Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages (original) (raw)
Abstract
Mesenchymal stem cells (MSCs) have been widely studied for their applications in stem cell-based regeneration. During myocardial infarction (MI), infiltrated macrophages have pivotal roles in inflammation, angiogenesis and cardiac remodeling. We hypothesized that MSCs may modulate the immunologic environment to accelerate regeneration. This study was designed to assess the functional relationship between the macrophage phenotype and MSCs. MSCs isolated from bone marrow and bone marrow-derived macrophages (BMDMs) underwent differentiation induced by macrophage colony-stimulating factor. To determine the macrophage phenotype, classical M1 markers and alternative M2 markers were analyzed with or without co-culturing with MSCs in a transwell system. For animal studies, MI was induced by the ligation of the rat coronary artery. MSCs were injected within the infarct myocardium, and we analyzed the phenotype of the infiltrated macrophages by immunostaining. In the MSC-injected myocardium, the macrophages adjacent to the MSCs showed strong expression of arginase-1 (Arg1), an M2 marker. In BMDMs co-cultured with MSCs, the M1 markers such as interleukin-6 (IL-6), IL-1β, monocyte chemoattractant protein-1 and inducible nitric oxide synthase (iNOS) were significantly reduced. In contrast, the M2 markers such as IL-10, IL-4, CD206 and Arg1 were markedly increased by co-culturing with MSCs. Specifically, the ratio of iNOS to Arg1 in BMDMs was notably downregulated by co-culturing with MSCs. These results suggest that the preferential shift of the macrophage phenotype from M1 to M2 may be related to the immune-modulating characteristics of MSCs that contribute to cardiac repair.
Keywords: immunologic environment, macrophage, mesenchymal stem cell, myocardial infarction
Introduction
Despite the rapid progress in therapeutic development, ischemic heart disease remains a leading cause of mortality. Mesenchymal stem cells (MSCs) are stromal cells with cardiac regenerative properties that have been demonstrated to reverse cardiac dysfunction and enhance angiogenesis in damaged heart tissue. In addition to their transdifferential capacity, MSCs possess the immunomodulatory capacity to inhibit lymphocyte proliferation, induce regulatory T cells and regulate the differentiation of dendritic cells. Recently, several studies demonstrated that functional interactions occur between MSCs and macrophages.1, 2, 3 In recent years, it has become clear that MSCs also regulate the function of macrophages. Co-culturing with MSCs induces macrophages to adapt an enhanced regulatory phenotype via the expression of increased levels of interleukin-10 (IL-10) and reduced levels of tumor necrosis factor-α (TNF-α), IL-12, low co-stimulatory molecule CD86, and human leukocyte antigen class II molecules.4
Macrophages exist in almost all tissues and have important roles in the maintenance of tissue homeostasis,5 they are an essential component of innate immunity and have a central role in inflammation and host defense.6 In response to signals derived from microbes, damaged tissues or activated lymphocytes,7, 8 monocytes/macrophages undergo reprogramming, which leads to the emergence of a spectrum of distinct functional phenotypes. Mirroring the Th1/Th2 nomenclature, macrophages undergo two different polarization states, the classically activated M1 phenotype and the alternatively activated M2 phenotype.9, 10 M1 macrophages (classically activated) exert proinflammatory activities that have been known to be induced by interferon (IFN) alone or in concert with microbial stimuli lipopolysaccharides (LPSs) as well as cytokines such as TNF and granulocyte-macrophage colony-stimulating factor. IL-4 and IL-13 were subsequently found to be more than simple inhibitors of macrophage activation and to induce an alternatively activated M2 macrophage, which are involved in inflammation resolution.11
In this study, we found that MSCs alternatively modulate the macrophage phenotype and may contribute to regeneration and immune-tolerance in injured cardiac tissue.
Materials and methods
Cytokines and reagent
Recombinant human IL-4 and recombinant human IFN-γ were purchased from Life Technologies (Grand Island, NY, USA), LPS was purchased from Sigma-Aldrich (St Louis, MO, USA), and macrophage colony-stimulating factor was obtained from Prospec (Rehovot, Israel).
Animals
Male inbred BALB/C mice, C57BL/6J mice (6–8 weeks old for isolation of MSCs, 8–10 weeks old for bone marrow-derived macrophage (BMDM) differentiation), and Sprague–Dawley rats (aged 8–10 weeks) were purchased from ORIENTBIO (Seong-nam, Korea). All experiments were performed after approval by our local ethical committee at Chonnam National University Medical School (CNU IACUC-H02011-11).
The isolation and culturing of MSCs
The MSCs were isolated and cultured using standard protocols.12 Bone marrow cells from C57BL/6 mice or rats were collected by flushing the femurs and tibias and were cultured with MesenPRO RS basal with MesenPRO RS growth supplement (Life Technologies, Grand Island, NY, USA), L-glutamine (Life Technologies), 100 IU ml–1 penicillin and 100 mg ml–1 streptomycin (Life Technologies). Culture medium was changed on day 3 to remove non-adherent cells. The medium was subsequently replaced for 4 days, and the MSCs were used within four passages.
