Impact of a New Fusion Receptor on PD-1–Mediated Immunosuppression in Adoptive T Cell Therapy (original) (raw)
Abstract
Background:
Adoptive T cell transfer (ACT) is currently under investigation for the treatment of metastatic cancer. Recent evidence suggests that the coinhibitory PD-1-PD-L1 axis plays a major role in ACT failure. We hypothesized that a new fusion receptor reverting PD-1–mediated inhibition into CD28 costimulation may break peripheral tolerance.
Methods:
Different PD-1-CD28 fusion receptor constructs were created and retrovirally transduced into primary T cell receptor transgenic murine CD8+ T cells specific for ovalbumin (OT-1). Cytokine release, proliferation, cytotoxicity, and tumor recognition were analyzed in vitro. Antitumor efficacy and mode of action were investigated in mice bearing subcutaneous tumors induced with the pancreatic carcinoma cell line Panc02 expressing the model antigen ovalbumin (Panc-OVA). For antitumoral efficacy, six to eight mice per group were used. All statistical tests are two-sided.
Results:
Transduction of the PD-1-CD28 receptor constructs mediated enhanced cytokine release, T cell proliferation, and T cell–induced lysis of target tumor cells. The PD-1-CD28 receptor function was dependent on two of the CD28-signaling motifs and IFN-γ release. Treatment of mice with established Panc-OVA tumors with fusion receptor–transduced OT-1 T cells mediated complete tumor regression. Mice rejecting the tumor were protected upon subsequent rechallenge with either ovalbumin-positive or -negative tumors, indicative of a memory response and epitope spreading in nine of 11 mice vs none of the six naïve mice (P < .001). Treatment efficacy was associated with accumulation of IFN-γ–producing T cells and an increased ratio of CD8+ T cells to immunosuppressive myeloid-derived suppressor cells in the tumors.
Conclusions:
Transduction of T cells with this new PD-1-CD28 receptor has the potential of breaking the PD-1-PD-L1–immunosuppressive axis in ACT.
Adoptive T cell therapy (ACT) is a powerful approach to treat even advanced stages of metastatic cancer (1). For ACT, antigen-specific T cells are isolated or engineered and are expanded in vitro prior to reinfusion to the patient (2). In clinical trials, unparalleled response rates in some cancer patients have been achieved by ACT in conjunction with total body irradiation. However, the majority of patients do not respond to this treatment (3,4). Tumor-induced immunosuppression that is not counteracted by total body irradiation has been implicated in this resistance to therapy (5). Recently, inhibitory receptors upregulated on activated T cells and their respective ligands expressed within the tumor milieu have shown to contribute to T cell therapy failure (6). They may thus represent attractive targets to improve ACT.
Among the inhibitory receptors, the programmed death receptor–1 (PD-1) plays a central role, given that recent studies have identified PD-1 expressed on tumor antigen–specific T cells in tumors (7). The interaction of PD-1 with its ligand PD-L1 suppresses TCR signaling and T cell activation and thus prevents effective activation upon target recognition (7–10). The clinical weight of these mechanisms is underlined by therapeutic studies combining ACT or gene-modified T cells with antibody-based PD-1 blockade that result in a marked improvement of antitumor activity (11,12).
The systemic application of PD-1- or PD-L1–blocking antibodies has the disadvantage of potentially targeting T cells of any reactivity and thus of inducing systemic side effects (13,14). Moreover, ACT by itself bears considerable risk of toxicity, as recently seen in phase I studies (15,16). The combination with indiscriminate PD-1 blockade carries the risk of potentiating side effects of either therapy alone.
A potential strategy to pursue PD-1-PD-L1 blockade without nonselective T cell activation is to limit its effect to the tumor reactive T cells.
PD-1 and CD28 belong to the CD28 superfamily. The principal compatibility of signaling between a CD28 extracellular and a PD-1 intracellular domain has been demonstrated (17,18). We thus hypothesized that fusing the extracellular portion of PD-1 to the intracellular portion of CD28 may protect the transduced T cells from PD-L1–induced T cell inhibition and may turn an inhibitory signal into the required costimulation signal for optimal T cell function. Since CD28 signaling is dependent on previous TCR engagement, T cell activation would only occur when the chimeric receptor–transduced T cell attaches to its specific tumor target. This conditional signaling could considerably improve safety and potentially also efficacy of ACT.
Methods
Generation of New Fusion Constructs
All constructs were generated by overlap extension polymerase chain reaction (PCR) and recombinant expression cloning into the retroviral pMP71 vector, as follows: the PD-1–transmembrane construct (PTM) consists of murine PD-1 (mPD-1) (Uniprot Entry Q02242 amino acids 1–190) and murine CD28 (mCD28) (Uniprot Entry P31041 AA 178–218); the CD28-transmembrane construct (CTM) consists of mPD-1 (AA 1–169) and mCD28 (AA 151–218); and the CD28 extra- and transmembrane construct (CEX) consists of mPD-1 (AA 1–169) and mCD28 (AA 115–218). PD-1 deletion mutant consists of mPD-1 (AA 1–247) (19). The PTM variants were generated from PTM by point mutations as follows: mutation of YMNM (AA 189–192) to FMNM (PTM-FMNM), mutation of PYAP (AA 206–209) to AYAA (PTM-AYAA) and the double mutant PTM-FMNM-AYAA.
Animal Experiments
Mice transgenic for a T cell receptor specific for ovalbumine (OT-1) were obtained from the Jackson laboratory (Bar Harbor, ME) (stock number 003831) and were bred in our animal facility under SPF conditions. OT-1 mice were crossed to CD45.1 congeneic marker mice (obtained from the Jackson laboratory, stock number 002014) and to CD90.1 congeneic marker mice (a kind gift from Reinhard Obst, PhD, Institute of Immunology, Munich, Germany) to generate CD45.1-OT-1 and CD90.1-OT-1 mice, respectively. Wild-type C57Bl/6 mice were purchased from Janvier, (St. Berthevin, France). Tumors were induced by subcutaneous injection of 2 x 106 tumor cells, and mice were treated by IV injection of T cells as indicated. For rechallenge experiments, mice were injected subcutaneously with 0.5 x 106 cells in the flank opposite to the site of the previously rejected tumor. All experiments were randomized and blinded. For neutralization experiments, anti-IFN-γ antibody R4-6A2 or isotype control (BioXcell, West Lebanon, NH) was applied IP at a dose of 200 µg per animal every three days for four doses. Tumor growth and condition of mice were monitored every other day. For antitumoral efficacy, six to eight mice per group were used. All animal experiments were approved by the local regulatory agency (Regierung von Oberbayern) and adhere to the National Institutes of Health guide for the care and use of laboratory animals.
