Opinion: Interactions of innate and adaptive lymphocytes (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 16.
Published in final edited form as: Nat Rev Immunol. 2014 Aug 18;14(9):631–639. doi: 10.1038/nri3726
Abstract
Innate lymphocytes, including natural killer (NK) cells and the recently discovered innate lymphoid cells (ILCs) have crucial roles during infection, tissue injury and inflammation. Innate signals regulate the activation and homeostasis of innate lymphocytes. Less well understood is the contribution of the adaptive immune system to the orchestration of innate lymphocyte responses. We review our current understanding of the interactions between adaptive and innate lymphocytes, and propose a model in which adaptive T cells function as antigen-specific sensors for the activation of innate lymphocytes to amplify and instruct local immune responses. We highlight the potential role of regulatory and helper T cells in these processes and discuss major questions in the emerging area of crosstalk between adaptive and innate lymphocytes.
Introduction
Different types of immune cells cooperate to achieve a finely balanced state of the immune system that maintains tolerance to self antigens, beneficial microsymbionts and nutrients, but enables the elimination or neutralization of pathogens, tumours, allergens and xenobiotics. It is now being appreciated that innate lymphocytes, including natural killer (NK) cells [G] and recently discovered innate lymphoid cells (ILCs) [G] are strategically positioned in many tissues of the body to exert crucial functions during infection, tissue injury and inflammation. These functions include direct cytotoxicity, the secretion of tissue-protective factors and the production of cytokines that help to orchestrate protective immune responses (Figure 1) (for review see 1–3).
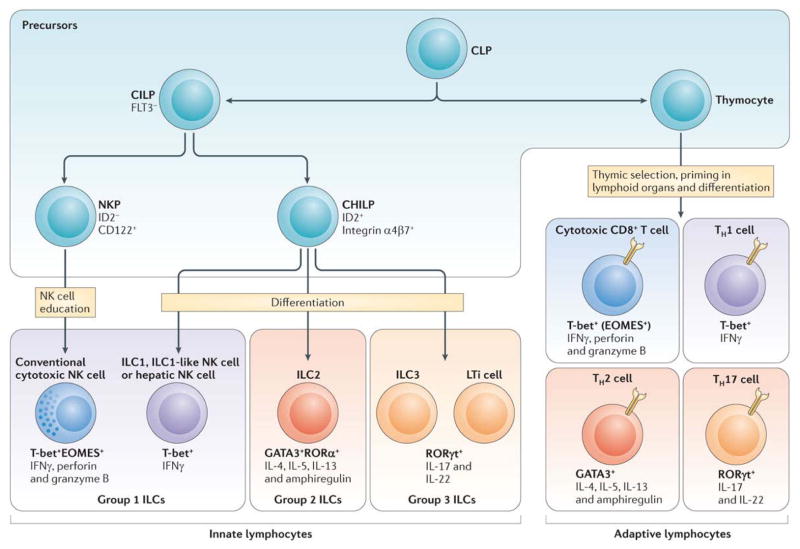
Figure 1. Innate and adaptive lymphocyte subsets.
A common lymphoid progenitor (CLP) in the bone marrow gives rise to precursors of T cells, NK cells and innate lymphoid cells (ILC). T cell precursors enter the thymus where they develop into naive T cells that harbor rearranged antigen-receptors and then seed the secondary lymphoid organs. Once stimulated by cognate antigen and polarizing innate cytokines, T cells undergo effector differentiation guided by key transcription factors and acquire the capacity to secret “hallmark” cytokines that orchestrate immune responses against intracellular pathogens (IFNγ), extracellular parasites (IL-4, -5, -13) or bacteria and fungi (IL-17). These T cells are frequently found in non-lymphoid organs as short-lived effector cells whereas some of them can become long-lived resident memory cells. Innate lymphocytes have been categorized based on their expression pattern of the aforementioned “master” transcription factors and “hallmark” cytokines that resemble T cell subsets. In contrast to T cells, ILC differentiate from the CLP through a common precursor in the bone marrow and developmentally acquire an “effector phenotype” reflected by their ability to seed peripheral organs and to produce the above-mentioned “helper” cytokines without further differentiation. Regulatory T cells are characterized by the expression of the lineage-specifying transcription factor FOXP3 (not depicted). Regulatory T cells can co-express FOXP3 and transcription factors specifying distinct helper T cell types which enables suppression of the respective classes of the immune response 40. So far, innate lymphocytes have not been found to express FOXP3. Not depicted are follicular helper T cells and a recently described ILC subset, both of which interact with B cells 23. Lymphoid tissue inducer (LTi) cells represent a subset of innate lymphocytes that interacts with stromal cells to facilitate the development of lymphoid organs. TH = T helper cell, NKP = NK cell precursor, CILP = Common ILC precursor, CHILP = Common “helper-like” ILC precursor.
NK cells and ILCs may have evolved to provide a rapid response to environmental challenges. Myeloid and epithelial cell-derived cytokines and alarmins [G], such as IL-12, IL-23 and IL-33, can directly activate these innate lymphocytes without the need for further differentiation (Box 1). The ease of activation of these cells has to be balanced by stringent control mechanisms, because excessive activation may contribute to a loss or impairment of tissue function and facilitate inflammatory processes. Indeed, innate lymphocytes have recently been implicated in inflammatory disorders including diabetes, allergic asthma, atopic dermatitis, inflammatory bowel diseases, organ fibrosis and cancer 4–14. Insufficient function of innate lymphocytes can lead to tissue dysfunction, barrier breach and severe pathology during local infection 15,16. The mechanisms regulating the activation of innate lymphocytes are therefore highly relevant for a broad range of physiological and pathological immune responses.
Box 1. Innate regulation of innate lymphocytes.
