ANTIANGIOGENESIS STRATEGIES REVISITED: FROM STARVING TUMORS TO ALLEVIATING HYPOXIA (original) (raw)
. Author manuscript; available in PMC: 2015 Nov 10.
Abstract
Ten antiangiogenic drugs targeting VEGF or its receptors are approved for cancer treatment. However, these agents, intended to block tumors’ blood supply, may cause hypoxia, which may fuel tumor progression and treatment resistance. Emerging clinical data suggest that patients whose tumor perfusion or oxygenation increases in response to these agents may actually survive longer. Hence, strategies aimed at alleviating tumor hypoxia while improving perfusion may enhance the outcome of radiotherapy, chemotherapy, and immunotherapy. Here, I summarize lessons learned from pre-clinical and clinical studies over the past decade and propose strategies for improving antiangiogenic therapy outcomes for malignant and nonmalignant diseases.
Introduction
Tumors acquire blood supply via multiple mechanisms: angiogenesis (sprouting new vessels from existing vessels), co-option, intussusception, vasculogenesis, vascular mimicry and trans-differentiation of cancer cells into endothelial cells (Carmeliet and Jain, 2011). More than 40 molecules have been identified to play a critical role in blood vessel recruitment, but most studies to date have focused on VEGF and its receptors. In fact, since 2004, 10 drugs that target VEGF or its receptors have been approved for the treatment of various malignant diseases (Table 1), with many more in clinical trials. Unfortunately, these agents – used as monotherapy or in combination with chemotherapy – have only provided survival benefits on the order of weeks to months in some tumor types, and have not been efficacious at all in others. Multiple mechanisms underlie these incremental benefits. In this Perspective, I will discuss these mechanisms and speculate on how we can better utilize current antiangiogenic (AA) agents and develop new ones to improve benefit to patients with cancer or other diseases with abnormal vasculature. Instead of reviewing the entire literature, I will focus on the underlying principles – inspired by the works of many in this field, but relying heavily on insights gained from our own pre-clinical and clinical studies.
Table 1. Survival Benefits from Anti-VEGF/VEGFR Drugs.
| Drug | Approved Indication | Improvementin RR (%) | Improvementin PFS(months) | Improvementin OS (months) | Ref. |
|---|---|---|---|---|---|
| Bevacizumab | Metastatic colorectalcancer (withchemotherapy) | 10 | 4.4 | 4.7 | (Kindler et al., 2010) |
| 0 | 1.4 | 1.4 | (Saltz et al., 2008) | ||
| 14.1 | 2.6 | 2.1 | (Giantonio et al., 2007) | ||
| Metastatic non-squamous NSCLC(with chemotherapy) | 20 | 1.7 | 2 | (Sandler et al., 2006) | |
| 16.2 and 13 | 0.4 and 0.6 | NS | (Reck et al., 2010) | ||
| Metastatic breastcancer (withchemotherapy)* | 15.7 | 5.9 | NS | (Miller et al., 2007) | |
| 9 and 18 | 0.8 and 1.9 | NS | (Miles et al., 2010) | ||
| 11.8 and 13.4 | 1.2 and 2.9 | NS | (Robert et al., 2011) | ||
| 9.9 | 2.1 | NS | (Brufsky et al., 2011) | ||
| Metastatic RCC (withIFNα) | 18 | 4.8 | NS | (Rini et al., 2010) | |
| 12.4 | 3.3 | NS | (Escudier et al., 2010) | ||
| Advanced cervicalcancer (withchemotherapy) | 12 | 2.3 | 3.7 | (Tewari et al., 2014) | |
| Sunitinib | Metastatic RCC | 35 | 6 | 4.6 | (Motzer et al., 2007) |
| GIST | 6.8 | 4.5 | NS | (Demetri et al., 2006) | |
| PNET | 9.3 | 4.8 | ? | (Raymond et al., 2011) | |
| Sorafenib | Metastatic RCC | 8 | 2.7 | NS | (Escudier et al., 2007) |
| Unresectable HCC | 1 | NS | 2.8 | (Llovet et al., 2008) | |
| Unresectable HCC | 2 | 1.4 | 2.3 | (Cheng et al., 2009) | |
| Pazopanib | Metastatic RCC | 27 | 5 | N/A | (Sternberg et al., 2010) |
| Advanced soft tissuesarcoma | 6 | 3 | NS | (van der Graaf et al., 2012) | |
| Vandetanib | Advanced medullarythyroid cancer | 43 | 6.2 | N/A | (Wells et al., 2012) |
| Axitinib | Advanced RCC | 10 | 2 | N/A | (Motzer et al., 2013) |
| Regorafenib | Chemo-refractorymetastatic colorectalcancer | 0.6 | 0.2 | 1.4 | (Grothey et al., 2013) |
| Aflibercept | Chemo-refractorymetastatic colorectalcancer | 8.7 | 2.2 | 1.4 | (Van Cutsem et al., 2012) |
| Cabozantinib | Advanced medullarythyroid cancer | 25 | 7.2 | NS | (Elisei et al., 2013) |
| Ramucirumab | Metastatic gastric andgastroesophagealjunction (GEJ) cancers | 0.8 | 0.8 | 1.4 | (Fuchs et al., 2014) |
| Metastatic GEJcancers (withchemotherapy) | 12 | 1.5 | 2.3 | (Wilke et al., 2014) | |
| Metastatic NSCLC(with chemotherapy) | N/A | N/A | N/A | g |
Solid tumors develop resistance to targeted therapies including AA therapies
Millions of advanced cancer patients worldwide have benefited from molecularly targeted therapeutics – whether these agents target oncogenic pathways in cancer cells, angiogenic pathways in blood vessels, or both. However, some tumors are intrinsically resistant to these agents while others develop resistance after an initial response, thus limiting overall survival benefits to months (Table 1). An important feature that distinguishes the AA drugs from other targeted therapies is that AA agents are typically given to unselected patients for the approved indications, whereas cancer cell targeted therapeutics are given to only subsets of patients selected on the basis of biomarkers. Thus, informed selection of patients likely to benefit from AA drugs could significantly improve benefits from these agents. For example, recent studies show that recurrent and newly diagnosed glioblastoma (GBM) patients whose tumor blood perfusion or oxygenation increases after the initiation of AA therapy survive 6-9 months longer than those whose tumor perfusion does not change or instead decreases (Batchelor et al., 2013; Emblem et al., 2013; Sorensen et al., 2012). These emerging data suggest that we should be able to improve overall survival with a more personalized use of existing AA agents and by developing novel hypoxia-alleviating agents.
Why alleviating hypoxia is critical for improving cancer treatment
The imbalance between pro- and anti-angiogenic signaling as well as physical compression leads to abnormal vessels and impaired blood perfusion in tumors (Jain 2005; Jain 2013). The degree of blood flow impairment varies with tumor growth stage and location, and can differ among tumor regions (Movie S1, embedded in Figure 1) or between a primary tumor and its metastases. This progressively worsening heterogeneity in blood perfusion as tumors grow raises an interesting conundrum: if a tumor needs blood vessels to grow and to metastasize, how does it keep growing when growth impairs the very blood supply that brings the required nutrients and removes waste products? This apparent paradox can be understood by thinking about how reduced blood supply can impart a survival advantage to these renegade cells by creating an abnormal microenvironment, characterized by hypoxia and acidosis (Figure 1).
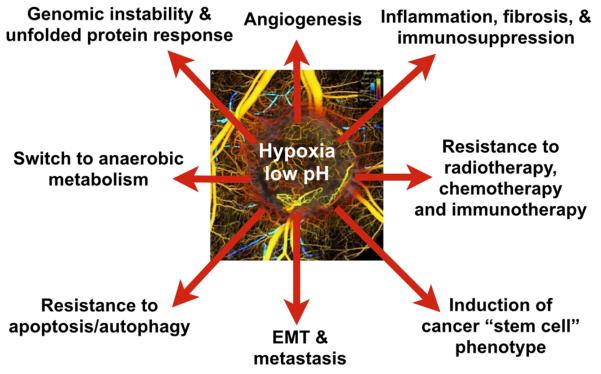
Figure 1. Hypoxia and acidosis resulting from impaired perfusion fuel tumor progression and treatment resistance.
Adverse consequences of hypoxia and acidosis and some of the molecular players contributing to these: 1) Induction of cancer “stem cell” phenotype (e.g., Akt/β-catenin, OCT4) (Lee and Simon, 2012); 2) Resistance to radiotherapy, chemotherapy and immunotherapy (e.g., fewer oxygen radicals, cell cycle arrest) (Huang et al., 2013; Neri and Supuran, 2011; Wilson and Hay, 2011); 3) Tumor growth and genomic instability: Expression of growth factors (e.g., IGF1, TGF-α), oncogenes, tumor suppressor genes (Bindra et al., 2007; Bristow and Hill, 2008; Wilson and Hay, 2011); 4) Epithelial to mesenchymal transition (EMT), invasion and metastasis (e.g., CXCR4, Snail, Lox, cMET) (Finger and Giaccia, 2010; Semenza, 2014); 5) Inflammation, immunosuppression and fibrosis (e.g., IL-6, TGF-β, SDF1α, TAM polarization, Tregs, MDSCs) (Casazza et al., 2013; Chen et al., 2014; Colegio et al., 2014; Motz and Coukos, 2013; Palazón et al., 2012; Semenza, 2014); 6) Abnormal angiogenesis (e.g., HIFs/VEGF, Ang2) (Carmeliet and Jain, 2011); 7) Resistance to apoptosis/autophagy (e.g., BNIP3) (Semenza, 2014); and 8) Switch to anaerobic metabolism (e.g., Glut1, LDHA, PGK1) (Semenza, 2014; Vander Heiden et al., 2009). Many of these consequences are dependent on HIF1α while others are not. Therefore, improving tumor perfusion may alleviate these adverse consequences. Inset shows heterogeneous perfusion in a tumor leading to hypoxic and acidic regions. [Inset reproduced from (Vakoc et al., 2009)]. See also Movie S1.
