Regulation of oxygen delivery to the body via hypoxic vasodilation (original) (raw)
The respiratory system has traditionally been thought of as a two-gas model: hemoglobin (Hb) within red blood cells (RBC) binds oxygen in the lungs, delivers the oxygen to peripheral tissues, and binds carbon dioxide, which is returned to the lungs, released, and expired. However, fine-tuning of the system is required so that blood flow is preferentially shunted to tissues that have a greater need for oxygen. This mechanism, hypoxic vasodilation, is defined as the prompt vascular response to increased local demand for oxygen because of a change in metabolic activity in the absence of injury or disease (1, 2). This process involves detection of blood oxygen content by sensors and then a rapid transduction of the signal into a vasodilatory bioactivity (2). Emerging data suggest that Hb in the RBC not only functions as a vehicle to carry adequate amounts of oxygen to tissues, but also functions as an oxygen sensor and oxygen-responsive nitric oxide (NO) signal transducer, thereby regulating vascular tone (2, 3).
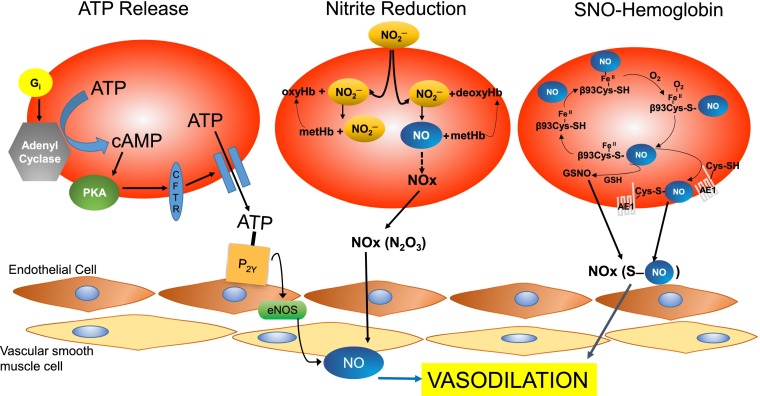
In the past several decades, emerging findings suggest that the respiratory system is mediated by a third gas, NO, which regulates hypoxic vasodilation (2). This concept remains controversial, and three mechanisms have been proposed for RBC-dependent hypoxic vasodilation: adenosine triphosphate (ATP) release and subsequent activation of endothelial nitric oxide synthase (eNOS) (4–7); nitrite reduction to NO by deoxyhemoglobin (8, 9); and S-nitrosohemoglobin (SNO-Hb)-dependent bioactivity (1, 2, 10) (Fig. 1). In PNAS, Zhang et al. (11) present novel information adding a definitive genetic layer of proof supporting the third mechanism, the SNO-Hb pathway in RBC-dependent hypoxic vasodilation. Importantly, the phenotype of the mouse model used in this work illustrates the critical physiological importance this mechanism has in regulating tissue oxygenation.
Fig. 1.
Three proposed mechanisms underlying hypoxic vasodilation. ATP release: Activation of Gi results in increased adenylyl cyclase activity, resulting in increased cAMP followed downstream by increased ATP release. ATP binds to endothelial purinergic receptors on endothelial cells and activates eNOS to stimulate NO-dependent vasodilation. Nitrite reduction: Nitrite entering RBCs reacts with both oxyHb (forming nitrate and metHb) and deoxyHb (forming NO and metHb). The reaction with deoxyHb is favored at low oxygen saturation. Formation of dinitrogen trioxide (N2O3) allows for NO-bioactivity to escape the RBC and mediate vasodilation. SNO-hemoglobin pathway: Hemoglobin becomes S-nitrosylated on a specific and conserved cysteine residue on the β-chain (β93Cys) as RBCs get oxygenated in the lungs. In the R-state, the SNO-Hb remains relatively stable. Upon deoxygenation, SNO-Hb reacts with RBC thiols, such as GSH and anion exchanger-1, to transmit a vasodilatory signal out of the RBC leading to vasodilation. AE-1, anion exchanger-1; ATP, Adenosine triphosphate; cAMP, Cyclic adenosine monophosphate; CFTR, Cystic fibrosis transmembrane regulator; eNOS, endothelial nitric oxide synthase; GSH, glutathione; GSNO, S-nitrosoglutathione; NO, nitric oxide; NO2−, nitrite; NO3−, nitrate; N2O3, dinitrogen trioxide; P2Y, purinergic receptors; PKA, Protein Kinase A.
