Human Papillomavirus Type 16 E6 Amino Acid 83 Variants Enhance E6-Mediated MAPK Signaling and Differentially Regulate Tumorigenesis by Notch Signaling and Oncogenic Ras (original) (raw)
Abstract
Oncogenically high-risk human papillomaviruses (HPVs) are causally associated with the progression of major human neoplasia-like cancers of the cervix. Several studies have defined functions of the key E6 and E7 oncoproteins in epithelial cell immortalization. The roles of these oncogenes in the progression of immortalized epithelial cells to invasive tumors are still poorly understood. Here, we establish a novel link between the E6 oncoprotein and activation of mitogen-activated protein kinase (MAPK) signaling and show that this signaling involves Rap1. We find that activated MAPK signaling cooperates with deregulated Notch1 signaling to recreate features of HPV-driven invasive cervical carcinomas. We extend our analysis to evaluate an E6 (amino acid [aa] 83) variant that has been linked to invasive tumors. The variant enhances MAPK signaling and cooperative transformation with deregulated Notch1 signaling. Unlike E6, this variant surprisingly inhibits oncogenic Ras-mediated transformation. Our data reveal that the quantitative differences in activation of MAPK signaling by E6 and its variant correlate with differences in cooperative transformation with other signaling pathways, thus suggesting that thresholds of MAPK activation may define permissive conditions for other signaling pathways in tumorigenesis. Epidemiological studies have suggested the importance of E6 aa 83 variants in invasive carcinomas; our data support a key deterministic role for this variant in human cervical tumorigenesis. These observations, along with our recent data showing that deregulated Notch signaling activates phosphatidylinositol 3-kinase signaling, strengthen the possibility of the existence of Ras-independent mechanisms to recreate signaling through classical Ras effector pathways.
Human papillomaviruses (HPVs) of the oncogenically high-risk category (types 16, 18, and 31) are causally associated with cancer of the cervix, a major subset of human neoplasia (46). HPVs have complex replication and assembly strategies linked to epidermal differentiation (22). Two key viral genes, E6 and E7, principally regulate this link with epidermal differentiation and in addition are sufficient to immortalize human epithelial cells (47). Several laboratories have identified cellular proteins that interact with the E6 and E7 oncoproteins, and attempts have been made to link these interactions with the life cycle of the virus and cellular processes like DNA replication and regulation of cell death (48). Considerable progress has been achieved in terms of identifying functions of E6 and E7 that are essential and that also cooperate with each other to immortalize human epithelial cells. Interactions of E6 and E7 with p53 and pRb, respectively, along with transcriptional activation of hTERT (human telomerase reverse transcriptase) by E6 are believed to cooperatively immortalize human epithelial cells (19, 25).
The E6 and E7 oncogenes are persistently expressed during all stages of HPV-mediated cervical cancers. A range of mechanisms has been identified that may contribute to the upregulation of expression of these oncogenes during tumor progression (46). Consistent with the persisting expression of E6 and E7 genes in cervical tumors, the inhibition of expression and function of the corresponding proteins in cervical tumor-derived cell lines leads to growth inhibition (38, 40). However, the functions of these oncogenes in the progression of immortalized epithelial cells to invasive tumors are currently poorly understood.
The generation of invasive tumors from high-grade precursor lesions has been recently associated with the accumulation of variants of E6. These epidemiological studies have shown remarkable association between the progression of high-grade precursor lesions to invasive tumors and the accumulation of an HPV type 16 (HPV-16) variant harboring a T-to-G transition at nucleotide 350 of the E6 oncogene corresponding to amino acid L83V (1, 30, 45). As yet, there are no studies which have analyzed the impact of this single amino acid (amino acid [aa] 83) change in prototype E6 in the progression to invasive carcinomas.
Models of tumor progression have placed signaling by oncogenic Ras as a central event in the transformation of immortalized human epithelial cells (9, 13). Expression of oncogenic Ras results in cells acquiring a complete neoplastic phenotype with all the hallmarks of cancerous cells (15). The key effector pathways downstream of oncogenic Ras, the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)/Akt and mitogen-activated protein kinase (MAPK) pathways, have been linked to cell survival and proliferation, respectively (10, 32), and they can complement each other in cellular transformation (23). Several observations suggest that cooperative signaling from host cellular factors, along with E6 and E7, are essential in driving complete tumor progression in vivo (47). With the relatively low frequency of oncogenic Ras mutations in cervical cancers (3), the identity and nature of these cooperative events are of enormous interest. Following the observations of Zagouras and colleagues, demonstrating intracellular forms of Notch1 in invasive cervical tumors (44), we have investigated the functional implications of these observations. Notch1 is part of a family of transmembrane receptors that are believed to signal principally by regulated cleavage and subsequent intracellular localization of the cleaved activated forms (41). It has previously been shown that such intracellular forms accumulate during the progression of high-grade precursor lesions (8). In concordance with a potential role for Notch signaling in driving the progression of cervical tumors expressing HPV-16 oncogenes, it was found that activated forms of Notch1 complement the functions of E6 and E7 oncogenes in the transformation of immortalized epithelial cells (31). Briefly, our data reveal that activated Notch1 signaling generates an antiapoptotic signal through the PI3K-PKB/Akt pathway. Activated Notch1 signaling through PI3K-PKB/Akt generates resistance to anoikis (apoptosis induced on matrix withdrawal) and also blocks p53-induced cell death (28, 31).
In this study, we have analyzed the role of the E6 oncogene in promoting the transformation of immortalized human epithelial cells. To define the role of E6 in this process, we have studied the effect of this oncogene on the activation of key Ras effector pathways. We establish a link between E6 and MAPK signaling through a Ras-independent, Rap1-dependent mechanism. Our data reveal that aa 83 variants markedly enhance MAPK signaling. Though aa 83 variants enhance cooperation with activated Notch signaling, they inhibit oncogenic Ras-mediated transformation, thus defining events that are potentially permissive for cervical tumor progression. Collectively, these results define both mechanisms and permissive interactions that may mediate tumor progression in the context of HPV-16 oncogenes.
MATERIALS AND METHODS
DNA constructs.
The following plasmid cDNA expression constructs were used: pcDNA3-Neo (Invitrogen); pcDNA3-E6, pcDNA3-AcN1, and pcDNA3-E7 (31); pLXSN-Neo and pLXSN-E6 (gifts of D. Galloway); pLXSN-S82DL83W E6, pcDNA3-L83V E6 (subcloned from pBSK-L83V E6; gifts of M. Tommasino); pSG5-V12Ras (a gift of J. Downward); pCHA-MEK1 S218/222D (a gift of K. Wong); pSG5-Neo and pSG5-E6TP1 (gifts of V. Band); pSG5-16 E6, pSG5-18 E6, and pSG5-31 E6 (gifts of L. A. Laimins); and pCDNA3-6b E6 (a gift of K. Raj). Glutathione _S_-transferase (GST)-fused Ras binding domain of Raf1 and GST-fused Rap1 binding domain of RalGDS cloned in pGEX vectors (Pharmacia) were gifts of J. L. Bos. Prototype HPV-16 E6 is referred to as E6 unless otherwise mentioned.
Generation of cell lines.
HaCaT, HeLa, CaSki, and SiHa cells were grown in Dulbecco modified Eagle medium (GIBCO) supplemented with 10% fetal calf serum (GIBCO) unless otherwise mentioned. d1DR cells were grown in keratinocyte serum-free medium supplemented with epidermal growth factor, bovine pituitary extract (GIBCO), 5% fetal calf serum, and 0.5 mM CaCl2. All transfections were carried out with LipofectAMINE 2000 (Invitrogen), as specified by the manufacturer, by using QIAGEN purified plasmid DNA. HaCaT cells (a gift of N. Fusenig) or d1DR cells (a gift of L. A. Pirisi-Creek) were transfected either in transient assays or to generate stable lines as described previously (31) by using the plasmids mentioned above.
Immunocytochemistry.
