Multi-scale Multi-mechanism Design of Tough Hydrogels: Building Dissipation into Stretchy Networks (original) (raw)
. Author manuscript; available in PMC: 2015 Feb 7.
Published in final edited form as: Soft Matter. 2014 Feb 7;10(5):672–687. doi: 10.1039/C3SM52272E
Abstract
As swollen polymer networks in water, hydrogels are usually brittle. However, hydrogels with high toughness play critical roles in many plant and animal tissues as well as in diverse engineering applications. Here we review the intrinsic mechanisms of a wide variety of tough hydrogels developed over past few decades. We show that tough hydrogels generally possess mechanisms to dissipate substantial mechanical energy but still maintain high elasticity under deformation. The integrations and interactions of different mechanisms for dissipating energy and maintaining elasticity are essential to the design of tough hydrogels. A matrix that combines various mechanisms is constructed for the first time to guide the design of next-generation tough hydrogels. We further highlight that a particularly promising strategy for the design is to implement multiple mechanisms across multiple length scales into nano-, micro-, meso-, and macro-structures of hydrogels.
Keywords: hydrogel, interpenetrating polymer network, hybrid crosslinkers, high-functionality crosslinkers, fiber reinforcement
1. Introduction
As aggregations of polymer networks and water, hydrogels are abundant in plant and animal tissues, with examples ranging from xylems and phloems to muscles and cartilages.1, 2 Owning to their unique integration of solid and liquid properties, hydrogels are also extensively explored and widely used in diverse applications such as contact lenses, wound dressings, cosmetics, absorbents in waste managements, media for electrophoresis, scaffolds for tissue engineering, vehicles for drug delivery, coatings for medical devices, extracellular matrices for biological studies, packers in oilfields, and sensors and actuators in soft machines.3–16
Natural hydrogels in plant and animal tissues usually need to be sufficiently robust to support mechanical loads from surrounding components. Similarly, many applications of hydrogels require them to maintain physical integrity over time, such as in contact lenses, wound dressings, drug delivery, and coatings; other applications even require hydrogels to carry significant mechanical loads and/or accommodate substantial deformation, such as in artificial load-bearing tissues, oilfield packers, and hydrogel-based actuators and soft machines.
Swelling of polymer networks in water usually reduces their mechanical strengths, leading to relatively brittle hydrogels. However, due to tremendous demands for tough hydrogels in various applications, intensive researches have been going on to improve mechanical strengths of hydrogels over the last few decades. Many hydrogels have shown significant enhancements of fracture toughness over their conventional counterparts (Fig. 1). Examples include poly (vinyl alcohol) hydrogels with crystalline domains17–19, double-network hydrogels20, 21, hydrogels with hybrid chemical and physical crosslinkers21–27, hydrogels with crosslinkers of high functionalities28–32, hydrogels with transformable domains33, 34, topological hydrogels with sliding crosslinkers35–37, and tetra-arm polymer hydrogels38, 39. Many tough hydrogels have also been made biocompatible for biomedical applications40–44 or responsive to external stimuli for actuators and soft machines45–48. A number of review articles have summarized the progresses of various types of tough hydrogels.45, 49–57
Figure 1. Examples of hydrogels with enhanced mechanical strengths.
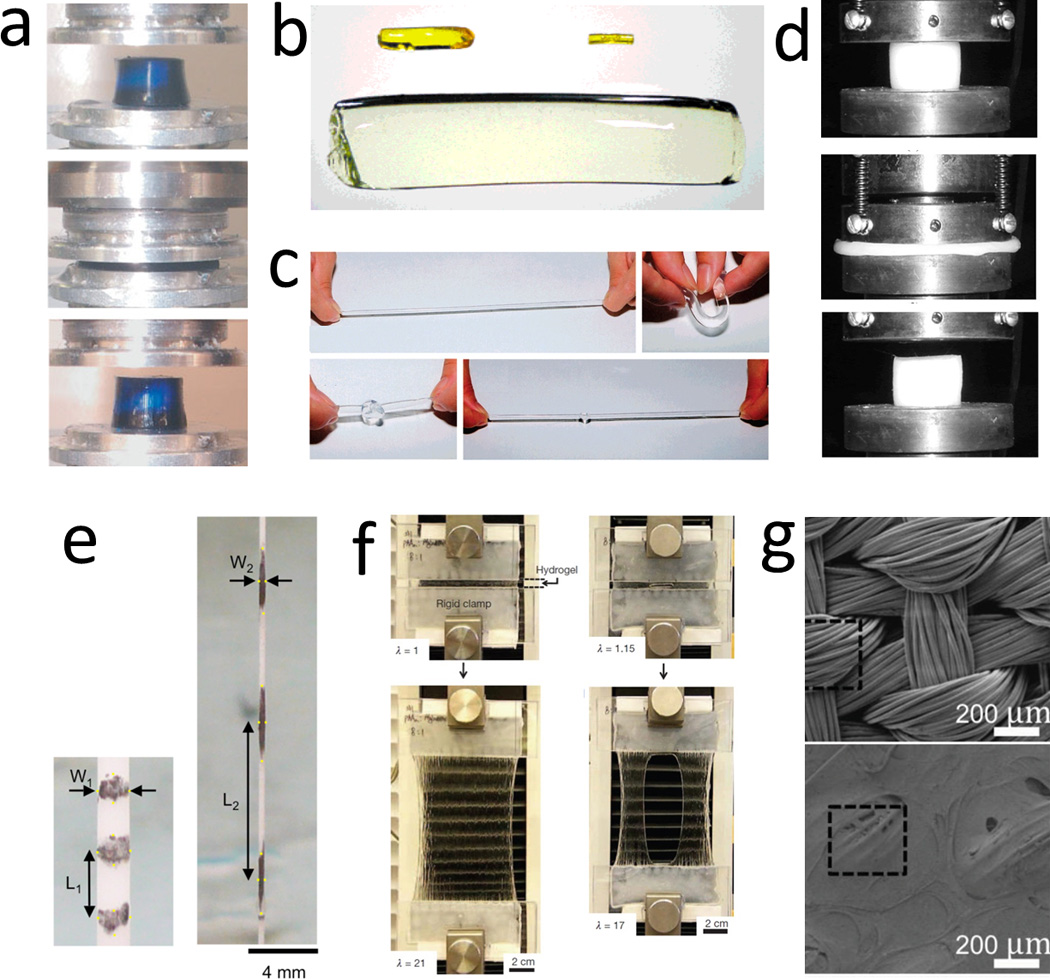
a. double-network hydrogel, reproduced with permission20, b. topological hydrogel with sliding crosslinkers, reproduced with permission35, c. hydrogel with nano-clay crosslinkers, reproduced with permission32, d. hydrogel with micro-sphere crosslinkers, reproduced with permission31, e. fibrin hydrogel with folded protein domains, reproduced with permission33, f. hydrogel with hybrid physical and chemical crosslinkers, reproduced with permission21, and g. fiber-reinforced hydrogel, reproduced with permission130.
Despite these successes, the developments of tough hydrogels have generally relied on an empirical and trial-and-error approach, whereas the intrinsic relations between various mechanisms for toughness enhancements have not been systematically discussed or explored.54, 55, 58 For instance, hydrogels with microscopic interpenetrating polymer networks20 and hydrogels with macroscopic fiber-reinforcements59 have both achieved impressive levels of fracture toughness. These hydrogels have very different compositions and structures and therefore different toughness-enhancement mechanisms at different length scales. However, it is not clear whether there exists a general principle underlying various toughness-enhancement mechanisms for hydrogels. Furthermore, the mechanical properties of existing tough hydrogels may still suffer from limitations. For example, some double-network hydrogels are susceptible to fatigue under cyclic loadings, due to the damage of polymer networks under large deformation; and there are substantial demands for new hydrogels with further enhanced mechanical properties, such as anti-fatigue hydrogels. Therefore, a general principle together with practical guidelines for the design of tough hydrogels will be of critical importance to the development of next-generation hydrogels as well as the fundamental understanding of soft materials.
The objectives of the current review are: i) to present a general principle that underlies various toughness-enhancement mechanisms in existing tough hydrogels, ii) provide a matrix of guidelines for the design of tough hydrogels, and iii) propose new strategies for the development of next-generation hydrogels with extraordinary mechanical properties. Using the theory of dissipation-induced toughening60–63, we will demonstrate that a general principle for the design of tough hydrogels is to implement mechanisms into hydrogels to dissipate significant amounts of mechanical energy under large deformation and to maintain their original configurations after deformation. While the developments of various tough hydrogels have indeed followed this general principle, we will show that different hydrogels have resorted to different mechanisms to dissipate mechanical energy or to maintain high elasticity. Consequently, a combination of the mechanisms for dissipating energy and maintaining elasticity naturally provides a matrix that can guide the design of nano-, micro-, meso-, and macro-structures of hydrogels to achieve high toughness. Thereafter, we will show that many possible combinations on the design matrix have not been explored for tough hydrogels. We will finally propose that integrating different mechanisms across multiple length scales represents a particularly promising strategy for the design of next-generation hydrogels with extraordinary mechanical properties.
2. General principle for the design of tough hydrogels
2.1. Fracture energy to characterize toughness of hydrogels
Since hydrogels generally undergo nonlinear and large deformation, the commonly used fracture toughness for linear elastic materials is generally not applicable to hydrogels. As a result, the toughness of hydrogels has been traditionally measured with a number of parameters, including Young’s and shear modulus, swelling ratio, fracture stress in tension, compression and shear; fracture strain in tension, compression and shear, and fracture energy. A tough hydrogel should be able to sustain relatively high levels of both mechanical load (i.e., stress) and deformation (i.e., strain), regardless of defects in it. However, modulus only characterizes stiffness of hydrogels under small deformation; swelling ratio only gives hydrogels’ water-retaining capacity; and fracture stress or strain only reflect the hydrogels’ capability of sustaining either mechanical load or deformation, respectively. In addition, the measured values of fracture stress and strain usually depend on the nature of defects in hydrogels. In this regard, fracture energy is a more adequate parameter to characterize fracture toughness of hydrogels than others.64
To clearly illustrate the importance of fracture energy, let’s consider the classical pure-shear test for measuring fracture energy of soft materials such as elastomers and gels.65–68 As illustrated in Fig. 2, two identical pieces of a hydrogel are fabricated with the same thickness T, width W and high H, where _W_>> H_>>T. Both pieces of samples are clamped along their long edges (i.e., along the width direction) with rigid plates. A notch with a length of ~0.5_W is introduced into the first sample, which is then gradually pulled to a stretch of λc times of its undeformed length until a crack begins to propagate from the notch (Fig. 2a). Thereafter, the second sample without notch is uniformly stretched to the same critical stretch λc, with the applied force F recorded as a function of the stretch λ (Fig. 2b). Since _W_>>H in the first sample, regions of hydrogel far away from the crack tip are either fully relaxed or uniformly deformed. Therefore, propagation of the crack can be regarded as transition of a uniformly-deformed region into a fully-relaxed region with the same width (Fig. 2a). The energy required to advance the notch by a unit area at the undeformed state (i.e., the fracture energy) can be calculated as Γ=(H∫1λcFdλ)/WT, where F and λ have been measured in the second sample (Fig. 2b). Furthermore, since the nominal stress along the applied force in the second sample is s = F /(WT), we can express the measured fracture energy of the hydrogel as65, 66
Figure 2. Schematics of the pure-shear test for measuring fracture energy of hydrogels.
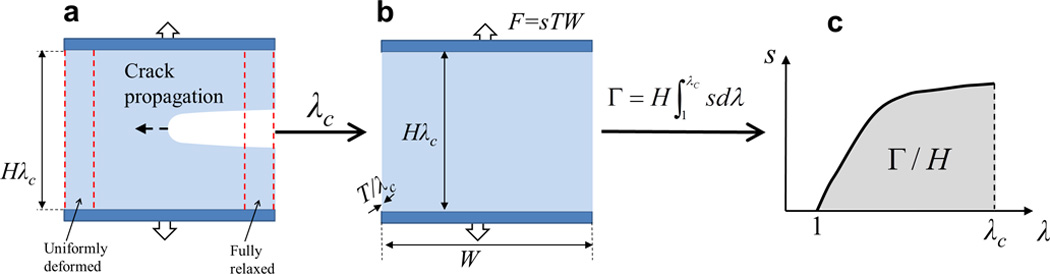
a. a piece of a hydrogel with a notch was stretched to a critical stretch of λ_c_ until the crack propagates; b. the same piece of hydrogel but without notch was stretched to λ_c_ with the nominal stress s recorded; and c. the fracture energy of the hydrogel can be calculated as Γ=H∫1λcsdλ.
From Eq. (1), it can be seen that a hydrogel with higher fracture energy tends to be able to sustain higher levels of both stress and strain, and therefore has a higher toughness. Furthermore, the measured fracture energy is independent of defects in hydrogels, because the size of the notch is much larger than any defect in the samples. Owning to these merits, fracture energy has been widely used as a critical parameter to characterize fracture toughness of hydrogels. It should be noted that many other methods can also be used to measure fracture energies of gels such as the trouser tear test65 and single-edge notch test69, and the measured fracture energies with different methods are generally consistent with one another21.
