T Cell–Mediated Host Immune Defenses in the Lung (original) (raw)
. Author manuscript; available in PMC: 2014 Feb 4.
Abstract
Evidence has increasingly shown that the lungs are a major site of immune regulation. A robust and highly regulated immune response in the lung protects the host from pathogen infection, whereas an inefficient or deleterious response can lead to various pulmonary diseases. Many cell types, such as epithelial cells, dendritic cells, macrophages, neutrophils, eosinophils, and B and T lymphocytes, contribute to lung immunity. This review focuses on the recent advances in understanding how T lymphocytes mediate pulmonary host defenses against bacterial, viral, and fungal pathogens.
Keywords: pneumonia, inflammation, coinfection
INTRODUCTION
Many pulmonary diseases are associated with or directly result from aberrant immunological responses. Most, if not all, of these immunological disorders are directly or indirectly involved with host defense pathways. The common causes of major pulmonary diseases listed by the American Lung Association and their relation to host defenses are summarized in Table 1.
Table 1.
List of lung diseases from the American Lung Association and their relations to host defense
| Name of the lung disease | Definition of disease | Relation to host defense |
|---|---|---|
| Acute bronchitis | Inflammation of the bronchial tubes | Usually direct |
| Asbestosis | Scarring of lung tissue as a result of breathing in asbestos fibers | Pathway involved |
| Asthma | A lung disease that makes it harder to move air in and out | Pathway involved |
| Bronchiolitis | An inflammation of the bronchioles | Pathway involved |
| Bronchopulmonary dysplasia | A lung disease that occurs most often in premature babies | Indirect |
| Byssinosis | A lung disease caused by exposure to dusts from cotton processing, hemp, and flax | Pathway involved |
| Chronic bronchitis | Chronic inflammation of the airways or bronchial tubes | Pathway involved |
| Coccidioidomycosis | An infection of the lungs caused by inhaling spores of the fungus Coccidioides immitis | Direct |
| Chronic obstructive pulmonary disease | Also known as emphysema and chronic bronchitis | Pathway involved |
| Cryptogenic organizing pneumonia | A disease in which the bronchioles and alveoli become inflamed with connective tissue | Pathway involved |
| Cystic fibrosis | An inherited disease that causes thick, sticky mucus in the lungs, pancreas, and other organs | Pathway involved |
| Emphysema | A lung disease that makes it hard to breathe | Pathway involved |
| Hantavirus pulmonary syndrome | A disease that comes from contact with infected rodents or their urine, droppings, or saliva | Direct |
| Histoplasmosis | An infection in the lungs caused by inhaling the spores of the fungus Histoplasma capsulatum | Direct |
| Human metapneumovirus | Infections that cause colds, pneumonia or bronchitis | Direct |
| Hypersensitivity pneumonitis | A disease in which lungs become inflamed when a patient breathes in certain fungal dusts | Pathway involved |
| Influenza | A serious respiratory illness caused by infection with three influenza virus families: A, B, or C | Direct |
| Lung cancer | The second most commonly diagnosed cancer and the most common cause of cancer death | Pathway involved |
| Lymphangiomatosis | A disease of the lymphatic system | Indirect |
| Mesothelioma | An uncommon form of cancer that involves the mesothelium | None |
| Nontuberculous mycobacterium | An infection caused by mycobacteria that are found in water and soil and only infect some people | Direct |
| Pertussis | A respiratory infection caused by the bacteria Bordetella pertussis | Direct |
| Pneumoconiosis | An occupational lung disease caused by inhaling coal dust | Indirect |
| Pneumonia | A common lung infection caused by bacteria, a virus, or fungi | Direct |
| Primary ciliary dyskinesia | Blockage and infections caused when mucus accumulates due to cilia dysfunction | Direct |
| Pulmonary fibrosis | A disease marked by scarring in the lungs | Direct |
| Pulmonary vascular disease | A disease that affects the blood circulation in the lungs | None |
| Respiratory syncytial virus | The most common cause of bronchiolitis and pneumonia in children younger than age one in the United States | Direct |
| Sarcoidosis | A disease caused by small areas of inflammation | Direct |
| Severe acute respiratory syndrome | A disease caused by a group of viruses called the coronaviruses | Direct |
| Silicosis | A disease caused by inhaling tiny bits of silica | Direct |
| Tuberculosis | A common infectious disease caused by various mycobacteria, usually Mycobacterium tuberculosis | Direct |
T cells play critical roles in the immune system: CD4+ (helper) T cells help B cells to mount antibody responses, provide feedback to dendritic cells (DCs) via costimulatory molecules and the elaboration of cytokines, and enhance and maintain responses of CD8+ (cytotoxic) T cells. Moreover, CD4+ T cells have direct effector activity, including performing cytotoxic functions (1), mediating macrophage activation, and inducing genes in mucosal tissues that contribute to host defense. CD8+ T cells contribute to cytokine production and more importantly kill viral-infected cells directly. γδ T cells and invariant natural killer T (iNKT) cells as well as the newly discovered innate lymphoid-like cells (ILCs) play critical roles in early responses to many pulmonary infections as well. Both CD4+ and CD8+ T cells can form immunological memory and benefit the host when the host encounters secondary challenge from the same or related pathogens.
The massive surface area of the lung epithelium and the intimate association of an extensive capillary network to facilitate gas exchange also places the lung in a potentially vulnerable position in relation to the external environment. As a consequence, the respiratory tract is constantly exposed to the external environment and can serve as a major portal of entry for many pathogens. The immune system of the respiratory tract consists of a specialized network of cells including secretory Clara cells, ciliated cells, and mucus-producing goblet cells as well as submucosal glands. The major resident myeloid-derived cell on the luminal side of the lung is the highly phagocytic alveolar macrophage. This system effectively controls many pulmonary pathogens and protects the host. However, many pathogens that escape these innate defenses require the T cell subsets mentioned above for ultimate control of the infection.
T cells play critical roles in pulmonary host defense against bacterial, viral, and fungal pathogens, as discussed in this review. In general, an insufficient T cell response in the course of an ongoing infection renders the host susceptible to infection and delayed clearance of the pathogen. Moreover, insufficient T cell immunity may also increase the likelihood of pathogen dissemination from the lung. In contrast, dysregulation of T cell responses during an immune response could also damage host tissues and have detrimental effects for the host itself. T cell responses in the lung can be dynamically regulated through many mechanisms such as the local environmental cytokine milieu, expression of costimulatory/coinhibitory molecules, and epigenetic regulation of genomic loci encoding cytokines and transcription factors. Dysregulated T cell responses have been associated with many chronic lung diseases, and thus T cell subsets are attractive targets for pharmaceutical intervention. In contrast, the generation of mucosal pathogen-specific T cell responses may be desired for advances in vaccine development.
T CELLS IN PULMONARY HOST DEFENSE
CD4+ T Cells
CD4+ T cells are a major T cell subset that plays a central role in immune system function when naive CD4+ T cells differentiate into effector and/or memory cells after encountering their cognate antigen via antigen-presenting cells (APCs). The phenotypes of effector CD4+ T cells differ depending on the stimulating conditions and can be categorized into various lineages. In the lungs, DCs bridge innate and adaptive immunity, and depending on context, they also induce various CD4+ T cell responses to infectious agents. During steady state, the lung contains two major subsets of CD11chi conventional (c)DCs (CD103− CD11b+ cells in the lamina propria and CD103+CD11b− cells in the epithelial layer). A population of CD11cdim plasmacytoid (p)DCs can also be found in the conducting airways (reviewed in Reference 2). When inflammation occurs, additional CD11b+ monocyte-derived DCs, expressing Ly6C and FcεRI, are recruited to the lungs (reviewed in Reference 3). In response to pathogen inhalation or in the case of concomitant exposure to harmless antigen with inhaled environmental adjuvants such as cigarette smoke, fine particulate matter, or bacterial contaminants, lung DCs produce IL-12p70, leukotriene C4, IL-6, IL-23p19, TGF-β, or IL-1β. MHC class II–restricted presentation of cognate antigen to CD4+ T cells in these different cytokine milieus can initiate different T cell responses. T cell activation is thought to be initiated in the lung-draining lymph nodes after lung DCs endocytose antigen and travel to that location. However, lymphotoxin-α−/− mice, which lack peripheral lymph nodes (4), are able to generate antigen-specific CD8+ T cell responses as well as to produce high titers of influenza-specific IgM, IgG, and IgA antibodies (5). Moreover, these mice clear low-dose influenza infection (5), which supports the argument that the initiation of T cell activation in the lung can occur outside of lymph nodes. Further support comes from the infection of CCR7−/− mice with Mycobacterium tuberculosis. These mice have defective DC trafficking from the lung to the lung-draining lymph nodes, yet they exhibit ectopic proliferation of _M. tuberculosis_–specific CD4+ T cells in the lungs (6).
CD4+ T cells represent an important component in pulmonary host defense against various pathogens, and perhaps the best example for this is demonstrated in HIV. HIV targets CD4+ T cells, causing the patient to progress to AIDS, which is defined either by CD4+ T cell numbers below 200 cells per μL or by the occurrence of specific diseases in association with an HIV infection. Many of these AIDS-defining illnesses are pulmonary infections, including Pneumocystis jiroveci pneumonia, tuberculosis (TB), candidiasis of the lung, histoplasmosis, or recurrent bacterial pneumonia (7). More recently, investigators have shown an increased susceptibility of AIDS patients to influenza viruses, Streptococcus pneumoniae, Haemophilus influenzae (reviewed in Reference 8), and Aspergillus fumigatus (reviewed in Reference 9).
Evidence of distinctive lineages of effector CD4+ T cells first came from the analysis of mouse CD4+ T cell clones by Mosmann & Coffman (10). Two distinct populations of CD4+ T cells were then designated as T helper (Th)1 and Th2 cells, distinguished mainly by the effector cytokines they produce but also by their expression of different patterns of cell surface molecules and transcription factors. The signature cytokine of Th1 cells is interferon (IFN)-γ, but they are also potent IL-2 producers (11). Moreover, these cells can also coexpress TNF-α (11). In contrast, Th2 cells fail to produce IFN-γ and produce the signature cytokines IL-4, IL-5, and IL-13. The Th1/Th2 paradigm dominated the field of T cell immunology for about 15 years, until 2003–2005, when a third distinctive effector lineage of CD4+ T cells, termed Th17 cells, was first demonstrated in mouse models of autoimmune encephalitis (12–17), although an IL-17-producing CD4+ T cell population, distinct from Th1 and Th2, was first demonstrated in 2000 (18).
In addition to their ability to differentiate into effector T cells, CD4+ T cells can also become cells with a regulatory function to suppress ongoing effector T cell responses (often referred to as inducible Treg cells or iTregs, discussed further below) (20–23). More recently, the CD4+ T cell subset found in the germinal center, T follicular helper (Tfh) cells, which help in antigen-specific antibody production, have emerged as a possible fifth lineage of CD4+ T cells (23).
