p27Kip1 modulates cell migration through the regulation of RhoA activation (original) (raw)
Abstract
The tumor suppressor p27Kip1 is an inhibitor of cyclin/cyclin-dependent kinase (CDK) complexes and plays a crucial role in cell cycle regulation. However, p27Kip1 also has cell cycle-independent functions. Indeed, we find that p27Kip1 regulates cell migration, as p27Kip1-null fibroblasts exhibit a dramatic decrease in motility compared with wild-type cells. The regulation of motility by p27Kip1 is independent of its cell-cycle regulatory functions, as re-expression of both wild-type p27Kip1 and a mutant p27Kip1 (p27CK–) that cannot bind to cyclins and CDKs rescues migration of p27–/– cells. p27–/– cells have increased numbers of actin stress fibers and focal adhesions. This is reminiscent of cells in which the Rho pathway is activated. Indeed, active RhoA levels were increased in cells lacking p27Kip1. Moreover, inhibition of ROCK, a downstream effector of Rho, was able to rescue the migration defect of p27–/– cells in response to growth factors. Finally, we found that p27Kip1 binds to RhoA, thereby inhibiting RhoA activation by interfering with the interaction between RhoA and its activators, the guanine–nucleotide exchange factors (GEFs). Together, the data suggest a novel role for p27Kip1 in regulating cell migration via modulation of the Rho pathway.
Keywords: p27Kip1, Rho, migration, actin stress fibers, Ras, ROCK
Cell migration is a complex and integrated phenomenon that plays a central role in a variety of physiological and pathological processes such as embryonic development, wound healing, immune response, angiogenesis, and tumor metastasis. Migration is a multistep process during which a cell needs to coordinately extend protrusions and form new adhesions at the leading edge, translocate its cell body, and release cell adhesions and retract at the rear of the cell (Webb et al. 2002; Ridley et al. 2003).
GTPases of the Rho family play an important role in the regulation and coordination of the remodeling of the cytoskeleton required for these events. GTPases cycle between an active GTP-bound state and an inactive GDP-bound state. When in the active conformation, the GTPases can interact with and activate a variety of effector proteins. Families of positive and negative regulators of Rho GTPases provide a tight regulation of their activation state. The exchange of GDP for GTP is facilitated by guanine–nucleotide exchange factors (GEFs), hence activating Rho proteins (Schmidt and Hall 2002). Conversely, GTPase-activating proteins (GAPs) accelerate the rate of GTP hydrolysis, thus returning them to their inactive state.
Of the 20 Rho family members described to date in mammalian cells, RhoA, Rac1, and Cdc42 have been the most studied (Etienne-Manneville and Hall 2002). Each has specialized roles in cell migration. Cdc42 is involved in the establishment of cell polarity during directed cell migration (Etienne-Manneville and Hall 2002). Rac1 drives cell migration by promoting the extension of lamellipodia at the leading edge of the cell (Ridley et al. 1992; Nobes and Hall 1999). Rac1 function is mediated, at least in part, by its ability to activate the p21-activated kinases (PAKs). PAK1 activation is accompanied by lamellipodia formation and by disassembly of stress fibers and focal adhesions (Manser et al. 1997; Frost et al. 1998). RhoA promotes the formation of actin stress fibers and focal adhesions through the recruitment and activation of its effectors mDia and the Rho-kinases, ROCK1 and ROCK2 (Ridley and Hall 1992; Leung et al. 1996; Amano et al. 1997). Rho and ROCK also control actin-myosin contractility, thus driving translocation of the cell body, and the retraction of the rear of the cell (Kimura et al. 1996; Webb et al. 2002; Ridley et al. 2003).
Although Rho activity is important for motility, its role in the formation of stress fibers and focal adhesions can lead to an inhibition of cell migration by increasing cellular adhesion to its substrate and preventing focal adhesion turnover (Ren et al. 2000; Arthur and Burridge 2001; Cox et al. 2001; Sahai et al. 2001; Vial et al. 2003). Consistent with a need for modulating Rho activity during migration, proper cell spreading on extracellular matrix requires the transient inhibition of RhoA activity by an integrin-mediated activation of p190–RhoGAP by c-Src (Ren et al. 1999; Arthur et al. 2000; Arthur and Burridge 2001). Overall, Rac and Rho play antagonistic and complementary roles during cell movement, and their respective activities need to be carefully balanced. In addition to their role in the regulation of the actin cytoskeleton, Rho GTPases also participate in the control of gene transcription, cell cycle progression, microtubule dynamics, and enzymatic activities (Etienne-Manneville and Hall 2002).
The tumor suppressor p27Kip1 is a member of the Cip/Kip family of cyclin/CDK (cyclin/cyclin-dependent kinase) inhibitors (CKIs) and plays a critical role in regulating the progression through the G1 and S phases of the cell cycle (Sherr and Roberts 2001). Inactivation of the cell-cycle inhibitory function of p27Kip1 is commonly observed in many cancers, either through loss of the p27Kip1 protein or by sequestering p27Kip1 in the cytoplasm, away from cyclin/CDK complexes (Slingerland and Pagano 2000; Philipp-Staheli et al. 2001; Bloom and Pagano 2003). Thus, the cell-cycle regulatory activity of p27Kip1 is regulated by changes in the abundance of the protein and in its subcellular localization, with relocalization of p27Kip1 in the cytoplasm triggered by its phosphorylation on Ser 10 (Rodier et al. 2001; Boehm et al. 2002; Ishida et al. 2002). However, p27Kip1 may also have cell cycle-independent functions (Coqueret 2003). In particular, transduction of a TAT–p27Kip1 fusion protein in HepG2 hepatocellular carcinoma cells elicited a migratory response similar to that induced by hepatocyte growth factor (HGF; Nagahara et al. 1998). It was subsequently found that HGF treatment induced nuclear export of p27Kip1 to the cytoplasm through its phosphorylation on Ser 10, and that a region in the C-terminal half of p27Kip1 was required to stimulate migration (McAllister et al. 2003). In addition, p27–/– 3T3 fibroblasts were refractory to HGF-induced migration (McAllister et al. 2003). However, the mechanism by which p27Kip1 regulates cell migration remains unknown.
In the present study, we used mouse embryonic fibroblasts (MEFs) derived from p27-null and wild-type embryos to dissect the role of p27Kip1 during cell migration. Motility of MEFs lacking p27Kip1 was impaired, likely due to an excess in RhoA activity, as these cells displayed increased numbers of stress fibers and focal adhesions, and elevated levels of Rho–GTP. Moreover, ROCK inhibition rescued migration in these cells. Furthermore, cotransfection experiments in HEK 293T cells revealed that p27Kip1 binds to RhoA and also interferes with Rho binding to its GEFs, but not to its effectors. Thus, we find that p27Kip1 regulates cell migration by preventing Rho activation.
Results
Migration is impaired in cells lacking p27Kip1
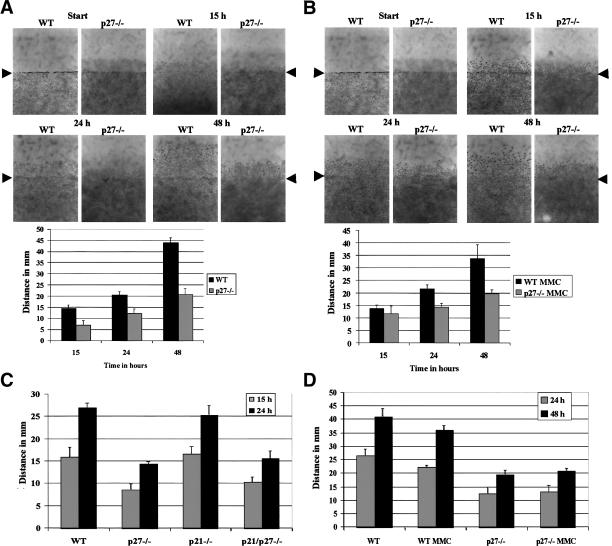
To further characterize the function of p27Kip1 during cell migration, we measured the motility of MEFs derived from p27–/– and wild-type embryos following wounding of a confluent cell monolayer, in response to various stimuli. Cell migration in this assay system is dependent upon reorganization of the actin cytoskeleton and the assembly and disassembly of focal adhesion complexes, processes which are governed by the Rho family GTPases Rho, Rac, and Cdc42 (Nobes and Hall 1999). In normal growth medium (10% serum), immortalized p27–/– MEFs exhibited a marked reduction in migration compared with cells derived from wild-type littermates (Fig. 1A), consistent with previously published results (McAllister et al. 2003). In contrast, primary MEFs lacking p21Waf1/Cip1 migrated equivalently to the wild-type cells, whereas the p27–/– and double knockout p21/p27–/– cells were equally impaired in their ability to migrate (Fig. 1C). We ruled out the possibility that the difference in motility was due to differences in proliferation, by pretreating the cells with mitomycin C (MMC) to block cell division. Migration was comparable with or without MMC pretreatment, both with immortalized and primary MEFs (Fig. 1B,D, respectively).
