The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node (original) (raw)
. Author manuscript; available in PMC: 2016 Jun 6.
Abstract
ICAM-1 (CD54) plays an important role in the cell-cell interaction and migration of leukocytes. Previous studies have shown that ICAM-1 is involved in inflammatory reactions and that a defect in ICAM-1 gene inhibits allergic contact hypersensitivity. This study indicates that the migration of hapten presenting Langerhans cells into the regional lymph nodes was significantly reduced in ICAM-1-deficient mice compared to wild-type C57BL/6 mice. The reduced number of dendritic cells in regional lymph nodes did not result from abnormal migration of Langerhans cells into the skin of ICAM-1-deficient mice. The concentration and distribution of Langerhans cells in the naïve skin of ICAM-1-deficient mice was equal to that of wild-type mice. Following hapten sensitization, Langerhans cell migration out of the skin and recruitment of fresh Langerhans cells back to the epidermis was not affected in ICAM-1-deficient mice. Further experiments demonstrated that ICAM-1 deficiency on lymphatic endothelium rather than on dendritic cells was responsible for the reduced migration of Langerhans cells into draining lymph nodes. This study indicates that ICAM-1 regulates the migration of dendritic cells into regional lymph nodes but not into or out of the skin.
Keywords: Dendritic cell, ICAM-1 molecule, Migration
1 Introduction
The indispensable role of dendritic cells (DC) in the development of immune responses is well accepted. These cells originate in the bone marrow and migrate through the blood to peripheral tissues where they reside for long periods of time. Langerhans cells, a subpopulation of immature DC present in the suprabasal layer of the epidermis, are strategically located to initiate immune responses to antigens encountered in the skin. Upon antigen stimulation, Langerhans cells capture antigens and migrate into the regional lymph nodes where they activate antigen specific T cells [1, 2]. The migration of DC from the bone marrow into peripheral tissues and then to lymphoid organs following encounter with antigen is a complex series of events that are regulated by an assortment of chemokines, cytokines and adhesion molecules, many of which are induced or increased by encounter with antigen [3, 4]. The adhesion molecules, E-cadherin [5], CLA, P- and E-selectins [6], β1 integrins [7] and ICAM-1 (CD54) [8] have all been implicated in DC migration into, out of, or retention in the skin.
ICAM-1 is a cell surface glycoprotein that is a member of the immunoglobulin superfamily. It is constitutively expressed on vascular and lymphatic endothelial cells, and monocytes and can be induced by the cytokines IL-1, TNF-α and interferon-γ on epidermal keratinocytes and a multiplicity of other cells [9, 10]. The induction of ICAM-1 on keratinocytes is attributed to the recruitment of leukocytes into the skin and development of cutaneous inflammation [11]. Low levels of ICAM-1 are present on Langerhans cells in the skin. As they mature following encounter with antigens, ICAM-1 expression dramatically increases [12]. Through its interaction with the ligand CD11a and CD11b, ICAM-1 mediates adhesive interactions between leukocytes to the vascular and lymphatic endothelium, a process which facilitates their transendothelial migration into tissues [9, 10, 13]. Inflammatory stimuli increase the expression of ICAM-1, which is one of mechanisms for the infiltration of leukocytes in inflammatory sites [14].
In ICAM-1-deficient mice, hapten induced contact hypersensitivity responses in the skin are reduced [15]. Multiple mechanisms may contribute to this defect in the cutaneous inflammation. The migration of hapten carrying Langerhans cells, the initial step for the induction of contact hypersensitivity, may be one factor. To address this issue, ICAM-1-deficient mice were used to examine its role in the migration of Langerhans cell precursors in the skin and draining lymph nodes following contact with hapten. The results indicate that ICAM-1 regulates Langerhans cell migration into the draining lymph nodes, but does not affect their transit into the skin, suggesting different mechanisms for the migration into these two sites.
