Roles and Regulation of Gastrointestinal Eosinophils in Immunity and Disease (original) (raw)
. Author manuscript; available in PMC: 2015 Aug 1.
Published in final edited form as: J Immunol. 2014 Aug 1;193(3):999–1005. doi: 10.4049/jimmunol.1400413
Abstract
Eosinophils have been considered to be destructive end-stage effector cells that have a role in parasitic infections and allergy reactions by the release of their granule-derived cytotoxic proteins. However, an increasing number of experimental observations indicate that eosinophils also are multifunctional leukocytes involved in diverse inflammatory and physiologic immune responses. Under homeostatic conditions, eosinophils are particularly abundant in the lamina propria of the gastrointestinal tract where their involvement in various biological processes within the gastrointestinal tract has been posited. In this review, we summarize the molecular steps involved in eosinophil development and describe eosinophil trafficking to the gastrointestinal tract. We synthesize the current findings on the phenotypic and functional properties of gastrointestinal eosinophils and the accumulating evidence that they have a contributory role in gastrointestinal disorders, with a focus on primary eosinophilic gastrointestinal disorders. Finally, we discuss the potential role of eosinophils as modulators of the intestinal immune system.
Introduction
Eosinophils are multifunctional pro-inflammatory leukocytes involved in the pathogenesis of allergic disorders and implicated in the protection against helminth infections (1, 2). Eosinophils are generally thought of as act pro-inflammatory cells due to their release of pleotropic cytokines, chemokines, and lipid mediators, as well as toxic cytoplasmic granule constituents including major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin1 (3, 4). Although eosinophils are recognized as circulating cells, composing 1–5% of peripheral blood leukocytes, they are primarily resident in the lamina propria of the small intestine, where they compose a substantial fraction (e.g. 20–30%) of the cellular population (2, 5). Recently, a standard protocol for the isolation of murine eosinophils from the intestinal lamina propria using eosinophil-specific surface markers has been established (5). Additionally, development of eosinophil-deficient mouse strains has expanded the understanding of the role of intestinal eosinophils from dogmatic anti-parasitic effector cells to immune modulatory cells. Herein, we discuss emerging advances in the understanding of intestinal eosinophils at baseline and during inflammatory gastrointestinal disorders, including primary eosinophilic gastrointestinal disorders2 such as eosinophilic esophagitis3 (6).
Developmental properties of eosinophils
Eosinophils develop in the bone marrow from pluripotent stem cells that become eosinophil progenitors marked by cluster of differentiation4 34+CD125+ expression. Eosinophil lineage specification is determined by the interplay of several transcription factors, including the zinc finger transcription factor GATA-binding protein 15, the E26 transformation-specific family member PU.1, interferon consensus sequence binding protein, and CCAAT/enhancer-binding protein family members (2, 7, 8), as well as by regulation by microRNAs6 including miR-21 and miR-223 (9, 10). Of the transcription factors, GATA-1 is the most important; targeted deletion of the high-affinity double palindromic GATA-1 binding site in the Gata1 promoter results in eosinophil-depleted mice referred to as ΔdblGATA mice (11). The ΔdblGATA mice, along with another eosinophil-deficient strain generated using a promoter of the eosinophil peroxidase gene to drive expression of cytocidal diphtheria toxin A (referred to as PHIL mice) (12), are now being used to uncover the function of eosinophils (13). Eosinophil development is guided by signals from the aforementioned transcription factors, and subsequently, permissive proliferation and differentiation are regulated primarily by IL-5, although IL-3 and GM-CSF can also contribute (2). Of these cytokines, IL-5 is the most specific for selective differentiation of eosinophils, stimulating their migration from the bone marrow to the circulation (2, 14). Eosinophils released into the blood migrate into the thymus, mammary gland, uterus, and the gastrointestinal tract, with the latter having the highest eosinophil levels under homeostatic conditions (2). Levels of gastrointestinal eosinophils have been estimated to be at least 10-fold higher than in the circulation (2, 3). Examination of the whole gastrointestinal tract reveals that only the esophagus is devoid of baseline eosinophils and that eosinophil levels progressively increase from the stomach to the colon, where they can be fairly high, with as many as 50 eosinophils/high-power microscopic field (400X) (15).