Rat model of myocardial infarction
For myocardial infarction (MI) induction, the rats were anesthetized with an intramuscular injection of ketamine (50 mg kg–1 body weight) and xylazine (5 mg kg–1 body weight), intubated and mechanically ventilated. The proximal left anterior descending coronary artery was ligated. Finally, the heart was repositioned in the chest, and the chest was closed. The animals remained in a supervised setting until becoming fully conscious. One week after MI induction, the rats were randomly divided into two groups: one group was injected with only phosphate-buffered saline (PBS; _n_=5), and the other group was injected with MSCs (_n_=4). The MSCs (5 × 105 diluted in 100 μl of PBS) were directly injected into the peri-infarct area. To visualize the injected MSCs for immunohistochemical examination, the cells were pre-stained with DAPI (4′,6-diamidino-2-phenylindole, 50 μg ml–1, Sigma-Aldrich) for 4 h and washed out with PBS before injection. Finally, the heart was repositioned in the chest, and the chest was closed. The animals remained in a supervised setting until becoming fully conscious.
Cardiac function measurement
Cardiac function was assessed by echocardiography. After 2 weeks of MSC injection, the animals were anesthetized, intubated and mechanically ventilated. Echocardiographic studies were performed with a 15-MHz linear array transducer system (iE33 system, Philips Medical Systems, Amsterdam, Netherland) by an expert who was not aware of the experimental conditions to exclude bias.
Immunohistochemistry
For immunohistochemical analysis, at 7 days after MSC injection, the heart was harvested, fixed in formalin and embedded in a paraffin block. Fluorescence immunohistochemistry was performed to identify the injected MSCs and infiltrated macrophages in the infarct myocardium.
DAPI-loaded MSCs were discovered in the peri-infarct zone, and double staining with CD68 (BMA Biomedicals, Augst, Switzerland) and arginase-1 (Arg1, Abcam, Cambridge, MA, USA) was performed. The slides were treated with 3% hydrogen peroxide in PBS for 10 min at room temperature to block endogenous peroxidase activity. After blocking nonspecific binding with 5% normal goat serum (Sigma-Aldrich), the slides were incubated with primary antibodies for 18 h at 4 °C. The sections were washed with PBS three times and then incubated for 1 h with Alexa-Fluor 488 or 594 secondary antibodies. The images were detected using a Carl-Zeiss confocal microscope, and the images were obtained using Zeiss LSM version 3.2 SP2 software (Carl Zeiss, Oberkochen, Germany). Cardiac fibrosis was measured by Masson's Trichrome staining, and fibrotic changes were evaluated by measuring blue-stained fibrotic deposits using Eclipse-80i microscope and NIS-Elements software (Nikon, Tokyo, Japan).
The isolation of mouse BMDMs
BMDMs were isolated and differentiated using standard protocols.13, 14 Primary macrophages were derived from bone marrow cells and were cultured for 7 days in RPMI-1640-containing macrophage colony-stimulating factor (100 ng ml–1, R&D Systems, Minneapolis, MN, USA) or macrophage differentiation media (30% L929 cell-conditioned medium, 20% fetal bovine serum and 50% RPMI-1640). L929 cell-conditioned medium was prepared by growing L929 cells in RPMI-1640-containing 10% fetal bovine serum for 10 days. The medium containing macrophage colony-stimulating factor secreted by the L929 cells was harvested and passed through a 0.22-μm filter.
Co-culturing of BMDMs and MSCs
For transwell co-culturing, differentiated BMDMs were seeded into a six-well plate. The next day, the 0.4-μm-pore size Corning transwell inserts (Sigma-Aldrich) containing 2 × 105 MSCs were placed into the six-well plate with the macrophages that were initially seeded. Co-cultures were incubated for 5, 24 or 48 h with or without 100 ng ml–1 LPS+30 ng ml–1 IFN-γ or IL-4 (20 ng ml–1).15
Real-time polymerase chain reaction
Overall, total RNA from BMDMs co-cultured with MSCs were extracted with TRIzol reagent (Life Technologies), and the RNA samples were converted into complementary DNA using an Applied Biosystems High-Capacity cDNA Reverse transcription Kit (Life Technologies) according to the manufacturer's instructions. Real-time PCR was performed using a QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA, USA) and Corbett Research Rotor-Gene RG-3000 Real-Time PCR System (Qiagen). The primers used in the PCR are described in Table 1.
Table 1. Primer sequences for real-time PCR analysis.
