Global dissemination of a multidrug resistant Escherichia coli clone (original) (raw)
Significance
Escherichia coli sequence type 131 (ST131) is a globally disseminated multidrug-resistant clone associated with human urinary tract and bloodstream infections. Here, we have used genome sequencing to map the temporal and spatial relationship of a large collection of E. coli ST131 strains isolated from six distinct geographical regions across the world. We show that E. coli ST131 strains are distinct from other extraintestinal pathogenic E. coli and arose from a single progenitor strain prior to the year 2000.
Keywords: bacterial evolution, genomics, phylogeography, genomic epidemiology
Abstract
Escherichia coli sequence type 131 (ST131) is a globally disseminated, multidrug resistant (MDR) clone responsible for a high proportion of urinary tract and bloodstream infections. The rapid emergence and successful spread of E. coli ST131 is strongly associated with several factors, including resistance to fluoroquinolones, high virulence gene content, the possession of the type 1 fimbriae FimH30 allele, and the production of the CTX-M-15 extended spectrum β-lactamase (ESBL). Here, we used genome sequencing to examine the molecular epidemiology of a collection of E. coli ST131 strains isolated from six distinct geographical locations across the world spanning 2000–2011. The global phylogeny of E. coli ST131, determined from whole-genome sequence data, revealed a single lineage of E. coli ST131 distinct from other extraintestinal E. coli strains within the B2 phylogroup. Three closely related E. coli ST131 sublineages were identified, with little association to geographic origin. The majority of single-nucleotide variants associated with each of the sublineages were due to recombination in regions adjacent to mobile genetic elements (MGEs). The most prevalent sublineage of ST131 strains was characterized by fluoroquinolone resistance, and a distinct virulence factor and MGE profile. Four different variants of the CTX-M ESBL–resistance gene were identified in our ST131 strains, with acquisition of CTX-M-15 representing a defining feature of a discrete but geographically dispersed ST131 sublineage. This study confirms the global dispersal of a single E. coli ST131 clone and demonstrates the role of MGEs and recombination in the evolution of this important MDR pathogen.
Many multidrug-resistant (MDR) bacterial strains are now recognized as belonging to clones that originate in a specific locale, country, or even globally. Escherichia coli sequence type 131 (ST131) is one such recently emerged and globally disseminated MDR pandemic clone responsible for community and hospital-acquired urinary tract and bloodstream infections. E. coli ST131 was identified in 2008 as a major clone linked to the spread of the CTX-M-15 extended-spectrum β-lactamase (ESBL) resistance (1–3). Since then, E. coli ST131 has also been strongly associated with fluoroquinolone resistance, and coresistance to aminoglycosides and trimethoprim-sulfamethoxazole (4–6). Alarmingly, strains of E. coli ST131 resistant to carbapenems have also been reported (7, 8), further limiting treatment options for this clone.
E. coli ST131 belongs to the B2 phylogenetic subgroup I, with most isolates characterized as serotype O25b:H4 (1). Epidemiology studies using pulse-field gel electrophoresis (PFGE) have demonstrated that E. coli ST131 strains exhibit diversity, with some dominant PFGE pulsotypes including the UK epidemic strain A (9) and pulsotype 968 (10, 11) widely distributed across the globe. More recently, a typing scheme using the type 1 fimbriae fimH adhesin gene revealed that a large subclonal lineage of E. coli ST131 strains possess the FimH30 allele, which is also associated with specific mutations in the gyrA and parC genes that confer resistance to fluoroquinolones (12).
Several whole genome (13–16) and PCR (1, 17–20) studies have revealed that E. coli ST131 strains possess a variable complement of genes encoding established virulence factors commonly associated with extraintestinal pathogenic E. coli (ExPEC). Indeed, few virulence genes appear to be uniformly present in E. coli ST131 and, thus, it is likely that differences in virulence gene content contribute to the variable virulence potential that has been reported. For example, although some ST131 strains cause rapid death in a mouse sepsis infection model (21), this phenotype is not consistent among all strains (22). The E. coli ST131 strain EC958, which is a representative of the FimH30-fluoroquinolone resistant subgroup, has been characterized at the molecular level (15). E. coli EC958 contains an insertion in the type 1 fimbriae regulator gene fimB (15) that is also common to other strains in the FimH30 subgroup (23) and colonizes the mouse bladder in a type 1 fimbriae-dependent manner (15). In mice, E. coli EC958 establishes acute and chronic urinary tract infection (UTI), forms intracellular bacterial communities in the bladder (24), and causes impairment of ureter contractility (25). E. coli EC958 is also resistant to the bactericidal action of human serum (26).
The rapid global dissemination of E. coli ST131, combined with its MDR phenotype and the lack of new antimicrobial drugs in the developmental pipeline, highlights the urgent need to understand this pathogen and combat its spread. Here, we sequenced the genomes of 95 E. coli ST131 strains from six geographical regions (isolated from 2000 to 2011) to examine the spatial and temporal relationships of E. coli ST131. Our data supports the rapid and recent global dispersal of E. coli ST131 as a single clone.
Results and Discussion
A Global Collection of E. coli ST131 Strains.
A collection of 99 E. coli strains defined as ST131, using a described PCR test specific for the O25b rfb gene and allele 3 of the pabB gene (27), were isolated between 2000 and 2011 from six countries (Australia, Canada, India, Spain, United Kingdom, New Zealand) (Dataset S1). The strains were obtained from several clinical sources and included isolates from urine (n = 53), blood (n = 21), peritoneal fluid (n = 1), abdominal abscess (n = 1), surgical wound (n = 2), and rectal swabs (n = 11). The strains were selected with an endeavor to encompass diversity with respect to geographic origin, date of isolation, and clinical source. The strains possessed a range of antibiograms, including variable resistance to aminoglycosides, second and third generation cephalosporins, fluoroquinolones, penicillins, and sulfonamides (Dataset S1). All strains were sequenced by using the Illumina HiSeq, assembled using Velvet, and in silico multilocus sequence typing (MLST) was performed to confirm the sequenced strains were ST131. Four strains originally defined as ST131 by rfb and pabB PCR actually belonged to ST95 (Dataset S1), thus reducing the final number of ST131 strains examined to 95.
