Insulin Use Early in the Course of Type 2 Diabetes Mellitus: The ORIGIN Trial (original) (raw)
Abstract
There has been a recent shift from a uniform treatment targeting HbA1c to a patient centered approach due to disappointing results of intensified glucose control in mega-trials such as VADT, ADVANCE, and ACCORD. In addition, morbidity and mortality has been substantially reduced since the UKPDS leading to an overestimation of the absolute risk for cardiovascular complications in randomized controlled trials. With substantial progress in prevention of cardiovascular complications, patients with type 2 diabetes now survive long enough to face diabetes-related complications and cancer risk. This requires rethinking of antidiabetic treatment strategies as exemplified by a recent consensus statement of the EASD and ADA, calling for a more patient centered treatment. Within this context the value of early insulin initiation was reinforced, the clinical utility of which has been demonstrated in the recent ORIGIN trial. ORIGIN demonstrated neutral results for the primary endpoint, but reduced microangiopathy in patients with an HbA1c value of ≥6.4 % with basal insulin glargine. After 5 years of follow-up 77 % of the patients in the glargine arm and 66 % with standard care remained at an HbA1c <7 %. An ongoing long-term follow-up (ORIGINALE) will clarify whether this also translates into a reduction of macrovascular events and mortality.
Keywords: Type 2 diabetes, Insulin, Consensus, Patient centered, ORIGIN trial
Introduction
We face a substantial change in the treatment of type 2 diabetes mellitus based on the results of recent clinical trials and the advent of new treatment options such as incretins and SGLT-2 inhibitors. Further treatment options are under clinical development and new treatment strategies are being tested. These taken all together may result in antidiabetic treatment being more effective and safe in the future than before.
Within this concise review we aim to explain how antidiabetic pharmacotherapy has refocused in recent years leading to trials such as ORIGIN (Outcome Reduction with Initial Glargine Intervention) [1••] which was, 90 years after the introduction of insulin as a life-saving treatment in type 1 diabetes, the first to evaluate the effect of early treatment with basal insulin compared with standard treatment in a large multinational multi-center randomized trial of patients with pre-diabetes and early type 2 diabetes on high cardiovascular risk.
Glucose Centered Randomized Clinical Trials
VADT [2], ADVANCE [3], and ACCORD [4], started early this century, shared the common goal of improving cardiovascular outcomes by means of improved HbA1c control. Diabetes related complications and safety including cancer risk were secondary endpoints. Compared with the UKPDS where treatment was initiated early (in newly diagnosed patients), the patient populations in VADT and ACCORD had treatment that was intensified late in the course of disease after many years of poor control. Results of the more recent trials were largely disappointing: In the VADT [2] intensive glucose control in patients with poorly controlled type 2 diabetes had no significant effect on the rates of major cardiovascular events, death, or microvascular complications. In ADVANCE [3] intensive glucose control that lowered HbA1c to 6.5 % yielded a 10 % relative reduction in the combined outcome of major macrovascular and microvascular events (non-significant). The risk reduction in ADVANCE was mainly driven by a 21 % relative reduction in nephropathy which corresponded to a reduced progression of albuminuria in VADT (P = 0.01). In ACCORD [4] cardiovascular mortality even increased in the intensified treatment arm by 21 % (HR 1.21; 95%CI 1.02–1.44) which led to the premature halt of this trial [4]. Using the cause of mortality in the ACCORD study [4] (Table 1) as an example, it is evident that myocardial infarction whose reduction was a primary treatment objective has a less important quantitative role than cancer and other non-cardiovascular diseases which are 2–3 times more frequent. Moreover, in sensitivity analyses, the incidence of myocardial infarction was not reduced with intensive glucose control. This is in contrast to a recent meta-analysis of 5 prospective randomized controlled trials published by Ray in which a 17 % reduction of non-fatal infarcts was observed in favor of an intensified treatment regimen (OR 0.83; 95%CI 0.75–0.93) [5], while total mortality was equal (OR 1.02; 95%CI 0.87–1.19).
Table 1.