Innate cytokines and alarmins have a major role in regulating the homeostasis and function of ILCs. Myeloid cells produce many soluble factors that activate innate lymphocytes, for example type-I interferons (IFNs), IL-12, IL-18 and IL-15, which can activate and induce the proliferation of NK cells and ILC1 [G]; IL-25 and the alarmin IL-33, which trigger ILC2 [G] responses; and IL-23 and IL-1β, which activate ILC3 [G]. Upon infection or tissue damage some of these factors (for example type-I IFN, IL-1β, IL-18 and IL-33) are also released by non-haematopoietic epithelial and stromal cells. Additional stroma-derived factors include IL-7, which is required for the development and homeostasis of ILCs, and TSLP, which can directly activate ILC2. Although the regulation of ILCs by innate cytokines is well established and has recently been reviewed elsewhere 73 (Figure 2), a major question is whether ILCs also integrate environmental cues through activating and inhibitory receptors. In analogy to established models of “missing-self”, “altered-self” and “non-self” recognition by NK cells 81, ILCs may express receptors that recognize epithelial or microbial ligands, and signals through these receptors may be crucial for tolerance and ILC function at mucosal sites. The interactions of innate lymphocytes with other innate lymphocytes remain currently largely unexplored.
Current research has largely focused on the role of innate cytokines produced by myeloid, epithelial and stromal cells in regulating the homeostasis and function of innate lymphocytes 1–3 (BOX 1 and Figure 2). Although less well studied so far, the adaptive immune system may also contribute to the activation of innate lymphocytes and the regulation of their responses. One hallmark of allergic and auto-inflammatory diseases, as well as of recurrent or persisting infections, is the sensitization of adaptive T cells to allergens and tissue or pathogen-derived antigens, respectively. Upon re-exposure, these T cells initiate immunity to a large extent by further recruiting innate effector cells of the myeloid lineages and orchestrating their responses (reviewed in 17). ILCs and NK cells have recently been found to participate in shaping and regulating adaptive immune responses 18–21, but little is known about how adaptive immunity instructs innate lymphocytes. In this article, we review our current understanding of the interactions between adaptive and innate lymphocytes. We focus on NK cells and ILCs and do not discuss “innate-like” T cells (such as γ δ-, natural killer or NK-like “innate” CD8+ T cells) or the interaction of innate lymphocytes with B cells, although some of the principles discussed here may also apply for these cells 22,23. We propose a model in which adaptive T cells function as peripheral antigen-specific sensors that recruit and activate innate lymphocytes to amplify and orchestrate local immune responses. We focus on the potential role of regulatory (FOXP3+) and CD4+ helper T cells in these processes. Finally, we highlight major questions and challenges in the emerging area of crosstalk between adaptive and innate lymphocytes.
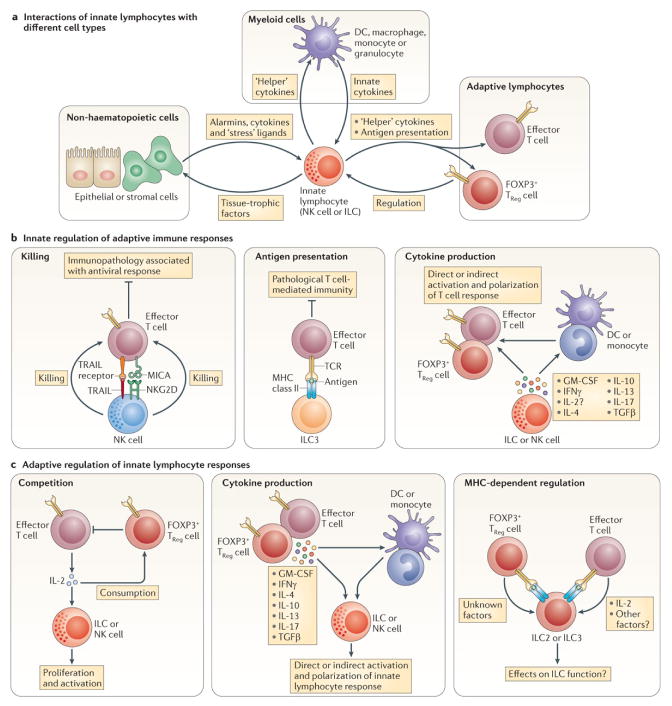
Figure 2. Interactions of innate lymphocytes.
(A) Innate lymphocytes interact with three major cell types: non-hematopoietic stromal and epithelial cells, myeloid cells and other lymphocytes. Many of these interactions are bidirectional. For example, epithelial derived alarmins can activate ILCs to secrete factors that stimulate epithelial regeneration. With the exception of NK cells, little is known about the receptors and ligands that mediate contact-dependent interactions of innate lymphocytes. While the communication of innate lymphocytes with myeloid cells is relatively well understood, much needs to be learned about the interactions of innate lymphocytes with other lymphocytes, including both innate and adaptive lymphocytes. In this regard, part (B) highlights known and putative interactions of innate lymphocytes with T cells. NK cells can limit immunopathology during antiviral T cell responses through TRAIL- or NKG2D-mediated killing of T cells. ILC3 can process antigens and present them on MHC class II molecules to limit pathological T cell immunity at mucosal sites. NK cells and ILCs produce classical T helper cell cytokines that are known to modulate the numbers and activity of myeloid cells, including neutrophils, eosinophils, macrophages and DCs, but can also act directly on T cells. Local conditioning of the cytokine milieu by innate lymphocytes may, therefore, directly and/or indirectly contribute to the initiation and polarization of the adaptive response. Similarly, the production of immunosuppressive cytokines (e.g. IL-10 or TGFβ) by innate lymphocytes may directly and/or indirectly modulate T cell responses. Part (C) summarizes the putative mechanisms by which T cells could modulate innate lymphocytes. T cells and innate lymphocytes may compete for common resources, such as growth factors and metabolites. In this regard, T cell-derived IL-2 can “help” the expansion and activation of NK cells, and potentially other subsets of ILC. TReg cells can restrain this cellular cross-talk by limiting T cell activation, or by competing with innate lymphocytes for IL-2 (see also Figure 3). T cell secretion of classical helper cytokines or immunosuppressive cytokines may directly and/or indirectly, via the modulation of myeloid cells, instruct innate lymphocyte responses. T cells and ILC can also engage in T cell receptor/MHC class 2 dependent interactions that may modulate the function of both cell types.