Rapid Global Dispersal of E. coli ST131 as a Single Clone.
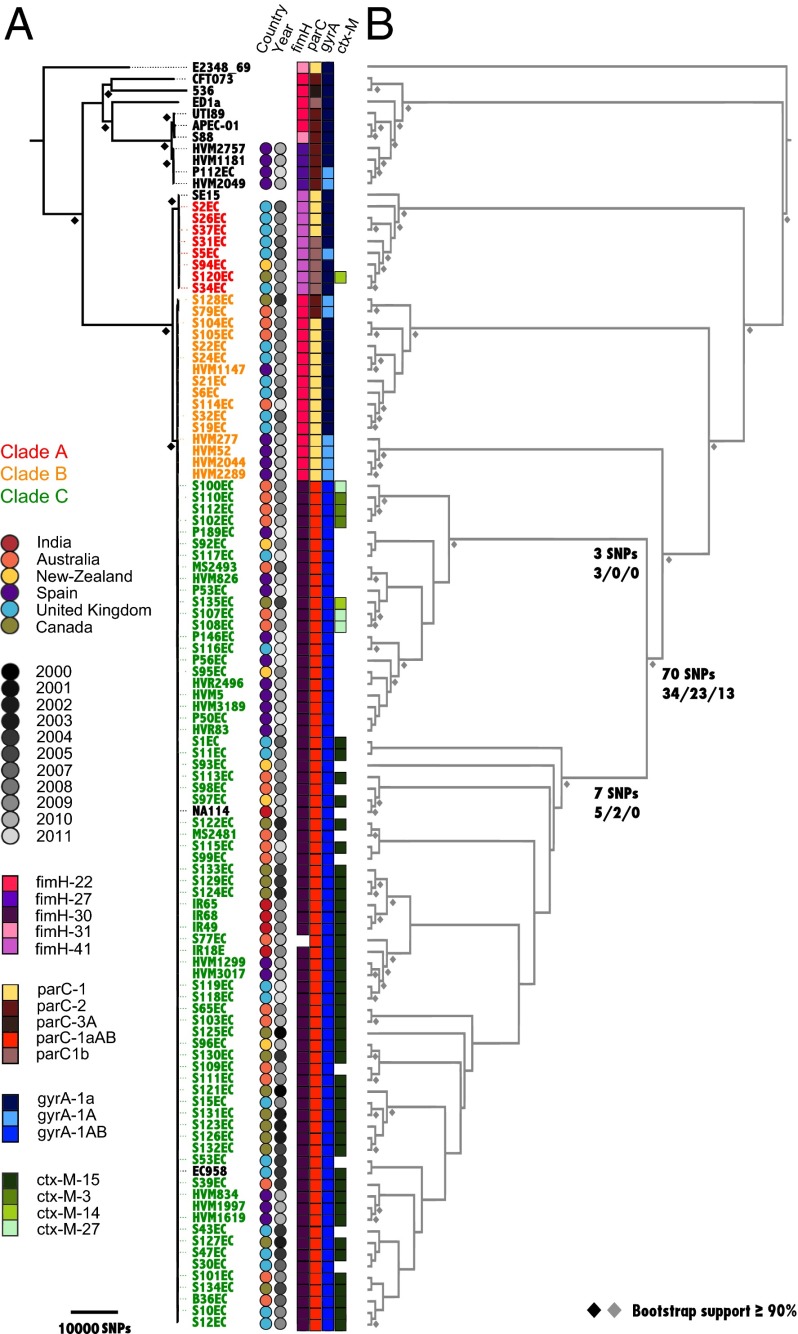
Phylogenetic analysis of the 95 E. coli ST131 strains was carried out by using whole genome alignment and single-nucleotide polymorphism (SNP) analysis using the completely sequenced ST131 representative strain SE15 (13). A maximum likelihood (ML) tree built using all 142,750 SNPs confirmed that all ST131 strains belonged to phylogroup B2, subgroup I and showed that ST131 clustered into three well-supported clades that we refer to as A, B, and C (SI Appendix, Fig. S1_A_). ML trees based on the 3,186,979-bp core alignment of the assembled sequence data supported this topology (SI Appendix, Fig. S2_A_). Recombination is the primary contributor to interclade diversity with only 70 nucleotide substitutions found to distinguish clades B and C after removal of recombinant regions (Fig. 1_A_ and Dataset S2). Neither temporal nor geographical clustering between the major clades could be observed (Fig. 1_A_); however, each clade is comprised of at least two well-supported sublineages and smaller clusters of closely-related strains that exhibit some geographical association (Fig. 1_B_ and Dataset S2). This data suggested an evolutionary history more complex than a standard geographical clonal expansion, as exemplified by many occurrences of nearly identical strains isolated in different countries and continents and over different periods of time. Similar phylogeographic patterns have been observed for other successful MDR global lineages such as Staphylococcus aureus ST239 and the PMEN1 pneumococcal lineages (28, 29), whereas a contrasting example of clonal expansion with more defined geographical clustering has been reported for Shigella sonnei (30).
Fig. 1.
Phylogenetic relationship of ST131 strains. (A) ML phylogram with triangles indicating bootstrap support of >90% from 1,000 replicates. The tree is rooted by using the outgroup phylogroup D strain UMN026; branch lengths correspond to the number of SNPs difference (scale bar bottom left). The phylogram was built from 119,514 substitution-only SNPs determined by read-mapping using E. coli SE15 as reference excluding recombinant regions, as defined by BRATNextGen analysis (34). The taxa labels for sequenced ST131 strains are colored red (clade A), orange (clade B) and green (clade C). Previously sequenced reference strains are colored black. Colored circles next to each strain correspond to country and year of isolation (see key). Squares indicate allelic profiling for fimH, parC, gyrA, and CTX-M (see key). A missing square indicates the gene is absent. (B) Several well-supported subclades are evident in the ST131 phylogeny, with the CTX-M-15 gene confined to the second subclade of clade C. The topology-only cladogram (not to scale) corresponding to the phylogram in A is shown in gray, with node support of >90% depicted as gray diamonds. The number of SNPs that define clade C and sublineages C1 (Upper) and C2 (Lower) are shown below relevant branches (nonsynonymous, synonymous, intergenic); refer to Dataset S2 for full list of SNPs and consequences.