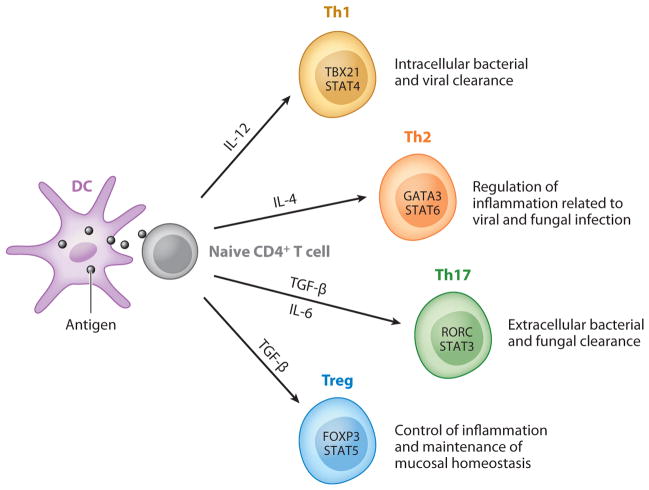
All the above-mentioned T helper lineages are important in pulmonary host defenses. Figure 1 summarizes the cytokines required for differentiation, lineage-specific transcription factors, and the functions in pulmonary host defense for each T helper lineage.
Figure 1.
Major CD4+ T cell subsets in pulmonary host defense. Naive CD4+ T cells differentiate into Th1, Th2, Th17, and Treg cells after antigen encounter presented by DCs followed by lineage specification that is controlled by certain cytokine environments (IL-12, IL-4, TGF-β/IL-6, or TGF-β, respectively) that regulate the expression of lineage-specific transcription factors (depicted within the respective T helper lineage cells). These effector T cells play critical roles in mediating pulmonary host defense, as noted in the figure.
Th1 Cells
Th1 cells are characterized by the production of their signature cytokine, IFN-γ. The differentiation of Th1 cells requires the cytokine IL-12, the master transcription factor TBX21, and the signaling transducer and activator of transcription STAT4 (see Figure 1). Humans carrying mutations in the IFNGR1 subunit of the IFN-γ receptor are susceptible to mycobacterial disease (24). Humans with a deleterious mutation in IL-12B (encoding IL-12p40) (7, 25) and IL12RB1 (encoding IL-12Rβ1) (26, 27) can suffer from infections with Mycobacterium bovis and M. avium. These bacteria are usually nonpathogenic in healthy individuals, but patients with these mutations suffer a defect in IFN-γ response that leads to infection. Thus, genetic evidence from both mouse and human demonstrates that many defects in the IFN-γ and IL-12 pathways can lead to profound mycobacterial susceptibility (reviewed in Reference 28). The WHO estimates the risk of developing TB to be between 20 and 37 times greater in people living with HIV than among those without HIV infection due to the loss of CD4+ T cells and consequent lack of IFN-γ response. HIV infection is one of the most powerful risk factors for progressing from TB infection to disease (29), which underscores the importance of CD4+ T cells in developing TB. IFN-γ secretion was significantly decreased in a study of HIV and TB dually infected patients when compared to healthy subjects and HIV− TB+ patients (30). Taken together, these data illustrate the critical role of Th1 cells and IFN-γ in M. tuberculosis resistance. Th1 cells are considered to be indispensable and play an essential role in combating TB.
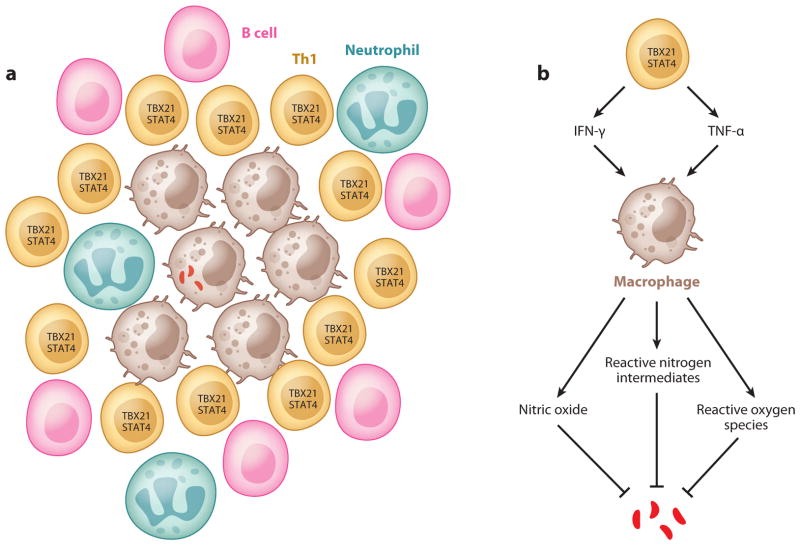
M. tuberculosis, an intracellular bacterium, is transmitted via aerosol droplets and primarily targets the respiratory system. Once the host inhales the bacterium, M. tuberculosis is phagocytosed by alveolar macrophages and myeloid DCs (mDCs), which actively circulate around the mucosal environment (31). Upon infection, mDCs migrate to the draining lymph nodes and initiate the _M. tuberculosis_–specific adaptive immune responses (31, 32). A delay in the priming phase of the adaptive immune response can lead to the establishment of bacterial persistence (32, 33). A successful priming of the adaptive immune response by DCs triggers the expansion and phenotypic and functional maturation of _M. tuberculosis_–specific T cells, which enter the circulation and home to the site of infection. Matured _M. tuberculosis_–specific T cells activate infected macrophages through the secretion of the Th1 signature cytokines IFN-γ and TNF-α (34, 35). Studies in _Ifng_-disrupted mice show defects in the induction of nitric oxide synthase, reactive nitrogen intermediates (26), and reactive oxygen species (25) (see Figure 2). These cytokines are important mediators for sequestering intraendosomal M. tuberculosis growth, achieving microbial killing, and perhaps more importantly, aiding the formation and maturation of the granuloma, a cellular aggregate found in _M. tuberculosis_–infected patients that has long been considered necessary for containment of infection (36, 37). The structure of a granuloma and the function of Th1 cells are summarized in Figure 2.
Figure 2.
Th1 cells and granuloma formation. (a) Schematic representation of granuloma structure: An infected macrophage is surrounded by many cell types such as uninfected macrophages, Th1 cells, B cells, and neutrophils. (b) Functions of Th1 cells in tuberculosis: Th1 cells activate macrophages via cytokines such as TNF-α and IFN-γ. Activated macrophages kill bacteria through nitric oxide, reactive nitrogen intermediates, and reactive oxygen species (see text for details).
IFN-γ can also inhibit IL-17 production by CD4+ T cells and may reduce pathogenic neutrophil recruitment in M. tuberculosis infection (38). Although many other cell types, such as macrophages, CD8+ T cells, NKT cells, and γδ T cells, can also produce IFN-γ, the IFN-γ production, especially from CD8+ T cells, is CD4+ dependent, underscoring the importance of Th1 cells in M. tuberculosis infection (39). Recent studies have suggested that rapid _M. tuberculosis_–specific Th1 cell depletion may contribute to the early onset of TB in individuals with latent M. tuberculosis reactivation (40). Th1 cells, mainly through the production of their signature cytokine, IFN-γ, and in certain cases TNF-α as well, are also involved in controlling pulmonary infection from intracellular bacteria such as Francisella tularensis (41) and Chlamydia pneumoniae (42) and from extracellular bacteria such as Klebsiella pneumoniae (43, 44). Several small clinical trials with inhaled recombinant IFN-γ have shown that the cytokine is well tolerated and associated with reduced organism burden in sputum (19, 45). A randomized placebo-controlled trial has also been conducted of parenteral IFN-γ treatment (1 × 106 IU of recombinant human IFN-γ given intramuscularly, daily for one month and then three times per week up to six months as adjuvant to daily oral azithromycin, ciprofloxacin, ethambutol, and rifampin) in pulmonary atypical mycobacterial infection. This trial showed faster resolution of symptom scores and a trend toward faster microbiological and radiological improvement (46). IFN-γ is also critical for the restriction of Legionella pneumophila infection in vivo; however, the important cellular sources appear to be natural killer (NK) cells (27). In mice, exogenous administration or transient transgenic expression of IFN-γ resulted in improvement in bacterial clearance (47), and in humans, peripheral blood mononuclear cells (PBMCs) from patients who had recovered from Legionnaire’s disease released reduced amounts of IFN-γ after lipopolysaccharide stimulation (48), suggesting that impaired IFN-γ production may contribute to susceptibility to L. pneumophila infection.
Th1 cells are also essential for host defense against many viruses. Influenza viruses are an important cause of respiratory tract infections and are responsible for 3–5 million clinical infections and 250,000–500,000 fatal cases annually (49). Infections with influenza virus trigger robust host immune responses that result ultimately in controlling and eradicating virus replication. Although it is commonly thought that viral-specific memory CD8+ T cells are critical for heterosubtypic immunity in immune-competent healthy individuals, the importance of CD4+ T cells in vaccine-induced heterosubtypic protection has been proven in murine models (50, 51), likely through IFN-γ- and IL-2-dependent mechanisms.
Respiratory syncytial virus (RSV) is another clinically significant virus and is an important cause of acute respiratory tract infections in infants and the elderly, causing significant morbidity and mortality. The WHO estimates the global burden of RSV disease at 64 million cases and 160,000 deaths annually. In the United States, RSV is responsible for 85,000–144,000 infant hospitalizations annually (52). Infection by RSV can cause extensive inflammation, mucus hypersecretion, airway obstruction, and lung damage in susceptible hosts (such as preterm infants or infants exposed to secondhand tobacco smoke) because of a Th2-biased immune response that can lead to persistent airway hyperresponsiveness in the host (6). An effective induction of Th1 immunity against RSV during childhood is thought to counteract this Th2-like immune response triggered by the natural RSV infection. Indeed, in mouse models, prevention of the immunopathology caused by RSV can be achieved through the generation of RSV-specific Th1 cells by a recombinant vaccine (53) or through the enhancement of Th1 responses by downregulation of IL-4Rα, a receptor expressed on Th2 cells (54). Data from these models support the hypothesis that Th1 cells play a protective role in host defense against RSV. Intranasal treatment with recombinant mouse IFN-γ in experimental RSV could also enhance classic activation of neonatal alveolar macrophages, reduce lung viral titers, and improve weight gain with no detectable increase in CD4+ or CD8+ T cell infiltration (55). This finding suggests that intranasal IFN-γ is a viable treatment option and should be considered for further studies in neonatal RSV disease.
Protective immunity to fungi also requires a robust Th1 response. Experimental data have shown the deleterious effects of the ablation of IL-12 (a crucial mediator for Th1 induction) or of IFN-γ during fungal infections (reviewed in Reference 56). For example, functional IFN-γ signaling is required for mice to confine the cryptococcal infection, given that IFN-γR−/− mice do not have the ability to resolve the cryptococcal infection (57); neutralization of IL-12 also prevented lung leukocyte production of IFN-γ in response to the infection (58). By producing IFN-γ and providing help for the generation of opsonizing antibodies, the activation of Th1 cells is also instrumental in the optimal activation of phagocytes at sites of infection. The roles of IFN-γ and TNF-α in fungal infections in macrophage activation do not differ from the roles already described in other infectious diseases. Th1 cytokines promote the release of nitric oxide and reactive oxygen species, both crucial cellular effectors against fungi. Not surprisingly, Th1 cells are beneficial, if not indispensable, in many fungal infections in the lungs, such as Aspergillus. In patients with clinical evidence of invasive aspergillosis, a favorable response to antifungal therapy correlates with higher IFN-γ production from Aspergillus extract–stimulated PBMCs (59). Although aerosol administration of recombinant murine IFN-γ reduced the intensity of Pneumocystis infection in CD4-depleted mice (60), IFN-γ-deficient mice can clear Pneumocystis, suggesting that the role of Th1 cells in Pneumocystis infection is dispensable (61). In a murine model of Coccidioides immitis infection, treatment of the susceptible BALB/c mice with recombinant IFN-γ significantly protected mice against systemic challenge, and neutralization of IFN-γ significantly decreased the capacity to control disease by the resistant DBA/2 mice (62). Administration of recombinant IL-12 to the susceptible mouse strain also resulted in a reduction in the fungal load in the lungs (63), suggesting a crucial role of Th1 cells in host defense against Coccidioides.