Role of innate lymphocytes in adaptive lymphocyte responses
Innate lymphocytes could directly or indirectly influence adaptive immune responses through cell-contact dependent interactions and soluble mediators, or by acting on accessory cells including antigen-presenting cells (APCs) or stromal cells. One can envision that NK cells and ILCs, which have also been dubbed “innate helper cells”, could orchestrate and polarize major types of the adaptive immune response owing to their ability to produce classical T helper cell cytokines (Figure 1). These cytokines are known to modulate the numbers and activity of myeloid cells, including neutrophils, eosinophils, macrophages and DCs, but can also act directly on T cells. Local conditioning of the cytokine milieu by ILCs may, therefore, directly and/or indirectly contribute to the initiation and polarization of the adaptive response (Figure 2B).
Best studied in this regard are NK cells: the early production of IFNγ by NK cells can modulate APC function and promote the differentiation of TH1 cells directly, and indirectly by inhibiting TH2 cell and TH17 cell differentiation (for review see 24). The “ILC-1-like” NK cells (Box 2) that are present in secondary lymphoid organs and produce increased amounts of IFNγ might be particularly well suited for this purpose 25–28. Similarly, ILC2-derived IL-4 and IL-13 may reinforce the priming of TH2 cells and potentially inhibit TH1 cell polarization. Consistent with this idea, ILC2-derived IL-13 was recently shown to be crucial for the initiation of TH2 cell-mediated allergic responses 12. ILC2 can also directly stimulate TH2 cell responses in vitro 29 and facilitate antigen-specific T cell responses during helminth infection 30. A similar function for ILC3 in promoting TH17 cells is conceivable, because the early production of IL-17 by γδ T cells also functions as a feed-forward mechanism to increase TH17 cell differentiation 31,32. ILC3 are also a source of GM-CSF that can support TReg cell homeostasis and generation in the gut 33. Furthermore, the functions of ILCs in lymphoid tissue organogenesis might also be relevant for adaptive immunity. In this regard, lymphoid tissue inducer (LTi) cell [G]-like ILCs have been proposed to facilitate the maintenance of CD4+ T cell memory 34 and to contribute to chronic T cell activation through lymphoid tissue neogenesis during chronic inflammatory diseases (reviewed in 35).
Box 2. NK cells and ILC1.
Group 1 ILC are a heterogeneous group of cells. The developmental relationship between individual ILC1 subsets and their distinguishing phenotypic features and functions are currently being defined. In C57BL/6 mice, NK cells typically express NK1.1, a marker that is also found on ILC1 and some ILC3, as well as CD4+ NKT cells and subsets of CD8+ T cells. Recent studies suggest that the expression of EOMES distinguishes conventional NK (cNK) cells from cells that were originally described as “phenotypically immature” 82, but that are now thought to be an independent cell lineage 58,83. Although they have been best characterized in the liver, Lin− EOMES− NK1.1+ cells have also been identified in the thymus, lymph nodes, spleen, bone marrow, skin, lung and gut 59,66. When compared with EOMES+ cNK cells, these EOMES− cells are highly responsive to IL-12, produce increased amounts of cytokines and are characterized by high levels of expression of CD90, CD127, CD69 and CXCR3 and a paucity of expression of Ly49 NK cell receptors; this surface phenotype is reminiscent of ILC1 5,59. Recent studies indicate that ILC1 and hepatic EOMES− NK cells develop from a common precursor distinct from the cNK cell precursor 58,59,84. The emerging view is that Lin− NK1.1+ cells represent at least three different cell types: EOMES+ cNK cells (which develop in an IL-15-dependent manner from an Id2− CD122+ NK cell precursor), EOMES− ILC1 (which develop in an IL-15-dependent manner from an Id2+ α4β7+ precursor of “helper-like” ILCs), and EOMES− cells that have a history of RORyt expression and may therefore be related to ILC3 (which develop in an IL-7-dependent manner from the Id2+ α4β7+ “helper-like” ILC precursor) 58,59,84,85. Based on their IL-15-dependent development from the “helper-like” ILC precursor we refer to EOMES− NK cells as ILC1-like NK cells. It is important to note, however, that these cells exhibit heterogeneity at different anatomical sites 58,65,66. Future studies are needed to address the differentiation, physiological functions and interactions of these cells in their specific tissue environment.
In addition to cytokine-mediated “help” for the initiation and polarization of T cell responses, recent studies have identified direct cell contact-dependent interactions of NK cells and ILCs with T cells, at least some of which have been suggested to inhibit T cell responses. NK cells have several means to regulate adaptive immunity 24, including the cytolytic elimination of CD8+ 21 and CD4+ T cells 19, which directly and indirectly, respectively, restrained CD8+ T cell-mediated tissue pathology during chronic infection with lymphocytic choriomeningitis virus (LCMV). Human NK cells isolated from chronically hepatitis B virus (HBV)-infected patients use the surface molecule TNF-related apoptosis-inducing ligand (TRAIL) to kill highly activated TRAIL-receptor-expressing autologous HBV-specific CD8+ T cells 36. Interestingly, TRAIL is also expressed by murine hepatic and splenic ILC1-like NK cells that accumulate during chronic LCMV infection 37, which raises the possibility that these NK cells might have regulatory roles during infection.
The idea that innate lymphocytes may be involved in the regulation and tuning of T cell responses in specific environments is further supported by the intriguing observation that RORyt+ ILC3 can process antigens and present them on MHC class II molecules. Mice in which ILC3 lacked MHC class II expression developed CD4+ T cell-dependent intestinal inflammation that was abrogated by antibiotic treatment 18. Hence, ILCs might limit pathology induced by adaptive immune responses to commensal microbiota at mucosal sites.
