DNMT3AR882H mutant and Tet2 inactivation cooperate in the deregulation of DNA methylation control to induce lymphoid malignancies in mice (original) (raw)
. Author manuscript; available in PMC: 2016 Sep 22.
Published in final edited form as: Leukemia. 2016 Feb 15;30(6):1388–1398. doi: 10.1038/leu.2016.29
Abstract
TEN-ELEVEN-TRANSLOCATION-2 (TET2) and DNA-METHYLTRANSFERASE-3A (DNMT3A), both encoding proteins involved in regulating DNA methylation, are mutated in hematological malignancies affecting both myeloid and lymphoid lineages. We previously reported an association of TET2 and DNMT3A mutations in progenitors of patients with angioimmunoblastic T-cell lymphomas (AITL). Here, we report on the cooperative effect of _Tet2_-inactivation and DNMT3A mutation affecting arginine 882 (DNMT3AR882H) using a murine bone marrow transplantation assay. Five out of 18 primary recipients developed hematological malignancies with one mouse developing an AITL-like disease, 2 mice presenting acute myeloid leukemia (AML)-like and 2 others T cell acute lymphoblastic leukemia (T-ALL)-like diseases within 6 months following transplantation. Serial transplantations of DNMT3AR882H Tet2−/− progenitors led to a differentiation bias toward the T-cell compartment, eventually leading to AITL-like disease in 9/12 serially transplanted recipients. Expression profiling suggested that DNMT3AR882H Tet2−/− T-ALLs resemble those of NOTCH1 mutant. Methylation analysis of DNMT3AR882H Tet2−/− T-ALLs showed a global increase in DNA methylation affecting tumor suppressor genes and local hypomethylation affecting genes involved in the Notch pathway. Our data confirm the transformation potential of DNMT3AR882H Tet2−/− progenitors and represent the first cooperative model in mice involving _Tet2_-inactivation driving lymphoid malignancies.
INTRODUCTION
DNA methylation of CpG dinucleotides is one of the major epigenetic marks in mammals and is known to play crucial roles in cellular processes including imprinting, gene expression regulation and control of differentiation. Mammalian enzymes catalyzing the conversion of cytosine to 5-methylcytosine (5-mC) by transferring a methyl group on the carbon 5 of cytosine belong to the DNA methyltransferase (DNMT) family including DNMT1, DNMT3A, DNMT3B and DNMT3L. Somatic heterozygous mutations in the DNMT3A gene have been reported in myeloid1, 2 and lymphoid malignancies3, and frequently target Arg882 (R882). The functional consequences of this mutant have been analyzed in murine embryonic stem (ES) cells, in which Dnmt3aR878H inhibits wildtype (WT) Dnmt3a and Dnmt3b4. In human, the DNMT3AR882H mutated protein presents as well a dominant-negative activity against DNMT3AWT by preventing the active homotetramer formation5. _Dnmt3a_-null mice present a hematopoietic stem/progenitor cells (HSPC) expansion and differentiation bias toward B-cell compartment upon serial transplantations, those B-lymphocytes retaining high expression of HSC-associated genes6.
Ten-Eleven-Translocation (TET) 2 catalyzes the conversion of 5-mC to 5-hydroxymethylcytosine (5-hmC) and may lead to active DNA demethylation. Loss of function mutations affecting TET2 gene have been described in human hematological malignancies affecting both myeloid and lymphoid lineages7-9. Studies in _Tet2_-inactivated mice revealed a cell-autonomous competitive advantage of hematopoietic progenitors followed by Chronic Myelomonocytic Leukemia (CMML) in a fraction of the animals10. This late malignant development in _Tet2_-inactivated context suggests that cooperative mutations are necessary for full-blown malignancy. Recently such cooperations have been experimentally demonstrated in myeloid transformations in mice11-17.
We previously reported a significant co-occurrence of TET2 and DNMT3A mutations in peripheral T-cell lymphoma (PTCL) patients; particularly frequent in angioimmunoblastic T-cell lymphoma (AITL)3, 18, 19. _TET2_-mutated PTCL show a follicular helper T cell (Tfh) signature and correlate with adverse outcome in patients20.
Here, we explore the functional consequences of TET2 and DNMT3A mutations cooperation in hematopoiesis using a bone marrow transplantation assay (BMT) in which mutant DNMT3AR882H is expressed in _Tet2_-inactivated (Tet2−/−) HSPC. Tet2 inactivation and DNMT3AR882H expression induced T-ALL or AML 6 months after transplantation. T-ALL is associated with hypermethylation and down-regulation of tumor suppressor genes and hypomethylation and up-regulation of Notch1 oncogene. The majority of serially transplanted mice developed an AITL-like disease closely resembling the human disease. Our data constitutes the first cooperative murine model of T-cell malignancies involving Tet2 inactivation.
METHODS
Plasmid construction
Full-length human DNMT3AR882H, NOTCH1L1601PΔP and TCL1A cDNA were subcloned into MSCV-GFP backbone. Retroviral preparations and transduction were performed as previously published21.
Murine bone marrow transplantation
Bone marrow transplantation using 3 months old C57BL/6 WT and Tet2−/− donors were performed as described previously8 leading to MSCV Tet2+/+, DNMT3AR882H Tet2+/+, MSCV Tet2−/− (n=20), and DNMT3AR882H Tet2−/− (n=18) mice. For serial transplantation, HSPC were flow-sorted from whole marrow 16 weeks after transplantation, using GFP+ Lin− Kit+ gating and engrafted with supplemented with 2.5×105 total marrow in lethally irradiated recipients (n=10). Animal experiments were approved by the Gustave Roussy animal care and use committees, according to ARRIVE guidelines.
Cell culture and western blotting
Culture of MO467, R152 and R338 cell lines, western blotting protocols and antibodies are described in Supplementary Methods.
Cell purification and cytometry
Total white blood cells from hematopoietic organs were stained in PBS supplemented with 2% FBS with fluorochrome-conjugated mouse antibodies against specific hematopoietic lineage markers. For the analysis of transplant-receiving mice, WBM was stained with fluorochrome-conjugated mouse antibodies to discriminate cells derived from competitors and donors (CD45.1+ and CD45.2+ respectively) and GFP expression was used to precisely define donor-derived cells (GFP+ CD45.2+). Fluorochrome-conjugated mouse antibodies were obtained from Becton Dickinson (Streptavidin/PeCy5, CD117/PeCy7, CD45.1/PE, CD4/PE or PB, CD8/PeCy7 or APC, CD19/APC, B220/APCCy7, TCRβ/PE, CD279/BV421, CD11b/PerCP-Cy5.5, Annexin V/APC, Ki67/PE) and eBiosciences (CD117/APCCy7, Sca-1/APC, CD45.2/APC, Gr1/PE). Hoescht 33342 was obtained from Invitrogen. APC BrdU Flow kit (Becton Dickinson) was used according to the manufacturer’s instruction. Cell sorting was performed either on a MoFlow (Beckman Coulter) or Influx (Becton Dickinson) cell sorter and analysis on a Canto II (Becton Dickinson). FACS data were analyzed by FlowJo Software (v8.8.7).
Methylation and Hydroxymethylation analyses by MeDIP and hMeDIP sequencing
5-mC and 5-hmC DNA immunoprecipitations of genomic DNA were performed as described22. 200-500 bp genomic DNA fragments were obtained using the bioruptor (Diagenode) and adaptor ligation was performed with the NEBNext DNA sample Prep Master Mix. One μg of adaptor ligated DNA was heat denatured and incubated with an IgG control antibody or with polyclonal 5-hmC22 or monoclonal 5-mC (Eurogentec) antibody. Dynabeads (Invitrogen) were added before immunoprecipitation and elution of DNA was obtained with proteinase K digestion. PCR amplification of immunoprecipitated DNA was performed using index Illumina multiplex primers and single-end sequenced on HiSeq-2000. Reads were aligned to mouse genome mm10 with BWA aln (v0.7.3a) and peak calling assessed with the R package MEDIPS (v1.10.0). Differential analysis of (hydroxy)methylation was done with edgeR and annotation with HOMER (v4.7.2). Differentially (hydroxy)methylated regions with a p-value <0.001 and a FC>1.5 were considered as significant. HOMER was also used for transcription factor binding motif discovery.
