Potentiation of TRPV3 Channel Function by Unsaturated Fatty Acids (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 6.
Published in final edited form as: J Cell Physiol. 2006 Jul;208(1):201–212. doi: 10.1002/jcp.20648
Abstract
Transient receptor potential vanilloid (TRPV) channels are polymodal detectors of multiple environmental factors, including temperature, pH, and pressure. Inflammatory mediators enhance TRPV function through multiple signaling pathways. The lipoxygenase and epoxygenase products of arachidonic acid (AA) metabolism have been shown to directly activate TRPV1 and TRPV4, respectively. TRPV3 is a thermosensitive channel with an intermediate temperature threshold of 31–39°C. We have previously shown that TRPV3 is activated by 2-aminoethoxydiphenyl borate (2APB). Here we show that AA and other unsaturated fatty acids directly potentiate 2APB-induced responses of TRPV3 expressed in HEK293 cells, Xenopus oocytes, and mouse keratinocytes. The AA-induced potentiation is observed in intracellular Ca2+ measurement, whole-cell and two-electrode voltage clamp studies, as well as single channel recordings of excised inside-out and outside-out patches. The fatty acid-induced potentiation is not blocked by inhibitors of protein kinase C and thus differs from that induced by the kinase. The potentiation does not require AA metabolism but is rather mimicked by non-metabolizable analogs of AA. These results suggest a novel mechanism regulating the TRPV3 response to inflammation, which differs from TRPV1 and TRPV4, and involves a direct action of free fatty acids on the channel.
Transient receptor potential (TRP) channels have emerged as cellular sensors of physical and chemical changes inside and outside cells (Clapham, 2003). Six TRP channels have been shown to be involved in temperature sensing in sensory neurons and skin. TRPA1 and TRPM8 are involved in detecting cool to cold temperatures while TRPV1, V2, V3, V4 are responsible for sensing warm to noxious heat. In addition to thermosensation, these channels also respond to inflammatory mediators, implicating their involvement in inflammatory pain and tissue injury-induced thermal hyperalgesia. For example, TRPV1 is activated by bradykinin, nerve growth factor, and ATP through signaling pathways mediated by activation of their respective receptors (Cesare et al., 1999; Chuang et al., 2001; Tominaga et al., 2001) and by tissue acidosis as a consequence of inflammation and malignant tumor growth (Reeh and Kress, 2001). Hypotonicity-induced activation of TRPV4 in primary afferent nociceptive nerve fibers is enhanced by prostaglandin E2 (Alessandri-Haber et al., 2003). TRPA1 is activated by bradykinin through activation of phospholipase C (Bandell et al., 2004). In the inflamed tissues, arachidonic acid (AA) is either released from the infiltrating lymphocytes or produced within the sensory fibers or skin cells following the activation of receptors by other inflammatory mediators. While the lipoxygenase products of AA directly activate TRPV1 (Hwang et al., 2000; Shin et al., 2002), the epoxygenase products are responsible for the stimulatory effect of AA on TRPV4 (Watanabe et al., 2003).
TRPV3 may be involved in the sensation of warm to noxious heat. The reported temperature threshold values for TRPV3 ranged from 31 to 39°C (Peier et al., 2002; Smith et al., 2002; Xu et al., 2002) and the channel was continuously activated up to 50°C (Xu et al., 2002). Expression of TRPV3 protein has been shown in mouse skin keratinocytes (Chung et al., 2003, 2004a) and in sensory neurons of human dorsal root ganglia (Smith et al., 2002). Knockout of TRPV3 gene from mice led to impaired responses to innocuous and noxious heat, which are believed to be due to a defect in thermasensation in the skin cells (Moqrich et al., 2005). Recently, we, and others, showed that TRPV3 channels are activated by 2-aminoethoxydiphenyl borate (2APB) (Hu et al., 2004; Chung et al., 2004b). With the use of 2APB, TRPV3-mediated heat-sensitive currents were detected in primary keratinocytes isolated from _TRPV4_−/− mice (Chung et al., 2004b). Because of the sequence homology and functional similarities between TRPV3 and other heat-sensitive channels, it has been speculated that TRPV3 also plays a role in thermal hypersensitivity induced by tissue injury and inflammation (Smith et al., 2002). Here we show that TRPV3 channel function is greatly enhanced by AA via a mechanism that does not require the oxidation of the fatty acid and the effect can be mimicked by other unsaturated fatty acids, including non-metabolizable analogues of AA. The potentiation by fatty acids is not mediated through protein kinase C (PKC) even though activation of PKC also causes an increase of TRPV3 activity. Parts of the results have appeared in an abstract form (Hu et al., 2005).
MATERIALS AND METHODS
DNA constructs, cells, and transfections
cDNA for murine TRPV1, TRPV2, and TRPV3 were obtained and placed in the pAGA3 or pIRES2EGFP vector as previously described (Hu et al., 2004). HEK293 cells were grown at 37°C, 5% CO2 in Dulbecco’s minimal essential medium containing 4.5 mg/ml glucose, 10% heat-inactivated fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. For intra-cellular Ca2+ measurements and electrophysiological studies, cells were seeded in 35-mm culture dishes and transfected with the desired DNA in the pIRES2EGFP vector using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the protocol provided by the manufacturer.
The 308 keratinocytes were kindly provided by Dr. S. Yuspa (National Cancer Institute) and cultured in a 3:1 (v/v) mixture of Dulbecco’s minimal essential medium and Ham’s F-12 medium supplemented with 10% heat-inactivated fetal bovine serum, 5 μg/ml insulin, 0.4 μg/ml hydrocortisone, 5 μg/ml transferrin, 2 nM 3,3–5′ triiodo-L-thyroxine, 0.1 nM cholera toxin, 10 ng/ml epidermal growth factor, 60 μg/ml penicillin, and 25 μg/ml gentamicin as described by Chung et al. (2003).
Intracellular Ca2+ measurements
Cells were seeded in wells of a 12-well plate that contained a 10-mm round coverslips pretreated with polyornithine (MW >30,000, 20 μg/ml, Sigma-Aldrich, St Louis, MO). After culturing for about 20 h, cells were washed once with an extracellular solution (ECS) containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose, and 15 Hepes, pH 7.4 and then incubated in 500 μl ECS supplemented with 0.1% bovine serum albumin, 2 μM fura2/AM (TEFLabs, Austin, TX) and 0.05% Pluronic F-127 (Molecular Probes, Eugene, OR) at 37°C for 30 min. At the end of the incubation, cells were washed three times with ECS and placed in 1 ml of the same solution.
Changes in intracellular Ca2+ concentrations ([Ca2+]i) were measured at 22–24°C using a PTI (Photon Technology International, Lawrenceville, NJ) photometry system attached to a Nikon Eclipse TE200 inverted microscope with alternating excitation wavelengths of 340 and 380 nm and the emission wavelength of 510 nm. Fluorescence intensities of a group of 10–20 cells were read by a photomultiplier attached to the microscope. 2APB and AA were dissolved in DMSO and then diluted to the final concentration with ECS, and applied through perfusion. Bovine serum albumin was omitted from all experiments that used free fatty acids.
Electrophysiological studies
Whole cell recordings of transiently transfected HEK293 cells, cRNA synthesis from TRPV1 and TRPV3 in pAGA3 vector, and cRNA injection in Xenopus oocytes were performed at room temperature (22–24°C) as described previously (Hu et al., 2004). Solutions used for whole-cell recordings of HEK293 cells were: pipette solution (in mM): 140 CsCl, 0.6 MgCl2, 1 or 10 BAPTA, 10 Hepes, pH 7.2; bath solution (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 Hepes, pH 7.4. For inside-out patches, the pipette solution contained (in mM) 140 CsCl and 10 Hepes, pH 7.4 and the bath contained 140 CsCl, 5 EGTA, 10 Hepes, pH 7.4. For outside-out patches, the pipette solution contained (in mM) 140 CsCl, 5 EGTA, 10 Hepes, pH 7.4 and the bath contained 140 CsCl and 10 Hepes, pH 7.4. The excised patches were held constantly at desired potentials while 2APB and AA were applied to the bath through perfusion. Single channel currents were recorded at 5 or 10 kHz for more than 1 min under each condition.