Unique characteristics of intestinal eosinophils
Until recently, no detailed phenotypic analysis of intestinal eosinophils had been performed on account of the difficulties inherent in identifying and/or isolating sufficient numbers of these cells from the gastrointestinal tract. However, phenotypic characterization of murine eosinophils in the intestinal lamina propria has been recently reported by several groups (5, 16, 17). For detection of eosinophils in mice, there are several available markers, including CCR3, sialic acid–binding immunoglobulin-like lectin7 F (homolog of siglec-8 in humans), and CD125, which encodes for the IL-5 receptor alpha8 (18–20). Although these markers are primarily expressed on eosinophils, CCR3 also is expressed on mast cells and Th type 29 cells (21), Siglec-F has been detected on alveolar macrophages (22), and IL-5Rα is also expressed on peritoneal B-1 cells (20). None of these markers, therefore, can be considered to represent a definite specificity for eosinophils. However, as eosinophils contain a dense concentration of cytoplasmic granules, their side scatter patterns under flow cytometry are readily distinguishable from that of other lineages. Thus, a combination of the relative expression of CCR3, Siglec-F, and/or IL-5Rα with their side scatter patterns can be used to delineate eosinophil subsets in the intestine (Fig. 1A) (5, 16, 17). In addition to these markers, intestinal eosinophils of mice express higher levels of myeloid marker CD11b than their blood counterparts and are positive for CD11c, a surface marker used to identify intestinal dendritic cells (Fig. 1A) (5). Meanwhile, small-intestinal eosinophils isolated from mice are negative for other markers associated with intestinal dendritic cells, such as MHC II, CD80, CD103, and CD205 (DEC-205) (5). Considering that CD11c expression also occurs on murine eosinophils in the thymus and uterus but not in the blood (5), it seems plausible that it is affected by the local microenvironment rather than being indicative of their antigen-presenting capacity. Along with CD11c, Siglec-F expression also is observed at higher levels in eosinophils of the small intestine (5). It remains to be determined whether the relative expressions of CD11c and Siglec-F in murine eosinophils from different sources correlate with their functional differences in specific tissues. CD22, a B cell–specific Siglec belonging to the inhibitory receptor family, is highly expressed on the surface of murine, small-intestinal eosinophils but is undetectable on eosinophils from the blood (Fig. 1A) (17). As the small intestine is a milieu rich in substances that stimulate eosinophils (23–25), CD22’s abundant expression on intestinal eosinophils might prevent overactivation of intestinal eosinophils under homeostatic conditions. Notably, CD22 expression on small-intestinal eosinophils is decreased under various inflammatory conditions such as bacterial colonization, systemic IL-5 overexpression, and ovalbumin-induced gastrointestinal inflammation (17). Therefore, it can be posited that small-intestinal eosinophils receive negative feedback signals from highly expressed inhibitory CD22 under the steady state but are ready for conversion to pro-inflammatory cells via down-regulation of CD22.
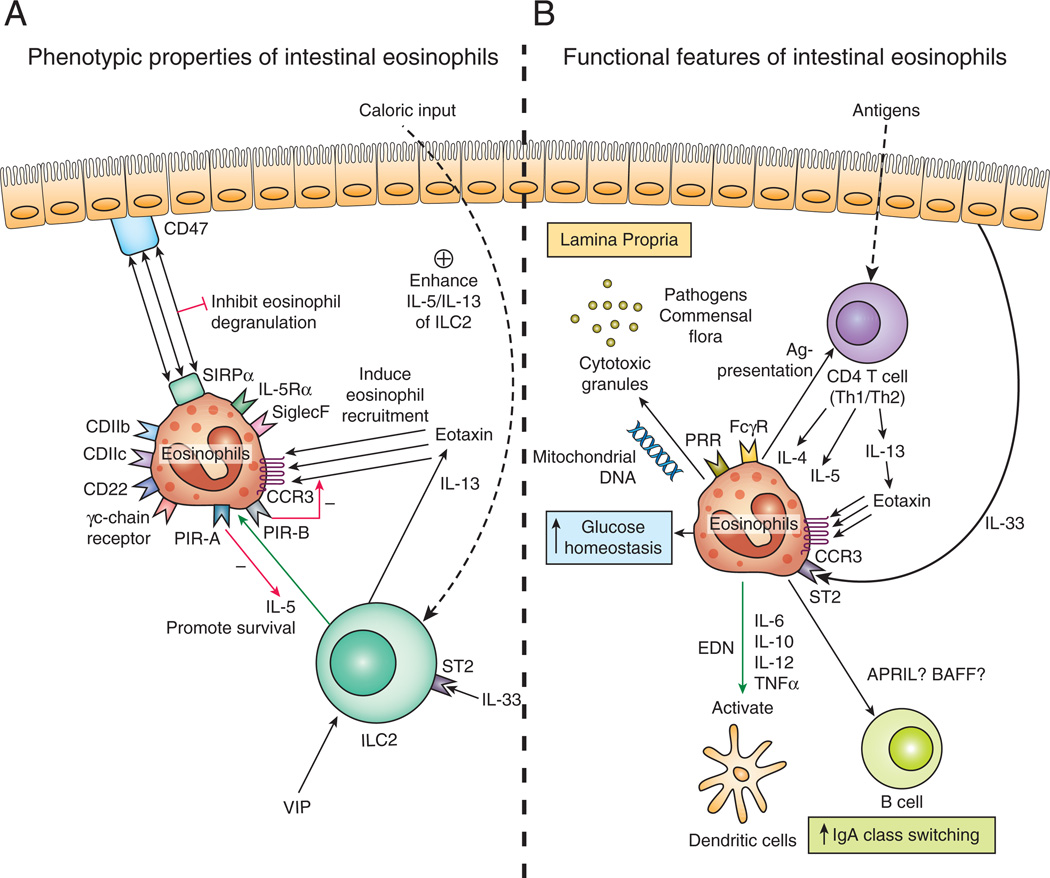
Figure 1. Phenotypic and functional properties of gastrointestinal eosinophils.
(A) In healthy conditions, eosinophils develop in the bone marrow and migrate to the lamina propria of gastrointestinal tract by a process regulated by CCR3/eotaxin-1. IL-5 secreted by ILC2 and cytokine signaling through the γc-chain receptors increases the life-span of small-intestinal eosinophils. Coexpression of IL-5 and IL-13 by ILC2 is enhanced by nutrient uptake, VIP stimulation, and IL-33. Eosinophil SIRP-α inhibits degranulation by interaction with membranous protein CD47, thus promoting eosinophil survival. IL-5 and eotaxin signaling is regulated by the opposing actions of PIR-A and PIR-B.(B) Eosinophils express a broad range of pattern-recognition (PRR) and Fc gamma receptors (FcγR), allowing them to be stimulated by various pathogens including bacteria, virus, and antibody-coated pathogens including helminths. Cytoplasmic granules and eosinophilic mitochrondrial DNA secreted by gastrointestinal eosinophils mediate tissue pathology and particulate in host clearance of pathogens. Eosinophils stimulated by Th2 type cytokines and epithelium-derived IL-33 mediate tissue inflammation in a variety of primary eosinophilic gastrointestinal disorder. Eosinophils may also modulate T cell–mediated immune responses, IgA class switching, and glucose homeostasis.
