Green Tea Extract Rich in Epigallocatechin-3-Gallate Prevents Fatty Liver by AMPK Activation via LKB1 in Mice Fed a High-Fat Diet (original) (raw)
Abstract
Supplementation with epigallocatechin-3-gallate has been determined to aid in the prevention of obesity. Decaffeinated green tea extract appears to restore a normal hepatic metabolic profile and attenuate high-fat diet (HFD)-induced effects, thereby preventing non-alcoholic fatty liver disease in mice. Mice were maintained on either a control diet (CD) or HFD for 16 weeks and supplemented with either water or green tea extract (50 mg/kg/day). The body mass increase, serum adiponectin level, and lipid profile were measured over the course of the treatment. Furthermore, the AMPK pathway protein expression in the liver was measured. From the fourth week, the weight gain in the CD + green tea extract (CE) group was lower than that in the CD + water (CW) group. From the eighth week, the weight gain in the HFD + water (HFW) group was found to be higher than that in the CW group. Moreover, the weight gain in the HFD + green tea extract (HFE) group was found to be lower than that in the HFW group. Carcass lipid content was found to be higher in the HFW group than that in the CW and HFE groups. Serum analysis showed reduced non-esterified fatty acid level in the CE and HFE groups as compared with their corresponding placebo groups. Increased adiponectin level was observed in the same groups. Increased VLDL-TG secretion was observed in the HFW group as compared with the CW and HFE groups. Increased protein expression of AdipoR2, SIRT1, pLKB1, and pAMPK was observed in the HFE group, which explained the reduced expression of ACC, FAS, SREBP-1, and ChREBP in this group. These results indicate that the effects of decaffeinated green tea extract may be related to the activation of AMPK via LKB1 in the liver of HFD-fed mice.
Introduction
It is well known that a high-fat diet (HFD) rich in saturated fat and low in dietary fiber can lead to obesity. Obesity, as a systemic and multifactorial disease, causes more damage than just adipocyte hypertrophy [1,2]. Charlton et al. [3] considered that non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of obesity and predicted that within 20 years, non-alcoholic steatohepatitis (NASH) will be the leading cause of liver cirrhosis requiring a transplant.
Insulin resistance in visceral adipose tissues in obesity has been shown to lead to an increased activation of the lipolytic signaling pathway [4,5], which further enhances non-esterified fatty acid (NEFA) uptake into the liver. The high hepatic influx of NEFA increases the secretion of very low density lipoproteins (VLDLs) and apolipoprotein B in the circulation, contributing to an increased hepatic glucose production by gluconeogenesis [6] and the activation of the de novo lipogenesis pathway [7]. NEFA overload induces an increase in triacylglycerol (TAG) level, exceeding the capacity of VLDL-TG synthesis, thereby promoting TAG accumulation in hepatocytes and contributing to the initiation of NAFLD [8,9].
Research on HFD animal models have shown that AMP-activated protein kinase (AMPK) phosphorylation via liver kinase B1 (LKB1) may be regulated by dietary patterns [10,11]. Furthermore, HFD per se may reduce adiponectin level, resulting in the reduction of the phosphorylation of AMPK, which can be activated by this adipokine [12,13]. LKB1 phosphorylation appears to be required for AMPKα activation. The role of adiponectin in LKB1 activation is controversial because a study [14] demonstrated its stimulation, whereas another study [10] did not. Further studies are needed to understand these mechanisms.
The complex formed by LKB1 and AMPK plays a key role in the regulation of hepatic fatty acid metabolism [15]. This complex is activated via phosphorylation. Several molecules activate LKB1 in the liver; one of them is SIRT1 [16]. Studies have shown that when activated by phosphorylation, this process regulates pLKB1 upstream phosphorylation of AMPK [17,18]. Activated pAMPK has the ability to modulate lipogenesis. The phosphorylation of AMPK leads to the phosphorylation and inactivation of acetyl-CoA carboxylase (ACC), which is an important regulatory enzyme in the synthesis of fatty acids by de novo lipogenesis [19,20]. ACC catalyzes the conversion of acetyl-CoA to malonyl-CoA via fatty acid synthase (FAS), an enzyme used in the synthesis of fatty acids. The inhibition of ACC by pAMPK reduces substrate flow for FAS, leading to a decrease in the activity of FAS [21].
Moreover, the NAFLD model demonstrated that AMPKα is a negative regulator of sterol element-binding protein 1-c (SREBP 1-c) and carbohydrate response element-binding protein (ChREBP). The increased phosphorylation of AMPKα appears to lead to a decrease in nuclear SREBP 1-c and ChREBP levels. This suggests the existence of a counter regulatory relationship between AMPKα/SREBP 1-c and ChREBP [13,22,23].
The effects of green tea (Camellia sinensis) in the prevention and treatment of obesity have been examined [24,25]. Epigallocatechin-3-gallate (EGCG) is the most abundant and biologically active catechin in green tea [26]. Studies have demonstrated that EGCG can reduce the risk of obesity and has possible protective effects against liver damage [2,27–29].
Banerjee et al. [18] report that a single dose of green tea extract was capable of increasing hepatic AMPK level and phosphorylation of LKB1, demonstrating the potent effects of green tea on these molecules. Studies with similar bioactive compounds have suggested that EGCG could prevent peripheral insulin resistance induced by the increase in NEFA level and that this protective effect may be associated with the inhibition of oxidative stress and AMPK signaling activation [30–36]. Stimulation of AMPK phosphorylation, which in turn inhibits ACC and FAS activity, could reduce the accumulation of hepatic TAGs.
Considering the lack of studies regarding the effect of decaffeinated green tea extract on the activation of the AMPK via LKB1, the purpose of this study was to investigate the effects of a green tea extract supplement on the activation of enzymes and factors related to hepatic de novo lipogenesis, concurrent with VLDL-TG secretion in HFD-fed mice.
Materials and Methods
Animal experiments
All animals experiments were performed according to protocols approved by the Experimental Research Committee of Universidade Federal de São Paulo (CEUA n° 975418) respecting the standards established by the Brazilian Guideline for Care and Use of Animals for Scientific Purposes and Teaching imposed by the National Council of Animal Experimentation—CONCEA in 2013.[37]. A total of 54 male Swiss mice at 30 days old were used. To total number of samples, the experimental protocol was performed twice, to prove the replicability of our model.
The mice were maintained in collective polypropylene cages in isolated room with controlled temperature (25± 2°C), humidity (60 ± 5%) and lighting (12-h light/dark cycle) and received water and diet ad libitum during all experimental period. After one week of acclimatization, the mice were divided evenly into four groups: Control diet + water (CW) (n = 14); Control diet + green tea extract rich in EGCG (CE) (n = 14); High-fat diet + water (HFW) (n = 13) and High-fat diet + green tea extract rich in EGCG (HFE) (n = 13). The mice were fed control diet (AIN-93) [38] or high-fat diet (AIN-93 adapted) and water, both ad libitum during the next 16 weeks (Table 1).