Figure 1.
p27-null MEFs have a migration deficit compared with wild-type MEFs. (A) Migration of immortalized wild-type (WT) and p27–/– MEFs following wounding of a confluent cell monolayer. Migration distances were measured as described in Materials and Methods. (B) Asin A except that cells were pretreated for 3 h with 10μg/mL MMC, to block cell proliferation. (C) Migration of primary wild-type (WT), p27–/–, p21–/–, and p21/p27–/– MEFs. (D) Migration of primary wild-type (WT) and p27–/– MEFs that were treated, or not, with MMC.
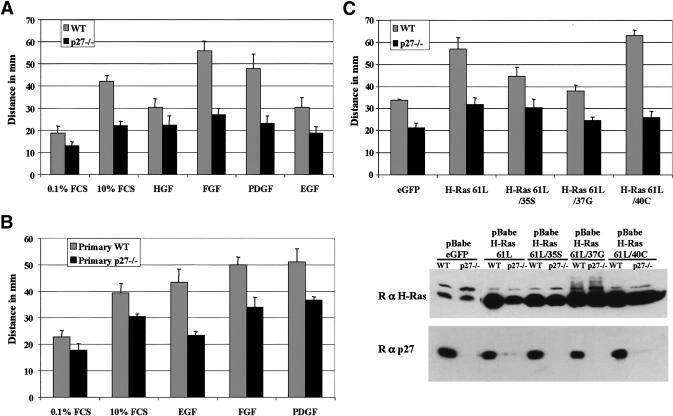
The migration defect of p27-null cells could not be completely overcome by stimulating motility using growth factors such as epidermal growth factor (EGF), fibroblast growth factor-2 (FGF2), platelet-derived growth factor-BB (PDGF-BB), HGF, or serum (Fig. 2A,B). In contrast, these growth factors were all able to induce robust migration of wild-type MEFs, albeit with variable potencies.
Figure 2.
p27–/– MEFs are refractory to induction of migration by growth factors or activated Ras. (A,B) Migration of immortalized (A) and primary (B) wild-type (WT) and p27–/– MEFs in the presence of growth factors (HGF, FGF2, PDGF-BB, or EGF) at a final concentration of 25 ng/mL. Cells were allowed to migrate for 48 h. (C) Wild-type (WT) and p27–/– were infected with retroviruses expressing the indicated forms of activated Ras. Cells were allowed to migrate for 48 h, and their migration was measured as in Figure 1. (Lower panel) The respective H-Ras and p27Kip1 protein levels of the cells used for the migration assay.
Ras activates many downstream signaling pathways, and is known to be a potent inducer of cell motility (Nobes and Hall 1999; Bar-Sagi and Hall 2000). To determine whether Ras activation could rescue migration in cells lacking p27Kip1, we introduced, by retroviral infection of wild-type and p27–/– MEFs, an activated H-Ras allele (H-Ras-61L) or activated H-Ras mutants that activate only a specific effector pathway (McFall et al. 2001). The H-Ras expression levels achieved following infection are displayed in Figure 2C (lower panel). H-Ras activation only marginally induced migration of p27–/– MEFs, whereas it dramatically increased motility in wild-type cells (Fig. 2C). Analogous results were obtained using K-Ras-V12 (data not shown). Interestingly, H-Ras-61L/40C, which activates only phosphatidylinositol 3-kinase (PI 3-Kinase), was the most potent inducer of wild-type cell migration, and produced a response similar to that of H-Ras-61L (Fig. 2C). In contrast, H-Ras-61L/37G, which activates only RalGDS, did not have a significant effect on cell motility. H-Ras-61L/35S, which activates only Raf, produced an intermediate response (Fig. 2C).
The effect of p27Kip1 deficiency on cell migration was confirmed in a second cell type by using antisense methods to inhibit p27Kip1 expression in human glioblastoma cells. A migration assay was performed, in the absence or presence of phorbol 12-myristate 13-acetate (PMA), a potent inducer of migration in these cells (Besson et al. 2002). The motility of U251N glioma cells transfected with an antisense p27Kip1 construct was markedly reduced compared with the parental line or the empty vector transfected cells (Supplementary Fig. 1). These results suggest that the regulation of cell migration by p27Kip1 may be a general phenomenon applicable to different cell types.
Overall, the data indicate that migration in response to a variety of stimuli is inhibited in different cell types in the absence of p27Kip1. These results suggest the existence of a p27Kip1-dependent event downstream of both growth factor receptors and Ras that is required for efficient cell migration.
Increased numbers of actin stress fibers and focal adhesions in p27–/– MEFs
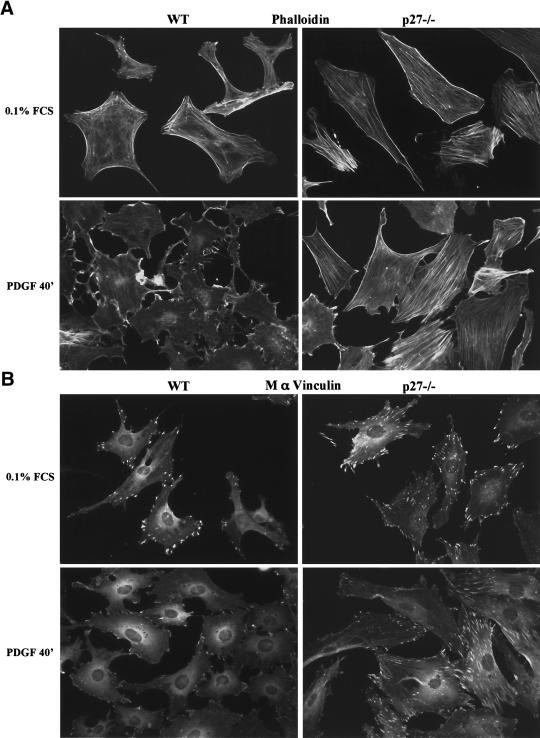
Changes in focal adhesions and the actin cytoskeleton are hallmarks of migrating cells (Webb et al. 2002; Ridley et al. 2003). We therefore surveyed by immunocytochemistry the actin cytoskeleton and focal adhesions of primary MEFs, using phalloidin and vinculin, respectively, in an attempt to identify morphological traits that could help clarify the role of p27Kip1 in cell migration. Wild-type MEFs had few actin stress fibers in serum-starved conditions, and PDGF-BB stimulation evoked a dramatic rearrangement of the actin cytoskeleton, with the loss of stress fibers and formation of membrane ruffles and lamellipodia, consistent with previous observations (Fig. 3A; Ridley et al. 1992). In contrast, p27–/– cells had an extensive network of stress fibers in the absence of serum, and PDGF stimulation largely failed to induce the actin rearrangement observed in wild-type cells, although some changes were observed at later time points (Fig. 3A; data not shown). Similarly, p27-null MEFs had an increased number of focal adhesions (visualized with a vinculin antibody) compared with wild-type cells and mostly failed to rearrange these upon PDGF stimulation (Fig. 3B). These results were recapitulated using immortalized MEFs (Supplmentary Fig. 2A,B). In addition, the localization of integrins αv and β3 at focal adhesions was increased in p27–/– MEFs (Supplementary Fig. 2C,D).
Figure 3.
Cells lacking p27Kip1 have increased numbers of actin stress fibers and focal adhesions. Primary MEFs were seeded on glass coverslips, allowed to grow for 16 h, then starved for 48 h in 0.1% FCS, or starved and stimulated for 40 min with 25 ng/mL PDGF-BB (PDGF). (A) Actin was visualized with phalloidin–rhodamine (1/500). (B) Mouse anti-vinculin (1/1000).
Collectively, the features displayed by p27–/– MEFs are reminiscent of cells in which the Rho pathway is activated, as actin stress fibers and focal adhesion assembly are two processes controlled by Rho and its effectors ROCK and mDia (Ridley and Hall 1992; Leung et al. 1996; Amano et al. 1997; Etienne-Manneville and Hall 2002; Webb et al. 2002; Ridley et al. 2003). Additionally, the localization of integrins at focal adhesions is also regulated by Rho and requires Rho-stimulated contractility (Chrzanowska-Wodnicka and Burridge 1996).