ST131 clade A contains the previously-sequenced SE15 strain and is the most divergent clade (∼7,000 and ∼8,900 SNPs from clades B and C, respectively) characterized by the fimH41 allele and different gyrA and parC variants. ST131 clade B is very similar to clade C (distinguished by ∼2,900 SNPs) and is characterized by an intact fimB, the _fimH_22 allele, and gyrA and parC variants that are consistent with their fluoroquinolone sensitivity (Fig. 1_A_ and Dataset S1). ST131 clade C strains make up 79% of the ST131 strains sequenced in this study and are distinguished by possession of the _fimH_30 allele and the fluoroquinolone resistance alleles _gyrA_1AB and _parC_1aAB (Fig. 1_A_). All but one of the clade C strains contained an insertion within the fimB gene as we originally observed in the clade C strain EC958 (15). These isolates were collected in six countries from 2000 to 2011, indicating that the dominant clade C ST131 lineage originated from a single clone before the year 2000 (Fig. 1_A_). Although we cannot rule out the possibility of a bias in our strain collection, we note that the dominant group among another large collection of ST131 strains was also found to share the same fimH30-gyrA1AB-parC1aAB allelic profile (12).
Analysis of the density of all SNPs along the SE15 reference chromosome revealed a nonhomogeneous distribution, with many core-genome regions associated with a density ∼8.5-fold higher than the expected average (Fig. 2). Because discrete regions with a high-density of SNPs may be the result of recombination events, as opposed to mutational hotspots (31, 32), we inferred the recombination across ST131 genomes by using a Bayesian clustering approach that was previously successfully applied to S. aureus and Streptococcus pneumoniae (33, 34). We found that recombination has introduced 76.6% of the 16,424 SNPs and 2,050 small indels that differentiate the strains within the ST131 lineage (SI Appendix, Fig. S3 and Dataset S3). Phylogenetic analysis using only SNPs found in recombinant regions also clustered the ST131 strains into the same three-clades structure (SI Appendix, Figs. S1_B_ and S2_B_). Overall these results reflect the significant role that recombination has played in shaping the three major ST131 lineages with subsequent point mutations driving the fine-scale diversity within each clade.
Fig. 2.

Distribution of ST131-only core SNPs in recombinant versus nonrecombinant regions. (A) Comparison of the linear genome arrangement of the clade C strain EC958 (Upper) and the clade A strain SE15 (Lower). Solid dark-blue lines between EC958 and SE15 indicate BLAST match of ≥99% nucleotide identity between the two genomes. Genomic features of interest are highlighted for both strains as follows: prophages (pink); ST131 characteristic ROD1, ROD2, and ROD3 (yellow); previously characterized genomic islands (blue); and other regions of interest (turquoise). Labels refer to the ST131-characteristic regions defined in the genome of EC958 (15). (B) Heatmap showing the density of 16,424 ST131-only core SNPs along the SE15 chromosome: Syn_NR (synonymous, nonrecombinant); NSyn_NR (nonsynonymous, nonrecombinant); Syn_R (synonymous, recombinant); and NSyn_R (nonsynonymous, recombinant). ST131-only core SNPs were defined as bases called from the mapping data in all strains of the dataset with polymorphisms specific to the ST131 lineage. Recombinant region coordinates were delineated by using BratNextGen. The SNP density heatmap with (number of SNPs per 1 kbp nonoverlapping bin) is indicated by the color key. The x axis at the bottom of the figure represents the SE15 reference chromosome coordinates.
Antibiotic Resistance Is Associated with ST131 Clade C.
Besides the major contribution of recombination events to the between-clade diversity of ST131, we also observed differences in the distribution of SNPs between recombinant and nonrecombinant regions (Fig. 2). SNP density across all strains combined was higher in recombinant regions with an estimated 1.19 × 10−2 SNPs per site compared with 1.39 × 10−3 SNPs per site in nonrecombinant regions. Despite the lower density of SNPs, nonrecombinant regions were characterized by a relatively higher ratio of nonsynonymous to synonymous SNPs (0.05 and 0.07 SNPs per kilobase, respectively) compared with recombinant regions (0.2 and 0.89 SNPs per kilobase, respectively). This difference was significant (χ2 = 1,045.8, P < 0.00001) and is consistent with a pairwise comparison of ST131 clade A and clade C strains (23).
Fluoroquinolone resistance is one of the major determining features of the ST131 clone and is associated with point mutations in the gyrA and parC genes (12) (Fig. 1). The three major gyrA alleles found in our ST131 dataset were attributed to vertically transmitted point mutations, with unique gyrA mutations also found in clade A strain S5EC (A669T) and clade C strain B36EC (Q453R), respectively. In contrast, the _parC_1aAB allele was introduced into clade C via recombination, replacing the _parC_1 allele and surrounding Rec_089 region that is conserved in most clade A and B strains (Dataset S3). Multiple, overlapping recombination events continue to shape the ST131 lineage as evidenced by two independent replacements of Rec_089 in subgroups of clade A (encompassing _parC_2) and clade B (_parC_3A), with a further two partial replacements of a 1.8-kb Rec_089 subfragment immediately upstream of parC in two clade C strains (S101EC and S113EC). Among the 34 nonsynonymous and nonrecombinant substitutions that define clade C, we could map nine to crystal structures of homologs, several of which encode amino acid changes that may impact their function (SI Appendix, Fig. S5 and Dataset S2). For example, there is a mutation in the gene encoding the MukB chromosome partition protein, a known interacting partner of ParC (35). In addition to established fimH, parC and gyrA mutations in clade C strains, our identification of further genes with clade C-specific mutations paves the way for more targeted investigations to identify key evolutionary events that underpin the success of E. coli ST131.
