Epigenetic perturbations by Arg882-mutated DNMT3A potentiate aberrant stem cell gene expression program and acute leukemia development (original) (raw)
. Author manuscript; available in PMC: 2017 Jul 11.
SUMMARY
DNA methyltransferase 3A (DNMT3A) is frequently mutated in hematological cancers; however, the underlying oncogenic mechanism remains elusive. Here, we report that DNMT3A mutational hotspot at Arg882 (DNMT3AR882H) cooperates with NRAS mutation to transform hematopoietic stem/progenitor cells and induce acute leukemia development. Mechanistically, DNMT3AR882H directly binds to and potentiates transactivation of stemness genes critical for leukemogenicity including Meis1, Mn1 and Hoxa gene cluster. DNMT3AR882H induces focal epigenetic alterations, including CpG hypomethylation and concurrent gain of active histone modifications, at cis-regulatory elements such as enhancers to facilitate gene transcription. CRISPR/Cas9-mediated ablation of a putative Meis1 enhancer carrying DNMT3AR882H-induced DNA hypomethylation impairs Meis1 expression. Importantly, DNMT3AR882H-induced gene expression programs can be repressed through Dot1l inhibition, providing an attractive therapeutic strategy for _DNMT3A_-mutated leukemias.
Graphical Abstract

eTOC blurb/In Brief
Lu et al. establish that Arg882-mutated DNMT3A contributes to acute myeloid leukemia (AML) pathogenesis through epigenetic activation of leukemia-related genes. Inhibition of Dot1l reverses mutant DNMT3A-induced gene expression, indicating a potential therapeutic strategy for AMLs harboring this mutation.
INTRODUCTION
DNA methylation provides a critical epigenetic means for defining cellular identity and regulating functional output of gene-regulatory elements such as promoters and enhancers (Jones, 2012; Schubeler, 2015). Recently, DNA methyltransferase 3A (DNMT3A), a de novo DNA methyltransferase gene, was found mutated in ~20–30% of human acute myeloid leukemias (AMLs) and ~10–20% of various other hematological cancers (Cancer Genome Atlas Research, 2013; Ley et al., 2010; Patel et al., 2012; Yan et al., 2011; Yang et al., 2015). DNMT3A mutations also associate well with clonally derived hematopoiesis at premalignant stages (Genovese et al., 2014; Jaiswal et al., 2014; Shlush et al., 2014; Xie et al., 2014) and often coexist with a secondary lesion that ‘hits’ either the FLT3-RAS kinase pathway, an epigenetic regulator (IDH1/2, TET2) or NPM1 in AML patients (Cancer Genome Atlas Research, 2013; Ley et al., 2010; Patel et al., 2012; Yang et al., 2015). These clinical findings suggest that DNMT3A mutation acts as a founder lesion and requires additional genetic event to induce malignant development. Consistently, mice with Dnmt3a knockout in the bone marrow produced phenotypically normal hematopoietic stem cells (HSCs); only after rounds of transplantation did _Dnmt3a-_null HSCs display self-renewal advantages (Challen et al., 2012). Mice with Dnmt3a mutation alone did not develop frank AML but showed increased susceptibility to malignant development upon acquisition of additional mutations (Celik et al., 2015; Chang et al., 2015; Mayle et al., 2015; Xu et al., 2014).
Mutational hotspot at Arg882 (R882), a residue located within the homodimerization interface of DNMT3A, accounts for the majority (~60%) of DNMT3A mutations found in AMLs (Ley et al., 2010; Yang et al., 2015). Due to a primarily heterozygous nature of DNMT3A R882 mutation, it was thought to act in a dominant-negative and/or haploinsufficient manner (Holz-Schietinger et al., 2012; Kim et al., 2013; Russler-Germain et al., 2014). Clinical evidence supports this notion as AML patients with DNMT3A R882 mutation exhibited focal DNA hypomethylation (Russler-Germain et al., 2014). Despite these advances, there is a lack of relevant AML animal models for studying DNMT3A R882 mutation. Molecular pathways and mechanisms by which DNMT3A mutation contributes to AML pathogenesis remain undefined. Targeted approaches for the treatment of _DNMT3A_-mutated AMLs remain to be developed.
RESULTS
DNMT3A hotspot mutation enhances sensitivity of hematopoietic stem/progenitor cells (HSPCs) to transformation in vitro
Previous reports indicate that hotspot mutations of DNMT3A such as DNMT3AR882H act in a dominant-negative manner by disrupting formation of a DNMT3A-associated tetramer complex required for efficient DNA methylation (Holz-Schietinger et al., 2012; Kim et al., 2013; Russler-Germain et al., 2014). These studies prompted us to ask whether ectopic expression of human DNMT3AR882H in murine HSPCs could establish a transformation phenotype in a colony-forming unit (CFU) and replating assay (Figure S1A). Initially, we found a lack of CFU-promoting effect by DNMT3AR882H alone (Figures 1A–C). We then asked if DNMT3AR882H could enhance sensitivity of HSPCs to transformation in the presence of a second oncogenic lesion. Toward this end, we used a bicistronic retroviral system to coexpress either wild-type (WT) or R882H-mutant (RH) DNMT3A, together with other mutations known to coexist with DNMT3A mutation in human AMLs: either NRAS (NRASG12D), NPM1 (NPM1c), or IDH1 (IDH1R132H) (Figure S1A) (Ley et al., 2010; Patel et al., 2012; Shih et al., 2012). Following viral transduction and drug selection, we obtained highly pure HSPCs with comparable levels of oncogene expression for CFU assays (Figures 1C and S1B–C). We did not observe CFU-promoting effect of DNMT3AR882H in the presence of NPM1c or IDH1R132H (Figure S1D). However, a significant increase of CFUs was seen post-replating of HSPCs coexpressing DNMT3AR882H and NRASG12D (hereinafter referred to as “RH-RAS”), relative to those with either oncogene alone (Figures 1A–B). In contrast to DNMT3AR882H, DNMT3AWT did not promote colony formation (Figure 1A–B). Post-replating, HSPCs expressing NRASG12D alone produced tiny and diffuse colonies of differentiated cells whereas those with RH-RAS gave rise to large, compact colonies that mainly comprised undifferentiated progenitors (Figures 1A insert, 1B and S1E–F). Importantly, cells expressing RH-RAS as derived from serially replated colonies were able to propagate and maintain their immature progenitor status in long-term liquid culture (Figures 1D and S1G), suggesting acquisition of indefinite self-renewal capability by these cells. These data have shown that, in contrast to DNMT3AWT_,_ R882-mutated DNMT3A promotes aberrant self-renewal of HSPCs and enhances their sensitivity to transformation in vitro. In addition, NRASG12D genetic background provides a useful platform for dissecting the role of DNMT3A mutation in AML development.
Figure 1. DNMT3AR882H acts in concert with mutant RAS to transform murine HSPCs ex vivo and induce AMLs in vivo.
(A) Colony-forming unit (CFU) assay using murine HSPCs expressing empty control (EV), wild-type (WT) or R882H mutant (RH) DNMT3A in combination with GFP or NRASG12D (RAS) Insert, a typical colony expressing RH-RAS at the 4th replating. Scale bar, 1 mm.
(B) Images of CFU assay at the 4th replating.
(C) Immunoblot of DNMT3A (Myc-tagged) and NRAS (Flag-tagged) in HSPCs post-infection.