Table 1. Composition of experimental diet CD and HFD (AIN-93 modified) [38], growth (G) and maintenance (M).
| Ingredients | CD (G/M) | HFD (G/M) |
|---|---|---|
| Cornstarch (%) | 62. 95 / 72.07 | 40. 92 / 40.87 |
| Casein (%) | 20. 0 / 14.0 | 13. 95 / 14.0 |
| Soybean Oil (%) | 7 0.0 / 4.0 | 7.0 / 4.0 |
| Lard (%) | - | 28. 08 / 31.2 |
| Cellulose (%) | 5 | 5 |
| Mixture of Vitamins (%) | 1.0 | 1.0 |
| Mixture of Mineral (%) | 3.5 | 3.5 |
| L-cystine (%) | 0. 3 / 0.18 | 0.18 / 0.18 |
| Cholinebitartrate (%) | 0.25 | 0.25 |
| Hydroquinone (g/kg) | 0.014 / 0.008 | 0.014 / 0.008 |
| Energy (Kcal/kg) | 3948 / 3.802 | 5.352 / 5.362 |
To ensure accurate use of the established dose of green tea extract, administration of green tea was performed by gavage. Thereby the HFE and CE groups received treatment green tea extract (0.1 mL green tea extract 98% EGCG - 50mg/kg/day). To guarantee that all animals pass by the same conditions of stress, manipulation and physical injury, the animals belonging to the placebo group (HFW and CW) were also submitted to gavage received filtered water in equal volume to that given for the groups treated with green tea extract (0.1 mL of water by gavage / day). The animals of placebo groups were weighed once a week, the weight of the green tea extract treatment groups were measured every day during the experimental period to adjust the daily dose to weight.
At the end of the experimental period, the mice were euthanized by beheading in the morning period between eight and ten o'clock, after 12 hours fasting. Blood and liver were collected for analyzes, the hepatic tissues were weighed and all samples were stored at −80°C. All experimental procedures described below were performed using single samples, sample pooling has not been necessary.
Experimental Procedures
Carcass lipid and protein content
The carcass lipid and protein content was determined in all experimental groups. The carcasses were eviscerated, and the retroperitoneal, epididymal and mesenteric white adipose tissue, gastrocnemius muscle and liver were removed. The remaining carcasses were weighed and stored at −20°C. The lipid content was measured as described by Stansbie et al.[39] and standardized using the method described by Oller do Nascimento and Williamson [40]. Briefly, the eviscerated carcass was autoclaved at 120°C for 90 min and homogenized with water at a volume twice the carcass mass. Triplicate aliquots of this homogenate were weighed and digested in 3 mL of 30% KOH and 3 mL of ethanol for ≥2 h at 70°C in capped tubes. After cooling, 2 mL of 12 N H2SO4was added, and the samples were washed three times with petroleum ether to extract the lipids. The results are expressed as grams of lipid per 100 g of carcass. To measure the protein content, aliquots of the same homogenate (approximately 1 g) were heated to 37°C for 1 h in 0.6 N KOH with constant shaking. After clarification by centrifugation, the protein content was measured using the Bradford assay (Bio-Rad, Hercules, California) with bovine serum albumin as a reference.
Serum parameters
The serum glucose, total cholesterol (TC), HDL-cholesterol and triacylglycerol (TAG) were analyzed by colorimetric method using commercial kits (Labtest ®). The serum concentration of adiponectin (R&D Systems) and Non-esterified fatty acids (Zen-bio, Inc.) were also evaluated. LDL-cholesterol level was estimated indirectly by Friedewald equation [LDL-c = total cholesterol-(HDL-c)-(TG/5)][41].
In vivo VLDL-triacylglycerol secretion
Considering the interfering effects that anesthesia can exert on other parameters that would be evaluated, an exclusive group of mice were treated for this trial to determine the in vivo VLDL-TAG secretion from the liver, and these mice were excluded from other analyses. The required sample size was five mice per experimental group (n = 5). After the 16-week intervention period, mice were fasted for 4 h to minimize the contribution by intestinal absorption of serum lipoproteins. They were anesthetized using ketamine (80 mg/kg) and xylazine (10 mg/kg) and infused through the jugular vein a Tyloxapol solution (400 mg/kg, Triton WR 1339, Sigma) dissolved in saline solution (300 mg/mL) and heated at 37°C. Blood samples were collected prior to injection and at again at 30, 60, 90, and 120 min after injection for the determination of TAG level. Hepatic VLDL-TAG secretion was then estimated by calculating the area under the curve (AUC). After the experimental procedure, the animals were euthanized by exsanguination trough the jugular vein access used for collecting blood samples during the experiment.
Western blot analysis
Hepatic tissue were homogenized in lyses buffer containing 100mM Tris–HCl (pH 7.5), 1% Triton X-100, 10% sodium dodecyl sulfate (SDS), 10mM EDTA, 100mM sodium fluoride, 10mM sodium pyrophosphate, 10mM sodium orthovanadate, 2.0mM phenylmethylsulphonyl fluoride (PMSF), and 0.1mg aprotinin/mL. The homogenate was centrifuged at 20,800 x g for 45 min at 4°C, and the supernatant was collected. The total protein concentration was measured with Bradford Reagent (Bio-Rad Laboratories, Inc.). Proteins in the lysates were electrophoretically separated in 10% SDS polyacrylamide gel and transferred to nitrocellulose membrane. Were used precast gels in 4–15% (Mini-PROTEAN®TGX TM Precast Gels) of Bio-Rad Laboratories, Inc. The membranes were blocked in 1% bovine serum albumin overnight at room temperature, and then incubated overnight with the following primary antibodies: pAMPK α1/2 (sc-33524), AMPK α1/2 (sc-25792), ACCα (sc-30212), FAS (sc-20140), SREBP-1 (sc-8984), ChREBP (sc-21189), SIRT-1 (sc-15404) purchased from Santa Cruz Biotechnology, Inc.(Santa Cruz, CA, USA). The β-tubulin, pLKB1 (S428), pACC (S79), pSREBP-1c (Ser372) antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Adiponectin Receptor-2 (AdipoR2) was purchased from ABCAM (Cambridge, UK) and LamininR-67KDa (NBP1-33002) was from Novus Biologicals (Littleton, CO, USA).The membranes were next incubated with horseradish-peroxidase-conjugated secondary antibodies during 1 hour at room temperature. The bands were visualized with enhanced chemiluminescence scanned at UVITec (Cambridge) after adding the ECL reagent (GE Healthcare Bio-Sciences AB, UK), and the intensities of the bands were quantified in ImageJ software (ImageJ, National Institute of Health, Maryland, USA). Performing calculations of each band obtained for analysis in proteins of interest for this study were normalized using β-tubulin levels of the respective membrane individually.
Statistical Analyses
Data were subjected to quality tests such as Shapiro–Wilk (normality), Levenne (homogeneity), and/or Mauchly (sphericity). In cases of non-sphericity, corrected values F (Greenhouse–Geisser) were used. If necessary, data were standardized according to the Z score. The descriptive analysis was performed using the mean ± SEM. To verify the interactions between groups, we used two-way ANOVA followed by Tukey’s post hoc test. The level of significance was p ≤ 0.05. For statistical analyses, the software SPSS Version 17.0 was used.