Our hypothesis is that impaired blood supply and the resulting abnormal tumor microenvironment help cancer cells evade the immune system, increase their invasive and metastatic potential, and apply selective survival pressures to which a cancer cell populations adapt (Figure 1). Under physiological conditions, immune cells constantly patrol tissues to identify and destroy pathogens, foreign antigens, and abnormal cells. In contrast, a hypoxic and acidic microenvironment reprograms the resident macrophages (phagocytes) – whose job is to recognize, engulf and remove dying cells – into a protumorigenic and immunosuppressive phenotype (Casazza et al., 2013; Colegio et al., 2014; Finger and Giaccia, 2010; Hanahan and Coussens, 2011; Keith et al., 2012; Motz and Coukos, 2013; Noy and Pollard, 2014; Palazón et al., 2012; Semenza, 2014; Wilson and Hay, 2011). Hypoxia and acidosis can also attenuate the killing potential of immune effector cells within the tumor microenvironment. Specifically, growth factors and chemokines (e.g., TGFβ, VEGF) induced by hypoxia or acidosis suppress the activity of T lymphocytes, and inhibit the ability of dendritic cells to process tumor antigens and present them to lymphocytes (Barsoum et al., 2014; Calcinotto et al., 2012; Gabrilovich et al., 2012; Palazón et al., 2012). In addition, hypoxia can directly up-regulate – via HIF1α activation – the expression of the immune check-point protein PD-L1 by myeloid-derived suppressor cells, dendritic cells and cancer cells to aid immune-suppression and evasion (Noman et al., 2014).
In addition to protection from the immune system, hypoxia may select for more malignant cells – as cells that respond to physiological cues normally undergo apoptosis under hypoxic conditions (Wilson and Hay, 2011). Hypoxia can increase the invasive potential of cancer cells by inducing the production of pro-migratory proteins (e.g., SDF1α, HGF) and pro-invasive extracellular matrix molecules (Finger and Giaccia, 2010; Semenza, 2014). Hypoxia also provides a niche for so-called cancer stem cells and facilitates inflammation, while also conferring resistance to radiation and many widely used therapeutic agents (Wilson and Hay, 2011). Collectively, these observations may explain why intratumoral hypoxia correlates with poor prognosis in many human cancers (Wilson and Hay, 2011). Elevated interstitial fluid pressure (IFP) resulting from compression of lymphatic vessels also fuels tumor progression and resistance to treatment, but via distinct mechanisms (Jain, 2013). Tumor vessel leakiness worsens interstitial hypertension, causes edema and leads to sluggish blood flow due to clogging of red blood cells concentrated by the leakage of plasma (Jain, 1988; Netti et al., 1996; Sevick and Jain, 1989). Tumor vessel leakiness and compression thereby collaborate in creating a vicious cycle responsible for both acute and chronic hypoxia as well as acidosis. Thus, normalizing the tumor microenvironment by repairing the function of tumor vessels may be a promising strategy to slow tumor progression and enhance cancer treatment.
Antiangiogenic agents can normalize the tumor vasculature and alleviate hypoxia
The role of blood vessels in tumor progression has been investigated for more than a century [for a review, see (Carmeliet and Jain, 2000)]. However, the development of AA agents was catapulted by the groundbreaking hypothesis – put forward in 1971 by the late Dr. Judah Folkman – that starving tumors by blocking angiogenesis would slow tumor progression and improve patient survival (Folkman, 1971). The cloning of VEGF by Napoleone Ferrara and his team was a turning point for the field (Ferrara, 2002). Initially discovered as Vascular Permeability Factor (VPF) by Harold Dvorak and colleagues (Dvorak, 2002), VEGF turned out to be a key survival factor for endothelial cells. This discovery propelled the development of both small [e.g., tyrosine kinase inhibitors (TKIs), peptides] and large molecular weight (e.g., antibodies, receptor-bodies) inhibitors of VEGF or its downstream signaling (Ferrara, 2002). As anticipated, these inhibitors alone decreased both blood vessel density and growth of tumors.
In contrast to most preclinical studies, monotherapy with bevacizumab – an anti-VEGF monoclonal antibody – failed to show overall survival benefit in patients (Jain, 2005). In multiple randomized phase III trials, bevacizumab conferred a survival benefit only when given in combination with chemotherapy (Table 1). These clinical findings seemed paradoxical: why do drugs designed to destroy tumor blood vessels benefit patients only by improving the efficacy of therapeutics that rely on those very blood vessels to reach their target?
We tried to resolve this paradox by proposing that judicious use of AA agents could transiently “normalize” the abnormal tumor vasculature, resulting in improved blood perfusion (Figure 2A). The latter would decrease hypoxia (known to confer resistance to radio-, chemo- and immune therapies) and increase drug accessibility. Thus, therapies given during the window of normalization might achieve greater efficacy (Jain, 2001). The normalized vessels would also resist shedding of cancer cells from the primary tumor, potentially decreasing metastases (Jain, 2005). This hypothesis, while controversial, offered a potential explanation for why bevacizumab can improve the outcome of chemotherapeutics, and more importantly, offered guidelines to improve such combination therapies (Jain et al., 2006). Other hypotheses, though not mutually exclusive, also offered potential reasons for combining AA agents with chemotherapies (Carmeliet and Jain, 2011). For example, some antiangiogenic agents may directly kill cancer cells and sensitize endothelial cells to cytotoxic drugs. Additionally, anti-cancer agents may also directly kill endothelial cells. Finally, killing of tumor cells by cytotoxic and/or antiangiogenic agents may transiently decompress blood vessels resulting in improved perfusion.
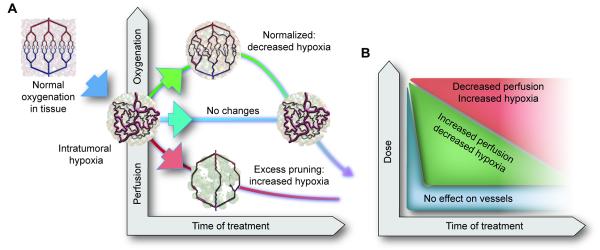
Figure 2. Effect of vascular normalization on tumor perfusion/oxygenation.
A, In a normal tissue, the blood vessels have normal structure and function due to balance of the signals downstream of the pro-angiogenic molecules (e.g., VEGF, Ang2) and antiangiogenic molecules (e.g., sVEGFR1, thrombospondins, semaphorins). In contrast, tumor vessels are structurally and functionally abnormal due to imbalance between pro- and antiangiogenic signals. This creates an abnormal microenvironment in tumors – characterized by hypoxia, acidosis and elevated fluid pressure – which fuels tumor progression and treatment resistance via multiple mechanisms shown in Figure 1. Inhibiting pro-angiogenic signaling or enhancing antiangiogenic signaling can prune some abnormal vessels and remodel the rest resulting in a “normalized vasculature”. Depending upon the extent of normalization versus pruning, tumor perfusion/oxygenation may increase, remain unchanged or decrease. Some tumors might be intrinsically resistant to a given AA agent and others may switch to non-sprouting mechanisms of vessels recruitment (e.g., vessel cooption) that are refractory to the given AA agent and continue to make abnormal vessels again. [Adapted and updated from Jain, 2001 and Sorensen et al, 2012]. See also Movie S2.
B, The window of increased perfusion from vascular normalization depends on the dose and potency of antiangiogenic therapy. High doses may cause excessive pruning of tumor vessels resulting in a shorter window, and may starve a tumor of oxygen and other nutrients. High doses may also increase toxicity – including some fatal. [Adapted and updated from Jain, 2013].
A variety of pre-clinical studies using direct and indirect AA agents supported the normalization hypothesis (Izumi et al., 2002; Jain et al., 1998; Tong et al., 2004; Winkler et al., 2004; Yuan et al., 1996). These studies also revealed that blockade of VEGF signaling or up-regulation of thrombospondin transiently pruned the immature and leaky vessels of tumors in mice and actively remodeled the remaining vasculature so that it more closely resembled the normal vasculature. The morphological changes were accompanied by functional improvements: decreased IFP, decreased tumor hypoxia, and improved penetration of macromolecules from these vessels in these tumors. Radiation therapy had a better outcome when given during the normalization window compared to prior to or after the normalization window (Winkler et al., 2004). We also discovered that Tie-2 activation contributed to the increased pericyte coverage and an increase in matrix metalloproteinase (MMP) activity that, in turn, repaired the basement membrane (Winkler et al., 2004).
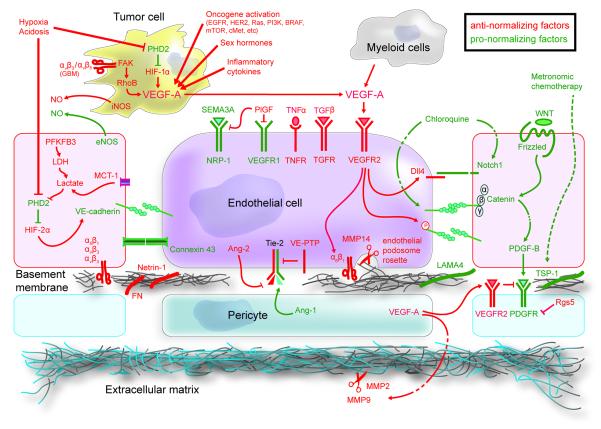
While focus of this Perspective is primarily on VEGF-inhibition because of its clinical relevance, a number of other molecular targets – present in cancer and a variety of host cells – that facilitate or hinder vascular normalization have also been investigated (Goel et al, 2011) (Figure 3 and Table 2). Several agents that target these pathways are now in clinical trials (e.g., Ang-2/Tie-2, FGFR) (Vasudev and Reynolds, 2014). Most recently, chloroquine, an anti-malarial drug being tested in cancer patients, has been shown to induce vascular normalization – leading to improved blood perfusion in tumors and reduced metastasis – by enhancing Notch signaling in endothelial cells (Figure 3) (Maes et al., 2014).
Figure 3. Pathways that facilitate or hinder vascular normalization.
Although most studies on vascular normalization have focused on VEGF, a number of molecular players can facilitate (green) or hinder normalization (red). Please note that the outcome may be dose and context-dependent. Table 2 provides further details about each of these molecular players. (Tumor cell is depicted near endothelial cells to save space.) [Adapted and updated from Goel et al, 2011].
