Formation and Maturation of Phase Separated Liquid Droplets by RNA Binding Proteins (original) (raw)
. Author manuscript; available in PMC: 2016 Oct 15.
Abstract
Eukaryotic cells possess numerous dynamic membrane-less organelles, RNP granules, enriched in RNA and RNA binding proteins containing disordered regions. We demonstrate that the disordered regions of key RNP granule components, and the full-length granule protein hnRNPA1, can phase separate in vitro, producing dynamic liquid droplets. Phase separation is promoted by low salt concentrations or RNA. Over time, the droplets mature to more stable states, as assessed by slowed fluorescence recovery after photobleaching and resistance to salt. Maturation often coincides with formation of fibrous structures. Different disordered domains can co-assemble into phase-separated droplets. These biophysical properties demonstrate a plausible mechanism by which interactions between disordered regions, coupled with RNA binding, could contribute to RNP granule assembly in vivo through promoting phase separation. Progression from dynamic liquids to stable fibers may be regulated to produce cellular structures with diverse physiochemical properties and functions. Misregulation could contribute to diseases involving aberrant RNA granules.
Introduction
Eukaryotic cells contain non-membrane bound organelles. Many of these structures are enriched in RNA and proteins, and play roles in modulating RNA-protein complexes. Such organelles are generically referred to as RNP granules and include the nucleolus, Cajal bodies, stress granules and P-bodies (Spector, 2006). RNP granules have higher protein concentration than the surrounding cytoplasm or nucleoplasm (Handwerger et al., 2005; Souquere et al., 2009; Yang et al., 2004). Fluorescence recovery after photobleaching (FRAP) analyses showed that proteins can associate, dissociate and move within RNP granules on timescales of seconds to minutes, although there is variability from structure to structure and protein to protein (Buchan and Parker, 2009). Recent live cell imaging has suggested that P-granules and the nucleolus behave as phase separated liquids, being round in shape, undergoing cycles of fusion and fission, and distorting in response to shear forces (Brangwynne et al., 2009; Brangwynne et al., 2011; Weber and Brangwynne, 2015; Wang et al., 2015).
Multiple interactions are important for the formation of RNP granules and/or the recruitment of molecules into them. Granule assembly typically requires a pool of RNA molecules, which can bind to the RNA binding domains of numerous granule proteins (e.g. Teixeira et al., 2005). Redundant protein-protein interactions are also necessary for formation of the micron-scale structures observed by light microscopy. Such interactions include those between well-folded domains, as in Edc3 dimerization in yeast P-body assembly (Ling et al., 2008; Decker et al., 2007), as well as between short linear motifs (SLiMs) in disordered regions of RNA binding proteins and other well-folded domains (reviewed in Jonas and Izaurralde, 2013). Some proteins, such as Dcp2 in P-bodies, contain multiple SLiMs, suggesting these interactions can crosslink multiple complexes to form higher order assemblies in vivo, and phase separation in model systems in vitro (Fromm et al., 2014; Li et al., 2012).
RNP granule proteins often contain both RNA binding domains as well as sequences that have been variously termed prion-related, low complexity or intrinsically disordered (we use the most generic term, intrinsically disordered region, IDR). These sequences were originally identified by their similarity to those of the known prions, human prion protein and Sup35p (Gilks et al., 2004; Riejns et al., 2008; Decker et al., 2007), but also include those containing repeated G/S-F/Y-G/S motifs (Kato et al., 2012; Nott et al., 2015; Updike et al., 2011; King et al., 2012; Sun et al., 2011). These sequences can be important for targeting to, and/or formation of, RNP granules. For example, a prion-like domain of Tia1 targets Tia1 to mammalian stress granules (Gilks et al., 2004). Similarly, P-bodies in yeast assemble through redundant interactions of the Edc3 protein and by a prion domain of Lsm4 (Decker et al., 2007), while an mRNP granule in the fungus Ashbya is driven by a polyQ region in the Whi3 protein (Lee et al., 2013). The P-granules in C. elegans are dependent on the Pgl family of proteins for their assembly, which contain an XFG repeat structure (Updike et al., 2011). In addition, the RNA binding protein Fus localizes to yeast and mammalian stress granules through its N-terminal IDR, and mutation of tyrosines in multiple GYG motifs in this region prevents this accumulation (Kato et al., 2012).
It remains unresolved how IDRs promote RNP targeting and formation. Some of these sequences can drive aggregation in vivo (Reijns et al., 2008). In vitro, several form amyloidlike fibers (Sun et al., 2011, Kato et al., 2012, Kim et al., 2013). The IDR of Fus forms filament-containing hydrogels in vitro, and recruitment of Fus IDR mutants into these hydrogels correlates with recruitment into stress granules in cells (Kato et al., 2012). These Fus filaments contain cross-beta structure, similar to classical amyloid filaments formed by the amyloid beta protein (Kato et al., 2012), suggesting that granules may consist of fiber-containing hydrogels (Kato et al., 2012; Han et al., 2012). A potentially alternative model is based on the observation that certain proteins and protein complexes can undergo liquid-liquid phase separation (LLPS), producing structures with physical behaviors similar to P granules and nucleoli (e.g. being round, dynamic, highly concentrated in protein, undergoing fusion and deforming under stress). For example, the disordered protein elastin has been known for decades to undergo LLPS as an important first step in generating elastic extracellular filaments needed for tissue stability (Yeo et al., 2011). Recently the IDR of Ddx4, a protein that resides in maternal mRNP granules referred to as nuage, was shown to undergo LLPS in vitro and in cells in a salt- and temperature-dependent manner (Nott et al., 2015). Phase separation of Ddx4 and elastin is thought to be driven by interactions between multiple, weakly adhesive elements of the proteins. In a related process, interactions between multivalent proteins and their multivalent ligands (both protein and RNA) can also produce LLPS, concomitant with assembly into large oligomers/polymers (Li et al., 2012; Banjade et al., 2014). In these systems, factors that increase crosslinking of the interacting species can promote LLPS (Li et al., 2012; Banjade et al., 2015). Thus, one hypothesis is that for proteins containing both RNA binding domains and IDRs, RNA-protein and IDR-IDR interactions may act cooperatively to promote LLPS. In IDR-containing systems the relationship between the molecular interactions that promote phase separation and those that promote fiber formation remains unknown. In either model, traditional protein-protein interactions that generate discrete multi-component complexes will also contribute to granule formation.
Herein, we examine the phase separation behaviors of IDRs from a series of engineered and natural RNA binding proteins in vitro. We demonstrate that some RNA binding proteins, or their IDRs, can rapidly phase separate on their own, or with RNA, to produce dynamic, liquid-like structures. On a slower time scale, the IDR elements mature to a less dynamic state, which can coincide with formation of fibrous structures. Different IDRs can co-assemble into phase-separated droplets to different degrees, indicating the presence of heterotypic interactions. We also observe analogous phase separation, maturation and heterotypic interactions for the full length RNA binding protein, hnRNPA1. Our data suggest that multivalent and weak interactions among disordered regions on RNA binding proteins, coupled with RNA-protein interactions, could contribute to RNP granule assembly by promoting LLPS. Moreover, such a view joins phase separation and fiber formation into a unified model by positing that the progression from dynamic liquid to more stable fibers could be regulated in cells to produce structures with varying physical properties and chemical compositions, according to particular biological needs. Aberrant regulation could explain the basis for the formation of pathological stress granules in certain diseases.
Results
Strategy
To examine the behavior of IDRs in vitro we recombinantly produced a panel of six IDRs taken from known components of RNP granules: Lsm4, which is known to play a role in P-body assembly in yeast (Decker et al., 2007); Tia1 and its yeast ortholog Pub1, whose IDR has been suggested to play a role in mammalian stress granule assembly (Gilks et al., 2004); TIF4632 (eIF4GII), a yeast stress granule component with an N-terminal IDR (Buchan et al., 2008); as well as human hnRNPA1 and Fus, which are abundant RNA binding proteins that localize to, and can contribute to, stress granule assembly (Schwartz et al., 2014; Guil et al., 2006). To visualize components by fluorescence microscopy, we fused all proteins to an N-terminal SNAP tag, which was then coupled to the SNAP-Surface 488 or SNAP-Surface 649 fluorophores. In some cases the proteins were fused to PTB, an RNA binding protein involved in splicing that contains four RNA-binding RRM domains. We previously reported that interactions between PTB and a single-stranded RNA containing five RRM-binding elements resulted in LLPS (Li et al., 2012). This collection of reagents enabled us to compare various IDRs, RNA binding elements and the interplay between domain- and IDR-promoted LLPS. To determine whether the behaviors of these simple engineered systems are manifest in more complicated natural protein, we generated recombinant full-length hnRNPA1, a mammalian granule protein that contains both folded RNA binding domains and IDR elements. We indicate the IDR element of a given protein with a subscripted IDR (e.g. Pub1IDR), and use no indication for full-length proteins. We examined whether these proteins could undergo LLPS either at low salt concentration, which can promote phase separation of some IDRs (Nott et al., 2015), or in the presence of RNA binding partners, and how the properties of the resulting droplets varied over time and between proteins.