Results
Effects of green tea extract on the mass gain and body composition
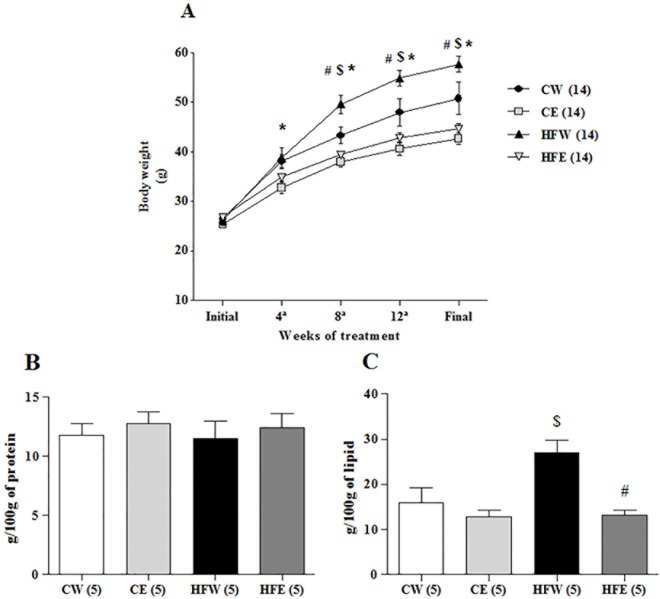
There were no statistically significant differences among the experimental groups when they were initially weighed. Nevertheless, the body weight of mice belonging to the CE group was significantly lower than those belonging to the CW group, starting at the fourth week until the end of experimental treatment (p < 0.001). The effects of HFD on weight gain were observed from the eighth week of experimental treatment, with an increase in mass in the HFW group as compared with the CW group (p < 0.001). Starting at the eighth week, the HFE group also had statistically significant lower weight gain as compared with the HFW and placebo groups (p < 0.001; Fig 1A).
Fig 1. Body composition: body mass gain; lean body mass; and subcutaneous fat accumulation.
(A) body mass gain over the 16 weeks of treatment with HFD; (B) lean body mass; (C) subcutaneous fat accumulation obtained from the carcass of different experimental groups. Data are expressed in mean ± s.e.m. *p<0.05 Control diet and EGCG (CE) group versus Control diet and Water (CW) group. $p<0.05 High-fat and Water (HFW) group versus CW group. #p<0.05 High-fat diet and EGCG (HFE) group versus HFW group.
On determining the body composition using the carcass analysis, differences in protein content among the groups were not observed. We observed an increase in the lipid content of carcasses in the HFW group as compared with the CW group (p = 0.005). On the other hand, the green tea extract supplement was able to reduce the lipid content in the HFE group as compared with the HFW group (p < 0.001; Fig 1B and 1C).
Effects of green tea extract on serum parameters
The serum lipid profiles, NEFA and adiponectin levels are shown in Table 2. Serum TAG (p = 0.049), TC (p = 0.001), and LDL-c (p = 0.010) levels were increased in the HFW group as compared with the CW group. LDL-c level was also reduced in the HFE group as compared with the HFW group (p = 0.011). NEFA level was reduced in the HFE group as compared with the HFW group (p < 0.001) as well as in the CE group as compared with the CW group (p < 0.001). Adiponectin presented a significantly higher level in the HFE group as compared with the HFW group (p = 0.031) and in the CE group as compared with the CW group (p = 0.046). The HDL-c assessment did not show differences among the experimental groups.
Table 2. Serum parameter assessed in different groups.
| Parameters | CW | CE | HFW | HFE |
|---|---|---|---|---|
| TC (mg/dL) | 136.9 ± 2.9 | 129.7 ± 2.7 | 163.5 ± 6.2 $ | 155.7 ± 4.17 |
| TAG (mg/dL) | 131.96 ± 3.3 | 129.89 ± 4.4 | 163.55 ± 8.5 $ | 147.87 ± 7.1 |
| HDL-c(mg/dL) | 54.28 ± 2.76 | 52.16 ± 4.04 | 61.61 ± 3.75 | 58.77 ± 2.44 |
| LDL-c (mg/dL) | 63.07 ± 4.18 | 51.28 ± 9.20 | 91.39 ± 8.23 $ | 78.97 ± 4.48 |
| NEFA (mM) | 4.67 ±0.27 | 2.97 ±0.14 * | 4.89 ±0.38 | 3.19 ±0.15 # |
| Adiponectin(μg/mL) | 8.38 ± 0.47 | 10.38 ± 0.66 * | 6.90 ± 1.01 | 8.73 ± 0.73 # |
Effect of green tea extract on VLDL-TG secretion
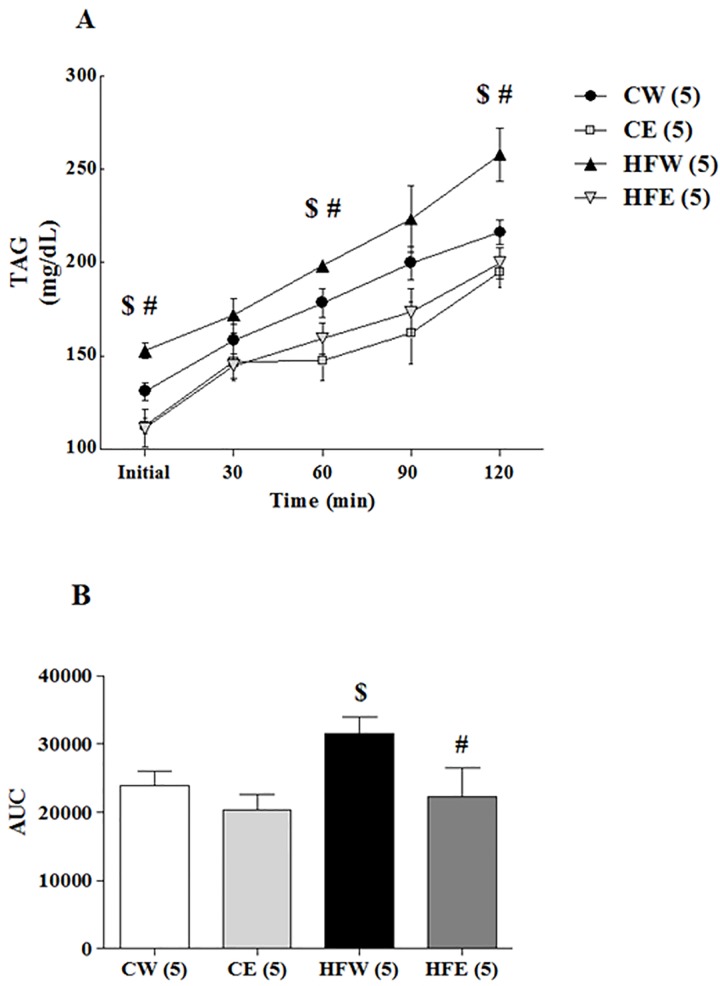
High-fat feeding in mice increases hepatic VLDL-TG secretion, and in the study we tested whether green tea extract can lower the secretion of VLDL-TG. The TAG concentration over time increased in the HFW group as compared with the CW and HFE groups at baseline (p = 0.037 and 0.019, respectively), 60 min (p = 0.028 and p = 0.033, respectively), and 120 min (p = 0.042 and p < 0.001, respectively) after tyloxapol injection. The AUC analysis affirmed the differences in the HFW group compared with the CW group (p = 0.012) and HFE group (p = 0.003; Fig 2A and 2B).
Fig 2. In vivo triacylglycerol production rate. After 16 week of treatment.
After 16 week of treatment, Triton WR1339 (400 mg/kg BW) was administered intravenously to 4 hours fasted mice. Samples collected at time 0, 30, 60, 90 and 120 min after injection. Plasma samples from each time point were used to determine plasma triacylglycerol levels over time (A). Triacylglycerol production (B) was estimated by calculated the AUC. Data are expressed in mean ± s.e.m.*p<0.05 Control diet and EGCG (CE) group versus Control diet and Water (CW) group. $p<0.05 High-fat and Water (HFW) group versus CW group. #p<0.05 High-fat diet and EGCG (HFE) group versus HFW group.