For two-electrode voltage clamp recordings, cRNA-injected oocytes were placed in a RC-3Z Oocyte Recording Chamber (Warner Instruments, Hamden, CT) and perfused with a bath solution that contained (in mM) 100 NaCl, 2.5 KCl, 1 MgCl2, 5 Hepes, pH 7.4. The oocytes were impaled with two intracellular glass electrodes filled with 3 M KCl connected to an OC-725C Oocyte Clamp amplifier (Warner Instruments). Two methods were used to record TRPV-mediated currents. In the first one, voltage commands were made from the Pulse +Pulse Fit program (HEKA Instruments, Southboro, MA) via an ITC-18 Computer Interface (Instrutech Co. Port Washington, NY). Oocytes were clamped at −20 mV, stepped to −100 mV for 20 ms, followed by a voltage ramp of 200 ms from −100 mV to +100 mV once every second. Currents were recorded at the sampling rate of 1 kHz. In the second method, oocytes were constantly held at −40 mV using the command from the amplifier. Currents were simultaneously recorded using a chart recorder (Astro Med, West Warwick RI) and digitized at 100 Hz using a PowerLab Data Acquisition System (ADInstruments, Colorado Springs, CO). For both methods, drugs were diluted to the final concentration and applied through perfusion.
Critical micelle concentration (CMC) measurements
AA was diluted at concentrations from 1.3 μM to 9 mM in 100 μl bath solution used for whole cell recording of HEK293 cells or that used for two-electrode voltage-clamp recording of Xenopus oocytes. The diluted AA was incubated for 1 h at 24°C in the presence of 5 μM N-phenyl-1-naphthylamine in wells of a black-wall 96-well plate. During the incubation, fluorescence (ex = 346 nm, em = 420 nm) was read in a plate reader at 1 min intervals. 2APB was included in some samples at a final concentration of 100 μM. CMCs were estimated from abscissa intercepts after fitting the data points (averages of fluorescence intensity during the last 10 min of incubation) that are significantly higher than the background by least squares linear regression as described (Brito and Vaz, 1986; Chirita et al., 2003).
Chemicals
Rev-5901, baicalein, cinnamyl-3,4-dihydroxy-cyanocinnamate (CDC), esculetin, 17-octadecynoic acid (17-ODYA), and SKF525A were purchased from BioMol (Plymouth Meeting, PA). Linoleic acid, docosahexaenoic acid, α-linolenic acid, γ-linolenic acid, 5,8,11,14-eicosate traynoic acid (ETYA), nordihydroguaiaretic acid (NDGA), and 2APB were purchased from Cayman Chemical Inc. (Ann Arbor, MI). 4-[(2_S_)-2-[(5-isoqui-nolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazi-nyl)propyl] phenyl isoquinolinesulfonic acid ester (KN-62) was from Tocris Cookson Inc. (Ellisville, MO). Ro318220 and Go6976 were purchased from EMD Bioscience (La Jolla, CA). All other fatty acids and chemicals were from Sigma-Aldrich.
All free fatty acids were dried under a stream of nitrogen gas and resuspended in argon-purged DMSO at 10 or 100 mM. The stock solutions in DMSO were stored in small aliquots at −80°C and used only once after thawing. Working solutions of free fatty acids were used within 4 h after diluting into aqueous solutions. The final concentration for DMSO was less than 0.24% for all experiments unless otherwise indicated.
Data analyses
Statistical analyses were performed by Student’s t test using Microcal Origin. Summary data are expressed as means ± SEM.
RESULTS
AA enhanced 2APB-evoked Ca2+ signal in mouse keratinocytes and HEK293 cells that expressed TRPV3
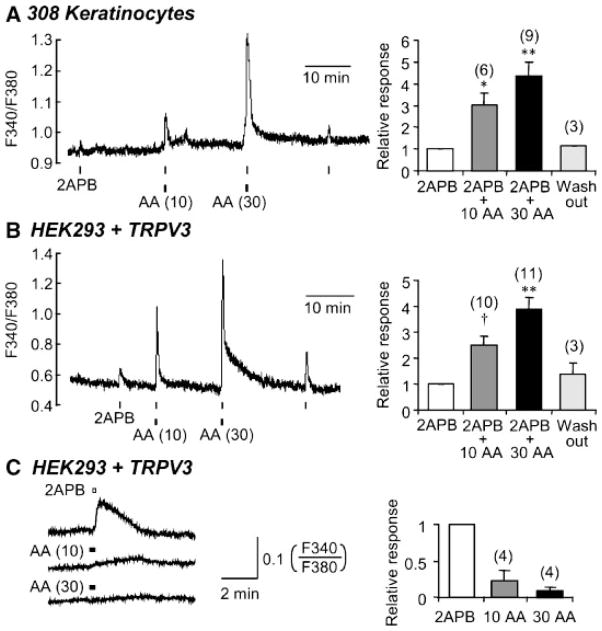
The expression and function of native TRPV3 channels in mouse keratinocytes have been reported (Chung et al., 2003, 2004a,b; Moqrich et al., 2005). It appeared that heating alone could not reliably activate the native channels but with the use of 2APB, the channel activity was consistently detected in these cells. In fura2-loaded mouse 308 keratinocytes, 300 μM 2APB elicited a small increase in [Ca2+]i at 22°C (Fig. 1A). Since the 308 cells express both TRPV3 and TRPV4 (Chung et al., 2003) and TRPV4 can be activated by AA through its epoxygenase products (Watanabe et al., 2003), we treated the cells with 10 or 30 μM AA. AA alone caused a small rise in [Ca2+]i in the keratinocytes; however, a much stronger increase in [Ca2+]i was detected when 2APB was added in the presence of AA and the increase was dependent on the dosage and duration (not shown) of the AA exposure (Fig. 1A).
Fig. 1.
Potentiation of TRPV3 function by AA in mouse keratinocytes and TRPV3-transfected HEK293 cells. Keratinocytes (A) or HEK293 cells expressing TRPV3 (B and C) were loaded with fura2 and monitored using Ca2+ photometry at 22–24°C as described in Materials and Methods. 2APB (300 μM for (A) and 100 μM for (B) and (C) and AA (10 or 30 μM as indicated) were applied through perfusion for 10 and 20 sec, respectively. Left, representative traces; right, summaries of relative responses for the number of measurements indicated in parentheses. *P <0.05, †P <0.005, **P <0.001, different from control response to 2APB.
Since only TRPV3 but not TRPV4 can be activated by 2APB (Hu et al., 2004; Chung et al., 2004b), the potentiation of the 2APB-elicited response by AA could either result from a synergistic activation of both TRPV3 and TRPV4 or from the effect of AA on TRPV3 channels. To determine if AA could act at TRPV3 without the presence of TRPV4, we repeated this experiment in HEK293 cells that expressed cloned murine TRPV3 channels. These cells do not endogenously express TRPV3 or TRPV4. The response to 2APB (100 μM) in the TRPV3-expressing HEK293 cells was potentiated by the pretreatment with AA to a similar degree as that in keratinocytes (Fig. 1B). At the dosage and duration (20 sec total) of the AA treatment, the [Ca2+]i increase elicited by the fatty acid alone was small, highly variable, and not correlated with its concentration (Fig. 1C), although longer (60 sec) incubation with 10–30 μM AA indeed induced more robust Ca2+ signal, perhaps through the endogenous AA-regulated channels (Mignen and Shuttleworth, 2001; Wu et al., 2002). However, the AA-induced endogenous response occurred more slowly than the 2APB-evoked activation of TRPV3 so that at the peak of the 2APB response, the contribution from the AA-regulated channels is negligible. Nonetheless, the slow decay at 30 μM AA in Figure 1B could be attributed to the endogenous channels.