ATP Regulation
Release of the endothelium-dependent vasodilator, ATP, was one of the first mechanisms proposed for hypoxic vasodilation (6, 7). In vivo studies support that ATP release contributes to increased local blood flow during hypoxia and exercise in tissues, such as skeletal muscle (4) and heart (5). However, the release of ATP in response to these sustained changes in oxygen saturation happens within minutes, whereas the effects of SNO-Hb occur within seconds, commensurate with the time it takes blood to transit the capillary bed (2). Thus, SNO-Hb and ATP may serve complementary roles in acute local and prolonged systemic hypoxia, respectively.
Nitrite Reductase
One hypothesis for the involvement of NO in hypoxic vasodilation is the nitrite reductase mechanism where nitrite is transformed to NO by a deoxyhemoglobin (deoxyHb)-mediated reduction. This mechanism involves the transport of nitrite into RBC, where it reacts with both oxyHb and deoxyHb, but it is only the reaction with deoxyHb that produces NO in hypoxic conditions (8, 9). NO is then eased out of the RBC via a localized reaction with deoxyHb and nitrite at the membrane (12) or by forming intermediate neutral or anion species, such as N2O3 (13). Several studies in humans (8, 9) have supported the involvement of a nitrite-dependent mechanism in hypoxic vasodilation. However, there are several studies that have reported that both Hb and RBC in fact block nitrite-mediated vasodilation (14). One major issue with this mechanism is that free NO is highly reactive and has a short half-life in blood, because of scavenging molecules in the RBC (oxyHb and deoxyHb) and the plasma. Accordingly, this finding suggests that a nitrite reductase mechanism is unlikely to be the primary pathway involved in hypoxic vasodilation.
SNO-Hemoglobin
In 1996, Jia et al. proposed a third mechanism, an elegant three-gas model for hypoxic vasodilation (10). Hb undergoes covalent S-nitrosylation on a specific and conserved cysteine residue on the β-chain (βCys93) as the RBC becomes oxygenated in the lungs, forming SNO-Hb (1, 2, 10). The highly conserved nature of this cysteine throughout evolution (10) provides strong support for the central role of SNO-Hb in hypoxic vasodilation. S-nitrosylation of Hb is governed in part by the state of the Hb molecule, which undergoes an allosteric shift from an R, or relaxed state, to the T, or tense state, during passage in the circulatory system. In the R-state, SNO-Hb remains relatively unreactive. When blood is subsequently deoxygenated in the microcirculation, Hb switches to the T-state, which triggers the release of NO from SNO-Hb. The formation of SNO-Hb is facilitated in the R-state by the internal orientation of βCys93, whereas βCys93 points out toward the protein surface in the T-state, facilitating the release of NO from SNO-Hb (15). The export of NO activity from the RBCs is not via free NO, but involves NO group transfer from SNO-Hb to other low-molecular or protein-bound thiols (transnitrosation), such as glutathione (GSH) or anion exchanger-1, respectively (10).
Using a knockin mouse model, where RBCs contained either WT human Hb or human Hb in which the βCys93 residue was replaced with alanine, Isbell et al. (16) concluded that SNO-Hb was not essential for hypoxic vasodilation based on the systemic blood pressure and time-to-fatigue in exercise not significantly changing in the βCys93 mutant and WT mice. This conclusion has been disputed (17) because neither blood pressure nor time to fatigue has any bearing on hypoxic vasodilation. Furthermore, these in vitro experiments examined pulmonary arteries in which hypoxia normally causes vasoconstriction, whereas systemic vessels would have been a better model (17). Further analysis suggests that these mice show defects in tissue perfusion, in that the concentration of Hb and S-nitrosothiols were elevated, which correlates with impaired hypoxic vasodilation. Thus, the study by Isbell et al. (16) does not rule out the possibility of βCys93 and SNO-Hb being major players in RBC-mediated hypoxic vasodilation.
