Autocrine Transforming Growth Factor-β1 Promotes in vivo Th17 Cell Differentiation (original) (raw)
. Author manuscript; available in PMC: 2013 Jun 23.
Summary
TGFβ1 is a regulatory cytokine that has an important role in controlling T cell differentiation. T cell-produced TGFβ1 acts on T cells to promote Th17 cell differentiation and the development of experimental autoimmune encephalomyelitis (EAE). However, the exact TGFβ1-producing T cell subset required for Th17 cell generation and its cellular mechanism of action remain unknown. Here we showed that deletion of the Tgfb1 gene from activated T cells and Treg cells, but not Treg cells alone, abrogated Th17 cell differentiation resulting in almost complete protection from EAE. Furthermore, differentiation of T cells both in vitro and in vivo demonstrated that TGFβ1 was highly expressed by Th17 cells and acted in a predominantly autocrine manner to maintain Th17 cells in vivo. These findings reveal an essential role for activated T cell-produced TGFβ1 in promoting the differentiation of Th17 cells and controlling inflammatory diseases.
Introduction
CD4+ T helper (Th) cells are central regulators of adaptive immune responses. Upon recognition of their cognate antigen in context of the associated environmental cues, naïve CD4+ T cells differentiate into Th1, Th2 and Th17 cells characterized by the secretion of signature cytokines IFN-γ, IL-4 and IL-17 respectively (Korn et al., 2009; Zhu et al., 2010). The various subsets of Th cells orchestrate host defense responses against a wide range of pathogens. However, deregulated Th cell responses result in immunopathology and the development of autoimmune diseases and atopic syndromes (Gutcher and Becher, 2007; Veldhoen, 2009).
How Th cell differentiation is initiated and regulated is an area of active research. Cytokines that activate the STAT family of transcription factors have been shown to play crucial roles in the induction and maintenance of Th cell differentiation (Zhu et al., 2010). Activation of the transcription factors STAT1 and STAT4 by IFN-γ and IL-12, respectively, instructs naïve T cells to develop into Th1 cells. Subsequently, IFN-γ produced by Th1 cells functions via an autocrine route to stabilize Th1 cell differentiation. IL-4 plays an analogous role in the initiation and stabilization of Th2 cell differentiation through the activation of STAT6. Th17 cell development is dependent on STAT3. STAT3 activators, IL-6 and IL-23, are produced by innate immune cells, and have critical functions in promoting the early and late stages of Th17 cell differentiation. Intriguingly, another STAT3 activator, IL-21 is preferentially expressed by Th17 cells, and is involved in the maintenance of Th17 cell differentiation (Korn et al., 2007; Nurieva et al., 2007; Wei et al., 2007). Thus, it appears that all three Th cell types produce unique sets of STAT-activating cytokines to maintain the heritable developmental programs of their respective lineages.
Transforming growth factor-β1 (TGFβ1) is a regulatory cytokine with a pivotal role in controlling T cell homeostasis and differentiation (Li and Flavell, 2008). Mice with complete TGFβ1 deficiency develop a T cell-dependent multifocal inflammatory disease that leads to their early demise at 3–4 weeks of age (Kulkarni et al., 1993; Shull et al., 1992). The importance of TGFβ signaling in T cells has been shown using transgenic mice with a T cell-specific deletion of TGFβ-receptor II (TGFβRII) or expression of a dominant negative TGFβRII (Gorelik and Flavell, 2000; Li et al., 2006a; Marie et al., 2006). Similar to complete TGFβ1 deficiency, mice with abrogated TGFβ1 signaling in T cells succumb to severe inflammatory disease associated with spontaneous T cell activation, and Th1 and Th2 cell differentiation. Contrasting with its role in inhibiting Th1 and Th2 cell differentiation, TGFβ signaling has been shown to promote the generation of Th17 cells. In the presence of IL-6, TGFβ1 induces the development of Th17 cells that have been shown to drive autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) (Bettelli et al., 2006; Langrish et al., 2005; Mangan et al., 2006; Veldhoen et al., 2006a). As such, diminished TGFβ signaling in T cells prevents Th17 cell generation, and results in resistance to EAE (Veldhoen et al., 2006b).
TGFβ1 is produced by multiple cell types and requires intracellular processing by the proprotein convertase furin for maturation (Dubois et al., 2001). Recent studies using mice with T cell-specific deletion of the Tgfb1 or the Furin gene revealed that T cells are the essential source of TGFβ1 required for controlling T cell tolerance and differentiation (Li et al., 2007; Pesu et al., 2008). In the absence of T cell-produced TGFβ1, conventional T cells differentiate into Th1 and Th2 cells, whereas CD4+Foxp3+ regulatory T (Treg) cells undergo high rates of proliferation. As a result, T cell-specific TGFβ1-deficient mice develop immunopathology in multiple organs and succumb to Th1 cell-mediated colitis. In addition, these mice are resistant to EAE, which is associated with abrogated Th17 cells in the central nervous system. These findings are in line with the observation that local but not systemic administration of neutralizing TGFβ1 antibody inhibits Th17 cell generation implicating autocrine or paracrine sources of TGFβ1 as key for regulating Th17 cell development (Veldhoen et al., 2006b). However, the exact T cell source or sources of TGFβ1 and the cellular mechanisms required for its regulation of Th17 cell differentiation remain unknown. Although early studies have shown that activated T cells can produce TGFβ1 (Kehrl et al., 1986), it has more recently been demonstrated that Treg cells induce the development of Th17 cells in the presence of lipopolysacharride (LPS) in vitro (Veldhoen et al., 2006a). Furthermore, in models of graft-versus-host disease, the co-transfer of Treg cells enhances IL-17 production by T cells (Lohr et al., 2006; Vokaer et al., 2010). These data point toward a role for Treg cell-produced TGFβ1 in inhibiting Th1 cell differentiation and promoting Th17 cell development and, moreover, they suggest a paracrine mechanism of TGFβ1 signaling in regulating T cell tolerance and Th17 cell differentiation.
In this study, we specifically deleted the Tgfb1 gene in activated T cells and Treg cells or in Treg cells alone to determine the exact T cell source of TGFβ1 for regulating T cell tolerance and differentiation. We found that abrogation of TGFβ1 in activated T cells and Treg cells, but not Treg cells alone, protected mice from EAE associated with compromised encephalitogenic Th17 cell differentiation. Analysis of Th cell subsets demonstrated that Th17 cells were the main producers of TGFβ1 both in vitro and in vivo. Furthermore, immunization of mixed bone marrow-chimeric mice demonstrated that TGFβ1 acted predominantly in an autocrine manner to promote Th17 cell differentiation in vivo. These results indicate that similar to the STAT3-activating cytokine IL-21, TGFβ1 produced by Th17 cells is necessary for stabilizing the commitment of the Th17 cell lineage.