Causes of death in the ACCORD trial
| Causes of death from randomization until end of study | Intensive treatment n (%) | Standard treatment n (%) |
|---|---|---|
| Any | 391 (7.6) | 327 (6.4) |
| Cardiovascular disease | ||
| Unexpected or presumed CV disease | 124 (2.4) | 103 (2.0) |
| Fatal myocardial infarction | 24 (0.5) | 14 (0.3) |
| Fatal congestive heart failure | 32 (0.6) | 25 (0.5) |
| Fatal procedure for CV disease | 14 (0.3) | 7 (0.1) |
| Fatal arrhythmia | 6 (0.1) | 18 (0.4) |
| Fatal procedure for non-CV disease | 2 (<0.1) | 4 (0.1) |
| Fatal stroke | 13 (0.3) | 17 (0.3) |
| Other CV disease | 11 (0.2) | 10 (0.2) |
| Cancer | 102 (2.0) | 101 (2.0) |
| Condition other than cancer or CV disease | 84 (1.6) | 60 (1.2) |
| Undetermined | 12 (0.2) | 21 (0.4) |
| Identified through National Death Index | 6 (0.1) | 1 (<0.1) |
In all of these studies hypoglycemia was an important treatment related complication, in particular with intensified glucose control (OR 2–3; Table 2). Authors however were not able to demonstrate a direct link between hypoglycemia and cardiovascular complications in this multimorbid patient population overall [6, 7]. Subsequent analyses showed that there are subgroups within the overall heterogenous, multimorbid study population in which a high mortality rate was associated with a high risk of hypoglycemia [6]. In a recently published prospective study that took into account the heterogeneity of the clinical series by using the Charlson Comorbidity Index it was demonstrated that anamnestic severe hypoglycemia was the most important predictor of cardiovascular and total mortality with an OR of 3.4 (95%CI 1.5–7.4) [8].
Table 2.
Clinic, HbA1c, and risk of hypoglycemia in the intervention arm of mega-trials
| ACCORD [4] | VADT [2] | ADVANCE [3] | ORIGIN [1••] | |
|---|---|---|---|---|
| n | 10,251 | 1791 | 11,140 | 12,612 |
| Age (y) | 62 | 60 | 66 | 64 |
| Diabetes duration (y) | 10 | 11.5 | 8 | 5 |
| Pre-diabetes (%) | 0 | 0 | 0 | 12 |
| Macrovascular complications (%) | 35 | 40 | 32 | |
| Baseline HbA1c (%) | 8.1 | 9.4 | 7.5 | 6.4 |
| Intensive TX target | A1c <6 % | A1c <6 % | A1c ≤6.5 % | FPG ≤5.3 mmol/L |
| Intervention | Multiple drugs | Multiple drugs | Gliclazide (± others) | Glargine (± others) |
| HbA1c (%) after Tx (Intervention arm) | 6.4 | 6.9 | 6.3 | 6.2 |
| Weight (kg) | +2.0 | +8.2 | −0.1 | +1.6 |
| Severe hypoglycemia | 3.1 | 3.8 | 0.6 | 1.0 |
| Annual mortality rate (%/y) | Intense: 1.41 | Intense: 2.04 | Intense: 1.78 | IGlar: 2.57 |
| Standard: 1.14 | Standard: 1.89 | Standard: 1.92 | Standard: 2.60 |
The disappointing results of these glucocentric large randomized trials led to a critical re-evaluation of the benefits and risks of blood glucose lowering drugs. Furthermore it led to the recognition that, based on more recent epidemiological longitudinal studies, morbidity and mortality has substantially changed since the UKPDS leading to an overestimation of the true risk for cardiovascular complications. This appears to be the result of improved blood pressure control, the frequent use of statins, the abundant use of blockers of the renin-angiotensin system, and new treatment options for antithrombotic treatment and anticoagulation. The benefits of a treatment effective in controlling cholesterol and blood pressure despite an HbA1c > 7 % have impressively been shown in the Steno-2 Study [9, 10]. Furthermore there is substantial improvement of glycemic control in the majority of patients in most countries. For example the mean HbA1c in Germany is 7 % [11, 12]. On the other hand patients with diabetes now survive long enough to face diabetes-related complications and increased risk of cancer.
Patient Centered Treatment
As a result of the disappointing results of a guideline compliant treatment in mega-trials and based on the recognition of the aforementioned change in patient profiles and expectations the “Consensus statement on patient centered treatment” developed by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) was a logical and overdue step towards a rational treatment that also considers clinical heterogeneity. This is especially true for a risk-adjusted intervention that takes into account the comorbidities, risks of hypoglycemia, and remaining life expectancy of vulnerable patients [13]. Derived from this, individualized treatment goals for HbA1c and the intensity of glycemic control were proposed.