IDRs Can Undergo LLPS
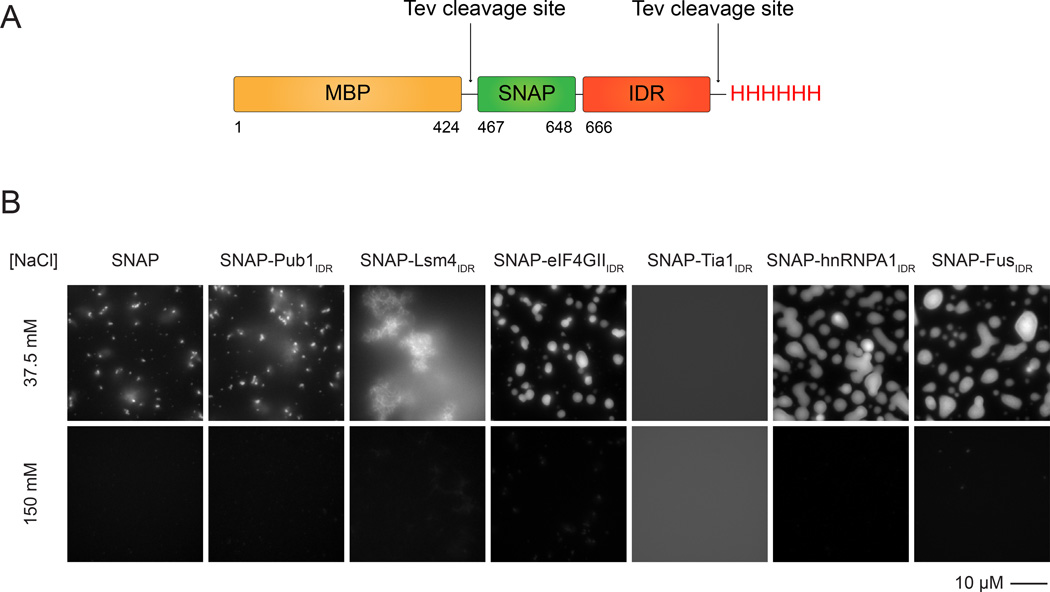
We initially asked whether IDRs alone can undergo LLPS when fused to the SNAP-tag. In all cases examined (Pub1IDR, eIF4GIIIDR, Lsm4IDR, Tia1IDR, FusIDR and hnRNPA1IDR), solutions of these proteins remained clear at room temperature under physiologic salt conditions (100–150 mM NaCl), and when examined by light microscopy, only diffraction-limited puncta were observed settled onto the glass slide surface, which appear to represent a low level of aggregated protein. However, when the NaCl concentration was diluted to 37.5 mM, solutions of hnRNPA1IDR, eIF4GIIIDR, and FusIDR(6–33 µM protein, see Figure 1 legend) became opalescent, and brightly fluorescent, micron sized spherical structures were observed by light microscopy. Over time, these structures settled onto the slide, where they sometimes spread into irregular shapes (Figure 1B). These structures appeared to be phase separated liquids based on several criteria. They: a) were spherical in solution, b) could flow and fuse (Movie S1), c) showed concentration dependence in their total volume (data not shown and see below), and d) disassembled rapidly when returned to high salt after 30' of low salt treatment (see below). Solutions of SNAP-Lsm4IDR, Pub1IDR and Tia1IDR either rapidly formed fiber-like structures (Lsm4) or remained as a single phase when fused to the SNAP tag at low salt. Thus, IDRs can alternatively form both fibers and phase-separated droplets under low salt conditions.
Figure 1. Particular IDRs Are Sufficient to Drive LLPS at Low Salt Concentration.
(A) Schematic of SNAP-IDR proteins. MBP, maltose binding protein. SNAP, SNAP-tag used for fluorophore labeling. IDR, intrinsically disordered region. TEV protease removes MBP and His tags.
(B) Fluorescence microscopy images of the macroscopic structures formed by SNAP-IDRs at 37.5 and 150 mM NaCl. SNAP-hnRNPA1IDR, 6.925 µM; SNAP-FusIDR, 10.75 µM; all the other proteins, 32.75 µM. Proteins were labeled with SNAP-Surface 649.
To determine how intracellular crowding might affect LLPS driven by IDRs, we examined the hnRNPA1IDR in the presence of 10% PEG. We observed that in the presence of crowding agents hnRNPA1IDR underwent LLPS at 150 mM NaCl and at a concentration of 1 µM (Figure S1B), which is below the concentration of hnRNPA1 in cells (1.17×l07 molecules per cell or approximately 5–10 µM; Beck et al., 2011). Thus crowding strongly promotes LLPS of hnRNPA1IDR.
RNA Can Promote LLPS of IDR Proteins
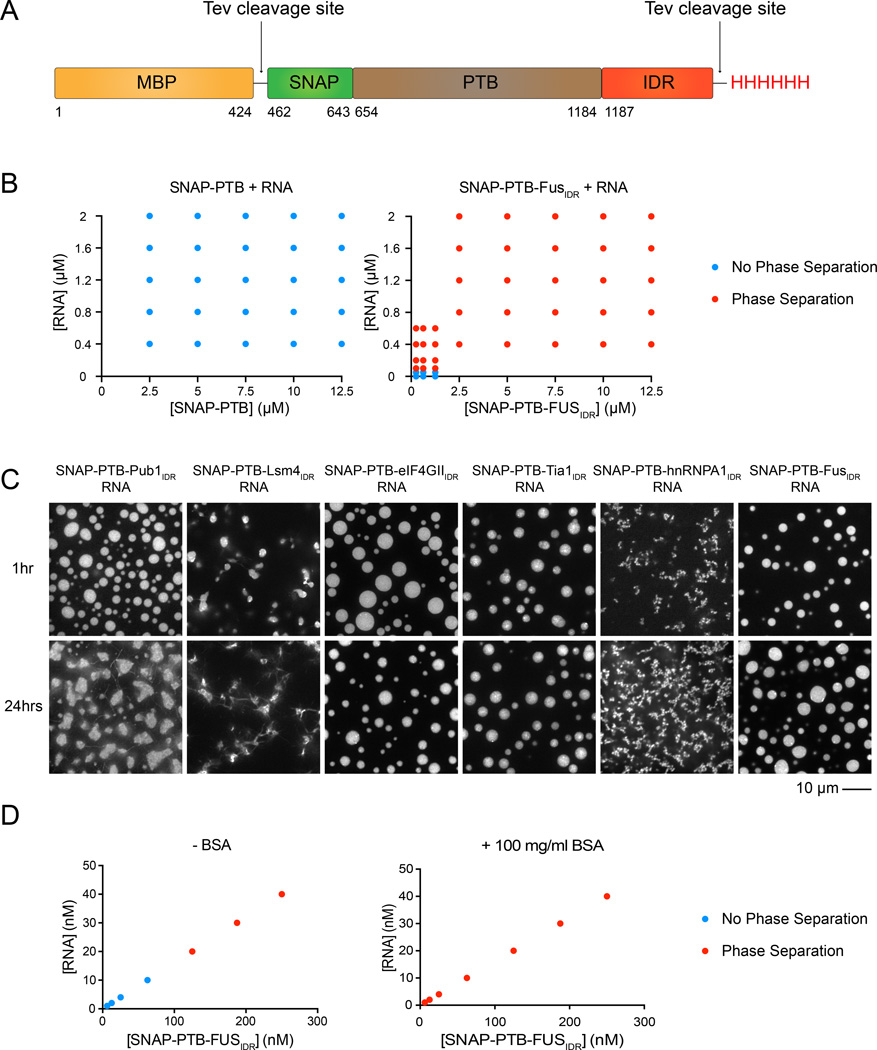
In cells, RNP granule assembly is dependent on the concentration of specific RNAs (Teixeira et al., 2005; Buchan and Parker, 2009). We previously showed that interactions between the four RNA-binding RRM domains of PTB and an RNA molecule containing multiple RRM-binding motifs can promote LLPS, independent of any IDR elements (Li et al., 2012). Thus, we asked whether RNA could promote LLPS in proteins containing both PTB and IDRs. At 100 mM NaCl and protein concentrations of 1.25–2.5 µM all six SNAP-PTB-IDR fusions phase separated upon addition of 0.4–0.8 µM RNA, producing droplets that concentrated both molecules (Figure 2 and Figure S2A, showing RNA enrichment). In contrast, SNAP-PTB only phase separated at 50 µM concentration in the presence of ≥ 2 µM RNA (not shown). Performing the experiment in the presence of 100 mg/ml BSA to mimic protein crowding effects within the cell, allowed the detection of RNA driven LLPS of the SNAP-PTB-FusIDR at concentrations below 10 nM (Figure 2D), which is below the cellular concentration of many of the abundant components of mRNP granules (Beck et al., 2011).
