Mitochondrial dynamics and division in budding yeast (original) (raw)
. Author manuscript; available in PMC: 2013 Sep 28.
Abstract
Mitochondria adopt a variety of different shapes in eukaryotic cells, ranging from multiple, small compartments to elaborate tubular networks. The establishment and maintenance of different mitochondrial morphologies depends, in part, on the equilibrium between opposing fission and fusion events. Recent studies in yeast, flies, worms and mammalian cells indicate that three high-molecular-weight GTPases control mitochondrial membrane dynamics. One of these is a dynamin-related GTPase that acts on the outer mitochondrial membrane to regulate fission. Recently, genetic approaches in budding yeast have identified additional components of the fission machinery. These and other new findings suggest a common mechanism for membrane fission events that has been conserved and adapted during eukaryotic evolution.
Although it is generally accepted that mitochondria play key roles in apoptosis, the cell stress response, aging and various genetically inherited diseases, it is only recently that the regulation of mitochondrial morphology has been recognized as an important factor controlling cell function. It is now known that three conserved GTPases, called Dnm1/Drp1/Dlp1, Fzo/mitofusin and Mgm1/Opa1/Msp1, regulate mitochondrial fission, fusion and membrane structure in many organisms. Moreover, a recent study demonstrated that inhibition of mammalian Drp1 function blocks cell death, suggesting that mitochondrial fission plays an essential role in apoptosis [1]. This finding raises the possibility that Drp1 (and perhaps Fzo- and Mgm1-type) GTPases are targets of signals that determine how mitochondrial membrane morphology changes in response to intracellular and extracellular cues.
A comprehensive list of the genes and proteins implicated in mitochondrial membrane dynamics can be found in several excellent reviews [2–4]. Here, we describe what is known about the three GTPases that regulate mitochondrial membrane dynamics and highlight new studies in budding yeast that provide insight into the mechanism of mitochondrial fission.
Mitochondrial morphology and membrane dynamics
Although the mitochondrion is always surrounded by a double membrane, contains a DNA genome and specializes in the synthesis of ATP, studies using vital dyes and the green fluorescent protein indicate that the morphology of this organelle is different in different cell types and organisms [5–7]. In some cells, mitochondria appear as small, bean-shaped compartments dispersed throughout the cytoplasm. In others, the organelles form elongated tubules or a single, highly branched reticulum. Mitochondria continuously grow, divide and fuse throughout the life of a cell and it is the frequency of fission events relative to fusion events that largely determines steady-state organelle morphology.
Owing to its many experimental advantages, the budding yeast Saccharomyces cerevisiae has emerged as a powerful system to study mitochondrial dynamics. The cells of most organisms cannot survive after mitochondrial DNA (mtDNA) is mutated or lost, and oxidative phosphorylation and ATP production are blocked. By contrast, yeast cells without functional mtDNA can be propagated and studied as long as they maintain a mitochondrial compartment and are provided with a fermentable carbon source such as glucose. Because many nuclear mutations that alter mitochondrial morphology also cause mtDNA loss, S. cerevisiae is one of the few experimental organisms in which the effects of these mutations can be examined and the corresponding genes characterized in detail.