Among the SNPs that have arisen in individual ST131 clade C strains or subgroups, there are a number within potential antibiotic resistance genes that may have been selected in response to antibiotic treatment (Dataset S2). Each ST131 clade C strain (minus NA114) has between 0 and 50 (mean = 13, SD = 11) unique, nonrecombinant SNPs, 49% of which are nonsynonymous. There are numerous examples of nonsynonymous SNPs within genes that encode homologs of multidrug resistance proteins or other putative transporters that may affect antimicrobial uptake or efflux (Dataset S2). There are also several SNPs in genes encoding penicillin-binding proteins (e.g., ECSF_2363/PBP1C, ECSF_0094/PBP3), other cell wall modifying enzymes (e.g., ECSF_2495 lytic murein transglycosylase B) and examples of cell division genes (e.g., ECSF_2198), or essential genes that may be important for intrinsic resistance development. Although the majority of ST131 clade C SNPs are unique to the strain in which they are found, or exhibit patterns of descent consistent with the inferred phylogeny, we identified genes in which the same mutation appeared to have been acquired independently (Dataset S2). For example, the dihydrofolate reductase gene (ESCF_0053) acquired the trimethoprim resistance L28R mutation in two phylogenetically separated clade C strains (S116EC and S11EC), with several other nonsynonymous mutations in this gene present in different strains.
The majority of clade C strains also possess the CTX-M-15 gene (36 of 42 strains in sublineage C2), with seven other clade C strains containing different CTX-M alleles (3, 14, or 27) (Fig. 1_A_ and Dataset S1). The CTX-M-15–positive strains cluster within a discrete, but temporally and geographically dispersed, sublineage within clade C (Fig. 1_B_). Although the pattern of CTX-M-15 distribution within this sublineage is suggestive of an ancestral acquisition of the CTX-M-15 gene and subsequent loss by some individual strains, this allele does not associate with any particular plasmid incompatibility group defined by sequence-based typing (SI Appendix, Fig. S4). Furthermore, the CTX-M-15 gene is found on assembled contiguous fragments (contigs) ranging in size from 1.4 kb to 10 kb with variable adjacent gene content (many of which have been previously identified on plasmids), suggesting that the CTX-M-15 gene has been independently acquired several times or that it has translocated between different plasmids or the chromosome. Both scenarios are consistent with previous reports of different types of ST131 plasmids that harbor CTX-M-15 (37).
E. coli ST131 Contains Many ExPEC-Associated Genes.
The complement of virulence-associated genes was determined in the 95 E. coli ST131 strains by examining for the presence of genes encoding chaperone-usher (CU) fimbriae (38), autotransporter (AT) proteins (39), siderophore receptors (40), toxins, colicins (41), and other genes often assessed by PCR in ExPEC (1) (SI Appendix, Fig. S6). The E. coli ST131 strains contained genes encoding type 1, Mat (ECP), Yde, ECSF_0166, EC958_4610, and Yeh fimbriae; other CU fimbriae genes including Afa and P fimbriae were variable. The complement of AT-encoding genes was highly conserved, with most strains containing genes encoding antigen 43, UpaB, UpaC, YfaL, and Sat. The ECSF_4014 AT gene was uniquely present in E. coli ST131 strains. Most E. coli ST131 strains contained a number of genes associated with iron acquisition; of note, the Yersiniabactin receptor (ECSF_1835) was found to be widely prevalent but highly diverse with 17 independent substitutions (14 nonsynonymous) confined to clade B and C strains, strongly suggesting that, like fimH, this gene may be under positive selection. Approximately 15% of E. coli ST131 strains contained genes encoding the HlyA and Cnf1 toxins. In all but clade C strain S115EC, these genes were colocated on the chromosome, which is consistent with their presence on the same genomic island in other ExPEC strains such as CFT073. In general, 131 UPEC-specific genes present in CFT073, UTI89, 536, and F11 (42) were also conserved, with only the gene encoding the putative regulator c0765 absent from all ST131 strains.
Diversity Within the ST131 Lineage Is Primarily due to Mobile Genetic Elements and Recombination of Associated Regions.
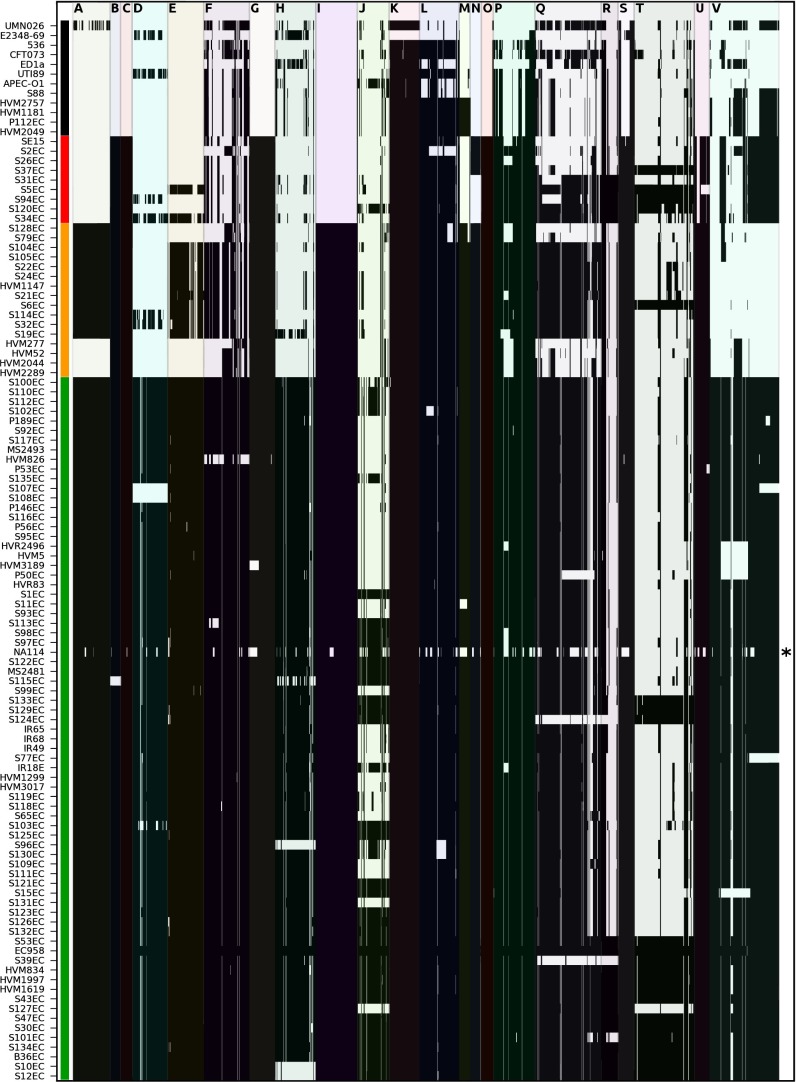