It was since the UKPDS trial results became available that metformin became the first-line drug for the treatment of type 2 diabetic patients in international guidelines [5, 14–16]. Accordingly metformin is recommended in the consensus statement as the drug of choice at the diagnosis of diabetes (Fig. 1). UKPDS was the only relevant outcome study to compare 3 different treatment options: metformin, sulfonylurea, and insulin. After 11 years of follow-up there was a significant reduction of microvascular complications in type 2 diabetic patients newly diagnosed at the time of enrollment. Cardiovascular events and total mortality was reduced in the metformin arm while HbA1c was not statistically different at the end of the treatment phase [17, 18].
Fig. 1.
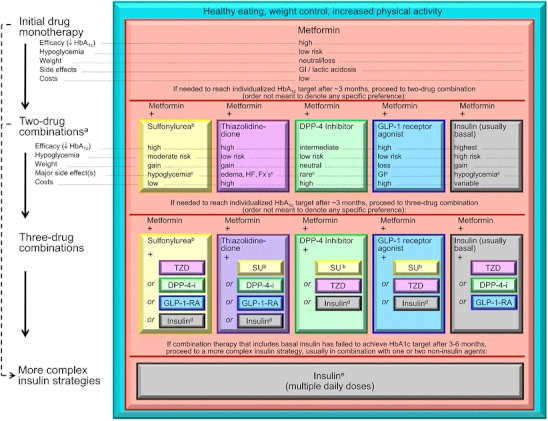
EASD/ADA 2012 Consensus statement. DPP-4-i, DPP-4 inhibitor; Fx’s, bone fractures; GI, gastrointestinal; GLP-1-RA, GLP-1 receptor agonist; HF, heart failure; SU, sulfonylurea. a, Consider beginning at this stage in patients with very high HbA1c (eg, ≥ 9 %). b, Consider rapid-acting, nonsulfonylurea secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on sulfonylureas. c, See Table 1 of the consensus statement for additional potential adverse effects and risks, under “Disadvantages.” d, Usually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents. e, Certain noninsulin agents may be continued with insulin (see text). Consider beginning at this stage if patient presents with severe hyperglycemia (≥16.7–19.4 mmol/L [≥300–350 mg/dL]; HbA1c ≥10.0 %–12.0 %) with or without catabolic features (weight loss, ketosis, etc). (With permission from: Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79. [48••]
When 2-drug combinations are necessary the consensus statement leaves it up to the treating physician to select from sulfonylureas, thiazolidinediones, DPP-4 inhibitors, GLP-1 receptor agonists, and (usually basal) insulin. Since there is no evidence-based outcome data for combination therapies, the decision for 1 of the combinations has to be made individually, based on an assessment of benefits and risks and taking into account expectations and the actual situation of the patient.
Pathophysiologic Rationale for Early Insulin Substitution
Normal fasting glucose homeostasis involves the hormonal regulation of glucose utilization and production (insulin, glucagon) and the filtration and reabsorption of glucose by the kidney [19].
A deficit of insulin secretion is not only pathognomonic for patients with type 1 but also type 2 diabetes. Delayed and defective insulin secretion in type 2 diabetes coupled with defects in insulin action and hyperglucagonemia leads to excessive hepatic glucose release and decreased peripheral glucose uptake. The resulting hyperglycemia and the increase of free fatty acids cause a further loss of β-cell function and increased apoptosis (gluco- and lipotoxicity) [20, 21]. Autopsy studies have shown that even before diabetes is diagnosed, more than 40 % of β-cell mass is lost in patients with Impaired Fasting Glucose (IFG) [22].
The decreased reabsorption of glucose is 1 potential approach to reduce hyperglycemia and in turn insulin resistance and glucose toxicity. The first such agent, phlorizin was already isolated in 1835 and reduced hyperglycemia by non-selective binding to both SGLT-2 and -1 [23, 24]. Phlorizin normalized insulin sensitivity in diabetic rats (Rossetti et al 1987). It resulted in glycosuria, which normalized both fasting and fed plasma glucose levels and completely reversed insulin resistance. Upon discontinuation hyperglycemia and insulin resistance recurred. These data demonstrated that hyperglycemia alone can lead to the development of insulin resistance via glucose toxicity [25]. Its development into an antidiabetic drug was later abandoned in favor of more selective SGLT2 inhibitors.