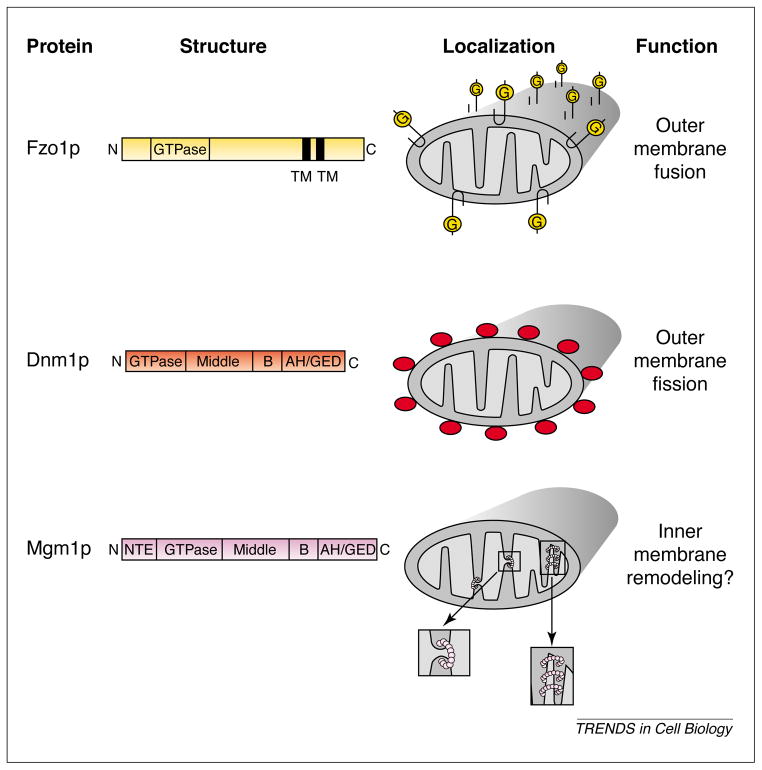
In wild-type yeast cells, mitochondria form an extensive tubular reticulum near the plasma membrane (Fig. 1a). This morphology is maintained by balanced fission and fusion events that occur approximately every two minutes (Fig. 1b) [7]. The fission and fusion of the inner and outer mitochondrial membranes occur in tandem, although the mechanisms by which this coordination is accomplished are not understood. Fission can occur within a tubule or at a branchpoint in the reticulum and is regulated by a GTPase called Dnm1p [8–10]. Fusion always occurs between two mitochondrial tips or between the tip and the side of a tubule, and is regulated by the transmembrane GTPase Fzo1p [11,12]. A third GTPase, called Mgm1p, localizes to the intermembrane space and plays an important, but as yet undetermined, role in mitochondrial compartment remodeling [13–15]. The domain structure, localization and function of all three GTPases are depicted in Fig. 2.
Fig. 1.

Yeast mitochondria form a dynamic reticulum. Wild-type yeast cells producing mitochondrion-targeted green fluorescent protein (mito-GFP) were grown to log phase in media containing galactose at 30°C A 3 μl aliquot was placed on a microscope slide under a coverslip and sealed with nail polish. (a) Stereo pair of a mito-GFP-labeled, unbudded yeast cell. Three-dimensional images were acquired using wide-field fluorescence microscopy. (b) Time-resolved images from a single optical section of 0.2 μm. Time increases from left to right in 3 min increments as indicated. Arrows on the 3 and 9 min images mark fusion events; the arrow on the 21 min image marks a fission event. Bar, 2 μm.
Fig. 2.
Three large GTPases control mitochondrial morphology in yeast. The predicted domain structures of Fzo1p, Dnm1p and Mgm1p, and their submitochondrial localization patterns are shown here. The known (Fzo1p, Dnm1p) or postulated (Mgm1p) function of each GTPase in mitochondrial membrane dynamics is indicated. The magnified views below the Mgm1p localization diagram show possible roles of Mgm1p in inner membrane fission (left) or cristae formation (right).
This view of mitochondrial membrane dynamics has been validated by studies of yeast mutants in which mitochondrial fission or fusion has been selectively blocked. In dnm1 mutant cells, mitochondrial fission is abolished but fusion of mitochondrial tips continues, resulting in the formation of elaborate ‘net-like’ structures (Fig. 3, left) [9,10]. By contrast, mitochondrial networks rapidly fragment in fzo1 mutant cells because mitochondrial fusion is blocked but fission continues unopposed (Fig. 3, right) [11,12].
Fig. 3.

Dnm1p and Fzo1p act in opposing fission and fusion pathways to maintain the yeast mitochondrial network. Ongoing fission and fusion events maintain a tubular mitochondrial network in wild-type yeast cells (middle). Loss of the Dnm1p GTPase (_dnm1_Δ) leads to net formation owing to unopposed mitochondrial tip fusion (left). Loss of the Fzo1p GTPase (_fzo1_Δ) leads to fragmentation owing to unopposed mitochondrial fission (right).
Large GTPases regulate mitochondrial membrane remodeling
The first known molecular regulator of mitochondrial fusion, Fzo, was discovered in Drosophila melanogaster, where it is required for a developmentally regulated mitochondrial fusion event during spermatogenesis [16]. The function of Fzo in mitochondrial fusion has been conserved during evolution as homologs have been identified in a range of other organisms including yeasts, worms, plants and humans [16]. Detailed analysis of yeast Fzo1p provided a direct demonstration that this GTPase regulates mitochondrial fusion. First, loss of Fzo1p function results in the rapid fragmentation of mitochondrial tubules, as described above, because fusion is blocked but fission continues unopposed [11,12]. Second, the mitochondrial fusion that normally occurs after two haploid yeast cells mate is blocked in the absence of Fzo1p function [11]. Recently, two additional Fzo homologs, termed ‘mitofusins’, were identified in Homo sapiens and implicated in mitochondrial fusion, confirming the important role that mitochondrial fusion plays in eukaryotic organisms [17]. Other components are likely to be involved in Fzo1p-dependent mitochondrial fusion. Indeed, a novel outer mitochondrial membrane protein called Ugo1p was recently identified in yeast and shown to be required for this process [18]. The exact role of Ugo1 in fusion and its functional relationship to Fzo1 are currently not known.
