Rapid cell death is preceded by amyloid plaque-mediated oxidative stress (original) (raw)
Abstract
Neuronal loss is the ultimate outcome in a variety of neurodegenerative diseases and central nerve system disorders. Understanding the sequelae of events that leads to cell death would provide insight into neuroprotective approaches. We imaged neurons in the living brain of a mouse model of Alzheimer’s disease that overexpresses mutant human amyloid precursor protein and presenilin 1 and followed the death of individual neurons in real time. This mouse model exhibited limited neurodegeneration and atrophy, but we were able to identify a small fraction of vulnerable cells that would not have been detectable by using standard approaches. By exploiting a genetically encoded reporter of oxidative stress, we identified susceptible neurons by their increased redox potential. The oxidative stress was most dramatic in neurites near plaques, propagated to cell bodies, and preceded activation of caspases that led to cell death within 24 h. Thus, local oxidative stress surrounding plaques contributes to long-range toxicity and selective neuronal death in Alzheimer’s disease.
Keywords: in vivo imaging, reduction-oxidation sensitive GFP
Alzheimer’s disease (AD) is underscored by neurodegeneration and is the most common form of dementia. The pathological hallmarks of this disease include amyloid plaques, neurofibrillary tangles, and neuronal loss. Early-onset familial AD is caused by genetic mutations of amyloid precursor protein (APP) or presenilin 1 and 2 (PS1 and PS2). Although recent genetic studies have revealed risk factors for late onset AD, the pathogenic pathways for sporadic AD remain largely unknown. The development of mouse models of AD that develop senile plaques similar to those found in AD patients was a critical step in identifying the role of amyloid β (Aβ) on neuronal function. A major disappointment of most of the mouse models is the lack of overt neuronal loss that is a hallmark of the human disease. Many, in fact, have used this lack of neuronal death as evidence that amyloid is not relevant to dementia in AD. We and others (1–4) have identified structural and functional alterations of neurons in the brains of APP mice that implicate amyloid-mediated toxicity, but we have never detected neuronal death. The ability to monitor cell death in an experimental model provides the opportunity to intervene with neuroprotective agents that could be applied to the spectrum of neurodegenerative diseases and CNS disorders.
We were able to identify vulnerable cells by quantitatively imaging the redox potential of neurons in the living brain. Our hypothesis was that amyloid-mediated increases in oxidative stress are the initiators of the toxic cascade that leads to cell loss. Accumulating evidence supports a role for oxidative stress in the pathogenesis of neuronal degeneration and death in AD (5–8). The evidence supporting oxidative stress in AD comes largely from postmortem samples and includes increased lipid peroxidation, decreased polyunsaturated fatty acids (9–12), increased protein oxidation (13, 14), and DNA oxidation (15, 16). The addition of exogenous Aβ also increases formation of free radicals and oxidative injury in in vitro studies (17, 18). However, there is no direct in vivo evidence of increased cellular oxidative stress in AD. Therefore, we translated a fluorescent indicator for oxidative stress for use with in vivo multiphoton microscopy imaging to address directly the question of whether oxidative stress is increased in living AD mouse brain and, if so, whether the oxidative stress is linked to neuronal degeneration.