(D) Microscopic image and Wright-Giemsa staining of RH-RAS_-_coexpressing cells derived from the 4th replating after long-term culture with the SCF cytokine in vitro. Scale bar, 10 μm.
(E) Kaplan-Meier survival curve of mice after bone marrow transplantation (BMT) of HSPCs freshly transduced with indicated genes. The p values were calculated by log-rank test.
(F–G) Spleen size (panel F, n = 3) and weight (panel G, n = 4–7) of indicated cohorts 3–4 weeks post-BMT. The p values were calculated by Student’s t-test.
(H) Wright–Giemsa staining of bone marrow (upper) and H&E staining of spleen (bottom) of indicated cohorts 4 weeks post-BMT. Scale bar, 10 μm (upper) and 200 μm (bottom).
(I) White blood cell (WBC) counts in peripheral blood of indicated cohorts (n = 6–13) 4 weeks post-BMT. The p values were calculated by Student’s t-test.
(J) Immunoblot of DNMT3A (Myc) and NRAS (Flag) proteins in bone marrow (BM) and spleen (SP) cells from mice with leukemia induced by RH-RAS coexpression. The first 2 lanes were loaded with samples of in vitro infected HSPCs.
(K) FACS analysis of Mac-1 and c-Kit with bone marrow and spleen cells of indicated cohorts 4 weeks post-BMT.
Error bar, +/− SD; ***, p < 0.001; ****, p < 0.0001.
See also Figure S1 and Table S1.
DNMT3AR882H acts in concert with activated RAS to induce murine AMLs in vivo
The observed in vitro effect of DNMT3AR882H on aberrant HSPC self-renewal and immortalization indicates that it could cooperate with NRASG12D to cause malignant transformation in vivo. Thus, we transplanted murine HSPCs freshly transduced with DNMT3A (either WT or RH) and/or NRASG12D to syngeneic mice. NRASG12D alone induced a myeloproliferative disease with incomplete penetrance (Figures 1E and S1H). DNMT3AR882H alone did not cause detectable diseases over a 12-month monitoring period; however, in the presence of NRASG12D, it significantly accelerated development of leukemia with a shorter latency phenotype and full penetrance (Figure 1E). RH-RAS-induced leukemia was also characterized by hepatosplenomegaly (Figures 1F–G and S1I), leukemic infiltration to bone marrow, spleen and liver (Figures 1H and S1J), and elevated counts of peripheral white blood cells and blasts (Figures 1I, S1K–O and Table S1). Leukemia induced by RH-RAS expressed virally transduced genes at a level comparable to progenitors immortalized by RH-RAS in vitro (Figure 1J) and displayed an immature myeloid (AML) immunophenotype (Mac-1+/c-Kitlow/Cd34low/Gr1−/Cd3e−/Cd19−/Ter119−; Figures 1K, S1P–Q and Table S1). Whole-exome sequencing of 3 independent murine AMLs identified no recurrent mutation of additional genes (Figure S1R), suggesting that DNMT3AR882H and NRASG12D are sufficient to drive AML development. Interestingly, unlike DNMT3AR882H, DNMT3AWT suppressed leukemogenesis in vivo (Figure 1E), suggesting that normal DNMT3A activities oppose AML pathogenesis.
DNMT3A hotspot mutation produces leukemia-initiating stem cells (LSCs) ex vivo in the presence of NRASG12D
To further verify cell transformation effect of RH-RAS, we used a previously described liquid cultivation system (Wang et al., 2007; Wang et al., 2009) and were able to recapitulate HSPC immortalization with RH-RAS only, and not either oncogene alone or coexpression of DNMT3AWT with NRASG12D (Figure S2A). Similar to those derived from CFU assays, RH-RAS-immortalized progenitors stably maintained their progenitor identity in vitro in the presence of SCF or Flt3 ligand, presented with expression of immature myeloid (c-Kit+/Mac-1low/Gr1−) and stem cell antigens (Cd34low/Flt3+/Sca1low/−) as well as a lack of other lineage markers (Figures 2A–B and S2B–C). Exposure of these progenitors to myeloid-promoting cytokines decreased cell proliferation (Figure S1G) and induced terminal myeloid differentiation (c-Kit−/Mac-1high/F4–80high; Figures 2B and S2D), demonstrating their myeloid differentiation capability. Engraftment with each of 3 independent RH-RAS-immortalized progenitor lines induced murine AMLs (Figures S2E–H) that can be propagated in vivo with sequential transplantation (Figure 2C). Importantly, as few as 50–500 of these cells were sufficient to cause AML (Figure 2D), illustrating their LSC characteristic (hereinafter called “LSCsRH-RAS”). To further characterize LSCsRH-RAS, we profiled their transcriptome and genome-wide occupancy of H3K4me1, a histone mark demarcating lineage-specific enhancers (Lara-Astiaso et al., 2014). Unsupervised clustering of H3K4me1 profiles of LSCsRH-RAS and various hematopoietic cell lineages revealed a similarity of LSCsRH-RAS to HSPCs such as HSC and myeloid progenitors, when compared to differentiated cell types (Figures 2E and S2I); similar results were found in their transcriptome comparison (Figures 2F and S2J). Notably, a closer similarity was seen when comparing LSCsRH-RAS to leukemic progenitors we and others previously produced using either HOXA9 plus MEIS1 (Wang et al., 2005), MLL translocation (Bernt et al., 2011), NUP98-NSD1 (Wang et al., 2007) or NUP98-JARID1A (Wang et al., 2009) (Figures 2E and S2I–J), implying a commonality of pathways underlying leukemogenicity by these oncogenes.
Figure 2. R882-mutated DNMT3A establishes leukemia-initiating stem cells (LSCs) ex vivo in the presence of activated RAS.
(A) FACS analysis of in vitro immortalized progenitors by RH-RAS using a liquid culture system.
(B) Wright–Giemsa staining (upper) and FACS analysis of RH-RAS-immortalized progenitors 14 days post-cultivation with indicated cytokines. FACS control, non-specific IgG (grey, open); Scale bar, 10 μm.
(C) Kaplan-Meier curve of mice receiving primary or secondary BMT with RH-RAS induced leukemia.
(D) Kaplan-Meier curve of mice (n = 5–6) receiving BMT of the indicated numbers of RH-RAS immortalized cells.
(E) Hierarchical clustering of genome-wide H3K4me1 profiles of LSCsRH-RAS_,_ AML-causing LSC lines produced by overexpressed HOXA9 plus MEIS1 (HOXA9-MEIS1), and various normal blood cell types. LT-HSC, long-term HSC; ST-HSC, short-term HSC; MPP, multipotent progenitor; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; GMP, granulocyte-monocyte progenitor; MEP, megakaryocyte-erythroid progenitor; Mac, macrophage; Mono, monocyte; GN, granulocyte; B, B220+/CD19+ B-cell; CD4/8, CD4/8+ T-cell; NK, natural killer cell; EryA and EryB, Ter119+/CD71+ erythroid cell with high and low forward scatter, respectively.
(F) Principal component (PC) analysis of transcriptome profiles of LSCsRH-RAS and various normal blood cell types. Besides what is described in panel E, CD34−KLS, Cd34−/c-Kit+/Lin−/ScaI+ HSC; MPP1, Flk2− multipotent progenitor; MPP2, Flk2+ multipotent progenitor; NKT, natural killer T-cell; Ery, erythroid cell.
See also Figure S2.