Adaptive control of innate lymphocytes
Recent studies suggesting a role for ILCs in the instruction and regulation of adaptive immunity 12,18,20 are intriguing as they go beyond the frequent use of lymphopenic mice to study ILCs, and they provide experimental evidence for physiological functions of ILCs in the presence of a functional adaptive immune system. The understanding that innate lymphocytes and T cells can respond to the same context-dependent stimuli (such as IL-12, IL-18, IL-1β, IL-23 and IL-33) raises the possibility that these cells may collaborate during immune responses and may be coordinately regulated. In addition to the ability of both ILCs and T cells to produce hallmark cytokines (such as IFNγ, IL-5, IL-13 and IL-17) and tissue-trophic effector molecules (such as amphiregulin and IL-22) the innate and adaptive lymphocyte lineages will likely have specific, non-redundant functions. Well recognized functions of T cells include the regulation and orchestration of multicellular immune responses 17,38,39. CD4+ T cells activate DCs and macrophages and “help” the induction of CD8+ T cell responses. CD4+FOXP3+ TReg cells counterbalance inflammatory responses and are crucial for immune system homeostasis 40. While the interaction of T cells with innate myeloid cell lineages is well established, it is currently largely unknown, whether T cells may similarly help and regulate innate lymphocytes. Here we discuss emerging evidence indicating that T cells may contribute to the control of NK cells and innate lymphoid cell lineages (Figure 2C).
Conventional NK cells
The best-studied members of group 1 ILCs are conventional NK (cNK) cells. TH and TReg cells have been shown to regulate NK cell homeostasis and responses in tumours 41–44, and during autoimmune challenge 8,14, transplant rejection 45 and infection 46–48. Mechanistically, it has been proposed that TReg cells suppress NKG2D-mediated NK cell cytotoxicity through TGFβ, potentially through a contact-dependent mechanism 41,42. T cell help for NK cells was mediated either indirectly, by IL-15-trans-presenting DCs 49, or directly, by T cells secreting IL-2. This T cell-derived cytokine has emerged as a crucial factor mediating the crosstalk between NK cells, TH cells and TReg cells. IL-2 can activate and induce the proliferation of NK cells and early work suggested that NK cells compete with T cells for IL-2 50,51. T cell-derived IL-2 facilitates the expansion of NK cells during infection 46, and the release of IL-2 from antigen-specific T cells activates human NK cells in blood samples from vaccinated individuals 52,53. The innate cytokines IL-12 and IL-18 induce the expression of the high-affinity IL-2Rα (CD25), which may enable NK cells to compete for IL-2 in vivo 37,43,48. Furthermore, CD25-deficient Ly49H+ NK cells proliferate significantly less than their wild-type counterparts during MCMV infection (unpublished observation, G.G. and A.R.). TReg cells limit the availability of IL-2 by restraining the activation of T cells. Furthermore, TReg cells, which constitutively express high levels of CD25, may actively deplete IL-2 through the “consumption” of the cytokine 54. In addition to its function in promoting NK cell proliferation, IL-2 was shown to increase NK cell cytotoxicity through translation-dependent mechanisms 51,55. Adding to the pleiotropic effects of IL-2, we recently found an unexpected immediate role for this cytokine in lowering the activation threshold of NK cells by increasing their ability to adhere to and engage their target cells 56. By restraining the availability of IL-2, TReg cells seem to increase the activation threshold of NK cells, a mechanism that might be of particular relevance to the restraint of NK cell mediated tissue damage in inflamed tissues containing highly activated T cells. Consistent with this idea, excessive amounts of IL-2 rapidly activated NK cells and exacerbated diabetes in TReg cell-depleted pre-diabetic mice 8. Autoimmunity facilitated by the unrestrained activation of NK cells may therefore be triggered when the IL-2 buffering capacity of TReg cells is compromised or exceeded 57. Of benefit, NK cell cytotoxicity may be rapidly increased by therapeutic strategies that enable NK cells to compete for IL-2 by increasing CD25 expression 43 or through the targeted delivery of IL-2.
ILC1-like NK cells
The factors defining the heterogeneity, and the specific functions and cellular interactions of particular subsets of innate lymphoid cells are currently being investigated. Recent studies indicate that expression of EOMES distinguishes cNK cells from ILC1-like NK cells 58,59 (Box 2). Interestingly, adaptive immune responses associated with chronic inflammation can drive the expansion of a phenotypically related, IL-2-responsive CD127+ NK cell subset 37. These ILC1-like NK cells accumulated in the spleens of TReg cell-depleted mice, as well as in tumour-bearing and chronically infected mice. The innate cytokine IL-12 induced the expression of CD25 on CD127+ ILC1-like NK cells, but not on cNK cells, and therefore enabled the preferential expansion of CD25+ CD127+ ILC1-like NK cell populations in a manner dependent on CD4+ T cells and IL-2 37. Like EOMES− cells in the liver (Box 2), these CD127+ ILC1-like NK cells expressed TRAIL, a molecule that has been implicated in the control of T cell responses (see above). It is therefore possible that the IL-2-dependent expansion of ILC1-like cells could be part of a regulatory feedback loop, in which T cells trigger the expansion of innate lymphocyte populations that function to restrain the T cell response. Interestingly, the IL-2-responsive ILC1-like NK cells in mice have some similarity to human CD56hi NK cells. Both cell types are characterized by Ly49lo/KIRlo, CD94hi, NKG2Ahi expression and high levels of IFNγ production, yet lack potent cytotoxic activity 25,26. Human CD56hi NK cells can also express CD25, and low-dose IL-2 treatment of patients with cancer or healthy volunteers preferentially expanded this subset 60,61. The observed similarities between murine ILC1-like NK cells and human CD56hi NK cells raise the possibility that the latter might also proliferate in response to CD25-dependent IL-2 signalling. Interestingly, human CD56hi NK cells, in parallel with their potential mouse counterparts, are present at increased numbers at sites of chronic inflammation in patients with tuberculosis, sarcoidosis, rheumatoid arthritis and cancer 62–64. These observations highlight the need for future studies addressing the heterogeneity, physiological functions and cellular interactions of ILC1 and ILC1-like cells in specific contexts, including the secondary lymphoid organs, the thymus, liver, mucosal barrier sites, tumours and settings of chronic inflammation 28,37,58,65,66.