| Gene | Primer |
|---|---|
| GAPDH | Forward: 5′-TGTGATGGGTGTGAACCACG-3′ |
| Reverse: 5′-CAGTGAGCTTCCCGTTCACC-3′ | |
| iNOS | Forward: 5′-TCACCTTCGAGGGCAGCCGA-3′ |
| Reverse: 5′-TCCGTGGCAAAGCGAGCCAG-3′ | |
| Arg1 | Forward: 5′-GATTATCGGAGCGCCTTTCT-3′ |
| Reverse: 5′-CCACACTGACTCTTCCATTCTT-3′ | |
| IL-6 | Forward: 5′-ATCCAGTTGCCTTCTTGGGACTGA-3′ |
| Reverse: 5′-TTGGATGGTCTTGGTCCTTAGCCA-3′ | |
| IL-1β | Forward: 5′-GGTGTGTGACGTTCCCATTA-3′ |
| Reverse: 5′-TCCTGACCACTGTTGTTTCC-3′ | |
| MCP-1 | Forward: 5′-CTCACCTGCTGCTACTCATTC-3′ |
| Reverse: 5′-TTACGGCTCAACTTCACATTCA-3′ | |
| CD206 | Forward: 5′-CTGCAGATGGGTGGGTTATT-3′ |
| Reverse: 5′-GGCATTGATGCTGCTGTTATG-3′ | |
| IL-4R | Forward: 5′-CTAGCTCCGTGCCCTTATTTAC-3′ |
| Reverse: 5′-GGTTGGCTTCTGGTGGTATT-3′ | |
| IL-10 | Forward: 5′-ACTGGCATGAGGATCAGCAG-3′ |
| Reverse: 5′-CTCCTTGATTTCTGGGCCAT-3′ |
Western blotting
The cells were washed with ice-cold PBS, lysed in lysis buffer (20 mM Tris-HCl pH 7.4, 0.1 mM EDTA, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride and 1 mg ml–1 leupeptin) on a rotation wheel for 1 h at 4 °C. After centrifugation at 10 000 × g for 10 min, the supernatant was prepared as a protein extract. Equal concentrations of proteins were fractionated by electrophoresis on 8% or 10% acrylamide gels and were transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA) membrane, followed by blotting with antibodies against monocyte chemoattractant protein-1 (MCP-1, Santa Cruz Biotech, Dallas, TX, USA), inducible nitric oxide synthase (iNOS, Cell Signaling Technology, Danvers, MA, USA), Arg1 (Cell Signaling Technology) and β-actin (Sigma Aldrich) followed by secondary staining with horseradish peroxidase-conjugated immunoglobulin G. Protein expression was detected using an Image Reader (LAS-3000 Imaging System, Fuji Photo Film, Tokyo, Japan). The expression level was quantified with ImageJ (NIH, Bethesda, MD, USA).
Analysis of the enzyme-linked immunosorbent assay
BMDMs alone or co-cultured with BM-MSCs for 24 h were incubated for 24 h with or without LPS (100 ng ml–1)+IFN-γ (30 ng ml–1) or IL-4 (20 ng ml–1). The secreted IL-10, IL-6 and IL-1β in the supernatants were evaluated using an enzyme-linked immunosorbent assay kit (e-Bioscience, San Diego, CA, USA).
Measurement of nitric oxide
The nitrite accumulation in the culture media was determined as an indicator of nitric oxide (NO) as previously described.16 Briefly, the culture media, obtained at designated time points, were used to measure the amount of NO production. Culture supernatants were collected by centrifugation (10 000 × g for 5 min) and subjected to an assay for NO production using the Griess reagent according to the manufacturer's instructions (Promega, Madison, WI, USA).
Arg1 activity assay
Intracellular Arg1 activity was assessed by measuring the amount of urea produced via the metabolism of L-arginine by Arg1 according to the manufacturer's directions (Quantichrome Urea Assay Kit, Bioassay Systems, Hayward, CA, USA).
Cytokine antibody array
Culture samples were analyzed with a cytokine antibody array, specifically the RayBio Mouse Cytokine Antibody Array 3 (RayBiotech, Inc., Norcross, GA, USA), according to the manufacturer's instructions. Briefly, cytokine array membranes were blocked in 2 ml of blocking buffer for 30 min and then incubated with 1 ml of the samples at room temperature for 2 h. The samples were then decanted from each container, and the membranes were washed three times with 2 ml of wash buffer I, followed by two washes with 2 ml of 1 × wash buffer II at room temperature with shaking. The membranes were then incubated in 1:250-diluted biotin-conjugated primary antibodies at room temperature for 2 h and washed as described above before incubation in 1:1000-diluted horseradish peroxidase-conjugated streptavidin. After incubation in horseradish peroxidase-conjugated streptavidin for 1 h, the membranes were washed thoroughly and exposed to a peroxidase substrate for 5 min in the dark before imaging.
Statistical analysis
All data are expressed as the mean±s.e.m. from at least three independent experiments. The differences between experimental and control groups were analyzed with the two-tailed unpaired Student's _t_-test using SPSS (SPSS Inc., Chicago, IL, USA). A value of P<0.05 was considered statistically significant.