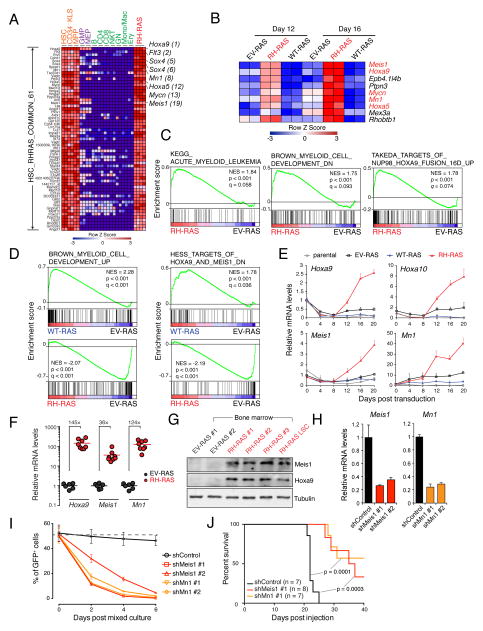
R882-mutated DNMT3A potentiates abnormal transcription of stem cell genes including a Meis1-Mn1-Hoxa regulatory node
Next, we sought to understand the molecular basis underlying indefinite self-renewal shown by LSCsRH-RAS. First, we asked if they carry self-renewal or stemness gene expression programs, a known feature of LSCs (Abramovich et al., 2005; Eppert et al., 2011; Krivtsov et al., 2006). By transcriptome analysis, we identified 54 genes uniquely expressed in LSCsRH-RAS and primitive HSPCs with self-renewal capabilities, relative to differentiating and mature hematopoietic cell types (Figure 3A and Table S2). The stem cell signature genes expressed in LSCsRH-RAS are only part of HSC stemness gene programs (~10%, Figure S3A); we further verified enrichment of the LSCRH-RAS stemness signature in self-renewing HSCs with independent datasets (Figures S3B–C). The top LSCRH-RAS stemness genes included Hoxa9, Mn1, Hoxa5 and Meis1 (Figure 3A), which encode a set of transcription factors (TFs) and cofactors crucial for sustaining self-renewal of normal HSCs and leukemic LSCs (Heuser et al., 2011; Huang et al., 2012; Wang et al., 2006; Wong et al., 2007). Gene targets of Meis1-Mn1-Hoxa, Flt3 and Sox4 (Heuser et al., 2011; Huang et al., 2012; Wang et al., 2005), were also among top stemness genes identified (Figure 3A), indicating activity of this TF regulatory circuitry in LSCsRH-RAS. Moreover, activation of Meis1 and Hoxa in LSCsRH-RAS was found comparable to that in LSCs defined by other deregulated chromatin factors such as MLL-AF9, NUP98-JARID1A or NUP98-NSD1_,_ while Mn1 and Mycn showed unique expression in LSCsRH-RAS (Figures S3D–E).
Figure 3. DNMT3AR882H potentiates aberrant activation of stemness genes including a critical Meis1-Mn1-Hoxa regulatory node.
(A) Heatmap of 61 probes (54 genes) showing unique expression in both self-renewing HSPCs (HSC, Cd34−KLS and MPP) and LSCsRH-RAS but not in differentiating (purple) or mature (green) blood cell types. Probes are ranked by higher expression in LSCsRH-RAS relative to differentiating and mature cells. Example genes are highlighted along with their respective rankings.
(B) Of the 54 self-renewal genes, genes showing consistently higher expression in HSPCs 12 and 16 days post-transduction of RH-RAS relative to EV-RAS.
(C) GSEA shows enrichment of AML-associated genes (left), genes downregulated upon myeloid differentiation (middle) and NUP98-HOXA9 targets (right) in HSPCs with RH-RAS versus EV-RAS.
(D) GSEA shows enrichment of differentiation gene sets in WT-RAS or RH-RAS HSPCs relative to EV-RAS. Left, myeloid differentiation genes; right, genes downregulated upon activation of HOXA9 and MEIS1.
(E) RT-qPCR of indicated genes in murine HSPCs post-transduction of EV-RAS, WT-RAS or RH-RAS.
(F) RT-qPCR of indicated genes in mouse bone marrow 21 days post-BMT of HSPCs with EV-RAS (n = 6) or RH-RAS (n = 8).
(G) Immunoblot of Meis1 and Hoxa9 in bone marrow of mice 21 days post-BMT of HSPCs with EV-RAS or RH-RAS. The last lane was loaded with LSCRH-RAS samples.
(H) RT-qPCR showing shRNA-mediated Meis1 or Mn1 knockdown in LSCsRH-RAS.
(I) Relative proliferation of indicated shRNA-expressing LSCsRH-RAS (GFP+) versus parental cells (GFP−). These GFP− and GFP+ cells were mixed in a 1:1 ratio at day 0, followed by measurement of % of GFP+ cells.
(J) Kaplan-Meier curve of mice engrafted with indicated shRNA-expressing LSCsRH-RAS. The p values were calculated by log-rank test.
Error bar, +/− SD.
See also Figure S3 and Table S2.
As LSCsRH-RAS carry both DNMT3A and NRAS mutations_,_ we next asked what stemness gene signatures are dependent on DNMT3AR882H. We performed microarray studies using HSPCs post-transduction of NRASG12D alone or with coexpressed DNMT3A_,_ either WT or R882H-mutant (hereinafter refer as EV-RAS, WT-RAS or RH-RAS). These HSPCs were collected 12 and 16 days after viral transduction when their proliferation rates were comparable (Figure S2A). Among the 54 LSCRH-RAS stemness genes, 9 were found upregulated by DNMT3AR882H at both time points, including Meis1, Mn1 and Hoxa (Figures 3B and S3F). Consistently, Gene Set Enrichment Analysis (GSEA) found that gene sets associated with AML development, undifferentiated myeloid cells, and NUP98-HOXA9 targets were significantly enriched in HSPCs with RH-RAS (Figure 3C). Conversely, gene sets associated with myeloid differentiation showed reduced expression in HSPCs expressing RH-RAS, relative to EV-RAS (Figure 3D), whereas the same gene sets showed enhanced expression in HSPCs expressing WT-RAS (Figure 3D), thus suggesting opposite effects of WT and R882-mutated DNMT3A on regulating genes crucial for HSPC self-renewal versus differentiation. We verified unique upregulation of Meis1, Hoxa and Mn1 in RH-RAS HSPCs (Figures 3E and S3G) and their induced AMLs (Figures 3F–G and S3H). To functionally assess whether the activated Meis1-Mn1-Hoxa circuitry is essential for RH-RAS_-_induced AML development, we introduced independent small hairpin RNAs (shRNAs) of Meis1 or Mn1 into LSCsRH-RAS (Figure 3H) and found that knocking down either gene significantly impaired in vitro growth of LSCsRH-RAS (Figures 3I and S3I) as well as their in vivo leukemogenic function (Figures 3J and S3J–K).
Together, these data reveal a role of R882-mutated DNMT3A in potentiating abnormal activation of stemness genes such as Meis1, Mn1 and Hoxa, which are required for mutant DNMT3A-mediated AML progression.