Figure 2. RNA Can Promote LLPS of IDR Proteins.
(A) Schematic of SNAP-PTB-IDR proteins. PTB, polypyrimidine tract-binding protein, containing four RRM RNA binding domains. TEV protease removes MBP and His tags.
(B) Phase diagram of SNAP-PTB and SNAP-PTB-FusIDR plus RNA. Red dots indicate phase separation; blue dots indicate no phase separation.
(C) Fluorescence microscopy images of the macroscopic structures formed at 100mM NaCl by SNAP-PTB-4GIIIDR 1.25 µM and RNA 0.4 µM; SNAP-PTB-FusIDR 1.25 µM + RNA 0.4 µM; for the rest of droplets, SNAP-PTB-IDR 2.5 µM, RNA 0.8 µM. Proteins were labeled with SNAP-Surface 649. Images were taken at 1 hour and 24 hours after the initiation of phase separation by RNA addition.
(D) Phase diagram of SNAP-PTB-FusIDR plus RNA in the absence and presence of 100 mg/ml bovine serum albumin (BSA).
To better understand how RNA promotes phase separation, we examined whether RNA could stimulate LLPS of the SNAP-IDR proteins lacking PTB. A range of RNA concentrations (0.1–10 µM) did not promote phase separation of the SNAP-IDR proteins, up to protein concentrations of 10 – 30 µM. The only exception to this behavior was provided by SNAP-eIF4GIIIDR, which did phase separate upon addition of RNA (Figure S2B), and was able to bind RNA directly according to a gel shift assay (data not shown). Thus, for all proteins except eIF4GII, RNA-induced phase separation required both the IDR and RNA binding to PTB. We interpret these results as indicating that PTB-RNA and IDR-IDR interactions act synergistically to promote LLPS.
Although all six SNAP-PTB-IDR proteins phase separated with RNA, the resulting structures differed significantly in their morphologies (Figure 2C). At 1 hour after RNA addition, droplets formed by the eIF4GIIIDR, Pub1IDR and FusIDR fusions were relatively large, round, and separated into discrete structures. By contrast, the Lsm4IDR, Tia1IDR and hnRNPA1IDR fusions created droplets that were smaller, and often attached to each other in long irregular chains, as though coalescence into larger structures had been aborted. These behaviors mirrored those observed for the SNAP-IDR structures induced by low salt, where SNAP-eIF4GIIIDR formed liquid droplets, while SNAP-Lsm4IDR formed fibers (Figure 1B). Thus, different IDRs can create phase-separated droplets with different physical properties.
Phase Separated Droplets Mature Over Time
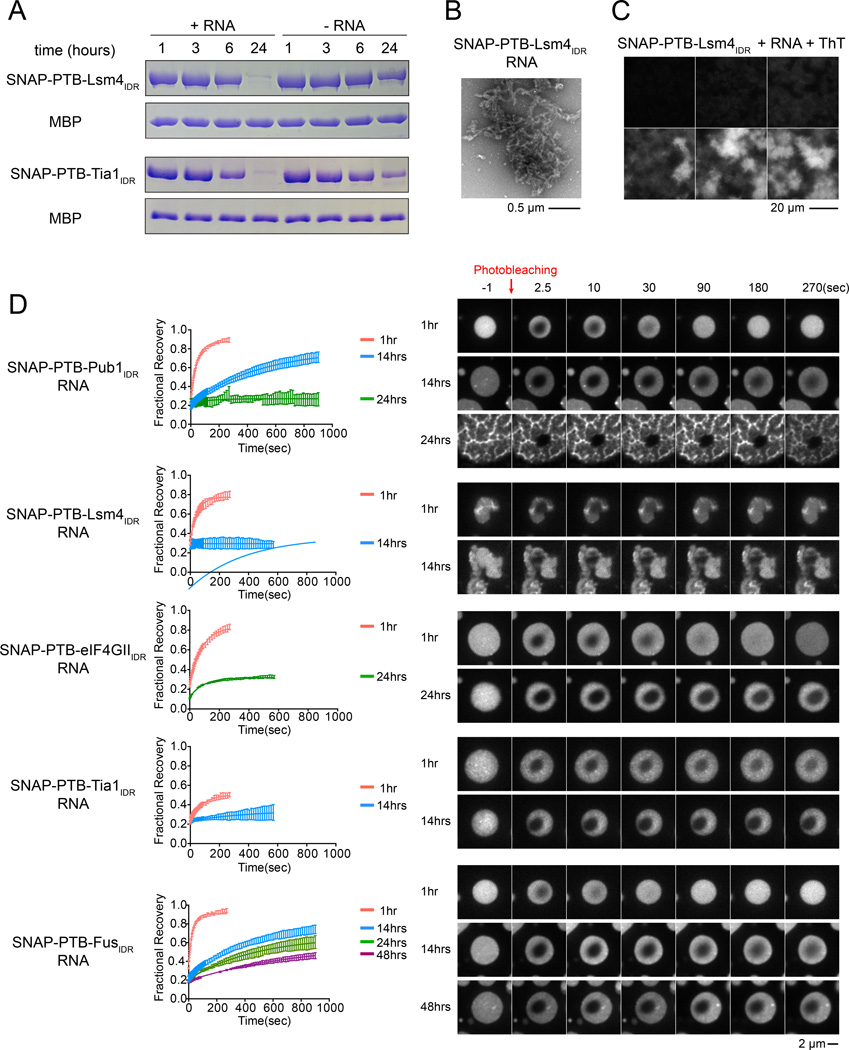
We next monitored the SNAP-PTB-IDR+RNA droplets over time after initiating their formation. In all cases, the droplets changed over time according to three measures. First, their gross appearance changed. When examined over 24 hours, droplets from most of the IDRs changed from round to irregularly shaped. Many also formed filamentous structures that extended outside of the droplet bodies, and became less homogeneously fluorescent (Figure 2C). The only exception to this behavior was SNAP-PTB-FusIDR, whose droplets remained round and homogeneous to 72 hours (data not shown). For SNAP-PTB-Lsm4IDR and SNAP-PTB- Tia1IDR, formation of filaments was greatly accelerated by LLPS in a biochemical assay, where RNA addition caused most of the protein to become insoluble (and filamentous in nature in electron micrographs) after 24 hours, while most remained soluble in solutions without RNA (Figure 3A and B). We also assessed the relative stability of the SNAP-PTB-Lsm4IDR and SNAP-PTB- TIA1IDR fibers by the addition of SDS prior to centrifugation. We observed that the SNAP-PTB-Lsm4IDR fibers were SDS resistant while the SNAP-PTB- Tia1IDR fibers were SDS sensitive (Figure S3C). This differential sensitivity to SDS indicates that IDRs will form fibers of different biochemical properties, tunable to their biological role.
Figure 3. Phase Separated Droplets of SNAP-PTB-IDRs Plus RNA Mature Over Time.
(A) SDS-PAGE of the high-salt soluble species present at different time points after the initiation of phase separation by RNA addition. MBP-SNAP-PTB-Lsm4IDR (5µM) or MBP-SNAP-PTB-TIA1IDR (5µM) were mixed with RNA (1.6µM) (phase separation) or buffer (no phase separation). At the indicated times, NaCl was raised to 500mM total concentration followed by 5 minutes of centrifugation, and the supernatant analyzed by SDS PAGE; gels were stained with coomassie blue. TEV protease was also present to remove MBP, which serves as an internal loading control.
(B) Transmission electron micrographs of the high-salt insoluble species in a solution of SNAP-PTB-Lsm4IDR plus RNA after 24 hours incubation.
(C) Representative images of increase over time in Thioflavin T (ThT) fluorescence for droplets of SNAP-PTB-Lsm4IDR(5 µM) plus RNA (1.6 µM) shown in the same intensity scale.
(D) The liquid droplets of SNAP-PTB-IDR proteins plus RNA become less dynamic over time. Left panels show FRAP recovery curves. Data are reported as mean ± SD. Right panels show a representative droplet for each protein at different time points. See also Table S1.
A second property that changed over time was resistance of the phase-separated droplets to salt. Phase separation of SNAP-hnRNPA1IDR and SNAP-FusIDR induced by low salt was largely reversible in the first 22 minutes after initiation. But with increasing time the remaining assemblies became salt resistant, with full resistance seen at 24 hours (Figure S3A and B).