In individuals with immune deficiency, histoplasmosis is a severe and potentially fatal disease. IFN-γ is the critical cytokine in activating macrophages to kill the organism (64). Mice that had an IFN-γ deficiency or that had been treated with an IFN-γ-neutralizing antibody were susceptible to Histoplasma capsulatum infection (65), indicating an indispensable role for IFN-γ in host defense against H. capsulatum.
Th2 Cells
In vitro, Th2 cell differentiation requires the cytokine IL-4 and is controlled by master transcription factors GATA3 and STAT6. Th2 immune responses are characterized by the production of IL-4, which can serve as an autocrine factor for Th2 differentiation and can stimulate activated B cells and promote differentiation of B cells into plasma cells. Th2 cells also produce IL-5, a key mediator of eosinophilopoiesis and eosinophil activation. IL-13, a product of Th2 cells, has some overlapping functions with IL-4 but in the lung is a critical inducer of airway hyperresponsiveness, goblet cell metaplasia, and mucus hypersecretion. IL-4 and IL-13 are also produced in abundance by mast cells, eosinophils, basophils, and alternatively activated macrophages (reviewed in Reference 66). Recently, cytokines regulating IL-4 and IL-13, such as epithelial-derived thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, have received attention for their role in allergic inflammation. TSLP is induced during influenza A infection and augments viral-specific CD8+ T cell responses by affecting DC function (67). IL-33 is also induced by influenza virus in murine lungs (4); however, it acts on type 2 ILCs to promote IL-13 production and induce airway hyperreactivity (68). Airway epithelial cell–derived IL-25 augments Th2 responses in NK cell–deficient mice during RSV infection (69).
Th2 cell cytokines can mediate host protective responses against helminth parasites. Parasitic pneumonia is a rare cause of pneumonia, occurring almost exclusively in immunocompromised persons, but HIV infection and use of immunosuppressive drugs have resulted in a resurgence of parasitic lung infections (70). Schistosome cercariae and hookworms typically migrate into the lungs transiently and cause lung inflammation before ultimately reaching other tissue sites for adult development (71). A recent study has suggested that the helminth-induced Th2-type response—which results when parasitic nematode larvae transiently migrate through the lung—mediates acute wound healing and control of inflammation that is initiated by IL-17 through IL-4 receptor signaling (72). This finding indicates a beneficial role for pulmonary Th2 responses in host defense against parasites, albeit in an indirect manner. Th2 cytokines, specifically IL-4, regulate the induction of cross-reactive IgE antibodies to aeroallergens by parasitic infection, suggesting that the immune responses to parasites can induce allergic disease (73). Pulmonary bacterial infections generally do not evoke strong Th2 responses. However, preexisting helminth infection inhibits anti-TB defense through the IL-4 receptor pathway (74).
Viral clearance and long-term humoral immunity to viral reinfections are largely dependent on the generation of neutralizing antibodies. Thus, investigators originally thought that IL-4-producing Th2 cells were required for optimal B cell and antibody responses against viral infection. However, the fact that Th2 cells were dominated by Th1 cells during viral infection was somewhat surprising (75). Nevertheless, the significance of Th2 immune responses in influenza infection is likely critical. On the one hand, Th2 cells drive the expansion, differentiation, and isotype switching of B cells and ultimately lead to the production of neutralizing antibodies (76, 77). These antibodies, at least in murine models of disease, are essential in viral clearance and protection from reinfection (78, 79). On the other hand, influenza virus–induced Th2 immune responses can also contribute to immunopathology in primary infection similar to RSV infection (80). In fact, a Th2-biased response to certain vaccine candidates, such as the formalin-inactivated RSV vaccine tested in the 1960s, leads to exacerbated pulmonary disease upon wild-type viral challenge (81).
The Th2 lineage effector cells in fungal infections are considered to be deleterious in part because they dampen the protective Th1 cell responses (82). The detrimental Th2 responses in pulmonary fungal infections cause significant clinical problems because Th2 responses are closely related to allergic inflammation and asthma. One example of fungal Th2 responses in the lung is allergic bronchopulmonary aspergillosis (ABPA), which can result from exposure to inhaled fungal spores and a skewed Th2 immune response to Aspergillus spp. ABPA causes airway inflammation, which can ultimately be complicated by bronchiectasis. Several genes have been implicated in the development of ABPA, including surfactant protein A2, HLA-DR, and mutations in the cystic fibrosis (CF) transmembrane regulator. In fact, ABPA can occur in 4–15% of patients with CF. This may be due to prolonged retention of Aspergillus spp. in the lung as well as to diminished Treg cell response to fungal antigens. Vitamin D deficiency has also been noted as an independent risk factor for ABPA (83). The dampening of Th2 responses can be achieved by the addition of vitamin D in vitro, which alters the Treg/Th2 balance in the T cells isolated from ABPA patients by reducing DC expression of a costimulatory molecule, OX40L, and increasing DC expression of TGF-β (83). Furthermore, IL-4-deficient mice are resistant to invasive pulmonary aspergillosis owing to enhanced IL-12 and IFN-γ production; however, IFN-γ-deficient mice are highly susceptible to experimental invasive pulmonary aspergillosis (84), underscoring the potential deleterious effects of Th2 responses and protective effects of the Th1 response.
However, Th2 cell–dependent humoral immune responses could confer some protection in immunocompromised hosts against opportunistic fungal pathogens such as Pneumocystis (85). Indeed, animals with defective secretory IgM production have compromised fungal recognition and showed delayed Pneumocystis clearance, which is associated with suboptimal Th2 as well as Th17 responses (86). However, more data support the notion that a predominant Th2 response during fungal infection is generally associated with detrimental effects to the host.
Th17 Cells
Although Th17 cells, as the third T helper lineage, were discovered in a mouse model of autoimmune disease, the pivotal roles of IL-17 and IL-17 receptor signaling in pulmonary host defense were well recognized and appreciated even before the discovery of the Th17 lineage (87). Mice deficient in either IL-17 or IL-17RA have increased susceptibility to gram-negative bacteria, such as Klebsiella pneumoniae (87) and Mycoplasma pneumoniae (88). In the K. pneumoniae model, IL-17RA knockout mice showed decreases in pulmonary G-CSF production, granulopoiesis, and neutrophil recruitment, resulting in higher bacterial burdens in the lung as well as in increased systemic dissemination to the spleen (87). Early IL-17 production in response to K. pneumoniae requires signaling via TLR4 and IL-23 production (66). Mice that do not express IL-23p19 have decreased levels of IL-17 and exhibit a similar phenotype to IL-17RA knockout mice, with diminished production of G-CSF and CXCL1, 2, and 5 (89). In IL-23p19 knockout mice, treatment with recombinant IL-17 restores chemokine production and neutrophil recruitment and results in reduced bacterial burden both locally and systemically (89). Similar results have also been observed in M. pneumoniae infection (88).
In contrast, data suggest that IL-23 and IL-17 are dispensable for protection against primary infection by the intracellular pathogen Mycobacterium tuberculosis. Genetic deletion of neither IL-17RA nor IL-23p19 in mice perturbs clearance of M. tuberculosis (90, 91). However, IL-23p19 expression is induced by M. tuberculosis, and use of IL-23 as a vaccine adjuvant in M. tuberculosis vaccination results in a more robust antigen-specific Th1 response (92–94). Repeated exposure of _M. tuberculosis_–infected animals to mycobacterial antigens can initiate immune-mediated pathology. Specifically, the exposure of _M. tuberculosis_–infected guinea pigs to either live mycobacteria or mycobacterial antigens results in necrotic inflammation at the site of challenge, a response that has been termed the Koch phenomenon (95). In a mouse model of the Koch phenomenon, _M. tuberculosis_–infected mice were repeatedly vaccinated subcutaneously with BCG (bacillus Calmette-Guérin) and developed an IL-23p19- and IL-17-dependent immunopathologic response, indicating a possible pathological consequence of vaccine-induced Th17 responses (96).
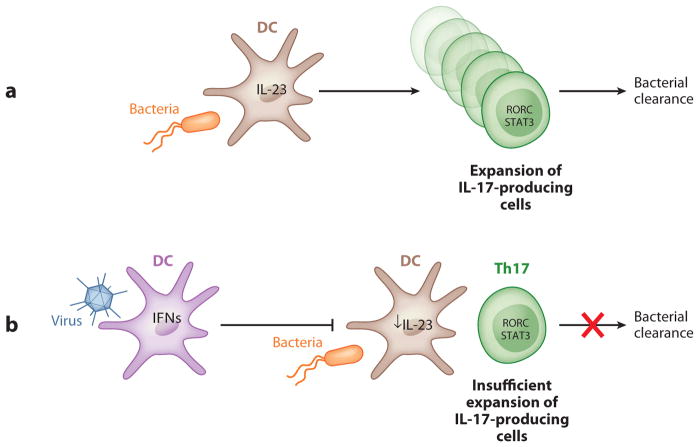
Interestingly, IL-17 is required for host defense against primary infection with a different intracellular pathogen, Francisella tularensis. This organism is a weak inducer of IL-12p70 in lung macrophages and DCs, and IL-17 expression is required for IL-12p70 production in DCs and macrophages. This IL-17-mediated induction of IL-12p70 is critical for the generation of Th1 responses that ultimately bring the infection under control (97). The IL-23/IL-17 axis is also critical in regulating lung neutrophil recruitment in response to mucoid Pseudomonas aeruginosa, but IL-23 was dispensable for bacterial clearance in the lung (98). IL-23 and IL-17 are also important in controlling methicillin-sensitive Staphylococcus aureus infection in the lung (99). S. aureus is also a known complication of pulmonary influenza infection, and one potential mechanism is the suppression of IL-23 and IL-17 by type I IFNs. Indeed, susceptibility to S. aureus infection can be restored by genetic deletion of the type I IFNAR in mice or by exogenous IL-23 to restore lung IL-17 responses. Figure 3 shows a schematic representation of the suppression of antibacterial Th17 responses by preexisting antiviral responses.
Figure 3.
Suppression of antibacterial Th17 responses by preexisting antiviral responses. (a) Under normal conditions, infection by a bacterial pathogen induces IL-23 production by dendritic cells (DCs) and leads to the generation and expansion of Th17 cells, which are essential for bacterial clearance. (b) In a coinfection condition, viral infection induces type I interferon (IFN) production by DCs, which can inhibit IL-23 production by these cells. This viral-induced reduction in IL-23 results in compromised IL-17 responses due to insufficient expansion of IL-17-producing cells, leading to reduced host defenses against bacterial infection.