Table 2. Molecules linked with tumor angiogenesis and vascular normalization*.
| Molecule | Complete name | Cell type | Localization | References |
|---|---|---|---|---|
| Ang1 | Angiopoietin 1 | Pericyte | Soluble factor | (Huang et al., 2010;Winkler et al., 2004) |
| Ang2 | Angiopoietin 2 | Endothelial | Soluble factor | (Huang et al., 2010;Nasarre et al., 2009) |
| BRAF | Proto-oncogene B-Raf | Tumor | Intracellular | (Bottos et al., 2012;Goel et al., 2012) |
| c-Met | Hepatocyte growthfactor receptor (HGFR) | Tumor | Transmembrane | (Goel et al., 2012;You et al., 2011) |
| Connexin-43 | Connexin 43 | Endothelial | Transmembrane | (Zhang et al., 2014) |
| Dll4 | Delta-like ligand 4 | Endothelial | Transmembrane | (Hellstrom et al., 2007) |
| EGFR | Epidermal growth factorreceptor | Tumor | Transmembrane | (Izumi et al., 2002) |
| eNOS | Endothelial NOS | Endothelial | Intracellular | (Fukumura et al., 2006) |
| FAK | Focal adhesion kinase | Tumor,endothelial | Intracellular | (Skuli et al., 2009) |
| FN | Fibronectin | ECM | Extracellular | (Chiang et al., 2009) |
| Frizzled | Frizzled | Endothelial | Transmembrane | (Elisei et al., 2013) |
| HIF | Hypoxia-inducible factor | Tumor,endothelial | Intracellular | (Semenza, 2014) |
| iNOS | Inducible nitric oxidesynthases | Tumor cells | Intracellular | (Fukumura et al., 2006; Kashiwagi et al., 2008) |
| Lactate | Lactate | Endothelial | Intracellular | (Goveia et al., 2014;Vegran et al., 2011) |
| LAMA4 | Laminin α4 | ECM | Extracellular | (Seano et al., 2014a;Zhou et al., 2004) |
| LDH | Lactate dehydrogenase | Endothelial | Intracellular | (Goveia et al., 2014;van Beijnum et al., 2006) |
| MCP-1 | Monocarboxylatetransporter 1 | Endothelial | Transmembrane | (Goveia et al., 2014) |
| MMP14 | Matrixmetallopotreinases 14 | Tumor,endothelial | Transmembrane | (Ager et al., 2014;Seano et al., 2014b) |
| MMP2 | Matrixmetallopotreinases 2 | Tumor,endothelial | Soluble factor | (Fang et al., 2000;Winkler et al., 2004) |
| MMP9 | Matrixmetallopotreinases 9 | Tumor,endothelial | Soluble factor | (Jodele et al., 2005;Winkler et al., 2004) |
| Netrin-1 | Netrin 1 | ECM | Extracellular | (Castets and Mehlen, 2010) |
| Notchl | Notch homolog 1 | Endothelial | Transmembrane | (Hellstrom et al., 2007; Phng and Gerhardt, 2009) |
| NRP-1 | Neuropilin 1 | Endothelial | Transmembrane | (Maes et al., 2014;Maione et al., 2009) |
| PDGF-B,C | Platelet-derived growthfactor B, C | Endothelial | Soluble factor | (Abramsson et al., 2003; di Tomaso et al., 2009) |
| PDGFR | PDGF receptor | Pericyte | Transmembrane | (Abramsson et al., 2003; di Tomaso et al., 2009) |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | Endothelial | Intracellular | (De Bock et al., 2013; Goveia et al., 2014) |
| PHD2 | HIF-prolyl hydroxylase 2 | Tumor,endothelial | Intracellular | (Mazzone et al., 2009) |
| PI3K | Phosphatidylinositolbisphosphate 3-kinase | Tumor | Intracellular | (Karar and Maity, 2011) |
| PIGF | Placental growth factor | Endothelial | Soluble factor | (Fischer et al., 2008) |
| Ras | Ras family | Tumor | Intracellular | (Karar and Maity, 2011; Zhang et al., 2001) |
| Rgs5 | Regulator of G-proteinsignaling 5 | Pericyte | Intracellular | (Hamzah et al., 2008) |
| RhoB | Ras homolog genefamily, member B | Tumor cells | Intracellular | (Skuli et al., 2009) |
| SEMA3A | Semaphorin 3A | Endothelial | Transmembrane | (Maione et al., 2009) |
| TGFR | TFG receptors | Endothelial | Transmembrane | (Liu et al., 2012) |
| TGFβ | Transforming growthfactor β | Tumor | Soluble factor | (Liu et al., 2012) |
| Tie2 | TIE 2 | Endothelial | Transmembrane | (Huang et al., 2010;Winkler et al., 2004) |
| TNFR | TNF receptors | Endothelial | Transmembrane | (Johansson et al., 2012) |
| TNFα | Tumor necrosis factor α | Tumor | Soluble factor | (Calcinotto et al., 2012;Johansson et al., 2012) |
| TSP-1 | Thrombospondin 1 | ECM | Extracellular | (Lawler and Lawler, 2012) |
| VE-cadherin | Vascular endothelialcadherin | Endothelial | Transmembrane | (Maes et al., 2014;Orsenigo et al., 2012) |
| VEGF-A | Vascular endothelialgrowth factor A | Tumor,endothelial | Soluble factor | (Batchelor et al., 2007; Ferrara, 2002;Winkler et al., 2004) |
| VEGFR2 | VEGF receptors | Endothelial | Transmembrane | (Ferrara, 2002;Sitohy et al., 2012;Winkler et al., 2004) |
| VE-PTP | VE-protein tyrosinephosphatase | Endothelial | Transmembrane | (Goel et al., 2013) |
| WNT | WNT family | Endothelial | Soluble factor | (Reis et al., 2012;Zhang et al., 2001) |
| α5β1 integrin | α5β1 integrin | Endothelial | Transmembrane | (Magnussen et al., 2005) |
| α6β1 integrin | α6β1 integrin | Endothelial | Transmembrane | (Seano et al., 2014a;Seano et al., 2014b) |
| αvβ3 integrin | αvβ3 integrin | Tumor,endothelial | Transmembrane | (Seano et al., 2014b;Skuli et al., 2009) |
| αvβ5 integrin | αvβ5 integrin | Tumor,endothelial | Transmembrane | (Skuli et al., 2009) |
| β-catenin | β-catenin | Endothelial | Intracellular | (Reis et al., 2012) |
Increased tumor perfusion/oxygenation appears to confer survival benefit
A phase I/II trial in rectal carcinoma patients revealed that a single dose of bevacizumab decreased vessel density, increased pericyte coverage and lowered the interstitial fluid pressure in tumors (Willett et al., 2004). A subsequent trial in recurrent GBM (rGBM) patients demonstrated that cediranib, an oral pan-VEGFR TKI, normalized tumor vessels, and alleviated edema (Batchelor et al., 2007). However, the improvement in PFS and OS correlated with the extent of normalization (Sorensen et al., 2009). Notably, cediranib transiently increased perfusion and oxygenation in a subset of recurrent and newly diagnosed GBM patients, and only these patients survived longer (Batchelor et al., 2013; Emblem et al., 2013; Sorensen et al., 2012). Similar findings on increased oxygenation and pathological response have been reported with bevacizumab in patients with locally advanced breast cancera.
These clinical trials suggest that for a given dose/schedule of AA agents, some patients whose tumor perfusion/oxygenation increases benefit while others do not. However, these findings need to be tested in prospective randomized trials. If validated, increased perfusion/oxygenation early in treatment could serve as a potential predictive biomarker for AA therapy. The next important challenge is to determine why for the same dose of AA agents, tumor perfusion goes up only in some patients and not in others.
Benefits of vascular normalization are AA-dose and drug-size dependent
As originally hypothesized, the extent of vascular normalization in a primary or metastatic lesion is likely dependent on the dose of anti-VEGF/R drugs relative to the level of VEGF in that lesion (Figure 2B). Very high doses of anti-VEGF/R agents could cause a rapid reduction in blood perfusion for a tumor with a certain level of VEGF by excessive vessel pruning and might not improve the outcome of concurrent therapies. High doses may increase hypoxia resulting in increased metastasis and enrichment of cancer stem cells as reported in pre-clinical studies (Chung et al., 2012). In contrast, lower doses might improve perfusion and outcome. Indeed, low doses of an anti-VEGFR2 antibody (10 or 20 mg/kg) increased perfusion compared to a high dose (40 mg/kg) or control IgG in a breast cancer model (Huang et al., 2012).
There are no clinical data directly comparing the dose effect of anti-VEGF agents on perfusion or oxygen levels. However, a study showed decreased perfusion and uptake of docetaxel in the non-small cell lung cancer in patients after administration of 15 mg/kg dose of bevacizumab (Van der Veldt et al., 2012). Unfortunately, unlike the GBM trials (Batchelor et al., 2013; Sorensen et al., 2012), this study did not look at the time course of perfusion or drug uptake. Nor did it examine whether these two parameters correlated with the treatment outcome. So, although this clinical study does show a decrease in perfusion with 15 mg/kg dose, it does not reveal if this reduction in perfusion translated into survival benefit for these patients. If starving tumors were the key mechanism of benefit from bevacizumab, as generally hypothesized, one would expect lower PFS and OS with lower dose of bevacizumab, assuming the lower dose will not saturate the VEGF target. However, there was no statistically significant difference in PFS or OS in non-small cell lung cancer patients treated with 15 mg or 7.5 mg/kg bevacizumab with chemotherapy (Reck et al., 2010). Whether lowering the dose below 7.5 would have increased PFS or OS in these patients is not known. These results collectively argue for tailoring the dose and schedule of anti-VEGF agents for individual patients using imaging or other biomarkers, including the levels of VEGF and its receptors in the primary tumor and metastatic lesions.
The size of concurrently administered drugs is also important, as anti-VEGF therapy is likely to lower size of pores in tumor vessels, thus lowering extravasation of drugs (Hobbs et al., 1998). For example, in a number of preclinical studies and a clinical study, bevacizumab has been shown to decrease the uptake of antibodies (e.g., trastuzumab, cetuximab)b (Arjaans et al., 2013; Heskamp et al., 2013; Pastuskovas et al., 2012). In contrast, in a breast cancer model in mice, low dose anti-VEGFR2 antibody (5 or 10 mg/kg, compared to the standard dose of 40 mg/kg) improved the delivery of antibody-sized nanoparticles (~12nm) but not larger nanoparticles (60 or 120 nm), while higher doses did not improve delivery of either (Chauhan et al., 2012). Furthermore, combination therapy with a low dose of anti-VEGFR2 antibody (5 mg/kg) improved the efficacy of 10 nm nab-paclitaxel (Abraxane®) but not 100 nm liposomal doxorubicin (Doxil®) (Chauhan et al., 2012). Thus, the dose of anti-VEGF agents may need to be tailored for the size of concurrently administered therapeutics and may compromise the delivery of therapeutics beyond a certain size for a given tumor. All this, of course, makes the optimal use of AA agents more complex.