Reduced Representation Bisulfite Sequencing (RRBS)
RRBS libraries were prepared as described previously23 with minor modifications. Genomic DNA (50-200ng) was digested for 5 hours with MspI, end-repaired, A tailed and ligated with T4 DNA ligase (Fermentas) to methylated Illumina adaptors. 150-400 bp fragments were gel-purified, bisulfite treated (EpiTect Bisulfite kit, Qiagen) and RRBS libraries were amplified by 15 cycles of PCR with PfUTurbo Cx hotstar DNA polymerase (Agilent) and indexed PE Illumina primers. The libraries were paired-end sequenced (2×75bp) on a HiSeq-2000 to an average of 30 million pairs of reads per sample. Raw reads were cleaned with Trim Galore (v0.2.1) and aligned to the mm10 genome with BSMAP (v2.74). We identified DMRs with the eDMR algorithm from the methylKit R package with the following criteria: at least 3 CpGs, a difference in methylation greater than 20% and an adjusted p-value <0.01. DMRs were annotated using the RefSeq mm10 transcript annotation.
RNAseq
RNA was extracted with the AllPrep kit (Qiagen), quantified as above and checked with Bioanalyzer (Agilent). SureSelect Automated Strand Specific RNA Library Preparation Kit was used according to manufacturer's instructions. Expression analysis was performed by counting the number of reads per gene using HTSeq-count and differential expression was assessed using the R package DESeq. P-values < 0.05 and −2 2 were used as thresholds for differentially expressed genes.
ChIPseq
ChIP analyses were performed according to the MagnaChIP kit (Millipore). Briefly, 10 × 106 MO467 or R152 cells were prepared as previously published24 with normal rabbit IgG (Millipore), H3K4me3 (Diagenode) and H3K27me3 (Millipore) antibodies. Adaptor-ligated libraries were prepared from 10ng of immunoprecipitated DNA using SPRIworks (Beckman Coulter). After indexed PCR amplification, the libraries were pooled and subjected to single-end sequencing. Reads were aligned to mouse genome mm10 with BWA aln (v0.7.5a) and peak calling assessed using MACS2 (v2.0.10.20131216) with a q-value cut-off <0.05. Annotation has been done with HOMER (v4.7.2).
Data access
(h)MeDIP, RRBS, RNAseq and ChIPseq data have been deposited at ArrayExpress under accession numbers E-MTAB-4157 to E-MTAB-4161.
Statistics
Data are depicted as mean ± s.e.m as precised. P-values were calculated with the two-tailed unpaired Student’s t test. P-values for survival curves were calculated using a Log-rank (Mantel-Cox) test. All statistical analyses were performed with Prism software version 6.0 (GraphPad). The size of animal cohorts was based on our previous studies as well as published literature. Neither randomization, nor blinding was used since all animal experiments were performed with homogeneous strain, age, and similar variance.
RESULTS
Impairment of DNMT3AR882H Tet2−/− HSPCs differentiation in vivo
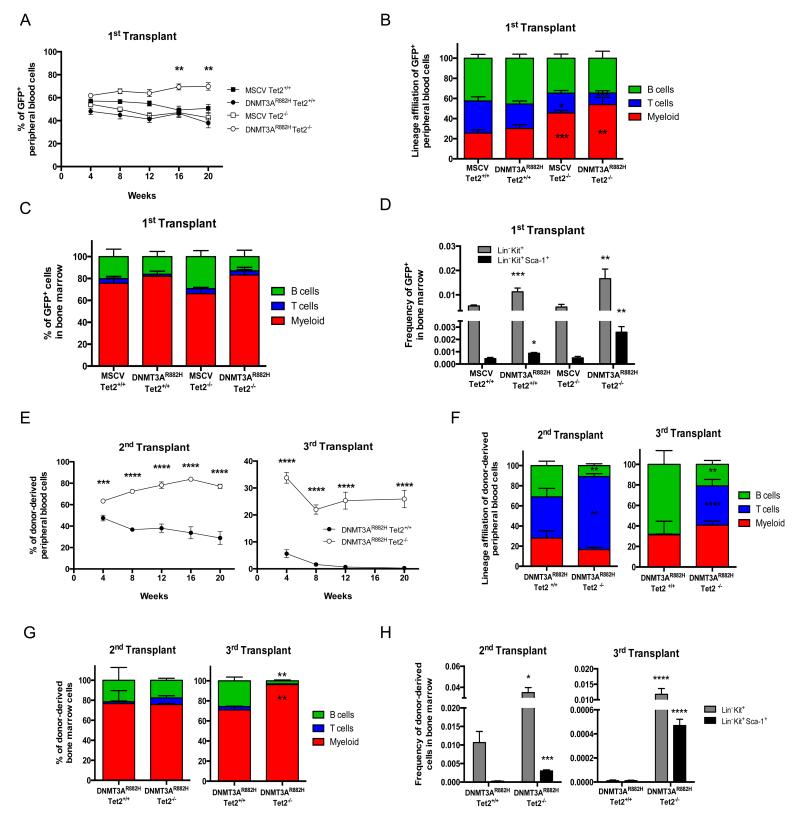
We performed BMT assays using Tet2 wild type (Tet2+/+) or _Tet2_−/− murine HSPC, transduced with _DNMT3AR882H_-expressing retroviruses. Monthly sampling of recipient mice revealed a mild growth advantage for _DNMT3AR882H Tet_2−/− cells over non-transduced Tet2−/− cells, as shown by the constant increase of GFP+ cells in recipients. In contrast, empty vector or DNMT3AR882H expression alone did not confer growth advantage over untransduced cells in a Tet2+/+ context (figure 1A). Engrafted cells showed efficient contribution to mature T-, B- and myeloid cells (figure 1B) although myeloid cells expanded in _Tet_2−/−, as compared to Tet2+/+ context. Contribution to the T-cell lineage was slightly decreased when DNMT3AR882H was expressed as compared to MSCV in the _Tet_2−/− context. The analysis of marrow and thymus of 3 mice per group 4 months after transplantation did not reveal significant difference in lineages proportions (figure 1C) nor in thymic population repartition (figure S1A). However, an increased proportion of Lin− Kit+ (LK) and Lin− Sca+ Kit+ (LSK) cells was observed when DNMT3AR882H is expressed (figure 1D), in keeping with previous reports showing increased contribution of _Dnmt3a_-inactivated cells to the stem/progenitor fraction6.
Figure 1. Proliferative advantage and impaired differentiation potential of DNMT3AR882H Tet2−/− HSPC.
Contribution of 1ary control (MSCV Tet2+/+), DNMT3A mutated (DNMT3AR882H Tet2+/+), _Tet2_-inactivated (MSCV Tet2−/−) and DNMT3A mutated and _Tet2_-inactivated (DNMT3AR882H Tet2−/−) GFP+ HSPC in first transplant to: (A) blood cells showing significant enrichment for GFP+ DNMT3AR882H Tet2−/− after 16 weeks (n= 8 to 20 mice per group); to (B) mature blood lineages at 20 weeks post-transplantation (n = 10 per group) and (C) to lineages at 20 weeks post-transplantation in bone marrow of 1ary transplanted mice (n = 3 per group). Shown are percentages of GFP+ myeloid cells (Mac1+ Gr1+), B cells (CD19+ B220+) and T cells (CD4+ and CD8+) (D) Frequencies of GFP+ Lin− Kit+ and Lin− Kit+ Sca-1+ progenitors in 1ary recipient mice (n = 3 per group). (E) Contribution of donor-derived GFP+ CD45.2+ HSPC to blood cells in 2ary (n = 9) and 3ary (n = 10) transplanted mice after 16 weeks. (F) Lineages contribution of GFP+ HSPC at 20 weeks post-transplantation in blood in 2ary and 3ary transplanted mice (n = 7). (G) Lineage contribution of GFP+ HSPCs at 20 weeks post-transplantation in bone marrow in 2ary and 3ary transplanted mice (n = 3). (H) Frequencies of GFP+ Lin− Kit+ and Lin− Kit+ Sca-1+ progenitors in 2ary and 3ary transplanted mice (n = 3). Mean ± SEM values are shown in graphs. An unpaired Student’s t test was used for statistical analyses. Significant differences in comparison to MSCV Tet2+/+ (figure 1B-D) and DNMT3AR882H Tet2+/+ (figure 1E-H) are indicated with *p< 0.05; **p< 0.01; ***p< 0.001; ****p< 0.0001.