In primary infection, cellular sources of IL-17 have been carefully examined in various models, and Th17 cells are only one source of IL-17. γδ T cells and newly discovered ILCs are also major sources of IL-17 and IL-22, another Th17 signature cytokine. In contrast, in the vaccinated host, Th17 cells can be the dominant source of IL-17 (92, 100). In M. tuberculosis, vaccination induces Th17 cells that populate the lung earlier than Th1 cells and, after challenge, trigger the production of the chemokines CXCL9 and CXCL10 (ligands for CXCR3) that recruit Th1 cells, which ultimately restrict bacterial growth. However, in K. pneumoniae, Th17 cells proliferate and contract in response to conserved outer membrane proteins to a much greater extent than do Th1 cells. Vaccine-induced Th17 cells are CD44hiCD62L− and are capable of conferring serotype-independent protection against bacteria with differing polysaccharide capsules. Th17-mediated serotype-independent protection has also been observed in mouse models of Streptococcus pneumoniae (101) and Pseudomonas aeruginosa (102). Taken together, these data support the hypothesis that antigen-specific memory Th17 cells confer a host advantage by providing heterologous mucosal immunity independent of serotype-specific antibodies.
Th17 and IL-17 responses in the lung are not always protective. In the setting of experimental primary influenza infection with PR8, IL-17 responses are detrimental to the host. In experiment models, elevated expression of IL-17 has been observed in IFN-γ knockout mice after influenza challenge and is associated with more weight loss and a longer time to recovery (103). IL-17RA knockout mice also showed significant reduction in weight loss, in neutrophil migration, in oxidized phospholipid generation, and in tissue myeloperoxidase upon lethal challenge of influenza (104), indicating a pathological role of IL-17RA ligands in host defense against influenza. Time-dependent increases in pulmonary IL-6, IL-23, and IL-17 expression were observed in a mouse model of RSV infection (105). Blockade of the IL-17 pathway by neutralizing antibody or gene knockout resulted in significant inhibition of mucus production, reduced inflammation, and decreased viral load, which is associated with significantly increased RSV-specific CD8+ T cells (105), supporting a pathogenic role of IL-17 during RSV infections.
The critical role of IL-17RA in antifungal host defense was first demonstrated in a murine model of systemic candidiasis (106). IL-17RA knockout mice had increased mortality, impaired peripheral neutrophil responses, and impaired neutrophil migration to the site of infected organs. Although there is a significant amount of evidence demonstrating a beneficial role for IL-17 in host defense against systemic and oral candidiasis (107), a similar phenomenon has not been seen in pulmonary fungal infections. A study using a mouse model of pulmonary Cryptococcus infection suggested that IL-17A may play a role in the early immune response to Cryptococcus neoformans but is dispensable for classical macrophage activation and control of fungal burden (108). However, the development of Th1 cells is crucial for long-term immunity following pulmonary C. neoformans infection, as demonstrated in _Ifng_−/− mice that had increased pulmonary fungal burden compared with wild-type mice (109). In humans, Aspergillus fumigatus induced a very limited Th17 response in PBMCs, and very low IL-17 levels were observed in bronchoalveolar lavage fluid and serum of patients with invasive aspergillosis (110). However, robust antigen-specific Th17 responses have been seen in the lungs of CF patients when stimulated with antigens from Pseudomonas aeruginosa and Aspergillus spp., although understanding the exact function of these cells requires further characterization (111). Furthermore, intranasal infection of mice with A. fumigatus induced detrimental Th17 responses because of the negative regulation of protective Th1 responses. Neutralization of IL-23 or IL-17 greatly increased resistance to Aspergillus infections (112). In a mouse model of chronic granulomatous disease (CGD), IL-17 produced by γδ T cells contributed to pathogenic inflammation (96). The IL-17 pathway may play a protective role in Pneumocystis murina infection (113). An IL-23 message was induced in alveolar macrophages with stimulation of P. murina organisms, and neutralization of IL-23 or IL-17 increased fungal burden in the lung during Pneumocystis infection in wild-type mice.
Overall, these data indicate that Th17 responses induced by fungal pathogens can be both protective and deleterious to the host depending on the context of infection, particularly the integrity of the downstream effector responses. For example, in the CGD model, neutrophils lack fungicidal activity and therefore fail to clear antigen. This failure may be important in driving the IL-17 pathology in this model (96). Recently, IL-23-dependent pathogenic Th17 cells have been discovered to emerge in the absence of TGF-β (114). Careful phenotyping of pathogenic and nonpathogenic Th17 cells in above-mentioned models may reconcile the discrepancies. However, another important issue that may regulate protective versus pathogenic Th17 responses is host factors, including the integrity of downstream effectors of IL-17R signaling in the epithelium or the integrity of neutrophil function. Thus, the structural integrity of the mucosa may be important in the outcome of fungal-induced Th17 responses.
Although data conflict regarding whether Th17 cells are protective or detrimental in primary fungal infection, vaccine-induced Th17 responses were necessary and sufficient to protect against the three major systemic mycoses (Coccidioides posadasii, Histoplasma capsulatum, and Blastomyces dermatitidis) in North America (115). Antifungal Th17 memory responses were further examined using a TCR transgenic mouse (116) that was generated with CD4+ T cells that specifically recognize an antigen from B. dermatitidis. These TCR transgenic CD4+ T cells recognize and proliferate in response to evolutionarily related systemic dimorphic fungi based on rRNA homology. When polarized to Th17 cells, these transgenic T cells can provide protection not only against B. dermatitidis but also against related fungi such as H. capsulatum. These fungi presumably express conserved antigens.
In the context of pulmonary host defense, the advantage of T cell–encoded effector cytokines could be threefold, as illustrated in Figure 4:
Figure 4.
Current hypotheses of the requirement for T cell immunity in the lung. (a) T cell responses can be turned on and off quickly through clonal expansion and apoptosis. (b) T cells can migrate to a distal site such as bone marrow to mobilize neutrophils through CXCL1 and G-CSF. (c) Memory T cells recognize conserved bacterial antigen and provide serotype-independent protection.
- T cells can rapidly divide and undergo apoptosis, providing a mechanism for rapid amplification and termination of Th1, Th2, and Th17 responses. This fulfills the requirements of massive cytokine production upon pathogen invasion and prevents collateral damage once pathogens are cleared from the respiratory tract (Figure 4_a_).
- T cells can traffic to and from mucosal sites to provide immune reconnaissance. This can be achieved by chemotaxis between the interactions of chemokine receptors on T cells and the chemogradient induced by infection. This ability to traffic between mucosal sites is particularly important in pulmonary infection because one critical effector cell population, the neutrophil, matures and resides in the bone marrow. T cells serve as excellent candidate cells to emigrate outside the lung to control systemic responses to infection (Figure 4_b_). Supporting evidence comes from a mouse model of Klebsiella pneumoniae, in which migration of IL-17-producing cells to the bone marrow via the CXCL12/CXCR4 axis was partially responsible for controlling bacterial-induced G-CSF expression (M. Zheng & J.K. Kolls, unpublished observations).
- The generation of memory T helper cells might also confer an advantage to the host above and beyond what a pathogen-specific antibody can provide. We provide evidence (100) that outer membrane proteins conserved across several serotypes of K. pneumoniae are responsible for antigen-specific Th17 cell priming in mediastinal lymph nodes during vaccination, resulting in long-term protection against strains that are not effectively neutralized by antibodies, suggesting a possible evolutionary advantage for the acquisition of IL-17 expression by CD4+ T cell subsets. Immunization with pneumococcal cell wall antigen also confers cross-serotype protection in mice infected with Streptococcus pneumoniae (117), and the protection is Th17 (101) and neutrophil (118) dependent, suggesting a potential novel antibody-independent immunization strategy against S. pneumoniae (Figure 4_c_).
IL-22, another major effector cytokine produced by Th17 cells, plays a critical role in pulmonary host defense, although the cellular sources can be more than just Th17 cells. In response to K. pneumoniae infection, IL-22 from CD90+ T cells increased lung epithelial cell proliferation and increased transepithelial resistance to injury (119). IL-22 expression was also found in the lung of _M. tuberculosis_–infected patients as well as in _M. tuberculosis_–infected rhesus macaques. However, mice lacking IL-22 are unaffected in their ability to control M. tuberculosis (11). Thus, the exact role of IL-22 in M. tuberculosis remains to be explored. IL-22 is also essential in tissue repair after influenza infection, and both conventional NK cells (120, 121) and iNKT cells (122) are IL-22 producers, based on reports that used different strains and dosages of viruses as well as various time points after infection. IL-22 appeared to be critical for host defense against A. fumigatus, given that mice deficient in IL-22 or receiving neutralization antibody against IL-22 demonstrated a higher lung fungal burden after A. fumigatus challenge (123).
T Regulatory Cells
Two main types of regulatory T (Treg) cells have been identified: Extrathymically derived adaptive (or induced) CD4+Foxp3+ regulatory T (iTreg) cells can be phenotypically and functionally distinguished from thymus-derived natural Foxp3+ regulatory T (nTreg) cells. Both play significant roles in tuning down effector immune responses, however. Mutations of the FOXP3 gene in humans cause IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome), a rare disorder that usually results in death in early infancy or childhood. Lung pathology has been revealed by postmortem examination of IPEX patients (124).
Generation of iTreg cells depends on productive antigen presentation by APCs in a microenvironment rich in TGF-β and IL-2 (125). Depending on the type and stage of infection, suppression of inflammatory responses by iTreg cells can be both beneficial and deleterious in host defense. In the lung, these cells play a critical role in mediating tolerance to inhaled antigen (68).
The immune response must eradicate the invading pathogens with the minimal cost of damage to self-tissues. The average adult takes 15 to 20 breaths a minute—over 20,000 breaths a day. The average adult at rest inhales and exhales 7 or 8 liters of air per minute, or approximately 11,000 liters of air in a day. Air at sea level contains 78% nitrogen and 21% oxygen, but inspired air can also contain large concentrations of antigens, including organic dusts, inorganic particles, and biological agents such as bacteria, viruses, and fungal spores. In most cases, these antigens are cleared by mucociliary clearance and coughing. However, if the antigens manage to persist in the lung long enough, they will be detected by the immune system and trigger immune responses. To maintain a tolerogenic environment that protects the host from inflammatory damage, the immune response in the lungs must be tightly regulated.
Treg cells serve as critical cells in controlling excess inflammation and in maintaining and restoring a homeostatic environment. In Chlamydia pneumoniae infection, which is associated with induction and exacerbation of asthma, Treg cells seem to play a protective role because depletion of Treg cells in a mouse model exacerbated allergic airway sensitization and eosinophilic airway inflammation (126). In mice acutely infected with Pseudomonas aeruginosa, depletion of Treg cells with anti-CD25 antibody did not affect airway neutrophil infiltration or overall survival, indicating that Treg cells do not contribute significantly to host defense against Pseudomonas (127).