Results
Macrophages adjacent to MSCs strongly expressed Arg1 in the infarcted myocardium
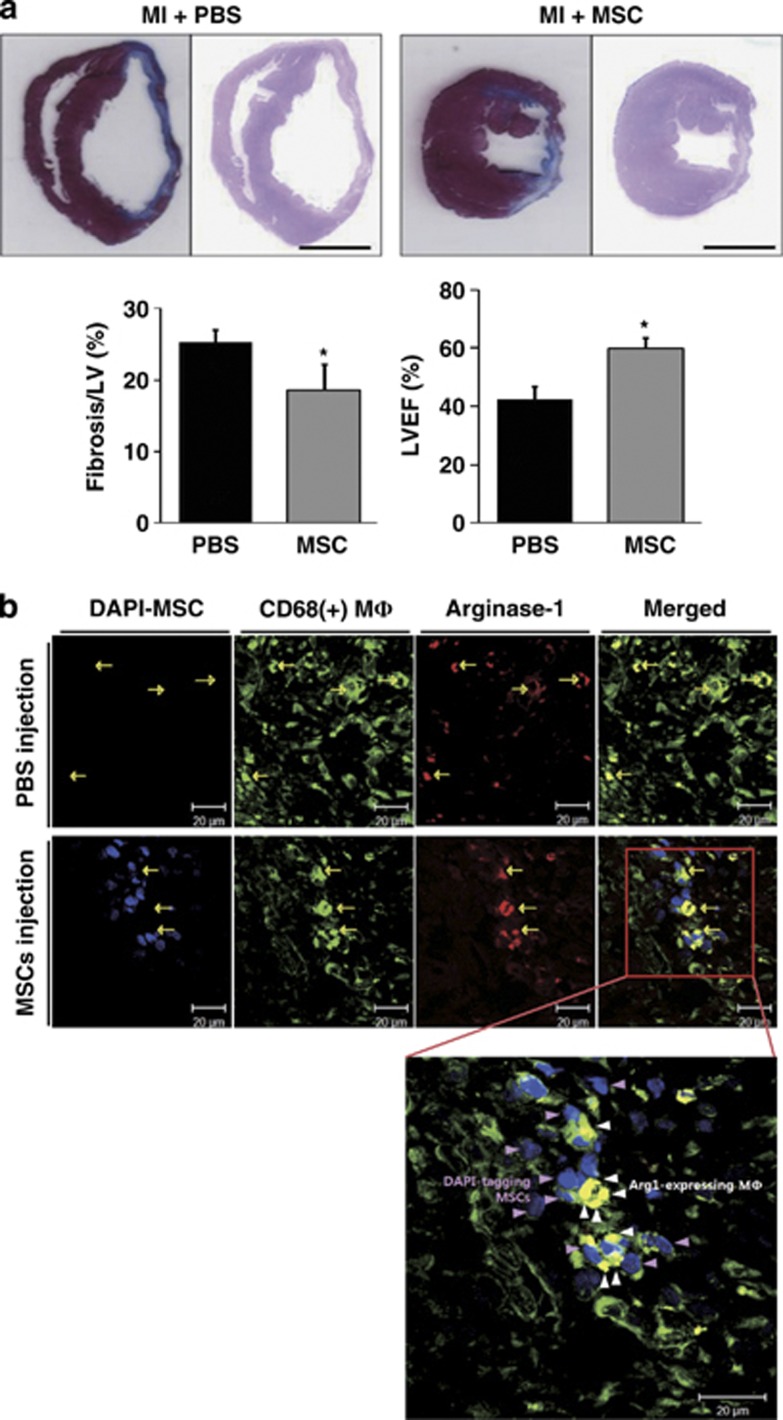
Cardiac fibrosis and function were assessed to evaluate the regeneration capacity of MSCs. Cardiac fibrosis was 25.29±1.83% in the PBS injection group and 18.62±3.63% in the MSC injection group (P<0.05, Figure 1a). The left ventricular ejection fraction was 42.58±4.20% in the PBS injection group and 59.92±3.75% in the MSC injection group (P<0.05, Figure 1a). These results showed the functional recovery from MIs by MSC administration.
Figure 1.
The identification of arginase-1 (Arg1)-expressing macrophages in infarct myocardium. (a) Representative images showed that the fibrotic area was smaller in the mesenchymal stem cell (MSC) group compared with the phosphate-buffered saline (PBS) group. The left ventricular ejection fraction was better in the MSC group compared with the PBS group. Scale bar=500 μm. (b) Immunocytochemistry images of 4′,6-diamidino-2-phenylindole (DAPI)-labeled MSCs or PBS injected in the hearts of infarct rats at 7 days post-cell injection. Representative images showed immunofluorescent staining for the macrophage marker CD68 (green) and Arg1 (red) in the infarct myocardium. In the high-magnification view of the rectangle, Arg1-expressing CD68 (+) macrophages (yellow) near DAPI-labeled MSCs (blue) were observed in the infarct zone. Pink arrowheads indicate injected MSCs; white arrowheads indicate macrophages with Arg1 expression. Scale bar=20 μm. *P<0.05, compared with each PBS group. LVEF, left ventricular ejection fraction.
Heart tissues from MI-induced rats injected with MSCs or PBS were analyzed by coimmunofluorescence for the macrophage marker CD68 (green) and the M2 marker Arg1 (red) to identify the polarization status of macrophages. As shown in Figure 1b, the MSCs were labeled by nuclear staining with DAPI before injection and were detected in the MSC-injected myocardium. Infiltrated CD68(+) macrophages were primarily localized in the infarcted area (green, yellow arrows). Merged high-power images showed that the exclusively strong expression of Arg1 was observed in CD68(+) macrophages (white arrowheads) adjacent to engrafted MSCs (pink arrowheads). Based on this finding, we hypothesized that MSCs regulate the macrophage phenotype to exert beneficial outcomes.