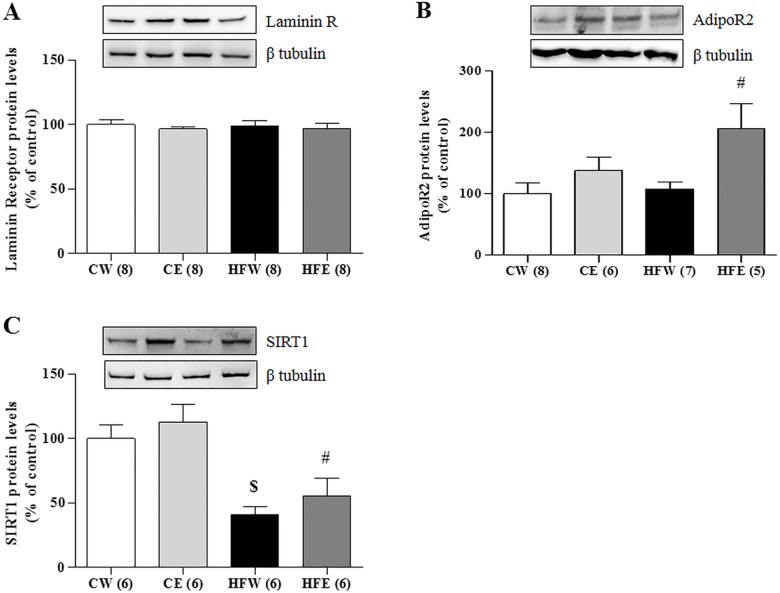
Effect of green tea extract on liver protein expression
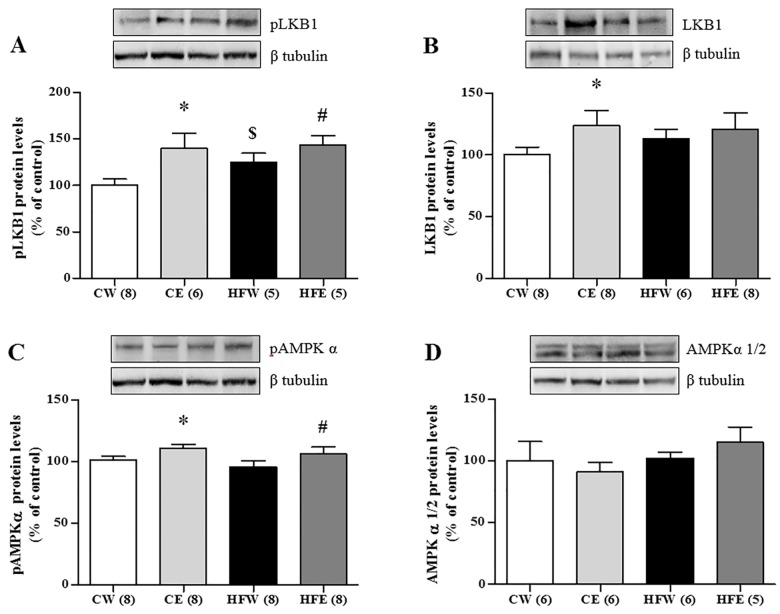
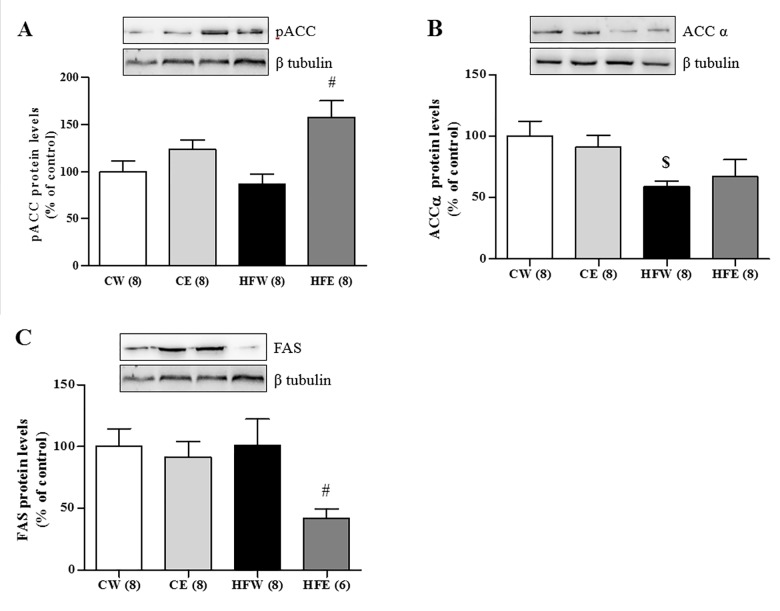
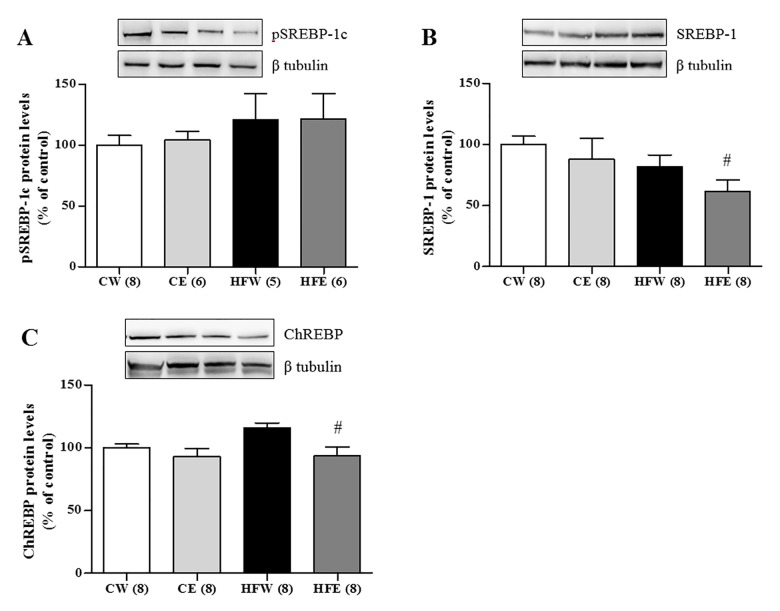
The protein expression of the laminin membrane receptors did not differ among the groups, but AdipoR2 expression in the liver was increased in the HFE group as compared with the HFW group (p = 0.006). SIRT1 expression was reduced in the HFW group compared as with the CW group (p = 0.004). Green tea extract improved SIRT1 expression in the HFE group as compared with the HFW group (p = 0.004; Fig 3A–3C). Green tea extract treatment also caused a specific increase in the phosphorylation of LKB1 in the HFE group (p = 0.047) as compared with the HFW group as well as in the CE (p = 0.034) and HFW (p = 0.027) groups in relation to the CW group. LKB1 was also elevated individually in the CE group compared with the CW group (p = 0.046). The activation through pAMPKα 1/2 increased in the CE group as compared with the CW group (p = 0.037) and in the HFE group (p = 0.034) as compared with the HFW group. The total fraction of AMPKα 1/2 did not differ among the groups (Fig 4A–4D). The lipogenic enzyme ACCα in its active form (not phosphorylated) was activated and higher in the HFW group as compared with the HFE group (p = 0.001) and the enzyme pACC in its inactive form (phosphorylated) was higher in the HFE group as compared with the HFW group (p = 0.017; Fig 5A and 5B). FAS and ChREBP were reduced in the HFE group as compared with the HFW group (p = 0.012 and p = 0.008, respectively). The total protein expression SREBP-1c that represents the active isoform was lower in the HFE group as compared with the HFW group (p = 0.007); on the other hand, the pSREPB-1c that represents the inactive isoform (phosphorylated) did not change among the groups (Figs 5C and 6A–6D).