While a number of preclinical studies have shown increased perfusion and drug uptake in tumors with a variety of AA agents (Goel et al., 2011; Maes et al., 2014), there are no clinical studies to date that have measured both time-course of perfusion and drug uptake along with survival benefit. The closest to drug uptake kinetics has been the measurement of oxygen supply – which similar to perfusion kinetics seems to support the benefit of vascular normalization (Emblem et al., 2013). It is possible that the benefit of vascular normalization may come primarily from improved tumor oxygenation resulting from improved perfusion – rather than improved drug uptake – for various reasons discussed earlier (Figure 1).
Overcoming resistance to AA therapy using biomarkers
Because tumors use multiple pathways for recruiting vessels, it is not surprising that blocking VEGF/R alone has inconsistent or incomplete effects on tumor vasculature. For example, non-sprouting mechanisms may be predominant in some treatment naïve-tumors (e.g., vessel cooption in the metastatic legions in the lungs, liver, lymph nodes) or may become operative as a means of escape from anti-VEGF therapy in other tumors (e.g., vessel cooption in GBM) (Movie S2). Some of these mechanisms may be triggered by molecules produced in response to increased hypoxia (e.g., HGF, SDF1α, Ang2), resulting from excessive pruning by longer treatment-duration and/or higher doses of anti-VEGF agents. Some treatment-naïve tumors may have a majority of blood vessels invested in pericytes and thus remain resistant to VEGF blockade (Sitohy et al. 2013). Other tumors may have excessive amount of endogenous anti-VEGF molecules (e.g., sVEGFR1, NRP1, thrombospondin), and thus not respond to exogenous VEGF blockade (Duda et al., 2010). Finally, cellular mechanisms involving various immune cell populations (Gr-1+ myeloid cells, T helper 17 cells) and fibroblasts have been implicated in resistance to AA therapy (Chung et al., 2013; Hanahan and Coussens, 2012; Noy and Pollard, 2014; Ohlund et al, 2014).
To gain insight into the molecular players involved in intrinsic and evasive resistance to anti-VEGF agents, we and other investigators have measured a panel of molecules in the tumor-tissue or circulation of patients undergoing AA therapies. Correlating the levels of these tissue/circulating biomarker candidates with treatment outcome has revealed candidate pathways potentially involved in treatment resistance to anti-VEGF therapies. For example, elevated levels of sVEGFR1 prior to treatment were associated with poor outcome from bevacizumab in rectal carcinoma, triple-negative breast cancer, hepatocellular carcinoma (HCC), and metastatic colorectal carcinoma patients (Duda et al., 2010; Meyerhardt et al., 2012; Raut et al., 2012; Willett et al., 2009; Zhu et al., 2013)c. Additionally, high levels of sVEGFR1 were also associated with fewer side effects in rectal, breast and liver cancer patients (Duda et al., 2010; Zhu et al., 2013)c. Finally, a retrospective analysis has shown that a genetic polymorphism in the VEGFR1 gene correlates with increased VEGFR1 expression and poor outcome of bevacizumab treatment in metastatic renal cell carcinoma and pancreatic ductal adenocarcinoma patients (Lambrechts et al., 2012). Similarly, elevated levels of NRP1 were associated with poor outcome in some trials (Lambrechts et al., 2013). It is possible that VEGFR1 and NRP1 function as endogenous VEGF-traps. Thus, adding an external anti-VEGF agent may not have significant biologic effects in patients with high sVEGFR1/NRP1 levels (Jain, 2013). Additionally, increased VEGFR1 levels may induce increased pro-angiogenic signaling by PlGF when VEGF is blocked (Lambrechts et al., 2012). These hypothesis-generating findings need to be tested prospectively, and if validated, alternate pathways need to be targeted in patients with elevated sVEGFR1/NRP1 levels. Along these lines, baseline level of the short form of VEGF (VEGF-A121) is a predictive biomarker in some studies, but not in others (Lambrechts et al., 2013), and is being tested prospectively in a breast cancer trial (ClinicalTrials.gov Identifier: NCT01663727).
As an example of evasive resistance, circulating levels of the chemokine SDF1α rise in patients who evade various anti-VEGF therapies, including rectal carcinoma with bevacizumab, GBM with cediranib, HCC with sunitinib and soft-tissue sarcoma with sorafenib (Duda et al., 2011). The SDF1α/CXCR4 pathway is involved in vessel co-option, vasculogenesis, fibrosis, lymphocyte trafficking and cancer cell invasion – depending on the tumor and treatment. For example, in HCC this pathway appears to increase fibrosis while in GBM it appears to facilitate invasion of cancer cells and co-option of host vessels by invading cancer cells (Chen et al., 2014)d (Movie S2). The latter finding has led to a clinical trial with AMD3100 (an anti-CXCR4 drug) plus bevacizumab in recurrent GBM patients (ClinicalTrials.gov Identifier: NCT01339039).
Other evasive pathways include Ang2/Tie2/VE-PTP and HGF/cMET, which play important roles in vascular structure and function (pericyte coverage, permeability) and cell invasion (Figure 3) (Sennino et al., 2012; Vasudev and Reynolds, 2014)e. In addition, cellular mechanisms of resistance involve the participation of local or bone marrow-derived populations of immune cells (e.g., Gr-1+ myeloid cells, Th17 cells) or pericyte coverage, which promote resistance through direct support of paracrine interactions with the endothelial cells (Carmeliet and Jain, 2011; Chung et al., 2013). A number of agents that target these evasive pathways are now in clinical trials (Vasudev and Reynolds, 2014).
Targeting VEGF versus VEGFR2 may have a different outcome
Since VEGFR2 is thought to be the main receptor conveying the pro-angiogenic signals downstream of VEGF, it is generally assumed that targeting VEGFR2 would have similar biological effects as targeting the ligand. However, this is not the case in some malignancies. For example, whereas bevacizumab monotherapy has not improved overall survival in any phase III trial, the anti-VEGFR2 antibody ramucirumab led to an OS advantage of 1.4 months in advanced gastric or gastroesophageal junction (GEJ) adenocarcinomas (Table 1). Interestingly, when added to paclitaxel, ramucirumab also increased OS by 2.3 months in patients with GEJ tumors (Table 1). When combined with chemotherapy, both bevacizumab and ramucirumab failed to improve OS in metastatic breast cancer, yet both improved survival in non-small cell lung cancer (NSCLC) (Table 1). It is tempting to assume that blood vessels of GEJ tumors are highly or even exclusively dependent on VEGFR2-signaling for their survival, and hence ramucirumab’s benefits result from starving these tumors – in support of the original anti-angiogenesis hypothesis. However, the starvation hypothesis does not explain the failure of bevacizumab in the same tumor type.
Blood vessels are not the only target of antiangiogenic agents
As pointed out above, the targets of AA agents include not only blood vessels, but also subsets of cancer and stromal cells. For example, VEGF can serve as a survival factor, promote epithelial-mesenchymal transition (EMT), and support stem-cell phenotype in cancer cells. VEGF can also block the maturation of dendritic cells (Goel and Mercurio, 2013). Similarly, PlGF – a member of the VEGF family – functions as a survival factor for medulloblastoma cells and facilitates their spread through the cerebrospinal fluid via neuropilin 1 (NRP1) signaling (Snuderl et al., 2013). Other angiogenic molecules, including angiocrines, such as PDGF, angiopoietins, SDF1α, TGFβ, also support the survival, proliferation and migration of various types of cancer and stromal cells (Butler et al., 2010). Similarly, sunitinib targets both VEGFR and c-KIT – which is commonly mutated in GIST (gastrointestinal stromal tumor) cells. Thus, AA agents and especially multi-receptor TKIs may affect tumor growth and metastasis by multiple mechanisms, making the task of deciphering their primary mechanism of action or identifying predictive biomarkers more complex. Future studies that incorporate tissue, circulating and imaging biomarkers are needed to resolve these outstanding issues.
Agents targeting oncogenic pathways can also normalize tumor vessels
Although agents targeting endothelial or perivascular cells can directly induce vascular normalization, inhibition of oncogenic signaling can have the same effect indirectly. In 1998, we showed that the initial effects of castration upon androgen-dependent carcinoma are primarily vascular (preceding tumor cell death), due to an indirect mechanism of hormone-depletion that suppresses tumor cell production of angiogenic factors (Jain et al., 1998). We subsequently showed that inhibition of Human Epidermal Growth Factor Receptor-2 (HER2) signaling in breast cancer cells using trastuzumab normalizes breast tumor vessels by modulating the expression of at least four pro- and AA molecules (Izumi et al., 2002). Moreover, several other reports describe similar effects from inhibiting oncogenic pathways (e.g., Ras, PI3K, AKT, EGFR, BRAF), which can lower the expression of VEGF and other pro-angiogenic molecules (Goel et al., 2012). Hence, such agents have the potential to improve tumor oxygenation via dual mechanisms: improved perfusion through normalization of vessels and reduced oxygen consumption by dying cancer cells.
Combining antiangiogenic agents with drugs that target oncogenic pathways
Combining AA agents with agents targeting oncogenic pathways – similar to chemotherapeutic agents – has led to some unexpected results. For example, despite promising pre-clinical results from combining VEGF and EGFR targeted agents in colorectal and NSCLC models, all phase III trials combining these targeted agents failed (Tol et al., 2009). Similarly, phase III trials combining VEGF and HER2-targted therapies in HER2+ breast cancer patients also failed (Gianni et al., 2013). A potential mechanism for these failures, as suggested above, is that the dose of bevacizumab used may have decreased the size of pores in the tumor vessel walls and compromised the delivery of antibodies (Chauhan et al., 2012). This hypothesis is consistent with elevated baseline plasma VEGF concentrations being associated with greater bevacizumab benefit. It is also consistent with the recent randomized phase II trial showing benefit of combining bevacizumab with a smaller drug – erlotinib – in EGFR-mutant NSCLC patients (Seto et al., 2014).
Unfortunately, in all of these trials, patients with central nervous system (CNS) metastases were excluded. We discovered that treatment of HER2+ breast tumors in the mouse brain with trastuzumab leads to increased VEGF production by host cells in the brain (Izumi et al., 2002). To this end, we combined HER2-targeted drugs (trastuzumab and lapatinib) with an anti-VEGFR2 antibody and demonstrated a significant improvement in survival of mice bearing HER2+ tumors in their brain (Kodack et al., 2012). Moreover, a phase II clinical trial with dual HER2-blockade and bevacizumab showed encouraging results in heavily pretreated HER2+ breast cancer patients with brain metastases (Falchook et al., 2013). Whether this will translate into increased OS in brain metastasis patients in a phase III trial remains to be seen.