Results
TGFβ1-GFP knockin mice reveal TGFβ1 expression in thymic and peripheral T cells
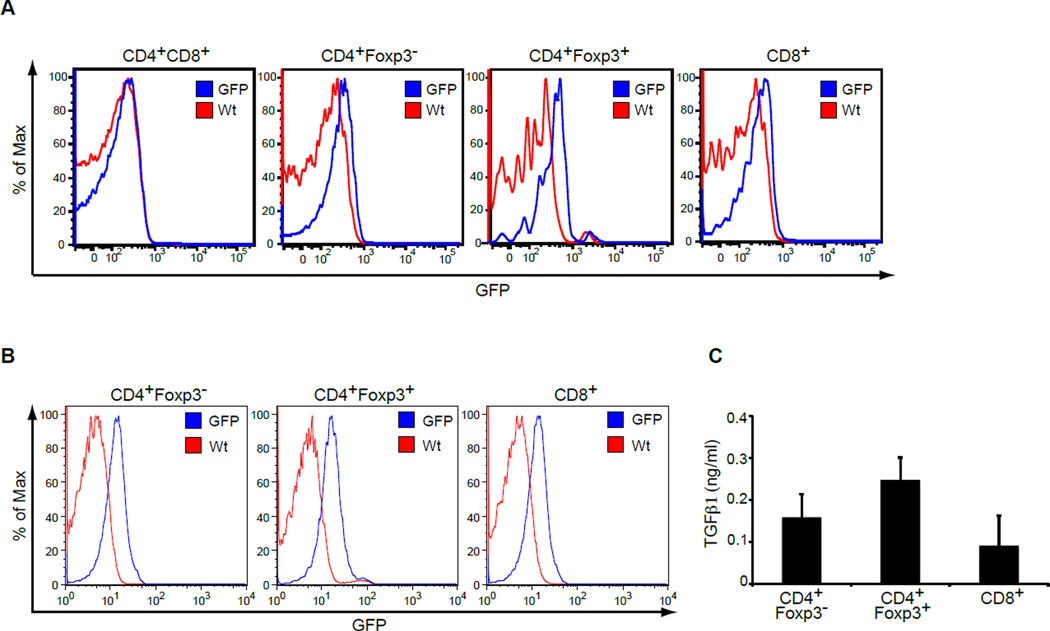
To study TGFβ1 expression in vivo, we have generated a mouse strain in which the coding region of green fluorescent protein (GFP) is inserted to the first exon of the Tgfb1 gene (Figure S1) thereby generating a Tgfb1 null allele. Mice hemizygous for the mutant allele (hereafter referred to as TGFβ1-GFP mice) were devoid of the immunopathology associated with TGFβ1 deficiency, and allowed the expression of TGFβ1 to be characterized by GFP upregulation. To determine the expression of TGFβ1 in the T cell compartment, we further crossed TGFβ1-GFP mice with Foxp3-RFP mice in which alleles of red fluorescent protein (RFP) are contained in the Foxp3 locus (Wan and Flavell, 2005). Examination of immature CD4+CD8+ and mature TCRβhiCD4+ and TCRβhiCD8+ thymocytes by flow cytometry demonstrated that GFP expression was negligible in CD4+CD8+ cells but was upregulated upon maturation of thymocytes to conventional CD4+Foxp3− and CD8+ T cells (Figure 1A). Furthermore, GFP expression was particularly enhanced in thymic CD4+Foxp3+ Treg cells (Figure 1A). Characterization of naive peripheral T cells showed increased GFP expression in comparison to thymic T cells (Figure 1B). The mean fluorescent intensity was similar in peripheral CD4+Foxp3− (MFI = 13.9) and CD8+ T cells (MFI = 13.5) and moderately increased in Treg cells (MFI = 18.6). To confirm that GFP expression reflects TGFβ1 upregulation, we tested the amount of TGFβ1 protein in the supernatant of the T cell subsets. TGFβ1 was produced by all three subsets but was consistently higher in the supernatant of stimulated Treg cells (Figure 1C). Thus, TGFβ1-GFP mice can be used as a tool to analyze cellular TGFβ1 expression and TGFβ1 is expressed by all T cell subsets, especially Treg cells.
Figure 1. TGFβ1-GFP knockin mice reveal expression of TGFβ1 in thymic and peripheral T cells.
(A) GFP expression in CD4+CD8+ double-positive (DP), CD4+Foxp3−, CD4+Foxp3+ and CD8+ thymocytes of TGFβ1-GFP Foxp3-RFP mice was determined by flow cytometric analysis. GFP-negative Wt mice were used as a control. Shown are representative results of three mice per group analyzed.
(B) GFP expression in peripheral lymph node CD4+Foxp3−, CD4+Foxp3+ and CD8+ T cells of TGFβ1-GFP Foxp3-RFP mice was analyzed by flow cytometric analysis. There results are representative of three mice per group.
(C) CD4+Foxp3−, CD4+Foxp3+ and CD8+ T cells purified from Foxp3-RFP mice were stimulated with CD3 and CD28 antibodies in the presence of IL-2 for 24 hr. TGFβ1 amounts in culture supernatant were determined by ELISA.
TGFβ1 deletion from OX40-positive T cells results in Treg cell expansion and Th1 cell differentiation
TGFβ1 produced by T cells is essential for controlling T cell tolerance and differentiation (Li et al., 2007). Because the _Tgfb1f/n Cd4_-cre mice used in that previous study deleted the Tgfb1 gene from both CD4+ and CD8+ T cells, we investigated the precise TGFβ1-producing T cell subset that is required for the regulation of T cell responses. To do so, we used mice with floxed/null alleles (hereafter called _Tgfb1_f/n mice) that express a Tgfb1 floxed allele and a TGFβ1-GFP knockin allele as previously reported (Li et al., 2007). We crossed these mice with Tnfrsf4 (encoding OX40)-cre mice that contain the gene that encodes Cre recombinase driven by the Tnfrsf4 promoter, which results in its expression in both the Treg and activated CD4+ T cell compartments (Klinger et al., 2009). To confirm recombination of the Tgfb1 locus in Tnfrsf4-cre mice, we analyzed yellow fluorescent protein (YFP)-positive and -negative cells from Tnfrsf4-cre-YFP mice that carry a Cre-dependent YFP reporter allele. As expected, YFP expression was present in more than 88% of Treg cells and 65% of activated CD4+ T cells of the lymph nodes (LNs) whereas it was expressed by less than 8% of naïve CD4+ T cells and no more than 5% of naive and activated CD8+ T cells (Figure S2A). Deletion of the loxp-flanked Tgfb1 allele was detected in the majority of Treg cells and activated CD4+ T cells whereas its deletion in naïve T cells was partial (Figure S2B). Thus Tnfrsf4-cre induces efficient ablation of the Tgfb1 allele in Treg cells as well as activated CD4+ T cells.
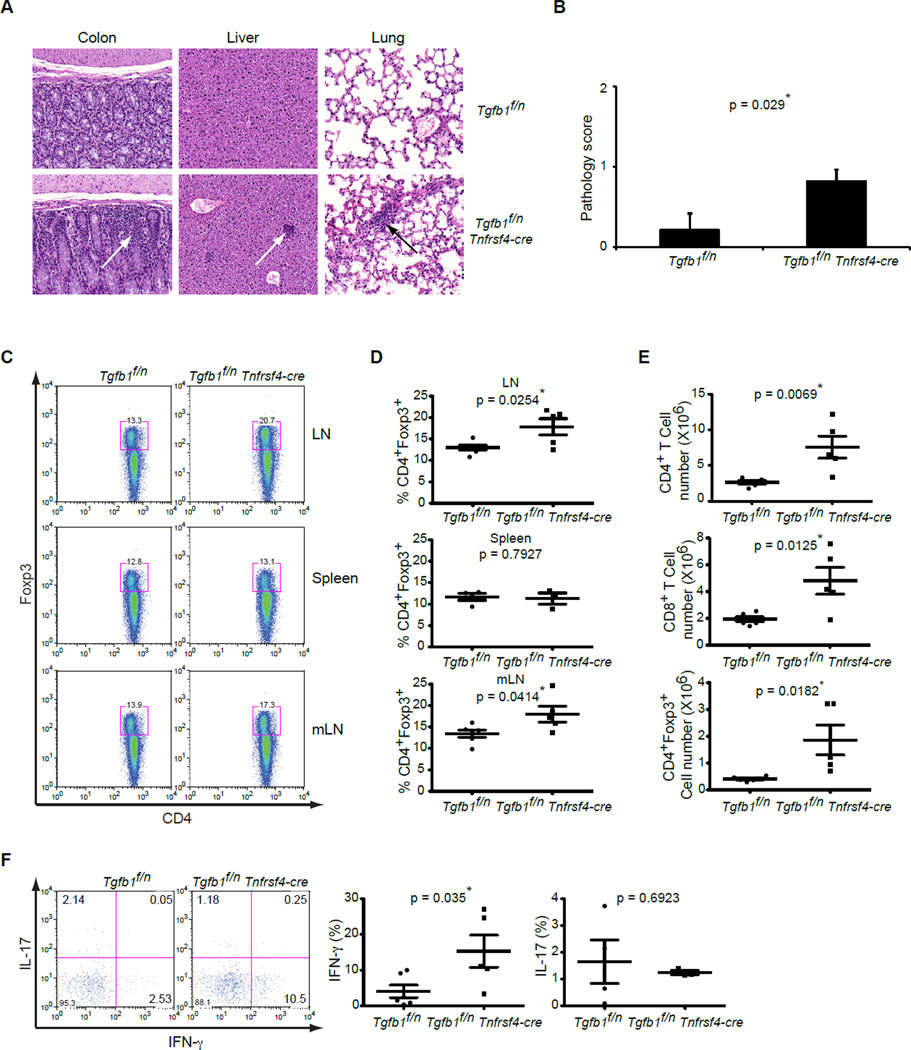
In the absence of T cell-produced TGFβ1, mice develop a severe wasting disease that is characterized by heavy mononuclear infiltrates in the colon as well as in the liver and lungs (Li et al., 2007). Compared to _Tgfb1_f/n Cd4-cre mice reported earlier, milder signs of wasting disease and colitis were observed in _Tgfb1_f/n Tnfrsf4-cre mice beginning at around 5 months of age. Consistent with this phenotype, histological examination demonstrated mononuclear cell infiltrates in the mucosal lamina propria of the colon in these mice (Figure 2A, left panels). Quantification of these sections confirmed that _Tgfb1_f/n Tnfrsf4-cre mice developed mild inflammation with slight epithelial hyperplasia and increased numbers of leukocytes in the mucosa (Figure 2B). We also observed small foci of infiltrating mononuclear cells in the liver parenchyma (Figure 2A, middle panels) and lungs of _Tgfb1_f/n Tnfrsf4-cre mice (Figure 2A, right panels), which were absent in _Tgfb1_f/n littermates. Therefore TGFβ1 produced by Treg and/or activated CD4+ T cells is required to protect mice from the development of an inflammatory disorder.
