Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function (original) (raw)
. Author manuscript; available in PMC: 2015 Aug 7.
Published in final edited form as: Cell Stem Cell. 2014 Aug 7;15(2):123–138. doi: 10.1016/j.stem.2014.07.012
Abstract
Respiratory disease is the third leading cause of death in the industrialized world. Consequently, the trachea, lungs, and cardiopulmonary vasculature have been the focus of extensive investigations. Recent studies have provided new information about the mechanisms driving lung development and differentiation. However, there is still much to learn about the ability of the adult respiratory system to undergo repair and to replace cells lost in response to injury and disease. This review highlights the multiple stem/progenitor populations in different regions of the adult lung, the plasticity of their behavior in injury models, and molecular pathways that support homeostasis and repair.
INTRODUCTION
The reparative behavior of adult tissues falls along an injury-response spectrum. At one end of the scale are tissues such as the epidermis, intestine, and hematopoietic system with a constitutively high rate of cell turnover and a well-delineated stem/progenitor cell hierarchy. At the other end are organs like the heart and brain that contain few stem cells and cannot repair efficiently, resulting in scarring after injury. In between these two extremes are tissues such as the lung, liver, and pancreas that have a low steady state cell turnover yet can respond robustly after injury to replace damaged cells. This remarkable capacity has prompted studies into the mechanisms that mediate inducible repair, as well as strategies to harness them therapeutically. This review, written by members of the NIH funded Lung Repair and Regeneration Consortium (LRRC; www.lungrepair.org) has three goals: first, to provide an overview of the stem/progenitor cells that build the respiratory system and their descendants that repair the adult organ, second, to survey some of the molecular pathways regulating lung stem/progenitor populations, and, third, to highlight recent discoveries in lung regeneration biology, including bioengineering of the lung.
STEM/PROGENITOR POPULATIONS IN LUNG DEVELOPMENT
The mammalian respiratory system consists of a tree-like arrangement of branched airway tubes connected to a single trachea and terminating in millions of delicate and highly vascularized gas-exchange units known as alveoli (Figure 1). The epithelium lining the whole system is continuous, and initially arises from a small region of anterior ventral foregut endoderm, marked by the transcription factor Nkx2.1. By the time the organ is mature the epithelium differs significantly along the proximal-distal axis, both in cellular composition and structural organization and, related to this, in stem cell composition and strategies for repair. Most of the lung mesenchyme likewise arises from a small population of mesoderm cells that will generate airway and vascular smooth muscle, cartilage, myofibroblasts, lipofibroblasts, and pericytes. The development and patterning of lung endoderm and mesoderm has been the topic of several comprehensive reviews (Cardoso and Whitsett, 2008; Herriges and Morrisey, 2014; Morrisey and Hogan, 2010; Ornitz and Yin, 2012; Shi et al., 2009), and only recent highlights are discussed here.
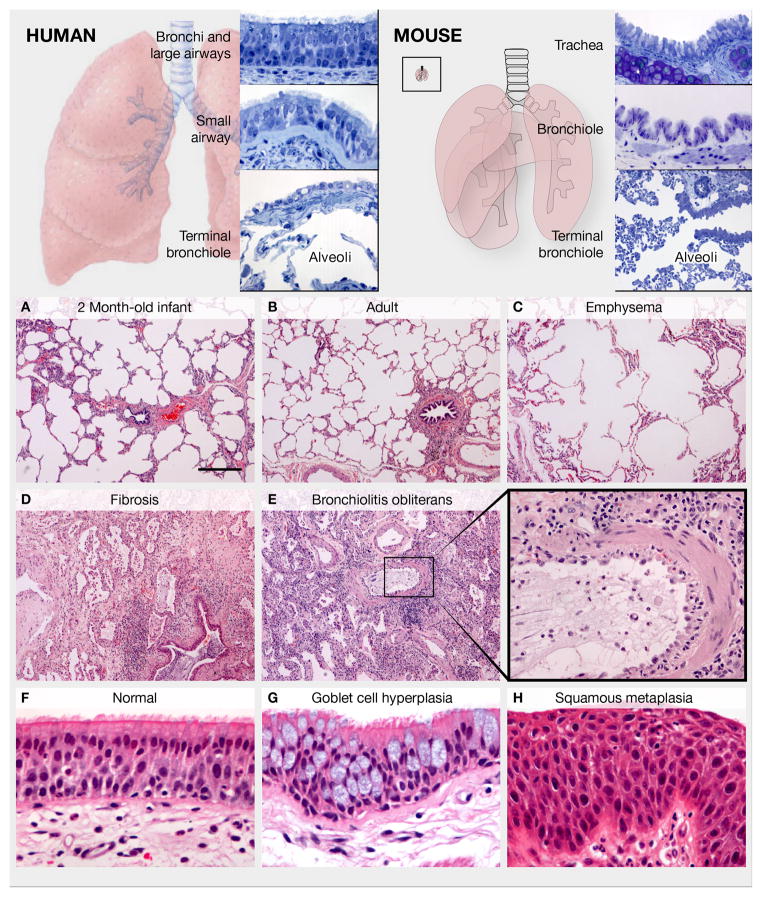
Figure 1. Anatomy of the adult human and mouse lung and examples of human lung pathology.
Upper panels: Regional epithelial histology in human and mouse. Left panel: The human trachea, bronchi and bronchioles >1 mm in diameter are lined by a pseudostratified epithelium with basal, multiciliated and secretory cells. Mucous goblet cells predominate in the larger airways, and Club cells in the smaller airways. Individual neuroendocrine cells and neuroendocrine bodies (NEBs) are scattered in the larger airways and increase distally. Cartilage, smooth muscle and stromal cells are associated with intralobar airways down to the small bronchioles. The simple cuboidal epithelium lining the terminal bronchioles leading into the alveoli is poorly characterized. The alveoli are lined by squamous AEC1s and cuboidal AEC2s. Right panel: In the mouse, only the trachea and mainstem bronchi have cartilage and a pseudostratified mucociliary epithelium with basal cells. The smaller bronchi and bronchioles are lined by a simple epithelium with multiciliated and Club cells, and fewer neuroendocrine cells and NEBs. The inset illustrates a mouse lung to the same scale as the human lung in left panel. Lower panels: Normal and pathologic human lung. (A, B) Images of the alveolar region in a 2 month-old infant and a normal adult illustrate that alveolar number increases postnatally through secondary alveolar septal crest formation. (C) In pulmonary emphysema, septal destruction and loss of alveolar cells results in alveolar enlargement. (D) In pulmonary fibrosis, the terminal bronchioles are plugged with mucus, alveolar epithelial morphology is abnormal, and alveolar architecture is dramatically altered by fibroblastic deposition of extracellular matrix. (E, E′) Bronchiolitis obliterans syndrome showing massive infiltration of immune cells, severe disruption of the small airway epithelium, and thickening of the underlying smooth muscle and stroma (boxed region magnified in E′). (F) Normal pseudostratified mucociliary bronchial epithelium from a lung transplant donor. (G, H) Goblet secretory cell hyperplasia and squamous metaplasia, respectively, in chronic obstructive lung disease. A–E and F–H, respectively, are the same magnification. Scale bar in A is 400 um.
From the point of view of regenerative biology there are multiple reasons why studying lung development is important. For example, some preterm babies are born at the stage of lung development when progenitors of alveolar stem cells are being laid down (Blackwell et al., 2011). Perinatal infections and inflammation that disrupt alveologenesis and cause bronchopulmonary dysplasia (BPD) may therefore have long-term consequences that might be avoided if we knew more about underlying mechanisms. More detailed information about the molecular identity of different cell types and their lineage specification can also inform strategies for generating lung cells ex vivo from pluripotent stem cells and provide new tools to mark and follow the behavior of stem/progenitor cells in models of human lung disease.
Branching morphogenesis and proximal-distal patterning of the epithelium occur early in lung development
Perhaps the best-studied phase in lung development to date is the process of branching morphogenesis by which the two primary lung buds that arise around E(embryonic) day 9.5 in the mouse and 4–5 weeks gestation in the human give rise to the airway tree. The buds are composed of a simple endodermal epithelium, surrounded by mesoderm and a vascular plexus. These tissues are encased in a thin layer of mesothelium that makes a transient early contribution to mesenchymal lineages (Dixit et al., 2013). The buds extend and branch in a pattern that is initially very stereotypic but becomes less so as development proceeds (Metzger et al., 2008; Morrisey and Hogan, 2010; Short et al., 2013). All lung endodermal cells initially express Nkx2.1 and this marker persists into the adult. However, as the primary buds extend and branch, distinct patterns of gene expression emerge in the endoderm of the stalks versus the buds. This proximal-distal difference is exemplified by the expression domains of Sox2 and Sox9 - two transcription factors required for early lung development. Sox2 expression is confined to the proximal stalks, while Sox9 is dynamically expressed in the more highly proliferative cuboidal cells of the distal buds (Chang et al., 2013; Rockich et al., 2013). Many other genes are differentially expressed in the tips, including Id2 encoding a bHLH transcription factor (Alanis et al., 2014; Herriges et al., 2012; Rawlins et al., 2009a). Original lineage labeling experiments suggested a model in which the Id2+ tip cells are a population of multipotent progenitors (Rawlins et al., 2009a). Early in development the tip cells generate daughters that translocate into the stalks and give rise to Sox2+ precursors of all the cell types in the intrapulmonary bronchi and bronchioles. These progeny include neuroendocrine cells, multiciliated cells, and dome shaped secretory cells (originally called Clara cells but now known as Club cells), and evidence suggests that notch signaling plays a key role in the patterning and specification of these different cell types (Morimoto et al., 2012; Tsao et al., 2009). Later in development the Id2+ tip cells give rise to alveolar cell types. More recent lineage tracing studies with a Shh-CreER allele support the idea that tip cells are multipotent progenitors, although whether activity of this cre driver is restricted to the distal tip at this stage of development was not directly tested (Desai et al., 2014). Future studies using Sox9-CreER and other progenitor cell-specific drivers should further refine our understanding of the multipotency of distal tip cells.