Fig 3. Liver protein expression in different experimental groups of Laminin R, AdipoR2 and SIRT1.
Western Blotting analysis of protein expression in the liver on different experimental groups of membrane receptors (A) Laminin Receptor, (B) AdipoR2, and (C) SIRT1. Image shows demonstrative bands of the analyzed proteins and respective housekeeping protein (β-tubulin) in liver. Data are expressed in mean ± s.e.m. *p<0.05 Control diet and EGCG (CE) group versus Control diet and Water (CW) group. #p<0.05 High-fat diet and EGCG (HFE) group versus HFW group. $p<0.05 HFW versus CW.
Fig 4. Liver protein expression in different experimental groups of LKB1 / AMPK pathway.
Western blotting analysis of protein expression in the liver on different experimental groups of AMPK—LKB1 pathway (A) pLKB1, (B) LKB1, (C) pAMPKα, (D) AMPKα 1/2. Image shows demonstrative bands of the analyzed proteins and respective housekeeping protein (β-tubulin) in liver. Data are expressed in mean ± s.e.m. *p<0.05 Control diet and EGCG (CE) group versus Control diet and Water (CW) group. #p<0.05 High-fat diet and EGCG (HFE) group versus HFW group. $p<0.05 HFW versus CW.
Fig 5. Liver protein expression in different experimental groups of lipogenic enzymes.
Western Blotting analysis of protein expression in the liver on different experimental groups of enzymes responsible for synthesis of fatty acids and de novo lipogenesis. (A) pACC, (B) ACCα and (C) FAS. Image shows demonstrative bands of the analyzed proteins and respective housekeeping protein (β-tubulin) in liver. Data are expressed in mean ± s.e.m. *p<0.05 Control diet and EGCG (CE) group versus Control diet and Water (CW) group. #p<0.05 High-fat diet and EGCG (HFE) group versus HFW group. $p<0.05 HFW versus CW.
Fig 6. Liver protein expression of nuclear transcription factors of lipids metabolism in different experimental groups.
Western Blotting analysis of protein expression in the liver on different experimental groups of nuclear transcription factors involved in promotion of enzymes responsible for synthesis of fatty acids and de novo lipogenesis. (A) pSREBP-1c, (B) SREBP-1, (C) ChREBP. Image shows demonstrative bands of the analyzed proteins and respective housekeeping protein (β-tubulin) in liver. Data are expressed in mean ± s.e.m. *p<0.05 Control diet and EGCG (CE) group versus Control diet and Water (CW) group. #p<0.05 High-fat diet and EGCG (HFE) group versus HFW group. $p<0.05 HFW versus CW.
Discussion
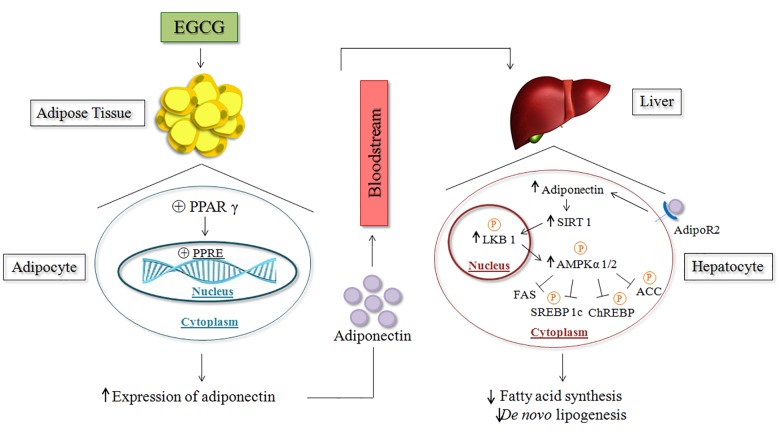
The main contribution of this study was new, additional information suggesting that decaffeinated green tea extract rich in EGCG may exert reduction on the de novo lipogenesis and secretion of VLDL. Our data suggest that green tea extract seems to have an influence on the increase of adiponectin production, which in turn could represent one mechanism of AMPK activation by LKB1. This may reduce the level of de novo lipogenesis and secretion of VLDL, suggesting a potential therapy for the prevention of liver damage in HFD-associated obesity.
After analyzing the data regarding body composition in this study, we can see the effectiveness of our obesity model. The ad libitum supply of HFD led to an increase in weight gain as well as an increase in the carcass fat content, and this was maintained throughout the treatment until the end of the experimental period (Fig 1C). Based on these parameters, it was also possible to observe the activity of green tea extract in reducing the effects of obesity (Fig 1A). Our results are widely supported by previous studies in which the same effects were observed following the administration of HFDs [5,42–46]. Similar effects of EGCG supplementation in preventing the deleterious effects of HFD have also been reported [24,25,47–52].
Some authors have described anti-nutritional effects of the components of green tea, including EGCG, in inhibiting the absorption of nutrients [53–55]. HFD, in some situations, has also been shown to cause sarcopenia [56–59]. The gastrocnemius relative weight (data not shown) and analysis of protein concentrations of carcasses showed that there was no depletion of lean body mass among the groups as compared with the control (Fig 1B). The absence of obesity-related sarcopenia means that our HFD model met the physiological and nutritional requirements for the maintenance of lean body mass. It also shows that the reduction in weight gain observed with green tea extract supplementation is not related to anti-nutritional factors of catechins or lack of nutrient absorption, which can be verified by the normal lean body mass seen in all experimental groups.
Another metabolic characteristic linked to obesity occurs in modifying serum parameters. For the classification of the metabolic syndrome, changes in serum lipid profile are used in addition to weight gain [60]. It is a common initiator of dyslipidemia: an increase in TAG, TC and LDL-c levels and a decrease in HDL-c level. The results based on these parameters show that our experimental model of obesity was effective in inducing obesity-linked metabolic changes, confirmed by a worsening of the TAG, TC, and LDL-c levels; however, there was no change in HDL-c level (Table 2).
Being multifactorial diseases, obesity and the metabolic syndrome have systemic, deleterious effects. Of all the comorbidities attributed to obesity, NAFLD is the only hepatic manifestation of obesity. Because the liver is an organ of vital importance for the maintenance of body homeostasis, it is extremely important to undertake measures to prevent and combat the initiation of NAFLD [2,3].
With increasing visceral adiposity, there is increased portal NEFA delivery to the liver. It has been demonstrated that the fraction of NEFA delivered to the liver from visceral fat is positively correlated to the visceral fat area, and it is approximately 5%–10% in normal-weight subjects and 20%–30% in obese subjects [61]. Hepatic uptake of NEFA is proportional to the rate of delivery. In the liver, NEFA is either oxidized or esterified to form TAGs. The TAG is stored in the cytosol prior to being incorporated into VLDLs and then secreted. When TAG production exceeds NEFA oxidation and VLDL production and secretion, TAGs accumulate in the liver, resulting in the development of NAFLD [62,63]. In our results, NEFA level was increased in the HFW group, and the green tea extract supplementation was able to reduce NEFA level, contributing to the prevention of NAFLD development (Table 2). When in vivo VLDL-TG secretion was analyzed (Fig 2), it was clear that green tea extract reduced the secretion rate of VLDL-TG (in agreement with the NEFA data) and decreased the amounts of this lipoprotein in the blood circulation.
After reviewing the literature, we had indications that Laminin Receptor 67 kDa could be increased by EGCG [64–68]. However, our results showed no change in protein expression of this receptor in the liver. This finding suggests that the dose chosen for our experimental treatment was physiologically adequate to exert beneficial effects without stimulating the increase of membrane receptor synthesis because this receptor can be found in large quantities in cancer cells [69].