The predicted protein domains of the S. cerevisiae Fzo1 GTPase are shown in Fig. 2. Like all the Fzo family members, yeast Fzo contains an N-terminal GTPase domain that is essential for fusion activity and two transmembrane domains near the C-terminus. The transmembrane domains anchor the protein in the outer mitochondrial membrane, leaving most of the N-terminus, the GTPase domain and the C-terminal tail extending into the cytoplasm (Fig. 2). Two strongly predicted α-helical domains near the N- and C-termini of the protein (not shown) have the potential to form coiled-coil structures and might stabilize interactions between Fzo1 proteins and/or as-yet-unidentified protein binding partner(s). Between the two transmembrane domains, a ten amino acid loop extends into the intermembrane space. Several studies suggest that this loop contacts the inner membrane [11,12] and might play a role in coordinating the behavior of the inner and outer membranes during fusion [19].
In contrast to Fzo1p, Dnm1p and Mgm1p are structurally related to the dynamin GTPase, which has a well-established role in membrane fission and vesicle release during endocytosis [20]. Like dynamin, Dnm1p and Mgm1p have an N-terminal GTPase domain that is required for function, a middle domain of ~150 amino acids, a divergent sequence called insert B and a C-terminal α-helical (AH) domain or GTPase effector domain (GED) (Fig. 2) [21]. The Mgm1 protein is presumably targeted to the mitochondrial compartment via a unique N-terminal extension. Homologs of both Mgm1 and Dnm1 have been identified in a range of other organisms including humans. Although defects in the function of the mammalian Dnm1p homolog have not yet been linked to human disease, mutations in a human homolog of Mgm1p (encoded by the OPA1 gene) are linked to a form of dominant optic atrophy that causes childhood blindness [22,23].
Studies in budding yeast indicate that the Mgm1p GTPase plays a complex, but as yet undefined, role in regulating mitochondrial membrane dynamics. Uncertainty about the role of Mgm1p in mitochondrial structure stems, in part, from a debate about its localization. In S. cerevisiae Mgm1p was reported to be an outer mitochondrial membrane protein exposed to the cytoplasm [14], whereas Schizosaccharomyces pombe Mgm1p/Msp1 was reported to be a matrix protein [24]. Recently, however, new studies using both functional hemagglutinin-tagged forms [15] and the native form (E.D. Wong, S.W. Gorisch, A.D. Mozdy, J.M. Shaw and J. Nunnari, unpublished) of the S. cerevisiae Mgm1 protein demonstrated that the bulk of the polypeptide chain resides in the intermembrane space. Additional immunoelectron microscopy experiments revealed that Mgm1p is associated with the inner membrane, concentrated along inner mitochondrial membrane cristae [15]. Based on this localization and on the structural similarities between Mgm1p and dynamin, it is reasonable to postulate that Mgm1p assembles around mitochondrial inner membranes and mediates inner membrane fission (Fig. 2). Alternatively, Mgm1p assembled around portions of the inner membrane might play an important role in generating or maintaining mitochondrial cristae (Fig. 2). Finally, because Mgm1p is in a position to interact with fission and fusion molecules that extend from the inner and outer mitochondrial membranes into the intermembrane space, this GTPase might play a unique role in coordinating the behavior of both membranes during the fission and fusion reactions [15].
