The C. elegans CSR-1 Argonaute pathway counteracts epigenetic silencing to promote germline gene expression (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 23.
SUMMARY
Organisms can develop adaptive sequence-specific immunity by re-expressing pathogen-specific small RNAs that guide gene silencing. For example, the C. elegans PIWI-Argonaute/piRNA pathway recruits RNA-dependent RNA polymerase RdRP to foreign sequences to amplify a trans-generational small RNA-induced epigenetic silencing signal (termed RNAe). Here we provide evidence that in addition to an adaptive memory of silenced sequences, C. elegans can also develop an opposing adaptive memory of expressed/self mRNAs. We refer to this mechanism, which can prevent or reverse RNAe as RNA-induced epigenetic gene activation (RNAa). We show that CSR-1, which engages RdRP-amplified small RNAs complementary to germline-expressed mRNAs, is required for RNAa. We show that a transgene with RNAa activity also exhibits accumulation of cognate CSR-1 small RNAs. Our findings suggest that C. elegans adaptively acquires and maintains a trans-generational CSR-1 memory that recognizes and protects self mRNAs, allowing piRNAs to recognize foreign sequences innately, without need for prior exposure.
INTRODUCTION
Epigenetics is often defined as the stable transmission of gene expression programs through mitotic or meiotic cell division without alteration in the DNA sequence (Bird, 2007). In eukaryotic cells epigenetic inheritance can be driven by covalent modifications to chromatin, often referred to as chromatin marks or simply epigenetic marks (Grewal and Elgin, 2007; Henderson and Jacobsen, 2007; Lippman and Martienssen, 2004; Strome and Lehmann, 2007).
An emerging theme in epigenetic regulation is the frequent involvement of non-coding RNAs (Daxinger and Whitelaw, 2012; Grewal and Elgin, 2007; Henderson and Jacobsen, 2007; Lessing and Lee, 2013; Lim and Brunet, 2013). In many organisms, epigenetic silencing has been linked to RNAi-related mechanisms, which involve small non-coding RNAs termed short-interfering (si) RNAs (see Ghildiyal and Zamore, 2009). Interestingly, the best-studied examples of RNAi-related epigenetic silencing also involve chromatin marks and their associated enzymatic mediators (Grewal and Elgin, 2007; Lippman and Martienssen, 2004), suggesting that RNAi and chromatin-modifying mechanisms reinforce and synergize with each other. Whereas the propagation of chromatin marks occurs in cis, RNAi can propagate in trans, allowing coordinate regulation of alleles on sister chromatids or of whole gene families such as transposons dispersed throughout the genome.
The core effectors of all RNAi-related pathways are Argonaute proteins. Argonautes present their guide RNAs for base pairing with target sequences and, upon binding, can cleave the target RNA and/or recruit cofactors that mediate post-transcriptional or transcriptional silencing (Ghildiyal and Zamore, 2009; Kuhn and Joshua-Tor, 2013). Although much less common, there are several examples of small-RNA pathways that appear to activate gene expression. For example, studies in human cultured cells have implicated small RNAs and/or Argonautes in gene activation, a phenomenon referred to as RNAa (Janowski et al., 2007; Li et al., 2006; Place et al., 2008). In these examples, targeting is thought to occur within the promoter region of the gene, perhaps acting on nascent promoter-derived transcripts, and is correlated with the induction of chromatin marks characteristic of gene activation. In plants small dsRNAs have been implicated in the activation of the Petunia pMADS3 homeotic gene and are thought to act by promoting DNA-methylation at a CpG site within an intronic cis-promoter element (Shibuya et al., 2009).
Two major groups of Argonaute proteins, the AGO proteins and the PIWI proteins, are encoded by animal genomes. PIWI Argonautes are expressed abundantly in the germline where they engage small-RNA species termed piwi-interacting (pi) RNAs (for review, see Juliano et al., 2011). In C. elegans, the PIWI Argonaute PRG-1 engages over 30,000 distinct genomically-encoded piRNA species (Batista et al., 2008; Das et al., 2008; Gu et al., 2012). Recent studies have shown that PRG-1 initiates silencing of transgenes containing foreign, non-C. elegans sequences (Shirayama et al., 2012), and suggest that it does so while allowing imperfect base pairing with target sequences (Bagijn et al., 2012; Lee et al., 2012; Shirayama et al., 2012). Upon recognition of foreign sequences PRG-1 is thought to recruit a cellular RNA-dependent RNA polymerase (RdRP), which in turn amplifies the silencing signal by producing antisense siRNAs perfectly complementary to the foreign sequences. These amplified siRNAs are loaded onto members of an expanded clade of worm-specific Argonautes (termed WAGO Argonautes), which are implicated in both cytoplasmic and nuclear gene silencing (Buckley et al., 2012; Gu et al., 2009; Guang et al., 2008; Yigit et al., 2006). The result is a remarkably stable mode of epigenetic silencing, termed RNA-induced epigenetic silencing (RNAe) (Shirayama et al., 2012). Alleles that are silenced by RNAe send trans-acting Argonaute-small-RNA signals that act in a sequence-specific manner to induce the permanent trans-generational silencing of their targets (Shirayama et al., 2012). The maintenance of RNAe requires chromatin factors, including heterochromatin protein 1 (HP1) and multiple histone methyltransferases (Ashe et al., 2012; Luteijn et al., 2012; Shirayama et al., 2012). Given the high numbers and the sequence diversity of C. elegans piRNAs, the allowance of two or three mismatches during target recognition should suffice, in principle, for piRNA to bind virtually any foreign RNA sequence. However, piRNAs should also recognize endogenous RNAs and therefore the piRNA surveillance model requires that “self” RNA be protected from RNAe (Shirayama et al., 2012).