The role of p27Kip1 in migration is independent of its cell cycle regulatory function
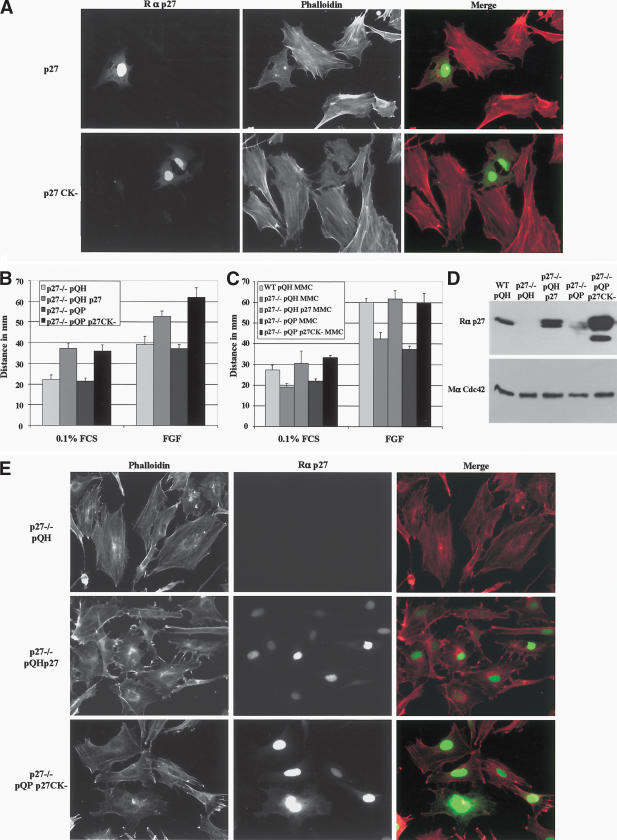
To ascertain whether the loss of p27Kip1 was responsible for the differences observed in migration and the cytoskeleton, p27Kip1 was re-expressed in p27–/– MEFs. Reexpression of p27Kip1, either transiently or stably, was accompanied by a decrease in the number of stress fibers in these cells (Fig. 4A,E, respectively). Interestingly, expression of p27CK–, a mutant p27Kip1 that can no longer bind to cyclins and CDKs (Vlach et al. 1997), induced an analogous response in p27–/– cells (Fig. 4 A,E). In addition, p27Kip1 and p27CK– were equally potent in rescuing the migratory defect of p27–/– cells (Fig. 4B,C). Thus, the loss of p27Kip1 is directly responsible for the phenotypes of p27–/– cells. Moreover, the function of p27Kip1 in the regulation of migration appears to be independent of its role as an inhibitor of cyclin/CDK complexes, consistent with findings by others (McAllister et al. 2003).
Figure 4.
Re-expression of p27Kip1 or a mutant form that cannot bind to cyclin/CDK (p27CK–) rescues the migration and decreases the number of actin stress fibers in p27-null MEFs. (A) Transient transfection of p27–/– MEFs with p27Kip1 (upper row) and p27CK– (lower row). Cells were plated on glass coverslips, incubated overnight prior to transfection, and fixed 36 h posttransfection. Cells were stained for p27Kip1 (green) and phalloidin (red). (B_–_E) Immortalized wild-type (WT) and p27–/– MEFs were infected with retroviruses encoding wild-type p27 (pQHp27), or p27CK– (pQPp27CK–), or the corresponding empty vectors. (B) Migration assays in the presence or absence of 25 ng/mL FGF2. (C) Migration assay with cells pretreated for 3 h with 10 μg/mL MMC. (D) Immunoblot to indicate p27Kip1 levels following retroviral infection in the cells used for the migration assays. The membrane was stripped and reprobed with a monoclonal antibody to Cdc42 to indicate protein loading. Fifty micrograms of protein was loaded per lane. (E) Retrovirally infected p27–/– MEFs were plated on coverslips for 24 h in 10% FCS DMEM, fixed, and stained for p27Kip1 (green) and phalloidin (red).
In hepatocellular carcinoma cells, the role of p27Kip1 in migration was associated with its export from the nucleus via Ser 10 phosphorylation (McAllister et al. 2003). In NIH3T3 fibroblasts, phosphorylation of p27Kip1 on Ser 10 increases following serum stimulation and is required for its export from the nucleus (Rodier et al. 2001; Boehm et al. 2002; Ishida et al. 2002). We analyzed by immunocytochemistry the subcellular localization of p27Kip1 in MEFs at various times following serum stimulation. Although most p27Kip1 was nuclear, a fraction of p27Kip1 could be detected in the cytoplasm. The nuclear stain was progressively lost during transit from G1 to S phase, whereas the cytoplasmic stain remained (Supplementary Fig. 3).
Elevated activation of the Rho pathway is responsible for the migration defect of cells lacking p27Kip1
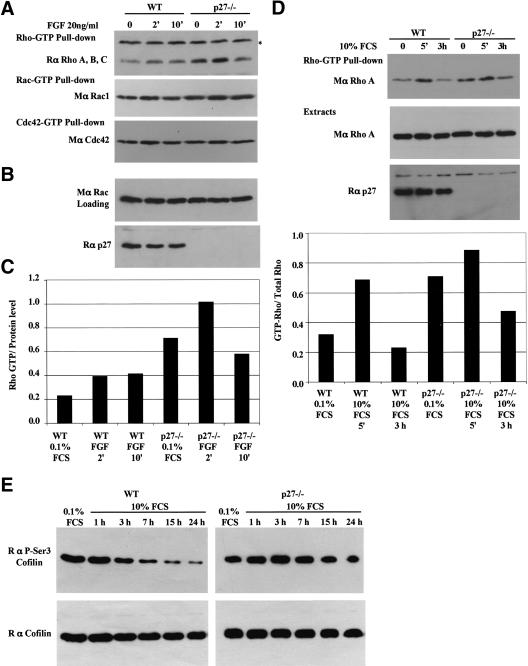
Because p27–/– cells displayed characteristics consistent with Rho activation, we measured the amount of GTP-loaded forms of Rho, Rac, and Cdc42 in wild-type and p27-null primary MEFs. Rho–GTP loading was modestly increased following FGF2 stimulation of wild-type MEFs. In contrast, although the amount of Rho–GTP also increased following FGF2 stimulation, Rho–GTP levels were elevated at all times in p27–/– cells (Fig. 5A,C). The loading of Rac and Cdc42 was not significantly different between p27–/– cells and their wild-type counterparts (Fig. 5A). Comparable results were obtained using serum and EGF to stimulate the MEFs (Fig. 5D; data not shown).
Figure 5.
Increased Rho–GTP levels in p27–/– MEFs. (A) Cells were stimulated with FGF2 (25 ng/mL) for the indicated time, and Rho–GTP levels measured using pull-down assays (see Materials and Methods for details). GST-C21 (Rho–GTP) pull-downs were probed with a rabbit anti-RhoA, anti-RhoB, anti-RhoC antibody. GST–PAK1-CD (Rac-GTP and Cdc42–GTP) pull-downs were probed with a mouse anti-Rac1. The membrane was stripped and reprobed with a mouse anti-Cdc42 antibody. The asterisk refers to a nonspecific band in the top panel. (B) A fraction (1/30) of the extracts was used to determine the amount of protein (probed for Rac), and the membrane was reprobed for p27Kip1 after stripping. (C) Quantification of Rho–GTP normalized to the loading control (Rac). (D) Rho–GTP pull-downs with cells starved for 48 h and stimulated with DMEM containing 10% FCS. The graph shows the amount of Rho–GTP in the pull-downs normalized to the total amount of Rho present in the loading control. Similar results were obtained in four independent experiments (E) Increased phosphorylation of cofilin on Ser 3 in p27–/– MEFs. Immortal wild-type (WT) and p27–/– MEFs were starved for 48 h and stimulated for the indicated time with 10% FCS. Ten micrograms of protein was loaded per well. Membranes were probed with a polyclonal antibody to phospho-Ser 3-cofilin, stripped, and reprobed with a polyclonal antibody to cofilin.
Activation of the Rho pathway was also evaluated using phosphospecific antibodies to the F-actin severing protein cofilin. Cofilin is inactivated by phosphorylation by LIM-kinase, itself activated by the Rho/ROCK pathway. We found that phospho-cofilin levels progressively decrease in wild-type MEFs after serum stimulation, whereas they remain elevated in p27–/– cells, consistent with elevated Rho activity (Fig. 5E).
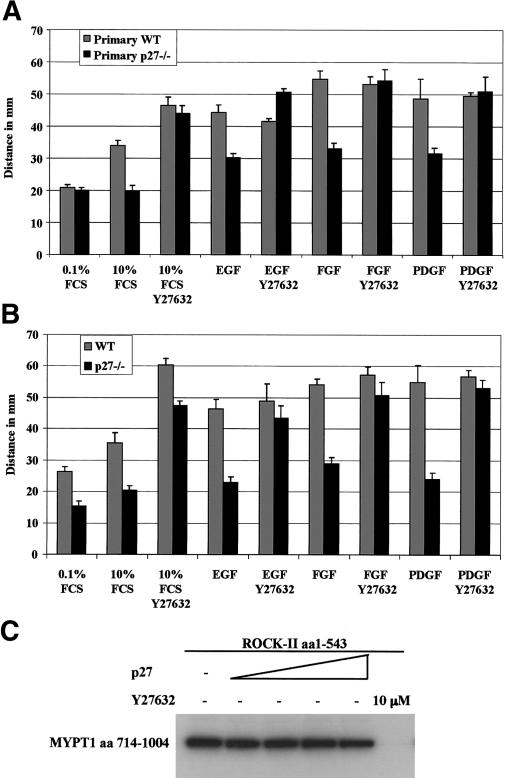
To further analyze the involvement of the Rho pathway, we compared the effect of the ROCK inhibitor Y27632 on wild-type and p27-null MEFs in migration assays. The migration defect of primary and immortalized p27–/– MEFs was completely rescued by Y27632 treatment (Fig. 6A,B). These results indicate that reduction of Rho signaling rescues the migration of cells lacking p27Kip1 and that p27Kip1 likely acts upstream of ROCK to regulate migration.