Over the last few years, several authors have described that green tea polyphenols and isolated EGCG are effective in raising serum adiponectin level and protein expression of its receptors (AdipoR1 and AdipoR2) in humans and HFD-fed mice [25,70–72]. Adiponectin gene expression is mainly regulated by the PPARγ nuclear transcription factor. PPARγ binds directly to the functional element PPAR-responsive element (PPRE) in the adiponectin promoter and increases the transcription of the adiponectin gene [73]. Tian et al. [74] demonstrated that green tea polyphenols can inhibit the activation of ERK1/2, increase the expression of PPARγ, and reduce the phosphorylation of PPARγ. Based on these findings, we suggest that in our study, green tea extract was responsible for the increase in the adiponectin level (Table 2).
Increased adiponectin and AdipoR2 levels have been shown to correlate with the activation of SIRT1 [75–77]. Civitarese et al. [78] demonstrated that adiponectin significantly increased SIRT1 protein expression but had no effect on SIRT1 mRNA, an indicator of post-transcriptional regulation. It was also demonstrated that knockout adiponectin receptors (-R1 and -R2 isoforms) reduced the amount of SIRT1 protein in primary human myotubes, indicating that adiponectin could directly upregulate SIRT1. These findings support our hypothesis that adiponectin, through its most abundant receptor in the liver (AdipoR2), stimulated the activation of SIRT1 in the HFE group (Fig 3B and 3C).
SIRT1 appears to activate AMPKα via a LKB1-dependent pathway in HFD. This has been reported in human hepatocytes [16] and reinforced by our study. Results suggests that AdipoR2 activates SIRT1, which in turn is responsible for the deacetylation of Lys48 residues on nuclear LKB1 [79,80]. When activated LKB1 migrates from the hepatocyte nucleus to the cytoplasm, where it induces phosphorylation of the AMPK α subunit [81]. The AMPK heterotrimeric molecule conformation is composed of three subunits, which exert different biological functions. It presents two regulatory subunits (AMPKβ and γ) and the catalytic subunit AMPKα is mainly responsible for the AMPK activity in rodent liver [82]. LKB1 can specifically phosphorylate the catalytic subunit (AMPKα 1/2), as shown in our results, particularly in the HFD group (Fig 2A–2D). The subunits AMPKα 1a and AMPKα 2 were the most discussed in the literature concerned with the effectiveness of AMPK activation [83,84].
ChREBP and SREBP 1-c are transcription factors that regulate the expression of hepatic enzymes involved in lipogenesis. These factors can bind to the transcription promoter genes of lipogenic proteins such as ACC, FAS, and fatty acid converting enzymes such as stearoyl CoA desaturase (SCD1) and fatty acid elongases such as fatty acid elongase 6 [85,86]. Both ChREBP and SREBP 1-c remain in the cytoplasm attached to an inactive phosphate group. The dephosphorylated (active) form has shown the ability to translocate to the nucleus and stimulate the synthesis of lipogenic enzymes [80,87]. Some studies reported that activated AMPK has a role in maintaining the phosphorylated SREBP1c and CHREBP forms in the cytoplasm and preventing them from reaching the nucleus [13,22,23,32,88,89]. These results agree with ours, wherein there was a reduction in the active forms of ChREBP and SREBP1c in the HFE group as compared with the HFW group. This led to reduced protein expression of lipogenic enzymes in the liver (Fig 6A–6C).
Activated AMPK demonstrated the ability to reduce the activity of the lipogenic enzymes, ACC and FAS [90]. ACC catalyzes the conversion of acetyl-CoA to malonyl-CoA, which is used in the synthesis of fatty acid de novo lipogenesis [85]. In our study, when phosphorylated, ACC was in its inactive form and we clearly observed that supplementation with green tea extract increased pACC level as well as reduced the FAS level, indicating that there was a reduction in fatty acid synthesis by the de novo lipogenesis pathway (Fig 5A–5C).
Conclusion
Overall, our results indicate that green tea extract supplementation was able to improve hepatic metabolism and reduce NEFA uptake into the liver; this led to a decrease in hepatic VLDL-TG secretion, decreased lipid synthesis, and improvement of adiponectin in HFD-fed mice. Highlighting its beneficial effects, it was noted that green tea extract supplementation has also been shown to stimulate the activation of the AMPK via LKB1 through adiponectin in HFD-fed mice and to regulate essential enzymes involved in the de novo lipogenesis pathway in the liver. This beneficial effect may contribute to the prevention and treatment of NAFLD (Fig 7).
Fig 7. EGCG through adiponectin and LKB1 / AMPK pathway on lipid metabolism—possible mechanism.
Statement from mechanism by which EGCG promotes increase in adiponectin and performs activation on the signaling of AMPK via LKB1 inhibiting mediators responsible for the synthesis of fatty acids and de novo lipogenesis. (Liver and DNA- from FreeDigitalPhotos.net).
Acknowledgments
This research was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, 2014/19508-7). Dream Designs gently provided liver image, and the DNA image was courtesy of Cool Design both from FreeDigitalPhotos.net.
Data Availability
Data are available from Figshare at http://dx.doi.org/10.6084/m9.figshare.1580067.
Funding Statement
This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo 2014/19508-7 - grant to: Dr Lila Missae Oyama and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - grant to Aline Boveto Santamarina.