AA-induced potentiation of TRPV3 currents in HEK293 cells
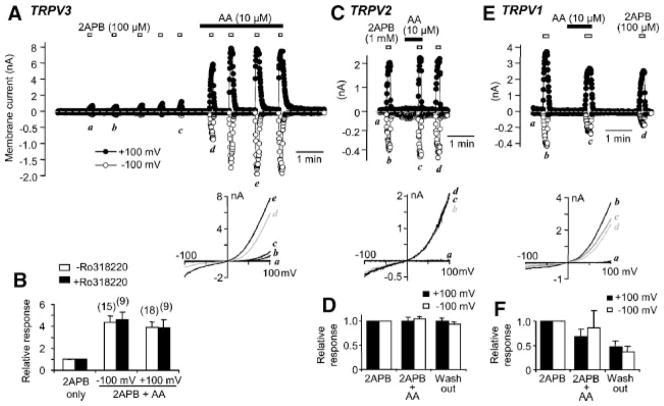
To confirm the potentiating action of AA on 2APB-evoked activation of TRPV3 channels, we studied the whole cells currents in TRPV3-transfected HEK293 cells. Exposure to 10 μM AA caused about threefold increase in 2APB-evoked currents at both the positive and negative membrane potentials with the potentiation at −100 mV being slightly larger than at +100 mV (Fig. 2A,B). In addition to TRPV3, 2APB also activates TRPV1 and TRPV2 (Hu et al., 2004; Chung et al., 2005). However, different from TRPV3, AA did not significantly increase the 2APB-evoked currents in cells that expressed TRPV1 or TRPV2 (Fig. 2C–F). It should be noted that although TRPV1 is activated by the lipoxygenase products of AA, most cells do not express enough lipoxygenases to sufficiently convert exogenously applied AA for activating TRPV1 unless exogenous 12-lipoxygenase is overexpressed (Shin et al., 2002). Thus, among the three 2APB sensitive TRPV channels, only TRPV3 is potentiated by exogenously applied AA.
Fig. 2.
AA potentiates 2APB-evoked currents in HEK293 cells expressing TRPV3 but not those expressing TRPV1 or TRPV2. A: TRPV3-transfected HEK293 cells were stimulated with 2APB multiple times to establish a basal response level to 2APB. Then AA was applied continuously while 2APB was added as indicated. Cells were held at 0 mV in whole-cell mode and currents were recorded using voltage ramps from −100 to +100 mV at 2 Hz. BAPTA was 1 mM in the pipette for all experiments. Upper, membrane currents at +100 (filled circles) and −100 mV (open circles). Note: different scales are used. Lower, I–V curves obtained by the voltage ramps at the indicated time points. B: Summary of relative responses to 100 μM 2APB with or without AA treatment. Cells were pretreated (filled bars) or not (open bars) with 1 μM Ro318220 for >20 min. C: Similar experiment as in (A) except that the cell was transfected with cDNA for TRPV2 and that the concentration of 2APB was 1 mM. D: Summary of relative responses for TRPV2-transfected cells n = 4. E: Similar experiment as in (A) except that the cell was transfected with cDNA for TRPV1. Note: desensitization occurred for TRPV1 during repetitive stimulation. F: Summary of relative responses for TRPV1-transfected cells n = 6.
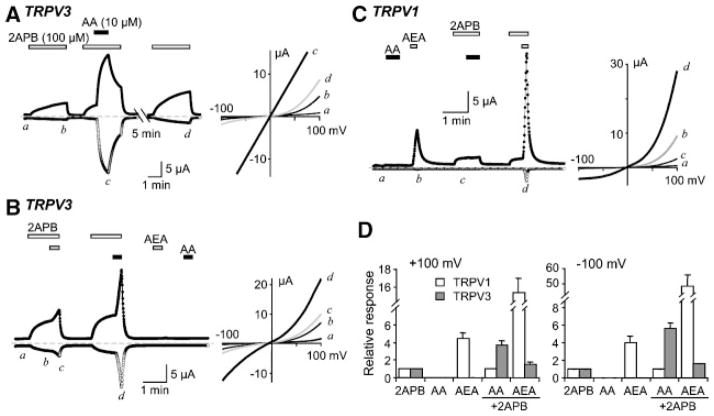
Ruthenium red (RR, 10 μM), a common TRPV channel blocker, suppressed the inward but increased the outward current activated by 2APB plus AA (Fig. 3A–D). The degree of inhibition at −100 mV and that of increase at +100 mV are not different from the changes caused by RR in cells stimulated with 2APB only (Fig. 3C,D). This peculiar behavior of RR has been shown for TRPV3 currents (Hu et al., 2004; Chung et al., 2004b, 2005). Moreover, increasing BAPTA concentration in the pipette solution from 1 to 10 mM did not prevent the AA-induced potentiation, indicating that changes in [Ca2+]i are not required for this modulation.
Fig. 3.
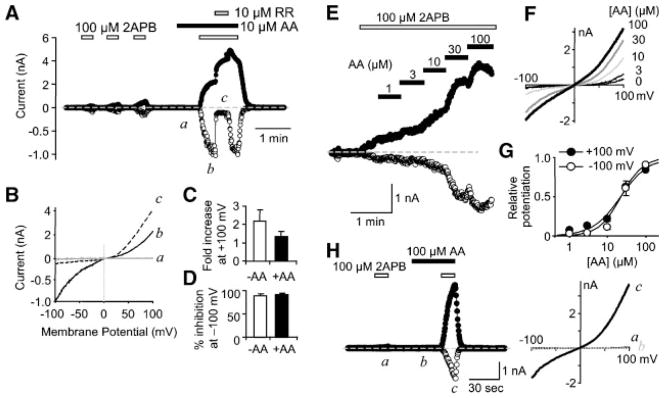
Properties of AA-potentiated TRPV3 currents. A–D, effects of 10 μM ruthenium red (RR) on the currents evoked by AA and 2APB in TRPV3-transfected cells. Shown are representative traces for membrane currents (A) at +100 (filled circles) and −100 mV (open circles) and I–V curves (B) during the application of AA (a), AA +2APB (b), and AA +2APB +RR (c). Dashed line (A) indicates zero current. Different scales are used to enlarge the current at negative potentials. (C, D) Summaries of fold increase at +100 mV (C) and % inhibition at −100 mV (D) induced by 10 μM RR for TRPV3 currents evoked by 2APB alone (open bars, n = 6) or 2APB +AA (filled bars, n = 8). E–G: Dose-dependence of AA-induced potentiation of 2APB-evoked TRPV3 currents. E: Membrane currents at +100 and −100 mV during continued application of 2APB and AA was added with increasing concentrations as indicated. F: I–V curves obtained at AA concentrations as indicated (in μM). G: Dose-response curves obtained after subtraction of the initial response to 2APB alone. Shown are normalized responses at +100 (filled circles) and −100 mV (open circles) from four cells. Curves are fits to the Hill equation. H: Membrane currents before and during preincubation with 100 μM AA for 30 sec and subsequent addition of 2APB in a TRPV3-transfected cell. I–V curves are shown to the right for time points as indicated. Similar results were obtained from two more cells. BAPTA was 10 mM in the pipette solution for all experiments.