The CSR-1 Argonaute engages antisense siRNAs complementary to the majority (perhaps all) endogenous germline-expressed genes (Claycomb et al., 2009; Gu et al., 2009). This finding, and the fact that its targets do not appear to exhibit CSR-1-dependent silencing, make this Argonaute a candidate for a self-RNA recognition factor. Paradoxically, however, CSR-1 protein has been shown to exhibit slicer activity in vitro (Aoki et al., 2007), and csr-1 mutants are partially deficient in dsRNA induced silencing (Claycomb et al., 2009; Yigit et al., 2006). The siRNAs that engage CSR-1, like those that engage WAGO Argonautes, are RdRP products. C. elegans RdRP products are often referred to as 22G-RNAs because they exhibit a predominant length of 22 nucleotides and a strong bias for a 5′ guanosine.
Evidence for a trans-activating signal that can counteract RNAe was discovered in crosses between an RNAe transgene and homologous actively expressed transgenes (Shirayama et al., 2012). Because this process involves the epigenetically transmitted, RNA-induced trans-activation of a silent allele (see below), we refer to the phenomenon as “RNAa” for RNA-induced epigenetic gene activation. Transgene alleles that are capable of sending the activating signal are designated as RNAa alleles; for example, oma-1::gfp(RNAa).
Here we show that CSR-1 is required for RNAa and that the ability of a foreign sequence to direct transactivation is correlated with acquisition of CSR-1-associated small RNAs antisense to the foreign sequence. In contrast to previously studied RNAa phenomena, the CSR-1-associated activating small-RNAs target sequences present in the mature mRNA rather than promoter or intron sequences. We show that propagation of an RNAe and an RNAa allele together for multiple generations results in a gradual transfer of a stable, expressed state to the formerly silent transgene. Finally, consistent with the idea that RNAa counteracts PRG-1 recognition, we show that re-silencing of a trans-activated RNAe allele depends on PRG-1 activity. Our findings suggest that CSR-1 small RNAs constitute a memory of previous germline-gene expression that protects endogenous genes from piRNA recognition. This self-memory system allows foreign sequences to be recognized innately without the need for prior exposure. Taken together, these findings and previous work on RNAe suggest that the C. elegans germline employs Argonaute-small-RNA complexes as trans-generational binary signals that program and reinforce the ON/OFF expression state for thousands of germline genes.
RESULTS
CSR-1 is required for RNAa
As a first test of whether trans-activation depends on CSR-1 activity, we crossed oma-1::gfp(RNAa) to gfp::cdk-1(RNAe) and exposed newly hatched F1 cross progeny to either csr-1(RNAi) by feeding, or to a control RNAi. Since OMA-1::GFP is expressed uniformly in oocyte cytoplasm (Lin, 2003), transactivation in this assay is evidenced by accumulation of the nuclear GFP::CDK-1 gene product (as shown in Figure 1A, B and C). When cross progeny were exposed to a control RNAi directed against sel-1, an abundant germline gene with a function unrelated to small RNA pathways, we found that 100% (n=66) of the F1s exhibited trans-activation of gfp::cdk-1(RNAe) (Figure S1A). In contrast, we found that 0% (n=80) of F1s exposed to csr-1(RNAi) exhibited GFP::CDK-1 nuclear expression (Figure S1A). These findings suggest that CSR-1 activity is required in the zygote for transactivation of an RNAe allele.
Figure 1. CSR-1 is required for RNAa.
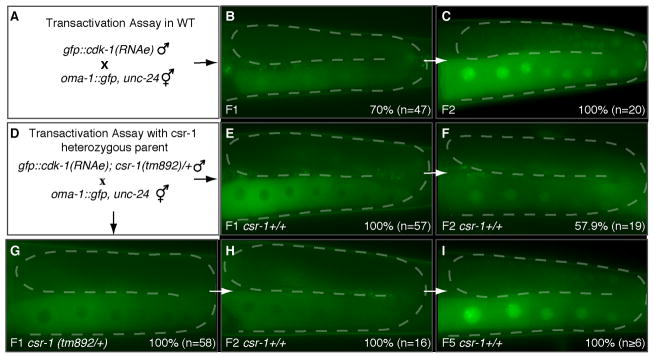
(A and D) Schematic diagrams of crosses between silenced (RNAe) and licensed (RNAa) GFP transgenic strains as indicated. B, C, E–I, Epifluorescence images of representative germlines (outlined with dashes) in first (F1) and subsequent (F2, F3, F5) generations. The cytoplasmic fluorescence signal is OMA-1::GFP; the nuclear signal is GFP::CDK-1. The percentages indicate the number of animals exhibiting the shown phenotype in this and the subsequent figures. See also Figure S1.