Results
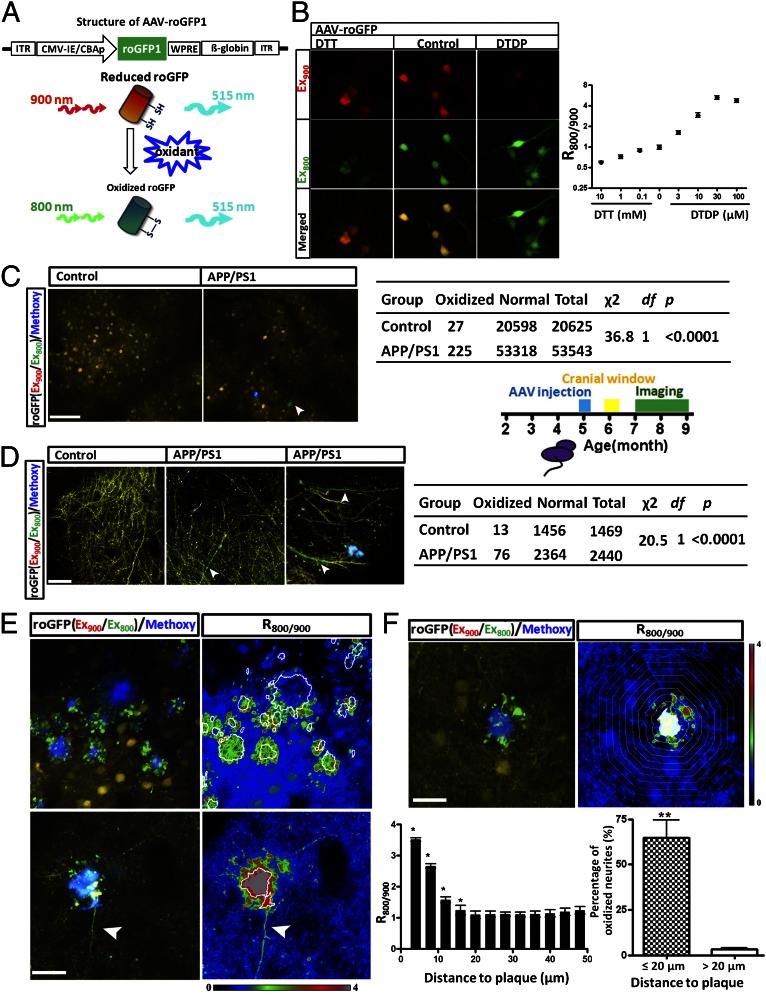
Redox-sensitive variants of the green fluorescent protein (roGFP) have been developed that allow the monitoring of oxidation/reduction potentials in cells by using ratiometric methods (19, 20). The probes were constructed by placing two cysteine residues onto neighboring strands of the β-barrel of GFP in positions favorable for disulfide bond formation. The formation of the Cys147-Cys204 disulfide caused by oxidants leads to a fluorescence increase when excited at 395 nm and a decrease when excited at 475 nm (19, 20). We mimicked these dual excitation wavelengths by using multiphoton excitation at 800 nm and 900 nm, respectively. Thus, the fluorescence (emission measured at 515 nm) ratio of excitation at 800 nm and 900 nm was used to monitor the oxidation/reduction status of roGFP in cells (Fig. 1_A_). We generated adeno-associated virus (AAV, sterotype 2/8) expressing roGFP1 and infected cultured mouse neocortical neurons. To calibrate the sensitivity of roGFP1 to various cellular redox conditions, we incubated primary neurons with the reducing agent DTT, and the oxidant, 4,4′-dithiodipyridine (DTDP), at varying concentration for 30 min. In neurons, the roGFP1 ratio decreased from 0.98 ± 0.03 to 0.59 ± 0.01 with application of 10 mM DTT (n ≥ 246 neurons), whereas the ratio increased to 5.21 ± 0.15 (n ≥ 287 neurons) when treated with 30 µM DTDP (Fig. 1_B_). Thus, the sensitivity of roGFP1 to redox changes in cultured neocortical neurons is similar to the published reports (19, 20) and confirms our roGFP1-AAV construct serves as an accurate indicator for intracellular redox potential.
Fig. 1.
In vivo imaging of cellular oxidative stress with roGFP in APP/PS1 mice and control littermates. (A) Schematic depicting the construct of AAV-roGFP and imaging of reduced or oxidized roGFP by multiphoton excitation. The ratio of the emission fluorescence excited at 800 nm and 900 nm was used as an index of the redox status of roGFP. (B) Redox sensitivity of roGFP in primary neurons. DTT decreased the roGFP ratio (_R_800/900), whereas DTDP increased the ratio (n ≥ 246 neurons). (C) Oxidative stress in neuronal soma in APP/PS1 transgenic mice and control wild-type mice. χ2 test showed significant increased oxidative stress in APP/PA1 mice than in control group (n = 20,625 neurons from eight control mice and n = 53,543 neurons from 13 APP/PS1 mice). Arrowhead shows an oxidized neuron. (D) Increased oxidative stress in neurites in APP/PS1 transgenic mice compared with control wild-type mice (n = 1,469 neurites from six control mice and n = 2,440 neurites from eight APP/PS1 mice). roGFP signals were measured in the areas distant (>20 µm) to Aβ plaques in APP/PS1 transgenic mice. Arrowheads showing oxidized neurites. (E) Images of oxidized neurites surrounding Aβ plaques. (E, Left) Multiphoton images of plaques surrounding areas. (E, Right) Heat-map image of the roGFP ratio surrounding plaques, based on Left. The arrowhead in Lower indicates the propagation (∼100 µm) of oxidative stress in a neurite. (F) Spatial impact of Aβ plaques on surrounding neurites. (F, Upper) An Aβ plaque results in an increased roGFP ratio in the surrounding area. Each band represents a 4-µm concentric area from the plaque edge. (F, Lower Left) Quantitative analysis of roGFP ratio versus the distance to plaques (n = 12 plaques from three mice). (F, Lower Right) Remarkable increase of oxidative stress in neurites closed (≤20 µm) to Aβ plaques. (Scale bars: B and F, 20 µm; C, 100 µm; D, 30 µm; E, 40 µm.)