Alveologenesis- a critical stage in preparing the lung for gas-exchange
Around E15.0, branching morphogenesis slows and important changes takes place in the distal epithelium (Figure 2). The tubes become narrower or “canalicular” and more closely associated with surrounding vasculature. Cells in the distal part of the tubules begin to express genes characteristic of the two epithelial cell types that make up the mature alveoli, namely the type 1 cells (AEC1s, that express Hopx, Pdpn and AGER) and type 2 cells (AEC2s, that express proteins associated with high levels of surfactant production and secretion)(Treutlein et al., 2014). Subsequently, the tubules enter the “saccular” phase that involves the “budding” of tiny peripheral sacs separated by primary septa. Postnatally, these sacs are further subdivided by secondary septa (Figure 2). Lineage tracing suggests the presence of bipotential cells in the tips of the distal tubules that can give rise to both AEC1 and AEC2 cells (Desai et al., 2014). There has been progress in defining more precisely the intermediate progenitors in the lineages between bipotential cells and mature AEC1 and AEC2 cells and single cell RNA-seq analysis has emerged as a powerful tool in this endeavor (Chang et al., 2013; Desai et al., 2014; Treutlein et al., 2014). A major question for the future is the nature of the signals that control the onset of the canalicular and saccular stages. Roles for signals from the adjacent mesoderm have been proposed, including glucocorticoids, retinoic acid, Fgf, parathyroid hormone, and insulin like growth factor 1 (Alanis et al., 2014; Chang et al., 2013; El Agha et al., 2014; Epaud et al., 2012; Moreno-Barriuso et al., 2006; Ramirez et al., 2000). Signals from the vasculature may play important roles too, given the close association between endothelium and endoderm at this time (Figures 2).
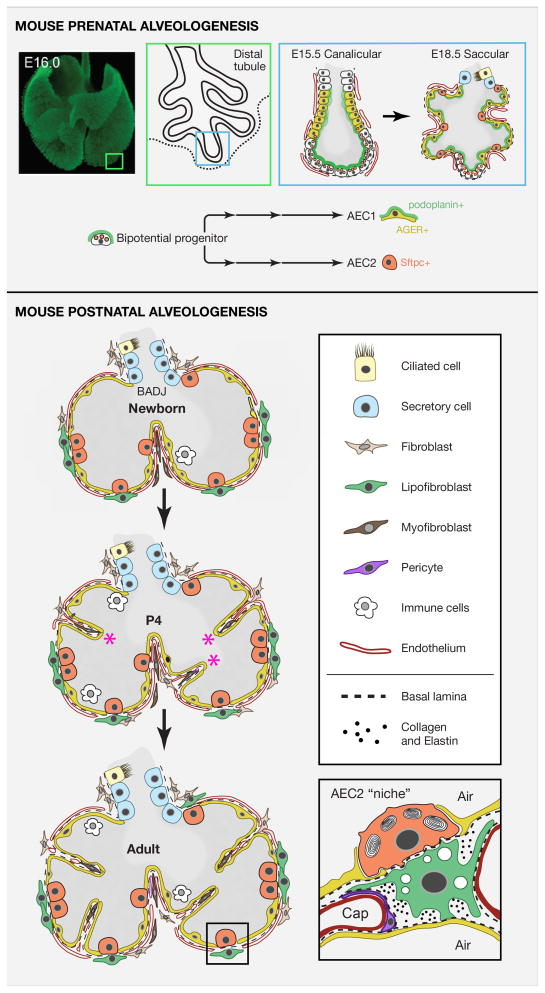
Figure 2. Pre- and postnatal alveologenesis in the mouse.
Upper panel: Schematic of the canalicular and saccular stages indicating that precursors of alveolar epithelial cells are laid down before birth. Image of whole mount E16 lung immunostained for e-cadherin provided by Ross Metzger. A distal canalicular tubule adjacent to the outer mesothelium is shown in greater detail (boxes). Evidence supports a model in which the population of distal tip cells (that are Sox9+ Id2+) is bipotential, expresses markers of both mature AEC2 and AEC1 cells, and gives rise to AEC2s (Sftpc+, orange) and AEC1s (podoplanin+, green and AGER+, yellow) through a series of intermediate progenitors (Chang et al., 2013; Desai et al., 2014; Rawlins et al., 2009a; Treutlein et al., 2014). During the saccular stage the distal tubules begin to bud into multiple sacs that are in close contact with vascular endothelial cells. A few Sox9+ Id2+ putative bipotential progenitors remain at this stage. Lower panel: Schematic representation of postnatal changes in lung architecture. The newborn lung has only primary septa. Around P4 secondary septa (asterisks) develop from crests of tissue containing capillary and stromal cells that migrate in from the walls and subdivide alveoli. The stromal population is incompletely defined but includes pericytes, fibroblasts, lipofibroblasts, and myofibroblasts. The latter are thought to be the main producers of the elastin deposited at the tip of each septum and in the walls, forming an integrated fibroelastic network. Inset (redrawn from Sirianni et al 2003) shows a schematic of the putative “niche” of an AEC2 stem cell. This includes AEC1s, lipofibroblasts, endothelial cells, pericytes and extracellular matrix (dashed line represents basal lamina, dots are collagen and elastin and many other components).
Development of the alveoli does not stop at birth; their number and surface area increases dramatically postnatally and continues for weeks (mice) and months (humans) with the formation of new secondary septa (Figure 1B and 2). The secondary septa arise from ridges on the wall of the alveolar sacs and the capillaries within them undergo remodeling by mechanisms that are poorly understood. The capillary meshwork also increases in size by a process known as intussusceptive microvascular growth (Burri et al., 2004). Only a very thin layer of matrix, a shared basal lamina, eventually separates AEC1s and the capillary endothelial cells. Stromal cells also move into the septa and differentiate into pericytes, lipofibroblasts, myofibroblasts, and other poorly defined lineages. Lipofibroblasts appear to associate closely with AEC2s while myofibroblasts lay down elastin fibers at the tip of the septa and in the stalks. These fibers form an integrated network throughout the alveolar region, providing a flexible and elastic scaffold that is critical for maintaining lung function and for keeping the small terminal airways and alveolar ducts open (Weibel, 2013). Mechanical forces and physical stress are emerging as key regulators of alveolar development, maintenance, repair and regrowth (reviewed in (Hsia et al., 2004; Wirtz and Dobbs, 2000).
Embryonic mesoderm contributes to lung development and promotes epithelial differentiation
The mesodermal component of the lung primordium plays multiple roles in lung development (reviewed in (Herriges and Morrisey, 2014). The cells around the distal tips function as a critical signaling population producing Fgf10 and other signaling molecules that drive outgrowth of the distal buds and branching morphogenesis. This early mesoderm also contains progenitors for specific adult cell populations of the adult lung. A major focus of recent studies is to understand how these mesodermal derivatives contribute to adult lung function and to regeneration and repair. Recent cell lineage tracing experiments have demonstrated that the early mesoderm harbors a cardiopulmonary mesoderm progenitor (CPP) that expresses Wnt2/Gli1/Isl1 and can generate both cardiac and lung mesodermal derivatives including cardiomyocytes, endocardium, pulmonary vascular and airway smooth muscle, and pulmonary Pdgfrβ+ pericyte like cells (Peng et al., 2013). As development proceeds, CPPs lose their ability to contribute to the various lung mesodermal derivatives and by the end of gestation can only make Pdgfrβ+ pericyte-like cells but not smooth muscle or endothelium. This same study also showed that the bulk of the vascular endothelial plexus in the alveolar region is derived from preexisting embryonic endothelium, likely through an angiogenic process (Peng et al., 2013). Since Wnt2+ and Gli1+ cells persist in the adult lung, an important question is whether these cells play a role in regeneration after injury. Gli1 is a down-stream effector of hedgehog signaling and components of this pathway have been associated in GWAS studies with chronic obstructive pulmonary disease (COPD)(Pillai et al., 2009; Wang et al., 2013a; Wilk et al., 2009). Therefore, the hedgehog pathway and Gli1+ cells may play a role in adult lung homeostasis and injury response by controlling mesodermal expansion.
Lineage tracing studies have also shown that the Fgf10 producing cells of the distal lung give rise to multiple mesodermal components in the adult, including airway smooth muscle and alveolar lipofibroblasts (El Agha et al., 2014). There is evidence that Fgf10 is reactivated in parabronchial smooth muscle after injury, including exposure to naphthalene and may promote the reparative process (Volckaert et al., 2011).
Previous work has established a requirement for Pdgfrα expressing cells in the distal embryonic lung for the development of alveolar myofibroblasts (Bostrom et al., 1996). Recent studies have indicated that Pdgfrα is dynamically expressed in alveolar myofiboblasts during regrowth after pneumonectomy (Chen et al., 2012b). Pdgfrα also marks a population of lipofibroblast-like cells that are spatially associated with AEC2 cells and likely form part of the alveolar stem cell niche (Figure 2; (Barkauskas et al., 2013)). Studies such as these have begun to address some of the outstanding questions about the origin, heterogeneity and function of lung mesodermal cells and how they interact with the various epithelial components to establish and maintain normal lung function and contribute to repair.
Applying developmental pathways to derive lung progenitors from pluripotent stem cells
One area of research where developmental studies are having a big impact is in the derivation of lung cells from pluripotent stem cells (PSCs). Several groups have demonstrated that step wise application of signaling factors in a manner that mimics the sequence of events during early anterior foregut and lung development is critical for deriving lung endoderm progenitors from mouse and human PSCs. Key pathways include those downstream of activin/nodal, Wnts, Bmps, and Fgfs (Ghaedi et al., 2013; Huang et al., 2014; Longmire et al., 2012; Mou et al., 2012; Wong et al., 2012). PSC-derived lung endoderm can be used for basic studies of diseases such as cystic fibrosis and potentially for cell-based therapies. This area of research will undoubtedly continue to be an important focus in lung regeneration research.
REGIONAL EPITHELIAL STEM/PROGENITOR CELL POPULATIONS MEDIATING ADULT LUNG HOMEOSTASIS AND REGENERATION
Three interrelated concepts have emerged from recent studies on adult lung reparative cell biology. The first is that depending on the composition and organization of the respiratory epithelium distinct regions of the lung contain different populations of epithelial cells that function as adult stem cells, as defined by their ability to undergo long term self-renewal and give rise to different cell types during homeostatic turnover or cell replacement after injury. The second is that lung cells exhibiting stem/progenitor activity are not necessarily undifferentiated. While Trp63+ basal cells in the pseudostratified mucociliary epithelium are morphologically simple, AEC2 cells in the alveoli and Club cells in mouse bronchioles, both of which function as long-term stem cells, also express genes associated with specialized functions, such as surfactant protein synthesis and secretion and glycoprotein production, respectively. The third concept is that in response to tissue damage, epithelial cells that express markers of one differentiated cell type can, under certain circumstances, change their phenotype and give rise to cells that either transiently or stably express markers characteristic of another cell type. In some cases lineage tracing experiments have shown that this phenotypic switching or plasticity involves a process of “de-differentiation” to a less specialized, multipotent and proliferative intermediate, followed by re-differentiation. In other cases the precise steps involved have not yet been identified and it remains possible that the phenotypic switch is “direct” and does not involve a specific undifferentiated and proliferative intermediate. Phenotypic plasticity is not unique to the lung and de-differentiation or trans-differentiation apparently occur quite frequently in response to adverse events in various tissues (Blanpain and Fuchs, 2014; Fuhrmann et al., 2013). Importantly, the process can be strongly influenced by the particular kind of damage sustained, whether it is acute or chronic, and whether it involves inflammation or immune modulation.