Figure 6.
The Rho-kinase inhibitor Y27632 rescues the migration defect of p27-null MEFs. (A) Migration of primary wild-type (WT) and p27–/– MEFs pretreated for 3 h with 10 μg/mL MMC, and then stimulated with growth factors (FGF2, PDGF-BB, and EGF) at 25 ng/mL in the presence or absence of the Rho-kinase inhibitor Y27632 (10 μM). Cells were allowed to migrate for 48 h. (B) Migration of immortalized wild-type (WT) and p27–/– MEFs, performed as in A except that the cells were not treated with MMC. (C) p27Kip1 does not inhibit ROCK2 in an in vitro kinase assay. Kinase activity of ROCK2 (5.72 nM) was measured in the presence of increasing amounts of recombinant p27Kip1 (2.16 μM, 4.32 μM, 10.81 μM, 21.63 μM, respectively) or the ROCK inhibitor Y27632 (10 μM), using MYPT1 as a substrate.
A closely related CKI, p21Waf1/Cip1, was recently found to directly inhibit ROCK activity in the cytoplasm (Tanaka et al. 2002; Lee and Helfman 2003). We therefore tested in an in vitro kinase assay using recombinant proteins whether p27Kip1 could inhibit the kinase activity of ROCK2 using myosin phosphatase targeting subunit-1 (MYPT1) as a substrate. Increasing amounts of p27Kip1 were unable to inhibit ROCK2 activity (Fig. 6C). In addition, recombinant p27Kip1 had no effect on the kinase activity of immunoprecipitated full-length ROCK1 overexpressed in HEK 293T cells (data not shown).
Taken together, the data suggest that increased Rho activity in p27–/– cells induces actin stress fiber formation and stabilizes focal adhesions, thus exercising a negative effect on cell motility. In addition, these results indicate that p27Kip1 affected the Rho pathway upstream of ROCK, because ROCK inhibition rescued the migration of p27–/– cells, and p27Kip1 did not directly inhibit ROCK activity.
p27Kip1 binds RhoA, thus preventing its interaction and activation by Rho–GEFs
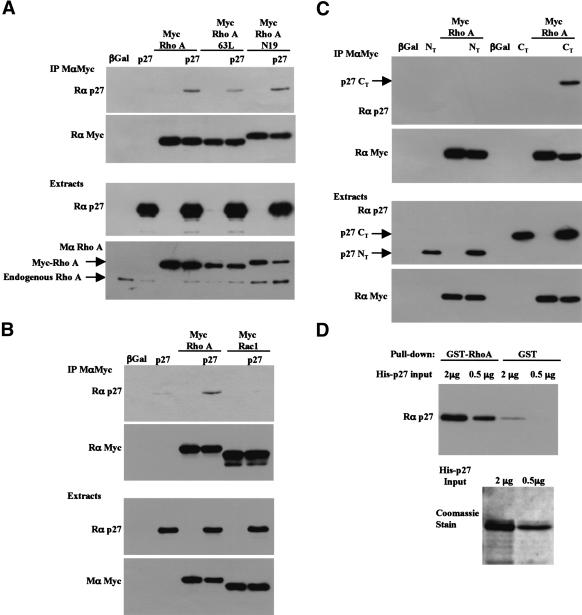
Because p27Kip1 seems to act upstream of ROCK to regulate cell motility, we tested whether p27Kip1 could interact with RhoA in coimmunoprecipitation experiments. Indeed, p27Kip1 was coprecipitated with RhoA in HEK 293T cells overexpressing both proteins (Fig. 7A,B). On the other hand, Rac1 did not interact with p27Kip1 (Fig. 7B). p27Kip1 could also interact with both activated (RhoA-63L) and dominant-negative RhoA (RhoA-N19; Fig. 7A). The interaction of p27Kip1 with both Rho–GTP and Rho–GDP is unlike that of most Rho-interacting proteins, which preferentially interact with one form of Rho or another. To delineate which part of p27Kip1 was mediating the interaction with RhoA, similar experiments were carried out using either the N-terminal half or the C-terminal half of p27Kip1. Only the C-terminal half of p27Kip1 could be coimmunoprecipitated with RhoA (Fig. 7C). These results are in agreement with the fact that the cyclin/CDK binding domains of p27Kip1, located at the N terminus of the protein, did not seem to play a role cell migration (Fig. 4), and that the “scatter domain” of p27Kip1 was located in the C-terminal part of the protein (McAllister et al. 2003). Finally, in vitro binding experiments revealed that the RhoA/p27 interaction was direct, as GST–RhoA bound to His-tagged p27Kip1 in pull-down assays (Fig. 7D).
Figure 7.
The C-terminal half of p27Kip1 interacts with RhoA in HEK 293T cells. (A) HEK 293T cells were transfected with full-length p27Kip1 and/or Myc-tagged RhoA constructs. RhoA was immunoprecipitated using a monoclonal Myc antibody (9E10). The immunoprecipitates were probed with a p27Kip1 antibody (rabbit, C19; top panel), and the membrane was stripped and reprobed for Myc (rabbit, A14) to indicate the amount of myc-RhoA immunoprecipitated. The amounts of p27Kip1 and RhoA in the cell extracts are shown in the lower panels. (B) As in A except that cells were transfected with p27Kip1 and/or Myc-tagged RhoA or Myc-tagged Rac1 to test the specificity of the interaction of p27 with RhoA. (C) As in A except the N-terminal half (amino acids 1–85, NT) or the C-terminal half (amino acids 86–198, CT) of p27Kip1 were cotransfected with Myc-RhoA. Only the C-terminal half of p27Kip1 was detected with a mix of N- and C-terminal p27Kip1 antibodies (rabbit, C19 and N20) in the Myc immunoprecipitates. A fraction of the lysates (1/12th of the amount used for immunoprecipitations) was probed for p27Kip1 and Myc to indicate the levels of the transfected proteins (lower panel). (D) In vitro binding assay. The indicated amounts of recombinant His-tagged p27Kip1 were incubated with an excess of GST–RhoA or GST beads. The amount of His-p27Kip1 recovered in the pull-downs was detected by immunoblot using a p27Kip1 antibody. (Lower panel) The amounts of His-p27Kip1 used in the pull-downs, stained with Coomassie blue.
The interaction between RhoA and p27kip1 suggested that p27Kip1 could inhibit the Rho pathway either by preventing the binding of active Rho (Rho–GTP) to its downstream effectors, or by interfering with the activation of Rho (exchange of GDP for GTP) by Rho–GEFs. To discriminate between the two, we utilized unique properties of RhoA mutants: The RhoA-63L mutation abolishes GTPase activity and results in a constitutively active Rho that is always bound to its effectors. On the other hand, the RhoA-N19 mutation has a decreased affinity for GTP and increased affinity for Rho–GEFs, thus acting as a dominant-negative by sequestering Rho–GEFs from endogenous Rho (Seasholtz et al. 1999).
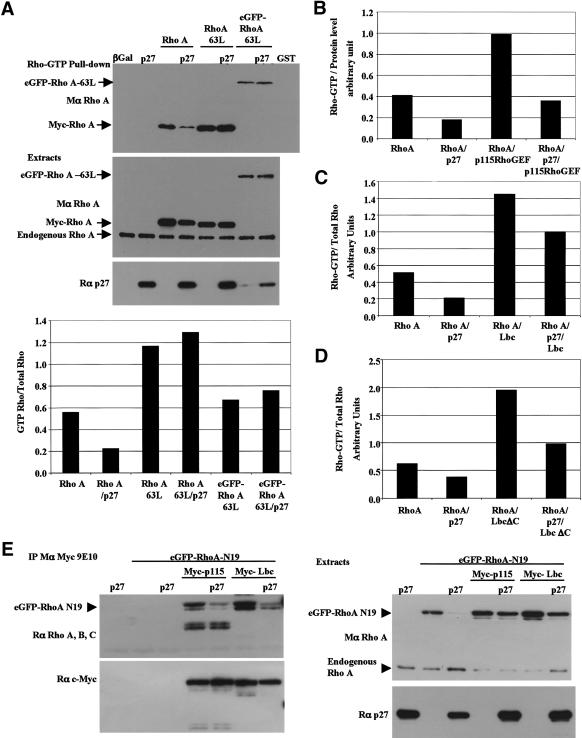
To determine whether p27Kip1 inhibited the Rho–GTP/effector interaction, Rho–GTP pull-downs were performed in HEK 293T cells transfected with p27Kip1 and either wild-type RhoA or activated RhoA-63L. Rho–GTP pull-down assays are based on the ability of GTP-bound Rho to bind to the Rho-binding domain of Rhotekin, an effector of Rho (Sander et al. 1998; Ren et al. 1999). Therefore, if p27Kip1 inhibits the Rho/effector interaction, then it should affect both wild-type and constitutively active RhoA equally. The amount of active wild-type RhoA in the pull-downs was reduced in the presence of p27Kip1, whereas that of constitutively activated RhoA-63L was unaffected (Fig. 8A). These results suggest that p27Kip1 interferes with Rho activation rather than with the binding of Rho–GTP to its effectors. However, we cannot rule out that the interaction of RhoA with p27Kip1 may affect the binding of RhoA to effectors that utilize different binding sites on Rho.
