Deletion of Autophagy-related 5 (Atg5) and Pik3c3 Genes in the Lens Causes Cataract Independent of Programmed Organelle Degradation (original) (raw)
Background: The role of autophagy-dependent quality control in the lens remains unclear.
Results: Deletion of Atg5 and Pik3c3/Vps34 in the lens does not affect programmed organelle degradation but causes cataract and a developmental defect, respectively.
Conclusion: These genes are important for quality control and development of the lens.
Significance: This study provides new insights into biology and age-related pathology of the lens.
Keywords: Autophagy, Cataract, Lens, Protein Degradation, Ubiquitin, Organelle Degradation, Quality Control
Abstract
The lens of the eye is composed of fiber cells, which differentiate from epithelial cells and undergo programmed organelle degradation during terminal differentiation. Although autophagy, a major intracellular degradation system, is constitutively active in these cells, its physiological role has remained unclear. We have previously shown that _Atg5_-dependent macroautophagy is not necessary for lens organelle degradation, at least during the embryonic period. Here, we generated lens-specific Atg5 knock-out mice and showed that Atg5 is not required for lens organelle degradation at any period of life. However, deletion of Atg5 in the lens results in age-related cataract, which is accompanied by accumulation of polyubiquitinated and oxidized proteins, p62, and insoluble crystallins, suggesting a defect in intracellular quality control. We also produced lens-specific Pik3c3 knock-out mice to elucidate the possible involvement of _Atg5_-independent alternative autophagy, which is proposed to be dependent on Pik3c3 (also known as Vps34), in lens organelle degradation. Deletion of Pik3c3 in the lens does not affect lens organelle degradation, but it leads to congenital cataract and a defect in lens development after birth likely due to an impairment of the endocytic pathway. Taken together, these results suggest that clearance of lens organelles is independent of macroautophagy. These findings also clarify the physiological role of Atg5 and Pik3c3 in quality control and development of the lens, respectively.
Introduction
The lens of the eye is composed of epithelial cells and fiber cells (1, 2). During embryogenesis, primary fiber cells are differentiated from epithelial cells at the posterior surface of the lens vesicle, whereas secondary fiber cells are subsequently differentiated from newly proliferated epithelial cells at the edges of the anterior epithelium. In the process of terminal differentiation of these fiber cells, membrane-bound organelles such as nuclei, the endoplasmic reticulum (ER),2 and mitochondria are degraded, forming the organelle-free zone (OFZ) (3–5). Organelle degradation begins during the embryonic period in primary fiber cells and continues throughout life in secondary fiber cells.
Although epithelial-to-fiber cell differentiation continues throughout life, the rate and speed decrease with age (6, 7). In rodents, the entire process, from epithelial mitosis to final differentiation, requires only 1 week if initiated during the embryonic period, but it takes 9 months from 5 months of age (6). Because these differentiating fiber cells are metabolically active but quiescent like neurons (8), they require an efficient intracellular quality control system until terminally differentiated, especially the slowly differentiating fiber cells of the adult lens. However, how the intracellular quality in the lens cells is maintained in vivo has not been fully understood.
Macroautophagy (referred to as “autophagy” hereafter) is one of the major intracellular degradation pathways along with the ubiquitin-proteasome system (9). Cytoplasmic proteins and organelles are enclosed by the autophagosome and then delivered to the lysosome by autophagy. Genetic studies in yeast have identified a set of autophagy-related (ATG) genes that are essential for autophagy. Several ATG genes, including Atg5, are well conserved in higher eukaryotes (10). Reverse genetic techniques have revealed various physiological functions of autophagy in mammals such as adaptive responses to starvation, quality control of intracellular proteins and organelles, embryonic development, tumor suppression, and elimination of intracellular microbes (9).
The presence of autophagic vacuoles in differentiating fiber cells was reported using in vitro culture systems (11). We also showed that autophagy is constitutively active in the in vivo mouse lens (12, 13). However, contrary to our expectation, the results of our study using conventional Atg5 knock-out mice showed that autophagy is not essential for organelle degradation at least in primary fiber cells (13). Nonetheless, as Atg5 knock-out mice die soon after birth (14), the importance of autophagy in organelle degradation in secondary fiber cells remains unclear. In addition, the role of autophagy in intracellular quality control of lens cells, particularly slowly differentiating fiber cells in the adult lens, has not been determined.
Recently, “alternative autophagy,” which is independent of Atg5 and Atg7, was reported and suggested to have a potential role in removal of mitochondria in reticulocytes (15). This type of autophagy was shown to be dependent on some of the upstream Atg factors such as Ulk1, FIP200, Beclin 1, and Pik3c3 (the class III phosphatidylinositol 3-kinase (PtdIns3K), also known as Vps34) (15). These factors are mostly multifunctional; for example, Pik3c3 is important for endocytosis and multivesicular body formation as well as autophagy (16–18). If alternative autophagy is involved in lens organelle degradation, we might have missed it in our previous study using Atg5 knock-out mice (13).
The aim of this study was to generate lens-specific Atg5 and Pik3c3 knock-out mice to define their physiological role in the lens. We found that neither _Atg5_-dependent nor _Pik3c3_-dependent autophagy was essential for lens organelle degradation. However, _Atg5_-dependent autophagy was essential for intracellular quality control and suppression of age-related cataract. We also demonstrated that Pik3c3 was required for development of the lens after birth and suppression of congenital cataract.
EXPERIMENTAL PROCEDURES
Mice
Experimental procedures to produce Atg5flox/flox (19), MLR10-Cre transgenic (20), and GFP-LC3 transgenic mice (12) have been described previously. Methods to produce Pik3c3flox/flox mice will be presented elsewhere.3 Briefly, two loxP sequences were introduced into introns 19 and 21 of the Pik3c3 gene to flank exons 20 and 21. Upon Cre-mediated recombination, the two exons encoding the kinase domain essential for phosphorylation of PtdIns are deleted. Wild-type C57BL/6 mice were obtained from Japan SLC, Inc. All mice were fed ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University (no. 0130091C).
Antibodies
Rabbit polyclonal antibodies against Atg5 (SO4) (21), LC3 (22), and Pik3c3 (23) for immunoblotting have been previously described. Mouse monoclonal anti-LC3 antibody (4E12) for immunohistochemistry was purchased from MBL. We also purchased the following: mouse monoclonal antibodies against KDEL (StressGen); polyubiquitin (FK2, MBL); β-actin and pan-cadherin (CH-19, Sigma); rabbit polyclonal antibodies against Tom20 (FL-145), p53 (FL-393), β-crystallin (FL-252), and γ-crystallin (FL-175, Santa Cruz Biotechnology); rat monoclonal antibody against Lamp-1 (1D4B, Santa Cruz Biotechnology); and guinea pig polyclonal antibody against p62 (PROGEN). Alexa 488-conjugated anti-mouse and rat IgG, Alexa 568-conjugated anti-rabbit IgG, Alexa 568-conjugated anti-guinea pig IgG secondary antibodies, and Hoechst 33342 were purchased from Molecular Probes.
Immunoblotting
Lenses from both eyes dissected from neonatal and adult mice were homogenized in 0.1 and 1.0 ml, respectively, ice-cold 0.25 m sucrose buffer (50 mm Tris-HCl (pH 7.5), 1 mm EDTA, and Complete EDTA-free protease inhibitor (Roche Applied Science)). For cell fractionation, homogenates were treated with 1% Triton X-100 and centrifuged at 15,000 × g for 15 min to separate the supernatant (Triton X-100-soluble fraction) and pellet fractions. The pellets were resuspended in 1% SDS in phosphate-buffered saline (PBS) (Triton X-100-insoluble fraction). Protein extracts were boiled in sample buffer and subjected to SDS-PAGE and immunoblotting. The amount of protein was quantified by densitometric measurements using ImageJ software.
Proteasomal Activity Assay
Lenses from both eyes dissected from mice at 8 months old were homogenized in 1 ml of ice-cold 0.25 m sucrose buffer containing 1% Triton X-100. The chymotryptic activity of the proteasome was measured by mixing the lysate with an assay buffer containing 100 μm fluorogenic peptide substrate succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Peptide Institute, Inc.) in 50 mm Tris-HCl (pH 8.0) and 1 mm DTT in the presence or absence of 50 μm MG132. After incubation for 30 min at 37 °C, hydrolysis of the synthetic peptides was measured at excitation and emission wavelengths of 355 and 460 nm, respectively, using an ARVO MX Plate Reader (PerkinElmer Life Sciences). MG132-sensitive activity was considered to be proteasome-specific.