Combining antiangiogenic agents with vessel decompressing agents
Diminished blood perfusion and hypoxia in tumors results not only from the abnormal structure and leakiness of tumor vessels, but also from compression of vessels by extravascular components in tumors (Chauhan et al., 2014; Chauhan et al., 2013; Jain, 1988; Jain, 2014; Padera et al., 2004; Stylianopoulos et al., 2012). This is evident in highly desmoplastic tumors where a large fraction of vessels are compressed and may contribute to the failure of AA therapies in these patients (e.g., pancreatic ductal adenocarcinomas, a subset of breast cancers) (Kindler et al., 2010). Moreover, some tumors begin to produce more extracellular matrix in response to VEGF-blockade – partly from increased hypoxia – and become treatment-resistant (Aguilera et al., 2014; Chen et al., 2014). Thus, strategies that can alleviate compressive forces exerted by stromal cells and/or extracellular matrix in desmoplastic tumors should decompress tumor vessels and sensitize these tumors to AA agents. In fact, when Shh-blockade improves perfusion in desmoplastic pancreatic ductal adenocarcinomas in mice, these tumors become responsive to an anti-VEGFR2 antibody (Rhim et al, 2014). Additionally, agents that can normalize both desmoplastic stroma and abnormal blood vessels may be effective in these treatment-resistant tumors (Stylianopoulos and Jain, 2013).
Our laboratory has recently discovered that widely prescribed anti-hypertensive drugs – angiotensin receptor blockers (ARBs) and ACE-inhibitors collectively known as renin-angiotensin system (RAS) inhibitors – can inhibit cancer-associated fibroblast activity to decrease the production of collagen I and hyaluronan, reduce compressive forces in tumors, open up blood vessels in desmoplastic breast and pancreatic ductal adenocarcinomas in mice, and improve the delivery and efficacy of chemotherapeutics (Chauhan et al., 2013). Other anti-fibrotic agents (e.g., pirfenidone) may also benefit the treatment of desmoplastic tumors (Kozono et al., 2013). However, given the heterogeneity of stromal cells, the choice of molecular target for depleting stroma is critical. For example, a recent study utilized genetic ablation of SMA+ cells to deplete stroma in desmoplastic pancreatic tumors (Ozdemir et al., 2014). Since pericytes – required to maintain vessel integrity – are also SMA+, this strategy destroyed many blood vessels and increased hypoxia. As expected (Figure 1), this genetic SMA+ cell depletion approach induced EMT, stem-cell phenotype, invasion, metastasis, inflammation, and immunosuppression by increasing hypoxia in these tumors.
Although there are no prospective clinical data on the combination of RAS-inhibitors with standard therapies, retrospective studies show that metastatic renal cell carcinoma patients survive 7 months longer when they receive RAS inhibitors in combination with sunitinib compared to sunitinib alone (30 vs 23 months) (Keizman et al., 2011). Similarly, another retrospective study of 2,277 advanced lung cancer patients showed better overall survival when RAS inhibitors were given concurrently with chemotherapy alone or chemotherapy and bevacizumabf. Since a significant fraction of cancer patients develop hypertension during the course of AA therapy, and because RAS inhibitors are fairly safe and relatively inexpensive, it would be worthwhile to test this hypothesis prospectively. Besides RAS inhibitors, it would also be of interest to test if alleviation of desmoplasia by nintedanib, an AA agent that targets VEGFR, FGFR and PDGFR and proven to be effective in idiopathic pulmonary fibrosis – contributed to the survival benefit in combination with docetaxel in the lung adenocarcinoma patients (Reck et al., 2014; Richeldi et al., 2014).
Vascular normalization can improve immunotherapy
Recently approved immune checkpoint inhibitors have led to unprecedented improvements in overall survival in melanoma patients (Wolchok et al., 2013). However, a subset of patients even in this highly responsive disease does not benefit. Additionally, the first FDA approved therapeutic vaccine sipuleucel-T (Sip-T) – where autologous dendritic cells are exposed to a fusion protein consisting of GM-CSF and prostatic acidic phosphatase and then infused back into the body – demonstrated a modest survival benefit of a few months. Finally, various vaccine and adoptive T cell therapies – including with chimeric antibody receptors (CAR) – have shown promise in various malignancies (Ogino et al., 2011). We hypothesize that the normalizing the tumor microenvironment will improve the outcome of all these different immunotherapies and potentially allow lowering the dose of immunotherapeutic agents that, in turn, may decrease their toxicity (Huang et al., 2013). As stated earlier, the abnormal microenvironment of tumors helps tumors evade immune response through multiple mechanisms, including impairment of lymphocyte infiltration, up-regulation of immune check-point protein expression via hypoxia, recruitment of Tregs, and establishment of an immunosuppressive tumor microenvironment that impairs the function of the resident and transiting immune effector cells (Figure 4) (Huang et al., 2013; Motz et al., 2014). Our laboratory has demonstrated that normalizing doses of anti-VEGFR2 antibody (DC101) can alleviate hypoxia, improve the delivery of immune effector cells into the tumor, convert the immunosuppressive microenvironment of tumors into an immunostimulatory one and improve survival from a vaccine therapy (Huang et al, 2012). There are also a number of pre-clinical studies that show the benefit of combining AA agents with various immunotherapies (Table 3). Whether vascular normalization played any role in these results remains unknown. Regardless, clinical trials combining immune checkpoint blockers and other immunotherapies with AA agents have begun (Table 3).
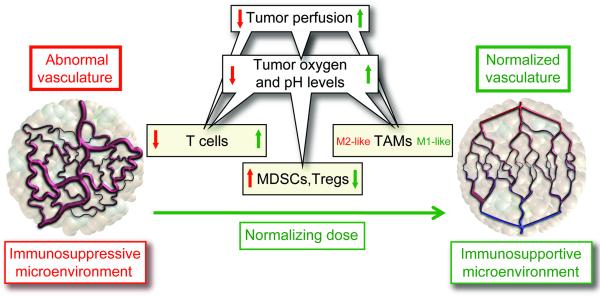
Figure 4. Vascular normalization can reprogram the tumor microenvironment from immunosuppressive to immunosupportive.
The abnormal tumor vasculature can impede T-effector cell infiltration into tumors, and create a hypoxic and acidic tumor microenvironment, which up-regulates PD-L1 on myeloid-derived suppressor cells (MDSCs), dendritic cells and cancer cells, impairs T-effector cell and polarizes tumor-associated macrophages (TAMs) to the immune inhibitory M2-like phenotype to suppress T-effector cell function. Hypoxia can also up-regulate multiple immune-suppressive growth factors and cytokines (e.g., VEGF, TGFβ). Vascular-normalization with appropriate dose and schedule of antiangiogenic treatment can normalize the tumor vasculature and generate a more homogeneous distribution of perfused tumor vessels, facilitating the infiltration of T-effector cells while reducing MDSC accumulation. In addition, alleviation of hypoxia and acidity by improved vascular perfusion polarizes TAMs to an immunostimulatory M1-like phenotype. [Adapted and updated from Huang et al, 2013].
Table 3. Combination of immunotherapies with antiangiogenic agents.
| Antiangiogenic | Immunotherapy | Tumor models | Results |
|---|---|---|---|
| Preclinical studies | |||
| Anti-VEGFR2 mAb | Whole tumor cell vaccine(secreting GM-CSF) | Breast carcinoma(Neu-expressing) | ↑ trafficking of CD8+ T cells↑ regression of tumor in FVBmice (Manning et al., 2007) |
| Anti-VEGFR2 mAb | Whole tumor cell vaccine(Mitomycin treated) | Breast carcinoma | ↑ recruitment of CD4+ andCD8+ T cells↓ MDSCs and Tregs↑ survival (Huang et al., 2012) |
| Adenoviral deliveryof sVEGFR1/R2 | Whole tumor cell vaccine(secreting GM-CSF) | Colon carcinomaMelanoma | ↑ infiltration of CD4+ and CD8+T cells.↓ MDSCs and Tregs.↑ survival (Li et al., 2006) |
| VEGF peptide mimic | HER-2 B cell epitopevaccine | Breast carcinoma | ↑ High affinity HER-2 nativeantibodies.↑anti-tumor andantiangiogenic effects.↓ tumor growth (Foy et al., 2012) |
| SU 6668 | Whole tumor cell vaccine(irradiated) andrecombinant B7.2-IgGfusion protein | Breast carcinoma | ↑ recruitment of CD8+ T cells(Huang et al., 2002) |
| Sunitinib | Pox-virus based vaccineexpressing carcinoembryonic antigen(CEA) and costimulatorymolecules | Colon carcinoma | ↑ intratumoral T cells↓ MDSCs and Tregs↓ tumor volume and ↑ survival(Farsaci et al., 2012) |
| Sorafenib | Anti-PD-1 antibody with aCXCR4 inhibitor(AMD3100) | Hepatocellularcarcinoma | ↑ intratumoral T cells↓ MDSCs and Tregs↓ primary and metastatic tumorvolume and ↑ apoptosis h |
| Anti-mouse VEGFmAb | Peptide-pulsed DCs | Sarcoma | ↑ DC number and function.↑ tumor growth delay(Gabrilovich et al., 1999) |
| Anti-mouse VEGFmAb | Anti-gp100 pmel-1 Tcells, gp100 vaccine, IL-2after lymphodepletion | Melanoma | ↑ immune cell infiltration↑ tumor growth delay↑ survival (Shrimali et al., 2010) |
| VEGFR-1 CAR-ModifiedT cells | Lung carcinoma | ↓ endothelial tube formation in_vitro_↑ tumor growth delay and ↓metastasis (Wang et al., 2013) | |
| Anti-VEGFR2 | Anti-PD-1 antibody | Colon carcinoma | ↑ inhibition of tumorneovascularization.↑ T cell infiltration.↑ expression of cytokines(Yasuda et al., 2013) |
| Clinical Studies | |||
| N/A | Peptide vaccine(VEGFR1, VEGFR2,URLC10, TTK or CDCA1) | NSCLC | ↑ T cell response↑ Stable disease for 2 months(Suzuki et al., 2013) |
| N/A | Antiangiogenic peptidevaccine | Different solid tumors | ↑ activation of T cells.Anti-tumor activity beingevaluated (Hayashi et al., 2013) |
| Sunitinib | Adoptive T cell transfer | RCC | ↓ number and function ofMDSCs and Tregs (Ko et al., 2009) |
| Bevacizumab | IFN-aplha2A | Metastatic RCC | ↑ progression free survival(Escudier et al., 2010) |
| Bevacizumab | Ipilimumab | Advanced melanoma | ↑ T cell infiltration(Hodi et al., 2014) |
| Bevacizumab | Nivolumab | NSCLC | (clinicaltrials.gov identifier:NCT01454102) |
| Bevacizumab | Nivolumab | GBM | (clinicaltrials.gov identifier:NCT02017717) |
Targeting endothelial cell metabolism can inhibit tumor angiogenesis
Until recently, only molecular signals such as VEGF and others have been demonstrated to regulate angiogenesis. However, endothelial cells require energy and new biomass to proliferate and migrate during new vessel formation. Carmeliet and colleagues have recently discovered increased glycolysis via the glycolytic activator PFKFB3 in the leading endothelial cells – known as the tip cells – during sprouting angiogenesis (De Bock et al., 2013; Schoors et al., 2014). PFKFB3-driven glycolysis is also required for proliferating stalk endothelial cells that elongate the vascular sprout. These studies show that endothelial cell metabolism plays a pivotal role in vessel sprouting. Furthermore, pharmacological blockade of PFKFB3 inhibited pathological angiogenesis with modest side effects. These studies provide a paradigm shift in previous anti-glycolytic strategies, which aimed to block glycolysis maximally and permanently, however at the cost of causing severe toxicity. Thus, targeting tumor endothelial cell metabolism opens up new possibilities for AA therapy. Whether targeting PFKFB3 also blocks non-sprouting modes of vessel recruitment is not known.
