Inhibition of influenza virus production in virus-infected mice by RNA interference (original) (raw)
Abstract
Influenza A virus infection is a major source of morbidity and mortality worldwide. Because the effectiveness of existing vaccines and antiviral drugs is limited, development of new treatment modalities is needed. Here, we show that short interfering RNAs (siRNAs) specific for conserved regions of influenza virus genes can prevent and treat influenza virus infection in mice. Virus production in lungs of infected mice is reduced by siRNAs given either before or after initiating virus infection, by using slow i.v. administration of small volumes containing siRNAs in complexes with a polycation carrier. Similar effects also are observed when mice are given DNA vectors i.v. or intranasally, from which siRNA precursors can be transcribed. Development of delivery systems that may be compatible with human use demonstrates the potential utility of siRNAs for prophylaxis and therapy of influenza virus infections in humans.
Among the most common infections of the upper respiratory tract and lungs are those caused by influenza A virus (1). In a typical year, the virus infects 15–20% of the population, causing ≈36,000 deaths in the United States and >500,000 deaths worldwide (2, 3). Infections with unusually virulent strains of the virus can lead to millions of deaths, as during the 1918 influenza pandemic (4). Although vaccination with killed virus, recombinant surface glycoproteins, or cold-adapted live virus can prevent illness in ≈70–80% of healthy adults, a vaccine protects against only a limited range of strains and may not be effective against a new, potentially pandemic strain. Furthermore, the rate of protection is low (<30–40%) in high-risk groups (5–7), including infants, the elderly, pregnant women, and individuals with a weakened immune system because of various conditions. Because most of the deaths occur in high-risk groups, the vaccines are not particularly effective for those who most need them. Several drugs also can lower the incidence of influenza infection when used prophylactically and reduce the duration of symptoms when given within one or two days of infection (8). However, the widespread use of these drugs is limited by concerns about side effects, patient compliance, and the possible emergence of drug-resistant variants. Thus, there is an urgent need for new measures to prevent and treat influenza virus infection, especially in high-risk groups and during an influenza pandemic.
RNA interference is a process by which double-stranded RNA directs sequence-specific degradation of mRNA (9–11). In mammalian cells, RNA interference can be triggered by synthetic RNA duplexes that are 21–25 nt long, referred to as short interfering RNA (siRNA) (12, 13). Because siRNAs confer transient interference of gene expression in a sequence-specific manner, they represent a new class of molecules that are likely to have significant medical applications (14, 15). siRNAs have been shown to inhibit virus infection if they are transfected into cultured cells by electroporation or by using cationic lipids (16–20). Similarly, we have previously shown that siRNAs specific for conserved regions of the influenza A virus genes can inhibit virus production profoundly in cultured cells and embryonated chicken eggs (21). In particular, siRNAs specific for genes encoding nucleocapsid protein (NP) and components of RNA transcriptase (PA and PB1) of the influenza virus not only target the specific mRNAs for degradation but also block the accumulation of all viral RNAs, making them especially potent as influenza inhibitors. To develop siRNAs for human application, researchers have investigated siRNAs in animal models. To date, the most effective siRNA delivery in mice relies on rapid i.v. injection of a large volume of siRNA solution (hydrodynamic or high-pressure transfection) (22–25). However, this traumatic procedure is unlikely to be practical in humans. Thus, the full utilization of siRNAs' potential in medical application requires development of safe and effective delivery systems.
Because of the similarities between siRNA and DNA, carriers developed to promote entry of DNA into cells (gene therapy) also should promote intracellular uptake of siRNA. Among various DNA transfection agents (26), we have examined the ability of polyethyleneimine (PEI), a cationic polymer, in promoting siRNA delivery in mice for prophylaxis and therapy of influenza virus infection. We focused on PEI because it has been shown to mediate DNA transfection in animals when DNA–PEI complexes were given i.v. or instilled directly into the lungs (27–30). Here, we show (i) that RNA interference mediated by NP-, PA-, and PB1-specific siRNAs can partially control influenza virus in mice even when given after virus infection has begun; (ii) that short hairpin RNAs (shRNAs) expressed from DNA vectors also are effective, a modality that may provide long-lasting effects; and (iii) that delivery systems compatible with human use successfully can inhibit virus replication in vivo. These findings suggest that with the development of appropriate carriers and optimized formulations, the siRNAs used here may provide the basis for prophylaxis and therapy of influenza virus infections in human populations.
Materials and Methods
siRNAs. RNA oligonucleotides were synthesized by Dharmacon (Lafayette, CO) and were deprotected and annealed as described (21). siRNA sequences for NP (NP-1496), PA (PA-2087), PB1 (PB1–2257), respiratory syncytial virus (RSV) (RSV-P), GFP, and Luciferase (Luc) were described previously (21, 22, 31).
PEI-Mediated DNA Transfection in Mice. pCMV-luc DNA (Promega) was mixed with PEI (Qbiogene, Carlsbad, CA) at a nitrogen/phosphorus weight ratio (N/P ratio) of 10 at room temperature for 20 min. For i.v. administration, 200 μl of the mixture containing 60 μg of DNA was injected retroorbitally into 8-week-old male C57BL/6 mice (Taconic Farms). For intratracheal (i.t.) administration, 50 μl of the mixture containing 60 μg of DNA was instilled into the lungs of anesthetized mice.