Modern studies of stem cell behavior, including investigation of plasticity in the physiological context of an adult tissue undergoing repair, are heavily dependent on the technique of lineage tracing (Blanpain and Simons, 2013). In the following sections we summarize some of the recent findings about epithelial stem/progenitor cell lineages in different regions of the adult lung, focusing on studies based on rigorous lineage labeling strategies.
Stem and progenitor cells of the pseudostratified mucociliary airway epithelium contribute to repair and regeneration
Most of the airways of the human lung, down to about 1.0 –1.5 mM in diameter are lined by a pseudostratified mucociliary epithelium containing multiciliated, secretory, tuft and neuroendocrine cells, as well as a population of basal cells (BCs) that are tightly attached to the basal lamina through hemidesmosomes containing α6β4 integrin. The height of the luminal cells and properties such as the proportion of goblet and different secretory cells and the density of basal cells, vary along the proximal-distal axis. Underlying the epithelium are blood vessels, smooth muscle, cartilage, stromal fibroblasts and nerves. As shown in Figure 1 and schematically in Figure 3, the normal organization of the mucociliary epithelium is disrupted in common pathological conditions. In the case of mucus hyperplasia there are many more goblet cells than normal, while in squamous metaplasia there are multiple layers of basal cells that give rise to keratinized squamous cells. Both mucus hyperplasia and squamous metaplasia are seen in the condition known as chronic obstructive pulmonary disease (COPD).
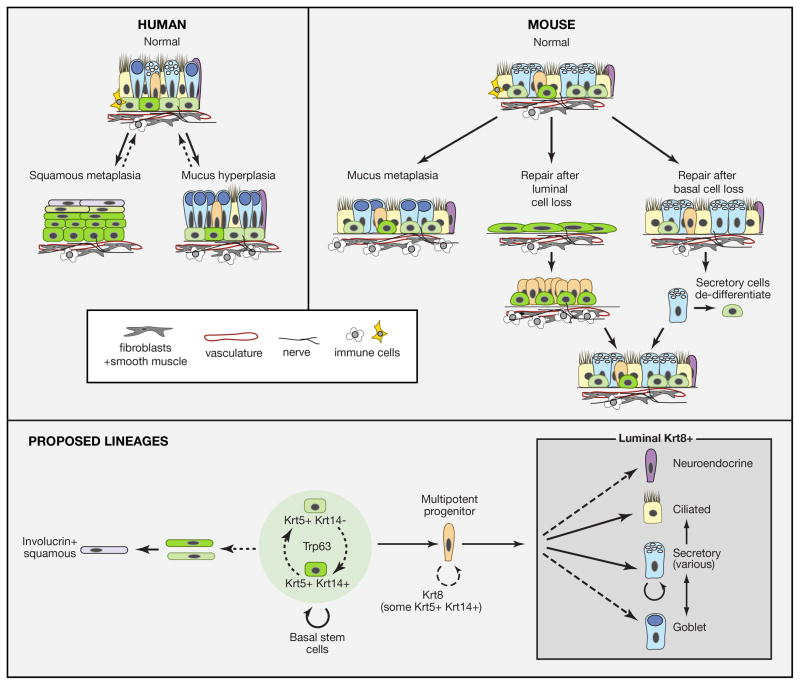
Figure 3. Homeostasis, repair and remodeling of pseudostratified mucociliary airway epithelium with basal cells.
Solid lines represent transitions or lineages that are generally accepted, while dotted lines are speculative lineages or relationships. Curved arrows represent self-renewal. Upper panel: Schematic representation of pseudostratified mucociliary epithelium of the human lung. The density of BCs and height and composition of luminal cells varies along the main axis. A few “intermediate” cells are present which may represent immediate undifferentiated progeny of BCs. In mucus hyperplasia the number of goblet cells increases either by proliferation of existing goblet cells or by differentiation of other secretory cells. In squamous metaplasia, BCs change their behavior so that the pool of proliferative Krt5+ Krt14+ BCs expands and stratifies and upper layers differentiate into keratinized squamous cells. Both conditions may be reversible. Middle panel: Repair and remodeling in the mouse trachea and primary bronchi. Since few goblet cells are normally present in mouse airways, their increase in number in response to immune stimuli is called metaplasia rather than hyperplasia. Genetic lineage tracing has shown that Scgb1a1+ cells are the predominant source of goblet cells in response to allergen exposure (Chen et al., 2009). If luminal cells are killed by SO2, surviving BCs spread and proliferate. They give rise to a population of Krt8+ progenitors that then differentiate into ciliated and secretory cells. There is transient influx of immune cells into the underlying stroma. If basal cells are killed genetically, secretory cells lineage-labeled with a driver for differentiated cell products (Scgb1a1 or Atpv1b) give rise to some of the regenerated BCs that continue to function as stem cells (Tata et al., 2013). Inset shows some of the non-epithelial components of the BC “niche”. In addition to the basal lamina these include fibroblasts, vasculature, and immune cells. Lower panel: Summary of lineage relationships from studies with both mouse and human airways. In both tissues BCs are heterogeneous for expression of Krt14; whether these BCs are interconvertable and/or whether Krt14+ cells have a higher probability of differentiation rather than self-renewal is not known. The existence of an intermediate progenitor cell is inferred from studies on repair. This cell is Krt8+ but may transiently express Krt5/Krt14. BCs and their immediate daughters give rise to ciliated and secretory cells during repair (Rock et al., 2011b). Whether they directly give rise to neuroendocrine cells and goblet cells or whether goblet cells only arise from secretory cells is not known. Secretory cells include Scgb1a1+ Club cells as well as variant Club cells that predominantly express other products (see text). Scgb1a1+ cells can give rise to ciliated cells.
A similar basic organization of pseudostratfied mucociliary epithelium and underlying mesenchyme is found in the mouse trachea (which is about 1.5 mM in diameter), extralobar bronchi and the first 2–3 generation of branches along the intralobar main axial pathway. These regions therefore provide an experimental platform for modeling human airways (Rock et al., 2010) (Figure 3). The BCs of the mouse proximal airways characteristically express Trp63, Ngfr, podoplanin (Pdpn, also known as T1alpha), the alpha 1–3 gal epitope that binds GSIB4 lectin, and cytokeratin (Krt)5 (Paul et al., 2014; Rock et al., 2009; Tata et al., 2013). At steady state about 20% of BCs also express Krt14 (Cole et al., 2010). This cytokeratin is also constitutively expressed in BCs lining the ducts of submucosal glands (that are not considered further here) and is upregulated in most Krt5 BC cells during repair (Hong et al., 2004; Wansleeben et al., 2014). The number and proportion of Krt5 basal cells declines with age in the mouse trachea, along with other significant changes in epithelial architecture and composition of stromal immune cells (Wansleeben et al., 2014). The consensus from in vivo lineage tracing experiments is that BCs are stem cells that self-renew over the long term and give rise to ciliated and secretory luminal cells during postnatal growth, homeostasis and epithelial repair following loss of luminal cells (Figure 3). Different experimental models have been developed to induce this loss in the mouse. For example, exposing mice to toxic gases (SO2, or chlorine) or to detergent kills all proximal luminal cells, while systemic naphthalene kills Club cells in the trachea as well as the bronchioles. There are many questions about BCs that have not yet been fully answered. For example, we do not know whether all Trp63+/Krt5+ BCs are multipotent, with the same reparative capacity, or whether there are subsets that are quiescent (for example Krt5+ cells versus Krt5/Krt14+ cells) or have intrinsically different potentials. These questions await quantitative, single cell clonal analysis under both steady state and different reparative conditions. In addition, we do not know the precise mechanisms by which BCs give rise to ciliated and secretory cells and what environmental signals affect the fate decision. One model, based on studies of the intestinal stem cells of the Drosophila larval midgut (Lucchetta and Ohlstein, 2012; Ohlstein and Spradling, 2007) is that BCs give rise to Trp63−/Krt5+/Krt8+ early progenitors or “intermediate” cells that can proliferate and give rise to either secretory or ciliated cells depending on local signals, including the level of intracellular notch signaling (Guseh et al., 2009; Rock et al., 2011b). Recent studies in the human lung have described a powerful non-invasive lineage tracing methodology for addressing the potential of individual airway progenitor cells to self-renew and differentiate (Teixeira et al., 2013). The results are consistent with a model in which BCs are a population of mutipotent progenitors that self-renew and/or differentiate stochastically to maintain tissue homeostasis. However, they do not rule out the existence of a quiescent stem cell pool that is only activated after injury.
One question of practical relevance for regenerative therapies in the human lung, is whether BCs are the only cell that can efficiently repair the pseudostratified epithelium or whether differentiated cells can become BCs under certain conditions. This question has been addressed using the rodent trachea as a model. Pioneering studies by Randell and colleagues using denuded rat tracheas suggested that GSIB4 lectin negative luminal cells were just as able to regenerate the entire epithelium as lectin+ basal cells (Liu et al., 1994). Subsequent lineage tracing studies showed that secretoglobin 1a1 (Scgb1a1) expressing secretory cells can give rise to Krt5+ BCs after SO2 induced injury but the frequency of conversion was very low, probably because mostly BCs survive this injury (Rawlins et al., 2009b). To overcome this problem, Rajagopal and colleagues used a strategy to specifically kill Krt5+ BCs in the mouse trachea in vivo (Tata et al., 2013). Under these conditions they found that differentiated Scgb1a1+ or Atpv1b1+ lineage labeled secretory cells can undergo dedifferentiation into Trp63+/Krt5+ BCs. These BCs persist over the long term and behave like normal Krt5+ stem cells. An important question raised by all these studies concerns the mechanisms that normally constrain the potential plasticity of secretory cells. Data from the studies by Tata et al using in vitro culture experiments suggest that contact with BCs prevents luminal cells from dedifferentiation but the precise mechanisms driving reprogramming and subsequent stem cell function need further study.