Under homeostatic conditions, most eosinophils produced in the bone marrow migrate to the small intestine; this process is regulated by eosinophil expression of CCR3 and α4β7 integrin, as their cognate ligands (eotaxin and mucosal vascular address in cell adhesion molecule 1, respectively) are constitutively expressed in the intestine (15, 26). β7 integrin appears to be particularly important in the large intestine, whereas eotaxins mediate eosinophil homing in the large and small intestine of mice (26, 27). Gastrointestinal eosinophils are post mitotic and have limited survival in the absence of survival-promoting cytokine signals (28); conversely, cytokine signaling through the common γchain increases the life-span of murine small-intestinal eosinophils (Fig. 1A) (5). Therefore, in combination with a specialized influx mechanism, the prolonged survival of eosinophils (at least 14 days) contributes to their predominance in the gastrointestinal tract (5). In addition, signal regulatory protein α10, highly expressed in small-intestinal eosinophils, inhibits degranulation of eosinophils by interaction with their ligand CD47, thus promoting eosinophil survival (Fig. 1A) (16). Also, type 2 innate lymphoid cells11 have been identified as a specialized cell population supporting the maintenance of murine intestinal eosinophils by secreting IL-5 and IL-13, which promote eosinophil survival and recruitment to the small intestine, respectively, via upregulation of eotaxin (29). Notably, the coexpression of IL-5 and IL-13 by ILC2 is enhanced after caloric intake and regulated by vasoactive intestinal peptide, which stimulates ILC2 through vasoactive intestinal peptide receptor type 2 to release IL-5 via a circadian rhythm (Fig. 1A) (29). However, under nutrient deprivation, ILC2 and ILC2–derived IL-5 and IL-13 are also increased in the gut (30), thus suggesting complex interactions between eosinophil survival and intestinal nutritional conditions.
Migratory properties of intestinal eosinophils
Most eosinophils generated in the bone marrow migrate to all segments of the gastrointestinal tract except the esophagus, coming to reside in the intestinal lamina propria under baseline conditions (2). Prenatal mice have comparable numbers of eosinophils in the gastrointestinal tract as adult mice; thus, at least relative to other leukocytes, eosinophil homing to the gastrointestinal tract seems to be independent of the enteric flora (15), likely mediated by constitutive ILC2 and eotaxin-1 (29). The recruitment of murine eosinophils to the gastrointestinal tract under the steady state is regulated primarily by eotaxin-1, which is localized in Ly6chighCCR2+F4/80+CD11b+ cells that are under the regulation of calprotectin (S100a8/S100a9) (31, 32). The importance of eotaxin-1 in regulating the baseline level of eosinophils in the gastrointestinal tract is underscored by the severe deficiency of eosinophils in the intestinal mucosa, without any significant decrease of bone marrow and peripheral blood eosinophils, in eotaxin-1–deficient mice (15, 33). By genomic analyses, two additional chemokines, designated eotaxin-2 and eotaxin-3, have been identified according to their eosinophil-selective chemoattractant activity (2). The specific activity of the eotaxin subfamily of chemokines is mediated by the G protein–coupled receptor CCR3, which is primarily expressed on eosinophils (Fig. 1A) (34, 35). Accordingly, deficiency of gastrointestinal eosinophils in mice, with the targeted deletion of CCR3, supports the critical role of eotaxin-1 in the maintenance of intestinal eosinophils under homeostatic conditions (36). Although eotaxin-1 is the major chemokine required for the baseline level of eosinophils in the gastrointestinal tract, eotaxin-1 alone is unlikely to be sufficient, as eotaxin-1 is abundantly expressed in the upper gastrointestinal segments (e.g. tongue, esophagus), and eosinophils are not normally present in these locations (15). Paired immunoglobulin-like receptor12 B, which is highly expressed in eosinophils and can suppress eotaxin/CCR3-mediated eosinophil migration to the small intestine, may be an inhibitory checkpoint for esophageal eosinophil trafficking (Fig. 1A) (37). While inhibiting eosinophil migration, PIR-B supports IL-5–induced expansion of murine eosinophils by suppressing PIR-A–induced apoptosis of bone marrow eosinophils (38).
Eosinophils also express a number of adhesion molecules involved in cell trafficking, including integrin α4β7, integrin α4β1 (VLA-4), and the β2-integrin family (the CD18 family) (2, 26). Integrin α4β1 interacts with the endothelium via VCAM-1 and fibronectin, the CD18 family of molecules binds to ICAM-1, and α4β7 integrin interacts with the mucosal vascular addressin cell adhesion molecule 1 that is expressed by the vascular endothelium in the intestinal tract (2, 26). These integrins have prominent roles in eosinophil trafficking during inflammation rather than during homeostasis, as demonstrated by the reduced number of small-intestinal eosinophils after oral allergen stimulation in β7 gene–targeted mice compared to non-deficient mice (26). Furthermore, in β7-deficient mice, eosinophil accumulation in the small intestine after Trichinella spiralis infection is delayed compared to in non-deficient mice (39). The trafficking of eosinophils to inflammatory sites also involves a number cytokines, particularly those of the Th2 type such as IL-4, IL-5, and IL-13, of which only IL-5 is implicated in selective tissue distribution (2). Under baseline conditions, eosinophils in the Peyer’s patch are barely detected in mice; however, after IL-5 overexpression, they substantially localize to the inter-follicular regions via eotaxin-1– and IL-5–dependent mechanisms (40).