Further considering the mechanistic origins of fracture energy, we can generally divide the fracture energy of a hydrogel into two parts, i.e.
where Γ0 is the intrinsic fracture energy of the hydrogel and ΓD the fracture energy due to mechanical dissipation in regions around the crack (Fig. 3).
Figure 3. The fracture energy of a hydrogel can be divided into two parts.
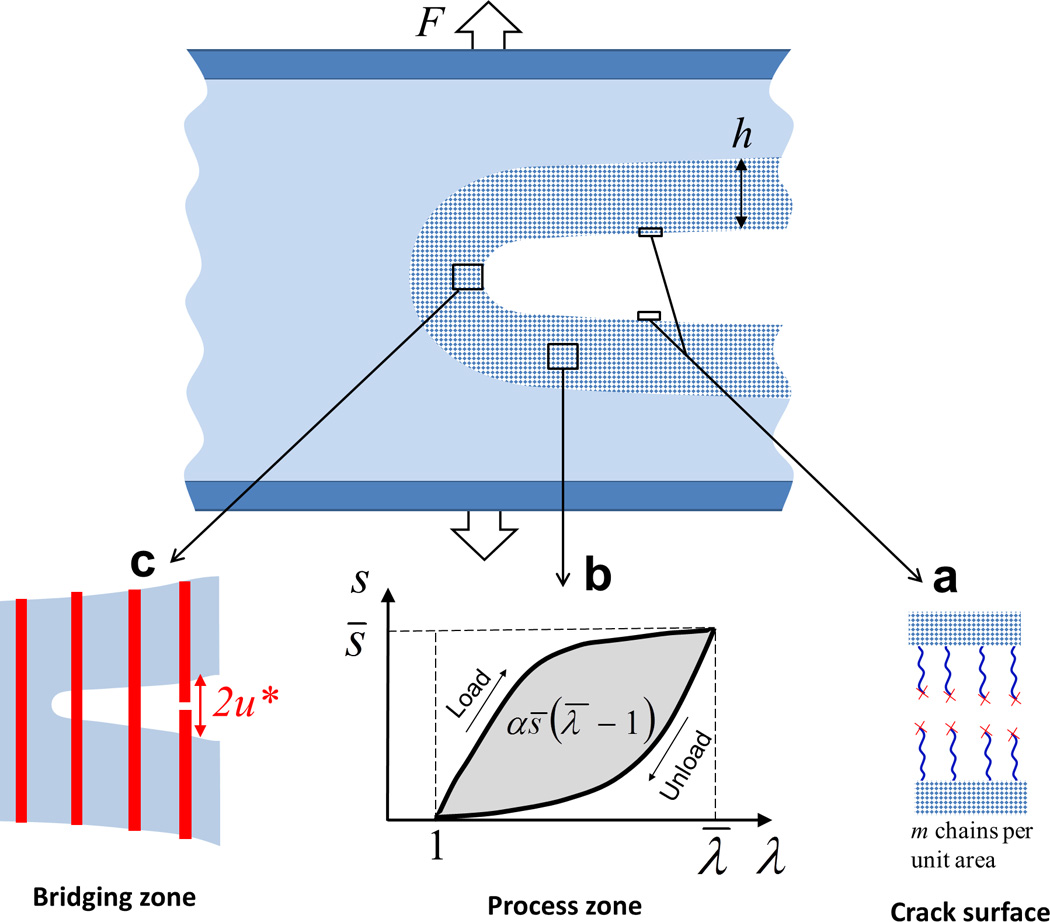
a. the intrinsic fracture energy by rupturing polymer chains along the crack plane, and b. the mechanical energy dissipated by loading and unloading hydrogel in process zone and c. by pullout of fibers and fillers in bridging zone.70
2.2. Intrinsic fracture energy of hydrogels
The intrinsic fracture energy is the energy required to break polymer chains lying across the crack plane by a unit area (Fig. 3a)66. According to the Lake-Thomas theory, the intrinsic fracture energy of a hydrogel can be calculated as66
where Uf is the energy required to rupture a polymer chain in the hydrogel, and m the number of chains across a unit area of the hydrogel in the undeformed state (Fig. 3a). Further denoting the number of chains across a unit area of the corresponding elastomer in the dry and undeformed state as mdry and the volume concentration of the polymer in the hydrogel as C, we have
The intrinsic fracture energy for common elastomers (i.e., Ufmdry) is ~50 Jm−2 66. Therefore, for corresponding hydrogels with ~90% water, the intrinsic fracture energy can be evaluated to be ~10 Jm−2, which is a relatively low value. Furthermore, since hydrogels are usually fabricated with dilute polymers which can rarely be crosslinked as tightly as in elastomers, Eq. (4) tends to overestimate the intrinsic fracture energy of hydrogels. Therefore, hydrogels with only intrinsic fracture energy are usually very brittle.
2.3. Fracture energy due to mechanical dissipation
In many hydrogels, particularly tough hydrogels, the propagation of a crack will not only break polymer chains lying across the crack plane, but also induce dissipation of mechanical energy in regions around the crack. As illustrated in Fig. 3b, when a hydrogel with a notch is stretched, the region around the crack tip will be first loaded and then unloaded as the crack propagates. In addition, for hydrogels reinforced with meso-/macro-scale fibers or fillers, the fibers or fillers behind the crack tip will be pulled out upon crack extension (Fig. 3c). During these processes, mechanical dissipation may result from different mechanisms such as rupture of polymer chains or crosslinkers, transformation of chains or crosslinkers, and friction of fillers and fibers with hydrogel matrices. These mechanisms will be discussed in detail in Sec 3.
Inspired by previous studies on high-toughness ceramics70, we divide the dissipation mechanisms of tough hydrogels into two categories: i). stress-strain hysteresis of hydrogels deformed and undeformed in a process zone around the crack (Fig. 3b), and ii). pullout of meso/macro-scale fibers or fillers in a bridging zone behind the crack tip (Fig. 3c).
2.3.1. Mechanical dissipation from process zone
Most of existing tough hydrogels use energy dissipation by deforming and undeforming hydrogels in process zones to enhance their toughness. The fracture energy due to mechanical dissipation in a process zone can be expressed as
where wD is the mechanical energy dissipated per unit volume of a hydrogel element in the process zone at reference state, h the width of the process zone at reference state, y the vertical coordinate of the hydrogel element, and V volume fraction of the hydrogel in the process zone. In Eq. (5), wD is given by the areas of hysteresis loops in stress-strain (or nominal stress-stretch) curves from deforming and undeforming the hydrogel element (Fig. 3b), i.e.
where λ_i_ are the principal stretches in three directions, si the corresponding principal stresses, and ∮ represents integration over the hysteresis loop. Since wD is dependent on the location of the hydrogel element, Eq. (5) and Eq. (6) usually need to be calculated with numerical models such as finite-element71–73 and phase-field74 models.
To illustrate the physical ideas of mechanical dissipation from process zone, we will not carry out the numerical calculations in the current paper. Instead, we denote a typical hydrogel element at location yD of the process zone, so that ∫0hwDdy=wD(yD)h. Since hydrogel around the crack tip is dominantly stretched in the direction along the applied force in pure shear tests, we further denote λ̅ as the maximum stretch along the applied force in the typical hydrogel element (at yD) and s̄ as the corresponding nominal stress in the typical element (Fig. 3b). Therefore, we can approximately express the fracture energy due to mechanical dissipation in process zone as
From Eq. (7), it is clear that a hydrogel that can sustain higher levels of stress and strain with larger stress-strain hysteresis and process zone will lead to higher fracture energy. For example, a hydrogel with V = 1, α ≈ 50%, s̄ ≈ 1 MPa, λ̅ ≈ 2 (i.e., 100% strain), and h ≈ 100 µm can readily achieve fracture energy ~100 Jm−2, much higher than the intrinsic fracture energy of hydrogels. Indeed, the fracture energies of many tough hydrogels have far exceeded 100 Jm−2, due to higher values of s̄, λ̅, α and/or h20, 21, 54, 59.
2.3.2. Mechanical dissipation from bridging zone
While most of existing tough hydrogels rely on toughening by mechanical dissipation from process zone, only a few fiber/filler-reinforced hydrogels have been recently developed.59, 75–77 These hydrogels generally rely on energy dissipation by pulling out meso/macro-scale fibers/fillers in bridging zones to enhance fracture toughness. The fracture energy due to mechanical dissipation in a bridging zone can be expressed as70, 78
where 2_u_ is the crack opening, 2_u_* the opening at the edge of the bridging zone as shown in Fig. 3c, T the normal stress in the fiber or filler, and A the area fraction of fibers or fillers on the crack plane. Denoting T̅ as a typical value of the normal stress in the fiber or filler, we can approximately express the fracture energy due to mechanical dissipation in bridging zone as
Using typical values of A =10%, T̅ =1 MPa, and u*= 500µm~1mm, we can evaluate the fracture energy from the bridging zone to be on the order of 100 Jm−2, which is also much larger than the intrinsic fracture energy of hydrogels.
Notably, the simultaneous operation of process zone and bridging zone may generate fracture energy much higher than the summation of fracture energy from either zone individually, due to the coupling effects between process zone and bridging zone.70 We will discuss the coupling effects in Sec. 6.
2.4. Design principle for tough hydrogels: energy dissipation and high stretchability
Since the intrinsic fracture energies of hydrogels are relatively low (i.e.,~10Jm−2) and almost impossible to be significantly increased, the enhancement of hydrogels’ fracture energy will basically rely on mechanical energy dissipated in regions around the cracks. Therefore, in order to design a tough hydrogel, we need to implement one or multiple mechanisms into the hydrogel to dissipate a substantial amount of mechanical energy in process and/or bridging zones upon crack propagation. From Eq. (7) and Eq. (9), we can see that the mechanical dissipation requires the hydrogel’s process zone to have high levels of stress (s̄) and strain (λ̅), large stress-strain hysteresis (α), and substantial size (h) (Fig. 3b); and/or requires its bridging zone to accommodate high traction (T) and large crack opening (u*) for pulling out fibers or fillers (Fig. 3c).
Evidentially, the typical strain (λ̅) and size (h) of the process zone and the crack opening (u*) in the bridging zone will monotonically increase with the stretchability of hydrogel. Therefore, to achieve substantial mechanical dissipation, it is also critical for the hydrogel in the process and bridging zones to be able to sustain relatively high levels of deformation while maintaining integrity. In particular, since the stress levels in hydrogels usually cannot exceed a few megapascals, enhancing the stretchability of hydrogels is an effective way to increase their fracture energy and toughness. For example, the tough polyacrylamide-alginate hydrogel developed by Sun et al can sustain a stretch of ~17 even with a notch in the sample and therefore gives fracture energy as high as ~9000 Jm−2 21. Besides the requirement of high stretchability in process and bridging zones, the bulks of tough hydrogels also need to maintain original shape and geometry after large deformation. Otherwise, the hydrogels can develop plastic deformation under loads (Fig. 4b), which is undesirable in applications of tough hydrogels. For example, many tough hydrogels have demonstrated full recovery of their original configurations after compressive strains over 90% (e.g., Fig. 1). Based on the above analyses, we can conclude that the general principle for the design of tough hydrogels is two-fold:
- to dissipate significant amounts of mechanical energy in hydrogels upon crack propagation, and
- to retain original configurations of hydrogels after large deformation.
Figure 4. Typical stress-strain curves of different types of hydrogels.
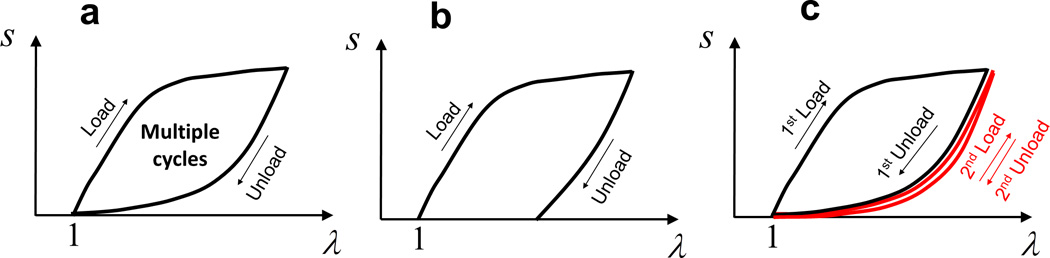
a. potentially tough and anti-fatigue hydrogels, b. hydrogel susceptible to plastic deformation, and c. hydrogel susceptible to fatigue under multiple cycles of large deformation.
While the developments of tough hydrogels have followed the general principle, different tough hydrogels have nevertheless relied on different mechanisms to dissipate mechanical energy and to maintain high elasticity. In Sec 3 and 4, we will discuss various mechanisms for energy dissipation (Fig. 5) and maintaining elasticity (Fig. 6) in hydrogels, respectively.
Figure 5. Mechanisms for dissipating mechanical energy in hydrogels.