Figure 8.
p27Kip1 inhibits RhoA activation by interfering with the binding of RhoA to its GEFs. (A) p27Kip1 does not affect the Rho/effector interaction. HEK 293T cells were cotransfected with p27Kip1 and the indicated RhoA expression vectors. (Top panel) The amount of Rho–GTP was determined in a pull-down assay, as described (see Fig. 5; Materials and Methods). (Bottom two panels) The amount of RhoA and p27Kip1 in the cellular extracts was determined by immunoblot. The graph represents the amount of GTP–RhoA normalized to the total amount of transfected RhoA protein (GTP–Rho/Total Rho). Similar results were obtained in three independent experiments. (B_–_D) p27Kip1 interferes with the activation of RhoA by its GEFs. As in A, except that cells were cotransfected with Myc-RhoA and/or p27Kip1, and/or Myc-p115RhoGEF (B), Myc-Lbc (C), and Myc-LbcΔC (D). The graphs indicate the amount of GTP–RhoA normalized to the total amount of transfected RhoA protein. For each GEF, similar results were obtained in at least two independent experiments. (E) p27Kip1 inhibits RhoA binding to its GEFs. HEK 293T cells were cotransfected with the indicated expression vectors. (Left panel, top) The Myc-tagged GEFs were immunoprecipitated using a Myc antibody (mouse, 9E10), and the amount of eGFP–RhoA-N19 coprecipitated with the GEFs was determined by immunoblot. Note that the lower band in the Myc-p115 lanes are nonspecific. The membrane was reprobed with a Myc antibody (rabbit, A14) to indicate the amount of GEF immunoprecipitated. The expression levels of eGFP–RhoA-N19 and p27Kip1 are indicated on the right panel (note that expression of eGFP–RhoA-N19 was very low in the control lane 3). Similar results were obtained in four independent experiments.
The ability of p27Kip1 to interfere with Rho activation was confirmed by the finding that p27Kip1 was able to inhibit, at least partially, the activation of RhoA by the Rho-specific GEFs p115–RhoGEF, Lbc, and a constitutively active form of Lbc, LbcΔC (Fig. 8B–D). We next tested whether p27Kip1 prevented the activation of RhoA by interfering with the Rho/GEF interaction. For this, RhoA-N19, which has an increased affinity for Rho–GEFs, was cotransfected with a Rho–GEF, either p115–RhoGEF or Lbc, in the presence or absence of p27Kip1. The amount of RhoA-N19 coprecipitated with either p115–RhoGEF or Lbc was markedly reduced in the presence of p27Kip1, indicating that p27Kip1 interferes with the interaction of RhoA with its GEFs, thereby preventing RhoA activation (Fig. 8E).
Discussion
In the present study, we discovered that p27Kip1, an inhibitor of cyclin-dependent kinases, has another function, which is to inhibit RhoA. Fibroblasts with an engineered deletion of the p27 gene had elevated amounts of endogenous Rho–GTP under all conditions assayed. This was associated with an exaggeration of Rho-dependent cellular phenotypes, including increased numbers of focal adhesions and actin stress fibers, increased phosphorylation of cofilin (which is a target of the Rho pathway), and a marked decrease in motility. Moreover, whereas control fibroblasts reorganized their actin cytoskeleton and focal adhesions in response to growth factors, p27-null fibroblasts proved largely refractory to these stimuli.
We have further defined the molecular mechanism by which p27Kip1 regulates cell migration. We first showed that p27Kip1 acted downstream of Ras and growth factor receptors but upstream of ROCK. Neither growth factor stimulation nor ectopic expression of activated Ras could correct the migration defect of p27-null fibroblasts. On the other hand, inhibition of ROCK restored cell motility. These results suggested that p27Kip1 might affect Rho itself. Indeed, p27Kip1 was able to interact with RhoA via its C terminus in coprecipitation experiments. Although p27Kip1 did not interfere with the ability of active Rho to interact with its effectors, it was able to block the binding of RhoA to its activators, the Rho–GEFs, and decreased Rho–GEF-stimulated accumulation of Rho–GTP. We concluded that p27Kip1 inhibits the Rho pathway by blocking the GEF-mediated activation of Rho. We propose that p27Kip1 modulates cell migration by reducing Rho–GTP levels.
Rho GTPases are critical regulators of the actin cytoskeleton and cell migration. Rho and its effector ROCK regulate stress fiber formation and focal adhesion assembly, as well as actin-myosin contractility (Ridley and Hall 1992; Kimura et al. 1996; Etienne-Manneville and Hall 2002; Webb et al. 2002). On the other hand, Rac and its effector PAK induce actin rearrangements, including stress fiber and focal adhesion disassembly, that control the formation of lamellipodia and new focal contacts at the cell edges (Ridley et al. 1992; Manser et al. 1997; Frost et al. 1998; Webb et al. 2002; Ridley et al. 2003). Efficient cell migration requires that the activities of Rho and Rac be tightly balanced; the dynamic activation and inhibition of both GTPases are spatially and temporally coordinated. Thus, insufficient levels of Rho–GTP will inhibit migration by preventing cells from achieving the level of adhesiveness and traction they need to move forward (Nobes and Hall 1999; Worthylake et al. 2001). Conversely, excessive Rho activity can also inhibit cell migration by increasing adhesion and preventing focal adhesion turnover (Ren et al. 2000; Arthur and Burridge 2001; Cox et al. 2001; Sahai et al. 2001; Vial et al. 2003).
Our major conclusion is that p27Kip1 inhibits RhoA activation. The effect this has on cellular phenotypes is likely to be cell type-specific. For example, in some cell types p27Kip1 increases cell motility, whereas in others it decreases motility. Our data suggest that fibroblasts lacking p27Kip1 migrate poorly due to increased Rho activation, and it has been shown that ectopic overexpression of p27Kip1 stimulates the migration of hepatocellular carcinoma cells (Nagahara et al. 1998; McAllister et al. 2003). In contrast, p27Kip1 inhibits migration in other cell types, including vascular smooth muscle cells, umbilical vein endothelial cells, and oral cancer cells (Goukassian et al. 2001; Sun et al. 2001; Diez-Juan and Andres 2003; Supriatno et al. 2003). These differences possibly originate from cell type-specific variation in the relative balance between Rho and Rac activity. Thus, in some cell types (e.g., fibroblasts) this balance could be slightly tilted towards Rho activation so that a decrease of Rho activity would stimulate cell migration. Conversely, in other cell types (e.g., endothelial cells), if the balance were tilted towards Rac activation, then a decrease in Rho activity might have an opposite effect. In addition, cell type-specific differences in the molecular interactions and subcellular localization of RhoA may also contribute to the different phenotypic consequences of altering Rho activation levels.
In transformed cells, one could expect that increased Rho activity stimulated by constitutive activation of upstream signaling pathways would result in inhibition of migration. This is not what is observed, however, as cancer cells are usually highly motile and invasive. In fact, tumor cells have found ways to uncouple distinct effector pathways downstream of Rho. For instance, in Ras-transformed cells, elevated levels of Rho activity are selected for to permit the down-regulation of p21Waf1/Cip1 and allow cell-cycle progression, whereas high levels of ERK signaling uncouple Rho from inducing actin stress fibers and focal adhesions by down-regulating ROCK1 and ROCK2 (Sahai et al. 2001). Alternatively, it has been proposed that Ras activation induces the cytoplasmic localization of p21Waf1/Cip1 where it inhibits ROCK activity, thereby uncoupling Rho from stress fiber formation (Lee and Helfman 2003).
Interestingly, it appears that all of the members of the Cip/Kip family of CKIs may play a role in the regulation of the Rho pathway, albeit acting at distinct levels in the pathway. We found that p27Kip1 regulates Rho activation. Cytoplasmic p21Waf1/Cip1 directly inhibits ROCK, resulting in increased neurite outgrowth (Tanaka et al. 2002; Lee and Helfman 2003). However, in our experiments the loss of p21Waf1 in MEFs had no effect on their migratory ability. p57Kip2 binds to LIM-kinase and induces its translocation to the nucleus, thereby inhibiting its activity (Yokoo et al. 2003). LIM-kinase phosphorylates and inactivates the actin depolymerization factor cofilin, and is itself directly activated by ROCK phosphorylation. Therefore, although they act differently, all of the Cip/Kip proteins seem to negatively regulate the Rho signaling pathway when in the cytoplasm. An analogous situation has been described in budding yeast, where the CKI Far1 regulates the Rho family protein Cdc42. Far1 binds to and sequesters Cdc24, a GEF for Cdc42, in the nucleus. Activation of Cln/Cdc28 in the G1 phase leads to phosphorylation and degradation of Far1, thereby releasing Cdc24. Cdc24 is then exported to the cytoplasm, where it regulates cytoskeletal organization and cell polarity (Shimada et al. 2000). It is tempting to speculate that the regulation of different proteins in the Rho pathway by CKIs could provide new levels of regulation of Rho-mediated processes, because the abundance and subcellular localization of CKIs are regulated throughout the cell cycle.
