Treatment options in recurrent ovarian cancer: latest evidence and clinical potential (original) (raw)
Abstract
Ovarian cancer (OC) is the fifth most common cause of cancer death in women. Although significant progress has been made in the treatment of OC, the majority of patients experience disease recurrence and receive second-line and sometimes several lines of treatment. Here we review the options available for the treatment of recurrent disease and discuss how different agents are selected, combined and offered in a rationale sequence in the context of multidisciplinary care. We reviewed published work between 1990 and 2013 and meeting abstracts related to the use of chemotherapy and surgery in patients with recurrent ovarian cancer. We discuss treatment regimens, efficacy endpoints and safety profiles of the different therapies. Platinum-based drugs are the most active agents and are selected on the basis of a probability of response to retreatment. Nonplatinum-based chemotherapy regimens are usually given in the ‘platinum-resistant’ setting and have a modest effect on outcome. Molecular targeted therapy of ovarian cancer given alone or integrated with chemotherapy is showing promising results. Many patients are now receiving more than one line of therapy for recurrent disease, usually platinum based until platinum resistance emerges. The sequential use of chemotherapy regimens and the incorporation of molecularly targeted treatments, either alone or in combination with chemotherapy, have over the last decade significantly extended the median survival of patients with ovarian cancer.
Keywords: bevacizumab, poly-ADP ribose polymerase inhibitors, platinum resistant, platinum sensitive, relapsed ovarian cancer
Introduction
Ovarian cancer (OC) is the fifth most common cause of cancer death in women. The results of primary treatment with surgery and chemotherapy, usually paclitaxel and carboplatin, have improved very little over the last two decades. The progression-free survival (PFS) has remained fairly constant at about 18 months. Approximately 80% of women with advanced OC will have tumour progression, or more commonly a recurrence. Although this is usually eventually fatal due to the emergence of drug resistance, there have been significant advances in treatment that over the last decade have, for example in England, led to a significant prolongation in 5-year survival while the incidence of the disease has remained constant [Trent Cancer Registry, 2012]. Here we review the variety of options available for the treatment of recurrent disease and discuss how different agents are selected, combined and offered in a rationale sequence, in the context of multidisciplinary care.
Platinum sensitivity
The choice of chemotherapy for recurrent OC is based on the ‘platinum-free interval’ (PFI), the interval between the completion of last platinum-based treatment and the detection of relapse. The term PFI is regarded as one of the most reliable predictors of response to subsequent chemotherapy [Gore et al. 1990; Markman et al. 1991; Eisenhauer et al. 1997]. The original definition identified two categories: ‘platinum sensitive’ with a PFI of at least 6 months and ‘platinum resistant’ if the PFI was less than 6 months. More recently, a widely accepted categorization particularly for the design of clinical trials defines ‘platinum refractory’ as disease progressing during therapy or within 4 weeks after the last dose; ‘platinum resistant’ as disease progressing within 6 months of platinum-based therapy; ‘partially platinum sensitive’ as disease progressing between 6 and 12 months; and ‘platinum sensitive’ as disease progressing with an interval of more than 12 months [Friedlander et al. 2011; Stuart et al. 2011].
Although these categories remain clinically relevant they are based on historic empirically defined observational data in patients with clinical evidence of relapse. The terminology has been extended to include patients with asymptomatic relapse, based on a rising CA125 and radiographic changes. However, the results of retreatment with platinum-based chemotherapy in early CA125 and radiographic relapse may be different from the originally defined categories. The categorization is used extensively for clinical trials but because of variations in the interpretation of ‘platinum sensitivity’, therapeutic decisions are often guided by a more flexible interpretation of these terms. Furthermore, the definitions above did not distinguish ‘platinum sensitivity’ in different subtypes of OC. For example, clear cell or mucinous tumours have a much lower sensitivity to platinum in both the primary and relapsed settings. Platinum resistance should not be regarded as absolute unless the tumour progresses on platinum therapy, but rather as a continuous time variable (see below). However, attempts to define the probability of the response to platinum treatment by molecular criteria are complex [Martin et al. 2008]. ‘Platinum sensitivity’ was originally defined for second-line therapy, but it is now used for subsequent therapies and seems to apply to at least three lines of relapse treatment [Hanker et al. 2012]. Simpler definitions of resistance have been proposed based on the demonstration of true resistance to platinum [Markman, 1998] but whilst the search for a molecular definition continues, the categorical observations above continue to be used in clinical practice and trials.
Role of surgery for relapsed OC
The role of surgery in the management of relapsed OC is uncertain. It is usually performed in women with a recurrence in only a few anatomical sites, usually after a long treatment-free interval. However, the extent to which this procedure adds to PFS or overall survival (OS) is unclear. The data demonstrating a benefit are derived from retrospective studies [Harter et al. 2009; Zang et al. 2011]. The German AGO (Arbeitsgemeinschaft Gynaekologische Onkologie) group defined a group of patients who benefit most based on the presence of at least two of the three following criteria: complete resection at first surgery, good performance status and absence of ascites [Harter et al. 2009]. The benefit of surgery can only be determined by a randomized trial and two ongoing randomized trials led by AGO and the Gynecologic Oncology Group may provide an answer to the question of whether surgery improves the outcome of OC in first recurrence.