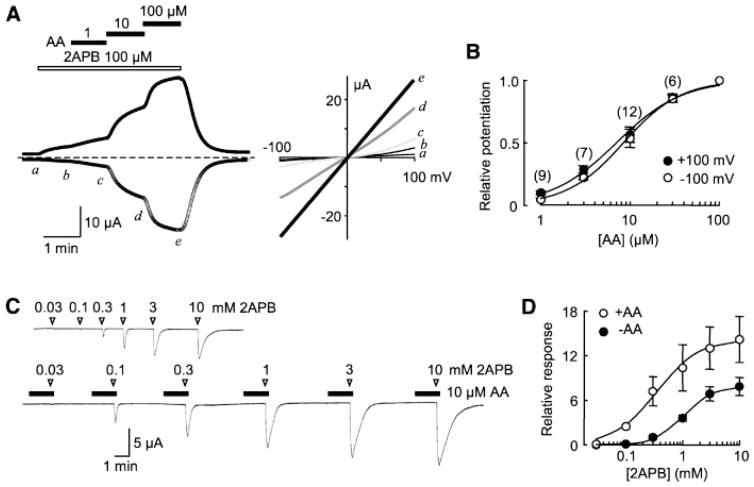
The AA-induced potentiation of TRPV3 currents was also observed when cells were continuously stimulated with 2APB (Fig. 3E,F). The degree of potentiation was dependent on AA concentrations and approached the maximum at ~100 μM. The EC50 values of AA were estimated to be 21.9 ± 4.5 μM (Hill coefficient, nh = 1.2 ± 0.5) at 100 mV and 22.6 ± 2.5 μM (nh = 1.5 ± 0.3) at −100 mV (Fig. 3G). At high AA concentrations (30 and 100 μM) the inward currents increased more strongly than the outward ones, giving rise to less rectifying current-voltage (I–V) curves, consistent with the observation that strongly activated TRPV3 currents had a linear I–V relationship (Chung et al., 2005). Notably, the potentiation obtained with this protocol is less than when cells were preincubated with AA and stimulated with 2APB afterwards, indicative of a relatively slow action of the fatty acid. This could result in a higher estimate of the EC50 values. Furthermore, at high concentrations, AA may form micelles that could cause membrane disruption and thus a non-specific leak. However, this does not seem to be the case as preincubation with 100 μM AA for 30 sec did not cause any obvious current in TRPV3-expressing cells, but it strongly increased the currents evoked by 2APB (Fig. 3H). The critical micelle concentration (CMC) for AA varies from 30 to 236 μM in the literature (Attwell et al., 1993; Wilson and Binder, 1997; Chirita et al., 2003). These differences are likely due to the fact that CMCs are highly dependent on salt concentrations, temperature, pH, and other factors employed for different experiments. To examine whether micelle formation could contribute to the increased current at high AA concentrations, we have measured the CMC of AA in the same bath solution we used for whole-cell experiments of HEK293 cells and obtained a value of 173 ± 24 μM (n = 6), which is higher than the highest AA concentration (100μM) we have used. Addition of 100μM 2APB to the bath solution did not change the CMC value (182 ± 20 μM, n = 6), indicating that 2APB does not promote micelle formation of the fatty acid. Therefore, it is unlikely that micelle formation contributes to the potentiating effect of AA on TRPV3.
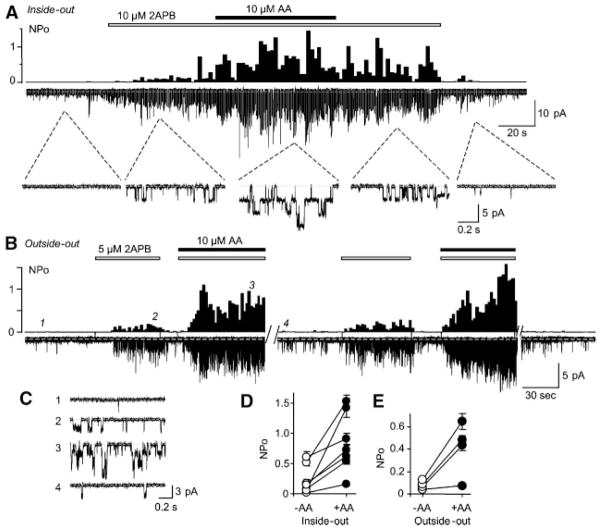
In excised inside-out patches, single channel currents were activated by 5 or 10 μM 2APB from cells that expressed TRPV3 but not control untransfected cells. Addition of 10 μM AA increased the open probability (NPo) 9.1 ± 3.9 fold (n = 7) (Fig. 4A,D). Similarly, in outside-out patches, the 2APB-evoked TRPV3 single channel activity was also potentiated by 10 μM AA with an average increase of NPo of 4.8 ± 1.0 fold (n = 4) (Fig. 4B,C,E).
Fig. 4.
Potentiation by AA of single channel activity in patches excised from HEK293 cells expressing TRPV3. A: Activity in an inside-out patch held at −100 mV. 2APB and AA were added as indicated. Shown are NPo in 2-sec bins (upper), raw current trace (middle), and in an expanded time scale (lower). B: Activity in an outside-out patch held at −50 mV. 2APB and AA were added as indicated. Note: this patch was stimulated twice with the same protocol. The two breaks between traces were 11 and 3 sec. Shown are NPo in 2-sec bins (upper) and raw current traces (lower). C: Expanded current traces at the time points indicated in (B). (D and E): Changes in average NPo in individual patches of inside-out (D) and outside-out (E) before (−AA) and after (+AA) the addition of AA.
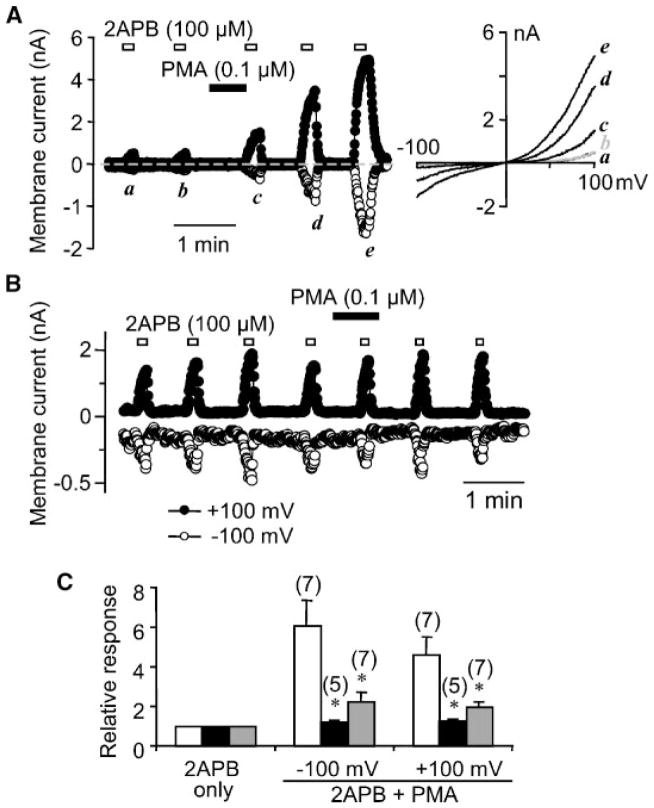
A similar potentiating effect was obtained with the use of phorbol-12-myristate-13-acetate (PMA), indicating that TRPV3 activity is also enhanced by PKC phosphorylation (Fig. 5A). As expected, the PMA-induced potentiation was blocked by pretreatment of the TRPV3-expressing cells with PKC inhibitors Ro318220 (1 μM) and Go6976 (0.3 μM) (Fig. 5B,C). However, even though unsaturated fatty acids are known to activate PKC (Murakami and Routtenberg, 1985; Seifert et al., 1988), the potentiating effect of AA on TRPV3 activity is not dependent on PKC because Ro318220 (1 μM) failed to block the action of AA (Fig. 2B).
Fig. 5.
Potentiation of 2APB-evoked TRPV3 currents by PMA in HEK293 cells. A: Similar experiment as in Figure 2A except the TRPV3-expressing cell was treated with 0.1 μM PMA for 40 sec as indicated. B: Similar to (A) except the cell was pretreated with 1 μM Ro318220 for 30 min. C: Summary of relative responses to 100 μM 2APB with or without PMA treatment. Cells were untreated (open bars) or pretreated with 1 μM Ro318220 (filled bars) or 0.3 μM Go6976 (gray bars) for >20 min. *P <0.05, significantly different from untreated cells by unpaired t test. BAPTA was 1 mM in the pipette solution.