Figure 2. TGFβ1 deletion from OX40-positive T cells results in Treg cell expansion and Th1 cell differentiation.
(A) Hematoxylin and Eosin staining of colon, liver and lung sections (original magnification, 20x) of Tgfb1f/n and Tgfb1f/n Tnfrsf4-cre mice at 5 months of age. These are representative results of five mice per group analyzed.
(B) Quantification of histological grading of colitis in Tgfb1f/n and Tgfb1f/n Tnfrsf4-cre mice at 5 months of age. These are results of five mice per group analyzed. The p values between the two groups are shown. * depicts significant difference.
(C, D) Flow cytometric analysis of Foxp3 expression in peripheral lymph node, spleen and mesenteric lymph node CD4+ T cells from Tgfb1f/n and Tgfb1f/n Tnfrsf4-cre mice.
(C) Representative plots of peripheral lymph node (n=5–6), spleen (3–4) and mesenteric lymph node (n=5–6) are presented. (D) Percentage of Foxp3-expressing Treg cells in the two groups is indicated. The p values between the two groups are shown. * depicts significant difference.
(E) Numbers of CD4+Foxp3−, CD4+Foxp3+ and CD8+ T cells in the mesenteric lymph nodes of Tgfb1f/n and Tgfb1f/n Tnfrsf4-cre mice (n=5–6). The p values of cell numbers between the two groups of mice are indicated. * depicts significant difference.
(F) Cytokine production of intraepithelial CD4+ T cells of the gut of Tgfb1f/n and Tgfb1f/n Tnfrsf4-cre mice. Intraepithelial lymphocytes were stimulated with PMA and ionomycin for 4 hr and were analyzed for IFN-γ and IL-17 expression. These are representative profiles of five-six mice per group analyzed. The p values of cytokine amounts between the two groups are indicated.* depicts significant difference.
We have previously reported that T cell-produced TGFβ1 is essential for the inhibition of T cell activation and their differentiation into Th1 and Th2 cells (Li et al., 2007). Unlike in _Tgfb1_f/n Cd4-cre mice, there was only marginally enhanced activation and differentiation of T cells in the peripheral lymph nodes (pLNs), spleen and mesenteric (mLNs) lymph nodes of _Tgfb1_f/n Tnfrsf4-cre mice (Figure S2C and data not shown). We have also reported that T cell-produced TGFβ1 is required to control the proliferation of Treg cells in the peripheral lymphoid organs (Li et al., 2007). Analysis of pLNs and mLNs showed that, in comparison to their _Tgfb1_f/n littermates, there was a higher frequency of CD4+Foxp3+ Treg cells in _Tgfb1_f/n Tnfrsf4-cre mice (Figure 2C and 2D), which is similar to that previously observed in Tgfb1f/n Cd4-cre mice (Li et al., 2007). Increased numbers of Treg as well as CD4+Foxp3− and CD8+ T cells were also detected in the mLNs of _Tgfb1_f/n Tnfrsf4-cre mice in comparison to control mice (Figure 2E). These findings reveal that in the microenvironment of the mLNs, which are the draining LNs of the intestine, TGFβ1 deletion in OX40-postive cells results in the expansion of Treg and conventional T cells.
Our data indicate that the absence of TGFβ1 from activated CD4+ T cells and Treg cells did not substantially affect T cell activation in LNs and spleen. We therefore decided to examine the differentiation of T cells in the gut where there is an increased frequency of activated T cells (Ivanov et al., 2008) thereby allowing the analysis of TGFβ1 deletion in both the Treg and effector CD4+ T cell compartments. Intraepithelial lymphocytes (IELs) from the small intestine showed a significantly higher frequency of IFN-γ-producing Th1 cells and a trend toward reduced percentage of IL-17-expressing Th17 cells in _Tgfb1_f/n Tnfrsf4-cre mice compared to _Tgfb1_f/n controls (Figure 2F). Collectively, these findings reveal that TGFβ1 produced by Treg cells and/or activated CD4+ T cells is required to inhibit Treg cell expansion, and is essential for inhibiting Th1 cell differentiation in the gut.
TGFβ1 abrogation from OX40-positive cells compromises Th17 cell generation
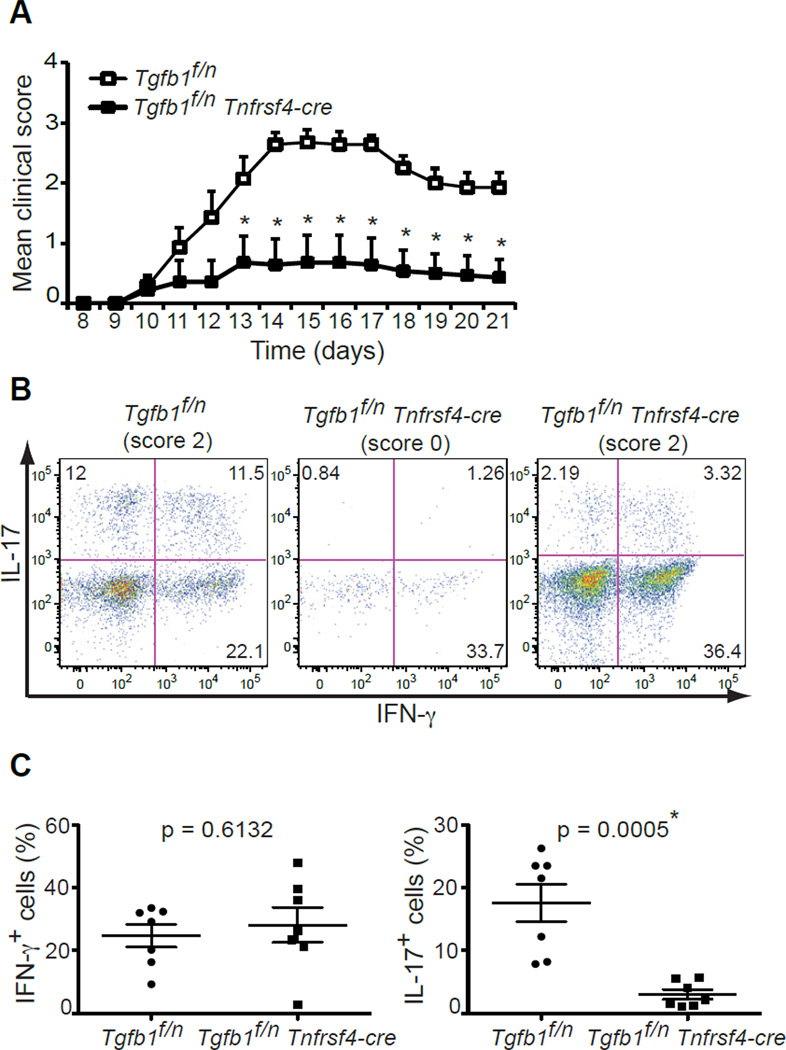
Together with IL-6, TGFβ1 is required for the de novo differentiation of Th17 effector cells that are implicated in autoimmune pathogenesis (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006a). Our previous studies in Tgfb1f/n Cd4-cre mice have demonstrated that T cells are the critical source of TGFβ1 for the generation of encephalitogenic Th17 cells, and consequently disease development, during MOG35–55-induced EAE (Li et al., 2007). We therefore wanted to determine if TGFβ1 produced by effector T cells and Treg cells is required for Th17 cell differentiation and disease development in the context of EAE. We first immunized Tnfrsf4-cre-YFP mice in order to analyze the cellular specificity of recombination of the floxed Tgfb1 gene during MOG35–55-induced EAE. Flow cytometry of infiltrating cells from the spinal cord of diseased mice demonstrated that YFP is expressed in most CD4+ T cells (over 80%) and a minor population of CD8+ T cells (about 20%) (Figure S2D). We immunized _Tgfb1_f/n Tnfrsf4-cre mice and their Wt littermates with MOG35–55 in complete Freund’s adjuvant (CFA), which showed that, similar to Tgfb1f/n Cd4-cre mice, _Tgfb1_f/n Tnfrsf4-cre mice were almost completely resistant to the development of EAE (Figure 3A). Five out of seven mice did not demonstrate any symptoms associated with EAE whereas the remaining 2 mice developed EAE with a clinical onset and severity almost identical to that of _Tgfb1_f/n mice. At day 21 post-immunization, we isolated the infiltrating leukocytes from the CNS of immunized mice and performed intracellular cytokine staining to determine if EAE resistance in _Tgfb1_f/n Tnfrsf4-cre mice is associated with reduced frequency of Th17 cells. As expected, IL-17- and IFN-γ-producing CD4+ T cells were found in the CNS of _Tgfb1_f/n mice with EAE (Figure 3B). In contrast, there was a significant decrease in the presence of IL-17-producing CD4+ T cells in _Tgfb1_f/n Tnfrsf4-cre mice as well as a small, but insignificant, increase in the percentage of Th1 cells (Figure 3B and 3C). Interestingly, in _Tgfb1_f/n Tnfrsf4-cre mice that had developed EAE symptoms, infiltrating CD4+ T cells mostly produced large amounts of IFN-γ whereas the percentage of Th17 cells present was minimal (Figure 3B, right panel). This is similar to what we have previously demonstrated in Tgfb1f/n Cd4-cre mice that develop mild EAE (Li et al., 2007). Taken together, these date show that the production of TGFβ1 from Treg cells and/or effector CD4+ T cells is indispensable for the differentiation of Th17 cells and the induction of EAE.