A third key observation was that the dynamics of the SNAP-PTB-IDR+RNA droplets changed substantially over time as assessed by FRAP of the SNAP-Surface 649 label (Figure 3D). In the first hour after initiation, the fluorescence of a small region in the center of larger droplets would recover after photobleaching with half-lives ranging from 19 to 64 seconds, and recovery fractions ranging from 0.3 to 0.9. Over 14–48 hours the half-lives for each fusion protein increased and the fractional recoveries steadily decreased, such that fluorescence of most of the IDRs no longer recovered at the latest time point. The progression from dynamic to static reflected the changes in droplet morphology: droplets that remained rounder and longer remained dynamic longer (e.g. Fus and eIF4GII), and vice versa (e.g. Lsm4 and Tia1). Since droplets from both the IDRs alone and PTB-IDR+RNA showed similar changes in morphology, we attribute these effects primarily to the IDR elements rather than PTB or RNA. Our interpretation of these results is that the high concentration of the IDR in the phase separated droplets (370 µM and 310 µM for SNAP-PTB-Pub1IDR and SNAP-PTB-eIF4GIIIDR, respectively; Figure S3D and E) leads over time to the formation of kinetically trapped and stable structures. This maturation may involve the formation of amyloid-like fibers, since the filamentous structures of SNAP-PTB-Lsm4IDR + RNA that become more numerous with time stained strongly with Thioflavin-T, (Figure 3C).
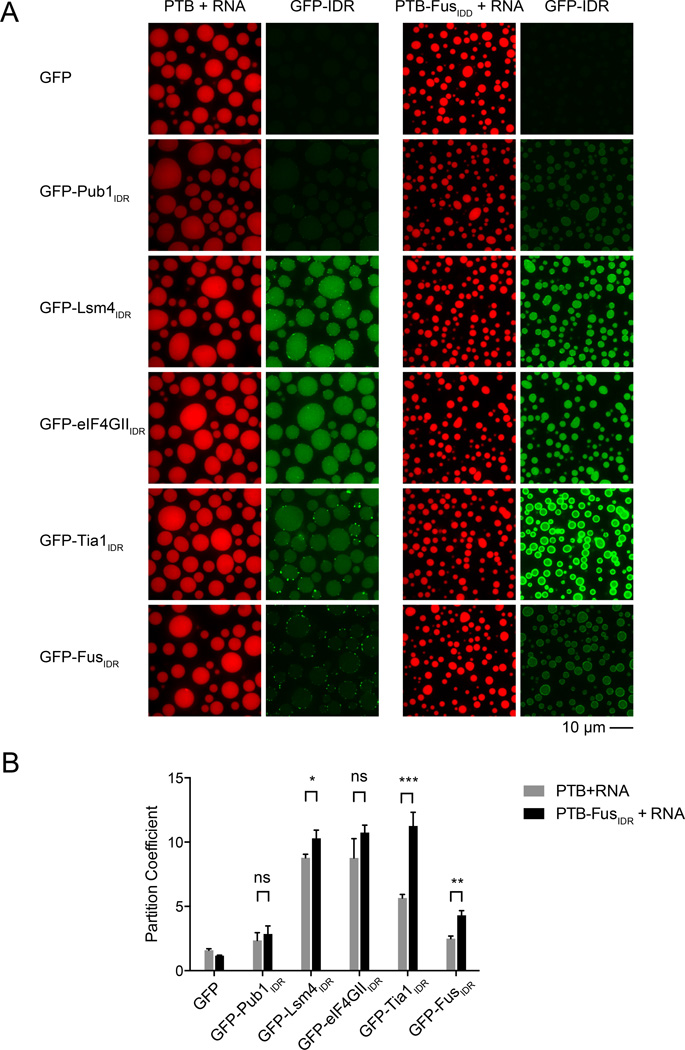
Phase Separated Droplets Can Recruit IDRs Through Multiple Types of Interactions
In vivo RNP granules contain both RNA and multiple proteins with IDRs. This raises the possibility that heterotypic interactions between IDRs, folded domains and/or RNA might recruit proteins with IDRs into these assemblies. To test this idea in vitro, we examined the ability of PTB-FusIDR+RNA droplets to recruit GFP fusions of the other IDRs. GFP alone was not selectively recruited into, or excluded from, any of the droplets, with partition coefficients ({droplet concentration}/{bulk concentration}, quantified from fluorescence intensities related to a calibration curve) of approximately 1 in all cases. All of the GFP-IDR proteins were recruited into the PTB-FusIDR+RNA droplets, with partition coefficients ranging from approximately 3 to 12 (Figure 4). All proteins were also recruited into PTB+RNA droplets. But for GFP-Lsm4IDR, GFP-Tia1IDR and GFP-FusIDR, recruitment into the PTB- FusIDR+RNA droplets was significantly higher (Figure 4B), suggesting that interactions dependent on the FusIDR can enhance hetero- and homotypic recruitment of IDRs into the phase separated droplets. Consistent with FusIDR promoting heterotypic recruitment, the difference between recruitment into PTB-FusIDR+RNA droplets and PTB+RNA droplets was even larger for SNAP-IDR proteins than for EGFP-IDR proteins (Figure S4).
Figure 4. IDR Dependent Phase Separated Droplets Recruit Heterotypic IDRs.
(A) Representative images showing the partitioning of GFP-IDR probes (100nM, green) into liquid droplets (red) of PTB or PTB-FusIDR plus Cy3-labeled RNA.
(B) Quantification of the GFP-IDR partition coefficients in experiments from panel A. The partition coefficients are plotted as mean ± SD, from three independent measurements each of which averaged all the droplets across four random slide regions. ns. not significant, p > 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001. P values were determined by unpaired t test.
The recruitment of GFP-Pub1IDR, GFP-Lsm4IDR and GFP-eIF4GIIIDR into the PTB+RNA droplets could be explained by weak interactions of these IDRs with RNA. Consistent with this possibility we note all of these IDRs have high predicted pI values (Pub1 11.5; Lsm4 11.0; eIF4GII, 9.6). Moreover, we observed by gel shift assays that Lsm4IDR and eIF4GIIIDR had stable interactions with RNA (data not shown). Analogously, GFP-Tia1IDR and GFP-FusIDR, which both are acidic (IDR pI values of 5.8 and 4.7, respectively) may be recruited through interactions with PTB, which is quite basic (pI = 9.2). Taken together, these observations suggest that IDR containing proteins could be recruited to RNA-protein granules by both IDR dependent interactions and by other interactions, which could include binding to RNA.
Natural RNP Granule Proteins Show the Same Behaviors as the Engineered Proteins
Using a series of engineered proteins, we have able to systematically explore the behaviors of different IDRs in combination with different RNA binding domains. We found that A) the IDRs can undergo LLPS at low salt, B) LLPS by IDRs is promoted under physiologic salt conditions by RNA, C) the IDR-containing phase separated droplets mature over time to become less dynamic and D) droplets formed by one IDR can recruit other IDRs to different degrees. We next asked whether these same behaviors are also manifest in natural RNA binding proteins.