References
- 1.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29: 415–45. 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 2.Darnton-Hill I, Nishida C, James W. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr. 2007;7: 101–121. 10.1079/PHN2003584 [DOI] [PubMed] [Google Scholar]
- 3.Charlton MR, Burns JM, Pedersen R, Watt KD, Heimbach JK, Dierkhising R. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. Elsevier Inc.; 2011;141: 1249–53. 10.1053/j.gastro.2011.06.061 [DOI] [PubMed] [Google Scholar]
- 4.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116: 1793–1801. 10.1172/JCI29069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7: 14–24. 10.1007/s11684-013-0262-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115: 1343–1351. 10.1172/JCI200523621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigouroux C, Caron-Debarle M, Le Dour C, Magré J, Capeau J. Molecular mechanisms of human lipodystrophies: from adipocyte lipid droplet to oxidative stress and lipotoxicity. Int J Biochem Cell Biol. Elsevier Ltd; 2011;43: 862–76. 10.1016/j.biocel.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Byrne CD. Ectopic fat, insulin resistance and non-alcoholic fatty liver disease. Proc Nutr Soc. 2013; 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci. 2013;1281: 106–22. 10.1111/nyas.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shearn CT, Smathers RL, Jiang H, Orlicky DJ, Maclean KN, Petersen DR. Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. J Nutr Biochem. Elsevier B.V.; 2013;1: 1–10. 10.1016/j.jnutbio.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Kahn BB, Shi H, Xue B-Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285: 19051–9. 10.1074/jbc.M110.123620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval C, Thissen U, Keshtkar S, Accart B, Stienstra R, Boekschoten MV, et al. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57Bl/6 mice. Diabetes. 2010;59: 3181–3191. 10.2337/db10-0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo H-Y, Kim M-K, Jung Y-A, Jang BK, Yoo E-K, Park K-G, et al. Clusterin decreases hepatic SREBP-1c expression and lipid accumulation. Endocrinology. 2013;154: 1722–30. 10.1210/en.2012-2009 [DOI] [PubMed] [Google Scholar]
- 14.Blagih J, Krawczyk CM, Jones RG. LKB1 and AMPK: central regulators of lymphocyte metabolism and function. Immunol Rev. 2012;249: 59–71. 10.1111/j.1600-065X.2012.01157.x [DOI] [PubMed] [Google Scholar]
- 15.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9: 563–575. 10.1038/nrc2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou X, Xu S, Maitland-Toolan K, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283: 20015–20026. 10.1074/jbc.M802187200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan R-Y, Li H-B. Recent Progress on Liver Kinase B1 (LKB1): Expression, Regulation, Downstream Signaling and Cancer Suppressive Function. Int J Mol Sci. 2014;15: 16698–16718. 10.3390/ijms150916698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S, Ghoshal S, Porter TD. Phosphorylation of hepatic AMP-activated protein kinase and liver kinase B1 is increased after a single oral dose of green tea extract to mice. Nutr Res. 2012;32: 985–990. 10.1016/j.nutres.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasenour CM, Berglund ED, Wasserman DH. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol Cell Endocrinol. Elsevier Ireland Ltd; 2013;366: 152–62. 10.1016/j.mce.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song C-Y, Shi J, Zeng X, Zhang Y, Xie W-F, Chen Y-X. Sophocarpine alleviates hepatocyte steatosis through activating AMPK signaling pathway. Toxicol In Vitro. 2013;27: 1065–71. 10.1016/j.tiv.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 21.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. 2004;114. 10.1172/JCI200422422.The [DOI] [PMC free article] [PubMed]
- 22.Iizuka K. Recent progress on the role of ChREBP in glucose and lipid metabolism. Endocr J. 2013;60: 543–55. 10.1507/endocrj [DOI] [PubMed] [Google Scholar]
- 23.O’Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol Cell Endocrinol. Elsevier Ireland Ltd; 2013;366: 135–51. 10.1016/j.mce.2012.06.019 [DOI] [PubMed] [Google Scholar]
- 24.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138: 1677–1683. 10.1016/j.bbi.2008.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, et al. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. Elsevier Inc.; 2014;25: 1–18. 10.1016/j.jnutbio.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa S, Utan A, Cervellati R, Speroni E, Guerra MC. Catechins: natural free-radical scavengers against ochratoxin A-induced cell damage in a pig kidney cell line (LLC-PK1). Food Chem Toxicol. 2007;45: 1910–7. 10.1016/j.fct.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 27.Xiao J, Ho CT, Liong EC, Nanji A, Leung TM, Lau TYH, et al. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 2014;53: 187–199. 10.1007/s00394-013-0516-8 [DOI] [PubMed] [Google Scholar]
- 28.Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol (Tokyo). 1986;32: 613–622. 10.3177/jnsv.32.613 [DOI] [PubMed] [Google Scholar]
- 29.Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002;83: 109–116. 10.1016/S0378-8741(02)00217-9 [DOI] [PubMed] [Google Scholar]
- 30.Davaatseren M, Hur HJ, Yang HJ, Hwang JT, Park JH, Kim HJ, et al. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem Toxicol. Elsevier Ltd; 2013;58: 30–36. 10.1016/j.fct.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Xiong Y, Wang DQ-H, Howles P, Basford JE, Wang J, et al. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54: 1430–8. 10.1194/jlr.M035907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. Elsevier Inc.; 2011;14: 612–22. 10.1016/j.cmet.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zang M, Xu S, Maitland-Toolan K, Zuccollo A, Hou X, Jiang B, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55: 2180–91. 10.2337/db05-1188 [DOI] [PubMed] [Google Scholar]
- 34.Latif R. Chocolate/cocoa and human health: a review. Neth J Med. 2013;71: 63–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/23462053 [PubMed] [Google Scholar]
- 35.Kobayashi-Hattori K, Mogi A, Matsumoto Y, Takita T. Effect of caffeine on the body fat and lipid metabolism of rats fed on a high-fat diet. Biosci Biotechnol Biochem. 2005;69: 2219–2223. 10.1271/bbb.69.2219 [DOI] [PubMed] [Google Scholar]
- 36.Kurimoto Y, Shibayama Y, Inoue S, Soga M, Takikawa M, Ito C, et al. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. J Agric Food Chem. 2013;61: 5558–5564. 10.1021/jf401190y [DOI] [PubMed] [Google Scholar]
- 37.BRAZIL C. Diretriz brasileira para o cuidado e a utilização de animais para fins científicos e didáticos [Internet]. Brasília DF—Brazil; 2013 pp. 1–50. Available: http://www.cobea.org.br/arquivo/download?ID_ARQUIVO=20
- 38.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123: 1939–51. Available: http://www.ncbi.nlm.nih.gov/pubmed/8229312 [DOI] [PubMed] [Google Scholar]
- 39.Stansbie D, Denton RM, Bridges BJ, Pask HT, Randle PJ. Regulation of Pyruvate Dehydrogenase and Pyruvate Dehydrogenase Phosphate Activity in Rat Epididymal Fat-Pads. Biochem J. 1976;154: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oller do Nascimento CM, Williamson DH. Evidence for conservation of dietary lipid in the rat during lactation and the immediate period after removal of the litter. Decreased oxidation of oral [1-14C]triolein. Biochem J. 1986;239: 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18: 499–502. 10.1177/107424840501000106 [DOI] [PubMed] [Google Scholar]
- 42.An R, Burd N. Change in daily energy intake associated with pairwise compositional change in carbohydrate, fat and protein intake among US adults, 1999–2010. Public Health Nutr. 2014; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carillon J, Romain C, Bardy G, Fouret G, Feillet-Coudray C, Gaillet S, et al. Cafeteria diet induces obesity and insulin resistance associated with oxidative stress but not with inflammation: improvement by dietary supplementation with a melon superoxide dismutase. Free Radic Biol Med. Elsevier; 2013;65: 254–61. 10.1016/j.freeradbiomed.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 44.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56: 1638–48. 10.1007/s00125-013-2913-1 [DOI] [PubMed] [Google Scholar]
- 45.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J, et al. Defining high-fat-diet rat models: Metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006; [DOI] [PubMed] [Google Scholar]