Another level of complexity is added by the fact that Rho can negatively regulate the levels of the p27Kip1 and p21waf1 proteins (Weber et al. 1997; Olson et al. 1998; Hu et al. 1999; Vidal et al. 2002). Thus, Rho and p21Waf1 and p27Kip1 could participate in a negative feedback loop: while CKIs may negatively regulate the Rho pathway, Rho signaling induces the down-regulation of these CKIs. This network of interactions among CDK inhibitors and elements of the Rho pathway suggests that this may represent a basic mechanism for coordinating cytoskeletal functions with cell division.
The effect of p27Kip1 on the Rho pathway could be genetically separated from its role in regulating cell proliferation. Indeed, wild-type p27Kip1 and a mutant that cannot bind to cyclins and CDKs were equally potent in decreasing the number of actin stress fibers and rescuing the migration defect of p27-null fibroblasts. Moreover, although the cyclin and CDK binding domains of p27Kip1 are located in the N-terminal of the protein, we found that the C-terminal half of p27Kip1 was mediating the interaction with RhoA. The C-terminal half of p27Kip1 may therefore constitute a versatile protein interaction domain and may promote the binding with additional cellular targets. It is of note that the adaptor protein Grb2 can also interact with p27Kip1 via this region (Sugiyama et al. 2001; Moeller et al. 2003). In doing so, p27Kip1 competes with the Ras-GEF Sos for binding to Grb2, thereby preventing Ras activation (Moeller et al. 2003). However, the physiological relevance of this interaction remains to be investigated. Ras activates multiple signaling cascades and is a potent inducer of cell motility (Bar-Sagi and Hall 2000). We ruled out that p27Kip1 was regulating cell migration by modulating Ras activity by overexpressing activated H-Ras and K-Ras alleles. Whereas wild-type cells were induced to migrate, activated Ras was unable to rescue migration of p27-null cells, indicating that p27Kip1 acted downstream of Ras.
p27Kip1 is a well characterized tumor suppressor and is frequently inactivated in malignancies. Loss of p27Kip1 usually correlates with increased tumor aggressiveness and poor clinical outcome (Slingerland and Pagano 2000; Philipp-Staheli et al. 2001; Bloom and Pagano 2003). However, unlike other tumor suppressors, the p27 gene is never mutated in tumors. Instead, p27Kip1 is inactivated primarily by down-regulation of the protein or by exclusion from the nucleus, where it exercises its cyclin/CDK inhibitory function (Slingerland and Pagano 2000; Philipp-Staheli et al. 2001). Interestingly, in subsets of certain types of tumors, high p27Kip1 levels correlate with high tumor grade, poor prognosis, and increased metastasis. This has been observed, for instance, in various types of carcinomas (breast, cervix, esophagus, and uterus) and some types of lymphomas and leukemias (Anayama et al. 1998; Dellas et al. 1998; Vrhovac et al. 1998; Saez et al. 1999; Sanchez-Beato et al. 1999; Shiozawa et al. 2001; Kouvaraki et al. 2002; Watanabe et al. 2002). These observations suggest that deregulation of p27Kip1 in tumors may serve to uncouple it from its cell cycle-inhibitory function, possibly by being excluded from the nucleus. Once in the cytoplasm, p27Kip1 could exert other functions, such as the regulation of cell migration, thereby promoting tumor progression and invasiveness. Thus p27Kip1 may be acting as both a tumor suppressor and an oncogene, depending on its subcellular localization, possibly explaining its unusual regulation in cancers.
Material and methods
Constructs, antibodies, and reagents
Retroviral vectors pBabe-puro H-Ras61L, H-Ras61L/35S, H-Ras61L/37G, and H-Ras 61L/40C were kindly provided by Channing Der (University of North Carolina, Chapel Hill, NC; McFall et al. 2001). The pCMV–Rho-A63L and RhoA-N19, and peGFP–RhoA-63L and RhoA-N19 were gifts from William Arthur (Fred Hutchinson Cancer Research Center, Seattle, WA). The pGEX3X-C21 and pGEX2TK-PAK-CD were kindly provided by John Collard (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Myc-tagged RhoA, Rac1, p115Rho–GEF, Lbc, and LbcΔC were cloned in the pRK5 vector.
Reagents were purchased from the following sources: EGF, FGF2, HGF, and PDGF-BB, PMA, Y27632, puromycin, and hygromycin (Calbiochem); polyclonal antibodies to c-Myc (A14), p27 (C19), p27 (N20), H-Ras (C20), and monoclonal antibodies to c-Myc (9E10) and RhoA (26C4; Santa Cruz Biotechnology); monoclonal antibodies to Rac1, and Cdc42 (BD-Biosciences); polyclonal antibodies to phospho-Ser 3-cofilin and cofilin (Cell Signaling Technology); polyclonal antibody to RhoA, RhoB, RhoC, and recombinant ROCKII (amino acids 1–543) and MYPT1 (amino acids 714–1004; Upstate Biotechnology); mitomycin-C and the monoclonal antibody to vinculin (hVIN-1; Sigma); cyanin-3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories); phalloidin–rhodamine and Alexa 488-conjugated secondary antibodies (Molecular Probes); horseradish peroxidase-conjugated secondary antibodies (Amersham).
Tissue culture and transfections
All cells were grown in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (HyClone), 0.1 mM nonessential amino acids, 0.1% dextrose, 2 μg/mL penicillin-streptomycin, 1 mM sodium pyruvate, and 2 mM L-glutamine (Life Technologies). Primary MEFs were prepared as described (Spector 1997) and used for experiments between passages 2 and 5. Immortal MEFs used in this study were immortalized using the 3T3 protocol (Todaro and Green 1963).
HEK 293T cells were transfected by the calcium-phosphate method for 24–30 h, with a typical efficiency of 50%–60%, routinely monitored with a pBabe-puro-eGFP vector. The amount of DNA transfected was normalized for each plate using a pcDNA3.1Hygro-LacZ vector (Invitrogen). MEF transient transfections were performed using Fugene 6 (Roche) as described by the manufacturer, and p27 and p27CK– cDNAs were cloned in a pcDNA3.1 vector. p27CK– was generated by site-directed mutagenesis as described (Vlach et al. 1997). For retroviral infections, the human p27Kip1 or p27CK– cDNA was inserted in the pQCXIP and pQCXIH vectors (Clontech). Viruses were produced using Phenix ecotropic cells, and MEFs were subjected to two rounds of infection, with an infection efficiency of ∼80%. Selection for infected cells started 48 h postinfection using 4 μg/mL puromycin (pQCXIP and pBabe-Puro constructs) or 250 μg/mL hygromycin (pQCXIH constructs). Cells were kept under selection at all times.
Migration assays
Cells were seeded at 80% confluence in 60-mm dishes and grown for an additional 24 h (for experiments using growth factors, cells were starved for 48–72 h at this point). Where indicated, cells were incubated in presence of 10 μg/mL MMC for 3 h prior to the beginning of the experiment. A linear scratch, ∼1 cm wide, was done using a rubber policeman across the diameter of the plate, and rinsed with phosphate-buffered saline (PBS). Cells were fed with growth medium supplemented (or not) with serum or the appropriate growth factor at a final concentration of 25 ng/mL. Cells were incubated for the indicated time, rinsed with PBS, and fixed for 5 min in 95% ethanol/5% acetic acid at room temperature. Fixed dishes were then stained with Harris' hematoxylin overnight and rinsed with H2O. For each plate, pictures were taken on a dissection microscope (Zeiss) at a magnification of 32×. The distance migrated from the scratch line by the cells at each time point was then measured (in millimeters) on the prints.
Immunofluorescence
Cells were grown on glass coverslips for 24 h before treatment. Following the appropriate treatment, cells were rinsed in PBS and fixed in 1% paraformaldehyde at 37°C for 20 min. Coverslips were stored in PBS at 4°C until stained. Cells were permeabilized for 3 min in 0.2% Triton-X100, rinsed three times in PBS, and incubated for 1 h with primary antibodies diluted in antibody dilution buffer (PBS complemented with 3% bovine serum-albumin, 0.05% Tween-20, and 0.08% sodium azide) at 37°C. The coverslips were rinsed three times in PBS. Incubation with secondary antibodies (at a 1/500 dilution in antibody dilution buffer) was for 30 min at 37°C. The coverslips were rinsed three times in PBS and mounted on glass slides. Images were obtained on a Nikon Eclipse 800 microscope with a 40× objective using a Spot charge-coupled-device camera (Diagnostic Instruments).