In chronic infection, Treg cells can be exploited by the pathogen, such as in M. tuberculosis infection, to suppress protective immune responses and benefit its survival in the host. Treg cells are increased in TB patients and may contribute to suppression of Th1-mediated immune responses (128). In a C57BL/6 mouse model, depletion of Treg cells early after infection resulted in a small increase in cytokine production but did not alter the course of either M. tuberculosis or BCG infections (129). Moreover, Treg inactivation by CD25 neutralizing antibody during BCG vaccination did not affect protection against virulent challenge (130). However, in BALB/c mice, the attenuation of Treg subsets during BCG vaccination had a positive impact on the protective capacity of this vaccine against M. tuberculosis infection (131). A recent study found that Treg cells recognizing M. tuberculosis antigens suppressed protective immune response during TB, supporting a pathogenic role for Treg cells in TB (3). The observed discrepancies in the roles of Treg cells in two different mouse strains can likely be explained by different host genetic backgrounds (132).
Influenza A virus infection results in the robust induction of a Treg response in mice (133, 134), and virus-specific Treg cells can be detected long after viral clearance in humans (135), suggesting a potential role for Treg cells in limiting excessive inflammation induced by influenza A virus infection. Furthermore, adoptive transfer of polyclonal Treg cells into influenza-infected, lymphocyte-deficient mice prolongs survival and reduces the innate inflammatory response (136). However, depletion of Treg cells by anti-CD25 antibody did not alter influenza A virus–induced mortality, weight loss, viral clearance, or cellularity within the lung (134). In RSV infection models, Treg cells limit immunopathology by influencing the trafficking and effector function of virus-specific CD8+ T cells in the lungs and draining lymph nodes (137, 138). Similar beneficial roles of Treg cells have been observed in infections with fungal pathogens such as Paracoccidioides brasiliensis. In a mouse model of paracoccidioidomycosis, the absence of TLR4 signaling did not increase mortality rates but resulted in less severe inflammatory reactions in the lung, which is associated with increased numbers of Treg cells (139). Mouse models of Pneumocystis clearly demonstrate that adoptive transfer of CD4+CD25+ Treg cells can inhibit pulmonary inflammation caused by CD4+CD25− effector cells and enhance survival of the host (140, 141).
Some HIV patients experience immune reconstitution inflammatory syndrome (IRIS) as a result of pathological responses induced during immune restoration following the initiation of highly active antiretroviral therapy (HAART). The presence of antigenic stimulus, whether infectious or noninfectious, is reportedly a prerequisite to developing IRIS (142). Interestingly, elevation of IL-6, IL-10, and IFN-γ expression in IRIS patients has been observed (143), suggesting a potential link between IRIS and Treg cells.
A comprehensive analysis of Treg cells in peripheral blood has been carried out with a large cohort of HIV-infected patients (n = 131) (144). In this study, investigators observed a significantly increased proportion of Treg cells in the remaining CD4+ T cells of HIV-positive patients compared with that of healthy controls, suggesting that Treg cells might be relatively more resistant to HIV depletion. Additionally, the relative frequency of Treg cells directly correlated with HIV viral load and inversely with CD4+ T cell counts, indicating a deleterious role for Treg cells in antiviral responses. However, the absolute Treg cell number was reduced in HIV-infected patients compared with healthy controls, and this reduction could be what leads to the lack of anti-inflammatory responses in IRIS patients.
Thus, although not involved in clearing pulmonary pathogens directly, Treg cells orchestrate their functions by modulating other lineages of CD4+ T cells, CD8+ T cells, and myeloid cells as well.
T Follicular Helper Cells
Tfh cells express the chemokine receptor CXCR5 to home to developing germinal centers, where they instruct B cells to class switch. However, their roles in pulmonary host defense have been underexplored. A recent study has shown that IL-21, a cytokine produced by Tfh, promotes the pathogenic inflammatory effect of pneumovirus in mice (145). The exact role of Tfh in this model has yet to be defined. Tfh cells can be induced in mice infected with influenza A virus (146). The development of B cell responses to influenza A was impaired by high-dose IL-2, and this was associated with impaired Tfh responses (147). However, whether Tfh cells are required for viral clearance has not been examined. A recent study suggests that Tfh and Th1 cells share a transient Tfh-like cell stage, raising the question of whether Tfh cells are a distinct lineage of CD4+ T cells (148).
CD8+ T Cells
CD8+ T cells are cytotoxic T cells and are also major producers of IFN-γ, TNF-α, and IL-2. Generation of strong antigen-specific CD8+ T cell responses to protective dominant epitopes of influenza in the lungs occurs after infection or immunization and is critical for clearance of the virus (149). However, the release of cytotoxic molecules (e.g., granzyme and perforin) and antiviral cytokines (e.g., TNF-α and IFN-γ) can also contribute to lung pathology (150). Transgenic mice with an elevated precursor frequency of anti-influenza CD8+ T cells are protected against low-dose viral challenge but succumb to high viral dose owing to exacerbation in viral pathology. This difference suggests a critical antigen threshold in CD8+ T cell activation that determines protection versus immunopathology during influenza (151). In RSV infection, CD8+ T cells are induced and recruited into the lungs but show significantly impaired expression of effector activity (152). Indeed, priming a RSV-specific CD8+ T cell response using recombinant vaccinia virus encoding the immunodominant RSV CD8+ T cell epitope inhibited formalin-inactivated RSV vaccine–induced pulmonary eosinophilia and disease exacerbation (153). These data support a requirement for sufficient viral-specific CD8+ T cell priming in RSV vaccine development.
The protective role that CD8+ T cells play in immunity to M. tuberculosis has also been well appreciated. Antigen-specific CD8+ T cells recognize and lyse _M. tuberculosis_–infected cells and produce cytokines such as TNF-α and IFN-γ, both of which are critical for macrophage activation (reviewed in Reference 154). In a mouse model of Streptococcus pneumoniae infection, CD8−/− mice exhibited significantly increased bacterial dissemination, enhanced lung inflammation, and significantly higher mortality compared with wild-type mice. Perforin−/− and IFN-γ−/− mice also exhibited enhanced lethality compared with wild-type mice (155), underscoring the importance of CD8+ T cells and potentially their effector molecules in bacterial pneumonia. In a cohort of HIV-infected women, lower CD8+ T cell counts were associated with increased risk for bacterial pneumonia and all-cause mortality (156), further supporting the protective role of CD8+ T cells in host defense against pulmonary bacterial infections.
CD8+ T cells in the naturally occurring host response to fungal pathogens in the lung have not been well documented, although _Aspergillus_-specific CD8+ T cells have been shown to be present in mice (157) as well as in healthy humans (158). Lack of activation of the protective memory CD8+ T cell population in TLR3−/− mice is associated with higher susceptibility to pulmonary aspergillosis (159), suggesting a protective role of CD8+ T cells in pulmonary antifungal immunity. CD8+ T cells are also recruited to the lung in large numbers in response to Pneumocystis infection, and they are associated with some level of host defense (160) as well as with lung injury (161). This discrepancy can be explained at least partially by the heterogeneity of antigen-specific CD8+ T cells induced by Pneumocystis infection because CD8+ T cells with a T-cytotoxic 1 (Tc1) phenotype seem to be protective, whereas non-Tc1 cells seem to contribute to immunopathology. Recently, IL-17-producing CD8+ T cells (Tc17) were shown to provide host protection against both pulmonary viral and fungal pathogens. Tc17 cells can be found in the lung following primary influenza challenge, and in vitro–generated Tc17 cells expand after transfer to naive recipients following viral challenge and confer protection against lethal influenza infection (162). In CD4+ T cell–depleted mice, Tc17 cells are induced upon vaccination and mediate protection against Blastomyces dermatitidis and Histoplasma capsulatum in a neutrophil-dependent manner (163). IL-17- and IL-22-producing CD8+ T cells are also found in SIV-infected rhesus macaques (K. Chen & J.K. Kolls, unpublished data). Taken together, these data suggest that Tc17 cells can confer protection in HIV patients and can also be exploited as a vaccine target for pulmonary host defense in immunocompromised individuals.
γδ T, Invariant NKT, and Innate Lymphoid Cells
γδ T cells, iNKT cells, and ILCs are all capable of responding immediately to cytokines produced by DCs and macrophages in the course of infection in the lung. These three cell types are critical for producing CD4+ T cell– and CD8+ T cell–derived cytokines before an adaptive immune response fully develops, bridging the innate and adaptive systems and optimizing the adaptive immune response against various pathogens.
γδ T cells represent a small subset of T cells that have a T cell receptor composed of one γ chain and one δ chain, as opposed to conventional T cells that have a T cell receptor made up of α and β chains. γδ T cells are a major source of IFN-γ, TNF-α, and IL-17 in mice infected with Klebsiella (100, 164), and mice lacking γδ T cells exhibit higher bacterial dissemination and poor survival (164). γδ T cells are also the dominant source of IL-17 during Mycobacterium tuberculosis infection (165) and a critical cellular source of TNF-α in Streptococcus pneumoniae infection (166). iNKT cells share properties of both T cells and NK cells and recognize antigens presented by the CD1d molecule. iNKT cells express limited T cell receptors: Vα14 for mouse and Vα24 for human. iNKT cells can be activated by an innate cytokine-and antigen-driven pathway, produces IFN-γ, and play a role in numerous bacterial infections in the lung including S. pneumoniae and Pseudomonas aeruginosa (reviewed in Reference 167). In response to viral infection, such as influenza, iNKT cells produce IL-22 and participate in the repair of the lung epithelium (122) and influence CD8+ T cell responses by promoting respiratory DC maturation (168). γδ T cells produce IL-17 in influenza-infected mice and seem to contribute to pathogenic inflammation (104), whereas a subset of human γδ T cells produces IFN-γ after influenza stimulation (169). Unlike iNKT and γδ T cells, ILCs found in the lung in response to influenza only produce type 2 cytokines and participate in tissue repair and allergic inflammation (170).
Little is known about the role of ILCs in antifungal immunity in the lung. The roles of γδ T and iNKT cells in host defense against Cryptococcus have been investigated. Activation of iNKT cells by their natural ligand, α-galactosylceramide, results in enhanced IFN-γ production and protective Th1 responses (171), whereas γδ T cells seem to downmodulate this protective Th1 response, given that γδ T cell–deficient mice have increased IFN-γ production and reduced fungal burden (172). γδ T cell–deficient mice also show a more rapid and complete resolution of Pneumocystis infection than do C57BL/6 controls. This augmented resolution is associated with elevated IFN-γ levels in bronchoalveolar lavage fluid predominantly produced by CD8+ T cells (173).
T CELL–MEDIATED HOST DEFENSE IN COINFECTIONS, CHRONIC OBSTRUCTIVE PULMONARY DISEASE, AND CYSTIC FIBROSIS
The normal lung microbiome remains to be defined. However, there is significant evidence that some bacterial infections occur in otherwise healthy hosts in the setting of a prior or concomitant viral infection. Recently, several studies have shed light on the immunological mechanisms that underlie these coinfections.
Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus were the most common bacterial coinfection during the 2009 H1N1 influenza pandemic and were associated with a higher risk of morbidity and mortality (174). In a murine coinfection model, severe bronchopneumonia with massive hemorrhage in influenza- and _Streptococcus pneumoniae_–coinfected mice was associated with augmented type 1 proinflammatory responses (175). Pulmonary IFN-γ produced during T cell responses to influenza infection can also suppress innate immunity against S. pneumoniae by downregulating the expression of the class A scavenger receptor MARCO on alveolar macrophages (91). Furthermore, antiviral responses to influenza can also sensitize hosts to secondary bacterial pneumonia by inhibiting the production of neutrophil chemoattractants CXCL1 and CXCL2 (176). In another mouse model of coinfection of influenza and S. aureus, influenza A was shown to strongly induce type I IFN and subsequently suppress the Th17 response, which was required for S. aureus clearance (99). These studies suggest that alteration of the normal T cell response by influenza likely compromises the host defense mechanism and worsens the outcome of subsequent bacterial infection. Coinfection of RSV and bacteria such as S. pneumoniae, H. influenzae, and S. aureus have also been reported (177). However, the immune responses in these patients are currently underexplored, and animal models to study these coinfections are also limited.
Viral and bacterial infections are often observed in chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and CF. In fact, coinfections are associated with most severe COPD exacerbations requiring hospitalization, and the presence of infection is related to exacerbation severity (178). Interestingly, blunted γδ T lymphocyte responses have been observed in COPD patients (179), suggesting that γδ T cells may be critical for host defense in COPD. Although the Th1 immune response to gram-negative bacterial infections is impaired in COPD [because PBMCs from COPD patients had reduced IFN-γ production upon lipopolysaccharide stimulation (180)], the Th17 response augmented by cigarette smoke seems to involve disease pathogenesis through induction of CCL2 and matrix metalloproteinase (2). Furthermore, the induction of osteopontin expression on lung APCs seemed to be responsible for the cigarette smoke–induced pathological Th17 responses in emphysema (181). Rhinovirus is responsible for the common cold and is currently the most important trigger of COPD exacerbations (182). The mechanisms of virus-induced exacerbations are not well understood. Although currently there is no direct evidence that rhinovirus infection triggers IL-17 production, IL-1β, a Th17-promoting cytokine, is released by human bronchial epithelial cells after infection with rhinovirus (183). IL-17A also synergistically enhances human rhinovirus-16-induced epithelial production of CXCL8, a neutrophil chemoattractant (184), which may contribute to viral-induced exacerbation. COPD exacerbations are often associated with increased levels of mediators including TNF-α, IL-6, and CXCL8 and inflammatory cells such as neutrophils and eosinophils (reviewed in Reference 111). All of these mediators are related to Th1 and Th17 responses, which suggests that T cells may play an important role in viral-induced COPD exacerbation.
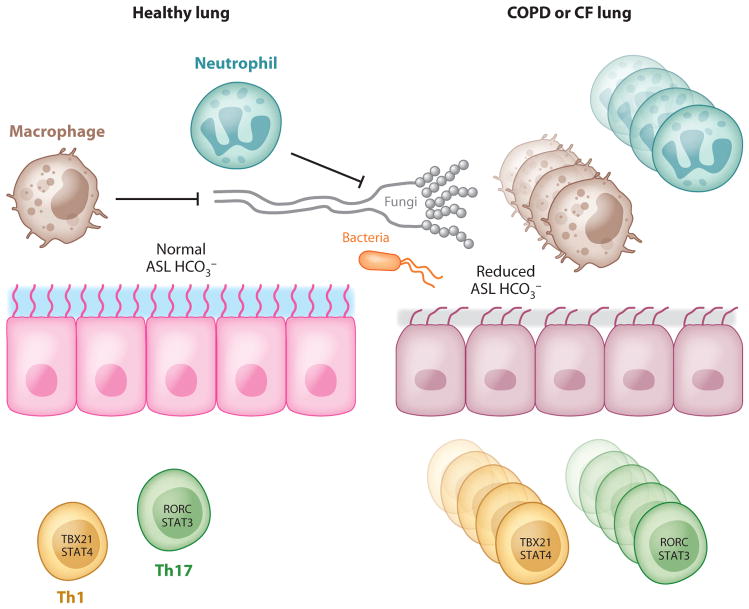
In CF, significantly elevated levels of the Th17 cytokines IL-17A and IL-17F were found in the sputum of patients who were colonized with Pseudomonas aeruginosa at the time of pulmonary exacerbation, and the levels declined with therapy directed against P. aeruginosa (185). Th17 cells are also found in the airway submucosa in CF (186), suggesting a pathogenic role of Th17 cells in that disease. However, the inability of dysfunctional epithelial cells to respond to Th17 cytokines in CF can also contribute to Pseudomonas colonization and lead to disease exacerbation. In normal lungs, where infection can be resolved in a timely fashion, the development of a memory response is protective and results in more efficient clearance of the pathogen with subsequent infections. In CF patients, the inability to clear Pseudomonas aeruginosa because of abnormalities in the airway surface liquid (ASL) and ciliary functions leads to persistent activation of inflammatory pathways that promote chronic IL-17 production and the development of a Th17 phenotype (Figure 5). Despite persistent neutrophilia in the lung, this response cannot effectively clear the pathogen (187). One hypothesis is that this may be partially due to an intrinsic defect in CF neutrophils (188). Failure of pathogen eradication would lead to further neutrophil recruitment, and thus chronic lung injury would be promoted over time (189). More recently, a defect in airway epithelial HCO3− secretion has been observed in a porcine CF model, which leads to a decrease of ASL pH and the inhibition of antimicrobial protein activity (190). Moreover, IL-17A induces bicarbonate secretion in human bronchial epithelial cells in a CFTR-dependent manner (191), suggesting that despite robust Th17 responses in the CF lung, a defect in bicarbonate secretion may render this Th17 response ineffective (Figure 5). T cell responses in asthma have been recently reviewed extensively (192) and are not discussed here.
Figure 5.
Proposed model of T cell responses in chronic lung disease. In the context of COPD or CF, lung epithelial cells have reduced anion transport (Cl− and HCO3−), leading to reduced airway surface liquid (ASL) volume and reduced antimicrobial activity. This can result in pathogen persistence and constitutive T cell activation and proliferation, leading to parenchymal lung damage.
CONCLUSIONS
Th1 and CD8+ T cells mainly contribute to successful host defense against viral pathogens such as influenza and RSV, whereas Th2 and Th17 responses are often detrimental and exacerbate diseases in RSV and influenza, respectively. Th1 and Th17 cells as well as γδ T cells are essential components of the armamentarium against bacterial and fungal infections. Treg cells benefit the host by repressing excessive inflammation during infection. γδ T cells, iNKT cells, and ILCs are also important mediators in pulmonary host defense that allow immediate responses to pathogens.
Investigators have long appreciated T cell diversity, but there is now increased recognition of T cell plasticity (193). It is challenging but promising to utilize the plastic nature of T cells to manipulate immune responses in the lung and ultimately balance immunological activation and tolerance in host defense and inflammation.
ILCs have emerged as a major immune modulator in the mucosal site, and their role seems to parallel that of the known T helper subsets: Th1, Th2, and Th17. Currently, only type 2 ILCs have been identified in the lung (170). Whether other types of ILCs are present in the lung and whether ILCs show plasticity remain to be addressed. The roles of Th17 cells in diseased lung are still controversial. A clear understanding of the Th17 pathway is critical to determining whether therapy targeting Th17 cells will be helpful in preventing inflammation-mediated damage. The challenges of developing safe and effective immunosuppressive therapy are significant.
Th17-based mucosal vaccines against extracellular bacterial and fungal pathogens seem promising. However, because of the proinflammatory nature of Th17 cells, safety issues have to be carefully addressed. The discovery of Tc17 cells opens up opportunities for development of a CD8+ T cell–based mucosal vaccine against pulmonary pathogens for immunocompromised people, especially HIV-infected patients.
Acknowledgments
The authors acknowledge support from the following US Public Health Service grants: P50HL084932, R37-HL079142, and 5R01HL061271.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol. 2011;2011:954602. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1(6):442–50. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 3.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207(7):1409–20. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, et al. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol. 2011;45(6):1125–32. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 5.Lund FE, Partida-Sánchez S, Lee BO, Kusser KL, Hartson L, et al. Lymphotoxin-α-deficient mice make delayed, but effective, T and B cell responses to influenza. J Immunol. 2002;169(9):5236–43. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- 6.Olmos S, Stukes S, Ernst JD. Ectopic activation of Mycobacterium tuberculosis-specific CD4+ T cells in lungs of CCR7−/− mice. J Immunol. 2010;184(2):895–901. doi: 10.4049/jimmunol.0901230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altare F, Lammas D, Revy P, Jouanguy E, Doffinger R, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guérin and Salmonella enteritidis disseminated infection. J Clin Investig. 1998;102(12):2035–40. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raju R, Peters BS, Breen RA. Lung infections in the HIV-infected adult. Curr Opin Pulm Med. 2012;18(3):253–58. doi: 10.1097/MCP.0b013e32835213d3. [DOI] [PubMed] [Google Scholar]
- 9.van de Veerdonk FL, Netea MG. T-cell subsets and antifungal host defenses. Curr Fungal Infect Rep. 2010;4(4):238–43. doi: 10.1007/s12281-010-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–57. [PubMed] [Google Scholar]
- 11.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187(10):5402–7. doi: 10.4049/jimmunol.1101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–14. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 13.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–48. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–57. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park H, Li Z, Yang XO, Chang SH, Nurieva R, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 19.Suárez-Méndez R, García-García I, Fernández-Olivera N, Valdés-Quintana M, Milanés-Virelles MT, et al. Adjuvant interferon gamma in patients with drug-resistant pulmonary tuberculosis: a pilot study. BMC Infect Dis. 2004;4:44. doi: 10.1186/1471-2334-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Jin W, Hardegen N, Lei KJ, Li L, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu S, Zhang N, Yopp AC, Chen D, Mao M, et al. TGF-β induces Foxp3+ T-regulatory cells from CD4+ CD25− precursors. Am J Transplant. 2004;4(10):1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 22.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, et al. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172(9):5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 23.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J Immunol. 2004;172(9):5213–21. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 24.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, et al. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335(26):1941–49. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 25.Elloumi-Zghal H, Barbouche MR, Chemli J, Bejaoui M, Harbi A, et al. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis bacille Calmette-Guérin infection. J Infect Dis. 2002;185(10):1468–75. doi: 10.1086/340510. [DOI] [PubMed] [Google Scholar]
- 26.de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–38. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 27.Sakai T, Matsuoka M, Aoki M, Nosaka K, Mitsuya H. Missense mutation of the interleukin-12 receptor β1 chain-encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood. 2001;97(9):2688–94. doi: 10.1182/blood.v97.9.2688. [DOI] [PubMed] [Google Scholar]
- 28.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanal O, et al. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet. 2002;32(1):97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 29.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–7. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 30.Bal AM, Lakhashe SK, Thakar MR, Tripathy SP, Paranjape RS. Dysregulation of proinflammatory and regulatory cytokines in HIV infected persons with active tuberculosis. Cytokine. 2005;30:275–81. doi: 10.1016/j.cyto.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179(4):2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 32.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci USA. 2008;105(31):10961–66. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chackerian AA, Perera TV, Behar SM. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect Immun. 2001;69(4):2666–74. doi: 10.1128/IAI.69.4.2666-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, et al. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34(5):807–19. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205(10):2359–68. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reece ST, Kaufmann SH. Floating between the poles of pathology and protection: can we pin down the granuloma in tuberculosis? Curr Opin Microbiol. 2012;15(1):63–70. doi: 10.1016/j.mib.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, et al. A γ interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7(5):e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nandi B, Behar SM. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208(11):2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shams H, Wizel B, Weis SE, Samten B, Barnes PF. Contribution of CD8+ T cells to γ interferon production in human tuberculosis. Infect Immun. 2001;69(5):3497–501. doi: 10.1128/IAI.69.5.3497-3501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198(11):1590–98. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzger DW, Bakshi CS, Kirimanjeswara G. Mucosal immunopathogenesis of Francisella tularensis. Ann N Y Acad Sci. 2007;1105:266–83. doi: 10.1196/annals.1409.007. [DOI] [PubMed] [Google Scholar]