Immunostaining with primary antibody HPV-16/HPV-18 (HPV-16/18) E6 (Oncogene Research) and appropriate tetramethyl rhodamine isothiocyanate (TRITC)-conjugated secondary antibody (DAKO) at dilutions recommended by the manufacturers was performed. Cells grown on coverslip dishes were fixed with 4% paraformaldehyde for 10 min at −20°C. Cells were washed three times with 0.1% Triton X-100 in phosphate-buffered saline and blocked with 0.1% bovine serum albumin for 45 min. Primary antibody (diluted in 0.1% bovine serum albumin) was added to the cells and left overnight at 4°C. After the cells were washed three times with 0.1% Triton X-100 in phosphate-buffered saline, they were incubated with diluted secondary antibody for 45 min. Cells were then visualized and imaged by confocal microscopy.
Confocal microscopy.
A laser-scanning confocal microscope (model MRC1024; Bio-Rad) attached to a Nikon inverted microscope (Eclipse TE300) and Lasersharp acquisition software were used for imaging. Laser power at 30% was used in all studies and a 60× oil immersion objective (numerical aperture of 1.4) was used. TRITC excitation or emission was achieved with a filter set (568 nm/605 nm) designed for TRITC detection. Images were processed (Adobe Photoshop software) by using identical values for contrast and brightness.
Immunoblotting.
For immunoblotting, 30 μg of total protein per cell lysate was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted, and probed with the primary antibodies mentioned in the figures, as reported previously (27). Antibodies against HPV-16/18 E6 (Abcam Limited); unphosphorylated and phosphorylated forms of MAPK 1 and 2 (MAPK1/2), MEK1/2, and Akt antibodies (Cell Signaling Technologies); anti-cRaf1, anti-BRaf, anti-H-Ras, anti-Rap1, anti-Fas, and anti-FasL antibodies (Santa Cruz Biotechnologies); and anti-Serine (Sigma) were used at dilutions recommended by the manufacturers.
Northern blot analysis.
RNA was isolated from cells by using Trizol (GIBCO) per the manufacturer's instructions. The 15 μg of isolated RNA from different cell lines was subjected to electrophoresis on 1% formaldehyde gels and transferred to Hybond plus nylon membranes (Amersham) for 12 to 16 h. The blots were washed, cross-linked, prehybridized, and hybridized according to standard protocols. A 32P (Brit, India)-labeled probe with high specific activity, prepared by a standard random primed reaction kit (Boheringer Mannheim), was used for hybridization. Autoradiography was done to determine the desired signal. For probe generation, the E6 gene, already cloned into the pLXSN vector, was amplified with 30 PCR cycles (94°C for 1 min [denaturation], 55°C for 1 min [annealing], and 72°C for 1 min [extension]). The primers used were E6 forward primer 5′ ATGCACCAAAAGAGAACTGC 3′ and E6 reverse primer 5′ TTACAGCTGGGTTTCTCTAC 3′.
Measurement of GTP-bound Ras-like GTPases by activation-specific probes.
An assay measuring GTP-bound Ras-like GTPases was done as previously reported (49). Briefly, bacterially expressed, GST-fused, activation-specific probes were incubated with glutathione-agarose beads. The conjugated beads were then incubated with cell lysates to precipitate the GTP-bound GTPases. The precipitated GTPases on the beads were washed, heated, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted.
Soft agar colony formation assay.
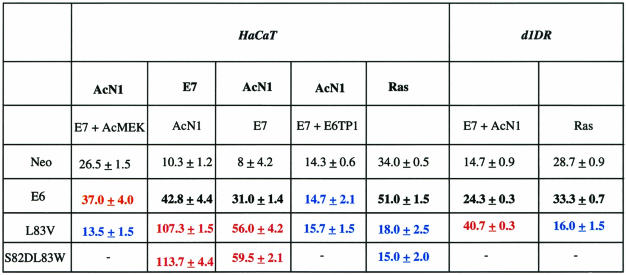
Soft agar colonies were generated as described previously (31). Briefly, stable HaCaT Neo, AcN1, E7, Ras or Jagged1, or d1DR cells were transiently transfected with the plasmid combinations mentioned in Table 1 and grown on soft agar for 21 days. Resulting colonies were counted under the microscope at 10× magnification from 10 random fields. The increase in colonies generated depends on the combination of genes expressed, irrespective of whether a gene is expressed under stable or transient conditions.
TABLE 1.
HPV-16 E6 aa 83 variants specifically enhance transformation mediated by AcN1 in vitro on soft agar colony formation assaysa
Tumor formation in nude mice.
HaCaT or d1DR cells grown in 92-mm2 dishes were transiently transfected with the combination of plasmids (total of 10 μg) mentioned in the figures. After 36 h of transfection, the cells were trypsinized and seeded in 175-mm2 flasks and grown for a further 24 h. The cells were harvested, counted, resuspended in the least quantity (∼200 μl) of 1× Dulbecco modified Eagle medium, and injected into nude mice (BALB/c-Nu or N:NIH-Swiss) subcutaneously in the flanks. A total of 107 cells were injected per mouse (between 6 and 8 weeks of age). The mice were checked every 4 days, and tumor size was measured after every 7 days. After 15 days, the mice were sacrificed, and the tumors were taken out, formalin fixed, and paraffin embedded. Tumor volumes were calculated by measuring mean diameters and using the formula 4/3π_r_3, where r is the radius of the tumor.
Organotypic raft cultures.
Raft cultures were prepared as described previously (6). Briefly, HaCaT cells stably transfected with vectors containing E6, S82DL83W, or mock (Neo) plasmids were seeded onto collagen matrices containing human fibroblast feeder cells DPC and, when confluent, raised onto steel grids and lifted to the air-liquid interface. All cultures were formalin fixed and paraffin embedded after being cultured for 9 days at the air-medium interface.
Immunohistochemistry.
Immunohistochemistry was performed on formalin-fixed, paraffin embedded tissue sections (5 to 7 μm) as previously described (6) by using the following antibodies at manufacturer-recommended dilutions: unphosphorylated and phosphorylated anti-MAPK1/2 and anti-Akt antibodies (Cell Signaling Technologies), anti-HPV-16/18 E6 (Oncogene Research), anti-involucrin and anti-cytokeratin10/13 antibodies (DAKO), and anti-cytokeratin 5/8 antibody (Research Diagnostics, Inc.).
Apoptosis assay.
Cells were subjected to anoikis as described previously (31). Briefly, CaSki cells were cotransfected with vectors expressing green fluorescent protein and the gene of interest with a plasmid ratio of 1:5. Thirty-six hours posttransfection, the cells were harvested and seeded in 35-mm tissue culture dishes either left uncoated or coated with 12 mg of polymethyl-methacrylate/ml in chloroform to prevent the deposition of extracellular matrix. After 8 h, cells were harvested and stained with Hoechst 33342 dye. Green fluorescent protein-expressing cells undergoing apoptosis were identified by chromatin condensation and fragmentation and scored by microscopic visualization using a filter in the UV range.
RESULTS
E6 activates the MAPK signaling pathway, and E6 aa 83 variants enhance this effect.
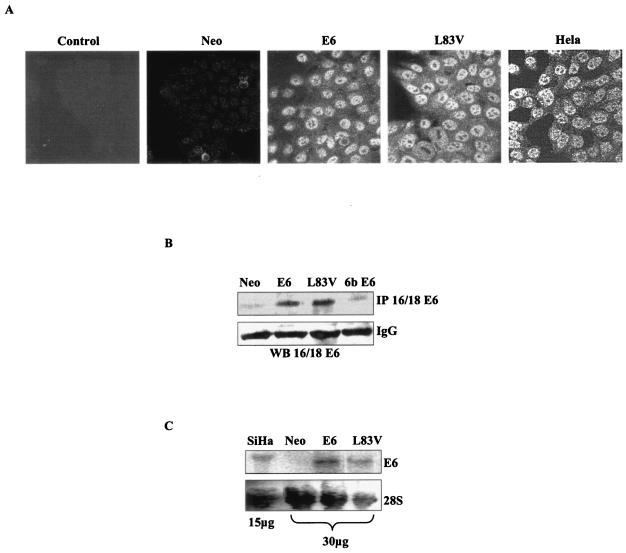
HaCaT cells, of an HPV-negative immortalized human epithelial cell line (5), were stably transfected with E6 or aa 83 variants (Fig. 1). Immunocytochemical analysis showed diffused cytoplasmic and distinct nuclear localization of E6 protein in HaCaT E6 and L83V cells, which is similar to HeLa cells, an HPV-18-derived cervical carcinoma cell line (Fig. 1A). HaCaT E6 and L83V cells subjected to immunoprecipitation and Western blot analysis showed comparable levels of expression of the E6 protein (Fig. 1B). HaCaT Neo and HPV-6b E6 cells were used as control cells to confirm the specificity of the antibody to only high-risk HPVs. In parallel, transcript levels of E6 mRNA showed expression levels comparable to those of SiHa cells, an HPV-16-derived cervical carcinoma cell line, by Northern blot analysis (Fig. 1C).