AA-induced potentiation of TRPV3 currents in Xenopus oocytes
The Xenopus oocyte system provides an alternative method to study ectopically expressed channels and to verify whether the regulation is dependent on host cell types. Compared to the conventional whole-cell recording technique for mammalian cells, the two-electrode voltage clamp method used to record the channel current in oocytes is less invasive and is not accompanied with the dilution of cellular contents. For TRPV3, the oocyte system has an additional advantage in that it allows repetitive stimulation of the channel under Ca2+-free and nominally Ca2+-free conditions for a long time (>2 h) without any apparent sensitization or desensitization. Sensitization to repetitive stimulation has been known to be a unique property of TRPV3 in mammalian systems (Xu et al., 2002; Chung et al., 2004a,b, 2005) and could contribute to the increase in response to 2APB in the experiments described above. In Xenopus oocytes injected with cRNA for TRPV3, 100 μM 2APB evoked an outwardly rectifying current that reversed at 0 mV (Fig. 6A). Addition of 10 μM AA greatly potentiated the 2APB-evoked current. At the peak of the potentiation, the I–V relationship became linear (Fig. 6A, right). This linear I–V relationship is also observed in Ca2+-free bath solutions for TRPV3 expressed in HEK293 cells when the channel is maximally activated (HH and MXZ, unpublished observations). The washout of the AA effect from the oocytes was slow. After 7 min, more than 80% of the potentiation was removed (Fig. 6A). However, complete washout usually took more than 20 min (not shown). The AA-induced potentiation was also observed during continuous recordings with cells held constantly at −40 mV. In average, 10 μM AA increased the current evoked by 300 μM 2APB 10.7 ± 1.6 fold (n = 60).
Fig. 6.
Potentiation of 2APB-evoked TRPV3 currents by AA in Xenopus oocytes. A: Consecutive responses of an oocyte that expressed TRPV3 to 3 min stimulations with 100 μM 2APB (open bars). AA (10 μM, black bar) was included for 1 min during the second stimulation. The oocyte was held at −20 mV and currents were recorded using voltage ramps from −100 to 100 mV at 1 Hz. Left, membrane currents at +100 (filled circles) and −100 mV (open circles). Right, I–V curves obtained by the voltage ramps at the indicated time points. B: Similar to (A) for a different oocyte, except 2APB (100 μM) was applied for 1.5 min and 10 μM AA or 10 μM AEA (gray bars) was applied for 30 sec. Note: the period of AA application was shorter than in (A) and the potentiation was truncated before it reached peak. Therefore, the I–V curve did not become completely linear. C: Similar to (B) but the oocyte expressed TRPV1. AA and AEA, both at 10 μM, were applied for 30 and 15 sec, respectively. D: Summary of responses relative to 100 μM 2APB alone at +100 (left) and −100 mV (right) for oocytes that expressed TRPV1 (white bars, n = 5) and TRPV3 (gray bars, n = 7). Recording conditions were the same as in (B) and (C).
Again, the AA-induced potentiation of the response to 2APB was specific to TRPV3. A similar treatment with AA did not increase the response to 2APB in oocytes injected with TRPV1 cRNA (Fig. 6C,D). As a control, anandamide (AEA) was used to activate TRPV1. When applied individually, both 2APB (100μM) and AEA (10μM) weakly activated TRPV1. When the two drugs were applied together, the response was more than additive, indicative of synergy between these drugs (Fig. 6C,D). By contrast, AEA only slightly increased the 2APB-evoked current in TRPV3-expressing cells (Fig. 6B,D), which could be due to a partial conversion of AEA to AA (Watanabe et al., 2003). Similarly, N-arachidonoyl dopamine, another endogenous TRPV1 agonist and an AA derivative, also failed to potentiate the 2APB response of TRPV3, while it activated TRPV1 under the same condition (not shown). These results indicate that the free carboxylate head group of AA is critical for the potentiating effect on TRPV3.
A dose-dependence analysis showed that the potentiation of the 2APB-elicited TRPV3 current can be detected with as low as 1 μM AA and approaches the maximum at ~100 μM (Fig. 7A,B). The EC50 values of AA were estimated to be 7.1 ± 0.6 μM (Hill coefficient, nh = 1.2 ± 0.1) at 100 mV and 8.5 ± 0.5 μM (nh = 1.4 ± 0.1) at −100 mV. Note the CMC of AA in the oocyte bath solution (nominally Ca2+ free) was determined to be 112 ± 15 μM (n = 3).
Fig. 7.
Concentration dependence of 2APB-evoked TRPV3 currents and its potentiation by AA in Xenopus oocytes. A: Representative traces showing the dose-dependent potentiation by AA of the response to 100 μM 2APB in an occyte that expressed TRPV3. B: Dose response curves were obtained as in Figure 3G. Shown are normalized responses from numbers of cells indicated in parentheses. C: Dose response relationships for 2APB without (upper) or with (lower) the pretreatment of 10 μM AA in the same oocyte that expressed TRPV3. The oocyte was held at −40 mV and current continuously recorded at 100 Hz. D: Dose response curves for 2APB-induced activation of TRPV3 at −40 mV in the absence (filled circles) or presence (open circles) of 10 μM AA. Data are fitted with Hill equation. For (C) and (D), the highest DMSO concentration used was 2.4% (for 10 mM 2APB). However, even at 10%, DMSO did not activate any current in the presence and absence of 10 μM AA and it did not increase the current activated by 300 μM 2APB in TRPV3-expression oocytes.
To better understand the effect of AA on 2APB-evoked TRPV3 function, we established the dose response curves for 2APB in the absence and presence of 10 μM AA. As shown in Figure 7C,D, both the sensitivity and the maximal response of TRPV3 to 2APB were increased by the pretreatment of AA. At −40 mV, the EC50 values of 2APB were 1060 ± 42 μM, (nh = 1.7 ± 0.2, n = 6) without AA and 348 ± 37 μM (nh = 1.2 ± 0.1, n = 5) with AA. Note, about 10-fold higher EC50 values have been found for capsaicin for the activation of TRPV1 in oocytes than in mammalian cells (McIntyre et al., 2001; Wang et al., 2004). Therefore, the EC50 value of 2APB for the activation of TRPV3 in oocytes is also expected to be higher than in mammalian cells (Chung et al., 2004b; Hu et al., 2004).
The potentiating effect of AA on TRPV3 is not dependent on its metabolism
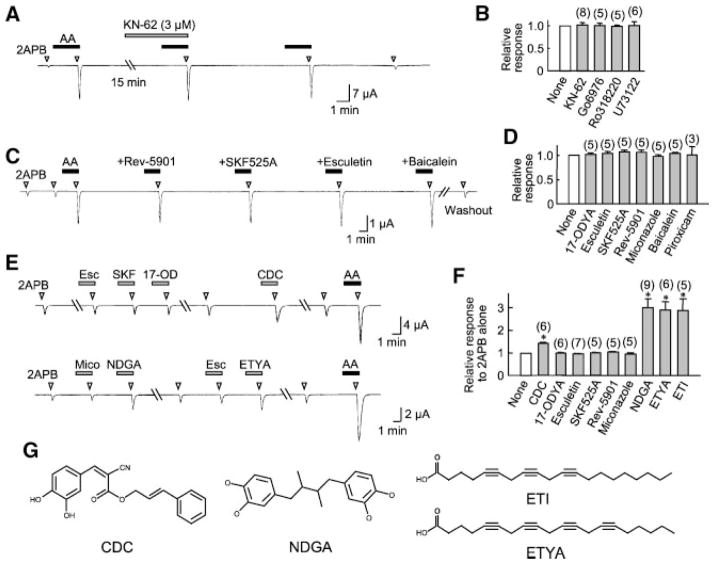
Just as in HEK293 cells, the potentiating effect of AA on TRPV3 expressed in oocytes was not affected by the PKC inhibitors, Ro318220 (1 μM) and Go6976 (0.3 μM). KN-62, an inhibitor of calmodulin-dependent kinases, also failed to affect the action of AA (Fig. 8A,B), ruling out the possibility that AA enhanced the channel activity through Ca2+ mobilization and in turn the activation of calmodulin-dependent kinases. Furthermore, preincubation of the oocytes with 10 μM U73122, a blocker of phospholipase C, for 15 min, had no effect on the potentiation by AA (Fig. 8B), indicating that activation of the phospholipase C cascade is not required.
Fig. 8.