Functional characteristics of intestinal eosinophils
Eosinophils have long been considered effector cells that have a protective role against parasitic helminth infection. However, eosinophils also are associated with numerous gastrointestinal disorders, such as EoE, eosinophilic gastritis, eosinophilic enteritis, and eosinophilic colitis, which are collectively referred to as EGID, as well as inflammatory bowel diseases13 (6). On the other hand, numerous lines of evidence indicate that eosinophils are multifunctional leukocytes involved in germane biologic processes in the gastrointestinal tract. Hereafter, the functional features of eosinophils as effector cells against pathogenic infections, as pathologic players in EGID and IBD, and as modulators of gastrointestinal immune responses, will be discussed.
Beneficial role of eosinophils
The anti-parasitic functionality of eosinophils is based primarily on their increase in the circulation and affected tissues during helminth infections (41), as well as their ability to mediate in vitro antibody-dependent cellular toxicity against helminthes (42, 43). Murine models of parasitic infection have demonstrated eosinophil recruitment to infected tissues and parasite death mediated by the release of toxic cytoplasmic granules, such as major basic protein (Fig. 1B) (44). Indeed, impaired resistance against secondary infection with intestinal Nippostrongylus brasiliensis is observed in ΔdblGATA mice (45). However, _Schistosoma mansoni_–infected ΔdblGATA and PHIL mice have normal disease progression despite the recruitment of large numbers of eosinophils in the affected liver (46). In _T. spiralis_–infected ΔdblGATA mice, marked death of T. spiralis muscle larvae by inducible NO synthase–producing neutrophils and macrophages is observed, and excessive host inflammatory responses are linked to pathologic changes of infected muscle (47). As transfer of eosinophils into ΔdblGATA mice restores larvae survival, eosinophils have now been paradoxically implicated in parasite survival, specifically through the promotion of Th2 cell recruitment and the prevention of macrophage- and neutrophil-induced parasite death (47). Collectively, these data suggest that under some conditions, there may be a symbiotic association between gastrointestinal eosinophils and parasites, which could contribute to the maintenance of tissue homeostasis by allowing parasites to reside in the host tissues with limited consequences of such infection. Accordingly, we conclude that the eosinophil response to parasitic infection may vary by both helminth species and the specific tissue infected. Although murine models provide an opportunity to delineate the role of the eosinophil in parasite infection, it is important to point out that murine eosinophils are less effective than rat eosinophils in killing schistosomes (48) and do not bind IgE, which may be an effector mechanism for human eosinophils against parasites (49). Additionally, experimental infection with parasites in mice is unlikely to adequately mimic natural infections in human. Therefore, cautious interpretation of the murine results is warranted. Eosinophils express a broad range of pattern-recognition receptors, which supports their potential role in responses against viral and bacterial infections (50). RNA viruses such as respiratory syncytial virus are susceptible to the antiviral activities of eosinophils, particularly those mediated by eosinophil granule ribonucleases (e.g. eosinophil cationic protein and EDN) (51). IL-5 transgenic mice have improved clearance of Pseudomonas aeruginosa (52); however, it is possible that IL-5 mediates antibacterial effects independently of eosinophils (53). Even so, a previously unrecognized antibacterial function of eosinophils has been demonstrated and involves eosinophils releasing their mitochondrial DNA in response to LPS from gram-negative bacteria (Fig. 1B) (54). Together with granule proteins, the secreted eosinophil-derived mitochondrial DNA binds and kills bacteria in the extracellular matrix of the mice intestine (54). Considering the abundant numbers of eosinophils in the intestinal lamina propria, trapping bacteria with the DNA complexes of eosinophils (mitochondrial DNA nets) could be a highly effective mechanism for protecting the gastrointestinal tract against pathogenic bacterial invasion.