- 42.Geng Y, Berencsi K, Gyulai Z, Valyi-Nagy T, Gonczol E, et al. Roles of interleukin-12 and γ interferon in murine Chlamydia pneumoniae infection. Infect Immun. 2000;68(4):2245–53. doi: 10.1128/iai.68.4.2245-2253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng JC, Moore TA, Newstead MW, Zeng X, Krieg AM, et al. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J Immunol. 2004;173(8):5148–55. doi: 10.4049/jimmunol.173.8.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng JC, Zeng X, Newstead M, Moore TA, Tsai WC, et al. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J Immunol. 2004;173(6):4075–83. doi: 10.4049/jimmunol.173.6.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-γ via aerosol. Lancet. 1997;349(9064):1513–15. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 46.Milanés-Virelles MT, García-García I, Santos-Herrera Y, Valdés-Quintana M, Valenzuela-Silva CM, et al. Adjuvant interferon γ in patients with pulmonary atypical Mycobacteriosis: a randomized, double-blind, placebo-controlled study. BMC Infect Dis. 2008;8:17. doi: 10.1186/1471-2334-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng JC, Tateda K, Zeng X, Standiford TJ. Transient transgenic expression of γ interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect Immun. 2001;69(10):6382–90. doi: 10.1128/IAI.69.10.6382-6390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lettinga KD, Weijer S, Speelman P, Prins JM, Van Der Poll T, et al. Reduced interferon-γ release in patients recovered from Legionnaires’ disease. Thorax. 2003;58(1):63–67. doi: 10.1136/thorax.58.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO. Influenza. WHO; Geneva: 2009. Apr, Fact Sheet No. 211. http://www.who.int/mediacentre/factsheets/fs211/en/index.html. [Google Scholar]
- 50.Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol. 2011;85(1):448–55. doi: 10.1128/JVI.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 2009 influenza and secondary bacterial infections. J Immunol. 2011;186(2):987–93. doi: 10.4049/jimmunol.1002664. [DOI] [PubMed] [Google Scholar]
- 52.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, et al. Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guérin promotes virus clearance and protects from infection. J Immunol. 2010;185(12):7633–45. doi: 10.4049/jimmunol.0903452. [DOI] [PubMed] [Google Scholar]
- 54.Ripple MJ, You D, Honnegowda S, Giaimo JD, Sewell AB, et al. Immunomodulation with IL-4Rα antisense oligonucleotide prevents respiratory syncytial virus-mediated pulmonary disease. J Immunol. 2010;185(8):4804–11. doi: 10.4049/jimmunol.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Empey KM, Orend JG, Peebles RS, Jr, Egaña L, Norris KA, et al. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of respiratory syncytial virus infection. PLoS ONE. 2012;7(7):e40499. doi: 10.1371/journal.pone.0040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin Microbiol Rev. 1997;10(4):611–36. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen GH, McDonald RA, Wells JC, Huffnagle GB, Lukacs NW, et al. The γ interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun. 2005;73(3):1788–96. doi: 10.1128/IAI.73.3.1788-1796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herring AC, Lee J, McDonald RA, Toews GB, Huffnagle GB. Induction of interleukin-12 and γ interferon requires tumor necrosis factor α for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect Immun. 2002;70(6):2959–64. doi: 10.1128/IAI.70.6.2959-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100(13):4521–28. doi: 10.1182/blood-2002-01-0265. [DOI] [PubMed] [Google Scholar]
- 60.Beck JM, Liggitt HD, Brunette EN, Fuchs HJ, Shellito JE, et al. Reduction in intensity of Pneumocystis carinii pneumonia in mice by aerosol administration of γ interferon. Infect Immun. 1991;59(11):3859–62. doi: 10.1128/iai.59.11.3859-3862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun. 1997;65(12):5052–56. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magee DM, Cox RA. Roles of γ interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun. 1995;63(9):3514–19. doi: 10.1128/iai.63.9.3514-3519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magee DM, Cox RA. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect Immun. 1996;64(9):3609–13. doi: 10.1128/iai.64.9.3609-3613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu-Hsieh BA, Howard DH. Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine γ interferon. Infect Immun. 1987;55(4):1014–16. doi: 10.1128/iai.55.4.1014-1016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clemons KV, Darbonne WC, Curnutte JT, Sobel RA, Stevens DA. Experimental histoplasmosis in mice treated with anti-murine interferon-γ antibody and in interferon-γ gene knockout mice. Microbes Infect. 2000;2(9):997–1001. doi: 10.1016/s1286-4579(00)01253-3. [DOI] [PubMed] [Google Scholar]
- 66.Williams CM, Rahman S, Hubeau C, Ma HL. Cytokine pathways in allergic disease. Toxicol Pathol. 2012;40(2):205–15. doi: 10.1177/0192623311430694. [DOI] [PubMed] [Google Scholar]
- 67.Yadava K, Sichelstiel A, Luescher IF, Nicod LP, Harris NL, Marsland BJ. TSLP promotes influenza-specific CD8+ T-cell responses by augmenting local inflammatory dendritic cell function. Mucosal Immunol. 2012;6(1):83–92. doi: 10.1038/mi.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–38. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185(8):4681–90. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 70.Vijayan VK. How to diagnose and manage common parasitic pneumonias. Curr Opin Pulm Med. 2007;13(3):218–24. doi: 10.1097/MCP.0b013e3280f31b58. [DOI] [PubMed] [Google Scholar]
- 71.Kolosionek E, Crosby A, Harhay MO, Morrell N, Butrous G. Pulmonary vascular disease associated with schistosomiasis. Expert Rev Anti-Infect Ther. 2010;8(12):1467–73. doi: 10.1586/eri.10.124. [DOI] [PubMed] [Google Scholar]
- 72.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18(2):260–66. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bush RK. Parasites and allergic disease: another piece of the puzzle. J Allergy Clin Immunol. 2012;130(1):257–58. doi: 10.1016/j.jaci.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 74.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, et al. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208(9):1863–74. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136–48. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marshall D, Sealy R, Sangster M, Coleclough C. TH cells primed during influenza virus infection provide help for qualitatively distinct antibody responses to subsequent immunization. J Immunol. 1999;163(9):4673–82. [PubMed] [Google Scholar]
- 77.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24(1):157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69(4):2075–81. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–86. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 80.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–55. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 82.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4(1):1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 83.Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Investig. 2010;120(9):3242–54. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cenci E, Mencacci A, Del Sero G, Bacci A, Montagnoli C, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180(6):1957–68. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 85.Kling HM, Shipley TW, Patil SP, Kristoff J, Bryan M, et al. Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection. Infect Immun. 2010;78(10):4320–30. doi: 10.1128/IAI.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, et al. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med. 2010;207(13):2907–19. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9(1):78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202(6):761–69. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J Immunol. 2005;175(2):788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 91.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat Med. 2008;14(5):558–64. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 92.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 93.Wozniak TM, Ryan AA, Britton WJ. Interleukin-23 restores immunity to Mycobacterium tuberculosis infection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J Immunol. 2006;177(12):8684–92. doi: 10.4049/jimmunol.177.12.8684. [DOI] [PubMed] [Google Scholar]
- 94.Wozniak TM, Ryan AA, Triccas JA, Britton WJ. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect Immun. 2006;74(1):557–65. doi: 10.1128/IAI.74.1.557-565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koch R. A further communication on a remedy for tuberculosis. Br Med J. 1891;1(1568):125–27. doi: 10.1136/bmj.1.1568.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207(8):1609–16. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, et al. Interleukin-23-mediated inflammation in Pseudomonas aeruginosa pulmonary infection. Infect Immun. 2012;80(1):398–409. doi: 10.1128/IAI.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186(3):1666–74. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, et al. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35(6):997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9(2):158–65. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, et al. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharideheterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181(7):4965–75. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown DM, Lee S, de Garcia-Hernandez ML, Swain SL. Multifunctional CD4 cells expressing γ interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86(12):6792–803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301–10. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, et al. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179(1):248–58. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190(3):624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 107.Gaffen SL, Hernandez-Santos N, Peterson AC. IL-17 signaling in host defense against Candida albicans. Immunol Res. 2011;50(2–3):181–87. doi: 10.1007/s12026-011-8226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hardison SE, Wozniak KL, Kolls JK, Wormley FL., Jr Interleukin-17 is not required for classical macrophage activation in a pulmonary mouse model of Cryptococcus neoformans infection. Infect Immun. 2010;78(12):5341–51. doi: 10.1128/IAI.00845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huffnagle GB, Lipscomb MF. Cells and cytokines in pulmonary cryptococcosis. Res Immunol. 1998;149(4–5):387–514. doi: 10.1016/s0923-2494(98)80762-1. [DOI] [PubMed] [Google Scholar]
- 110.Chai LY, van de Veerdonk F, Marijnissen RJ, Cheng SC, Khoo AL, et al. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130(1):46–54. doi: 10.1111/j.1365-2567.2009.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, et al. Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.05.036. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37(10):2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 113.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75(6):3055–61. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Investig. 2011;121(2):554–68. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wuthrich M, Hung CY, Gern BH, Pick-Jacobs JC, Galles KJ, et al. A TCR transgenic mouse reactive with multiple systemic dimorphic fungi. J Immunol. 2011;187(3):1421–31. doi: 10.4049/jimmunol.1100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74(4):2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Investig. 2009;119(7):1899–909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary natural killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol. 2010;84(15):7750–59. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.49. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287(12):8816–29. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect Immun. 2012;80(1):410–17. doi: 10.1128/IAI.05939-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Myers AK, Perroni L, Costigan C, Reardon W. Clinical and molecular findings in IPEX syndrome. Arch Dis Child. 2006;91(1):63–64. doi: 10.1136/adc.2005.078287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 126.Crother TR, Schroder NW, Karlin J, Chen S, Shimada K, et al. Chlamydia pneumoniae infection induced allergic airway sensitization is controlled by regulatory T-cells and plasmacytoid dendritic cells. PLoS ONE. 2011;6(6):e20784. doi: 10.1371/journal.pone.0020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carrigan SO, Yang YJ, Issekutz T, Forward N, Hoskin D, et al. Depletion of natural CD4+CD25+ T regulatory cells with anti-CD25 antibody does not change the course of Pseudomonas aeruginosa-induced acute lung infection in mice. Immunobiology. 2009;214(3):211–22. doi: 10.1016/j.imbio.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 128.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173(7):803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 129.Quinn KM, McHugh RS, Rich FJ, Goldsack LM, de Lisle GW, et al. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol Cell Biol. 2006;84(5):467–74. doi: 10.1111/j.1440-1711.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- 130.Quinn KM, Rich FJ, Goldsack LM, de Lisle GW, Buddle BM, et al. Accelerating the secondary immune response by inactivating CD4+CD25+ T regulatory cells prior to BCG vaccination does not enhance protection against tuberculosis. Eur J Immunol. 2008;38(3):695–705. doi: 10.1002/eji.200737888. [DOI] [PubMed] [Google Scholar]