2 Results
2.1 The migration of hapten-labeled Langerhans cells into the draining lymph nodes is inhibited in ICAM-1-deficient mice
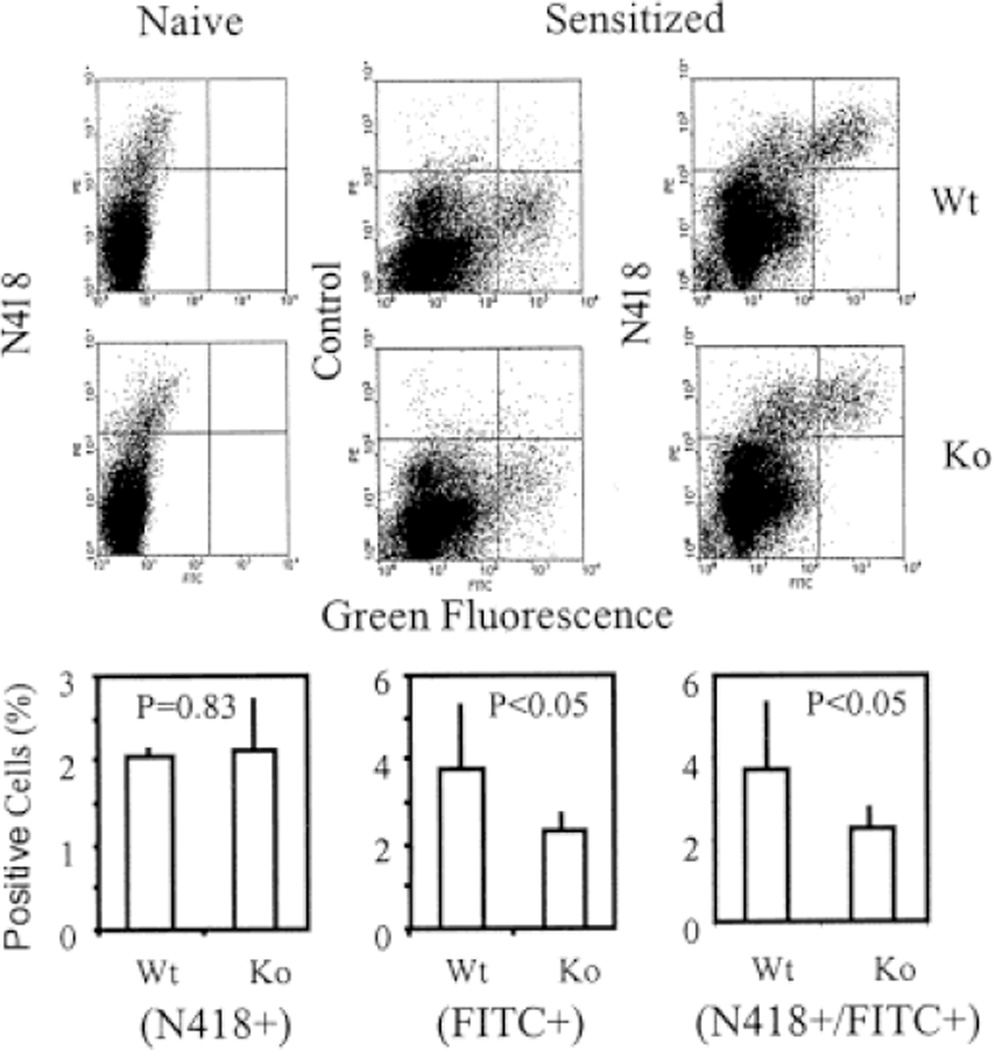
Preliminary studies confirmed previous reports that sensitization of ICAM-1-deficient mice with haptens DNFB and FITC resulted in a deficient contact hypersensitivity response (data not shown). To determine whether the migratory pattern of dendritic cells might in part be responsible for the altered immune reactivity of these mice, ICAM-1-deficient and wild-type C57BL/6 mice were sensitized with FITC and the draining lymph nodes were taken 22–24 h later for analysis of FITC-labeled Langerhans cells by flow cytometry. ICAM-1-deficient mice had a significantly lower level of FITC-labeled cells in the draining lymph nodes than the wild-type mice (2.3±0.45% vs. 3.8±1.5%, p < 0.05). Most of the FITC-labeled cells were stained by anti-CD11c antibody N418, indicating that the hapten-labeled cells were mature DC (Fig. 1). The level of N418/FITC-double-positive cells was also significantly lower in ICAM-1-deficient mice than in the wild-type mice (2.3±0.5% _vs._ 3.7±1.7%, p <.05). The number of N418-positive cells in the lymph node of naive ICAM-1-deficient and wild-type mice was equivalent (2.14±0.6% _vs._ 2.06±0.1%, _p_ > 0.05).
Fig. 1.
Flow cytometry analysis on hapten-labeled and resident DC in the regional lymph nodes of ICAM-1-deficient and wild-type mice. Lymph node cells of FITC-sensitized or naive mice were stained with PE-labeled CD11c (N418) or control antibodies. The profiles indicate N418(PE)+ resident DC (upper left), hapten FITC-labeled cells (lower right), and PE/FITC-double-positive cells (upper right). The bar graphs indicate the average percentage of the positive cells in each experimental group with at least six mice. The statistical analysis indicates that the number of FITC-positive and FITC/PE-double-positive cells was significantly reduced in hapten-sensitized ICAM-1-deficient mice (p < 0.05).
Kinetics of DC migration into draining lymph nodes was compared in ICAM-1-deficient and wild-type mice following hapten sensitization. As shown in Table 1, the number of hapten-labeled migratory DC in the draining lymph nodes of both mouse strains peaked at 24 h and rapidly declined at 48 h. In our hands, hardly any N418-positive hapten-labeled DC could be detected in the draining lymph nodes after 48 h. Although there was variation, the difference in migration between ICAM-1-deficient and wild-type mice was seen at all tested time points. A change in the kinetic of DC migration in ICAM-1-deficient mice was not evident.
Table 1.
Kinetics of Langerhans cell migration into draining lymph nodesa)
| Mice | Positive cell number/Lymph node (× 104) | ||
|---|---|---|---|
| 24 h | 36 h | 48 h | |
| C75BL/6 | 12.6 ± 0.25 | 11.7 ± 1.6 | 6.8 ± 0.65 |
| ICAM-1Ko | 8.8 ± 0.25 | 9.25 ± 0.9 | 4.6 ± 1.05 |
2.2 The migration of Langerhans cells into and out of the skin is normal in ICAM-1-deficient mice
To determine whether the reduced migration of hapten-labeled Langerhans cells in the regional lymph nodes resulted from a reduced number of Langerhans cells in the skin of ICAM-1-deficient mice, the epidermis of wild-type and ICAM-1-deficient mice was stained with anti-MHC class II antibody and the number of positive cells was determined. The result indicates that the density of Ia-positive Langerhans cells in the skin of naive ICAM-1-deficient and wild-type mice was comparable (1,311±94.6 vs. 1,151±70.7 cells/mm2, p >.05). In addition, the morphology of Langerhans cells in the skin of ICAM-1-deficient mice was normal (Fig. 2). These findings indicate that the migration of Langerhans cells into the epidermis under naïve conditions does not require ICAM-1.
