Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy (original) (raw)
. Author manuscript; available in PMC: 2016 Dec 15.
Abstract
Indoleamine 2, 3-dioxygenase 1 (IDO1), IDO2 and tryptophan 2, 3-dioxygenase (TDO) comprise a family of enzymes that catalyze the first- and rate-limiting step associated with the catabolic conversion of tryptophan (Trp) into kynurenine (Kyn). Through subsequent enzymatic and spontaneous reactions, Kyn is further converted into the energetic substrates, NAD+ and ATP to fuel cellular metabolic functions. Coincidently, the depletion of Trp and accumulation of Kyn has been demonstrated to induce effector T cell apoptosis/dysfunction and immunosuppressive regulatory T cell induction, respectively. Similar to other immune checkpoints, IDO1 and TDO are suggested to be important targets for immunotherapeutic intervention. This is represented by the recent growth of efforts to inhibit the Trp to Kyn pathway as a means to control immunosuppression. Inhibitors currently in clinical trials, INCB024360, GDC-0919, Indoximod and an IDO1 peptide-based vaccine, are being evaluated for their efficacy against a wide range of cancers including melanoma, glioblastoma, non-small-cell lung-, pancreatic- and/or breast-cancer, as well as metastatic disease. Despite the rapid development of potent clinical-grade inhibitors, strategic questions remain. Here, we review the state of the literature with respect to current therapeutic inhibitors of tryptophan catabolism, evaluation of those efforts, preclinically and clinically, compensatory changes that occur with therapeutic targeting, as well as newly recognized signaling features that raise critical questions to the field. Given the rapidly evolving interest in determining how IDO1/TDO, and to an unknown extent, IDO2, can be targeted for increasing cancer immunotherapeutic efficacy, we present a brief but comprehensive analysis that addresses critical questions, while highlighting the mechanics that remain to be explored.
Background
Cancer immunology and immunotherapy
The immune system is composed of an immediate-acting innate arm comprised principally of granulocyte- and myeloid-lineage cells that quickly respond to cues of inflammation and/or injury, in addition to an adaptive arm, principally comprised of B and T cells that provide specificity and memory. Under normal circumstances, these immunological arms are mutually-dependent on one another for providing defense against infection, injury and/or malignancy. T cells, which primarily mature following immunological challenge(s), include CD4+ and CD8+ T lymphocytes that express a wide variety of cytokines based on the context of priming stimuli. Included in the CD4+ T cell compartment are highly immunosuppressive regulatory T cells (Treg; CD4+CD25+FoxP3+CTLA-4+) that mature naturally in the thymus (nTreg) or are post-thymically induced from naïve CD4+Foxp3− cells into Foxp3-expressing cells (iTreg) (1–3). With respect to solid cancer(s), immunosuppressive mechanisms utilized to evade anti-tumor immunity include Treg accumulation (4, 5) effector T cell expression of the PD-1 receptor (6), as well as high PD-L1 levels that localize to multiple types of cells in the tumor microenvironment (7, 8). Therefore, an active effort both clinically and preclinically are to develop strategies that re-active a productive antitumor effector T cell response, while simultaneously inhibiting immunosuppressive mechanisms.
Recent studies have demonstrated great promise at targeting immunosuppression in cancer, including clinical trials aimed at inhibiting PD-1, PD-L1 and/or CTLA-4 in patients diagnosed with late-stage melanoma, non-small-cell lung cancer and/or renal-cell cancer (9–12). Follow-up studies have also shown that the benefit of combined PD-1/CTLA-4 inhibition is not restricted to those patients previously treated with systemic therapy (13). Preclinical work using multiple tumor models in immunocompetent mice further confirm that these immune checkpoint-targeted therapies require effector T cells for antitumor activity, with several studies reporting a coincident neutralization of tumor-infiltrating Treg (14–16). These clinical studies, combined with extensive preclinical validation of combinatorial approaches confirm that, immunotherapy is a high-value strategy for treating patients with aggressive and immunosuppressive malignancies.