Infasurf-Mediated DNA Transfection in Mice. Plasmid DNA at the indicated amounts was mixed with 40 μl of Infasurf (provided by Edmund Egan, ONY, Amherst, NY) and instilled into the lungs of anesthetized mice. For the no-treatment group, mice were instilled with the same volume of 5% glucose.
PEI-Mediated siRNA Delivery in Mice. siRNAs were mixed with PEI at an N/P ratio of 5 at room temperature for 20 min. For i.v. administration, 200 μl of the mixture containing the indicated amounts of siRNA was injected retroorbitally.
Luc Assay. At indicated times after pCMV-luc DNA administration, lungs, spleen, liver, heart, and kidney were harvested and homogenized in Cell Lysis Buffer (Marker Gene Technologies, Eugene, OR). Luminescence was analyzed by using the Luciferase Assay System (Promega) and measured by using Opto-comp I (MGM Instruments, Hamden, CT). The protein concentrations in homogenates were measured by the BCA assay (Pierce).
Influenza Virus Infection and Madin–Darby Canine Kidney (MDCK)/Hemagglutinin Assay of Lung Virus Titers. Influenza A/Puerto Rico/8/34 (PR8, subtype H1N1) was provided by Peter Palese (Mount Sinai School of Medicine, New York). For infection, 30 μl of PBS containing the indicated doses of infectious virus was instilled into anesthetized mice through nostrils. At different times after infection, the lungs were harvested and homogenized, and the homogenates were frozen and thawed twice to release the virus. To titer the virus, flat-bottom, 96-well plates were seeded with 3 × 104 MDCK cells per well, and 24 hr later, the serum-containing medium was removed. Twenty-five micro-liters of lung homogenates, either undiluted or diluted from 10- to 107-fold (in 10-fold steps), was added into triplicate wells. After incubation for 1 hr, 175 μl of infection medium containing 4 μg/ml trypsin was added to each well. After incubation for 48 hr at 37°C, the presence or absence of virus in the culture supernatants was determined by hemagglutination of chicken red blood cells (Charles River Breeding Laboratories). The virus titers were determined by interpolation of the dilution endpoint that infected 50% of wells. The virus titers are shown as log10 tissue culture infectious dose (TCID50). Student's t test was performed as indicated.
shRNA-Expressing Lentivirus Vector. The lentivirus vector pLL3.7 was provided by Luk Van Parijs (Massachusetts Institute of Technology). Oligonucleotides encoding various shRNAs were inserted between the U6 promoter and termination sequences. To produce lentivirus, plasmid DNA and helper plasmids (pM-DLg/pRRE, pVSV.G, pRSV-rev, provided by Van Parijs) were transfected into 293T cells. Forty-eight hours later, culture supernatants were collected, spun at 2,000 rpm for 7 min at 4°C, and then filtered through a 0.45-μm filter. To infect Vero cells with lentivirus, Vero cells were seeded at 1 × 105 per well in 24-well plates. After overnight culture, culture supernatants containing lentivirus (either 0.25 or 1.0 ml) were added to the wells in the presence of 8 μg/ml Polybrene. The plates then were centrifuged at 2,500 rpm at room temperature for 1 hr and returned to culture. Twenty-four hours after infection, the resulting Vero cell cultures (Vero-NP-0.25 and Vero-NP-1.0) were analyzed for GFP expression by flow cytometry along with parental (noninfected) Vero cells.
**IFN-**α Assay. Mice were injected i.v. with siRNA–PEI complexes. Twenty-four hours later, lung homogenates were prepared, and the level of IFN-α in the homogenates was measured by ELISA (PBL Biomedical Laboratories, Piscataway, NJ).
Results
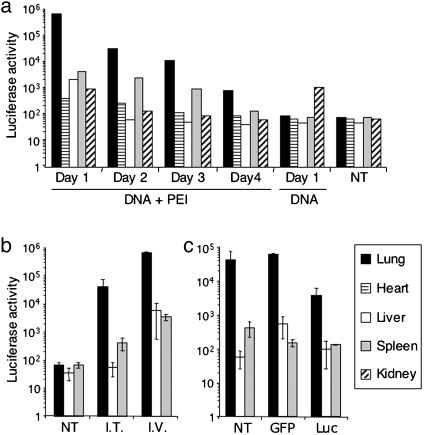
PEI Promotes Preferential Delivery of Nucleic Acid into the Lungs. For effective siRNA-mediated prevention and treatment of influenza, it is necessary to deliver siRNAs into epithelial cells of the respiratory tract, where productive influenza infection occurs (1). To determine the tissue distribution of PEI-mediated nucleic acid delivery in mice, Luc-expressing DNA–PEI complexes were injected i.v., and 24 hr later, Luc activity was measured in various organs. Consistent with previous findings (28, 32), activity was highest in the lungs, where Luc activity was detected for at least 4 days (Fig. 1_a_), whereas in heart, liver, spleen, and kidney, levels were 100–1,000 times lower and were detected for a shorter time after injection. When DNA–PEI complexes were instilled i.t., significant Luc activity also was detected in the lungs, although at a lower level than after i.v. administration (Fig. 1_b_).