How basal and luminal cells repopulate an airway denuded of epithelium by injury has considerable relevance to proposals to bioengineer replacement lungs or airway segments by seeding cells into de-cellularized lung “scaffolds” (see later section). Evidence from in vivo injury/repair models suggests that if the basal lamina is not completely covered by BCs the underlying stroma proliferates out of control and gives rise to granulation tissue containing fibroblasts and immune cells and blocks the airways (O’Koren et al., 2013). This finding suggests that there is normally a tight interplay between the airway epithelium and the underlying stroma that keeps fibrosis in check. Identifying the factors that mediate this signaling may not only inform strategies for bioengineering replacement parts but also provide insights into respiratory disorders in which fibrosis restricts small airways in the human lung such as bronchiolitis obliterans (Figure 1).
Lung injury models reveal differential regenerative capacities of epithelial cells of the mouse bronchioles
The small intralobar airways of the mouse lung are called bronchioles; they do not have cartilages or a dedicated systemic blood supply and are surrounded by airway smooth muscle and fibroblasts (Figures 1 and 4). The epithelial lining is a simple cuboidal epithelium containing Foxj1+ multiciliated cells and neuroendocrine cells that are usually clustered into neuroendocrine bodies (NEBs). The bronchioles also contain a heterogeneous population of secretory cells that are still not fully defined. The best studied are called Club cells (previously known as Clara cells) and in the mature state have a characteristic domed appearance with vesicles containing the secretoglobin Scgb1a1 (also called CCSP or CC10). There has been recent progress in identifying new markers for Club cells that can be used to follow their development and functional heterogeneity (Guha et al., 2014). Club cells immediately adjacent to NEBs are resistant to naphthalene-induced depletion and characteristically express low levels of Scgb1a1 but high levels of Scgb3a2 and uroplakin 3a (Upka3) (Guha et al., 2012).
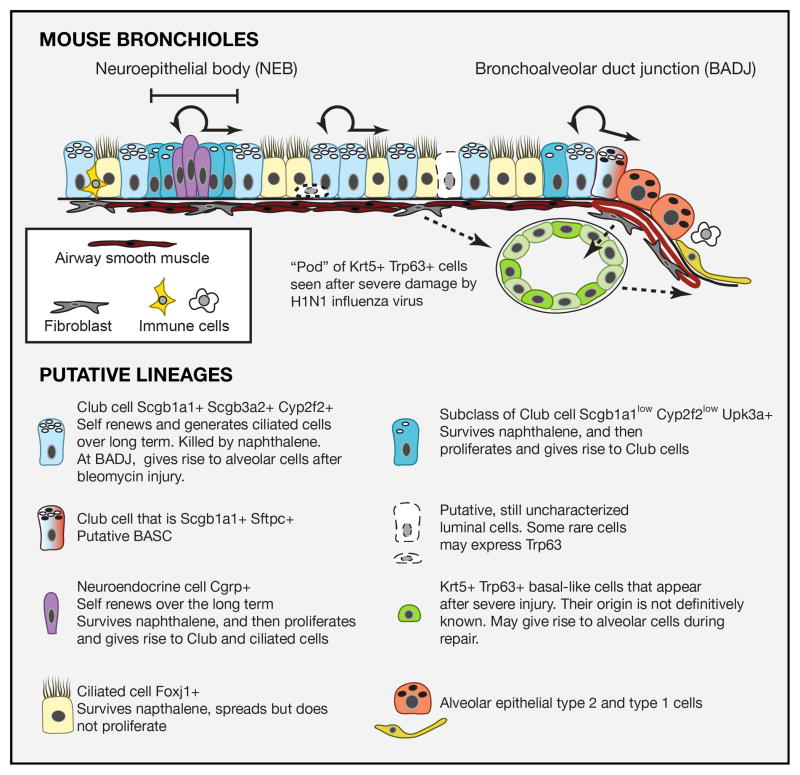
Figure 4. Epithelial cells of mouse bronchioles and the cellular responses to injury.
Schematic representation of different cell types in the mouse bronchiolar epithelium. All cells are likely attached to the basal lamina through integrin alpha6beta4. The full heterogeneity of epithelial cell types is still under investigation and the presence of rare Trp63+ cells is controversial. Shown with dashed arrows are putative cells-of-origin of the Krt5+ Trp63+ basal-like cells present in “pods” in the lungs of mice after infection with H1N1 influenza virus. There is some evidence that these Krt5+ cells contribute to the regeneration of damaged alveoli but more lineage tracing data are required.
Using standard immunohistochemical analysis, the bronchioles of the mouse lung do not appear to contain basal cells, but the presence of rare Trp63+ cells has not been ruled out. The transition from bronchioles to alveolar sacs in the mouse lung is known as the bronchioalveolar duct junction (BADJ). It contains a few cells (<1 per BADJ) that co-express Scgb1a1 and surfactant protein C (Sftpc) proteins and are proposed as putative bronchioalveolar stem cells (BASCs) (Kim et al., 2005) (Figures 3 and 4). It should be noted that the transition between terminal bronchioles and alveoli is quite different between mouse and human lungs (Figure 1)
Turnover of mouse bronchiolar epithelium is normally quite low but lineage tracing studies have established that Scgb1a1+ cells do self-renew and give rise to ciliated cells over the long term (Rawlins et al., 2009b). Thus, the Scgb1a1+ population meets the definition of long-term stem cells, although demonstrating a differentiated phenotype. Moreover, as discussed here and in the next section there is now evidence that the proliferation and phenotype of Scgb1a1+ Club cells can change in response to certain injury/repair models (Figures 4 and 5).
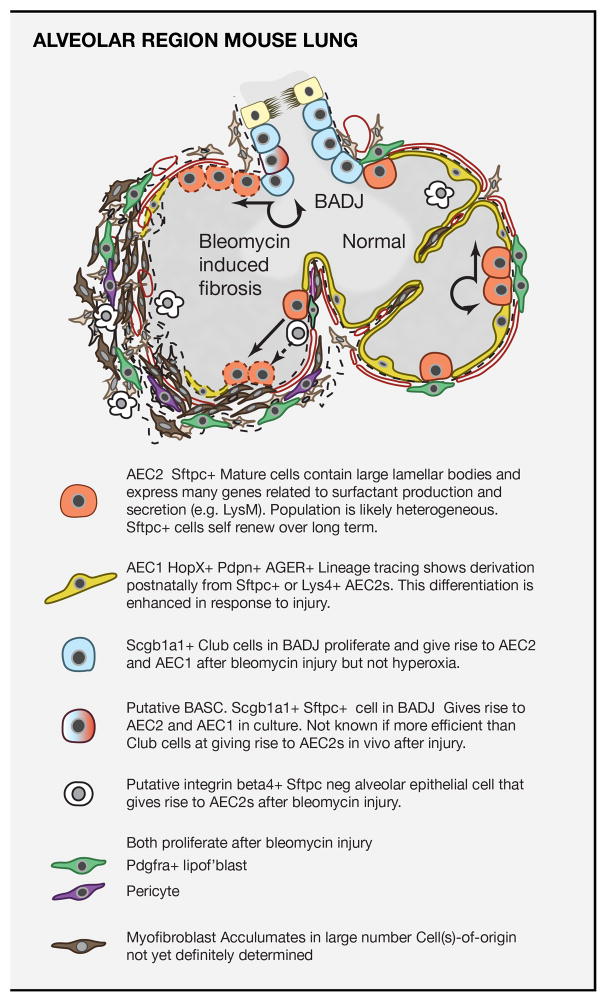
Figure 5. Stem cells of the mouse alveolar region and their role in response to injury.
Upper panel: Schematic of normal mouse alveolar region and changes elicited by exposure to bleomycin. At steady state there is little cell turnover and AEC2s self renew and give rise to AEC1s with low frequency. Bleomycin damages multiple alveolar cell types resulting in the exposure of denunded basal lamina and matrix (dashed lines) and influx of immune cells. Various mesenchymal cells proliferate and give rise to myofibroblasts and large amounts of extracellular matrix. In this model evidence argues against myofibroblasts being derived from epithelial cells and the fibrosis is transient (Rock et al., 2011a). Both AEC2s and Scgb1a1+ cells in the BADJ proliferate and give rise to the majority of the AEC2 and AEC1 cells (dotted cells) in the fibrotic regions (Barkauskas et al., 2013). Epithelial cells in the alveolar region that are Sftpc- integrin beta4+ may also be a source of reparative AEC2s (Chapman et al., 2011). Lower panel: Schematic of different cell types involved in alveolar turnover and repair in the bleomycin injury model. A detailed inventory of markers has been compiled from single cell RNA-seq studies (Treutlein et al., 2014).
Systemic treatment of mice with naphthalene kills Club cells that express the cytochrome Cyp2f2. Ciliated cells spread to cover denuded matrix but do not proliferate and the epithelium is restored by the proliferation of naphthalene resistant Club cells, including those adjacent to NEBs and in the BADJ (Giangreco et al., 2002; Guha et al., 2014). It has been suggested that the NEBs provide a specific niche for reparative Club cells. However, deletion of neuroendocrine cells did not affect the ability of Club cells to regenerate after naphthalene injury (Song et al., 2012). Lineage tracing studies using a Cgrp-CreER allele have provided evidence that differentiated neuroendocrine cells can proliferate after naphthalene-induced loss of Club cells and give rise to lineage labeled Club and ciliated cells. This conclusion is tempered by the fact that the wash-out period between tamoxifen administration and naphthalene treatment was very short, so that in these experiments Club cells up-regulating Cgrp might have been lineage labeled (Song et al., 2012).
The damage caused by naphthalene treatment is relatively specific and appears to be rapidly repaired. However, much more extensive damage is caused in the mouse lung by H1N1 influenza viral infection (Kumar et al., 2011). Although there is clinical evidence for repair of the human lung after acute influenza infection (Toufen et al., 2011) precise mechanisms are still poorly understood. In the mouse it appears that a quite unexpected level of plasticity can be evoked in epithelial cells in response to signals expressed following such viral infection. Immunohistochemistry of mouse lung following infection shows the presence of epithelial “pods” containing Krt5+ Trp63+ basal-like cells both around the bronchioles and within the alveoli in the areas of greatest damage (Kumar et al., 2011) (Figure 3). Lineage tracing experiments using an Scgb1a1-CreER allele have suggested that the “pods” arise from Club cells. However, in these experiments the wash-out period between administration of Tamoxifen and viral infection was again quite short (7 days). Consequently, an Scgb1a1 negative cell activated by the injury might have been labeled if it transiently up-regulated Scgb1a1 early during the repair process (Zheng et al., 2014). Alternatively, the pods may arise from as yet uncharacterized progenitor populations in the bronchioles and/or from AEC2 cells in the alveoli (Xian and McKeon, 2012). Krt5+ “pods” have been identified in mouse lungs following bleomycin injury (Zheng et al., 2014) but, again, their origin is not yet resolved. Given the high relevance of influenza infection to human health this is an important area of research. More information is needed about the extent to which the Krt5 reparative cells do in fact contribute to alveolar repair, how this is achieved, and whether the same cell type(s) plays a role in the human lung.