The sensitivity of roGFP1 was further examined in the somatosensory cortex of mice in vivo. Two months after AAV injection, we observed roGFP1 fluorescence in both apical tufts in layers I/II and neuronal soma in layers II–IV (Movie S1). DTT or DTDP were topically applied to the cortex, and fluorescence ratio changes of roGFP1 were examined. Similar to cultured neurons, the changes in roGFP1 ratio in both neuronal soma and neurites were correlated with DTT or DTDP treatment in the brain (Fig. S1_A_). The maximal ratio change using 2 mM DTDP increased the roGFP1 ratio from 1.01 ± 0.01 to 1.78 ± 0.04 (n ≥ 59 imaging sites from three mice); whereas 2 mM DTT decreased the resting roGFP ratio (0.90 ± 0.01, n ≥ 20 imaging sites from three mice). Although the dynamic range of roGFP is reduced in the living brain compared with in vitro preparations, as with other genetically encoded reporters (2), it remains a sensitive reporter of changes in redox potential. Thus, this approach provides a quantitative functional readout of intracellular oxidative stress in cortical neurons in living mouse brain.
We next examined the intracellular redox status in APP/PS1 transgenic mice of both apical tufts in layers I/II and neuronal soma in layer II–IV. We used age-matched (7–9 mo) littermates as controls. To avoid the effects of senile plaques and address the question of whether soluble oligomeric Aβ species would result in oxidative stress in neurons in vivo, only cell bodies and neurites that were 20 µm or more away from plaques were measured in APP/PS1 mice. No significant differences in the average roGFP ratio was detected in either neuronal soma or neurites (Fig. S1_B_), suggesting that soluble Aβ did not significantly increase oxidative stress in most of the neurons in the APP/PS1 mice. Detailed analysis, however, showed that a small fraction of somas and neurites showed increased oxidation (_R_800/900 ≥ 1.5) in both control and APP/PS1 mice. Importantly, APP/PS1 mice showed many more neurons and neurites with increased redox potential than control mice: 0.13% neurons in control mice (Fig. 1_C_; n = 20,625 neurons from eight mice) and 0.42% neurons in APP/PS1 mice (Fig. 1_C_; n = 53,543 neurons from 13 mice) showed oxidative stress in the soma. A much larger fraction of neurites with elevated roGFP ratios in the brain were detected (Fig. 1_D_; 0.88%, in control mice, n = 1,469 neurites from six mice; 3.12% in APP/PS1 mice, n = 2,440 neurites from eight mice). Taken together, these results suggest that the increased levels of soluble Aβ in the APP/PS1 mice did not significantly alter the redox status in the majority of neurons, but led to the emergence of a small population of neuronal cell bodies and neurites in the brain with increased oxidative stress.
Next, the effect of individual senile plaques on oxidative stress was investigated in APP/PS1 mice. Strikingly, in contrast to the neurites away from Aβ plaques, the majority of neurites close to Aβ plaques (≤20 µm) showed evidence of oxidative stress (64.9 ± 9.7%, Fig. 1 E and F and Movie S2). Most of these oxidized neurites surrounding Aβ plaques formed dystrophic varicosities, which are likely to be axonal abnormalities (21). Oxidation in dystrophic neurites has been reported by using immunohistochemistry and includes lipid peroxidation and the accumulation of a variety of mitochondrial stress markers (22, 23). Furthermore, structural alteration of dendrites that included a beaded appearance was also observed in oxidized neurites near Aβ plaques (Fig. S2). The finding of intracellular oxidative stress localized to the immediate vicinity of plaques suggests that Aβ plaques might be a focal source of oxidative stress. In rare cases, we found oxidative stress in long neurite segments near plaques that extended up to 100 µm away from individual senile plaques (Fig. 1_E_; n = 9 neurites from eight mice), suggesting a spatial influence much larger than the plaque itself.
To quantitatively analyze the spatial impact of Aβ plaques on oxidative stress, the roGFP ratio in concentric bands spaced at 4-µm intervals from the border of individual plaques was measured. The oxidative stress “burden” within the range of 16 µm from the boundary of plaques was significantly increased compared with that in the areas far from the plaques (n = 12 plaques from three mice; Fig. 1_F_). In contrast to Aβ plaques, cerebrovascular amyloid angiopathy showed no effects on the redox potential in both neuronal soma and neurites close to affected vessels (Fig. S3). Taken together, and in comparison with other brain areas, the rich accumulation of oxidized neurites surrounding Aβ plaques demonstrates the role of Aβ plaques as a central source for oxidative stress and accompanied neuritic degeneration.
We next sought to examine the cause of the oxidative stress. We topically applied the reducing agent DTT (10 mM, 30 min) to the cortex of APP/PS1 mice. We observed that the roGFP ratio in oxidized neurites decreased dramatically from 2.52 ± 0.01 to 1.56 ± 0.06 after DTT treatment (n = 6 plaques from three mice; Fig. S4_A_), suggesting that the cellular oxidation can be at least partially reversed with a strong reducing agent. This experiment also demonstrates that oxidation of the roGFP probe is reversible in vivo. Our previous reports demonstrated that _N_-tert-butyl-phenylnitrone (PBN), a free radical spin trap, could reduce the extracellular free radicals associated with Aβ plaques and reduce extracellular plaque associated oxidative stress (24). However, the cellular oxidation in both oxidized neurites and normal neurites was not affected by 1- to 4-wk treatment with PBN (100 mg/kg, i.p. daily; Fig. S4_B_), suggesting that extracellular free radicals associated with Aβ plaques might not contribute to the cellular oxidation in surrounding neurites or that PBN is not a strong enough antioxidant to overcome the intracellular stress.