Phenotypic analyses of mgm1 mutant cells indicate that the Mgm1 protein also controls mitochondrial membrane dynamics. When Mgm1 function is impaired in yeast, mitochondrial tubules rapidly fragment [15] and mtDNA is subsequently lost [14,15,25]. Mitochondrial fragmentation in mgm1 cells could be the result of a block in fusion or, alternatively, activation of Dnm1p-dependent fission. Both of these possibilities are consistent with the observation that mitochondrial fragmentation is blocked in mgm1 mutant strains in which fission has been abolished by mutation of DNM1 [15,32]. The role of Mgm1p in fusion has also been examined by monitoring mitochondrial fusion during mating between yeast strains containing mutant mgm1 alleles [15]. To avoid indirect effects resulting from the fragmentation of mitochondrial membranes, fusion was examined in mgm1 zygotes in which mitochondrial tubules were restored by deletion of the DNM1 gene. Surprisingly, these studies revealed that the effect of mgm1 mutations on mitochondrial fusion is allele specific. Although an mgm1 temperature-sensitive allele supported mitochondrial fusion in zygotes even after prolonged exposure to the non-permissive temperature [15], fusion was blocked in mgm1 null zygotes (E.D. Wong et al., unpublished; A.D. Mozdy et al., unpublished). One explanation for these disparate observations is that, in _mgm1_-null cells, mitochondrial ultrastructure is disrupted to such an extent that mitochondrial fusion is blocked secondarily. Alternatively, these findings might indicate that the temperature-sensitive mgm1 allele tested is a hypomorph and that Mgm1p is somehow required during the mitochondrial fusion event. More studies are currently under way to elucidate the function of Mgm1p in membrane dynamics.
Fission and the outer membrane GTPase Dnm1p
The role of the Dnm1p GTPase in mitochondrial dynamics was first uncovered in a screen for yeast mutants with defective mitochondrial morphology [8]. Subsequent studies (described above) suggested that loss of Dnm1p function blocked mitochondrial fission but allowed the fusion of mitochondrial tips to continue, resulting in the formation of elaborate net-like structures [9,10]. Most importantly, the mitochondrial fragmentation induced by a fzo1 mutation no longer occurred in cells containing a dnm1 mutation [9,10]. This result provided a direct demonstration that mitochondrial fragmentation in fzo1 was caused by Dnm1p-mediated fission.
Mutations in several Dnm1p homologs including mammalian Drp1 [26] and Caenorhabditis elegans DRP-1 [27] were also shown to block mitochondrial fission. Conversely, overproduction of Dnm1p [28] and C. elegans DRP-1 [27] increases the number of mitochondrial fragments, suggesting that the rate of fission is limited by the steady-state level of this GTPase. Although the behavior of the inner and outer mitochondrial membranes during fission is coupled in yeast cells, division of the inner mitochondrial membrane in C. elegans is not affected by mutations that block outer mitochondrial membrane fission [27]. This finding raises the possibility that the coupling of the inner and outer mitochondrial membrane dynamics might be regulated differently in different organisms.
Biochemical analyses detect a cytoplasmic pool of Dnm1p in yeast cells [8] and of Drp1 in mammalian cells [26]. In vivo, however, a large proportion of these proteins assembles into punctate structures on the outer mitochondrial membrane [8,26]. Immunogold labeling in yeast revealed Dnm1p clustering at constricted sites on mitochondrial tubules that appear to be dividing [9]. Time-lapse imaging of GFP-tagged Dnm1p in S. cerevisiae [10], DRP-1 in C. elegans [27] and Drp1 in mammalian cells [26] showed that mitochondrial tubules divide at sites where these punctate structures are found. Together, these studies provide compelling evidence that cytoplasmic Dnm1p/DRP-1/Drp1 assembles at discrete sites on the outer mitochondrial membranes of all three species, where it plays a direct role in mitochondrial fission.
Mammalian dynamin is a tetramer that assembles into collars around the necks of clathrin-coated pits at the plasma membrane [20]. GTP hydrolysis catalyzed by dynamin in these collars is required for the efficient fission and release of clathrin-coated vesicles [20]. Although Dnm1p is related to dynamin, there is currently no ultrastructural evidence that this yeast protein assembles into higher-order structures. However, two recent studies showed that mammalian Drp1 (also called Dlp1) [26,29] assembles to form rings in vitro. This ring formation induces tubulation of synthetic liposomes, raising the possibility that the mitochondrial dynamin family members form rings around mitochondrial membranes in vivo before organelle fission [29]. Clearly, more efforts must be made to determine whether Dnm1p forms rings and whether the rings formed in vitro are functionally and structurally equivalent to the punctate structures that regulate mitochondrial fission in vivo. In addition, it should be noted that the diameter of mitochondrial tubules is significantly larger than either the neck of a clathrin-coated pit or a synthetic tubule formed in vitro. Thus, the Dnm1p-containing structures visualized on mitochondrial tubules in vivo might contain additional molecules that accommodate this structural difference (for example, Mdv1p, see below). (To facilitate discussion, yeast Dnm1p and Mgm1p are depicted as forming rings around mitochondrial outer or inner membranes in Figs 2, 4 and 5.) How conformational changes in Dnm1p- or Mgm1p-containing structures lead to mitochondrial membrane constriction and fission is not currently understood.