Non-beneficial role of eosinophils in gastrointestinal disorders: primary EGID and IBD
The most common primary EGID, EoE, is a worldwide emerging disease representing the second most common cause of chronic esophagitis (55, 56). Although infiltration of eosinophils into the esophageal mucosa is the hallmark of EoE, accumulation of activated immune cells such as mast cells, B cells, and T cells, as well as APC, is also observed in active EoE (56, 57). Along with inflammatory cell infiltration, hyperplasia of esophageal epithelial cells is a general histologic characteristic of EoE (58). Although not fully understood, immune sensitization to a variety foods and Th2-polarized allergic inflammation in the esophageal mucosa has been posited as the critical immunologic aspect of EoE development (57). In the absence of eosinophils, disease features such as tissue remodeling (e.g. epithelial hyperplasia), collagen accumulation, and gastric motility are attenuated, implicating eosinophils as key effector cells, at least in these animal models (59, 60). Eosinophil-derived TGF-β is implicated in tissue remodeling of EoE and also induces expression of periostin in patient biopsies, an extracellular matrix protein that increases eosinophil infiltration in the mucosal layer, thus further promoting disease pathogenesis (56, 61). Genetic susceptibility in humans has been linked to sequence variants at genetic locus 5q22 (encoding thymic stromal lymphopoietin), CRLF2 (the thymic stromal lymphopoietin receptor), FLG (filaggrin), and CCL26 (eotaxin-3), consistent with the complex interplay of epithelial cell gene products and Th2 immunity (62–64). Indeed, the Th2 cytokines IL-4, IL-5, and IL-13 are elevated in the esophageal mucosa, with IL-13 having a particularly important role in EoE pathogenesis (Fig. 1B) (56). IL-13 drives marked up-regulation of eotaxin-3, thus promoting the chemoattraction of CCR3+ eosinophils; further, IL-13 induces an EoE-like transcriptome in primary esophageal epithelial cell cultures (65) and triggers production of periostin in primary esophageal cultures (61). Impaired barrier function of the esophageal epithelium has also been indicated as a potential pathophysiological mechanism, as verified at least in part by the decrease of desmosomal cadherin desmoglein1, an intercellular adhesion molecule, in active EoE (66, 67). In fact, down-regulation of desmoglein 1 by IL-13 not only induces impaired barrier function of the esophageal epithelium, but also initiates a pro-allergic transcriptional response including POSTN (periostin) expression (67). Considering periostin can directly enhance eosinophil adhesion, decreased expression of desmoglein 1 may further potentiate inflammatory response of EoE by increasing migration of eosinophils.
IBD are characterized by chronic inflammation of the intestine and elevated levels of eosinophils have been observed in IBD which correlates with disease severity (68, 69). Murine models of IBD have provided important insight about the role of eosinophils in the pathogenesis of IBD. Increased numbers and degranulation of eosinophils are indeed observed in chemical–induced models of IBD, and are attenuated in eosinophil–deficient mice and eotaxin-1 deficient mice which exhibit reduced clinical scores and pathology (70, 71). Progression of colonic inflammation is also attenuated with depletion of eosinophils by administration of anti-IL-5 or CCR3 antibodies (72, 73). The tissue immune microenvironment is suggested to influence the downstream immune consequences mediated by eosinophils, leading either to exacerbation of local inflammatory responses or maintenance of tissue homeostasis (74).
Eosinophils as modulators of intestinal immune responses: interaction with T cells
Accumulating evidence suggests a role for eosinophils as modulators of T cell–mediated immune responses. Several studies have found that eosinophils can express MHC II and costimulatory molecules, which suggests a capacity to function as APC. Despite blood eosinophils not expressing MHC II in the steady state, human blood eosinophils stimulated with IL-3, IL-4, GM-CSF, and IFN-γ express MHCII molecules (75, 76). Although the antigen-presenting capabilities of small-intestinal eosinophils have not been examined in depth, intestinal eosinophils isolated from mice have been found to express only relatively low levels of CD86 and MHC II, which implies an incapacity to present antigens to naïve CD4+ T cells under homeostatic conditions (77). Notably, eosinophils secrete an array of cytokines such as IL-2, IL-4, IL-6, IL-10, and IL-12, along with TNF-α, that are capable of activating dendritic cells (2). Moreover, it has been reported that EDN can activate dendritic cells by stimulating the TLR-2 signaling pathway of those cells and inducing Th2 polarization capacity of dendritic cells in mice (78). On the basis of these observations, eosinophils may constitute a portion of non-conventional APC that promote the activity of dendritic cells at least in the murine gastrointestinal tract (Fig. 1B).
Eosinophils as modulators of intestinal immune responses: role in mucosal IgA class switching
The gastrointestinal tract, exposed to potentially harmful commensals and airborne and ingested pathogens, protects itself via production of IgA, the most abundant antibody isotype in the human body for neutralization of microbes in a non-inflammatory manner (79). Murine eosinophils in the bone marrow are known to support the survival of plasma cells by secreting APRIL (a proliferation-including ligand)14 (80). Together with B cell–activating factor, APRIL is known to induce IgA class switching in a T cell–independent manner (81). Therefore, small-intestinal eosinophils may have a role in the gastrointestinal tract’s T cell–independent IgA production, specifically by producing APRIL. Although the involvement of intestinal eosinophils in IgA class switching has not yet been directly examined, the impaired IgA production reported in CD47-deficient mice suggests a potential role in IgA synthesis (82). As noted above, small-intestinal eosinophils highly express SIRP-α, a cognate receptor for CD47, and SIRP-α/CD47 signaling contributes to the prolonged survival of murine intestinal eosinophils by regulation of their degranulation (16). The impaired production of IgA in CD47-deficient mice may correlate with reduced viability of small-intestinal eosinophils. The constitutive presence of eosinophils in the intestinal tissues suggests their potential role in IgA class switching in this location (Fig. 1B). In the healthy state, eosinophils are barely present in the Peyer’s patch or mesenteric lymph nodes (40), where T-cell–dependent IgA class switching takes place. Therefore, it is more likely that eosinophils contribute to T-cell–independent IgA class switching, which mainly occurs in the small-intestinal lamina propria, where abundant numbers of eosinophils reside. However, eosinophils produce TGF-β, a cytokine critical for the antibody class switching toward IgA in response to T-cell–dependent antigens (2, 83). Therefore, in terms of TGF-β secretion, eosinophils can be posited to play a supportive role in the induction of IgA class switching in organized lymphoid tissue. Notably, we have observed an IgA deficiency in the intestinal lavage and serum of genetically modified eosinophil-deficient mice (unpublished data), which implicates gastrointestinal eosinophils as a regulator of IgA class switching in the intestine.