Next, we examined the involvement of soluble Aβ aggregates in the generation of cellular oxidation. We imaged roGFP in primary neuronal cultures obtained from Tg2576 mice. At 14–18 d in vitro (DIV), we were not able to detect oxidative stress in neurites (Fig. S5 A_–_C). We also applied conditioned media harvested from primary neuronal cultures derived from Tg2576 mice to naive neuronal cultures. We were not able to detect intracellular oxidative stress (Fig. S5_D_). roGFP was imaged in 2.5- to 3-mo-old APP/PS1 mice, at an age with soluble Aβ species, but before plaque deposition. Although rare examples of oxidized neurites were detected, there was no difference between transgenic and nontransgenic animals at this age (Fig. S6). Finally, we used the monoclonal antibody 3D6, recognizing amino acids 1–5 of Aβ, that has been shown to lead to the clearance of amyloid deposits in vivo (25, 26). We applied 3D6 directly to the cortical surface of APP/PS1 mouse brain and compared its effect acutely at 1 h and 1 wk after application. No significant changes in the roGFP ratio in both oxidized and normal neurites (Fig. S4_C_) were observed at either time point. Although it is not appropriate to overinterpret this negative result, it is known that 3D6 binds to both soluble and aggregated Aβ, and we would predict that acute application would “neutralize” all species of amyloids to some extent. Together with the observation that the average cellular redox potential far from plaques was not elevated in APP/PS1 mice, these results further suggest that soluble Aβ in the APP/PS1 mouse brain may not directly induce cellular oxidative stress. Thus, clearance of extracellular Aβ by 3D6 or reactive oxygen species by PBN did not rescue the cellular oxidative stress in neurites surrounding Aβ plaques in APP/PS1 mouse. This non-rescue event suggests that once the redox potential is increased within cells that it no longer needs the continued presence of Aβ, and is severe enough that extracellular antioxidants are ineffective at buffering the oxidative stress. This non-rescue event implies that prevention therapies will be more effective than treatment therapies or that longer durations of treatment will be necessary.
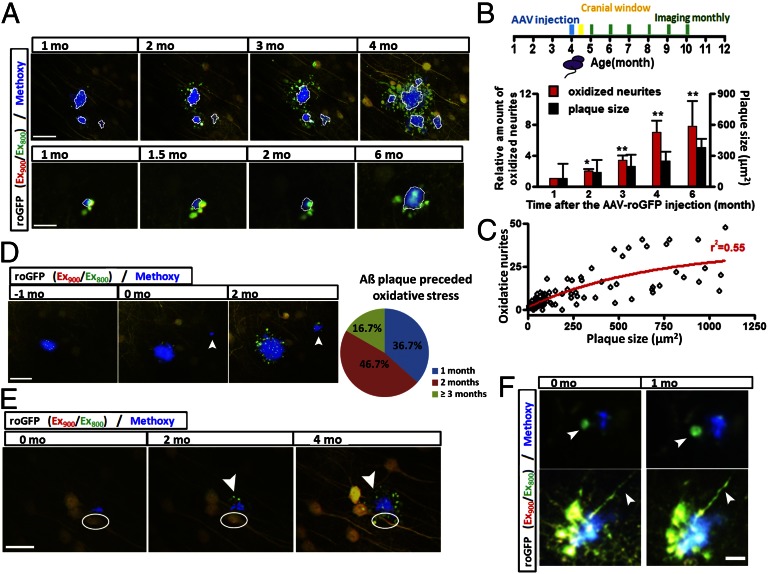
We next explored the relationship between the generation of Aβ plaques and oxidative stress by injecting AAV-roGFP in younger (4-mo-old) APP/PS1 mice, when Aβ plaques begin to accumulate. Cranial windows were implanted shortly (2 wk) after the injection of virus and mice were imaged 2 wk after surgery. Thus, imaging sessions began at 5 mo of age and were repeated monthly (Fig. 2_B_). First, we found that the cellular oxidative stress in neurites surrounding Aβ plaques propagated to larger areas month by month (Fig. 2_A_). The oxidized neurites surrounding Aβ plaques survived for weeks, in some cases, longer than 1 mo (Fig. 2_F_ and Fig. S7_B_). By following images of 30 Aβ plaques with oxidized neurites longitudinally, we found that in the APP/PS1 mice, the total amount of oxidized neurites increased over sevenfold within 5 mo (Fig. 2_B_). Imaging over these long intervals also revealed a tendency for some plaques to increase in size (Fig. 2_B_). Fig. 2_C_ reflects the observation that the number of oxidized neurites increased with the size of amyloid plaques. Interestingly, during the 5 mo of imaging, Aβ plaques always preceded the detection of oxidative stress in surrounding neurites (Fig. 2_D_ and Fig. S7_A_). A total of 33 newly formed Aβ plaques were identified in four mice. The time delay between the emergence of Aβ plaques and the oxidation of surrounding neurites was approximately 1–3 mo (Fig. 2_D_). Finally, during the accumulation of plaques, we were surprised to observe neuronal death in rare situations (n = 6 cells from four mice) in areas adjacent to Aβ plaques, which were surrounded by oxidized neurites (Fig. 2_E_).