Figure 3. TGFβ1 abrogation from OX40-positive T cells compromises Th17 cell generation.
(A) EAE disease course in Tgfb1f/n and Tgfb1f/n Tnfrsf4-cre mice (n=7). Disease scores are plotted as mean ± SEM. * depicts significant difference.
(B, C) Cytokine production by CD4+ T cells isolated from the CNS day 21 after disease induction. The cells were stimulated with PMA and ionomycin for 4 hr and were analyzed for IFN-γ and IL-17 expression. Five out of seven Tgfb1f/n Tnfrsf4-cre mice did not develop any clinical sign of disease (score 0), whereas the remaining 2 developed disease similar to Tgfb1f/n littermates (final score 2). The representative plots of diseased and healthy Tgfb1f/n and Tgfb1f/n Tnfrsf4-cre mice are shown in (B). The frequency of IFN-γ- and IL-17-producing CD4+ T cells are shown in (C). The p values of percentiles of cells producing IFN-γ or IL-17 between the two groups are indicated. * depicts significant difference.
Treg cell-produced TGFβ1 is essential for the inhibition of Treg cell expansion
To further narrow down the T cell source of TGFβ1 that is required for controlling T cell homeostasis and differentiation, we crossed _Tgfb1_f/n mice with Foxp3-cre mice to delete TGFβ1 in Treg cells. Foxp3-cre mice have been shown to delete floxed genes efficiently and specifically in Treg cells (Rubtsov et al., 2008). Using Foxp3-cre-YFP mice, we confirmed the recombination of the Tgfb1 allele in almost all CD25+CD4+ Treg cells in comparison to minor deletion in CD25−CD4+ T cells (Figure S2E).
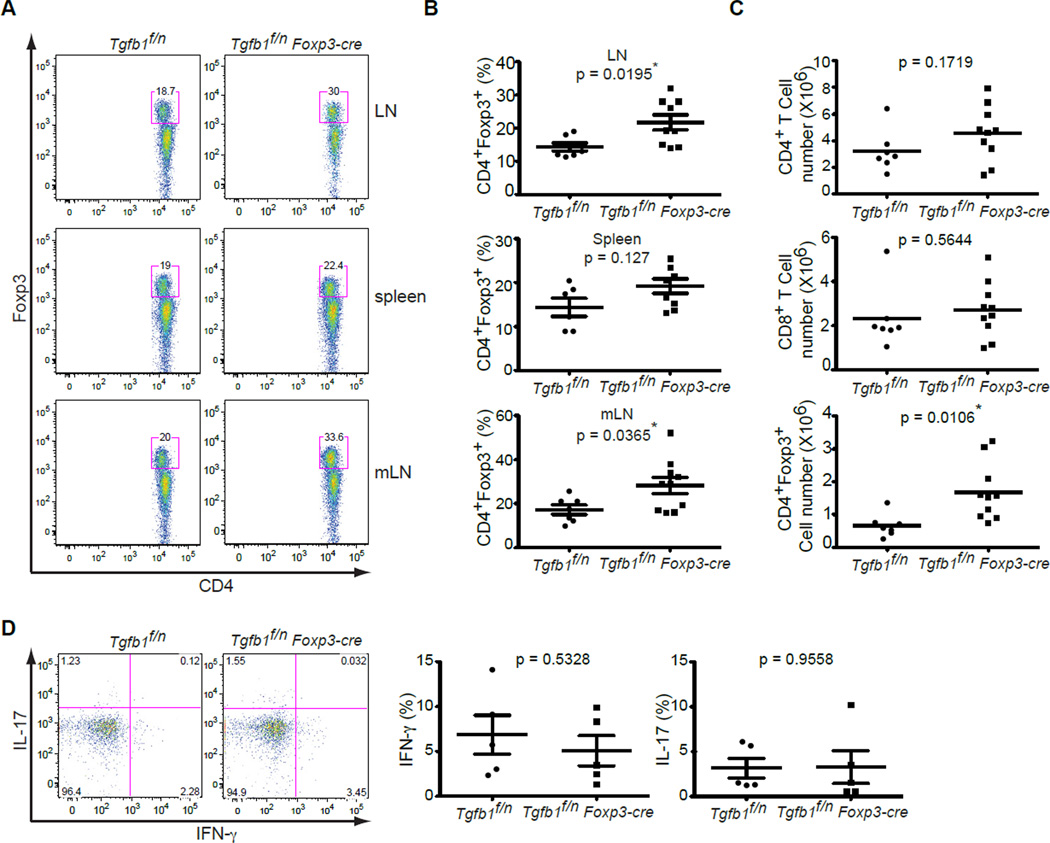
In contrast to the wasting disease that developed in _Tgfb1_f/n Tnfrsf4-cre mice (Figure 2A), _Tgfb1_f/n Foxp3-cre mice remained healthy and showed no signs of inflammation even at the age of 9 months (data not shown). To determine the impact of Treg cell-specific TGFβ1 deletion on T cell homeostasis, we analyzed the T cell compartment in the peripheral lymphoid organs. Similar to the results in _Tgfb1_f/n Tnfrsf4-cre mice (Figure 2C), _Tgfb1_f/n Foxp3-cre mice had significantly increased frequencies of CD4+Foxp3+ Treg cells in the pLNs and mLNs but not in the spleen (Figure 4A and 4B). Furthermore, there was a more than 2-fold increase in the number of mesenteric Treg cells in _Tgfb1_f/n Foxp3-cre mice (Figure 4C). In contrast to _Tgfb1_f/n Tnfrsf4-cre mice, however, there was no increase in CD4+Foxp3− and CD8+ T cell numbers in the mLNs of _Tgfb1_f/n Foxp3-cre mice (Figure 4C). Analysis of T cells in the peripheral lymphoid organs of _Tgfb1_f/n Foxp3-cre mice showed no spontaneous activation and differentiation of T cells (data not shown). These results demonstrate that TGFβ1 produced by Treg cells alone is specifically required for inhibiting Treg cell proliferation.
Figure 4. Treg cell-produced TGFβ1 is essential for the inhibition of Treg cell expansion.
(A, B) Flow cytometric analysis of Foxp3 expression in CD4+ T cells from peripheral lymph nodes, spleen and mesenteric lymph nodes of Tgfb1f/n and Tgfb1f/n Foxp3-cre mice. Representative results are presented in (A) and percentage of Treg cells in peripheral lymph nodes, spleen and mesenteric lymph nodes are shown in (B). The p values of Treg cell numbers between the two groups are indicated. * depicts significant difference.
(C) Number of CD4+Foxp3−, CD4+Foxp3+ and CD8+ T cells in the mesenteric lymph nodes of Tgfb1f/n and Tgfb1f/n Foxp3-cre mice (n=7–10). The p values of cell numbers between the two groups of mice are indicated. * depicts significant difference.