FIG. 1.
Generation and characterization of HaCaT cells stably expressing HPV-16 E6 and its aa 83 variant. (A) HaCaT cells stably expressing E6 and L83V were checked for E6 expression by using anti-HPV16/18 E6 antibody and compared with HeLa cells. HaCaT cells stably transfected with mock vector (Neo) were used as the control. HaCaT E6 cells stained with isotype control are labeled as “control.” (B) Expression levels of E6 and L83V were also confirmed by immunoprecipitation (IP) followed by immunoblotting with anti-HPV-16/18 E6 antibody. Cell lysates from HaCaT Neo and HPV-6b E6 were used as the control for specificity of the antibody to high-risk (HPV-16/18) E6. Immunoglobulin G (IgG) levels in the lysates were used as the loading control. WB, Western blot. (C) Northern blot analysis of the stable cells was done to confirm the transcript levels of E6 and its aa 83 variant. Total RNA from SiHa cells was used as the control. Agarose gels were stained with ethidium bromide to show the 28S RNA species. pcDNA3 vector backbone has been used in all the mentioned plasmids.
The resistance of cells to anoikis and serum starvation-induced apoptosis along with sustained proliferation define key features of transformation. HaCaT cells expressing activated alleles of Notch1 (AcN1) were previously used to show the activation of PI3K-PKB/Akt (31) and induction of resistance to anoikis. The expression of E6 in HaCaT cells leads to the generation of resistance to serum starvation (27).
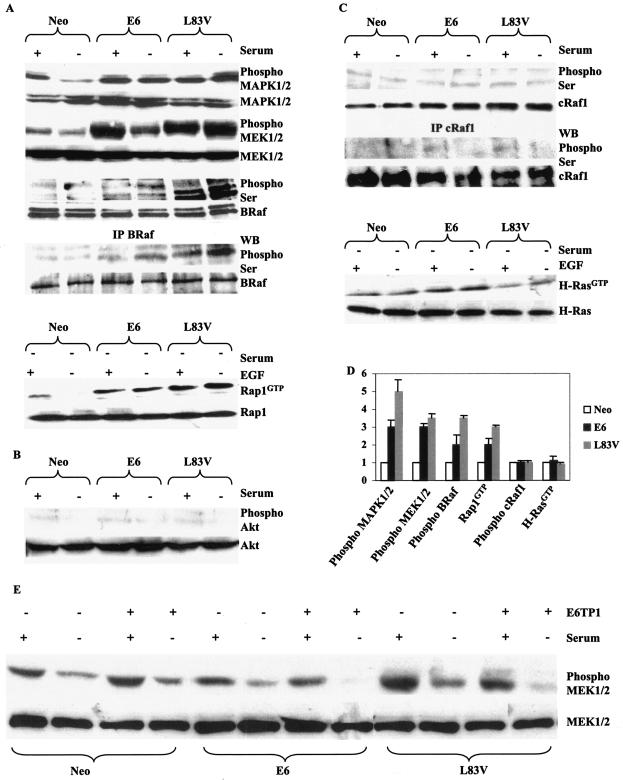
In contrast to AcN1, the expression of E6 and aa 83 variants does not affect PI3K-PKB/Akt activation in cells, and the levels of phosphorylated Akt (phospho-Akt) are virtually undetectable in HaCaT E6 and L83V cells (Fig. 2B).
FIG. 2.
E6 and aa 83 variants activate MAPK signaling cascade. (A) HaCaT cells stably expressing Neo, E6, or L83V E6 were checked for the endogenous levels of phosphorylated (phospho) or unphosphorylated forms of the primary proteins involved in the MAPK pathway by Western blot analysis to detect the activity of these proteins. The levels of total BRaf, MEK1/2, and MAPK1/2 were comparable across all the cell lines. The activation status of Rap1 was biochemically analyzed by assaying for the amount of Rap1GTP, which was deprived of serum for 24 h (−) or stimulated with 20 ng of epidermal growth factor/ml (+) for 30 min after 24 h of serum withdrawal. (B) Active phosphorylated levels of Akt were analyzed. (C) Similar levels of cRaf1 phosphorylated on Ser were detected across the cell lines. The activation status of H-Ras was determined by estimating levels of RasGTP; cells were grown in conditions similar to those mentioned for Rap1GTP. (D) Difference (fold) in activation of various components of the MAPK signaling pathway. (E) Effect of E6TP1 on MAPK signaling. The cell lines analyzed were grown in either the presence (+) or absence (−) of serum unless otherwise mentioned. A Western blot (WB) representative of five experiments is depicted in each case. IP, immunoprecipitation.
We assessed the activation status of MAPK1/2 and the best-characterized effectors upstream of MAPK1/2, viz., MEK1/2 and Raf family members. On serum deprivation, E6 and L83V led to about 3.0- and 5.0-fold increases in phospho-MAPK1/2 and phospho-MEK1/2 levels, respectively, in comparison to levels of HaCaT Neo control cells grown under similar conditions (Fig. 2A and D). We found that phospho-Ser levels on BRaf in HaCaT E6 and L83V cells were almost 2.0- and 3.5-fold higher than that detected in HaCaT Neo control cells, respectively (Fig. 2A and D). BRaf is preferentially activated by the Ras homologue Rap1 (4, 43). Consistent with these reports, we show that Rap1GTP levels also show about 2.0- and 3.0-fold increases in HaCaT E6 and L83V cells, respectively, in comparison with HaCaT Neo controls (Fig. 2A and D). Similar activation of the MAPK signaling pathway is obtained by using C33A cells, an HPV-negative human cervical tumor-derived cell line (data not shown). The data in Fig. 2 show that E6 and aa 83 variants activate MAPK1/2 downstream of Rap1-BRaf-MEK1/2. Increased activation of the MAPK signaling pathway as detected by increased levels of phospho-MAPK1/2 and phospho-MEK1/2 is also detected in the presence of S82DL83W, a laboratory-generated mutant (21), compared with prototype E6 (data not shown). The aa 83 variants enhance E6-mediated activation of the MAPK pathway at all the steps that we have examined. The inability to detect an increase in activated H-Ras and cRaf1 is consistent with a Rap1-dependent mechanism of MAPK activation (Fig. 2C and D).
Recent studies have led to the identification of a novel E6 binding protein, E6TP1, a Rap1 GTPase-activating protein (GAP) homologue with similar activity (11, 34). Furthermore, mutations in E6 that inhibit binding to and degradation of E6TP1 reduce the immortalization potential of E6. The introduction of E6TP1 into HaCaT E6 and L83V cells leads to reduced levels of phospho-MEK1/2 (Fig. 2E). This result is consistent with a possible E6- and L83V-induced degradation of E6TP1 mediating activation of Rap1 signaling.
