Glucose Counterregulatory Responses to Hypoglycemia (original) (raw)
. Author manuscript; available in PMC: 2013 Aug 28.
Published in final edited form as: Pediatr Endocrinol Rev. 2011 Sep;9(1):463–475.
Abstract
The brain relies almost exclusively on glucose for fuel. Therefore, adequate uptake of glucose from the plasma is key for normal brain function and survival. Despite wide variations in glucose flux (i.e. fed state, fasting state, etc), blood glucose is maintained in a very narrow range. This is accomplished by a series of hormonal and physiologic responses. As a result, hypoglycemia is a rare occurrence in normal individuals. However, glucose counterregulatory responses are altered in patients with diabetes treated with insulin especially after repeated hypoglycemia or antecedent exercise.
Keywords: Hypoglycemia, Counterregulation
Glucose is an essential fuel for the brain. Therefore, adequate uptake of glucose from the plasma is key for normal brain function and survival. For this reason, glucose homeostasis is tightly regulated by a series of hormones and physiologic responses. As a result, hypoglycemia is a rare occurrence in normal individuals, but occurs commonly in patients with diabetes mellitus treated with insulin. Severe hypoglycemia results in cognitive dysfunction including obtundation and seizures; if prolonged it can lead to coma and death. In healthy subjects, the thresholds for counterregulatory hormone production are highly reproducible. However, in diabetic patients, many of these responses are blunted and thresholds may vary depending on their glycemic control. The severe consequences of hypoglycemia and the blunting of counterregulatory and symptom responses make hypoglycemia the limiting factor in the glycemic management of diabetes (1).
DEFINITION OF HYPOGLYCEMIA
Under normal circumstances plasma glucose concentration is maintained in a relative narrow range, 4.0–8.0 mmol/L (72–144 mg/dl) in healthy subjects. This results from a fine balance between glucose influx (exogenous glucose delivery and endogenous glucose production) and glucose efflux (glucose utilization by insulin sensitive tissues such as the skeletal muscle and insulin insensitive tissues, particularly the brain) (2). A series of hormones, neurotransmitters and substrate factors are involved in the regulation of glucose metabolism, and the levels of most of these factors change in response to falling plasma glucose levels (3–5). Hypoglycemia results from an imbalance between glucose influx and glucose efflux due to either excessive glucose removal from the circulation, deficient glucose delivery into the circulation, or both. During conditions of increased glucose utilization such as exercise, pregnancy, and sepsis, blood glucose levels are sustained in the normal range by the capacity of the normal liver (and kidneys) to increase glucose production by gluconeogenesis and glycogenolysis. Therefore, hypoglycemia occurs in instances of defective counterregulatory hormone regulation, elevated circulating insulin levels due to excessive secretion of insulin or iatrogenic hyperinsulinemia; deficiency of counterregulatory hormones; gluconeogenic enzymatic defects or failure to mobilize or utilize gluconeogenic substrates.
The diagnosis of a hypoglycemic disorder or an iatrogenic hypoglycemic episode is more convincing when the Whipple’s triad is documented: (6) a measured low plasma glucose level, symptoms compatible with hypoglycemia, and the recovery of symptoms when the plasma glucose level is raised to normal. The plasma glucose level that defines hypoglycemia has been controversial in children (7). Among term neonates, the plasma glucose level to define hypoglycemia has ranged from <1.0 mmol/L (18mg/dL) to <4.0 mmol/L (70mg/dL), with a modal value of 2.0 mmol/L (36mg/dL) according to a survey of pediatricians in the United Kingdom (8). Lower levels (<1.1 mmol/L [20 mg/dL]) were used in low-birth weight infants. Aynsley-Green suggested that the use of a lower plasma glucose levels in low-birth weight infants stems from studies in the 1960s which demonstrated a fall in plasma glucose level immediately after birth in all neonates which was more pronounced and prolonged in those weighing <2.5 kg; this would include both pre-term and small for gestational age infants. At that time feeding or intravenous glucose support was often delayed for hours (9). However, Lucas et al. followed more than 600 preterm infants and demonstrated that although plasma glucose levels <2.6 mmol/L (46 mg/dL) were common in the newborn period, if those plasma glucose levels persisted for 5 or more days there was an increase in cerebral palsy and developmental delay (10).
Current data suggest a cutoff of plasma glucose level ≤2.5 mmol/L (45 mg/dL) in infants <24 h of life and a plasma glucose level ≤2.8 mmol/L (50 mg/dL) after 24h of life as operational thresholds for hypoglycemia (7) (11). Venous plasma glucose concentrations greater than 3.9 mmol/L (70 mg/dL) after an overnight fast are clearly normal in healthy children and adults. Those between 3.0 and 3.9 mmol/L (54–70 mg/dL) are borderline, and those less than 3.0 mmol/L (54 mg/dL) indicate postabsorptive hypoglycemia in older children and adult subjects without diabetes mellitus. However, in patients with diabetes, glycemic control is limited by hypoglycemia and its associated risks including altered mental status, seizure, coma and even death. Thus, a plasma glucose level of less than 3.9 mmol/L (70 mg/dL) has been recommended as an alert and intervention value for hypoglycemia in patients with diabetes(12). Repeated episodes of hypoglycemia result in hypoglycemia unawareness, impaired glucose counterregulation and predisposition to severe hypoglycemia (12).
GLYCEMIC THRESHOLDS FOR HYPOGLYCEMIC RESPONSES
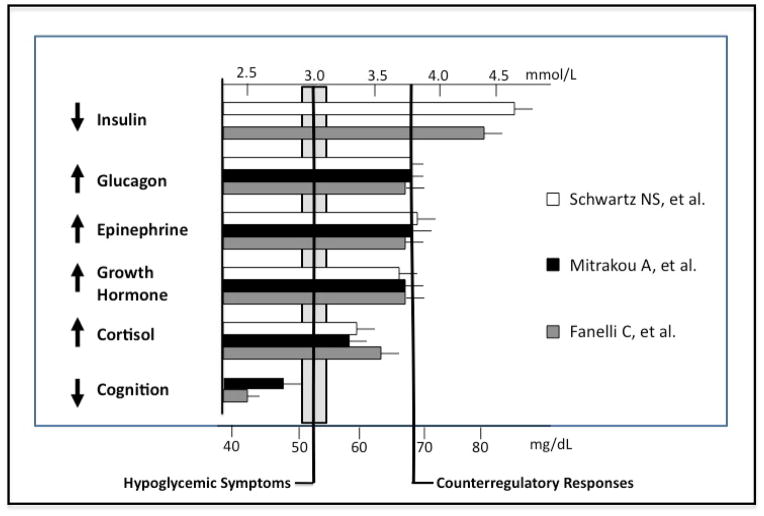
Progressively declining plasma glucose concentrations elicit a sequence of physiological and behavioral responses, with defined glycemic thresholds, in healthy individuals (Fig. 1) (13–15). As plasma glucose fall within the physiologic range (4.4–4.7 mmol/L [80–85 mg/dL]), insulin secretion by the pancreatic β-cells decreases, favoring increased glucose production and decreasing glucose utilization by tissues other than the brain. If plasma glucose levels continue to fall just below the physiologic range (3.6–3.9 mmol/L [65–70 mg/dL]), counterregulatory hormones are secreted. These include glucagon, which is secreted by the pancreatic α-cells and stimulates hepatic glycogenolysis and favors gluconeogenesis, and epinephrine that stimulates hepatic glycogenolysis, mobilizes precursors for hepatic and renal gluconeogenesis and limits glucose utilization by insulin-sensitive tissues. Lower plasma glucose concentrations (2.8–3.0 mmol/L [50–55mg/dL]) cause neurogenic and neuroglycopenic hypoglycemic symptoms, and ultimately, brain dysfunction at levels ~<2.8 mmol/L (50 mg/dL). These thresholds shift to higher plasma glucose concentrations in people with poorly controlled diabetes and to lower plasma glucose concentrations in people who suffer recurrent hypoglycemia, such as those with well-controlled diabetes or with endogenous hyperinsulinism (16–18).
Figure 1.