ChIP-Seq reveals context-dependent targeting of R882-mutated DNMT3A into the LSC genome
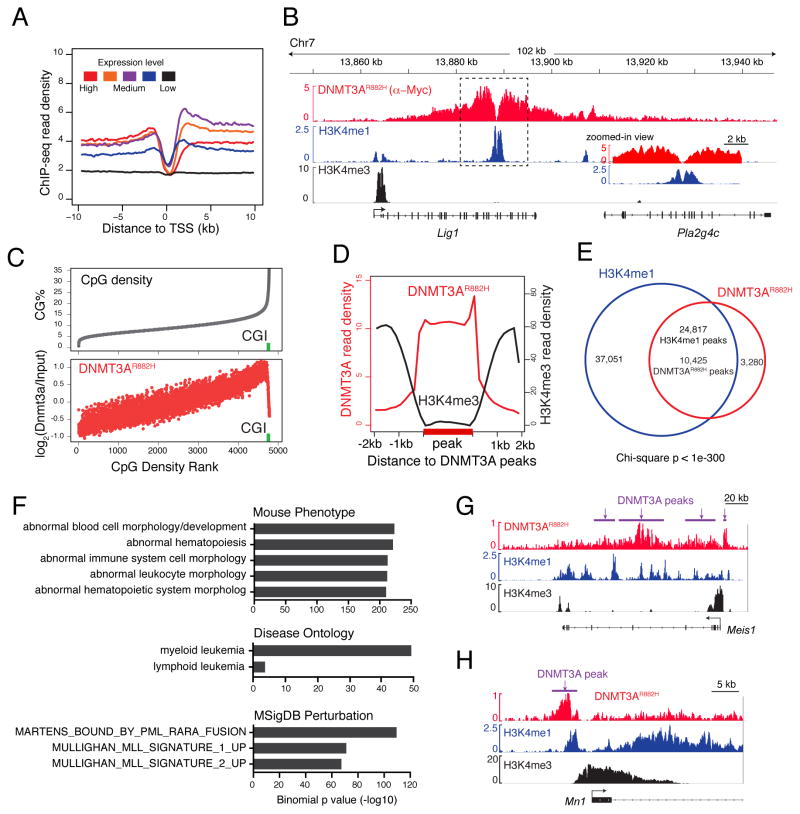
The LSCRH-RAS cellular model described above provides an ideal system for dissecting the molecular mechanism underlying DNMT3AR882H-mediated oncogenesis. Mutant DNMT3A proteins are exclusively nuclear (Figure S4A), thus, we first mapped their genome-wide occupancy in LSCsRH-RAS by ChIP-Seq using antibodies against the Myc tag fused to DNMT3AR882H (Figure S1A). Myc-DNMT3AR882H ChIP-Seq gave robust and specific signals (Figure 4A); as a negative control, Myc ChIP-Seq using cells without Myc-DNMT3AR882H expression did not detect any peaks (Figure S4B). We identified 13,705 genomic regions with significant DNMT3AR882H binding (i.e. DNMT3AR882H peaks, Table S3) in LSCsRH-RAS, which spread over promoter and inter- or intra-genic regions (Figure S4C). DNMT3AR882H exhibited a broad binding pattern with an average peak size of ~17 kb (Figures S4D and 4B, with an example peak at Lig1). Interestingly, DNMT3AR882H binding was stronger at intermediately transcribed genes, relative to low or highly expressed genes (Figure 4A), and positively correlated to CpG dinucleotide density except at CpG islands (CGIs) where DNMT3AR882H has a sharp drop in overall binding (Figure 4C). DNMT3AR882H binding regions also showed depletion of H3K4me3 (Figures 4D), a histone modification known to suppress DNMT3A binding due to an intrinsic histone H3-‘reader’ activity of DNMT3A’s ADD domain (Guo et al., 2015; Noh et al., 2015). Intriguingly, 76.1% of DNMT3AR882H peaks were found in close proximity to and significantly overlapped with peaks of H3K4me1, a histone mark demarcating enhancer elements (observed/expected = 10.2, p < 1e-300; Figure 4E), as exemplified by those identified in an intron region of Lig1 and an intergenic region of Vegfa (Figures 4B, insert, and S4E, boxed areas). Ontology analysis of DNMT3AR882H peaks revealed their significant enrichment at genes related to normal and malignant hematopoiesis, PML-RARα targets and MLL rearrangement-associated genes (Figures 4F and S4F). Notably, key AML-promoting or stemness genes upregulated by DNMT3AR882H such as Meis1, Mn1, Hoxa and Mycn were all found directly bound by DNMT3AR882H (Figures 4G–H and S4G–H). Collectively, our genome-wide profiling of DNMT3AR882H has revealed a CpG content and ‘histone code’-dependent targeting of R882-mutated DNMT3A into cancer cell genomes; we have also identified a previously unappreciated overlap of DNMT3AR882H with putative enhancer and cis-regulatory sites (marked by H3K4me1) at numerous developmental genes including a Meis1-Mn1-Hoxa node.
Figure 4. ChIP-Seq reveals chromatin context-dependent binding of R882-mutated DNMT3A to genomic regions, including stemness genes such as a Meis1-Mn1-Hoxa node.
(A) DNMT3AR882H ChIP-Seq profiles across transcription start site (TSS) of genes with different expression levels in LSCsRH-RAS.
(B) Example ChIP-Seq profiles for DNMT3AR882H, H3K4me1 and H3K4me3 at the Lig1 gene. Box, a zoomed-in view of dashed box region showing overlap of DNMT3AR882H and H3K4me1 peaks.
(C) Correlation of DNMT3AR882H binding and CpG density. Shown is % of CpG density (grey) and DNMT3AR882H ChIP-Seq reads (red) at 1-kb windows of the entire genome ranked by CpG density. Green square, CpG island (CGI).
(D) Plot of averaged DNMT3AR882H (red) and H3K4me3 (black) ChIP-Seq signals at DNMT3AR882H peaks (labeled in bold on x-axis) and surrounding regions (+/−2 kb).
(E) Venn diagram shows significant overlap of DNMT3AR882H and H3K4me1 peaks in LSCsRH-RAS.
(F) Genomic Regions Enrichment of Annotations Tool (GREAT) analysis shows enrichment of indicated gene signatures among DNMT3AR882H peaks.
(G–H) ChIP-Seq profiles of DNMT3AR882H, H3K4me1 and H3K4me3 at Meis1 (panel G) and Mn1 (panel H). Purple bars, DNMT3AR882H peak calls.
See also Figure S4 and Table S3.
R882-mutated DNMT3A induces focal hypomethylation of CpG sites enriched with gene-regulatory elements
We next aimed to delineate DNMT3AR882H-induced epigenetic perturbations during AML progression. By enhanced Reduced Representation Bisulfite Sequencing (eRRBS), we mapped global DNA methylation patterns of murine HSPCs 16 days post-transduction of EV-RAS, RH-RAS or WT-RAS. Analysis of eRRBS data, which had 11–12× coverage for 6.5 million CpGs in all samples, revealed no significant changes in global CpG methylation (Figure S5A–C) except a moderate change at CpG shores (Figure S5C). By pairwise comparison of CpG methylation, we identified 12,889 differentially methylated CpG sites (DMCs) in HSPCs expressing RH-RAS relative to EV-RAS, with most of DMCs (80.8%) hypomethylated (Figure 5A, left; hereinafter called as “DNMT3AR882H-associated hypo-DMCs”); in contrast, DMCs associated with DNMT3AWT are largely hypermethylated (hyper-DMCs, 80.6%; Figure 5A, right). DNMT3AR882H-associated hypo-DMCs were found mainly in intron, intragenic and promoter regions, while DNMT3AWT_-_induced hyper-DMCs were enriched in promoters and CGIs (Figures S5D–E). Importantly, DNMT3AR882H-associated hypo-DMCs were significantly enriched at genomic regions with H3K4me1 (Figure 5B) or with DNMT3AR882H binding (Figure 5B–C). DNMT3AR882H-associated hypo-DMCs were also found enriched with the binding site of the ETS family of TFs (Erg and Spi1/PU.1) and other hematopoietic TFs (Runx1 and Mycn; Figure 5D). In contrast, DNMT3AR882H-associated hyper-DMCs exhibited none of these features and, instead, correlated negatively to DNMT3AR882H binding (Figures 5B–5D), suggesting that creation of hyper-DMCs is due to indirect effect of DNMT3AR882H.