Eosinophils as modulators of intestinal immune responses: role in adipose tissue metabolism
The incidence of obesity has rapidly increased worldwide, and obesity-associated diseases including insulin resistance, type 2 diabetes, fatty liver disease, atherosclerosis, and stroke constitute major health problems (84). Inflammation is a key feature of obesity and infiltration of pro-inflammatory macrophages, neutrophils, CD8+ T cells, CD4+ T cells, and mast cells is observed in visceral adipose tissue with obesity (85). Meanwhile, in non-obese normal visceral tissue of mice, eosinophils and alternatively activated macrophages are observed, suggesting a role of eosinophils in adipose tissue metabolism (86). Alternatively activated macrophages in adipose tissue improve insulin sensitivity (glucose homeostasis) and eosinophils are necessary for that specific subset of macrophages (86, 87). Furthermore, the absence of eosinophils directly leads to adiposity and systemic insulin resistance of mice (88), implying a protective role of eosinophils against development of type 2 diabetes (Fig. 1B). Maintenance of eosinophils in visceral adipose tissue is dependent on IL-5 and IL-13, and ILC2 expressing both of these cytokines promote accumulation of eosinophils in visceral adipose tissue (88). Therefore, interaction between ILC2 and eosinophils not only supports survival of intestinal eosinophils as previously noted, but also seems to be implicated in metabolic homeostasis.
Conclusions
Eosinophils have been considered to be end-stage effector cells that are involved in host protection against parasitic infection and the development of inflammatory disorders. However, accumulating evidence now indicate that eosinophils are multifunctional leukocytes that have a broader role in host responses against a wide range of infections (bacteria and viruses) and are potentially key modulators of the intestinal immune system. Likely, they may intimately interact and/or regulate commensal intestinal microflora, especially in view of the antibacterial effect of their granule proteins (7) and ability to modify innate immunity. The recent recognition of EGID, which are rapidly growing in prevalence, calls attention to the potential importance of translating the molecular and cellular immunological knowledge focused on gastrointestinal eosinophils into clinical treatment strategies. Indeed, the development of anti–eotaxin1 (bertilimumab), anti–IL-5 (mepolizumab, reslizumab) and eosinophil-depleting anti-CD125 (benrazumab) humanized antibodies provides an opportunity to test the role of eosinophils in a variety of human gastrointestinal disorders and to better uncover the physiological role of gastrointestinal eosinophils in humans.
Acknowledgments
NIH grants are U19 AI066738 and R37 A1045898 and U19 AI070235.
Footnotes
1
EDN, eosinophil-derived neurotoxin
2
EGID, eosinophilic gastrointestinal disorder(s)
3
EoE, eosinophilic esophagitis
4
CD, cluster of differentiation
5
GATA-1, GATA-binding protein 1
7
Siglec, sialic acid–binding immunoglobulin-like lectin
8
IL-5Rα, interleukin 5 receptor alpha
9
Th2, T helper cell type 2
10
SIRP-α, signal regulatory protein α
11
ILC2, type 2 innate lymphoid cells
12
PIR, Paired immunoglobulin-like receptors
13
IBD, inflammatory bowel disease(s)
14
A proliferation-inducing ligand, APRIL
References
- 1.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv. Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Hogan SP. The eosinophil. Annu. Rev. Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg ME. Eosinophilia. N. Engl. J. Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils. Immunol. Rev. 2001;179:139–155. doi: 10.1034/j.1600-065x.2001.790114.x. [DOI] [PubMed] [Google Scholar]
- 5.Carlens J, Wahl B, Ballmaier M, Bulfone-Paus S, Forster R, Pabst O. Common gamma-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J. Immunol. 2009;183:5600–5607. doi: 10.4049/jimmunol.0801581. [DOI] [PubMed] [Google Scholar]
- 6.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J. Allergy Clin. Immunol. 2004;113:11–28. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Hogan SP, Waddell A, Fulkerson PC. Eosinophils in infection and intestinal immunity. Curr Opin Gastroenterol. 2013;29:7–14. doi: 10.1097/MOG.0b013e32835ab29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milanovic M, Terszowski G, Struck D, Liesenfeld O, Carstanjen D. IFN consensus sequence binding protein (Icsbp) is critical for eosinophil development. J. Immunol. 2008;181:5045–5053. doi: 10.4049/jimmunol.181.7.5045. [DOI] [PubMed] [Google Scholar]
- 9.Wong CK, Lau KM, Chan IH, Hu S, Lam YY, Choi AO, Lam CW. MicroRNA-21* regulates the prosurvival effect of GM-CSF on human eosinophils. Immunobiology. 2013;218:255–262. doi: 10.1016/j.imbio.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Lu TX, Lim EJ, Besse JA, Itskovich S, Plassard AJ, Fulkerson PC, Aronow BJ, Rothenberg ME. MiR-223 deficiency increases eosinophil progenitor proliferation. J. Immunol. 2013;190:1576–1582. doi: 10.4049/jimmunol.1202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 13.Svensson-Frej M. Immunobiology of intestinal eosinophils - a dogma in the changing? J. Innate Immun. 2011;3:565–576. doi: 10.1159/000328799. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 15.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verjan Garcia N, Umemoto E, Saito Y, Yamasaki M, Hata E, Matozaki T, Murakami M, Jung YJ, Woo SY, Seoh JY, Jang MH, Aozasa K, Miyasaka M. SIRPalpha/CD172a regulates eosinophil homeostasis. J. Immunol. 2011;187:2268–2277. doi: 10.4049/jimmunol.1101008. [DOI] [PubMed] [Google Scholar]