Follow-up strategies and retreatment
Elevated levels of CA125, a serum tumour marker, are found in over 85% of patients with advanced OC. An elevation of this marker following a response to primary chemotherapy has been used by the Gynaecological Cancer Inter Group to define recurrence [Rustin et al. 2011]. Some clinicians have used a CA125-defined relapse to initiate second-line therapy. The value of this was questioned in the MRC OV05-EORTC 55955 collaborative trial, in which patients were randomly divided into two groups: in one group a raised CA125 was notified to the clinician to start chemotherapy; and in the other group raised CA125 levels were concealed until there was clinical suspicion of relapse. In this group, treatment was delayed by a median of 4.8 months with no detriment to survival [hazard ratio (HR) 1.01; 95% confidence interval (CI) 0.82–1.25; p = 0.91] [Rustin et al. 2010]. Furthermore, quality of life was worse in the group receiving early therapeutic intervention. There have been several interpretations of the results but perhaps the least controversial one is that when confirming relapse by CA125 it is safe not to immediately initiate treatment unless there is a clear clinical indication to do so.
Whilst some variation in clinical practice continues, most patients still undergo routine testing of CA125 at intervals of approximately 3 months for the first 2 years. CT imaging is triggered by an elevation in CA125 or clinical symptoms. The routine use of more complex imaging with positron emission tomography computed tomography (PET-CT) to pick up disease earlier is unlikely to be cost effective. The principal role of PET-CT is to identify occult disease that may contraindicate surgery.
Treatment of recurrent OC
‘Platinum-sensitive’ recurrence
Platinum-based therapy continues to be the principal regimen used to treat tumours that recur at least 6 months after prior therapy [Markman et al. 1991]. Carboplatin monotherapy is very convenient to administer, well tolerated and produces relatively high response rates (RRs). However, the response usually lasts for only a few months and with each subsequent course of therapy the treatment-free interval usually becomes shorter until the tumour is declared ‘platinum resistant’. Several studies have combined platinum-based drugs with other agents. Combinations increase the tumour RR and extend the PFS [Bolis et al. 2001; Parmar et al. 2003; Pfisterer et al. 2006; Alberts et al. 2008; Pujade-Lauraine et al. 2010].
A recent trial meta-analysis comparing platinum monotherapy with platinum combinations showed a benefit in PFS and OS [Raja et al. 2013]. In this meta-analysis the reduction in risk of relapse in patients with a treatment-free interval of over 12 months or 6–12 months was similar. A benefit of combination therapy was also seen in patients who had received more than one prior line of therapy. However, combination chemotherapy leads to increased toxicity and a possible detrimental effect on quality of life. The effect of combination chemotherapy on the quality of life of patients with recurrent OC has not been satisfactorily investigated in clinical trials. Guidelines offer several treatment options [Ledermann et al. 2013b; Morgan et al. 2013]. The choice between single agent platinum and a platinum doublet needs to be made following discussion with individual patients.
ICON4/OVAR 2.2 was the first large-scale randomized phase III trial to compare the addition of a second drug to platinum-based therapy. Paclitaxel added to platinum, most commonly carboplatin, extended both the PFS and OS of patients with recurrent OC [Parmar et al. 2003]. Median PFS was increased significantly from 10 to 13 months. The benefit in OS was smaller, corresponding to an absolute difference of 7% at 2 years (57% compared with 50%). This regimen has frequently been used as a reference treatment for newer comparative trials. However, the inclusion of paclitaxel increases toxicity, notably, hair loss and more peripheral neuropathy, and these side effects are of greater concern when the length of benefit gained by combination chemotherapy is relatively short. This led to a trial in which carboplatin and paclitaxel were compared with carboplatin and pegylated liposomal doxorubicin (PLD). The trial, CALYPSO, was designed as a noninferiority study. The toxicity profile was shown to be better than with carboplatin and paclitaxel and there was an increase in the PFS [Pujade-Lauraine et al. 2010]. However, on further follow up there was no improvement in OS [Wagner et al. 2012]. Interestingly, the incidence of carboplatin allergy, a relatively common occurrence when retreating patients with relapsed disease, was lower when the drug was combined with PLD compared with paclitaxel [Bafaloukos et al. 2010; Wagner et al. 2012].
It is generally accepted that the probability of responding to platinum rechallenge depends on the ‘platinum-free’ interval. It has been suggested that artificially extending this interval may increase the subsequent response to platinum-based chemotherapy [Tanguay et al. 2009; Bryant et al. 2011]. In vitro and clinical data have shown that platinum resistance is not necessarily a stable phenomenon; it is inducible and perhaps reversible [Kavanagh et al. 1995; Horowitz et al. 2004]. A subgroup analysis of the OVA 301 trial which compared PLD with or without trabectedin, a DNA minor groove binding drug, suggested there was a benefit in delaying the PFI by using a nonplatinum combination therapy. Patients with ‘partially platinum-sensitive’ relapse had an improved PFS and a 41% reduction in the risk of death compared with those treated with PLD alone [Monk et al. 2010]. It is hypothesized that treatment with nonplatinum drugs to extend the PFI can render cells more sensitive to platinum drugs and this may partially explain the survival benefit observed in the partially platinum-sensitive population following reintroduction of subsequent platinum therapy [Kaye et al. 2011]. This needs confirmation in a prospective trial. Two trials that compare the use of nonplatinum therapy with platinum-based treatment followed by crossover at next progression are in progress. One of these uses the combination of trabectedin and PLD (INOVATYON trial) [ClinicalTrials.gov identifier: NCT01379989], and the other compares PLD with carboplatin and paclitaxel, crossing over to the other regimen on progression (MITO-8) [ClinicalTrials.gov identifier: NCT00657878].