MSCs shifted from the M1 to the M2 phenotype in activated BMDMs
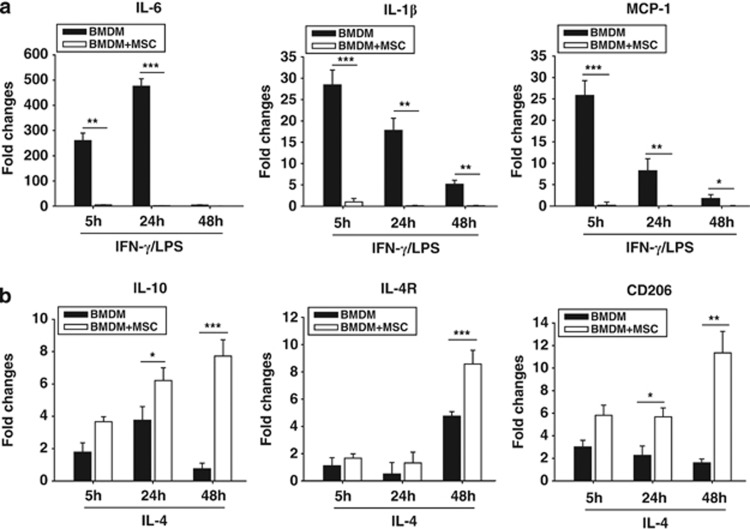
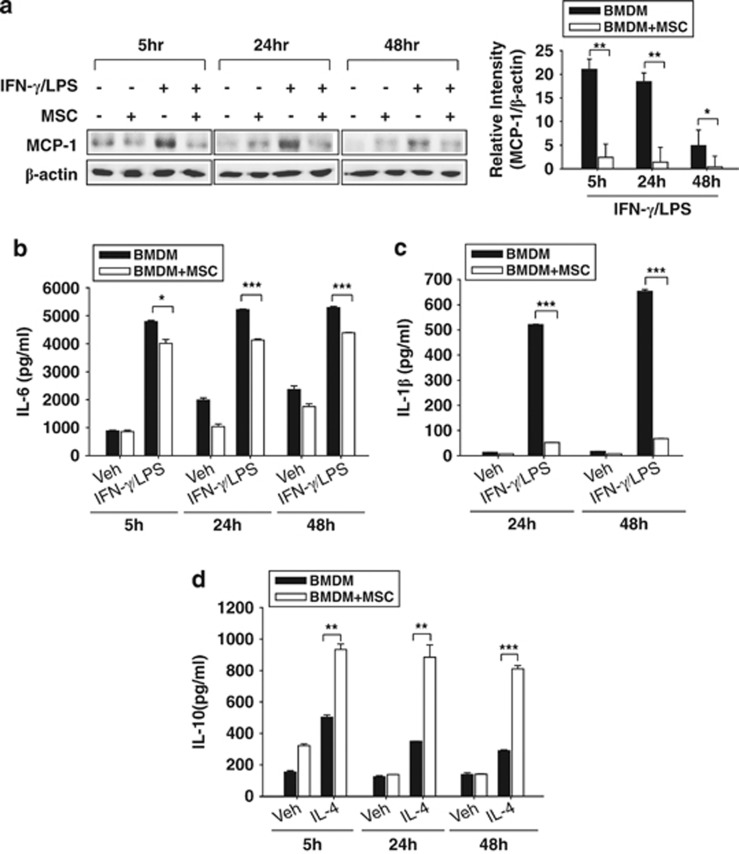
To assess the effect of MSCs on the macrophage phenotype, we isolated bone marrow to culture MSCs or to differentiate into macrophages. Both the M1 and M2 markers were analyzed in the BMDMs. BMDMs were stimulated with IFN-γ (30 ng ml–1)/LPS (100 ng ml–1) or with IL-4 (20 ng ml–1) for 5, 24 or 48 h in the presence or absence of co-culturing with MSCs in the transwell system. M1 markers such as IL-6, IL-1β and MCP-1 were expressed in IFN-γ/LPS-stimulated BMDMs; however, their mRNA levels were significantly decreased by co-culturing with MSCs (Figure 2a). IL-10, IL-4R and CD206 were analyzed in IL-4-stimulated BMDMs as M2 markers. Their induction was strongly enhanced by co-culturing with MSCs within 48 h (Figure 2b). Then, we measured the protein levels of IL-1β, IL-6 and IL-10 by enzyme-linked immunosorbent assay and MCP-1 by western blotting in activated BMDMs. IFN-γ/LPS-induced MCP-1 and IL-6 proteins were decreased by co-culturing with MSCs (Figures 3a and b). Released IL-1β markedly increased but then decreased by co-culturing with MSCs (Figure 3c). In IL-4-activated BMDMs, IL-10 induction was enhanced in BMDMs co-cultured with MSCs (Figure 3d). These results showed that co-culturing with MSCs leads to inhibitory responses on M1 markers and stimulatory effects on M2 markers.
Figure 2.
The modulatory effect of mesenchymal stem cells (MSCs) on the phenotype of bone marrow-derived macrophages (BMDMs) at the transcriptional level. (a) Real-time PCR analyses showed that transcriptions of interleukin-6 (IL-6), IL-1β and monocyte chemoattractant protein-1 (MCP-1) in interferon-γ (IFN-γ)/lipopolysaccharide (LPS)-stimulated BMDM were inhibited by co-culturing with MSCs. (b) Real-time PCR analyses showed that the transcription levels of IL-10, IL-4R and CD206 were enhanced by co-culturing with MSCs in IL-4-stimulated BMDMs. *P<0.05, **P<0.01, ***P<0.001 compared with each BMDM group.
Figure 3.