Fig. 4.
Mdv1p and Fis1p regulate Dnm1p-mediated mitochondrial fission. (a) Predicted domain structures and mitochondrial localization patterns of Dnm1p, Mdv1p and Fis1p. (b) The dependence of Dnm1p’s mitochondrial localization pattern on FIS1 and MDV1 function. The localization pattern of Dnm1p (red circles) is shown on the outer mitochondrial membrane in (from left to right) wild-type cells, fis1 mutant cells, mdv1 mutant cells and mdv1 fis1 mutant cells. The fission phenotype observed is indicated below each cell type. (c) The dependence of Mdv1p’s mitochondrial localization pattern on DNM1 and FIS1 function. The localization pattern of Mdv1p (blue circles) is shown on the outer mitochondrial membrane in (from left to right) wild-type cells, fis1 mutant cells, dnm1 mutant cells and dnm1 fis1 mutant cells. In dnm1 fis1 mutant cells, Mdv1p localizes to the cytoplasm and is not shown (far right). The fission phenotype observed is indicated below each cell type.
Abbreviations: AH, α-helical domain; NTE, N-terminal extension; TM, transmembrane domain.
Fig. 5.
Molecular model of mitochondrial division. T op: mitochondrial division is depicted as a linear pathway with the relative rates of each step (1–3) indicated. Red circles represent Dnm1p-containing complexes. Bottom: the postulated molecular interactions occurring between Dnm1p, Fis1p and Mdv1p during steps 1 and 2 of the pathway. Mitochondrial tubules are drawn in cross section and a single complex is shown containing the three molecules. Dnm1p, Mdv1p and Fis1p are shown in red, blue, and green, respectively. Abbreviation: IMS, intermembrane space.
New components of the outer membrane fission machinery
Recently, a genetic approach was used to identify novel molecules, called Mdv1/Fis2/Gag3/Net2 [30–33] and Fis1/Mdv2 [30,31], that work together with the Dnm1p GTPase during mitochondrial fission. Several lines of evidence show that Mdv1p (mitochondrial division 1) and Fis1p (fission 1) regulate fission. First, like dnm1 mutations, mdv1 and fis1 mutations block fission and result in the formation of interconnected mitochondrial nets [30–33]. Second, fis1 and mdv1 mutations block mitochondrial fragmentation and mtDNA loss in the fzo1 fusion mutant [30,31,33]. Finally, the fis1 and mdv1 mutations are specific for fission and do not interfere with the fusion of mitochondrial membranes during yeast mating [30,31,33].
The MDV1 gene encodes a soluble 80 kDa protein with an N-terminal extension, a central coiled-coil domain and seven C-terminal WD repeats (Fig. 4a). Mdv1p interacts with Dnm1p in a yeast two-hybrid assay [30,33,34] and co-localizes with Dnm1p in punctate structures on the outer mitochondrial membrane [30,33], suggesting that the two proteins are part of a complex that marks future division sites. Interestingly, homologs of MDV1 have not been identified in higher eukaryotic genomes, suggesting that Mdv1p function is specific for yeast mitochondrial division or has been replaced by another gene product in multicellular eukaryotes.
The 17 kDa Fis1 protein spans the outer mitochondrial membrane, with its N-terminus exposed to the cytoplasm and its C-terminus facing the intermembrane space [31]. Unlike the punctate structures formed by Dnm1p and Mdv1p, Fis1p is distributed evenly over the entire mitochondrial surface [31] (Fig. 4a). Homologs of FIS1 have been identified in a wide range of eukaryotes including worms, mammals and plants, suggesting that its role in mitochondrial division has been conserved [31].