Fig. 1.
PEI promotes DNA and siRNA uptake in the lungs. (a) PEI mediates DNA transfection in the lungs after i.v. injection. Luc-expressing DNA (60 μg) was mixed with PEI at an N/P ratio of 10. The mixture was then injected into mice i.v. As controls, mice were not injected with anything (NT, no treatment) or injected with the same amount of naked DNA. One, 2, 3, and 4 days after injection, the indicated organs were harvested and homogenized. Luc activity and amount of protein in the homogenates were assayed. Shown are Luc activities in relative light units in 0.5 mg of protein in different organs over 4 days. Data shown are from one of two experiments. (b) PEI mediates DNA transfection in the lungs after i.t administration. The assays were done in the same way as in a, except that some mice were given DNA–PEI complexes i.t. Shown are average Luc activities in 0.5 mg of protein in the indicated organs of three mice per group 24 hr after DNA administration. Error bars indicate standard deviation. (c) PEI promotes siRNA uptake in the lungs after i.v. administration. Mice were given Luc-expressing DNA (60 μg)-PEI complexes i.t. and then promptly injected i.v. with siRNA–PEI complexes. Sixty micrograms of GFP- or Luc-specific siRNAs was used per mouse (at an N/P ratio of 5). For the NT group, mice were given the same volume of 5% glucose. Twenty-four hours later, Luc activity was assayed in homogenates of lungs, liver, and spleen. Shown are average Luc activity values in 0.5 mg of protein for three mice per group. Error bars indicate standard deviation.
To test the ability of PEI to promote uptake of siRNAs by the lungs, mice were first given Luc-expressing DNA–PEI complexes i.t., followed by i.v. injection of Luc-specific siRNA or control GFP-specific siRNA in complexes with PEI. Twenty-four hours later, Luc activity in the lungs was 17-fold lower in mice that received Luc siRNA than in those given GFP siRNA or nothing (Fig. 1_c_). Because Luc siRNA can inhibit Luc expression only in the same lung cells that were transfected with the DNA vector, these results indicate that i.v. injection of siRNA–PEI complexes promotes entry of siRNA into cells in the lungs.
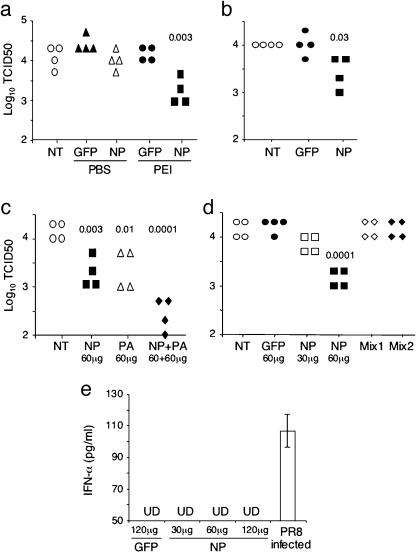
siRNAs Can Prevent Influenza Virus Infection. To test the prophylactic activity of influenza-specific siRNAs, mice first were injected i.v. with a small volume (200 μl) of NP-specific siRNA in PBS or in complexes with PEI. As controls, mice were not treated or injected i.v. with GFP-specific siRNA in PBS or as complexes with PEI. Three hours later, mice were infected intranasally with influenza virus (PR8 strain). Twenty-four hours after infection, virus titers in the lungs were measured by MDCK/hemagglutinin assay. The virus titers were high if mice were not treated or given GFP-specific siRNA, whereas virus titers were reduced ≈10-fold in mice that were given NP siRNA–PEI complexes (Fig. 2_a_). Significant reduction of virus titers still was observed when the NP siRNA–PEI complexes were given 12 hr before virus infection (data not shown) or when the lungs were harvested 2 days after virus infection (Fig. 2_b_). PA-specific siRNA–PEI complexes, injected i.v., also inhibited virus production in mice (Fig. 2_c_). When NP- and PA-specific siRNAs were injected together, in PEI complexes, virus titers in the lungs were reduced further. Because the reduction in virus titer depended on the amount of NP siRNA injected (Figs. 2_d_ and 3_a_), the more severe reduction when both NP and PA siRNAs were used could reflect the use of a larger total amount of siRNA. Nevertheless, the simultaneous use of two or more siRNAs, specific for different influenza virus genes, is likely to be important for preventing the emergence of viruses resistant to a single siRNA.