The modulatory effect of mesenchymal stem cells (MSCs) on the phenotype of bone marrow-derived macrophages (BMDMs) at the translational level. (a) The protein level of MCP-1 was measured by western blotting and was reduced by co-culturing with MSCs at 5, 24 and 48 h. Released interleukin-6 (IL-6; b), IL-1β (c) and IL-10 (d) were measured by an enzyme-linked immunosorbent assay (ELISA) at 5, 24 and 48 h. Co-culturing with MSCs inhibited the release of both IL-6 and IL-1β in interferon-γ (IFN-γ)/lipopolysaccharide (LPS)-stimulated BMDMs. In contrast, IL-10 release was enhanced by MSC co-culturing in IL-4-stimulated BMDMs. The relative density of bands was measured and was presented as graphs. *P<0.05, **P<0.01, ***P<0.001 compared with each BMDM group.
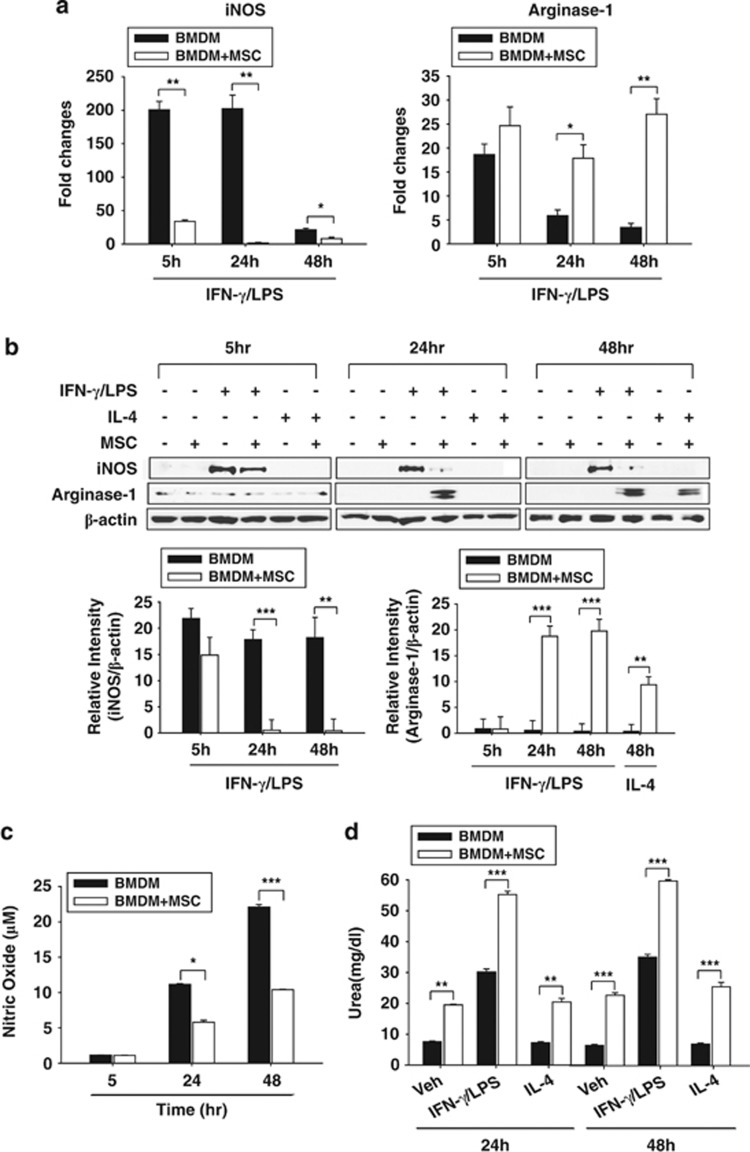
MSCs regulated iNOS and Arg1 reciprocally in activated BMDMs
iNOS and Arg1 are known to utilize L-arginine as a common substrate; however, they represent the M1 and M2 phenotypes, respectively. We analyzed the expression and function of both iNOS and Arg1 in activated BMDMs with or without co-culturing with MSCs. IFN-γ/LPS-induced iNOS mRNA was remarkably decreased by co-culturing with MSCs. In contrast, Arg1 mRNA was highly induced in IFN-γ/LPS-stimulated BMDMs co-cultured with MSCs (Figure 4a).
Figure 4.
Mesenchymal stem cell (MSC) co-culturing skewed the bone marrow-derived macrophage (BMDM) phenotype to M1 by elevating the ratio of inducible nitric oxide (iNOS) to arginase-1 (Arg1). The iNOS mRNA level decreased; however, the Arg-1 level remarkably increased in BMDMs co-cultured with MSCs (a). Intracellular iNOS and Arg1 protein levels were evaluated by western blotting. The protein expression pattern was similar to mRNA pattern (b). In addition to quantitative changes, iNOS (c) and Arg1 (d) enzyme activities were assessed. iNOS activity was reduced in BMDMs, whereas Arg1 activity increased in BMDMs co-cultured with MSCs. The relative density of the bands was measured and was presented as graphs. *P<0.05, **P<0.01, ***P<0.001 compared with each BMDM group.
Then, we examined the protein levels of iNOS and Arg1 in BMDMs by western blotting. IFN-γ/LPS-induced iNOS protein was barely detected; however, the Arg1 level markedly increased in both IFN-γ/LPS- or IL-4-stimulated BMDMs (Figure 4b). The expression ratio of iNOS to Arg1 was calculated and is shown in Table 2.