Targeted therapy of ‘platinum-sensitive’ recurrent OC
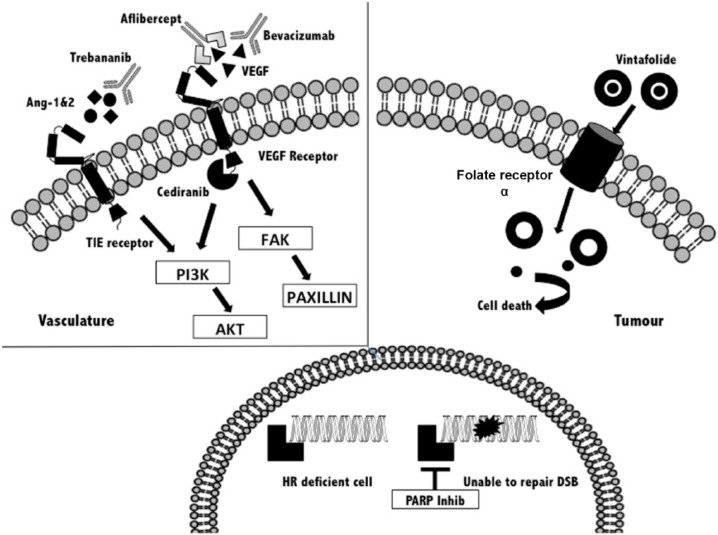
There are now several molecularly directed drugs that are being tested in patients with OC (Figure 1). Clinical studies have been conducted in patients at different stages of the treatment pathway. Two classes of drug are being extensively explored in the ‘platinum-sensitive’ group. These are inhibitors of angiogenesis, an important driver of tumour growth [Burger, 2011] and poly-ADP ribose polymerase (PARP) inhibitors that are active in patients with BRCA gene mutations, and those deficient in the repair of DNA damage through homologous recombination [Yap et al. 2011].
Figure 1.
Overview of new emerging compounds in the treatment of recurrent ovarian cancer. AKT, Protein Kinase B;, Ang-1&2, angiopoietins 1 and 2; DSB double-strand break; FAK, focal adhesion kinase; HR, homologous repair; PARP, poly-ADP ribose polymerase; PI3K phosphatidylinositol-4,5-bisphosphate 3 kinase; TIE, tyrosine kinase with immunoglobulin-like and EGF-like domains; VEGF, vascular endothelial growth factor.
Vascular endothelial growth factor and vascular endothelial growth factor receptor inhibitors
The greatest clinical experience has been gained from studies with a humanized monoclonal antibody, bevacizumab, directed against circulating vascular endothelial growth factor (VEGF) A, an important ligand that binds to the VEGF receptor (VEGFR), stimulating angiogenesis. The key study in ‘platinum-sensitive’ recurrent OC (OCEANS trial) compared the combination carboplatin and gemcitabine with or without bevacizumab in 484 patients [Aghajanian et al. 2012]. The antibody was administered until progression or unacceptable toxicity. The addition of bevacizumab significantly improved the PFS (12.4 versus 8.4 months; HR 0.484; 95% CI 0.388–0.605; p < 0.0001); furthermore the addition of bevacizumab to chemotherapy increased the objective response rate (78.5% versus 57.4%; p < 0.0001). However, there has not been any demonstrable benefit in OS. Many patients went on to receive multiple further lines of chemotherapy and more than 30% of patients on placebo received bevacizumab at some time during treatment for further progression. Treatment was generally well tolerated. Grade 3 hypertension (0.4% versus 17.4%) and proteinuria (0.9% versus 8.5%) were the most notable drug-related side effects and no patients developed a gastrointestinal perforation while on the trial. As an increasing number of women in Europe receive bevacizumab with ‘first-line’ therapy there is a need to establish whether the benefit of bevacizumab is carried over into subsequent line therapy. There is some evidence to support this hypothesis [McCann et al. 2012], and the Italian MITO group are now examining this in a randomized trial (MITO-16/MANGO2b) [ClinicalTrials.gov identifier: NCT01802749].
Other vascular targeting strategies have been tested in patients with recurrent OC. For example, trebananib (AMG 386), a peptibody, which is a Fc-peptide fusion molecule that inhibits angiopoietin 1 and 2. Preliminary data from TRINOVA-1 are now available. The trial compared weekly paclitaxel with or without weekly intravenous trebananib, and trebananib maintenance in a group of women with ‘platinum-resistant’ or ‘partially platinum-sensitive’ relapse. There was a significant prolongation of the median PFS in patients receiving trebananib (7.2 months versus 5.4 months; HR 0.66; p < 0.001) [Monk et al. 2013]. There was no significant hypertension. The most common notable side effect was local oedema.
Small molecule oral VEGF receptor tyrosine kinase (VEGFR) inhibitors have been studied in relapsed OC. Most of these VEGFR inhibitors have a broad spectrum of activity, inhibiting other receptor tyrosine kinases such as platelet-derived growth factor receptor and fibroblast growth factor receptor. The result of a randomized trial with cediranib, whose main action is on VEGFR2, was recently reported [Ledermann et al. 2013a]. Cediranib or placebo was given with platinum-based chemotherapy to women with first ‘platinum-sensitive’ relapsed OC. This was a three-arm study in which the primary aim was to compare the effect of cediranib with chemotherapy and as maintenance to chemotherapy and placebo. A third arm contained patients given cediranib with chemotherapy, switching to placebo during the maintenance phase. The trial enrolled 456 patients and the primary analysis showed that cediranib significantly improved PFS in the maintenance arm (12.5 versus 9.4 months; HR 0.57; 95% CI 0.45–0.74; p = 0.00001). There was a significant trend in improvement across all three arms, indicating that some benefit occurred when cediranib was given only with chemotherapy. There was also a significant improvement in OS between the two arms in the primary analysis (HR 0.70; 95% CI 0.51–0.99; p = 0.042). Hypertension was the main side effect and the most common symptomatic toxicities were fatigue, nausea and diarrhoea.