It is well known that in patients with type 1 diabetes a “honeymoon” can be observed after the introduction of effective insulin treatment which is accompanied by an improved residual β-cell function. Thus, the question arose whether the correction of gluco- and lipotoxicity with insulin recovers β-cell function, despite the loss already established with respect to β-cell mass. From earlier in vitro studies it is known that hyperglycemia leads to increased production of free radicals that inhibit the expression of insulin genes in glucotoxic β-cells. This glucotoxic effect develops along a continuum starting in the pre-diabetic range (IFG) and reversible by near normal glucose control. A very important finding was that the recovery of β-cell function was improved with a shorter glucotoxic exposure time [26, 27]. The term “metabolic memory” today summarizes the complex process of denaturation and glycosylation of functional proteins, in particular of the mitochondrial respiratory chain, leading to persisting damage evoked by prolonged hyperglycemia [28].
Clinical Rationale for Early Insulin Substitution
Insulin First-Line Treatment
Pivotal evidence for using insulin as a first-line treatment was provided by large scale Chinese studies, that demonstrated that near-normal glycemic control in patients with a new diagnosis of type 2 diabetes, a massive derailment of glucose, and HbA1c levels > 9 % led to a long-lasting remission of type 2 diabetes [29]. Newly diagnosed type 2 diabetic patients were treated with 2-week continuous subcutaneous insulin infusion (CSII) by Xu Wen et al [30••] to achieve normoglycemia. Patients had no acute insulin response prior to initiation of CSII. After 14 days of CSII treatment acute insulin response was reestablished and the HOMA-IR was reduced from 8.61 to 3.91. The most remarkable result was that, after 2 years, about 50 % of patients with intermittent insulin treatment were still in remission. The results were the better, the shorter the history of diabetes and the closer to normal the initial glycemic control was.
A large retrospective analysis of the long-term results of early insulin treatment in newly diagnosed type 2 diabetic patients, conducted by Chon et al [31], demonstrated that initial insulin therapy provided better long-term remissions than initiation with oral antidiabetic drugs. The authors concluded that early insulin therapy improved β-cell function and made long-term control of diabetes easier. Accordingly, in the Canadian [32] and Saxonian guidelines [33] initial insulin treatment is recommended for newly diagnosed type 2 diabetes with an HbA1c > 9 %.
Insulin Added to Metformin
According to the consensus statement of the EASD / ADA (Fig. 1) insulin is 1 out of a number of treatment options beyond sulfonylureas, thiazolidinediones, DPP-4 inhibitors, GLP-1 receptor agonists when metformin monotherapy fails. This is however not common practice and sulfonylureas are often used instead [34]. Insulin is frequently only used when HbA1c rises beyond 7.5 % to 8.0 % despite dual or even triple combinations of antidiabetic treatments. It is regarded to be an ultima ratio “complex insulin treatment strategy” which is usually installed on top of initial oral antidiabetic drug treatment.
As Pennartz et al [35] demonstrated in a recent study in type 2 diabetic patients being inadequately controlled with metformin, early use of basal insulin is associated with a significant improvement in residual β-cell function. In the EARLY observational trial, the use of basal insulin glargine in 1438 patients with maximal dose metformin treatment reduced HbA1c from 8.7 % to 7.4 % within 24 weeks with a low risk for hypoglycemia while weight slightly decreased. Upon multivariable analysis the results were the better, the shorter the history of diabetes and the higher the HbA1c at baseline [36].
Fonseca et al confirmed these results in a recent meta-analysis demonstrating the benefits of early addition of basal insulin to metformin compared with a prior combination of metformin and a sulfonylurea [37]. Patients on metformin monotherapy and add-on glargine insulin achieved a greater reduction in HbA1c with less severe hypoglycemia and less weight gain compared with those with baseline metformin plus sulfonylurea.
The ORIGIN Study Results
Pro or Con Early Insulin Therapy?
ORIGIN was the first to evaluate the effect of early treatment with basal insulin compared with standard treatment in a multinational, multi-center randomized study [1••]. The study included patients with type 2 diabetes but also 12 % of patients with pre-diabetes (Table 2). These were substantially different from those of other recent large RCTs in diabetic patients with respect to diabetes duration and HbA1c and complied with the requirements of an early insulin treatment. According to the inclusion criteria they represented, however, a group of patients with high cardiovascular risk. With 66 % of patients having had a prior major cardiovascular event these patients were rather similar to those of the PROactive study [38] and had an increased risk compared with ADVANCE [3], ACCORD [4], and VADT [2].