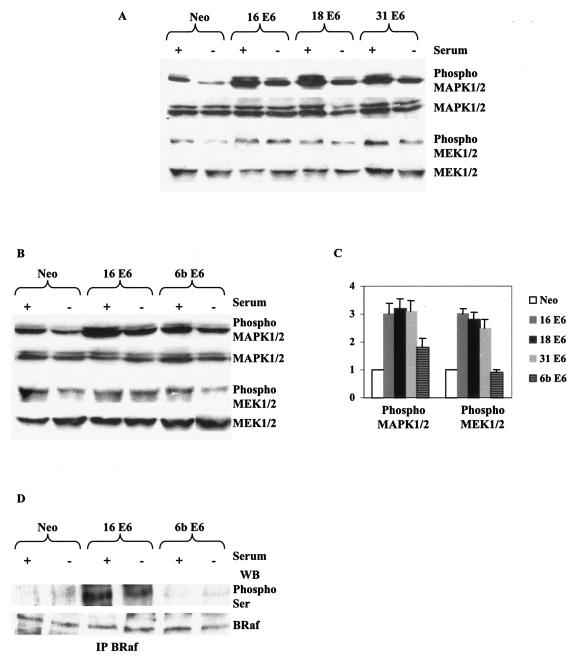
To further establish the involvement of E6TP1 in the activation of MAPK signaling in the presence of E6, we evaluated the activation of this pathway in the presence of high-risk HPV-18 and -31 E6 and also low-risk HPV-6b E6 (Fig. 3). High-risk HPV E6 is capable of better binding and degradation of E6TP1 than low-risk HPV E6 (11). HaCaT cells expressing HPV-18 and -31 E6 have phospho-MAPK1/2 and phospho-MEK1/2 levels comparable to that of HaCaT cells expressing HPV-16 E6, which is about threefold more than that expressed by control cells (Neo) (Fig. 3A and C). HaCaT cells expressing HPV-6b E6 have phospho-MAPK1/2 and phospho-MEK1/2 levels less than that of HPV-16 E6 and comparable to those of control Neo cells (Fig. 3B and C). The negligible expression levels of BRaf phosphorylated on Ser residues in HPV-6b E6-expressing cells that are comparable to those of the control cells (Fig. 3D) further emphasizes the activation of the MAPK signaling pathway downstream of BRaf in the presence of E6.
FIG. 3.
E6 from high-risk, and not low-risk, HPVs activates MAPK signaling cascade. (A) HaCaT cells transiently expressing Neo, HPV-16 E6, HPV-18 E6, or HPV-31 E6 were checked for levels of phosphorylated (phospho) or unphosphorylated forms of MAPK1/2 and MEK1/2 by Western blot analysis to detect the activity of these proteins. The levels of total MAPK1/2 and MEK1/2 were comparable across all the cell lines. pSG5 vector backbone has been used in all the plasmids used for transient transfections. (B) HaCaT cells stably expressing Neo, HPV-16 E6, or HPV-6b E6 were checked for levels of phosphorylated or unphosphorylated forms of MAPK1/2 and MEK1/2 by Western blot analysis to detect the activity of these proteins. The levels of total MAPK1/2 and MEK1/2 were comparable across all the cell lines. pcDNA3 vector backbone has been used in all the plasmids used. (C) Difference (fold) in activation of MAPK1/2 and MEK1/2 in the various cell lines. (D) Cell lysates similar to those used in panel B were checked for activation status of BRaf by immunoprecipitation and immunoblotting. Total BRaf levels are comparable across the cell lines.
E6 aa 83 variants selectively enhance synergistic effects of E6 with AcN1 and E7 on colony formation assays.
The expression of either AcN1 or constitutively active Akt complements HPV-16 E6 and E7 genes to typically yield three- to fivefold increases in the number of colonies formed on soft agar assays with HaCaT cells (31). Based on the activation of MAPK signaling by E6 and aa 83 variants, we evaluated the ability of activated MAPK signaling to complement E7 and AcN1. Constitutively active MEK (AcMEK) cooperates with E7 and AcN1 to yield a 2.5-fold increase in colony numbers with HaCaT cells (Table 1). To evaluate the possibility that enhancing MAPK activation may increase cooperative transformation, we compared L83V to prototype E6. Expression of L83V, instead of E6, along with E7 and AcN1 increases the colony-forming efficiency from 3- to 5- to 8- to 10-fold. Similar assays undertaken in HaCaT cells with the expression of the Notch ligand Jagged1 generate comparable increases in numbers of colonies in soft agar (data not shown). The laboratory-generated mutant S82DL83W yields data similar to those obtained with L83V, suggesting that a loss of leucine at aa 83 is the key mutational change responsible for bringing about the dramatic differences in cooperation with other oncogenes. Strengthening the notion that Rap1/MAPK signaling complements E7 and AcN1 in these assays, the expression of E6TP1 inhibits transformation by both E6 and L83V (Table 1).
E7, along with prototype E6, has previously been reported to cooperate with oncogenic Ras on transformation assays. Oncogenic Ras is sufficient to transform HaCaT cells in soft agar assays; the addition of E6 and E7 yields a modest increase (Table 1) (31). Surprisingly, both L83V and S82DL83W block transformation by oncogenic Ras (Table 1). In parallel, the expression of AcMEK, which enhances cooperative transformation by prototype E6, inhibits colony formation in the presence of L83V (Table 1) and oncogenic Ras (data not shown).
The two key observations from the above set of experiments are that aa 83 variants enhance cooperative transformation by E7 and AcN1 and inhibit colony formation in the presence of oncogenic Ras. We further verified these results with d1DR cells, primary keratinocytes immortalized with HPV-16 DNA (42). We found a pattern of increase in colony numbers in d1DR cells in the presence of E6 or its variant similar to that of HaCaT cells (Table 1). While exogenous expression of E6, E7, and AcN1 in d1DR cells leads to a 1.5-fold increase in colony numbers, the L83V E6 variant along with E7 and AcN1 increases the colony numbers by 2.7-fold over the control. Furthermore, the expression of oncogenic Ras along with the E6 variant leads to a similar decrease in the number of colonies formed, as observed with HaCaT cells. d1DR cells, though immortalized with HPV-16 DNA, need exogenous expression of the viral oncogenes, as their endogenous levels of expression are very low and probably insufficient for complete transformation (data not shown).
Collectively, these data show that E6 aa 83 variants enhance transformation by activated Notch1; however, they inhibit oncogenesis by Ras. Furthermore, the complementation analysis suggests that AcMEK can substitute for prototype E6.
E6, E7, and AcN1 synergize to form tumors in nude mice, and E6 variants form more aggressive tumors.
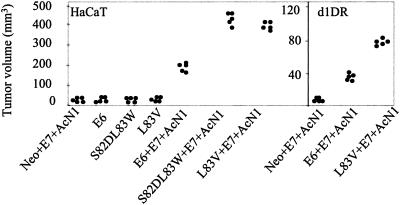
To further corroborate the in vitro results, we evaluated the ability of the above-mentioned transfected cells to form tumors in vivo. The expression of AcN1 in HaCaT cells along with HPV-16 E6 and E7 oncogenes sustains tumor formation in vivo. Typically, AcN1 with E6 and E7 generates nonregressing tumor volumes greater than 150 mm3 at 2 weeks. At a similar time point, aa 83 variants with E7 and AcN1 generate nonregressing tumor volumes greater than 350 mm3 (Fig. 4). Expression of E6 or aa 83 variants (S82DL83W or L83V) alone or with E7 in HaCaT cells generated nodule-like structures with volumes of less than 10 mm3 at 2 weeks (Fig. 4). d1DR cells expressing AcN1 or mock vector Neo did not form tumors. However, the coexpression of E6 or L83V with AcN1 and E7 generated tumor volumes greater than 35 and 75 mm3, respectively.
FIG. 4.
HPV-16 oncogenes cooperate with AcN1 to form tumors in nude mice. HaCaT or d1DR cells were transiently transfected with the given gene combinations and injected. Graphs show tumor volumes at 2 weeks. Each dot represents a tumor from one mouse. Tumors of negligible volumes are taken as 0.1 mm3 for convenience of representation on the graphs. All the tested gene combinations were significant compared to the controls with Neo. Student's t test was used to obtain statistical significance (P < 0.001). Corresponding vector backbones carrying Neo and E6 were used depending on whether pcDNA3-L83V or pLXSN-S82DL83W was transfected in the mentioned cell lines.