Figure 5. DNMT3AR882H induces focal CpG hypomethylations enriched at H3K4me1-demarcated, gene-regulatory sites in HSPCs.
(A) Distribution of DMCs in the genome of murine HSPCs transduced with RH-RAS or WT-RAS, relative to EV-RAS.
(B) Heatmap showing enrichment of DNMT3AR882H-associated DMCs at indicated genomic regions or ChIP-Seq peaks in comparison to genome average. The enrichment value was calculated as log2(observed/expected) of the DMC numbers. CGI, CpG island; CGS, CpG shore.
(C) Distribution of DNMT3AR882H-associated DMCs across DNMT3AR882H ChIP-Seq peaks (shown in a bold bar on x_-_axis). Y_-_axis shows % of DMCs located at 100-bp window of genomic regions centered on DNMT3AR882H peaks.
(D) Enrichment of indicated TF binding motifs in DNMT3AR882H-associated hypo-DMCs and hyper-DMCs.
(E) Summary of DMRs identified in the HSPCs with RH-RAS or WT-RAS, relative to EV-RAS.
(F) Venn diagram showing overlap of DNMT3AR882H and DNMT3AWT-associated DMRs.
(G) GREAT annotation of DNMT3AR882H-associated hypo-DMRs.
(H) H3K4me1 profiles at DNMT3AR882H-associated hypo-DMRs, hyper-DMRs, and random control regions. Plotted across DMRs (labeled in a bold line on x_-_axis) were averaged H3K4me1 ChIP-Seq read densities in EV-RAS cells.
(I) Scatter plots showing methylation changes of selected CpGs in human AMLs with DNMT3A R882 mutation relative to DNMT3A WT AMLs. Mean methylation differences (y_-_axis) and p value (x_-_axis) for each CpG between two AML patient groups were plotted. Left, CpGs in the human genome homologous to DNMT3AR882H-associated hypo-DMRs identified in murine HSPCs; right, randomly picked CpG controls.
(J) DNA methylation profiles of Meis1 in indicated murine HSPCs and MEIS1 in human AMLs with WT (n = 50) or R882-mutated (n = 20) DNMT3A. Faded points show individual CpG methylation beta values and connected lines indicate the mean methylation levels at each CpG site. Grey box, a hypo-DMR in intron 6.
(K) Bisulfite sequencing of the Meis1 intron 6 DMR in indicated murine HSPC samples.
(L) Box plots of methylation beta values of all CpGs (shown as dots in boxplot) at MEIS1 intron 6 in human AMLs with R882-mutated DNMT3A (n = 20) relative to AMLs with either non-R882 mutated (n = 15) or WT (n = 50) DNMT3A. Horizontal line, median; box, interquartile range; whiskers extend to 1.5× the interquartile range. The p values were calculated by Mann-Whitney U test.
See also Figure S5 and Table S4 and S5.
Consistent with DMCs, differentially methylated regions (DMRs) identified in HSPCs co-transduced with DNMT3AR882H relative to control were mainly hypomethylated (hereinafter called “DNMT3AR882H-associated hypo-DMRs”; Table S4, n = 1,199) while DNMT3AWT-associated DMRs were mainly hypermethylated (hyper-DMRs) (Figures 5E and S5F). These two sets of DMRs showed a significant overlap, including those found at DNMT3AR882H-deregulated stemness genes (Meis1, Mn1, Hoxa7 and Mycn), further highlighting that WT and R882-mutated DNMT3A have opposing effects on DNA methylation of crucial AML-promoting genes (Figure 5F). In addition, DNMT3AR882H-associated hypo-DMRs were enriched at genes related to transcriptional regulation, hematopoietic development and cancer (Figures 5G and S5G). Consistent with results in DMCs, H3K4me1 and DNMT3AR882H binding were significantly enriched at DNMT3AR882H-associated hypo-DMRs (Figures 5H and S5H). Taken together, our results show that R882_-_mutated DNMT3A is sufficient to induce CpG hypomethylation at putative cis-regulatory sites of key stemness genes that we have functionally validated as essential for AML progression in murine models.
DNMT3AR882H–induced DNA hypomethylation identified in murine models mirrors what was seen in human AMLs with DNMT3A R882 mutation
A focal CpG hypomethylation phenotype seen in the above murine model is reminiscent of what was observed in human AMLs with DNMT3A mutation (Russler-Germain et al., 2014). To assess whether our murine model mimics human disease, we first identified regions in the human genome that are homologous (i.e., conserved) to DNMT3AR882H-associated hypo-DMRs defined in the murine model. We then found that, relative to randomized control, CpGs located in such conserved human genomic sites showed a significant reduction in their methylation levels among human AML samples with DNMT3A R882 mutation, relative to those with normal DNMT3A (Figure 5I; p < 2.2e-16). Despite a relatively limited coverage of CpGs by the 450K-array platform used in the human AML study (Russler-Germain et al., 2014), genes with hypo-DMRs identified in AML patients carrying DNMT3A R882 mutation also had significant overlap with those that gain DNMT3AR882H-associated hypo-DMRs in our murine model (Table S5; p < 0.05). We identified 119 genes showing CpG hypomethylation in both human AMLs and murine LSC models, which again include stemness and AML-promoting genes MEIS1, HOXA7 and MN1 (Figures 5J and S5I). We subsequently verified differential CpG methylation of DMRs at these genes in murine cells by direct bisulfite sequencing (Figures 5K and S5J), and further showed that a consistent hypomethylation pattern exists at conserved DMRs in human AMLs with DNMT3A R882 mutation, relative to those with non-R882 mutated or normal DNMT3A (Figures 5L and S5K).
Hypo-DMRs induced by DNMT3AR882H facilitate gain of histone acetylation at gene-regulatory sites
Because DNMT3AR882H binding and induced hypo-DMRs showed significant overlap with H3K4me1, a histone mark demarcating gene-regulatory regions such as enhancers and proximal elements close to promoters (Rada-Iglesias et al., 2011), we performed ChIP-Seq profiling of H3K27ac, a histone modification correlating to enhancer/promoter activity, with the samples we used for eRRBS. Intriguingly, we found that introducing DNMT3AR882H to HSPCs caused an overall gain of H3K27ac at DNMT3AR882H-associated hypo-DMRs (Figure 6A, left) whereas no overall change in H3K4me1 was seen for these hypo-DMRs (Figure 6B, left); in contrast, expression of DNMT3AWT decreased overall H3K27ac and H3K4me1 at these hypo-DMRs (Figures 6A and 6B, left). As a control, DNMT3AR882H-associated hyper-DMRs did not show such changes (Figures 6A and 6B, right). Consistently, similar histone modification changes were seen at regions in close proximity to DNMT3A-associated DMCs (Figures S6A and S6B). Importantly, DMRs at key stemness or AML genes such as Meis1, Mn1, Hoxa and Mycn all exhibited significant gain of H3K27ac in DNMT3AR882H-expressing HSPCs as well as loss of H3K27ac in DNMT3AWT-expressing HSPCs at their putative cis-regulatory sites (Figures 6C and S6C–S6H). By ChIP-qPCR, we verified the observed changes of H3K27ac and H3K4me1 at a panel of DMRs post-transduction of DNMT3AR882H versus DNMT3AWT into HSPCs (Figures 6D and S6I). Furthermore, expression of DNMT3AR882H enhanced binding of the H3K27 acetyltransferase p300 to hypo-DMRs at stemness genes (Figure 6E), suggesting that CpG hypomethylation facilitates recruitment of H3K27ac ‘writers’. In addition, overall gain of H3K27ac at hypo-DMRs was found significant regardless of expression changes of their associated genes (Figure S6J), indicating that H3K27ac gain at hypo-DMRs is not merely a consequence of gene activation, as exemplified by that found at hypo-DMRs of Kdm2b, Sirt4 and Pax5 (Figures S6K–S6M).