E. coli ST131 strain EC958 contains several mobile genetic elements (MGEs) and other genomic regions not found in completely sequenced non-ST131 UPEC strains (i.e., CFT073, 536, UTI89, UMN026, IAI39), including seven prophage elements (Phi1-7), the Flag-2 lateral flagellar locus, the O-antigen loci, the _ratA_-like toxin encoding gene, the type VI secretion locus, the capsular locus, and four genomic islands (GI) in chromosomal integration hotspots (GI-pheV, GI-selC, GI-leuX, and GI-thrW) (15). The majority of these regions were highly conserved in strains from clade C but were fully or partly absent in strains from clades A and B (Fig. 3 and SI Appendix, Fig. S6). Exceptions included the Phi6, GI-selC, and capsular loci regions, which were not exclusively associated with a particular clade, suggesting a more complex evolutionary history. The Flag-2 locus was completely absent in strains from clade A and in four Spanish strains of clade B (HVM277, HVM52, HVM2044, HVM2289), replaced by the fhiA-mbhA scar found in E. coli K12 strains (43). Interestingly, these four Spanish strains form a discrete sublineage (Fig. 1_B_) and also lack prophage and genomic islands that are present in other clade B strains. In contrast to the O25b serotype of most clade B and C strains, the LPS core biosynthesis region (specifically wbbJ-rfbE) of clade A strains was the same as in SE15, which has been reported as serotype O150 (13). Three regions of difference (ROD) > 10 kb in length were shown to be unique in ST131 strains EC958 and SE15 (15). Although the functions of genes encoded by ROD1 are unclear, ROD1 is conserved in all ST131 strains. Similarly, ROD2 (which contains several sugar metabolism genes) was also ubiquitous but contained deletions in at least three ST131 strains (SI Appendix, Fig. S7). ROD3 is also conserved across all ST131 strains except for clade A. The absence of several regions within the NA114 genome that are otherwise present in closely related clade C strains such as S97EC (Fig. 3) is consistent with the assembly of this genome, which was performed by concatenating ordered contigs to produce a single pseudomolecule without gap closure and finishing (14).
Fig. 3.
Selected regions of interest in ST131 strains. ST131-characteristic regions previously defined in the genome of EC958 (15) are shown along the x axis with strain identifiers listed on the y axis according to the phylogenetic tree order displayed in Fig. 1_A_. Regions A–M are shown to scale in order of their location relative to the SE15 chromosome (Fig. 2) and correspond to: A, flag2 flagellar region (38.1 kb); B, GI-ThrW genomic island; C, ROD1; D, Phi1 prophage; E, Phi2 prophage; F, Phi3 prophage; G, ROD2; H, Phi4 prophage; I, Phi5 prophage; J, Phi6 prophage; K, High-Pathogenicity Island; L, cryptic prophage; M, O-antigen 1 region (wbbJ-rfbE); N, Phi7 prophage; O, RatA-like region; P, T6SS region; Q, GI-PheV genomic island; R, capsule region; S, O-antigen2 region; T, GI-SelC genomic island; U, ROD3; V, GI-LeuX. Black shading indicates a match of ≥95% nucleotide identity in a minimum window of 200 bp calculated by comparing the query sequence to the assembled contigs or the consensus from mapped reads for each strain, as implemented in seqfindr (http://github.com/mscook/seqfindr).
A total of 137 regions were defined as recombinant within the ST131 lineage (Dataset S3), with a clear propensity to be located adjacent to predicted MGEs (SI Appendix, Fig. S3). These recombinant regions totalled 0.94 Mb, or nearly one-fifth of the entire E. coli SE15 genome and include the aforementioned fimH and parC genes, which are found on recombinant regions Rec_137 (92.3 kb) and Rec_089 (18.5 kb), respectively. Although the majority of regions are less than 1,000 bp in length, ∼80% of the recombinant bases are contained within 24 large recombinant regions that range in size from 10.2 kb to 166.2 kb. We could define the lineage on which the recombination event occurred in the majority of cases; however, the larger fragments, such as Rec_088, Rec_089, and Rec_137, have a more complex evolutionary history with evidence for multiple blocks of different origin, reflecting sequential, overlapping recombination events within the same region (SI Appendix, Fig. S3 and Dataset S3). When considering the repertoire of recombinant regions that distinguish each clade, clade A was the most distant, with a total of 0.52 and 0.6 Mb differing from clade B and C, respectively. Fewer recombinant regions distinguish clade B from clade C, with the majority of differences contained in regions upstream of Phi3, and upstream and downstream of GI-pheV and GI-leuX, respectively (Dataset S3).
A striking feature of the recombination distribution along the chromosome is that the majority of large recombinant regions were associated with the sites of insertion of prophage and genomic island MGEs (SI Appendix, Fig. S3). Statistical evaluation of 10,000 replicates of the Kolmogorov–Smirnov test confirmed that the distribution of the observed distances between recombinant regions and MGEs was significantly negatively skewed compared with randomly selected regions (K–S test, mean D = 0.370, SD = 0.049, mean P = 6.082 × 10−6, SD = 8.004 × 10−5) (SI Appendix, Fig. S8). This phenomenon has been observed in a comparison using the E. coli ST131 SE15 and NA114 genomes, for which our analysis agrees with 20 of 22 recombination regions (23), and in other comparisons of closely related E. coli genomes (44). In contrast, a reduced role for recombination was reported in a study comparing 12 ST131 genomes and 50 publicly available E. coli reference genomes (45).