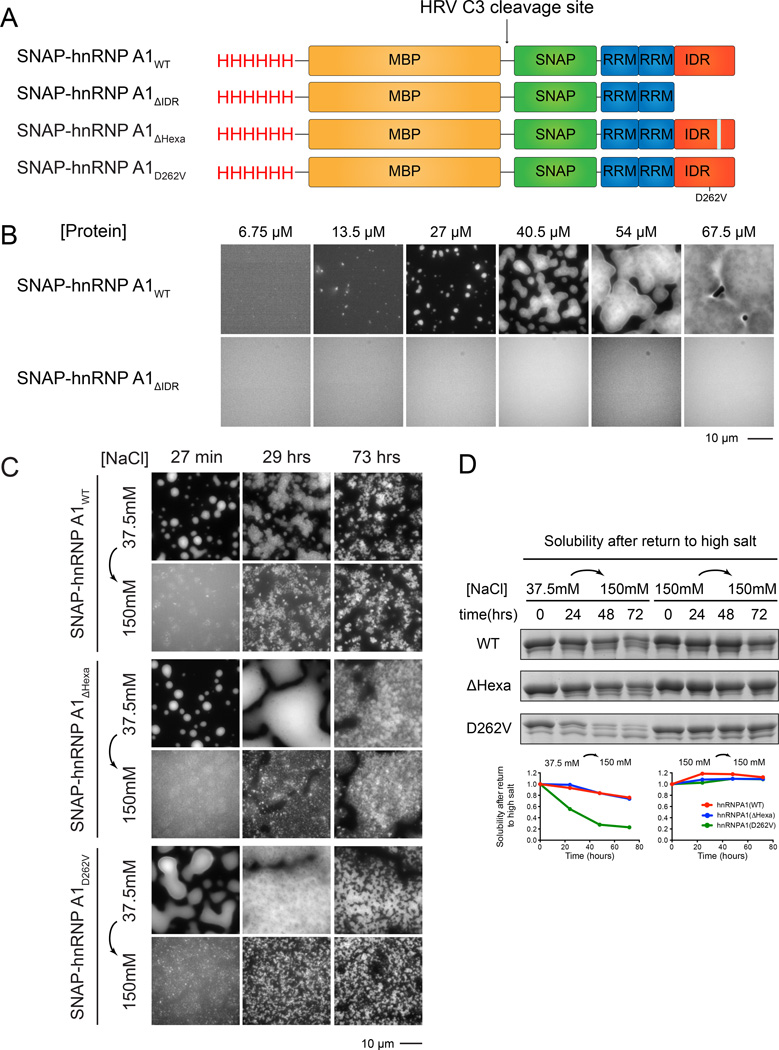
hnRNPA1 is component of stress granules in mammalian cells (Guil et al., 2006), whose mutations can be causative in inclusion body myopathies such as ALS and FTLD, and can increase stress granule formation (Kim et al., 2013). The protein is composed of two N-terminal RNA-binding RRM domains, and an ~200 residue C-terminal IDR that is enriched in G/S-Y/F-G/S elements (Figure 5A). We expressed full-length hnRNPA1 in E. coli with an N-terminal SNAP-tag and purified it to homogeneity. Under physiologic salt conditions (150 mM NaCl), the protein was soluble and existed as a single-phase solution up to concentrations as high as 300 µM at room temperature. However, upon transfer to low salt (37.5 mM NaCl), SNAP-hnRNPA1 rapidly coalesced into micron sized, brightly fluorescent spherical structures at concentrations above 13.5 µM (Figure 5B). As with the engineered proteins, these structures were spherical, could flow and fuse (Movie S3), were concentration dependent (Figure 5B) and disassembled rapidly when returned to high salt after 30 minutes of low salt treatment, indicating that they were phase separated liquids (Figure 5C). This phase separation was dependent on the IDR since an hnRNPA1 construct lacking this C-terminal element (hnRNPA1AΔIDR) did not phase separate under salt and protein concentrations where the full-length protein generated droplets (Figure 5B). Similar to the model systems, we observed that macromolecular crowding in the presence of PEG led to LLPS of full length hnRNPA1 under physiological concentrations of protein as low as 1 µM at 37.5 mM NaCl (Figure S5A). Thus, hnRNPA1 can undergo IDR-dependent LLPS on its own at low ionic strength.
Figure 5. Full-length hnRNPA1 Undergoes IDR Dependent Phase Separation.
(A) Schematic of domain architecture of SNAP-hnRNPA1 proteins. HRVC3 removes His and MBP tags.
(B) Fluorescence microscopy images of the macroscopic structures formed by SNAP-hnRNPA1WT or SNAP-hnRNPA1ΔIDR at 37.5 mM NaCl, Images are shown in different intensity scale to highlight morphological changes.
(C) Fluorescence microscopy images of structures formed by hnRNPA1WT, hnRNPA1ΔHexa, and hnRNPA1D262V (all 25 µM) at 37.5 mM NaCl. At indicated time points, NaCl was raised to 150 mM total concentration and structures that remained were imaged. Images are shown in different intensity scale to highlight morphological changes.
(D) SDS-PAGE assays of the amount of high-salt soluble species present at different time points after the initiation of phase separation at 37.5 mM NaCl. hnRNPA1WT, hnRNPA1ΔHexa, and hnRNPA1D262V (all 25 µM) were incubated at 37.5 mM NaCl for the indicated time period before raising total NaCl concentration to 150 mM followed by centrifugation. The supernatant was then analyzed by SDS-PAGE with Coomassie staining. Quantification of the relative intensities of the bands is shown in the lower panel.
The phase separated hnRNPA1 droplets also matured over time. When examined from 27 minutes to 73 hours after initiation of LLPS in low salt, the droplets changed from uniformly round and homogeneously fluorescent to irregularly shaped and heterogeneously fluorescent (Figure 5C, top row). Further, while phase separation was largely reversible by returning to high salt after 27 minutes, with increasing time the remaining assemblies became salt resistant (Figure 5C, second row). Thus, as with the engineered systems, the phase separated hnRNPA1 droplets become more stable over time. Since the IDR of hnRNPA1 is known to form amyloid-like filaments (Kato et al., 2012; Kim et al 2013), it seems likely that formation of amyloid fibers contributes to this maturation of the droplets over time. We note that our data showing that hnRNPA1 can phase separate, and that phase separation promotes formation of structures that likely resemble amyloids have also been described (Molliex et al., 2015, submitted).
To better understand the relationship between fiber formation and phase separation by hnRNPA1, we examined how mutations that affect the propensity of the protein to form amyloid structures (Kim et al., 2013) affect phase separation and droplet maturation. A hexapeptide deletion of hnRNPA1 (hnRNPA1ΔHexa) removes the predicted amyloid-forming region of the protein and abolishes hnRNPA1 fiber formation in vitro (Kim et al., 2013). Oppositely, a mutation in the amyloid core (D262V) is known to induce ALS-like neurodegeneration in vivo and amyloid hyper-assembly in vitro (Kim et al., 2013). We observed that both the variants of hnRNPA1ΔHexa and hnRNPA1D262V produced droplets similarly to wild-type hnRNPA1, indicating that the ability to phase separate is not strictly coupled to amyloid formation (Figure 5C). Interestingly, hnRNPA1D262V became salt resistant much faster than the wild-type and the Δhexapeptide hypo-assembly mutant (Figure 5D). Consistent with the ability of hnRNPA1D262V to form amyloid fibers, we observed that SDS-resistant fibers were visible after 29 hours in solutions containing the hnRNPA1D262V droplets (Figure S5C). Similar fibers were also seen in the hnRNPA1D262V protein preparation with continued incubation at high salt where no phase separation occurs, but only after 54 hours (Figure S5D). Thus, phase separation is not required to generate fibers, although as in the engineered systems it appears to increase the rate of fiber formation, presumably due to the high concentration of protein in the droplets. Consistent with these hnRNPA1 fibers having amyloid-like features, fibers formed in either high or low salt stained positive with Thioflavin-T (Figure S5B).
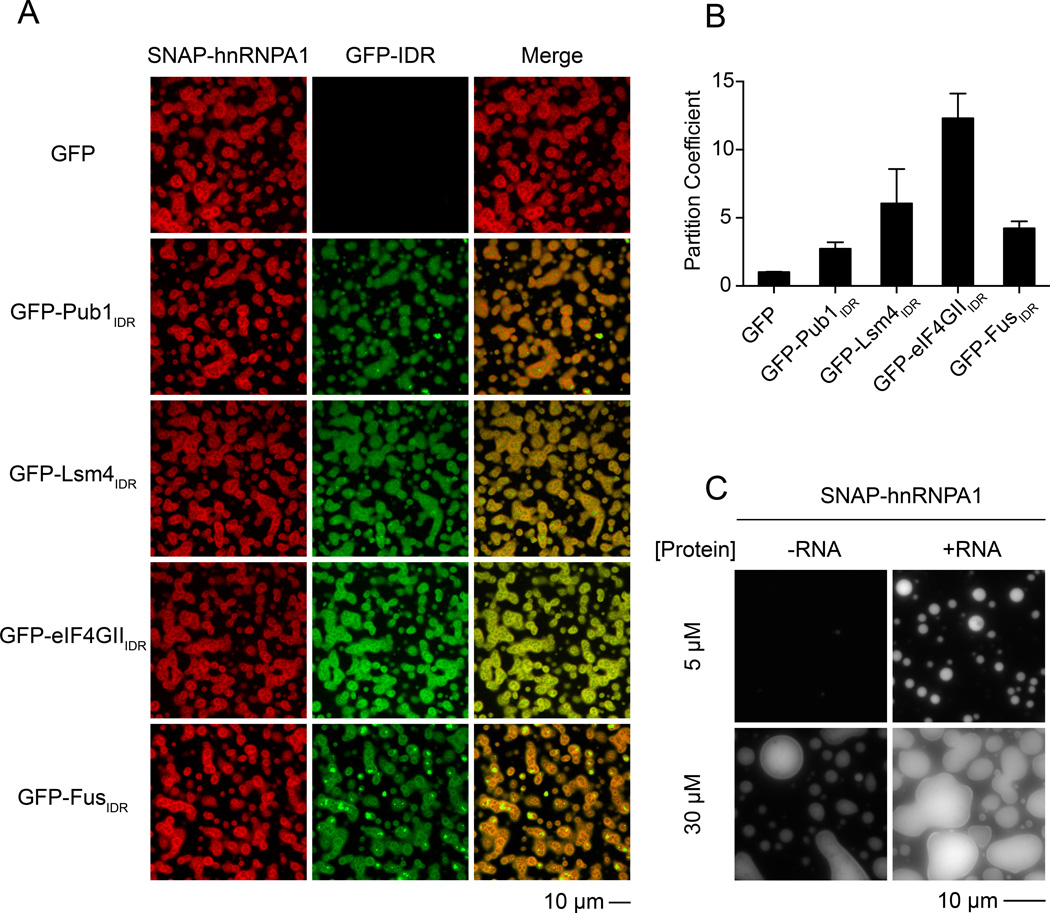
We also observed that phase separated droplets of hnRNPA1 could recruit other IDRs. Specifically, the GFP-FusIDR, GFP-eIF4GIIIDR and GFP-Lsm4IDR proteins were similarly recruited into droplets produced from the hnRNPA1 protein in low salt, while GFP alone was not (Figure 6A and B). This indicates that the heterotypic recruitment of IDR proteins into droplets can occur in a protein dependent manner.