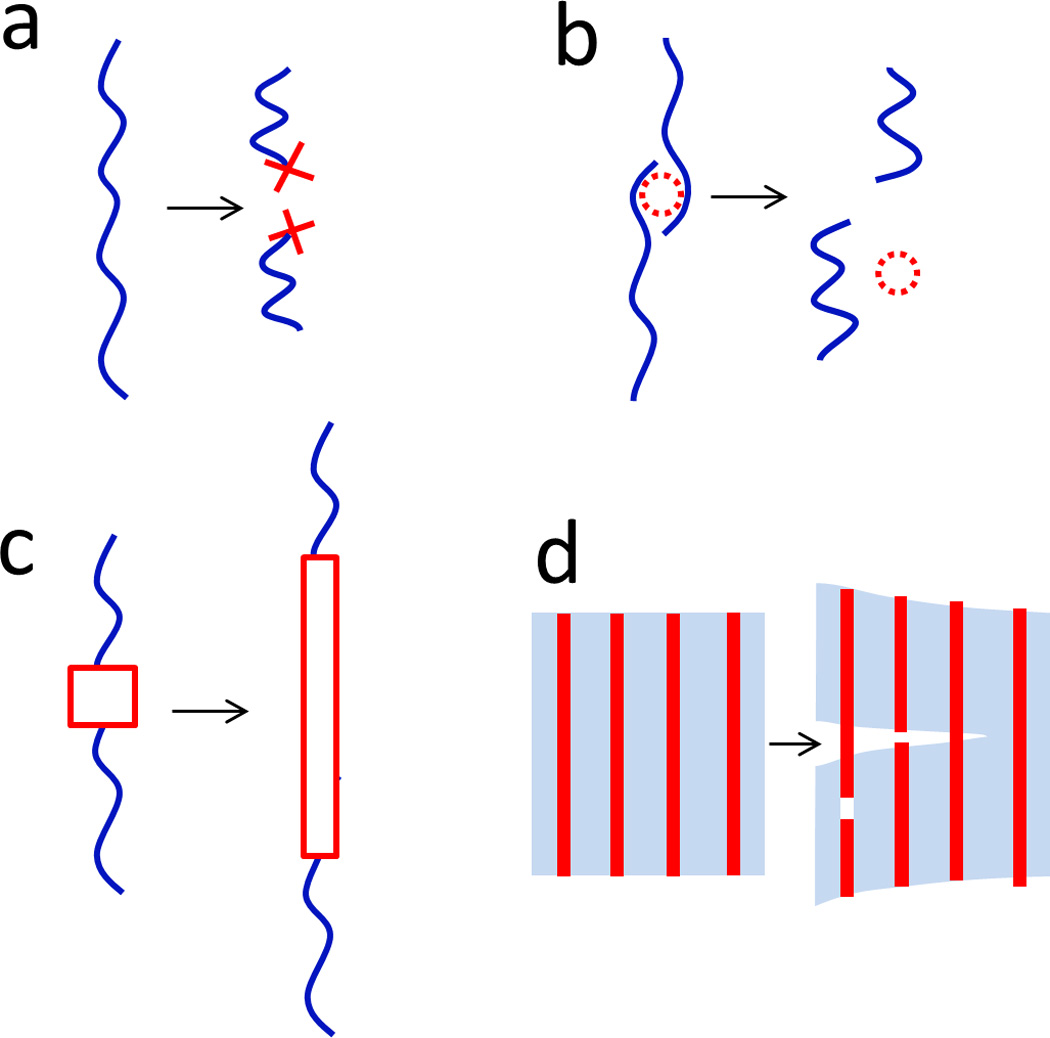
a. fracture of polymer chains, b. reversible crosslink of polymer chains, c. transformation of domains in polymer chains or crosslinkers, and d. pullout of fibers or fillers.
Figure 6. Mechanisms for maintaining high elasticity of hydrogels.
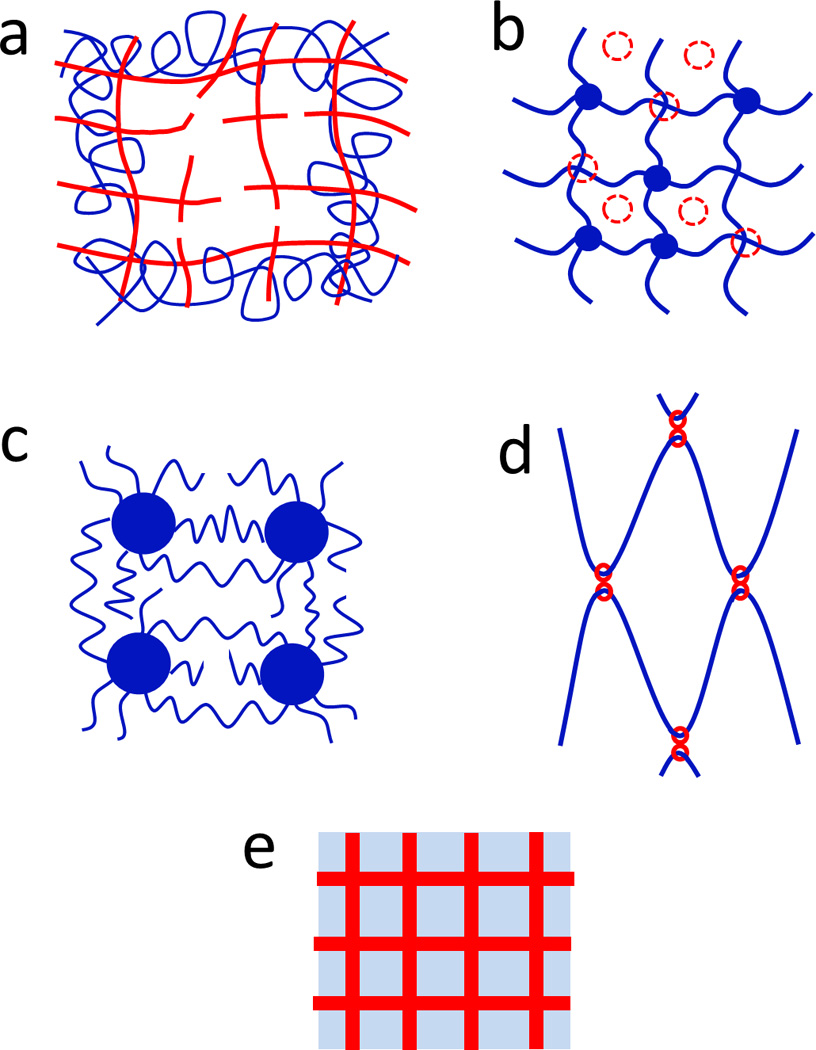
a. interpenetration of long-chain network, b. hybrid physical and chemical crosslinkers, c. high-functionality crosslinkers, d. network with long monodisperse polymer chains, and e. meso-/macro-scale composites.
In addition, it should be noted that if a hydrogel decreases its stress-strain hysteresis after the first or first few loading-unloading cycles, it will reduce the capability of energy dissipation (i.e., reduce α in Eq. (7)) and decrease fracture energy over multiple cycles of large deformation (Fig. 4c). Therefore, in order to develop tough hydrogels that are also anti-fatigue under large deformation, the stress-strain hysteresis of the hydrogels need to be repeatable over multiple loading-unloading cycles (Fig. 4a).
3. Mechanisms for dissipating energy in tough hydrogels
3.1. Fracture of polymer chains
Following the pioneering work by Gong et al on double-network hydrogels20, 43, 54, 55, 64, 79–90, fracture of polymer chains has been widely used as a mechanism to dissipate mechanical energy in hydrogels (Fig. 5a). As a polymer chain is fractured, the mechanical energy stored in the chain is dissipated. According to the Lake-Thomas theory, the fracture energy due to rupture of polymer chains in a process zone can be evaluated as66
where Uf is the energy required to rupture a polymer chain in the hydrogel, Nf the number of polymer chains fractured per unit volume of the process zone, and h the width of the process zone.
The chain-fracture mechanism requires that polymer chains in process zones can be effectively fractured. In order to promote fracture, a large number of polymer chains with relatively short lengths are usually incorporated into hydrogels. Even when the hydrogel is undeformed, these short chains can be highly stretched due to swelling of the hydrogel.72, 91 As the hydrogel is deformed, the short chains can be ruptured to dissipate mechanical energy. Previous studies have shown that the chain-fracture mechanism can be implemented with a wide variety of highly crosslinked polymers, such as poly(2-acrylamido-2-methylpropanesulfonic acid)20, glycidyl methacrylated hyaluronan92, poly(acrylic acid)93, agarose94, poly(vinyl alcohol)95, gellan gum methacrylate42, methacrylated chondroitin sulfate96. For example, Fig. 7a shows a damage zone around the crack in the poly(2-acrylamido-2-methylpropanesulfonic acid)-polyacrylamide double-network hydrogel, due to the fracture of polymers chains in the short-chain network of poly(2-acrylamido-2-methylpropanesulfonic acid) 97, 98.
Figure 7. Examples of mechanisms for energy dissipation in tough hydrogels.
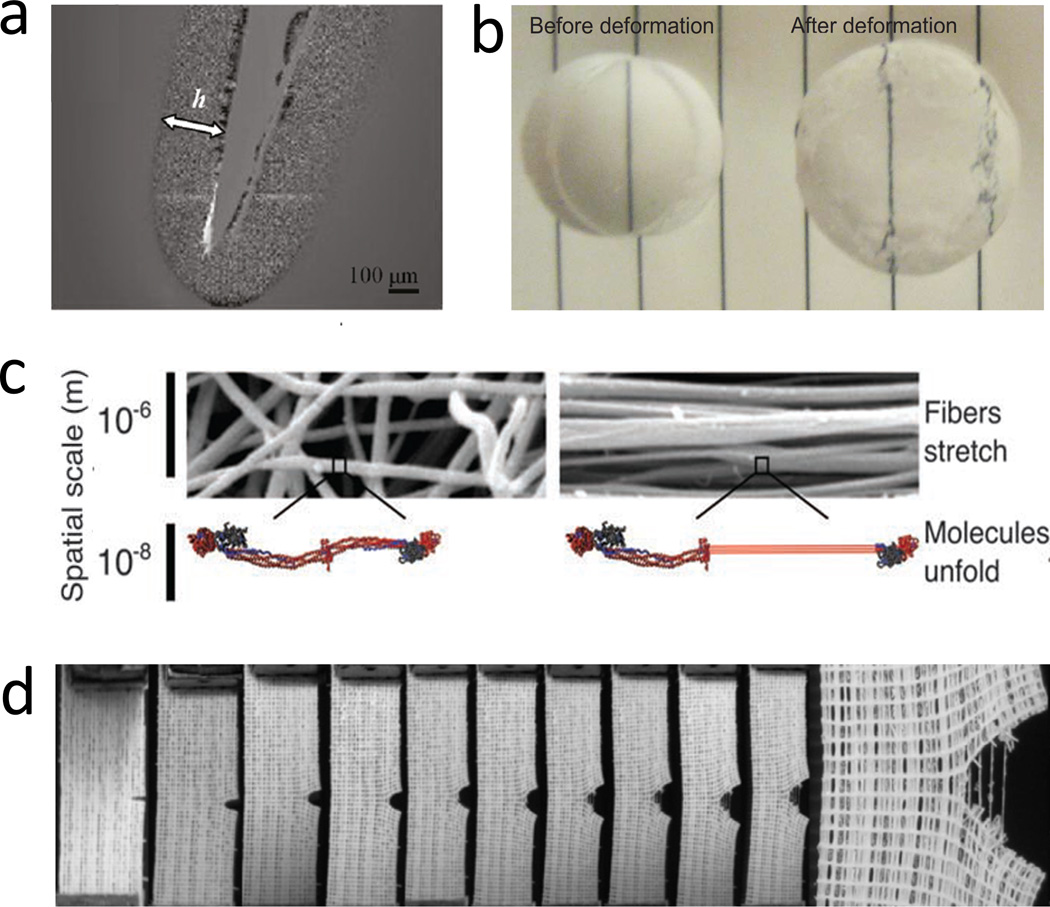
a. damage zone around a crack in a double-network hydrogel due to the fracture of the short-chain network, reproduced with permission97, b. plastic deformation in an ionically crosslinked hydrogel under compression due to reversible crosslink of polymer chains from ionic crosslinkers, reproduced with permission102, c. unfolding of folded domains in a fibrin hydrogel under deformation, reproduced with permission33, and d. fracture and pullout of fibers upon crack propagation in a fiber-reinforced hydrogel, reproduced with permission59.
