Advances in Chimeric Antigen Receptor Immunotherapy for Neuroblastoma (original) (raw)
. Author manuscript; available in PMC: 2014 Jul 22.
Published in final edited form as: Discov Med. 2013 Dec;16(90):287–294.
Abstract
Neuroblastoma (NBL) is the most common extracranial pediatric solid tumor and has heterogeneous biology and behavior. Patients with high-risk disease have poor prognosis despite complex multimodal therapy; therefore, novel curative approaches are needed. Immunotherapy is a novel therapeutic approach that harnesses the inherent activity of the immune system to control and eliminate malignant cells. One form of immunotherapy uses chimeric antigen receptors (CAR) to target tumor-associated antigens. CARs are derived from the antigen-binding domain of a monoclonal antibody (MAb) coupled with the intracellular signaling portion of the T cell receptor. CARs can combine the specificity and effectiveness of MAbs with the active bio-distribution, direct cytotoxicity, and long-term persistence of T cells. NBL provides an attractive target for CAR immunotherapy as many of its tumor-associated antigens are not expressed at significant levels on normal tissues, thus decreasing potential treatment related toxicity. Two previous clinical trials utilizing L1-cell adhesion molecule (L1-CAM) and disialoganglioside (GD2) specific CARs (GD2-CAR) have demonstrated safety and anti-tumor efficacy in heavily pretreated relapsed/refractory neuroblastoma patients. Based on these promising results and on improved techniques that can further potentiate CAR therapies, two clinical trials are currently investigating the use of GD2-CARs in children with NBL. Several approaches may further enhance anti-tumor activity and persistence of CAR modified cells, and if these can be safely translated into the clinic, CAR-based immunotherapy could become a viable adjunct or potential alternative to conventional treatment options for patients with NBL.
Introduction
Neuroblastoma (NBL) is the most common extracranial solid tumor in children and the 3rd most common cause of pediatric cancer deaths (Smith et al., 2010). NBL is heterogeneous in biology and behavior: spontaneous regression can be seen in neonates with either local or systemic disease, while metastatic disease in patients older than 18 months of age poses a treatment challenge despite the combined use of surgical, radiotherapy, chemotherapy, and immunotherapy (Nuchtern et al., 2012; Baker et al., 2010; Matthay et al., 1999; Yu et al., 2010). Despite the use of multimodal therapy, there is a significant treatment failure rate, so that novel therapeutic options are necessary.
Although the immune system can recognize and kill malignant cells, cancers often escape this surveillance, spread, and ultimately cause death. Immunotherapy is intended to redirect the immune system to target tumors and tumor-associated antigens, leading to the elimination of malignant cells. Multiple forms of immunotherapy are being used in the clinic, including monoclonal antibodies (MAbs) targeted to tumor cell surface antigens or that disrupt the normal checkpoints that inhibit anti-tumor immune responses, cytokines that modify innate or adaptive immunity, tumor vaccines, and adoptive cellular therapies (Cheung et al., 2012; Yu et al., 2010; Hank et al., 1990; Brahmer et al., 2012; Topalian et al., 2012; Wolchok et al., 2013; Bowman et al., 1998; Brenner et al., 2000; Pule et al., 2008; Louis et al., 2011; Park et al., 2007). Neuroblastoma provides an attractive target for immunotherapy as it is derived from embryonic neuroectoderm and expresses certain antigens not widely detected in non-embryonic tissues, such as the L1-cell adhesion molecule (L1-CAM), disialoganglioside (GD2), B7H3, and o-acetyl GD2 (Schonmann et al., 1986; Cheung, 1991; Zhao and Cheung, 1995; Modak et al., 2001; Alvarez-Rueda et al., 2011).
Clinically, MAb treatment targeting GD2 antigens improves event-free and overall survival in patients with NBL (Yu et al., 2010; Cheung et al., 2012), but the anti-tumor effects are limited by passive bio-distribution of the antibody, the half-life of the antibody, and its dependence on other effector mechanisms (i.e., complement dependent lysis or antibody dependent cytotoxicity) to destroy tumor cells. Tumor infiltrating lymphocytes (TILs) are capable of cytotoxic activity in a major histocompatibility complex (MHC) dependent manner, and have been used successfully to treat patients with cancer (Morgan et al., 2006; Hunder et al., 2008). Unfortunately, many solid tumors, including neuroblastoma, may have abnormal MHC expression and antigen processing function, thus limiting the applicability of this approach (Raffaghello et al., 2005; del Campo et al., 2012).