Figure 6. Phase Separated Droplets of Full-length hnRNP Al Recruit GFP-IDRs and Are Promoted by RNA.
(A) Images showing the partitioning of GFP-IDR probes (100 nM, green) into the liquid droplets (red) of SNAP-hnRNP A1 (30 µM) at 37.5 mM NaCl. SNAP-hnRNPA1 was labeled with SNAP-Surface 649.
(B) Quantification of the GFP-IDR partition coefficients in experiments from panel A. The partition coefficients are plotted as mean ± SD, from three measurements each of which averaged all the droplets across three slide regions.
(C) Fluorescence microscopy images of SNAP-hnRNP A1 (2 or 20 µM) with or without RNA(2: 1 molar ratio of 5XA1 RNA : hnRNPA1) at 175 mM NaCl, 100 mg/ml PEG 3350.
Initial attempts to observe LLPS upon RNA addition to hnRNPA1 under physiologic salt conditions were unsuccessful. However, if we added PEG as a crowding agent, we observed phase separation of hnRNPA1 without RNA at high protein concentrations (Figure 6C), and stimulation of LLPS with RNA addition at lower protein concentrations (Figure 6C), demonstrating that RNA can promote LLPS of hnRNPA1. Similar results were observed with Ficoll as a crowding agent (Figure S6A and B).
Thus, the basic behaviors that we observed for droplet formation, maturation and partitioning are common among a large group of engineered and natural RNA binding proteins, suggesting they are likely common to proteins of this type that contain both RNA binding domains and IDRs.
Discussion
Disordered Regions Can Promote Phase Separation
Our observations demonstrate that IDRs on a number of RNA binding proteins can phase separate alone or in concert with RNA binding domains and RNA. Further, the different types of interactions in these systems, IDR-IDR and folded domain-RNA, act synergistically to promote LLPS. How might this occur? Interactions between RNA and RNA binding proteins are often multivalent due to both multiple binding sites on RNA and multiple RNA binding domains in the proteins. IDR interactions are also likely to be multivalent through weak binding of multiple sequence motifs in the disordered chains to each other (Nott et al., 2015). This property enables each to promote oligomerization and concomitant phase separation (Li et al., 2012). In PTB-IDR fusion proteins, the PTB-RNA and IDR interactions could act together to produce larger oligomeric structures than either would alone, thereby promoting LLPS at lower concentrations (Flory, 1953). Analogous behaviors are seen for proteins such as the γ-crystalins, whose crosslinked oligomers undergo LLPS at progressively lower concentrations as their size increases (Pande et al., 1995; Asherie et al., 1998). This suggests that RNP granule assembly will be driven in part by mRNA providing multivalent sites for RNA binding proteins and then hetero- and homotypic interactions between IDRs on these proteins creating larger oligomers generated from two inherent forms of multivalency.
IDR Dependent Phase Separated Droplets Mature to a Less Dynamic State
Based on salt resistance, morphology and FRAP analysis phase-separated droplets promoted by IDRs mature over time to more stable, less dynamic assemblies. Maturation appears to be driven, at least in part, by the formation of amyloid-like fibers, since A) over time many of the IDR proteins form filaments that can be observed by light- and/or electron microscopy (Figures 2C and 3B), B) these fibers can stain with Thioflavin-T and in some cases are SDS resistant (Figures S3C and S5C), and C) droplets formed by a pathogenic mutant of hnRNPA1 that has a greater propensity to form fibers (Kim et al., 2013) mature more rapidly (Figure 5D). For SNAP-PTB-Lsm4IDR, SNAP-PTB-Tia1IDR and full length hnRNPA1 (Figures 3A and 5D) filaments form more rapidly in phase separated solutions likely due to the high concentration of molecules in the droplets, as expected by analogy to other amyloid systems (Eisenberg and Junker, 2012). Thus, the high concentration of IDRs produced by LLPS can increase the rate of amyloid fiber formation, thereby leading to droplet maturation. This suggests that hydrogels with a high concentration of fibrous structures could be highly analogous, if not identical, to a matured phase separated droplet containing specific IDRs.
IDR Dependent Phase Separated Droplets Recruit Other IDR Proteins
Several observations demonstrate that phase separated droplets promoted by one IDR can effectively recruit proteins containing the same, or different IDRs. First, PTB-FusIDR+RNA droplets effectively recruit GFP-IDR and SNAP-IDR molecules (Figures 4 and S4). IDR recruitment appears to depend on a combination of interactions with PTB/RNA and also the Fus IDR. Second, hnRNPA1 droplets generated in low salt effectively recruit the IDRs of Lsm4, Pub1, eIF4GII and Fus (Figure 6). The ability of a given phase separated droplet to recruit diverse IDRs could provide a mechanism for the assembly of multiple RNA binding proteins with IDR domains into a single mRNP granule. Moreover, the presence of heterotypic interactions between IDRs on RNA binding proteins might also increase the relevant concentration available to trigger phase separation in vivo.
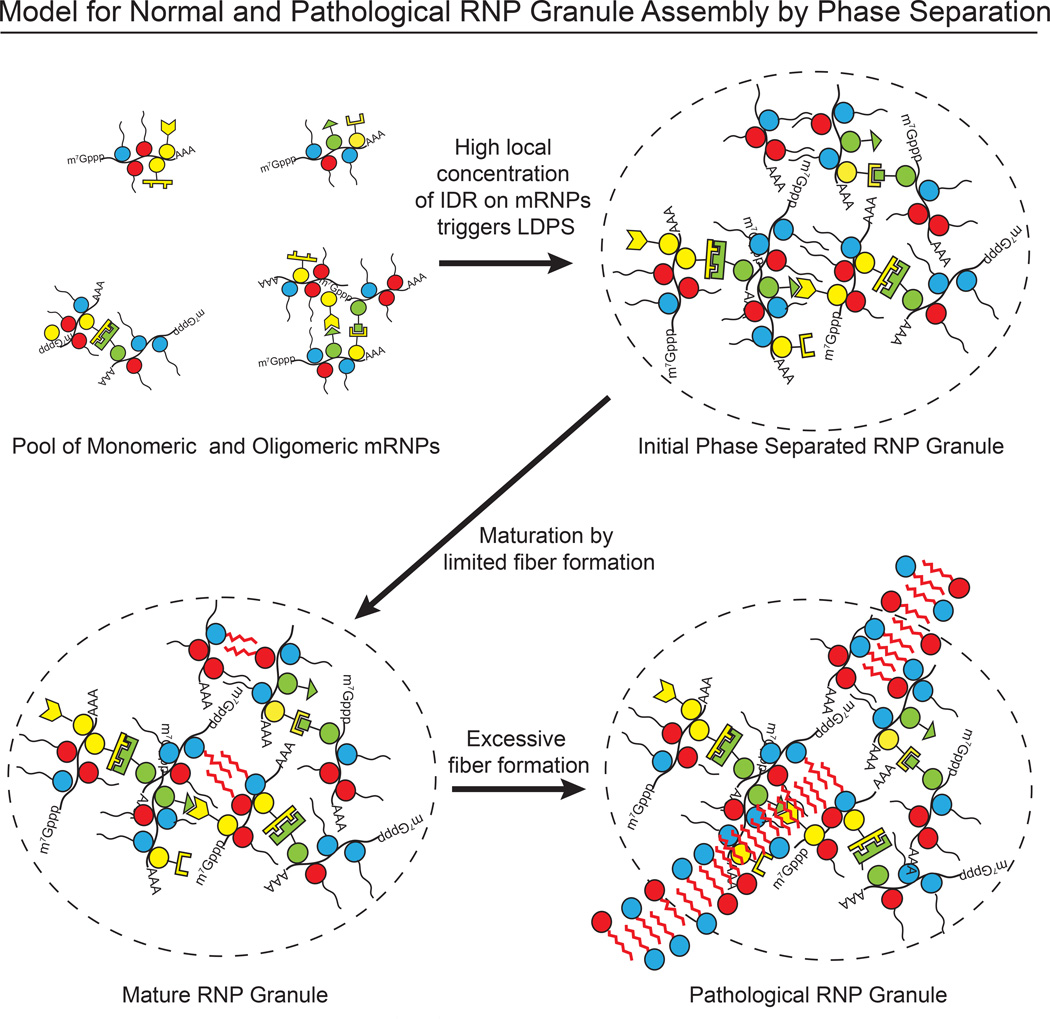
A Model for RNP Granule Assembly
Our data suggest a molecular mechanism by which LLPS could contribute to RNA granule formation through synergistic, multivalent interactions of defined RNA binding domains, IDR elements and RNA. Since IDR interactions may be of relatively low specificity, the high concentration of heterotypic IDRs on an assembled mRNP, or mRNPs oligomerized by traditional protein-protein interactions, could create a high local IDR concentration to initiate phase separation (Figure 7). The liquid-like structures formed by these processes would be distinct from traditional macromolecular assemblies in at least two important ways. First, unlike canonical multicomponent complexes (e.g. the ribosome) a granule formed by LLPS would not be stereochemically defined. Rather, beyond the scale of its individual proteins or RNA-protein complexes, the elements would be randomly organized and dynamically rearranging. Second, because phase separated droplets are >90 % water by mass (based on protein concentrations ~300 µM), macromolecules could enter, diffuse within and exit easily. Indeed, 40 kDa dextrans readily enter P-granules in nematodes, although 155 kD dextrans are excluded (Updike et al., 2011). Together, these properties conspire to enable molecules to be concentrated within granules, but move rapidly and freely within them.