Statistical Analysis
For statistics, GraphPad Prism software version 5.0b was used. All variables reported are continuous. Differences between experimental conditions were analyzed using the unpaired two-sided Student’s t test. For comparison of experimental conditions of individual mice, the Mann-Whitney test was used. P values under .05 were considered statistically significant. For in vivo experiments, differences between groups were analyzed using two-way analysis of variance with correction for multiple testing by the Bonferroni method. Overall survival was analyzed by log-rank test. Survival is defined in days from tumor induction until natural death or until mice were killed because one of the following predefined criteria was reached: tumor size greater than 225mm2, weight loss greater than 15%, or severe distress. Data are shown as mean values ± SD of a minimum of three biological replicates or independent experiments, as indicated. All statistical tests were two-sided.
All other methods are described in detail in the Supplementary Methods (available online).
Results
Rationale and Design of a New PD-1-CD28 Fusion Receptor
Efficacy of adoptive transfer of OVA-specific CD8+ T cells was assessed in mice bearing established OVA-expressing Panc02 (Panc02-OVA) tumors. Transferred T cells failed to reject tumors in most mice. This was paralleled by upregulation of PD-1 on the transferred T cells infiltrating the tumor (Supplementary Figure 1, A and B, available online). Given that Panc02-OVA cells express the ligand for PD-1 (PD-L1), which is upregulated by IFN-γ (Supplementary Figure 1, C and D, available online), this points to a relevant role of the PD-1-PD-L1 axis in suppressing the antigen-specific T cell response in the tumor. We reasoned that protecting the transferred T cells from PD-1–mediated suppression may enhance the efficacy of adoptive T cell therapy. Because PD-1 is a member of the CD28/CTLA-4 family, it appeared possible that receptor signaling could be compatible and that a fusion PD-1-CD28 receptor construct could turn engagement of PD-1 by PD-L1 into CD28 costimulatory activity (scheme in Supplementary Figure 1E, available online). We therefore designed a fusion receptor consisting of the extra- and transmembrane portion of PD-1 with the intracellular domain of CD28 for transduction in primary murine T cells.
Functional Analysis of Transduced T Cells In Vitro
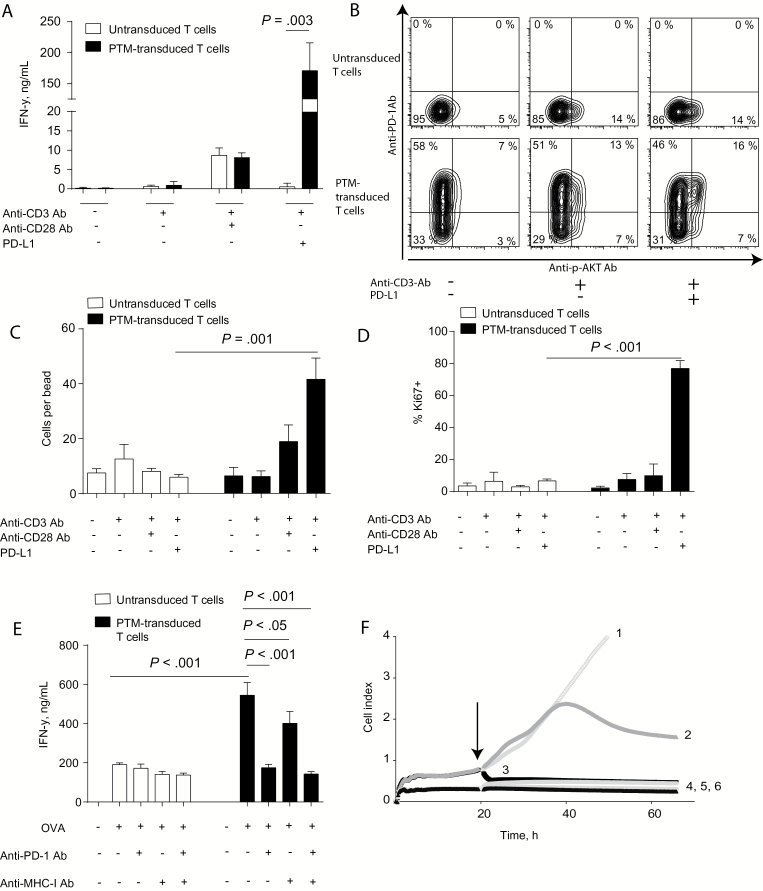
To test the functionality of the novel PD-1–transmembrane PD-1-CD28 receptor (PTM), we transduced primary murine T cells and stimulated them with agonistic anti-CD3 antibodies and recombinant PD-L1. PTM-transduced T cells showed markedly increased IFN-γ (170 +/- 26 vs 0.5 +/- 0.5ng/mL, P < .003) (Figure 1A) and IL-2 induction as compared with untransduced T cells (Supplementary Figure 1F, available online). Additional stimulation with anti-CD28 antibody further boosted cytokine production (Supplementary Figure 2A, available online). Cytokine induction was paralleled by downstream phosphorylation of AKT upon PD-L1 engagement (Figure 1B), demonstrating CD28 signaling in transduced T cells. Activation of the PTM receptor statistically significantly enhanced the number of viable cells as compared with untransduced T cells (42 +/- 4 vs 6 +/- 1 cells per bead, P = .001) (Figure 1C). This increase in cell numbers was associated with strong ki67 upregulation by the transduced T cells (Figure 1D), indicating strong mitotic activity. When coculturing PTM receptor–transduced OT-1 T cells with Panc02-OVA-PD-L1 or Panc02-PD-L1 cells, strong costimulatory activity was observed, as evidenced by IFN-γ-release in transduced as compared with untransduced T cells (545 +/- 37 vs 191 +/- 0.5ng/mL, P < .001) (Figure 1E). The costimulatory activity of the PTM receptor was dependent on the presence of PD-L1, on OVA expression by the tumor cells and on TCR engagement, as evidenced by MHC-I blocking (Figure 1E) and by coculture with OVA-negative Panc02-PD-L1 cells (Supplementary Figure 2B, available online). Anti-CD3 antibody– and PD-L1–prestimulated PTM receptor–transduced T cells mediated immediate and complete lysis of tumor cells, whereas untransduced T cells were ineffective (P < .001 from 22 hours) (Figure 1F). Together, these findings indicate that PTM receptor–transduced T cells have become resistant to PD-1-PD-L1–mediated anergy. These results demonstrate the functionality and the therapeutic potential of the novel PD-1-CD28 fusion receptor in vitro.