We next wished to explore the consequences of reducing the dose of csr-1 activity. To do this we conducted the transactivation assay using heterozygous csr-1(tm892) null mutant animals (Figure 1D), which exhibit wild-type fertility. Interestingly, we found that trans-activation failed to occur when either transgenic parent was heterozygous for csr-1(tm892) (Figure 1E and G; Figure S1A and B). We found that 100% of the F1 cross progeny failed to activate gfp::cdk-1(RNAe) when the csr-1(tm892) mutant was introduced from the father (n=115) or from the mother (_n=_15). This parental effect indicates that zygotic expression of CSR-1, although necessary, as suggested by the RNAi studies above, is not sufficient for trans-activation: Even F1 progeny homozygous for wild-type csr-1(+) activity failed to exhibit trans-activation if either parent was heterozygous for csr-1(tm892) (Figure 1E). As expected, when F1 wild-type csr-1(+) hermaphrodites were allowed to self cross, we observed trans-activation in the germlines of their F2 progeny (57.9%, n=19; Figure 1F). In contrast, heterozygous csr-1(tm892) hermaphrodites produced self progeny that failed to exhibit trans-activation (0%, n=16; Figure 1H) and transactivation was only restored among their wild-type progeny in subsequent generations (100%, n≥6; Figure 1I).
RNAa activity correlates with the accumulation of CSR-1 22G-RNAs
A previous study indicated that 22G-RNAs targeting cdk-1::gfp, a neutral transgene that is expressed but sensitive to silencing via RNAe, are present at very low levels, much lower for example than the level of CSR-1 22G-RNAs targeting the endogenous cdk-1 portion of the transgene (Shirayama et al., 2012). The genetic analysis of RNAa described above suggest that transactivation of an RNAe allele is acutely sensitive to the dose of CSR-1 activity. We therefore wondered if small RNAs targeting GFP in the oma-1::gfp(RNAa) strain might be enriched to levels similar to an endogenous germline-expressed gene and whether they depend on CSR-1 activity. To explore this possibility, we first analyzed total small-RNA levels targeting oma-1::gfp in wild-type animals and in mutants defective in RNAa, csr-1(tm892), or defective in RNAe, rde-3(ne3370). In wild-type and rde-3 mutant animals, we found that 22G-RNAs targeting gfp exhibited levels similar to 22G-RNAs targeting oma-1 itself (Figure 2B and 2C). Conversely, and consistent with the idea that these _gfp_-targeted 22G-RNAs are in the CSR-1 pathway, we found that small RNAs targeting gfp were reduced by 73% in csr-1(tm892) mutants, a reduction similar to that observed for small RNAs targeting oma-1 and other germline-expressed RNAs (Figure 2D and data not shown).
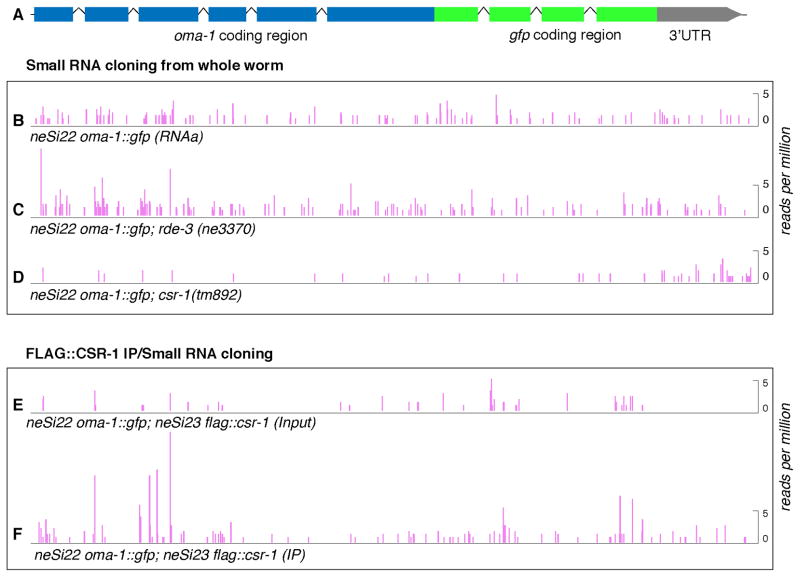
Figure 2. CSR-1-associated small RNAs targeting GFP in neSi22 oma-1::gfp(RNAa).
(A) Schematic of oma-1::gfp transgene. The exon-intron structure is indicated with boxes and lines, respectively. (B–F) Plots showing the density of antisense small RNAs mapping along oma-1::gfp in wild-type (B) and mutant strains rde-3 (C) and csr-1(D). In (E and F) the histograms show read densities of small RNAs obtained from the same lysate before (Input) and after FLAG::CSR-1 Immunoprecipitation (IP). The height of each peak corresponds to the number of RNA reads that begin at that position per million total reads. See also Figure S2.