Figure 7. Possible Model for How Phase Separation Contributes to RNP Granule Assembly.
Initially the macromolecular interactions within the phase separated droplets are transient, and without long-range structural organization, affording the droplets liquid-like properties. But the high protein concentrations within the droplets would accelerate the natural tendency of IDRs to form amyloid-like fibers. Further experiments will be necessary to determine whether the interactions that promote LLPS and those that promote fiber formation are mediated by the same or different polypeptide structures and/or chemical elements in the IDR chains. Either way, fiber formation would naturally lead over time to more static structures that behave as solids. In cells, the balance between disordered, dynamic liquid and ordered, more static fibers is likely regulated to produce a range of RNP granules with different physical and chemical properties according to specific functional needs. More generally, the ability of LLPS to rapidly generate a concentrated biochemical compartment within cells that favors downstream interactions is likely to be a fundamental principle of these transitions, which cells use in numerous contexts to rapidly establish and then maintain cellular structures.
The lengths and/or numbers of fibers within a granule could be controlled through factors that enhance or inhibit fiber nucleation and growth, or that actively disassemble fibers, such as the ATP-driven disaggregase machines VCP/Cdc48 and Hsp70/Hsp40 complexes, which are known to control RNA granule lifetimes and turnover (Buchan et al., 2013; Walters et al., 2015). When this regulation is aberrant, for example due to mutations that increase fiber propensity or decrease disaggregase activity, fiber formation can become extreme, leading to disease. Such a model could explain how increased or prolonged stress granules can promote the formation of pathological aggregates that occur in various degenerative diseases (Ramaswami et al., 2013; Li et al., 2013). The unique ability of IDRs to populate highly distinct structural and dynamic states under physiologic conditions would make them uniquely suited to control the physical and chemical properties of RNP granules, perhaps explaining the abundance of IDR elements in RNA binding proteins.
Experimental Procedures
Materials
Proteins were expressed from the pMal-c2 vector (NEB), except for full length hnRNPA1 and related mutants, which were cloned into a modified pet11a vector (Novagen)(see Supplemental Experimental Procedures). Proteins were expressed in E. coli BL21(DE3) and purified with Ni-NTA and/or amylose resin. SNAP-PTB-IDRs were further purified through a Superdex200 column (GE Healthcare). Proteins were fluorescently-labeled with SNAP-Surface 488 or SNAP-Surface 649 (NEB) according to the manufacturer’s protocols. Amino acid residues of all proteins and nucleotide sequences of all RNAs are listed in Table S2.
Droplet Assembly
For SNAP-IDRs and SNAP-hnRNPA1 (~2% fluorescently labeled), droplet assembly was initiated by diluting solutions to 37.5 mM NaCl, 20 mM Tris pH 7.4, 1 mM DTT. For SNAP-PTB-IDRs, proteins and RNA were mixed at the indicated concentrations (including 100 nM SNAP-PTB-IDRs labeled with SNAP-Surface 649) in 100 mM NaCl, 20 mM imidazole pH 7.0, 1 mM DTT, 10% glycerol. N-terminal purification tags of SNAP-hnRNPA1 were removed by HRV C3 protease (EMD Milipore) during the dye conjugation step. N-terminal MBP and C-terminal His tags of SNAP-IDRs and SNAP-PTB-IDRs were cleaved during droplet assembly with TEV protease (Promega ProTEV). Reactions were performed in glass-bottom chambers pre-coated with 3% BSA. Thioflavin T incorporation was measured by including 10–25 µM of the reagent in droplet forming reactions. Imaging parameters can be found in Supplemental Experimental Procedures.
Fluorescence Microscopy
All images of SNAP-IDR and SNAP-hnRNPA1 were acquired on a Delta Vision epi-fluoresence microscope, equipped with a SCMOS camera. The EGFP-IDR recruitment assay was performed on a Nikon AR1 LSM confocal microscope. All images of SNAP-PTB-IDR were acquired on a Leica-based spinning disk confocal microscope (EMCCD digital camera, ImagEM X2, Hamamatsu; confocal scanner unit, CSU-X1, Yokogawa).
Fluorescence Recovery After Photobleaching
The proteins were labeled with SNAP-Surface 649 and bleached with a 405 nm laser line. Droplets were > 5 µm in diameter and the bleaching area was ~ 2 µm in diameter. Time-lapse images were acquired at 637 nm. Images were processed in ImageJ. Background intensity was subtracted and an image of a homogeneous solution was used to correct for uneven illumination. At each time point, fluorescence intensity within the bleaching spot was divided by the intensity of a neighboring unbleached area of the same size to correct for changes in illumination. The corrected intensities were fit to a single exponentia1 growth curve to yield the half time and the ratio of recovery ([Imax-Imin]/[I0-Imin]) using GraphPad Prism 5 (GraphPad Software), as shown in Table S1. Data are reported as mean ± SEM, n≥2.
SDS-PAGE Assay for High-Salt Soluble Species in Droplets
Identical samples were prepared to contain 5 µM MBP-SNAP-PTB-Lsm4 (or 5 µM MBP-SNAP-PTB-Tia1) plus 1.6 µM RNA(phase separation) or buffer(no phase separation). TEV protease was present to remove MBP, which served as an internal loading control. At the indicated time points, NaCl was raised to 500 mM total concentration to disassemble the droplets. After incubation for 1 minute followed by centrifugation at 15,000 g for 5 minutes, the supernatant was carefully removed and loaded on an SDS-PAGE gel. Gels were stained with coomassie blue. The pellet was examined by TEM (see below). For SNAP-hnRNPA1WT, SNAP-hnRNPA1ΔHexa, SNAP-hnRNPA1D262V, 25 µM proteins were first treated with HRV C3 at room temperature for 5 hours to cleave MBP completely. Proteins were diluted to 37.5 mM NaCl to initiate phase separation. At indicated time points, NaCl was raised to 150 mM concentration. After incubation for 5 minutes followed by centrifugation at 15,000 g for 5 minutes, the supernatant was carefully removed and assessed by SDS-PAGE gels stained with coomassie blue. Intensities of the bands were measured in Image J and normalized by the intensities of MBP.
Transmission Electron Microscopy
The pellet after high salt wash (see previous section) at 24 hours was resuspended in buffer (100 mM NaCl, 20 mM imidazole pH 7.0, 1 mM DTT) by brief sonication, and directly transferred to a TEM grid (FCF300-Cu grid, Electron Microscopy Sciences) and stained with 5 µl of 1% (w/v) PTA (phosphotungstic acid, pH adjusted to 8.0 with NaOH) for 1 minute. After the removal of PTA, the grid was air-dried. The images were obtained on FEI Tecnai transmission electron microscope.