(D) Cytokine production by intraepithelial CD4+ T cells of the small intestine of Tgfb1f/n and Tgfb1f/n Foxp3-cre mice. Intraepithelial lymphocytes were stimulated with PMA and ionomycin for 4 hr and were analyzed for IFN-γ and IL-17 expression. These are representative profiles of five mice per group analyzed. The p values of cytokine amounts between the two groups are indicated.
Treg cells are highly abundant in gut-associated tissues (Ivanov et al., 2008). Previous studies have demonstrated that the TGFβ pathway is required for Treg cell inhibition of inflammation in a transfer model of colitis (Fahlen et al., 2005; Li et al., 2007; Powrie et al., 1996). Using TGFβ1-deficient Treg cells, we could show that Treg cell-produced TGFβ1 contributes to the inhibition of Th1 cell differentiation and the ensuing development of colitis (Li et al., 2007). As TGFβ1 produced by activated CD4+ T cells and Treg cells was necessary to prevent spontaneous Th1 cell differentiation in the intestine (Figure 2E), we wondered whether this inhibition depends solely on Treg cell-produced TGFβ1. There was no difference in the frequency of IFN-γ-producing Th1 cells as well as IL-17-producing Th17 cells in the IELs of _Tgfb1_f/n Foxp3-cre mice in comparison to _Tgfb1_f/n mice (Figure 4D). Therefore Treg cell-produced TGFβ1 is dispensable for inhibiting the development of Th1 cells in the peripheral immune system as well as in the intestinal mucosa in the steady state.
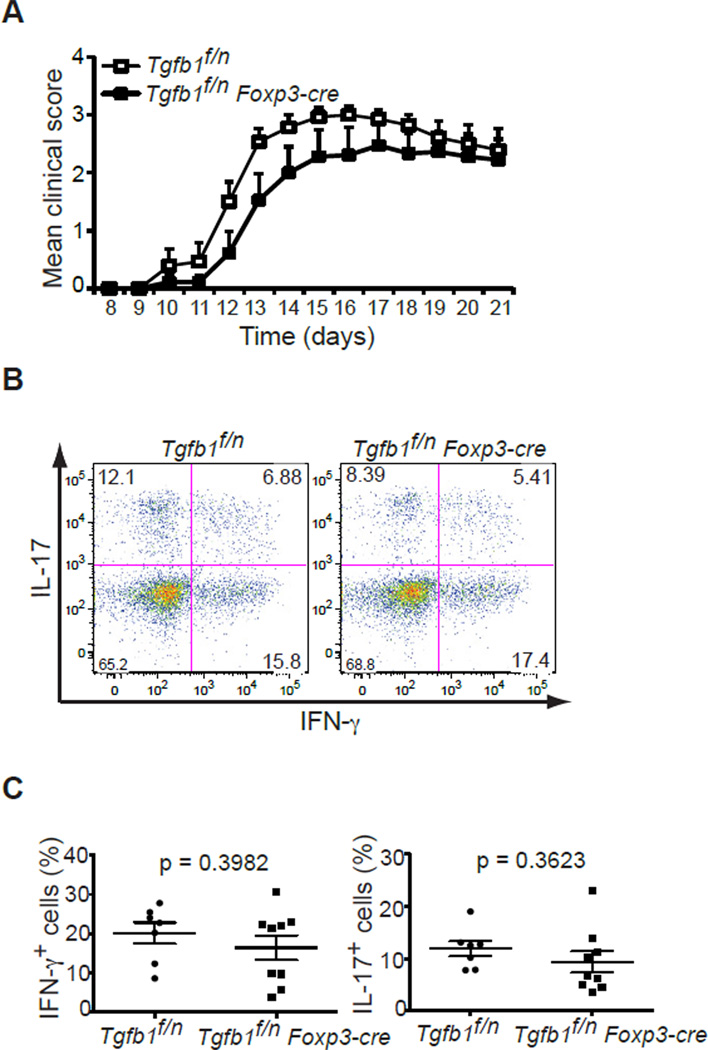
Treg cell-produced TGFβ1 is inessential for effector Th17 cell differentiation
Treg cells have been shown to promote Th17 cell differentiation both in vitro and in models of systemic autoimmune disease and graft-versus-host disease (Lohr et al., 2006; Veldhoen et al., 2006a; Vokaer et al., 2010). Absence of TGFβ1 production by OX40-positive cells abrogated the generation of Th17 cells during MOG35–55-induced EAE (Figure 3B and C). We therefore sought to determine the role of TGFβ1 produced by Treg cells alone in promoting Th17 cell differentiation during EAE. Immunization of _Tgfb1_f/n Foxp3-cre mice with MOG35–55 demonstrated that they were susceptible to EAE development and furthermore they showed a clinical onset and severity that was similar to that of Tgfb1f/n littermates (Figure 5A). In accordance with their susceptibility to disease, there was no defect in the ability of _Tgfb1_f/n Foxp3-cre mice to generate IL-17-producing CD4+ T cells (Figure 5B) and indeed the mean percentages of IFN-γ- and IL-17-producing T cells was comparable to that of _Tgfb1_f/n mice (Figure 5C). We have previously shown through fate-mapping experiments that a small fraction of Th17 cells in the intestine are derived from T cells that have expressed Foxp3 at some point during their development (Zhou et al., 2008). To investigate TGFβ1 deletion in Th17 cells of _Tgfb1_f/n Foxp3-cre mice, we analyzed recombination of the Tgfb1 allele in both _in vitro_-differentiated Th17 cells and CCR6+CD4+ T cells that were enriched for Th17 cells (see below) isolated from the CNS of _Tgfb1_f/n Foxp3-cre mice with EAE. There was minimal deletion of Tgfb1 allele in Th17 cells, as compared to Treg cells, from _Tgfb1_f/n Foxp3-cre mice both in vitro and in vivo (Figure S3), supporting specific Cre expression in Treg cells in these mice. Altogether these data indicate that TGFβ1 produced by Treg cells is not essential for Th17 cell differentiation in the EAE model.
Figure 5. Treg cell-produced TGFβ1 is dispensable for effector Th17 cell differentiation.
(A) EAE disease course in Tgfb1f/n and Tgfb1f/n Foxp3-cre (n=7–9). Disease scores are plotted as mean ± SEM.
(B, C) Cytokine production by CD4+ T cells isolated from the CNS day 21 after disease induction. The cells were stimulated with PMA and ionomycin for 4 hr and were analyzed for IFN-γ and IL-17 expression. The representative plots of diseased Tgfb1f/n and Tgfb1f/n Foxp3-cre mice with a final score of 2 are shown in (B). The frequency of IFN-γ- and IL-17-producing CD4+ T cells are shown in (C). The p values of percentiles of cells producing IFN-γ or IL-17 between the two groups are indicated.
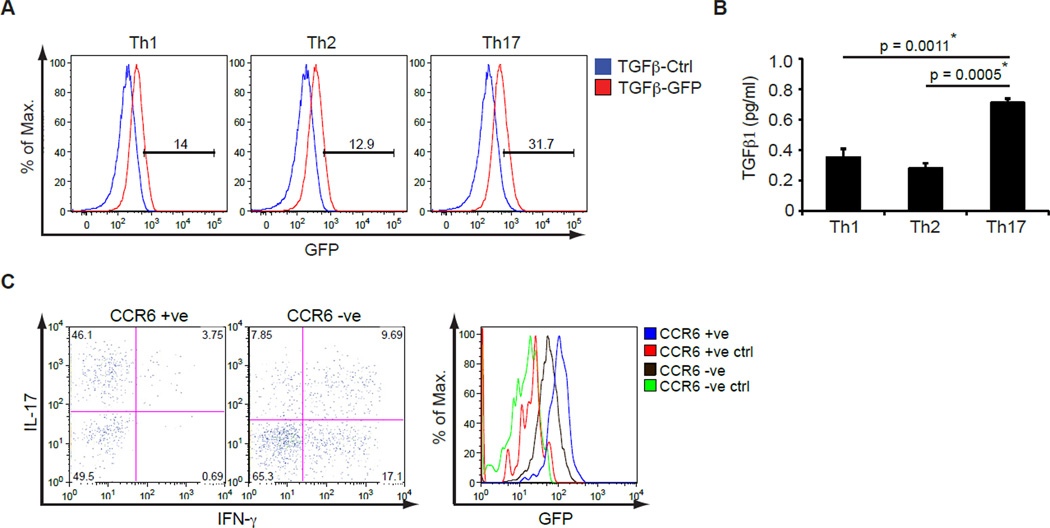
TGFβ1 is highly expressed by effector Th17 cells
Given that TGFβ1 secretion by activated CD4+ T cells and Treg cells, but not Treg cells alone, is an absolute requirement for Th17 cell regulation in the EAE model, we wanted to determine the expression of TGFβ1 by different effector CD4+ T cell subsets. To do so, we analyzed the expression of GFP by naïve CD4+ T cells from hemizygous TGFβ1-GFP knockin mice differentiated towards Th1, Th2 and Th17 cell subsets in vitro for 3 days. GFP was upregulated to the same extent by Th1 and Th2 cell subsets, but was most highly upregulated in Th17 cells (Figure 6A). We next confirmed the expression of TGFβ1 at the protein level by restimulating differentiated CD4+ T cells with CD3 antibody in serum-free medium for 24 hours and performing TGFβ1 ELISA of the supernatant. TGFβ1 was produced by all helper T cell subsets however there were significantly higher levels of TGFβ1 in the supernatant of effector Th17 cells (Figure 6B). Therefore, under in vitro T cell differentiation conditions, effector Th17 cells are the major producers of TGFβ1.