Fig. 2.
siRNAs inhibit influenza virus production in the lungs when given before virus infection. (a) Influenza-specific siRNAs inhibit virus production in the lungs. Sixty micrograms of GFP- or NP-specific siRNAs was mixed with PEI at an N/P ratio of 5. The mixtures were injected i.v. into groups of four mice. As controls, mice were not injected with anything (NT) or were injected with the same amounts of siRNAs in PBS. Three or 12 (not shown) hr later, mice were infected with 12,000 plaque-forming units (pfu) of PR8 virus intranasally. The lungs were harvested 24 hr after infection, and virus titers in the lung homogenates were measured by MDCK/hemagglutinin assays. The virus titers are shown as log10 tissue culture infectious dose (TCID50). Each data point represents one mouse, and the number indicates P values between GFP siRNA–PEI-treated and NP siRNA–PEI-treated mice. Data shown are from one of two experiments. (b) Significant reduction in virus titers is detected 2 days after virus infection. The experiments were performed in the same way as in a, except that 1,500 pfu was used to infect the mice, and virus titers were assayed in the lungs 2 days after infection. Data shown are from one of two experiments. (c) Simultaneous use of two different siRNAs results in stronger inhibition of virus production. Sixty micrograms of NP, PA, or both NP and PA siRNA at the same amount were mixed with PEI and then injected into mice i.v. The rest of the experiments were performed as in a. Numbers indicate P values between nontreated and treated mice. Data shown are from one of two experiments. (d) Residual siRNAs in the lung homogenates do not inhibit virus production in MDCK/hemagglutinin assays. The assays were done as in a, except one group of mice was given 30 μg of NP siRNA, and another group of mice was injected with NP siRNA (60 μg)–PEI complexes but was not infected with virus. The lung (Mix1) or liver (Mix2) homogenates from the latter group of mice were mixed at a 1:1 ratio with the lung homogenates from the infected mice that were not treated with any siRNA (NT). The virus titers in the same amount of the mixed homogenates were then measured by MDCK/hemagglutinin assay. The number indicates P values between GFP siRNA–PEI treated and NP siRNA–PEI treated mice. Data shown are from one of three experiments. (e) NP siRNA does not induce IFN-α production in mice. Mice were given siRNA–PEI complexes i.v. (at the indicated siRNA amounts), and 24 hr later the levels of IFN-α in lung homogenates were measured by ELISA. Lung homogenates of PR8-infected mice were used as a positive control. The detection limit of the ELISA was 50 pg/ml. Representative data from one of two experiments are shown. UD, undetectable.
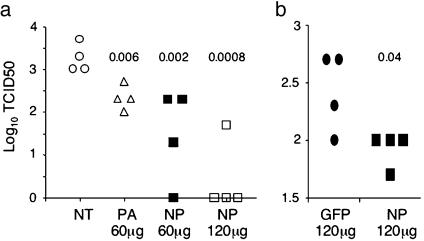
Fig. 3.
siRNAs given after infection inhibit influenza virus production. (a) Groups of four mice were infected intranasally with PR8 virus (3,000 pfu per mouse). Five hours later, siRNAs at the indicated amounts in complexes with PEI were injected into the infected mice i.v. Virus titers in the lungs were measured 33 hr after infection. Each data point represents one mouse, and numbers are P values between nontreated and siRNA-treated mice. Data shown are from one of two experiments. (b) Mice were infected intranasally with PR8 virus (1,500 pfu per mouse). Twenty-four hours later, 120 μg of GFP or NP siRNA in PEI complexes was injected i.v. into the infected mice. Virus titers in the lungs were measured 52 hr after infection. Data shown are from one of two experiments.
In the assay used to measure virus titers in the lungs, MDCK cells were incubated with lung homogenates, followed by measurement of virus titers in the culture supernatants by hemagglutination of chicken red blood cells. A possible source of error is that residual siRNA in the lung homogenates might have inhibited virus production in the MDCK cells. To evaluate this possibility, we mixed equal amounts of lung homogenates from virus-infected mice that were not given siRNA with lung or liver homogenates from uninfected mice that were given NP siRNA–PEI complexes. The mixture was then used to infect MDCK cells as in the standard MDCK/hemagglutinin assay. Virus titers in lung homogenates of infected but untreated mice were not reduced by the addition of lung or liver homogenates from uninfected, NP siRNA-treated mice (Fig. 2_d_). Thus, the NP siRNA-mediated inhibition of virus production observed in infected mice occurred in their lungs, not during assay in the MDCK cells.
Studies by whole genome expression profiling have shown that siRNA-mediated gene silencing is gene-specific (33, 34). However, other reports indicate a nonspecific effect of siRNAs (35), including induction of type I IFN (36). A recent report suggests that IFN induction is limited to siRNAs synthesized by phage polymerase, not chemically synthesized siRNAs (as used in this report), because of the presence of 5′-triphosphate in biosynthesized siRNAs (37). Based on our previous study indicating that NP and PA siRNAs potently inhibited influenza virus production in Vero cells, which are deleted of IFN-α, β, and ω genes (21), the observed inhibition of influenza virus titers in the lungs is unlikely due to an indirect effect by siRNA-induced IFN. To exclude this possibility, we assayed IFN-α in lung homogenates 24 hr after i.v. injection of siRNA–PEI complexes. No significant levels of IFN-α were detected whether GFP-siRNAs or different amounts of NP-siRNAs were used (Fig. 2_e_), whereas IFN-α was detected readily in the lung homogenates 24 hr after PR8 virus infection.
siRNAs Can Treat an Ongoing Influenza Virus Infection. To determine whether siRNAs also are effective therapeutically, mice first were infected with virus and 5 hr later were injected with PA or NP siRNA–PEI complexes, or nothing. Twenty-four hours later, virus titers in the lungs were high in untreated mice and were reduced in mice that were given 60 μg of PA or NP siRNA (Fig.3_a_). Especially notable was that when the infected mice were given 120 μg of NP siRNA, virus titers in the lungs of three of four mice were below the detection limit, representing a >1,000-fold reduction. Even when infected mice were given NP siRNA 24 hr after initiation of virus infection, virus titers in the lungs still were reduced significantly (Fig. 3_b_). These results suggest that siRNAs may be used to treat ongoing influenza virus infection.