Figure 1.
In vitro characterization of the novel PD-1-CD28 fusion receptor (PD-1 transmembrane domain, PTM receptor). A) PTM receptor–transduced or –untransduced primary murine T cells were either stimulated with anti-CD3 antibody, anti-CD3 plus anti-CD28 antibodies or with anti-CD3 antibody plus recombinant PD-L1 and resulting IFN-γ release was measured by enzyme linked immunosorbent assay (ELISA). B) PTM receptor–transduced or –untransduced primary murine T cells were either left unstimulated or stimulated with anti-CD3 antibody or with anti-CD3 antibody plus recombinant PD-L1 and phosphorylation of AKT was measured by flow cytometry. C) PTM receptor–transduced or –untransduced primary murine T cells were either stimulated with anti-CD3 antibody, anti-CD3 plus anti-CD28 antibodies or with anti-CD3 antibody plus recombinant PD-L1 and cell numbers were normalized to standardized counting beads. D) PTM receptor or untransduced primary murine T cells were either stimulated with anti-CD3 antibody, anti-CD3 plus anti-CD28 antibodies, or with anti-CD3 antibody plus recombinant PD-L1 for 24 hours and stained intracellularly for the mitosis marker ki67. E) PTM receptor or untransduced OT-1 T cells were cocultured with Panc02-OVA-PD-L1 in the presence or absence of anti-PD-1 antibody or anti-mouse H2kb SIINFEKL antibody and resulting IFN-γ production was measured by ELISA. F) PTM receptor or untransduced OT-1 T cells were prestimulated with anti-CD3 antibody and with recombinant PD-L1. In the meantime, Panc02-OVA-PD-L1 cells were seeded and grown prior to the addition of prestimulated T cells (arrow). The conditions are as follows Panc02-OVA-PD-L1 only (1), Panc02-OVA-PD-L1 + prestimulated untransduced T cells (2), Panc02-OVA-PD-L1 + prestimulated PTM-receptor transduced T cells (3), PTM receptor (4) and untransduced T cells (5) and medium (6). Panc02-OVA cell viability was measured by impedance-based measurement. Experiments (A to E) are representative of at least three independent experiments each performed in triplicates. Experiment (F) is representative of three independent experiments performed in duplicates for technical reasons. Bars represent SD and P values from Student’s t test are shown. All tests are two-sided.
Functional Comparison of Different PD-1-CD28 Fusion Receptors
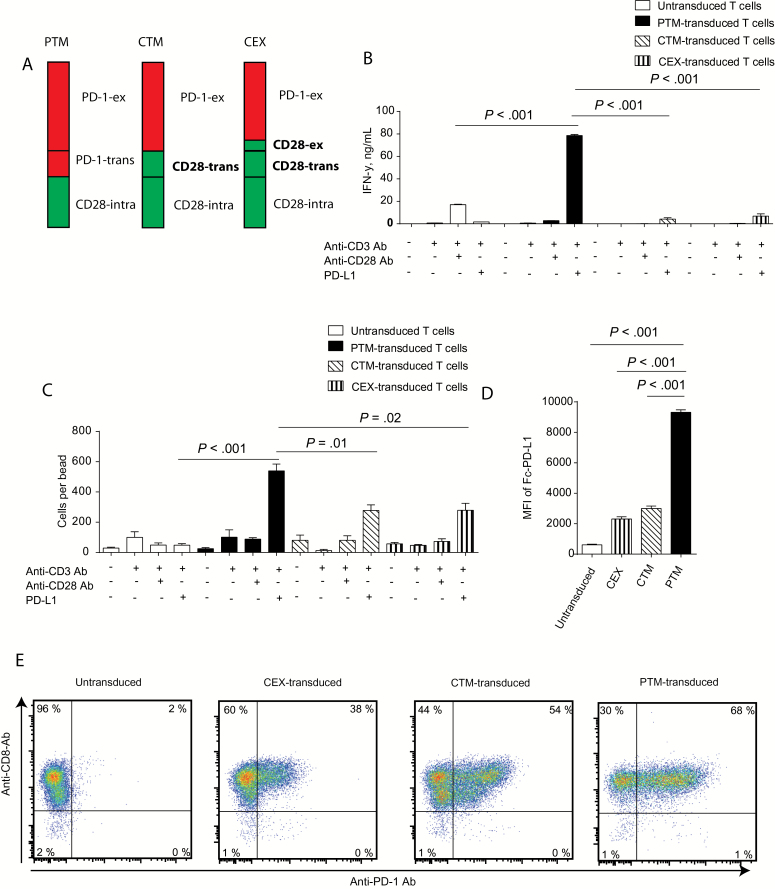
Previously, two PD-1-CD28 fusion receptors have been described with up to two-fold cytokine induction, little proliferative activity, and some cytolytic potential (20,21). Given the strong effects observed with our PTM receptor, we next asked whether the difference to our results is related to the structure of the fusion receptor. We thus generated additional constructs for PD-1-CD28 fusion receptors containing the CD28 transmembrane domain (CTM) or the CD28 transmembrane domain plus part of the CD28 extracellular domain (CEX) (Figure 2A). When stimulated with anti-CD3 antibodies and recombinant PD-L1, all receptors were functional as assessed by IFN-γ release (79 +/- 0.9 vs 4 +/- 1 vs 7 +/- 2ng/mL, P < .001) (Figure 2B) and by induction of proliferation (540 +/- 45 vs 278 +/- 37 vs 279 +/- 46 cells per bead, P < .01 and .02, respectively) (Figure 2C). The PTM receptor, however, was far superior to the CTM and CEX receptors in terms of both IFN-γ secretion and proliferation. Mechanistically, the enhanced activity was paralleled by enhanced binding of PD-L1 to the PTM receptor as opposed to the CTM and CEX receptors (MFI 9315 +/- 165 vs 2311 +/- 144 vs 2997 +/- 167, P < .001) (Figure 2D). The enhanced binding of the PTM receptor can only partly be explained by increased surface expression of this construct, as expression on CD8-T cells by flow cytometry was not largely superior for all constructs (Figure 2E). The enhanced binding of the PTM receptor may be responsible for its markedly superior functional activity in comparison to the other fusion constructs.