The importance of injury models for studying stem and progenitor cells of the alveolar compartment
The major epithelial cell types of the gas exchange region are cuboidal AEC2s specialized for surfactant protein production and secretion, and flat, highly extended AEC1s, specialized for gas exchange (Figure 5). Cell turnover is normally very low, but numerous studies have shown changes in cell behavior, including proliferation, normal differentiation and phenotypic plasticity, in response to a variety of injury models. These include exposure to nitric oxide, high levels of oxygen (hyperoxia), the chemotherapy drug bleomycin, cigarette smoke, irradiation, and viral infection.
Alveolar epithelial type 2 cells (AEC2)
Studies more than 40 years ago with H3 thymidine labeling showed that AEC2s in adult monkeys and rats proliferate in response to injury by hyperoxia and nitric oxide and give rise to AEC1s (Evans et al., 1975; Kaplan et al., 1969). This capacity for self-renewal and differentiation of adult AEC2s has been confirmed by recent in vivo genetic lineage tracing studies using Cre recombinase driven by genes associated with differentiated functions such as Sftpc and Lyz2 (LysM) (Barkauskas et al., 2013; Desai et al., 2014). In steady state, there is relatively little clonal expansion of individual AEC2s and very little differentiation into AEC1s. After injury of the alveolar region by bleomycin (Rock et al., 2011a) and by hyperoxia (Desai et al., 2014), the rate of differentiation of lineage labeled AEC2s into AEC1s is much higher. To follow this regenerative capacity in the absence of confounding fibrosis a new model of lung injury was developed in which about 50% of the AEC2 cells are specifically killed by induced expression of diphtheria toxin. Lineage tracing, coupled with new imaging techniques, showed that surviving AEC2 cells undergo clonal expansion, with daughter cells dispersing among neighboring alveoli, most likely by active migration (Barkauskas et al., 2013). The rate of differentiation into AEC1s is low, presumably because this cell population is not damaged.
These observations raise several interesting questions. For example, is there heterogeneity in the AEC2 population or do they all have the same capacity for self-renewal and differentiation? Some evidence for heterogeneity comes from the fact that about 10% of adult AEC2s are lineage labeled in vivo using an _Scgb1a1-_CreER “knock-in“ allele and give rise to adenocarcinomas after Kras activation(Xu et al., 2012). In addition a recent report suggests that an Sftpc−/integrin beta 4+ cell type exists in the alveoli that can generate Sftpc+ AEC2 and AEC1 cells after injury (Chapman et al., 2011). Another important question is the nature of the niche in which AEC2s reside and whether it produce specific survival and homing signals after loss of AEC2s. To address this question, assays have been developed for clonal expansion of lineage labeled AEC2s in 3D organoid culture. In this assay, AEC2s only proliferate in the presence of Pdgfrα+ stromal cells and they form “alveolopheres” containing lineage labeled AEC1s expressing a number of markers, including podoplanin, Hopx, AGER, and Aquaporin 5 (Barkauskas et al., 2013). Similar assays are being used to identify factors made by other mesodermal lineages such as endothelial cells that together with the fibroblasts likely comprise the alveolar stem cell niche (Lee et al., 2014). In turn, understanding how AEC2s regulate the behavior of the niche is also important and recent studies suggested a role for BMP4 and thrombospondin in the reciprocal interaction between epithelial progenitors and endothelial cells (Lee et al., 2014).
It is has been hypothesized that AEC2s that are stressed due to cellular damage or aging are unable to undergo self-renewal and generation of AEC1s but instead signal fibroblastic proliferation, or at least fail to keep stromal proliferation in check. In the human, stress may be caused by genetic mutations resulting in unfolded surfactant proteins. In the disease dyskeratosis congenita, environmental stimuli such as smoking or dust combined with a relative stem cell deficiency due to lack of telomerase components results in pulmonary fibrosis (Noble et al., 2012). Identification and characterization of the signals generated by AEC2s that keep stromal populations in a quiescent state will be important for future development of therapies for fibroproliferative diseases such as idiopathic pulmonary fibrosis and bronchiolitis obliterans.
Alveolar epithelial type 1 cells (AEC1)
Previous studies have suggested that AEC1s normally have a limited proliferative capacity in vivo and can modulate their identity when cultured in vitro (Danto et al., 1995; Evans et al., 1973). In the future, novel tools including inducible Cre lines made using genes such as Hopx and AGER expressed in mature AEC1s (Barkauskas et al., 2013; Takeda et al., 2013; Treutlein et al., 2014) may allow the ability of these cells to proliferate and give rise to AEC2 cells in specific injury models to be addressed unambiguously. The potential role of changes in AEC1s, AEC2s and mesenchymal cells in the development of smoking- and age-related emphysema, in which there is a decline in the number of AEC2s and simplification of alveoli (Figure 1), is also an important avenue of inquiry.
REMODELING AND REGROWTH OF THE ALVEOLAR REGION AFTER PNEUMONECTOMY AND BLEOMYCIN INJURY
Upon reaching adulthood, cessation of rib cage expansion coincides with the cessation of lung growth in large mammals. Thereafter, re-initiation of lung growth/regeneration requires changes in mechanical signals and the availability of space for the lung to grow. This can be provided experimentally by the process of unilateral pneumonectomy (PNX)(Dane et al., 2013; Ravikumar et al., 2013; Ravikumar et al., 2014). In this procedure in mice the single left lobe is removed, leaving the four right lobes intact. This results in the regrowth or “re-alveolarization” of the remaining lungs. New lobes are not added but there is a dramatic increase in alveolar number through addition of new septa and a restoration in respiratory capacity in the remaining lung, within 2–3 weeks for rodents. New alveolar tissue is preferentially laid down at the periphery of the lung (Foster et al., 2002; Massaro and Massaro, 1993) where mechanical strain of the septa is accentuated due to a relative lack of bronchovascular support (Yilmaz et al., 2011). Evidence exists for proliferation and changes in the behavior of multiple cell lineages in the PNX model, including bronchiolar cells, AEC2s, endothelial cells and Pdgfrα+ stromal cells (Chen et al., 2012b; Eisenhauer et al., 2013; Hoffman et al., 2010; Hsia, 2004; Thane et al., 2014; Voswinckel et al., 2004).
Evidence points to the increase in proliferation following PNX being regulated by a combination of signaling pathways, matrix composition and remodeling, especially related to elastin, and mechanical forces. Early studies suggested that EGF and FGF signaling promote post-PNX alveolar regeneration (Kaza et al., 2002; Kaza et al., 2000) and this has been supported by later work focusing on the role of pulmonary capillary endothelial (PCE) cells in producing paracrine growth factors (Ding et al., 2011). After PNX, there is increased proliferation of PCEs, and their activation of VEGFR-2 and upregulation of FGFR1 and MMP-14 are necessary for the restoration of lung mass and function. Despite these intriguing observations, the precise cellular mechanisms that result in the dramatic increase in alveolar number and lung capacity remain unclear. Detailed anatomical studies, including scanning electron microscopy of vascular casts, suggest that alveolar number increases by the ingrowth of crests of tissue containing capillaries and stromal cells from the walls of preexisting alveoli, a process that mimics normal postnatal development (Figure 2) (Ackermann et al., 2013). Elucidation of the mechanisms that drive regrowth, and how they are attenuated as lung capacity is restored, may suggest potential therapeutic interventions for when repair does not occur properly. However, it will first be important to translate findings with small rodent models to larger animal models with different mechanical and growth conditions in their lungs (Hsia, 2004; Thane et al., 2014).
An injury model that is widely used in research is exposing mice to intratracheal bleomycin, a chemotherapy drug that causes damage to multiple cell types in the alveoli, including AEC1 and endothelial cells. One of the consequences of bleomycin exposure is a transient disruption of alveolar architecture and fibrosis, together with the appearance many myofibroblasts and AEC2s with abnormal morphology (Figure 5). Lineage tracing studies in this injury model have shown two main sources of reparative AEC2s and AEC1s. Some of them are derived from Sftpc+ AEC2s in the alveoli, while others are derived from differentiated Scgb1a1+ Club cells in the terminal bronchioles (Barkauskas et al., 2013; Chen et al., 2012a; Rock et al., 2011a; Tropea et al., 2012). Dual positive Scgb1a1+/Sftpc+ cells that reside in the BADJ, and which have been termed bronchoalveolar stem cells or BASCs, also contribute to this phenotypic conversion. However, whether they are more efficient at this than neighboring Club cells will require cell-specific lineage tracing tools. Significantly, Club cells do not give rise to AEC2 and AEC1 cells following injury to the alveoli by hyperoxia. Thus, while Club cells can be considered a facultative stem cell population capable of extensive expansion and phenotypic flexibility during regeneration, this does not occur in all injury/repair models.
PATHWAYS THAT PROMOTE LUNG REPAIR AND REGENERATION
A major goal in the field of lung repair is to define the molecular pathways that regulate the activation, expansion, and differentiation of stem and progenitor lineages in response to injury and repair. One strategy is to focus on interactions between epithelial and mesenchymal cells in the reparative niche since cross-talk between these populations is known to be an essential process for proper development of the lung. A recent example of this approach is a study showing that thrombospondin-1, expressed by lung endothelial cells, controls the differentiation of a subpopulation of Sca1+ self-renewing lung epithelial cells (Lee et al., 2014). Bmp4 activated expression of thrombospondin-1 in lung endothelial cells, which in turn regulated the differentiation of Sca1+ lung epithelial cells. However, the source of Bmp4 in the adult lung in vivo was not determined in these studies (Lee et al., 2014).