These trials clearly show that the addition of antiangiogenic agents to chemotherapy can improve PFS. Most studies have failed to demonstrate an OS benefit. The reasons for this are complex and include factors such as crossover and a long postprogression survival. Nevertheless, VEGF and VEGFR inhibitors have activity in patients with ‘platinum-sensitive’ relapsed OC and more work is needed to define better which patients benefit and where in the treatment pathway of OC they should best be used. Bevacizumab is licensed for the treatment of recurrent OC in Europe but not in the USA.
PARP inhibitors
PARP inhibitors have been shown to be a highly active class of drugs in patients with germ-line BRCA mutations. These mutations occur in up to 17% patients with high-grade serous cancer, the commonest subtype of OC [Alsop et al. 2012]. Mutations in BRCA1 and BRCA2 genes result in deficiency of homologous recombination repair (HRD) of DNA damage. Cells deficient in BRCA function are more reliant on base-excision repair pathways that are inhibited by blocking the PARP enzymes. PARP inhibition in tumours that have HRD prevents the repair of single-strand DNA breaks, leading to an accumulation of double-strand breaks. This results in synthetic lethality as cells with HRD are unable to repair spontaneous DNA damage. Even in multiply pretreated patients, significant tumour responses and disease stabilization were seen with single-agent therapy using olaparib [Fong et al. 2009]. It is now known that HRD may occur by mechanisms other than germ-line BRCA mutation, including somatic mutation, BRCA1 gene methylation and mutation or malfunction of other genes [Bell et al. 2011]. This suggests PARP inhibitors may have a wider role, and olaparib, the most extensively tested PARP inhibitor, has been shown to be active in high-grade serous OC without a BRCA mutation, particularly in those women with a ‘platinum-sensitive’ relapse [Gelmon et al. 2011].
This led to a randomized trial in which 265 patients with recurrent high-grade serous cancer were given olaparib or placebo as maintenance therapy after responding to platinum-based therapy for relapse. Patients were treated until disease progression. Olaparib reduced the risk of progression by 65% (HR 0.35; 95% CI 0.25–0.49; p < 0.001), increasing the median time to progression on maintenance therapy from 4.8 to 8.4 months [Ledermann et al. 2012]. In a more recent analysis, it was found that 51% of patients in the trial had a BRCA mutation. Olaparib showed the greatest effect in this subgroup, reducing the risk of progression by 82% (HR 0.18; 95% CI 0.11–0.31; p < 0.00001) and with a median PFS of 11.2 versus 4.3 months respectively. A benefit was also seen in the BRCA wild type group, but the difference was less marked (HR 0.54; 95% CI 0.33–0.84; p = 0.007). The OS data are still not mature; there is currently no significant difference between the two groups [Ledermann et al. 2014].
Studies have been performed adding PARP inhibitors to chemotherapy, but most of the ongoing phase III trials with olaparib and other PARP inhibitors are being done in the maintenance phase of treatment following a response to platinum-based chemotherapy. Two PARP inhibitor trials with niraparib or rucaparib include patients without BRCA mutations. There is ongoing work to develop tests that will predict which patients without a BRCA mutation will respond to PARP inhibitors.
PARP inhibitors are usually well tolerated and can be given for a prolonged period. Fatigue, low-grade nausea and anaemia are the main side effects [Audeh et al. 2010]. Some patients on PARP inhibitor trials have remained on treatment for several years without further progression of their recurrent OC [Ledermann et al. 2014]. It is anticipated that these drugs will soon be licensed for treatment and they may to lead significant changes in clinical practice. As a new class of treatment it will be important to continue to investigate other opportunities for their use, for example in ‘platinum-resistant’ OC.
‘Platinum-resistant’ recurrence
‘Platinum resistance’ eventually occurs in virtually all patients with recurrent OC. It includes patients with a very heterogeneous group of tumours; those who do not respond to first-line therapy (platinum refractory), relapse within 6 months of treatment, or relapse within 6 months of several lines of treatment for recurrent disease. Clinical trials in ’platinum-resistant’ disease often include patients from some or all of these categories. Furthermore, the biological behaviour of tumours in these groups can be different as some patients have rapidly progressive symptomatic disease whilst others are asymptomatic and may have slow-growing disease. As a result, even in randomized trials interpretation of the results can be difficult. It is important that trials in ‘platinum-resistant’ OC include other endpoints such as patient-reported outcome.
Platinum for ‘platinum-resistant’ recurrence?
Whilst it might be thought that the definition of ‘platinum resistance’ might preclude the further use of platinum-based therapy there are many examples of patients with ‘platinum-resistant’ disease (as opposed to ‘platinum refractory’) who respond to further treatment with platinum-based therapy. Examples include a dose-dense schedule of platinum, either cisplatin or carboplatin given weekly with the addition etoposide or paclitaxel [Van Der Burg et al. 2002; Sharma et al. 2009], or platinum combined with gemcitabine in a two out of three week schedule [Rose et al. 2003]. However, the extra value of the second agent has never been tested against single agent platinum therapy in a randomized trial.