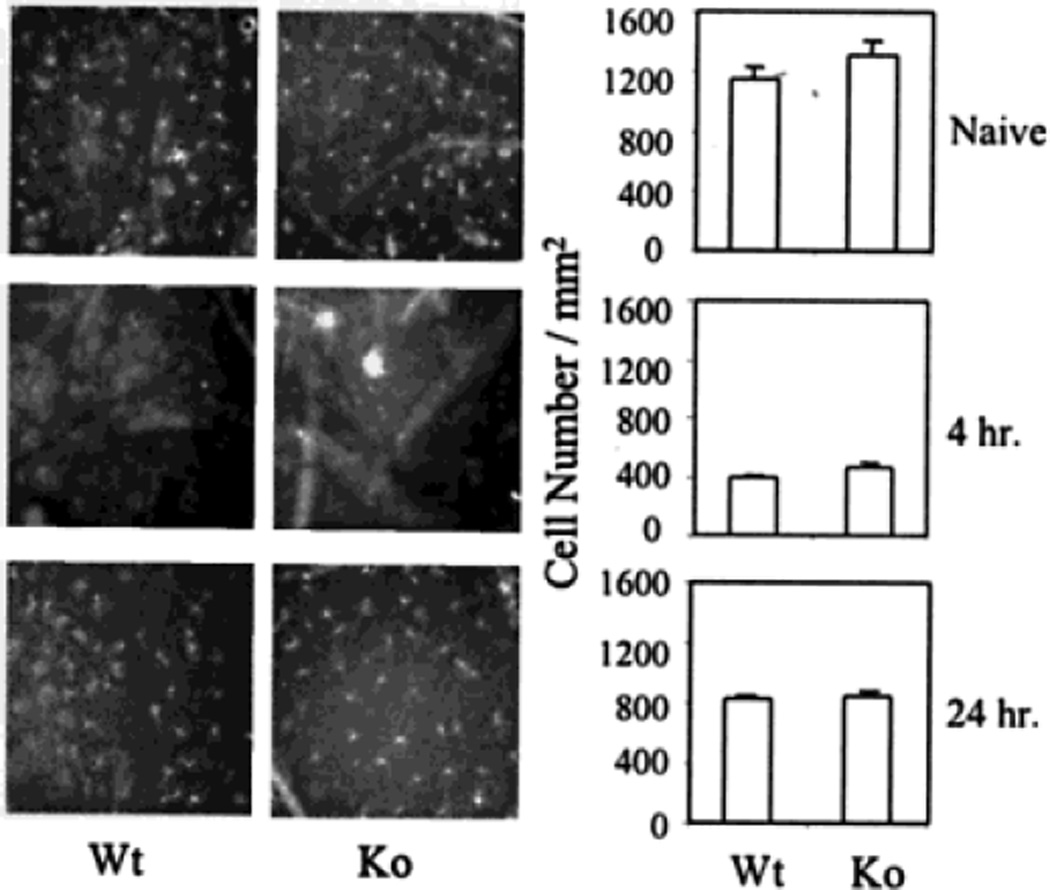
Fig. 2.
Immunohistochemical staining of epidermal Langerhans cells. The epidermis of naive or hapten-treated ICAM-1-deficient and wild-type mice was stained with FITC-labeled anti-Iab antibody. The photos indicate MHC class II+ Langerhans cells in the naïve and hapten-treated epidermis at 4 or 24 h following hapten application. The bar graphs indicate the average number of MHC class II+ Langerhans cells per mm2 in each experimental group with six mice. The difference between wild-type C57BL/6 and ICAM-1-deficient mice is not statistically significant at the indicated times.
Studies were next conducted to determine whether the reduction in lymph node DC in ICAM-1-deficient mice was caused by an inability of Langerhans cells to emigrate out of the skin. Wild-type and ICAM-1-deficient mice were hapten-sensitized, after which the density of Langerhans cells in the epidermis was assessed by immunohistochemistry. Preliminary studies revealed that the greatest reduction in epidermal Langerhans cells in normal mice occurred at four hours (data not shown). No significant difference was observed in epidermal Ia-positive Langerhans cells between ICAM-1-deficient and wild-type mice (463±31.1 vs. 394±17.7 cells/mm2, p >.05). Other time points were also examined to exclude the possibility that ICAM-1 interfered with the kinetics of emigration of hapten-sensitized Langerhans cells out of the epidermis. No significant differences in Langerhans cell densities were noted at these other time points either. Thus, the reduction in the densities of DC in regional lymph nodes in ICAM-1-deficient mice could not be attributed to an inability of Langerhans cells to migrate out of the epidermis.
Further experiments were conducted to examine the effect of ICAM-1 deficiency on the re-population of Langerhans cells in the epidermis after hapten-sensitization. Mice were sensitized with DNFB and the epidermis was taken for analysis of Ia-positive Langerhans cells 24 h later. As shown in Fig. 2, the density of Ia-positive Langerhans cells in the epidermis had significantly recovered by 24 h. There was no significant difference in the number of Langerhans cells in ICAM-1-deficient and wild-type mice 24 h after hapten sensitization (854±27.7 vs. 831±22.7 cells/mm2, p >.05). This result provides additional evidence that the migration of Langerhans cells into the epidermis is not regulated by ICAM-1 under either naive or inflammatory conditions.
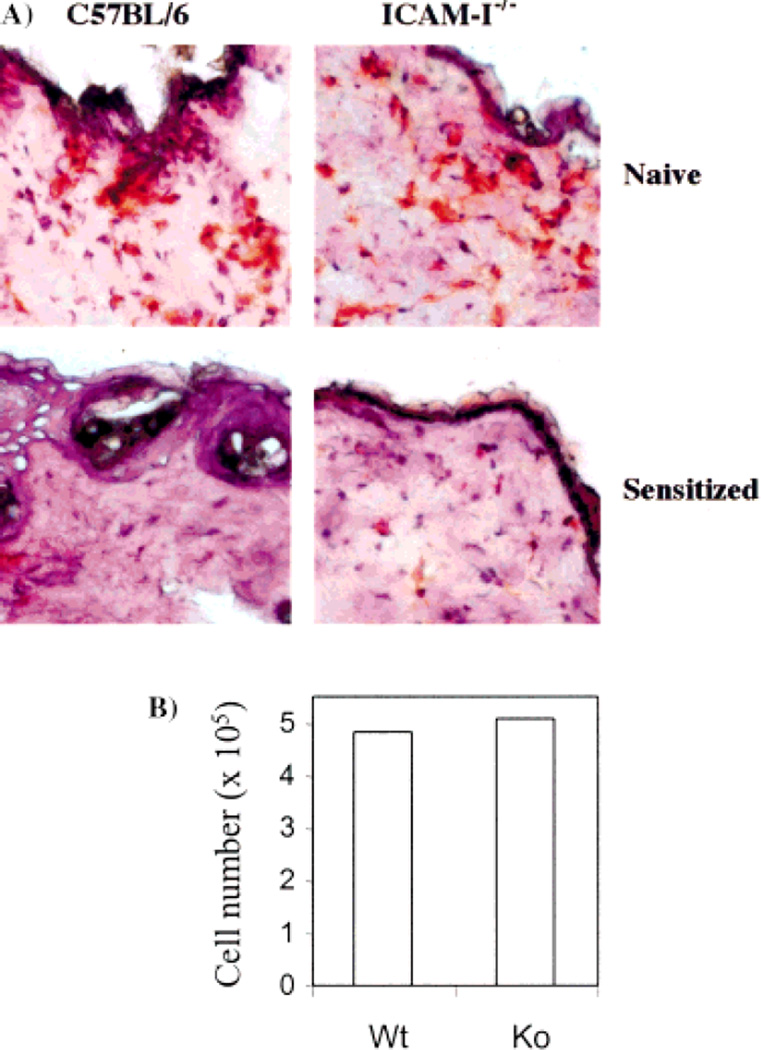
Finally, the migration of Langerhans cells in the dermis was examined. Following hapten sensitization, the number of class II-positive cells in the dermis was greatly reduced at 4 h, like in the epidermis. No significant difference was found in ICAM-1-deficient and wild-type mice (Fig. 3A), suggesting that Langerhans cells were not retained in the dermis after leaving the epidermis following hapten sensitization.