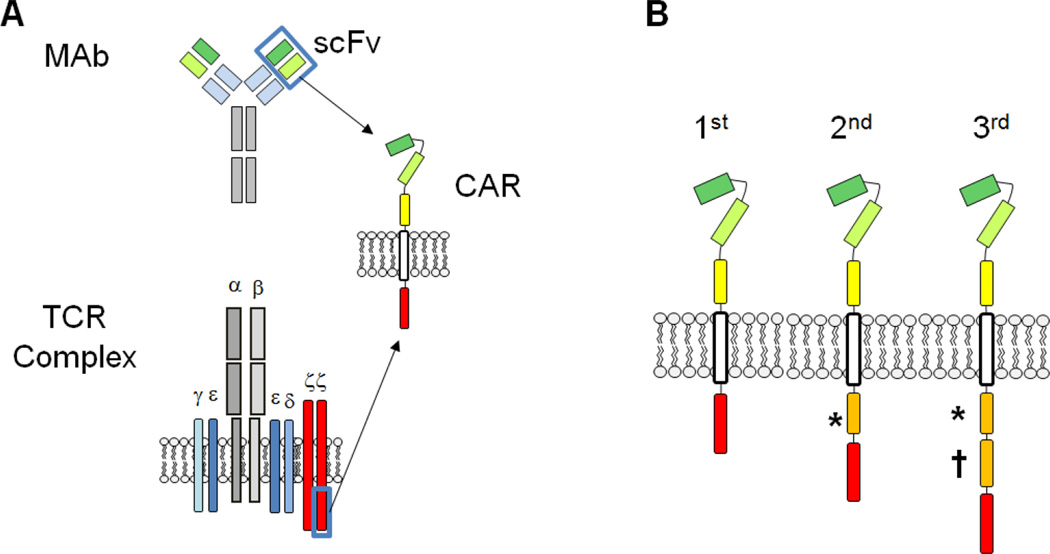
Effector lymphocytes can be genetically modified to express chimeric antigen receptors (CARs), which bind to tumor-associated antigens and lead to anti-tumor activity in an MHC-unrestricted manner. CARs are usually generated by joining the heavy and light chain variable regions of a monoclonal antibody with a linker to form a single-chain variable fragment (scFv). The scFv is then attached most commonly to the transmembrane and cytoplasmic portions of the T cell receptor (TCR) ζ chain, via a flexible hinge region, to form a fully functional 1st generation CAR (Figure 1A). Engagement of the scFv results in tyrosine phosphorylation of immunereceptor activation motifs present in the cytoplasmic domain (Savoldo and Dotti, 2013), and nuclear signaling, leading to T cell activation, cytokine secretion, and cellular proliferation. In addition to TCR ζ chain phosphorylation, effective T cell activation requires costimulatory signals from the tumor or professional antigen presenting cells (APCs). The tumor microenvironment lacks fully functional APCs and solid tumors typically do not express costimulatory surface molecules. Therefore, to provide costimulatory signals for optimal activation and survival of adoptively transferred cells, intracellular endodomains from costimulatory receptors such as CD28, OX40, 4-1BB, or inducible T-cell costimulator (ICOS) have been added to the 1st generation CAR constructs (Figure 1B). The 2nd and 3rd generation CAR products have improved anti-tumor effects, proliferation, and cytokine release in both preclinical and early phase clinical studies (Krause et al., 1998; Pule et al., 2005; Brentjens et al., 2007; Zhong et al., 2010; Kalos et al., 2011; Porter et al., 2011; Savoldo and Dotti, 2013).
Figure 1.