Other signaling pathways known to play important roles in stem cell self-renewal and differentiation such as Wnt and Notch also play key roles in lung repair and regeneration. Wnt signaling is an essential regulator of early lung endoderm specification and development and has been implicated in regulating regenerative responses. Using Wnt reporter lines, several groups have demonstrated activation of canonical Wnt signaling in various compartments in the lung undergoing active regrowth and regeneration (Al Alam et al., 2011; Aumiller et al., 2013; Flozak et al., 2010; Hashimoto et al., 2012; Zhang et al., 2008). In the airway epithelium, Wnt signaling is activated upon secretory cell depletion by naphthalene treatment. Activation of Wnt signaling through loss of the critical transcription factor Gata6 leads to expansion of the putative bronchioalveolar stem cell population after naphthalene injury (Zhang et al., 2008). However, postnatal deletion of β-catenin (Ctnnb1) in this model did not inhibit secretory cell regeneration, suggesting that canonical Wnt signaling is not essential in this model of lung regeneration (Zemke et al., 2009).
Wnt signaling is also pro-fibrotic and increased Wnt signaling has been demonstrated in idiopathic fibrosis lesions (Chilosi et al., 2003). Loss of Ctnnb1 in postnatal alveolar epithelium leads to increased fibrosis and alveolar epithelial cell death (Tanjore et al., 2013). Moreover, blocking Wnt signaling pharmacologically can reduce fibrosis caused by bleomycin treatment in mice, although whether this is an effect on alveolar epithelial or meseodermal lineages is unknown (Henderson et al., 2010). Thus, the ability of Wnt signaling to promote proper repair and regeneration after injury is context dependent and chronic activation could lead to increased fibrosis.
The role for Notch signaling in promoting the secretory cell phenotype over the ciliated cell phenotype during lung endoderm development (Guseh et al., 2009; Tsao et al., 2009), is recapitulated in the response of BCs after airway injury. Notch signaling is essential for BC self-renewal and differentiation of these cells into the secretory cell lineage after SO2 mediated airway epithelial injury. Increased Notch activation can expand the secretory lineage at the expense of the ciliated lineage (Rock et al., 2011a; Xing et al., 2012). In recent studies it has been found that reactive oxygen species (ROS) activates Notch signaling by activating Nrf2 (Paul et al., 2014). The ROS-Notch pathway is important for BC self-renewal through regulation of cell proliferation, which may be critical for maintaining the correct number of BCs in the upper airways of mouse and human lungs.
Histone acetylation and deacetylation activities are also altered in lung diseases such as asthma and COPD. Asthmatic bronchial epithelium displays increased HAT activity and decreased HDAC activity (Gunawardhana et al., 2014). Corticosteroid treatment of asthma induces acetylation and activation of anti-inflammatory genes, and also recruitment of HDAC2 complexes to deacetylate and silence pro-inflammatory genes (Ito et al., 2002). COPD patient biopsies show a correlation between disease progression and loss of HDAC2 expression and activity, but lower HDAC activity is resistant to anti-inflammatory steroid therapy (Ito et al., 2005). In mice, genetic deletion of HDAC1/2 in secretory Club cells prevented normal epithelial repair after naphthalene injury (Wang et al., 2013b). Loss of HDAC1/2 in the secretory epithelium lead to the induction of tumor suppressors Rb1, p21/Cdkn1a, and p16/Ink4a after injury, which resulted in reduced proliferation and poor epithelial regeneration (Wang et al., 2013b). This loss in regeneration was persistent, indicating that HDAC1/2 are required for regeneration of secretory epithelium after naphthalene induced depletion in the lung. HDAC function may also be important for regulating the proper balance of AEC1 and AEC2 cells during development and regeneration. Hopx, expressed by AEC1s and by alveolar progenitors, recruits HDAC2 to negatively regulate AEC2-specific gene expression (Yin et al., 2006).
HDACs are also known to bind to non-histone proteins. The forkhead transcription factors Foxp1/2/4 are expressed at high levels in the developing lung epithelium (Lu et al., 2002; Shu et al., 2007). The interaction between Foxp1/2 and HDAC activity plays an important role in lung injury and regeneration, as demonstrated by the resistance of Foxp1/HDAC2 double heterozygous animals to hyperoxic injury mediated by the regulation of the cytoprotective cytokine IL-6 (Chokas et al., 2010). The combined loss of Foxp1/4 in the postnatal Scgb1a1+ secretory lineage results in spontaneous differentiation of Scgb1a1+ cells into Muc5a/c+ goblet cells (Li et al., 2012). Importantly, this abnormal secretory epithelium lacking Foxp1/4 is unable to regenerate after naphthalene induced injury suggesting that acquisition of a goblet like phenotype impairs airway secretory cell regeneration (Li et al., 2012).
Developmental studies in the lung have revealed critical roles for multiple species of non-coding RNAs, including miRNAs and long non-coding RNAs (lncRNAs), in epithelial branching and differentiation. Two miRNA clusters play important roles in lung epithelial endoderm proliferation and differentiation. miR17-92 and miR302-367 are both highly expressed during early lung endoderm development but are significantly down-regulated or extinguished by birth (Tian et al., 2011; Ventura et al., 2008). Several of the miRNAs in these two clusters share common seed sequences. Over-expression of these miRNA clusters results in increased lung epithelial proliferation but inhibition of differentiation with increased expression of progenitor markers (Lu et al., 2007; Tian et al., 2011). These data suggest that these miRNAs promote the early lung progenitor phenotype that is characterized by a highly proliferative, undifferentiated state. Since miRNAs can be used as small molecule therapeutics, such capabilities could be harnessed to promote lung regeneration via therapeutic use of miRNA mimics or antimiRs. A recent study identified hundreds of lncRNAs expressed in the developing and postnatal lung (Herriges et al., 2014). A significant subset of these lncRNAs is located near protein coding genes including important transcription factors such as Nkx2.1 and Foxf1. One of these lncRNAs, called NANCI, regulates Nkx2.1 and is essential for its expression as well as targets of Nkx2.1 function including Sftpc and Scgb1a1. Given the important role for Nkx2.1 in lung development and postnatal homeostasis, lncRNAs such as NANCI likely play a similarly important role in postnatal lung homeostasis and repair.
IMPORTANCE OF THE IMMUNE RESPONSE TO LUNG INJURY REPAIR
The lung contains an important population of mesodermal cells that arise from the hematopoeitic lineage but reside for significant periods within the lung parenchyma and within alveoli. Significant evidence is emerging for a complex orchestration by these cells during repair and regeneration. Recent live-imaging approaches have highlighted resident populations of neutrophils and monocytes at sites of alveolar injury and highly localized damage in response to either ischemia reperfusion or response to TLR signaling (Kreisel et al., 2010; Looney et al., 2011). While immediate damage is likely a product of neutrophil extravasation and perhaps myeloid-mediated breakdown of alveolar epithelium, the immune response is also likely protective. For example, alveolar CD11c positive cells (macrophages and dendritic cells) utilize Connexin 43-mediated tight junctions to communicate with the epithelium and participate in calcium waves that propagate across damaged epithelium. Interactions of some CD11c cells in this way are apparently protective as CD11c-driven deletion of Cx43 resulted in augmented damage in response to TLR signals (Westphalen et al., 2014). It remains to be determined whether myeloid cells with a more ‘M2’ phenotype associated with TGFβ and IL10 production have specific roles in injury/repair and indeed whether macrophage function in the lung falls under the control of the adaptive immune response. Early data using mice that are partially depleted for regulatory T cells indicated that these cells regulated the resolution of lung injury but the mechanism and extent of this remains unclear (D’Alessio et al., 2009)
BIOENGINEERING LUNG TISSUE
The current clinical approach for replacement of diseased lung tissue is allogeneic lung transplantation. However, this procedure remains limited due to a relative shortage of donor organs, immunologic rejection by the recipient, and complications due to intense immunosuppression necessary to avoid rejection (Lau et al., 2004). In the United States, 5-year patient survival following lung transplantation is approximately 50% compared to about 70% for liver (Wang et al., 2014).
Engineered Tracheal Replacements
Engineered airways (trachea and bronchi) have been in use for over a decade, the first being designed to repair a tracheobronchial anastomosis following surgery for malignancy (Macchiarini et al., 2004). Since this initial report, fully-engineered tracheal segments have been made using cadaveric donor decellularized matrices, upon which recipient-derived cells were cultured to generate an autologous airway graft to repair tracheobronchial malacia (Macchiarini et al., 2008). Synthetic scaffolds have also been explored for use as tracheal replacements. Clinical trials of solid prostheses have included materials such as stainless steel (Cotton et al., 1952), steel coil (Beattie et al., 1956), silicone (Neville et al., 1990), polyethylene (Clagett et al., 1952), Teflon (Ekestrom and Carlens, 1959), and hydroxylapatite (Hirano et al., 1989). However, most solid prostheses eventually fail to become well integrated with the surrounding tissue and cause problems with infection, dislodgement, migration, and obstruction with granulation tissue (Grillo, 2002).
Whole Lung Engineering
More recently, whole-lung engineering has been attempted, albeit in animal models only (Ott et al., 2010; Petersen et al., 2010). These approaches make use of whole-lung decellularization strategies (Price et al., 2010) followed by reseeding airways and vessels with primary epithelial and vascular endothelial cells from syngenic animals, respectively. Following culture within a bioreactor, the organs were re-implanted into syngeneic recipients, and demonstrated efficient gas exchange (Ott et al., 2010; Petersen et al., 2010). However, the lungs ultimately fail after several hours to days due to a combination of intravascular coagulation (likely due to incomplete endothelialization of the decellularized vasculature) and defects in barrier function leading to exudation of fluid into the airways.
More recently, strategies for decellularizing whole lungs from pigs, non-human primates (Bonvillain et al., 2013) and humans (Booth et al., 2012) have been established, that pave the way for recellularization studies (Gilpin et al., 2013; Nichols et al., 2013; Wagner et al., 2014). Although these scaffolds provide anatomic access to the airway and vascular compartments, one key challenge will be delivery of mesenchymal populations to the interstitium and particularly the delicate septa. Regionally specific mesenchymal populations, coupled with distinct extracellular matrix cues (Hinenoya et al., 2008; Sannes, 1984) may significantly enhance the outcomes of epithelial repopulation of decellularized matrices. However, the complexity of human lung will make this a daunting task for many years to come.
CONCLUSIONS
Much progress has been made in recent years in defining the cell lineages that contribute towards lung repair and regeneration. The focus of most of this work has been on the epithelium, given its essential function in the lung. However, other cellular constituents, including the lymphatic and vascular systems as well as the immune system, almost certainly play important roles in repair and regeneration, and determining their contribution is an important area of future research.