Since _Dnmt3a_-inactivation has been shown to impair HSPC differentiation upon serial transplantation6, we sorted GFP+ LK progenitors of 3 DNMT3AR882H Tet2−/− or Tet2+/+ mice 16 weeks post-transplantation and serially transplanted them into 10 secondary mice. GFP+ LK progenitors from 3 secondary mice were engrafted in 10 tertiary recipients. DNMT3AR882H Tet2−/− cells showed efficient participation to mature blood cells in both secondary and tertiary experiments, indicating their self-renewal capacity, whereas DNMT3AR882H Tet2+/+ progenitors were progressively diluted (figure 1E). Detailed analyses showed that the contribution of the donor-derived cells to mature lineages differs between Tet2 contexts in both secondary and tertiary transplantations (figure 1F). Expression of DNMT3AR882H in Tet2+/+ context induced an increase in the B-cell fraction, in keeping with the reported B-cell bias of _Dnmt3a_-inactivated HSPCs6. Expression of DNMT3AR882H in Tet2−/− progenitors led to an expansion of the T-cell compartment in blood in both secondary and tertiary transplantations. However, DNMT3AR882H Tet2−/− marrow examination did not show significant differences in lineage contribution in secondary transplant (figure 1G, left) while 3ary transplants showed an accumulation of mature myeloid cells in marrow as compared with DNMT3AR882H Tet2+/+ mice (figure 1G, right). As expected for Tet2−/− cells, the proportion of DNMT3AR882H Tet2−/− HSPC in the bone marrow was significantly higher, compared to DNMT3AR882H Tet2+/+ HSPC in secondary and tertiary transplanted recipients (figure 1H). No difference in thymic populations repartition between DNMT3AR882H Tet2−/− and DNMT3AR882H Tet2+/+ secondary recipients was observed (figure S1B). Overall, these results indicate cooperation of the DNMT3AR882H mutant with _Tet2_-inactivation leading to differential accumulation of DNMT3AR882H Tet2−/− cells in hematopoietic organs, with myeloid cells primarily in the marrow and T-cells in the blood.
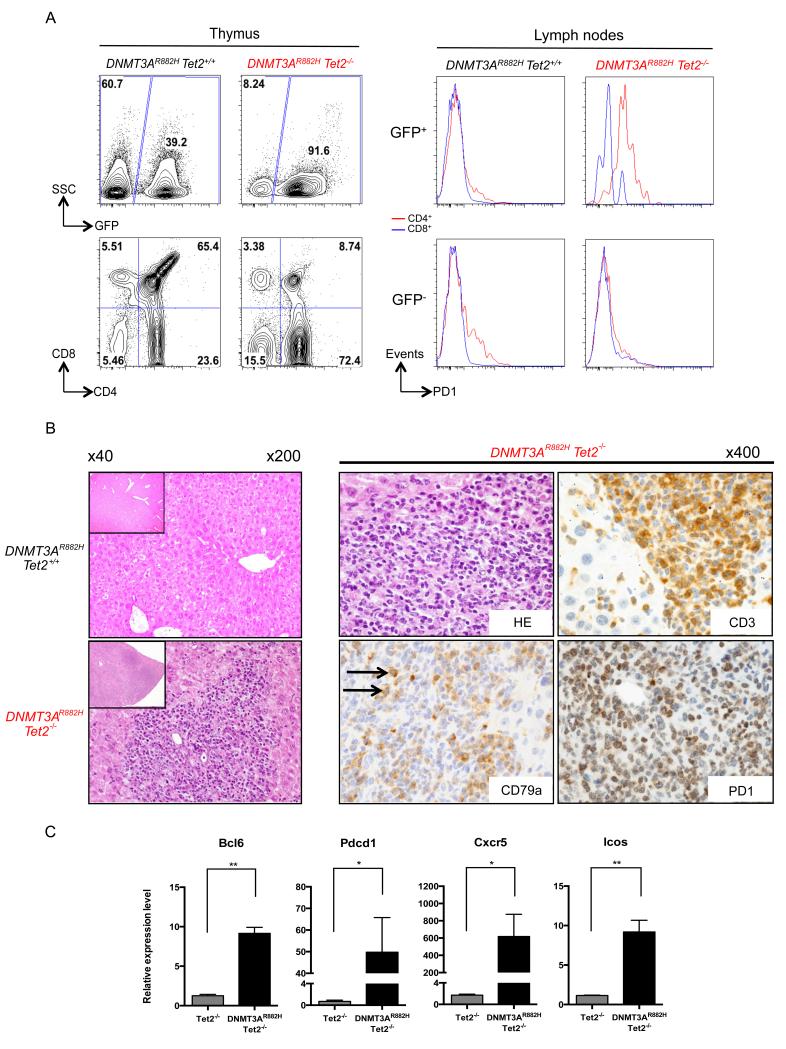
AITL-like development in DNMT3AR882H Tet2−/− serially transplanted engrafted mice
During the course of the monitoring (10 to 16 months after transplantation), we sacrificed 4 (out of 6 secondary) and 5 (out of 6 tertiary) DNMT3AR882H Tet2−/− mice, based on peripheral cytopenia and splenomegaly (Table 1). Analyses identified enlarged thymus, along with an expansion of the CD4+ T-cell population in the thymus (figure 2A, left) and in all hematopoietic organs investigated (data not shown). Since human AITL is associated with high PD1 expression levels, we investigated its expression in DNMT3AR882H Tet2−/− T-cell populations. Cell surface expression of PD1 was observed on GFP+ CD4+ cells in every tissue, including lymph nodes (figure 2A, right). Histopathological analysis of DNMT3AR882H Tet2−/− symptomatic mice showed an infiltration of both the splenic white pulp and the liver by pleomorphic predominantly small to medium lymphoid cells accompanied by eosinophils, plasma cells and a few scattered larger cells resulting in alteration of the normal liver architecture with some focused perivascular infiltrations (figure 2B). The proliferation was essentially composed of CD3+ and PD1+ atypical T-cells, whereas CD79a staining highlighted recruitment of a few large B-lymphocytes in addition to the plasmocytes around T-cell infiltrates, therefore mimicking the pathological and phenotypic features of human AITL. The AITL phenotype was further underscored by the high expression of Bcl6, Pdcd1, Cxcr5 and Icos genes25, 26, in GFP+ CD4+ DNMT3AR882H Tet2−/− cells as compared with normal Tet2+/+ CD4+ thymocytes (figure 2C).
Table 1. Characteristics of symptomatic (AITL) and moribund (T-ALL and AML) mice.