Structure and design of chimeric antigen receptors (CARs). A) The extracellular, antigen binding domain of a CAR is typically derived from the single chain variable fragment (scFv) of a monoclonal antibody (MAb). The scFv segment is further linked to a transmembrane domain and an endodomain -- most commonly the signaling moiety of the T cell receptor (TCR) zeta chain. B) CARs are classified into 1st, 2nd, or 3rd generation products depending on the number of costimulatory endodomains (*, †) added to the construct. The most commonly used costimulatory endodomains in clinical and preclinical studies are CD27*, CD28*, inducible T-cell costimulator (ICOS)*, 4-1BB†, and OX40†.
Here, we will review the past, present, and future of chimeric antigen receptor based neuroblastoma therapies.
Previous Clinical Trials with Chimeric Antigen Receptors
The first phase 1 clinical trial using CARs for children with NBL investigated the feasibility, safety, and antitumor efficacy of a first generation CAR targeting the cell adhesion molecule L1-CAM in patients with relapsed/refractory disease (Park et al., 2007). L1-CAM is a glycoprotein belonging to the immunoglobulin superfamily, located on chromosome Xp28. L1-CAM expression is relatively specific to neuroblastoma, although the molecule is also found on some cells of the central nervous system, sympathetic ganglia, and adrenal medulla, as well as on other types of cancer cells (Euer et al., 2005; Arlt et al., 2006; Fogel et al., 2003; Thies et al., 2002; Izumoto et al., 1996). The L1-CAM specific 1st generation CAR was constructed using the scFv from the CE7 MAb. Ten heavily pre-treated patients were enrolled in this study. Two patients became ineligible prior to cell infusion, and two cell products did not meet release criteria due to duplicate CAR gene insertion and low level endogenous T cell receptor expression; thus 6 patients received a total of 12 infusions. The characteristics of these patients are summarized in Table 1. Grade III toxicities included lymphopenia, neutropenia, low hemoglobin, bacteremia, and pneumonitis, but there were no Grade IV or V toxicities. Based on the International Neuroblastoma Response Criteria (Brodeur et al., 1993), an anti-tumor response was detectable in one patient who had minimal residual disease prior to infusion. The patient first had a mixed and then partial response, 35 and 56 days after cell infusion, respectively, and the infused cells were detectable in the peripheral blood up to 56 days after adoptive transfer. None of the remaining patients had an anti-tumor response and all had shorter persistence of CAR T cells. The authors suggest that the decreased persistence could have been due to an immune response directed toward the fusion protein CE7R although no anti-CE7R serum activity was ever detected (Park et al., 2007).
Table 1.
Patient Characteristics.
| Study | Patient# | Age | Gender | Disease Status | Cell Dose | Responseat Day 56 | OverallResponse | Outcome1 |
|---|---|---|---|---|---|---|---|---|
| L1-CAM CAR T cell trial2 | 1 | 9 | M | Relapsed, bone, bone marrow | 108, 109 | PD | CR | DOD |
| 2 | 12 | M | Relapsed, bulky | 108, 109 | PD | PD | DOD | |
| 3 | 7 | F | Relapsed, bone | 108, 108 | PR | PD | DOD | |
| 4 | 9 | F | Relapsed, bone, LN | 108 | PD | PD | DOD | |
| 5 | 16 | F | Relapsed, bulky | 108, 109 | PD | SD | DOD | |
| 6 | 10 | M | Relapsed, bulky | 108, 108, 108 | PD | PD | DOD | |
| GD2-CAR EBV-CTL/ATC trial3 | 1 | 9 | M | NED | 2×107/m2 | NED | NED | NED |
| 2 | 5 | M | NED | 2×107/m2 | NED | NED | NED | |
| 3 | 4 | M | NED | 2×107/m2 | NED | NED | NED | |
| 4 | 20 | F | Relapsed, NED | 2×107/m2 | NED | NED | AWD | |
| 5 | 7 | M | Relapsed, NED | 2×107/m2 | NED | NED | AWD | |
| 6 | 4 | F | Relapsed, bone | 2×107/m2 | PD | PD | DOD | |