It should also be noted that fracture of polymer chains usually induces irreversible damage of polymer networks in hydrogels. As a result, the stress-strain hysteresis loops generally decrease over repeated deformation in tough hydrogels that use the chain-fracture mechanism (Fig. 4c)20. Therefore, tough hydrogels relying on the chain-fracture mechanism will be susceptible to fatigue under multiple cycles of large deformation, unless other mechanisms are introduced to heal the ruptured polymer chains.99–101
3.2. Reversible crosslinking of polymer chains
Physical crosslinkers in polymer networks are generally weaker than chemical crosslinkers based on covalent bonds. Polymer chains can be detached from physical crosslinkers under mechanical loads and/or agitations from environments such as temperature, pH, and ionic strength (Fig. 5b). The detachment relaxes stretched polymer chains, and therefore dissipates mechanical energy in the polymer network. Furthermore, these physical crosslinkers in hydrogels can usually be recovered after decrosslinking. The reversible crosslinking of polymer chains has been widely used as a mechanism to induce mechanical dissipation in hydrogels (Fig. 5b). The mechanical energy dissipated due to decrosslinking of polymer chains in a process zone can be evaluated as
where Udc is the mechanical energy dissipated per decrosslinked polymer chain, Ndc the number of decrosslinked polymer chains per unit volume of the dissipation zone, and h the width of the dissipation zone. The parameter Udc in Eq. (11) should be not greater than the fracture energy of the chain Uf.
Physical crosslinkers commonly used for the reversible-crosslinking mechanism in hydrogels include ionic interaction21, 102–106, ligand-receptor interaction107, 108, hydrogen bond31, 109–115, and hydrophobic interaction116–122. Since these physical crosslinkers in hydrogels can usually be recovered after decrosslinking, it is possible to maintain the stress-strain hysteresis of hydrogels the same over cyclic loadings, potentially leading to anti-fatigue hydrogels (Fig. 4a)26, 115, 123–126. On the other hand, since the recovered crosslinkers are usually not at their original locations, hydrogels that only use the reversible-crosslinking mechanism can deform plastically under loads (Fig. 4b). For example, Zhao et al observed plastic deformation in ionically-crosslinked alginate hydrogels under compression, but not in the covalently-crosslinked ones (Fig. 7b).102 Therefore, when the reversible-crosslinking mechanism is used, it is critical to introduce another mechanism to maintain elasticity of hydrogels, which will be discussed in Sec 4.
In addition, the reversible crosslinking of different types of crosslinkers usually occurs at different time scales. Therefore, hydrogels that use the reversible-crosslinking mechanism usually demonstrate rate-dependent viscoelasticity, which is commonly associated with the mechanical dissipation and toughening of these hydrogels26. However, so far only few measurements on fracture energies of tough hydrogels were carried out at different strain rates. Therefore, it is still a critical task to measure the capacity of existing reversible crosslinkers in dissipating mechanical energy under different strain rates. Such information will guide the design of viscoelastic hydrogels that can maintain high toughness under a required range of strain rates.
3.3. Transformation of domains in polymer chains or crosslinkers
Polymer chains and crosslinkers may contain certain domains that transform between different configurations under mechanical loads (Fig. 5c).127 During the transformation, mechanical energy can be dissipated. Furthermore, the transformation may also change the lengths of polymer chains and crosslinkers, and induces effects such as variation of swelling ratio or color change of the hydrogels.33, 34 Examples of transformable domains included folded proteins and polysaccharides in biological polymers and mechanophores -- molecular units that can be chemically activated by mechanical forces -- in synthetic polymers.127–129 For example, Brown et al demonstrated that fibrin hydrogels can sustain high stretches up to 2.7, owing to protein unfolding in fibrin fibers (Fig. 7c).33 The protein unfolding also induces expulsion of water, varying swelling ratio of the hydrogel. As another example, Gossweiler et al incorporated spiropyran mechanophores into polydimethylsiloxane elastomer matrices34. Although elastomers instead of hydrogels were tested, Gossweiler et al showed that mechanical deformation can activate spiropyran mechanophores, which not only dissipate mechanical energy but also change the elastomer’s color from transparent into pure.
Interestingly, while transformable domains are rarely incorporated into synthetic polymers, they exist in a wide variety of biological polymers, such as fibrin, collagen, silk, keratin, and polysaccharides.127 Although the fracture energy of biological hydrogels can reach relatively high values (e.g., 1000 Jm−2 for cartilage), only a few tough hydrogels have been developed with biological polymers. Nevertheless, designing hydrogels based on biological polymers with transformable domains indeed represents a promising approach in development of tough hydrogels.
3.4. Pullout of fibers or fillers
The dissipation mechanisms discussed above generally depend on molecular-scale nano-/micro-structures of polymer networks, and are generally applied in process zones of hydrogels. On the other hand, meso-/macro-scale fibers and fillers can also be embedded in hydrogel matrix. Fracture and pullout of the fibers and fillers in a bridging zone in front of the crack can significantly dissipate mechanical energy in the hydrogel (see Fig. 5d and Eq.(8) and (9)).59, 75, 130
Examples of fibers and hydrogel matrices that use the fiber-pullout mechanism include polyurethane-fiber reinforced epoxy-amine hydrogel (Fig. 7d)59; polycaprolactone-fiber reinforced poly(ethylene glycol)75, agarose76, and alginate-polyacrylamide130 hydrogels; and polyglycolic-acid-fiber reinforced agarose and fibrin hydrogels77.
4. Mechanisms for maintaining elasticity of tough hydrogels
In addition to energy dissipation, high stretchability is another critical property for hydrogels to achieve high fracture energy and toughness. In this section, we will discuss various mechanisms that enable stretchability and elasticity of hydrogels. The stretch limit of a polymer chain with n monomers is λlim=n131–133. Swelling a polymer network in water or polymer solution will pre-stretch polymer chains in the network. The effective stretch limit of a polymer chain in a hydrogel from the swollen state (as the reference state) to a deformed state can be expressed as131–133
where C is the volume concentration of the polymer network in the hydrogel. From Eq. (13), it is clear that longer polymer chains (i.e., higher n) can give higher effective stretch limit and accommodate higher water concentration in hydrogels. Therefore, in order to achieve high stretchability of hydrogels, relatively long polymer chains usually need to be incorporated into the hydrogels through different mechanisms (Fig. 6).
4.1. Interpenetration of long-chain network
Polymer networks with relatively long polymer chains can be interlaced with other networks with relatively short chains on a molecular scale to form interpenetrating polymer networks. While the short-chain networks may be fractured or physically decrosslinked under deformation, the long-chain networks can still maintain high elasticity of the interpenetrating networks (Fig. 6a). Since 2003, Gong group have interpenetrated different types of polymer networks, and found that long-chain networks are indeed critical components for various double-network hydrogels to achieve high toughness.20, 43, 54, 55, 64, 79–88 Owning to the broad choices of polymers, interpenetration of long-chain network has been widely used as a mechanism to achieve high elasticity of tough hydrogels. The most common candidates for the long-chain polymer networks include polyacrylamide20, 111, poly(N-isopropylacrylamide)112, poly(ethylene glycol)116, poly(N,N’-dimethylacrylamide)92, 114, poly(acrylic acid)134, 135, and gelatin20, 42.
4.2. Hybrid physical and chemical crosslinkers
While decrosslinking of physically crosslinked networks dissipates mechanical energy, hydrogels with only physical crosslinkers can develop irreversible plastic deformation under mechanical loads (Fig. 4b and Fig. 7b). To maintain elasticity of hydrogels, chemical crosslinkers based on covalent bonds or other strong crosslinkers such as crystalline domains can be used to loosely crosslink polymers to give long-chain networks. Meanwhile, physical crosslinkers can be incorporated into the same polymer networks to increase the overall crosslinking density. The resultant hybrid-crosslinked networks enable hydrogels to maintain high elasticity when the networks are partially decrosslinked under large deformation (Fig. 6b).
Owning to its simple fabrication, hybrid crosslinking has been widely used as a mechanism to maintain high elasticity of tough hydrogels (Fig. 6b). Polymer networks that enable the hybrid-crosslinking mechanism usually can be crosslinked both chemically and physically. Examples of polymer networks with hybrid crosslinkers for tough hydrogels include alginate25, 102, chitosan22, and polyacrylamide with hydrophobic modification117, 119, 136.
4.3. High-functionality crosslinkers
Following Flory, we define the number of polymer chains that can be crosslinked by a crosslinker as the functionality of the crosslinker.131 Common physical and chemical crosslinkers usually have relatively low functionalities (e.g., less than 10), and there is usually a single polymer chain bridging between two adjacent common crosslinkers. When polymer chains are ruptured under deformation, the connections between crosslinkers are eliminated, potentially leading to fracture of the network. In order to achieve high elasticity of hydrogels, large crosslinkers with very high functionality (e.g., over 100) can be incorporated into polymer networks. In these networks, there are multiple polymer chains that connect two adjacent high-functionality crosslinkers, and these chains usually have non-uniform lengths. Therefore, as the polymer networks are deformed, relatively short chains may be ruptured or detached from the high-functionality crosslinkers but the long chains can still maintain the elasticity of the hydrogels (Fig. 6c).
Examples of high-functionality crosslinkers used in tough hydrogels include crystalline domains in polymer networks such as poly(vinyl alcohol)17, 18; exfolidated nano-clays that can crosslink various polymers such as polyacrylamide, poly(N-isopropylacrylamide) and poly(ethylene glycol)28–30; glassy spheres of poly(methyl methacrylate) that crosslink poly(methyl methacrylate)-poly(n-butyl acrylate) copolymers137; microspheres made from mixtures of styrene, butyl acrylate, and acrylic acid31; chitosan nanofibers that crosslink polyacrylamide138; graphene oxide that crosslinks polyacrylamide139, 140; and lamellar bilayer structure of surfactant that crosslinks polyacrylamide124.
4.4. Networks with long monodisperse polymer chains
Polymer networks are usually constituted of polydisperse polymer chains, due to the nature of polymerization. When such a polymer network is deformed, the shorter chains are more susceptible to rupture, which can initiate damages in the network. Although polymer networks with long polydisperse chains may still give relatively high stretchability, it may be more desirable to use networks with monodisperse chains to design tough hydrogels (Fig. 6d).
To achieve polymer networks with monodisperse chains, Sakai et al crosslinked tetrahedron-like macromonomers of poly(ethylene glycol) with well-defined sizes38, 39. The resultant poly(ethylene glycol) networks were proven to be extremely uniform by small-angle neutron scattering and light scattering measurements141, 142. As another example, Okumura and Ito developed a special crosslinker with the shape of eight, which not only interlocks two polymer chains but also can slide along the chains35. When a polymer network with the sliding crosslinkers is deformed, polymer chains can automatically adjust their lengths due to relocation of crosslinkers to give relatively homogeneous networks under loads (Fig. 6d). Poly(ethylene glycol) hydrogels with sliding crosslinkers indeed gave very high swelling ratio and strethability.
Notably, mechanisms for energy dissipation have not been implemented into polymer networks with monodisperse chains developed so far, as indicated by the negligible stress-strain hysteresis loops of these hydrogels.35–39 Furthermore, to our knowledge, the fracture energy of polymer networks with monodisperse chains has not been measured either.
4.5. Meso-/Macro-scale composites
The mechanisms discussed above generally rely on nano-/micro-structures of polymer networks to maintain high elasticity of hydrogels. On the other hand, meso-/macro-scale fibers and fillers can also be embedded in hydrogel matrices to form composites. As discussed in Sec 3.4, the fracture and pullout of the fibers or fillers can dissipate mechanical energy in hydrogels. Furthermore, if the fibers or fillers are interwoven into three-dimensional networks, they can also maintain the elasticity of hydrogel composites under deformation (Fig. 6e).59 In addition, since the networks of fibers or fillers constrain the swelling or deswelling of hydrogels, they can also control water concentration in hydrogel composites.59 Examples of tough fiber-reinforced hydrogel composites have been discussed in Sec 3.4.
5. A design matrix for tough hydrogels
As discussed above, the general principle for the design of tough hydrogels is to implement mechanisms into hydrogels to dissipate mechanical energy and to maintain high elasticity. We have discussed various mechanisms for dissipating energy and maintaining elasticity of hydrogels in Sec 4 and 5, respectively. Combinations of the two sets of mechanisms naturally provide a matrix that guides the design of tough hydrogels (Table 1). A number of combinations on the design matrix have been intensively explored over past few decades, which will be discussed in this section.
Table 1.
A matrix for the design of tough hydrogels by combining mechanisms for dissipating mechanical energy and maintaining elasticity, and references that used the combined mechanisms. Note that many promising combinations on the matrix have not been explored.