Fig. 2.
Time series imaging of Aβ plaques and surrounding cellular redox potential reveals the generation and propagation of cellular oxidation. (A) An existing Aβ plaque results in the propagation of oxidative stress in surrounding neurites (n = 30 plaques from four mice). (B) Increase in the number of oxidized neurites and size of amyloid plaques over time. (C) Correlation between the number of oxidized neurites and size of amyloid plaques. The fit curved was created by GraphPad Prism 4. (D) Aβ plaque preceded oxidative stress in surrounding neurites (n = 30 plaques from four mice). Arrowhead showing the formation of a new plaque and appearance of oxidized neurites 2 mo later. (E) Aβ plaque caused oxidative stress in neurites and subsequent neuronal death. Arrowheads showing oxidative stress occurred 2 mo after the appearance of the plaque; white circles showing neuronal death occurred after the accumulation of oxidative stress (n = 6 cells from four mice). (F) Long-term survival of oxidized neurites surrounding Aβ plaques. Arrowheads show oxidized neurites. (Scale bars: A, Upper and E, 40 µm; A, Lower, 15 µm; D, 30 µm; F, 10 µm.)
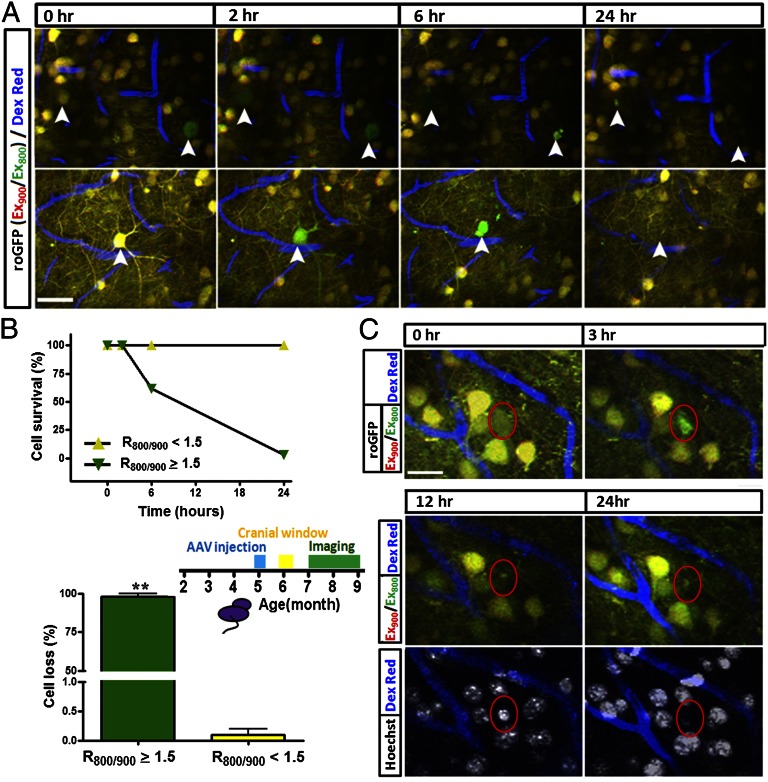
We looked at this neuronal death more closely, using longitudinal imaging over a 24-h period. We stereotaxically injected AAV-roGFP1 in 5-mo-old transgenic mice. A cranial window was implanted 1 mo later, and mice were allowed to recover for 1 mo after the surgery before the beginning of imaging. In the initial imaging session, we observed a small fraction of neurons with an increased oxidation status (_R_800/900 ≥ 1.5) in both soma and surrounding dendrites (n = 34 neurons from five mice) (Fig. 3 A and B) as described above. At subsequent time points, we observed that in those identified neurons, the roGFP1 ratio increased from 1.86 ± 0.05 to 2.98 ± 0.15 within 2 h; 38.24% of those oxidized neurons died within 6 h; 97.06% of which died within 24 h (Fig. 3_B_), whereas none of the neurons with low redox potential indicated by roGFP1 disappeared. This result demonstrates a strong correlation between oxidative stress and neuronal death. Interestingly, we observed that some neurons (n = 13) first showed morphological alterations in dendrites followed by the formation of apoptotic body-like structures in the soma at approximately 4–6 h after oxidation was detected (Fig. 3_A_ and Fig. S8_A_). As a control, the remaining neurons with a roGFP1 ratio of 1.01 ± 0.01 (n ≥ 11,334 neurons from five mice) did not show any changes within 24 h (Fig. 3_B_).
Fig. 3.