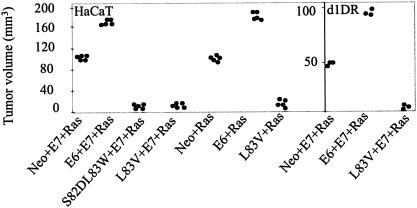
HaCaT cells expressing Ras either alone or along with E7 generate tumor volumes of around 100 mm3 (Fig. 5). The expression of prototype E6 enhances this oncogenic Ras effect and generates tumor volumes of around 200 mm3. Likewise, in d1DR cells, the coexpression of Ras with E6 and E7 generates tumor volumes of around 100 mm3, twofold more than those obtained with Neo, E7, and Ras. However, the expression of E6 aa 83 variants completely blocks tumor formation mediated by oncogenic Ras in both HaCaT and d1DR cells. Consistent with the in vitro results, while E6 aa 83 variants support transformation in the presence of AcN1 they inhibit tumor formation mediated by oncogenic Ras.
FIG. 5.
HPV-16 aa 83 variants do not cooperate with oncogenic Ras to form tumors in nude mice. HaCaT or d1DR cells were transiently transfected with the given gene combinations and injected. Graphs show tumor volumes at 2 weeks. Each dot represents a tumor from one mouse. Tumors of negligible volumes are taken as 0.1 mm3 for convenience of representation on the graphs. All the tested gene combinations were significant compared to the controls with Neo. Student's t test was used to obtain statistical significance (P < 0.001). Corresponding vector backbones carrying Neo and E6 were used depending on whether pcDNA3-L83V or pLXSN-S82DL83W was transfected in the mentioned cell lines.
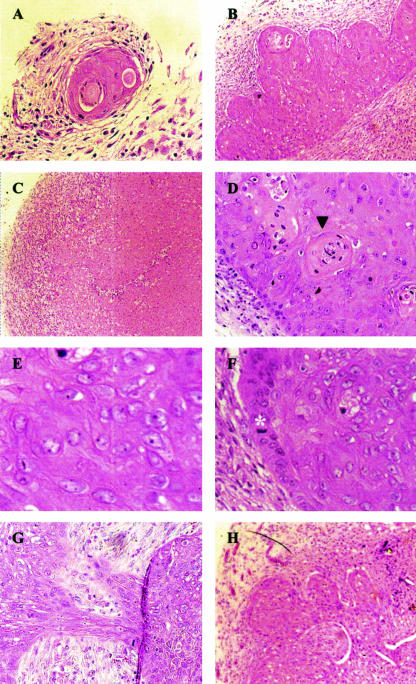
Generated tumors were stained with hematoxylin and eosin to analyze their histomorphological features (Fig. 6). Subcutaneous lumps generated in the presence of E6 or E6 aa 83 variants alone have large areas of necrosis, are full of “foamy cells” (tumor cells engulfed by infiltrating macrophages), lack potential to grow into frank tumors (Fig. 6A and C), and are referred to as necrotic lesions. In contrast, tumors generated by HaCaT cells transfected with E6, E7, and AcN1 are vascular, well differentiated as distinguished by the presence of keratin pearls, and do not have areas of necrosis (Fig. 6B and D). Tumors generated with HaCaT cells expressing either of the E6 aa 83 variants along with E7 and AcN1 are poorly differentiated. These tumors have large pleiomorphic nuclei, prominent nucleoli, and several mitotic figures per high-powered field (Fig. 6E and F). They are also more vascular than the tumors generated with E6, E7, and AcN1 (Fig. 6H). Stromal fibroblasts present in these tumor masses are easily distinguishable in the tumor sections by their elongated morphology, normal nuclei, and pink-staining collagen deposition in the surrounding matrix. Mouse stromal fibroblasts are closely intermingled with epithelial tumor cells throughout the tumor mass, suggesting an active participation of these normal fibroblasts in tumor formation (Fig. 6G).
FIG. 6.
Histology of subcutaneous tumors. All panels were stained with hematoxylin and eosin. (A) Necrotic lesion expressing prototype E6. (B) Well-differentiated tumor expressing E6, E7, and AcN1. (C) Lesion generated in the presence of S82DL83W showing foamy cells. (D) Squamous differentiation in an E6-, E7-, and AcN1-expressing tumor as depicted by the presence of a keratin pearl (▾). (E) Poorly differentiated S82DL83W-, E7-, and AcN1-expressing tumor with cells having large pleiomorphic nuclei and prominent nucleoli. (F) Abundance of mitotic figures in the same tumor (asterisk). (G) Close intermingling of epithelial cells and stromal fibroblasts. Epithelial cells are the larger, more pinkish-stained cells, whereas the fibroblasts are paler-stained cells. (H) Abundance of blood vessels in an S82DL83W-, E7-, and AcN1-expressing tumor. Photomicrographs are representative of five experiments. Panels A, B, C, G, and H were taken at 20× magnification, while D, E, and F were taken at 40× magnification.
Status of signaling cascades in vivo.
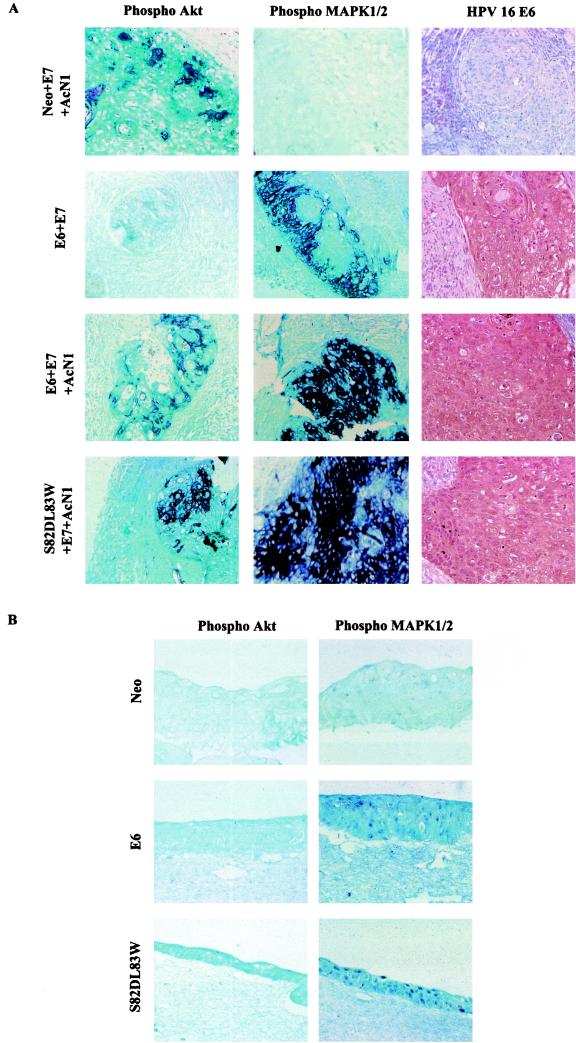
The necrotic lesions generated by E6 or E6 variant alone or in the presence of E7 have phospho-MAPK1/2 but not phospho-Akt (Fig. 7A, left and middle columns). The nodule-like structure formed by HaCaT containing Neo, E7, and AcN1 shows the presence of phospho-Akt but not phospho-MAPK1/2. This result shows that the activation of the MAPK signaling pathway occurs specifically in the presence of E6 (Fig. 7A, right column), as is also evident in the immunohistochemical analysis of organotypic raft cultures (Fig. 7B). The rafts generated by HaCaT Neo cells are well differentiated, unlike those with E6 or variants of E6, which exclusively contain layers of proliferating cells (data not shown). Rafts generated with HaCaT cells expressing E6 or S82DL83W have a strong detection of phospho-MAPK1/2 staining. The HaCaT Neo rafts showed an almost negligible pattern of expression of phospho-MAPK1/2 (Fig. 7B, right column). Staining with antibody against phospho-Akt was not detected in any of the raft sections (Fig. 7B, left column).
FIG.7.
Activation of MAPK signaling pathway in mouse tumors and rafts. Microphotographs of a tumor generated at 2 weeks by HaCaT cells expressing the shown gene combinations (A) or rafts generated from HaCaT cells stably expressing Neo, E6, and S82DL83W (B) were immunostained by using antibodies against phospho-MAPK1/2 and phospho-Akt. Tumors in panel A were also stained with anti-HPV-16/18 E6 antibody to confirm the expression of E6. Microphotographs were taken under 20× magnification.