Survival indicates the number of survival days referring to subsequent transplant. Hemoglobin (HB) levels, white blood cell (WBC), red blood cell (RBC) and platelet (PLT) counts are indicated. Infiltration of tissues by GFP+ lymphoid CD4+ CD8+, myeloid Mac1+ Gr1+ Kit+ tumoral of GFP+ CD4+ PD1+ populations was determined by flow cytometry.
| Frequence | Round oftransplantation | Survival(days) | WBC(Kcells/μL) | HB(g/dL) | RBC(Kcells/μL) | PLT(Kcells/μL) | GFP+ tumoralpopulation | Disease | Autopsy |
|---|---|---|---|---|---|---|---|---|---|
| 5/18 | 1ary | 179 | 12,73 | 10,9 | 6,95 | 155 | Mac1+ Gr1+ Kit+ | AML | Hepatosplenomegaly adenopathy |
| 180 | 8,15 | 13 | 8,77 | 723 | Mac1+ Gr1+ Kit+ | AML | Hepatosplenomegaly adenopathy | ||
| 181 | 8,21 | 12,6 | 9,21 | 689 | CD4+CD8+ | T-ALL | Thymoma | ||
| 215 | 1,47 | 15 | 10,08 | 547 | CD4+CD8+ | T-ALL | Mediastinal tumor | ||
| 363 | 4,08 | 14,9 | 6,64 | 456 | CD4+ PD1+ | AITL | Hepatosplenomegaly adenopathy | ||
| 4/6 | 2ary | 302 | 5,45 | 14,2 | 5,91 | 497 | CD4+ PD1+ | AITL | Splenomegaly adenopathy |
| 302 | 3,49 | 14,5 | 7,96 | 449 | CD4+ PD1+ | AITL | Splenomegaly adenopathy | ||
| 462 | 10,55 | 2,8 | 1,57 | 52 | CD4+ PD1+ | AITL | Splenomegaly adenopathy | ||
| 497 | 3,42 | 10,2 | 5,12 | 204 | CD4+ PD1+ | AITL | Splenomegaly adenopathy | ||
| 5/6 | 3ary | 416 | 5,21 | 9,8 | 4,99 | 152 | CD4+ PD1+ | AITL | Splenomegaly adenopathy |
| 461 | 3 | 11,4 | 5,28 | 253 | CD4+ PD1+ | AITL | Splenomegaly adenopathy | ||
| 491 | 5,95 | 11,7 | 5,72 | 855 | CD4+ PD1+ | AITL | - | ||
| 491 | 1,93 | 12,8 | 6,41 | 866 | CD4+ PD1+ | AITL | Enlarged thymus | ||
| 491 | 4,27 | 13,3 | 6,25 | 793 | CD4+ PD1+ | AITL | - |
Figure 2. Development of AITL-like disease in mice serially transplanted with DNMT3AR882H Tet2−/− HSPC.
(A) Flow cytometry analysis of the thymic and nodal cells from 2ary recipient mice. Frequencies of donor-derived GFP+ cells are represented (top, left). CD4+ population is expanded in the GFP+ fraction of DNMT3AR882H Tet2−/− in comparison to DNMT3AR882H Tet2+/+cells (bottom, left). Analysis of nodal cells of 2ary recipient mice show surface expression of PD1 in DNMT3AR882H Tet2−/− GFP+ CD4+ (red) but not in CD8+ (blue) cells (top, right). GFP− cells are shown as controls (bottom, right) (B) Hematoxylin-Eosin staining of liver sections from a 10 months symtomatic DNMT3AR882H Tet2−/− (bottom, left) animal and a DNMT3AR882H Tet2+/+ (top, left) control (original magnification 200). Insets show a smaller magnification of the same section and highlight the disorganized liver structure (original magnification 40). Pathological analysis of liver section from a symptomatic DNMT3AR882H Tet2−/− mouse with Hematoxylin-Eosin (top, middle) showing a dense atypical lymphoid infiltrate, containing a prominent CD3+ T-cell population (top, right) expressing PD1 (bottom, right) admixed with scattered CD79a+ B-cells and plasmocytes (bottom, middle) (original magnification 400). Arrows point at scattered large B-cells recruited around T cells. (C) qRT-PCR validation of Bcl6, Pdcd1, Cxcr5 and Icos genes overexpression in GFP+ CD4+ of AITL mice (n = 2) as compared to CD4+ Tet2−/− thymocytes (n = 3). Each value represents the mean ± SEM. An unpaired Student’s t test was used for statistical analyses. Significant differences between groups are indicated *p< 0.05; **p< 0.01; ***p< 0.001; ****p< 0.0001.
Together, these data showed that 75% (9/12) of mice serially engrafted with DNMT3AR882H Tet2−/− progenitors developed an AITL-like disease.
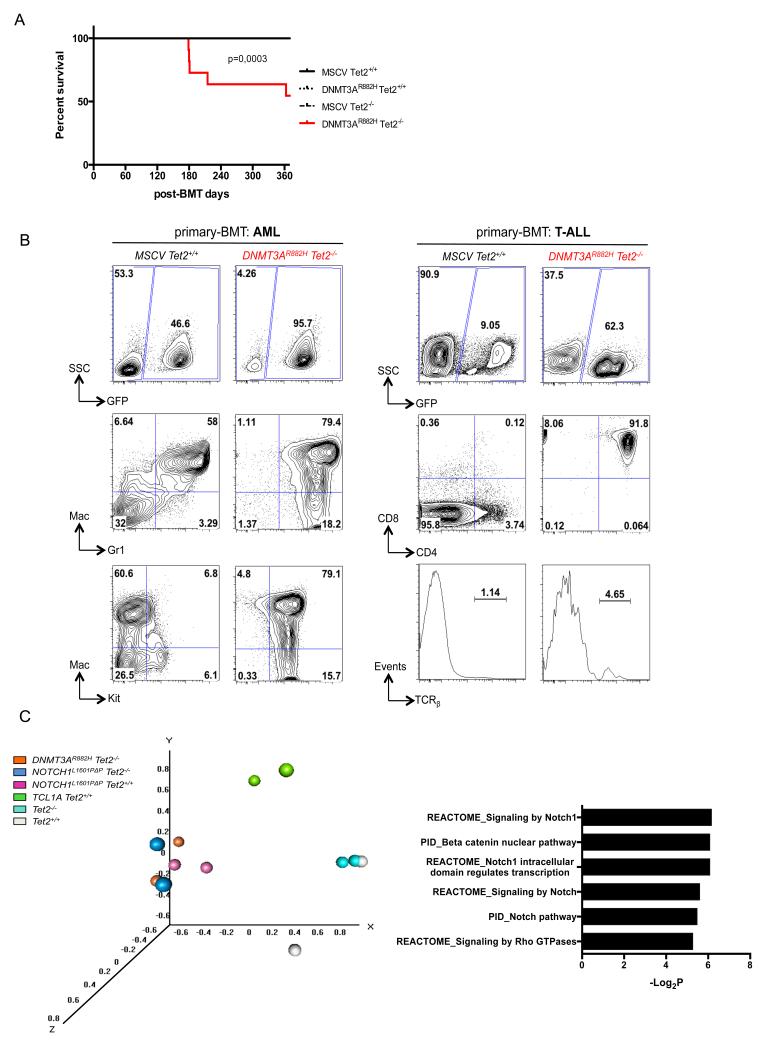
Mice transplanted with DNMT3AR882H Tet2−/− HSPCs die prematurely and develop acute leukemias
We monitored disease development in primary recipients for one year. Tet2+/+, Tet2−/− and DNMT3AR882H Tet2+/+ transplanted mice remained healthy throughout the period, whereas among the 18 DNMT3AR882H Tet2−/− primary engrafted mice, 1 showed symptoms of AITL-like disease, 4 developed AML-like or T-ALL-like diseases, and were euthanized around 6 months post-engraftment (figure 3A and Table 1).
Figure 3. Development of acute leukemia in mice transplanted with DNMT3AR882H Tet2−/− HSPC.
(A) Kaplan-Meier survival curve of 1ary control (MSCV Tet2+/+), DNMT3A mutated (DNMT3AR882H Tet2+/+), _Tet2_-inactivated (MSCV Tet2−/−) and DNMT3A mutated and _Tet2_-inactivated (DNMT3AR882H Tet2−/−) mice (n = 8 to 15 per group). (B) Representative flow cytometry analysis of bone marrow of a 1ary recipient control and an AML mouse (left). Top, percentage of GFP+ cells, middle, Mac1+ and Gr1+ expression in GFP+ cells showing myeloid populations and bottom, Kit+ expression in GFP+ populations are represented. Flow cytometry analysis of bone marrow of a 1ary recipient control and a T-ALL mouse are represented as well (right). Top, percentage GFP+, middle, CD4+ and CD8+ expression in GFP+ cells were further gated showing T-lymphoid cells invasion. Bottom, TCRβ expression in total GFP+ population is represented (C) Principal component analysis of expression profile from T-ALL samples compared to Tet2−/− and Tet2+/+ thymocytes (left) and pathways analyses of genes differentially expressed between DNMT3AR882H Tet2−/− T-ALL and Tet2+/+ thymocytes (right). Complete list provided in Table S2.