Effects of kinase and AA metabolic blockers on 2APB-elicited TRPV3 current in Xenopus oocytes. A: A representative trace showing that KN-62 did not affect the potentiation effect of AA in oocytes that expressed TRPV3. Cells were held at −40 mV and treated with 300 μM 2APB with or without preincubation with 10 μM AA in the absence or presence of the inhibitor as indicated. B: Relative potentiation of the response to 300 μM 2APB by 10 μM AA without (open bar) or with (gray bars) the pretreatment with kinase inhibitors, KN-62 (3 μM), Go6979 (0.3 μM), and Ro318220 (1 μM) or the phospholipase C inhibitor, U73122 (10 μM), for >15 min. Shown are responses normalized to the value obtained with 2APB plus AA only. (C and D): Similar to (A) and (B) but AA metabolic blockers were used as indicated. All inhibitors were tested at 10 μM except for piroxicam, which was 60 μM. E: Representative traces showing the direct effects of some AA metabolic inhibitors on the 2APB-evoked TRPV3 current. Esc, SKF, 17-OD, and Mico denote esculetin, SKF525A, 17-ODYA, and miconazole, respectively. Cells were held at −40 mV and stimulated with 300 μM 2APB with or without pretreatment of 10 μM AA metabolic blockers. F: Summary of responses normalized to that obtained with 2APB alone. *P <0.01 different from 1. G: Structures of CDC, NDGA, ETI, and ETYA.
In order to know whether AA acts on TRPV3 directly or through its oxidized products, as in the case of TRPV1 and TRPV4, we applied metabolic blockers of AA, including cyclooxygenase inhibitor piroxicam (60 μM), lipoxygenase inhibitors NDGA (10 μM), 5,8,11-eicosa-triynoic acid (ETI, 10 μM), Rev-5901 (10 μM), baicalein (10 μM), CDC (10 μM), and esculetin (10 μM), and epoxygenase inhibitors 17-ODYA (10 μM), miconazole (10 μM), and SKF525A (10 μM), as well as the non-metabolizable AA analogue ETYA (1–100 μM). None of these drugs blocked the potentiating effect of AA (Fig. 8C,D), indicating that oxidation of AA is not required for the modulation of TRPV3 function. On the other hand, when applied without AA, while most drugs had no significant effect on the 2APB-elicited TRPV3 current, some (CDC, ETI, ETYA, and NDGA) also enhanced the channel activity by themselves (Fig. 8E,F). ETYA and ETI are triple-bond analogous of AA and another polyunsaturated fatty acid, 5,8,11-eicosatrienoic acid (20:3 n9, mead acid), respectively (Fig. 8G). The fact that these non-metabolizable fatty acids also potentiated the TRPV3 activity further supports the idea that the channel is regulated directly by free fatty acids but not their oxidation products.
A dose response analysis of ETYA on the potentiation of 2APB-evoked TRPV3 current at −40 mV indicates that the non-metabolizable fatty acid has a slightly lower affinity as compared to AA (EC50 = 21 ± 4 μM, n = 6), and its efficacy is three times lower than that of AA at the concentration (100 μM) that gave the maximal potentiation. The average TRPV3 current evoked by 300 μM 2APB at −40 mV increased from −1.4 ± 0.3 μA (n = 13) to −45 ± 9 μA with 100 μM AA (n = 7) and −11 ± 1 μA with 100 μM ETYA (n = 6). This difference is statistically significant (P = 0.006, by unpaired t test).
The 2APB-evoked TRPV3 response is enhanced by other unsaturated fatty acids
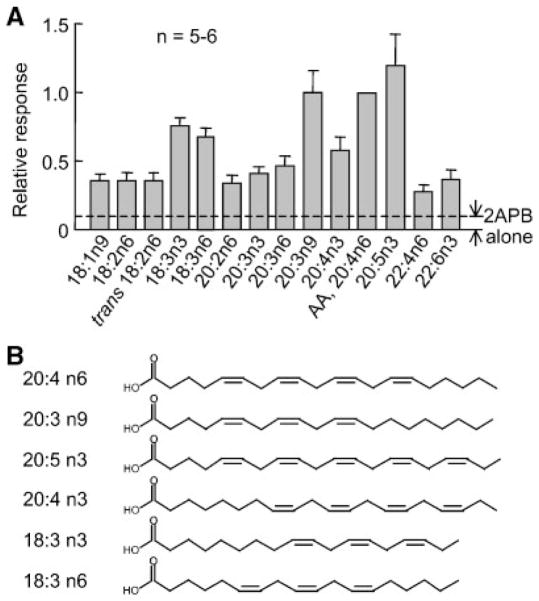
Next, we tested a number of fatty acids for their effects on TRPV3 activity. These included a saturated fatty acid, palmitic acid (16:0), trans unsaturated fatty acids, elaidic (trans 18:1 n9) and linoelaidic (all trans 18:2 n6) acids, and many cis unsaturated fatty acids of the Δ9, ω-3, and ω-6 desaturase families. At 10 μM, none of the fatty acids directly activated TRPV3. Except for palmitic and elaidic acids (n = 11 for each), all other fatty acids potentiated the TRPV3 current evoked by 300 μM 2APB (Fig. 9A). Palmitic acid also failed to enhance the TRPV3 activity at up to 500 μM (n = 3, data not shown), suggesting that only unsaturated fatty acids are able to potentiate TRPV3 channel function. Noticeably for unsaturated fatty acids, those with the double bonds starting at the fifth position from the carboxylated head group, 5,8,11-eicosatrienoic acid (20:3 n9), 5,8,11,14-eicosatetraenoic acid (AA, 20:4 n6), and 5,8,11,14,17-eicosapentaenoic acid (20:5 n3) (see structures in Fig. 9B), had the strongest effect on the potentiation of the 2APB response (~10-fold at −40 mV). The two linolenic acids (18:3 n3 and 18:3 n6) and ω-3 AA (20:4 n3) had intermediate effect (~5- to 7-fold) while the rest of them showed a more moderate effect with 2- to 3-fold increase of the 2APB-induced response. Interestingly, the potency order does not correlate with the number of double bonds but rather to their positions. At least for those with a moderate effect, the configuration of the unsaturated bonds did not matter. For example, 10 μM linoelaidic (all trans 18:2 n6) and linoleic (all cis 18:2 n6) acids (Fig. 9A) as well as ETI and ETYA (Fig. 8F) all caused about 2–3 fold increase of the 2APB response. The monounsaturated fatty acids appeared to be exempted from this rule since only oleic (cis 18:1 n9) but not elaidic (trans 18:1 n9) acid or 17-ODYA enhanced the 2APB-evoked TRPV3 current.
Fig. 9.
Effect of different free fatty acids on 2APB-evoked TRPV3 current in Xenopus oocytes. A: Oocytes expressing TRPV3 were held at −40 mV and stimulated with 300 μM 2APB without or with the pretreatment of 10 μM different free fatty acid as indicated. Shown are current amplitudes normalized to that obtained with 2APB plus 10 μM AA. Dashed line shows the response level to 2APB alone, based on the average increase induced by 10 μM AA for 300 μM 2APB at −40 mV. B: Structures of selected free polyunsaturated fatty acids.
DISCUSSION
Thermosensitive channels are not only important for temperature sensing but also involved in inflammatory pain and tissue injury-induced thermal hyperalgesia. It has become clear that temperature is only one of the factors that regulate the heat-activated TRPV channels. A growing number of natural and synthetic compounds as well as endogenous ligands have been identified for TRPV1 and, to a lesser extent, for TRPV2–4 (Caterina et al., 1997; Hwang et al., 2000; Smart et al., 2000; Huang et al., 2002; Watanabe et al., 2002, 2003; Chu et al., 2003; Hu et al., 2004; Chung et al., 2004b, 2005). In addition, both TRPV2 and TRPV4 are activated by hypotonicity-induced cell swelling (Liedtke et al., 2000; Strotmann et al., 2000; Nilius et al., 2001; Muraki et al., 2003), showing that these channels are polymodal detectors of noxious or innocuous heat, pressure, and various chemical stimuli.