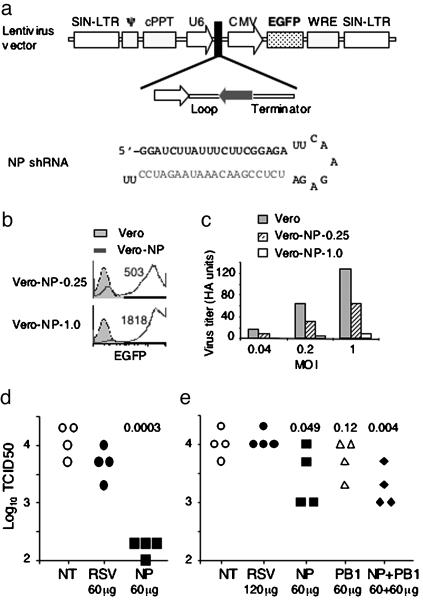
shRNAs Transcribed from DNA Vectors also Inhibit Influenza Virus Infection. We also have investigated whether administration of a DNA vector, from which shRNA can be transcribed and processed into siRNAs (38), can inhibit influenza virus production in mice. For this purpose, oligonucleotides encoding NP or other shRNA were cloned into a lentivirus vector that also expressed enhanced GFP (EGFP) (Fig. 4_a_) (39). To test the ability of NP shRNA transcribed from the lentivirus vector to inhibit influenza virus production, we transfected the DNA vector into lentivirus-packaging cells. Different amounts of the lentivirus culture supernatants then were incubated with Vero cells overnight. When 0.25 ml of supernatant was used, most cells (≈95%) were EGFP positive, and the mean fluorescence intensity (MFI) was ≈500 (Vero-NP-0.25, Fig. 4_b_). When 1 ml of supernatant was used, almost all cells became EGFP positive, and the MFI was increased to >1,800 (Vero-NP-1.0), suggesting infection of Vero cells by the lentivirus. The parental and lentivirus-infected Vero cells then were infected with influenza virus at a multiplicity of infection of 0.04, 0.2, and 1, and virus titers in the culture supernatants were measured 48 hr after virus infection. With increasing multiplicity of infection, virus titers in the supernatants of parental Vero cell cultures increased (Fig. 4_c_). In contrast, virus titers in the supernatants of Vero-NP-1.0 cell cultures remained very low, indicating that influenza virus production was markedly inhibited in these cells. Influenza virus production in Vero-NP-0.25 cell cultures also was inhibited, although to a lesser extent. These results suggest that NP shRNA expressed from a lentivirus vector can be processed into siRNA to inhibit influenza virus production in Vero cells. The extent of inhibition was roughly proportional to the extent of lentivirus infection as indicated by the EGFP intensity.
Fig. 4.
shRNAs transcribed from DNA vectors inhibit influenza virus production in mice. (a) Schematic diagram of lentivirus vector expressing shRNA. Transcription of shRNA is driven by the U6 promoter. EGFP expression is driven by the cytomegalovirus (CMV) promoter. SIN-LTR, ψ, cPPT, and WRE are lentivirus components. Sequence of NP shRNA is shown. (b) Expression of NP shRNA from lentivirus vector in Vero cells. Lentiviruses were produced by cotransfecting DNA vectors encoding NP shRNA and packaging vectors into 293T cells. Culture supernatants (0.25 or 1.0 ml) were used to infect Vero cells overnight. The resulting Vero cell lines (Vero-NP-0.25 and Vero-NP-1.0) were analyzed for GFP expression by flow cytometry. Numbers indicate GFP mean fluorescence intensity of Vero-NP-0.25 and Vero-NP-1.0 cells. (c) Influenza virus production in Vero cells is inhibited by NP shRNA transcribed from lentivirus. Parental and NP shRNA-expressing, lentivirus-infected Vero cells were infected with PR8 virus at a multiplicity of infection of 0.04, 0.2 and 1. Virus titers in the culture supernatants were measured by hemagglutination of chicken red blood cells 48 hr after infection. Vero cells infected with lentiviruses expressing RSV shRNA did not inhibit influenza virus production (not shown). (d) Influenza virus production in mice is inhibited by i.v. administration of DNA vectors that express the NP shRNA. Sixty micrograms of plasmid DNA encoding shRNA specific for NP or RSV was mixed with PEI at an N/P ratio of 10. The mixtures were injected i.v. into groups of four mice. Fifteen hours later, the mice were infected intranasally with 12,000 pfu of PR8 virus. The lungs were harvested 24 hr after infection, and the virus titers were measured. Each data point represents one mouse. The number indicates P values between mice that were given RSV shRNA-expressing DNA and mice that were given NP shRNA-expressing DNA. Data shown are from one of two experiments. (e) Influenza virus production is inhibited in mice by intranasal administration of DNA vectors that express influenza-specific shRNAs. DNA encoding RSV-, NP-, PB1-, or both NP- and PB1-shRNA at the indicated amounts were mixed with Infasurf and administered into the lungs of the mice by instillation. For NT group, mice were instilled with the same volume of 5% glucose. Thirteen hours later, the mice were infected intranasally with PR8 virus (2,000 pfu per mouse). The virus titers in the lungs were measured 24 hr after infection. Numbers indicate P values between mice that were given RSV shRNA-expressing DNA and mice that were given influenza shRNA-expressing DNA. Data are from one of two experiments.