Recombinant Regions Have Shaped the ST131 Lineage.
Fimbrial adhesins and bacterial motility genes were significantly overrepresented in recombinant fragments (SI Appendix, Fig. S9). A prime example was the fliC-fliY flagellar locus encoded on the recombinant fragment Rec_051 (ECSF_1762 to ECSF_1776). In SE15 and other ST131 clade A strains, the fliC allele corresponds to the H5 serogroup. In contrast, clade B and C strains possess an H4 fliC allele within Rec_051, a 12.6-kb recombinant fragment that is adjacent to the Phi5 insertion in EC958. The fim operon containing the type 1 fimbrial fimH gene resides within Rec_137, which at 92.6 kb is one of the largest and most complex recombinant fragments within the ST131 lineage (Dataset S3). The subfragment of Rec_137 that encodes the region fimC to uxuR displayed characteristic recombination patterns, introducing a clade-specific fimH allele (_fimH_41 in clade A, _fimH_22 in clade B, and _fimH_30 in clade C). Interestingly, these three fimH alleles were also identified as the major signatures in a small collection of mainly American isolates, and the same recombinant region was deduced from the comparison of SE15 and NA114 (23). As observed in EC958 (15), an insertion within fimB was found in clade C strains, although it is not clear if this insertion was acquired by homologous recombination concomitant with the acquisition of the _fimH_30 subfragment, or subsequent to this event. The only exception in our collection was the ST131 clade C strain S77EC, which contained a large deletion encompassing part of the 3′ end of the adjacent GI-leuX island (Fig. 3) and the fim locus.
Several regions containing putative virulence genes, namely Rec_087 (ECSF_2626 to ECSF_2634) and part of Rec_088 (ECSF_2784 to ECSF_2804), which contain genes related to a Type 6 Secretion System (T6SS) and a Type 2 Secretion System (T2SS), respectively, have also undergone gene conversion. Clade B and C strains carry T6SS alleles that are distinct from clade A strains. In contrast, the T2SS locus in clade C strains appears to have been subjected to several independent recombination events, consistent with its location in a recombination hotspot downstream of the GI-pheV island (SI Appendix, Fig. S3). Between the T2SS region and GI-pheV, the Rec_088 recombinant fragment also encodes the group II capsule synthesis locus (ECSF_2771 to ECSF_2783). Several variant region 2 gene clusters were observed between region 1 (kpsFEDUCS) and region 3 (kpsTM) of ST131 genomes, consistent with multiple instances of replacement since divergence of ST131 clades A and C with corresponding differences in K-antigen serotype (46). As described above, differences in the LPS core biosynthesis locus within the 70.3-kb Rec_069 recombinant fragment suggest that the O25b serotype is also associated with divergence of clades B and C from clade A (13).
Several less-well characterized genomic regions that could differentiate clade C strains from other ST131 strains were also identified. Two regions with the most distinctive recombination profiles that clearly distinguished all three clades were Rec_131 (ECSF_4099 to ECSF_4159) upstream of GI-leuX, and part of Rec_137 (ECSF_4277 to ECSF_4338). The Rec_131 region contains the tamAB genes, which encode a recently described translocation and assembly module that contributes to the secretion of some AT proteins (47), whereas the Rec_137 region contains genes associated with salt resistance (osmY), siderophore-based iron transport (fhuF), and regulation (creBC). When the impact of recombination on major gene functions independent of virulence was considered, significant differences were observed in genes encoding transporters, fructose-mannose metabolism, histidine metabolism, and the pentose-phosphate pathway (SI Appendix, Fig. S9). The impact of these sequence changes remains to be determined.
Conclusion
Our whole-genome phylogenetic analysis indicates that ST131 has arisen from a single progenitor E. coli that diverged into three sublineages some time before the year 2000 with acquisition of multiple mobile genetic elements, associated recombination events, and point-mutations jointly responsible for the emergence of the most prevalent clade C strains. In addition to the known fimH, fimB, parC, and gyrA alleles that characterize ST131 clade C, we have defined several additional genes and regions that may be important for adaptive diversification in response to host or antibiotic resistance pressures. These results also provide a framework for future PCR-based assays to rapidly classify ST131 strains and monitor their evolution. Further molecular analysis of the clade defining variants and MGEs identified in this study will help to elucidate the mechanisms that have led to ST131 colonization of the urinary tract and other clinical sites, and the rapid global dispersal of this important group of ExPEC.
Materials and Methods
Genome Sequencing and Assembly.
Draft genomes were generated by using 100-bp paired-end Illumina HiSeq 2000 reads and assembled with Velvet (48). Contigs ≥200 bp were ordered against the EC958 draft genome (BioProject: PRJEA61443) by using Mauve (49). Sequencing reads are available at the European Nucleotide Archive under study number ERP001354, accessions in Study ERP001354 (ERS126551–ERS126646) (see Dataset S1 for accession numbers) with draft genomes available at http://github.com/BeatsonLab-MicrobialGenomics/ST131_99. See also SI Appendix, SI Materials and Methods.
Genome Analysis.
Alignment of the ST131 draft genome assemblies and three ST131 reference genomes (SE15, NA114, and EC958), plus completely sequenced non-ST131 genomes belonging to the E. coli B2 phylogenetic group (CFT073, UTI89, E2348/69, ED1a, 536, S88, APEC O1), was performed by using Mugsy (50) and GBLOCKS (51) with a minimum syntenic block of 5 kbp. Recombination in the ST131 sequences was estimated by using BratNextGen, which implements a Bayesian clustering algorithm for detection of recombinant fragments in closely related sequences (34). See also SI Appendix, SI Materials and Methods.
Read Mapping and SNP Analysis.