ILC2
The role of T cells for the function of innate lymphoid cells is currently less well understood. Interestingly, however, several recent studies suggest that IL-2 may also be important for Type-2 innate lymphoid cells. ILC2 have been found in the dermis, lung, liver, visceral adipose tissue and gut, and typically express high levels of CD25. Activation of ILC2 with IL-33 increases CD25 expression 7, and IL-2 has been used to activate and to expand ILC2 populations in vitro and in vivo 16,67,68. ILC2 express receptors for the STAT5-activating cytokines IL-2, IL-7 and TSLP, as well as the NF-κB-activating cytokines IL-25 and IL-33. IL-25 or IL-33 alone can activate ILC2 to produce IL-5 and/or IL-13, but the presence of a STAT5-activating cytokine further increases cytokine production by ILC2 in vitro 67. Accordingly, IL-2 has been shown to facilitate IL-9 production by pulmonary ILC2 in a model of papain-induced airway hyper-reactivity 13. The prolonged treatment of RAG-2−/− mice with IL-2 resulted in the expansion and activation of dermal ILC2 and induced allergic skin disease 6. In addition to a co-activating role for ILC2, IL-2 might also modulate the homeostasis, expansion and survival of ILC2. Despite these important findings, it is currently unclear to what extent ILC2 access the IL-2 that is produced under physiological conditions. Our recent studies indicate that CD25 expression confers a competitive advantage to ILC2 during homeostasis and that TReg cells restrain the IL-2-dependent proliferation of ILC2 (G.G. and A.Y.R, unpublished observations). These findings raise the possibility that interactions with T cells are crucial for the function and homeostasis of ILC2, and that IL-2 may be an important mediator of these interactions. A role for T cell dependent “help” to ILC2 is also supported by the observation that ILC2 fail to protect against infection with Nippostrongylus brasiliensis in the absence of T cells in RAG-2−/− mice 30. ILC2 and T cells can engage in reciprocal interactions, in which ILC2 appear to stimulate TH2 cell responses through contact- and MHC class 2 dependent mechanisms, and T cells reinforce ILC2 function through the secretion of IL-2 29and Oliphant et al.. Reciprocal regulation may potentially also occur between ILC2 and TReg cells, because fewer TReg cells are found in the visceral adipose tissue of mice depleted of ILC2 upon the conditional expression of the catalytic diphtheria toxin A (DTA) subunit induced by Cre recombinase driven by either IL-13 or IL-5 regulatory elements (A. Molofsky/R. Locksley, personal communication). Although future studies are required to unambiguously dissect ILC2-TReg cellular crosstalk, this observation raises the intriguing possibility that reciprocal interactions may regulate innate and adaptive lymphocytes in the adipose tissue, where both ILCs and TReg cells have important effects on metabolic status 7,69.
ILC3
To the best of our best knowledge, ILC3 have not been reported to respond to IL-2 in vivo, and the control of ILC3 function by T cells has not been studied so far. It is noteworthy, however, that ILC3 may control T cell responses in the gut (see above), raising the possibility of reciprocal interactions 18.
In summary, a growing number of studies support the idea that a tri-cellular crosstalk between TH cells, TReg cells and innate lymphocytes may be central to the regulation and function of innate effector lymphocytes and that IL-2 is an important mediator of this adaptive–innate cross-talk. This concept also raises a number of important questions that we discuss in the next section.
Major questions
Emerging data indicate that innate lymphocytes and T cells can either help or restrain each other’s activation. Largely unanswered questions are how the IL-2 dependent crosstalk occurs at the cellular, molecular and spatio-temporal level, and which IL-2-independent mechanisms can potentially mediate adaptive control of innate lymphocytes.
The source and the sensing of IL-2
Although T cells are clearly a predominant source of IL-2 in the immune system, other cell types have been proposed to secrete IL-2 as well. In particular cNK cells, EOMES− hepatic NK cells and ILC2 can produce IL-2 when stimulated under certain conditions 16,58. IL-2 secretion by innate lymphocytes has been proposed 16,58,66, but its potential physiological relevance is currently lacking. For example, it is unclear whether IL-2 secreted by innate lymphocytes might function in an autocrine or in a paracrine manner, as a potential early source of IL-2 for effector T cell activation or TReg cell homeostasis. It has been estimated that only cells in a very close proximity would be able to “help” or to “regulate” each other through the paracrine provision or competitive deprivation of IL-2, respectively 70,71. Therefore, a major question is whether T cells and innate lymphocyte subsets interact in a spatiotemporal manner that would enable direct IL-2-dependent help and regulation. Intriguingly, only IL-2-producing CD4+ T cells could “help” the NK cell response during experimental Leishmania infection, but unambiguous evidence for a direct paracrine effect is lacking 46,72. Attempts to discriminate direct, cell-intrinsic effects of IL-2 from indirect effects mediated by additional cell types are complicated by the lack of studies using conditional alleles of IL-2 and IL-2R as well as the shared use of common receptor subunits by different cytokines: CD122 binds IL-2 and IL-15, and CD132 signals in response to IL-2, IL-4, IL-7, IL-9 and IL-15. Nevertheless, a role for cell-intrinsic CD25-dependent IL-2 signals in vivo was demonstrated for splenic ILC1-like NK cells 37, and has been observed for conventional NK cells during MCMV infection, as well as for ILC2 during homeostasis (G.G. and A.Y.R, unpublished observations). In summary, although several studies suggest that IL-2 is a crucial cytokine for the regulation of innate lymphocytes, and that T cells and TReg cells might regulate innate lymphocyte activation and proliferation by modulating the availability of IL-2, a formal demonstration of the relevant cellular sources of IL-2, the immediate sensors, as well as where and when these interactions take place is currently lacking. Addressing these questions is required to better understand the IL-2-dependent adaptive–innate lymphocyte crosstalk.