Table 2. The ratio of iNOS to Arg1 in IFN-γ/LPS-stimulated BMDMs with or without co-culturing with MSCs.
| | 5 h | 24 h | 48 h | | | --------- | ------------- | --------------- | --------------- | | mRNA | | | | | BMDM | 10.78±4.50 | 34.45±7.34 | 6.35±2.31 | | BMDM+MSC | 1.38±0.59** | 0.18±0.28*** | 0.30±0.62** | | | | | | | | Protein | | | | | BMDM | 25.50±1.90 | 22.50±1.87 | 23.50±1.42 | | BMDM+MSC | 16.71±2.38* | 0.51±1.97*** | 0.80±2.24*** |
Next, the enzymatic activities of iNOS and Arg1 were analyzed to confirm the authentic effect of MSCs on the iNOS/Arg1 ratio. iNOS is primarily responsible for NO generation in macrophages, and NO was measured in activated BMDMs. IFN-γ/LPS-induced NO production was significantly inhibited in BMDMs by co-culturing with MSCs (Figure 4c). In contrast, Arg1 activity was enhanced both in IFN-γ/LPS- and IL-4-stimulated BMDMs co-cultured with MSCs (Figure 4d).
MSCs suppressed inflammatory cytokines and enhanced anti-inflammatory cytokines
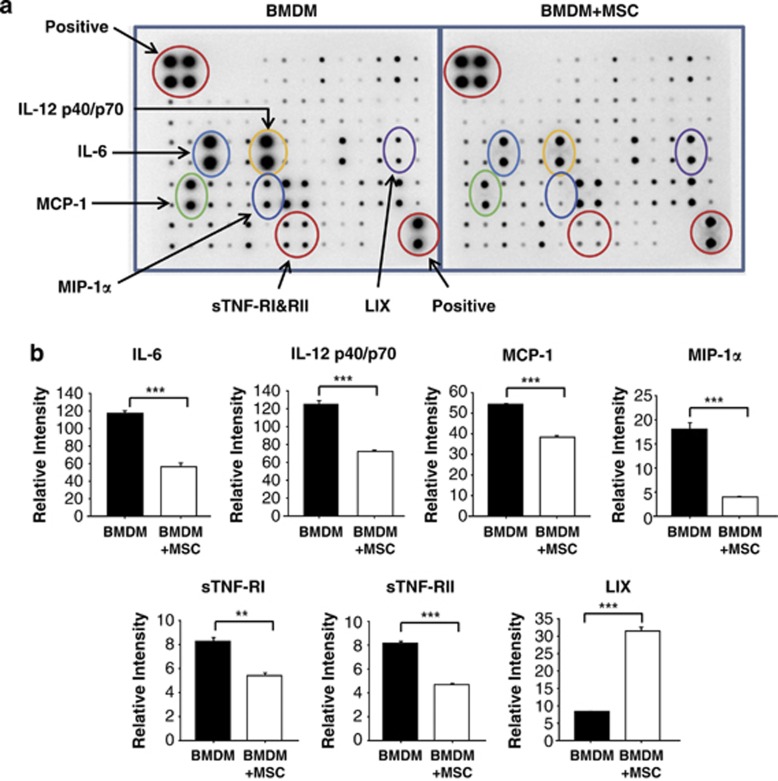
Our results show that MSCs switch macrophages from the M1 phenotype to the M2 phenotype. In addition, MSC-mediated immune-regulation in a transwell cell culture system primarily acted through the secretion of soluble molecules that are downregulated or induced following cross-talk with macrophages. To examine the responsible soluble factors, we arrayed the inflammation-related cytokines. Antibody arrays showed the differences in the secretion of a panel of inflammation-related cytokines and chemokines in BMDMs after 24 h of stimulation with IFN-γ/LPS (Figure 5a).
Figure 5.
Inflammation-related cytokine secretions from bone marrow-derived macrophages (BMDMs) were analyzed by protein array. (a) Supernatants from interferon-γ (IFN-γ)/lipopolysaccharide (LPS)-activated BMDMs with or without co-culturing with mesenchymal stem cells (MSCs) were assayed to determine the cytokine levels. The BMDMs were cultured for 24 h with IFN-γ/LPS in the presence or absence of MSC co-culturing, and the resulting culture supernatants were analyzed by cytokine protein array. The image of the spot signal on the membrane because of each cytokine is shown. (b) The relative density of spots was measured and was presented as graphs. **P<0.01, ***P<0.001 compared with each BMDM group.
The secretion of IL-6, IL-12, MCP-1, macrophage inflammatory protein-1α, soluble TNF receptor I (sTNF-RI) and sTNF-RII was all reduced; however, the secretion of soluble LIX 1 was increased in BMDMs co-cultured with MSCs compared with BMDMs not co-cultured with MSCs (Figure 5b).
Discussion
In this study, we investigated the role of MSCs in regulating the phenotypes in activated macrophages and found that MSCs preferentially polarized macrophages to the M2 anti-inflammatory phenotype.
Circulating monocytes travel to the injured myocardium where they differentiate and contribute to various parts of the healing process. Macrophages are central mediators of the inflammatory response, contributing both to the initiation and the resolution of inflammation. Monocytes and macrophages infiltrate injured tissue in large numbers early after ischemia17 and participate in tissue repair.18 Macrophages undergo classical activation (pro-inflammatory M1) or alternative activation (anti-inflammatory M2) in response to various signals and have characteristic markers and cytokine profiles.10, 11 Nahrendorf et al. investigated the function of macrophages in heart repair.19, 20, 21 They showed that two subsets of macrophages, pro-inflammatory Ly-6Chigh and anti-inflammatory Ly-6Clow cells, localized to area MI to participate in cardiac healing and to the nonischemic remote myocardium to be participate in the remodeling process after MI.