Figure 2.
Functional comparison of different PD-1-CD28 fusion receptors. A) Schematic overview of the structure of the different receptors: PTM: PD-1 extracellular domain (PD-1-ex) and transmembrane domain (PD-1-trans) fused to the CD28 intracellular domain (CD28-intra). CTM: PD-1 extracellular domain fused to CD28 transmembrane (CD28-trans) and intracellular domain; and CEX: PD-1 extracellular domain fused to CD28 extracellular segment (CD28-ex) and CD28 transmembrane and intracellular domain. B) PTM-, CTM-, CEX- or untransduced primary murine T cells were stimulated with anti-CD3 antibody, anti-CD3 plus anti-CD28 antibodies, or anti-CD3 antibody plus recombinant PD-L1 and IFN-γ production was measured by ELISA. C) PTM-, CTM-, CEX receptor–transduced or –untransduced primary murine T cells were stimulated with anti-CD3 antibody, anti-CD3 plus anti-CD28 antibodies, or anti-CD3 antibody plus recombinant PD-L1, and resulting cell numbers were normalized to counting beads. D) PTM-, CTM-, CEX receptor–transduced or –untransduced T cells were incubated with recombinant PD-L1, and PD-L1 binding was measured by flow cytometry. E) Representative costaining for CD8 and PD-1 expression of PTM-, CTM-, CEX- or untransduced T cells. All experiments are representative of at least three independent experiments each performed in triplicates. Bars represent SD and P values from Student’s t test are shown. All tests are two-sided.
Functional Domains Required for PTM Fusion Receptor Function
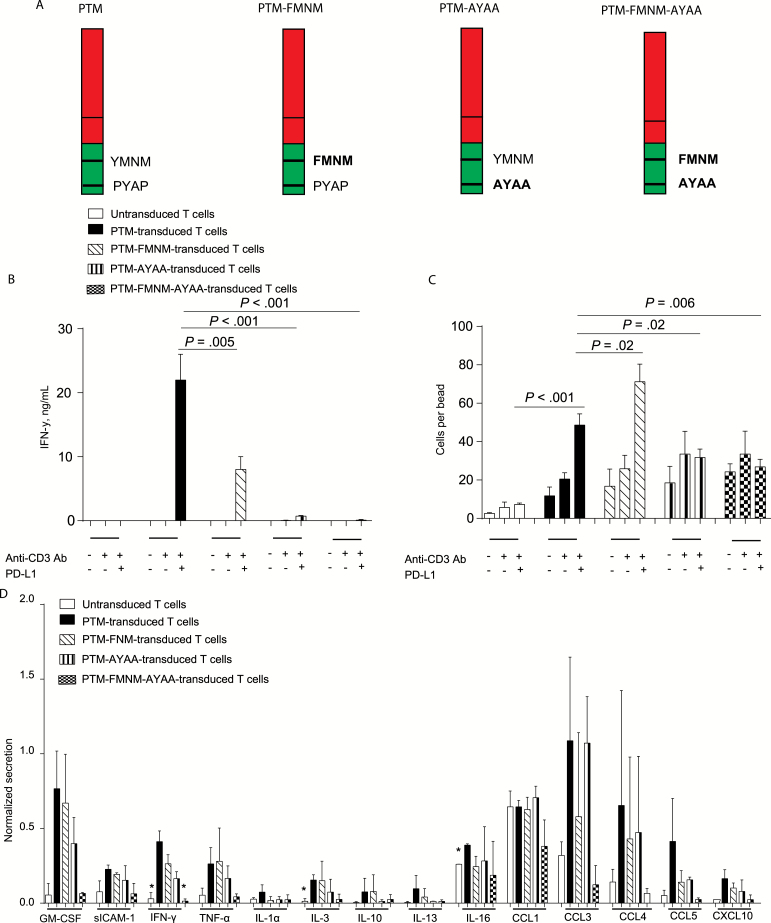
To further dissect the mechanisms underlying the activity of PTM, we generated mutant receptors where the signaling domains of CD28 were rendered nonfunctional. The YMNM motif of the intracellular CD28 domain is required for optimal cytokine secretion upon CD28 activation, and the PYAP motif is essential for both cytokine production and cell proliferative activity (22). We generated a PTM-FMNM mutant construct, a PTM-AYAA mutant construct, and a PTM-FMNM-AYAA double mutant construct for expression in primary murine T cells (Figure 3A). T cells expressing the PTM construct or one of the three mutant constructs were stimulated with anti-CD3 antibodies and recombinant PD-L1. PTM receptor–transduced T cells produced statistically significantly more IFN-γ than PTM-FMNM, PTM-AYAA, or PTM-FMNM-AYAA (22 +/- 2 vs 8 +/- 1 vs 1 +/- 0.07 vs 0.1 +/- 0.05ng/mL, P < .001) (Figure 3B). PTM receptor engagement induced proliferation in a PYAP-dependent manner, while YMNM was dispensable for the proliferative effect (Figure 3C). In contrast, production of various cytokines and chemokines by PTM receptor engagement seems to be dependent on both motifs, since mutant constructs were weaker inducers compared with native PTM receptor (Figure 3D).
Figure 3.