Nonplatinum-based therapy
OC responds to many different cytotoxic agents and there are many publications of phase II trials demonstrating a tumour response. Most of these studies are not randomized and may suffer from selection bias, making it difficult to interpret the value of published results. Four drugs are most commonly used in the setting of ‘platinum resistance’. These are PLD, paclitaxel, topotecan and gemcitabine. Randomized phase III trials have reported similar rates of tumour response (range 10–15), PFS of 3–4 months and OS of about 12 months [Ten Bokkel Huinink et al. 1997; Piccart et al. 2000; Gordon et al. 2001; Buda et al. 2004; Bozas et al. 2007; Mutch et al. 2007; Meier et al. 2009; Monk et al. 2010]. Nonplatinum combination therapy has not been shown to be superior to sequential single agent treatment.
Paclitaxel is one of the most active nonplatinum drugs used in this setting. The standard schedule is a 3 h infusion every 3 weeks. However, the drug may be more effective when given weekly. Part of this additional benefit may be due to an antiangiogenic effect [Kerbel and Kamen, 2004]. The most compelling data are from a first-line study that showed that PFS and OS were significantly increased by weekly paclitaxel [Katsumata et al. 2013]. Several studies in the ‘platinum-resistant’ setting have reported high RR to weekly paclitaxel although a single randomized trial comparing weekly with 3-weekly paclitaxel failed to show a benefit over the weekly regimen [Rosenberg et al. 2002]. Nevertheless, weekly paclitaxel is considered as a standard regimen in ‘platinum-resistant’ disease and is being used as the ‘backbone’ regimen in combination with a variety of molecularly targeted agents.
Over the last 5 years, trials have been conducted with several new cytotoxic compounds in patients with ‘platinum-resistant’ disease. Examples include the epothilones, novel microtubule inhibitors [Rustin et al. 2011; Colombo et al. 2012] and pemetrexed, a multitargeted antifolate agent that inhibits thymidylate synthase [Vergote et al. 2009]. However, none of these trials has led to the licencing of a new drug in this phase of the disease. The management of ‘platinum-resistant’ disease remains challenging and better strategies to overcome drug resistance are needed [Pinato et al. 2013].
Targeted therapies: focus on emerging compounds
Antiangiogenic agents play an important role in all phases of treatment, including ‘platinum-resistant’ disease. The original studies with bevacizumab showed that as a single agent, tumour response occurred in 16% of patients when used alone or in combination with low-dose cyclophosphamide [Burger et al. 2007; Garcia et al. 2008]. Recently, the randomized AURELIA trial showed that bevacizumab given as chemotherapy (PLD, weekly paclitaxel or topotecan) and then as maintenance treatment to prevent progression significantly improved the RR of tumours (11.8% versus 27.3%) and PFS (HR 0.48; 95% CI 0.38–0.60) in women with ‘platinum-resistant’ OC [Pujade-Lauraine et al. 2014]. Furthermore, in this group of women who often had significant symptoms, the addition of bevacizumab led to improvements in symptom control and quality of life [Stockler et al. 2013]. Other strategies, such as the drug aflibercept, a VEGFR trap, have proved useful in controlling ascites [Gotlieb et al. 2012]. Several of the VEGFR inhibitors that have been tested in earlier phases of treatment (nintedanib; pazopanib; cediranib) were first shown to have single agent activity in ‘platinum-resistant’ OC. However, there are no published randomized trials of these agents in the setting of ‘platinum-resistant’ disease. Another strategy under development is to target the α folate receptor (FR). High expression is seen in several tumour types, including OC with low levels in noncancerous tissues. A randomized phase II trial, PRECEDENT, compared PLD with PLD and vintafolide (EC 145), a folic acid–desacetyl vinblastine conjugate that targets FR-positive cells. The combination increased the median PFS from 2.7 to 5.0 months (HR 0.63; 95% CI 0.41–0.96; p = 0.031). Patients underwent SPECT imaging before treatment with Tc99m etarfolatide to identify FR-positive tumour deposits and the greatest benefit of the therapy conjugate was seen in patients with 100% of lesions positive for FR (median PFS of 5.5 compared with 1.5 months for PLD alone; HR 0.38; 95% CI 0.17–0.85; p =0 .013) [Naumann et al. 2013]. A phase III trial is in progress. This approach offers the opportunity of patient selection through real-time in vivo imaging of tumours. The focus of future research is likely to be directed at targeting aberrant pathways or mutations that are being identified through molecular analyses of tumours [Bell et al. 2011; Han et al. 2012; Verhaak et al. 2013]. This information can be used to develop and evaluate new signal-pathway inhibitors. An overview of emerging compounds in the treatment of OC is provided in Figure 1.
Conclusions
OC is one of the most chemosensitive epithelial tumours at initial diagnosis and unlike most other tumours frequently displays chemosensitivity to multiple lines of chemotherapy. Whilst the rate of cure has increased little over the last two decades, there has been a significant prolongation of survival through the careful sequential use of active drugs. The interval between different lines of therapy is used to guide the selection of drugs. The most active agents in OC are platinum-based drugs and these are usually given as a platinum doublet until ‘platinum resistance’ develops. Clinical trials have identified several nonplatinum drugs that can be used during this phase of the disease, but their activity is less than platinum. Many of the molecularly targeted drugs that have been shown to have activity in a variety of solid tumours have demonstrable therapeutic efficacy in OC. These include drugs that target the tumour vasculature as well as those that inhibit intracellular signalling or DNA repair processes. Choosing these or other molecular therapies will need to be made by identifying markers that are predictive of a response. This avenue of research is being actively explored to optimize the care of patients with recurrent OC.