IDO1, TDO, and the Trp→Kyn catabolic pathway
L-tryptophan (L-Trp) is used in a variety of anabolic/catabolic processes and metabolized into serotonin, melatonin, protein and Kyn. IDO1 and TDO are the primary enyzmes that catalyze the rate-limiting cleavage of the Trp indole ring 2,3-double bond and incorporation of molecular oxygen. The product of this reaction is _N_-formylkynurenine, which is rapidly and spontaneously converted into L-Kyn. The latter catabolite is further converted into downstream intermediates, including 3-hydroxy-L-kynurenine (3-HK), 3-hydroxyanthranilate (3-HAA) and quinolinic acid (Quin), which also impact immune responses (17).
Although IDO1 and TDO both catalyze Trp, their quaternary structures (18, 19), expression in normal versus transformed tissue (20, 21) and regulation (22, 23) are quite distinct. While monomeric IDO1 acts on a broad range of substrates and is capable of cleaving both D- and L-Trp, homotetrameric TDO is enantiomer-specific and only catabolizes L-Trp (24). IDO1 expression in adults is relatively limited to lymphoid tissues and placenta (20), whereas TDO is constitutively expressed in liver and brain (25, 26), likely reflecting their primarily immunomodulatory or energy regulating roles, respectively. Until 2007, IDO1 was the only known indoleamine dioxygenase acting at the 2,3 double bond. Three independent groups then identified the novel paralog, IDO2 (27–29). While the IDO1 and IDO2 genes are 43% homologous and found directly adjacent to one another on chromosome 8, the Km of human IDO1 and IDO2 for L-Trp is 20.90 ± 3.95μM and 6809 ± 917μM, respectively, indicating a substantial decrease in activity for the latter enzyme (30). This is particularly interesting given that the residues required for tryptophan catalytic activity are present in both gene products (27). Also notable is that mouse IDO2 has been shown to possess higher enzymatic activity than the human homolog, although the genetic depletion of mouse IDO2 has no impact on systemic Kyn levels (31); a dramatic contrast to the impact of IDO1-deficiency (32).
IDO1 and the stress response
Due to IDO1 expression induced in response to infection, it was originally thought that it serves as an innate immune effector to restrict the amount of Trp required for microbial growth (33). This initial hypothesis was revised by Munn and Mellor who demonstrated that the in vivo administration of an IDO1 inhibitor, 1-methyl tryptophan (1-MT), led to T cell-dependent fetal allograft rejection (34). Subsequent work demonstrated that IDO1-expressing-macrophages, -dendritic cells (DC) and -tumor cells mediate the inhibition of T cell proliferation (35–38). IDO1 responses were found to be mediated by downstream stress-response pathways including general control non-depressible 2 (GCN2) and mTOR; both important regulators that sense amino acid sufficiency (Figure 1). The GCN2 pathway is activated when amino acid deficiency increases overall uncharged tRNA levels, resulting in GCN2 kinase phosphorylation of the alpha subunit of translation initiation factor 2 alpha (eIF2α) and subsequent inhibition of translation. It was first discovered that GCN2-activated plasmacytoid DC could suppress T cell proliferation in vivo by an IDO1-dependent mechanism (39). It was later discovered that the genetic deletion of IDO1, but not GCN2, prevented skin carcinogenesis in a mouse papilloma model, suggesting that additional critical pathways were downstream of IDO1 activity (40). In support of these findings, Metz et al. identified that IDO1-mediated Trp depletion suppressed mTOR, a critically important immunoregulatory kinase (40) that could be reactivated by treatment with D-1-MT, a Trp mimetic, in vitro.