Antiangiogenic agents cause toxicities
Similarly to most cancer therapeutics, AA agents may lead to cardiovascular and non-cardiovascular adverse effects, some of which may be fatal (~1.5-2.5% of patients). These toxicities are dependent not only on the class of AA agents (targeting VEGF versus VEGFR versus VEGFR-TKI), but also on specific AA agent. Cardiovascular toxicities include hypertension, thromboembolic disease, left ventricular dysfunction, myocardial ischemia, and prolongation of the QTc interval. Non-cardiovascular toxicities include proteinuria, bleeding, delayed wound healing, gastrointestinal perforation, fatigue, thyroid dysfunction, stomatitis, myelosuppression, cutaneous effects (including hand-foot syndrome) and dysphonia. Other rare and AA agent-specific adverse effects include reversible posterior leukoencephalopathy, osteonecrosis of the jaw, microangiopathic hemolysis, pancreatic enzyme elevations, hypoglycemia. Some of these adverse effects are dose-dependent and are even reversible, while others are not. Retrospective studies have shown association between some of these (e.g., hypertension) and the survival benefit, but none have been proven prospectively. Some of the adverse effects appear contradictory – such as hemorrhage and thrombosis – which makes their management challenging and deciphering the underlying mechanisms complex. (For a comprehensive recent review on these adverse effects and their management, please see http://www.uptodate.com/contents/toxicity-of-molecularly-targeted-antiangiogenic-agents-cardiovascular-effects and http://www.uptodate.com/contents/toxicity-of-molecularly-targeted-antiangiogenic-agents-non-cardiovascular-effects). It is tempting to postulate that reducing the dose of AA agents would not only reduce toxicity, but may also increase the window of normalization and delay the onset of hypoxia with all its negative consequences. However, there are no phase III randomized trials to date that compare the effect of high versus low dose of the same AA agent on the efficacy or toxicity.
Animal models of cancer and experimental design need to be improved
A major challenge in AA therapy of cancer has been the discordance between the pre-clinical and clinical results. There are many potential reasons for this, including limitations of available animal models as well as the experimental design. First, the pre-clinical tumor models used generally grow rapidly and are more sensitive to anti-VEGF agents than their human counterparts (with the notable exception of renal cell carcinomas). Genetically engineered mouse models (GEMMs) are less genetically complex than human tumors and rarely metastasize similarly to the human disease. Patient-derived xenograft (PDX) models are improving with the development of mice with “more” humanized immune system. Second, almost all AA agents have been approved for metastatic disease, while most pre-clinical studies examine the effect on primary tumors (corresponding to the neoadjuvant setting in the clinic). Although better murine models of advanced disease are now being developed, pre-clinical studies are rarely carried out in the adjuvant or metastatic settings (Francia et al., 2011). It is worth noting that the US FDA will provide accelerated approval upon demonstration of a substantial increase in the pathological complete response rate for patients with aggressive breast cancers (e.g., triple-negative), with full approval conditional on eventual demonstration of improvements in disease-free and OS rates, as well as acceptable toxicity (Prowell and Pazdur, 2012). Third, while both bevacizumab and aflibercept have shown improved OS only when combined with chemo- or immune-therapeutics, most pre-clinical studies tested these agents as monotherapies. Fourth, although bevacizumab does not recognize mouse VEGF, many investigators use bevacizumab in their murine studies, thus not addressing the contribution of host VEGF in the outcome. Fifth, in many pre-clinical studies, the dose of AA agents has been unusually high, potentially leading to misleading results (Chung et al., 2012). Sixth, many studies using GEMMs in which the relevant gene is knocked-out in the embryo represent prevention rather than intervention studies. Hence, the findings may not translate to the treatment setting, and can even derail treatment strategies. Seventh, murine models tend to underestimate toxicity. We need to take these limitations into account in both experimental design and data interpretation.
The normalization strategy can benefit patients with nonmalignant diseases
Abnormal vessels are a hallmark of not only cancer, but also a number of nonmalignant diseases that afflict more than half a billion people worldwide. These include wet age-related macular degeneration (AMD) and diabetic macular edema. Vascular normalization seems to be a major mechanism of benefit from the approved anti-VEGF agents (Jain, 2005). Neurofibromatosis-2 (NF2)-associated schwannomas also harbor abnormal vessels causing edema, which may contribute to hearing loss by disrupting auditory nerve function. Additionally, inflammatory molecules produced as a result of hypoxia may also trigger hearing loss (Roosli et al., 2012). Indeed, low dose bevacizumab improved hearing in 60% of NF2 patients in a phase I trial (Plotkin et al., 2009). A follow-up phase II study showed hearing benefit in approximately 40% patients (Plotkin S., personal communication). Bevacizumab is now approved for NF2-related schwannomas in UK. Other potential applications of vascular normalization include controlling plaque-rupture, neurovascular complications stemming from radiation therapy and tuberculosis (Jain et al., 2007; Solano et al., 2007). Unfortunately, similar to cancer, the nonmalignant diseases also become resistant to anti-VEGF therapies. Fortunately, the benefit may last years in the latter compared to only a couple of months in the former (Table 1). One cause of failure may be fibrosis – presumably instigated by hypoxia resulting from prolonged VEGF-blockade. Additionally, some vessels may be refractory to VEGF-blockade due to pericyte coverage. A phase II data suggests that prevention of fibrosis and pruning of resistant vessels with a PDGF inhibitor may prolong the benefit of ranibizumab in patients with wet AMD and has led to a phase III trial (ClinicalTrials.gov Identifier: NCT01940900). A number of trials that target PDGF or Ang2 along with VEGF to prolong the benefit to patients are planned or ongoing (Ratner, 2014).
Summary and Perspective
In conclusion, AA therapy, despite being given to unselected patients, has benefited numerous patients worldwide who had no other alternative treatment options. Similar to various therapeutic approaches that looked straightforward in the beginning, antiangiogenesis has turned out to be more complex and nuanced than originally envisaged for multiple reasons: First, a major part of the complexity in AA therapy stems from multiple mechanisms employed by tumors to recruit blood vessels. These mechanisms seem to vary not only spatially and temporally within a tumor, but also between a primary tumor and its metastases, and among tumor types. Moreover tumors may switch from one mechanism to another during growth and in response to treatment. While VEGF seems to be a central player in sprouting angiogenesis, our knowledge of the molecular players in other mechanisms is still in its infancy. Understanding these mechanisms in more detail will allow development of novel agents to target all types of tumor vessels and augment responses to currently available AA agents.
Second, the initial focus of antiangiogenic therapy was to target endothelial cells, pericytes and/or the basement membrane they are invested in. Now we know that these cells interact not only with each other and cancer cells, but also with the extracellular matrix and other stromal cells in the tumor microenvironment, including the resident and transiting immune cells, cancer stem cells as well as fibroblasts/myofibroblasts. This interaction is not only chemical in nature, but also physical. While our understanding of the biochemical microenvironment has grown exponentially, our understanding of the physical microenvironment is in its early stages (Jain et al., 2014). The physical forces exerted by the tumor stroma can directly induce cancer cell invasion (Tse et al, 2011), and compress blood and lymphatic vessels. As discussed earlier, the resulting hypoxia, acidosis and interstitial hypertension can fuel tumor heterogeneity, progression and treatment resistance. Furthermore, forces exerted by plasma and interstitial fluid can also affect vessel formation and function (Song and Munn, 2011). Thus, strategies to control these forces are likely to yield new ways of alleviating hypoxia, slowing tumor progression and reducing treatment resistance.
Third, not only blood vessels, but also other components of the tumor microenvironment are abnormal and all these abnormalities in concert seem to fuel tumor progression and treatment resistance. Thus, we need to develop therapeutic agents that normalize the entire tumor microenvironment, including immune and other stromal cells, and not just the tumor blood vessels. Limited preclinical as well as retrospective clinical studies suggest targeting the rennin-angiotensin system as a promising approach for normalizing CAFs in desmoplastic tumors – which account for about 25% of human tumors. Similarly, a number of agents that aim to normalize the immune microenvironment have been approved and others are being tested. In the long run, a judicious combination of these agents is likely to yield significant benefit to patients while reducing their toxicity.
Finally, unlike cancer cell targeted therapies, many AA agents are not directly cytotoxic, but directly affect the vascular permeability. This makes interpreting contrast-enhanced images complicated. Hence, the search for biomarkers has been challenging. Most biomarker studies have focused on circulating biomarkers that are unable separate the response of host from that of neoplastic lesions. Tissue biomarkers – based generally on a limited sample(s) of tumors – do not account for the heterogeneity inherent in all malignancies. Advanced imaging techniques can provide both spatial and temporal information, but are expensive and use protocols that may not be standardized across multiple institutions. A limited number of correlative trials have used all three approaches and have provided powerful insights into the mechanisms of response and resistance. However, these trials have been small. Hence, these findings need to be validated in prospective, randomized trials. Importantly, future trials with novel agents need to integrate all three types of biomarkers.
Addressing these challenges and judiciously using existing and newly developed AA agents, alone or with other emerging therapeutic approaches, is likely to increase survival benefits in selected patients, while sparing other patients from unnecessary and expensive treatments.