Glycemic thresholds for physiological responses to hypoglycemia. Adapted from Cryer PE. Hypoglycemia. Pathophysiology, Diagnosis and Treatment. New York: Oxford University Press, 1997, pp. 184, with permission from the author and publisher.
GLUCOSE METABOLISM
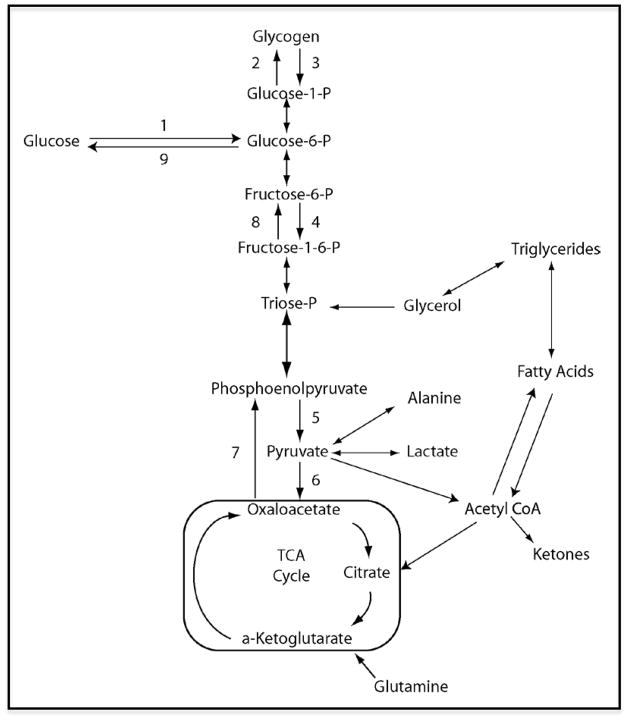
Plasma glucose is derived from intestinal absorption of dietary carbohydrates or endogenous glucose production by either glycogenolysis, or gluconeogenesis (Figure 2). Glucose is transported into the cellular compartment by different glucose transporters. It enters down a concentration gradient across cellular plasma membranes of myocytes, adipocytes and across the blood brain barrier by GLUT-1; and into hepatocytes and pancreatic β cells by GLUT-2. Myocytes and adipocytes also express GLUT-4 in their cytoplasm, which translocates to the cell membrane in response to insulin. Once glucose enters the cell, it is phosphorylated by a hexokinase (glucokinase in hepatocytes and β cells) and then either stored as glycogen or metabolized through glycolysis. Glycolysis is the conversion of glucose-6-phosphate to pyruvate and generation of adenosine triphosphate (ATP). The pyruvate that is produced can be reduced to lactate under anaerobic conditions or it can be oxidized via the tricarboxylic acid (Krebs) cycle, resulting in greater amounts of ATP.
Figure 2.
Schematic representation of glucose metabolism. 1: Hexokinase/glucokinase, 2: Glycogen synthase, 3: Phosphorylase, 4: Phosphofructokinase, 5: Pyruvate kinase, 6: Pyruvate carboxylase, 7: Phosphoenolpyruvate carboxykinase, 8: Fructose-1,6-biphosphatase, 9: Glucose-6-phosphatase.
Gluconeogenesis is the conversion of pyruvate derived from precursors including lactate and amino acids (especially alanine and glutamine) to glucose. Most tissues express the enzymes required for glycogenolysis (phosphorylase) and gluconeogenesis (including the critical gluconeogenic enzymes pyruvate carboxylase, phosphoenolpyruvate carboxykinase, and fructose-1,6-bisphosphatase), but only the liver and kidneys express the enzyme necessary for the release of glucose into the circulation (glucose-6-phosphatase) to contribute to the systemic glucose pool (19). The liver is the major source of net endogenous glucose production (20,21).
Insulin, glucagon and epinephrine regulate the rates of transcription of the key enzymes and regulatory steps involved in endogenous glucose production (gluconeogenesis and glycogen synthesis), and glucose utilization (glycolysis and glycogenolysis). Insulin, which is secreted by pancreatic β cells in response to increases in plasma glucose levels, suppresses glucose production and increases glucose utilization by sensitive tissues such as muscle, fat and liver. Glucagon, which is stimulated by low plasma glucose levels, increases hepatic glucose production primarily by stimulating glycogenolysis. Epinephrine increases glucose production directly by mobilizing the gluconeogenic precursors such as alanine and lactate from muscle and glycerol from fat. It also decreases glucose clearance by insulin-sensitive tissues and limits insulin secretion.
PHYSIOLOGICAL RESPONSES IN THE FED STATE
After a meal the blood glucose concentration increases depending on the amount of carbohydrate ingested, its rate of transit through and absorption from the GI tract, and release of insulin. In the β-cell, glucose is taken up via GLUT2 and phosphorylated to glucose-6-phosphate via an islet-specific glucokinase. Glucose-6-phosphate is then metabolized to produce ATP. Increased levels of ATP result in closure of the ATP-dependent potassium channels (KATP), depolarization of the cell, calcium influx, and release of insulin from secretory granules into the circulation. Basal insulin secretion is pulsatile occurring every 9–14 minutes. There is also a cephalic insulin secretion phase that occurs in response to the sight, smell and taste of food and is mediated by parasympathetic cholinergic innervation (22). Rapid increases in serum glucose, such as following an intravenous bolus of glucose, result in an early (first-phase) burst of insulin secretion that peaks in 3–5 minutes and subsides within 10 minutes. If elevated glucose concentrations are sustained, a second phase of insulin secretion occurs with release of both stored and newly-synthesized insulin. Insulin decreases serum glucose concentrations by three mechanisms: a) inhibition of hepatic glucose production b) suppression of glucagon production by alpha cells in the pancreatic islets and c) stimulation of glucose uptake by myocytes and adipocytes by inducing translocation of GLUT4 to the cell surface. The rate of glucose uptake and utilization by the peripheral tissues subsequently exceeds the appearance of exogenous glucose, resulting in a fall of glucose levels close to the preprandial level. This decline in plasma glucose level results in a decrease in insulin secretion and a resumption of glucagon secretion. Thus, systematic glucose balance is maintained, hypoglycemia does not occur and glucose delivery to the brain is preserved.
PHYSIOLOGICAL RESPONSES IN THE POSTABSORPTIVE STATE
The postabsorptive state begins 4–6 hours after a meal when nutrient absorption from the proceeding meal has subseeded. In this condition plasma glucose levels are maintained in the normal physiologic range and rates of glucose production and utilization average about 2.2 mg/kg/minute in adults(23). This is higher in children and even higher in infants, presumably due to their larger brain mass as a proportion of their body weight. Low insulin levels favor hepatic glycogenolysis and hepatic and renal gluconeogenesis during an overnight fast. As fasting period is prolonged, glycogen stores are depleted and plasma glucose levels decline. This stimulates the secretion of glucagon, epinephrine, growth hormone, and cortisol, which promote hepatic gluconeogenesis, lipolysis and ketogenesis. If fasting is prolonged 24–48 hours, glucose utilization by fat and muscle decreases significantly; insulin levels are suppressed and lipolysis and ketogenesis increase and ketones become the significant fuel source for the brain.
PHYSIOLOGICAL RESPONSES TO HYPOGLYCEMIA
With intact counterregulatory factors, a drop in plasma glucose results in the key physiological defenses against falling plasma glucose concentrations: 1) a decrease in pancreatic β-cell insulin secretion, 2) an increase in pancreatic α-cell glucagon secretion and 3) an increase in adrenomedullary epinephrine secretion. It also results in the perception of hypoglycemic symptoms that are largely sympathetic neural and which prompt carbohydrate ingestion.
Insulin and Glucagon
The first response to falling glucose levels is decreased insulin secretion. As plasma glucose continues to fall (3.6–3.9 mmol/L [65–70 mg/dL]), glucagon is released (13,24) through incompletely understood mechanisms. Studies of isolated rat α-cells, isolated islets, the perfused pancreas and rodents in vivo suggest that it is regulated by intrinsic, paracrine and neuronal mechanisms on both β-cells and α-cells. Alpha cells, like beta cells, express glucokinase and KATP channels so they can sense low glucose concentrations directly. One paracrine effect is insulin inhibition of glucagon secretion from alpha cells(25). Another is somatostatin-mediated inhibition. There is evidence that gut incretin and CNS factors are also involved.