Analysis of Oxidized Proteins
Carbonyl-oxidized proteins were detected using the OxyBlot protein oxidation detection kit (Millipore) according to the manufacturer's instructions. In brief, Triton X-100-soluble and -insoluble fractions were first denatured with 6% SDS and then treated with either 2,4-dinitrophenylhydrazine solution or with derivatization control solution (negative control) for 15 min. After neutralization, the samples were subjected to SDS-PAGE and immunoblotting using an antibody specific to the 2,4-dinitrophenol moiety.
Preparation of NaOH-soluble and -insoluble Fractions
The sequential preparation of water-soluble, NaOH-soluble, and NaOH-insoluble fractions was performed as described previously (24). In brief, lens homogenates were first centrifuged at 15,000 × g for 15 min and separated into supernatant (water-soluble) and pellet fractions. The pellets were treated with 20 mm NaOH, centrifuged at 15,000 × g for 15 min, and further separated into supernatant (NaOH-soluble) and pellet fractions. The resultant pellets were washed with 1 mm Na2CO3 three times and resuspended in sample buffer containing 10 mm DTT (NaOH-insoluble fractions).
Immunohistochemistry
Lenses were fixed with 4% paraformaldehyde (PFA) in PBS (pH 7.4) overnight and treated with 15% sucrose in PBS for 4 h and then with 30% sucrose solution overnight. Lens samples were embedded in Super Cryoembedding Medium compound (SECTION-LAB, Japan) and stored at −80 °C. Mid-sagittal lens slices were sectioned at a thickness of 7 μm with a cryostat (CM3050 S, Leica) using adhesive film (Cryofilm type IIC9, SECTION-LAB) according to a previously described method (25). Cryosections were stained with hematoxylin and eosin and photographed using a microscope (BX51, Olympus) equipped with a digital camera (DP70, Olympus). For immunohistochemistry, after treatment with 0.1% Triton X-100 for 15 min and blocking in 5% bovine serum albumin in PBS for 30 min, lens sections were incubated with primary antibodies for 1 h, followed by further incubation for 1 h with secondary antibodies. For staining polyubiquitin and p62, lens sections were first prepared without fixation, followed by fixation with 4% PFA for 10 min. Sections were analyzed using a microscope (IX81, Olympus) equipped with a CCD camera (ORCA ER, Hamamatsu Photonics). Images were acquired using MetaMorph version 7.0 (Molecular Devices Japan).
Electron Microscopy
The lens from adult mice was fixed with 2.5% glutaraldehyde and 4% PFA in 0.1 m cacodylate buffer (pH 7.4) at room temperature overnight. The lens from neonatal mice was fixed with 2.5% glutaraldehyde and then post-fixed with 1.5% OsO4 in 0.1 m sodium phosphate buffer (pH 7.4) for 2 h. Tissues were dehydrated in a graded series of ethanol and embedded in epoxy resin. Ultra-thin sections were made using an ultramicrotome (Reichert). Sections were stained with uranyl acetate and lead citrate and observed under an H7100 electron microscope (Hitachi).
Quantitative RT-PCR
To prepare fiber cells, lenses from at least six mice were dissected by forceps into fiber cells and capsules that included adhering epithelial cells and vascular cells (the tunica vasculosa lentis). Total RNA was extracted from fiber cells using ISOGEN (Nippon Gene) and reverse-transcribed using ReverTraAce (Toyobo). PCRs were performed in triplicate using SYBR Premix Ex Taq (Takara Bio) and were monitored by a Thermal Cycler Dice TP800 (Takara Bio). Expression level of Pik3c3 was normalized to that of Actb. Primers used are listed as follows: Pik3c3, 5′-TGTCAGATGAGGAGGCTGTG-3′ and 5′-CCAGGCACGACGTAACTTCT-3′; Actb, 5′-TCCCTGGAGAAGAGCTACGA-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′.
Lens Growth and Cataract Analysis
Dark field images of the dissected lens in PBS were acquired using a microscope (SZX12, Olympus) and photographed. Lens equatorial diameter was traced on digitized images using Adobe Photoshop CS5. The presence of cataract was detected by gross examination and defined as any opacity in the lens of either one or both eyes. Cataract was also confirmed using microscopy after dissecting the lens in most representative cases.
Statistical Analysis
All numerical data represent the mean ± S.E. Statistical comparisons were made using the two-tailed Student's t test. The incidence of cataract was statistically validated using the χ2 test.
RESULTS
Atg5-deficient Lens Develops Age-related Cataract
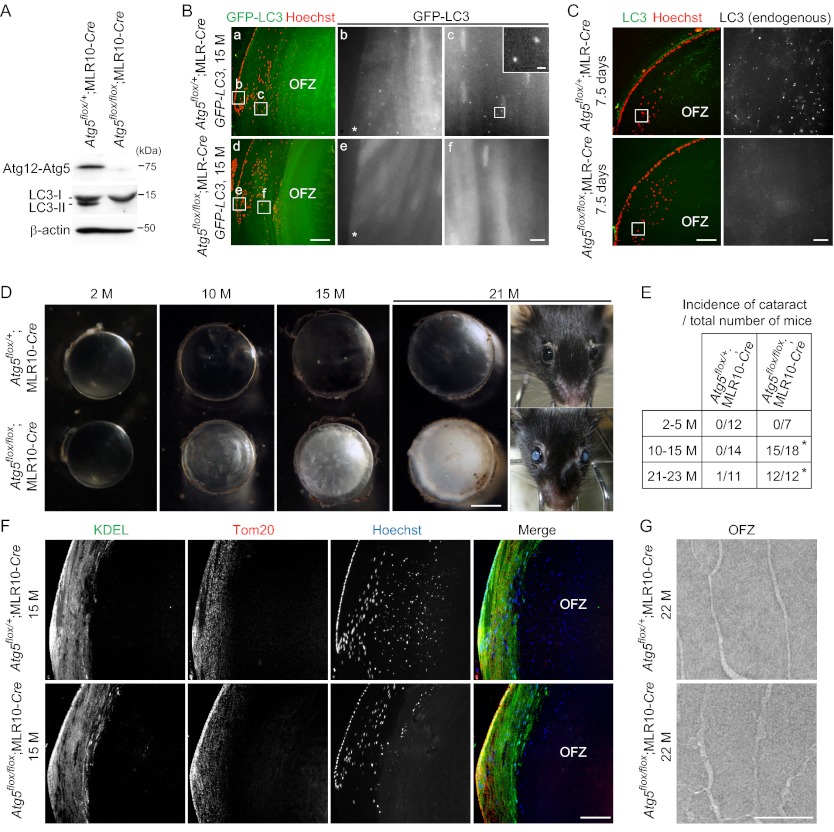
To investigate the physiological function of autophagy in the lens, we generated lens-specific Atg5_-deficient mice. Mice bearing an Atg5flox allele (19) were crossed with MLR10-Cre transgenic mice expressing Cre recombinase under the control of a modified αA-crystallin promoter (20). Expression of Atg5 (detected as the Atg12 to Atg5 conjugate) and the Atg5-dependent conversion of LC3-I to LC3-II (21) were completely inhibited in the lens of 15-month-old Atg5flox/flox;MLR10-Cre mice (Fig. 1A). To confirm autophagy inhibition in the lens, we crossed these mice with autophagy-indicator mice carrying a GFP-LC3 transgene, a specific marker of the autophagosome (12). In the control lens from 15-month-old Atg5flox/+;MLR10_-Cre;GFP-LC3 mice, a number of GFP-LC3 puncta were observed in differentiating secondary fiber cells at the superficial lens cortex (referred to hereafter as the “cortical region”) outside the OFZ (Fig. 1B, panels a–c) but not in differentiated fiber cells in the OFZ (data not shown), suggesting that autophagy is constitutively active only during the period of fiber cell differentiation. By contrast, Atg5flox/flox;MLR10_-Cre_;GFP-LC3 mice demonstrated almost no GFP-LC3 puncta in lens fiber cells (Fig. 1B, panels d–f). We also detected endogenous LC3-positive puncta in secondary fiber cells of Atg5flox/+;MLR10-Cre mice but not Atg5flox/flox;MLR10-Cre mice at 7.5 days after birth (Fig. 1C). These results suggest that autophagosome formation is suppressed in the _Atg5_-deficient lens.
FIGURE 1.