Recent evidence suggests that lung epithelial lineages that undergo long term self-renewal and differentiation are themselves functionally differentiated cells. Examples are Scgb1a1+ Club cells and Sftpc+ AEC2s. Moreover, these and other epithelial cells can undergo phenotypic switches in response to tissue damage and the response can vary according to the type and severity of injury. These concepts have had an important impact on our understanding of stem/progenitor biology, not only in the lung but in other tissues as well (Blanpain and Fuchs, 2014). How this plasticity is regulated is an exciting area of inquiry.
The identification of stem/progenitor lineages and activity in the adult lung has proceeded much faster than our understanding of the molecular pathways that regulate their cell behavior. The concept that developmental pathways are reactivated and play important roles in lung repair and regeneration requires additional testing, especially in more physiologically or clinically relevant models such as influenza injury. These studies will require additional information on how structures are formed in the lung, in particular the process of alveologenesis, which is still poorly understood. This is a topic that will greatly benefit from additional research into the development and maturation of the lung. Importantly, the advent of new ex vivo models of lung stem cell activity such as tracheospheres and alveolospheres should allow for testing of factors that can promote either stem/progenitor self-renewal or differentiation.
New techniques such as a functional engraftment assay are also critical for testing the true ability of different stem/progenitor lineages that have been and will continue to be identified in the lung. This will entail a better understanding of the niches that lung stem/progenitor cells reside in, which will include defining the role of extracellular matrix and an improved understanding of cell-cell signaling mechanisms important for regional cell behavior after injury or in disease. All of these outstanding issues will require a renewed commitment to basic science as well as the ongoing push to better understand how animal models can be used to understand human lung disease and regeneration. Much has been gained from an investment into basic lung development and there remains much still to be discovered. Only by combining the strength of such basic studies along with exploration of how the human lung responds to injury and insult, can the field develop new strategies and therapies for combating human lung disease.
Acknowledgments
The authors apologize for the omission of any references due to the space constraints of this review and wish to thank members of their laboratories for input and helpful criticism of this review. The authors wish to acknowledge the important support from the National Institutes of Health including the Lung Repair and Regeneration Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann M, Houdek JP, Gibney BC, Ysasi A, Wagner W, Belle J, Schittny JC, Enzmann F, Tsuda A, Mentzer SJ, et al. Sprouting and intussusceptive angiogenesis in postpneumonectomy lung growth: mechanisms of alveolar neovascularization. Angiogenesis. 2013 doi: 10.1007/s10456-013-9399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Alam D, Green M, Tabatabai Irani R, Parsa S, Danopoulos S, Sala FG, Branch J, El Agha E, Tiozzo C, Voswinckel R, et al. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS One. 2011;6:e23139. doi: 10.1371/journal.pone.0023139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanis DM, Chang DR, Akiyama H, Krasnow MA, Chen J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat Commun. 2014;5:3923. doi: 10.1038/ncomms4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller V, Balsara N, Wilhelm J, Gunther A, Konigshoff M. WNT/beta-catenin signaling induces IL-1beta expression by alveolar epithelial cells in pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2013;49:96–104. doi: 10.1165/rcmb.2012-0524OC. [DOI] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. The Journal of clinical investigation. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EJ, Jr, Blades B, Keshishian JM. Tracheal reconstruction. The Journal of thoracic surgery. 1956;32:707–725. discussion, 725–707. [PubMed] [Google Scholar]
- Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol. 2011;187:2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science (New York, NY) 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Simons BD. Unravelling stem cell dynamics by lineage tracing. Nature reviews Molecular cell biology. 2013;14:489–502. doi: 10.1038/nrm3625. [DOI] [PubMed] [Google Scholar]
- Bonvillain RW, Scarritt ME, Pashos NC, Mayeux JP, Meshberger CL, Betancourt AM, Sullivan DE, Bunnell BA. Nonhuman primate lung decellularization and recellularization using a specialized large-organ bioreactor. Journal of visualized experiments : JoVE. 2013:e50825. doi: 10.3791/50825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Developmental dynamics : an official publication of the American Association of Anatomists. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Whitsett JA. Resident cellular components of the lung: developmental aspects. Proceedings of the American Thoracic Society. 2008;5:767–771. doi: 10.1513/pats.200803-026HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD, Chen J. Lung epithelial branching program antagonizes alveolar differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18042–18051. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. The Journal of clinical investigation. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. The Journal of clinical investigation. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem cells (Dayton, Ohio) 2012a;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor alpha expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol. 2012b;47:517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. The American journal of pathology. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokas AL, Trivedi CM, Lu MM, Tucker PW, Li S, Epstein JA, Morrisey EE. Foxp1/2/4-NuRD interactions regulate gene expression and epithelial injury response in the lung via regulation of interleukin-6. J Biol Chem. 2010;285:13304–13313. doi: 10.1074/jbc.M109.088468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett OT, Moersch HJ, Grindlay JH. Intrathoracic tracheal tumors: development of surgical technics for their removal. Annals of surgery. 1952;136:520–532. doi: 10.1097/00000658-195209000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal Basal cells: a facultative progenitor cell pool. The American journal of pathology. 2010;177:362–376. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton BH, Hills B, Penido JR. Resection of the trachea for carcinoma; report of two cases. The Journal of thoracic surgery. 1952;24:231–245. [PubMed] [Google Scholar]
- D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. The Journal of clinical investigation. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane DM, Yilmaz C, Estrera AS, Hsia CC. Separating in vivo mechanical stimuli for postpneumonectomy compensation: physiological assessment. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 2013;114:99–106. doi: 10.1152/japplphysiol.01213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 1995;12:497–502. doi: 10.1165/ajrcmb.12.5.7742013. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014 doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Ai X, Fine A. Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development. 2013;140:4398–4406. doi: 10.1242/dev.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer P, Earle B, Loi R, Sueblinvong V, Goodwin M, Allen GB, Lundblad L, Mazan MR, Hoffman AM, Weiss DJ. Endogenous distal airway progenitor cells, lung mechanics, and disproportionate lobar growth following long-term postpneumonectomy in mice. Stem cells (Dayton, Ohio) 2013;31:1330–1339. doi: 10.1002/stem.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekestrom S, Carlens E. Teflon prosthesis in tracheal defects in man. Acta chirurgica Scandinavica Supplementum Suppl. 1959;245:71–75. [PubMed] [Google Scholar]
- El Agha E, Herold S, Alam DA, Quantius J, Mackenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141:296–306. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epaud R, Aubey F, Xu J, Chaker Z, Clemessy M, Dautin A, Ahamed K, Bonora M, Hoyeau N, Flejou JF, et al. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One. 2012;7:e48071. doi: 10.1371/journal.pone.0048071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. The American journal of pathology. 1973;70:175–198. [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Experimental and molecular pathology. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, Mutlu GM, Budinger GR, Gottardi CJ. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. The Journal of biological chemistry. 2010;285:3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Yan X, Bellotto DJ, Moe OW, Hagler HK, Estrera AS, Hsia CCW. Expression of epidermal growth factor and surfactant proteins during postnatal and compensatory lung growth. American Journal of Physiology Lung Cell and Molecular Physiology. 2002;283:L981–990. doi: 10.1152/ajplung.00053.2002. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Experimental eye research. 2013 doi: 10.1016/j.exer.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. The Journal of clinical investigation. 2013;123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. The American journal of pathology. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. The Journal of Heart and Lung Transplantation. 2013 doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Grillo HC. Tracheal replacement: a critical review. The Annals of thoracic surgery. 2002;73:1995–2004. doi: 10.1016/s0003-4975(02)03564-6. [DOI] [PubMed] [Google Scholar]
- Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J, Li G, Dickel L, Johnson JE, Kimura S, et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12592–12597. doi: 10.1073/pnas.1204710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A, Vasconcelos M, Zhao R, Gower AC, Rajagopal J, Cardoso WV. Analysis of Notch signaling-dependent gene expression in developing airways reveals diversity of Clara cells. PLoS One. 2014;9:e88848. doi: 10.1371/journal.pone.0088848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardhana LP, Gibson PG, Simpson JL, Powell H, Baines KJ. Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clin Exp Allergy. 2014;44:47–57. doi: 10.1111/cea.12168. [DOI] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Chen H, Que J, Brockway BL, Drake JA, Snyder JC, Randell SH, Stripp BR. beta-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. Journal of Cell Science. 2012;125:932–942. doi: 10.1242/jcs.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges JC, Yi L, Hines EA, Harvey JF, Xu G, Gray PA, Ma Q, Sun X. Genome-scale study of transcription factor expression in the branching mouse lung. Developmental dynamics : an official publication of the American Association of Anatomists. 2012;241:1432–1453. doi: 10.1002/dvdy.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges MJ, Swarr DT, Morley MP, Rathi KS, Peng T, Stewart KM, Morrisey EE. Long noncoding RNAs are spatially correlated with transcription factors and regulate lung development. Genes Dev. 2014;28:1363–1379. doi: 10.1101/gad.238782.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinenoya N, Naito I, Momota R, Sado Y, Kumagishi K, Ninomiya Y, Ohtsuka A. Type IV collagen alpha chains of the basement membrane in the rat bronchioalveolar transitional segment. Arch Histol Cytol. 2008:185–194. doi: 10.1679/aohc.71.185. [DOI] [PubMed] [Google Scholar]
- Hirano M, Yoshida T, Sakaguchi S. Hydroxylapatite for laryngotracheal framework reconstruction. The Annals of otology, rhinology, and laryngology. 1989;98:713–717. doi: 10.1177/000348948909800910. [DOI] [PubMed] [Google Scholar]
- Hoffman AM, Shifren A, Mazan MR, Gruntman AM, Lascola KM, Nolen-Walston RD, Kim CF, Tsai L, Pierce RA, Mecham RP, et al. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. American journal of physiology Lung cellular and molecular physiology. 2010;298:L158–168. doi: 10.1152/ajplung.90594.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. The American journal of pathology. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia CC. Signals and mechanisms of compensatory lung growth. Journal of applied physiology. 2004;97:1992–1998. doi: 10.1152/japplphysiol.00530.2004. [DOI] [PubMed] [Google Scholar]