IDR Recruitment Assay
Droplets were formed by either 10 µM PTB plus 3.2 µM RNA (note that PTB phase separates at lower concentrations than SNAP-PTB) or 1.25 µM PTB-FusIDR plus 0.4 µM RNA. Droplets were labeled with 10 nM 3’-Cy3 RNA and 100 nM GFP-IDR was used as probe. MBP tag was removed with TEV protease. After a 1 hour incubation, images were acquired at 637 nm and 561 nm simultaneously. Background intensity was subtracted and an image of a homogeneous solution was used to correct for uneven illumination. Droplet intensities were measured by averaging the intensities at the center (with diameter 2.5 µm smaller than that of the droplets) of droplets (~ 5 0–100 total number) from at least three different areas. For bulk intensities, identical samples were prepared in microcentrifuge tubes for 1 hour and centrifuged at 21,130 g for 5 minutes. Supernatants were transferred to glass-bottom chambers and the intensities were measured identically. Intensities were converted to concentrations through a GFP standard curve. The partition coefficient is defined as [GFP]droplet / [GFP]bulk, and shown as mean ± SD from three measurements. P values were obtained using the unpaired t-test. A representative image for each condition was chosen and the same brightness and contrast were used to show the relative intensities. For recruitment in droplets of SNAP-hnRNPA1FL,_30 µM SNAP-Surfac4 649 tagged SNAP-hnRNPA1 with purification tags removed was mixed with 100 nM GFP-IDR or GFP alone in the presence of TEV protease. After incubation for 30 minutes at room temperature, samples were diluted with 20 mM Tris pH 7.4 to indicated protein and salt concentrations. Three aliquots were plated into glass-bottomed chambers. After 45 minutes the other half of the reaction was centrifuged at 16,300 g for 3 minutes, and 3 aliquots of the supernatant were transferred to the chamber. Droplet and supernatant wells were imaged with different instrument parameters due to the small dynamic range of the camera. The reported relative enrichment is defined as Idroplet / Ibulk.
Supplementary Material
1
2
3
4
5
6
Highlights.
Intrinsically disordered regions (IDRs) of RNA binding proteins can phase separate.
RNA, crowding agents, and low salt promote IDR phase separation.
Phase separated droplets stabilize over time by formation of amyloid-like fibers.
Multiple IDRs can be recruited to phase separated droplets.
ACKNOWLEDGMENTS
This work was initiated and supported by an HCIA grant from the Howard Hughes Medical Institute, and we thank all members of the HHMI/MBL Summer Institute for their intellectual contributions. We thank Laura Mizoue for assistance with protein preparations. D.P was supported by T32 GM063235. Work in RP's laboratory was supported by GM045443 and the Howard Hughes Medical Institute. Work in MKR's lab was supported by the Howard Hughes Medical Institute and grants from the NIH (R01-GM56322), and Welch Foundation (1-1544).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Y. L. and D. P. conducted all the experiments and contributed to their design, interpretation, and writing of the manuscript. M.R. and R.P. assisted with the experimental design and manuscript writing.
Refrences
- Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherie N, Pande J, Lomakin A, Ogun O, Hanson SR, Smith JB, Benedek GB. Oligomerization and phase separation in globular protein solutions. Biophysical Chemistry. 1998;75:213–227. doi: 10.1016/s0301-4622(98)00208-7. [DOI] [PubMed] [Google Scholar]
- Banjade S, Rosen MK. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife. 2014;3:04123. doi: 10.7554/eLife.04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S, Wu Q, Mittal A, Peeples W, Pappu RV, Rosen MK. A conserved interdomain linker promotes phase separation of the multivalent adaptor protein Nek. Proc. Natl. Acad. Sci. 2015 doi: 10.1073/pnas.1508778112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R. The quantitative proteome of a human cell line. Mol Syst Biol. 2011;7:549. doi: 10.1038/msb.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Hyman AA, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proceedings of the National Academy of Sciences. 2011;108:4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Kolaitis RM, Taylor JP, Parker R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell. 2013;153:1461–1474. doi: 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Molecular Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. The Journal of Cell Biology. 2008;183:441–455. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Teixeira D, Parker R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. The journal of Cell Biology. 2007;179:437–449. doi: 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CCH, Eckmann CR, Myong S, Brangwynne CP. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proceedings of the National Academy of Sciences. 2015;112:7189–7194. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory PJ. Principles of polymer Chemistry. Cornell University Press; 1953. [Google Scholar]
- Fromm SA, Kamenz J, Nöldeke ER, Neu A, Zocher G, Sprangers R. In Vitro Reconstitution of a Cellular Phase-Transition Process that Involves the mRNA Decapping Machinery. Angewandte Chemie International Edition. 2014;53:7354–7359. doi: 10.1002/anie.201402885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Molecular Biology of the Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil S, Long JC, Caceres JF. hnRNP Al relocalization to the stress granules reflects a role in the stress response. Molecular and Cellular Biology. 2006;26:5744–5758. doi: 10.1128/MCB.00224-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, McKnight SL. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012;149:768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Handwerger KE, Cordero JA, Gall JG. Cajal bodies, nucleoli, and speckles in the Xenopus oocyte nucleus have a low-density, sponge-like structure. Molecular Biology of the Cell. 2005;16:202–211. doi: 10.1091/mbc.E04-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S, Izaurralde E. The role of disordered protein regions in the assembly of decapping complexes and RNP granules. Genes & Development. 2013;27:2628–2641. doi: 10.1101/gad.227843.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, McKnight SL, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, Kanagaraj AP, Taylor JP, et al. Mutations in prion-like domains in hnRNPA2Bl and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Research. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Zhang H, Baker AE, Occhipinti P, Borsuk ME, Gladfelter AS. Protein aggregation behavior regulates cyclin transcript localization and cell-cycle control. Developmental Cell. 2013;25:572–584. doi: 10.1016/j.devcel.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, Russo PS, Jiang QX, Nixon BT, Rosen MK. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. The Journal of Cell Biology. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SH, Decker CJ, Walsh MA, She M, Parker R, Song H. Crystal structure of human Edc3 and its functional implications. Mol Cell Biol. 2008;28:5965–5976. doi: 10.1128/MCB.00761-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschi M, Leishman CC, Berardone N, Boccaccio GL. Dynein and kinesin regulate stress-granule and P-body dynamics. Journal of cell science. 2009;122:3973–3982. doi: 10.1242/jcs.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Kim HJ, Coughlin M, Kanagaraj AP, Mittag T, Taylor JP. Phase separation mediated by low-complexity domains underlies stress granule assembly and drives pathological fibrillization. Submitted. 2015 doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, Baldwin AJ. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Molecular Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande J, Lomakin A, Fine B, Ogun O, Sokolinski I, Benedek G. Oxidation of gamma II-crystallin solutions yields dimers with a high phase separation temperature. Proceedings of the National Academy of Sciences. 1995;92:1067–1071. doi: 10.1073/pnas.92.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Taylor JP, Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell. 2013;154:727–736. doi: 10.1016/j.cell.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijns MA, Alexander RD, Spiller MP, Beggs JD. A role for Q/N-rich aggregation-prone regions in P-body localization. Journal of Cell Science. 2008;121:2463–2472. doi: 10.1242/jcs.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov AN, Rubinstein M. Thermoreversible gelation in solutions of associating polymers. 1. Statics. Macromolecules. 1998;31:1373–1397. [Google Scholar]
- Schwartz JC, Podell ER, Han SS, Berry JD, Eggan KC, Cech TR. FUS is sequestered in nuclear aggregates in ALS patient fibroblasts. Molecular Biology of the Cell. 2014;25:2571–2578. doi: 10.1091/mbc.E14-05-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquere S, Mollet S, Kress M, Dautry F, Pierron G, Weil D. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. Journal of Cell Science. 2009;122:3619–3626. doi: 10.1242/jcs.054437. [DOI] [PubMed] [Google Scholar]
- Spector DL. Snapshot: Cellular bodies. Cell. 2006;127:1071. doi: 10.1016/j.cell.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, Gitler AD. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. Plos Biology. 2011;9:e1000614. doi: 10.1371/journal.pbio.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. The Journal of Cell Biology. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Updike DL, Hachey SJ, Kreher J, Strome S. P granules extend the nuclear pore complex environment in the C. elegans germ line. The Journal of Cell Biology. 2011;192:939–948. doi: 10.1083/jcb.201010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R, Muhlrad D, Garcial J, Parker R. DifferenTia1 effects of Ydjl and Sisl on Hsp70 mediated clearance of stresssgranules inSaccharomyces cerevisiae. RNA. 2015 doi: 10.1261/rna.053116.115. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Smith J, Chen BC, Schmidt H, Rasoloson D, Paix A, Lambrus BG, Calidas D, Betzig E, Seydoux G. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. Elife. 2015;3:e04591. doi: 10.7554/eLife.04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SC, Brangwynne CP. Inverse size scaling of the nucleolus by a concentration-dependent phase transition. Current Biology. 2015;25:641–646. doi: 10.1016/j.cub.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Jakymiw A, Wood MR, Eystathioy T, Rubin RL, Fritzler MJ, Chan EK. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. Journal of Cell Science. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- Yeo GC, Keeley FW, Weiss AS. Coacervation of tropoelastin. Advances in Colloid and Interface Science. 2011;167:94–103. doi: 10.1016/j.cis.2010.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5
6