Figure 6. TGFβ1 is highly expressed by effector Th17 cells.
(A) Naïve CD4+ T cells from TGFβ1-GFP knockin mice were differentiated to Th1, Th2 and Th17 cells in vitro for 3 days and then analyzed for upregulation of GFP expression by flow cytometry. These are representative results of three independent experiments.
(B) Naïve CD4+ T cells from C57BL/6 mice were differentiated to Th1, Th2 and Th17 cells in vitro for 3 days and 2x106 cells were restimulated with CD3 antibody for 24 hr. TGFβ1 amounts in the supernatants were determined by ELISA. A representative of three independent experiments is shown. The p values between the groups are shown. * depicts significant difference.
(C) GFP expression in CCR6+ and CCR6− CD4+ T cells isolated from the CNS of MOG 35–55-immunized TGFβ1-GFP knockin mice. TGFβ1-GFP knockin mice were immunized with MOG35–55 and infiltrating mononuclear cells were isolated from the CNS after disease onset and stimulated with PMA and ionomycin for 4 hours to analyze IL-17 and IFN-γ expression in CCR6+ and CCR6− CD4+ T cells (left panels). Histogram shows GFP expression in TGFβ1-GFP knockin mice in comparison to GFP-negative controls (right panel). Data are representative of three TGFβ1-GFP knockin mice analyzed.
To determine whether TGFβ1 is predominantly produced by Th17 cells in vivo, we wished to analyze the expression of TGFβ1 in IL-17-positive and -negative cells. TGFβ1-GFP knockin mice were immunized with MOG35–55 in CFA and the CNS-infiltrating cells were isolated upon disease onset. In order to identify infiltrating Th17 cells, we assessed the T cells for expression of the chemokine receptor CCR6, which is preferentially expressed on differentiated Th17 cells (Reboldi et al., 2009; Yamazaki et al., 2008). We confirmed that CCR6-positive cells expressed high amounts of IL-17 whereas CCR6-negative cells contained a mix of both Th1 and Th17 cells although they were predominantly IFN-γ producers (Figure 6C). Gating on CCR6-positive and CCR6-negative CD4+ T cells demonstrated that there was an almost 2-fold increase in the mean fluorescence intensity of GFP expression in CCR6-positive Th17 cells versus CCR6-negative T cells (Figure 6C). These observations demonstrate that TGFβ1 is expressed by all effector Th subsets however it is especially upregulated by differentiated Th17 cells both in vitro and in vivo.
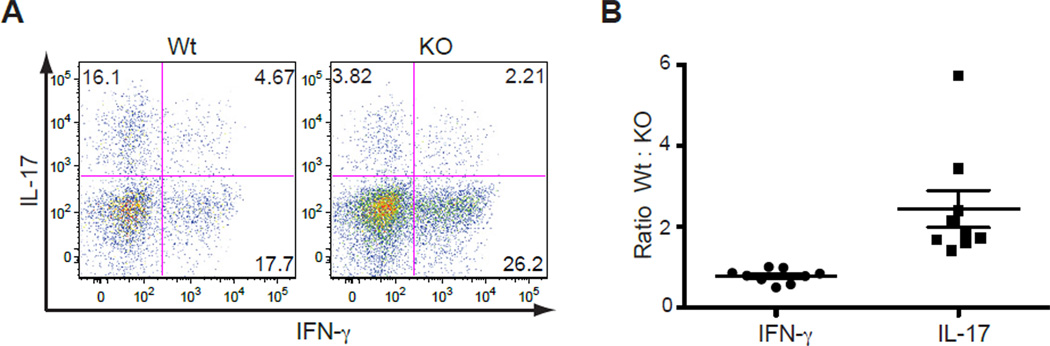
T cell-produced TGFβ1 acts in an autocrine manner to promote Th17 cell differentiation
As TGFβ1 was highly produced by Th17 cells, we wanted to assess whether T cell-produced TGFβ1 functions in an autocrine or paracrine manner to control Th17 cell differentiation. To test this hypothesis, we generated mixed bone marrow-chimeric mice. _Rag1_−/− recipient mice were sublethally irradiated and reconstituted with a 1:1 mix of Wt and _Tgfb1_f/n Cd4-cre bone marrow on the CD45.1 and CD45.2 congenic backgrounds, respectively. After 6 weeks, mice were immunized with MOG35–55 to induce EAE. At the peak of disease, CNS-infiltrating CD4+ T cells were isolated and the frequencies of Th17 and Th1 cells in the CD45.1 and CD45.2 populations were tested by flow cytometry. As expected, Wt (CD45.1) CD4+ T cells had differentiated into both IL-17- or IFN-γ-expressing effector T cells (Figure 7A, left panel). In contrast, there was a substantial decrease in the frequency of Th17 cells and a slight increase in Th1 cells among the _Tgfb1_f/n Cd4-cre (CD45.2) CD4+ T cell population in the same mouse (Figure 7A, right panel). This resulted in ratios of 2.44 and 0.7882 for Wt : _Tgfb1_f/n Cd4-cre Th17 and Th1 cells, respectively (Figure 7B). The decreased frequency of IL-17-producing _Tgfb1_f/n Cd4-cre CD4+ T cells suggests that T cell-produced TGFβ1 is required to function predominantly in an autocrine manner to regulate the maintenance of Th17 cells (Figure S4).
Figure 7. T cell-produced TGFβ1 acts in an autocrine manner to promote Th17 cell differentiation.
(A, B) Cytokine expression of Wild-type (Wt) and Tgfb1f/n Cd4-cre (KO) CD4+ T cells isolated from the CNS of mixed BM-chimeric mice immunized with MOG35–55. Upon disease onset, CD4+ T cells were isolated from the CNS, stimulated with PMA and ionomycin for 4 hr and analyzed for IFN-γ and IL-17 expression. Depicted in (A) is one representative plot of nine mice analyzed. The ratios of all Wt:KO CD4+ T cells that produce IFN-γ or IL-17 are depicted in (B).
Discussion
TGFβ1 is a regulatory cytokine that is secreted by the majority of cell types and has a critical role in controlling T cell differentiation (Li and Flavell, 2008; Li et al., 2006a; Marie et al., 2006). In this report, we generated mice with TGFβ1 deletion in both activated CD4+ T cells and Treg cells or in Treg cells alone by breeding floxed Tgfb1 mice with Tnfrsf4-cre or Foxp3-cre transgenic mice, respectively. With these transgenic models, we showed that TGFβ1 produced specifically by Treg cells is essential for hindering expansion of peripheral Treg cells. In addition, TGFβ1 deletion from activated CD4+ T cells and Treg cells, but not Treg cells alone, abrogated the generation of encephalitogenic Th17 cells during MOG35–55-induced EAE. Using TGFβ1-GFP knockin mice and by generating BM chimeric mice, we could show that TGFβ1 is highly upregulated in effector Th17 cells and functions predominantly in an autocrine loop to promote Th17 cell differentiation during autoimmunity. These results uncover the precise cellular sources of CD4+ T cell-produced TGFβ1 for controlling T cell differentiation and provide the cellular mechanism required for maintaining the commitment of the Th17 cell lineage.