We next examined the physical association of _gfp_-directed 22G-RNAs by sequencing RNAs recovered in Argonaute protein immunoprecipitation (IP) complexes. To do this we conducted IP assays using epitope-tagged Argonaute proteins, FLAG::CSR-1 and FLAG::WAGO-9/HRDE-1 (Ashe et al., 2012; Buckley et al., 2012; Shirayama et al., 2012). Consistent with their genetic dependence on csr-1, we found that 22G-RNAs antisense to gfp were enriched (3.14-fold) in the FLAG::CSR-1 IP from oma-1::gfp transgenic animals (Figure 2E and F), and were not enriched in the FLAG::WAGO-9/HRDE-1 IP (Figure S2A and B). For comparison we also performed IP studies in a gfp::cdk-1(RNAe) strain. As expected, we found a reciprocal relationship in this silent strain; 22G-RNAs targeting gfp were depleted (3.35-fold) in the FLAG::CSR-1 IP relative to input (Figure S2C and E), and were enriched (1.75-fold) in the FLAG::WAGO-9/HRDE-1 IP (Figure S2D).
Thus we have shown that in three small-RNA Seq libraries independently prepared from csr-1(+) animals, 22G-RNAs targeting gfp were present at levels similar to CSR-1 22G-RNAs targeting the _oma-1-_derived portion of the RNAa transgene. Furthermore, we have shown that these gfp 22G-RNAs were depleted in csr-1 mutants and were enriched in the CSR-1 IP. In contrast, an RNAe transgenic strain exhibited gfp 22G-RNAs that were enriched in the WAGO-9 IP and were depleted in the CSR-1 IP. Finally, a strain with a neutral transgene (sensitive to RNAe) exhibited very low levels of gfp 22G-RNAs relative to the levels of CSR-1 22G-RNA targeting the endogenously-derived portion of the transgene (Shirayama et al., 2012). Taken together, these findings indicate that the RNAa activity of oma-1::gfp correlates with the accumulation CSR-1 22G-RNAs targeting the foreign, gfp, sequences of the transgene.
Multi-generational exposure to RNAa can gradually license an RNAe allele
The above findings indicate that C. elegans transgenes can adopt at least three different states: i) a dominant-acting trans-silencing state (RNAe); ii) a neutral, expressed state that is sensitive to trans-silencing; and (iii) a dominant trans-activating state (RNAa). Previous studies have shown that an RNAe allele can transfer the silent state to a neutral allele. We therefore wished to know whether transient exposure to an RNAa allele could stably activate (or license) the expression of an RNAe allele. To explore this possibility, we set up a series of crosses between an RNAa transgene and a number of distinct RNAe transgenes. After establishing the double transgenic lines, we outcrossed the strains to wild-type to separate the two transgenes again and then monitored expression and RNAa or RNAe status. We found that different transgenes behaved differently in these crosses. For example, gfp::cdk-1(RNAe) was activated in the presence of oma-1::gfp(RNAa) (Figure 3A), but was immediately silenced after crossing away the RNAa transgene (Figure 3B) (Shirayama et al., 2012). In contrast, a cdk-1::gfp(RNAe) allele remained stably expressed after transient exposure to the RNAa transgene (Shirayama et al., 2012). Finally, a gfp::csr-1(RNAe) transgene was never activated upon exposure to oma-1::gfp(RNAa). Instead, each allele maintained its expression status in the double homozygote – silent gfp::csr-1(RNAe) and active oma-1::gfp(RNAa) (data not shown).
Figure 3. RNAa counteracts Piwi-dependent silencing and acts over multiple generations to establish an active epigenetic gene-expression state.
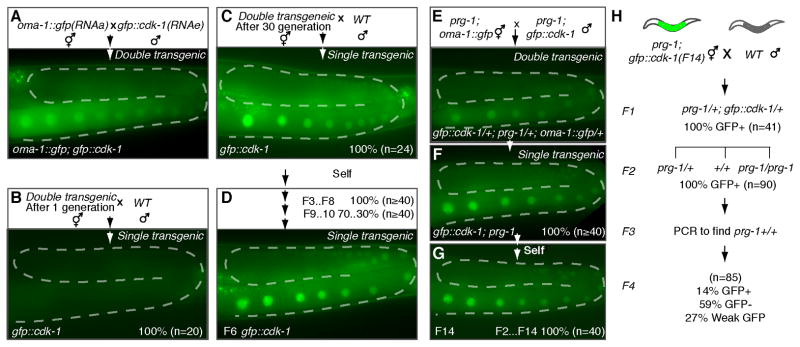
A–H, Genetic crosses with corresponding epifluorescence images showing representative germlines of resulting progeny. The percentages of animals expressing gfp::cdk-1 (nuclear GFP signal) at each generation and the number of animals scored ‘n’ are indicated. A–D, Analysis of RNAa exposure on the durability of gene activation in wild-type animals. Newly trans-activated F2 double transgenic animals (A), were outcrossed to wild-type (WT), either immediately or after propagating as a double transgenic strain for 30 generations (F30), to obtain gfp::cdk-1 “single transgenic” animals shown in B and C, respectively. Siblings of animals shown in (C) were allowed to produce self progeny (D) for multiple generations, and GFP fluorescence was scored in each generation as indicated. E–H, Analysis of the genetic influence of Piwi (prg-1) on transactivation. RNAa and RNAe transgenes established in a wild-type background were crossed into prg-1 prior to conducting the trans-activation assay shown in (E). After one generation, oma-1::gfp was segregated away to yield gfp::cdk-1 single transgenic animals assayed in (F). Siblings of animals shown in (F) were allowed to produce self progeny for multiple generations and GFP fluorescence was scored in each generation (G) as indicated. In (H) _gfp::cdk-1_was outcrossed from the prg-1(tm872) mutant background and the animals were scored for GFP in subsequent generations as indicated.