Fig. 3.
The effect of ICAM-1 deficiency on DC migration out of the skin. (A) Immunohistochemical staining on skin cross-sections with anti-Iab antibody. Mice were sensitized with hapten and the skin was taken 4 h later to prepare frozen cross-sections. The photos show representative results of four mice in each group. (B) DC migration out of skin explants in cultures. Mice were sensitized on ears and hapten-treated skin samples were taken 2 h later and placed in cultures. The migratory cells were harvested and counted 3 days after the culture. The result shows the total cell number from five mice (ten ears) of each group.
In further experiments, mice were hapten-sensitized on ears and the skin explants were prepared from the treated dorsal side of ears. The explants were placed in culture and the number of Langerhans cells that migrated out of the explant was determined. These cells contained about 70% CD11c–positive DC. Fig. 3B shows the result of ten mice in each group. Langerhans cells migrated out of the skin explants at a similar level in both mouse strains. This further confirms that ICAM-1 deficiency has no effect on Langerhans cell migration out of the skin.
2.3 ICAM-1 in lymph nodes is important for migration of Langerhans cells
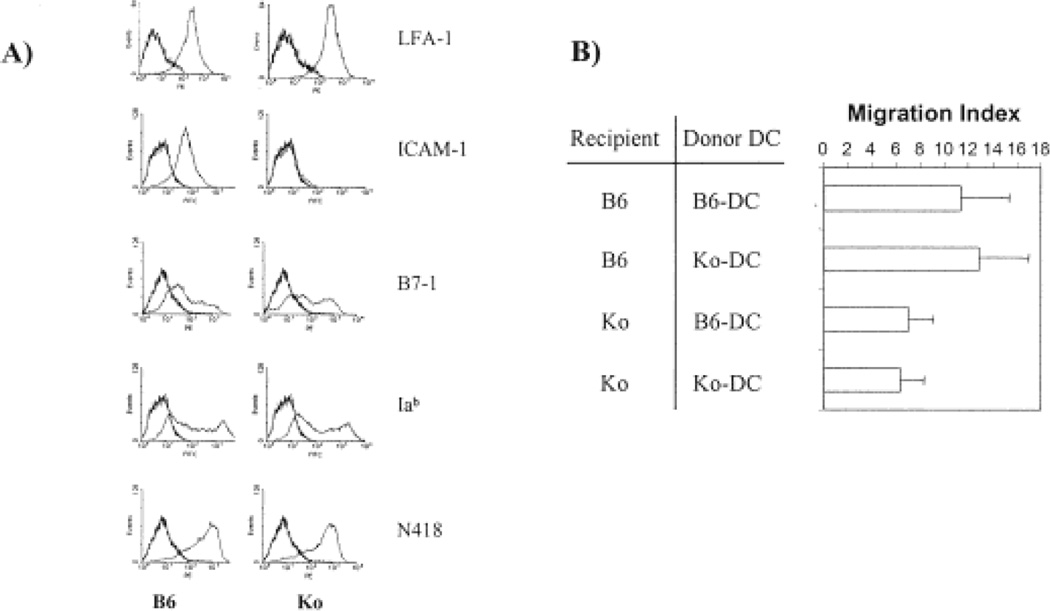
Langerhans cells express ICAM-1 and LFA-1. Both molecules are up-regulated during maturation [12, 16]. To determine whether ICAM-1 expressed by DC or lymphatic endothelium is critical for the migration of Langerhans cells into lymph nodes, bone marrow-derived DC were generated for an in vivo migration assay. Bone marrow-derived DC were employed instead of Langerhans cells because the number and purity of isolated Langerhans cells were insufficient for the assay. By using a well-established in vitro culture system, a large number of bone marrow-derived DC that contained > 90% N418-positive DC were generated. Flow cytometry analysis demonstrated that the phenotype of DC from ICAM-1-deficient and wild-type mice was identical with respect to the expression of CD11a, CD11c, MHC class II, and B7–1 (Fig. 4A). These cells were labeled with 51Cr and injected into the footpad of mice. The radioactivity of the draining popliteal lymph node was quantitated and standardized from each individual animal as described in Sect. 4. The migration of wild-type DC into the regional lymph node of ICAM-1-deficient recipients was significantly reduced in comparison to that of wild-type recipient mice (Fig. 4B, migration index: 7±2.1 vs. 11.3±4.03, p < 0.05). The migration index of wild-type and ICAM-1-deficient DC was similar in ICAM-1-deficient recipient mice (7±2.1 vs. 6.4±1.9). In wild-type recipients, the migration index of ICAM-1-deficient DC is not significantly different from that of wild-type DC (12.8±4.2 vs. 11.3±4.03). The data strongly suggest that ICAM-1 expression in the regional lymphatics rather than on Langerhans cells is important for the migration of hapten-labeled Langerhans cells into the regional lymph node.
Fig. 4.
In vivo migration assay for determination of DC migration into the regional lymph node. Bone marrow-derived dendritic cells were used for the in vivo migration assay. (A) The phenotype of bone marrow-derived DC from C57BL/6 and ICAM-1 knockout mice. (B) The migration index of 51Cr-labeled DC in the draining popliteal lymph node at 18–20 h after injection. The migration index is calculated as described in Sect. 4 and the bar graph shows the data of eight mice in each group. The difference in the migration index between C57BL/6 and ICAM-1-deficient recipient mice is significant (p < 0.05) regardless of donor DC.