The 2 AML-like DNMT3AR882H Tet2−/− mice exhibited hepatosplenomegaly and additional adenopathy (Table 1) consistent with 80% of Mac1+ Gr1+ Kit+ blastic myeloid population in the marrow (figure 3B, left). Secondary transplantation experiments performed with one of the AML sample also confirmed the malignant nature of the tumor (data not shown). Bone marrow from T-ALL-like DNMT3AR882H Tet2−/− recipients exhibited more than 90% of abnormal GFP+ TCRβ− CD4+ CD8+ (double positive, DP) T-cells (figure 3B, right) which induced a similar T-ALL to transplanted recipients (figure S2A). Histopathological analysis showed alteration of the normal liver architecture with significant diffuse T-cell infiltration accompanied with focused perivascular infiltrations (figure S2B). These findings show that DNMT3AR882H Tet2−/− progenitors are predisposed to both myeloid and lymphoid acute transformation in vivo.
Concomitant ablation of Tet2 and overexpression of DNMT3AR882H induce both global and local changes of 5-hmC and 5-mC epigenomic patterns
As the amount of material available from AITL-like mice was not sufficient, we then used DNMT3AR882H Tet2−/− T-ALL models to investigate the mechanism underlying T-cell transformation using expression and methylation analyses. First, we performed transcriptome profiling of DNMT3AR882H Tet2−/− T-ALL samples as well as T-ALL generated by BMT using known T-cell oncogenes: TCL1A27 and NOTCH1L1601PΔP 28. We used DP thymocytes from Tet2−/− and Tet2+/+ mice as a reference (Table S1). Principal component analysis (PCA) based on global expression profiles identified 3 groups: 1- non-transformed Tet2+/+ and Tet2−/− thymocytes, 2- TCL1A-induced T-ALLs and 3-DNMT3AR882H Tet2−/− and NOTCH L1601PΔP T-ALLs (figure 3C, left). Differentially expressed genes between DNMT3AR882H Tet2−/− and Tet2+/+ thymocytes (Table S2) presented a marked overlap with known Notch pathway signatures (figure 3C, right), consistent with their clustering close to NOTCH L1601PΔP T-ALLs samples in the PCA.
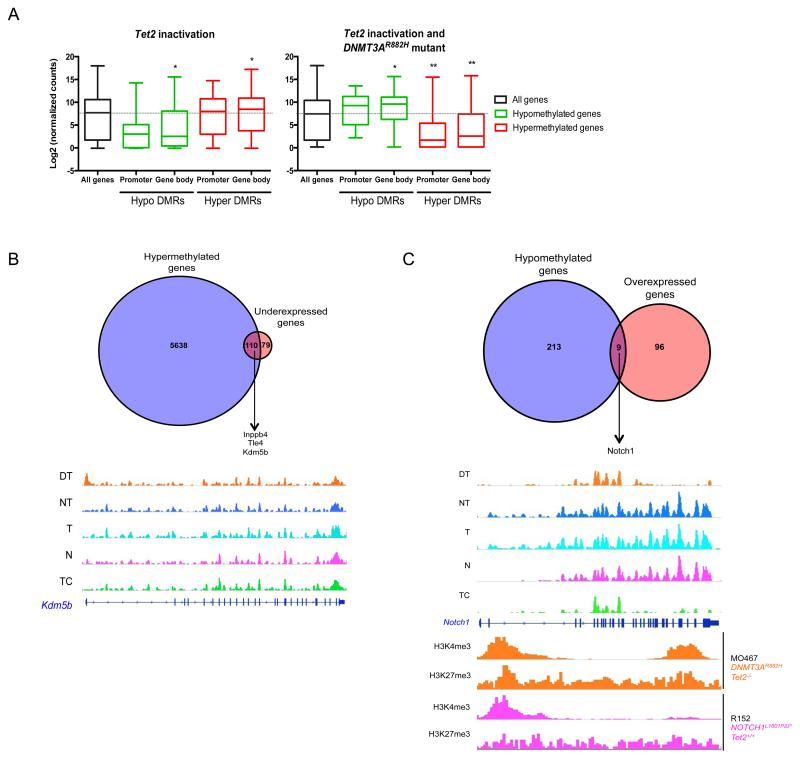
Next, we compared DNA (hydroxy)methylation profiles between DNMT3AR882H Tet2− T-ALLs, normal Tet2−/− thymocytes and other murine T-ALL samples (Table S1). Analyses of the combination of these samples allowed us to associate DNA (hydroxy)methylation changes to _Tet2_-inactivation, DNMT3AR882H mutant or the cooperation of _Tet2_-inactivation and DNMT3AR882H mutant. First, we analyzed hydroxymethylated and methylated DNA immunoprecipitation (hMeDIP and MeDIP, respectively) sequencing data (Table S3 and S4). Global hydroxymethylation is modified upon _Tet2_-inactivation, with almost all differentially hydroxymethylated regions (DhMRs) being hypo-hydroxymethylated (figure 4A, top) and located in intergenic regions (figure S3A) as expected from the proposed function of Tet2 in regulating enhancer activity15, 29. _Tet2_-inactivation was also associated with both hyper (n=1115) and hypo (n=90) differentially methylated regions (DMRs) (figure 4A, bottom). DNMT3AR882H was not associated with hypo-DhMR but with some hyper-DhMRs, suggesting deregulation of the control of DNA hydroxymethylation. Both hyper and hypo DMRs were in greater numbers in DNMT3AR882H than for _Tet2_-inactivation (2929 and 347 respectively), and frequently located in promoter regions for hypermethylation and in gene bodies for hypomethylation. The cooperation between those two previous conditions led to further deregulation of hydroxymethylation, some regions presenting less 5-hmC and some others more. The combination of both _Tet2_-inactivation and DNMT3AR882H mutant presented almost the same number of DMR as compared with DNMT3AR882H mutant alone, and did not markedly affect the repartition within the different genome annotations.
Figure 4. Genome-wide methylation and hydroxymethylation changes in tumoral DNMT3AR882H Tet2−/− cells.
(A) Number of differentially hydroxymethylated regions (DhMRs) (top) and differentially methylated regions (DMRs) (bottom) upon _Tet2_-inactivation, DNMT3AR882H expression and compound _Tet2_-inactivation with DNMT3AR882H mutant expression. (B) Normalized CpGi centered tag density profiles for 5-hmC (top) and 5-mC (bottom) ± 5kb flanking regions upon _Tet2_-inactivation (left) and DNMT3AR882H mutant expression (right). This plot represents the frequency of 5-(h)mC on CpGi among the several T-ALL genotypes and highlights both a hypo-hydroxy and hypermethylation for DNMT3AR882H Tet2−/− samples. Two replicates per genotype were used.
We then specifically analyzed the methylation at CpG islands (CpGi) regions through the mouse genome, by representing the coverage of reads obtained from hMeDIP and MeDIP (figure 4B). _Tet2_-inactivation was not associated with marked changes in 5-hmC or 5-mC contents whereas DNMT3AR882H mutant expression was associated with higher 5-hmC levels and a strong increase in 5-mC. To obtain a more precise analysis of CpG methylation in DNMT3AR882H Tet2−/− tumors, we performed Reduced Representation Bisulfite Sequencing (RRBS) sequencing on the same samples (Table S1). CpG methylation was increased in DNMT3AR882H Tet2−/− samples with respect to other T-ALLs and Tet2−/− samples (figure S3B) and DMRs could be identified between samples (Table S5).