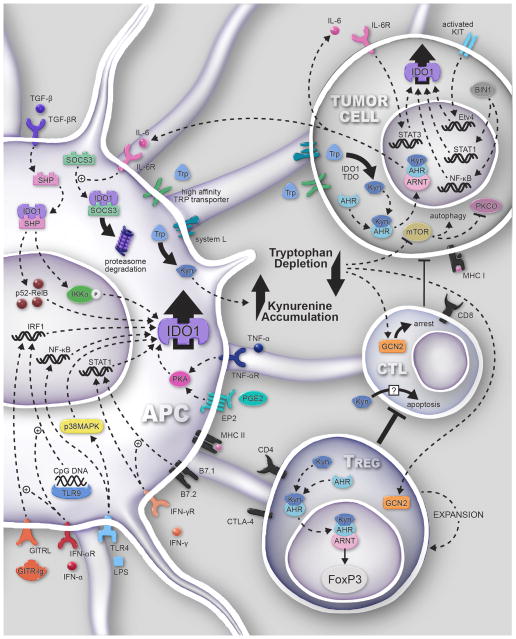
Figure 1. Signaling pathways associated with tryptophan (Trp) dioxygenases and cancer.
The high expression of active IDO1 leads to a commensurately high rate of tryptophan conversion and depletion. This induces cell cycle arrest and/or anergy in the effector cytotoxic lymphocyte (CTL) compartment via the eIF2a kinase-dependent GCN2 pathway. Simultaneously, this mechanism also contributes to the activation/maturation of Treg in association with CTLA4-mediated CD80/CD86 co-inhibition. Kynurenine (Kyn) directly induces the apoptosis of CTL by an uncharacterized mechanism, while interacting with the aryl hydrocarbon receptor (AhR) in naïve CD4+ T cells, resulting in the induction of FoxP3+ iTreg. AhR interacts with the aryl hydrocarbon receptor nuclear translocator (ARNT) to mediate the specific transcriptional programming. Coincidently, IDO1 non-enzymatically enforces immunosuppression through two intrinsic immunoreceptor tyrosine-based inhibitory motifs (ITIM) in antigen presenting cells (APC). TGF-β signaling results in the phosphorylation of the IDO1 ITIM, triggering non-canonical NF-κB activation and phosphorylation of IKKα, followed by nuclear translocation of the NF-κB subunits, p52 and RelB and autocrine reinforcement of IDO1 and TGF-β expression. The high affinity Trp transporter is expressed by APC and tumor cells, with the majority of agonists leading to IDO1 activity demonstrated in APC. Similarly, the oncogene, c-KIT, and tumor-suppressor gene, Bin1, as well as the IL-6/AhR/STAT-3 signaling loop, have also been shown to impact the regulation of IDO1 in tumor cells. (39, 69–76). Notably, in the presence of IDO1/IDO2/TDO, Kyn accumulation simultaneously contributes to Treg activation, promoting the disabling and/or apoptosis of CTL, thereby supporting tumor outgrowth by virtue of an unproductive antitumor response. TGF-βR: transforming growth factor-beta receptor; IL-6R: interleukin-6 receptor; IRF1: interferon response factor 1; GITRL: glucocorticoid-induced TNFR-related protein ligand; IFN-αR: interferon alpha receptor; TLR4: toll-like receptor 4; LPS: lipopolysaccharide; MHC I/II: major histocompatibility complex I/II; EP2: prostaglandin receptor E2; PGE2: prostaglandin E2; TNF-αR: tumor necrosis factor alpha; SHP: SH2 domain containing protein tyrosine phosphatase; SOCS3: suppressor of cytokine signaling 3; mTOR: mammalian target of rapamycin; Etv4: ETS translocation variant 4; STAT: signal transducer and activator of transcription; PKCθ: protein kinase C theta; Bin1: Myc box-dependent-interacting protein 1; GCN2: general control nonderepressible 2; PKA: protein kinase A.