- 131.Jaron B, Maranghi E, Leclerc C, Majlessi L. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PLoS ONE. 2008;3(7):e2833. doi: 10.1371/journal.pone.0002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paula MO, Fonseca DM, Wowk PF, Gembre AF, Fedatto PF, et al. Host genetic background affects regulatory T-cell activity that influences the magnitude of cellular immune response against Mycobacterium tuberculosis. Immunol Cell Biol. 2011;89(4):526–34. doi: 10.1038/icb.2010.116. [DOI] [PubMed] [Google Scholar]
- 133.Betts RJ, Prabhu N, Ho AW, Lew FC, Hutchinson PE, et al. Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp3+ regulatory T cell response. J Virol. 2012;86(5):2817–25. doi: 10.1128/JVI.05685-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Betts RJ, Ho AW, Kemeny DM. Partial depletion of natural CD4+CD25+ regulatory T cells with anti-CD25 antibody does not alter the course of acute influenza A virus infection. PLoS ONE. 2011;6(11):e27849. doi: 10.1371/journal.pone.0027849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Piersma SJ, van der Hulst JM, Kwappenberg KM, Goedemans R, van der Minne CE, van der Burg SH. Influenza matrix 1-specific human CD4+ FOXP3+ and FOXP3− regulatory T cells can be detected long after viral clearance. Eur J Immunol. 2010;40(11):3064–74. doi: 10.1002/eji.200940177. [DOI] [PubMed] [Google Scholar]
- 136.Antunes I, Kassiotis G. Suppression of innate immune pathology by regulatory T cells during Influenza A virus infection of immunodeficient mice. J Virol. 2010;84(24):12564–75. doi: 10.1128/JVI.01559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fulton RB, Meyerholz DK, Varga SM. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol. 2010;185(4):2382–92. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruckwardt TJ, Bonaparte KL, Nason MC, Graham BS. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J Virol. 2009;83(7):3019–28. doi: 10.1128/JVI.00036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Loures FV, Pina A, Felonato M, Araujo EF, Leite KR, et al. Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells. Infect Immun. 2010;78(3):1078–88. doi: 10.1128/IAI.01198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32(5):1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 141.McKinley L, Logar AJ, McAllister F, Zheng M, Steele C, et al. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J Immunol. 2006;177(9):6215–26. doi: 10.4049/jimmunol.177.9.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Worsley CM, Suchard MS, Stevens WS, Van Rie A, Murdoch DM. Multi-analyte profiling of ten cytokines in South African HIV-infected patients with Immune Reconstitution Inflammatory Syndrome (IRIS) AIDS Res Ther. 2010;7:36. doi: 10.1186/1742-6405-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schulze Zur Wiesch J, Thomssen A, Hartjen P, Toth I, Lehmann C, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. 2011;85(3):1287–97. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spolski R, Wang L, Wan CK, Bonville CA, Domachowske JB, et al. IL-21 promotes the pathologic immune response to pneumovirus infection. J Immunol. 2012;188(4):1924–32. doi: 10.4049/jimmunol.1100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boyden AW, Legge KL, Waldschmidt TJ. Pulmonary infection with influenza a virus induces site-specific germinal center and T follicular helper cell responses. PLoS ONE. 2012;7(7):e40733. doi: 10.1371/journal.pone.0040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36(5):847–56. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35(6):919–31. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang J, Fisher EM, Murasko DM. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res Rev. 2011;10(4):422–27. doi: 10.1016/j.arr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peiris JS, Hui KP, Yen HL. Host response to influenza virus: protection versus immunopathology. Curr Opin Immunol. 2010;22(4):475–81. doi: 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188(2):223–32. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8(1):54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 153.Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol. 2007;179(8):5415–24. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- 154.Woodworth JS, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev Immunol. 2006;26(4):317–52. doi: 10.1615/critrevimmunol.v26.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weber SE, Tian H, Pirofski LA. CD8+ cells enhance resistance to pulmonary serotype 3 Streptococcus pneumoniae infection in mice. J Immunol. 2011;186(1):432–42. doi: 10.4049/jimmunol.1001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gohil SK, Heo M, Schoenbaum EE, Celentano D, Pirofski LA. CD8+ T cells and risk for bacterial pneumonia and all-cause mortality among HIV-infected women. J Acquir Immune Defic Syndr. 2012;60(2):191–98. doi: 10.1097/QAI.0b013e31824d90fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Templeton SP, Buskirk AD, Law B, Green BJ, Beezhold DH. Role of germination in murine airway CD8+ T-cell responses to Aspergillus conidia. PLoS ONE. 2011;6(4):e18777. doi: 10.1371/journal.pone.0018777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chaudhary N, Staab JF, Marr KA. Healthy human T-cell responses to Aspergillus fumigatus antigens. PLoS ONE. 2010;5(2):e9036. doi: 10.1371/journal.pone.0009036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carvalho A, De Luca A, Bozza S, Cunha C, D’Angelo C, et al. TLR3 essentially promotes protective class I-restricted memory CD8+ T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood. 2012;119(4):967–77. doi: 10.1182/blood-2011-06-362582. [DOI] [PubMed] [Google Scholar]
- 160.McAllister F, Steele C, Zheng M, Young E, Shellito JE, et al. T cytotoxic-1 CD8+ T cells are effector cells against pneumocystis in mice. J Immunol. 2004;172(2):1132–38. doi: 10.4049/jimmunol.172.2.1132. [DOI] [PubMed] [Google Scholar]
- 161.Meissner NN, Lund FE, Han S, Harmsen A. CD8 T cell-mediated lung damage in response to the extracellular pathogen pneumocystis is dependent on MHC class I expression by radiation-resistant lung cells. J Immunol. 2005;175(12):8271–79. doi: 10.4049/jimmunol.175.12.8271. [DOI] [PubMed] [Google Scholar]
- 162.Hamada H, de Garcia-Hernandez ML, Reome JB, Misra SK, Strutt TM, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182(6):3469–81. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ, Klein BS. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog. 2012;8(7):e1002771. doi: 10.1371/journal.ppat.1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moore TA, Moore BB, Newstead MW, Standiford TJ. γδ-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165(5):2643–50. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 165.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδT cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177(7):4662–69. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 166.Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, et al. Accumulation of γ/δ T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9(3):251–58. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 167.Harding M, Kubes P. Innate immunity in the vasculature: interactions with pathogenic bacteria. Curr Opin Microbiol. 2012;15(1):85–91. doi: 10.1016/j.mib.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 168.Paget C, Ivanov S, Fontaine J, Blanc F, Pichavant M, et al. Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J Immunol. 2011;186(10):5590–602. doi: 10.4049/jimmunol.1002348. [DOI] [PubMed] [Google Scholar]
- 169.Qin G, Liu Y, Zheng J, Ng IH, Xiang Z, et al. Type 1 responses of human Vγ9Vδ2 T cells to influenza A viruses. J Virol. 2011;85(19):10109–16. doi: 10.1128/JVI.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Monticelli LA, Sonnenberg GF, Artis D. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Curr Opin Immunol. 2012;24(3):284–89. doi: 10.1016/j.coi.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, et al. Activation of Vα14+ natural killer T cells by α-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001;69(1):213–20. doi: 10.1128/IAI.69.1.213-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Uezu K, Kawakami K, Miyagi K, Kinjo Y, Kinjo T, et al. Accumulation of γδT cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol. 2004;172(12):7629–34. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 173.Steele C, Zheng M, Young E, Marrero L, Shellito JE, et al. Increased host resistance against Pneumocystis carinii pneumonia in γδ T-cell-deficient mice: protective role of γ interferon and CD8+ T cells. Infect Immun. 2002;70(9):5208–15. doi: 10.1128/IAI.70.9.5208-5215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blyth CC, Webb SA, Kok J, Dwyer DE, van Hal SJ. The impact of bacterial and viral co-infection in severe influenza. Influenza Other Respir Viruses. 2012 doi: 10.1111/j.1750-2659.2012.00360.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Seki M, Yanagihara K, Higashiyama Y, Fukuda Y, Kaneko Y, et al. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur Respir J. 2004;24(1):143–49. doi: 10.1183/09031936.04.00126103. [DOI] [PubMed] [Google Scholar]
- 176.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Investig. 2009;119(7):1910–20. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hishiki H, Ishiwada N, Fukasawa C, Abe K, Hoshino T, et al. Incidence of bacterial coinfection with respiratory syncytial virus bronchopulmonary infection in pediatric inpatients. J Infect Chemother. 2011;17(1):87–90. doi: 10.1007/s10156-010-0097-x. [DOI] [PubMed] [Google Scholar]
- 178.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respirat Crit Care Med. 2006;173(10):1114–21. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 179.Pons J, Sauleda J, Ferrer JM, Barcelo B, Fuster A, et al. Blunted γδ T-lymphocyte response in chronic obstructive pulmonary disease. Eur Respir J. 2005;25(3):441–46. doi: 10.1183/09031936.05.00069304. [DOI] [PubMed] [Google Scholar]
- 180.Knobloch J, Schild K, Jungck D, Urban K, Muller K, et al. The T-helper cell type 1 immune response to gram-negative bacterial infections is impaired in COPD. Am J Respirat Crit Care Med. 2011;183(2):204–14. doi: 10.1164/rccm.201002-0199OC. [DOI] [PubMed] [Google Scholar]
- 181.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, et al. Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci Transl Med. 2012;4(117):117ra9. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(2):115–20. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 183.Shi L, Manthei DM, Guadarrama AG, Lenertz LY, Denlinger LC. Rhinovirus-induced IL-1β release from bronchial epithelial cells is independent of functional P2X7. Am J Respir Cell Mol Biol. 2012;47(3):363–71. doi: 10.1165/rcmb.2011-0267OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wiehler S, Proud D. Interleukin-17A modulates human airway epithelial responses to human rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L505–15. doi: 10.1152/ajplung.00066.2007. [DOI] [PubMed] [Google Scholar]
- 185.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-α and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175(1):404–12. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, et al. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184(2):252–58. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dinwiddie R. Pathogenesis of lung disease in cystic fibrosis. Respir Int Rev Thorac Dis. 2000;67(1):3–8. doi: 10.1159/000029453. [DOI] [PubMed] [Google Scholar]
- 188.Painter RG, Bonvillain RW, Valentine VG, Lombard GA, LaPlace SG, et al. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol. 2008;83(6):1345–53. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dubin PJ, Kolls JK. IL-17 in cystic fibrosis: more than just Th17 cells. Am J Respir Crit Care Med. 2011;184(2):155–57. doi: 10.1164/rccm.201104-0617ED. [DOI] [PubMed] [Google Scholar]
- 190.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487(7405):109–13. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kreindler JL, Bertrand CA, Lee RJ, Karasic T, Aujla S, et al. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296(2):L257–66. doi: 10.1152/ajplung.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42(2):364–73. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012;24(3):297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]