Reads from each ST131 isolate and reads simulated in silico for the 10 complete genomes used in this study were mapped onto the reference genome SE15 (16) by using SHRIMP 2.0 (52). Nesoni (www.vicbioinformatics.com/software.nesoni.shtml) was used to perform SNP calling (conservative default parameters), small indel prediction, and coding effect SNP annotation. In addition, the Nesoni n-way pairwise comparison method was used to establish the list of all polymorphic positions conserved in all strains of the dataset. Polymorphic substitution-only sites were concatenated to produce an alignment that was used for phylogenetic tree construction. Analysis and visualization of SNP distribution across the collection were performed by using custom R scripts. See also SI Appendix, SI Materials and Methods.
Phylogeny.
ML phylogenetic trees were estimated by using RAxML 7.2.8 (53) for the inferred core genome and the SNP alignments (prerecombination and postrecombination filtering) under the GTR nucleotide substitution model with a gamma correction for ASRV. Recombination filtering was performed by collapsing the recombinant segment boundaries predicted for each strain into a unique list of 137 nonoverlapping segments and subsequently masking these regions from the alignment (Dataset S3). Support for nodes was assessed by using 1,000 random bootstrap replicates. See also SI Appendix, SI Materials and Methods.
Comparative Genomics.
Virulence factor profiles, and the presence of other regions in the draft genomes, were visualized by using seqfindr (http://github.com/mscook/seqfindr). Query sequences and their source are listed in Dataset S1 and with sequences available at http://github.com/BeatsonLab-MicrobialGenomics/ST131_99/. Comparisons between individual genomes and verification of seqfindr results were performed by using BLAST (54), Artemis Comparison Tool (55), Easyfig (56), and BRIG (57). See also SI Appendix, SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank Deborah Williamson, Karina Kennedy, and Hanna Sidjabat for their contribution of strains. We thank the Wellcome Trust Sanger Institute and Australian Genome Research Facility for sequencing. This work was supported by Australian National Health and Medical Research Council (NHMRC) Grants APP1012076 and APP1067455 (to M.A.S. and S.A.B.). N.K.P. and E.S. were supported by the Australian Infectious Diseases Research Centre. M.A.S. is supported by Australian Research Council (ARC) Future Fellowship FT100100662. M.T. is supported by ARC Discovery Early Career Researcher Award DE130101169. M.D. is supported by NHMRC Training Fellowship GNT0635250. J.R.-B. and A.P. are supported by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, the Spanish Network for Research in Infectious Diseases RD12/0015; Fondo de Investigación Sanitaria Grants 070190, 10/02021, 10/0195, and 10/00795; and Junta de Andalucía Grants 0048/2008 and CTS-5259.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Database deposition: The sequences reported in this paper have been deposited in the European Nucleotide Archive under study nos. ERP001354 and ERP004358. For a list of accession numbers, see Dataset S1.
References
- 1.Nicolas-Chanoine MH, et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother. 2008;61(2):273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 2.Coque TM, et al. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg Infect Dis. 2008;14(2):195–200. doi: 10.3201/eid1402.070350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau SH, et al. Major uropathogenic Escherichia coli strain isolated in the northwest of England identified by multilocus sequence typing. J Clin Microbiol. 2008;46(3):1076–1080. doi: 10.1128/JCM.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers BA, Sidjabat HE, Paterson DL. Escherichia coli O25b-ST131: A pandemic, multiresistant, community-associated strain. J Antimicrob Chemother. 2011;66(1):1–14. doi: 10.1093/jac/dkq415. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JR, et al. Escherichia coli sequence type ST131 as an emerging fluoroquinolone-resistant uropathogen among renal transplant recipients. Antimicrob Agents Chemother. 2010;54(1):546–550. doi: 10.1128/AAC.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida Y, et al. Clonal spread in Eastern Asia of ciprofloxacin-resistant Escherichia coli serogroup O25 strains, and associated virulence factors. Int J Antimicrob Agents. 2010;35(5):444–450. doi: 10.1016/j.ijantimicag.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Peirano G, Schreckenberger PC, Pitout JD. Characteristics of NDM-1-producing Escherichia coli isolates that belong to the successful and virulent clone ST131. Antimicrob Agents Chemother. 2011;55(6):2986–2988. doi: 10.1128/AAC.01763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris D, et al. Detection of OXA-48 carbapenemase in the pandemic clone Escherichia coli O25b:H4-ST131 in the course of investigation of an outbreak of OXA-48-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56(7):4030–4031. doi: 10.1128/AAC.00638-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau SH, et al. UK epidemic Escherichia coli strains A-E, with CTX-M-15 beta-lactamase, all belong to the international O25:H4-ST131 clone. J Antimicrob Chemother. 2008;62(6):1241–1244. doi: 10.1093/jac/dkn380. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JR, et al. MASTER Investigators Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967-2009. Emerg Infect Dis. 2012;18(4):598–607. doi: 10.3201/eid1804.111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee R, et al. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol. 2013;34(4):361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JR, et al. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J Infect Dis. 2013;207(6):919–928. doi: 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toh H, et al. Complete genome sequence of the wild-type commensal Escherichia coli strain SE15, belonging to phylogenetic group B2. J Bacteriol. 2010;192(4):1165–1166. doi: 10.1128/JB.01543-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avasthi TS, et al. Genome of multidrug-resistant uropathogenic Escherichia coli strain NA114 from India. J Bacteriol. 2011;193(16):4272–4273. doi: 10.1128/JB.05413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Totsika M, et al. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: Genome analysis and virulence mechanisms. PLoS ONE. 2011;6(10):e26578. doi: 10.1371/journal.pone.0026578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark G, et al. Genomic analysis uncovers a phenotypically diverse but genetically homogeneous Escherichia coli ST131 clone circulating in unrelated urinary tract infections. J Antimicrob Chemother. 2012;67(4):868–877. doi: 10.1093/jac/dkr585. [DOI] [PubMed] [Google Scholar]
- 17.Gibreel TM, et al. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J Antimicrob Chemother. 2012;67(2):346–356. doi: 10.1093/jac/dkr451. [DOI] [PubMed] [Google Scholar]