IDO1-mediated suppression of T cell activity is hypothesized to rely on the depletion of free Trp. This premise requires cell-specific transport mechanisms that includes both the transporter System L, which shuttles Trp and other large hydrophobic amino acids through a low affinity (Km = 20–30 μM) (41) interaction, as well as through an independent high affinity (Km = 200–300 nM) interaction. Interestingly, the high affinity transporter is upregulated in differentiated myeloid-derived macrophages (MDM) but not in T cells. In support of the requirement for transport, both Trp and the competitive inhibitor, L-1-MT, inhibit Trp uptake into cells, collectively suggesting that competitive IDO1 inhibitors target the transporter and enzyme, simultaneously.
Regulation of IDO1/IDO2/TDO
The literature is replete with redundant pathways that lead to IDO1 expression and activity. Pro-inflammatory signals including IFN-γ, CpG DNA and LPS are potent inducers of IDO1 expression (33, 42–44). Cytokines, including TNF-α, IL-6 and IL-1β, synergize with each other to dramatically increase IDO1 expression. Other IDO1 modulators include soluble GITR, prostaglandin E2, the oncogene, c-KIT, as well as the tumor suppressor, Bin1 (45). Interesting new data suggests that Wnt5α also mediates IDO1 activity through β-catenin signaling in DC (46), while maintaining continuous expression through an AhR-IL-6-STAT3 signaling loop in some cancer cells lines (47). Thus, based on the large number of pathways that modulate and/or sustain IDO1 expression/activity, the direct targeting of IDO1, rather than pathways that are up- or down-stream, will likely be the most effective modality for controlling the overall impact mediated by this Trp dioxygenase.
Similar to IDO1, TDO mRNA expression has also been found in human tumors (21). Dominant factors that affect TDO expression and/or activity include sex steroid hormones (48) and glucocorticoids (22). New preclinical data also suggest that tumor-infiltrating T cells may regulate TDO expression based on findings from intracranially-injected syngeneic murine brain tumors grown in Rag1−/− mice (15). Notably, intraperitoneally-injected mastocytoma cells overexpressing TDO induces potent immunosuppression that can be reversed with a pharmacological inhibitor of enzymatic activity, leading to immune-mediated tumor rejection (P<0.001) (21).
In contrast, the newest member of the tryptophan catabolic family, IDO2, has yet to be confirmed as a critical contributor to Kyn accumulation and tumor immunity. Notably, while mouse IDO2 possesses some capacity for Trp→Kyn conversion, the human ortholog is devoid of the same enzymatic capacity at physiological Trp levels (30). Furthermore, transcriptome analysis of 129 human tumor samples and 25 human tumor cell lines has demonstrated limited IDO2 expression (49). As IDO2 was originally cloned from liver (27), it is still unknown whether there are IDO2 splice variants specific to subtypes of differentiated- and/or transformed-tissues.
IDO1 and inflammation in tumors
It is notable to highlight the interaction between inflammation, IDO1 and cancer (50, 51), raising critical questions regarding how and when to optimally target tryptophan catabolism for therapeutic purposes. Furthermore, despite the presence of antigen-specific T cells within the microenvironment, tumors often escape, immunologically, without loss of antigen expression or presentation (MHC molecule) capacity. This effect is mediated, in-part, through the induction, upregulation and/or enhanced participation of immunosuppressive T cell-impairing ligands, CTLA-4 and PD-L1 (52). Similar to PD-L1, IDO1 expression also increases through a response to IFN-γ released in the tumor microenvironment (53) as a potent compensatory mechanism contributing to the resistance of productive antitumor immunity (54). Interestingly, only a subset of patients have a T cell infiltrating presence within the tumor microenvironment, an observation reported for head and neck- and bladder-cancer, as well as melanoma, lung adenoma and glioblastoma (55). A notable observation from those patients treated with the immune checkpoint inhibitor, PD-1, correlate a high degree of clinical response to the pre-existence of tumor-infiltrating T cells (56). This observation, paired with the association of IDO1 induction by T cell-derived, IFN-γ, leads to the hypothesis that IDO1 inhibitors will be most effective against T cell-inflamed tumors; either de novo or caused by immunotherapeutic intervention. Preclinical studies support this hypothesis, providing evidence that, combinatorial immune checkpoint blockade and IDO1-pathway inhibition provide potent reactivation of tumor-infiltrating T cells and/or decreased tumor-resident immunosuppressive regulatory T cells (P<0.01) (15, 57).