Specific transcriptional changes in tumoral DNMT3AR882H Tet2−/− cells
Mean expression in Tet2−/− context of DMR-associated gene showed statistical differences only when DMRs were located within gene bodies, hyper-DMR being associated with higher expression and hypo-DMR with low expression (figure 5A). With DNMT3AR882H mutant and _Tet2_-inactivation, hyper-DMRs were associated with lower gene expression, whereas hypo-DMRs were associated with higher expression.
Figure 5. Correlation between methylation and transcriptional profiles of DNMT3AR882H Tet2−/− tumoral cells.
(A) Correlation between methylation on promoters or gene bodies and gene expression profiles associated with _Tet2_-inactivation (left), and both _Tet2_-inactivation and DNMT3AR882H mutant expression (right). Significant differences in comparison to all genes are shown * 5e-02 < p > 1e-10; ** 1e-10 < p > 1e-100; *** p < 1e-100. (B) Venn diagram showing hypermethylated and underexpressed genes associated with _Tet2_-inactivation and DNMT3AR882H mutant (top) and Kdm5b promoter hypermethylation in DNMT3AR882H Tet2−/− sample (bottom). Methylation profiles of others T-ALLs and Tet2−/− non-transformed cells are used as controls (Table S1) (C) Venn diagram showing hypomethylated and overexpressed genes associated with Tet2 inactivation and DNMT3AR882H mutant (top) and Notch1 gene body hypomethylation in DNMT3AR882H Tet2−/− sample (bottom). Methylation profiles of others T-ALLs and Tet2−/− non-transformed cells are used as controls (Table S1). Representative ChIPseq H3K4me3 and H3K27me3 marks performed on DNMT3AR882H Tet2−/− cell line MO467 showing gain of H3K4me3 mark in gene body region of Notch1 locus, as compared with Notch1 L1601PΔP Tet2+/+ cell line R152. DT= DNMT3AR882H Tet2−/−, NT= NOTCH1 L1601PΔP Tet2−/−, T= Tet2−/−, N= NOTCH1 L1601PΔP Tet2+/+, TC= TCL1A Tet2+/+, WT= Tet2+/+ (Table S1).
We identified the genes that were statistically both hypermethylated and underexpressed in DNMT3AR882H Tet2−/− cells (Figure 5B, top). 215 methylated CpG regions lie within 110 genes (Table S6). The sequences of the methylated CpG regions significantly showed DNA binding sequences of T-cell transcription factors, Thpok (Zbtb7b), Tcf1 and Tcf7 (figure S4A). Thpok is a key regulator of CD4 lymphocytes differentiation and upregulated in DNMT3AR882H Tet2−/− cells as compared to Tet2−/− cells (data not shown). Tcf1 is a known tumor suppressor in T-cell transformation and a negative regulator of the Notch pathway30-32. Methylation of its target sites may mimic inactivation of the gene itself, as suggested here by the statistically significant down regulation of the β-catenin pathway (Figure 3C). Other tumor suppressor genes, known to be inactivated during hematopoietic transformation such as Inpp4b33, 34 and Tle435 were among the 110 genes that both were hypermethylated and downregulated. An additional example is Kdm5b, which encodes a H3K4 demethylase (figure 5B, bottom).
Nine genes were statistically both hypomethylated and overexpressed (figure 5C, top and Table S7). Two of them, Dtx1 and Notch1 belong to the Notch pathway, which is essential for both normal and malignant T-cell differentiation. The hypomethylated region was located in the 3′ part (figure 5C, bottom) and was associated with Notch1 overexpression in DNMT3AR882H Tet2−/− tumor cells (figure S4B, top). Regarding Dtx1 overexpression (figure S4B, bottom), the hypomethylated regions are also located in the 3′ part of the gene (figure S4C). To assess histone methylation marks associated with transcription activation or repression on Notch1 and Dtx1 loci, we performed ChIPseq on DNMT3AR882H Tet2−/− (MO467) or NOTCH1 L1601PΔP Tet2+/+ (R152) murine T-ALL established cell lines (Table S1). Hypermethylated regions were associated with less H3K4me3 whereas hypomethylated regions were associated with H3K4me3 enrichment (figure 5C bottom and S4D).
Altogether, these results highlight a synergistic effect of Tet2−/− inactivation and DNMT3AR882H overexpression on DNA methylation and gene expression, resulting in both down-regulation of tumor suppressor genes and up-regulation of oncogenes, including Notch pathway genes.
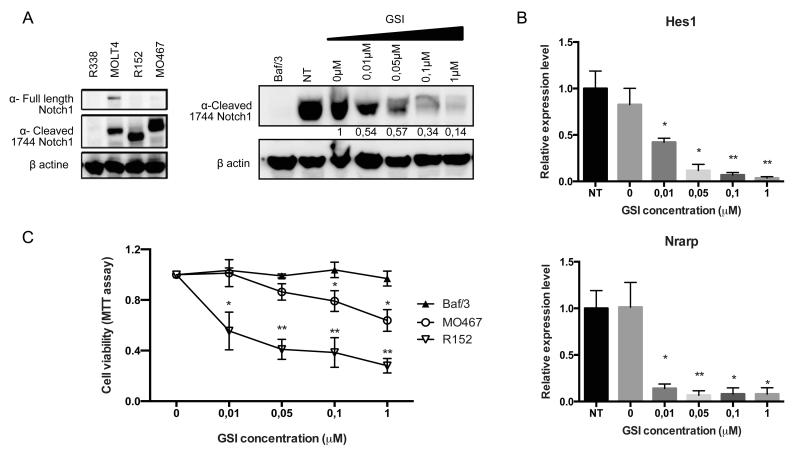
Notch dependency of tumor DNMT3AR882H Tet2−/− cells
To functionally assess the role of the Notch pathway in this transformation process, we studied Notch-dependency of MO467 (DNMT3AR882H Tet2−/−) and R152 (NOTCH1L1601PΔP Tet2+/+) established T-ALL cell lines. Activation of the Notch pathway in MO467 cells was confirmed by the presence of cleaved Notch1 proteins (figure 6A, left). Treatment of the cell lines with increasing amount of a γ-secretase inhibitor (GSI) led to decreased expression of the cleaved form of Notch1 (figure 6A, right) and decreased expression of known Notch1 target genes: such as Hes1 and Nrarp (figure 6B). GSI treatment led to reduced viability/proliferation of DNMT3AR882H Tet2−/− and NOTCH1L1601PΔP Tet2+/+ cells but not of Ba/F3 cells (figure 6C) and was associated with an increased proportion of cells in G0/G1 and decrease of cells in S-phase in both DNMT3AR882H Tet2−/− (figure S5A and 5B) and NOTCH1L1601PΔP Tet2+/+ (data not shown) cell lines. We confirmed these results by treating R158 (NOTCH1L1601PΔP Tet2−/−) and R467 (DNMT3AR882H Tet2−/−) primary cells cultured on MS5 and MS5-DL1 feeders, with another GSI (DAPT). DNMT3AR882H Tet2−/− T-ALL cells proliferate in a Notch-dependent manner similarly to NOTCH1L1601PΔP Tet2−/− T-ALL cells, although they do not respond to classical Notch activation by DL1 ligand (figure S5C). Furthermore, an increased proportion of both apoptotic and G0/G1 DNMT3AR882H Tet2−/− T-ALL cells is observed (figure S5D and E).
Figure 6. Notch dependency of tumoral DNMT3AR882H Tet2−/− cells.