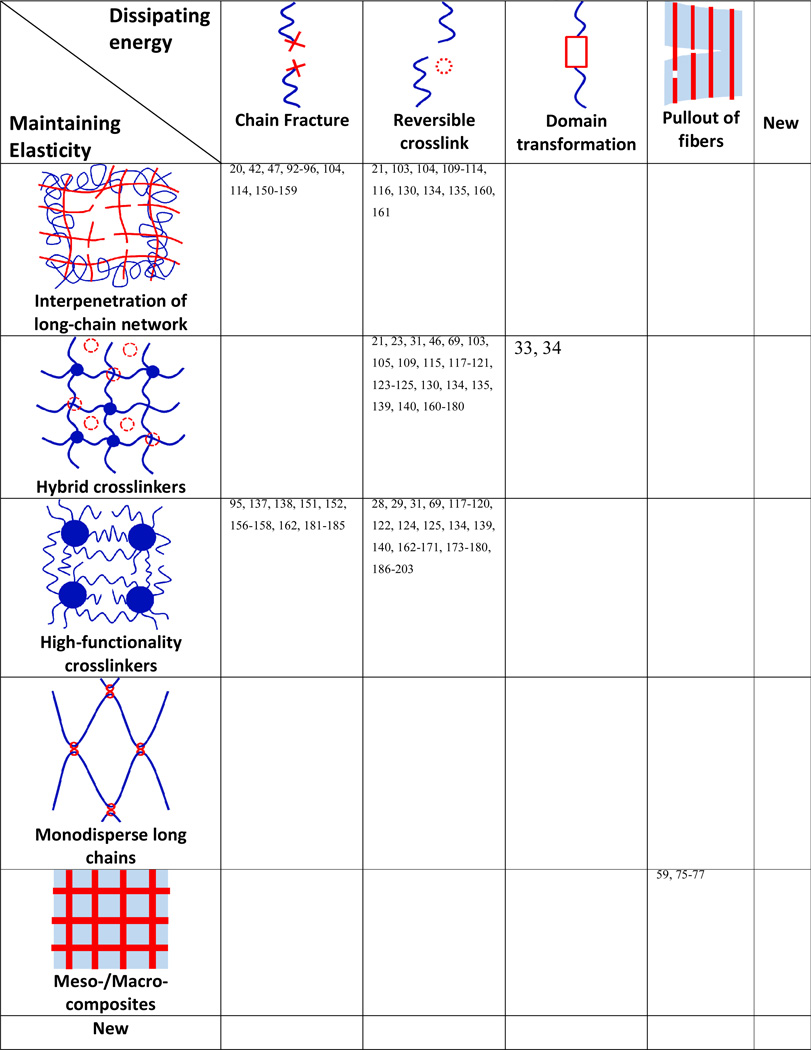
5.1. Chain fracture/reversible crosslinking plus interpenetration of long-chain network
From the design matrix (Table 1), it is clear that one common strategy to develop tough hydrogel is to interpenetrate polymer networks with relatively short and long polymer chains. Under deformation, the short-chain networks can be fractured and/or physically decrosslinked to dissipate mechanical energy (Sec 3.1 and Sec 3.2), while the long-chain networks will maintain the high elasticity of the hydrogels (Sec 4.1).
Gong et al carried out pioneering works in developing various tough hydrogels by combining mechanisms of chain fracture and interpenetration of long-chain network.20 For example, they interpenetrated short-chain network of poly(2-acrylamido-2-methylpropanesulfonic acid) with long-chain network of polyacrylamide. The resultant hydrogel gave a fracture energy over 1000 Jm−2, owning to the rupture of poly(2-acrylamido-2-methylpropanesulfonic acid) and the high elasticity of polyacrylamide (Fig. 1a).20, 54 Recently, Sun et al combined mechanisms of reversible crosslinking and interpenetration of long-chain network by interpenetrating ionically-crosslinked alginate and covalently-crosslinked polyacrylamide networks. Reversible crosslinking of the short-chain alginate and high elasticity of the long-chain polyacrylamide lead to a tough hydrogel with fracture energy over 9000 Jm−2 and uniaxial stretch over 21 (Fig. 1f). Remarkably, the alginate-polyacrylamide hydrogel can partially recover its stress-strain hysteresis after large deformation, due to the recovery of ionic crosslinkers in alginate. Furthermore, a number of theoretical and computational models have been developed for tough hydrogels using combined mechanisms of chain fracture/ reversible crosslinking and interpenetration of long-chain network.61, 62, 72, 143–145
5.2. Reversible crosslinking plus hybrid crosslinkers
Another commonly used strategy to design tough hydrogel is based on a single polymer network but with hybrid physical and chemical crosslinkers. The chemical crosslinkers alone give relatively long polymer chains that maintain high elasticity of hydrogels (Sec. 4.2), while the physical crosslinkers enable reversible crosslinking of polymer chains to dissipate mechanical energy (Sec. 3.2).
For example, Kong et al compared the fracture toughness of ionically and covalently crosslinked alginate hydrogels, and found that the ionic hydrogel has much higher toughness than the covalent one25. Zhao et al later observed plastic deformation in ionic alginate hydrogels under compression, validating the reversible crosslinking of ionic crosslinkers which dissipated mechanical energy in the hydrogel (Fig. 7b).102 Kersey et al incorporated metal-ligand complexes as weak reversible crosslinkers into a covalently-crosslinked hydrogel, which significantly improved the fracture toughness of the hydrogel.24 Miquelard-Garnier et al introduced hydrophobic groups into hydrogels to form weak crosslinkers, and found that the hydrogels’ mechanical dissipation under deformation increased due to the dissociation of hydrophobic groups.146–148 Hybrid crosslinking based on hydrophobic interactions was also investigated by Abdurrahmanoglu et al for tough hydrogels.117, 119, 136 Henderson et al introduced ionic crosslinkers into a triblock copolymer hydrogel that has been strongly crosslinked by glassy spheres.23 They found that ionic crosslinkers significantly increased fracture stress as well as stress-strain hysteresis of the hydrogel. Recently, Hui and Long developed a theoretical model for tough and self-healable hydrogels that use combined mechanisms of reversible crosslinking and hybrid crosslinkers.149
5.3. Chain fracture/ reversible crosslinking plus high-functionality crosslinkers
Another strategy to design tough hydrogel is based on large crosslinkers with high functionalities. Multiple polymer chains can be attached to a high-functionality crosslinker via covalent bonds and/or physical interactions. Furthermore, the lengths of polymer chains connecting two adjacent crosslinkers are generally non-uniform. As the hydrogel is deformed, relatively short chains across crosslinkers can be ruptured or physically decrosslinked to dissipate mechanical energy (Sec 3.1 and Sec 3.2), while relatively long chains can still bridge between the crosslinkers to maintain high elasticity of the hydrogel (Sec 4.3).
For example, in the pioneer works by Peppas, crystalline domains were introduced into poly(vinyl alcohol) using the freeze-thaw method to form high-functionality crosslinkers, which significantly enhance the toughness of hydrogels 17, 18. Haraguchi and Takehisa and others used exfoliated nano-clays as high-functionality crosslinkers, on which multiple types of polymers such as polyacrylamide, poly(N-isopropylacrylamide), poly(ethylene glycol) can be crossklinked through hydrogen bonds, ionic interactions, or covalent bonds.28–30 Seitz et al developed tough hydrogels based on poly(methyl methacrylate)-poly(n-butyl acrylate) copolymers, where the poly(methyl methacrylate) segments aggregate into glassy spheres as high-functionality crosslinkers.137
5.4. Pullout of fibers plus meso-/macro-scale composites
Fiber reinforcement is an emerging strategy to design tough hydrogels. Stretchy fiber networks embedded in hydrogel matrices can maintain the elasticity of hydrogels under deformation (Sec 4.5), while the fracture and pullout of fibers in bridging zones of the hydrogels can dissipate mechanical energy (Sec 3.4).
For example, Agrawal et al used rapid prototyping technique to print polyurethane fibers into three-dimensional networks to reinforce an epoxy-amine hydrogel. Fracture energy of the fiber-refined hydrogel was measured to range from 3 to 12 kJm−2, as the fiber density increased from 100 to 200 per inch.59 Moutos et al weaved polyglycolic-acid fibers into three-dimensional networks to reinforced agarose and fibrin hydrogels.77 Thereafter, Liao et al used woven polycaprolactone-fiber networks to reinforce an interpenetrating-network hydrogel based on alginate and polyacrylamide. Notably, multiple combinations of mechanisms were employed in Liao et al’s hydrogel, including reversible crosslinking of alginate plus hybrid crosslinkers and pullout of fibers plus meso-scale composite.130
6. Future directions
6.1. New combinations of mechanisms
While a number of combinations on the design matrix have been intensively explored over past few decades, there are still promising combinations that have not been well studied (Table 1). For example, Brown et al showed that a combination of domain transformation and hybrid crosslinking improved the mechanical strength and stretchability of fibrin hydrogels.33 Since a wide variety of biological polymers can give domain transformations for mechanical dissipation, interpenetrating these polymers with long-chain networks or crosslinking them with high-functionality crosslinkers may result in tough hydrogels, which may also possess bioactivities due to the biopolymers used.
As another example, polymer networks with long monodisperse polymer chains may be further crosslinked with reversible physical crosslinkers or interpenetrated with short-chain polymer networks. In this way, the uniform long-chain polymer networks will lead to high stretchability of the hydrogels while the reversible crosslinking or chain fracture dissipates mechanical energy, potentially resulting in tough hydrogels.
In addition, new mechanisms are being intensively developed for dissipating energy and maintaining high elasticity of hydrogels. These new mechanisms in combination with each other or with existing mechanisms can provide promising strategies for the design of future tough hydrogels (Table 1).
6. 2. Multi-scale multi-mechanism design for next-generation tough hydrogels
The mechanisms discussed in Sec 3, 4 and 5 are based on nano-, micro-, meso-, and macro-structures of hydrogels, which span length scales over multiple orders of magnitude (Fig. 8). The mechanisms of chain fracture, reversible crosslinking, interpenetration of long-chain network, hybrid crosslinkers, and monodisperse chains are generally implemented at length scales between 1nm and 100nm. The size of transformable domains ranges from 10nm to 1µm, and that of high-functionality crosslinkers from 100nm to 100µm. Furthermore, fiber or filler reinforcements of hydrogels usually rely on meso-/macro-structures of hydrogels with length scales ranging from 1µm to 1mm (Fig. 8).
Figure 8.
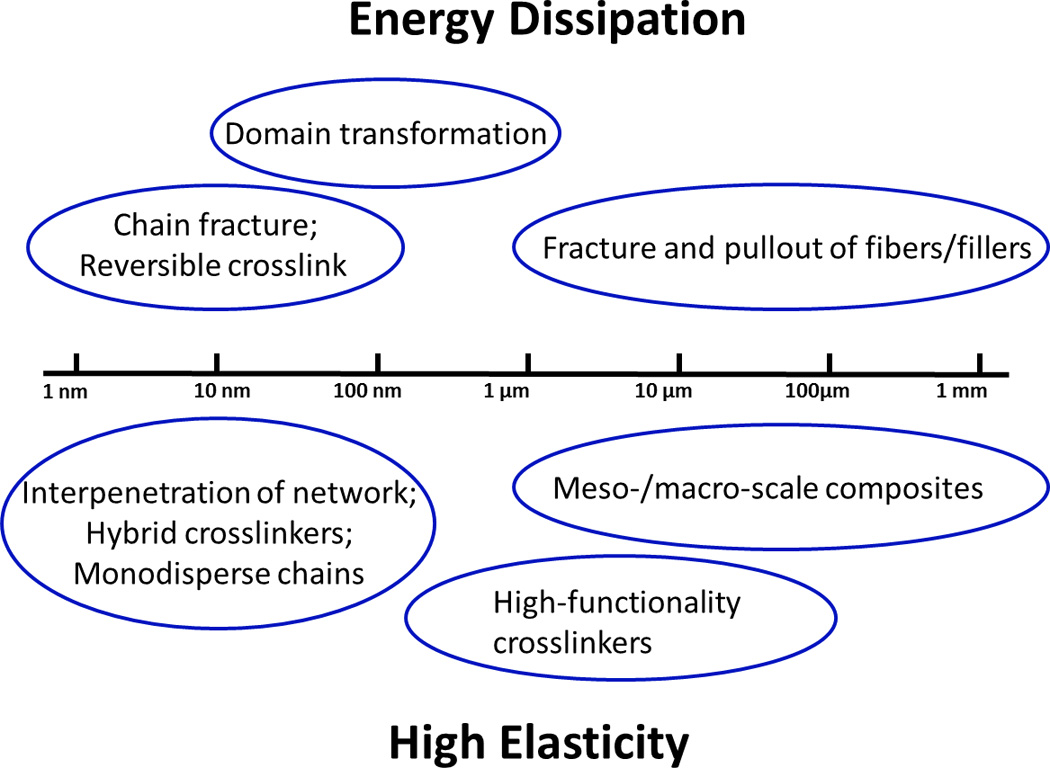
The mechanisms for dissipating mechanical energy and maintaining high elasticity of hydrogels span over multiple length scales ranging from nanometers to millimers
Despite the diverse mechanisms discussed above (Table 1), tough hydrogels developed so far usually use a single pair of mechanisms to dissipate mechanical energy and maintain high elasticity of hydrogels. However, these mechanisms may become ineffective in many situations, deteriorating the hydrogels’ toughness. For example, double-network hydrogels usually rely on the fracture of a short-chain network for energy dissipation and the interpenetration of a long-chain network for maintaining elasticity. However, if the short-chain network is already ruptured, the hydrogels’ fracture energy can be significantly reduced. In fact, the toughness of poly(2-acrylamido-2-methylpropanesulfonic acid)-polyacrylamide hydrogel decreases drastically, as the poly(2-acrylamido-2-methylpropanesulfonic acid) network is ruptured. As another example, hydrogels with hybrid crosslinkers usually rely on reversible crosslinking of physical crosslinkers for energy dissipation and the chemically crosslinked networks to maintain elasticity. However, if the change of a solution’s pH or ionic strength has eliminated those physical crosslinkers, the hybrid hydrogels will cease to be tough. Indeed, the alginate-polyacrylamide hydrogel with hybrid crosslinkers will significantly reduce its toughness, if the Ca2+ crosslinkers for alginate is chelated by EDTA.