Clinical-Translational Advances
No IDO1 inhibitor is currently approved by the US Food and Drug Administration. However, results of recent Phase I-II studies suggest that indoximod (D-1-MT), INCB024360 and/or IDO1-targeting vaccines are well tolerated by cancer patients, with clinical anticancer effects in a subset of patients (58, 59). Notably, the number of clinical trials focused on IDO1 has recently grown in size, with many coupling multiple modalities to test the combinatorial benefit (Table 1). These recent reports, in addition to preclinical data suggest that, combining tryptophan enzyme targeting with chemotherapy, radiotherapy and/or immunotherapy, may be an effective tool against a wide range of malignancies.
Table 1. Ongoing and historical clinical trials that target tryptophan catabolism in cancer.
Clinical trials were identified on the website clinicaltrials.gov as of 07/15/2015.
| Agent | Indication(s) | Phase | Status | Notes | Identifier |
|---|---|---|---|---|---|
| Indoximod (D- 1-MT) | Metastatic solid tumor | I | Completed | Combined with docetaxel | NCT01191216 |
| Solid Tumor | I | Completed | Single Agent | NCT00567931 | |
| I | Terminated | Single Agent | NCT00739609 | ||
| Malignant glioma | I/II | Recruiting | For recurrent glioma patients | NCT02052648 | |
| Metastatic Breast Cancer | I/II | Active, not recruiting | Combined with vaccine | NCT01042535 | |
| II | Recruiting | Combined with docetaxel | NCT01792050 | ||
| Melanoma | I/II | Recruiting | Combined with ipilimumab | NCT02073123 | |
| Metastatic Adenoma of Pancreas | I/II | Recruiting | Combined with gemcitabine and nab-paclitaxel | NCT02077881 | |
| Prostate carcinoma | II | Recruiting | Combined with sipuleucel-T | NCT01560923 | |
| NSCLC | II | Not yet recruiting | Combined with docetaxel and tergenpumatucel-L | NCT02460367 | |
| INCB024360 | Advanced neoplasms | I | Completed | As single agent | NCT01195311 |
| Myelodysplastic Syndromes | II | Active, not recruiting | As single agent | NCT01822691 | |
| Melanoma | I/II | Recruiting | Combined with ipilimumab | NCT01604889 | |
| II | Recruiting | Combined with a multipeptide-based vaccine | NCT01961115 | ||
| Reproductive tract tumors | II | Completed | Compared to tamoxifen | NCT01685255 | |
| I | Recruiting | As single agent | NCT02042430 | ||
| I/II | Withdrawn | Combined with vaccine therapy | NCT01982487 | ||
| I | Recruiting | Combined with adoptive transfer of NK cells and IL-2 | NCT02118285 | ||
| I/II | Recruiting | Combined with DC-targeted NY-ESO-1 and poly-ICLC | NCT02166905 | ||
| Solid tumors | I/II | Recruiting | Combined with a PDCD1 mAb | NCT02178722 | |
| I/II | Recruiting | Combined with MEDI4736 (PD-L1 mAb) | NCT02318277 | ||
| Prev. Tx NSCLC | I | Recruiting | Combined with MPDL3280A (PD-L1 mAb) | NCT02298153 | |
| GDC-0919 (formerly NLG-919) | Solid tumors | I | Recruiting | Single agent | NCT02048709 |
| Locally-advanced or metastatic solid tumors | I | Not yet open | Combined with MPDL3280A (PD-L1 mAb) | NCT02471846 | |
| IDO1 peptide | NSCLC | I | Completed | As single agent | NCT01219348 |
| Melanoma | I | Recruiting | Combined with ipilimumab or vemurafenib | NCT02077114 | |
| Melanoma | II | Recruiting | Combined with temozolomide, imiquimod, GM-CSF and survivin peptide | NCT01543464 |