Western blotting
Cells were lysed in immunoprecipitation buffer (50 mM HEPES at pH 7.5, 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 0.1% Tween-20, 1% Igepal 30; complemented with 1 mM dithiothreitol, 10 mM β-glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL pepstatin-A, and 1 mM phenylmethylsulfonylfluoride). Cellular debris was eliminated by centrifugation for 5 min at 14,000 rpm. Protein concentrations were determined using the Bio-Rad protein assay, and unless otherwise indicated, 40 μg of protein was loaded for SDS-PAGE on 12% gels. Proteins were transferred onto polyvinylidene difluoride membranes (Perkin Elmer). Membranes were blocked in PBS containing 0.1% Tween-20 and 10% milk for 1 h. Membranes were incubated with primary antibodies overnight at 4°C, and with secondary antibodies for 2 h at room temperature. All antibodies were diluted in PBS containing 0.1% Tween-20 and 5% milk. ECL (Amersham) was used for immunodetection.
Coimmunoprecipitations and pull-down assays
HEK 293T cells were lysed as described above, using 1 mL of immunoprecipitation (IP) buffer for a 100-mm plate. For each IP, 4 μg of the appropriate antibody, 100 μL of lysate, and 15 μL of protein A sepharose beads (Amersham) were incubated for 3 h at 4°C. Immunoprecipitates were rinsed three times in immunoprecipitation buffer, and 10 μL of 4× sample buffer was added to the bead pellet. The immunoprecipitates, as well as 10 μL of the corresponding lysates for protein loading, were subjected to Western blotting as described above.
Pull-down assays for Rho–GTPases were performed as described (Sander et al. 1998; Malliri et al. 2000). Briefly, cells were washed with ice-cold PBS, scraped off the plates in cell-lysis buffer (50 mM Tris-HCl at pH 7.4, 2 mM MgCl2, 1% Igepal 30, 10% glycerol, 100 mM NaCl, complemented with 1 mM dithiothreitol, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 10 μg/mL pepstatin-A) on ice. Lysates were centrifuged for 5 min at 14,000 rpm at 4°C. A fraction of the cleared lysates was incubated for 30 min at 4°C with 15 μL of GST–PAK-CD or GST–C21 bound to glutathione-coupled Sepharose beads (at ∼1 μg GST fusion protein per microliter of beads). The pellet of beads was washed three times with ice-cold cell lysis buffer, resuspended in 4× sample buffer, and subjected to SDS-PAGE as described above. For pull-down assays on MEFs, a 150-mm plate at 50% confluence was lysed in 800 μL of cell-lysis buffer; 600 μL was used for the pull-down assay, and 30 μL was loaded on SDS-PAGE for protein loading. For pull-downs on HEK 293T cells, a 100-mm plate was lysed in 1 mL of cell lysis buffer, the pull-down assay was carried out with 20 μL of lysate, and 8 μL was loaded on SDS-PAGE for protein loading.
In vitro RhoA/p27Kip1 binding assay
The indicated amount (2 or 0.5 μg) of His-tagged p27Kip1 was incubated with 10 μL of GST–RhoA or GST bound to glutathione beads in 500 μL of IP buffer for 2 h at 4°C. Bead pellets were rinsed three times in IP buffer, resuspended in 4× sample buffer, and subjected to SDS-PAGE as described above.
ROCKII kinase assays
Kinase assays were carried in a final volume of 25 μL, using 5μCi of γ32P-ATP. Twenty milli-units of recombinant ROCKII (amino acids 1–543; 5.72 nM) and the indicated amount of HIS-tagged p27Kip1 were added 5 min prior to the beginning of the reaction. One μg of MYPT1 was used as substrate for ROCK. Kinase assays were incubated for 15 min at 30°C, and the reaction was terminated by the addition of 10 μL of sample buffer. Samples were resolved on SDS-PAGE, and the gel slab was dried and subjected to autoradiography.
Acknowledgments
We are grateful to William Arthur, Channing Der, and John Collard for providing us with reagents. We thank the members of the Roberts lab for stimulating discussions and advice. A.B. is a Howard Hughes Medical Institute fellow of the Life Sciences Research Foundation. A.S. and A.H. were generously supported by a program grant from Cancer Research UK. J.M.R. is an investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1185504.
References
- Amano, A., Chihara, K., Kimura, K., Fukata, Y., Nakamura, N., Matsuura, Y., and Kaibuchi, K. 1997. Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science 275**:** 1308–1311. [DOI] [PubMed] [Google Scholar]
- Anayama, T., Furihata, M., Ishikawa, T., Ohtsuki, Y., and Ogoshi, S. 1998. Positive correlation between p27Kip1 expression and progression in human esophageal squamous cell carcinoma. Int. J. Cancer 79**:** 439–443. [DOI] [PubMed] [Google Scholar]
- Arthur, W.T. and Burridge, K. 2001. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 12**:** 2711–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, W.T., Petch, L.A., and Burridge, K. 2000. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10**:** 719–722. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi, D. and Hall, A. 2000. Ras and Rho GTPases: A family reunion. Cell 103**:** 227–238. [DOI] [PubMed] [Google Scholar]
- Besson, A., Wilson, T.L., and Yong, V.W. 2002. The anchoring protein RACK1 links protein kinase C ε to integrin β chains. J. Biol. Chem. 277**:** 22073–22084. [DOI] [PubMed] [Google Scholar]
- Bloom, J. and Pagano, M. 2003. Deregulated degradation of the CDK inhibitor p27 and malignant transformation. Semin. Cancer Biol. 13**:** 41–47. [DOI] [PubMed] [Google Scholar]
- Boehm, M., Yoshimoto, T., Crook, M., Nallamshetty, S., True, A., Nabel, G., and Nabel, E. 2002. A growth factor-dependent nuclear kinase phosphorylates p27Kip1 and regulates cell cycle progression. EMBO J. 21**:** 3390–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M. and Burridge, K. 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell. Biol. 133**:** 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coqueret, O. 2003. New roles for p21 and p27 cell cycle inhibitors: A function for each cell compartment. Trends Cell Biol. 13**:** 65–70. [DOI] [PubMed] [Google Scholar]
- Cox, E.A., Sastry, S.K., and Huttenlocher, A. 2001. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol. Biol. Cell 12**:** 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellas, A., Schultheiss, E., Leivas, M.R., Moch, H., and Torhorst, J. 1998. Association of p27Kip1, cyclin E and c-myc expression with progression and prognosis in HPV-positive cervical neoplasms. Anticancer Res. 18**:** 3991–3998. [PubMed] [Google Scholar]
- Diez-Juan, A. and Andres, V. 2003. Coordinate control of proliferation and migration by the p27Kip1/cyclin-dependent kinase/Retinoblastoma pathway in vascular smooth muscle cells and fibroblasts. Circ Res. 92**:** 402–410. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S. and Hall, A. 2002. Rho GTPases in cell biology. Nature 420**:** 629–635. [DOI] [PubMed] [Google Scholar]
- Frost, J.A., Khokhlatchev, A., Stippec, S., White, M.A., and Cobb, M.H. 1998. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J. Biol. Chem. 273**:** 28191–28198. [DOI] [PubMed] [Google Scholar]
- Goukassian, D., Diez-Juan, A., Asahara, T., Schratzberger, P., Silver, M., Murayama, T., Isner, J.M., and Andres, V. 2001. Overexpression of p27Kip1by doxycycline-regulated adenoviral vectors inhibits endothelial cell proliferation and migration and impairs angiogenesis. FASEB J. 15**:** 1877–1885. [DOI] [PubMed] [Google Scholar]
- Hu, W., Bellone, C., and Baldassare, J. 1999. RhoA stimulates p27Kip1 degradation through its regulation of cyclin E/CDK2 activity. J. Biol. Chem. 274**:** 3396–3401. [DOI] [PubMed] [Google Scholar]
- Ishida, N., Hara, T., Kamura, T., Yoshida, M., Nakayama, K., and Nakayama, K.I. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277**:** 14355–14358. [DOI] [PubMed] [Google Scholar]
- Kimura, K., Ito, M., Amano, M., Chihara, K., Fukata, Y., Nakafuku, M., Yamamori, B., Feng, J., Nakano, T., Okawa, K., et al. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273**:** 245–248. [DOI] [PubMed] [Google Scholar]
- Kouvaraki, M., Gorgoulis, V.G., Rassidakis, G.Z., Liodis, P., Markopoulos, C., Gogas, J., and Kittas, C. 2002. High expression levels of p27 correlate with lymph node status in a subset of advanced invasive breast carcinomas. Cancer 94**:** 2454–2465. [DOI] [PubMed] [Google Scholar]
- Lee, S. and Helfman, D.M. 2003. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/Cofilin pathway. J. Biol. Chem. 279**:** 1885–1891. [DOI] [PubMed] [Google Scholar]
- Leung, T., Chen, X.Q., Manser, E., and Lim, L. 1996. The p160 RhoA-binding kinase ROKa is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 16**:** 5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliri, A., ten Klooster, J.P., Olivio, C., and Collard, J.G. 2000. Determination of the activity of Rho-like GTPases in cells. Methods Mol. Biol. 189**:** 99–109. [DOI] [PubMed] [Google Scholar]