IL-2-independent mechanisms
ILC3 do not generally express the high-affinity IL-2 receptor CD25, and cNK cells require innate triggers to induce the up-regulation of CD25. As discussed above, TReg cells can also suppress cNK cells in a contact-dependent manner, or through the control of DC function 41,42,49. Furthermore, subsets of NK cells, ILC2 and ILC3 have been reported to express MHC class II molecules and may have direct interactions with CD4+ T cells 18 REF 18 and Oliphant et al.. These observations argue for additional IL-2-independent pathways by which T cells may either recruit and activate, or inhibit innate lymphocytes. Potential mechanisms involve the secretion of other cytokines and chemokines, direct contact-dependent interactions, and mechanisms that involve accessory cells (DCs or macrophages), which mediate the activation or inhibition of innate lymphocytes 73 (Fig. 2C). In addition, T cells and innate lymphocytes could potentially regulate each other through competition for cytokines (such as IL-7, IL-2, potentially IL-12, IL-18 and IL-33), nutrients, or access to DCs and macrophages. This competition for resources, which is a well-established principle for T cells, may be relevant in the context of T cell–innate lymphocyte interactions as well. Future studies are needed to define the additional pathways that mediate adaptive–innate lymphocyte crosstalk at mucosal sites. These studies would benefit from the development of genetic mouse models targeting innate lymphocytes.
T cells as antigen-specific sensors that regulate innate lymphocytes
As we have discussed, a growing body of evidence indicates that reciprocal interactions between innate and adaptive lymphocytes are likely to be important for immune responses at the sites at which innate lymphocytes typically reside. Effector T cells enter these tissues during acute injury or infection-induced inflammation. Although the majority of effector T cells disappear from the tissue upon resolution of inflammation, some differentiate into peripheral tissue-resident memory cells. These cells have important functions in the early defense against reinfection 74,75. Although it is not clear to what extent the capacity to accommodate memory T cells in non-inflamed peripheral niches is limited 76–78, it is probable that under homeostatic conditions, only few T cells with a given specificity are present at major pathogen entry sites such as the skin, lung, genital tract or the gut. A major question is, therefore, how a limited number of memory cells can confer initial protection against the invasion by pathogens that replicate and spread rapidly before large numbers of antigen-specific cells can be expanded and recruited from the lymph node.
We propose that T cells, in addition to their direct effector function, amplify their response by acting as antigen-specific sensors that activate and instruct tissue-resident innate lymphocytes to provide local protection from tissue damage and pathogen invasion. T cells are known to orchestrate the responses of other immune cells. For example, T cells facilitate the recruitment and activation of leukocytes (neutrophils, eosinophils, macrophages and monocytes) or additional T cells through cytokines and chemokines 17,79. Memory T cells, particularly tissue-resident ones, are activated early during acute injury and infections 74,75 and increase and accelerate innate immune cell recruitment and activation 39,79. This “alarming and sensing function” 79 might extend to the activation of tissue-resident innate lymphocytes. Thus, we suggest that upon antigen recognition, tissue-resident T cells modulate the responsiveness of innate lymphocytes, together with the cues provided by activating and inhibitory receptors and innate cytokines (Figure 3). In support of this idea, some of the innate cytokines that activate both innate lymphocytes and T cells (IL-12, IL-18 and IL-33) up-regulate the high-affinity IL-2 receptor CD25 on conventional NK cells, ILC1-like NK cells and ILC2 7,37,48. Increased CD25 expression renders the innate lymphocytes perceptive to IL-2, a prototypic T cell cytokine that is released early after TCR stimulation and co-activates these cells 6,8,13,46,56,72, and drives their proliferation 6,37,43,46,48. That innate cytokines poise ILCs to receive a T cell-derived activating signal provides the basis for a two-step model of the crosstalk between T cells and innate lymphocytes. Additional T cell-derived cytokines (such as IFNγ, IL-4, IL-5, IL-13 and IL-17) may also act on innate lymphocytes either directly or indirectly by activating tissue-resident macrophages and DCs. In support of this concept, IFNγ secreted by memory T cells during recall infection accelerated and potentiated the activation of innate cells, including monocytes, macrophages and NK cells 39. The rapid T-cell dependent “innate recall” of NK cells required IFNγ signaling in monocytes and macrophages, and was associated with accelerated IL-12 production. Intriguingly, these observations suggest, that activation of memory T cells can in fact precede the full activation of the studied innate cells.
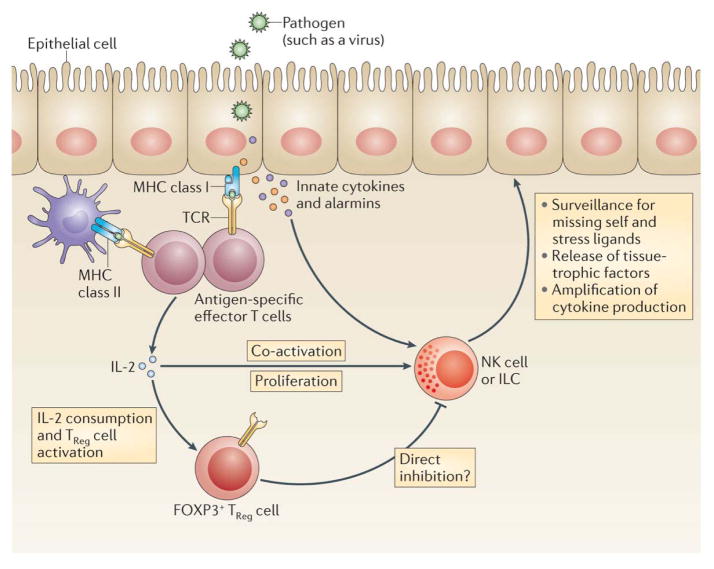
Figure 3. A model for IL-2-dependent adaptive–innate lymphocyte crosstalk.