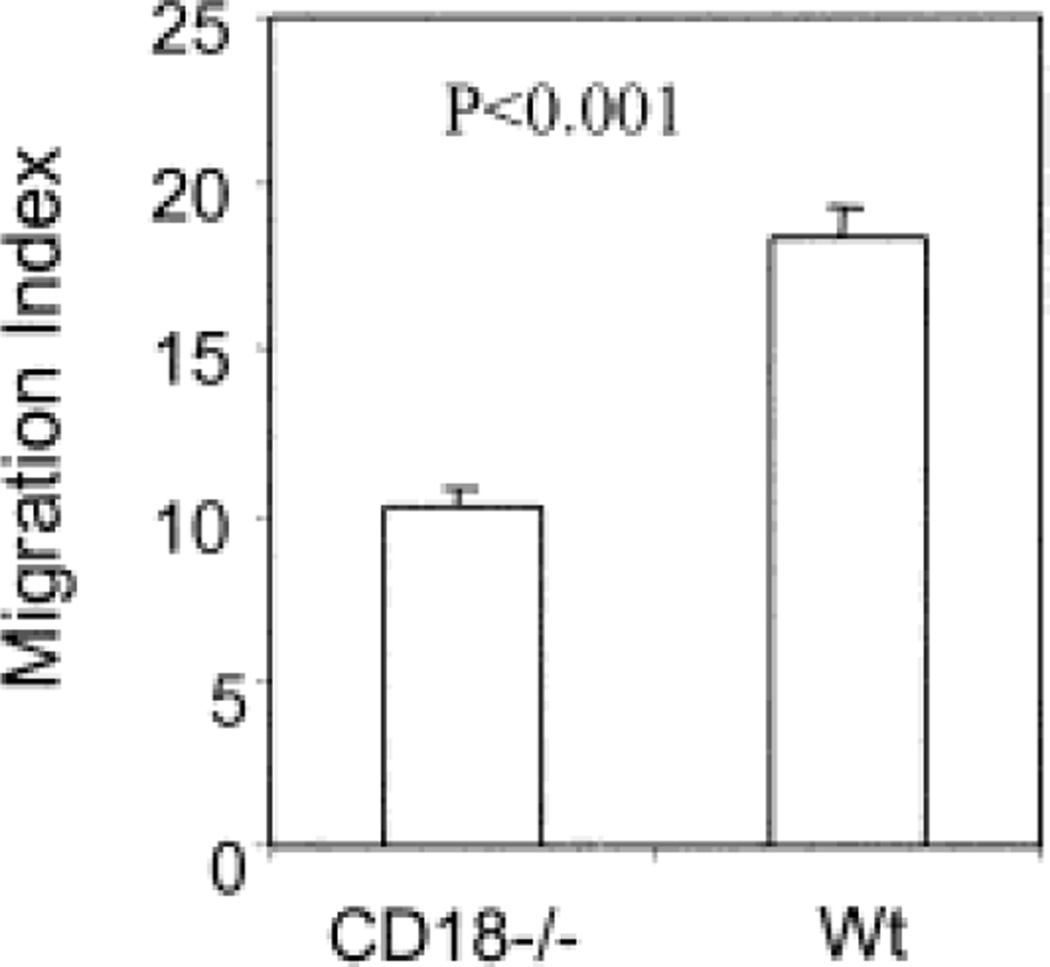
To further confirm the role of lymphatic ICAM-1 in regulation of DC migration, CD18-deficient DC were compared with wild-type DC in migration to draining lymph nodes of wild-type recipient mice. As shown in Fig. 5, the migration of CD18-deficient DC into draining lymph nodes was significantly reduced. The migration index of CD18-deficient was reduced about 50% compared to wild-type DC, similar to that seen between wild-type and ICAM-1-deficient mice (Fig. 4).
Fig. 5.
The effect of CD18 deficiency on DC migration into regional lymph nodes. Bone marrow DC from wild-type and CD18-deficient mice were generated, labeled with 51Cr and injected into naïve wild-type mice. The migration index of DC in the draining popliteal lymph node at 18 h after injection is exhibited. The result is representative of two experiments with six mice in each group.
3 Discussion
The cytokines and adhesion molecules responsible for localization of leukocytes at specific sites within inflamed and non-inflamed tissues is an area of active investigation. Of particular interest are the adhesion molecules that control the movement of DC, a process that is complex and incompletely understood. DC originate in the bone marrow, travel to peripheral tissues where they reside for variable lengths of time, and then after they have encountered antigen, must move out of peripheral tissues to lymphoid organs to activate T cells.
The experiments described here were prompted by the observation that contact hypersensitivity reactions are reduced in mice treated with anti-ICAM-1 antibodies and in strains of animals with a genetic deficiency in ICAM-1 [8, 15]. ICAM-1 is expressed on Langerhans cells, keratinocytes, and the vascular and the lymphatic endothelium [9, 12, 13]. Thus, there are ample opportunities for this adhesion molecule to be involved in migratory properties of Langerhans cells. In the current study, ICAM-1-deficient mice were used to examine this issue. The findings demonstrate that Langerhans cells utilize ICAM-1 for migration in a highly selective manner. Its expression is necessary for their entrance into lymph nodes, but it is not required for migration into or out of the skin. Thus, Langerhans cells utilize different adhesion molecules for migration depending on the location to which they are going. This seems reasonable given the fact the endothelial cells of blood and lymph vessels express different patterns of adhesion molecules [17] and therefore, the mechanism for cell migration through these two pathways may be different. A recent study demonstrated that the accumulation of DC in the lung was not significantly affected in ICAM-1-deficient mice [18].