After an average follow-up of 6.2 years about 84 % of patients in the glargine arm were still on treatment. The incidence of cardiovascular events as the primary endpoint and all-cause mortality were similar in both groups (all-cause mortality 2.57 %/year in the glargine and 2.60 % in the control arm) and corresponded well to those observed in the PROactive study (all-cause mortality 2.36 %/year in the pioglitazone and 2.45 % in the placebo arm). This is reassuring given the recent analyses of the FDA looking into MACE rates (CV death, non-fatal MI, or stroke) with insulin degludec (/aspart) and glargine, where an increased event rate was noted with degludec (HR 1.82; 95%CI 1.03–3.19) (FDA 2012). Both in the glargine arm and the 1 treated with standard therapy of ORIGIN HbA1c remained within the range of baseline values. For glargine HbA1c was 6.2 % at the end of follow-up and 6.5 % for standard therapy. As with the UKPDS, HbA1c it reached its minimum after 1 year in either strategy (5.9 % vs 6.2 %) and steadily increased thereafter. Fasting blood glucose in the glargine arm (95 mg/dL or 5.6 mmol) remained in the target range however, indicating a moderate worsening of postprandial blood glucose control [39, 40].
Microangiopathy as a secondary endpoint was less frequent in the glargine than in the standard therapy arm. Further noteworthy results relate to the risk of cancer and hypoglycemia. The risk of lethal (HR 0.94; 95%CI 0.77–1.15) and non-lethal (HR 1.00; 95%CI 0.88–1.13) cancer was virtually identical in both treatment arms, a result which questions the value of recent registry studies suggesting increased cancer rates with insulin glargine compared with human insulin [41, 42]. From a diabetologist’s perspective the low incidence of hypoglycemia with glargine in comparison with incidence rates seen in ACCORD is important. At an almost identical HbA1c (ORIGIN 6.2 %, ACCORD 6.4 %) severe hypoglycemia was infrequent in the glargine arm of ORIGIN but much more frequent in the intensified treatment arms of ACCORD (3.1 %) and VADT (3.8 %). This has to be interpreted however on the background of a longer diabetes duration (10 years in ACCORD and 11.5 years in VADT vs 5 yrs in ORIGIN) and high baseline HbA1c values (8.1 % in ACCORD, 9.4 % in VADT vs 6.4 % in ORIGIN) (Table 2). Weight gain was 1.6 kg and moderate compared with ACCORD (+2.0 kg) and VADT (+8.2 kg).
Results from a Patient-Centered Treatment Perspective
From a cardiologist’s perspective results are neutral. Patients with history of acute coronary syndrome had an identical outcome compared with those without. This corrects conclusions made based on the long-term results of DIGAMI-2 [43] and the European Heart Survey [44] in patients with acute coronary syndromes undergoing treatment with insulin and in which a higher cardiovascular mortality was observed.
From a diabetologist’s perspective ORIGIN was the first trial to demonstrate that an early close to normal glycemic control prevents diabetes progression for more than 5 years by keeping HbA1c within the treatment range of around 6.5 %. This halt of progression was on the other hand neither accomplished in the UKPDS [17] nor ADOPT [45]. With this in mind insulin appears to do better, but its clinical relevance can only be determined based on longer term follow-ups which have already been secured by ORIGINALE (and Legacy Effect). In a re-evaluation of glycemic control in the ORIGIN study, in a multivariate analysis with HbA1c <6.5 % as dependent variable, low HbA1c at baseline, and treatment with glargine were predictors of glycemic control within the target range [38]. For daily practice the study demonstrated, with compliance to insulin therapy of 84 % after 6 years, that basal insulin allows a well-accepted and safe treatment management, guided by fasting blood glucose determination.
Conclusions
The results of studies investigating an early intervention with close to normal glycemic control result in the urgent request for a preventive intervention as early as possible. The effectiveness and sustainability of an early intervention before an increase in HbA1c beyond the target range has been documented in ORIGIN. At HbA1c values between 6.5 and 8 % a significant slowing of the increase in HbA1c was observed in the ORIGIN study with both oral antidiabetic treatment options and Insulin, whereas the increase within the target range was less with insulin than with OADs. A clinically relevant benefit in favor of insulin was found only for microangiopathy within the observation period and at HbA1c values > 6.4 %. To demonstrate a potential added value for the reduction of cardiovascular endpoints and mortality requires longer follow-up periods as shown in UKPDS [46] and DCCT [47] legacy analysis.