The next-generation tough hydrogels should be able to maintain high toughness in various environments and loading conditions. However, as discussed above, hydrogels that rely on a single pair of mechanisms may lose their toughness due to environmental and loading effects. A promising strategy to design next-generation tough hydrogels is to integrate multiple pairs of mechanisms across multiple length scales into a hydrogel (Fig. 9). In this way, if one pair of mechanisms becomes ineffective, other pairs can still maintain high toughness of the hydrogel. For example, a tough hydrogel that integrates fiber-reinforcement at macro-/meso-scale, high-functionality crosslinkers at micro-scale, and hybrid crosslinkers at nano-scale may give consistently high fracture energy under various environments and loading conditions (Fig. 9). Recently, Liao et al integrated meso-scale fiber-reinforcement and nano-scale hybrid crosslinkers into a tough hydrogel. Despite the great promise, the multi-scale multi-mechanism strategy for the design of tough hydrogels is still in its initial stage and requires further researches.
Figure 9. A promising strategy to design next-generation tough hydrogels is to integrate multiple mechanisms across multiple length scales into a hydrogel.
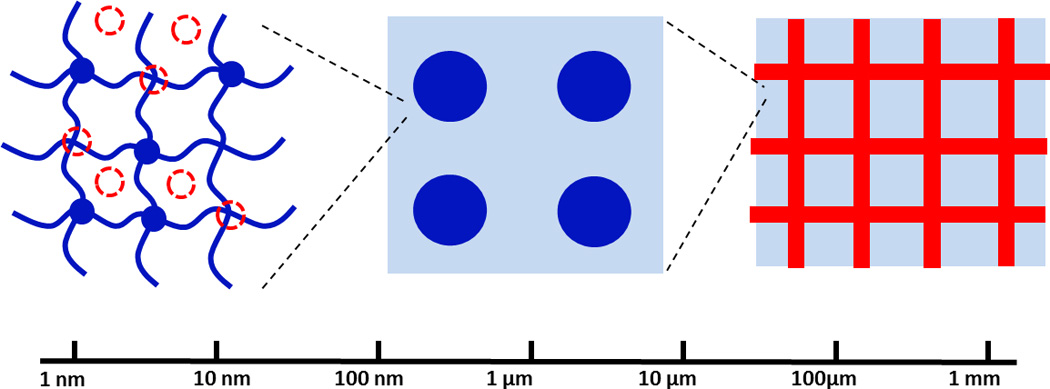
For example, a tough hydrogel may integrate fiber-reinforcement at macro-/meso-scale, high-functionality crosslinkers at micro-scale, and hybrid crosslinkers at nano-scale.
Furthermore, the fracture energy of a hydrogel based on multiple mechanisms may also be much higher than the summation of fracture energy for individual mechanism, due to the coupling between different mechanisms. For example, the pullout of fibers can significantly deform hydrogel along the fibers, potentially dissipating more mechanical energy in the hydrogel matrix than in the same hydrogel without fibers. In fact, the coupling effects between process zones and bridging zones have been widely used to enhance toughness of ceramics.70 Similarly, the coupling between various mechanisms in hydrogels may play an important role in the design of next-generation tough hydrogels.
Last but not least, in contrast to the extensive experimental works on various types of tough hydrogels (Table 1), theoretical models developed so far for tough hydrogels have been limited to those with interpenetrating networks61, 62, 72, 143–145 and hybrid crosslinkers149. However, multi-scale predictive models can greatly facilitate the design and development of next-generation tough hydrogels based on various mechanisms. For example, atomistic or molecular-dynamic models may be used to predict the values of physical parameters such as Uf in Eq. (10) and Udc in Eq. (11). Based on these parameters predicted by the nano/micro-scale models, meso/macro-scale models such as finite-element models72 can be used to further predict the energy dissipation and fracture process of tough hydrogels by calculating parameters such as α and h in Eq. (7). Furthermore, mechanism-based physical models can also provide fundamental understanding of the coupling effects between different pairs of mechanisms in tough hydrogels, rationally guiding the multi-scale multi-mechanism design of next-generation tough hydrogels.
7. Summary
While hydrogels have promising applications in diverse fields, the scope of hydrogel applications is often severely limited by their relatively low mechanical strengths. Over the last few decades, substantial progress has been made to develop hydrogels with high mechanical toughness. As a result, the fracture energy of various hydrogels has been enhanced from tens to thousands of joules per meter square, potentially exceling the fracture energy of tough natural hydrogels such as cartilage.
This review is aimed at elucidating the fundamental principle and mechanisms for toughness enhancement in various hydrogels, and proposing strategies for the design of future tough hydrogels. A general principle that underlies the development of tough hydrogels is to implement mechanisms into hydrogels to dissipate significant amounts of mechanical energy under large deformation and to maintain their original configurations after deformation. The mechanisms for dissipating mechanical energies in hydrogels include fracture of polymer chains, reversible crosslinking of polymer chains, domain transformation in polymers or crosslinkers, and pullout of fibers or fillers; and the mechanisms for maintaining high elasticity of hydrogels include interpenetration of long-chain network, hybrid crosslinkers, high-functionality crosslinkers, networks with long monodisperse chains, and meso-/macro-scale composites. Combinations of the two sets of mechanisms provide a matrix that can guide the design of next-generation tough hydrogels. A particularly promising strategy for the design is to implement multiple pairs of mechanisms across multiple length scales into nano-, micro-, meso-, and macro-structures of hydrogels.
Reference
- 1.Zwieniecki MA, Melcher PJ, Holbrook NM. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]
- 2.Mow VC, Kuei SC, Lai WM, Armstrong CG. Journal of Biomechanical Engineering-Transactions of the Asme. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 3.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Advanced Materials. 2006;18:1345–1360. [Google Scholar]
- 4.Lee KY, Mooney DJ. Chemical Reviews. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 5.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 6.Kopecek J. Journal of Polymer Science Part a-Polymer Chemistry. 2009;47:5929–5946. doi: 10.1002/pola.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida R. Current Organic Chemistry. 2005;9:1617–1641. [Google Scholar]
- 8.Schmidt JJ, Rowley J, Kong HJ. Journal of Biomedical Materials Research Part A. 2008;87A:1113–1122. doi: 10.1002/jbm.a.32287. [DOI] [PubMed] [Google Scholar]
- 9.Calvert P. Mrs Bulletin. 2008;33:207–212. [Google Scholar]
- 10.Murosaki T, Noguchi T, Hashimoto K, Kakugo A, Kurokawa T, Saito J, Chen YM, Furukawa H, Gong JP. Biofouling. 2009;25:657–666. doi: 10.1080/08927010903082628. [DOI] [PubMed] [Google Scholar]
- 11.Seliktar D. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 12.Calvert P. Advanced Materials. 2009;21:743–756. [Google Scholar]
- 13.Dong L, Agarwal AK, Beebe DJ, Jiang H. Nature. 2006;442:551–554. doi: 10.1038/nature05024. [DOI] [PubMed] [Google Scholar]
- 14.Cai S, Lou Y, Ganguly P, Robisson A, Suo Z. Journal of Applied Physics. 2010;107:103535. [Google Scholar]
- 15.Lewis G. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2012;100B:1702–1720. doi: 10.1002/jbm.b.32712. [DOI] [PubMed] [Google Scholar]
- 16.Hong W, Zhao X, Zhou J, Suo Z. Journal of the Mechanics and Physics of Solids. 2008;56:1779–1793. [Google Scholar]
- 17.Peppas NA, Merrill EW. Journal of Polymer Science Part a-Polymer Chemistry. 1976;14:441–457. [Google Scholar]
- 18.Peppas NA, Merrill EW. Journal of Biomedical Materials Research. 1977;11:423–434. doi: 10.1002/jbm.820110309. [DOI] [PubMed] [Google Scholar]
- 19.Stauffer SR, Peppas NA. Polymer. 1992;33:3932–3936. [Google Scholar]
- 20.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Advanced Materials. 2003;15:1155-+. [Google Scholar]
- 21.Sun J-Y, Zhao X, Illeperuma WRK, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. European Journal of Pharmaceutics and Biopharmaceutics. 2004;57:19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 23.Henderson KJ, Zhou TC, Otim KJ, Shull KR. Macromolecules. 2010;43:6193–6201. [Google Scholar]
- 24.Kersey FR, Loveless DM, Craig SL. J. R. Soc. Interface. 2007;4:373–380. doi: 10.1098/rsif.2006.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong HJ, Wong E, Mooney DJ. Macromolecules. 2003;36:4582–4588. [Google Scholar]
- 26.Sun TL, Kurokawa T, Kuroda S, Ihsan AB, Akasaki T, Sato K, Haque MA, Nakajima T, Gong JP. Nat Mater. 2013;12:932. doi: 10.1038/nmat3713. [DOI] [PubMed] [Google Scholar]
- 27.Shull KR. Nature. 2012;489:36–37. doi: 10.1038/489036a. [DOI] [PubMed] [Google Scholar]
- 28.Haraguchi K, Takehisa T. Advanced Materials. 2002;14:1120–1124. [Google Scholar]
- 29.Wang Q, Mynar JL, Yoshida M, Lee E, Lee M, Okuro K, Kinbara K, Aida T. Nature. 2010;463:339–343. doi: 10.1038/nature08693. [DOI] [PubMed] [Google Scholar]
- 30.Haraguchi K, Li HJ. Angewandte Chemie-International Edition. 2005;44:6500–6504. doi: 10.1002/anie.200502004. [DOI] [PubMed] [Google Scholar]
- 31.Huang T, Xu H, Jiao K, Zhu L, Brown HR, Wang H. Advanced Materials. 2007;19:1622-+. [Google Scholar]
- 32.Haraguchi K, Takehisa T, Fan S. Macromolecules. 2002;35:10162–10171. [Google Scholar]
- 33.Brown AEX, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Science. 2009;325:741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gossweiler G, Wang Q, Zhao X, Craig S. In preparation. 2013 [Google Scholar]
- 35.Okumura Y, Ito K. Advanced Materials. 2001;13:485-+. [Google Scholar]
- 36.Ito K. Polymer Journal. 2007;39:489–499. [Google Scholar]
- 37.Ito K. Current Opinion in Solid State & Materials Science. 2010;14:28–34. [Google Scholar]
- 38.Sakai T, Matsunaga T, Yamamoto Y, Ito C, Yoshida R, Suzuki S, Sasaki N, Shibayama M, Chung UI. Macromolecules. 2008;41:5379–5384. [Google Scholar]
- 39.Sakai T, Akagi Y, Matsunaga T, Kurakazu M, Chung U, Shibayama M. Macromolecular Rapid Communications. 2010;31:1954–1959. doi: 10.1002/marc.201000286. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe Y, Yasuda K, Azuma C, Taniguro H, Onodera S, Suzuki A, Chen YM, Gong JP, Osada Y. Journal of Materials Science-Materials in Medicine. 2008;19:1379–1387. doi: 10.1007/s10856-007-3255-7. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda K, Kitamura N, Gong JP, Arakaki K, Kwon HJ, Onodera S, Chen YM, Kurokawa T, Kanaya F, Ohmiya Y, Osada Y. Macromolecular Bioscience. 2009;9:307–316. doi: 10.1002/mabi.200800223. [DOI] [PubMed] [Google Scholar]