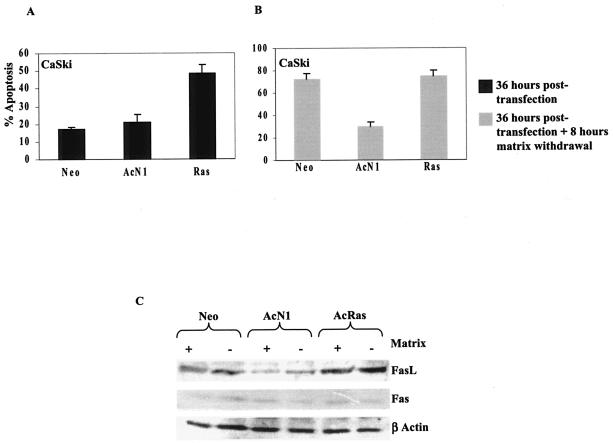
Exogenous expression of oncogenic Ras results in apoptosis of CaSki cells.
To evaluate the hypothesis that aa 83 variants are permissive for deregulated Notch1 signaling and not oncogenic Ras, we used CaSki cells, a cell line that harbors the L83V variant of E6 in addition to prototype E6 (24). The expression of oncogenic Ras in adherent CaSki cells leads to a twofold increase in apoptosis compared to mock vector-transfected cells (Fig. 8A). The expression of AcN1 does not induce apoptosis in these cells. This phenotypic difference is better defined in assays that involve matrix withdrawal. CaSki cells show an anoikis response (18); the expression of AcN1 but not oncogenic Ras, however, protects these cells against anoikis (Fig. 8B). An analysis of Fas expression shows comparable protein levels in matrix-attached and detached cell populations (Fig. 8C). CaSki cells expressing oncogenic Ras show high levels of FasL per se, with a marked increase on matrix withdrawal. In contrast, CaSki cells expressing AcN1 have relatively low levels of FasL and show a marginal increase in suspension (Fig. 8C). This result is consistent with the observations that in some cases, oncogenic Ras-mediated MAPK signaling and cell detachment trigger overexpression of FasL (33, 39), while in others, the inhibition of PI3K-PKB/Akt-mediated signaling enhances Fas-mediated apoptosis (29). Collectively, these data reiterate the notion that the expression of oncogenic Ras, but not AcN1, is detrimental for cells harboring the L83V variant of E6 and probably leads to apoptosis in these cells by upregulating the expression of FasL.
FIG. 8.
Expression of oncogenic Ras (V12Ras) in CaSki cells induces apoptosis. CaSki cells transfected with the mentioned plasmids were either scored for apoptotic nuclei 36 h posttransfection (A) or subjected to a further 8 h of matrix withdrawal (B). The expression of oncogenic Ras in Caski cells induces apoptosis. (C) Levels of FasL and Fas were checked in the mentioned cells, and levels of β-actin were used as a loading control. Thirty-six hours posttransfection, cells were either left attached to tissue culture dishes (+) or kept in suspension (−) for 8 h before they were lysed.
DISCUSSION
In this study, we have established a novel link between HPV-16 E6 and the activation of the MAPK signaling pathway. We report the detection of increased levels of active MAPK1/2, MEK1/2, and BRaf by using various biochemical and immunohistochemical analyses linking this key HPV oncogene to the MAPK pathway. In vitro complementation experiments with constitutively active MEK instead of E6 in the presence of E7 and AcN1 are also consistent with a role of the MAPK signaling pathway in HPV-mediated transformation. We found that under conditions of similar expression levels of protein (Fig. 1A and B) and mRNA (Fig. 1C), the aa 83 variant of E6 shows enhanced signaling through the MAPK pathway compared to prototype E6 (Fig. 2A and D). Furthermore, the activation of MAPK signaling in the presence of E6 occurs irrespective of the cell line used (Fig. 2 and data not shown). Increased MAPK signaling and enhanced oncogenic properties exhibited by the L83V E6 variant offer insights into mechanisms that may operate in human cervical neoplasia, given the higher frequency of its occurrence in the progression of high-grade precursor lesions to invasive carcinomas. The lack of activation of Akt by E6 is consistent with the hypothesis that Notch1-mediated signaling through PI3K-PKB/Akt (31) is the mechanism used to activate other Ras effector pathways in the context of HPV-induced tumorigenesis (Fig. 2B).
Three parallel observations helped us define a link between E6 and MAPK signaling through the Rap1 pathway. First, we were unable to determine an increase in levels of RasGTP and activated cRaf1, an archetypal effector of the MAPK pathway downstream of oncogenic Ras (Fig. 2C and D). Second, our data revealed an increase in levels of Rap1GTP and the accompanying Raf effector, activated BRaf (Fig. 2A and D). Third, we found that the expression of E6TP1, a Rap1 GAP, inhibited an E6-mediated increase in MEK1/2 activity (Fig. 2E). The expression of E6TP1 also eliminated the synergistic transformation of E6 with E7 and AcN1, suggesting that the degradation of this molecule may lead to endogenous activation of the Rap1 pathway. Moreover, the activation of the BRaf-MEK1/2-MAPK1/2 signaling pathway in the presence of only high-risk HPV E6 (Fig. 3A and C) and not low-risk HPV-6b E6 (Fig. 3B to D) further argues that degradation of E6TP1 (11) might be a key factor in mediating signaling through this pathway. As aa 83 is not part of the region that has been delineated in the degradation of E6TP1, a detailed analysis may be useful to evaluate the potential role of this amino acid in modulating E6-E6TP1 interactions and possible quantitative differences in degradation. Rap1 was originally identified as a molecule that could revert the transformation phenotype of K-Ras and was thereby called K-rev1. More recent studies suggest that it can activate MAPK signaling by recruiting BRaf, depending on the cellular context (4). Our observations are in keeping with reports suggesting the involvement of deregulated Rap1 signaling in malignancy (4, 17).
We focused our analysis on evaluating the contribution of MAPK signaling, induced by E6, to the progression of immortalized human epithelial cells to complete transformation in both in vitro soft agar assays and in vivo nude mice tumors. Analogous to activated forms of PKB/Akt that can replace AcN1 in cooperative transformation assays with E6 and E7 (31), we show that AcMEK can cooperate with E7 and AcN1 (Table 1, column 2). In the presence of E7 and AcN1, the expression of aa 83 variants alone generates increases in colony-forming efficiency similar to those with a combination of E6 and AcMEK. These results are consistent with MAPK activation complementing E7 and AcN1 functions in transformation. We also find that an increase in MAPK signaling enhances this synergistic transformation, supporting the argument necessitating the requirement of cooperative interaction between Ras effector pathways in transformation (23).
In the absence of an absolute correlation between in vitro transformation studies and in vivo tumor formation and growth (14, 26), we analyzed the tumor-forming potential of E6 and aa 83 variants along with other oncogenes in nude mice. Extending the results in vivo, we found that tumors are formed in nude mice with HaCaT cells in the presence of E6, E7, and AcN1 (Fig. 4). The expression of E6 aa 83 variants results in bigger (Fig. 4) and more aggressive (Fig. 6) tumors than those with prototype E6. Increased neoangiogenesis, mitotic figures, and better intermingling of tumor tissue with the host stroma characterize tumors generated in the presence of E6 variants (Fig. 6). We assessed the in vitro colony and in vivo tumor-forming ability by using another immortalized epithelial cell line, d1DR (Table 1 and Fig. 4). In addition to potential differences in genetic backgrounds that may influence the nature of cooperation between signaling pathways, it was important to ascertain whether the observations in HaCaT cells are likely to operate in the epithelial cells immortalized by HPV oncogenes. Furthermore, Talora et al. noted that very high levels of expression of Notch1 using adenoviruses may repress the endogenous E6/E7 promoter and that cervical tumor-derived cell lines have lowered Notch1 levels (37). Thus, we chose d1DR cells, as in some ways they mimic the stage corresponding to dysplastic human cervical cells better than HaCaT cells. We were surprised to find that these cells needed exogenous E6 and E7 (data not shown). However, it is already known that there are mechanisms which operate to increase the levels of E6 and E7 protein during the progression of cervical lesions (46). We have not examined the basis of low levels of E6 and E7 in d1DR cells, but it is unlikely to be dependent on Notch signaling, as Jagged1 is not expressed in these cells (data not shown). Importantly, we found that tumor progression and inhibition are similar to those of HaCaT cells transfected with AcN1 and oncogenic Ras, respectively. A recent report by Lathion and colleagues has shown that, unlike with high expression levels, moderate levels of activated Notch1 expression do not repress the HPV E6/E7 promoter (20). Our experiments were undertaken with plasmids that express moderate levels of AcN1 (data not shown) and support an oncogenic role for Notch signaling. In addition, Lathion et al. and our laboratory found that cervical tumor-derived cell lines express both transmembrane and activated forms of Notch1 (20, 39a).