We next wanted to explore whether prolonged exposure to RNAa could influence the tendency of gfp::cdk-1 to revert back to an RNAe status. Consistent with this idea, after propagating the oma::gfp; gfp::cdk-1 double transgenic strain for 10 generations and then outcrossing to wild-type to separate the two transgenes, we found that the gfp::cdk-1(+) transgene remained expressed for one full generation after separation before re-silencing. Interestingly, the period of sustained expression increased to nearly 10 generations when gfp::cdk-1 and oma-1::gfp(RNAa) were separated after 30 generations of co-propagation (Figure 3C and D). However we found that, even though expression of the formerly RNAe transgene was stabilized by long-term exposure to RNAa, the RNAa status was not transferred. Instead, the activated transgene remained sensitive to silencing when exposed through a genetic cross to gfp::csr-1(RNAe) (100%, n=24). Taken together these findings suggest that an RNAa transgene can, over time, influence the epigenetic stability of an RNAe allele. However, the transfer of RNAa status is either very slow or depends on other factors that remain to be identified.
RNAa counteracts PRG-1-dependent silencing
The PIWI Argonaute PRG-1 is required for the initiation of RNAe, but not for the maintenance of silencing (Shirayama et al., 2012). We therefore wondered whether PRG-1 activity is required to re-initiate silencing of an RNAe transgene after trans-activation. To test this possibility, we first crossed the gfp::cdk-1(RNAe) and oma-1:gfp(RNAa) transgenes into the prg-1(tm872) mutant background. As expected, we found that each transgene, singly, maintained its silent or active expression state in the prg-1 mutant background. We then repeated the trans-activation crosses by mating these prg-1 mutant strains (Figure 3E). As observed in the wild-type prg-1(+) background, the gfp::cdk-1(RNAe) transgene was activated in the F1 cross progeny (Figure 3E). We then allowed the two transgenes to segregate from one another. Strikingly, we found that 100% of the F2 through F14 gfp::cdk-1 transgenic animals examined maintained expression in the absence of the oma-1::gfp(RNAa) transgene (Figure 3F and G). Thus in the absence of prg-1 activity the RNAa allele is not required to maintain the activated status of the formerly RNAe transgene. We next crossed these actively expressing prg-1 mutant transgenic animals to wild-type to restore prg-1 activity. We found that, once homozygous for prg-1(+) activity, 85% of the animals examined (n=85) exhibited re-silencing of the transgene by the F4 generation (Figure 3H). These findings indicate that prg-1 is required to re-initiate silencing on an RNAe transgene, and that RNAa opposes this PRG-1 silencing activity.
DISCUSSION
A genome-wide mechanism for the epigenetic adaptation of gene expression
The term epigenetics is used to describe many diverse types of biological events, ranging from the activity of prions (Halfmann and Lindquist, 2010), to the transmission of heritable membrane structures (Harold, 2005), and extending even to cellular differentiation events (Goldberg et al., 2007). In a recent review, Adrian Bird suggested a compelling definition for chromatin-focused epigenetic events as “the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states” (Bird, 2007). A key element of this definition is that epigenetic chromatin marks are seen as responsive and adaptive: they help to canalize and buffer gene expression programs that may have more direct upstream triggers. Our findings are consistent with this adaptive view of epigenetic programming. They suggest how Argonaute small RNA pathways can work in concert with chromatin pathways to create heritable binary signals that communicate a memory of germline gene-expression from one generation to the next. In this system, small RNAs can both perpetuate expression states in cis and signal adapted gene-expression states to dispersed alleles of a gene.
While the work in this paper is focused on the role of Argonaute small RNA pathways in the control of transgene expression states, the same Argonaute pathways also act globally in the germline to target expressed (CSR-1-targeted) and silenced (WAGO-targeted) genes genome wide. A parallel paper by Conine et al. (Cell In Press) shows that CSR-1 is required to promote the expression of many male-specific germline genes. In the absence of paternal CSR-1 activity, males are initially fertile, but progressively become sterile over a period of 5 to 6 generations. This “germline mortal” phenotype is consistent with previous work on the loss of specific Argonaute-silencing pathways (Buckley et al., 2012) and may reflect a gradual loss of the “adapted” epigenetic state after the reinforcing activities of the small RNA pathways are lost.