Sympathoadrenal Response
In normal subjects, plasma glucose level of 3.6–3.9 mmol/L [65–70 mg/dL] increases catecholamine-mediated (adrenergic) and acetylcholine-mediated (cholinergic) neurotransmission in the peripheral autonomic nervous system (specifically the sympathoadrenal system) and in the CNS (13,24). The glucose level at which activation of catecholamine responses occur in children has been shown to be higher than in adults and it varies with the level of glycemic control (26). The sympathoadrenal response includes activation of the adrenal medulla to secrete epinephrine and norepinephrine as well as activation of the sympathetic nervous system to release norepinephrine and acetylcholine. DeRosa and Cryer demonstrated lack of increase in plasma epinephrine in response to hypoglycemia in adrenalectomized compared to control subjects, thus implicating the adrenal gland as the primary source of these counterregulatory hormones during hypoglycemia. In contrast, neurogenic symptoms (nervous, sweaty, and hungry) were similar in hypoglycemic control and adrenalectomized subjects suggesting that these are mediated by the sympathetic and parasympathetic neural system rather than adrenomedullary activation (27).
Epinephrine and norepinephrine act on the liver via beta-2 adrenergic receptors to increase hepatic glycogenolysis and gluconeogenesis (28). Several studies in fasted dogs demonstrate only an indirect effect of epinephrine on gluconeogenesis by increasing gluconeogenic substrate release in peripheral tissues (29,30). Increased gluconeogenesis in humans in response to epinephrine also appears to reflect increased lipolysis with generation of gluconeogenic precursors, glycerol and free fatty acids (31). As both glucagon and epinephrine are secreted at similar levels of hypoglycemia, it is important to consider the effect of these hormones in concert. Studies in dogs show an additive effect of epinephrine and glucagon on glucose production (32). Co-infusion of gluconeogenic precursors with glucagon and epinephrine further augmented this increase in hepatic glucose production by increasing gluconeogenesis after 60 minutes suggesting that peripheral production of gluconeogenic precursors is the limiting factor in hepatic gluconeogenesis induced by glucagon and epinephrine.
Growth Hormone and Cortisol
In contrast to the rapid effects of glucagon and epinephrine on glucose regulation, the effects of growth hormone and cortisol during hypoglycemia are delayed. In pharmacologically-induced suppression of all the glucose counterregulatory hormones in humans, and selective replacement by a pancreatic-adrenocortical-pituitary clamp with subcutaneous insulin infusion, lack of cortisol rise resulted in lower rates of glucose production and higher rates of glucose utilization after 6 hours when glucagon, insulin, and growth hormone were infused to maintain similar plasma concentrations in the two groups (33). Plasma glucose levels were also lower in the low cortisol group after 9.5 hours. In a similar study, the effect of growth hormone replacements on glucose utilization and production appeared to have a similar delay (34). Comparison of adult control subjects and untreated hypopituitarism patients with documented growth hormone and cortisol deficiency showed lower plasma glucose levels in the GH and cortisol deficient patients beginning at 2.5 hours after initiation of an insulin infusion which become statistically significant by 12 hours (35). Hypoglycemia corrected rapidly in both groups after stopping the insulin infusion suggesting no critical effect of GH or cortisol on correction of hypoglycemia.
Symptoms
Hypoglycemic symptoms commonly occur with plasma glucose levels < 3.0 mm/L (54 mg/dL). However, the absolute BG level at which signs and symptoms occur may vary among individuals and within the same individual at different times or situations. Particularly in patients with diabetes, it may vary depending on their glycemic control, or prior hypoglycemic episodes (36,37). Symptoms of hypoglycemia can be divided into neurogenic (autonomic) and neuroglycopenic categories (38–40). Neurogenic symptoms result from the sympathoadrenal discharge triggered by hypoglycemia. Adrenergic symptoms such as palpitations, tremor, and anxiety are mediated by norepinephrine from sympathetic postganglionic neurons, the adrenal medullae, or both, and epinephrine from the adrenal medullae. Cholinergic symptoms such as sweating, hunger and paresthesias are mediated by acetylcholine from sympathetic postganglionic neurons. Both are largely mediated by sympathetic neural, rather than adrenomedullary, activation (27). Neuroglycopenic symptoms result from CNS neuronal glucose deprivation and include behavioral changes, confusion, fatigue or weakness, visual changes, seizure, loss of consciousness, and, even death if hypoglycemia is severe and prolonged (38).
CENTRAL INTEGRATION OF GLUCOSE COUNTERREGULATION
Neuronal circuits both peripherally and centrally play an important role in integration of the response to glycemic stimuli. Glucose in the mouth activates taste receptors with projections to the brainstem, resulting in activation of the vagus nerve and induction of the cephalic phase of insulin secretion (22,41). Intestinal glucose stimulates the secretion of various intestinal hormones (glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) and activates enteric and autonomic neurons (42,43). Portal venous glucose activates afferents projecting to the hypothalamus and nucleus of the tractus solitarius among others resulting in simulation of glucose storage in the liver, muscle and fat as well as inhibition of counterregulation, cessation of eating and first phase insulin secretion (44–47).
Glucose sensing in CNS, particularly in the hypothalamus, plays an important roll in modulating counterregulatory hormones. In the central nervous system, both glucose-excited (activated by increased glucose concentrations) and glucose-inhibited (inhibited by increased glucose concentrations) neurons have been identified widely throughout the brain but are increased in the hypothalamus and brain stem (48). Intracarotid glucose infusion reduces secretion of counterregulatory hormones in response to systemic hypoglycemia (49,50). Similarly, intraventricular injection of 2-deoxyglucose, which competes with glucose for cellular uptake but is not metabolized (phosphorylated), increases secretion of glucagon resulting in systemic hyperglycemia (51). Similar to beta cells, glucose excited cells express GLUT2, which transports glucose into the cells. Metabolism of glucose increases the ATP: ADP ratio resulting in closure of the KATP channel, membrane depolarization, and neuronal activity (52). Activation of the hypothalamus and brainstem results in release of the appetite stimulating peptides NPY and AgRP as well as the appetite suppressing peptides POMC and CART (53,54). In addition glucose plays a direct role in regulation of feeding. A drop in glucose precedes initiation of feeding, and feeding is suppressed by an increase in plasma glucose levels (55,56). Although the ventral medial hypothalamus (VMH) has been considered the central integrator of the sympathoadrenal response to hypoglycemia, recent findings by Teves et al. (57), Arbelaez et al. (58) and the Amiel laboratory have shifted the focus to other brain regions (premedial frontal cortex, orbitoprefrontal cortex, thalamus and amygdala) as possible integrated cerebral networks that regulate physiologic responses to hypoglycemia (59).
DIAGNOSIS AND MANAGEMENT OF HYPOGLYCEMIA IN NONDIABETIC INFANTS AND CHILDREN
Diagnosis of hypoglycemia must be based on a laboratory glucose level due to variability of glucose meter readings. A critical sample should be obtained during a hypoglycemic episode or if the blood glucose level is ≤ 2.8 mmol/L (≤ 50 mg/dL) during a prolonged fast to evaluate the etiology of hypoglycemia. This critical sample includes blood glucose, serum bicarbonate (HCO3) to assess for acidosis, insulin, c-peptide, β-hydroxybutyrate, lactate, cortisol, growth hormone, free fatty acids and ammonia as well as urine ketones during the hypoglycemic episode. During a normal response to a blood glucose level below 50 mg/dL, the insulin level should be undetectable (≤ 2 μU/mL), β-hydroxybutyrate increased (≥ 2–5 mmol/L), lactate reduced (≤ 1.5 mmol/L), free fatty acids increased (≥ 1.5 mmol/L), and all the counterregulatory hormones increased (60). A glucagon challenge test (0.03 mg/kg to a maximum of 1 mg IV) is also useful to determine hyperinsulinism when blood glucose is ≤ 2.8 mmol/L and critical samples have already been obtained; an increase in glucose of >30 mg/dL within 15–30 minutes of the injection is highly suggestive, if not diagnostic of hyperinsulinism. Additional laboratory studies that may be useful include plasma free and total carnitine, urine organic acids, and acylcarnitine profile. These can be obtained at any time but are most sensitive during a hypoglycemic episode. An Acylcarnitine profile is suggested prior to a scheduled diagnostic fast, as disorders of fatty acid oxidation are not uncommon and can result in hypoglycemia, lethargy, seizures and SIDS associated with fasting.