This controversy is addressed by Zhang et al. (11), who advance the understanding of the role of βCys93 and SNO-Hb in hypoxic vasodilation by RBCs and thereby for blood flow autoregulation. Using the βCys93-mutant mice, but this time examining both local blood flow and tissue oxygenation, Zhang et al. clearly demonstrate that βCys93 plays an essential role in hypoxic vasodilation. Mice deficient in βCys93 showed impaired hindlimb peripheral blood flow and tissue oxygenation at baseline and when exposed to progressive global hypoxia. To confirm that the primary mechanism was through the SNO-Hb pathway, aortic rings from eNOS knockout mice (to eliminate the direct effect of ATP, NOS, and nitrite) were used to assess vasodilation at 1% oxygen by RBC from WT and βCys93-mutant mice in the presence or absence of GSH. GSH-mediated vasorelaxation was not different with the control RBCs, but was eliminated when RBCs from βCys93-mutant mice were used, further suggesting a primary role for SNO-Hb and βCys93 in local blood flow regulation. The authors next examined the effect of hypoxia on cardiac structure and function. βCys93-mutant mice showed left ventricular dilation and impaired myocardial contractility and cardiac output under hypoxia, and a greater and higher frequency of ST-wave elevation (indicative of acute ischemic injury) following transient progressive hypoxia. Furthermore, in room air, T-wave amplitude was significantly reduced in βCys93-mutant mice, indicating myocardial ischemia, a change that, (under normoxia) reflects a decrease in coronary blood flow. These findings establish an essential role for βCys93-derived NO bioactivity in both hypoxia- and flow-coupled response, and illustrate that SNO-Hb plays a central role in blood flow autoregulation.
What is the clinical relevance of these findings? Disordered delivery or release of NO bioactivity from RBC into microvessels because of defects in S-nitrosylation has been implicated in a variety of diseases, including diabetes, sepsis, sickle cell disease, preeclampsia, pulmonary hypertension, congestive heart failure, and as a major underpinning of the complications of blood transfusion, all of which have been characterized by impairments in tissue blood flow (1, 2, 18). βCys93 mutant mice had litters with approximately half as many pups as WT mice (11), suggesting a role for SNO-Hb in the regulation of fetal blood flow. Furthermore, βCys93 mutant mice exhibited myocardial infarction and cardiogenic shock under hypoxic conditions, suggesting that alterations in SNO-Hb and RBC play a role in ischemic coronary syndromes and heart failure (11). Thus, by understanding the mechanisms by which SNO-Hb mediates hypoxic vasodilation, better diagnostic strategies and therapeutic interventions for these pathological conditions can be gained. Myocardial ischemia at rest in this model shows the central physiologic role SNO-Hb plays in blood flow autoregulation and solidifies the notion of a three-gas model of the cardiovascular and respiratory systems.
Acknowledgments
J.M.H. is supported by National Institutes of Health Grants R01 HL110737, R01 HL107110, R01 HL084275, and 5UM HL113460, and grants from the Starr Foundation and Soffer Family Foundation.
Footnotes
Conflict of interest statement: J.M.H. discloses a relationship with Vestion that includes equity, board membership, and consulting.
See companion article on page 6425.
References
- 1.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med. 2009;15(10):452–460. doi: 10.1016/j.molmed.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 3.Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: Oxygen carriers and regulators of local blood flow. J Exp Biol. 2009;212(Pt 21):3387–3393. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- 4.González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: Role of circulating ATP. Circ Res. 2002;91(11):1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 5.Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: Possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288(4):H1586–H1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- 6.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269(6 Pt 2):H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278(4):H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 8.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 9.Crawford JH, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107(2):566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380(6571):221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, et al. Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci USA. 2015;112:6425–6430. doi: 10.1073/pnas.1502285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladwin MT, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291(5):H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3(12):785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 14.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: Evidence for an S-nitrosothiol-based signal. Circ Res. 2008;103(5):545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamler JS, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276(5321):2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 16.Isbell TS, et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14(7):773–777. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamler JS, Singel DJ, Piantadosi CA. SNO-hemoglobin and hypoxic vasodilation. Nat Med. 2008;14(10):1008–1009, author reply 1009–1010. doi: 10.1038/nm1008-1008. [DOI] [PubMed] [Google Scholar]
- 18.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114(14):1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]