A growing body of evidence suggests cooperation between innate and adaptive lymphocytes. In this context, T cells might function as antigen-specific sensors that amplify local immune responses by recruiting and modulating innate lymphocytes. The cytokine IL-2 provides one example of how a tri-cellular crosstalk between T cells, TReg cells and innate lymphocytes can be established. In this regard, local inflammatory mediators could function to pre-activate the three cell types, and to render innate lymphocytes more perceptive to IL-2, for example through the upregulation of CD25. Once T cells encounter their cognate antigens (presented e.g. by epithelial or myeloid cells, or potentially by ILC) they secrete IL-2 (and other soluble factors) that can co-activate innate lymphocytes, and decrease their activation threshold. Such a response-modulating function of T cells would function to “spread” the antigen-specific signal to surrounding innate lymphocytes, to amplify local cytokine production, increase the secretion of tissue-protective factors, and optimize NK cell-mediated immune surveillance for “missing-self” and stress-induced ligands. Regulatory T cells could balance this adaptive–innate crosstalk and prevent excessive activation of ILCs by competing for IL-2. Alternatively, IL-2 may activate TReg cells to directly inhibit innate lymphocytes, or to synergize with innate lymphocytes in maintaining tissue function and homeostasis.
The T cell-mediated activation of innate lymphocytes could amplify the corresponding protective antigen-specific response type and additionally reinforce beneficial specialized functions of innate lymphocytes (Figure 3). Examples of such specialized functions could be the production of tissue-protective factors by ILCs to prevent inflammation-induced impairment of organ function and to prevent pathogen invasion and secondary infections. Furthermore, we suggest that IL-2-induced enhancement of NK cell cytotoxicity against cells, in which pathogens or tumours induce MHC class I down-regulation, probably makes NK cells more efficacious at preventing immune evasion 56.
In addition to the amplification of ILC responses and numbers, antigen-dependent modulation by T cells would also link the full activation of innate lymphocytes to the elaborate mechanisms of tolerance and regulation of T cell responses. The latter would include the control of innate lymphocytes by TReg cells. In this regard, TReg cells could limit the “access” of innate lymphocytes to IL-2 by inhibiting IL-2 production by T cells, depriving the environment of IL-2 through consumption and inhibiting the expression of CD25 by ILC. Inhibiting CD25 expression could potentially be achieved by multiple means including inhibiting the production of CD25-inducing cytokines IL-12 and IL-18 by macrophages and DCs, competition for IL-12, IL-18 and IL-33, or displaying inhibitory cytokines (such as TGF-β) that might prevent the up-regulation of CD25 37. Therefore, one function of TReg cells might be to restrain T cell “help” for innate lymphocytes so as to prevent the excessive activation of these cells and subsequent impairment in tissue function (Figure 3).
Notably, TReg cells are enriched at some of the same sites as ILCs, including the skin, the visceral adipose tissue and the gut. Similarly to ILCs, which are important for tissue homeostasis 7,15,16, tissue-resident TReg cells express a distinct set of tissue-trophic factors 69,80. Thus, the two cell types may also cooperatively promote tissue function in some contexts. Such cooperation may potentially occur in the visceral adipose tissue where both ILCs and TReg cells have crucial functions affecting metabolic homeostasis, in the gut through the restraint of T cells recognizing microbial symbionts, or during the response to tissue injury 16,18,80.
Conclusion/Perspective
A growing number of studies indicate a cross-talk between adaptive and innate lymphocyte responses. Effector T cell-mediated help and regulatory T cell-mediated suppression may modulate the function of innate lymphocytes and vice versa through reciprocal interactions. We propose a model where innate lymphocytes, which have an important role in tissue homeostasis and barrier immunity, may be co-opted as effectors of adaptive responses initiated by antigen-specific “sentinel” T cells at the sites of pathogen entry or acute tissue injury. Interactions between T cells and innate lymphocytes may orchestrate tissue-specific immune responses upon repeated exposure to pathogen, cross-reactive heterologous immunity and inflammatory disorders. Better understanding the molecular and cellular mechanisms of the crosstalk between innate and adaptive lymphocytes and its context-dependent physiological relevance may allow novel means of therapeutic modulation of effector responses in infection, inflammatory diseases and cancer.
Acknowledgments
A.Y.R. is supported by an NIH grant (R37 AI034206) and Ludwig Center at Memorial Sloan-Kettering Cancer Center. A.Y.R. is an investigator at the Howard Hughes Medical Center. G.G. is an Irvington Fellow of the Cancer Research Institute. We would like to thank Joseph C. Sun and members of the Rudensky and Sun laboratories for helpful discussions. We would like to apologize to those investigators whose related work we were unable to discuss or quote due to space limitations.
Glossary
natural killer (NK) cells
Innate lymphocytes that can recognize and “kill” infected or cancerous cells. NK cells also produce IFNy, and may have immunoregulatory functions
Innate lymphoid cells (ILCs)
Recently discovered subsets of innate lymphocytes that seed peripheral organs and produce “helper” cytokines and tissue-protective factors that are crucial for barrier immunity
Alarmins
prefabricated molecules (for example IL-33) that are released upon cell and tissue damage by epithelial, stromal and myeloid cells to activate cells of the immune system. The potency of some alarmins is regulated locally, for example by proteolytic cleavage
ILC1
“innate helper” cells characterized by expression of the transcription factor Tbet and their ability to produce IFNy in response to IL-12. ILC1 may have crucial functions during infections with intracellular pathogens
ILC2
“innate helper” cells characterized by expression of the transcription factor GATA-3 and their ability to produce IL-5 and IL-13 in response to IL-25 and IL-33. ILC2 have important roles during asthma, parasitic infections, but also tissue homeostasis and fibrosis, for example through the secretion of amphiregulin
ILC3
“innate helper” cells characterized by expression of the transcription factor RORyt and their ability to produce IL-17 and IL-22 in response to IL-23. ILC3 have crucial functions during bacterial infections particularly in the intestine. ILC3 may also present antigens and contribute to immune tolerance against microbial symbionts
lymphoid tissue inducer (LTi) cell
Group 3 ILC characterized by expression of the transcription factor RORyt and the production of lymphotoxin α1β1. LTi cells are required for the development of secondary lymphoid organs, and may have functions during chronic inflammation and for T cell memory
References
- 1.Sanos SL, Diefenbach A. Innate lymphoid cells: from border protection to the initiation of inflammatory diseases. Immunol Cell Biol. 2013;91:215–224. doi: 10.1038/icb.2013.3. [DOI] [PubMed] [Google Scholar]