| 7 | 9 | F | Relapsed, bone | 2×107/m2 | CR | CR | CR | |
| 8 | 4 | F | Relapsed, bone | 2×107/m2 | PR | CR | CR | |
| 9 | 10 | M | Relapsed, bulky | 2×107/m2 | PD | PD | DOD | |
| 10 | 11 | M | Relapsed, bulky | 2×107/m2 | PD | PD | DOD | |
| 11 | 10 | F | Relapsed, NED | 5×107/m2 | NED | NED | NED | |
| 12 | 4 | F | Relapsed, NED | 5×107/m2 | NED | NED | DOD | |
| 13 | 15 | F | Relapsed, bone marrow | 5×107/m2 | CR | CR | DOD | |
| 14 | 9 | F | Relapsed, bulky | 5×107/m2 | PD | PD | DOD | |
| 15 | 3 | M | Relapsed, bulky | 5×107/m2 | SD | SD | DOD | |
| 16 | 9 | F | Relapsed, bulky | 5×107/m2 | Tumor necrosis | Tumor necrosis | DOD | |
| 17 | 7 | M | Relapsed, NED | 1×108/m2 | NED | NED | DOD | |
| 18 | 4 | F | Relapsed, bulky | 1×108/m2 | Tumor necrosis | Tumor necrosis | DOD | |
| 19 | 7 | M | Relapsed, bulky | 1×108/m2 | SD | PR | AWD |
This trial proved the feasibility of expanding T cell clones genetically modified to express a 1st generation CAR for the treatment of children with relapsed/refractory neuroblastoma. One reason for the limited antitumor efficacy seen with this product may have been suboptimal persistence of infused cells associated with inadequate activation and proliferation from the first generation CAR, or exhaustion after relatively long ex vivo culturing (Riddell et al., 1992).
The largest phase 1 clinical trial for patients with NBL used a GD2-specific 1st generation CAR and evaluated their safety and anti-tumor efficacy (clinicaltrials.gov ID: NCT00085930) (Pule et al., 2008; Louis et al., 2011). GD2 is a disialoganglioside expressed on tumors such as neuroblastoma, melanoma, and osteosarcoma. GD2 is also expressed on peripheral nerves and some cells of the cerebellum. The GD2-specific scFv of this CAR was generated from the 14g2a MAb (Rossig et al., 2000; 2001). The CAR was inserted into autologous activated T cells (ATCs) or Epstein-Barr virus specific cytotoxic T cells (CAR-CTLs) by retroviral transduction. The investigators hypothesized that persistence of CAR-CTLs may be superior to that of CAR-ATCs as CAR-CTLs would receive additional costimulation from binding at the native EBV-specific TCR. Both ATCs and EBV-CTLs were infused into each patient and peripheral blood was monitored for evidence of persistence and immunophenotypic changes (Pule et al., 2008; Louis et al., 2011).
On this clinical trial, 19 patients were treated and received a total of 40 products. 8/19 had no evidence of disease at the time of infusion (5/8 after treatment for relapse and 3/8 after treatment for high-risk disease). The infusions were well tolerated. There were no dose limiting toxicities and the only treatment-related adverse events were low-grade fever and mild pain at the sites of known disease. None of the patients developed neurologic abnormalities or peripheral neuropathy (Pule et al., 2008; Louis et al., 2011). Significant antitumor efficacy was detected including 3 complete remissions, 1 partial response, 1 stable disease, and 2 with tumor necrosis out of the 11 patients with evaluable disease at the time of enrollment. The median overall survival was 931 days and after a median follow-up of 329 days, 2 of the 3 complete response patients were disease free. When comparing CAR-CTLs with CAR-ATCs, the persistence of CAR-CTLs was superior during the first 6 weeks post-infusion; however, longer-term follow-up found that both types of gene modified cell product were at low levels for an extended period of time (up to 96 and 192 weeks, respectively, for CAR-CTL and CAR-ATCs). Importantly, detection of the CAR T cells in peripheral blood for 6 weeks or more was associated with a statistically prolonged time to progression (TTP) (Louis et al., 2011), and such detection correlated with the number of CD4 T helper cells and central memory cells in the infused product.