The expression of E6 in the epidermis in transgenic mouse models leads to hyperplastic epithelium (35). We found that E6 and its variants can inhibit cellular differentiation and promote proliferation on organotypic raft cultures (data not shown). The effect of MAPK1/2 on cell proliferation is well established (2). Detection of active MAPK1/2 in HaCaT E6 rafts correlates with the expansion of the proliferative layer and strengthens the link of this signaling cascade with regulating proliferation (Fig. 7B). Expression of E6 or aa 83 variants alone suffices to activate MAPK signaling in the necrotic lesions generated in nude mice (Fig. 7A). Nonregressing tumors generated in the presence of E7 and AcN1 along with either prototype E6 or the aa 83 variant show detectable levels of active forms of both MAPK1/2 and PKB/Akt. However, individual activation of the MAPK or the PI3K pathway alone is not sufficient to generate nonregressing tumors. This finding reconfirms the importance of combined or synchronous activation of both of these signaling cascades in the formation and maintenance of tumors.
An unexpected finding in the in vitro transformation and in vivo tumor formation assays is the striking difference in the cooperation of oncogenic Ras with prototype E6 and aa 83 variants (Table 1 and Fig. 5). While prototype E6 synergizes with both AcN1 and oncogenic Ras, E6 aa 83 variants cooperate with AcN1 but not with oncogenic Ras both in vitro (Table 1, columns 6 and 8) and in vivo (Fig. 4 and 5). These data suggest that aa 83 variants define the permissive cooperative pathways that mediate tumor progression. The only detectable biochemical differences that we have noted so far are the quantitative differences in the MAPK pathway. We examined the nature of cooperative transformation with AcMEK independently with aa 83 variants and oncogenic Ras (Table 1 and data not shown). While AcMEK enhances E6-mediated transformation, it inhibits transformation in the context of L83V (Table 1, column 2). Similarly, AcMEK inhibits oncogenic Ras-mediated transformation (data not shown). These observations fit the notion that increasing MAPK signaling beyond a threshold interferes with molecules like aa 83 variants and oncogenic Ras that have an inherently high level of MAPK activity. While the quantitative differences in MAPK signaling that we have identified may serve to inhibit oncogenic Ras, we cannot rule out other overlapping mechanisms. For example, Rap1 can attenuate Ras-mediated MAPK activation by competitive interference of cRaf1 (7, 16).
Extending these studies in the L83V E6-harboring HPV-16-positive cervical carcinoma cell line CaSki further reveals that AcN1 but not oncogenic Ras generates resistance to anoikis (Fig. 8B). The induction of apoptosis by oncogenic Ras in this cell line (Fig. 8A) is consistent with our observations of HaCaT and d1DR cells showing a lack of cooperation between L83V and oncogenic Ras; increased apoptosis correlates with enhanced FasL expression (Fig. 8C).
The selective cooperation of E6 aa 83 variants and AcN1 that we report in this study is of potentially immense interest, given that both the activation of Notch signaling and accumulation of L83V variants have been linked to the progression of high-grade precursor lesions. Gray and colleagues have reported the presence of the Notch1 ligand Jagged1 in cervical tumors (12). We find that Jagged1 can generate an antiapoptotic phenotype in HaCaT cells and sustain transformation by HPV-16 E6 and E7 oncogenes (39a), analogous to that obtained by the expression of AcN1 (31). Our analysis shows an enhanced transformation potential of Jagged1 in the presence of L83V compared with prototype E6 (data not shown). Furthermore, Jagged1 is upregulated in the transition of high-grade precursor lesions to invasive tumors and also in late passage W12 cells (Veeraghavalu et al., under review), a cell line that carries HPV-16 genomes (36). Consistent with these observations, the inhibition of Jagged1-mediated Notch signaling inhibits anoikis resistance in CaSki cells. Collectively, these data suggest that regulated changes in the expression of molecules that modulate Notch signaling may parallel the accumulation of aa 83 variants in the progression of cervical tumors.
The experiments undertaken in this study are based on models of tumor progression that emphasize the role of oncogenic Ras in the transformation of immortalized human epithelial cells (13). Our results on the contrasting cooperation of E6, L83V, and AcMEK1/2 with AcN1 and oncogenic Ras suggest that levels of MAPK signaling may define permissiveness of interactions between oncogenic pathways. Thus, balancing signaling mediated by various Ras effector pathways may be as important as their recruitment. The striking inhibition of oncogenic Ras by L83V is consistent with studies undertaken on cervical tumor precursor lesions and cancers that have revealed a fairly low frequency of oncogenic Ras mutations. An epidemiological analysis of the distribution of L83V and oncogenic Ras mutations would be an invaluable sequel to this study.
Acknowledgments
We thank UICC for ICRETT fellowships awarded to O.C., K.V., and V.T.
We thank N. Fusenig and L. A. Pirisi-Creek for cell lines and M. Tommasino, V. Band, K. Wong, J. L. Bos, L. A. Laimins, and K. Raj for constructs. We also thank animal maintenance facilities at Cambridge and NCBS. We thank Naval P. Shanware for assistance in this research and Deepa Subramanyam for critical reading of the manuscript.