- 17.Wen T, Mingler MK, Blanchard C, Wahl B, Pabst O, Rothenberg ME. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. J. Immunol. 2012;188:1075–1082. doi: 10.4049/jimmunol.1102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, Murphy PM, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J. Biol. Chem. 1996;271:7725–7730. doi: 10.1074/jbc.271.13.7725. [DOI] [PubMed] [Google Scholar]
- 19.Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82:521–530. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T, Ikuta K, Sugaya H, Maki K, Takagi M, Kanazawa H, Sunaga S, Kinashi T, Yoshimura K, Miyazaki J, Takaki S, Takatsu K. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira MM, Wells TN, Lukacs NW, Proudfoot AE, Kunkel SL, Williams TJ, Hellewell PG. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J. Clin. Invest. 1997;100:1657–1666. doi: 10.1172/JCI119690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, McGovern TW, Gleich GJ. Eosinophil infiltration and degranulation in normal human tissue. Anat. Rec. 1998;252:418–425. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Straumann A, Kristl J, Conus S, Vassina E, Spichtin HP, Beglinger C, Simon HU. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm. Bowel Dis. 2005;11:720–726. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 25.Motegi Y, Kita H, Kato M, Morikawa A. Role of secretory IgA, secretory component, and eosinophils in mucosal inflammation. Int. Arch. Allergy Immunol. 2000;122(Suppl 1):25–27. doi: 10.1159/000053627. [DOI] [PubMed] [Google Scholar]
- 26.Brandt EB, Zimmermann N, Muntel EE, Yamada Y, Pope SM, Mishra A, Hogan SP, Rothenberg ME. The alpha4bbeta7-integrin is dynamically expressed on murine eosinophils and involved in eosinophil trafficking to the intestine. Clin. Exp. Allergy. 2006;36:543–553. doi: 10.1111/j.1365-2222.2006.02456.x. [DOI] [PubMed] [Google Scholar]
- 27.Mishra A, Hogan SP, Brandt EB, Wagner N, Crossman MW, Foster PS, Rothenberg ME. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J. Biol. Chem. 2002;277:4406–4412. doi: 10.1074/jbc.M110424200. [DOI] [PubMed] [Google Scholar]
- 28.Fulkerson PC, Rothenberg ME. Origin, regulation and physiological function of intestinal oeosinophils. Best Pract. Res. Clin. Gastroenterol. 2008;22:411–423. doi: 10.1016/j.bpg.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr, Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, Wert SE, Rothenberg ME. Eotaxin is required for the baseline level of tissue eosinophils. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waddell A, Ahrens R, Tsai YT, Sherrill JD, Denson LA, Steinbrecher KA, Hogan SP. Intestinal CCL11 and eosinophilic inflammation is regulated by myeloid cell-specific RelA/p65 in mice. J. Immunol. 2013;190:4773–4785. doi: 10.4049/jimmunol.1200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J. Exp. Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J. Exp. Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J. Exp. Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munitz A, McBride ML, Bernstein JS, Rothenberg ME. A dual activation and inhibition role for the paired immunoglobulin-like receptor B in eosinophils. Blood. 2008;111:5694–5703. doi: 10.1182/blood-2007-12-126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baruch-Morgenstern NB, Shik D, Moshkovits I, Itan M, Karo-Atar D, Bouffi C, Fulkerson PC, Rashkovan D, Jung S, Rothenberg ME, Munitz A. Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development. Nat. Immunol. 2014;15:36–44. doi: 10.1038/ni.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artis D, Humphreys NE, Potten CS, Wagner N, Muller W, McDermott JR, Grencis RK, Else KJ. Beta7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur. J. Immunol. 2000;30:1656–1664. doi: 10.1002/1521-4141(200006)30:6<1656::AID-IMMU1656>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. Peyer's patch eosinophils: identification, characterization, and regulation by mucosal allergen exposure, interleukin-5, and eotaxin. Blood. 2000;96:1538–1544. [PubMed] [Google Scholar]
- 41.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 42.Haque A, Ouaissi A, Joseph M, Capron M, Capron A. IgE antibody in eosinophil- and macrophage-mediated in vitro killing of Dipetalonema viteae microfilariae. J. Immunol. 1981;127:716–725. [PubMed] [Google Scholar]
- 43.Kazura JW, Grove DI. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis. Nature. 1978;274:588–589. doi: 10.1038/274588a0. [DOI] [PubMed] [Google Scholar]
- 44.O'Connell AE, Hess JA, Santiago GA, Nolan TJ, Lok JB, Lee JJ, Abraham D. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect. Immun. 2011;79:2770–2778. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int. J. Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J. Immunol. 2012;188:417–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dent LA, Munro GH, Piper KP, Sanderson CJ, Finlay DA, Dempster RK, Bignold LP, Harkin DG, Hagan P. Eosinophilic interleukin 5 (IL-5) transgenic mice: eosinophil activity and impaired clearance of Schistosoma mansoni. Parasite Immunol. 1997;19:291–300. doi: 10.1046/j.1365-3024.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- 49.Jones RE, Finkelman FD, Hester RB, Kayes SG. Toxocara canis: failure to find IgE receptors (Fc epsilon R) on eosinophils from infected mice suggests that murine eosinophils do not kill helminth larvae by an IgE-dependent mechanism. Exp. Parasitol. 1994;78:64–75. doi: 10.1006/expr.1994.1006. [DOI] [PubMed] [Google Scholar]