Atg5 is required for suppression of age-related cataract but not for programmed organelle degradation in the lens. A, expression of Atg5 and LC3B in the lens of 15-month (M)-old Atg5flox/+;MLR10-Cre and Atg5flox/flox;MLR10-Cre mice. Triton X-100-soluble fractions of lens homogenates were prepared and subjected to immunoblot analysis using antibodies against Atg5 and LC3. β-Actin was used as a loading control. The positions of the Atg12-Atg5 conjugate, LC3-I and LC3-II (LC3-PE conjugate), are indicated. B, GFP-LC3 puncta formation in the lens of 15-month-old Atg5flox/+;MLR10-Cre;GFP-LC3 (panels a–c) and Atg5flox/flox;MLR10-Cre;GFP-LC3 (panels d–f) mice. The nuclei were stained with Hoechst 33342. GFP signals in the indicated regions in panels a and d are magnified and shown in panels b, c, e, and f. The GFP-LC3 puncta in panel c are further magnified and shown in the inset. Asterisks indicate lens epithelial cells. OFZ, organelle-free zone. Scale bars, 100 μm (panels a and d), 10 μm (panels b, c, e, and f), and 1 μm in inset (panel c). C, immunohistochemical analysis of the lens of 7.5-day-old Atg5flox/+;MLR10-Cre and Atg5flox/flox;MLR10-Cre mice. Slices were stained with anti-LC3 antibody and Hoechst 33342 (left). Endogenous LC3 signals in the indicated regions were magnified and are shown in the right panels. Scale bars, 100 μm (left) and 10 μm (right). D, representative dark field images of the lens of 2-, 10-, 15-, and 21-month-old mice. The appearance of a 21-month-old mouse is also shown. Scale bar, 1 mm. E, incidence of cataract at indicated ages. Cataract was defined as visible opacity by gross examination and microscopy. p < 0.0001, χ2 test comparing the two genotypes. F, immunohistochemical staining of the lens of 15-month-old mice using anti-KDEL (marker of the ER) and anti-Tom20 (mitochondrial marker) antibodies and Hoechst 33342. Data are representatives of four independent experiments. Scale bar, 100 μm. G, electron micrographs of fiber cells in the OFZ of 22-month-old mice. Scale bar, 1 μm.
The lens in Atg5flox/flox;MLR10-Cre mice showed normal morphogenesis and was transparent until around 5 months after birth (Fig. 1D). By 6–9 months, however, opacity appeared in the lens and progressively developed with age. After 21 months, all Atg5flox/flox;MLR10-Cre mice (n = 12) showed severe bilateral cataract, whereas only one of the 11 Atg5flox/+;MLR10-Cre mice showed cataract (unilateral) (Fig. 1E). These results suggest that Atg5 is required for suppression of age-related cataract.
Atg5 Is Not Required for Organelle Degradation in Fiber Cells
We next determined whether autophagy is dispensable for organelle degradation not only in primary fiber cells but also in secondary fiber cells in adult mice. In secondary fiber cells of Atg5flox/+;MLR10-Cre mice at 15 months of age, the ER, mitochondria, and nuclei were present in the cortical region but not in the central OFZ (Fig. 1F). Although Atg5flox/flox;MLR10-Cre mice developed cataract, the lens OFZ was normally generated in these mice. Electron microscopic analysis also confirmed the generation of an OFZ (Fig. 1G). Thus, these data suggest that autophagy is not required for lens organelle degradation throughout life and that the cause of age-related cataract development in the _Atg5_-deficient lens is not due to a defect in programmed organelle degradation.
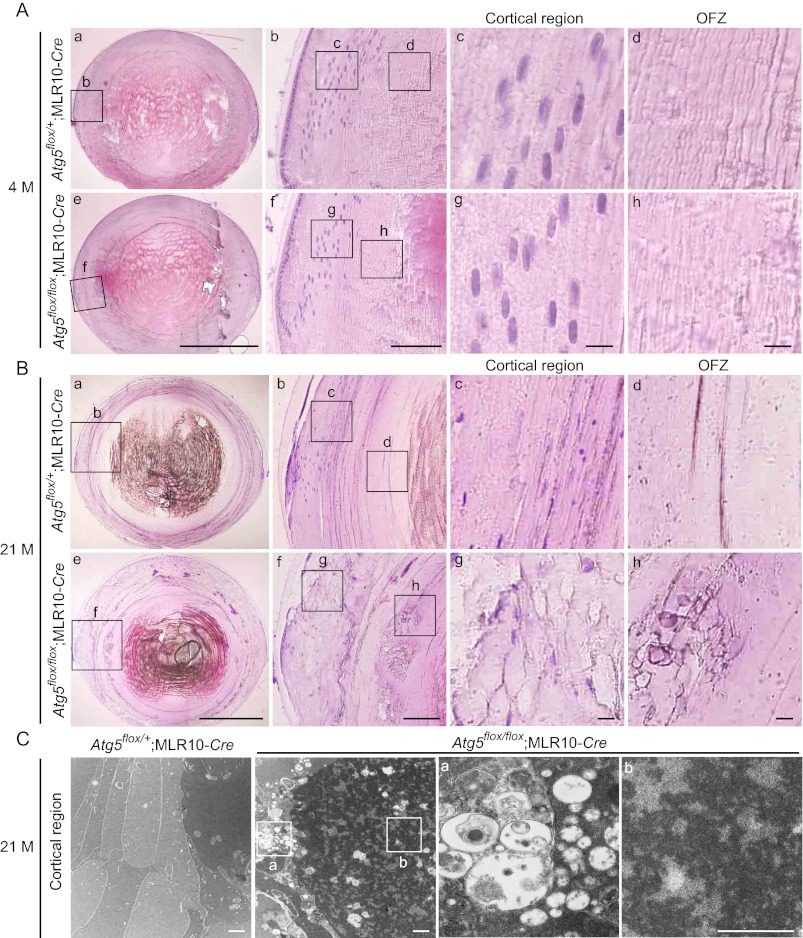
Lens Fiber Cells Are Disorganized in the Cortical Region in the Lens of Aged Lens-specific Atg5-deficient Mice
We next analyzed the phenotype of _Atg5_-deficient lens in more detail. Hematoxylin and eosin staining of the lens revealed no obvious morphological difference between Atg5flox/+;MLR10-Cre mice and Atg5flox/flox;MLR10-Cre mice at 4 months of age (Fig. 2A). At 21 months, control lens of Atg5flox/+;MLR10-Cre mice showed highly ordered structure of the fiber cells in the cortical region (Fig. 2B, panels a–d). By contrast, the fiber cells in this region were disorganized and swollen in Atg5flox/flox;MLR10-Cre mice (Fig. 2B, panels e–h). These changes were observed in almost all differentiating fiber cells in the cortical region (Fig. 2B, panel g) and some of the differentiated fiber cells that have lost organelles (Fig. 2B, panel h). Electron microscopic analysis of the lens of 21-month-old Atg5flox/flox;MLR10-Cre mice revealed accumulation of vacuoles of various sizes and high density deposits in the cytoplasm of differentiating fiber cells (Fig. 2C) but not in terminally differentiated cells in the central region of the lens (Fig. 1G). Taken together, these results suggest that age-related cataract development in _Atg5_-deficient lens is caused by disorganization and accumulation of abnormal materials in cortical fiber cells.
FIGURE 2.
Fiber cells are disorganized in the cortical region of the lens in aged lens-specific _Atg5_-deficient mice. A and B, hematoxylin and eosin staining of the lens of 4-month (M)-old (A) and 21-month-old (B) Atg5flox/+;MLR10-Cre (panels a–d) and Atg5flox/flox;MLR10-Cre (panels e–h) mice. Magnified images of the indicated cortical region (panels c and g) and OFZ (panels d and h) are shown. Scale bars, 1 mm (panels a and e), 100 μm (panels b and f), and 10 μm (panels c, d, g, and h). C, electron micrographs of fiber cells in the cortical region of the lens of 21-month-old mice. Magnified images of the indicated regions are shown in the right panels (panels a and b). Scale bars, 1 μm.