The seminal observation associating IDO1, immunosuppression and cancer utilized a polyclonal antibody to identify the immunohistochemical frequency of expression among different human malignancies (60). Unexpectedly, recent analyses utilizing a novel monoclonal anti-human IDO1 antibody has demonstrated distinct differences compared to those original observations (20). While it was initially reported that, 90–100% of human prostate and pancreatic tumors, as well as glioblastoma, were IDO1 positive, the latter study found only 42%, 38% and 8% of those malignancies positive, respectively. Since the antibodies were well-vetted in both investigations, these conclusions present a cautionary tale that likely reflect more than simple differences in antibody specificity, but more broadly, the potential for alternative splice variants and/or post-translational modifications resulting in antigenic variation. Thus, immunohistochemical studies associating IDO1 expression and survival should be interpreted carefully (61). Furthermore, these conflicting findings complicate strategies that would ideally use IDO1 IHC as a prognostic tool for selecting patients that would benefit most from IDO1 inhibition.
Recent work studying the Kyn/Trp ratio in patients with glioblastoma has suggested that analyzing a time point well after surgical tumor resection, 10+ weeks post-operative, may be prognostically valuable to clinicians planning to enroll patients in immunotherapy (62). While this finding requires further validation in a larger patient cohort, it suggests the possibility that IDO1 activity increases well after GBM patients are operated on, as well as highlights the potential relevance of utilizing a clinical inhibitor against IDO1, systemically. Similarly, the Kyn/Trp ratio was recently validated as a prognostic tool in cervical cancer patients whereby, low Trp levels indicated a tumor size greater than 4cm and metastatic spread to the lymph node (63). Accordingly, high Kyn/Trp ratios in patient sera were associated with lymph node metastasis, FIGO stage, tumor size, parametrial invasion and poor disease-specific survival, further suggesting the relevance of IDO1 targeting based on a tryptophan catabolic signature. Similar work was recently shown in a clinical study that identified higher Kyn/Trp ratios in T-cell lymphotropic virus type-1 asymptomatic carriers when compared to healthy controls (64). Importantly, the serum Kyn/Trp ratio was a significantly independent detrimental prognostic factor in patients with adult T-cell leukemia/lymphoma. These collective analyses have begun to elucidate the relevance of determining an IDO1 enyzmatic ‘signature’ in patient sera, which preliminarily, appears to be both prognostically valuable and clinical-informative.
Given that the majority of clinical studies aimed at IDO1 inhibition that are currently ongoing, have yet to report results, we can gain insight into preclinical analyses that have shown great potential targeting this immunosuppressive mediator. However, these models possess limited usefulness when considering the potential effects that standard of care treatment have on IDO1 activity and/or expression, as well as the potential change of expression between primary and recurrent tumors. Given that inflammation is a primary driver of IDO1 expression, it may be relevant to prognostically stratify tumors that possess a wide range of T cell infiltrating-heterogeneity, when compared to the primary versus relapsed malignancy (65, 66).