- Manser, E., Huang, H.Y., Loo, T.H., Chen, X.Q., Dong, J.M., Leung, T., and Lim, L. 1997. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 17**:** 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister, S.S., Becker-Hapak, M., Pintucci, G., Pagano, M., and Dowdy, S. 2003. Novel p27kip1 C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol. Cell. Biol. 23**:** 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall, A., Ulku, A., Lambert, Q.T., Kusa, A., Rogers-Graham, K., and Der, C.J. 2001. Oncogenic ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinositol 3-kinase. Mol. Cell. Biol. 21**:** 5488–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller, S., Head, E., and Sheaff, R. 2003. p27Kip1 inhibition of Grb2-Sos formation can regulate Ras activation. Mol. Cell. Biol. 23**:** 3735–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, H., Vocero-Akbani, A.M., Snyder, E.L., Ho, A., Latham, D.G., Lissy, N.A., Becker-Hapak, M., Ezhevsky, S.A., and Dowdy, S. 1998. Transduction of full length TAT fusion proteins into mammalian cells: TAT–p27Kip1 induces cell migration. Nat. Med. 4**:** 1449–1452. [DOI] [PubMed] [Google Scholar]
- Nobes, C. and Hall, A. 1999. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell. Biol. 144**:** 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, M.F., Paterson, H.F., and Marshall, C.J. 1998. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature 394**:** 295–299. [DOI] [PubMed] [Google Scholar]
- Philipp-Staheli, J., Payne, S.R., and Kemp, C.J. 2001. p27Kip1: Regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp. Cell Res. 264**:** 148–168. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., Kiosses, W.B., and Schwartz, M.A. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18**:** 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X.D., Kiosses, W.B., Sieg, D.J., Otey, C.A., Schlaepfer, D.D., and Schwartz, M.A. 2000. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113**:** 3637–3678. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J. and Hall, A. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70**:** 389–399. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., Paterson, H.F., Johnston, C.L., Diekmann, D., and Hall, A. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70**:** 401–410. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., Schwartz, M.A., Burridge, K., Firtel, R., Ginsberg, M., Borisy, G., Parsons, J.T., and Horwitz, A.F. 2003. Cell migration: Integrating signals from front to back. Science 302**:** 1704–1709. [DOI] [PubMed] [Google Scholar]
- Rodier, G., Montaglioni, A., Di Marcotullio, L., Coulombe, P., Draetta, G., Pagano, M., and Meloche, S. 2001. p27 cytoplasmic localization is regulated by phosphorylation on Ser 10 and is not a prerequisite for its proteolysis. EMBO J. 20**:** 6672–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez, A., Sanchez, E., Sanchez-Beato, M., Cruz, M.A., Chacon, I., Munoz, E., Camacho, F.I., Martinez-Montero, J.C., Mollejo, M., Garcia, J.F., et al. 1999. p27Kip1 is abnormally expressed in diffuse large B-cell lymphomas and is associated with an adverse clinical outcome. Br. J. Cancer 80**:** 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai, E., Olson, M.F., and Marshall, C.J. 2001. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 20**:** 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Beato, M., Camacho, F.I., Martinez-Montero, J.C., Saez, A., Villuendas, R., Sanchez-Verde, L., Garcia, J.F., and Piris, M.A. 1999. Anomalous high p27Kip1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27Kip1-cyclin D3 colocalization in tumor cells. Blood 94**:** 765–772. [PubMed] [Google Scholar]
- Sander, E.E., van Delft, S., ten Klooster, J.P., Reid, T., van Der Kammen, R.A., Michiels, F., and Collard, J.G. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell. Biol. 143**:** 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A. and Hall, A. 2002. Guanine nucleotide exchange factors for Rho GTPases: Turning on the switch. Genes & Dev. 16**:** 1587–1609. [DOI] [PubMed] [Google Scholar]
- Seasholtz, T.M., Majumdar, M., and Brown, J.H. 1999. Rho as a mediator of G protein-coupled receptor signaling. Mol. Pharmacol. 55**:** 949–956. [DOI] [PubMed] [Google Scholar]
- Sherr, C.J. and Roberts, J.M. 2001. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes & Dev. 13**:** 1501–1512. [DOI] [PubMed] [Google Scholar]
- Shimada, Y., Gulli, M., and Peter, M. 2000. Nuclear sequestration of the exchange factor Cdc24 by Far1 regulates cell polarity during yeast mating. Nat. Cell Biol. 2**:** 117–124. [DOI] [PubMed] [Google Scholar]
- Shiozawa, T., Shiohara, S., Kanai, M., Konishi, I., Fujii, S., and Nikaido, T. 2001. Expression of the cell cycle regulator p27Kip1 in normal squamous epithelium, cervical intraepithelial neoplasia, and invasive squamous cell carcinoma of the uterine cervix. Cancer 92**:** 3005–3011. [DOI] [PubMed] [Google Scholar]
- Slingerland, J. and Pagano, M. 2000. Regulation of the CDK inhibitor p27 and its deregulation in cancer. J. Cell Physiol. 183**:** 10–17. [DOI] [PubMed] [Google Scholar]
- Spector, D.L. 1997. Isolation of fibroblasts. In Cells: A laboratory manual (eds. D. Spector et al.), pp. 4.3–4.7. Cold Spring Harbor Laboratory Press, New York.
- Sugiyama, Y., Tomoda, K., Tanaka, T., Arata, Y., Yoneda-Kato, N., and Kato, J. 2001. Direct binding of the signal transduction adaptor Grb2 facilitates down-regulation of the cyclin-dependent kinase inhibitor p27Kip1. J. Biol. Chem. 276**:** 12084–12090. [DOI] [PubMed] [Google Scholar]
- Sun, J., Marx, S.O., Chen, H.J., Poon, M., Marks, A.R., and Rabbani, L.E. 2001. Role for p27Kip1 in vascular smooth muscle cell migration. Circulation 103**:** 2967–2972. [DOI] [PubMed] [Google Scholar]
- Supriatno, Harada, K., Kawaguchi, S., Yoshida, H., and Sato, M. 2003. Effect of p27Kip1 on the ability of invasion and metastasis of an oral cancer cell line. Oncol. Rep. 10**:** 527–532. [PubMed] [Google Scholar]
- Tanaka, H., Yamashita, T., Asada, M., Mizutani, S., Yoshikawa, H., and Tohyama, M. 2002. Cytoplasmic p21Cip1/Waf1 regulates neurite remodeling by inhibiting Rho-kinase activity. J. Cell. Biol. 158**:** 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro, G.J. and Green, H. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell. Biol. 17**:** 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial, E., Sahai, E., and Marshall, C.J. 2003. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 4**:** 67–79. [DOI] [PubMed] [Google Scholar]
- Vidal, A., Millard, S., Miller, J., and Koff, A. 2002. Rho activity can alter the translation of mRNA and is important for RasV12-induced transformation in a manner dependent on p27 status. J. Biol. Chem. 277**:** 16433–16440. [DOI] [PubMed] [Google Scholar]
- Vlach, J., Hennecke, S., and Amati, A. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 16**:** 5334–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrhovac, R., Delmer, A., Tang, R., Marie, J.P., Zittoun, R., and Ajchenbaum-Cymbalista, F. 1998. Prognostic significance of the cell cycle inhibitor p27Kip1 in chronic B-cell lymphocytic leukemia. Blood 91**:** 4694–4700. [PubMed] [Google Scholar]
- Watanabe, J., Sato, H., Kanai, T., Kamata, Y., Jobo, T., Hata, H., Fujisawa, T., Ohno, E., Kameya, T., and Kuramoto, H. 2002. Paradoxical expression of cell cycle inhibitor p27 in endometrioid adenocarcinomas of the uterine corpus with proliferation and clinicopathological parameters. Br. J. Cancer 87**:** 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, D.J., Parsons, J.T., and Horwitz, A.F. 2002. Adhesion assembly, disassembly and turnover in migrating cells—Over and over and over again. Nat. Cell Biol. 4**:** E97–E100. [DOI] [PubMed] [Google Scholar]
- Weber, J., Hu, W., Jefcoat, S.J., Raben, D., and Baldassare, J. 1997. Ras-stimulated extracellular signal-related kinase-1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27Kip1. J. Biol. Chem. 272**:** 32966–32971. [DOI] [PubMed] [Google Scholar]
- Worthylake, R., Lemoine, S., Watson, J., and Burridge, K. 2001. RhoA is required for monocyte tail retraction during transendothelial migration. J. Cell. Biol. 154**:** 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo, T., Toyoshima, H., Miura, M., Wang, Y., Iida, K.T., Suzuki, H., Sone, H., Shimano, H., Gotoda, T., Nishimori, S., et al. 2003. p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J. Biol. Chem. 278**:** 52919–52923. [DOI] [PubMed] [Google Scholar]