We found that ICAM-1 on the lymphatic endothelium is required for Langerhans cells to enter draining lymph nodes where interactions between contact allergen bearing DC and T cells takes place. This conclusion is based on the fact that there were reduced numbers of hapten-labeled Langerhans cells in the draining lymph nodes of contact-sensitized ICAM-1-deficient mice compared to wild-type mice. The number of DC, as determined by CD11c expression (N418), in the lymph nodes of naive ICAM-1-deficient mice was not different than that of wild-type mice, indicating that the deficiency in dendritic cell percentages following hapten administration was not caused by a preexisting disparity in percentages of DC in the lymph nodes of naive mice. Exactly why there was no difference in the percentage of lymph node DC in naive wild-type and ICAM-1-deficient mice is unclear. It is known that DC are a heterogeneous group of cells with several subpopulations [3]. The origination of resident DC in lymph nodes remains to be defined. It may be that the DC in lymph nodes of naive mice come directly from the bone marrow or develop in situ from precursor cells that do not need ICAM-1 for entry into the lymph nodes. Evidence indicates that some dendritic cell subpopulations develop from precursor cells in lymphoid organs [19, 20]. It is possible that these studies underestimate the total contribution of ICAM-1 since the mice used for these experiments, which do not express wild-type ICAM-1, have been shown to express alternatively spliced forms of ICAM-1 in various tissues following inflammatory stimulation [21]. Although it is not clear at this time whether these proteins can promote ICAM-1-dependent interactions in vivo, a possible effect cannot be excluded by this study.
The inhibition of Langerhans cell migration into the regional lymph nodes is not due to a change in the kinetic of DC migration in ICAM-1-deficient mice. The number of migratory DC in both mouse strains peaks at 24 h and declines rapidly at 48 h after haptensensitization while the difference between wild-type and ICAM-1-deficient animals remains unchanged. Migratory DC in the draining lymph nodes of both mouse strains have a short life span, which is in accordance with previous studies indicating that mature DC are short-lived [2, 22, 23].
In vivo migration assays indicated that the migration of radiolabeled DC into the draining lymph node was reduced in the ICAM-1-deficient recipient mice compared to normal mice. These studies not only provided additional evidence that ICAM-1 plays a key role in the relocation of Langerhans cells to the regional lymph nodes in inflammatory conditions, but also demonstrated that ICAM-1 on host cells, rather than on Langerhans cells, is responsible for the altered migration. The latter is further confirmed by reduced migration of CD18-deficient DC into draining lymph nodes of wild-type recipient animals. Obviously, neither ICAM-1 deficiency in lymph nodes nor CD18 deficiency on DC completely abrogated the migration of Langerhans cells to the regional lymph nodes. Both flow cytometry of hapten-labeled Langerhans cell in the regional lymph nodes and in vivo migration assays showed only a partial reduction, suggesting that molecules other than ICAM-1 and CD18 play a role in the regulation of Langerhans cell migration to regional lymph nodes.
ICAM-1 was not required for Langerhans cell migration out of the epidermis. Consistent with previous observations, there was a decline in epidermal Langerhans cell densities after application of hapten to the skin [24]. The magnitude of the reduction was similar in ICAM-1-deficient and normal mice. Moreover, similar numbers of class II-positive DC were present in the dermis of ICAM-1-deficient and wild-type mice before and after sensitization. The number of Langerhans cells migrating out of skin explants also exhibited no significant difference in these two strains of mice. It is interesting that DC do not accumulate around lymph vessels in the dermis of ICAM-1-deficient mice, suggesting that ICAM-1 is not required for DC entry to the lymph vessels in the skin. DC reside in immature form in the skin and maturate during migration through lymph into lymph nodes following antigen stimulation. Different mechanisms may regulate DC migration into lymph vessels in the skin and entry into lymph nodes since mature DC express different patterns of adhesion molecules than immature DC. LFA-1, the ligand for ICAM-1, is not expressed on epidermal Langerhans cells [16]. However, it can be induced during maturation and is abundantly expressed by lymph node DC [3]. It is important to note that the bone marrow-derived DC that were used in the in vivo migration assay experiments reported here were stimulated with TNF-α for maturation prior to harvest and expressed a high level of CD11a and thus were able to engage ICAM-1 on lymphatic endothelial cells. It has been reported that blocking VLA-6 (α6β1 integrin) with specific antibody inhibited the migration of Langerhans cells out of the skin following hapten sensitization, suggesting that β1 integrins may play a role in Langerhans cell migration in the skin [7]. Another factor responsible for Langerhans cell migration out of the epidermis may be down regulation of E-cadherin expression, which occurs following Langerhans cell activation [5, 25].
The fate of DC that migrate out of the skin but unable to enter into the regional lymph nodes in ICAM-1-deficient mice following antigen stimulation remains to be defined. Our assumption is that these DC either migrate through blood vessels to the spleen or die in the lymph due to the absence of contact with T cells. It is known that activated DC have short life span in the absence of help signals from activated T cells. CD40L and TRANCE that are expressed by activated T cells have been demonstrated to regulate DC maturation and survival [23, 26–28]. Obviously, more studies are required to clarify these issues.
A lack of ICAM-1 did not impair Langerhans cell migration into the epidermis, since normal concentrations of Langerhans cells were found in the epidermis of ICAM-1-deficient mice and the magnitude and kinetics of the repopulation of Langerhans cells into the epidermis after hapten application was virtually the same in the two strains. Migration into the skin is likely to be mediated by other adhesion molecules, such as P- and E-selectin, which were reported to regulate the migration of Langerhans cells into inflammatory sites in the skin [6]. The lack of an effect of ICAM-1 expressed by Langerhans cells on their migration does not mean that it does not important role in the pathogenesis of contact hypersensitivity. It has been shown that ICAM-1 on antigen presenting cells is important for them to adhere to and to activate T cells [29, 30]. In this way, Langerhans cell ICAM-1 may participates in allergic contact hypersensitivity reactions.
In summary, this study indicates that ICAM-1 plays a role in the migration of DC into regional lymph nodes following antigen stimulation. The incomplete inhibition of DC migration into lymph nodes in ICAM-1-deficient mice suggests that other adhesion molecules are also involved in the process. ICAM-1 has a minor effect on the migration and distribution of DC in the skin, indicating different mechanisms for DC migration in the skin and lymph nodes.