Studies on prg-1 mutants suggest that the default state for transgene expression is “ON”. Therefore a simple model for the CSR-1 pathway is that it prevents the incursion of silencing signals within its targeted sequence domain (Model, Figure 4). It is possible that CSR-1 prevents PRG-1 and WAGO silencing by using its slicer activity to destroy template RNAs engaged in RdRP transcription and WAGO loading. Understanding the mechanistic details of RNAa will require further exploration of how chromatin and small RNA pathways change as alleles switch from a silenced to expressed status, and will also require new tools for directly intervening in the feed-forward Argonaute and chromatin pathways. A recent study describes one such tool, a tethering system that recruits CSR-1 to target sequences through direct RNA binding, thus activating an RNAe allele without the need for a transactivating allele and its cognate small RNAs (Wedeles and Claycomb, in this issue).
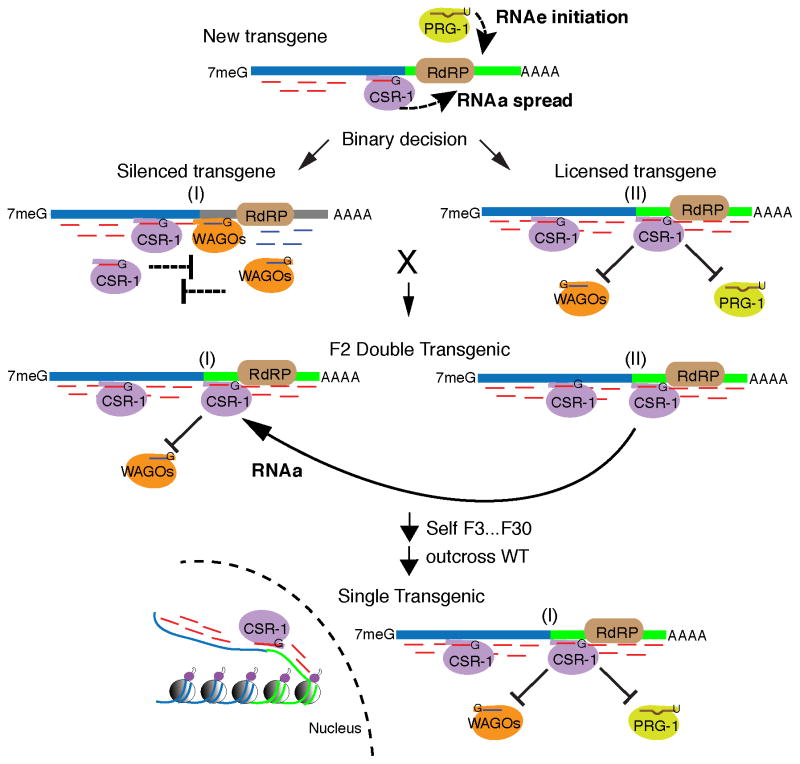
Figure 4. Model for transactivation by CSR-1.
See Discussion for details.
An innate sequence-specific genome-defense mechanism
The findings described here support a model for genome defense that employs a truly surprising strategy – one that permits a rapid “innate” and yet sequence-specific response without the need for prior exposure to a pathogenic sequence or for structural triggers of pathogen-specific activity such as the expression of long dsRNA. Instead, our findings suggest that the recognition of foreign sequences in C. elegans depends directly on the Piwi pathway, which scans for foreign sequences (Ashe et al., 2012; Lee et al., 2012; Shirayama et al., 2012), and indirectly on the CSR-1 pathway, which protects endogenous germline-expressed genes from piRNA-mediated recognition. Thus sequence specificity is achieved, not by capturing and remembering foreign sequences as in some systems (Khurana et al, 2011; Sorek et al., 2008), but rather by remembering all self sequences, thereby permitting the innate recognition of foreign sequences (Model, Figure 4).
Under some circumstances foreign sequences appear to be adopted as self. One possible model for this adoption process is that CSR-1 recognition can spread, in cis, from fused endogenous sequences within a transgene (Model, Figure 4). Targeting by CSR-1 within the endogenous sequences could promote the local recruitment of RdRP, leading to the de novo synthesis of CSR-1 22G-RNAs within the adjacent foreign sequences. Molecular spreading of this type has been observed in gene silencing in both plants and animals (Axtell et al., 2006; Pak and Fire, 2007; Sijen et al., 2001; Sijen et al., 2007). The decision to silence or license a newly introduced transgene would then be determined through a competition between cis-spreading of CSR-1 recognition and initial recognition by the PRG-1/21U-RNA pathway (Model, Figure 4). For some transgenes, such as oma-1::gfp(RNAa), this process leads to the “adoption” of the foreign sequences (through acquisition of CSR-1 targeting) permitting these gfp sequences to trans-activate homologous transgenes (Model, Figure 4).
Conclusion
Epigenetic pathways are diverse and can differ widely from organism to organism. This is particularly true with Argonaute pathways, which exhibit evidence of extensive gene duplication and pathway diversification in both plants and animals (Cerutti and Casas-Mollano, 2006; Ghildiyal and Zamore, 2009). The rapid evolution of these pathways could reflect selective pressure exerted in response to their targets, which in most organisms include a striking genomic load of transposons. While the details may differ from one system to another, the concepts revealed in one organism will likely be relevant in other systems. For example, it is now clear that a dynamic interplay between Argonaute/small RNA pathways and chromatin modifiers is involved both in the silencing of repetitive gene families and in essential chromosome functions such as kinetochore assembly and chromosome segregation in organisms as diverse as fungi, plants and animals (Grewal and Elgin, 2007).