This trial showed that the adoptive transfer of autologous, 1st generation GD2-specific CAR T cells (GD2-CAR) was safe and led to anti-tumor activity in children with high-risk or relapsed/refractory NBL. The results also confirmed that the presence of both central memory cells and CD4 T cell subsets in the T cell product is critical for long-term CAR persistence and that prolonged persistence was associated with improved tumor control.
Current Clinical Investigations with Neuroblastoma Specific CARs
Investigators have evaluated 2 distinct methods to increase persistence: lymphodepletion prior to adoptive transfer and utilization of 2nd or 3rd generation CARs. Lymphodepletion prior to cell infusions creates a better homeostatic environment for the transferred cells to expand (Muranski et al., 2006). Newer generation CARs improve both activation and costimulation after antigen binding (Figure 1) (Savoldo and Dotti, 2013). Based on these data, two recently open clinical trials are aiming to improve the effectiveness of CARs for children with NBL.
In one study, 1st generation GD2-CAR donor derived virus-specific CTLs are being used to treat children with relapsed/refractory NBL after allogeneic stem cell transplantation (ASCT) (clinicaltrials.gov ID: NCT01460901). Graft versus tumor (GVT) activity has been described after ASCT for NBL (Perez-Martinez et al., 2009; Matthay et al., 1994; Ladenstein et al., 2008). Unfortunately, the anti-tumor benefit was neutralized by transplant related mortality (TRM). Viral infections are one of the major cause of mortality after ASCT (Kennedy-Nasser et al., 2008) and virus-specific T cells (VSTs) targeting EBV, CMV, and adenovirus have been used successfully to both prevent and treat post-transplant viral infections (Watarai et al., 2008; Gerdemann et al., 2013; Leen et al., 2009). Therefore, investigators hypothesize that using VSTs expressing a 1st generation GD2 CAR may improve the survival of patients with relapsed/refractory NBL by 1) allowing CAR modified cells to take advantage of the lymphodepleted, post-transplant environment to grow and expand after infusion; 2) by providing an antigen-specific GVT effect; 3) decreasing viral-associated TRM by prophylactic adoptive transfer of CAR-expressing tri-virus specific T cells early post-transplant; and 4) by providing additional costimulation to the infused cells via binding at their native T cell receptor upon exposure to viral antigens. This single center phase 1 trial aims to determine the safety profile and the behavior of the infused CAR-VSTs.
The second currently approved study (GRAIN) is a dose escalation trial using ATCs transduced with a 3rd generation GD2-CAR and inducible caspase 9 (iC9) safety switch for children with relapsed/refractory disease (clinicaltrials.gov ID: NCT01822652). The incorporation of CD28 and OX40 costimulatory endodomains into the previously used 1st generation GD2-CAR improved cell survival and antitumor effect during preclinical evaluations (Pule et al., 2005). However, other investigators using 2nd and 3rd generation CARs had noted both increased efficacy and increased toxicity (cytokine storm or cytokine release syndrome) once these products were tested clinically (Morgan et al., 2010; Kalos et al., 2011; Brentjens et al., 2013). In order to add an additional layer of safety, the current neuroblastoma CAR-T cell study incorporates an inducible caspase 9 (iC9) safety switch within the construct. iC9 is a genetically modified molecule engineered to dimerize, trigger programmed cell death, and rapidly eliminate the gene modified cell when exposed to the small molecule AP1903. Activation of the iC9 construct after AP1903 infusion has been tested in a previous immunotherapeutic clinical trial for pediatric patients and led to the elimination of 90% of transduced cells within 30 minutes of the IV infusion (Di et al., 2011).
Future Directions
Future efforts will likely focus on increasing the activity of the infused CAR-T cells, improving their targeting, and reducing their sensitivity to the inhibitory microenvironment of the tumor.
To broaden the utility of CAR immunotherapy in the solid tumor setting, many research teams are looking for ways to further enhance anti-tumor efficacy while maintaining an acceptable toxicity profile. For example, alternative or additional costimulatory endodomains such as 4-1BB or ICOS may provide a CAR construct that provides superior survival and activation signals for anti-tumor activity in the immunosuppressive microenvironment fostered by NBL.