Figure 6. DNMT3AR882H-associated hypo-DMRs gain epigenetic alterations associated with gene activation.
(A–B) H3K27ac (panel A) and H3K4me1 (panel B) profiles at DNMT3AR882H-associated DMRs (bold on x-axis) and the surrounding regions. Averaged ChIP-Seq read densities in HSPCs with EV-RAS, RH-RAS or WT-RAS were plotted.
(C) H3K27ac and H3K4me1 profiles at Meis1 intron 6 in indicated HSPCs. Green bar, hypo-DMR.
(D–E) ChIP-qPCR of H3K27ac (panel D) and p300 binding (panel E) at hypo-DMRs in indicated HSPCs.
(F) Percentage of DNMT3AR882H-associated hypo-DMRs showing indicated H3K27ac changes in HSPCs with RH-RAS versus EV-RAS. Gain, increased H3K27ac; Loss, reduced H3K27ac; NC, no significant H3K27ac change. The total DMRs used for calculation were hypo-DMRs carrying H3K27ac (left) or H3K4me1 (right) in at least one cell condition.
(G) Percentage of DNMT3AR882H-associated hypo-DMRs (n = 1,199) showing H3K27ac gain in HSPCs with RH-RAS versus EV-RAS, when these hypo-DMRs are divided based on degree of DNA methylation reduction (x-axis) shown in the same samples.
(H) GSEA shows that genes with gain of H3K27ac at hypo-DMRs are enriched in HSPCs 16 days post-transduction of RH-RAS, relative to EV-RAS.
(I) Heatmap shows expression of genes in panel H ranked by higher expression in HSPCs with RH-RAS, relative to EV-RAS. The significantly upregulated genes in RH-RAS HSPCs are defined as “DNMT3AR882H signature genes” (n = 57), with selected ones listed along with their respective rankings (bottom).
(J) Quantification of expression-enhancing activity of DNMT3AR882H-associated hypo-DMRs with the embedded CpGs either non-methylated (CpG) or methylated (mCpG) using a CpG-free luciferase reporter system. The reporter without any DMR insertion was used as control. The p values were calculated by Student’s t-test.
(K) 3C assay shows looping interaction of the Meis1 intron 6 hypo-DMR (P4) to gene promoter (P0), relative to other tested sites.
(L–M) Scheme (panel L) and PCR validation (panel M) of CRISPR/Cas9-mediated deletion of the Meis1 intron 6 DMR. MOCK, parental LSCRH-RAS; Control, no sgRNA; sgMeis1, a pair of sgRNAs that target the DMR boundaries.
(N) Sequencing of the genomic PCR products from F2/R2 primers shows CRISPR/Cas9-induced deletion of the Meis1 intron 6 DMR.
(O) Expression levels of Meis1 in LSCRH-RAS lines shown in panel M. The p values were calculated by Student’s t-test by comparing to MOCK.
(P) Impact of DNA methylation levels in MEIS1 intron 6 in cytogenetically normal human AMLs grouped by DNMT3A WT (n = 45), non-R882 (n = 13) and R882 mutations (n =16). Plotted were mean methylation beta values of CpGs at MEIS1 intron 6 and log2-transformed expression values of RNA-Seq by Expectation-Maximization (RSEM). R2 and p values shown were determined with data of R882-mutant AMLs.
Error bar, +/− SD; **, p < 0.01; ***, p < 0.001; NS, not significant.
See also Figure S6 and Table S4 and S6.
Because DNMT3AR882H-induced hypo-DMRs can be found outside of gene-regulatory regions, we have focused on those overlapping with a peak of H3K4me1 (a total of 777 DMRs) or H3K27ac (333 DMRs) in at least one cell condition and found that, in either case, 9 to 11-fold more of DMRs showed enhanced H3K27ac levels than those with decreased H3K27ac (Figure 6F). These results indicate that DNA hypomethylation facilitates H3K27ac gain at gene-regulatory sites but also acts in a context-dependent manner. Consistently, more hypo-DMRs with gained H3K27ac were observed at regions showing a greater loss of CpG methylation (Figure 6G), supporting degree of DNA hypomethylation as a contributing factor that fine-tunes functional output of gene-regulatory sites. Moreover, genes with increased H3K27ac at their hypo-DMRs were found enriched in HSPCs expressing RH-RAS relative to EV-RAS (Figures 6H), from which we identified 57 genes as both epigenetically altered and transcriptionally activated by DNMT3AR882H (thus hereafter called as “DNMT3AR882H signature genes”, Figure 6I and Table S6). Notably, these DNMT3AR882H signature genes included DNMT3AR882-associated stemness genes we have studied above (a Meis1-Mn1-Hoxa node and Mycn) as well as other putative AML-promoting genes such as Id2, Bcl2 and Runx3 (Figure 6I).
The Meis1 intron 6 enhancer carrying DNMT3AR882H-induced CpG hypomethylation is crucial for Meis1 gene activation in LSCs
To demonstrate a causal role of DNMT3AR882H-induced focal DNA hypomethylation in gene expression regulation, we cloned sequences from a panel of hypo-DMRs into a CpG-free reporter system designed to assess putative gene-regulatory activity and its relationship to CpG methylation (Schmidl et al., 2009). We found all tested hypo-DMRs possess strong expression-enhancing activity in the absence of their CpG methylation (Figure 6J). CpG methylation of these hypo-DMRs completely abolished their expression-enhancing activities (Figure 6J), demonstrating a hypomethylation-dependent activation of cis-regulatory elements harbored within hypo-DMRs. To further verify DMR-associated enhancer activity in LSCsRH-RAS, we closely examined a hypo-DMR located in the intron 6 of Meis1 (Figure 6C, green bar) because Meis1 is a critical effector gene for DNMT3AR882H-associated AML progression (Figures 3H–3J) and this hypo-DMR is also found conserved in human AMLs with DNMT3A R882 mutation (Figures 5J–5L). Notably, this hypo-DMR is positive for H3K4me1 (Figure 6C) and has a significant overlap with a previously reported MEIS1 enhancer in human cells (Xiang et al., 2014). First, we carried out Chromosome Conformation Capture (3C), a surrogate assay for scoring enhancer usage and promoter association, and indeed detected a long-range looping interaction of the intron 6 hypo-DMR with the Meis1 promoter in LSCsRH-RAS (Figure 6K). To further determine the role of this putative intron 6 enhancer in DNMT3AR882H-induced Meis1 gene activation, we employed the CRISPR/Cas9-based genomic editing technology. Cas9 and a pair of single guide RNAs (sgRNA) targeting boundaries of the Meis1 hypo-DMR were transduced into LSCsRH-RAS (Figure 6L). PCR and direct sequencing confirmed sgRNA-mediated specific deletion of the hypo-DMR in five independent LSCRH-RAS lines (Figures 6M–6N and S6N). In all cases, ablation of this putative enhancer significantly reduced Meis1 expression (Figure 6O). Consistently, among human AMLs with DNMT3A R882 mutation, lower DNA methylation at the MEIS1 intron 6 correlated with higher expression of MEIS1 (Figure 6P). It is also worth noting that 54.5% (6/11) of DNMT3A WT AMLs display significant DNA methylation of MEIS1 intron 6 and yet express MEIS1 at high levels (Figure 6P), indicating different gene activation mechanisms exist in these AML cases. Together, using Meis1 as a paradigm example, we show that focal CpG hypomethylation induced by DNMT3A R882 mutation promotes enhancer activation and expression of key AML genes.