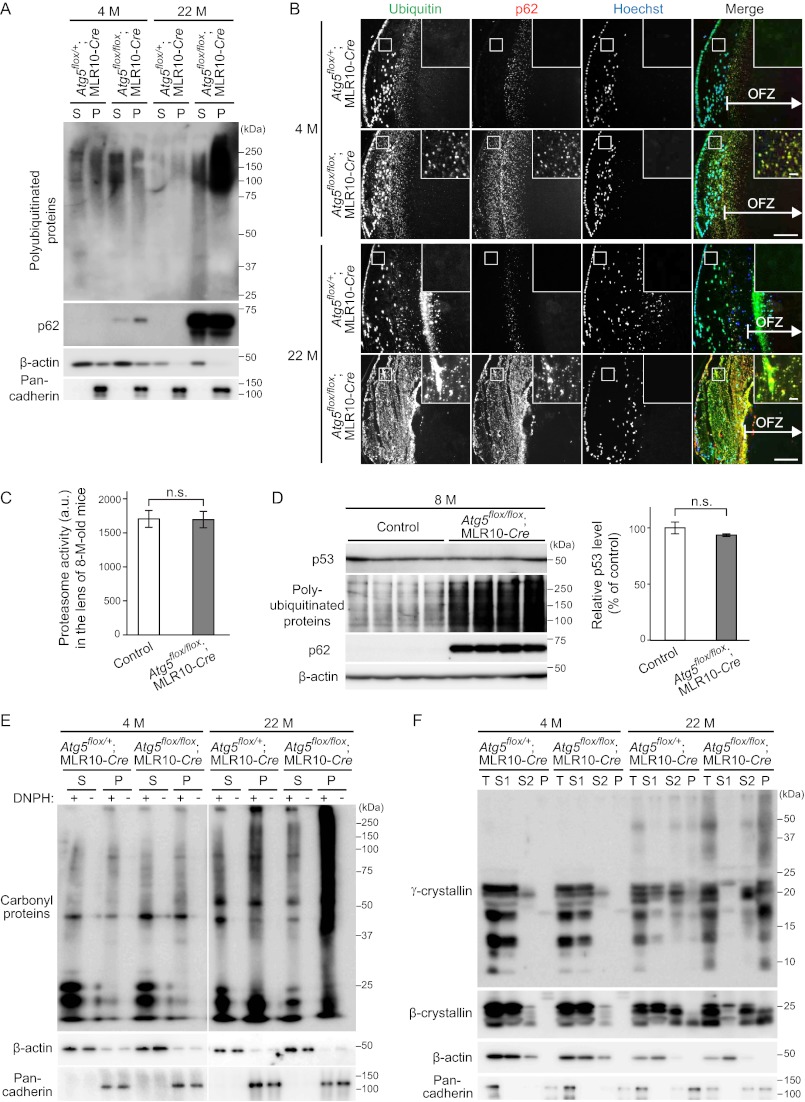
Polyubiquitinated Proteins and p62 Accumulate in Atg5-deficient Lens
As autophagy is important for intracellular quality control, autophagy defects lead to accumulation of abnormal proteins and organelles, which are often ubiquitinated (9). Although 4-month-old Atg5flox/flox;MLR10-Cre mice were free of cataracts, immunoblot analysis revealed a slight accumulation of polyubiquitinated proteins (Fig. 3A). p62, which is a selective substrate of autophagy and is known to aggregate under autophagy-deficient conditions (26, 27), also accumulated mainly in the Triton X-100-insoluble fraction in the lens (Fig. 3A). There was massive accumulation of both polyubiquitinated proteins and p62 in the cataractous lens of 22-month-old Atg5flox/flox;MLR10-Cre mice. These results suggest that, as observed in other tissues (9), autophagy is important for intracellular quality control in the lens.
FIGURE 3.
Atg5-dependent autophagy is required for quality control in lens cells. A, immunoblot analysis of polyubiquitinated proteins and p62 of the lens of 4-month (M)-old and 22-month-old mice. Lens homogenates separated into 1% Triton X-100-soluble (S) or -insoluble (P) fractions were analyzed by immunoblotting using anti-ubiquitin and anti-p62 antibodies. β-Actin and pan-cadherin were used as loading controls for the soluble and insoluble fractions, respectively. B, immunohistochemical analysis of ubiquitinated proteins and p62. Lenses were stained using anti-ubiquitin and anti-p62 antibodies and Hoechst 33342. The indicated regions are shown and magnified in the insets. Scale bars, 100 μm and 10 μm in inset. C, chymotryptic activities of the proteasome in the lens of 8-month (M)-old control (Atg5flox/flox and Atg5flox/+;MLR10-Cre) and Atg5flox/flox;MLR10-Cre mice. Lens homogenates were mixed with fluorogenic peptide substrate succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin in the presence or absence of MG132. MG132-sensitive activities were quantified (mean ± S.E.). n.s., not significant, unpaired Student's t test; n = 6. D, immunoblot analysis of p53, a proteasome substrate, in the lens of 8-month-old control and Atg5flox/flox;MLR10-Cre mice. Lens homogenates were analyzed by immunoblotting using anti-p53, anti-ubiquitin, and anti-p62 antibodies. β-Actin was used as a loading control. Relative protein levels of p53 to β-actin were quantified by densitometric analysis (mean ± S.E.). n.s., not significant, unpaired Student's t test; n = 4. E, immunoblot analysis of oxidized (carbonylated) proteins in the lens. Triton X-100-soluble and -insoluble fractions were treated with or without 2,4-dinitrophenylhydrazine to derivatize carbonyl groups in oxidized proteins. These samples were subjected to immunoblot analysis using anti-2,4-dinitrophenol (DNP) antibody. F, immunoblot analysis of crystallins using anti-γ- and anti-β-crystallin antibodies. Total lens homogenates (T) were first separated into water-soluble (S1) and -insoluble fractions. The latter was treated with NaOH and separated into NaOH-soluble (S2) and -insoluble (P) fractions. β-Actin and pan-cadherin were used as loading controls. Data are representatives of two (A, C, D, E, and F) or three (B) independent experiments.
We next determined where these polyubiquitinated proteins and p62 accumulated. In control lens of Atg5flox/+;MLR10-Cre mice, punctate signals positive for ubiquitin and/or p62 were observed in the outermost region of the OFZ at 4 and 22 months (Fig. 3B) and at 0.5 days of age (data not shown). This seems to be related to organelle and protein degradation as part of the normal developmental process, and it is consistent with previous findings that the ubiquitin-proteasome system is active during the process (28). However, in Atg5flox/flox;MLR10-Cre mice, aggregates containing ubiquitin and p62 were also observed in the cortical region of the lens outside the OFZ (Fig. 3B), where autophagy constitutively occurs in control mice (Fig. 1B). They were already detected in transparent lens of 4-month-old mice and became more massive in cataractous lens at 22 months. These additional aggregates were not generated in the control lens of Atg5flox/+;MLR10-Cre mice (Fig. 3B). No ubiquitin or p62 aggregates were observed in deeper regions of the OFZ of the lens of either Atg5flox/+;MLR10-Cre or Atg5flox/flox;MLR10-Cre mice (Fig. 3B). These results suggest that constitutive autophagy in slowly differentiating cortical fiber cells is important for prevention of accumulation of abnormal aggregates.
There was no difference in proteasomal chymotryptic activity in the lens between control and Atg5flox/flox;MLR10-Cre mice at 8 months of age (Fig. 3C), as reported previously in neuron-specific _Atg7_-deficient mice (29). The protein level of p53, a typical substrate of the proteasome (30), in the lens was also comparable between control and Atg5flox/flox;MLR10-Cre mice (Fig. 3D). As polyubiquitinated proteins and p62 already massively accumulated in the lens of Atg5flox/flox;MLR10-Cre mice at this age, these results suggest that the accumulation of ubiquitin aggregates in _Atg5_-deficient lens was not caused by a defect in the ubiquitin-proteasome system.
Insoluble Oxidized Proteins and Crystallins Accumulate in Cataractous Atg5-deficient Lens
Because age-related cataract is frequently associated with oxidative stress and/or a decrease in crystalline solubility (31), we next investigated whether these changes were found in the _Atg5_-deficient lens. Carbonylated proteins extensively accumulated in the insoluble fraction in the cataractous lens of 22-month-old but not in the transparent lens of 4-month-old Atg5flox/flox;MLR10-Cre mice (Fig. 3E); this suggests that oxidative stress is present in the aged _Atg5_-deficient lens. In general, crystallins are water- and NaOH-soluble in the normal lens but become insoluble under cataractous conditions (24). Indeed, γ- and β-crystallins, which are abundant proteins specifically expressed in lens fiber cells (32), were solubilized in water or NaOH in 4-month-old Atg5flox/flox;MLR10-Cre mice. However, in the lens of 22-month-old Atg5flox/flox;MLR10-Cre mice, the amount of water-soluble crystallins decreased, whereas that of NaOH-insoluble crystallins increased (Fig. 3F). There was no difference in the distribution of γ- and β-crystallins in the lens between Atg5flox/+;MLR10-Cre and Atg5flox/flox;MLR10-Cre mice at 22 months of age (data not shown). These data indicate that cataract in _Atg5_-deficient lens is accompanied by accumulation of insoluble oxidized proteins and crystallins.