Oxidative stress precedes neuronal death in living APP/PS1 transgenic mice. (A) Time-lapse images showing oxidative stress preceding neuronal death in an APP/PS1 mouse. Arrowheads show the dynamics of oxidative stress and neuronal death. Arrowheads show oxidative stress in the neuronal somas and neurites, both of which became fragmented around 4 h later. (B) Plot (Upper) shows the time course of neuronal death of oxidized neurons (n = 34 neurons) and nonoxidized neurons (n ≥ 11,334 neurons from five mice). Column bar (Lower) shows the percentage of cell died with 24 h in both oxidized and nonoxidized neurons (n = 34 mice). (C) Loss of neurons after the appearance of oxidative stress (n = 18 neurons from four mice). Red circle shows the occurrence of oxidative stress. Note that the loss of roGFP signal is accompanied with nuclear condensation at 12 h, and the same nucleus has disappeared within 24 h. (Scale bars: A, 30 µm; C, 20 µm.)
To further confirm the death of the oxidized neurons, the nuclear dye Hoechst 33342 was topically applied to the mouse brain. We found that in the oxidized neurons (n = 18 from four mice), nuclear condensation detected with Hoechst 33342 was found 9 h after the detection of oxidative stress in the soma (Fig. 3_C_). After 24 h, the condensed nucleus disappeared. In contrast, in neurons without oxidative stress (n ≥ 8,000 from four mice), we never observed nuclear condensation or loss within the same 24-h period (Fig. 3_C_).
To exclude the phototoxic effects of repeated laser scanning in the generation of oxidative stress and the acceleration of cell death, we minimized the imaging sessions to only image at 12 or 24 h (Fig. S8_B_). Consistent with previous results, all of the neurons (n = 14 neurons from four mice) identified with an increased redox potential proceeded to die within 24 h. To further exclude the surgical procedure as a source of oxidative stress, we imaged six mice at 0, 1, 2, and 4 mo after implanting the cranial window. Despite this large range of recovery times after the surgical procedure, the frequency of neurons identified as exhibiting an increased redox potential measured with roGFP1 remained constant (Fig. S9).
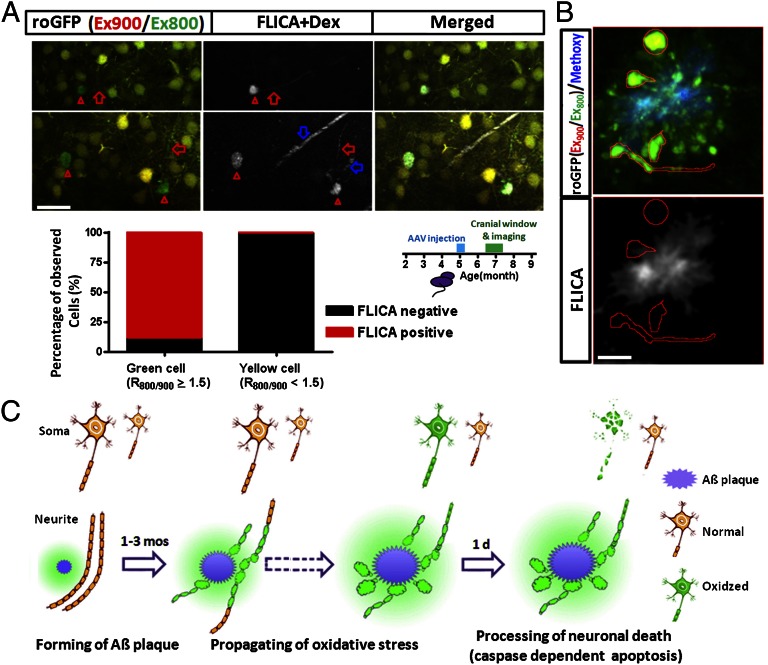
Next, we asked whether the neuronal death that was preceded by an increased redox potential followed a pathway of programmed cell death. To this end, a fluorescent indicator of caspase activation (FLICA) (27) was applied topically to the cortex of the brain in vivo and imaged with multiphoton microscopy. We found that most oxidized neurons were FLICA positive (39 of 44), suggesting a caspase-dependent programmed cell death in most of the neurons with oxidative stress (Fig. 4_A_; n = 8 mice). The five oxidized neurons that were FLICA negative recovered within 1 d with the roGFP ratio returning to normal levels, confirming that it is the activation of caspases that leads to ultimate cell death. With more frequent imaging, we found examples of caspase activation that preceded the oxidative stress by ∼1 h (Fig. S10_A_; n = 6 neurons from three mice), but we interpret this delay of observed oxidative stress as a limitation of the speed and sensitivity of the roGFP1 reporter (19, 28). Furthermore, caspase activation could persist along with the increased oxidative stress in the soma for up to 12 h with neuronal death being the ultimate outcome (Fig. S10_B_; n = 21 neurons from three mice). Taken together, we identified oxidative stress in a small number of neurons (∼0.4% of total neurons), the majority of which were undergoing apoptotic (caspase-dependent) programmed cell death within 24 h. Interestingly, no evidence for caspase activation (FLICA) was detected in the persistently oxidized neurites near plaques, which suggests that oxidation in the neurites alone was not sufficient for caspase activation and subsequent cell death (Fig. 4_B_).