To determine whether shRNAs expressed from DNA vectors were effective in mice, plasmid DNA was mixed with PEI, and the complexes were injected into mice i.v. Fifteen hours later, mice were infected with PR8 virus, and virus titers in the lungs were assayed 24 hr after infection. Compared with virus levels in untreated mice, the levels in mice that were given the DNA vector encoding NP shRNA were reduced ≈100-fold (Fig. 4_d_), whereas the levels of virus in mice that were given a control DNA vector, expressing an shRNA specific for RSV, were not significantly affected.
Infasurf, a surfactant extract from calf lungs used to prevent the development of respiratory distress syndrome in premature babies, is known to promote DNA transfection in the lungs (40). Plasmid DNA encoding different shRNAs were mixed with Infasurf, and the mixtures (40 μl) were instilled into the lungs of anesthetized mice. Thirteen hours later, mice were infected with PR8 virus intranasally, and 24 hr after infection, virus titers in the lungs were measured. Virus titers were high in mice that received no plasmid DNA or a control DNA vector expressing RSV shRNA (Fig. 4_e_), whereas the virus titers were decreased in mice that were given the DNA vector expressing NP shRNA. The virus titers were decreased more significantly if mice were given two DNA vectors: one expressing NP shRNA and the other expressing PB1 shRNA. The inhibition was less robust than when the corresponding DNA vectors were administered i.v. in PEI complexes, probably because Infasurf is less efficient than PEI in promoting DNA transfection. Together, these results show that shRNA transcribed from DNA vectors also can inhibit influenza virus production in mice.
Discussion
Here, we show that when given i.v. to mice either before or after virus infection, siRNA reduced influenza virus production in the lungs; that DNA vectors from which shRNAs can be transcribed also significantly inhibited virus production in the lungs when administered either i.t. or i.v.; and that the inhibitions were sequence-specific and dosage-dependent. Based on the findings that i.v. delivered Luc siRNA inhibited Luc expression from i.t. delivered DNA vector and that any residual siRNA in the lung homogenates had no inhibitory effect on virus production by MDCK cells used to measure lung virus titers, the siRNA-mediated effects observed likely occurred in cells of the lungs. Moreover, these effects most likely occurred in lung epithelial cells because it is only in these cells that productive infection by the influenza virus PR8 strain occurs (1).
The prevention and treatment of influenza virus infection in humans by siRNAs or DNA encoding shRNA requires safe and effective means of delivery. Several reports, including the accompanying report by Tompkins et al. (41), have shown that rapid injections of a large volume of siRNA in PBS i.v. can deliver siRNA to the lungs (and other organs) (22–25). Our findings demonstrate that conventional i.v. injections, involving small volumes delivered relatively slowly, also are effective and might be useful therapeutically under some circumstances. We also show that DNA vectors might provide the means for expressing shRNAs in the lungs for a prolonged period, especially considering that surfactants used for delivery are approved by the Food and Drug Administration. Alveolar epithelial cells are separated from the capillary circulation by a layer of endothelial cells and basement membrane(s). Precisely how i.v. injected siRNA–PEI complexes deliver siRNA to lung epithelial cells is not clear (28). The findings are consistent with reports indicating that the lungs are the most readily transfectable organs when DNA–carrier complexes are administered i.v., probably because lungs contain the first capillary beds traversed by i.v.-injected materials and are likely the most vascularized tissues in the body. Cationic polymers are thought to mediate DNA transfection by condensing large plasmid DNA molecules into smaller complexes and interacting with negatively charged sialic acid residues of cell surface glycoproteins (26). PEI might promote intracellular uptake of siRNA by similar mechanisms.