- Hsia CCW, Berberich MA, Driscoll B, Laubach VE, Lillehei CW, Massaro DJ, Perkett EA, Pierce RA, Rannels DE, Ryan RM, et al. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170:319–343. doi: 10.1164/rccm.200209-1062ST. [DOI] [PubMed] [Google Scholar]
- Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nature biotechnology. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Caramori G, Lim S, Oates T, Chung KF, Barnes PJ, Adcock IM. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med. 2002;166:392–396. doi: 10.1164/rccm.2110060. [DOI] [PubMed] [Google Scholar]
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. The New England journal of medicine. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Kaplan HP, Robinson FR, Kapanci Y, Weibel ER. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. I. Clinical and light microscopic studies. Laboratory investigation; a journal of technical methods and pathology. 1969;20:94–100. [PubMed] [Google Scholar]
- Kaza AK, Kron IL, Leuwerke SM, Tribble CG, Laubach VE. Keratinocyte growth factor enhances post-pneumonectomy lung growth by alveolar proliferation. Circulation. 2002;106:I120–124. [PubMed] [Google Scholar]
- Kaza AK, Laubach VE, Kern JA, Long SM, Fiser SM, Tepper JA, Nguyen RP, Shockey KS, Tribble CG, Kron IL. Epidermal growth factor augments postpneumonectomy lung growth. J Thorac Cardiovasc Surg. 2000;120:916–921. doi: 10.1067/mtc.2000.110460. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CL, Patterson GA, Palmer SM. Critical care aspects of lung transplantation. J Intensive Care Med. 2004;19:83–104. doi: 10.1177/0885066603261509. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung Stem Cell Differentiation in Mice Directed by Endothelial Cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang Y, Zhang Y, Lu MM, DeMayo FJ, Dekker JD, Tucker PW, Morrisey EE. Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development. 2012;139:2500–2509. doi: 10.1242/dev.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Nettesheim P, Randell SH. Growth and differentiation of tracheal epithelial progenitor cells. The American journal of physiology. 1994;266:L296–307. doi: 10.1152/ajplung.1994.266.3.L296. [DOI] [PubMed] [Google Scholar]
- Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. Stabilized imaging of immune surveillance in the mouse lung. Nature methods. 2011;8:91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MM, Li S, Yang H, Morrisey EE. Foxp4: a novel member of the Foxp subfamily of winged-helix genes co-expressed with Foxp1 and Foxp2 in pulmonary and gut tissues. Mech Dev. 2002;119(Suppl 1):S197–202. doi: 10.1016/s0925-4773(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchetta EM, Ohlstein B. The Drosophila midgut: a model for stem cell driven tissue regeneration. Wiley interdisciplinary reviews Developmental biology. 2012;1:781–788. doi: 10.1002/wdev.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Walles T, Biancosino C, Mertsching H. First human transplantation of a bioengineered airway tissue. J Thorac Cardiovasc Surg. 2004;128:638–641. doi: 10.1016/j.jtcvs.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Postnatal lung growth: evidence that the gas-exchange region grows fastest at the periphery. American Journal of Physiology. 1993;265:L319–322. doi: 10.1152/ajplung.1993.265.4.L319. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Barriuso N, Lopez-Malpartida AV, de Pablo F, Pichel JG. Alterations in alveolar epithelium differentiation and vasculogenesis in lungs of LIF/IGF-I double deficient embryos. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:2040–2050. doi: 10.1002/dvdy.20842. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139:4365–4373. doi: 10.1242/dev.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville WE, Bolanowski JP, Kotia GG. Clinical experience with the silicone tracheal prosthesis. J Thorac Cardiovasc Surg. 1990;99:604–612. discussion 612–603. [PubMed] [Google Scholar]
- Nichols JEE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, Lafrancesca S, Sakamoto J, Vega S, et al. Production and Assessment of Decellularized Pig and Human Lung Scaffolds. Tissue engineering. 2013 doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. The Journal of clinical investigation. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol. 2013;49:788–797. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science (New York, NY) 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Yin Y. Signaling networks regulating development of the lower respiratory tract. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nature medicine. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al. Dynamic Changes in Intracellular ROS Levels Regulate Airway Basal Stem Cell Homeostasis through Nrf2-Dependent Notch Signaling. Cell Stem Cell. 2014 doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science (New York, NY) 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5:e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue engineering Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MI, Chung UI, Williams MC. Aquaporin-5 expression, but not other peripheral lung marker genes, is reduced in PTH/PTHrP receptor null mutant fetal mice. Am J Respir Cell Mol Biol. 2000;22:367–372. doi: 10.1165/ajrcmb.22.3.3923. [DOI] [PubMed] [Google Scholar]
- Ravikumar P, Yilmaz C, Bellotto DJ, Dane DM, Estrera AS, Hsia CC. Separating in vivo mechanical stimuli for postpneumonectomy compensation: imaging and ultrastructural assessment. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 2013;114:961–970. doi: 10.1152/japplphysiol.01394.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar P, Yilmaz C, Dane DM, Bellotto DJ, Estrera AS, Hsia CC. Defining a Stimuli-Response Relationship in Compensatory Lung Growth Following Major Resection. Journal of applied physiology. 2014 doi: 10.1152/japplphysiol.01291.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009a;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009b;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BLM. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial-to-mesenchymal transition. Proceedings of the National Academy of Sciences of the United States of America. 2011a doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway Basal stem cells. Cell Stem Cell. 2011b;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Disease models & mechanisms. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, Kopp JL, Sander M, Wellik DM, Spence JR. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4456–4464. doi: 10.1073/pnas.1311847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannes PL. Differences in basement membrane-associated microdomains of type I and type II pneumocytes in the rat and rabbit lung. 1984:827–833. doi: 10.1177/32.8.6747274. jhcsagepubcom. [DOI] [PubMed]
- Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2009;6:558–563. doi: 10.1513/pats.200905-031RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K, Hodson M, Smyth I. Spatial mapping and quantification of developmental branching morphogenesis. Development. 2013;140:471–478. doi: 10.1242/dev.088500. [DOI] [PubMed] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17531–17536. doi: 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, Leboeuf MR, Padmanabhan A, Wang Q, Li L, Lu MM, Millar SE, Epstein JA. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development. 2013;140:1655–1664. doi: 10.1242/dev.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, Degryse AL, Crossno PF, Xu XC, McConaha ME, Jones BR, Polosukhin VV, Bryant AJ, Cheng DS, Newcomb DC, et al. beta-catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med. 2013;187:630–639. doi: 10.1164/rccm.201205-0972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira VH, Nadarajan P, Graham TA, Pipinikas CP, Brown JM, Falzon M, Nye E, Poulsom R, Lawrence D, Wright NA, et al. Stochastic homeostasis in human airway epithelium is achieved by neutral competition of basal cell progenitors. Elife. 2013;2:e00966. doi: 10.7554/eLife.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thane K, Ingenito EP, Hoffman AM. Lung regeneration and translational implications of the postpneumonectomy model. Transl Res. 2014;163:363–376. doi: 10.1016/j.trsl.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Tian Y, Zhang Y, Hurd L, Hannenhalli S, Liu F, Lu MM, Morrisey EE. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245. doi: 10.1242/dev.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufen C, Jr, Costa EL, Hirota AS, Li HY, Amato MB, Carvalho CR. Follow-up after acute respiratory distress syndrome caused by influenza a (H1N1) virus infection. Clinics (Sao Paulo) 2011;66:933–937. doi: 10.1590/S1807-59322011000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014 doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Alex Mitsialis S, Kourembanas S, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. American journal of physiology Lung cellular and molecular physiology. 2012;302:L829–837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. The Journal of clinical investigation. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voswinckel R, Motejl V, Fehrenbach A, Wegmann M, Mehling T, Fehrenbach H, Seeger W. Characterisation of post-pneumonectomy lung growth in adult mice. The European respiratory journal. 2004;24:524–532. doi: 10.1183/09031936.04.10004904. [DOI] [PubMed] [Google Scholar]
- Wagner DE, Bonenfant NR, Sokocevic D, Desarno MJ, Borg ZD, Parsons CS, Brooks EM, Platz JJ, Khalpey ZI, Hoganson DM, et al. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2013.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhou H, Yang J, Xiao J, Liang B, Li D, Zeng Q, Fang C, Rao Z, Yu H, et al. Association of HHIP polymorphisms with COPD and COPD-related phenotypes in a Chinese Han population. Gene. 2013a;531:101–105. doi: 10.1016/j.gene.2013.08.069. [DOI] [PubMed] [Google Scholar]
- Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of Liver Transplantation for Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:394–402. e391. doi: 10.1016/j.cgh.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tian Y, Morley MP, Lu MM, Demayo FJ, Olson EN, Morrisey EE. Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Dev Cell. 2013b;24:345–358. doi: 10.1016/j.devcel.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansleeben C, Bowie E, Hotten DF, Yu YR, Hogan BL. Age-related changes in the cellular composition and epithelial organization of the mouse trachea. PLoS One. 2014;9:e93496. doi: 10.1371/journal.pone.0093496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER. It takes more than cells to make a good lung. Am J Respir Crit Care Med. 2013;187:342–346. doi: 10.1164/rccm.201212-2260OE. [DOI] [PubMed] [Google Scholar]
- Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk JB, Chen TH, Gottlieb DJ, Walter RE, Nagle MW, Brandler BJ, Myers RH, Borecki IB, Silverman EK, Weiss ST, et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009;5:e1000429. doi: 10.1371/journal.pgen.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respiration Physiology. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian W, McKeon F. Adult stem cells underlying lung regeneration. Cell cycle (Georgetown, Tex) 2012;11:887–894. doi: 10.4161/cc.11.5.19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Li A, Borok Z, Li C, Minoo P. NOTCH1 is required for regeneration of Clara cells during repair of airway injury. Stem cells (Dayton, Ohio) 2012;30:946–955. doi: 10.1002/stem.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, Hogan BL, Onaitis MW. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz C, Tustison NJ, Dane DM, Ravikumar P, Takahashi M, Gee JC, Hsia CC. Progressive adaptation in regional parenchyma mechanics following extensive lung resection assessed by functional computed tomography. Journal of applied physiology. 2011;111:1150–1158. doi: 10.1152/japplphysiol.00527.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Gonzales L, Kolla V, Rath N, Zhang Y, Lu MM, Kimura S, Ballard PL, Beers MF, Epstein JA, et al. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. American journal of physiology Lung cellular and molecular physiology. 2006;291:L191–199. doi: 10.1152/ajplung.00385.2005. [DOI] [PubMed] [Google Scholar]
- Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, Stripp BR. beta-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol. 2009;41:535–543. doi: 10.1165/rcmb.2008-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Yin L, Chen J. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. American Journal of Respiratory Cell and Molecular Biology. 2014;50:595–604. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]