Concluding Remarks
Our substantial knowledge of the role and expression of IDO1 in cancer has continued to expand over the past 2 decades, yet critical questions regarding alternative functions regulated by posttranslational modifications, the role that IDO2/TDO plays in the absence or inhibition of IDO1, as well as the impact of tissue-specific alternative splicing, still remain. Most inhibitory strategies against IDO1 focus on disabling enzymatic activity. However, preclinical mouse tumor models suggest that this tactic, alone, will not lead to effective antitumor immunity; further suggesting that IDO1 inhibition is best suited for combinatorial therapeutic strategies. However, these findings also raise the intriguing, yet unproven possibility that, IDO1 subsumes a new/alternative immunosuppressive role when Trp catabolism is abrogated, in vivo. In support of this hypothesis, it’s notable that indoximod (D-1-MT), currently cast as an IDO1 pathway inhibitor, does not inhibit Trp to Kyn catabolism (67, 68) (Supplementary Table S1). This combination of reported observations and untested hypotheses paint a blurry picture of a highly immunosuppressive player in tumor immunity. Unmistakably, IDO1 is a critical mediator that, given the normal limited expression throughout the body, makes it an ideal target for cancer immunotherapy. The central question going forward, thus becomes, how can we best inhibit the activity of this pleiotropic target?
Supplementary Material
1
Acknowledgments
Funding: S. Spranger is supported by a Cancer Research Institute Postdoctoral Fellowship. D.A. Wainwright is supported by PHS grant number R00NS082381, awarded by the NINDS, U.S. Department of Health and Human Services; a Robert H. Lurie Comprehensive Cancer Center – Zell Scholar Program of the Zell Family Foundation Gift; and the Northwestern Brain Tumor Institute.
The authors thank Mr. Michael Gallagher for his expertise in medical illustration and contribution toward creation of Figure 1.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 2.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379–87. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 8.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–9. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–8. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Kang SA, Mukherjee T, Bale S, Crane BR, Begley TP, et al. Crystal structure and mechanism of tryptophan 2,3-dioxygenase, a heme enzyme involved in tryptophan catabolism and in quinolinate biosynthesis. Biochemistry. 2007;46:145–55. doi: 10.1021/bi0620095. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto H, Oda S, Otsuki T, Hino T, Yoshida T, Shiro Y. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc Natl Acad Sci U S A. 2006;103:2611–6. doi: 10.1073/pnas.0508996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015;3:161–72. doi: 10.1158/2326-6066.CIR-14-0137. [DOI] [PubMed] [Google Scholar]
- 21.Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109:2497–502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comings DE, Muhleman D, Dietz G, Sherman M, Forest GL. Sequence of human tryptophan 2,3-dioxygenase (TDO2): presence of a glucocorticoid response-like element composed of a GTT repeat and an intronic CCCCT repeat. Genomics. 1995;29:390–6. doi: 10.1006/geno.1995.9990. [DOI] [PubMed] [Google Scholar]
- 23.Dai W, Gupta SL. Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-gamma. Upstream control region discriminates between interferon-gamma and interferon-alpha. J Biol Chem. 1990;265:19871–7. [PubMed] [Google Scholar]
- 24.Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978;253:4700–6. [PubMed] [Google Scholar]
- 25.Haber R, Bessette D, Hulihan-Giblin B, Durcan MJ, Goldman D. Identification of tryptophan 2,3-dioxygenase RNA in rodent brain. J Neurochem. 1993;60:1159–62. doi: 10.1111/j.1471-4159.1993.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 26.Knox WE, Auerbach VH. The hormonal control of tryptophan peroxidase in the rat. J Biol Chem. 1955;214:307–13. [PubMed] [Google Scholar]
- 27.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJD, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Metz R, DuHadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound d-1-Methyl-Tryptophan. Cancer Res. 2007;67:7082–7. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 29.Yuasa H, Takubo M, Takahashi A, Hasegawa T, Noma H, Suzuki T. Evolution of vertebrate indoleamine 2,3-dioxygenases. J Mol Evol. 2007;65:705–14. doi: 10.1007/s00239-007-9049-1. [DOI] [PubMed] [Google Scholar]
- 30.Pantouris G, Serys M, Yuasa HJ, Ball HJ, Mowat CG. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids. 2014;46:2155–63. doi: 10.1007/s00726-014-1766-3. [DOI] [PubMed] [Google Scholar]
- 31.Metz R, Smith C, DuHadaway JB, Chandler P, Baban B, Merlo LMF, et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26:357–67. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagano J, Shimizu M, Hara T, Shirakami Y, Kochi T, Nakamura N, et al. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet-induced hepatic inflammation. PLoS One. 2013;8:e73404. doi: 10.1371/journal.pone.0073404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–22. [PubMed] [Google Scholar]