From a clinical practice perspective we would not be hesitant to recommend early insulin initiation in type 2 diabetic patients with an HbA1c > 9 % at diagnosis, patients with a fast increase of HbA1c after diagnosis and new onset symptoms, patients with multiple infections and patients with an HbA1c > 7 % despite maximal metformin treatment and diabetes-related complications.
Acknowledgments
Markolf Hanefeld and Peter Bramlage have developed the outline and Peter Bramlage has drafted the first draft of the manuscript. Both authors jointly revised and approved the final manuscript.
Disclosure
M.H. has received research funding and honoraria from a number of pharmaceutical companies producing antidiabetic drugs. He received honoraria for talks from Takeda, GSK, and Sanofi-Aventis, and is a member of the advisory board of GSK and Sanofi-Aventis. He also has board membership with Sanofi-Aventis. P.B. has received research funding and honoraria from a number of pharmaceutical companies producing antidiabetic drugs. He has received research funding and honoraria from Bristol-Myers Squibb, AstraZeneca, Novartis, and Sanofi-Aventis.
Contributor Information
Markolf Hanefeld, Email: hanefeld@gwtonline-zks.de.
Peter Bramlage, Email: peter.bramlage@ippmed.de.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 2.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 3.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMicm066227. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthaei S, Bierwirth R, Fritsche A, Gallwitz B, Haring HU, Joost HG, et al. Medical antihyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes. 2009;117:522–57. doi: 10.1055/s-0029-1239559. [DOI] [PubMed] [Google Scholar]
- 6.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomized controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 7.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 8.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–901. doi: 10.2337/dc11-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 10.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 11.Ott P, Benke I, Stelzer J, Kohler C. Hanefeld M [“Diabetes in Germany” (DIG) study. A prospective 4-year-follow-up study on the quality of treatment for type 2 diabetes in daily practice] Dtsch Med Wochenschr. 2009;134:291–7. doi: 10.1055/s-0028-1123994. [DOI] [PubMed] [Google Scholar]
- 12.Huppertz E, Pieper L, Klotsche J, Stridde E, Pittrow D, Bohler S, et al. Diabetes Mellitus in German primary care: quality of glycaemic control and subpopulations not well controlled - results of the DETECT study. Exp Clin Endocrinol Diabetes. 2009;117:6–14. doi: 10.1055/s-2008-1073127. [DOI] [PubMed] [Google Scholar]
- 13.Tschoepe D, Bramlage P, Binz C, Krekler M, Deeg E, Gitt AK. Incidence and predictors of hypoglycaemia in type 2 diabetes—an analysis of the prospective DiaRegis registry. BMC Endocr Disord. 2012;12:23. doi: 10.1186/1472-6823-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schernthaner G, Barnett AH, Betteridge DJ, Carmena R, Ceriello A, Charbonnel B, et al. Is the ADA/EASD algorithm for the management of type 2 diabetes (January 2009) based on evidence or opinion? A critical analysis. Diabetologia. 2010;53:1258–69. doi: 10.1007/s00125-010-1702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes A Standards of medical care in diabetes– 2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colagiuri S. Global Guideline for Type 2 Diabetes. 2012. http://www.idf.org/global-guideline-type-2-diabetes-2012 [DOI] [PubMed]
- 17.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 20.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–13. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med. 2009;26:1185–92. doi: 10.1111/j.1464-5491.2009.02847.x. [DOI] [PubMed] [Google Scholar]
- 22.Kjems LL, Kirby BM, Welsh EM, Veldhuis JD, Straume M, McIntyre SS, et al. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes. 2001;50:2001–12. doi: 10.2337/diabetes.50.9.2001. [DOI] [PubMed] [Google Scholar]
- 23.Chao EC. A paradigm shift in diabetes therapy-dapagliflozin and other SGLT2 inhibitors. Discov Med. 2011;11:255–63. [PubMed] [Google Scholar]
- 24.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 25.Freitas HS, Anhe GF, Melo KF, Okamoto MM, Oliveira-Souza M, Bordin S, et al. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology. 2008;149:717–24. doi: 10.1210/en.2007-1088. [DOI] [PubMed] [Google Scholar]