(A) Notch1 full length or cleaved protein levels in murine DNMT3AR882H Tet2−/− cell line MO467 show no full-length Notch1 protein as well as in Notch1 L1601PΔP Tet2+/+ cell line R152 (left). The human T-ALL cell line MOLT4 is used as a positive control and the murine T-ALL cell line R338 (from a _Ezh2_-deficient background) is used as a negative control. Notch1 cleaved protein levels in murine DNMT3AR882H Tet2−/− cell line MO467 show a reduction of protein level among increasing amount of γ-secretase inhibitor doses (right). The murine cell line Baf/3 is used as a negative control. GSI = Gamma secretase inhibitor. (B) qRT-PCR validation of selected Notch1 target genes Hes1 (top) and Nrarp (bottom) downregulation upon γ-secretase inhibitor treatment during 48h. NT= non-treated. Each value represents the mean ± SEM (n = 3) of three independent experiments. (C) MTT assay on murine DNMT3AR882H Tet2−/− cell line MO467 treated during 48h with increasing amount of γ-secretase inhibitor. The murine Baf/3 cell line is used as a negative control and the murine Notch1L1601PΔP Tet2+/+ cell line R152 is used as a positive control. Each value represents the mean ± SEM (n = 3) of three independent experiments. Significant differences in comparison to Baf/3 cells are indicated *p< 0.05; **p< 0.01.
DISCUSSION
Here, we report that concomitant DNMT3AR882H expression and loss of Tet2 in mouse HSPC leads to both myeloid and lymphoid hematological malignancies. The AML-like disease developed by 10% of _DNMT3AR882H Tet2_−/− mice is consistent with the recurrence of these mutations in human AML. The T-lymphoid diseases are more frequent, in keeping with the tight association between these mutations in human AITL. As the abnormal expansion of myeloid and T-cells is observed in different organs, marrow and blood respectively, the microenvironment may play an important role in determining the amplified linages36.
Our model represents the first cooperative murine model involving _Tet2_-inactivation in T-cell malignancies. In many aspects, these results are in accordance with _Dnmt3a_-null mice, which also develop both lymphoid and myeloid malignancies37, 38. We however did not observe B-cell malignancies, in keeping with the human situation: B-lymphoid malignancies essentially lack DNMT3A mutations in human.
The combination of Tet2 loss and DNMT3AR882H expression resulted in high hydroxymethylation and methylation disorder. _DNMT3AR882H_-associated hypermethylation, correlated with low expression of tumor suppressor loci and hypomethylation associated with high expression of oncogene. In our model, among the widespread DNA methylation aberrations, only few genes may have functional impact. This is exemplified by hypomethylation and overexpression of the Notch1 and Dtx1 genes, which results in the activation of the Notch pathway, a major oncogenic pathway in T-cell malignancies. Other abnormalities may sustain T-cell transformation, such as low expression of Tcf1, leading to the downregulation of the Wnt/β catenin pathway, as reported in a fraction of PTCL39, together with methylation of the promoters of target genes. Overexpression of Zbtb7b, may be involved in the generation of hyper DMRs and cellular transformation40.
Finally, the high frequency of DNMT3A mutations in adult T-ALL41, 42 may be associated with NOTCH1 hypomethylation causing high NOTCH1 expression during T-cell differentiation and subsequent occurrence of NOTCH1 activation mutation to induce T-ALL. With age, our mouse model consistently develops an AITL-like disease, a lymphoma occurring in the elderly in human and for which only few mouse models exist43, 44. Given that AITL cells could not be engrafted aside from subsequent progenitors transplantation along with a decrease in the GFP+ proportion, their isolation in sufficient number for functional analyses was precluded. The AITL phase is preceded by an expansion of the CD4+ T-cell compartment, and associated with high Notch1 and Dtx1 expression (data not shown). The link between early expansion of the CD4+ population and subsequent development of AITL-like disorder is at the moment speculative, but may rely on high expression Thpok and of Notch1 during thymocytes differentiation. Recently, immunohistochemical analyses of human samples uncovered NOTCH1 activation in up to 38% of peripheral T cell lymphoma samples45.
The relative low number of transformations observed in primary recipients likely reflects the insufficiency of TET2 and DNMT3A mutations to drive full transformation. The observed bias toward T-cell abnormalities might be due to impaired methylation, which has been suggested to protect from lymphoid differentiation46. Serial transplantations might facilitate epigenetic drift, leading to Notch1 overexpression and CD4+ T-cells accumulation.
Together, our data support the idea that Notch pathway activation represents an important survival signal for abnormal Tfh-cells. Interaction with B-lymphocyte and myeloid cells might substitute for Notch survival signals required by these abnormal Tfh-cells47 and/or for T-cells survival48.
Supplementary Material
Supplemental Information
TableS2
TableS3
TableS4
TableS5
ACKNOWLEDGEMENTS
The authors thank members of the Bernard lab for helpful discussions, Julie Mouillaux for her involvement in this project, Patrick Gonin from the Gustave Roussy animal facility for excellent mouse care as well as Yann Lecluse and Philippe Rameau from the Gustave Roussy Flow Cytometry Core Facility. We also thank the Gustave Roussy Genomic Platform for high throughput sequencing. Work in the lab was supported by grants from INSERM, Institut National du Cancer (INCA), 2013-1-PL BIO-09, INCa-DGOS-INSERM 6043, Ligue Nationale Contre le Cancer (LNCC), Fondation pour la Recherche Médicale (FRM) and Association Laurette Fugain. The work in the lab of KH was supported by the Danish Cancer Society, the European Research Council (294666_DNAMET), the Danish National Research Foundation (DNRF82), and the Novo Nordisk Foundation. LS is supported by fellowships from Cancéropôle Ile de France and Fondation Association pour la recherche sur le Cancer (ARC).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest
Supplementary Information is available at Leukemia’s website
REFERENCES
- 1.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010 Dec 16;363(25):2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nature genetics. 2011 Apr;43(4):309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 3.Couronne L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med. 2012 Jan 5;366(1):95–96. doi: 10.1056/NEJMc1111708. [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ, Zhao H, Hardikar S, Singh AK, Goodell MA, Chen T. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood. 2013 Dec 12;122(25):4086–4089. doi: 10.1182/blood-2013-02-483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russler-Germain DA, Spencer DH, Young MA, Lamprecht TL, Miller CA, Fulton R, et al. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer Cell. 2014 Apr 14;25(4):442–454. doi: 10.1016/j.ccr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012 Jan;44(1):23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009 May 28;360(22):2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 8.Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011 Jul 12;20(1):25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nature genetics. 2009 Jul;41(7):838–842. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 10.Scourzic L, Mouly E, Bernard OA. TET proteins and the control of cytosine demethylation in cancer. Genome Med. 2015;7(1):9. doi: 10.1186/s13073-015-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Schneider RK, Breyfogle LJ, Rosen EA, Poveromo L, Elf S, et al. Distinct effects of concomitant Jak2V617F expression and Tet2 loss in mice promote disease progression in myeloproliferative neoplasms. Blood. 2015 Jan 8;125(2):327–335. doi: 10.1182/blood-2014-04-567024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kameda T, Shide K, Yamaji T, Kamiunten A, Sekine M, Taniguchi Y, et al. Loss of TET2 has dual roles in murine myeloproliferative neoplasms: disease sustainer and disease accelerator. Blood. 2015 Jan 8;125(2):304–315. doi: 10.1182/blood-2014-04-555508. [DOI] [PubMed] [Google Scholar]