Here we have shown that C. elegans employs Argonautes to protect expressed genes from silencing. Interestingly, while the interaction between an RNAa allele and an RNAe allele resulted in a rapid reversal of the silenced state, conversion of the formerly silent allele to a state permissive of independent sustained gene expression required dozens of generations of continuous exposure to RNAa. This slow conversion of the RNAe allele is consistent with the adaptive definition of an epigenetic process (Bird, 2007) and could reflect a gradual elimination of either small RNAs or of chromatin marks that can stimulate re-silencing (or possibly a slow accumulation of chromatin marks that enforce expression). CSR-1 localizes on chromatin and immunoprecipitates with target DNA sequences (Claycomb et al., 2009). Thus CSR-1 could influence chromatin directly perhaps by engaging nascent transcripts at target genes. It will be interesting in the future to determine whether CSR-1 actively recruits chromatin modifiers to promote gene expression. Furthermore, CSR-1 and members of the WAGO family are abundantly expressed in both oocytes and mature sperm (Conine et al., submitted; Claycomb et al., 2009; Conine et al., 2010; Gu et al., 2009; Shirayama et al., 2012). Germline transmission of these Argonautes and their associated small RNAs may thus have genome-wide effects on epigenetic inheritance with potentially significant evolutionary implications.
Experimental Procedures
Genetics
The C. elegans strains used in this study (see Supplemental Experimental Procedures) were derived from the Bristol N2 strain and cultured as described (Brenner, 1974). Strain WM288 contains a single-copy oma-1::gfp transgene that was created using the MosSCI heat shock protocol combined with ivermectin selection as described previously (Shirayama et al., 2012).
Small RNA cloning and deep sequencing
Total RNA was extracted from 10 young adult worms (Shirayama et al., 2012). Small RNAs (18 – 40 nucleotides) were gel-purified, treated with TAP to generate monophosphate 5′ ends, ligated to 5′ and 3′ linkers and converted to cDNA (Gu et al., 2009; Shirayama et al., 2012). Illumina adapters were added by PCR (Gu et al., 2009; Shirayama et al., 2012). To clone CSR-1 associated small RNAs, M2 FLAG antibody (Sigma) was used to immunoprecipitate FLAG::CSR-1 from 20 mg of lysate from synchronous adult worms homogenized in a stainless steel dounce (Gu et al., 2009). Small RNAs were extracted from FLAG::CSR-1 immune complexes and processed for deep sequencing as described above. Libraries were sequenced in the UMass Medical School Deep Sequencing Core using an Illumina GAII instrument.
For AGO IP studies the relative enrichment was measured by calculating the (# of antisense GFP reads)/(total # of genome matching antisense reads) in the Input and the IP, and then dividing the two numbers.
Computational analysis
Deep sequencing data were processed and analyzed using Bowtie (version 0.12.7) (Langmead et al., 2009) and custom Perl scripts (Gu et al., 2009; Shirayama et al., 2012). Small RNA reads were mapped to WormBase WS215 and normalized to non-structural RNA reads or to the total number of small RNAs that map antisense to protein coding genes. CSR-1 small RNA targets were defined previously (Claycomb et al., 2009; Gu et al., 2009). All scripts are available upon request.
Microscopy
Transgenic worms expressing GFP were mounted on RITE-ON glass slides (Beckton Dickinson) in the presence of 0.2 mM levamisole. Epi-fluorescence and differential interference contrast (DIC) microscopy were performed using an Axioplan2 Microscope (Zeiss). Images were captured with an ORCA-ER digital camera (Hamamatsu) and Axiovision (Zeiss) software.
Supplementary Material
01
Highlights.
- C. elegans develops an adaptive memory of expressed/self mRNAs
- The CSR-1 Argonaute mediates RNA-induced epigenetic gene activation (RNAa)
- RNAa counteracts Piwi Argonaute-dependent epigenetic silencing (RNAe)
- Multi-generational exposure to RNAa adapts an RNAe allele for independent expression
Acknowledgments
We thank Ahmed Elewa, Colin Conine, the Mello lab and Victor Ambros for input and discussion, William Stanney III for technical assistance, Ellen Kittler and the UMass Deep Sequencing Core for assistance, and James Mello for critical reading of the manuscript. Some strains used in this study were obtained from the Caenorhabditis Genetic Center. M. Seth is a Howard Hughes Medical Institute International Student Research Fellow. C.C.M. is a Howard Hughes Medical Institute investigator and is supported by NIH grant GM058800.