The effects of inflammatory mediators on TRPV1 have been extensively studied. First, TRPV1 is activated by protons (Tominaga et al., 1998), implicating its role in tissue acidosis associated with inflammation. Second, bradykinin, ATP, prostaglandins, glutamate, and nerve growth factor activate or potentiate the activity of TRPV1 indirectly by triggering cell signaling events through their respective receptors (Chuang et al., 2001; Tominaga et al., 2001; Hu et al., 2002). Several protein kinase A- and PKC-dependent phosphorylation sites have been shown to be involved in the receptor-induced modulation of TRPV1 activity (Bhave et al., 2002, 2003; Numazaki et al., 2002). In addition, the breakdown of phosphatidylinositol 4,5-bisphosphate appears to be critical for the enhancement of TRPV1 activity elicited by the stimulation of phospholipase C (Chuang et al., 2001; Prescott and Julius, 2003). Furthermore, the release of AA following receptor activation could also contribute to the sensitization of TRPV1 during inflammation. Several lipoxygenase products of AA could stimulate TRPV1 at room temperatures (Hwang et al., 2000). Interestingly, this effect was not mimicked by exogenous application of AA unless 12-lipoxygenase was overexpressed (Shin et al., 2002). Consistent with this, our data also show that AA has no significant effect on TRPV1 expressed in Xenopus oocytes and HEK293 cells.
Here, we show that at least two components of the inflammatory response are capable of increasing TRPV3 channel function. First, TRPV3 activity is enhanced by a short treatment with PMA, suggesting that phosphorylation by PKC is involved in the regulation of this channel. Second, the activity was potentiated by unsaturated fatty acids, among which AA is one of the most potent. However, the effect of AA is not dependent on the protein kinases as it is not affected by kinase inhibitors. We show here that AA also potentiates the 2APB-elicited [Ca2+]i rise in mouse keratinocytes, which express functional TRPV3 endogenously (Chung et al., 2003, 2004a,b; Moqrich et al., 2005), indicating that just like the heterologously expressed channels, native TRPV3 channels are positively regulated by free unsaturated fatty acids. This enhancement of TRPV3 function by PKC and fatty acids may be involved in inflammatory responses other than inflammation-induced thermal hyperalgesia, which, however, was reportedly unaffected in TRPV3 knockout mice (Moqrich et al., 2005).
The physiological and pathophysiological relevance of fatty acid-induced potentiation of TRPV3 function depends on the overall concentration of total unsaturated fatty acids. On appearance, the AA concentration required for the maximal potentiation of TRPV3 activity may be very high. However, such a high level activation of TRPV3 may never be necessary in vivo as the degree of AA potentiation is so strong that 1 μM AA is sufficient to cause significant increase in channel activity. In addition, the potentiation is further increased by longer incubations with the fatty acid. The broad effective range of AA concentration, on the other hand, may be important for TRPV3 to work as a polymodal sensor in skin cells and, perhaps, sensory nerve terminals in pathological conditions, under which the fatty acid concentrations vary widely due to infiltration and breakdown of lymphocytes. For example, in the most severe case of involved psoriasis, which is an auto-immune disease affecting skin, the AA concentration in epidermis is about 100 μM. In uninvolved psoriasis, AA concentration would also reach as high as 13 μM (Hammarstrom et al., 1975; Brash, 2001). Under less severe conditions, the concentration for all free unsaturated fatty acids together is likely to be sufficient to cause potentiation of TRPV3, which may be activated by heat or an endogenous ligand(s) whose action is mimicked by 2APB. Thus, the regulation of TRPV3 by unsaturated fatty acids is of close relevance to human health. Not surprisingly, several other ion channels, for example, the two-pore domain K+ channels, are regulated by AA at a similar concentration range (Fink et al., 1998; Patel et al., 1998) and the effect of fatty acids on these channels does not saturate at 100 μM (Patel et al., 2001).
Unlike the action of AA on TRPV1 and TRPV4, the potentiation of TRPV3 function by AA does not require metabolization of the fatty acid. The finding that nearly all unsaturated fatty acids, including monounsaturated cis and polyunsaturated trans and triple-bond fatty acids, as well as certain lipoxygenase inhibitors (CDC and NDGA), are able to enhance TRPV3 channel activity suggests a more direct action by the fatty acids. This potentiation profile is remarkably similar to the agonist profile reported for Drosophila TRP and TRPL, which are also thought to be directly activated by the fatty acids (Chyb et al., 1999). The resistance to kinase and phospholipase C blockers and intracellular Ca2+ chelating by BAPTA, as well as the recapitulation of AA-induced enhancement in excised membrane patches, supports the idea that further metabolism and/or signal transduction are not required for the potentiating effect of AA on TRPV3. Metabolic stress has also been shown to activate Drosophila TRP and TRPL (Agam et al., 2000). This activation could occur through an inhibition of diacylglycerol kinase, providing more substrates for the generation of free unsaturated fatty acids (Hardie et al., 2003). The fact that AA also increased the 2APB-evoked TRPV3 activity in excised inside-out patches would appear to exclude any involvement of metabolic inhibition in the fatty acid-induced potentiation. Since 1-oleoyl-2-acetyl-sn-glycerol (up to 100 μM) neither activated nor potentiated the activity of TRPV3 (data not shown), a direct action of diacylglycerols on the TRPV3 channel is unlikely.
The mechanism of potentiation of TRPV3 activity by unsaturated fatty acids remains to be resolved. Fatty acids modulate ion channels either by interacting with channel protein or by partitioning into lipid bilayer (Patel et al., 2001). The non-specific detergent effect of fatty acids on membrane may be ruled out by the lack of function of palmitic and elaidic acids. Because of the potentiating effect of linoelaidic acid, it also seems that the potentiation does not result from an increase in membrane fluidity, which occurs with cis but not trans isomers of unsaturated fatty acids (Chyb et al., 1999). Membrane bending has been implicated in the activation of some mechanosensitive channels that are also activated by unsaturated fatty acids (Kung, 2005). Whether this explains the fatty acid-induced potentiation of TRPV3 is debatable since the mechanosensitive TRPV2 (Muraki et al., 2003) is not potentiated under the same conditions. Furthermore, the strength of the potentiation is not dependent on the length of the fatty acid chain or the number of unsaturated bonds. Among the fatty acids that potentiate TRPV3, the most potent ones, 20:3 n9, 20:4 n6; 20:5 n3, all contain double bonds that start at the fifth position from the carboxylated head group, indicative of the involvement of a common structural motif that binds, maybe, directly to channel protein. Thus, our data are consistent with a direct effect of unsaturated fatty acids on TRPV3 channel, although an action through changes in membrane properties cannot be completely ruled out at the present time.
The mechanism by which CDC and NDGA enhance 2APB-evoked activation of TRPV3 is unclear. These inhibitors are not structurally similar to AA (Fig. 8G). Their effect could be indirect through inhibition of lipoxidation of endogenous fatty acids like in the case of their activation of Drosophila TRP and TRPL channels (Chyb et al., 1999). This interpretation is questionable given that other lipoxygenase inhibitors, Rev-5901, baicalein, and esculetin, did not have a similar potentiating effect. However, it is possible that only those lipoxygenases, for example, 15-lipoxygenase, that are sensitive to CDC and NDGA are actively involved in AA metabolism under resting conditions in Xenopus oocytes and as such, only their inhibition results in accumulation of significant amount of free unsaturated fatty acids.
The current study suggests that distinct molecular mechanisms are involved in the regulation of TRPV channels by polyunsaturated fatty acids such as AA. An intriguing possibility is for TRPV channels to serve as sensors of AA metabolism. AA, in its fatty acid form, enhances the activity of TRPV3; its epoxygenase products stimulate TRPV4 (Watanabe et al., 2003); its lipoxygenase products activate TRPV1 directly (Hwang et al., 2000), while its cyclooxygenase products (prostaglandins) prolong TRPV1 activity indirectly through receptor-induced protein kinase A activation and phosphorylation of the channel protein (Bhave et al., 2002). Interestingly, it was recently demonstrated that a subset of 20-carbon polyunsaturated fatty acids are critically important for sustaining TRPV-mediated Ca2+ signaling in sensory neurons of C. elegans as well as the olfactory and nociceptive behaviors of the nematode that depend on TRPV signaling (Kahn-Kirby et al., 2004). Thus, modulation of TRPV channels by polyunsaturated fatty acids had occurred early during evolution. Another interesting finding is that the concentrations of the three polyunsaturated fatty acids (20:3 n9, 20:4 n6; 20:5 n3) that are most potent for enhancing TRPV3 activity were specifically decreased in the plasma from patients with chronic renal failure and a history of pruritus symptoms (Peck et al., 1996). Although how pruritus or itching is linked to plasma fatty acid regulation and TRPV3 function is unknown, these data highlight the uniqueness of these three polyunsaturated fatty acids in itch sensation, during which the downregulation of the fatty acid concentrations could help maintain a lower activity of TRPV3.
