesicles but not mixing of their lipids. Biophys J. 2002;82:2090–2100. doi: 10.1016/S0006-3495(02)75556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martens S, et al. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 81.Bretou M, et al. A fast mode of membrane fusion dependent on tight SNARE zippering. J Neurosci. 2008;28:8470–8476. doi: 10.1523/JNEUROSCI.0860-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]