Dot1l inactivation suppresses DNMT3AR882H-associated LSC properties and aberrant activation of stemness gene programs
To explore the potential strategy for reversing DNMT3AR882H-induced gene deregulation and thus treating _DNMT3A_-mutated leukemia, we conducted compound treatment studies with a collection of epigenetic regulator inhibitors and identified that LSCsRH-RAS showed a significantly higher sensitivity to a Dot1l inhibitor, SGC0946, relative to control cells without DNMT3A mutation, i.e. LSCs expressing NRASG12D plus oncogenic TFs (Figure S7A). Dot1l, a histone H3 lysine 79 (H3K79) methyltransferase, belongs to a transcription elongation regulatory complex that engages acetylated histones at cis-regulatory sites (Li et al., 2014). Genomic profiling of H3K79 dimethylation (H3K79me2) detected its overall elevation at DNMT3AR882H-associated hypo-DMRs in HSPCs (Figure 7A), as exemplified by those at Meis1, Hoxa, Mn1 and Mycn (Figures 7B and S7B). We confirmed H3K79me2 gain at these genes by ChIP-qPCR (Figure S7C). Next, we asked if pharmacological inhibition of Dot1l could reverse DNMT3AR882H-induced gene activation. We first confirmed SGC0946-mediated suppression of H3K79me2 in LSCsRH-RAS (Figure S7D), followed by microarray profiling. Notably, after SGC0946 treatment, we detected significant downregulation of DNMT3AR882H signature genes (Figures 7C and 7D) and concurrent upregulation of myeloid differentiation genes in LSCsRH-RAS (Figures 7D and S7E). Although Hoxa and Meis1 were shown as part of MLL-AF9 target genes that are dependent on Dot1l (Chen et al., 2015), the DNMT3AR882H signature genes displayed a greater sensitivity to Dot1l inhibitors than MLL-AF9 targets in LSCsRH-RAS (Figure 7E); conversely, the DNMT3AR882H signature genes do not show overall response to Dot1l inhibitors in MLL-AF9_-_transformed AML cells (Figure S7F). These analyses indicate that DNMT3A R882 mutation confers a unique dependency on the Dot1l enzymatic activity in AML. We further verified downregulation of DNMT3AR882H-associated stemness genes Hoxa, Meis1, Mn1 and Mycn after treatment with SGC0946 (Figures 7F and 7G and S7G) or knockdown of Dot1l (Figures 7H and S7H and S7I). In response to Dot1l inactivation, multiple murine and human AML lines bearing DNMT3A mutation showed suppressed in vitro growth (Figures 7I and S7J and S7K) and concurrent cell differentiation (Figures 7J and 7K and S7L–S7N). _DNMT3A_-mutated human AML lines also had decreased HOXA or MEIS1 expression upon DOT1L blockade (Figures S7O and S7P). In contrast, various murine and human leukemia lines established by oncogenic TFs were all insensitive to Dot1l inhibition (Figures 7I and S7K). Also, enforced expression of HOXA9 plus MEIS1 reversed sensitivity of LSCsRH-RAS to Dot1l inhibition (Figure 7L), demonstrating a crucial role of these TFs in DNMT3AR882H-mediated oncogenic effects. Importantly, knockdown of Dot1l in LSCsRH-RAS or their pre-treatment with Dot1l inhibitors significantly delayed in vivo AML progression and prolonged survival of engrafted mice (Figure 7M). Collectively, we show that expression of DNMT3AR882H confers Dot1l dependency in AML and that reversing DNMT3AR882H-induced gene activation by Dot1l inhibition may provide a potential therapeutic means for the treatment of AMLs with DNMT3A mutation.
Figure 7. Dot1l inhibition reverses DNMT3AR882H-mediated aberrant transactivation of stem cell genes, thereby suppressing acute leukemogenicity.
(A) Averaged H3K79me2 ChIP-Seq signals at DNMT3AR882H-associated hypo-DMRs and hyper-DMRs in HSPCs with RH-RAS or EV-RAS.
(B) H3K79me2 profiles at Meis1 and Hoxa in indicated HSPCs.
(C) GSEA shows downregulation of DNMT3AR882H signature genes in LSCsRH-RAS post-treatment with 1 μM SGC0946 for 4 days.
(D) Heatmap shows downregulation of DNMT3AR882H signature genes and upregulation of myeloid differentiation genes in SGC0946-treated LSCsRH-RAS versus mock-treated.
(E) Boxplots show relative expression of DNMT3A signature genes (n = 54), MLL-AF9 gene targets (n = 129) and all genes in the genome in SGC0946-treated LSCsRH-RAS, relative to mock-treated. Horizontal line, median; box, interquartile range; whiskers, 10 to 90 percentiles. The p values were calculated by Mann-Whitney U test.
(F–G) RT-qPCR (panel F) and immunoblot (panel G) of indicated genes and proteins in LSCsRH-RAS 6 days post-treatment with SGC0946.
(H) Expression of indicated genes in LSCsRH-RAS transduced with Dot1l shRNAs or vector control.
(I) Relative growth of LSCsRH-RAS and other AML lines established by MLL-AF9, Hoxa9 plus Meis1 (A9M), A9M plus NRASG12D (A9M-RAS), and Hoxb8 plus Meis2 (WEHI3B) after a 12-day treatment with SGC0946 versus DMSO.
(J–K) Wright–Giemsa staining (panel J) and FACS analysis (panel K) of LSCsRH-RAS 6 days post-treatment with DMSO or 1 μM SGC0946. Scale bar, 10 μm.
(L) Effect of SGC0946 on growth of LSCsRH-RAS transduced with vector or Hoxa9 plus Meis1 (A9M). Relative proliferation was normalized to DMSO-treated cells.
(M) Survival of mice engrafted with LSCsRH-RAS, either mock-treated, stably transduced with a Dot1l shRNA, or pre-treated with 1 μM SGC0946 ex vivo for 6 days. The p values were calculated by log-rank test.
Error bar, +/− SD.
See also Figure S7 and Table S6.