Mdv1p is required for Dnm1p function but not Dnm1p assembly
Localization studies revealed that Mdv1p is not required for the assembly of Dnm1p on mitochondrial tubules [30–33]. In wild-type DNM1 MDV1 FIS1 cells, Dnm1p and Mdv1p localize together in complexes on the outer mitochondrial membrane that catalyze membrane fission (Fig. 4b, c) [30,33]. In cells lacking the Mdv1 protein (DNM1 mdv1 FIS1), both the number and the distribution of Dnm1p complexes on the outer mitochondrial membrane appear wild type (Fig. 4b) [30–33]. However, these Dnm1p-containing complexes are unable to complete the mitochondrial division event. These complexes are likely to be productive intermediates because MDV1 expression from an inducible promoter in DNM1 mdv1 FIS cells is sufficient to restore mitochondrial fission by these structures [30]. These results indicate that Mdv1p is not required for the recruitment of Dnm1p to the mitochondrial membrane or for its assembly into punctate structures. Instead, Mdv1p acts late in the fission pathway, after Dnm1p assembly, by stimulating a potentially rate-limiting step in fission.
Fis1p is required for assembly of functional Dnm1p–Mdv1p complexes
The topology of Fis1p on the outer mitochondrial membrane suggests that this protein might be acting early in the fission pathway as a ‘receptor’ on which to assemble functional Dnm1p fission complexes. In vivo localization studies of Dnm1p in fis1 mutant cells confirmed this prediction (Fig. 4b). In contrast to both wild-type and _mdv1_-mutant cells, Dnm1p–Mdv1p division complexes fail to assemble correctly in the DNM1MDV1fis1 mutant and are instead present in a few large aggregates on the outer membrane (Fig. 4b, c) [30,31]. Because Dnm1p and Mdv1p associate within these aberrant structures, Fis1p is not required for the Dnm1p–Mdv1p interaction [30]. In addition, the role of Fis1p in the assembly of Dnm1p structures does not depend on Mdv1p function, because the aberrant Dnm1p localization pattern in the DNM1 MDV1 fis1 strain is identical to that found in DNM1 mdv1 fis1 cells [30,31]. These observations suggest that Fis1p acts early in the fission pathway to regulate the assembly and distribution of Dnm1p-containing fission complexes on the outer mitochondrial membrane. Although these results clearly demonstrate a role for Fis1p in assembling Dnm1p–Mdv1p complexes, the ability of Dnm1p to associate with mitochondria in the absence of both Fis1p and Mdv1p suggests that Dnm1p interacts with specific lipids and/or additional molecules in the outer mitochondrial membrane.
Fis1p is required for a second function
A second, late function for Fis1p was suggested by Mdv1p localization studies [30]. In wild-type cells, Mdv1p assembles with Dnm1p in punctate structures associated with mitochondria (Fig. 4b, c). In cells lacking Dnm1p (dnm1 MDV1 FIS1), Mdv1p is no longer observed in punctate structures but, interestingly, is still associated tightly with mitochondria and distributed evenly on the outer membrane (Fig. 4c) [30,32,33]. This interaction of Mdv1p with mitochondria depends on Fis1p function because, in cells that lack both Dnm1p and Fis1p (Fig. 4c, dnm1 MDV1 fis1), Mdv1p no longer associates with the mitochondrial membrane and is found in the cytoplasm [30]. Thus, Fis1p targets Mdv1p to the mitochondrial membrane in the absence of Dnm1p. These findings suggest that Fis1p performs a second function late in the fission pathway via a direct Mdv1p–Fis1p interaction. Alternatively, the late Fis1p function might facilitate an interaction between Mdv1p and another unidentified component to regulate membrane division [30]. Regardless of the exact molecular interactions, these combined localization studies suggest that Fis1p function is required at least twice during mitochondrial division: at an early stage to regulate Dnm1p assembly and at a later stage, together with Mdv1p, to facilitate Dnm1p-dependent mitochondrial membrane constriction and division.
Regulation by Dnm1p occurs via a multistep pathway
Based on the genetic and cellular studies described above, a molecular model for mitochondrial division can be proposed (Fig. 5). The pathway of mitochondrial division consists of slow and fast steps. Assembly of Dnm1p onto the cytoplasmic face of the outer mitochondrial membrane is currently the earliest recognizable event in the pathway (Fig. 5, top, step 1). Although Mdv1p is part of the Dnm1p complex that is assembled, Mdv1p is not required for the assembly event itself. By contrast, the integral membrane protein Fis1p is required for the assembly of functional Dnm1p complexes. Other important features of this assembly step remain unclear, including the signals that determine the position of complex assembly and the overall conformation of the structure formed (rings, collars, etc.). However, it is clear that Dnm1p complex assembly is not a rate-limiting step in the pathway, because the number of visible complexes at steady state is large compared with the rate of division [8], and time-lapse studies indicate that these complexes persist for a while before the fission event [10].