Functional comparison of different mutated PTM fusion receptors in their putative signaling domains. A) Schematic overview of the different PTM receptor mutants: PTM (YMNM-PYAP, wild-type), PTM-FMNM (tyrosine-mutated, Y to F), PTM -AYAA (prolin-mutated, P to A), and PTM-FMNM-AYAA (proline- and tyrosine-mutated). B) PTM, PTM tyrosine–mutated (PTM-FMNM), PTM proline–mutated (PTM-AYAA), and PTM tyrosine– and proline–mutated (PTM-FMNM-AYAA) or –untransduced T cells were stimulated with anti-CD3 antibody or with anti-CD3 antibody plus recombinant PD-L1, and IFN-γ production was measured by ELISA. C) Untransduced, PTM-, PTM-FMNM-, PTM-AYAA-, or PTM-FMNM-AYAA–transduced T cells were stimulated with anti-CD3 antibody or anti-CD3 antibody plus recombinant PD-L1, and the amount of viable cells was quantified by normalization to counting beads. D) Untransduced, PTM-, PTM-FMNM-, PTM-AYAA-, or PTM-FMNM-AYAA–transduced T cells were stimulated with anti-CD3 antibody plus recombinant PD-L1, and cytokine release was analyzed semiquantitatively using a murine cytokine array. The array screens for expression for a broad panel of 40 cytokines. P values below .05 are marked with * in Figure 3D. Experiments (B and C) are representative of at least three independent experiments performed in triplicates. Experiment (C) was performed three times in duplicates for technical reasons. Bars represent SD, and P values from Student’s t test are shown. All tests are two-sided.
Therapeutic Efficacy of PD-1-CD28 Fusion Receptor–Transduced OT-1 T Cells in a Murine Pancreatic Cancer Model
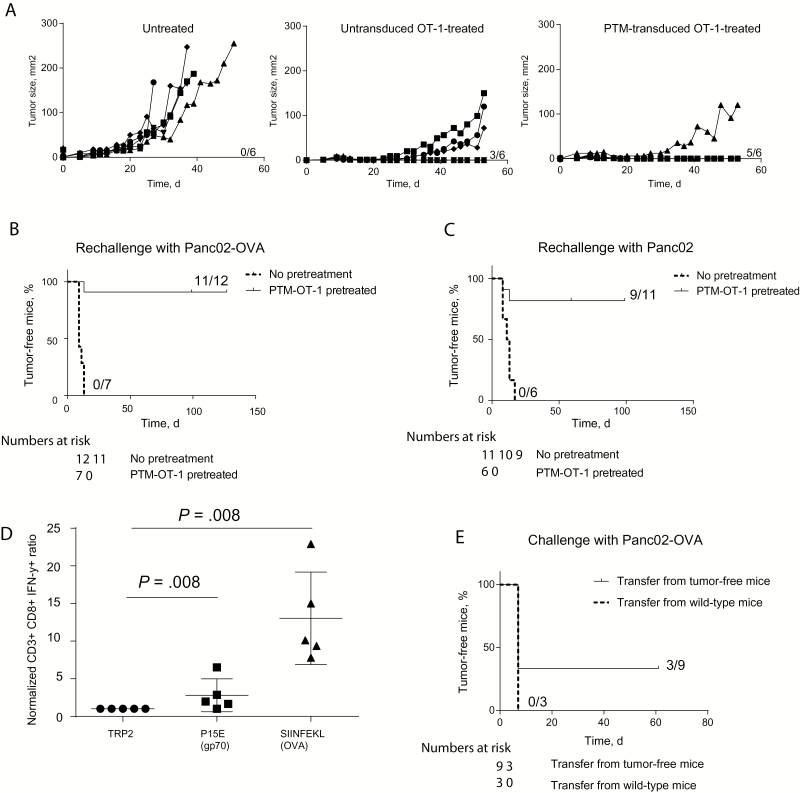
To further assess the potency of PD-1-CD28 (PTM) receptor–transduced antigen-specific T cells, we treated mice bearing subcutaneous Panc02-OVA tumors with untransduced OT-1 T cells or PTM receptor–transduced OT-1 T cells. PTM receptor–transduced T cells induced superior antitumor immunity as compared with mice receiving untransduced T cells (Figure 4A). Interestingly, PTM receptor–transduced OT-1 T cells retained their therapeutic potential in the Panc02-OVA-PD-L1 model, strongly overexpressing PD-L1, while the effect of untransduced OT-1 cells was almost completely abrogated (Supplementary Figure 2C, available online). When rechallenged with Panc02-OVA cells, 11 of 12 mice previously treated with PTM receptor–transduced OT-1 T cells remained tumor-free compared with 0% of control mice (P < .001) (Figure 4B). Moreover, when rechallenged with wild-type Panc02 cells, nine of 11 mice previously treated with PTM receptor–transduced OT-1 T cells remained tumor free vs none of the six naïve mice (P < .001) (Figure 4C). These results are suggestive of epitope spreading in cured mice leading to immunity against other Panc02-specific, tumor-associated antigens, such as p15E (23). Therefore, lymph nodes of tumor-free mice were analyzed for the presence of SIINFEKL (OVA)- and of p15E (gp70)-specific CD8+ T cells. A statistically significant increase in numbers of SIINFEKL-specific CTL cells were found in mice following transduced T cell transfer compared with CTL specific for control peptide (13 +/- 3 vs 1 +/- 0, P = .008) (Figure 4D). We also detected a small but statistically significant increase of p15E-specific CTL (3 +/- 1 vs 1 +/- 0, P = .008) (Figure 4D). The resulting immunity was transferrable as shown by tumor protection in three out of nine mice adoptively transferred with splenocytes from cured mice and delay in tumor outgrowth, compared with none out of three mice transferred with naïve splenocytes (Figure 4E and data not shown).
Figure 4.