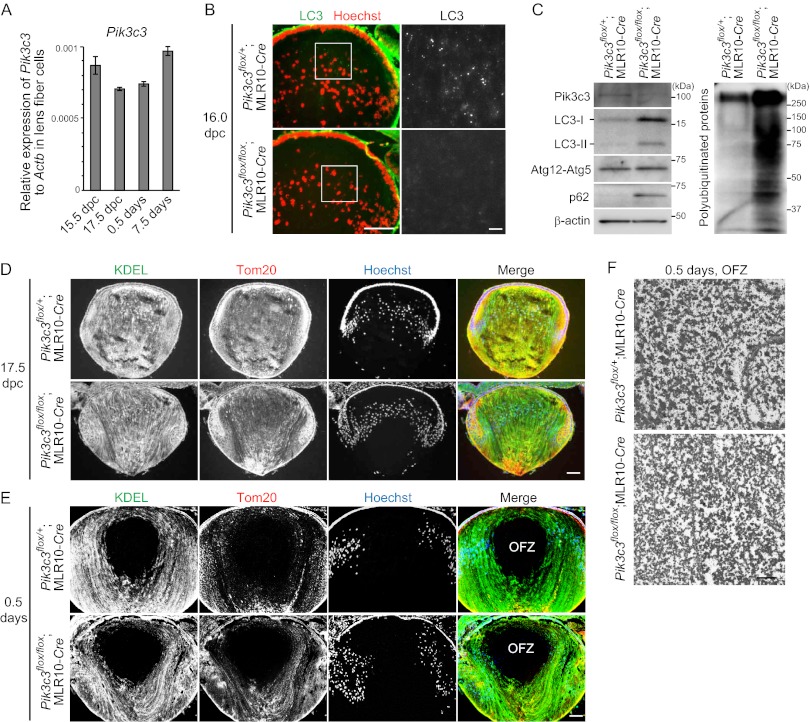
Pik3c3-dependent Autophagy Is Not Required for Lens Organelle Degradation
Following the recent report that alternative autophagy is dependent on Pik3c3 (15), we generated lens-specific _Pik3c3_-deficient mice to examine the possible involvement of _Atg5_-independent alternative autophagy in lens organelle degradation. Pik3c3 was constitutively expressed in lens fiber cells in wild-type embryos at 15.5 and 17.5 days postcoitum (dpc), and neonates at 0.5 and 7.5 days old (Fig. 4A). Whereas endogenous LC3 puncta were detected in the embryonic lens (at 16.0 dpc) of Pik3c3flox/+;MLR10-Cre mice, they were absent in the lens of Pik3c3flox/flox;MLR10-Cre embryos (Fig. 4B), suggesting that autophagy was inhibited at this time point. This is in line with previous reports using MLR10-Cre transgenic mice (33, 34), in which expression of Cre initiated around 10.5 dpc (20), and therefore Pik3c3flox/flox;MLR10-Cre mice can be used for functional analysis in both primary and secondary fiber cells as reported previously (33–36). Immunoblot analysis of the lens confirmed the absence of Pik3c3 proteins and accumulation of polyubiquitinated proteins and p62 in the 0.5-day-old Pik3c3flox/flox;MLR10-Cre neonates (Fig. 4C). LC3-I and LC3-II rather accumulated in _Pik3c3-_deleted lens, which is consistent with previous findings that Pik3c3 and Atg14, a subunit of the class III PtdIns3K complex, are not essential for LC3 conversion (37–39). Taken together, these data suggest that Pik3c3 was successfully deleted in the lens of Pik3c3flox/flox;MLR10-Cre embryos before initiation of organelle degradation in the lens.
FIGURE 4.
Pik3c3-dependent autophagy is not required for lens organelle degradation. A, quantitative RT-PCR analysis of Pik3c3 in lens fiber cells of embryos at 15.5 and 17.5 dpc and neonates at 0.5 and 7.5 days old. Relative mRNA expression of Pik3c3 to Actb is shown. B, immunohistochemical analysis of endogenous LC3 in the lens of Pik3c3flox/+;MLR10-Cre and Pik3c3flox/flox;MLR10-Cre embryos at 16.0 dpc. Sections were stained with anti-LC3 antibody and Hoechst 33342 (left panel). Magnified images of LC3 staining are shown in the right panels. Scale bars, 100 μm (left panel) and 10 μm (right panel). C, immunoblot analysis of Pik3c3, LC3, Atg5, p62, and polyubiquitinated proteins in the lens of 0.5-day-old neonates. Triton X-100-soluble fractions of lens homogenates were prepared and subjected to immunoblotting using the indicated antibodies. β-Actin was used as a loading control. D and E, immunohistochemical analysis of the lens of mice at 17.5 dpc (D) and 0.5 days after birth (E) using anti-KDEL (marker of the ER) and anti-Tom20 (mitochondrial marker) antibodies and Hoechst 33342. Data are representatives of seven independent experiments. Scale bar, 100 μm. F, electron micrographs of the OFZ in the lens of 0.5-day-old neonates. Scale bar, 1 μm.
Next, we investigated the requirement of Pik3c3 in lens organelle degradation during the embryonic period. Because organelles in primary fiber cells are degraded between 17.5 dpc and birth (13, 40), we analyzed this time period. At 17.5 dpc, the lens from both Pik3c3flox/+;MLR10-Cre and Pik3c3flox/flox;MLR10-Cre embryos contained an abundance of ER, mitochondria, and nuclei in primary fiber cells located in the central region (Fig. 4D). By contrast, the organelles in primary fiber cells were completely degraded, and the OFZ was formed in the lens of 0.5-day-old Pik3c3flox/+;MLR10-Cre neonates, and there were no significant differences in the lens of Pik3c3flox/flox;MLR10-Cre neonates (Fig. 4E). The absence of organelles was further confirmed by electron microscopy (Fig. 4F). These results suggest that Pik3c3 is not required for lens organelle degradation during the embryonic period in primary fiber cells and that neither conventional nor alternative macroautophagy is significantly involved in this process.
Lens-specific Pik3c3-deficient Mice Develop Congenital Cataract and Microphthalmia
Although Pik3c3flox/flox;MLR10-Cre mice showed normal organelle degradation in the lens during the embryonic period, they developed congenital cataract and microphthalmia (Fig. 5A). Pik3c3flox/flox;MLR10-Cre neonates at 0.5 days old showed bilateral lens opacity. The size of the lens in the Pik3c3flox/flox;MLR10-Cre mouse was smaller than that of the Pik3c3flox/+;MLR10-Cre mouse at 0.5 and 7.5 days and 2 months, suggesting that post-neonatal lens development was defective. In addition, the size of the entire eyeball was smaller in _Pik3c3_-deficient mice, compared with control mice; this was identifiable at birth and more pronounced at 2 months of age (Fig. 5B).
FIGURE 5.
Pik3c3 is required for secondary fiber cell differentiation and suppression of congenital cataract and microphthalmia. A, representative dark field images of dissected lens of Pik3c3flox/+;MLR10-Cre (control) and Pik3c3flox/flox;MLR10-Cre mice (Pik3c3 KO) at 0.5 and 7.5 days and 2 months. Scale bar, 1 mm. The equatorial diameter of the lens was quantified (mean ± S.E.). *, p < 0.05; **, p < 0.01, unpaired Student's t test; n = 6 (0.5 days), 6 (7.5 days), and 4 (2 months). B, representative images of eyeball and appearance of a 2-month (M)-old mouse. Scale bar, 1 mm. The equatorial diameter of the eyeball at 2 months was quantified (mean ± S.E.). *, p < 0.05, unpaired Student's t test comparing indicated pairs; n = 3. C, hematoxylin and eosin staining of the lens of 17.5-dpc Pik3c3flox/+;MLR10-Cre (control, panels a–c) and Pik3c3flox/flox;MLR10-Cre (Pik3c3 KO, panels d–f) embryos. Magnified images of secondary fiber (panels b and e) and primary fiber cells (panels c and f) in the indicated regions are shown in the right panels. Scale bars, 0.1 mm (panels a and d) and 20 μm (panels b, c, e, and f). D, hematoxylin and eosin staining of the lens from mice at 0.5 and 7.5 days and 2 months. Magnified images in the indicated regions are shown in the right (0.5 and 7.5 days) or lower (2 months) panels. Arrowheads indicate cellular aggregates. Scale bars, 0.1 mm. E, immunohistochemical analysis of crystallins using anti-β- and anti-γ-crystallin antibodies in the lens of 0.5-day-old neonates. Scale bar, 100 μm. F, immunoblot analysis of γ- and β-crystallins in the lens of 0.5-day-old neonates. Triton X-100-soluble fractions of lens homogenates were prepared and subjected to immunoblotting using the indicated antibodies. β-Actin was used as a loading control.