Further, as the affinity of CARs and/or TCRs increases with genetic modification, the likelihood of on- and off-target toxicity secondary to low level antigenic expression on normal tissues increases. Thus, further discovery and evaluation of tumor specific antigens, as well as improved methods of preclinical toxicity assessments will be critically important (Linette et al., 2013). L1-CAM and GD2 expression is not strictly restricted to NBL; however, the recently described o-acetyl-GD2 (oaGD2) has been found on 100% of NBL cells and its expression appears completely absent on peripheral nerves (Alvarez-Rueda et al., 2011). Using oaGD2 as a CAR target may maintain anti-tumor effects without increasing the risk of neuronal toxicity and preclinical testing is on-going. Improving tumor trafficking by adoptively transferred CAR cells may also increase their effectiveness (Di et al., 2011). For example, NBL produces CCL2 and genetic modification of GD2-CAR T cells with the receptor for this chemokine (CCR2b) improved T cell homing to the tumor and resulted in better in vivo and in vitro antitumor activity (Craddock et al., 2010).
A complex interplay exists between malignant NBL cells and non-malignant stromal cells that leads to an overall immunosuppressive microenvironment (Pistoia et al., 2013). Preclinical testing has shown that this inhibitory environment can be overcome by stronger activation of T cell intracellular survival pathways (i.e., constitutive activation of akt), the production of cytokines locally by CAR modified cells (i.e., designed to secrete IL-7 or IL-15), or genetic engineering of the cells with non-functional TGF-β receptors (Sun et al., 2010; Hoyos et al., 2010; Perna et al., 2013; Foster et al., 2008). Conversely, constitutive pathway activation or autocrine cytokine secretion may allow gene-modified cells to evade physiologic control of proliferation and survival. The use of a suicide gene or the development of transient expression of these modifications may be important alternatives to limit the risk of uncontrolled cell proliferation and maintain safety.
Lastly, investigators are also looking at ways to optimize the cellular product that is given to patients. For example, the presence of CD4 T helper cells and/or central memory T cells (CM) within the infused product has been associated with improved persistence and antitumor activity of CAR T cells in both preclinical and clinical studies (Louis et al., 2011; Klebanoff et al., 2012). With recent advances in GMP manufacturing, clinical scale expansion of either high purity CD4 T cells or CM T cells is possible and genetic engineering of these cells with an NBL-specific CAR may provide an effective treatment approach. Additionally, other cell types besides T cells can be genetically modified to express CARs. Natural killer T cells (NKTs) are an evolutionary conserved subset of innate lymphocyte expressing an invariant TCR (Valfa24, Jalfa18, paired with Vbeta11 in humans). They react with monomorphic CD1d on antigen presenting cells such as monocytes/ macrophages (Kronenberg and Gapin, 2002). NKTs control NBL growth by destroying cancer supporting tumor associated macrophages (TAMs) in the tumor microenvironment (Song et al., 2009) and the presence of NKTs in primary NBL tissues from patients with Stage 4 disease is associated with improved survival. NKTs genetically modified to express GD2-CARs are showing promising results in preclinical models by killing both NBL and TAMs in vitro and inducing significant anti-tumor effect in vivo (Heczey et al., 2013). Natural killer (NK) cells have innate antitumor properties and killer inhibitory receptor mismatched NK cells were shown to have anti-tumor effect post-autologous transplantation in children with NBL (Perez-Martinez et al., 2009). Genetic modification of NK cells with either a 14g2a scFv and the NK cell activating 2B4 intracellular domain, or 14.18 scFv and TCR ζ chain, have both shown promising preclinical activity (Altvater et al., 2009; Esser et al., 2012).
Conclusion
Previous studies using CARs to treat patients with NBL have shown safety and promising anti-tumor effects. A number of approaches have been developed to further enhance anti-tumor activity of CAR modified cells. If these enhancements can be safely translated into the clinic, using CARs to treat NBL can become a viable non-chemotherapeutic treatment alternative for patients diagnosed with this disease.
Acknowledgment
A.H. is supported by the NIH-NCI K12 CA090433. C.U.L. is supported by a Sidney Kimmel Translational Science Award, NIH RO1 CA142636, and NIH P01 CA094237. Research presented within this manuscript was supported by NIH RO1 CA142636 and NIH P01 CA094237.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose related to the project. The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene.