Therapeutic efficacy of PTM receptor–transduced OT-1 T cells in vivo and induction of immunological memory. A) Eighteen mice were injected subcutaneously with Panc02-OVA cells. Once the tumors were established, six mice each were randomly assigned either to no treatment, to adoptive transfer of untransduced OT-1 T cells, or to adoptive transfer of PTM receptor–transduced OT-1 T cells. Tumor size was measured in a blinded fashion every other day. The experiment is representative of three independent experiments with six mice per group. B) Surviving mice (n = 12) from two independent experiments were rechallenged with Panc02-OVA cells at the same time as tumor-naïve wild-type mice (n = 7). C) Surviving mice after first rechallenge (n = 11, from experiment depicted in panel [B]) and tumor-naïve wild-type mice (n = 6) were rechallenged with a sublethal dose of Panc02 cells. D) Lymph nodes from surviving mice from Panc02 rechallenge (experiment depicted in panel [C]) were stimulated in organ culture in vitro with either control peptide TRP2, P15E peptide, or SIINFEKL peptide. The number of IFN-γ–producing CD8+-T cells was analyzed by flow cytometry and was normalized to the number of IFN-γ–producing CD8+-T cells after TRP2 stimulation for each mouse. E) Splenocytes from mice having cleared Panc02-OVA tumors after transfer of PTM receptor–transduced OT-1 T cells or from wild-type mice were adoptively transferred on wild-type mice. These mice were challenged with Panc02-OVA cells. Transfer of splenocytes from tumor-free mice prevented tumor outgrowth in three of nine mice. Survival analysis was performed using the log-rank test. For comparison of experimental conditions of individual mice, the Mann-Whitney test was used. All tests are two-sided.
Distribution of Adoptively Transferred T Cells in Tumor-Bearing Mice
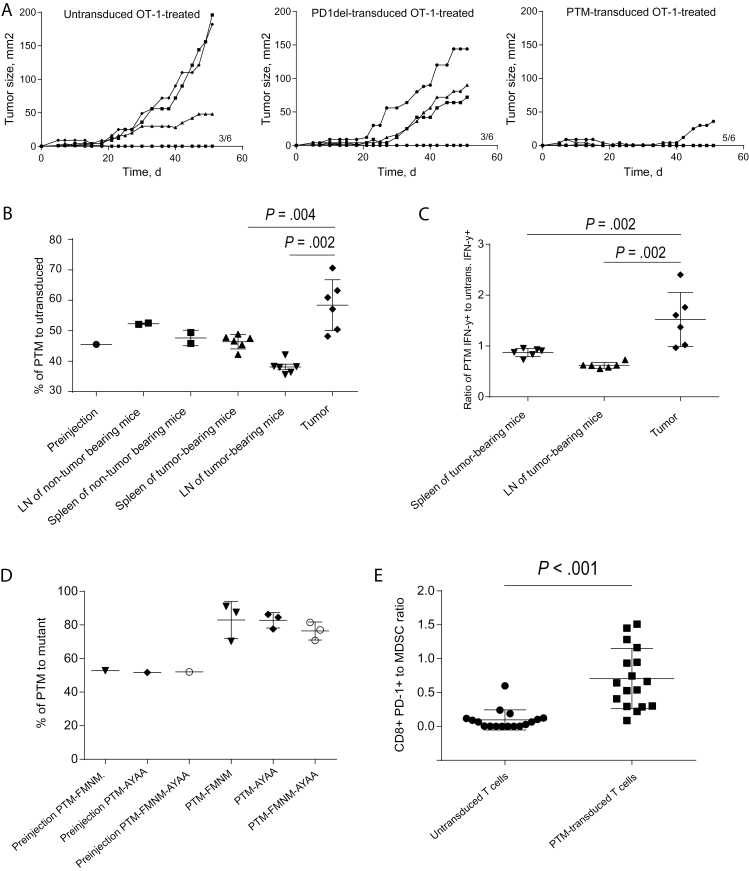
To differentiate whether the therapeutic efficacy of PTM receptor–transduced vs untransduced OT-1 T cells is because of the presence of the CD28 domain in the PTM receptor or merely to the expression of a nonsignaling PD-1 on the T cell surface, we expressed a new PD-1 deletion mutant, devoid of the intracellular portion of PD-1 (PD-1del). Injection of PD-1del–transduced OT-1 T cells did not improve the therapeutic efficacy compared with untransduced OT-1 T cells in the Panc02-OVA model, in contrast to injection of PTM receptor–transduced OT-1 T cells (Figure 5A). These results indicated dependency on the intracellular CD28 domain of the PTM fusion receptor. We next investigated the fate of PTM receptor–transduced vs untransduced OT-1 T cells in tumor-bearing mice. PTM receptor–transduced T cells showed enrichment in Panc02-OVA tumors compared with untransduced T cells (59 +/- 2 vs 49 +/- 1 %, P = .002). This effect was not observed in lymph nodes or in organs of non–tumor bearing mice (Figure 5B). In addition, the PTM receptor–transduced OT-1 T cells produced statistically significantly more IFN-γ than untransduced OT-1 T cells in the tumor compared with other organs or to non–tumor bearing mice (1.5 +/- 0.2 vs 0.6 +/- 0.02 vs 0.9 +/- 0.03 ratio of PTM IFN-γ + to untransduced IFN-γ + T cells, P = .002) (Figure 5C). Neutralization of IFN-γ in vivo almost completely abrogated the therapeutic impact of PTM–transduced OT-1 T cells, indicating the importance of this cytokine for the function of receptor (Supplementary Figure 2D, available online). To further dissect the signaling motifs responsible for the accumulation of PTM receptor–transduced OT-1 T cells in the tumor, we used the PTM-FMNM, PTM-AYAA, and PTM-FMNM-AYAA mutant construct–transduced T cells described above to compare their fate with PTM receptor–transduced OT-1 T cells in tumor-bearing animals. The T cell infiltration and persistence of PTM receptor–transduced T cells at the tumor site was dependent on both YMNM and PYAP motifs, because T cells carrying the mutants were found in lower amounts compared with T cells carrying the wild-type receptor (Figure 5D). The increase in infiltrating PTM receptor–transduced OT-1 T cells shifted the ratio of infiltrating CD8+ T cells to myeloid-derived suppressor cells (MDSCs) in favor of the PTM receptor–transduced OT-1 T cells (0.7 +/- 0.1 vs 0.1 +/- 0.03, P < .001, PTM-CD8+ T cells to MDSC ratio) (Figure 5E). A similar effect was observed for the ratio of CD8+ T cells to regulatory T cells (Supplementary Figure 2E, available online). Together, these findings indicate that adoptive transfer of PTM receptor–transduced T cells tipped the balance from immunosuppression towards productive immunity.