- 42.Shin H, Olsen BD, Khademhosseini A. Biomaterials. 2012;33:3143–3152. doi: 10.1016/j.biomaterials.2011.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasuda K, Gong JP, Katsuyama Y, Nakayama A, Tanabe Y, Kondo E, Ueno M, Osada Y. Biomaterials. 2005;26:4468–4475. doi: 10.1016/j.biomaterials.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Yin H, Akasaki T, Sun TL, Nakajima T, Kurokawa T, Nonoyama T, Taira T, Saruwatari Y, Gong JP. J. Mat. Chem. B. 2013;1:3685–3693. doi: 10.1039/c3tb20324g. [DOI] [PubMed] [Google Scholar]
- 45.Bin Imran A, Seki T, Takeoka Y. Polymer Journal. 2010;42:839–851. [Google Scholar]
- 46.Tang L, Liu WG, Liu GP. Advanced Materials. 2010;22:2652-+. doi: 10.1002/adma.200904016. [DOI] [PubMed] [Google Scholar]
- 47.Fei RC, George JT, Park J, Means AK, Grunlan MA. Soft Matter. 2013;9:2912–2919. doi: 10.1039/c3sm27226e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuart MAC, Huck WTS, Genzer J, Mueller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Nature Materials. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 49.Myung D, Waters D, Wiseman M, Duhamel PE, Noolandi J, Ta CN, Frank CW. Polymers for Advanced Technologies. 2008;19:647–657. doi: 10.1002/pat.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson JA, Turro NJ, Koberstein JT, Mark JE. Progress in Polymer Science. 2010;35:332–337. [Google Scholar]
- 51.Haraguchi K. Polymer Journal. 2011;43:223–241. [Google Scholar]
- 52.Shibayama M. Soft Matter. 2012;8:8030–8038. [Google Scholar]
- 53.Haque MA, Kurokawa T, Gong JP. Polymer. 2012;53:1805–1822. [Google Scholar]
- 54.Gong JP. Soft Matter. 2010;6:2583–2590. [Google Scholar]
- 55.Tanaka Y, Gong JP, Osada Y. Progress in Polymer Science. 2005;30:1–9. [Google Scholar]
- 56.Naficy S, Brown HR, Razal JM, Spinks GM, Whitten PG. Australian Journal of Chemistry. 2011;64:1007–1025. [Google Scholar]
- 57.Peak CW, Wilker JJ, Schmidt G. Colloid and Polymer Science. 2013 [Google Scholar]
- 58.Hassan CM, Peppas NA. In: Biopolymers/Pva Hydrogels/Anionic Polymerisation Nanocomposites. Abe A, editor. Vol. 153. 2000. pp. 37–65. [Google Scholar]
- 59.Agrawal A, Rahbar N, Calvert PD. Acta Biomaterialia. 2013;9:5313–5318. doi: 10.1016/j.actbio.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 60.McMeeking RM, Evans AG. Journal of the American Ceramic Society. 1982;65:242–246. [Google Scholar]
- 61.Brown HR. Macromolecules. 2007;40:3815–3818. [Google Scholar]
- 62.Tanaka Y. Epl. 2007;78:56005. [Google Scholar]
- 63.Irwin GR. Journal of Applied Mechanics. 1957;24:361. [Google Scholar]
- 64.Tanaka Y, Kuwabara R, Na YH, Kurokawa T, Gong JP, Osada Y. Journal of Physical Chemistry B. 2005;109:11559–11562. doi: 10.1021/jp0500790. [DOI] [PubMed] [Google Scholar]
- 65.Rivlin RS, Thomas AG. Journal of Polymer Science. 1953;10:291–318. [Google Scholar]
- 66.Lake GJ, Thomas AG. Proceedings of the Royal Society of London Series a-Mathematical and Physical Sciences. 1967;300:108-&. doi: 10.1098/rspa.1947.0042. [DOI] [PubMed] [Google Scholar]
- 67.Baumberger T, Caroli C, Martina D. Nature Materials. 2006;5:552–555. doi: 10.1038/nmat1666. [DOI] [PubMed] [Google Scholar]
- 68.Baumberger T, Caroli C, Martina D. European Physical Journal E. 2006;21:81–89. doi: 10.1140/epje/i2006-10048-6. [DOI] [PubMed] [Google Scholar]
- 69.Lin WC, Fan W, Marcellan A, Hourdet D, Creton C. Macromolecules. 2010;43:2554–2563. [Google Scholar]
- 70.Evans AG. Journal of the American Ceramic Society. 1990;73:187–206. [Google Scholar]
- 71.Hong W, Liu Z, Suo Z. International Journal of Solids and Structures. 2009;46:3282–3289. [Google Scholar]
- 72.Zhao X. Journal of the Mechanics and Physics of Solids. 2012;60:319–332. doi: 10.1016/j.jmps.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Hong W. Soft Matter. 2012;8:8171–8178. [Google Scholar]
- 74.Hong W, Wang X. Journal of the Mechanics and Physics of Solids. 2013;61:1281–1294. [Google Scholar]
- 75.Coburn J, Gibson M, Bandalini PA, Laird C, Mao HQ, Moroni L, Seliktar D, Elisseeff J. Smart Structures and Systems. 2011;7:213–222. doi: 10.12989/sss.2011.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang J, Oh H, Lee J, Song TH, Jeong YH, Cho DW. Applied Physics Letters. 2013;102:211914. [Google Scholar]
- 77.Moutos FT, Freed LE, Guilak F. Nature Materials. 2007;6:162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 78.Evans AG, McMeeking RM. Acta Metallurgica. 1986;34:2435–2441. [Google Scholar]
- 79.Na YH, Kurokawa T, Katsuyama Y, Tsukeshiba H, Gong JP, Osada Y, Okabe S, Karino T, Shibayama M. Macromolecules. 2004;37:5370–5374. [Google Scholar]
- 80.Nakayama A, Kakugo A, Gong JP, Osada Y, Takai M, Erata T, Kawano S. Advanced Functional Materials. 2004;14:1124–1128. [Google Scholar]
- 81.Kaneko D, Tada T, Kurokawa T, Gong JP, Osada Y. Advanced Materials. 2005;17:535-+. [Google Scholar]
- 82.Tsukeshiba H, Huang M, Na YH, Kurokawa T, Kuwabara R, Tanaka Y, Furukawa H, Osada Y, Gong JP. Journal of Physical Chemistry B. 2005;109:16304–16309. doi: 10.1021/jp052419n. [DOI] [PubMed] [Google Scholar]
- 83.Na YH, Tanaka Y, Kawauchi Y, Furukawa H, Sumiyoshi T, Gong JP, Osada Y. Macromolecules. 2006;39:4641–4645. [Google Scholar]
- 84.Huang M, Furukawa H, Tanaka Y, Nakajima T, Osada Y, Gong JP. Macromolecules. 2007;40:6658–6664. [Google Scholar]
- 85.Webber RE, Creton C, Brown HR, Gong JP. Macromolecules. 2007;40:2919–2927. [Google Scholar]
- 86.Tominaga T, Tirumala VR, Lee S, Lin EK, Gong JP, Wu W-L. Journal of Physical Chemistry B. 2008;112:3903–3909. doi: 10.1021/jp710284e. [DOI] [PubMed] [Google Scholar]
- 87.Nakajima T, Furukawa H, Tanaka Y, Kurokawa T, Osada Y, Gong JP. Macromolecules. 2009;42:2184–2189. [Google Scholar]
- 88.Tominaga T, Tirumala VR, Lin EK, Gong JP, Furukawa H, Osada Y, Wu WL. Polymer. 2007;48:7449–7454. [Google Scholar]
- 89.Nakajima T, Fukuda Y, Kurokawa T, Sakai T, Chung U-i, Gong JP. ACS Macro Lett. 2013;2:518–521. doi: 10.1021/mz4002047. [DOI] [PubMed] [Google Scholar]
- 90.Nakajima T, Kurokawa T, Ahmed S, Wu W-l, Gong JP. Soft Matter. 2013;9:1955–1966. [Google Scholar]
- 91.Myung D, Koh W, Ko J, Hu Y, Carrasco M, Noolandi J, Ta CN, Frank CW. Polymer. 2007;48:5376–5387. [Google Scholar]
- 92.Weng L, Gouldstone A, Wu Y, Chen W. Biomaterials. 2008;29:2153–2163. doi: 10.1016/j.biomaterials.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dai TY, Qing XT, Lu Y, Xia YY. Polymer. 2009;50:5236–5241. [Google Scholar]
- 94.DeKosky BJ, Dormer NH, Ingavle GC, Roatch CH, Lomakin J, Detamore MS, Gehrke SH. Tissue Engineering Part C-Methods. 2010;16:1533–1542. doi: 10.1089/ten.tec.2009.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang XY, Guo XL, Yang SG, Tan SX, Li XF, Dai HJ, Yu XL, Zhang XL, Weng N, Jian B, Xu J. Journal of Applied Polymer Science. 2009;112:3063–3070. [Google Scholar]
- 96.Suekama TC, Hu J, Kurokawa T, Gong JP, Gehrke SH. ACS Macro Lett. 2013;2:137–140. doi: 10.1021/mz3006318. [DOI] [PubMed] [Google Scholar]
- 97.Yu QM, Tanaka Y, Furukawa H, Kurokawa T, Gong JP. Macromolecules. 2009;42:3852–3855. [Google Scholar]
- 98.Liang SM, Wu ZL, Hu J, Kurokawa T, Yu QM, Gong JP. Macromolecules. 2011;44:3016–3020. [Google Scholar]
- 99.Cordier P, Tournilhac F, Soulie-Ziakovic C, Leibler L. Nature. 2008;451:977–980. doi: 10.1038/nature06669. [DOI] [PubMed] [Google Scholar]
- 100.White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S. Nature. 2001;409:794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 101.Brochu ABW, Craig SL, Reichert WM. Journal of Biomedical Materials Research Part A. 2011;96A:492–506. doi: 10.1002/jbm.a.32987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao X, Huebsch N, Mooney DJ, Suo Z. Journal of Applied Physics. 2010;107:63509. doi: 10.1063/1.3343265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Z, Chao T, Jiang SY. Journal of Physical Chemistry B. 2008;112:5327–5332. doi: 10.1021/jp710683w. [DOI] [PubMed] [Google Scholar]
- 104.Yang W, Furukawa H, Gong JP. Advanced Materials. 2008;20:4499–4503. [Google Scholar]
- 105.Friedrich T, Tieke B, Stadler FJ, Bailly C. Soft Matter. 2011;7:6590–6597. [Google Scholar]
- 106.Hunt JN, Feldman KE, Lynd NA, Deek J, Campos LM, Spruell JM, Hernandez BM, Kramer EJ, Hawker CJ. Advanced Materials. 2011;23:2327-+. doi: 10.1002/adma.201004230. [DOI] [PubMed] [Google Scholar]
- 107.Bell GI. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 108.Lee KY, Kong HJ, Larson RG, Mooney DJ. Advanced Materials. 2003;15:1828–1832. [Google Scholar]
- 109.Hao JK, Weiss RA. Polymer. 2013;54:2174–2182. [Google Scholar]
- 110.Liang SM, Wu JJ, Tian HF, Zhang LN, Xu J. ChemSusChem. 2008;1:558–563. doi: 10.1002/cssc.200800003. [DOI] [PubMed] [Google Scholar]
- 111.Hagiwara Y, Putra A, Kakugo A, Furukawa H, Gong JP. Cellulose. 2010;17:93–101. [Google Scholar]
- 112.Chang CY, Han K, Zhang LN. Polymers for Advanced Technologies. 2011;22:1329–1334. [Google Scholar]
- 113.Naficy S, Razal JM, Whitten PG, Wallace GG, Spinks GM. J. Polym. Sci. Pt. B-Polym. Phys. 2012;50:423–430. [Google Scholar]
- 114.Azuma C, Yasuda K, Tanabe Y, Taniguro H, Kanaya F, Nakayama A, Chen YM, Gong JP, Osada Y. Journal of Biomedical Materials Research Part A. 2007;81A:373–380. doi: 10.1002/jbm.a.31043. [DOI] [PubMed] [Google Scholar]
- 115.Zhang JL, Wang N, Liu WG, Zhao XL, Lu W. Soft Matter. 2013;9:6331–6337. [Google Scholar]
- 116.Myung D, Koh WU, Ko JM, Hu Y, Carrasco M, Noolandi J, Ta CN, Frank CW. Polymer. 2007;48:5376–5387. [Google Scholar]
- 117.Tuncaboylu DC, Sari M, Oppermann W, Okay O. Macromolecules. 2011;44:4997–5005. [Google Scholar]
- 118.Yang J, Shi FK, Gong C, Xie XM. Journal of Colloid and Interface Science. 2012;381:107–115. doi: 10.1016/j.jcis.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 119.Abdurrahmanoglu S, Can V, Okay O. Polymer. 2009;50:5449–5455. [Google Scholar]
- 120.Li WB, An HY, Tan Y, Lu CG, Liu C, Li PC, Xu K, Wang PX. Soft Matter. 2012;8:5078–5086. [Google Scholar]
- 121.Abdurrahmanoglu S, Cilingir M, Okay O. Polymer. 2011;52:694–699. [Google Scholar]