- 50.Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136:11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J. Leukoc. Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 52.Linch SN, Kelly AM, Danielson ET, Pero R, Lee JJ, Gold JA. Mouse eosinophils possess potent antibacterial properties in vivo. Infect. Immun. 2009;77:4976–4982. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linch SN, Danielson ET, Kelly AM, Tamakawa RA, Lee JJ, Gold JA. Interleukin 5 is protective during sepsis in an eosinophil-independent manner. Am. J. Respir. Crit. Care Med. 2012;186:246–254. doi: 10.1164/rccm.201201-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 55.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N. Engl. J. Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 56.Abonia JP, Rothenberg ME. Eosinophilic esophagitis: rapidly advancing insights. Annu. Rev. Med. 2012;63:421–434. doi: 10.1146/annurev-med-041610-134138. [DOI] [PubMed] [Google Scholar]
- 57.Mulder DJ, Justinich CJ. Understanding eosinophilic esophagitis: the cellular and molecular mechanisms of an emerging disease. Mucosal Immunol. 2011;4:139–147. doi: 10.1038/mi.2010.88. [DOI] [PubMed] [Google Scholar]
- 58.Steiner SJ, Kernek KM, Fitzgerald JF. Severity of basal cell hyperplasia differs in reflux versus eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2006;42:506–509. doi: 10.1097/01.mpg.0000221906.06899.1b. [DOI] [PubMed] [Google Scholar]
- 59.Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, Foster PS, Rothenberg ME. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat. Immunol. 2001;2:353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 60.Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, Blanchard C, Putnam PE, Rothenberg ME. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, Stringer K, Abonia JP, Molkentin JD, Rothenberg ME. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, Franciosi JP, Kushner JP, Abonia JP, Assa'ad AH, Kovacic MB, Biagini Myers JM, Bochner BS, He H, Hershey GK, Martin LJ, Rothenberg ME. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010;126:160–165. e3. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, Buckmeier BK, Jameson SC, Greenberg A, Kaul A, Franciosi JP, Kushner JP, Martin LJ, Putnam PE, Abonia JP, Wells SI, Rothenberg ME. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J. Immunol. 2010;184:4033–4041. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, Collins MH, Putnam PE, Wells SI, Rothenberg ME. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 66.Ravelli AM, Villanacci V, Ruzzenenti N, Grigolato P, Tobanelli P, Klersy C, Rindi G. Dilated intercellular spaces: a major morphological feature of esophagitis. J. Pediatr. Gastroenterol. Nutr. 2006;42:510–515. doi: 10.1097/01.mpg.0000215312.78664.b9. [DOI] [PubMed] [Google Scholar]
- 67.Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, Kemme KA, Costello MS, Mingler MK, Blanchard C, Collins MH, Abonia JP, Putnam PE, Dellon ES, Orlando RC, Hogan SP, Rothenberg ME. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 69.Bischoff SC, Mayer J, Nguyen QT, Stolte M, Manns MP. Immunnohistological assessment of intestinal eosinophil activation in patients with eosinophilic gastroenteritis and inflammatory bowel disease. Am. J. Gastroenterol. 1999;94:3521–3529. doi: 10.1111/j.1572-0241.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- 70.Ahrens R, Waddell A, Seidu L, Blanchard C, Carey R, Forbes E, Lampinen M, Wilson T, Cohen E, Stringer K, Ballard E, Munitz A, Xu H, Lee N, Lee JJ, Rothenberg ME, Denson L, Hogan SP. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J. Immunol. 2008;181:7390–7399. doi: 10.4049/jimmunol.181.10.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, Foster PS, Hogan SP. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J. Immunol. 2004;172:5664–5675. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 72.Takedatsu H, Mitsuyama K, Matsumoto S, Handa K, Suzuki A, Takedatsu H, Funabashi H, Okabe Y, Hara T, Toyonaga A, Sata M. Interleukin-5 participates in the pathogenesis of ileitis in SAMP1/Yit mice. Eur. J. Immunol. 2004;34:1561–1569. doi: 10.1002/eji.200324680. [DOI] [PubMed] [Google Scholar]
- 73.Masterson JC, McNamee EN, Jedlicka P, Fillon S, Ruybal J, Hosford L, Rivera-Nieves J, Lee JJ, Furuta GT. CCR3 Blockade Attenuates Eosinophilic Ileitis and Associated Remodeling. Am. J. Pathol. 2011;179:2302–2314. doi: 10.1016/j.ajpath.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc. Natl. Acad. Sci. U. S. A. 1989;86:1348–1351. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weller PF, Rand TH, Barrett T, Elovic A, Wong DT, Finberg RW. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J. Immunol. 1993;150:2554–2562. [PubMed] [Google Scholar]
- 77.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 78.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cerutti A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 81.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 82.Westlund J, Livingston M, Fahlen-Yrlid L, Oldenborg PA, Yrlid U. CD47-deficient mice have decreased production of intestinal IgA following oral immunization but a maintained capacity to induce oral tolerance. Immunology. 2012;135:236–244. doi: 10.1111/j.1365-2567.2011.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 84.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 85.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol. Metab. 2012;23:407–415. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 86.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]