- 13.Lobry C, Ntziachristos P, Ndiaye-Lobry D, Oh P, Cimmino L, Zhu N, et al. Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J Exp Med. 2013 Feb 11;210(2):301–319. doi: 10.1084/jem.20121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muto T, Sashida G, Oshima M, Wendt GR, Mochizuki-Kashio M, Nagata Y, et al. Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. J Exp Med. 2013 Nov 18;210(12):2627–2639. doi: 10.1084/jem.20131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen KD, Jia G, Johansen JV, Pedersen MT, Rapin N, Bagger FO, et al. Loss of TET2 in hematopoietic cells leads to DNA hypermethylation of active enhancers and induction of leukemogenesis. Genes Dev. 2015 May 1;29(9):910–922. doi: 10.1101/gad.260174.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih AH, Jiang Y, Meydan C, Shank K, Pandey S, Barreyro L, et al. Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer Cell. 2015 Apr 13;27(4):502–515. doi: 10.1016/j.ccell.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soucie E, Hanssens K, Mercher T, Georgin-Lavialle S, Damaj G, Livideanu C, et al. In aggressive forms of mastocytosis, TET2 loss cooperates with c-KITD816V to transform mast cells. Blood. 2012 Dec 6;120(24):4846–4849. doi: 10.1182/blood-2011-12-397588. [DOI] [PubMed] [Google Scholar]
- 18.Palomero T, Couronne L, Khiabanian H, Kim MY, Ambesi-Impiombato A, Perez-Garcia A, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014 Feb;46(2):166–170. doi: 10.1038/ng.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014 Feb;46(2):171–175. doi: 10.1038/ng.2872. [DOI] [PubMed] [Google Scholar]
- 20.Lemonnier F, Couronne L, Parrens M, Jais JP, Travert M, Lamant L, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012 Aug 16;120(7):1466–1469. doi: 10.1182/blood-2012-02-408542. [DOI] [PubMed] [Google Scholar]
- 21.Malinge S, Ragu C, Della-Valle V, Pisani D, Constantinescu SN, Perez C, et al. Activating mutations in human acute megakaryoblastic leukemia. Blood. 2008 Nov 15;112(10):4220–4226. doi: 10.1182/blood-2008-01-136366. [DOI] [PubMed] [Google Scholar]
- 22.Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011 May 19;473(7347):343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc. 2011 Apr;6(4):468–481. doi: 10.1038/nprot.2010.190. [DOI] [PubMed] [Google Scholar]
- 24.Thiollier C, Lopez CK, Gerby B, Ignacimouttou C, Poglio S, Duffourd Y, et al. Characterization of novel genomic alterations and therapeutic approaches using acute megakaryoblastic leukemia xenograft models. J Exp Med. 2012 Oct 22;209(11):2017–2031. doi: 10.1084/jem.20121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 26.de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007 Jun 1;109(11):4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 27.Virgilio L, Lazzeri C, Bichi R, Nibu K, Narducci MG, Russo G, et al. Deregulated expression of TCL1 causes T cell leukemia in mice. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3885–3889. doi: 10.1073/pnas.95.7.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang MY, Xu L, Shestova O, Histen G, L'Heureux S, Romany C, et al. Leukemia-associated NOTCH1 alleles are weak tumor initiators but accelerate K-ras-initiated leukemia. J Clin Invest. 2008 Sep;118(9):3181–3194. doi: 10.1172/JCI35090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsagaratou A, Aijo T, Lio CW, Yue X, Huang Y, Jacobsen SE, et al. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2014 Aug 12;111(32):E3306–3315. doi: 10.1073/pnas.1412327111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinke FC, Yu S, Zhou X, He B, Yang W, Zhou B, et al. TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4(+) T cell fate and interact with Runx3 to silence Cd4 in CD8(+) T cells. Nat Immunol. 2014 Jul;15(7):646–656. doi: 10.1038/ni.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S, Xue HH. TCF-1 mediates repression of Notch pathway in T lineage-committed early thymocytes. Blood. 2013 May 9;121(19):4008–4009. doi: 10.1182/blood-2013-01-477349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staal FJ, Clevers H. Tales of the unexpected: Tcf1 functions as a tumor suppressor for leukemias. Immunity. 2012 Nov 16;37(5):761–763. doi: 10.1016/j.immuni.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Li Chew C, Lunardi A, Gulluni F, Ruan DT, Chen M, Salmena L, et al. In Vivo Role of INPP4B in Tumor and Metastasis Suppression through Regulation of PI3K-AKT Signaling at Endosomes. Cancer Discov. 2015 Jul;5(7):740–751. doi: 10.1158/2159-8290.CD-14-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kofuji S, Kimura H, Nakanishi H, Nanjo H, Takasuga S, Liu H, et al. INPP4B Is a PtdIns(3,4,5)P3 Phosphatase That Can Act as a Tumor Suppressor. Cancer Discov. 2015 Jul;5(7):730–739. doi: 10.1158/2159-8290.CD-14-1329. [DOI] [PubMed] [Google Scholar]
- 35.Greif PA, Eck SH, Konstandin NP, Benet-Pages A, Ksienzyk B, Dufour A, et al. Identification of recurring tumor-specific somatic mutations in acute myeloid leukemia by transcriptome sequencing. Leukemia. 2011 May;25(5):821–827. doi: 10.1038/leu.2011.19. [DOI] [PubMed] [Google Scholar]
- 36.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer cell. 2008 Jun;13(6):483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV, et al. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood. 2015 Jan 22;125(4):629–638. doi: 10.1182/blood-2014-08-594648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters SL, Hlady RA, Opavska J, Klinkebiel D, Pirruccello SJ, Talmon GA, et al. Tumor suppressor functions of Dnmt3a and Dnmt3b in the prevention of malignant mouse lymphopoiesis. Leukemia. 2014 May;28(5):1138–1142. doi: 10.1038/leu.2013.364. [DOI] [PubMed] [Google Scholar]
- 39.Dorfman DM, Greisman HA, Shahsafaei A. Loss of expression of the WNT/beta-catenin-signaling pathway transcription factors lymphoid enhancer factor-1 (LEF-1) and T cell factor-1 (TCF-1) in a subset of peripheral T cell lymphomas. Am J Pathol. 2003 May;162(5):1539–1544. doi: 10.1016/s0002-9440(10)64287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HO, He X, Mookerjee-Basu J, Zhongping D, Hua X, Nicolas E, et al. Disregulated expression of the transcription factor ThPOK during T-cell development leads to high incidence of T-cell lymphomas. Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7773–7778. doi: 10.1073/pnas.1424104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossmann V, Haferlach C, Weissmann S, Roller A, Schindela S, Poetzinger F, et al. The molecular profile of adult T-cell acute lymphoblastic leukemia: mutations in RUNX1 and DNMT3A are associated with poor prognosis in T-ALL. Genes Chromosomes Cancer. 2013 Apr;52(4):410–422. doi: 10.1002/gcc.22039. [DOI] [PubMed] [Google Scholar]
- 42.Neumann M, Heesch S, Schlee C, Schwartz S, Gokbuget N, Hoelzer D, et al. Whole-exome sequencing in adult ETP-ALL reveals a high rate of DNMT3A mutations. Blood. 2013 Jun 6;121(23):4749–4752. doi: 10.1182/blood-2012-11-465138. [DOI] [PubMed] [Google Scholar]
- 43.Ellyard JI, Chia T, Rodriguez-Pinilla SM, Martin JL, Hu X, Navarro-Gonzalez M, et al. Heterozygosity for Roquinsan leads to angioimmunoblastic T-cell lymphoma-like tumors in mice. Blood. 2012 Jul 26;120(4):812–821. doi: 10.1182/blood-2011-07-365130. [DOI] [PubMed] [Google Scholar]
- 44.Muto H, Sakata-Yanagimoto M, Nagae G, Shiozawa Y, Miyake Y, Yoshida K, et al. Reduced TET2 function leads to T-cell lymphoma with follicular helper T-cell-like features in mice. Blood Cancer J. 2014;4:e264. doi: 10.1038/bcj.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kluk MJ, Ashworth T, Wang H, Knoechel B, Mason EF, Morgan EA, et al. Gauging NOTCH1 Activation in Cancer Using Immunohistochemistry. PLoS One. 2013;8(6):e67306. doi: 10.1371/journal.pone.0067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009 Nov;41(11):1207–1215. doi: 10.1038/ng.463. [DOI] [PubMed] [Google Scholar]
- 47.Auderset F, Schuster S, Fasnacht N, Coutaz M, Charmoy M, Koch U, et al. Notch signaling regulates follicular helper T cell differentiation. J Immunol. 2013 Sep 1;191(5):2344–2350. doi: 10.4049/jimmunol.1300643. [DOI] [PubMed] [Google Scholar]
- 48.Fasnacht N, Huang HY, Koch U, Favre S, Auderset F, Chai Q, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. J Exp Med. 2014 Oct 20;211(11):2265–2279. doi: 10.1084/jem.20132528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information
TableS2
TableS3
TableS4
TableS5