Footnotes
Funding: This research has not received any funding from a grant-funding agency or commercial sources.
Conflict of interest statement: J.A. Ledermann has over the last 3 years attended Advisory Boards for Roche, AstraZeneca, MSD/Merck, Boehringher Ingelheim, PharmaMar and Janssen. He was a member of the GSK Data Monitoring Group for AGO OVA-16 but has not received personal remuneration for any of this work. He has received a travel grant from Roche and AstraZeneca. D. Luvero and A. Milani declare no conflicts of interest.
Contributor Information
Daniela Luvero, UCL Hospitals London and Department of Obstetrics and Gynecology, University Campus Bio-Medico of Rome, Rome, Italy.
Andrea Milani, UCL Hospitals London and FPO, IRCCS, Candiolo Cancer Institute and Department of Oncology, University of Turin, Turin, Italy.
Jonathan A. Ledermann, UCL Cancer Institute, Cancer Research UK & UCL Cancer Trials Centre, 90 Tottenham Court Road, London W1T 4TJ, UK
References
- Aghajanian C., Blank S., Goff B., Judson P., Teneriello M., Husain A., et al. (2012) OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30: 2039–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts D., Liu P., Wilczynski S., Clouser M., Lopez A., Michelin D., et al. (2008) Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200). Gynecol Oncol 108: 90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop K., Fereday S., Meldrum C., Defazio A., Emmanuel C., George J., et al. (2012) BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Crit Oncol 30: 2654–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audeh M., Carmichael J., Penson R., Friedlander M., Powell B., Bell-Mcguinn K., et al. (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376: 245–251 [DOI] [PubMed] [Google Scholar]
- Bafaloukos D., Linardou H., Aravantinos G., Papadimitriou C., Bamias A., Fountzilas G., et al. (2010) A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group Study. BMC Med 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D., Berchuck A., Birrer M., Chien J., Cramer D., Dao F., et al. (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolis G., Scarfone G., Giardina G., Villa A., Mangili G., Melpignano M., et al. (2001) Carboplatin alone vs carboplatin plus epidoxorubicin as second-line therapy for cisplatin- or carboplatin-sensitive ovarian cancer. Gynecol Oncol 81: 3–9 [DOI] [PubMed] [Google Scholar]
- Bozas G., Bamias A., Koutsoukou V., Efstathiou E., Gika D., Papadimitriou C., et al. (2007) Biweekly gemcitabine and cisplatin in platinum-resistant/refractory, paclitaxel-pretreated, ovarian and peritoneal carcinoma. Gynecol Oncol 104: 580–585 [DOI] [PubMed] [Google Scholar]
- Bryant C., Kumar S., Spannuth W., Shah J., Munkarah A., Deppe G., et al. (2011) Feasibility of extension of platinum-free interval with weekly bolus topotecan and subsequent platinum retreatment outcomes in recurrent ovarian cancer. Arch Gynecol Obstet 283: 361–367 [DOI] [PubMed] [Google Scholar]
- Buda A., Floriani I., Rossi R., Colombo N., Torri V., Conte P., et al. (2004) Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian collaborative study from the Mario Negri Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) Group and I.O.R. (Istituto Oncologico Romagnolo) Group. Br J Cancer 90: 2112–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. (2011) Overview of anti-angiogenic agents in development for ovarian cancer. Gynecol Oncol 121: 230–238 [DOI] [PubMed] [Google Scholar]
- Burger R., Sill M., Monk B., Greer B., Sorosky J. (2007) Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25: 5165–5171 [DOI] [PubMed] [Google Scholar]
- Colombo N., Kutarska E., Dimopoulos M., Bae D., Rzepka-Gorska I., Bidzinski M., et al. (2012) Randomized, open-label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary pritoneal cancer. J Clin Oncol 30: 3841–3847 [DOI] [PubMed] [Google Scholar]
- Eisenhauer E., Vermorken J., Van Glabbeke M. (1997) Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: a multivariate analysis of 704 patients. Ann Oncol 8: 963–968 [DOI] [PubMed] [Google Scholar]
- Fong P., Boss D., Yap T., Tutt A., Wu P., Mergui-Roelvink M., et al. (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361: 123–134 [DOI] [PubMed] [Google Scholar]
- Friedlander M., Trimble E., Tinker A., Alberts D., Avall-Lundqvist E., Brady M., et al. (2011) Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer 21: 771–775 [DOI] [PubMed] [Google Scholar]
- Garcia A., Hirte H., Fleming G., Yang D., Tsao-Wei D., Roman L., et al. (2008) Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital Phase II Consortia. J Clin Oncol 26: 76–82 [DOI] [PubMed] [Google Scholar]
- Gelmon K., Tischkowitz M., Mackay H., Swenerton K., Robidoux A., Tonkin K., et al. (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12: 852–861 [DOI] [PubMed] [Google Scholar]
- Gordon A., Fleagle J., Guthrie D., Parkin D., Gore M., Lacave A. (2001) Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19: 3312–3322 [DOI] [PubMed] [Google Scholar]
- Gore M., Fryatt I., Wiltshaw E., Dawson T. (1990) Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol 36: 207–211 [DOI] [PubMed] [Google Scholar]
- Gotlieb W., Amant F., Advani S., Goswami C., Hirte H., Provencher D., et al. (2012) Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol 13: 154–62 [DOI] [PubMed] [Google Scholar]