Several reports suggest the immune engagement may be involved in cardiac regeneration via cellular interaction between MSCs and macrophages. A recent study reported that MSC survival increased when the cells were exposed to M2 macrophages compared with M1 macrophages.1 Adipose tissue-derived MSCs were reported to release IL-10, vascular endothelial growth factor and M2 inducers such as IL-4 and IL-13 when they were co-cultured with macrophages.22 Our previous report showed that MSC injection into infarcted myocardium significantly attenuated inflammation during functional recovery.23
We observed high expression of Arg1 in macrophages next to engrafted MSCs in infarcted myocardium compared with PBS-injected myocardium (Figure 1b). We discovered that the modulatory action of MSCs on macrophages accelerated the healing process in injured tissue. The data presented in this report show that the macrophage phenotype shifted from M1 to M2 in the presence of MSCs based on mRNA, protein and enzyme activity assays. Co-culturing with MSCs leads to distinct responses to both IFN-γ/LPS-induced M1 mediators and to IL-4-induced M2 mediators in activated BMDMs.
M1 macrophages express high levels of iNOS that compete with Arg1 for L-arginine, the common substrate of both enzymes. iNOS converts L-arginine to NO, competing with Arg1, which converts NO into urea and ornithine. By inhibiting iNOS, Arg1 may promote the M2 phenotype and contributes to the suppression of the M1 phenotype. Our data show the opposite contribution of the two enzymes, iNOS and Arg1, in activated BMDMs. As shown in Figure 4 and Table 2, the ratio of iNOS to Arg1 decreased radically by co-culturing with MSCs. Specifically, MSCs inhibited both iNOS expression and activity but increased Arg1 expression with its activity. The differential effects of macrophage populations may have implications for the pathogenesis and regeneration of various diseases.
To explore the soluble mediators responsible for interactions between MSCs and BMDMs, the cytokine array was performed (Figure 5). In BMDM-cultured media, IL-12, IL-6, MCP-1, macrophage inflammatory protein-1α, sTNF-RI and sTNF-RII were reduced; however, LIX was increased by co-culturing with MSCs. IL-12 stimulates T-cell growth and induces the production of IFN-γ and TNF-α from T and natural killer cells and reduces the IL-4-mediated suppression of IFN-γ.24 IL-6 and MCP-1 showed significant reduction as demonstrated in Figures 2 and 3. Macrophage inflammatory protein-1α, also known as chemokine (C-C motif) ligand 3, is an inflammatory chemokine and is involved in atherosclerotic lesion formation with an acute inflammatory state.25 sTNF-Rs, which are pro-inflammatory cytokines, have an affinity for TNF and compete with membrane-bound TNF-Rs to bind the ligand. They contribute to a counter-regulatory response to excessive TNF activity.26 LIX is known as a C-X-C motif chemokine 5 (CCL5) and as RNATES (regulated on activation, normal T-cell expressed and secreted). LIX is a chemotactic cytokine involved in recruiting leukocytes into inflammatory lesions.27
The present report demonstrates the regulation of the balance between M1 and M2 in macrophages by MSCs may result in a favorable environment to accommodate therapeutic MSCs.
Acknowledgments
This study was supported the National Research Foundation of Korea grant funded by the Korean Government (MEST), Republic of Korea (2010-0020261) and the Korean Health Technology R&D Project, Ministry of Health and Welfare, and Republic of Korea (HI12C0199).
The authors declare no conflict of interest.
References
- Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protocols. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM.The isolation and characterization of murine macrophages Curr Protocols Immunol 2008. Chapter 14: Unit 14 1. [DOI] [PMC free article] [PubMed]
- Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protocols. 2008;3:1–7. doi: 10.1101/pdb.prot5080. [DOI] [PubMed] [Google Scholar]
- Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beda N, Nedospasov A. A spectrophotometric assay for nitrate in an excess of nitrite. Nitric Oxide Biol Chem. 2005;13:93–97. doi: 10.1016/j.niox.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–163. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adutler-Lieber S, Ben-Mordechai T, Naftali-Shani N, Asher E, Loberman D, Raanani E, et al. Human macrophage regulation via interaction with cardiac adipose tissue-derived mesenchymal stromal cells. J Cardiovasc Pharmacol Therapeutics. 2013;18:78–86. doi: 10.1177/1074248412453875. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ahn Y, Kwon JS, Cho YK, Jeong MH, Cho JG, et al. Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury. Cells Tissues Organs. 2012;195:428–442. doi: 10.1159/000329234. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- de Jager SC, Bot I, Kraaijeveld AO, Korporaal SJ, Bot M, van Santbrink PJ, et al. Leukocyte-specific CCL3 deficiency inhibits atherosclerotic lesion development by affecting neutrophil accumulation. Arteriosclerosis Thrombosis Vasc Biol. 2013;33:e75–e83. doi: 10.1161/ATVBAHA.112.300857. [DOI] [PubMed] [Google Scholar]
- Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH. Chemokine-chemokine receptor network in immune cell trafficking. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4:343–361. doi: 10.2174/1568008043339712. [DOI] [PubMed] [Google Scholar]