Particular attention in the clinical history should be placed to the timing of hypoglycemic symptoms or signs in relation to the duration of fasting or time since last meal before hypoglycemia occurred. Hypoglycemia that occurs after a short fasting period (within 4 to 6 hours of fasting) is caused by either hyperinsulinemia or a glycogen storage disease. In contrast, fasting that takes 8 to 12 hours to provoke hypoglycemia usually implies a defect in gluconeogenesis, or counterregulatory hormone deficiencies, as occurs in hypopituitarism, growth hormone, or cortisol deficiency. Other entities that take more than 10 hours of fasting to manifest hypoglycemia include defects in gluconeogenesis, glycogen metabolism, and fatty acid oxidation and are unlikely to manifest in the newborn period (11).
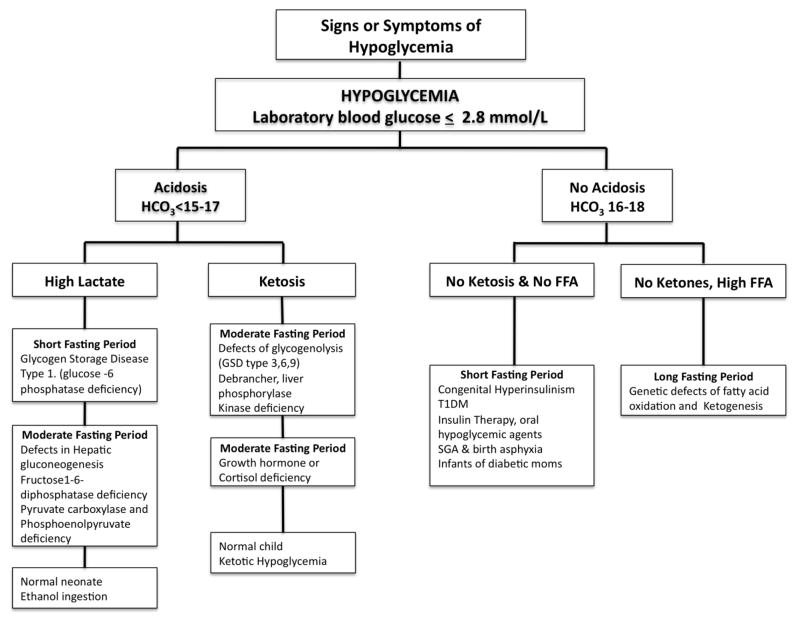
In non-diabetic pediatric patients, hypoglycemia can be classified based on the presence or absence of acidosis (HCO3 ≤ 15–17). (Figure 3). The acidosis is due to either lactic acidosis or ketosis. Hypoglycemia with lactic acidosis can be attributed to glycogen storage disease type 1 (glucose-6-phosphatase deficiency), defects in hepatic gluconeogenesis (fructose-1,6-bisphosphatase deficiency, pyruvate carboxylase or phosphoenolpyruvate deficiency), galactosemia, alcohol ingestion or it can be seen in normal newborns in the first 24 hours of life. Hypoglycemia with ketotic acidosis occurs in patients with glycogen storage disease types 3, 6, and 9 (debrancher, liver phosphorylase, and phosphorylase kinase deficiencies), cortisol deficiency, growth hormone deficiency, and ketotic hypoglycemia. Ketotic hypoglycemia occurs commonly in the toddler/preschool age group during periods of poor oral intake (intercurrent illness) or prolonged fast (10–12 hours); this entity is common, but should be a diagnosis of exclusion. Hypoglycemia without acidosis or ketosis is caused by conditions with elevated insulin levels, hypopiuitarism or disorders of fatty acid oxidation, the latter of which presents with elevated free fatty acids. Transient neonatal hyperinsulinism is found in infants of diabetic mothers, those with intrauterine growth retardation, perinatal stress such as hypoxic-ischemic injury and infants taking beta-blockers. Congenital hyperinsulinism is caused by mutations in potassium channel genes (SUR1 and KIR6.2), glutamate dehydrogenase, glucokinase and short-chain acyl CoA dehydrogenase (SCHAD) (61). Exogenous insulin or insulin secretagogues should also be considered (62).
Figure 3.
Diagnostic approach to hypoglycemia in infants and children. Adapted from Shepherd SP, Kalla A, Arbelaez AM. Endocrine Diseases, Chapter 14. In: Dusenbery SM, White A, eds. The Washington Manual of Pediatrics. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2009;198–222. Adapted from the original with permission from the publisher.
The initial treatment of hypoglycemia should be with feeding, IV dextrose or glucagon (if due to hyperinsulinism) to raise and maintain the plasma glucose ≥ 3.8 mmol/L (70 mg/dL) (63). Treatment and prevention of further hypoglycemia depends on the etiology. Patients are managed with frequent feedings, avoidance of fast, and, in older children, uncooked cornstarch can be used to facilitate an overnight fast. Patients with congenital hyperinsulinism may require high rates of glucose infusion (GIR 10–30 mg/kg/min) until definitive management can be initiated. Many of these patients respond to treatment with diazoxide. However, patients with mutations in potassium channel genes (SUR 1, Kir6.2) often do not respond to diazoxide and may require additional treatment, such as octreotide, until definitive surgical management either with focal or subtotal pancreatectomy can be done depending on the presence of focal or diffuse disease (64).
The major long-term effects of severe, prolonged hypoglycemia are cognitive impairment, recurrent seizure activity, or both (65,66). Subtle effects on personality are also possible but have been less clearly defined. Permanent neurologic sequelae are present in 25–50% of patients with severe recurrent symptomatic hypoglycemia who were hypoglycemic at younger than 6 mo of age (64). These sequelae may be reflected in pathologic changes characterized by atrophic gyri, reduced myelination in cerebral white matter, and atrophy in the cerebral cortex (10,67–69).
HYPOGLYCEMIA AND DIABETES
Hypoglycemia is the limiting factor in the glycemic management of insulin-treated diabetes (1). Absolute therapeutic insulin excess of sufficient magnitude can cause isolated episodes of iatrogenic hypoglycemia and in its extreme manifestations, hypoglycemia, can lead to permanent sequelae and even death (70–74). Most episodes in diabetic patients are the result of the interplay of relative or mild to moderate absolute therapeutic insulin excess and compromised physiologic defenses against falling blood glucose concentrations. The pathogenesis of hypoglycemia in insulin-treated diabetes typically involves no decrease in circulating insulin (which has been given exogenously), lack of glucagon secretion, and an attenuated increase in sympathoadrenal (both adrenomedullary and sympathetic neural) activity. In this setting, prior hypoglycemia, exercise and sleep result in the clinical syndromes of defective glucose counterregulation and hypoglycemia unawareness. These constitute the concept of hypoglycemia-associated autonomic failure (1). Defective glucose counterregulation is associated with a 25-fold (75) or greater (76) increased risk of severe iatrogenic hypoglycemia and hypoglycemia unawareness is associated with a 6-fold increased risk of severe hypoglycemia (77). Severe hypoglycemia has been defined as a blood sugar resulting in altered mental status resulting in the patient being unable to assist in their own care; this includes episodes associated with loss of consciousness or seizure requiring glucagon or parenteral glucose therapy (78). Severe hypoglycemia is more common among infants and adolescents and in those patients with lower glycosylated hemoglobin (HbA1c) and longer duration of the disease (79–81).
There is no consistent value of blood glucose used to define hypoglycemia for children with diabetes. Nevertheless, the ADA Workgroup suggested using a self-monitored glucose concentration of 3.9 mmol/L (70 mg/dL) as the definition in all age groups for research purposes in evaluating therapies designed to alter frequency of hypoglycemia (12). This level represents a glucose concentration near the lower limit of the post-absorptive range (3.9–6.1 mmol/L; (70–110 mg/dL)) and the threshold for activation of counterregulatory hormones (3.6–3.9 mmol/L; (65–70 mg/dL)) in non-diabetic patients, but is higher than the level of hypoglycemia required to produce symptoms and cognitive dysfunction in non-diabetic and well-controlled diabetic patients (78,82,83).