Fig. 4.
Caspase activation co-occurred with oxidative stress in neuronal soma, but not in dystrophic neurites surrounding Aβ plaques. (A) Caspase activation in oxidized neurons (green/oxidized cell, n = 44 neurons from eight mice; yellow/normal cell, n ≥ 16,000 neurons from eight mice). Red triangles show caspase activation (FLICA positive) in oxidized somas, and red arrows show caspase activation in oxidized neurites. Blue arrows show Texas Red dextran (70-kDa) angiogram. (B) Absence of caspase activation in the oxidized neurites surrounding Aβ plaques. (Scale bars: A, 30 µm; B, 10 µm.) (C) Summarized model for the time line of cellular oxidation, the formation of Aβ plaques and neuronal death.
Discussion
Longitudinal in vivo imaging using roGFP in APP/PS1 transgenic mice allowed the determination of the temporal relationship among Aβ plaque deposition, oxidative stress, and cell death. Our data demonstrate that plaques precede (1–3 mo) and lead to oxidative stress in surrounding neurites (Fig. 4_C_). The oxidative stress in neurites surrounding Aβ plaques propagates spatially over time, which ultimately leads to oxidation in neuronal soma. In contrast to the oxidized neurites, which survive for several weeks, oxidation in neuronal soma was associated with caspase-dependent apoptosis. Degeneration of neuronal soma was rapid and occurred within 24 h once the cellular oxidation propagated into the cell body and triggered caspase activation (Fig. 4_C_). These results implicate Aβ as the mediator of oxidative stress and subsequent neurodegeneration; however, it does not necessarily demonstrate that Aβ has a direct effect on neuronal toxicity. It is possible that intermediate cellular players, including astrocytes or microglia, respond to amyloid deposits with chemokine or cytokine signaling that, in turn, leads to oxidation in neurites.
Our experiments do not support a role of soluble, oligomeric Aβ in the generation of intracellular oxidative stress. Acute experiments in primary neuronal cultures, in young mice before plaques are present, or in brains treated with an anti-Aβ antibody failed to implicate nonfibrillar amyloid as a mediator of oxidative stress. These results, however, should be tempered with the caveats that the concentration and duration of soluble amyloid exposure may have been insufficient. Our chronic imaging experiments demonstrated that oxidative stress builds slowly in neurites in the proximity of senile plaques and eventually propagates to neuronal cell bodies.
Using in vivo imaging in a transgenic mouse model, we followed the death of individual neurons in real time. Although these results should be verified in other transgenic mouse models, we were able to identify vulnerable neurons by an increase in redox potential in the cell soma. Thus, we show direct evidence of the involvement of cellular oxidative stress in neuronal degeneration in living animals. Although the number of cells that died were a small fraction of neurons in the brain, the use of the roGFP indicator allowed us to identify and monitor the time course of cell death even though these events were rare. The delineation of this sequence of events leading to neuronal death highlights several potential avenues for therapeutic intervention to prevent or reverse the progression of AD. An effective treatment will likely include a combination of drugs that reduce oxidative stress and prevent amyloid accumulation. Our results also suggest that a preventative approach will be more effective than a treatment approach. Additionally, it is likely that the mechanisms of cell death are common to other neurodegenerative diseases or CNS disorders, allowing a generalization of neuroprotective strategies.
Materials and Methods
Animals and Surgery.
Animal experiments were performed under the guidelines of Institutional Animal Care and Use Committee (IACUC, protocol 2004N0000047). We used APP/PS1 double transgenic mice expressing mutant human APPswe/human PS1-ΔE9 along with age-matched nontransgenic littermate controls (29). Four- to 6-mo-old mice received intracortical injections of roGFP-AAV (sterotype 2/8) and cranial window implantation 2 wk, 1 mo, or 2 mo later. Intracortical injections and cranial window implantation have been described (26, 30). Time-lapse images of roGFP were taken monthly. For surgeries and imaging sessions, mice were anesthetized with 1.5% (vol/vol) isoflurane.
Neuronal Culture Preparation.
Primary neuronal cultures were prepared as described (2). In brief, mixed cortical neurons were generated from CD1 or Tg2576 at embryonic day 15–16. The neurons were maintained in Neurobasal media containing 2% B27 supplement (Invitrogen) for 6–12 DIV before roGFP-AAV infection.
roGFP-AAV Preparation.
roGFP1 (C48S/T65S/S147C/Q204C), obtained from J. Remington (University of Oregon, Eugene, OR), was introduced into the construct pAAV-CBA-roGFP1-woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) (30). The plasmid contained the AAV terminal repeats (ITRs), the only remaining feature of the wild-type AAVgenome. Flanked by the ITRs, the expression cassette included the following components: (i) a 1.7-kb sequence containing hybrid cytomegalovirus immediate-early enhancer/chicken β-actin promoter/exon1/intron; (ii) roGFP1; and (iii) WPRE. The virus titer was 1.0 × 10−12 viral genomes per milliliter.