In summary, we have demonstrated that TRPV3 activity is positively modulated by PKC and free unsaturated fatty acids. Activation of PKC in skin cells and sensory neurons is an important event downstream from receptor activation by a variety of inflammatory mediators and concentrations of free unsaturated fatty acids are elevated in inflammatory states. Our data suggest that TRPV3 might have involvement similar or complementary to that of TRPV1 in the production of the sensory hypersensitivity characteristic of inflammatory responses to tissue injury, infectious agents and of inflammatory diseases of intrinsic origin.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: R01-DK057075, R01-NS042183, and P30-NS045758.
The authors wish to thank Dr. Stuart Yuspa for the 308 keratinocytes, Dr. Jeff Kuret on the help for CMC measurements, Dr. Alexander Zholos for technical advice on single channel studies, and Ms. Dina Chuang-Zhu for technical assistance. This work was supported by the US NIH grants DK057075 (to J.D.W.) and NS042183 (to M.X.Z.). Additional support was provided by NIH grant P30-NS045758.
Abbreviations
2APB
2-aminoethoxydiphenyl borate
AEA
anandamide
AA
arachidonic acid
[Ca2+]i
intracellular Ca2+ concentrations
CDC
cinnamyl-3,4-dihydroxy-cyanocinnamate
CMC
critical micelle concentration
ECS
extracellular solution
ETI
5,8,11-eicosatriynoic acid
ETYA
5,8,11,14-eicosatetraynoic acid
I–V
current–voltage
nh
Hill coefficient
NDGA
nordihydroguaiaretic acid
NPo
open probability
17-ODYA
17-octade-cynoic acid
PKC
protein kinase C
PMA
phorbol-12-myristate-13-acetate
RR
ruthenium red
TRP
transient receptor potential
TRPV
vanilloid subfamily of TRP
LITERATURE CITED
- Agam K, von Campenhausen M, Levy S, Ben-Ami HC, Cook B, Kirschfeld K, Minke B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci. 2000;20:5748–5755. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., IV cAMP-dependant protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu H, Glauner K, Zhu W, Wang H, Brasier D, Oxford G, Gereau R., IV Protein kinase C phosporylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito RM, Vaz WL. Determination of the critical micelle concentration of surfactants using the fluorescent probe N-phenyl-1-naphthylamine. Anal Biochem. 1986;152:250–255. doi: 10.1016/0003-2697(86)90406-9. [DOI] [PubMed] [Google Scholar]
- Caterina M, Schumacher M, Tominaga M, Rosen T, Levine J, Julius D. The Capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proc Natl Acad Sci USA. 1999;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirita CN, Necula M, Kuret J. Anionic micelles and vesicles induce tau fibrillization in vitro. J Biol Chem. 2003;278:25644–25650. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- Chu C, Huang S, De Petrocellis L, Bisogno T, Ewing S, Miller J, Zipkin R, Daddario N, Appendino G, Di Marzo V, Walker J. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- Chuang H, Prescott E, Kong H, Sheilds S, Jordt S, Basbaum A, Chao M, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Chung M, Lee H, Caterina M. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004a;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004b;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Guler AD, Caterina MJ. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J Biol Chem. 2005;280:15928–15941. doi: 10.1074/jbc.M500596200. [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci USA. 1975;72:5130–5134. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Chyb S, Raghu P. Rescue of light responses in the Drosophila “null” phospholipase C mutant, norpAP24, by the diacylglycerol kinase mutant, rdgA, and by metabolic inhibition. J Biol Chem. 2003;278:18851–18858. doi: 10.1074/jbc.M300310200. [DOI] [PubMed] [Google Scholar]
- Hu H, Bhave G, Gereau R., IV Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: Potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Wang C, Colton CK, Tang J, Wood JD, Zhu MX. Regulation of TRPV3 function by polyunsaturated fatty acids. Biophys J. 2005;88:358A. Part 2 (abstract) [Google Scholar]
- Huang S, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros T, Krey J, Chu C, Miller J, Davies S, Geppetti P, Walker J, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Cho H, Kwak J, Lee S, Kang C, Jung J, Cho S, Min K, Suh Y, Kim D. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119:889–900. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom M, Bell A, Denis C, Sali A, Hudspeth A, Friedman J, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmosensor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P, McLatchie L, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Pang H, Bevan S, James I. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br J Pharmac. 2001;132:1089–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Shuttleworth TJ. Permeation of monovalent cations through the non-capacitative arachidonate-regulated Ca2+ channels in HEK293 cells. Comparison with endogenous store-operated channels. J Biol Chem. 2001;276:21365–21374. doi: 10.1074/jbc.M102311200. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Murakami K, Routtenberg A. Direct activation of purified protein kinase C by unsaturated fatty acids (oleate and arachidonate) in the absence of phospholipids and Ca2+ FEBS Lett. 1985;192:189–193. doi: 10.1016/0014-5793(85)80105-8. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Wissenbach U, Bodding M, Droogmans G. Differential activation of the volume-sensitive cation channel TRP12 (OTRPC4) and volume-regulated anion currents in HEK-293 cells. Pflugers Arch. 2001;443:227–233. doi: 10.1007/s004240100676. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cε and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honore E. Lipid and mechano-gated 2P domain K+ channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Peck LW, Monsen ER, Ahmad S. Effect of three sources of long-chain fatty acids on the plasma fatty acid profile, plasma prostaglandin E2 concentrations, and pruritus symptoms in hemodialysis patients. Am J Clin Nutr. 1996;64:210–214. doi: 10.1093/ajcn/64.2.210. [DOI] [PubMed] [Google Scholar]
- Peier A, Reeve A, Anderson D, Moqrich A, Earley T, Hergarden A, Story G, Colley S, Hogenesch J, Mcintyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Prescott D, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Reeh PW, Kress M. Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol. 2001;1:45–51. doi: 10.1016/s1471-4892(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Seifert R, Schachtele C, Rosenthal W, Schultz G. Activation of protein kinase C by cis- and trans-fatty acids and its potentiation by diacylglycerol. Biochem Biophys Res Commun. 1988;154:20–26. doi: 10.1016/0006-291x(88)90643-2. [DOI] [PubMed] [Google Scholar]
- Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Gunthorpe M, Jerman J, Nasir S, Gray J, Muir A, Chambers J, Randall A, Davis J. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Gunthorpe M, Kelsell R, Hayes P, Reilly P, Facer P, Wright J, Jerman J, Walhin J, Ooi L, Egerton J, Charles K, Smart D, Randall A, Anand P, Davis J. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant T. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina M, Malmberg A, Rosen T, Gilbert H, Skinner K, Raumann B, Basbaum A, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Hu H, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine trpv1 gene dominant-negatively modulates the activity of TRPV1 channels. J Biol Chem. 2004;279:37423–37430. doi: 10.1074/jbc.M407205200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Binder LI. Free fatty acids stimulate the polymerization of tau and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer’s disease. Am J Pathol. 1997;150:2181–2195. [PMC free article] [PubMed] [Google Scholar]
- Wu X, Babnigg G, Zagranichnaya T, Villereal ML. The role of endogenous human Trp4 in regulating carbachol-induced calcium oscillations in HEK-293 cells. J Biol Chem. 2002;277:13597–13608. doi: 10.1074/jbc.M110881200. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey S, Kotecha S, Moran M, Chong J, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano P, Curtis R, Clapham D. TRPV3 is a calcium-permeable temperature sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]