Although hypoglycemia is a rare disorder for healthy subjects it is a fact of life for people with T1DM or T2DM on insulin secretagogue or insulin therapy. They suffer untold numbers of episodes of asymptomatic hypoglycemia, an average of two episodes of symptomatic hypoglycemia per week, and one episode of severe hypoglycemia, often with seizure or coma, per year. Although the risk is low early in the course of T2DM, hypoglycemia becomes progressively more frequent, approaching the frequency in T1DM, as people with T2DM develop absolute endogenous insulin deficiency (1,84,85). With the new intensive insulin regimens and the use of insulin analogs, data suggest that despite continuously declining mean HbA1C levels the rate of severe hypoglycemia of hypoglycemia has decreased to 8–30 episodes per 100 patient-years of diabetes exposure and more recently plateau (79) (80,86–88). Hypoglycemia can interfere with school and social activities. It can be disabling presenting with recurrent seizures, cognitive dysfunction, particularly in those diagnosed before the age of 5 years (89) and can cause changes in the white and gray matter of developing brains (90). Hypoglycemia can be fatal. Recent reports suggest that 6 to 10% of people with T1DM die from hypoglycemia (91–93). Multiple cases of previously well patients with type 1 diabetes experiencing sudden, unexpected death, have occurred while the patient sleeps during the evening. This is known as the “dead in bed” syndrome. It has been thought to be due to cardiac arrhythmias, particularly prolonged QT (94). Mortality rates in T2DM are as yet unknown, but deaths due to hypoglycemia have been documented (1).
Hypoglycemia may be precipitated by missed snack/meal, wrong dose of insulin, exercise (during and several hours after), alcohol ingestion and sleep (95). The effect of exercise has been demonstrated in the Diabetes Research in Children Network study (DirecNet), which reported hypoglycemia during exercise in 22% of subjects and overnight following exercise in 42% compared to only 16% in patients after a sedentary day (96). The goal of treatment in hypoglycemia is to restore the blood glucose level to euglycemia (4.4–5.6 mmol/L (80–100 mg/dL)) and prevent recurrent hypoglycemia. During non severe hypoglycemic episodes patients require immediate treatment with oral, rapidly absorbed, simple carbohydrates and in severe hypoglycemia, patients can be treated with intravenous dextrose or intramuscular glucagon (0.5 mg for age <12 yr, 1.0 mg for ages >12 yr,). It is of key importance to determine the precipitating factors of the hypoglycemic episodes to prevent these from happening again. Comorbidities such as celiac disease or Addison’s disease may also increase the risk for hypoglycemia. Appropriate treatment of these may reduce the frequency of hypoglycemia (97,98). Short-term scrupulous avoidance of hypoglycemia reverses hypoglycemia unawareness and improves defective glucose counterregulation (99–102). Evidence from research studies of the use of continuous glucose monitoring (103) and potential pharmacologic interventions with KATP agonists, beta-adrenergic agonists, SSRIs, opioid agonists, may be shown to be effective in the prevention of hypoglycemia and HAAF (104–110)
Once the mechanism causing hypoglycemia is determined, it should be aimed to either correction of that disorder or if that is not possible, to attain measures to reduce the likelihood of recurrent hypoglycemia with its attendant morbidity and potential mortality.
Acknowledgments
The author is supported in part by National Institutes of Health grant UL1RR24992. We acknowledge the assistance of the following Washington University staff: Janet Dedeke and Amy Hauch, MSN, RN in helping prepare this manuscript and Drs. Philip E. Cryer and Neil H. White for their review of this manuscript.
Footnotes
Disclosures
J.E.S. and A.M.A. have nothing to declare.
References
- 1.Cryer PE. Hypoglycemia in diabetes. Pathophysiology, prevalence and prevention. American Diabetes Association; 2009. pp. 1–160. [Google Scholar]
- 2.Cryer PE. Glucose counterregulation: Prevention and correction of hypoglycemia in humans. Am J Physiol. 1993;264:E149–E155. doi: 10.1152/ajpendo.1993.264.2.E149. [DOI] [PubMed] [Google Scholar]
- 3.Clarke WL, Santiago JV, Thomas L, Ben-Galim E, Haymond MW, Cryer PE. Adrenergic mechanisms in recovery from hypoglycemia in man: Adrenergic blockade. Am J Physiol. 1979;236:E147–E152. doi: 10.1152/ajpendo.1979.236.2.E147. [DOI] [PubMed] [Google Scholar]
- 4.Gerich J, Davis J, Lorenzi M, Rizza R, Bohannon N, Karam J, Lewis S, Kaplan R, Schultz T, Cryer P. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979;236:E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- 5.Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979;64:62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whipple AO. The surgical therapy of hyperinsulinism. Journal of International Chirurgery. 1938;3:237–276. [Google Scholar]
- 7.Cornblath M, Hawdon JM, Williams AF, Aynsley-Green A, Ward-Platt MP, Schwartz R, Kalhan SC. Controversies regarding definition of neonatal hypoglycemia: Suggested operational thresholds. Pediatrics. 2000;105:1141–1145. doi: 10.1542/peds.105.5.1141. [DOI] [PubMed] [Google Scholar]
- 8.Koch T, Eyre JA, Aynsley-Green A. Neonatal hypoglycaemia: The controversy regarding definition. Arch Dis Child. 1988;63:1386–1388. doi: 10.1136/adc.63.11.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aynsley-Green A. Glucose, the brain and the paediatric endocrinologist. Horm Res. 1996;46:8–25. doi: 10.1159/000184971. [DOI] [PubMed] [Google Scholar]
- 10.Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297:1304–1308. doi: 10.1136/bmj.297.6659.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling MA, Menon RK. Differential diagnosis and management of neonatal hypoglycemia. Pediatr Clin North Am. 2004;51:703–723. x. doi: 10.1016/j.pcl.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Defining and reporting hypoglycemia in diabetes: A report from the american diabetes association workgroup on hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. 1987;79:777–781. doi: 10.1172/JCI112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260:E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 15.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, Divincenzo A, Annibale B, Lepore M, Lalli C, Delsindaco P, Brunetti P, Bolli GB. Relative roles of insulin and hypoglycemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycemia in male and female humans. Diabetologia. 1994;37:797–807. doi: 10.1007/BF00404337. [DOI] [PubMed] [Google Scholar]
- 16.Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone-release. Diabetes. 1988;37:901–907. doi: 10.2337/diab.37.7.901. [DOI] [PubMed] [Google Scholar]
- 17.Boyle PJ, Schwartz NS, Shah SD, Clutter WE, Cryer PE. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med. 1988;318:1487–1492. doi: 10.1056/NEJM198806093182302. [DOI] [PubMed] [Google Scholar]
- 18.Mitrakou A, Mokan M, Ryan C, Veneman T, Cryer P, Gerich J. Influence of plasma glucose rate of decrease on hierarchy of responses to hypoglycemia. J Clin Endocrinol Metab. 1993;76:462–465. doi: 10.1210/jcem.76.2.8432790. [DOI] [PubMed] [Google Scholar]