Labeling Procedures.
Methoxy-XO4 was injected (4 mg/kg i.p.) 1 d before the imaging to label amyloid plaques. To facilitate image alignment from section to section, Dextran Texas Red (Dex Red; 70,000 molecular weight; Invitrogen) was injected into a lateral tail vein to create a fluorescent angiogram. Caspase indicator (FLICA; Invitrogen) was applied topically (5× FLICA solution in sterile PBS) before sealing the craniotomy with a coverslip. Hoechst 33342 (10 μg/mL; Invitrogen) ws also applied topically to label nuclei.
In Vivo Imaging.
Images of roGFP expressing neurons, amyloid pathology, Texas Red Dextran filled aniograms, FLICA, and Hoechst staining were obtained by using one of two microscopes: (i) Bio-Rad 1024ES multiphoton microscope (Bio-Rad), mounted on an Olympus BX50WI upright microscope; (ii) Olympus Fluoview 1000MPE with prechirp optics and a fast AOM mounted on an Olympus BX61WI upright microscope. A wax ring was placed on the edges of the coverslip of the cortical window and filled with distilled water to create a well for an Olympus Optical 20× dipping objective (numerical aperture, 0.95). A mode-locked titanium/sapphire laser (Tsunami; Spectra-Physics) generated two-photon fluorescence with 700- to 960-nm excitation, and three photomultiplier tubes (Hamamatsu) collected emitted light in the range of 380–480, 500–540, and 560–650 nm (26). Neurites were typically sampled 0–100 µm below the surface of the brain, and cell soma were sampled 100–500 µm below the surface.
With single-photon excitation, roGFP has been reported to have two absorbance maxima at ∼395 and 475 nm. We mimicked these dual excitation wavelengths by using multiphoton excitation at 800 nm and 900 nm, respectively. Thus, the fluorescence (emission measured at 515 nm) ratio of excitation at 800 nm and 900 nm was used to monitor the oxidation/reduction status of roGFP. The excitation power of each wavelength was adjusted so that the resting ratio of live cells in vitro and in vivo was ∼1. Overall, the _R_800/900 was approximately 1.0 in both wild-type and transgenic mice.
Image Analysis.
Three-dimensional image stacks were processed by using ImageJ and IDL software (Research Systems). The boundary of dense-core plaques was determined as the position in which the fluorescence intensity was the half-maximum of methoxy-XO4 fluorescence intensity. Data are reported as mean ± SE. Statistical significance was determined by student t test, ANOVA or χ2 test where appropriate. Images presented in the figures are single slices or 2D maximum intensity image projections of the 3D volumes.
Supplementary Material
Supporting Information
Acknowledgments
We thank James Remington (University of Oregon) for providing the roGFP construct (proGFP-N1). This study was supported by National Institutes of Health Grants AG024688 (to B.J.B.), EB000768 (to B.J.B.), and NCSF31100776 (to H.X.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Koffie RM, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106(10):4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuchibhotla KV, et al. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59(2):214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer-Luehmann M, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451(7179):720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spires TL, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25(31):7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 7.Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23(1):134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 8.Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol Sci. 2008;29(12):609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45(8):1594–1601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 10.Marcus DL, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol. 1998;150(1):40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- 11.Praticò D, MY Lee V, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: Evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12(15):1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 12.Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J Neurochem. 1990;55(1):342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- 13.Butterfield DA, et al. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006;22(2):223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Smith CD, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36(5):747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem. 2006;96(3):825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- 17.Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM. Direct evidence of oxidative injury produced by the Alzheimer’s beta-amyloid peptide (1-40) in cultured hippocampal neurons. Exp Neurol. 1995;131(2):193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 18.Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21(12):4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon MB, Remington SJ. Redox-sensitive green fluorescent protein: Probes for dynamic intracellular redox responses. A review. Methods Mol Biol. 2008;476:51–65. doi: 10.1007/978-1-59745-129-1_4. [DOI] [PubMed] [Google Scholar]
- 20.Dooley CT, et al. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279(21):22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 21.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7(11):1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 22.Sayre LM, et al. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68(5):2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard V, et al. Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Exp Neurol. 2003;184(1):247–263. doi: 10.1016/s0014-4886(03)00252-8. [DOI] [PubMed] [Google Scholar]
- 24.McLellan ME, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci. 2003;23(6):2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bard F, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 26.Bacskai BJ, et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22(18):7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Calignon A, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464(7292):1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon MB, Remington SJ. Re-engineering redox-sensitive green fluorescent protein for improved response rate. Protein Sci. 2006;15(1):45–57. doi: 10.1110/ps.051734306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borchelt DR, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 30.Spires TL, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25(31):7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information