4 Materials and methods
4.1 Mice and reagents
C57BL/6 wild-type mice were purchased from Charles River Laboratories. The ICAM-1 knockout mice used in these studies were back-crossed 12 generations onto the C57BL/ 6 strain background [15]. CD18 null mice have been previously described [31]. Mice were maintained in the University of Alabama at Birmingham animal care facility in accordance with the animal protocol approved by the IACUC.
The hybridomas GK1.5 (anti-CD4), Lyt-2 (anti-CD8), AF6–120.1 (anti-Iab), 2.4G2 (anti-CD16/32) and N418 (anti-CD11c) were purchased from the ATCC. The antibodies were purified from culture supernatants by affinity chromatography through a protein-G-coupled Sepharose column (Gamma-Bind Plus, Pharmacia). The purified N418 antibody was biotinlynated in our lab according to the manufacturer’s instructions (Sigma). Fluorescence-labeled anti-CD4, CD8, TCRβ chain, Iab and ICAM-1, B220, B7-1, and isotope matched control antibodies were purchased from PharMingen. FITC- or PE-labeled streptavidin was from PharMingen.
GM-CSF and IL-4 were obtained from Sigma, and TNF-α from Genzyme. Dinitrofluorobezene (DNFB) and fluorescein isothiocyanate (FITC) were from Sigma.
4.2 Generation of bone marrow-derived DC
Bone marrow-derived DC were prepared as described [32]. Briefly, bone marrow cells were taken from femurs and tibias. Red blood cells were lysed with ACK buffer. The cells were incubated with a cocktail of antibodies against Iab, CD45R/B220, Lyt-2 and GK1.5 (2 µg/106 cells) on ice for 1 h and washed once with HBSS. Cells were suspended in 7 ml RPMI 1640 medium with 1 ml of 1 M Hepes buffer, 1 ml of 3% BSA and 1 ml Low-Toxin rabbit complement (Accurate Chemical & Scientific Corporation) and incubated at 37°C for 1 h. Cells were washed once with HBSS and cultured in 10% FCS RPMI 1640 media supplemented with recombinant mouse GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) in 24-well plates (5×105 cells/well). On day 6, half of the medium was replaced with fresh medium and recombinant mouse TNF-α was added at 100 U/ml. The cells were harvested on the following day for use.
4.3 Immunohistochemical staining of epidermal Langerhans cells
Mice were painted with 5 µl of 0.5% DNFB on the dorsal side of ears and killed at the indicated times for staining of epidermal Langerhans cells. The preparation and staining of epidermal Langerhans cells were performed as published with minor modifications [33]. Briefly, the dorsal ear skin of sensitized or naive mice was taken and incubated with the dermal side down in 0.5 M ammonium thiocyanate at 37°C for 20–30 min. The epidermal sheets were separated from the dermis and fixed immediately in cold acetone for 10 min. The epidermal sheets were rehydrated in PBS for 60 min and incubated in the blocking buffer (50 µg/ml 2.4G2 and 0.5% BSA in PBS) for 30 min at room temperature. After one wash, biotinylated anti-Iab antibody was added at 1 µg/ sheet and incubated overnight on a shaker at 4°C. The sheets were then washed three times in PBS at room temperature. Streptavidin-FITC (1:100, 200 µl/sheet) was added and incubated on a shaker at room temperature for 2 h. After washings as described above, epidermal sheets were mounted on slides with 90% glycerol. Three photographs were taken from each epidermal sheet using a Leiz microscope connected to a digital camera and saved in a computer with the IP Spectrum software. The result was evaluated with Photoshop. The number of positive cells was counted on each photograph and the mean positive cell number/mm2 was calculated for each experimental group with at least six mice per group.
4.4 Immunohistochemical staining on skin tissue sections
Hapten treated skin tissues were taken at 4 h after hapten sensitization with 0.5% DNFB and frozen immediately in liquid nitrogen. Frozen sections (5 µm thick) were fixed in cold acetone for 10 min and rehydrated. After incubation with blocking buffer as described for staining epidermal sheets, sections were then incubated with biotinylated anti-Iab antibody (1 µg/section) for 60 min followed by streptavidin-peroxidase (1:200) for 60 min at room temperature. The reaction was visualized by adding 3,3’-diaminobenzidine as substrate (Sigma, Fast DAB Tablet Sets). Sections were counter stained with hematoxylin and photographed under microscope.
4.5 Flow cytometry analysis
For examining hapten-labeled Langerhans cells in the draining lymph nodes, mice were painted with 200 l 0.5% FITC on the shaved abdomen and killed 22–24 h later. The draining inquinal and axillary lymph node cells were stained with anti-CD11c antibody N418 for Langerhans cells as described [34]. Briefly, up to 106 cells were incubated at 4°C for 30 min with 200 µl blocking buffer containing 50 µg/ml anti-CD16/CD32 antibody (2.4G2, ATCC) and 5% FCS to block Fc receptor and nonspecific binding. After one wash, biotinylated N418 antibody (2 µg/sample) was added and incubated at 4°C for 30 min. The cells were then washed three times and incubated with PE-labeled streptavidin (1:100 diluted, 100 µl/sample) at 4°C for 30 min. The cells were washed three times and then fixed in 1% formalin. Thirty thousand events were collected in a FACSCalibur with the software CellQuest (Becton Dickinson). The data was analyzed with the software WinMDI 2.8. Naive mice were used as a negative control. FITC-positive cells were hapten-labeled cells and PE-positive cells were DC. The double-positive cells represented hapten-labeled Langerhans cells. The quadrants were set to calculate the percentage of each cell population.
To characterize the bone marrow-derived DC preparation, cells were stained with FITC- or PE-labeled CD4, CD8, B220, TCR, ICAM-1, LFA-1, B7, Iab, N418 and isotope matched control antibodies. The cells were measured in a FACSCalibur and data were analyzed with the software WinMDI 2.8.
4.6 DC migration out of skin explants
This was performed as described previously with modifications [33]. Briefly, the dorsal side of mouse ears was painted with 5 µl 0.5% DNFB and harvested 2 h later. The hapten treated dorsal skin was peeled off and placed on the top of RPMI 1640 medium with 10% FCS in 24-well tissue culture plates with one ear/well. The migratory cells were harvested and counted after cultures. The peak number of migratory cells was observed at 3 days. Cells in suspension consisted of about 70% CD11c+ DC. The treatment of ears with hapten increased the yield of migratory cells two to three times.