- Han Y., Huang H., Xiao Z., Zhang W., Cao Y., Qu L., et al. (2012) Integrated analysis of gene expression profiles associated with response of platinum/paclitaxel-based treatment in epithelial ovarian cancer. PLoS One 7: e52745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker L., Loibl S., Burchardi N., Pfisterer J., Meier W., Pujade-Lauraine E., et al. (2012) The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol 23: 2605–2612 [DOI] [PubMed] [Google Scholar]
- Harter P., Hahmann M., Lueck H., Poelcher M., Wimberger P., Ortmann O., et al. (2009) Surgery for recurrent ovarian cancer: role of peritoneal carcinomatosis: exploratory analysis of the desktop I trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann Surg Oncol 16: 1324–1330 [DOI] [PubMed] [Google Scholar]
- Horowitz N., Hua J., Gibb R., Mutch D., Herzog T. (2004) The role of topotecan for extending the platinum-free interval in recurrent ovarian cancer: an in vitro model. Gynecol Oncol 94: 67–73 [DOI] [PubMed] [Google Scholar]
- Katsumata N., Yasuda M., Isonishi S., Takahashi F., Michimae H., Kimura E., et al. (2013) Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14: 1020–1026 [DOI] [PubMed] [Google Scholar]
- Kavanagh J., Tresukosol D., Edwards C., Freedman R., Gonzalez De Leon C., Fishman A., et al. (1995) Carboplatin reinduction after taxane in patients with platinum-refractory epithelial ovarian cancer. J Clin Oncol 13: 1584–1588 [DOI] [PubMed] [Google Scholar]
- Kaye S., Colombo N., Monk B., Tjulandin S., Kong B., Roy M., et al. (2011) Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer delays third-line chemotherapy and prolongs the platinum-free interval. Ann Oncol 22: 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R., Kamen B. (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4: 423–436 [DOI] [PubMed] [Google Scholar]
- Ledermann J., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G., et al. (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366: 1382–1392 [DOI] [PubMed] [Google Scholar]
- Ledermann J., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G., et al. (2014) Olaparib maintenance therapy in patients with platinum sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of a randomised phase 2 trial. Lancet Oncol 15: 852–861 [DOI] [PubMed] [Google Scholar]
- Ledermann J., Perren T., Raja F., Embleton A., Rustin G., Jayson G., et al. (2013a) Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: results of the ICON6 Trial. European Cancer Congress E J Cancer (Suppl. 3): LBA10 S5 [Google Scholar]
- Ledermann J., Raja F., Fotopoulou C., Gonzalez-Martin A., Colombo N., Sessa C., et al. (2013b) Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl. 6): vi24–32 [DOI] [PubMed] [Google Scholar]
- Markman M. (1998) ‘Recurrence within 6 months of platinum therapy’: an adequate definition of ‘platinum-refractory’ ovarian cancer? Gynecol Oncol 69: 91–92 [DOI] [PubMed] [Google Scholar]
- Markman M., Rothman R., Hakes T., Reichman B., Hoskins W., Rubin S., et al. (1991) Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 9: 389–393 [DOI] [PubMed] [Google Scholar]
- Martin L., Hamilton T., Schilder R. (2008) Platinum resistance: the role of DNA repair pathways. Clin Cancer Res 14: 1291–1295 [DOI] [PubMed] [Google Scholar]
- McCann G., Smith B., Backes F., Rath K., Chacko S., Salani R., et al. (2012) Recurrent ovarian cancer: is there a role for re-treatment with bevacizumab after an initial complete response to a bevacizumab-containing regimen? Gynecol Oncol 127: 362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier W., Du Bois A., Reuss A., Kuhn W., Olbricht S., Gropp M., et al. (2009) Topotecan versus treosulfan, an alkylating agent, in patients with epithelial ovarian cancer and relapse within 12 months following 1st-line platinum/paclitaxel chemotherapy. A prospectively randomized phase III trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol 114: 199–205 [DOI] [PubMed] [Google Scholar]
- Monk B., Herzog T., Kaye S., Krasner C., Vermorken J., Muggia F., et al. (2010) Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol 28: 3107–3114 [DOI] [PubMed] [Google Scholar]
- Monk B., Poveda A., Vergote I., Raspagliesi F., Fujiwara K., Bae D., et al. (2013) A phase III, randomized, double-blind trial of weekly paclitaxel plus the angiopoeitin 1 and 2 inhibitor, trebananib, or placebo in women with recurrent ovarian cancer: Trinova-1. European Cancer Congress. E J Cancer 49(Suppl. 3): LBA41 S18 [Google Scholar]
- Morgan R., Jr, Alvarez R., Armstrong D., Burger R., Chen L., Copeland L., et al. (2013) Ovarian cancer, version 2.2013. J Natl Compr Canc Netw 11: 1199–1209 [DOI] [PubMed] [Google Scholar]
- Mutch D., Orlando M., Goss T., Teneriello M., Gordon A., McMeekin S., et al. (2007) Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 25: 2811–2818 [DOI] [PubMed] [Google Scholar]
- Naumann R., Coleman R., Burger R., Sausville E., Kutarska E., Ghamande S., et al. (2013) Precedent: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol 31: 4400–4406 [DOI] [PubMed] [Google Scholar]
- Parmar M., Ledermann J., Colombo N., Du Bois A., Delaloye J., Kristensen G., et al. (2003) Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 Trial. Lancet 361: 2099–2106 [DOI] [PubMed] [Google Scholar]