Figure 5.
In vivo mode of action of PTM receptor–transduced OT-1 T cells. A) Eighteen mice were subcutaneously injected with Panc02-OVA cells. Once the tumors were established, the mice were randomly assigned to adoptive transfer of either untransduced OT-1 T cells or of T cells transduced with a deleted PD-1 receptor or with unmodified PTM fusion receptor. Tumor size was measured every other day in a blinded fashion. The experiment is representative of three independent experiments with six mice per group. B) T cells from CD45.1-OT-1 mice were transduced with PTM receptor, and T cells from CD90.1-OT-1 mice were left untransduced. T cells were coinjected in equal amounts in wild-type mice (n = 2) or in mice bearing Panc02-OVA tumors (n = 6). Four days later, T cells were analyzed in the different compartments and the ratio of PTM receptor–transduced to –untransduced OT-1-T cells was compared. The experiment is representative of three independent experiments with six mice per tumor-bearing group. C) Untransduced CD90.1-OT-1-T cells and PTM receptor–transduced CD45.1-OT-1 T cells were isolated from tumor, spleen, and lymph nodes obtained in experiment (B) and were analyzed for IFN-γ expression by flow cytometry. The experiment is representative of three independent experiments with six mice per tumor-bearing group. D) CD45.1 OT-1 T cells were transduced with PTM receptor, CD90.1 OT-1-T cells were transduced with either of the mutant receptors PTM-FMNM, PTM-AYAA, or PTM-FMNM-AYAA and were mixed in equal amounts with PTM-transduced CD45.1 OT-1 T cells prior to transfer to Panc02-OVA–tumor bearing mice (n = 3 per group). Four days after transfer, the ratio of PTM receptor–transduced T cells to mutant receptor–transduced T cells was analyzed by flow cytometry. The experiment is representative of three independent experiments with three mice per tumor-bearing group. E) PTM receptor–transduced or –untransduced OT-1 T cells were adoptively transferred in Panc02-OVA–tumor bearing mice (n = 17, respectively). One week later the number of tumor-infiltrating MDSC (CD45+, CD11b+, Ly6+, Gr-1 intermediate+) was analyzed. Data represent results of pooled mice from three independent experiments. Bars represent SD. Survival analysis was performed using the log-rank test. For comparison of experimental conditions of individual mice, the Mann-Whitney test was used. All tests are two-sided.
Discussion
This study demonstrates that the novel PD-1-CD28 fusion receptor (carrying the PD-1 transmembrane domain, PTM receptor) described is highly functional when transduced into primary murine T cells. Our results suggest that PTM receptor–transduced T cells are resistant to PD-1-PD-L1–mediated anergy. These effects are, however, only observed in the presence of the T cell–targeted antigen, adding specificity to the therapeutic concept. Of note, the PD-1-CD28 fusion receptor described here is functionally superior to previously described fusion receptors. The balance of stimulation and repression is critical for T cell function and constitutes an Achilles heel to adoptive T cell therapy (ACT) (24,25). Proof-of-concept studies have shown that antitumor effects can be achieved by introducing chimeric antigen receptors (CARs) into T cells prior to transfer, providing not only antigen recognition but also costimulation (26,27). These T cells are, however, still sensitive to inhibition by check point controllers such as PD-1. Additional strategies may be required to shield these T cells and unleash their full therapeutic potential (28). CTLA-4, PD-1, and CD28 belong to the same transmembrane receptor family. Their compatibility has been employed to boost the T cell response using a stimulatory CTLA-4-CD28 fusion receptor and to inhibit off-target T cell reactivity using inhibitory receptors with the signaling domains of PD-1 or CTLA-4 (29,30). Combining PD-1 ligation with costimulatory CD28 signaling is thus a reasonable strategy and its feasibility has previously been shown (20,21). However, the fusion receptors previously described showed only modest cytokine induction (two- to three-fold) and little or no difference in lytic activity when transduced into primary T cells. This is in marked contrast to the fusion receptor described in the present study, which achieved up to 300-fold increase in IL-2 and IFN-γ secretion and strong T cell proliferation as well as enhancement of tumor cell lytic activity in vitro and in vivo. We provide evidence that our new receptor may be superior to previously described constructs and that enhanced fusion receptor function may be dependent on the binding capacity for the ligand PD-L1, which was maximal for the PTM fusion receptor. These results demonstrate that the architecture of the fusion receptor is critical for its function.
We could dissect for the first time the mode of action of PD-1-CD28 fusion receptor–transduced T cells and could show that both intracellular signaling motifs in the CD28 domain, previously described in (22), are of central importance. For the PTM fusion receptor both motifs were necessary for cytokine release, while proliferation of T cells relied on the PYAP motif. These findings give structural clues to the mode of action of the PTM fusion receptor.
A hurdle to PD-1–targeted therapy is its critical role in immune homeostasis and the unselective expression of both PD-1 and its ligands PD-L1 and PD-L2 in the organism (31). The unspecific activation of the PTM receptor at sites where its ligand is expressed is a potential risk to the strategy pursued in the present study. We found that concomitant TCR activation is critical for PTM fusion receptor signaling. This suggests that administration of T cells transduced with the PTM fusion receptor is less likely to induce unwanted immune effects than antibody-based systemic PD-1 inhibition.
The importance of the tumor environment in cancer immunotherapy has been repeatedly demonstrated, especially in the context of adoptive T cell therapy. Regulatory T cells (Tregs) tip the balance in favor of anergy, limiting the effect of adoptively transferred T cells (32). Changes in the balance between Tregs and cytotoxic T cells have been shown to suffice for inducing antitumor efficacy (33,34). MDSCs play a similar, nonredundant role in limiting the effector function of adoptively transferred T cells and are also associated with failure of ACT (35). Targeting of suppressive immune cell populations may present a therapeutic strategy for enhancing ACT efficacy (12,36). We found an increase in the T cell to Treg and T cell to MDSC ratios, indicating that the adoptively transferred, fusion receptor–transduced T cells exert not only direct tumor cell killing, but also influence bystander immune cell populations in the tumor stroma. This has been deemed critical for successful ACT (37).