- 19.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest. 1995;96:2528–2533. doi: 10.1172/JCI118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boden G. Gluconeogenesis and glycogenolysis in health and diabetes. J Investig Med. 2004;52:375–378. doi: 10.1136/jim-52-06-31. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Ferranini E. Regulation of intermediary metabolism during fasting and feeding. In: DeGroot LJ, editor. Endocrinology. 3. Philadelphia, PA: WB Saunders; 1995. pp. 1389–1410. [Google Scholar]
- 22.Konturek SJ, Konturek JW. Cephalic phase of pancreatic secretion. Appetite. 2000;34:197–205. doi: 10.1006/appe.1999.0281. [DOI] [PubMed] [Google Scholar]
- 23.Cryer P. The prevention and correction of hypoglycemia. In: Jefferson LS, Goodman HM, editors. Handbook of physiology. Section 7, the endocrine system. Volume II, the endocrine pancreas and regulation of metabolism. Bethesda, MD: American Physiological Soc; 2001. pp. 1057–1092. [Google Scholar]
- 24.Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144–1152. doi: 10.1056/NEJM199504273321707. [DOI] [PubMed] [Google Scholar]
- 25.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes. 2010;59:2936–2940. doi: 10.2337/db10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amiel SA, Simonson DC, Sherwin RS, Lauritano AA, Tamborlane WV. Exaggerated epinephrine responses to hypoglycemia in normal and insulin-dependent diabetic children. J Pediatr. 1987;110:832–837. doi: 10.1016/s0022-3476(87)80393-1. [DOI] [PubMed] [Google Scholar]
- 27.DeRosa MA, Cryer PE. Hypoglycemia and the sympathoadrenal system: Neurogenic symptoms are largely the result of sympathetic neural, rather than adrenomedullary, activation. Am J Physiol Endocrinol Metab. 2004;287:E32–E41. doi: 10.1152/ajpendo.00539.2003. [DOI] [PubMed] [Google Scholar]
- 28.Exton JH. Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev. 1987;3:163–183. doi: 10.1002/dmr.5610030108. [DOI] [PubMed] [Google Scholar]
- 29.Chu CA, Sindelar DK, Neal DW, Cherrington AD. Direct effects of catecholamines on hepatic glucose production in conscious dog are due to glycogenolysis. Am J Physiol. 1996;271:E127–E137. doi: 10.1152/ajpendo.1996.271.1.E127. [DOI] [PubMed] [Google Scholar]
- 30.Chu CA, Sindelar DK, Neal DW, Allen EJ, Donahue EP, Cherrington AD. Comparison of the direct and indirect effects of epinephrine on hepatic glucose production. J Clin Invest. 1997;99:1044–1056. doi: 10.1172/JCI119232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufour S, Lebon V, Shulman GI, Petersen KF. Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am J Physiol Endocrinol Metab. 2009;297:E231–E235. doi: 10.1152/ajpendo.00222.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustavson SM, Chu CA, Nishizawa M, Farmer B, Neal D, Yang Y, Vaughan S, Donahue EP, Flakoll P, Cherrington AD. Glucagon’s actions are modified by the combination of epinephrine and gluconeogenic precursor infusion. Am J Physiol Endocrinol Metab. 2003;285:E534–E544. doi: 10.1152/ajpendo.00059.2003. [DOI] [PubMed] [Google Scholar]
- 33.Defeo P, Perriello G, Torlone E, Ventura MM, Santeusanio F, Brunetti P, Gerich JE, Bolli GB. Demonstration of a role for growth-hormone in glucose counterregulation. American Journal of Physiology. 1989;256:E835–E843. doi: 10.1152/ajpendo.1989.256.6.E835. [DOI] [PubMed] [Google Scholar]
- 34.Defeo P, Perriello G, Torlone E, Ventura MM, Fanelli C, Santeusanio F, Brunetti P, Gerich JE, Bolli GB. Contribution of cortisol to glucose counterregulation in humans. American Journal of Physiology. 1989;257:E35–E42. doi: 10.1152/ajpendo.1989.257.1.E35. [DOI] [PubMed] [Google Scholar]
- 35.Boyle PJ, Cryer PE. Growth hormone, cortisol, or both are involved in defense against, but are not critical to recovery from, hypoglycemia. Am J Physiol. 1991;260:E395–E402. doi: 10.1152/ajpendo.1991.260.3.E395. [DOI] [PubMed] [Google Scholar]
- 36.Cox DJ, Gonder-Frederick L, Antoun B, Cryer PE, Clarke WL. Perceived symptoms in the recognition of hypoglycemia. Diabetes Care. 1993;16:519–527. doi: 10.2337/diacare.16.2.519. [DOI] [PubMed] [Google Scholar]
- 37.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 38.Towler DA, Havlin CE, Craft S, Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes. 1993;42:1791–1798. doi: 10.2337/diab.42.12.1791. [DOI] [PubMed] [Google Scholar]
- 39.McAulay V, Deary IJ, Frier BM. Symptoms of hypoglycaemia in people with diabetes. Diabet Med. 2001;18:690–705. doi: 10.1046/j.1464-5491.2001.00620.x. [DOI] [PubMed] [Google Scholar]
- 40.McCrimmon RJ, Jacob RJ, Fan X, McNay EC, Sherwin RS. Effects of recurrent antecedent hypoglycaemia and chronic hyperglycaemia on brainstem extra-cellular glucose concentrations during acute hypoglycaemia in conscious diabetic bb rats. Diabetologia. 2003;46:1658–1661. doi: 10.1007/s00125-003-1231-4. [DOI] [PubMed] [Google Scholar]
- 41.Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 42.Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept. 2005;128:117–124. doi: 10.1016/j.regpep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi A, Shimizu N, Oomura Y, Kobashi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46:215–218. doi: 10.1016/0304-3940(84)90444-0. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu N, Oomura Y, Novin D, Grijalva CV, Cooper PH. Functional correlations between lateral hypothalamic glucose-sensitive neurons and hepatic portal glucose-sensitive units in rat. Brain Res. 1983;265:49–54. doi: 10.1016/0006-8993(83)91332-x. [DOI] [PubMed] [Google Scholar]
- 46.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking glp-1 and gip receptors. J Clin Invest. 2004;113:635–645. doi: 10.1172/JCI20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donovan CM, Bohland M. Hypoglycemic detection at the portal vein: Absent in humans or yet to be elucidated? Diabetes. 2009;58:21–23. doi: 10.2337/db08-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 2007;22:241–251. doi: 10.1152/physiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- 49.Biggers DW, Myers SR, Neal D, Stinson R, Cooper NB, Jaspan JB, Williams PE, Cherrington AD, Frizzell RT. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes. 1989;38:7–16. doi: 10.2337/diab.38.1.7. [DOI] [PubMed] [Google Scholar]
- 50.Frizzell RT, Jones EM, Davis SN, Biggers DW, Myers SR, Connolly CC, Neal DW, Jaspan JB, Cherrington AD. Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes. 1993;42:1253–1261. doi: 10.2337/diab.42.9.1253. [DOI] [PubMed] [Google Scholar]
- 51.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 52.Matschinsky FM. Banting lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 53.Luckman SM, Lawrence CB. Anorectic brainstem peptides: More pieces to the puzzle. Trends Endocrinol Metab. 2003;14:60–65. doi: 10.1016/s1043-2760(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 54.Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr Opin Clin Nutr Metab Care. 2004;7:471–478. doi: 10.1097/01.mco.0000134368.91900.84. [DOI] [PubMed] [Google Scholar]
- 55.Louis-Sylvestre J, Le Magnen J. Fall in blood glucose level precedes meal onset in free-feeding rats. Neurosci Biobehav Rev. 1980;4 (Suppl 1):13–15. doi: 10.1016/0149-7634(80)90041-x. [DOI] [PubMed] [Google Scholar]
- 56.Campfield LA, Brandon P, Smith FJ. On-line continuous measurement of blood glucose and meal pattern in free-feeding rats: The role of glucose in meal initiation. Brain Res Bull. 1985;14:605–616. doi: 10.1016/0361-9230(85)90110-8. [DOI] [PubMed] [Google Scholar]