- Pfisterer J., Plante M., Vergote I., Du Bois A., Hirte H., Lacave A., et al. (2006) Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 24: 4699–4707 [DOI] [PubMed] [Google Scholar]
- Piccart M., Green J., Lacave A., Reed N., Vergote I., Benedetti-Panici P., et al. (2000) Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: a randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology Group. J Clin Oncol 18: 1193–1202 [DOI] [PubMed] [Google Scholar]
- Pinato D., Graham J., Gabra H., Sharma R. (2013) Evolving concepts in the management of drug resistant ovarian cancer: dose dense chemotherapy and the reversal of clinical platinum resistance. Cancer Treat Rev 39: 153–160 [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E., Hilpert F., Weber B., Reuss A., Poveda A., Kristensen G., et al. (2014) Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol 32: 1302–8 [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E., Wagner U., Aavall-Lundqvist E., Gebski V., Heywood M., Vasey P., et al. (2010) Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol 28: 3323–3329 [DOI] [PubMed] [Google Scholar]
- Raja F., Counsell N., Colombo N., Pfisterer J., Du Bois A., Parmar M., et al. (2013) Platinum versus platinum-combination chemotherapy in platinum-sensitive recurrent ovarian cancer: a meta-analysis using individual patient data. Ann Oncol 24: 3028–3034 [DOI] [PubMed] [Google Scholar]
- Rose P., Mossbruger K., Fusco N., Smrekar M., Eaton S., Rodriguez M. (2003) Gemcitabine reverses cisplatin resistance: demonstration of activity in platinum- and multidrug-resistant ovarian and peritoneal carcinoma. Gynecol Oncol 88: 17–21 [DOI] [PubMed] [Google Scholar]
- Rosenberg P., Andersson H., Boman K., Ridderheim M., Sorbe B., Puistola U., et al. (2002) Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol 41: 418–424 [DOI] [PubMed] [Google Scholar]
- Rustin G., Reed N., Jayson G., Ledermann J., Adams M., Perren T., et al. (2011) A phase II trial evaluating two schedules of sagopilone (ZK-EPO), a novel epothilone, in patients with platinum-resistant ovarian cancer. Ann Oncol 22: 2411–2416 [DOI] [PubMed] [Google Scholar]
- Rustin G., van der Burg MEL., Griffin CL., Guthrie D., Lamont A., Jayson GC., et al. (2010) Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 376: 1155–63 [DOI] [PubMed] [Google Scholar]
- Rustin G., Vergote I., Eisenhauer E., Pujade-Lauraine E., Quinn M., Thigpen T., et al. (2011) Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer 21: 419–423 [DOI] [PubMed] [Google Scholar]
- Sharma R., Graham J., Mitchell H., Brooks A., Blagden S., Gabra H. (2009) Extended weekly dose-dense paclitaxel/carboplatin is feasible and active in heavily pre-treated platinum-resistant recurrent ovarian cancer. Br J Cancer 100: 707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockler M., Hilpert F., Friedlander M., King M., Wenzel L., Lee C., et al. (2013) Health-related quality of life (HRQOL) results from the Aurelia Trial Evaluating Bevacizumab (BEV) plus chemotherapy (CT) for platinum-resistant recurrent ovarian cancer (OC). J Clin Oncol 31(Suppl.): abstract 5542. [Google Scholar]
- Stuart G., Kitchener H., Bacon M., Dubois A., Friedlander M., Ledermann J., et al. (2011) 2010. Gynecologic Cancer Intergroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer 21: 750–755 [DOI] [PubMed] [Google Scholar]
- Tanguay J., Ansari J., Buckley L., Fernando I. (2009) Epithelial ovarian cancer: role of pegylated liposomal doxorubicin in prolonging the platinum-free interval and cancer antigen 125 trends during treatment. Int J Gynecol Cancer 19: 361–366 [DOI] [PubMed] [Google Scholar]
- Ten Bokkel Huinink W., Gore M., Carmichael J., Gordon A., Malfetano J., Hudson I., et al. (1997) Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 15: 2183–2193 [DOI] [PubMed] [Google Scholar]
- Trent Cancer Registry (2012) Overview of Ovarian Cancer in England: incidence, Mortality and Survival. www.ncin.org.uk
- Van Der Burg M., De Wit R., Van Putten W., Logmans A., Kruit W., Stoter G., et al. (2002) Weekly cisplatin and daily oral etoposide is highly effective in platinum pretreated ovarian cancer. Br J Cancer 86: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote I., Calvert H., Kania M., Kaiser C., Zimmermann A., Sehouli J. (2009) A randomised, double-blind, phase II study of two doses of pemetrexed in the treatment of platinum-resistant, epithelial ovarian or primary peritoneal cancer. Eur J Cancer 45: 1415–1423 [DOI] [PubMed] [Google Scholar]
- Verhaak R., Tamayo P., Yang J., Hubbard D., Zhang H., Creighton C., et al. (2013) Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest 123: 517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U., Marth C., Largillier R., Kaern J., Brown C., Heywood M., et al. (2012) Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. Br J Cancer 107: 588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap T., Sandhu S., Carden C., De Bono J. (2011) Poly(ADP-ribose) polymerase (PARP) inhibitors: exploiting a synthetic lethal strategy in the clinic. CA Cancer J Clin 61: 31–49 [DOI] [PubMed] [Google Scholar]
- Zang R., Harter P., Chi D., Sehouli J., Jiang R., Trope C., et al. (2011) Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer 105: 890–896 [DOI] [PMC free article] [PubMed] [Google Scholar]