- 46.Martins SV, Lopes P, Alfaia CM, Rodrigues PO, Alves SP, Pinto RM, et al. Serum adipokine profile and fatty acid composition of adipose tissues are affected by conjugated linoleic acid and saturated fat diets in obese Zucker rats. Br J Nutr. 2010;103: 869–78. 10.1017/S000711450999256X [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Cheung C, Reuhl K. Effects of green tea polyphenol (-)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J Agric Food Chem. 2011;59: 11862–71. 10.1021/jf2029016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, Bay B- H, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS One. 2014;9: e87161 10.1371/journal.pone.0087161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunha CA, Lira FS, Rosa Neto JC, Pimentel GD, Souza GIH, da Silva CMG, et al. Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low-grade inflammation in mice fed a high-fat diet. Mediators Inflamm. 2013;2013: 635470 10.1155/2013/635470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin Med. 2010;5: 13 10.1186/1749-8546-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PRC. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr. 2007;26: 396S–402S. Available: http://www.ncbi.nlm.nih.gov/pubmed/17906193 [DOI] [PubMed] [Google Scholar]
- 52.Lin J-K, Lin-Shiau S-Y. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006;50: 211–7. 10.1002/mnfr.200500138 [DOI] [PubMed] [Google Scholar]
- 53.Ma Q, Kim EY, Lindsay EA, Han O. Bioactive Dietary Polyphenols Inhibit Heme Iron Absorption in a Dose-Dependent Manner in Human Intestinal Caco-2 Cells. J Food Sci. 2011;76 10.1111/j.1750-3841.2011.02184.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim EY, Pai TK, Han O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal caco-2 cell monolayers. J Agric Food Chem. 2011;59: 3606–3612. 10.1021/jf104260j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48: 409–416. 10.1016/j.fct.2009.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bollheimer LC, Buettner R, Pongratz G, Brunner-Ploss R, Hechtl C, Banas M, et al. Sarcopenia in the aging high-fat fed rat: A pilot study for modeling sarcopenic obesity in rodents. Biogerontology. 2012;13: 609–620. 10.1007/s10522-012-9405-4 [DOI] [PubMed] [Google Scholar]
- 57.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2014; n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 58.De Rosa E, Santarpia L, Marra M, Sammarco R, Amato V, Onufrio M, et al. Preliminary evaluation of the prevalence of sarcopenia in obese patients from Southern Italy. Nutrition. 2014; [DOI] [PubMed] [Google Scholar]
- 59.Fellner C, Schick F, Kob R, Hechtl C, Vorbuchner M, Büttner R, et al. Diet-Induced and Age-Related Changes in the Quadriceps Muscle: MRI and MRS in a Rat Model of Sarcopenia. Gerontology. 2014;60: 530–538. 10.1159/000360289 [DOI] [PubMed] [Google Scholar]
- 60.Ratto E, Leoncini G, Viazzi F, Vaccaro V, Parodi A, Falqui V, et al. Metabolic syndrome and cardiovascular risk in primary hypertension. J Am Soc Nephrol. 2006;17: S120–2. 10.1681/ASN.2005121328 [DOI] [PubMed] [Google Scholar]
- 61.Stanhope KL, Havel PJ. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol. 2008;19: 16–24. 10.1097/MOL.0b013e3282f2b24a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blachnio-Zabielska A, Baranowski M, Zabielski P, Gorski J. Effect of high fat diet enriched with unsaturated and diet rich in saturated fatty acids on sphingolipid metabolism in rat skeletal muscle. J Cell Physiol. 2010;225: 786–91. 10.1002/jcp.22283 [DOI] [PubMed] [Google Scholar]
- 63.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178: 373–83. Available: http://www.ncbi.nlm.nih.gov/pubmed/16357064 [DOI] [PubMed] [Google Scholar]
- 64.Fujimura Y, Sumida M, Sugihara K, Tsukamoto S, Yamada K, Tachibana H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS One. 2012;7: e37942 10.1371/journal.pone.0037942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim H-S, Quon MJ, Kim J-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. Elsevier; 2014;2: 187–195. 10.1016/j.redox.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tachibana H. Green tea polyphenol sensing. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87: 66–80. 10.2183/pjab.87.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C-T, Chang H-H, Hsiao C-H, Lee M-J, Ku H-C, Hu Y-J, et al. The effects of green tea (-)-epigallocatechin-3-gallate on reactive oxygen species in 3T3-L1 preadipocytes and adipocytes depend on the glutathione and 67 kDa laminin receptor pathways. Mol Nutr Food Res. 2009;53: 349–60. 10.1002/mnfr.200800013 [DOI] [PubMed] [Google Scholar]
- 68.Hong Byun E, Fujimura Y, Yamada K, Tachibana H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol. 2010;185: 33–45. 10.4049/jimmunol.0903742 [DOI] [PubMed] [Google Scholar]
- 69.Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50: 188–210. 10.1002/mnfr.200500109 [DOI] [PubMed] [Google Scholar]
- 70.Derdemezis CS, Kiortsis DN, Tsimihodimos V, Petraki MP, Vezyraki P, Elisaf MS, et al. Effect of plant polyphenols on adipokine secretion from human SGBS adipocytes. Biochem Res Int. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimada M, Mochizuki K, Sakurai N, Goda T. Dietary supplementation with epigallocatechin gallate elevates levels of circulating adiponectin in non-obese type-2 diabetic Goto-Kakizaki rats. Biosci Biotechnol Biochem. 2007;71: 2079–2082. 10.1271/bbb.70174 [DOI] [PubMed] [Google Scholar]
- 72.Wu AH, Yu MC, Stanczyk FZ, Tseng C-C, Pike MC. Anthropometric, dietary, and hormonal correlates of serum adiponectin in Asian American women. Nutr Cancer. 2011;63: 549–557. 10.1080/01635581.2011.551986 [DOI] [PubMed] [Google Scholar]
- 73.Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARγ-dependent and -independent mechanisms. Am J Pathol. Elsevier Inc.; 2011;178: 2690–2699. 10.1016/j.ajpath.2011.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian C, Ye X, Zhang R, Long J, Ren W, Ding S, et al. Green Tea Polyphenols Reduced Fat Deposits in High Fat-Fed Rats via erk1/2-PPARγ-Adiponectin Pathway. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang X, Hu M, Rogers CQ, Shen Z, You M. Role of SIRT1-FoxO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanol-administered mice. Antioxid Redox Signal. 2011;15: 425–435. 10.1089/ars.2010.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Z, Zhou J, Zhou D, Zhu Z, Sun L, Nanji AA. The Adiponectin-SIRT1-AMPK Pathway in Alcoholic Fatty Liver Disease in the Rat. Alcohol Clin Exp Res. 2015;39: 424–433. 10.1111/acer.12641 [DOI] [PubMed] [Google Scholar]
- 77.Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, et al. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60: 3235–3245. 10.2337/db11-0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch W, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4: 485–494. 10.1371/journal.pmed.0040076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee J, Hong S-W, Rhee E-J, Lee W-Y. GLP-1 Receptor Agonist and Non-Alcoholic Fatty Liver Disease. Diabetes Metab J. 2012;36: 262–7. 10.4093/dmj.2012.36.4.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298: G364–G374. 10.1152/ajpgi.00456.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20: 98–105. 10.1097/MOL.0b013e328328d0a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ceddia RB. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol. Elsevier Ireland Ltd; 2013;366: 194–203. 10.1016/j.mce.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 83.Zhang W, Zhang X, Wang H, Guo X, Li H, Wang Y, et al. AMP-activated protein kinase α1 protects against diet-induced insulin resistance and obesity. Diabetes. 2012;61: 3114–25. 10.2337/db11-1373 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Viollet B, Guigas B, Leclerc J, Hébrard S, Lantier L, Mounier R, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf). 2009;196: 81–98. 10.1111/j.1748-1716.2009.01970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21: 507–11. Available: http://www.ncbi.nlm.nih.gov/pubmed/18360697 [PubMed] [Google Scholar]
- 86.Henriksen BS, Curtis ME, Fillmore N, Cardon BR, Thomson DM, Hancock CR. The effects of chronic AMPK activation on hepatic triglyceride accumulation and glycerol 3-phosphate acyltransferase activity with high fat feeding. Diabetol Metab Syndr. Diabetology & Metabolic Syndrome; 2013;5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13: 376–388. 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weng S-Y, Schuppan D. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J Hepatol. 2013;58: 619–21. 10.1016/j.jhep.2012.09.031 [DOI] [PubMed] [Google Scholar]
- 89.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond). 2013;124: 491–507. 10.1042/CS20120536 [DOI] [PubMed] [Google Scholar]
- 90.Seo MS, Kim JH, Kim HJ, Chang KC, Park SW. Honokiol activates the LKB1–AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicol Appl Pharmacol. Elsevier B.V.; 2015;284: 113–124. 10.1016/j.taap.2015.02.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from Figshare at http://dx.doi.org/10.6084/m9.figshare.1580067.