What, then, are the rate-limiting steps? Based on electron microscopic analyses indicating that mitochondrial membrane constriction is relatively rare [9], the first rate-limiting step is likely to be membrane or lipid remodeling events that generate a local constriction in the mitochondrial tubule (Fig. 5, top, step 2). Evidence that the GTPase cycle of Dnm1p might regulate this rate-limiting step in mitochondrial fission comes from mutational studies of the Dnm1p AH domain [28]. In previous in vitro studies of mammalian dynamin, the AH domain was shown to stimulate GTP hydrolysis by dynamin after the protein assembled to form higher-order structures [35,36]. Based on these findings, dynamin’s AH domain was renamed the GED [36]. Overproduction of a dynamin GED mutation predicted to prolong the GTP-bound state was shown to enhance the rate of endocytosis in vivo, consistent with the idea that dynamin–GTP regulates a rate-limiting step in endocytosis [36]. A later study established that this rate-limiting step was the formation of constricted coated pits [35]. The effect of Dnm1p’s AH/GED on the rate of GTP hydrolysis by assembled Dnm1p has not yet been measured. However, production of an equivalent Dnm1-GED mutant protein has been shown to increase the steady state level of mitochondrial fission in yeast cells [28]. By analogy with the dynamin studies, this result is consistent with the idea that the Dnm1p AH/GED region, and perhaps the Dnm1p GTPase cycle, modulate a rate-limiting membrane constriction step during mitochondrial division [28]. Most importantly, this study strongly suggests that Dnm1p functions as a classical GTPase, recruiting downstream partners required for membrane constriction when it is in its GTP-bound state (Dnm1p–GTP). Although Dnm1p might also perform a mechanochemical role during membrane constriction, there is currently no evidence for this.
At this point, models for the roles of Dnm1p, Mdv1p and Fis1p in mitochondrial constriction and fission are purely speculative. One possibility is that these three proteins form a complex that undergoes conformational changes that affect the Dnm1p GTPase cycle, the outer membrane constriction event or both. Possible molecular rearrangements of a hypothetical Dnm1p–Mdv1p–Fis1p complex during the membrane constriction step of mitochondrial division are shown in Fig. 5. Division might be initiated by the recruitment of cytoplasmic Dnm1p–GTP to the mitochondrion, where Fis1p regulates its assembly into higher-order structures at the surface of the outer membrane. Mdv1p either assembles with Dnm1p–GTP during the formation of these higher-order structures or associates with Dnm1p–GTP in these structures after their formation. A conformational change in this complex that brings Fis1p and Mdv1p into contact might initiate membrane curvature and constriction (Fig. 5 shows two possible conformational changes that bring Fis1p and Mdv1p into direct contact). Alternatively, Dnm1p–GTP might recruit additional components to this complex that either modify lipids in the outer membrane leaflet and induce membrane curvature or facilitate the mechanical constriction of the membrane tubule.
After membrane constriction is accomplished, GTP hydrolysis by Dnm1p might trigger disassembly of the Dnm1p–Mdv1p–Fis1p complex so that membrane fission can be completed (Fig. 5, step 3). Although genetic and cytological evidence support many of the molecular interactions proposed by this model, further biochemical studies are required to establish the existence of such a Dnm1p–Mdv1p–Fis1p complex and any molecular rearrangements this complex undergoes during mitochondrial division. Moreover, although it is clear that Dnm1p can bind and hydrolyze GTP in vitro, the stages of the mitochondrial division reaction that require GDP binding, GDP–GTP exchange and GTP hydrolysis must be determined. Despite these caveats, this model contains several testable features and should provide a useful framework for the molecular, genetic and biochemical analysis of the mitochondrial division reaction.
Concluding remarks
Now that some of the players are known and a basic pathway for mitochondrial division has been mapped out, studies aimed at dissecting the biochemical roles of Dnm1p, Mdv1p and Fis1p can begin. These investigations will require the development of in vitro assays that reconstitute individual steps in the fission pathway. Identification of the molecular machinery that coordinates the behavior of both membranes during fission and fusion will also be essential. It is clear that mitochondria have much to teach us about membrane dynamics in general. As the molecular secrets of mitochondrial fission and fusion are unraveled, we will gain insights into similar processes occurring at other cellular membranes.
Acknowledgments
We thank S. Sever for insightful discussions and past and present members of our labs for their hard work and contributions to this field. Research in the Shaw lab has been supported by grants from the NIH, ACS, NSF and the University of Utah Huntsman Cancer Institute. Research in the Nunnari lab has been supported by grants from the NIH and NSF.