The PTM fusion receptor described here may have applicability in different clinical settings because of its immune stimulating capacities and its dependence of TCR antigen recognition (ie, MHC restriction): one scenario would be to transduce donor lymphocytes (in the context of donor lymphocyte infusion, DLI) with PTM fusion receptor for the treatment of relapsing hematologic malignancies after allogeneic stem cell transplantation (38,39). A second option would be to combine PTM fusion receptor transduction with tumor targeting TCR transduction into autologous T cells in the setting of adoptive T cell therapy. TCR gene therapy is a field in need of approaches to enhance efficacy without potentiating side effects (40).
Our study is not without limitations. Although our work provides proof of concept for the use of such a receptor in combination with a TCR-based approach, the combinatorial capacity with the above T cell–based approaches as well as the clinical translation need to be demonstrated.
In summary, we describe a new PD-1-CD28 fusion receptor for transduction into T cells with superior effector function and enhanced antitumoral efficacy compared with previously described fusion receptors. We give an in-depth analysis of its in vitro and in vivo mode of action, warranting its further development as a strategy to enhance the efficacy of T cell–based therapy in cancer.
Funding
This work was supported by the Wilhelm Sander Stiftung (grant number 2014.018.1 to SE and SK), the international doctoral program “i-Target: Immunotargeting of cancer” funded by the Elite Network of Bavaria (to SK, MS, and SE), the Melanoma Research Alliance (grant number N269626 to SK and SE), the Graduiertenkolleg 1202 “Oligonucleotides in cell biology and therapy” funded by the Deutsche Forschungsgemeinschaft (to SG, CL, SK, MS, and SE), the Else Kröner-Fresenius-Stiftung (to SK), and the German Cancer Aid (to SK and MR). SG received a stipend from the German Cancer Aid.
Supplementary Material
Supplementary Data
The study funders had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
Parts of this work have been performed for the doctoral theses of SG, MC, CL, and YZ at the Ludwig-Maximilians-Universität München. The authors have no competing interests to declare.
References
- 1.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer—what clinicians need to know. Nat Rev Clin Oncol. 2011;8(10):577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Powell DJ, Jr, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. [DOI] [PubMed] [Google Scholar]
- 6.Abate-Daga D, Hanada K, Davis JL, et al. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood. 2013;122(8):1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding ZC, Lu X, Yu M, et al. Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T cell responses through the PD-1/PD-L1 axis. Cancer Res. 2014;74(13):3441–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karyampudi L, Lamichhane P, Scheid AD, et al. Accumulation of Memory Precursor CD8 T cells in Regressing Tumors Following Combination Therapy with Vaccine and Anti-PD-1 Antibody. Cancer Res. 2014;74(11):2974–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John LB, Devaud C, Duong CP, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–5646. [DOI] [PubMed] [Google Scholar]
- 12.Goding SR, Wilson KA, Xie Y, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190(9):4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley JL, June CH. The CD28 family: a T cell rheostat for therapeutic control of T cell activation. Blood. 2005;105(1):13–21. [DOI] [PubMed] [Google Scholar]
- 18.Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–954. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki T, Maeda A, Nishimura H, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98(24):13866–13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ankri C, Shamalov K, Horovitz-Fried M, et al. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol. 2013;191(8):4121–4129. [DOI] [PubMed] [Google Scholar]
- 21.Prosser ME, Brown CE, Shami AF, et al. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol. 2012;51(3–4): 263–272. [DOI] [PubMed] [Google Scholar]
- 22.Boomer JS, Green JM. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2(8):a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer C, Bauernfeind F, Sterzik A, et al. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut. 2007;56(9):1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Chacon JA, Li Y, Wu RC, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34(3):236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chacon JA, Wu RC, Sukhumalchandra P, et al. Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T cell therapy. PLoS One. 2013;8(4):e60031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hombach AA, Abken H. Of chimeric antigen receptors and antibodies: OX40 and 41BB costimulation sharpen up T cell-based immunotherapy of cancer. Immunotherapy. 2013;5(7):677–681. [DOI] [PubMed] [Google Scholar]
- 27.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales-Kastresana A, Labiano S, Quetglas JI, et al. Better performance of CARs deprived of the PD-1 brake. Clin Cancer Res. 2013;19(20):5546–5548. [DOI] [PubMed] [Google Scholar]
- 29.Shin JH, Park HB, Oh YM, et al. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T cell therapy in murine tumor models. Blood. 2012;119(24):5678–5687. [DOI] [PubMed] [Google Scholar]
- 30.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. [DOI] [PubMed] [Google Scholar]
- 32.Bauer CA, Kim EY, Marangoni F, et al. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. 2014;124(6):2425–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perna SK, Pagliara D, Mahendravada A, et al. Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T cell inhibition. Clin Cancer Res. 2014;20(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao X, Ahmadzadeh M, Lu YC, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119(24):5688–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosoi A, Matsushita H, Shimizu K, et al. Adoptive cytotoxic T lymphocyte therapy triggers a counter-regulatory immunosuppressive mechanism via recruitment of myeloid-derived suppressor cells. Int J Cancer. 2014;134(8):1810–1822. [DOI] [PubMed] [Google Scholar]
- 36.Kodumudi KN, Weber A, Sarnaik AA, et al. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol. 2012;189(11):5147–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schietinger A, Philip M, Liu RB, et al. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207(11):2469–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tischer J, Engel N, Fritsch S, et al. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transplant. 2014;49(7):895–901. [DOI] [PubMed] [Google Scholar]
- 39.Bachireddy P, Hainz U, Rooney M, et al. Reversal of in situ T cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123(9):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chodon T, Comin-Anduix B, Chmielowski B, et al. Adoptive Transfer of MART-1 T Cell Receptor Transgenic Lymphocytes and Dendritic Cell Vaccination in Patients with Metastatic Melanoma. Clin Cancer Res. 2014;20(9):2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data