There is much plasticity in the heritable development of Th17 cells, which results in the conversion of _in vitro_-generated Th17 cells to Th1 cells when transferred to recipient mice (Bending et al., 2009; Martin-Orozco et al., 2009; Zhou et al., 2009). Recent data demonstrating the loss of IL-17 expression in differentiated Th17 cells in the absence of TGFβ1 implicate a role for TGFβ1 in maintaining the differentiation of Th17 cells as well as inducing their development (Lee et al., 2009; Lexberg et al., 2008; Zhu et al., 2010). In this report, we were able to show, both in vitro and in vivo, that TGFβ1 is highly upregulated by differentiated Th17 cells. This is analogous to the upregulation of IFN-γ and IL-4 by Th1 and Th2 cells, respectively, which are the key inducers as well as the major stabilizers of those corresponding differentiation programs. The autocrine action of activated CD4+ T cell-produced TGFβ1 implicates a dual function for TGFβ1 in inducing the development and maintaining the commitment of Th17 cells. In contrast to other cytokines involved in effector T cell maintenance, TGFβ1 does not signal through the STAT pathway making it a novel cytokine for the maintenance of T cell differentiation.
The molecular mechanism of TGFβ1 signaling for polarization of Th17 cells is still unclear. TGFβ1 is superfluous in T cells that are deficient in Th1 and Th2 transcription factors implying that TGFβ1 promotes Th17 cell differentiation indirectly by inhibiting Th1 and/or Th2 cell differentiation (Das et al., 2009). Despite the abrogation of Th17 cells in _Tgfb1_f/n Tnfrsf4-cre mice upon EAE induction, we did not observe a marked increase in Th1 cell generation. This suggests that there are alternative molecular mechanisms controlling the initiation and maintenance of Th17 cell differentiation by TGFβ1. In vitro, the presence of TGFβ1 and IL-6 is sufficient for the differentiation of Th17 cells. During in vivo differentiation, a more complex network of cytokines, including IL-23, IL-21 and IL-1β, act to promote or maintain Th17 development in addition to TGFβ1 and IL-6 (Chung et al., 2009; Korn et al., 2007; Korn et al., 2009; Nurieva et al., 2007; Wei et al., 2007). It will therefore also be of interest to identify the precise Th17 cell-inducing cytokines responsible for the upregulation of TGFβ1 expression during Th17 cell development.
Th17 cells are characterized by their production of the signature cytokines IL-17, IL-17F and IL-22 (Harrington et al., 2005; Korn et al., 2009; Park et al., 2005; Veldhoen et al., 2006a). Our data suggest that, in addition to these cytokines, TGFβ1 can be considered as a cytokine preferentially produced by Th17 cells. Th17 cell cytokines perform a variety of effector functions with actions on both immune and non-immune cells (Korn et al., 2009). Likewise, TGFβ1 has pleiotropic effects on both hematopoietic and non-hematopoietic cells and plays an important role in fibrosis and wound healing (Li et al., 2006b). It is thus plausible that TGFβ1 production by Th17 cells is important for aiding tissue repair in the target tissue. The effector function of Th17-expressed TGFβ1 on non-immune cells warrants further investigation.
Treg cells promote Th17 cell polarization in vitro, and co-transfer of Treg cells enhances the frequency of Th17 cells in models of systemic autoimmunity and graft-versus-host disease (Lohr et al., 2006; Veldhoen et al., 2006b; Vokaer et al., 2010). We show here that though the absence of TGFβ1 from activated CD4+ T cells and Treg cells leads to abrogated Th17 cell generation and resistance to EAE, TGFβ1 produced by Treg cells alone is not essential for generating encephalitogenic Th17 cells. Treg cells may promote Th17 cell differentiation through TGFβ1-independent mechanisms. In support of this hypothesis, Chen et al (2011, in this issue) have demonstrated that requirement of Treg cells for the early polarization of Th17 cells relies on IL-2 consumption and is independent of TGFβ1 production by Treg cells.
We and others have previously shown in a co-transfer model of colitis that TGFβ1 derived from Treg cells is required for inhibiting Th1 cell differentiation and colitis development (Li et al., 2007; Powrie et al., 1996). However, we did not observe any signs of spontaneous colitis in _Tgfb1_f/n Foxp3-cre mice, nor was there an increase in colonic Th1 cells. This discrepancy may result from the difference in colitis models. In the co-transfer model, we found that TGFβ1 originating from naïve T cells contributes to Th1 cell inhibition as well (Li et al., 2007), implying that activated T cell-produced TGFβ1 is also required to inhibit spontaneous Th1 differentiation, and colitis development, in an autocrine manner. Indeed our experiments with mixed bone marrow chimeric mice showing an increased frequency of IFN-γ-producing KO CD4+ T cells support a role for autocrine TGFβ1 in limiting Th1 cell generation. In addition, it is possible that redundancy in TGFβ1 production by both Tregs and conventional CD4+ T cells results in protection from colitis in _Tgfb1_f/n Foxp3-cre mice. Interestingly, the colitis phenotype in _Tgfb1_f/n Tnfrsf4-cre mice was less severe than what we previously observed in _Tgfb1_f/n Cd4-cre mice (Li et al., 2007). This suggests that there is compensation by TGFβ1 produced by other T cell types, such as CD8+ T cells or naïve CD4+ T cells, which may limit Th1 cell differentiation and colitis.
T cell-produced TGFβ1 is required to inhibit the proliferation of peripheral Treg cells but is dispensable for their maintenance (Li et al., 2007). The analysis of Treg cells in the peripheral lymphoid organs of _Tgfb1_f/n Foxp3-cre mice showed that TGFβ1 produced by Treg cells is indispensable to limit their expansion. Therefore the autocrine action of TGFβ1 is not restricted to Th17 or Th1 cell differentiation but is also a mechanism used by Treg cells to control Treg cell homeostasis. TGFβ1 is secreted as a latent protein that requires liberation from the latent complex to be active (Li and Flavell, 2008). αvβ8 integrin expressed on dendritic cells (DCs) is essential for the activation of TGFβ1 involved in T cell regulation and Th17 cell differentiation (Acharya et al., 2010; Lacy-Hulbert et al., 2007; Melton et al., 2010; Travis et al., 2007). DCs interact closely with CD4+ T cells for antigen presentation during T cell priming but they are also crucial for mediating entry of autoreactive T cells into the CNS during EAE (Greter et al., 2005; McMahon et al., 2005). Furthermore, the ubiquitous expression of the TGFβ receptor suggests that TGFβ1 will be rapidly consumed by cells in the immediate vicinity of TGFβ1 activation thereby limiting its effects on other cells. We can thus speculate that the predominantly autocrine mode of TGFβ1 regulation of Tregs or effector CD4+ T cells results from both its requirement for activation by αvβ8-expressing DCs and its rapid consumption by the same TGFβ1-producing T cells.
In conclusion, we report that activated Th17 cells themselves are an essential source of TGFβ1, which functions in an autocrine manner to promote the stability of the Th17 cell lineage. These findings clarify our understanding of the cellular mechanisms involved in the control of Th17 cell differentiation that can be exploited for the immunotherapy of autoimmune disease.
Experimental Procedures
Mice
Mouse genomic DNA of the Tgfb1 gene was isolated from a 129SV BAC library (genome System). The coding sequence of gfp gene (encoding green fluorescent protein) and a stop codon was inserted after the start codon of Tgfb1 gene. We constructed the targeting vector by cloning three genomic fragments into plasmid pEasy-Flox. Linearized targeting vector was transfected into ES cells (TC1). Homologous recombinants were identified by Southern-blot analysis. Clones carrying the mutated allele of the Tgfb1 gene (TGFβ1-GFP) were injected into blastocysts and were implanted into foster mothers. Chimeric mice were bred to C57BL/6 mice, and the F1 generation was screened for germline transmission of the mutated allele. TGFβ1-GFP knockin mice were backcrossed to C57BL/6 background for 10 generations before use in experiments. Mice containing floxed Tgfb1, Cd4-cre, Tnfrsf4-cre, Foxp3-cre, and Foxp3-RFP alleles were already described (Klinger et al., 2009; Lee et al., 2001; Li et al., 2007; Rubtsov et al., 2008; Wan and Flavell, 2005). Rosa26-YFP reporter and Rag1−/− mice were obtained from the Jackson Lab. Treg cell-specific TGFβ1-deficient mice were created by crossing _Tgfb1_-floxed mice with the _Foxp3_-cre transgene. Treg and activated T cell-specific TGFβ1-deficient mice were created by crossing _Tgfb1_-floxed mice with the Tnfrsf4-cre transgene. For both strains of mice, the TGFβ1-GFP knockin allele was also used as a Tgfb1 null allele to compensate the deletion of LOC232987 gene in the floxed Tgfb1 allele as previously reported (Li et al., 2007). TGFβ1-GFP knockin mice were crossed with Foxp3-RFP mice, which mark Treg cells by red fluorescent protein expression. All mice were maintained under specific pathogen-free conditions and animal experimentation was conducted in accordance with institutional guidelines.