Contributor Information
Janet M. Shaw, Email: shaw@bioscience.biology.utah.edu, Dept of Biology, University of Utah, 257 S. 1400 E., Salt Lake City, UT 84112, USA.
Jodi Nunnari, Section of Molecular and Cellular Biology, University of California, Davis, CA, USA.
References
- 1.Frank S, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 2.Hermann GJ, Shaw JM. Mitochondrial dynamics in yeast. Annu Rev Cell Dev Biol. 1998;14:265–303. doi: 10.1146/annurev.cellbio.14.1.265. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe MP. The machinery of mitochondrial inheritance and behavior. Science. 1999;283:1493–1497. doi: 10.1126/science.283.5407.1493. [DOI] [PubMed] [Google Scholar]
- 4.Jensen RE, et al. Yeast mitochondrial dynamics: fusion, division, segregation and shape. Microsc Res Tech. 2001;51:573–583. doi: 10.1002/1097-0029(20001215)51:6<573::AID-JEMT7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LV, et al. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980;77:990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- 7.Nunnari J, et al. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol Biol Cell. 1997;8:1233–1242. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otsuga D, et al. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleazard W, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann GJ, et al. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J Cell Biol. 1998;143:359–374. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapaport D, et al. Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae. J Biol Chem. 1998;273:20150–20155. doi: 10.1074/jbc.273.32.20150. [DOI] [PubMed] [Google Scholar]
- 13.Guan K, et al. Normal mitochondrial structure and genome maintenance in yeast requires the dynamin-like product of the MGM1 gene. Curr Genet. 1993;24:141–148. doi: 10.1007/BF00324678. [DOI] [PubMed] [Google Scholar]
- 14.Shepard K, Yaffe M. The yeast dynamin-like protein, Mgm1p, functions on the mitochondrial outer membrane to mediate mitochondrial inheritance. J Cell Biol. 1999;144:711–719. doi: 10.1083/jcb.144.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong ED, et al. The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J Cell Biol. 2000;151:341–352. doi: 10.1083/jcb.151.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 17.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 18.Sesaki H, Jensen RE. UGO1 encodes an outer membrane protein required for mitochondrial fusion. J Cell Biol. 2001;152:1123–1134. doi: 10.1083/jcb.152.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritz S, et al. Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J Cell Biol. 2001;152:683–692. doi: 10.1083/jcb.152.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Bliek AM. Functional diversity in the dynamin family. Trends Cell Biol. 1999;9:96–102. doi: 10.1016/s0962-8924(98)01490-1. [DOI] [PubMed] [Google Scholar]
- 22.Alexander C, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 23.Delettre C, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 24.Pelloquin L, et al. Fission yeast Msp1 is a mitochondrial dynamin-related protein. J Cell Sci. 1999;112:4151–4161. doi: 10.1242/jcs.112.22.4151. [DOI] [PubMed] [Google Scholar]
- 25.Jones BA, Fangman WL. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 1992;6:380–389. doi: 10.1101/gad.6.3.380. [DOI] [PubMed] [Google Scholar]
- 26.Smirnova E, et al. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labrousse AM, et al. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 28.Fukushima NH, et al. The AH/GED sequence of the Dnm1p GTPase regulates self-assembly and controls a rate-limiting step in mitochondrial fission. Mol Biol Cell. 2001;12:2756–2766. doi: 10.1091/mbc.12.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon Y, et al. Mammalian dynamin-like protein Dlp1 tubulates membranes. Mol Biol Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tieu Q, Nunnari J. Mdv1p is a WD repeat protein that interacts with the dynamin-related GTPase, Dnm1p, to trigger mitochondrial division. J Cell Biol. 2000;151:353–365. doi: 10.1083/jcb.151.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozdy AD, et al. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–379. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fekkes P, et al. Gag3p, an outer membrane protein required for fission of mitochondrial tubules. J Cell Biol. 2000;151:333–340. doi: 10.1083/jcb.151.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerveny KL, et al. Division of mitochondria requires a novel DNM1-interacting protein, Net2p. Mol Biol Cell. 2001;12:309–321. doi: 10.1091/mbc.12.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uetz P, et al. A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:601–603. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 35.Sever S, et al. Dynamin: GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J Cell Biol. 2000;150:1137–1147. doi: 10.1083/jcb.150.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sever S, et al. Impairment of dynamin’s GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–486. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]