References
- Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, Campana D, Juergens H, Pule M, Rossig C. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15(15):4857–4866. doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Rueda N, Desselle A, Cochonneau D, Chaumette T, Clemenceau B, Leprieur S, Bougras G, Supiot S, Mussini JM, Barbet J, Saba J, Paris F, Aubry J, Birkle S. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PLoS One. 2011;6(9):e25220. doi: 10.1371/journal.pone.0025220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt MJ, Novak-Hofer I, Gast D, Gschwend V, Moldenhauer G, Grunberg J, Honer M, Schubiger PA, Altevogt P, Kruger A. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006;66(2):936–943. doi: 10.1158/0008-5472.CAN-05-1818. [DOI] [PubMed] [Google Scholar]
- Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A, Stram D, Castleberry RP, Shimada H, Sandler A, Shamberger RC, Look AT, Reynolds CP, Seeger RC, Matthay KK. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363(14):1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman L, Grossmann M, Rill D, Brown M, Zhong WY, Alexander B, Leimig T, Coustan-Smith E, Campana D, Jenkins J, Woods D, Kitchingman G, Vanin E, Brenner M. IL-2 adenovector-transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma. Blood. 1998;92(6):1941–1949. [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner MK, Heslop H, Krance R, Horowitz M, Strother D, Nuchtern J, Grilley B, Martingano E, Cooper K. Phase I study of chemokine and cytokine gene-modified autologous neuroblastoma cells for treatment of relapsed/refractory neuroblastoma using an adenoviral vector. Hum Gene Ther. 2000;11(10):1477–1488. doi: 10.1089/10430340050057549. [DOI] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177) doi: 10.1126/scitranslmed.3005930. 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La PK, Quintas-Cardama A, Larson SM, Sadelain M. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(18 Pt 1):5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De BB, Evans AE, Favrot M, Hedborg F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- Cheung NK. Immunotherapy. Neuroblastoma as a model. Pediatr Clin North Am. 1991;38(2):425–441. doi: 10.1016/s0031-3955(16)38085-3. [DOI] [PubMed] [Google Scholar]
- Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30(26):3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, Foster AE. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33(8):780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo AB, Carretero J, Aptsiauri N, Garrido F. Targeting HLA class I expression to increase tumor immunogenicity. Tissue Antigens. 2012;79(3):147–154. doi: 10.1111/j.1399-0039.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- Di SA, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, Aperlo-Iffland C, Huston JS, Uherek C, Schonfeld K, Tonn T, Huebener N, Lode HN, Koehl U, Wels WS. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16(3):569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euer NI, Kaul S, Deissler H, Mobus VJ, Zeillinger R, Weidle UH. Identification of L1CAM, Jagged2 and Neuromedin U as ovarian cancer-associated antigens. Oncol Rep. 2005;13(3):375–387. [PubMed] [Google Scholar]
- Fogel M, Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Smirnov A, Edler L, Ben-Arie A, Huszar M, Altevogt P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet. 2003;362(9387):869–875. doi: 10.1016/S0140-6736(03)14342-5. [DOI] [PubMed] [Google Scholar]
- Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31(5):500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, Martinez CA, Kennedy-Nasser A, Leung KS, Gottschalk SM, Krance RA, Brenner MK, Rooney CM, Heslop HE, Leen AM. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21(11):2113–2121. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, Sondel PM. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50(17):5234–5239. [PubMed] [Google Scholar]
- Heczey A, Liu D, Courtney A, Marinova E, Guo L, Gao X, Yvon E, Hicks J, Dotti G, Metelitsa LS. NKT Cells as a Novel Platform for Cancer Immunotherapy with Chimeric Antigen Receptors. American Society of Gell and Cell Therapy Annual Meeting. 2013 [Google Scholar]
- Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumoto S, Ohnishi T, Arita N, Hiraga S, Taki T, Hayakawa T. Gene expression of neural cell adhesion molecule L1 in malignant gliomas and biological significance of L1 in glioma invasion. Cancer Res. 1996;56(6):1440–1444. [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95) doi: 10.1126/scitranslmed.3002842. 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y, Liu H, Heslop HE, Brenner MK, Krance RA. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2008;14(11):1245–1252. doi: 10.1016/j.bbmt.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35(9):651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med. 1998;188(4):619–626. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2(8):557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- Ladenstein R, Potschger U, Hartman O, Pearson AD, Klingebiel T, Castel V, Yaniv I, Demirer T, Dini G. 28 years of high-dose therapy and SCT for neuroblastoma in Europe: lessons from more than 4000 procedures. Bone Marrow Transplant. 2008;41(Suppl 2):S118–S127. doi: 10.1038/bmt.2008.69. [DOI] [PubMed] [Google Scholar]
- Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell hv, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay KK, Seeger RC, Reynolds CP, Stram DO, O’Leary MC, Harris RE, Selch M, Atkinson JB, Haase GM, Ramsay NK. Allogeneic versus autologous purged bone marrow transplantation for neuroblastoma: a report from the Childrens Cancer Group. J Clin Oncol. 1994;12(11):2382–2389. doi: 10.1200/JCO.1994.12.11.2382. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341(16):1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61(10):4048–4054. [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol. 2006;3(12):668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchtern JG, London WB, Barnewolt CE, Naranjo A, McGrady PW, Geiger JD, Diller L, Schmidt ML, Maris JM, Cohn SL, Shamberger RC. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children’s Oncology Group study. Ann Surg. 2012;256(4):573–580. doi: 10.1097/SLA.0b013e31826cbbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, Jensen MC. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez A, Leung W, Munoz E, Iyengar R, Ramirez M, Vicario JL, Lassaletta A, Sevilla J, Gonzalez-Vicent M, Madero L, Diaz-Perez MA. KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr Blood Cancer. 2009;53(1):120–124. doi: 10.1002/pbc.21955. [DOI] [PubMed] [Google Scholar]
- Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B, Dotti G. Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes without enhancement of regulatory T-cell inhibition. Clin Cancer Res. 2013 Nov 26; doi: 10.1158/1078-0432.CCR-13-1016. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistoia V, Morandi F, Bianchi G, Pezzolo A, Prigione I, Raffaghello L. Immunosuppressive microenvironment in neuroblastoma. Front Oncol. 2013;3:167. doi: 10.3389/fonc.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell hv, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12(5):933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Prigione I, Bocca P, Morandi F, Camoriano M, Gambini C, Wang X, Ferrone S, Pistoia V. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene. 2005;24(29):4634–4644. doi: 10.1038/sj.onc.1208594. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer. 2001;94(2):228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- Rossig C, Nuchtern JG, Brenner MK. Selection of human antitumor single-chain Fv antibodies from the B-cell repertoire of patients immunized against autologous neuroblastoma. Med Pediatr Oncol. 2000;35(6):692–695. doi: 10.1002/1096-911x(20001201)35:6<692::aid-mpo45>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Savoldo B, Dotti G. Chimeric antigen receptors (CARs) from bench-to-bedside. Immunol Lett. 2013;155(1–2):40–42. doi: 10.1016/j.imlet.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonmann SM, Iyer J, Laeng H, Gerber HA, Kaser H, Blaser K. Production and characterization of monoclonal antibodies against human neuroblastoma. Int J Cancer. 1986;37(2):255–262. doi: 10.1002/ijc.2910370214. [DOI] [PubMed] [Google Scholar]
- Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O’Leary M, Smith FO, Reaman GH. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck YA, Seeger RC, Metelitsa LS. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119(6):1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, Spencer DM, Rooney CM. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 2010;18(11):2006–2017. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies A, Schachner M, Moll I, Berger J, Schulze HJ, Brunner G, Schumacher U. Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur J Cancer. 2002;38(13):1708–1716. doi: 10.1016/s0959-8049(02)00105-3. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. 2008;3(1):70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XJ, Cheung NK. GD2 oligosaccharide: target for cytotoxic T lymphocytes. J Exp Med. 1995;182(1):67–74. doi: 10.1084/jem.182.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]