Disruption of Pik3c3 Impairs Differentiation of Secondary Lens Fiber Cells with Accumulation of Vacuoles
At 17.5 dpc, secondary fiber cells in the outer cortical region, where differentiation is initiated, were disorganized and not elongated in Pik3c3flox/flox;MLR10-Cre embryos (Fig. 5C, panel e). These abnormalities were not detected in primary fiber cells (Fig. 5C, panel f). At 0.5 and 7.5 days after birth, these abnormal secondary fiber cells were still not elongated and had formed cellular aggregates (Fig. 5D). At 2 months, fiber cells were fragmented and markedly aggregated, and the entire lens structure eventually deteriorated. Other ocular abnormalities, including retinal folding and immature iris development, were also detected in these eyes (Fig. 5D). Expression of γ- and β-crystallins, which is a hallmark of early fiber cell differentiation (2), was reduced in the aggregated fiber cells in _Pik3c3_-deficient lens at 0.5 days of age (Fig. 5E), even though expression of these crystallins in whole lens was not significantly affected (Fig. 5F). These results suggest that defective secondary fiber differentiation is the major cause of the developmental defects in _Pik3c3_-deficient lens.
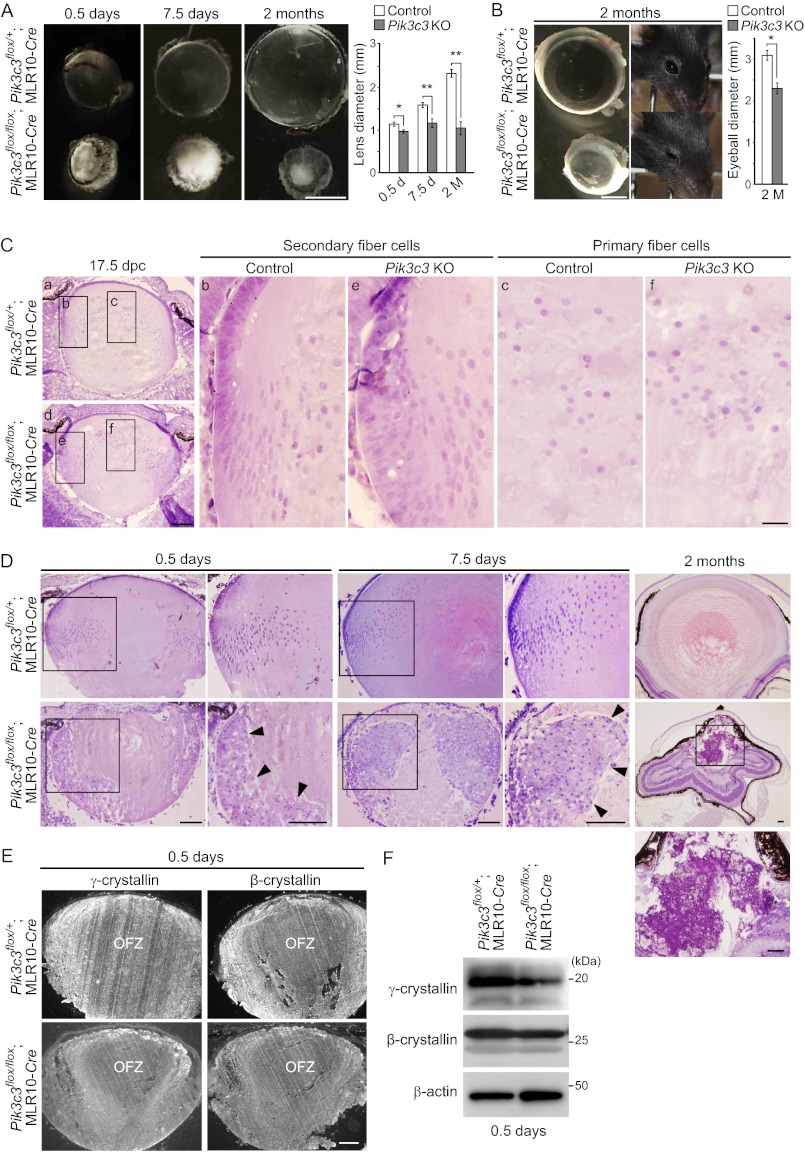
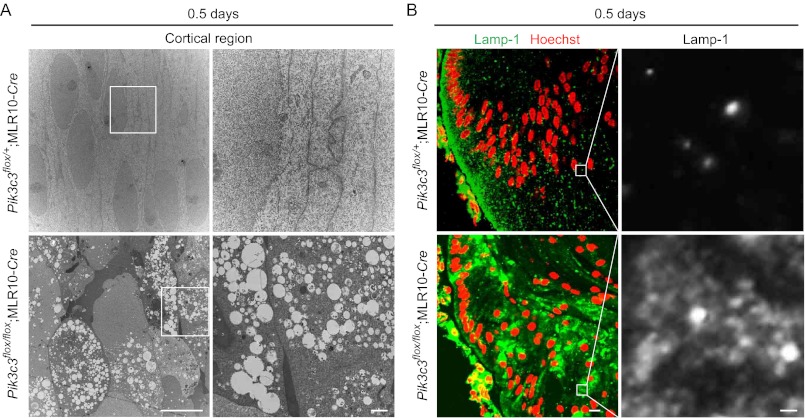
Electron microscopic analysis of the lens of 0.5-day-old neonates also showed disorganized alignment of swollen secondary fiber cells in the cortical region of the lens of Pik3c3flox/flox;MLR10-Cre but not Pik3c3flox/+;MLR10-Cre mice (Fig. 6A). A large number of vacuoles were observed in these disorganized secondary fiber cells (Fig. 6A), but not in the primary fiber cells in the central region of the lens (Fig. 4F). Many Lamp-1-positive structures accumulated in the disorganized fiber cells of Pik3c3flox/flox;MLR10-Cre mice (Fig. 6B), suggesting that the vacuoles are derived from the late endosome or lysosome. Taken together, these results suggest that Pik3c3 has an essential role in a pathway other than autophagy, probably the endocytic pathway, for development of the lens likely through differentiation of secondary fibers.
FIGURE 6.
Accumulation of vacuoles in _Pik3c3_-deficient secondary lens fiber cells. A, electron micrographs of fiber cells in the cortical region of the lens in 0.5-day-old mice. Magnified images in the indicated regions are shown in the right panels. Scale bars, 10 μm (left) and 1 μm (right). B, immunohistochemical analysis of Lamp-1 in 0.5-day-old mice. Lenses were stained using anti-Lamp-1 antibody and Hoechst 33342 (left). Magnified images in the indicated regions are shown in the right panels. Scale bars, 10 μm (left) and 1 μm (right).
DISCUSSION
In this study, we demonstrate that programmed organelle degradation in the lens requires neither Atg5 nor Pik3c3; this suggests that conventional macroautophagy and so-called “alternative” macroautophagy (15) are not primarily involved in this process. These results extend the findings of our previous study demonstrating a nonessential role of _Atg5_-dependent autophagy in organelle degradation in primary fiber cells (13). Although we could not analyze the role of Pik3c3 in secondary fiber cells due to the developmental defect, our data suggest that a yet uncharacterized degradation system is involved in lens organelle degradation. Several potential mechanisms have been reported, which include the ubiquitin-proteasome system (28, 41, 42), 15-lipoxygenase (43), and DNase II-like acid DNase (DLAD) (44). Among them, DLAD, a lysosomal DNase (45, 46), is required for degradation of nuclear DNA in the lens (44). Thus, it is likely that the lysosome is involved in degradation of nuclear DNA in a manner different from any known types of autophagy. Further analysis would be required to reveal the novel function of the lysosome.