- 57.Teves D, Videen TO, Cryer PE, Powers WJ. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A. 2004;101:6217–6221. doi: 10.1073/pnas.0307048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: A mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470–475. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunn JT, Cranston I, Marsden PK, Amiel SA, Reed LJ. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: A new player in hypoglycemia unawareness? Diabetes. 2007;56:2766–2773. doi: 10.2337/db07-0666. [DOI] [PubMed] [Google Scholar]
- 60.Stanley C, Thomson P, Finegold D, et al. Hypoglycemia in infants and neonates. In: Sperling M, editor. Pediatric endocrinology. 2. Philadelphia: WB Saunders; 2002. pp. 135–159. [Google Scholar]
- 61.De Leon DD, Stanley CA. Mechanisms of disease: Advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab. 2007;3:57–68. doi: 10.1038/ncpendmet0368. [DOI] [PubMed] [Google Scholar]
- 62.Stanley CA. Hypoglycemia in the neonate. Pediatr Endocrinol Rev. 2006;4 (Suppl 1):76–81. [PubMed] [Google Scholar]
- 63.Palladino AA, Bennett MJ, Stanley CA. Hyperinsulinism in infancy and childhood: When an insulin level is not always enough. Clinical Chemistry. 2008;54:256–263. doi: 10.1373/clinchem.2007.098988. [DOI] [PubMed] [Google Scholar]
- 64.Stanley CA. Advances in diagnosis and treatment of hyperinsulinism in infants and children. J Clin Endocrinol Metab. 2002;87:4857–4859. doi: 10.1210/jc.2002-021403. [DOI] [PubMed] [Google Scholar]
- 65.Menni F, de Lonlay P, Sevin C, Touati G, Peigne C, Barbier V, Nihoul-Fekete C, Saudubray JM, Robert JJ. Neurologic outcomes of 90 neonates and infants with persistent hyperinsulinemic hypoglycemia. Pediatrics. 2001;107:476–479. doi: 10.1542/peds.107.3.476. [DOI] [PubMed] [Google Scholar]
- 66.Dalgic N, Ergenekon E, Soysal S, Koc E, Atalay Y, Gucuyener K. Transient neonatal hypoglycemia--long-term effects on neurodevelopmental outcome. J Pediatr Endocrinol Metab. 2002;15:319–324. doi: 10.1515/jpem.2002.15.3.319. [DOI] [PubMed] [Google Scholar]
- 67.Kinnala A, Rikalainen H, Lapinleimu H, Parkkola R, Kormano M, Kero P. Cerebral magnetic resonance imaging and ultrasonography findings after neonatal hypoglycemia. Pediatrics. 1999;103:724–729. doi: 10.1542/peds.103.4.724. [DOI] [PubMed] [Google Scholar]
- 68.Alkalay AL, Flores-Sarnat L, Sarnat HB, Moser FG, Simmons CF. Brain imaging findings in neonatal hypoglycemia: Case report and review of 23 cases. Clin Pediatr (Phila) 2005;44:783–790. doi: 10.1177/000992280504400906. [DOI] [PubMed] [Google Scholar]
- 69.Vannucci RC, Vannucci SJ. Hypoglycemic brain injury. Semin Neonatol. 2001;6:147–155. doi: 10.1053/siny.2001.0044. [DOI] [PubMed] [Google Scholar]
- 70.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child. 1999;81:318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hannonen R, Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol. 2003;45:262–268. doi: 10.1017/s0012162203000501. [DOI] [PubMed] [Google Scholar]
- 72.Reichard P, Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the stockholm diabetes intervention study. Diabetes. 1994;43:313–317. doi: 10.2337/diab.43.2.313. [DOI] [PubMed] [Google Scholar]
- 73.Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: A 7-year prospective study. J Pediatr. 1999;134:503–506. doi: 10.1016/s0022-3476(99)70211-8. [DOI] [PubMed] [Google Scholar]
- 74.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- 75.White NH, Skor DA, Cryer PE, Levandoski LA, Bier DM, Santiago JV. Identification of type i diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med. 1983;308:485–491. doi: 10.1056/NEJM198303033080903. [DOI] [PubMed] [Google Scholar]
- 76.Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Benedetti MM, Santeusanio F, Gerich JE, Brunetti P. A reliable and reproducible test for adequate glucose counterregulation in type i diabetes mellitus. Diabetes. 1984;33:732–737. doi: 10.2337/diab.33.8.732. [DOI] [PubMed] [Google Scholar]
- 77.Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2008;25:501–504. doi: 10.1111/j.1464-5491.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 78.Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2009;10 (Suppl 12):134–145. doi: 10.1111/j.1399-5448.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 79.Jones TW, Boulware SD, Kraemer DT, Caprio S, Sherwin RS, Tamborlane WV. Independent effects of youth and poor diabetes control on responses to hypoglycemia in children. Diabetes. 1991;40:358–363. doi: 10.2337/diab.40.3.358. [DOI] [PubMed] [Google Scholar]
- 80.Rosilio M, Cotton JB, Wieliczko MC, Gendrault B, Carel JC, Couvaras O, Ser N, Bougneres PF, Gillet P, Soskin S, Garandeau P, Stuckens C, Le luyer B, Jos J, Bony-Trifunovic H, Bertrand AM, Leturcq F, Lafuma A. Factors associated with glycemic control. A cross-sectional nationwide study in 2,579 french children with type 1 diabetes. The french pediatric diabetes group. Diabetes Care. 1998;21:1146–1153. doi: 10.2337/diacare.21.7.1146. [DOI] [PubMed] [Google Scholar]
- 81.Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, Hamman RF, Klingensmith G. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287:2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- 82.Cryer PE. Preventing hypoglycaemia: What is the appropriate glucose alert value? Diabetologia. 2009;52:35–37. doi: 10.1007/s00125-008-1205-7. [DOI] [PubMed] [Google Scholar]
- 83.Ryan CM, Atchison J, Puczynski S, Puczynski M, Arslanian S, Becker D. Mild hypoglycemia associated with deterioration of mental efficiency in children with insulin-dependent diabetes mellitus. J Pediatr. 1990;117:32–38. doi: 10.1016/s0022-3476(05)82440-0. [DOI] [PubMed] [Google Scholar]
- 84.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 86.Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. The hvidore study group on childhood diabetes. Diabetes Care. 1997;20:714–720. doi: 10.2337/diacare.20.5.714. [DOI] [PubMed] [Google Scholar]
- 87.Tupola S, Rajantie J, Maenpaa J. Severe hypoglycaemia in children and adolescents during multiple-dose insulin therapy. Diabet Med. 1998;15:695–699. doi: 10.1002/(SICI)1096-9136(199808)15:8<695::AID-DIA651>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 88.Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. 2004;27:2293–2298. doi: 10.2337/diacare.27.10.2293. [DOI] [PubMed] [Google Scholar]
- 89.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics. 1985;75:921–927. [PubMed] [Google Scholar]
- 90.Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. 2007;30:2331–2337. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- 91.Genuth S, Nathan DM, Zinman B, Crofford O, Crandall J, Phillips M, Reid M, Brown-Friday J, Engel S, Sheindlin J, Martinez H, Shamoon H, Engel H, Dahms W, Palmert M, Mayer L, Pendegras S, Zegarra H, Miller D, Singerman L, Smith-Brewer S, Gubitosi-Klug R, Gaston P, Genuth S, Brillon D, Lackaye ME, Reppucci V, Lee T, Heinemann M, Whitehouse F, McLellan M, Kruger D, Carey JD, Angus E, Croswell M, Galpirn A, Bergenstal R, Johnson M, Spencer M, Morgan K, Etzwiler D, Kendall D, Noller D, Jacobson A, Golden E, Beaser R, Ganda O, Hamdy O, Rosenzweig J, Wolpert H, Sharuk PG, Arrigg P, Burwood A, Rand L, Nathan DM, Larkin M, Godine J, Moore D, Cagliero E, Lou P, Fritz S, Service J, Ziegler G, Pach J, Colligan R, Lopes-Virella M, Colwell J, Hermayer K, Brabham M, Soule J, Belvins A, Parker J, Lee D, Lindsey P, Bracey M, Lee K, Farr A, Elsing S, Thompson T, Selby J, Lyons T, Yacoub-Wasef S, Szpiech M, Wood D, Mayfield R, Molitch M, Schaefer B, Jampol L, Lyon A, Gill M, Mathura J, Strugula Z, Kaminski L, Shankle J, Astlesford P, Blackburn D, Ajroud-Driss S, Stone O, West C, Burnett-Zeigler I, Weinberg D, Kolterman O, Lorenzi G, Goldbaum M, Sivitz W, Bayless M, Weingeist T, Stone E, Boldt HC, Gehres K, Russell S, Bayless J, Kramer J, Long J, Zeither R, Hebdon M, Donner T, Johnsonbaugh S, Gordon J, Kowarski A, Ostrowski D, Donner T, Steidl S, Jones B, Counts D, Herman W, Martin C, Pop-Busui R, Vine AK, Elner S, Feldman E, Albers J, Greene D, Stevens MJ, Bantle J, Rogness B, Olsen T, Steuer E, Rath P, Johnston R, Hainsworth D, Hitt S, Giangiacom J, Goldstein D, Schade D, Canady J, Burge M, Schluter JM, Das A, Hornbeck D, Schwartz S, Bourne PA, Maschak-Carey BJ, Baker L, Braunstein S, Brucker A, Orchard T, Silvers N, Ryan C, Songer T, Doft B, Olson S, Bergren RL, Lobes L, Fineman M, Freisberg L, Lobes L, Rath PP, Becker D, Drash A, Morrison A, Vaccaro-Kish J, Bernal ML, Malone J, Pavan PR, Grove N, Iyer MN, Burrows AF, Tanaka EA, Berger C, Gstalder R, Dagogo-Jack S, Wigley C, Ricks H, Kitabchi A, Murphy MB, Moser S, Meyer D, Iannacone A, Chaum E, Bryer-Ash M, Schussler S, Lambeth H, Raskin P, Strowig S, He YG, Edwards A, Alappatt J, Wilson C, Park S, Zinman B, Barnie A, MacLean S, Devenyi R, Mandelcorn M, Brent M, Rogers S, Gordon A, Palmer J, Catton S, Brunzell J, Ginsberg J, Kinyoun J, Van Ottingham L, Dupre J, Harth J, Nicolle D, Canny C, May M, Lipps J, Agarwal A, Adkins T, Survant L, Lorenz R, Feman S, White N, Levandoski L, Boniuk I, Grand G, Thomas M, Burgess D, Joseph D, Blinder K, Shah G, Santiago J, Tamborlane W, Gatcomb P, Stoessel K, Taylor K, Dahms W, Trail R, Quin J, Gaston P, Palmert M, Lachin J, Cleary P, Kenny D, Backlund J, Sun W, Rutledge B, Waberski B, Klumpp K, Chan K, Diminick L, Rosenberg D, Petty B, Determan A, Williams C, Dews L, Hawkins M, Cowie C, Fradkin J, Siebert C, Eastman R, Danis R, Davis M, Hubbard L, Geithman P, Kastorff L, Neider M, Badal D, Esser B, Miner K, Wabers H, Glander K, Joyce J, Robinson N, Hurtenbach C, Hannon C, Steffes M, Bucksa J, Chavers B, O’Leary D, Funk L, Polak J, Harrington A, Crow R, Gloeb B, Thomas S, O’Donnell C, Prineas R, Campbell C, Ryan C, Sandstrom D, Williams T, Geckle M, Cupelli E, Thoma F, Burzuk B, Woodfill T, Low P, Sommer C, Nickander K, Detrano R, Wong N, Fox M, Kim L, Oudiz R, Weir G, Clark C, D’Agostino R, Espeland M, Klein B, Manolio T, Rand L, Singer D, Stern M, Lopes-Virella M, Garvey WT, Lyons TJ, Jenkins A, Klein R, Virella G, Jaffa AA, Lackland D, Brabham M, Mcgee D, Zheng D, Mayfield RK, Paterson A, Boright A, Bull S, Sun L, Scherer S, Zinman B, Brunzell J, Hokanson J, Marcovina S, Purnell J, Sibley S, Deeb S, Edwards K, Manning C, Ruocco A, Tinker J, Thomas JL, Shikhman M, Krishnamurthy L, Scott S, Baines T, Wong B, Nakash O, Baity M, Kotyk-Sellnow S, Swenson S, Devine D, Meyer T, Khanna M, Dreyer S, Randal D, Scott S, Kramer J, Bayless J, Kraft C, Welnel S, Swisher S, Pramuka M, Murphy L, Hunter C, Shaw J, Montague E, Scully J, Roger M, Gouchie C, Folley B, Perantie D, Warren S, Dorr C, Cuglietto D, Stone O, Boyd C, Spencer R, Kernen S, Wells K, Yanover T, Spencer A, Nathan DM, Grp DESR. Long-term effect of diabetes and its treatment on cognitive function. New England Journal of Medicine. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feltbower RG, Bodansky HJ, Patterson CC, Parslow RC, Stephenson CR, Reynolds C, McKinney PA. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: Results from the yorkshire register of diabetes in children and young adults. Diabetes Care. 2008;31:922–926. doi: 10.2337/dc07-2029. [DOI] [PubMed] [Google Scholar]
- 93.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in norway. Diabetologia. 2006;49:298–305. doi: 10.1007/s00125-005-0082-6. [DOI] [PubMed] [Google Scholar]
- 94.Heller SR. Sudden death and hypoglycemia. International Diabetes Monitor. 2009;21:234–241. [Google Scholar]
- 95.Ly TJ, Tim Hypoglycemia in children and adolescents. Diabetic Hypoglycemia. 2009;2:3–10. [Google Scholar]
- 96.Tsalikian E, Mauras N, Beck RW, Tamborlane WV, Janz KF, Chase HP, Wysocki T, Weinzimer SA, Buckingham BA, Kollman C, Xing D, Ruedy KJ. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147:528–534. doi: 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mohn A, Cerruto M, Iafusco D, Prisco F, Tumini S, Stoppoloni O, Chiarelli F. Celiac disease in children and adolescents with type i diabetes: Importance of hypoglycemia. J Pediatr Gastroenterol Nutr. 2001;32:37–40. doi: 10.1097/00005176-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 98.McAulay V, Frier BM. Addison’s disease in type 1 diabetes presenting with recurrent hypoglycaemia. Postgrad Med J. 2000;76:230–232. doi: 10.1136/pmj.76.894.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Divincenzo A, Modarelli F, Ciofetta M, Lepore M, Annibale B, Torlone E, Perriello G, Defeo P, Santeusanio F, Brunetti P, Bolli GB. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycemia, following institution of rational, intensive insulin therapy in iddm. Diabetologia. 1994;37:1265–1276. doi: 10.1007/BF00399801. [DOI] [PubMed] [Google Scholar]
- 100.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344:283–287. doi: 10.1016/s0140-6736(94)91336-6. [DOI] [PubMed] [Google Scholar]
- 101.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426–1434. doi: 10.2337/diab.43.12.1426. [DOI] [PubMed] [Google Scholar]
- 102.Amiel SA. Hypoglycemia: From the laboratory to the clinic. Diabetes Care. 2009;32:1364–1371. doi: 10.2337/dc09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hermanides J, DeVries JH. Sense and nonsense in sensors. Diabetologia. 2010;53:593–596. doi: 10.1007/s00125-009-1649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Briscoe VJ, Ertl AC, Tate DB, Davis SN. Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes. 2008;57:3315–3322. doi: 10.2337/db08-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raju B, Arbelaez AM, Breckenridge SM, Cryer PE. Nocturnal hypoglycemia in type 1 diabetes: An assessment of preventive bedtime treatments. J Clin Endocrinol Metab. 2006;91:2087–2092. doi: 10.1210/jc.2005-2798. [DOI] [PubMed] [Google Scholar]
- 106.Cooperberg BA, Breckenridge SM, Arbelaez AM, Cryer PE. Terbutaline and the prevention of nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2008;31:2271–2272. doi: 10.2337/dc08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Richardson T, Thomas P, Ryder J, Kerr D. Influence of caffeine on frequency of hypoglycemia detected by continuous interstitial glucose monitoring system in patients with long-standing type 1 diabetes. Diabetes Care. 2005;28:1316–1320. doi: 10.2337/diacare.28.6.1316. [DOI] [PubMed] [Google Scholar]
- 108.Smith D, Pernet A, Rosenthal JM, Bingham EM, Reid H, Macdonald IA, Amiel SA. The effect of modafinil on counter-regulatory and cognitive responses to hypoglycaemia. Diabetologia. 2004;47:1704–1711. doi: 10.1007/s00125-004-1513-5. [DOI] [PubMed] [Google Scholar]
- 109.McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, Zhu W, Gram DX, Sherwin RS. Activation of atp-sensitive k+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes. 2005;54:3169–3174. doi: 10.2337/diabetes.54.11.3169. [DOI] [PubMed] [Google Scholar]
- 110.Leu J, Cui MH, Shamoon H, Gabriely I. Hypoglycemia-associated autonomic failure is prevented by opioid receptor blockade. J Clin Endocrinol Metab. 2009;94:3372–3380. doi: 10.1210/jc.2009-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]