- 2.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews. Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 3.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nature reviews. Immunology. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 4.Kirchberger S, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–931. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs A, et al. Intraepithelial Type 1 Innate Lymphoid Cells Are a Unique Subset of IL-12- and IL-15-Responsive IFN-gamma-Producing Cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roediger B, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med. 2013;210:1153–1165. doi: 10.1084/jem.20122248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hams E, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111:367–372. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHedlidze T, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halim TY, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm C, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nature reviews. Immunology. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu J, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang PA, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorski SA, Hahn YS, Braciale TJ. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS pathogens. 2013;9:e1003615. doi: 10.1371/journal.ppat.1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magri G, et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15:354–364. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34:342–349. doi: 10.1016/j.it.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Strowig T, et al. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008;4:e27. doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehniger TA, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 27.Luther C, Warner K, Takei F. Unique progenitors in mouse lymph node develop into CD127+ NK cells: thymus-dependent and thymus-independent pathways. Blood. 2011;117:4012–4021. doi: 10.1182/blood-2010-07-298901. [DOI] [PubMed] [Google Scholar]
- 28.Vosshenrich CA, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 29.Mirchandani AS, et al. Type 2 Innate Lymphoid Cells Drive CD4+ Th2 Cell Responses. Journal of immunology. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 30.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Cui Y, et al. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. Journal of immunology. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Withers DR, et al. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J Immunol. 2012;189:2094–2098. doi: 10.4049/jimmunol.1201639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 36.Peppa D, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. 2013;210:99–114. doi: 10.1084/jem.20121172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med. 2013;210:1179–1187. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bevan MJ. Helping the CD8(+) T-cell response. Nature reviews. Immunology. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 39.Soudja SM, et al. Memory-T-Cell-Derived Interferon-gamma Instructs Potent Innate Cell Activation for Protective Immunity. Immunity. 2014;40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smyth MJ, et al. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 43.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maury S, et al. CD4+CD25+ regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci Transl Med. 2010;2:41ra52. doi: 10.1126/scitranslmed.3001302. [DOI] [PubMed] [Google Scholar]
- 45.Barao I, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bihl F, et al. Primed antigen-specific CD4+ T cells are required for NK cell activation in vivo upon Leishmania major infection. J Immunol. 2010;185:2174–2181. doi: 10.4049/jimmunol.1001486. [DOI] [PubMed] [Google Scholar]
- 47.Sungur CM, et al. Murine natural killer cell licensing and regulation by T regulatory cells in viral responses. Proc Natl Acad Sci U S A. 2013;110:7401–7406. doi: 10.1073/pnas.1218767110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SH, Fragoso MF, Biron CA. Cutting Edge: A Novel Mechanism Bridging Innate and Adaptive Immunity: IL-12 Induction of CD25 To Form High-Affinity IL-2 Receptors on NK Cells. J Immunol. 2012;189:2712–2716. doi: 10.4049/jimmunol.1201528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terme M, et al. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol. 2008;180:4679–4686. doi: 10.4049/jimmunol.180.7.4679. [DOI] [PubMed] [Google Scholar]
- 50.Su HC, et al. IL-2-dependent NK cell responses discovered in virus-infected beta 2-microglobulin-deficient mice. J Immunol. 1994;153:5674–5681. [PubMed] [Google Scholar]
- 51.Henney CS, Kuribayashi K, Kern DE, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291:335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 52.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 53.Horowitz A, et al. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–6052. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 54.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 55.Fehniger TA, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Gasteiger G, et al. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210:1167–1178. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long SA, et al. Rapamycin/IL-2 Combination Therapy in Patients With Type 1 Diabetes Augments Tregs yet Transiently Impairs beta-Cell Function. Diabetes. 2012;61:2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daussy C, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klose CS, et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 60.Caligiuri MA, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito S, et al. Ultra-low Dose Interleukin-2 Promotes Immune-modulating Function of Regulatory T Cells and Natural Killer Cells in Healthy Volunteers. Mol Ther. 2014;22:1388–1395. doi: 10.1038/mt.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46:1763–1772. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 63.Bauernhofer T, Kuss I, Henderson B, Baum AS, Whiteside TL. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol. 2003;33:119–124. doi: 10.1002/immu.200390014. [DOI] [PubMed] [Google Scholar]
- 64.Schierloh P, et al. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J Immunol. 2005;175:6852–6860. doi: 10.4049/jimmunol.175.10.6852. [DOI] [PubMed] [Google Scholar]
- 65.Klose CS, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 66.Sojka DK, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo L, Junttila IS, Paul WE. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 2012;33:598–606. doi: 10.1016/j.it.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mjosberg J, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 69.Cipolletta D, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Busse D, et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A. 2010;107:3058–3063. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofer T, Krichevsky O, Altan-Bonnet G. Competition for IL-2 between Regulatory and Effector T Cells to Chisel Immune Responses. Frontiers in immunology. 2012;3:268. doi: 10.3389/fimmu.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu W, Di Santo JP. Taming the beast within: regulation of innate lymphoid cell homeostasis and function. Journal of immunology. 2013;191:4489–4496. doi: 10.4049/jimmunol.1301759. [DOI] [PubMed] [Google Scholar]
- 74.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 75.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev. 2013;255:165–181. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vezys V, et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 77.Welsh RM, Selin LK. Attrition of memory CD8 T cells. Nature. 2009;459:E3–4. doi: 10.1038/nature08091. discussion E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huster KM, et al. Cutting edge: memory CD8 T cell compartment grows in size with immunological experience but nevertheless can lose function. Journal of immunology. 2009;183:6898–6902. doi: 10.4049/jimmunol.0902454. [DOI] [PubMed] [Google Scholar]
- 79.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burzyn D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–372. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gordon SM, et al. The Transcription Factors T-bet and Eomes Control Key Checkpoints of Natural Killer Cell Maturation. Immunity. 2012 doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fathman JW, et al. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood. 2011;118:5439–5447. doi: 10.1182/blood-2011-04-348912. [DOI] [PMC free article] [PubMed] [Google Scholar]