- 34.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 35.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–9. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 37.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 39.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–8. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segawa H, Fukasawa Y, Miyamoto K-i, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–51. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 42.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 43.Fallarino F, Volpi C, Zelante T, Vacca C, Calvitti M, Fioretti MC, et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol. 2009;183:6303–12. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- 44.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The Signal transducer and activator of transcription 1α and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-κB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–62. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 45.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holtzhausen A, Zhao F, Evans K, Tsutsui M, Orabona C, Tyler DS, et al. Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunol Res. 2015;3:1082–95. doi: 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–51. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li DD, Gao YJ, Tian XC, Yang ZQ, Cao H, Zhang QL, et al. Differential expression and regulation of Tdo2 during mouse decidualization. J Endocrinol. 2014;220:73–83. doi: 10.1530/JOE-13-0429. [DOI] [PubMed] [Google Scholar]
- 49.van Baren N, Van den Eynde BJ. Tryptophan-degrading enzymes in tumoral immune resistance. Frontiers in Immunology Front Immunol. 2015;6:34. doi: 10.3389/fimmu.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A. 2008;105:17073–8. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller AJ, DuHadaway JB, Chang MY, Ramalingam A, Sutanto-Ward E, Boulden J, et al. Non-hematopoietic expression of IDO is integrally required for inflammatory tumor promotion. Cancer Immunol Immunother. 2010;59:1655–63. doi: 10.1007/s00262-010-0891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. [Google Scholar]
- 53.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 56.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20:221–32. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 59.Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5:8136–46. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 61.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72:1031–8. doi: 10.1227/NEU.0b013e31828cf945. [DOI] [PubMed] [Google Scholar]
- 62.Zhai L, Dey M, Lauing KL, Gritsina G, Kaur R, Lukas RV, et al. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015 Aug 13; doi: 10.1016/j.jocn.2015.06.018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferns DM, Kema IP, Buist MR, Nijman HW, Kenter GG, Jordanova ES. Indoleamine-2,3-dioxygenase (IDO) metabolic activity is detrimental for cervical cancer patient survival. Oncoimmunology. 2015;4:e981457. doi: 10.4161/2162402X.2014.981457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masaki A, Ishida T, Maeda Y, Suzuki S, Ito A, Takino H, et al. Prognostic significance of tryptophan catabolism in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2015;21:2830–9. doi: 10.1158/1078-0432.CCR-14-2275. [DOI] [PubMed] [Google Scholar]
- 65.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gill BJ, Pisapia DJ, Malone HR, Goldstein H, Lei L, Sonabend A, et al. MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc Natl Acad Sci U S A. 2014;111:12550–5. doi: 10.1073/pnas.1405839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Löb S, Königsrainer A, Zieker D, Brücher BDM, Rammensee H-G, Opelz G, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153–7. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–4. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 69.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 70.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–8. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 71.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 72.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–50. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seymour RL, Ganapathy V, Mellor AL, Munn DH. A high-affinity, tryptophan-selective amino acid transport system in human macrophages. J Leukoc Biol. 2006;80:1320–7. doi: 10.1189/jlb.1205727. [DOI] [PubMed] [Google Scholar]
- 74.Silk JD, Lakhal S, Laynes R, Vallius L, Karydis I, Marcea C, et al. IDO Induces Expression of a Novel Tryptophan Transporter in Mouse and Human Tumor Cells. J Immunol. 2011;187:1617–25. doi: 10.4049/jimmunol.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T Cells. J Immunol. 2010;185:3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma MD, Baban B, Chandler P, Hou D-Y, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1