REFERENCES
- 1.Andersson, S., M. Alemi, E. Rylander, A. Strand, B. Larsson, J. Sallstrom, and E. Wilander. 2000. Uneven distribution of HPV 16 E6 prototype and variant (L83V) oncoprotein in cervical neoplastic lesions. Br. J. Cancer 83**:**307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, E. J., W. Clark, and D. A. Gillespie. 2000. Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr. Biol. 10**:**1119-1122. [DOI] [PubMed] [Google Scholar]
- 3.Bos, J. L. 1989. Ras oncogenes in human cancer: a review. Cancer Res. 49**:**4682-4689. [PubMed] [Google Scholar]
- 4.Bos, J. L., J. de Rooij, and K. A. Reedquist. 2001. Rap1 signalling: adhering to new models. Nat. Rev. Mol. Cell Biol. 2**:**369-377. [DOI] [PubMed] [Google Scholar]
- 5.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106**:**761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman, N., and M. A. Stanley. 1994. Analysis of HLA-DR expression on keratinocytes in cervical neoplasia. Int. J. Cancer 56**:**314-319. [DOI] [PubMed] [Google Scholar]
- 7.Cook, S. J., B. Rubinfeld, I. Albert, and F. McCormick. 1993. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 12**:**3475-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel, B., A. Rangarajan, G. Mukherjee, E. Vallikad, and S. Krishna. 1997. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J. Gen. Virol. 78**:**1095-1101. [DOI] [PubMed] [Google Scholar]
- 9.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15**:**50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke, T. F., D. R. Kaplan, and L. C. Cantley. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88**:**435-437. [DOI] [PubMed] [Google Scholar]
- 11.Gao, Q., S. Srinivasan, S. N. Boyer, D. E. Wazer, and V. Band. 1999. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol. Cell. Biol. 19**:**733-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray, G. E., R. S. Mann, E. Mitsiadis, D. Henrique, M. L. Carcangiu, A. Banks, J. Leiman, D. Ward, D. Ish-Horowitz, and S. Artavanis-Tsakonas. 1999. Human ligands of the Notch receptor. Am. J. Pathol. 154**:**785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400**:**464-468. [DOI] [PubMed] [Google Scholar]
- 14.Han, R., N. M. Cladel, C. A. Reed, and N. D. Christensen. 1998. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology 251**:**253-263. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100**:**57-70. [DOI] [PubMed] [Google Scholar]
- 16.Hu, C. D., K. Kariya, G. Kotani, M. Shirouzu, S. Yokoyama, and T. Kataoka. 1997. Coassociation of Rap1A and Ha-Ras with Raf-1 N-terminal region interferes with Ras-dependent activation of Raf-1. J. Biol. Chem. 272**:**11702-11705. [DOI] [PubMed] [Google Scholar]
- 17.Ishida, D., K. Kometani, H. Yang, K. Kakugawa, K. Masuda, K. Iwai, M. Suzuki, S. Itohara, T. Nakahata, H. Hiai, H. Kawamoto, M. Hattori, and N. Minato. 2003. Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1-deficient mice. Cancer Cell 4**:**55-65. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi, K., and S. Yasumoto. 1999. Retention of cell adhesion and growth capability in human cervical cancer cells deprived of cell anchorage. Jpn. J. Cancer Res. 90**:**867-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396**:**84-88. [DOI] [PubMed] [Google Scholar]
- 20.Lathion, S., J. Schaper, P. Beard, and K. Raj. 2003. Notch1 can contribute to viral-induced transformation of primary human keratinocytes. Cancer Res. 63**:**8687-8694. [PubMed] [Google Scholar]
- 21.Liu, Y., J. J. Chen, Q. Gao, S. Dalal, Y. Hong, C. P. Mansur, V. Band, and E. J. Androphy. 1999. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol. 73**:**7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCance, D. J. 1988. Human papillomavirus (HPV) infections in the aetiology of cervical cancer. Cancer Surv. 7**:**499-506. [PubMed] [Google Scholar]
- 23.McCubrey, J. A., J. T. Lee, L. S. Steelman, W. L. Blalock, P. W. Moye, F. Chang, M. Pearce, J. G. Shelton, M. K. White, R. A. Franklin, and S. C. Pohnert. 2001. Interactions between the PI3K and Raf signaling pathways can result in the transformation of hematopoietic cells. Cancer Detect. Prev. 25**:**375-393. [PubMed] [Google Scholar]
- 24.Meissner, J. D. 1999. Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J. Gen. Virol. 80**:**1725-1733. [DOI] [PubMed] [Google Scholar]
- 25.Munger, K., and P. M. Howley. 2002. Human papillomavirus immortalization and transformation functions. Virus Res. 89**:**213-228. [DOI] [PubMed] [Google Scholar]
- 26.Murakami, H., T. Nakayama, K. Nishijo, T. Hosaka, T. Nakamata, T. Aoyama, T. Okamoto, T. Tsuboyama, T. Nakamura, and J. Toguchida. 2002. Morphological and biological heterogeneity of three tumorigenic cell lines derived from a single p53−/− osteoblast-like cell line, MMC2. Cancer Lett. 182**:**203-211. [DOI] [PubMed] [Google Scholar]
- 27.Mythily, D. V., S. Krishna, and V. Tergaonkar. 1999. Pleiotropic effects of human papillomavirus type 16 E6 oncogene expression in human epithelial cell lines. J. Gen. Virol. 80**:**1707-1713. [DOI] [PubMed] [Google Scholar]
- 28.Nair, P., K. Somasundaram, and S. Krishna. 2003. Activated Notch1 inhibits p53-induced apoptosis and sustains transformation by human papillomavirus type 16 E6 and E7 oncogenes through a PI3K-PKB/Akt-dependent pathway. J. Virol. 77**:**7106-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osaki, M., S. Kase, K. Adachi, A. Takeda, K. Hashimoto, and H. Ito. 2004. Inhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastric carcinoma cell line, MKN-45. J. Cancer Res. Clin. Oncol. 130**:**8-14. [DOI] [PubMed] [Google Scholar]
- 30.Pillai, M. R., S. Sreevidya, B. H. Pollock, P. G. Jayaprakash, and B. Herman. 2002. Human papillomavirus type 16 E6 and E7 gene variations in Indian cervical cancer. Gynecol. Oncol. 87**:**268-273. [DOI] [PubMed] [Google Scholar]
- 31.Rangarajan, A., R. Syal, S. Selvarajah, O. Chakrabarti, S. Sarin, and S. Krishna. 2001. Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology 286**:**23-30. [DOI] [PubMed] [Google Scholar]
- 32.Roovers, K., and R. K. Assoian. 2000. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22**:**818-826. [DOI] [PubMed] [Google Scholar]
- 33.Rosen, K., W. Shi, B. Calabretta, and J. Filmus. 2002. Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand. J. Biol. Chem. 277**:**46123-46130. [DOI] [PubMed] [Google Scholar]
- 34.Singh, L., Q. Gao, A. Kumar, T. Gotoh, D. E. Wazer, H. Band, L. A. Feig, and V. Band. 2003. The high-risk human papillomavirus type 16 E6 counters the GAP function of E6TP1 toward small Rap G proteins. J. Virol. 77**:**1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song, S., H. C. Pitot, and P. F. Lambert. 1999. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J. Virol. 73**:**5887-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley, M. A., H. M. Browne, M. Appleby, and A. C. Minson. 1989. Properties of a non-tumorigenic human cervical keratinocyte cell line. Int. J. Cancer 43**:**672-676. [DOI] [PubMed] [Google Scholar]
- 37.Talora, C., D. C. Sgroi, C. P. Crum, and G. P. Dotto. 2002. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 16**:**2252-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan, T. M., and R. C. Ting. 1995. In vitro and in vivo inhibition of human papillomavirus type 16 E6 and E7 genes. Cancer Res. 55**:**4599-4605. [PubMed] [Google Scholar]
- 39.van den Brink, M. R., R. Kapeller, J. C. Pratt, J. H. Chang, and S. J. Burakoff. 1999. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J. Biol. Chem. 274**:**11178-11185. [DOI] [PubMed] [Google Scholar]
- 39a.Veeraraghavalu, K., M. Pett, R. V. Kumar, P. Nair, A. Rangarajan, M. A. Stanley, and S. Krishna. Papillomavirus-mediated neoplastic progression is associated with reciprocal changes in Jagged1 and Manic Fringe expression linked to Notch activation. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 40.von Knebel Doeberitz, M., C. Rittmuller, F. Aengeneyndt, P. Jansen-Durr, and D. Spitkovsky. 1994. Reversible repression of papillomavirus oncogene expression in cervical carcinoma cells: consequences for the phenotype and E6-p53 and E7-pRB interactions. J. Virol. 68**:**2811-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinmaster, G. 1997. The ins and outs of Notch signaling. Mol. Cell. Neurosci. 9**:**91-102. [DOI] [PubMed] [Google Scholar]
- 42.Woodworth, C. D., P. E. Bowden, J. Doniger, L. Pirisi, W. Barnes, W. D. Lancaster, and J. A. DiPaolo. 1998. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Res. 48**:**4620-4628. [PubMed] [Google Scholar]
- 43.York, R. D., H. Yao, T. Dillon, C. L. Ellig, S. P. Eckert, E. W. McCleskey, and P. J. Stork. 1998. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392**:**622-626. [DOI] [PubMed] [Google Scholar]
- 44.Zagouras, P., S. Stifani, C. M. Blaumueller, M. L. Carcangiu, and S. Artavanis-Tsakonas. 1995. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc. Natl. Acad. Sci. USA 92**:**6414-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zehbe, I., E. Wilander, H. Delius, and M. Tommasino. 1998. Human papillomavirus 16 E6 variants are more prevalent in invasive cervical carcinoma than the prototype. Cancer Res. 58**:**829-833. [PubMed] [Google Scholar]
- 46.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288**:**F55-F78. [DOI] [PubMed] [Google Scholar]
- 47.zur Hausen, H. 1999. Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin. Cancer Biol. 9**:**405-411. [DOI] [PubMed] [Google Scholar]
- 48.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2**:**342-350. [DOI] [PubMed] [Google Scholar]
- 49.Zwartkruis, F. J., R. M. Wolthuis, N. M. Nabben, B. Franke, and J. L. Bos. 1998. Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J. 17**:**5905-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]