- 26.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleason CE, Gonzalez M, Harmon JS, Robertson RP. Determinants of glucose toxicity and its reversibility in the pancreatic islet beta-cell line, HIT-T15. Am J Physiol Endocrinol Metab. 2000;279:E997–1002. doi: 10.1152/ajpendo.2000.279.5.E997. [DOI] [PubMed] [Google Scholar]
- 28.Ceriello A. Hypothesis: the “metabolic memory”, the new challenge of diabetes. Diabetes Res Clin Pract. 2009;86(Suppl 1):S2–6. doi: 10.1016/S0168-8227(09)70002-6. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Xu W, Liao Z, Yao B, Chen X, Huang Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27:2597–602. doi: 10.2337/diacare.27.11.2597. [DOI] [PubMed] [Google Scholar]
- 30.Xu W, Li YB, Deng WP, Hao YT, Weng JP. Remission of hyperglycemia following intensive insulin therapy in newly diagnosed type 2 diabetic patients: a long-term follow-up study. Chin Med J. 2009;122:2554–9. [PubMed] [Google Scholar]
- 31.Chon S, Oh S, Kim SW, Kim JW, Kim YS, Woo JT. The effect of early insulin therapy on pancreatic beta-cell function and long-term glycemic control in newly diagnosed type 2 diabetic patients. Korean J Intern Med. 2010;25:273–81. doi: 10.3904/kjim.2010.25.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharyya OK, Estey EA, Cheng AY. Update on the Canadian Diabetes Association 2008 clinical practice guidelines. Can Fam Physician. 2009;55:39–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Fachkommission Sachsen. [Diabetes mellitus Type 2]. 2012.
- 34.Bramlage P, Binz C, Gitt AK, Krekler M, Plate T, Deeg E, et al. Diabetes treatment patterns and goal achievement in primary diabetes care (DiaRegis)—study protocol and patient characteristics at baseline. Cardiovasc Diabetol. 2010;9:53. doi: 10.1186/1475-2840-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennartz C, Schenker N, Menge BA, Schmidt WE, Nauck MA, Meier JJ. Chronic reduction of fasting glycemia with insulin glargine improves first- and second-phase insulin secretion in patients with type 2 diabetes. Diabetes Care. 2011;34:2048–53. doi: 10.2337/dc11-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanefeld M, Fleischmann H, Landgraf W, Pistrosch F. EARLY Study: early basal insulin therapy under real-life conditions in type 2 diabetics. Diabetes, Stoffwechsel und Herz. 2012;21:91–7. [Google Scholar]
- 37.Fonseca V, Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab. 2011;13:814–22. doi: 10.1111/j.1463-1326.2011.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 39.Hanefeld M, Koehler C, Hoffmann C, Wilhelm K, Kamke W, Gerstein H. Effect of targeting normal fasting glucose levels with basal insulin glargine on glycaemic variability and risk of hypoglycaemia: a randomized, controlled study in patients with early Type 2 diabetes. Diabet Med. 2010;27:175–80. doi: 10.1111/j.1464-5491.2009.02915.x. [DOI] [PubMed] [Google Scholar]
- 40.Ceriello A. Basal insulin and cardiovascular and other outcomes. N Engl J Med. 2012;367:1762–3. doi: 10.1056/NEJMc1210553. [DOI] [PubMed] [Google Scholar]
- 41.Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–44. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, et al. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia. 2012;55:51–62. doi: 10.1007/s00125-011-2312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellbin LG, Malmberg K, Norhammar A, Wedel H, Ryden L. Prognostic implications of glucose-lowering treatment in patients with acute myocardial infarction and diabetes: experiences from an extended follow-up of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2 Study. Diabetologia. 2011;54:1308–17. doi: 10.1007/s00125-011-2084-x. [DOI] [PubMed] [Google Scholar]
- 44.Anselmino M, Malmberg K, Ohrvik J, Ryden L. Evidence-based medication and revascularization: powerful tools in the management of patients with diabetes and coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur J Cardiovasc Prev Rehabil. 2008;15:216–23. doi: 10.1097/HJR.0b013e3282f335d0. [DOI] [PubMed] [Google Scholar]
- 45.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 46.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 47.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]