4.7 DC migration assay
Bone marrow-derived DC contained >90% N418+ cells. 2×107-3×107 cells were labeled with 400 µCi 51Cr (Amer-sham) at 37°C for 60 min and washed three times in medium. One million cpm (about 106 cells) in 50 µl PBS were injected into the left footpad of mice anesthetized with pentobarbital and the mice were killed 16–18 h later. The draining popliteal lymph node, the tissue around the LN and the injected foot were collected for measurement of cpm in a gamma counter (Wallac). Approximately, 20% of the injected radioactivity was recovered from the injected foot and about 0.5% was found in the draining lymph node. The cpm of the surrounding tissues was usually lower than 5% of that of the draining LN.
The migration index was calculated according to the following formula: migration index = (cpm of LN–cpm of surrounding tissues)/cpm of injected foot.
4.8 Statistical analysis
The difference in the migration index between experimental groups was analyzed with the Student’s _t_-test with p < 0.05 considered being statistically significant.
Acknowledgments
The work is supported by NIH grants AR46256–01 (H.X.), AR46404 (D.B.), CA70396, CN-85083–57, and CA79820–02 (C.E.).
Abbreviation
DC
Dendritic cell
References
- 1.Steinman RM, Pack M, Inaba K. Dendritic cell development and maturation. Adv. Exp. Med. Biol. 1997;417:1–6. doi: 10.1007/978-1-4757-9966-8_1. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells [In Process Citation] Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J. Leukoc. Biol. 1999;66:252–262. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- 5.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Fuhlbrigge RC, Kieffer JD, Ayehunie S, Hynes RO, Cheng G, Grabbe S, von Andrian UH, Kupper TS. Interaction of dendritic cells with skin endothelium: A new perspective on immunosurveillance. J. Exp. Med. 1999;189:627–636. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price AA, Cumberbatch M, Kimber I, Ager A. Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J. Exp. Med. 1997;186:1725–1735. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Wang JH, Guo YJ, Sy MS, Bigby M. In vivo treatment with anti-ICAM-1 and anti-LFA-1 antibodies inhibits contact sensitization-induced migration of epidermal Langerhans cells to regional lymph nodes. Cell. Immunol. 1994;158:389–399. doi: 10.1006/cimm.1994.1285. [DOI] [PubMed] [Google Scholar]
- 9.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 10.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 11.McHale JF, Harari OA, Marshall D, Haskard DO. Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-alpha. J. Immunol. 1999;162:1648–1655. [PubMed] [Google Scholar]
- 12.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J. Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 14.Granger N, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J. Leu-koc. Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 15.Sligh JE, Jr, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anjuere F, Martinez del Hoyo G, Martin P, Ardavin C. Langerhans cells acquire a CD8+ dendritic cell phenotype on maturation by CD40 ligation. J. Leukoc. Biol. 2000;67:206–209. doi: 10.1002/jlb.67.2.206. [DOI] [PubMed] [Google Scholar]
- 17.Erhard H, Rietveld FJ, Brocker EB, de Waal RM, Ruiter DJ. Phenotype of normal cutaneous microvasculature. Immunoelectron microscopic observations with emphasis on the differences between blood vessels and lymphatics. J. Invest. Dermatol. 1996;106:135–140. doi: 10.1111/1523-1747.ep12329708. [DOI] [PubMed] [Google Scholar]
- 18.Schneeberger EE, Vu Q, LeBlanc BW, Doerschuk CM. The accumulation of dendritic cells in the lung is impaired in CD18−/− but not in ICAM-1−/− mutant mice. J. Immunol. 2000;164:2472–2478. doi: 10.4049/jimmunol.164.5.2472. [DOI] [PubMed] [Google Scholar]
- 19.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 20.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King P, Sandberg E, Selvakumar A, Fang P, Beaudet A, Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J. Immunol. 1995;154:6080–6093. [PubMed] [Google Scholar]
- 22.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J. Exp. Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Volo-godskaia M, Steinman RM, Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo . J. Exp. Med. 2000;191:495–502. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinlich G, Heine M, Stossel H, Zanella M, Stoitzner P, Ortner U, Smolle J, Koch F, Sepp NT, Schuler G, Romani N. Entry into afferent lymphatics and maturation in situ of migrating murine cutaneous dendritic cells. J. Invest. Dermatol. 1998;110:441–448. doi: 10.1046/j.1523-1747.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 25.Jakob T, Saitoh A, Udey MC. E-cadherin-mediated adhesion involving Langerhans cell-like dendritic cells expanded from murine fetal skin. J. Immunol. 1997;159:2693–2701. [PubMed] [Google Scholar]
- 26.McLellan A, Heldmann M, Terbeck G, Weih F, Linden C, Brocker EB, Leverkus M, Kampgen E. MHC class II and CD40 play opposing roles in dendritic cell survival. Eur. J. Immunol. 2000;30:2612–2619. doi: 10.1002/1521-4141(200009)30:9<2612::AID-IMMU2612>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J. Immunol. 1998;161:2094–2098. [PubMed] [Google Scholar]
- 28.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J. Exp. Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krutmann J, Khan IU, Zhang F, Rich EA, Wallis RS, Ellner JJ, Elmets CA. The cell membrane is a major locus for UVB-induced inhibition of monocyte accessory function. J. Clin. Invest. 1990;85:1529–1536. doi: 10.1172/JCI114600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang A, Udey MC. Inhibition of epidermal Langerhans cell function by low dose ultraviolet B radiation. Ultraviolet B radiation selectively modulates ICAM-1 (CD54) expression by murine Langerhans cells. J. Immunol. 1991;146:3347–3355. [PubMed] [Google Scholar]
- 31.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Wayne Smith C, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J. Immunol. 1998;161:5516–5524. [PubMed] [Google Scholar]
- 33.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J. Exp. Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Heeger PS, Fairchild RL. Distinct roles of B7-1 and B7-2 determinants during priming of effector CD8+ Tc1 and regulatory CD4+ Th2 cells for contact hypersensitivity. J. Immunol. 1997;159:4217–4226. [PubMed] [Google Scholar]