- 18.Coelho A, et al. Spread of Escherichia coli O25b:H4-B2-ST131 producing CTX-M-15 and SHV-12 with high virulence gene content in Barcelona (Spain) J Antimicrob Chemother. 2011;66(3):517–526. doi: 10.1093/jac/dkq491. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis. 2010;51(3):286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 20.Lavigne JP, et al. Virulence potential and genomic mapping of the worldwide clone Escherichia coli ST131. PLoS ONE. 2012;7(3):e34294. doi: 10.1371/journal.pone.0034294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clermont O, et al. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J Antimicrob Chemother. 2008;61(5):1024–1028. doi: 10.1093/jac/dkn084. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JR, Porter SB, Zhanel G, Kuskowski MA, Denamur E. Virulence of Escherichia coli clinical isolates in a murine sepsis model in relation to sequence type ST131 status, fluoroquinolone resistance, and virulence genotype. Infect Immun. 2012;80(4):1554–1562. doi: 10.1128/IAI.06388-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul S, et al. Role of homologous recombination in adaptive diversification of extraintestinal Escherichia coli. J Bacteriol. 2013;195(2):231–242. doi: 10.1128/JB.01524-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Totsika M, et al. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis. 2013;208(6):921–928. doi: 10.1093/infdis/jit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floyd RV, et al. Escherichia coli-mediated impairment of ureteric contractility is uropathogenic E. coli specific. J Infect Dis. 2012;206(10):1589–1596. doi: 10.1093/infdis/jis554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phan MD, et al. The serum resistome of a globally disseminated multidrug resistant uropathogenic Escherichia coli clone. PLoS Genet. 2013;9(10):e1003834. doi: 10.1371/journal.pgen.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clermont O, et al. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J Antimicrob Chemother. 2009;64(2):274–277. doi: 10.1093/jac/dkp194. [DOI] [PubMed] [Google Scholar]
- 28.Croucher NJ, et al. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011;331(6016):430–434. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris SR, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327(5964):469–474. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt KE, et al. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat Genet. 2012;44(9):1056–1059. doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martincorena I, Seshasayee AS, Luscombe NM. Evidence of non-random mutation rates suggests an evolutionary risk management strategy. Nature. 2012;485(7396):95–98. doi: 10.1038/nature10995. [DOI] [PubMed] [Google Scholar]
- 32.Touchon M, et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 2009;5(1):e1000344. doi: 10.1371/journal.pgen.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillo-Ramírez S, et al. Phylogeographic variation in recombination rates within a global clone of methicillin-resistant Staphylococcus aureus. Genome Biol. 2012;13(12):R126. doi: 10.1186/gb-2012-13-12-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marttinen P, et al. Detection of recombination events in bacterial genomes from large population samples. Nucleic Acids Res. 2012;40(1):e6. doi: 10.1093/nar/gkr928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, et al. Escherichia coli condensin MukB stimulates topoisomerase IV activity by a direct physical interaction. Proc Natl Acad Sci USA. 2010;107(44):18832–18837. doi: 10.1073/pnas.1008678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantón R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9(5):466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Woodford N, et al. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob Agents Chemother. 2009;53(10):4472–4482. doi: 10.1128/AAC.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurpel DJ, Beatson SA, Totsika M, Petty NK, Schembri MA. Chaperone-usher fimbriae of Escherichia coli. PLoS ONE. 2013;8(1):e52835. doi: 10.1371/journal.pone.0052835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells TJ, Totsika M, Schembri MA. Autotransporters of Escherichia coli: A sequence-based characterization. Microbiology. 2010;156(Pt 8):2459–2469. doi: 10.1099/mic.0.039024-0. [DOI] [PubMed] [Google Scholar]
- 40.Alteri CJ, Mobley HL. Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine. Infect Immun. 2007;75(6):2679–2688. doi: 10.1128/IAI.00076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruant G, et al. Development and validation of an oligonucleotide microarray for detection of multiple virulence and antimicrobial resistance genes in Escherichia coli. Appl Environ Microbiol. 2006;72(5):3780–3784. doi: 10.1128/AEM.72.5.3780-3784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd AL, Rasko DA, Mobley HL. Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J Bacteriol. 2007;189(9):3532–3546. doi: 10.1128/JB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren CP, Beatson SA, Parkhill J, Pallen MJ. The Flag-2 locus, an ancestral gene cluster, is potentially associated with a novel flagellar system from Escherichia coli. J Bacteriol. 2005;187(4):1430–1440. doi: 10.1128/JB.187.4.1430-1440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Didelot X, Méric G, Falush D, Darling AE. Impact of homologous and non-homologous recombination in the genomic evolution of Escherichia coli. BMC Genomics. 2012;13:256. doi: 10.1186/1471-2164-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNally A, Cheng L, Harris SR, Corander J. The evolutionary path to extraintestinal pathogenic, drug-resistant Escherichia coli is marked by drastic reduction in detectable recombination within the core genome. Genome Biol Evol. 2013;5(4):699–710. doi: 10.1093/gbe/evt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 47.Selkrig J, et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat Struct Mol Biol. 2012;19(5):506–510. doi: 10.1038/nsmb.2261. [DOI] [PubMed] [Google Scholar]
- 48.Zerbino DR, Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darling AE, Mau B, Perna NT. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5(6):e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angiuoli SV, Salzberg SL. Mugsy: Fast multiple alignment of closely related whole genomes. Bioinformatics. 2011;27(3):334–342. doi: 10.1093/bioinformatics/btq665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17(4):540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 52.David M, Dzamba M, Lister D, Ilie L, Brudno M. SHRiMP2: Sensitive yet practical SHort Read Mapping. Bioinformatics. 2011;27(7):1011–1012. doi: 10.1093/bioinformatics/btr046. [DOI] [PubMed] [Google Scholar]
- 53.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 54.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carver T, et al. Artemis and ACT: Viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24(23):2672–2676. doi: 10.1093/bioinformatics/btn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan MJ, Petty NK, Beatson SA. Easyfig: A genome comparison visualizer. Bioinformatics. 2011;27(7):1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information