PCR Typing
For detection of the floxed and deleted Tgfb1 alleles, DNA was isolated from different cell types and was analyzed by PCR with the following primer set: 5′-CTTCCTAACCCCAGAGGTGGA-3′, 5′-CACATTAAGTCGTGGCTAGGG-3′, and 5′-CCCAGGCTAGCCTTGAACTTCT-3′. To analyze germline transmission of the GFP knockin allele the following primers were used: 5′-CGCATCCCACCTTTGCCGAG-3′, 5′-GGCGTCAGCACTAGAAGCCA-3′ and 5′-GCCGTAGGTCAGGGTGGTCA-3′.
Flow Cytometry
Fluorescent-dye-labeled antibodies against cell surface markers CD4, TCR-β, CD62L, CD44, CD45.1 and CD45.2 were purchased from eBiosciences. Spleen and lymph node cells were depleted of erythrocytes by hypotonic lysis. Cells were incubated with specific antibodies for 20 min at 4°C in the presence of 2.4G2 mAb to block FcgR binding. All samples were acquired and analyzed with LSR II flow cytometer (Becton Dickenson) and FlowJo software (Tree Star). Intracellular Foxp3 staining was carried out with a kit from eBiosciences. For intracellular cytokine staining, spleen, lymph node CNS cells and IELs were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma). 1 µM ionomycin (Sigma) and Golgistop (BD Biosciences) for 4 hr. After stimulation, cells were stained with cell surface marker antibodies, fixed and permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) and stained with IFN-γ and IL-17 antibodies.
ELISA
To detect TGFβ1 cytokine amounts in the tissue-culture supernatant, latent TGFβ1 in the culture supernatant was activated by acid treatment and assayed with antibody pairs from R&D Systems (BAF240 and MAB1835).
Cell Purification and Culture
CD4+ and CD8+ T cells were enriched from spleen and lymph node cells by positive selection with anti-CD4 and anti-CD8 microbeads (Miltenyi biotec). Enriched T cells were further purified with a cell sorter (Becton Dickenson) by gating on RFP+CD4+ T cells for Treg cell isolation. Sorted and enriched CD4+Foxp3+, CD4+Foxp3− and CD8+ T cells were cultured at 1×106 cells/well on CD3-coated plates for 24 hours in medium supplemented with nutridoma-SP (Roche). Naïve CD4+ T cells were enriched from spleen and lymph nodes of Wt mice using CD4+CD62L+ T cell isolation kit (Miltenyi Biotec). For in vitro T cell differentiation, 1×106 naïve CD4+ T cells were added to a 24-well plate coated with 5 µg/ml CD3 antibody in complete medium and supplemented with 2 µg/ml CD28 antibody. T cells were differentiated for 3 days using the following cytokines and antibodies: 50 U/ml IL-2, 10 µg/ml anti-IFN-γ (XMG1.2) and anti-IL-4 (11B11), 10 ng/ml IL-12, 10 ng/ml IL-4, 1 ng/ml hTGFβ1, 50 ng/ml IL-6, 10 ng/ml IL-1β and 50 ng/ml IL-23. For detecting TGFβ1 production, differentiated T cells were counted and resuspended at the same concentration in medium supplemented with nutridoma-SP (Roche) in plates coated with CD3 (2 µg/ml) antibody for 24 hours.
EAE Induction and Disease Scoring
Mice were immunized subcutaneously with 50 µg/ml MOG35–55 peptide in 200 µl emulsion of CFA (IFA supplemented with 2.5 mg/ml Mycobacterium Tuberculosis) and were injected on days 0 and 2 with 200 ng/mouse pertussis toxin (List Biological Laboratories). The scoring system used was as follows: 1, limp tail; 2, partial hind limb paralysis; 3, total hind-limb paralysis; 4, hind-limb paralysis and 75% body paralysis; and 5, complete body paralysis/moribund.
Isolation of Mononuclear Cells from CNS and Small Intestine
Mononuclear cells from spinal cords, brain stem and cerebellum were isolated as previously described (Li et al., 2007). In brief, mice were perfused with 20 ml PBS with 2 mM EDTA. Brain stem and cerebellum were dissected and the spinal cord was flushed out with PBS, cut into pieces and digested in PBS supplemented with 10 mg/ml Collagenase D (Roche). The digested CNS was passed through a 70 µm cell strainer, washed and resuspended in 38% Percoll solution (Sigma) and pelleted for 20 min at 2000 rpm. Cells were washed in PBS and used in experiments. For intraepithelial lymphocyte isolation, the small intestine was dissected and, after removal of Peyer’s patches, was incubated two times with DMEM supplemented with 10% FBS and 1 mM DTT for 20 min at 37°C on a shaker. Supernatant was passed through a 70 µm cell strainer, pelleted and resuspended in 40% Percoll. Cells were collected from the interface of a 40% : 70% Percoll gradient after centrifugation at 2500 rpm for 20 min at room temperature. Cells were washed and used in experiments.
Generation of Bone Marrow Chimeras
Bone marrow cells isolated from 6- to 8-week old CD45.1+ congenic C57BL/6 (Wt) mice or CD45.2+ Tgfb1f/n Cd4-cre mice were depleted of erythrocytes by hypotonic lysis and of T cells and antigen-presenting cells by complement-mediated lysis. A 1:1 mix of Wt and Tgfb1f/n Cd4-cre bone marrow cells were injected i.v. into 6- to 8-week old sublethally irradiated (600 rad) Rag1−/− mice.
Histopathology
Tissues from sacrificed animals were fixed in Safefix II (Protocol) and embedded in paraffin. 5 µm sections were stained with hematoxylin and eosin. The histological grading system is as follows: 0, normal colonic crypt architecture with few leukocytes present and plentiful goblet cells; 1, mild inflammation: slight epithelial hyperplasia and increased numbers of leukocytes in the mucosa; 2, moderate colitis: pronounced epithelial cell hyperplasia, significant leukocyte infiltration of the mucosa and decreased numbers of goblet cells; 3, severe colitis: marked epithelial hyperplasia with extensive leukocyte infiltration of the mucosa, sub-mucosa and tunica muscularis, significant depletion of goblet cells, occasional ulceration or crypt abscesses; 4, very severe colitis: marked epithelial epithelial hyperplasia with extensive, dense trans-mural leukocyte infiltration from the submucosa through to the serosa, severe depletion of goblet cells, many crypt abscesses and severe ulceration.
Statistical Analysis
Student’s t test was used to calculate statistical significance for difference in a particular measurement between groups. A p value of < 0.05 was considered statistically significant.
Supplementary Material
01
Highlights.
- Treg cell-produced TGFβ1 is dispensable for Th17 cell differentiation
- Differentiated Th17 cells highly express TGFβ1 both in vitro and in vivo
- TGFβ1 promotes Th17 cell differentiation in an autocrine manner
Acknowledgements
We thank L. Evangelisti, C. Hughes, and J. Stein for their help in creating the TGFβ1-GFP mutant mice, N. Killeen for providing the Tnfrsf4-cre mouse strain. The projects described were supported by grants from the Rita Allen Foundation (M.O.L.), the National Institute of Arthritis, Musculoskeletal and Skin Diseases (KO1 AR053595 and RO1 AR060723, M.O.L.), the Arthritis Foundation (M.O.L.) and the National Research Fund, Luxembourg and the Marie Curie actions of the European Commission (FP7-COFUND, I.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement
The authors declare that they have no competing financial interests.
References
- Acharya M, Mukhopadhyay S, Paidassi H, Jamil T, Chow C, Kissler S, Stuart LM, Hynes RO, Lacy-Hulbert A. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Inves. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois CM, Blanchette F, Laprise MH, Leduc R, Grondin F, Seidah NG. Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme. Am J Pathol. 2001;158:305–316. doi: 10.1016/s0002-9440(10)63970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger M, Kim JK, Chmura SA, Barczak A, Erle DJ, Killeen N. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. 2009;182:4581–4589. doi: 10.4049/jimmunol.0900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexberg MH, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006a;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1 and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006b;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, Roebroek A, Belkaid Y, Creemers J, O'Shea JJ. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–250. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M. The role of T helper subsets in autoimmunity and allergy. Curr Opin Immunol. 2009;21:606–611. doi: 10.1016/j.coi.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006a;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006b;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- Vokaer B, Van Rompaey N, Lemaitre PH, Lhomme F, Kubjak C, Benghiat FS, Iwakura Y, Petein M, Field KA, Goldman M, Le Moine A, Charbonnier LM. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 2010;185:3417–3425. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01