When 60 μg of NP siRNA was used, an ≈10-fold reduction in lung virus titers was observed. When 120 μg of NP siRNA was used, >1,000-fold reduction in lung virus titers was observed in some mice. Although a 10-fold reduction seems modest, lung virus titer reductions of this magnitude have been shown to accompany survival of lethal challenges in vaccine development studies (42, 43). The significance of our findings is especially notable because only one dose of siRNA was administered, the stability of siRNA in vivo was not maximized, the transfection carriers and their ratios to siRNA were not optimized, and large challenge doses of virus were used (up to 12,000 pfu). The latter is likely far greater than those encountered in natural exposure of humans to influenza virus. With further optimization of transfection carriers and siRNAs and the use of multiple doses, siRNAs may prove to be potent inhibitors of influenza virus infection in humans. Consistent with this view, in an independent study by Tompkins et al. (41) using the same siRNAs, multidoses and multiroutes of administration significantly promoted the survival of mice after lethal challenge. It is notable that similar and complementary results were obtained in these two independent studies, using different carriers, doses, strains of mice, and virus strains. Together, the findings suggest that with the development of appropriate carriers and optimized formulations, the siRNAs used here may provide the basis for prophylaxis and therapy of influenza virus infections in human populations.
Acknowledgments
We thank Drs. Peter Palese and Adolfo Garcia-Sastre for kindly allowing Q.G. to visit their laboratories and learn various techniques for handling influenza virus; Dr. Luk Van Parijs for providing lentivirus vector, packaging cell line, and advice on the lentivirus system; and Dr. Daniel Anderson and members of the Chen and Eisen laboratories for helpful discussions. This work was supported in part by National Institutes of Health Grants AI50631 (to J.C.), AI56267 (to J.C.), and CA60686 (to H.N.E.) and a Cancer Center Core Grant (to Tyler Jacks). A.B. is supported partly by a postdoctoral fellowship from the Serono Foundation and the National Institutes of Health.
Abbreviations: siRNA, short interfering RNA; shRNA, short hairpin RNA; PEI, polyethyleneimine; NP, nucleocapsid protein; MDCK, Madin–Darby canine kidney; PR8, A/Puerto Rico/8/34; RSV, respiratory syncytial virus; i.t., intratracheal; pfu, plaque-forming unit; Luc, luciferase; EGFP, enhanced GFP.
References
- 1.Lamb, R. A. & Krug, R. M. (2001) in Fundamental Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott Williams & Wilkins, Philadelphia), pp. 725–769.
- 2.Thompson, W. W., Shay, D. K., Weintraub, E., Brammer, L., Cox, N., Anderson, L. J. & Fukuda, K. (2003) J. Am. Med. Assoc. 289**,** 179–186. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2003) Influenza: Report by the Secretariat to the Fifty-Sixth World Health Assembly (W.H.O., Geneva), A56/23.
- 4.Patterson, K. D. & Pyle, G. F. (1991) Bull. Hist. Med. 65**,** 4–21. [PubMed] [Google Scholar]
- 5.Patriarca, P. A., Weber, J. A., Parker, R. A., Hall, W. N., Kendal, A. P., Bregman, D. J. & Schonberger, L. B. (1985) J. Am. Med. Assoc. 253**,** 1136–1139. [PubMed] [Google Scholar]
- 6.Nichol, K. L., Wuorenma, J. & von Sternberg, T. (1998) Arch. Intern. Med. 158**,** 1769–1776. [DOI] [PubMed] [Google Scholar]
- 7.Glezen, W. P., Decker, M. & Perrotta, D. M. (1987) Am. Rev. Respir. Dis. 136**,** 550–555. [DOI] [PubMed] [Google Scholar]
- 8.Stiver, G. (2003) Can. Med. Assoc. J. 168**,** 49–56. [PMC free article] [PubMed] [Google Scholar]
- 9.Vaucheret, H., Beclin, C. & Fagard, M. (2001) J. Cell Sci. 114**,** 3083–3091. [DOI] [PubMed] [Google Scholar]
- 10.Sharp, P. A. (2001) Genes Dev. 15**,** 485–490. [DOI] [PubMed] [Google Scholar]
- 11.Brantl, S. (2002) Biochim. Biophys. Acta 1575**,** 15–25. [DOI] [PubMed] [Google Scholar]
- 12.Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E. & Mello, C. C. (1998) Nature 391**,** 806–811. [DOI] [PubMed] [Google Scholar]
- 13.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411**,** 494–498. [DOI] [PubMed] [Google Scholar]
- 14.Saksela, K. (2003) Trends Microbiol. 11**,** 345–347. [DOI] [PubMed] [Google Scholar]
- 15.Silva, J. M., Hammond, S. M. & Hannon, G. J. (2002) Trends Mol. Med. 8**,** 505–508. [DOI] [PubMed] [Google Scholar]