Supplementary Material
1
2
3
Acknowledgments
I would like to thank my colleagues and collaborators for their contributions to the area of tumor microenvironment and antiangiogenesis therapy for more than three decades. In addition, I would like to thank Dan G. Duda and Giorgio Seano for their help in manuscript preparation, Dan G. Duda for preparing Figure 1 and Table 1, Trupti Vardam for Table 3, Giorgio Seano for Table 2, Figures 2, 3 and 4, and James Baish, Tracy T. Batchelor, Ana Batista, Peter Carmeliet, Vikash Chauhan, Helen Chen, Elisabeth de Vries, Dai Fukumura, Steven Isakoff, Robert S. Kerbel, Lance L. Munn, Kamila Naxerova, Mei Rosa Ng, Timothy P. Padera, Sara Tolaney and Lei Xu for many helpful comments. I apologize to authors whose original work I could not cite due to limitations on the number of references.
I received consultant fees from Enlight, Ophthotech and SynDevRx; own equity in Enlight, Ophthotech, SynDevRx, and XTuit; and serve on the Board of Directors of XTuit and Boards of Trustees of H&Q Healthcare Investors, H&Q Life Sciences Investors, and Tekla Healthcare Opportunities Fund.
Footnotes
a
Garcia-Foncillas, J., Martinez, P., Lahuerta, A., Llombart Cussac, A., Gonzalez, M. G., Sanchez-Gomez, R. M., Alvarez, I., Anton, A., Illarramendi, J. J., De Juan, A., et al. (2012). Dynamic contrast-enhanced MRI versus 18F-misonidazole-PET/CT to predict pathologic response in bevacizumab-based neoadjuvant therapy in breast cancer. J Clin Oncol 30, (suppl; abstr 10512).
b
Oosting, S., Nagengast, W., Oude Munnink, T., Lub-de Hooge, M., Brouwers, A., and Glaudemans, A. (2012). 89 Zr-bevacizumab PET imaging in metastatic renal cell carcinoma patients before and during antiangiogenic treatment. J Clin Oncol. 30, abstr 10581.
c
Boucher, Y., Martin, J. D., Tolaney, S. M., Seano, G., Goel, S., Ancukiewicz, M., Isakoff, S. J., Winer, E. P., Krop, I. E., and Jain, R. K. (2013). Tissue biomarkers and interstitial fluid pressure in a phase II study of preoperative (preop) bevacizumab (bev) followed by dose-dense doxorubicin (A)/cyclophosphamide (C)/paclitaxel (T) in combination with bev in HER2-negative operable breast cancer (BC). Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6-10; Washington, DC Philadelphia (PA): AACR; Cancer Res 73, Abstract nr LB-76.
d
Kirkpatrick, N. D., and Jain, R. K. (2010). In vivo vessel co-option by glioma cells following chronic anti-angiogenic treatment. 2010 AACR Annual Meeting Proceedings, (abstr 368)
e
Hidalgo, M., Le Tourneau, C., Massard, C., Boni, V., Calvo, E., Albanell, J., Taus, A., Sablin, M.-P., Varga, A., Bahleda, R., et al. (2014). Results from the first-in-human (FIH) phase I study of RO5520985 (RG7221), a novel bispecific human anti-ANG-2/anti-VEGF-A antibody, administered as an intravenous infusion to patients with advanced solid tumors. 2014 ASCO Annual Meeting Proceedings, (abst 2525).
f
Menter, A. R., Carroll, N., Delate, T., Hornbrook, M. C., Kushi, L. H., Sakoda, L., Lee, V. S., Quinn, V. P., Adams, J. L., and Ritzwoller, D. P. Effect of angiotensin system inhibitors on survival in patients receiving chemotherapy for advanced non-small cell lung cancer. 2014 ASCO Annual Meeting Proceedings, (abstr 8069)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ager EI, Kozin S, Kirkpatrick ND, Seano G, Kodack DP, Askoxylakis V, Huang Y, Goel S, Snuderl M, Muzikansky A, et al. Blockade of MMP14 activity in murine breast carcinomas: implications for macrophages, vessels, and radiotherapy. J. Natl. Cancer Inst. 2014 doi: 10.1093/jnci/djv017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera KY, Rivera LB, Hur H, Carbon JG, Toombs JE, Goldstein CD, Dellinger MT, Castrillon DH, Brekken RA. Collagen Signaling Enhances Tumor Progression after Anti-VEGF Therapy in a Murine Model of Pancreatic Ductal Adenocarcinoma. Cancer Res. 2014;74:1032–1044. doi: 10.1158/0008-5472.CAN-13-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjaans M, Oude Munnink TH, Oosting SF, Terwisscha van Scheltinga AG, Gietema JA, Garbacik ET, Timmer-Bosscha H, Lub-de Hooge MN, Schroder CP, de Vries EG. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 2013;73:3347–3355. doi: 10.1158/0008-5472.CAN-12-3518. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. USA. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra RS, Crosby ME, Glazer PM. Regulation of DNA repair in hypoxic cancer cells. Cancer Metast. Rev. 2007;26:249–260. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- Bottos A, Martini M, Di Nicolantonio F, Comunanza V, Maione F, Minassi A, Appendino G, Bussolino F, Bardelli A. Targeting oncogenic serine/threonine-protein kinase BRAF in cancer cells inhibits angiogenesis and abrogates hypoxia. Proc. Natl. Acad. Sci. USA. 2012;109:E353–359. doi: 10.1073/pnas.1105026109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nature Rev. Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2011;29:4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nature Rev. Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2013;33:1743–1754. doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- Castets M, Mehlen P. Netrin-1 role in angiogenesis: to be or not to be a proangiogenic factor? Cell Cycle. 2010;9:1466–1471. doi: 10.4161/cc.9.8.11197. [DOI] [PubMed] [Google Scholar]
- Chauhan VP, Boucher Y, Ferrone CR, Roberge S, Martin JD, Stylianopoulos T, Bardeesy N, DePinho RA, Padera TP, Munn LL, Jain RK. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell. 2014;26:14–15. doi: 10.1016/j.ccr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang Y, Reiberger T, Duyverman AM, Huang P, Samuel R, Hiddingh L, Roberge S, Koppel C, Lauwers GY, et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by SDF1α/CXCR4 axis and Gr-1+ myeloid cell infiltration in mice. Hepatol. 2014;59:1435–1447. doi: 10.1002/hep.26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- Chi J-T, Wang Z, Nuyten DSA, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland Å, Børresen-Dale A-L, et al. Gene Expression Programs in Response to Hypoxia: Cell Type Specificity and Prognostic Significance in Human Cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1074–1079. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AS, Kowanetz M, Wu X, Zhuang G, Ngu H, Finkle D, Komuves L, Peale F, Ferrara N. Differential drug class-specific metastatic effects following treatment with a panel of angiogenesis inhibitors. J. Pathol. 2012;227:404–416. doi: 10.1002/path.4052. [DOI] [PubMed] [Google Scholar]
- Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes J-M, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- di Tomaso E, London N, Fuja D, Logie J, Tyrrell JA, Kamoun W, Munn LL, Jain RK. PDGF-C induces maturation of blood vessels in a model of glioblastoma and attenuates the response to anti-VEGF treatment. PLoS One. 2009;4:e5123. doi: 10.1371/journal.pone.0005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin. Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DG, Willett CG, Ancukiewicz M, di Tomaso E, Shah M, Czito BG, Bentley R, Poleski M, Lauwers GY, Carroll M, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emblem KE, Mouridsen K, Bjornerud A, Farrar CT, Jennings D, Borra RJ, Wen PY, Ivy P, Batchelor TT, Rosen BR, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat. Med. 2013;19:1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, Ravaud A, Golding S, Jethwa S, Sneller V. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J. Clin. Oncol. 2010;28:2144, 2150. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- Falchook GS, Moulder SL, Wheler JJ, Jiang Y, Bastida CC, Kurzrock R. Dual HER2 inhibition in combination with anti-VEGF treatment is active in heavily pretreated HER2-positive breast cancer. Ann. Oncol. 2013;24:3004–3011. doi: 10.1093/annonc/mdt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc. Natl. Acad. Sci. USA. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Intl. J. Cancer. 2012;130:1948–1959. doi: 10.1002/ijc.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Finger E, Giaccia A. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metast. Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat. Rev. Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Foy KC, Miller MJ, Moldovan N, Carson WE, Kaumaya PTP. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. OncoImmunol. 2012;1:1048–1060. doi: 10.4161/onci.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to Vascular Endothelial Growth Factor Enhance the Efficacy of Cancer Immunotherapy by Improving Endogenous Dendritic Cell Function. Clin. Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, et al. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J. Clin. Oncol. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB., 3rd Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat. Rev. Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Fukumura D, Jain RK. Normalization of the tumor vasculature through oncogenic inhibition: an emerging paradigm in tumor biology. Proc. Natl. Acad. Sci. USA. 2012;109:E1214. doi: 10.1073/pnas.1203794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Gupta N, Walcott BP, Snuderl M, Kesler CT, Kirkpatrick ND, Heishi T, Huang Y, Martin JD, Ager E, et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J. Natl. Cancer Inst. 2013;105:1188–1201. doi: 10.1093/jnci/djt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol. Med. 2014;6:1105–1120. doi: 10.15252/emmm.201404156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Grone HJ, Hammerling GJ, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Kurata T, Fujisaka Y, Kawakami H, Tanaka K, Okabe T, Takeda M, Satoh T, Yoshida K, Tsunoda T, et al. Phase I trial of OTS11101, an anti-angiogenic vaccine targeting vascular endothelial growth factor receptor 1 in solid tumor. Cancer Sci. 2013;104:98–104. doi: 10.1111/cas.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Heskamp S, Boerman OC, Molkenboer-Kuenen JDM, Oyen WJG, van der Graaf WTA, van Laarhoven HWM. Bevacizumab reduces tumor targeting of antiepidermal growth factor and anti-insulin-like growth factor 1 receptor antibodies. Intl. J. Cancer. 2013;133:307–314. doi: 10.1002/ijc.28046. [DOI] [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat. Rev. Cancer. 2010;10:575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- Huang X, Wong MK, Yi H, Watkins S, Laird AD, Wolf SF, Gorelik E. Combined Therapy of Local and Metastatic 4T1 Breast Tumor in Mice Using SU6668, an Inhibitor of Angiogenic Receptor Tyrosine Kinases, and the Immunostimulator B7.2-IgG Fusion Protein. Cancer Res. 2002;62:5727–5735. [PubMed] [Google Scholar]
- Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. An Indirect Way to Tame Cancer. Sci. Am. 2014;310:46–53. doi: 10.1038/scientificamerican0214-46. [DOI] [PubMed] [Google Scholar]
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- Jain RK, Finn AV, Kolodgie FD, Gold HK, Virmani R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nat. Clin. Pract. Cardivasc. Med. 2007;4:491–502. doi: 10.1038/ncpcardio0979. [DOI] [PubMed] [Google Scholar]
- Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Ann. Rev. Biomed. Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodele S, Chantrain CF, Blavier L, Lutzko C, Crooks GM, Shimada H, Coussens LM, Declerck YA. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc. Natl. Acad. Sci. USA. 2012;109:7841–7846. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S, Tsukada K, Xu L, Miyazaki J, Kozin SV, Tyrrell JA, Sessa WC, Gerweck LE, Jain RK, Fukumura D. Perivascular nitric oxide gradients normalize tumor vasculature. Nat. Med. 2008;14:255–257. doi: 10.1038/nm1730. [DOI] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizman D, Huang P, Eisenberger MA, Pili R, Kim JJ, Antonarakis ES, Hammers H, Carducci MA. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur. J. Cancer. 2011;47:1955–1961. doi: 10.1016/j.ejca.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, et al. Gemcitabine Plus Bevacizumab Compared With Gemcitabine Plus Placebo in Patients With Advanced Pancreatic Cancer: Phase III Trial of the Cancer and Leukemia Group B (CALGB 80303) J. Clin. Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, Farrar CT, Huang Y, Ager E, Kamoun W, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc. Natl. Acad. Sci. USA. 2012;109:E3119–3127. doi: 10.1073/pnas.1216078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, Mizumoto K, Tanaka M. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013;73:2345–2356. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Claes B, Delmar P, Reumers J, Mazzone M, Yesilyurt BT, Devlieger R, Verslype C, Tejpar S, Wildiers H, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012;13:724–733. doi: 10.1016/S1470-2045(12)70231-0. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J. Clin. Oncol. 2013;31:1219–1230. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect. Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KE, Simon MC. From stem cells to cancer stem cells: HIF takes the stage. Curr. Opin. Cell Biol. 2012;24:232–235. doi: 10.1016/j.ceb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, Prell R, VanRoey MJ, Simmons AD, Jooss K. Vascular Endothelial Growth Factor Blockade Reduces Intratumoral Regulatory T Cells and Enhances the Efficacy of a GM-CSF-Secreting Cancer Immunotherapy. Clin. Cancer Res. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl. Acad. Sci. USA. 2012;109:16618–16623. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26:190–206. doi: 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Magnussen A, Kasman IM, Norberg S, Baluk P, Murray R, McDonald DM. Rapid access of antibodies to alpha5beta1 integrin overexpressed on the luminal surface of tumor blood vessels. Cancer Res. 2005;65:2712–2721. doi: 10.1158/0008-5472.CAN-04-2691. [DOI] [PubMed] [Google Scholar]
- Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, Seano G, Serini G, Bussolino F, Giraudo E. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J. Clin. Invest. 2009;119:3356–3372. doi: 10.1172/JCI36308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EA, Ullman JGM, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA. A Vascular Endothelial Growth Factor Receptor-2 Inhibitor Enhances Antitumor Immunity through an Immune-Based Mechanism. Clin. Cancer Res. 2007;13:3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Ancukiewicz M, Abrams TA, Schrag D, Enzinger PC, Chan JA, Kulke MH, Wolpin BM, Goldstein M, Blaszkowsky L, et al. Phase I study of cetuximab, irinotecan, and vandetanib (ZD6474) as therapy for patients with previously treated metastastic colorectal cancer. PLoS One. 2012;7:e38231. doi: 10.1371/journal.pone.0038231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2010;28:3239, 3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- Nasarre P, Thomas M, Kruse K, Helfrich I, Wolter V, Deppermann C, Schadendorf D, Thurston G, Fiedler U, Augustin HG. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 2009;69:1324–1333. doi: 10.1158/0008-5472.CAN-08-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nature Rev. Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- Netti PA, Roberge S, Boucher Y, Baxter LT, Jain RK. Effect of transvascular fluid exchange on pressure-flow relationship in tumors: a proposed mechanism for tumor blood flow heterogeneity. Microvasc. Res. 1996;52:27–46. doi: 10.1006/mvre.1996.0041. [DOI] [PubMed] [Google Scholar]
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat. Rev. Clin. Oncol. 2011;8:711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- Palazón A, Aragonés J, Morales-Kastresana A, de Landázuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin. Cancer Res. 2012;18:1207–1213. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- Pastuskovas CV, Mundo EE, Williams SP, Nayak TK, Ho J, Ulufatu S, Clark S, Ross S, Cheng E, Parsons-Reponte K, et al. Effects of Anti-VEGF on Pharmacokinetics, Biodistribution, and Tumor Penetration of Trastuzumab in a Preclinical Breast Cancer Model. Mol. Cancer Ther. 2012;11:752–762. doi: 10.1158/1535-7163.MCT-11-0742-T. [DOI] [PubMed] [Google Scholar]
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev. Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Plotkin SR, Stemmer-Rachamimov AO, Barker FG, 2nd, Halpin C, Padera TP, Tyrrell A, Sorensen AG, Jain RK, di Tomaso E. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. New Engl. J. Med. 2009;361:358–367. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowell TM, Pazdur R. Pathological Complete Response and Accelerated Drug Approval in Early Breast Cancer. New Engl. J. Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- Ratner M. Next-generation AMD drugs to wed blockbusters. Nat. Biotechnol. 2014;32:701–702. doi: 10.1038/nbt0814-701. [DOI] [PubMed] [Google Scholar]
- Raut CP, Boucher Y, Duda DG, Morgan JA, Quek R, Ancukiewicz M, Lahdenranta J, Eder JP, Demetri GD, Jain RK. Effects of sorafenib on intra-tumoral interstitial fluid pressure and circulating biomarkers in patients with refractory sarcomas (NCI protocol 6948) PLoS One. 2012;7:e26331. doi: 10.1371/journal.pone.0026331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. New Engl. J. Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann. Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E, et al. Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J. Exp. Med. 2012;209:1611–1627. doi: 10.1084/jem.20111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. New Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J. Clin. Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- Roosli C, Linthicum FH, Jr., Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otol. Neurotol. 2012;33:473–480. doi: 10.1097/MAO.0b013e318248ee02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Seano G, Chiaverina G, Gagliardi PA, di Blasio L, Puliafito A, Bouvard C, Sessa R, Tarone G, Sorokin L, Helley D, et al. Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis. Nat. Cell Biol. 2014a doi: 10.1038/ncb3036. ePub on Sep 14, 2014 as doi: 10.1038/ncb3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seano G, Daubon T, Genot E, Primo L. Podosomes as novel players in endothelial biology. Eur. J. Cell Biol. 2014b doi: 10.1016/j.ejcb.2014.07.009. ePub on Aug 7, 2014 as doi: 10.1016/j.ejcb.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen Sensing, Hypoxia-Inducible Factors, and Disease Pathophysiology. Annu. Rev. Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- Sevick EM, Jain RK. Viscous resistance to blood flow in solid tumors: effect of hematocrit on intratumor blood viscosity. Cancer Res. 1989;49:3513–3519. [PubMed] [Google Scholar]
- Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, Cohen-Jonathan Moyal E. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–3316. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152:1065–1076. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano JM, Bakri SJ, Pulido JS. Regression of radiation-induced macular edema after systemic bevacizumab. Can. J. Ophthalmol. 2007;42:748–749. doi: 10.3129/i07-140. [DOI] [PubMed] [Google Scholar]
- Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69:5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. USA. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianopoulos T, Jain RK, et al. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci U S A. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Fukuhara M, Yamaura T, Mutoh S, Okabe N, Yaginuma H, Hasegawa T, Yonechi A, Osugi J, Hoshino M, et al. Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer. J. Transl. Med. 2013;11:97. doi: 10.1186/1479-5876-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, et al. Improved survival with bevacizumab in advanced cervical cancer. New Engl. J. Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. New Engl. J. Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. doi: 10.1073/pnas.1118910109. Epub 2011 Dec 27. [DOI] [PubMed] [Google Scholar]
- Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, Munn LL. Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A. 2012;109:911–916. doi: 10.1073/pnas.1118910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009;15:1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schoffski P, Aglietta M, Staddon AP, Beppu Y, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, Hendrikse NH, Eriksson J, Windhorst AD, Postmus PE, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21:82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Wang W, Ma Y, Li J, Shi HS, Wang LQ, Guo FC, Zhang J, Li D, Mo BH, Wen F, et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency. Gene Ther. 2013;20:970–978. doi: 10.1038/gt.2013.19. [DOI] [PubMed] [Google Scholar]
- Wells SA, Jr., Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY, et al. Efficacy, Safety, and Biomarkers of Neoadjuvant Bevacizumab, Radiation Therapy, and Fluorouracil in Rectal Cancer: A Multidisciplinary Phase II Study. J. Clin. Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov ON, Kim T-Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. New Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, Yagita H, Nakajima Y. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin. Exp. Immunol. 2013;172:500–506. doi: 10.1111/cei.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You WK, Sennino B, Williamson CW, Falcon B, Hashizume H, Yao LC, Aftab DT, McDonald DM. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71:4758–4768. doi: 10.1158/0008-5472.CAN-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc. Natl. Acad. Sci. USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, O’Carroll SJ, Henare K, Ching LM, Ormonde S, Nicholson LF, Danesh-Meyer HV, Green CR. Connexin hemichannel induced vascular leak suggests a new paradigm for cancer therapy. FEBS Letters. 2014;588:1365–1371. doi: 10.1016/j.febslet.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- Zhou Z, Doi M, Wang J, Cao R, Liu B, Chan KM, Kortesmaa J, Sorokin L, Cao Y, Tryggvason K. Deletion of laminin-8 results in increased tumor neovascularization and metastasis in mice. Cancer Res. 2004;64:4059–4063. doi: 10.1158/0008-5472.CAN-04-0291. [DOI] [PubMed] [Google Scholar]
- Zhu AX, Ancukiewicz M, Supko JG, Sahani DV, Blaszkowsky LS, Meyerhardt JA, Abrams TA, McCleary NJ, Bhargava P, Muzikansky A, et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: a phase II study. Clin. Cancer Res. 2013;19:1557–1566. doi: 10.1158/1078-0432.CCR-12-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3