Footnotes
ACCESSION NUMBERS
Illumina data are available from GEO under the accession number GSE49532.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki K, Moriguchi H, Yoshioka T, Okawa K, Tabara H. In vitro analyses of the production and activity of secondary small interfering RNAs in C. elegans. Embo J. 2007;26:5007–5019. doi: 10.1038/sj.emboj.7601910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, Jan C, Rajagopalan R, Bartel DP. A two-hit trigger for siRNA biogenesis in plants. Cell. 2006;127:565–577. doi: 10.1016/j.cell.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Bagijn MP, Goldstein LD, Sapetschnig A, Weick EM, Bouasker S, Lehrbach NJ, Simard MJ, Miska EA. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science. 2012;337:574–578. doi: 10.1126/science.1220952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, Kimble J, Fire A, Kennedy S. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489:447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti H, Casas-Mollano JA. On the origin and functions of RNA-mediated silencing: from protists to man. Curr Genet. 2006;50:81–99. doi: 10.1007/s00294-006-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, van Wolfswinkel JC, Chaves DA, Shirayama M, Mitani S, Ketting RF, et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139:123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine CC, Batista PJ, Gu W, Claycomb JM, Chaves DA, Shirayama M, Mello CC. Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107:3588–3593. doi: 10.1073/pnas.0911685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, et al. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol Cell. 2008;31:79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Grewal SI, Elgin SC. Transcription and RNA interference in the formation of heterochromatin. Nature. 2007;447:399–406. doi: 10.1038/nature05914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Lee HC, Chaves D, Youngman EM, Pazour GJ, Conte D, Jr, Mello CC. CapSeq and CIP-TAP identify Pol II start sites and reveal capped small RNAs as C. elegans piRNA precursors. Cell. 2012;151:1488–1500. doi: 10.1016/j.cell.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Shirayama M, Conte D, Jr, Vasale J, Batista PJ, Claycomb JM, Moresco JJ, Youngman EM, Keys J, Stoltz MJ, et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol Cell. 2009;36:231–244. doi: 10.1016/j.molcel.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang S, Bochner AF, Pavelec DM, Burkhart KB, Harding S, Lachowiec J, Kennedy S. An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science. 2008;321:537–541. doi: 10.1126/science.1157647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Lindquist S. Epigenetics in the extreme: prions and the inheritance of environmentally acquired traits. Science. 2010;330:629–632. doi: 10.1126/science.1191081. [DOI] [PubMed] [Google Scholar]
- Harold FM. Molecules into cells: specifying spatial architecture. Microbiol Mol Biol Rev. 2005;69:544–564. doi: 10.1128/MMBR.69.4.544-564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Jacobsen SE. Epigenetic inheritance in plants. Nature. 2007;447:418–424. doi: 10.1038/nature05917. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Chem Biol. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- Juliano C, Wang J, Lin H. Uniting germline and stem cells: the function of Piwi proteins and the piRNA pathway in diverse organisms. Annu Rev Genet. 2011;45:447–469. doi: 10.1146/annurev-genet-110410-132541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CD, Joshua-Tor L. Eukaryotic Argonautes come into focus. Trends Biochem Sci. 2013;38:263–271. doi: 10.1016/j.tibs.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D, Jr, Mello CC. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell. 2012;150:78–87. doi: 10.1016/j.cell.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessing D, Lee JT. X chromosome inactivation and epigenetic responses to cellular reprogramming. Annu Rev Genomics Hum Genet. 2013;14:85–110. doi: 10.1146/annurev-genom-091212-153530. [DOI] [PubMed] [Google Scholar]
- Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, Enokida H, Dahiya R. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JP, Brunet A. Bridging the transgenerational gap with epigenetic memory. Trends Genet. 2013;29:176–186. doi: 10.1016/j.tig.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. A gain-of-function mutation in oma-1, a C. elegans gene required for oocyte maturation, results in delayed degradation of maternal proteins and embryonic lethality. Dev Biol. 2003;258:226–239. doi: 10.1016/s0012-1606(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature. 2004;431:364–370. doi: 10.1038/nature02875. [DOI] [PubMed] [Google Scholar]
- Luteijn MJ, van Bergeijk P, Kaaij LJ, Almeida MV, Roovers EF, Berezikov E, Ketting RF. Extremely stable Piwi-induced gene silencing in Caenorhabditis elegans. Embo J. 2012;31:3422–3430. doi: 10.1038/emboj.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak J, Fire A. Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science. 2007;315:241–244. doi: 10.1126/science.1132839. [DOI] [PubMed] [Google Scholar]
- Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Fukushima S, Takatsuji H. RNA-directed DNA methylation induces transcriptional activation in plants. Proc Natl Acad Sci U S A. 2009;106:1660–1665. doi: 10.1073/pnas.0809294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee HC, Gu W, Ishidate T, Conte D, Jr, Mello CC. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell. 2012;150:65–77. doi: 10.1016/j.cell.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Sijen T, Steiner FA, Thijssen KL, Plasterk RH. Secondary siRNAs result from unprimed RNA synthesis and form a distinct class. Science. 2007;315:244–247. doi: 10.1126/science.1136699. [DOI] [PubMed] [Google Scholar]
- Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–393. doi: 10.1126/science.1140846. [DOI] [PubMed] [Google Scholar]
- Yigit E, Batista PJ, Bei Y, Pang KM, Chen CC, Tolia NH, Joshua-Tor L, Mitani S, Simard MJ, Mello CC. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006;127:747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01