- 16.Randall, G., Grakoui, A. & Rice, C. M. (2003) Proc. Natl. Acad. Sci. USA 100**,** 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, M. T., Coburn, G. A., McClure, M. O. & Cullen, B. R. (2003) J. Virol. 77**,** 11964–11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCown, M., Diamond, M. S. & Pekosz, A. (2003) Virology 313**,** 514–524. [DOI] [PubMed] [Google Scholar]
- 19.Yokota, T., Sakamoto, N., Enomoto, N., Tanabe, Y., Miyagishi, M., Maekawa, S., Yi, L., Kurosaki, M., Taira, K., Watanabe, M. & Mizusawa, H. (2003) EMBO Rep. 4**,** 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapadia, S. B., Brideau-Andersen, A. & Chisari, F. V. (2003) Proc. Natl. Acad. Sci. USA 100**,** 2014–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge, Q., McManus, M., Nguyen, T., Shen, C.-H., Sharp, P. A., Eisen, H. N. & Chen, J. (2003) Proc. Natl. Acad. Sci. USA 100**,** 2718–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCaffrey, A. P., Meuse, L., Pham, T. T., Conklin, D. S., Hannon, G. J. & Kay, M. A. (2002) Nature 418**,** 38–39. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, D. L., Hagstrom, J. E., Loomis, A. G., Wolff, J. A. & Herweijer, H. (2002) Nat. Genet. 32**,** 107–108. [DOI] [PubMed] [Google Scholar]
- 24.Song, E., Lee, S. K., Wang, J., Ince, N., Ouyang, N., Min, J., Chen, J., Shankar, P. & Lieberman, J. (2003) Nat. Med. 9**,** 347–351. [DOI] [PubMed] [Google Scholar]
- 25.McCaffrey, A. P., Nakai, H., Pandey, K., Huang, Z., Salazar, F. H., Xu, H., Wieland, S. F., Marion, P. L. & Kay, M. A. (2003) Nat. Biotechnol. 21**,** 639–644. [DOI] [PubMed] [Google Scholar]
- 26.Han, S.-O., Mahato, R. I., Sung, Y. K. & Kim, S. W. (2000) Mol. Ther. 2**,** 302–317. [DOI] [PubMed] [Google Scholar]
- 27.Zou, S. M., Erbacher, P., Remy, J. S. & Behr, J. P. (2000) J. Gene Med. 2**,** 128–134. [DOI] [PubMed] [Google Scholar]
- 28.Goula, D., Becker, N., Lemkine, G. F., Normandie, P., Rodrigues, J., Mantero, S., Levi, G. & Demeneix, B. A. (2000) Gene Ther. 7**,** 499–504. [DOI] [PubMed] [Google Scholar]
- 29.Bragonzi, A., Boletta, A., Biffi, A., Muggia, A., Sersale, G., Cheng, S. H., Bordignon, C., Assael, B. M. & Conese, M. (1999) Gene Ther. 6**,** 1995–2004. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari, S., Pettenazzo, A., Garbati, N., Zacchello, F., Behr, J. P. & Scarpa, M. (1999) Biochim. Biophys. Acta 1447**,** 219–225. [DOI] [PubMed] [Google Scholar]
- 31.Bitko, V. & Barik, S. (2001) BMC Microbiol. 1**,** 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goula, D., Benoist, C., Mantero, S., Merlo, G., Levi, G. & Demeneix, B. A. (1998) Gene Ther. 5**,** 1291–1295. [DOI] [PubMed] [Google Scholar]
- 33.Semizarov, D., Frost, L., Sarthy, A., Kroeger, P., Halbert, D. N. & Fesik, S. W. (2003) Proc. Natl. Acad. Sci. USA 100**,** 6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chi, J. T., Chang, H. Y., Wang, N. N., Chang, D. S., Dunphy, N. & Brown, P. O. (2003) Proc. Natl. Acad. Sci. USA 100**,** 6343–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persengiev, S. P., Zhu, X. & Green, M. R. (2004) RNA 10**,** 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sledz, C. A., Holko, M., de Veer, M. J., Silverman, R. H. & Williams, B. R. (2003) Nat. Cell Biol. 5**,** 834–839. [DOI] [PubMed] [Google Scholar]
- 37.Kim, D.-H., Longo, M., Han, Y., Lundberg, P., Cantin, E. & Rossi, J. (2004) Nat. Biotechnol. 22**,** 321–325. [DOI] [PubMed] [Google Scholar]
- 38.Brummelkamp, T. R., Bernards, R. & Agami, R. (2002) Science 296**,** 550–553. [DOI] [PubMed] [Google Scholar]
- 39.Rubinson, D. A., Dillon, C. P., Kwiatkowski, A. V., Sievers, C., Yang, L., Kopinja, J., Rooney, D. L., Ihrig, M. M., McManus, M. T., Gertler, F. B., et al. (2003) Nat. Genet. 33**,** 401–406. [DOI] [PubMed] [Google Scholar]
- 40.Weiss, D. J., Mutlu, G. M., Bonneau, L., Mendez, M., Wang, Y., Dumasius, V. & Factor, P. (2002) Mol. Ther. 6**,** 43–49. [DOI] [PubMed] [Google Scholar]
- 41.Tompkins, S. M., Lo, C.-Y., Tumpey, T. M. & Epstein, S. L. (2004) Proc. Natl. Acad. Sci. USA 101**,** 8682–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epstein, S. L., Tumpey, T. M., Misplon, J. A., Lo, C. Y., Cooper, L. A., Subbarao, K., Renshaw, M., Sambhara, S. & Katz, J. M. (2002) Emerg. Infect. Dis. 8**,** 796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang, S., Mozdzanowska, K., Palladino, G. & Gerhard, W. (1994) J. Immunol. 152**,** 1653–1661. [PubMed] [Google Scholar]