- 122.Jiang GQ, Liu C, Liu XL, Chen QR, Zhang GH, Yang M, Liu FQ. Polymer. 2010;51:1507–1515. [Google Scholar]
- 123.Bai T, Zhang P, Han YJ, Liu YA, Liu WG, Zhao XL, Lu W. Soft Matter. 2011;7:2825–2831. [Google Scholar]
- 124.Haque MA, Kurokawa T, Kamita G, Gong JP. Macromolecules. 2011;44:8916–8924. [Google Scholar]
- 125.Haque MA, Kurokawa T, Gong JP. Soft Matter. 2012;8:8008–8016. [Google Scholar]
- 126.Bin Ihsan A, Sun TL, Kuroda S, Haque MA, Kurokawa T, Nakajima T, Gong JP. J. Mat. Chem. B. 2013;1:4555–4562. doi: 10.1039/c3tb20790k. [DOI] [PubMed] [Google Scholar]
- 127.Miserez A, Guerette PA. Chemical Society Reviews. 2013;42:1973–1995. doi: 10.1039/c2cs35294j. [DOI] [PubMed] [Google Scholar]
- 128.Davis DA, Hamilton A, Yang J, Cremar LD, Van Gough D, Potisek SL, Ong MT, Braun PV, Martinez TJ, White SR, Moore JS, Sottos NR. Nature. 2009;459:68–72. doi: 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]
- 129.Black AL, Orlicki JA, Craig SL. Journal of Materials Chemistry. 2011;21:8460–8465. [Google Scholar]
- 130.Liao I-C, Moutos FT, Estes BT, Zhao X, Guilak F. Adv. Funct. Mater. 2013 doi: 10.1002/adfm.201300483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Flory PJ. Principles of Polymer Chemistry. Cornell University Press; 1953. [Google Scholar]
- 132.Gennes P-Gd. Scaling Concepts in Polymer Physics. Cornell University Press; 1979. [Google Scholar]
- 133.Rubinstein M, Colby RH. Polymer Physics. Oxford University Press; 2003. [Google Scholar]
- 134.Lin HR, Ling MH, Lin YJ. Journal of Biomaterials Science-Polymer Edition. 2009;20:637–652. doi: 10.1163/156856209X426448. [DOI] [PubMed] [Google Scholar]
- 135.Baskan T, Tuncaboylu DC, Okay O. Polymer. 2013;54:2979–2987. [Google Scholar]
- 136.Zhang C, Aung A, Liao LQ, Varghese S. Soft Matter. 2009;5:3831–3834. [Google Scholar]
- 137.Seitz ME, Martina D, Baumberger T, Krishnan VR, Hui C-Y, Shull KR. Soft Matter. 2009;5:447–456. [Google Scholar]
- 138.Zhou CJ, Wu QL. Colloids and Surfaces B-Biointerfaces. 2011;84:155–162. doi: 10.1016/j.colsurfb.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 139.Liu RQ, Liang SM, Tang XZ, Yan D, Li XF, Yu ZZ. Journal of Materials Chemistry. 2012;22:14160–14167. [Google Scholar]
- 140.Liu JQ, Chen CF, He CC, Zhao L, Yang XJ, Wang HL. Acs Nano. 2012;6:8194–8202. doi: 10.1021/nn302874v. [DOI] [PubMed] [Google Scholar]
- 141.Matsunaga T, Sakai T, Akagi Y, Chung U-i, Shibayama M. Macromolecules. 2009;42:6245–6252. [Google Scholar]
- 142.Matsunaga T, Sakai T, Akagi Y, Chung U-i, Shibayama M. Macromolecules. 2009;42:1344–1351. [Google Scholar]
- 143.Edgecombe S, Linse P. Polymer. 2008;49:1981–1992. [Google Scholar]
- 144.Wang X, Hong W. Soft Matter. 2011;7:8576–8581. [Google Scholar]
- 145.Okumura K. Europhysics Letters. 2004;67:470–476. [Google Scholar]
- 146.Miquelard-Garnier G, Hourdet D, Creton C. Polymer. 2009;50:481–490. [Google Scholar]
- 147.Miquelard-Garnier G, Demoures S, Creton C, Hourdet D. Macromolecules. 2006;39:8128–8139. [Google Scholar]
- 148.Miquelard-Garnier G, Creton C, Hourdet D. Macromolecular Symposia. 2007;256:189–194. [Google Scholar]
- 149.Hui CY, Long R. Soft Matter. 2012;8:8209–8216. [Google Scholar]
- 150.Wang XZ, Wang HL, Brown HR. Soft Matter. 2011;7:211–219. [Google Scholar]
- 151.Nakajima T, Takedomi N, Kurokawa T, Furukawa H, Gong JP. Polymer Chemistry. 2010;1:693–697. [Google Scholar]
- 152.Wang Q, Hou RX, Cheng YJ, Fu J. Soft Matter. 2012;8:6048–6056. [Google Scholar]
- 153.Nakajima T, Sato H, Zhao Y, Kawahara S, Kurokawa T, Sugahara K, Gong JP. Advanced Functional Materials. 2012;22:4426–4432. [Google Scholar]
- 154.Saito J, Furukawa H, Kurokawa T, Kuwabara R, Kuroda S, Hu JA, Tanaka Y, Gong JP, Kitamura N, Yasuda K. Polymer Chemistry. 2011;2:575–580. [Google Scholar]
- 155.Chen P, Wu RL, Wang JD, Liu Y, Ding CR, Xu SM. Journal of Polymer Research. 2012;19:9825. [Google Scholar]
- 156.Hu J, Kurokawa T, Nakajima T, Sun TL, Suekama T, Wu ZL, Liang SM, Gong JP. Macromolecules. 2012;45:9445–9451. [Google Scholar]
- 157.Hu J, Kurokawa T, Hiwatashi K, Nakajima T, Wu ZL, Liang SM, Gong JP. Macromolecules. 2012;45:5218–5228. [Google Scholar]
- 158.Hu J, Hiwatashi K, Kurokawa T, Liang SM, Wu ZL, Gong JP. Macromolecules. 2011;44:7775–7781. [Google Scholar]
- 159.Kishi R, Hiroki K, Tominaga T, Sano KI, Okuzaki H, Martinez JG, Otero TF, Osada Y. J. Polym. Sci. Pt. B-Polym. Phys. 2012;50:790–796. [Google Scholar]
- 160.Harrass K, Kruger R, Moller M, Albrecht K, Groll J. Soft Matter. 2013;9:2869–2877. [Google Scholar]
- 161.Bakarich SE, Pidcock GC, Balding P, Stevens L, Calvert P, Panhuis MIH. Soft Matter. 2012;8:9985–9988. [Google Scholar]
- 162.Qin XP, Zhao F, Liu YK, Wang HY, Feng SY. Colloid and Polymer Science. 2009;287:621–625. [Google Scholar]
- 163.Zhao J, Jiao KX, Yang J, He CC, Wang HL. Polymer. 2013;54:1596–1602. [Google Scholar]
- 164.Chang C-W, van Spreeuwel A, Zhang C, Varghese S. Soft Matter. 2010;6:5157–5164. [Google Scholar]
- 165.Fukasawa M, Sakai T, Chung UI, Haraguchi K. Macromolecules. 2010;43:4370–4378. [Google Scholar]
- 166.Shikinaka K, Koizumi Y, Osada Y, Shigehara K. Polymers for Advanced Technologies. 2011;22:1212–1215. [Google Scholar]
- 167.Yang J, Han CR, Duan JF, Ma MG, Zhang XM, Xu F, Sun RC. Cellulose. 2013;20:227–237. [Google Scholar]
- 168.Xu B, Li HJ, Wang YY, Zhang GZ, Zhang QS. Rsc Advances. 2013;3:7233–7236. [Google Scholar]
- 169.Zhu AF, Li G, Jiang JM. Journal of Macromolecular Science Part B-Physics. 2012;51:1002–1010. [Google Scholar]
- 170.Gaharwar AK, Dammu SA, Canter JM, Wu CJ, Schmidt G. Biomacromolecules. 2011;12:1641–1650. doi: 10.1021/bm200027z. [DOI] [PubMed] [Google Scholar]
- 171.Fan JC, Shi ZX, Lian M, Li H, Yin J. Journal of Materials Chemistry A. 2013;1:7433–7443. [Google Scholar]
- 172.Gao H, Wang N, Hu XF, Nan WJ, Han YJ, Liu WG. Macromolecular Rapid Communications. 2013;34:63–68. doi: 10.1002/marc.201200548. [DOI] [PubMed] [Google Scholar]
- 173.Rose S, Dizeux A, Narita T, Hourdet D, Marcellan A. Macromolecules. 2013;46:4095–4104. [Google Scholar]
- 174.Shen JF, Yan B, Li T, Long Y, Li N, Ye MX. Composites Part a-Applied Science and Manufacturing. 2012;43:1476–1481. [Google Scholar]
- 175.Gaharwar AK, Rivera C, Wu CJ, Chan BK, Schmidt G. Materials Science & Engineering C-Materials for Biological Applications. 2013;33:1800–1807. doi: 10.1016/j.msec.2012.12.099. [DOI] [PubMed] [Google Scholar]
- 176.Haraguchi K, Song L. Macromolecules. 2007;40:5526–5536. [Google Scholar]
- 177.Yang J, Han CR, Duan JF, Xu F, Sun RC. Acs Applied Materials & Interfaces. 2013;5:3199–3207. doi: 10.1021/am4001997. [DOI] [PubMed] [Google Scholar]
- 178.Carlsson L, Rose S, Hourdet D, Marcellan A. Soft Matter. 2010;6:3619–3631. [Google Scholar]
- 179.Li ZY, Su YL, Xie BQ, Wang HL, Wen T, He CC, Shen H, Wu DC, Wang DJ. J. Mat. Chem. B. 2013;1:1755–1764. doi: 10.1039/c3tb00246b. [DOI] [PubMed] [Google Scholar]
- 180.Zhao F, Qin XP, Feng SY. Polym. Compos. 2012;33:44–51. [Google Scholar]
- 181.Tan Y, Xu K, Wang PX, Li WB, Sun SM, Dong LS. Soft Matter. 2010;6:1467–1471. [Google Scholar]
- 182.Tan Y, Wang PX, Xu K, Li WB, An HY, Li LL, Liu C, Dong LS. Macromolecular Materials and Engineering. 2009;294:855–859. [Google Scholar]
- 183.Wu YT, Zhou Z, Fan QQ, Chen L, Zhu MF. Journal of Materials Chemistry. 2009;19:7340–7346. [Google Scholar]
- 184.Cha WI, Hyon SH, Ikada Y. Makromolekulare Chemie-Macromolecular Chemistry and Physics. 1992;193:1913–1925. [Google Scholar]
- 185.Kaneko T, Tanaka S, Ogura A, Akashi M. Macromolecules. 2005;38:4861–4867. [Google Scholar]
- 186.Lin L, Liu M, Chen L, Chen P, Ma J, Han D, Jiang L. Advanced Materials. 2010;22:4826-+. doi: 10.1002/adma.201002192. [DOI] [PubMed] [Google Scholar]
- 187.Liu Y, Zhu MF, Liu XL, Zhang W, Sun B, Chen YM, Adler HJP. Polymer. 2006;47:1–5. [Google Scholar]
- 188.Mujumdar SK, Siegel RA. Journal of Polymer Science Part a-Polymer Chemistry. 2008;46:6630–6640. doi: 10.1002/pola.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen P, Xu SM, Wu RL, Wang JD, Gu RB, Du J. Applied Clay Science. 2013;72:196–200. [Google Scholar]
- 190.Hu XB, Wang T, Xiong LJ, Wang CY, Liu XX, Tong Z. Langmuir. 2010;26:4233–4238. doi: 10.1021/la903298n. [DOI] [PubMed] [Google Scholar]
- 191.Abdurrahmanoglu S, Can V, Okay O. Journal of Applied Polymer Science. 2008;109:3714–3724. [Google Scholar]
- 192.Takeno H, Nakamura W. Colloid and Polymer Science. 2013;291:1393–1399. [Google Scholar]
- 193.Zhu M, Liu Y, Sun B, Zhang W, Liu X, Yu H, Zhang Y, Kuckling D, Adler H-JP. Macromolecular Rapid Communications. 2006;27:1023–1028. [Google Scholar]
- 194.Zhang W, Liu Y, Zhu M, Zhang Y, Liu X, Yu H, Jiang Y, Chen Y, Kuckling D, Adler H-JP. Journal of Polymer Science Part a-Polymer Chemistry. 2006;44:6640–6645. [Google Scholar]
- 195.Zhu AF, Shi Z, Jin JH, Li G, Jiang JM. Journal of Macromolecular Science Part B-Physics. 2012;51:2183–2190. [Google Scholar]
- 196.Wang JF, Lin L, Cheng QF, Jiang L. Angewandte Chemie-International Edition. 2012;51:4676–4680. doi: 10.1002/anie.201200267. [DOI] [PubMed] [Google Scholar]
- 197.Wu CJ, Gaharwar AK, Chan BK, Schmidt G. Macromolecules. 2011;44:8215–8224. [Google Scholar]
- 198.White JC, Stoppel WL, Roberts SC, Bhatia SR. Journal of Biomedical Materials Research Part A. 2013;101A:438–446. doi: 10.1002/jbm.a.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.He CC, Jiao KX, Zhang X, Xiang M, Li ZY, Wang HL. Soft Matter. 2011;7:2943–2952. [Google Scholar]
- 200.Yang J, Gong C, Shi FK, Xie XM. Journal of Physical Chemistry B. 2012;116:12038–12047. doi: 10.1021/jp303710d. [DOI] [PubMed] [Google Scholar]
- 201.Yang J, Deng LH, Han CR, Duan JF, Ma MG, Zhang XM, Xu F, Sun RC. Soft Matter. 2013;9:1220–1230. [Google Scholar]
- 202.Jiang GQ, Liu C, Liu XL, Zhang GH, Yang M, Liu FQ. Macromolecular Materials and Engineering. 2009;294:815–820. [Google Scholar]
- 203.Tuncaboylu DC, Argun A, Sahin M, Sari M, Okay O. Polymer. 2012;53:5513–5522. [Google Scholar]