Our findings instead revealed a critical role of autophagy in quality control of intracellular proteins and organelles, as observed in other tissues such as the liver, nervous system, heart, and glomerulus (9, 47). This function in the lens is important for prevention of age-related cataract. We detected abnormal accumulation of aggregates containing ubiquitin and p62 in differentiating fiber cells in the cortical region of the lens from aged lens-specific _Atg5_-deficient mice. There was no abnormal accumulation of these aggregates in the center of the OFZ, where autophagy cannot occur even in wild-type cells because of the lack of lysosomes. Thus, the importance of autophagy is cell type- or age-specific. It was reported that fiber cell differentiation requires 2, 4, and 9 months when initiated at 1, 2, and 5 months of age, respectively (6). This time is almost constant between 5 and 10 months of age (6). It would be reasonable to hypothesize that autophagic quality control is particularly important when fiber cell differentiation is very slow. Once fiber cells are terminally differentiated, autophagy may no longer be important. This could explain why cataract develops in _Atg5_-deficient lens between 5 and 10 months of age with a relatively intact central region.
An important question is whether impairment of autophagy is associated with cataracts in humans. Recently, mutations in the FYCO1 gene were identified in patients with autosomal-recessive congenital cataracts (48). It was reported that FYVE and coiled-coil domain containing 1 (FYCO1) protein interact with PtdIns(3)P, LC3, and Rab7 and are involved in positioning of autophagosomes (49). FYCO1 and several ATG genes are indeed expressed in the human lens (50). Furthermore, mutations in the gene encoding ectopic P-granules autophagy protein 5 homolog (EPG5), a higher eukaryote-specific gene required for lysosomal degradation of autophagosome (51), were recently identified in patients with recessively inherited congenital Vici syndrome, which demonstrates cataracts in addition to other multiple abnormalities such as callosal agenesis, immunodeficiency, and cardiomyopathy (52). Thus, it would be important to determine whether the congenital cataract seen in patients with these conditions is indeed caused by a defect in autophagy or in another function of FYCO1 (53) and EPG5 (54) and whether autophagic activity is affected in other types of human cataract.
We also revealed that Pik3c3 is essential for lens development after birth. The abnormal development of the entire eyeball, including the retina and iris in Pik3c3flox/flox;MLR10-Cre mice, may be a secondary result of impaired lens development, as similar phenotypes were reported in other mouse models with lens-specific deletion of fibroblast growth factor receptor (33), PKCλ (34), β1-integrin (35), β-catenin (36), and N/E-cadherin (55). Alternatively, it may be due to an off-target effect of MLR10-Cre expression in non-lens tissues in the eye. However, this is unlikely because MLR10-Cre is highly specific to the lens within the eye; only a few cells in the cornea demonstrate ectopic expression (20). As development of the lens in Atg5flox/flox;MLR10-Cre mice is normal, the impaired development of _Pik3c3_-deficient lens is likely to be due to a defect in an autophagy-independent pathway. As reported previously in other _Pik3c3_-deficient cells (17, 56, 57), we observed many enlarged vacuoles in the lens, which were likely positive for Lamp-1, suggesting a defect in the late endosome or lysosome. Another possibility is that the lens phenotype is due to a defect in alternative autophagy (15). However, the lack of specific markers for alternative autophagy hampered further investigation.
Our data suggest that fiber cell differentiation is defective in _Pik3c3_-deficient secondary but not primary lens fiber cells. A similar pattern is observed in the zebrafish lens opaque (lop) mutant, in which a gene encoding PtdIns synthase is mutated, and lens development is compromised in the secondary but not primary fiber cells (58). Thus, the contribution of PtdIns metabolism during differentiation might be different in these two fiber cell types. Mutations in the gene encoding oculocerebrorenal syndrome of Lowe 1 (OCRL1), a phosphoinositide 5-phosphatase, are responsible for Lowe syndrome, characterized by congenital cataracts, mental retardation, and renal Fanconi syndrome (59). Therefore, further studies on the role of Pik3c3 in PtdIns metabolism of the lens will provide a more general insight into the pathogenesis of congenital cataracts.
*
This work was supported, in whole or in part, by National Institutes of Health Grant R01EY012995 from NEI (to M. L. R.). This work was also supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, the Funding Program for Next Generation World-Leading Researchers (to T. S. and N. M.), the Takeda Science Foundation (to T. S. and N. M.), the Toray Science Foundation (to T. S.), grants for a research fellowship of the Japan Society for the Promotion of Science for Young Scientists (to H. M.), and Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency (to J. S.).
3
S. Eguchi, H. Kimura, J. Sasaki, and T. Sasaki, unpublished data.
2
The abbreviations used are:
ER
endoplasmic reticulum
OFZ
organelle free zone
LC3
microtubule-associated protein light chain 3
Atg5
autophagy-related 5
Pik3c3
phosphatidylinositol 3-kinase catalytic subunit type 3
Vps34
vacuolar protein sorting 34
PtdIns
Phosphatidylinositol
Cre
cyclization recombination enzyme
Lamp-1
lysosomal-associated membrane protein-1
PFA
paraformaldehyde
dpc
days postcoitum.
REFERENCES
- 1.Piatigorsky J. (1981) Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 19, 134–153 [DOI] [PubMed] [Google Scholar]
- 2.McAvoy J. W., Chamberlain C. G., de Iongh R. U., Hales A. M., Lovicu F. J. (1999) Lens development. Eye 13, 425–437 [DOI] [PubMed] [Google Scholar]
- 3.Kuwabara T., Imaizumi M. (1974) Denucleation process of the lens. Invest. Ophthalmol. 13, 973–981 [PubMed] [Google Scholar]
- 4.Bassnett S., Shi Y., Vrensen G. F. (2011) Biological glass: structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1250–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wride M. A. (2011) Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1219–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna C., O'Brien J. E. (1961) Cell production and migration in the epithelial layer of the lens. Arch. Ophthalmol. 66, 103–107 [DOI] [PubMed] [Google Scholar]
- 7.Rafferty N. S., Rafferty K. A., Jr. (1981) Cell population kinetics of the mouse lens epithelium. J. Cell. Physiol. 107, 309–315 [DOI] [PubMed] [Google Scholar]
- 8.Frederikse P. H., Kasinathan C., Kleiman N. J. (2012) Parallels between neuron and lens fiber cell structure and molecular regulatory networks. Dev. Biol. 368, 255–260 [DOI] [PubMed] [Google Scholar]
- 9.Mizushima N., Komatsu M. (2011) Autophagy: renovation of cells and tissues. Cell 147, 728–741 [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N., Yoshimori T., Ohsumi Y. (2011) The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 [DOI] [PubMed] [Google Scholar]
- 11.Walton J., McAvoy J. (1984) Sequential structural response of lens epithelium to retina-conditioned medium. Exp. Eye Res. 39, 217–229 [DOI] [PubMed] [Google Scholar]
- 12.Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui M., Yamamoto A., Kuma A., Ohsumi Y., Mizushima N. (2006) Organelle degradation during the lens and erythroid differentiation is independent of autophagy. Biochem. Biophys. Res. Commun. 339, 485–489 [DOI] [PubMed] [Google Scholar]
- 14.Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. (2004) The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 [DOI] [PubMed] [Google Scholar]
- 15.Nishida Y., Arakawa S., Fujitani K., Yamaguchi H., Mizuta T., Kanaseki T., Komatsu M., Otsu K., Tsujimoto Y., Shimizu S. (2009) Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654–658 [DOI] [PubMed] [Google Scholar]
- 16.Futter C. E., Collinson L. M., Backer J. M., Hopkins C. R. (2001) Human VPS34 is required for internal vesicle formation within multivesicular endosomes. J. Cell Biol. 155, 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson E. E., Overmeyer J. H., Gunning W. T., Maltese W. A. (2006) Gene silencing reveals a specific function of hVps34 phosphatidylinositol 3-kinase in late versus early endosomes. J. Cell Sci. 119, 1219–1232 [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T., Takasuga S., Sasaki J., Kofuji S., Eguchi S., Yamazaki M., Suzuki A. (2009) Mammalian phosphoinositide kinases and phosphatases. Prog. Lipid Res. 48, 307–343 [DOI] [PubMed] [Google Scholar]
- 19.Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 [DOI] [PubMed] [Google Scholar]
- 20.Zhao H., Yang Y., Rizo C. M., Overbeek P. A., Robinson M. L. (2004) Insertion of a Pax6 consensus binding site into the αA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest. Ophthalmol. Vis. Sci. 45, 1930–1939 [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N., Yamamoto A., Hatano M., Kobayashi Y., Kabeya Y., Suzuki K., Tokuhisa T., Ohsumi Y., Yoshimori T. (2001) Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 152, 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosokawa N., Hara Y., Mizushima N. (2006) Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett. 580, 2623–2629 [DOI] [PubMed] [Google Scholar]
- 23.Kihara A., Kabeya Y., Ohsumi Y., Yoshimori T. (2001) Beclin-phosphatidylinositol 3-kinase complex functions at the _trans_-Golgi network. EMBO Rep. 2, 330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong X., Li E., Klier G., Huang Q., Wu Y., Lei H., Kumar N. M., Horwitz J., Gilula N. B. (1997) Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91, 833–843 [DOI] [PubMed] [Google Scholar]
- 25.Kawamoto T. (2003) Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole animals, insects, and plants. Arch. Histol. Cytol. 66, 123–143 [DOI] [PubMed] [Google Scholar]
- 26.Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., Stenmark H., Johansen T. (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu M., Waguri S., Koike M., Sou Y. S., Ueno T., Hara T., Mizushima N., Iwata J., Ezaki J., Murata S., Hamazaki J., Nishito Y., Iemura S., Natsume T., Yanagawa T., Uwayama J., Warabi E., Yoshida H., Ishii T., Kobayashi A., Yamamoto M., Yue Z., Uchiyama Y., Kominami E., Tanaka K. (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 [DOI] [PubMed] [Google Scholar]
- 28.Zandy A. J., Bassnett S. (2007) Proteolytic mechanisms underlying mitochondrial degradation in the ocular lens. Invest. Ophthalmol. Vis. Sci. 48, 293–302 [DOI] [PubMed] [Google Scholar]
- 29.Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 [DOI] [PubMed] [Google Scholar]
- 30.Korolchuk V. I., Mansilla A., Menzies F. M., Rubinsztein D. C. (2009) Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol. Cell 33, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael R., Bron A. J. (2011) The ageing lens and cataract: a model of normal and pathological ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1278–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andley U. P. (2007) Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 26, 78–98 [DOI] [PubMed] [Google Scholar]
- 33.Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., Garcia C. M., Zhang H., Yu K., Ornitz D. M., Beebe D. C., Robinson M. L. (2008) Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiyama Y., Akimoto K., Robinson M. L., Ohno S., Quinlan R. A. (2009) A cell polarity protein aPKCλ is required for eye lens formation and growth. Dev. Biol. 336, 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simirskii V. N., Wang Y., Duncan M. K. (2007) Conditional deletion of β1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev. Biol. 306, 658–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez G., Wijesinghe M., Turner K., Abud H. E., Taketo M. M., Noda T., Robinson M. L., de Iongh R. U. (2009) Conditional mutations of β-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest. Ophthalmol. Vis. Sci. 50, 4794–4806 [DOI] [PubMed] [Google Scholar]
- 37.Itakura E., Kishi C., Inoue K., Mizushima N. (2008) Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 19, 5360–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong Y., Wang Q. J., Li X., Yan Y., Backer J. M., Chait B. T., Heintz N., Yue Z. (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaber N., Dou Z., Chen J. S., Catanzaro J., Jiang Y. P., Ballou L. M., Selinger E., Ouyang X., Lin R. Z., Zhang J., Zong W. X. (2012) Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc. Natl. Acad. Sci. U.S.A. 109, 2003–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrensen G. F., Graw J., De Wolf A. (1991) Nuclear breakdown during terminal differentiation of primary lens fibres in mice: a transmission electron microscopic study. Exp. Eye Res. 52, 647–659 [DOI] [PubMed] [Google Scholar]
- 41.Imai F., Yoshizawa A., Fujimori-Tonou N., Kawakami K., Masai I. (2010) The ubiquitin proteasome system is required for cell proliferation of the lens epithelium and for differentiation of lens fiber cells in zebrafish. Development 137, 3257–3268 [DOI] [PubMed] [Google Scholar]
- 42.Caceres A., Shang F., Wawrousek E., Liu Q., Avidan O., Cvekl A., Yang Y., Haririnia A., Storaska A., Fushman D., Kuszak J., Dudek E., Smith D., Taylor A. (2010) Perturbing the ubiquitin pathway reveals how mitosis is hijacked to denucleate and regulate cell proliferation and differentiation in vivo. PLoS One 5, e13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Leyen K., Duvoisin R. M., Engelhardt H., Wiedmann M. (1998) A function for lipoxygenase in programmed organelle degradation. Nature 395, 392–395 [DOI] [PubMed] [Google Scholar]
- 44.Nishimoto S., Kawane K., Watanabe-Fukunaga R., Fukuyama H., Ohsawa Y., Uchiyama Y., Hashida N., Ohguro N., Tano Y., Morimoto T., Fukuda Y., Nagata S. (2003) Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature 424, 1071–1074 [DOI] [PubMed] [Google Scholar]
- 45.Nakahara M., Nagasaka A., Koike M., Uchida K., Kawane K., Uchiyama Y., Nagata S. (2007) Degradation of nuclear DNA by DNase II-like acid DNase in cortical fiber cells of mouse eye lens. FEBS J. 274, 3055–3064 [DOI] [PubMed] [Google Scholar]
- 46.De Maria A., Bassnett S. (2007) DNase IIβ distribution and activity in the mouse lens. Invest. Ophthalmol. Vis. Sci. 48, 5638–5646 [DOI] [PubMed] [Google Scholar]
- 47.Menzies F. M., Moreau K., Rubinsztein D. C. (2011) Protein misfolding disorders and macroautophagy. Curr. Opin. Cell Biol. 23, 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J., Ma Z., Jiao X., Fariss R., Kantorow W. L., Kantorow M., Pras E., Frydman M., Pras E., Riazuddin S., Riazuddin S. A., Hejtmancik J. F. (2011) Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am. J. Hum. Genet. 88, 827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pankiv S., Alemu E. A., Brech A., Bruun J. A., Lamark T., Overvatn A., Bjørkøy G., Johansen T. (2010) FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol. 188, 253–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brennan L. A., Kantorow W. L., Chauss D., McGreal R., He S., Matucci L., Wei J., Riazuddin S. A., Cvekl A., Hejtmancik J. F., Kantorow M. (2012) Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol. Vis. 18, 1773–1786 [PMC free article] [PubMed] [Google Scholar]
- 51.Tian Y., Li Z., Hu W., Ren H., Tian E., Zhao Y., Lu Q., Huang X., Yang P., Li X., Wang X., Kovács A. L., Yu L., Zhang H. (2010) C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 141, 1042–1055 [DOI] [PubMed] [Google Scholar]
- 52.Cullup T., Kho A. L., Dionisi-Vici C., Brandmeier B., Smith F., Urry Z., Simpson M. A., Yau S., Bertini E., McClelland V., Al-Owain M., Koelker S., Koerner C., Hoffmann G. F., Wijburg F. A., ten Hoedt A. E., Rogers R. C., Manchester D., Miyata R., Hayashi M., Said E., Soler D., Kroisel P. M., Windpassinger C., Filloux F. M., Al-Kaabi S., Hertecant J., Del Campo M., Buk S., Bodi I., Goebel H. H., Sewry C. A., Abbs S., Mohammed S., Josifova D., Gautel M., Jungbluth H. (2013) Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat. Genet. 45, 83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mrakovic A., Kay J. G., Furuya W., Brumell J. H., Botelho R. J. (2012) Rab7 and Arl8 GTPases are necessary for lysosome tubulation in macrophages. Traffic 13, 1667–1679 [DOI] [PubMed] [Google Scholar]
- 54.Li W., Zou W., Yang Y., Chai Y., Chen B., Cheng S., Tian D., Wang X., Vale R. D., Ou G. (2012) Autophagy genes function sequentially to promote apoptotic cell corpse degradation in the engulfing cell. J. Cell Biol. 197, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pontoriero G. F., Smith A. N., Miller L. A., Radice G. L., West-Mays J. A., Lang R. A. (2009) Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev. Biol. 326, 403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou X., Wang L., Hasegawa H., Amin P., Han B. X., Kaneko S., He Y., Wang F. (2010) Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 9424–9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X., Takatoh J., Wang F. (2011) The mammalian class 3 PI3K (PIK3C3) is required for early embryogenesis and cell proliferation. PLoS One 6, e16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy T. R., Vihtelic T. S., Ile K. E., Watson C. T., Willer G. B., Gregg R. G., Bankaitis V. A., Hyde D. R. (2011) Phosphatidylinositol synthase is required for lens structural integrity and photoreceptor cell survival in the zebrafish eye. Exp. Eye Res. 93, 460–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe M. (2005) Structure and function of the Lowe syndrome protein OCRL1. Traffic 6, 711–719 [DOI] [PubMed] [Google Scholar]