DISCUSSION
In this study, we report a set of ex vivo LSC and in vivo murine AML model systems for studying functionality of DNMT3A R882 mutation in AML pathogenesis. Using these human disease-mimicking models, we have (1) defined a causal role of DNMT3AR882H in promoting AML transformation in vitro and in vivo; (2) identified DNMT3AR882H-deregulated gene pathways, including a Meis1-Mn1-Hoxa TF node that we functionally validated as essential for DNMT3AR882H-mediated AML progression; (3) shown that DNMT3AR882H directly binds to gene-regulatory sites, notably enhancers, inducing focal DNA hypomethylation and concurrent gain of histone acetylation; (4) determined a critical role of the epigenetically altered enhancer and cis-regulatory elements for DNMT3AR882H-associated gene activation; and (5) importantly, we have also demonstrated that pharmacological inhibition of Dot1l reverses the mutant DNMT3A-associated gene activation, thus providing a potential therapeutic avenue for the affected AMLs.
The molecular pathways identified in this study help explain several important biological phenomena related to DNMT3A mutation and hematological disease. First, as the Meis1-Mn1-Hoxa circuitry is crucial for both normal expansion of HSCs and malignant transformation of LSCs (Argiropoulos and Humphries, 2007; Heuser et al., 2011), deregulation of this TF node by R882-mutated DNMT3A provides a molecular explanation not only for malignant hematopoiesis but also for clonal hematopoiesis, a phenotype strongly associated with DNMT3A mutation (Genovese et al., 2014; Jaiswal et al., 2014; Xie et al., 2014). In addition, these findings help explain a mutually exclusive pattern for DNMT3A mutation and MLL rearrangement in AMLs (Cancer Genome Atlas Research, 2013; Patel et al., 2012) because the latter itself is a strong inducer of Meis1 and Hoxa activation (Chi et al., 2010).
Our results also demonstrate requirement of cooperation between DNMT3A mutation and the activated kinase such as RAS for AML induction. RAS mutation alone induces an hyper-proliferative phenotype but does not support self-renewal, which is in agreement with previous studies (Zhang et al., 2009); RAS activation was also known to induce cell senescence, a barrier of cancer development (Campisi and d’Adda di Fagagna, 2007). On the other hand, DNMT3A mutation confers aberrant HSPC self-renewal, blocks differentiation programs and yet lacks pro-proliferation effect; besides a Meis1-Mn1-Hoxa node we have functionally confirmed as essential for DNMT3AR882H-associated AML, other downstream targets of DNMT3AR882H, such as pro-survival (Bcl2), anti-differentiation (Id2) and stemness (Mycn) genes, might be equally crucial for AML progression. These findings suggest that synergy between DNMT3A and kinase mutations is likely due to their differential effects on pathways relating to AML development. However, it is also possible that the two mutations may affect distinctive sets as well as same sets of downstream effectors via genetic or epigenetic mechanisms. A similar synergy is most likely to exist between DNMT3A mutation and the activated FLT3, which acts upstream of RAS and coexists with the former in human AMLs as well.
Our studies clearly show that DNMT3A mutation-induced CpG hypomethylations are not random: they are significantly enriched at gene-regulatory sites, notably, putative enhancers marked by H3K4me1 as well as the binding sites of master hematopoietic TFs. Precise mechanisms by which CpG methylation of these cis-regulatory sites regulates gene expression remain to be fully studied. For example, despite a large number of DMCs found associated with either DNMT3A or TET2 mutation in AML, a relatively small number of genes show changes in their expression (Russler-Germain et al., 2014; Shih et al., 2015). A possible explanation is that effect of CpG methylation on gene expression is context dependent (Baubec and Schubeler, 2014): degree of CpG methylation change, density or genomic location of CpG, methyl-CpG “readers” and TF binding are all possible factors affecting the ultimate effect of DNA methylation on gene expression. Unlike histone (de)acetylation, CpG (de)methylation at distal cis-regulatory sites such as enhancers may act as a permissive mechanism influencing gene expression, rather than a strong and instructive one controlling levels of gene activation and transcription. Nevertheless, using reporter assays and CRISPR/Cas9-mediated enhancer editing, we have determined the role of select hypo-DMRs in activation of associated target genes such as Meis1.
This study also provides useful information on how to treat _DNMT3A_-mutated AMLs. Pharmacological blockade of Dot1l reversed DNMT3A mutation-induced gene activation resulting in an impaired AML pathogenesis. In the future, examination of other ‘druggable’ factors would likely identify additional therapeutic strategies for the treatment of _DNMT3A_-mutated AMLs. Therefore, in addition to elucidating the underlying oncogenic mechanisms, the ex vivo and in vivo model systems presented herein should be useful for exploring AML therapeutics.
EXPERIMENTAL PROCEDURES
In vitro colony-forming unit (CFU) assay with serial replating
Following lineage-negative (Lin−) enrichment and retroviral transduction, 30,000 of infected HSPCs were plated in the semi-solid methylcellulose cultivation system (Methocult, Stem Cell Technologies), followed by CFU counting and replating for every 10–14 days according to manufacturer’s protocol.
Animal studies and In vivo leukemogenic assay
All animal experiments were approved by and performed in accord with the guidelines of the Institutional Animal Care and Use Committee at the University of North Carolina (Chapel Hill, NC). Leukemogenic potentials of transduced HSPCs were evaluated by BMT into sublethally irradiated syngeneic mice. Briefly, 200,000 of bone marrow-derived Lin− HSCPs following procedures of cytokine stimulation, retroviral transduction and drug selection were injected via tail veil to recipient mice as described before (Wang et al., 2009).
Statistical Analysis
Data are presented as the mean ± SD for 3 independent experiments unless otherwise noted. Statistical analysis was performed with Student’s t-test for comparing two sets of data with assumed normal distribution. We used Mann-Whitney U test for data not showing a normal distribution, chi-square test for categorical variables, and log-rank test for Kaplan-Meier survival curves to determine statistical significance. A p value < 0.05 was considered significant.
The detailed procedures of plasmid construction, cell culture, antibody and immunoblot, flow cytometry, microarray analysis, ChIP-Seq, eRRBS, Exome-Seq, RT-qPCR, ChIP-qPCR, 3C-qPCR, shRNA-mediated knockdown, luciferase reporter assay, CRISPR/Cas9-mediated genomic editing, as well as the detailed information for computational and statistical analysis of deep sequencing data, are described in Supplemental Experimental Procedures.
Supplementary Material
1
2
3
4
SIGNIFICANCE.
Recurrent DNMT3A mutations at Arg882 are found in hematological malignancies and disorders; however, due to a lack of relevant disease models, molecular mechanisms by which DNMT3A mutations influence leukemogenesis remain largely undefined. Through establishment and characterization of murine leukemia and leukemia stem cell models, we show that DNMT3AR882H mutation potentiates transactivation of stemness genes required for acute leukemogenicity. Integrated epigenomic profiling of murine models further reveals the underlying epigenetic alterations induced by DNMT3AR882H, which are enriched at gene-regulatory sites and resemble those seen in human patients. Pharmacological inhibition of Dot1l suppresses DNMT3AR882H-associated gene activation and acute leukemogenesis. Our findings not only promote mechanistic understandings of DNMT3A mutation-associated clonal and malignant hematopoiesis but also provide a therapeutic avenue for _DNMT3A_-mutated leukemias.
Highlights.
- DNMT3AR882H promotes acute leukemogenicity in the presence of mutant NRAS
- DNMT3AR882H induces focal DNA hypomethylation at cis-elements of key stemness genes
- DNMT3AR882H potentiates stemness gene expression via enhancer/promoter activation
- DNMT3AR882H-induced gene activation programs are sensitive to Dot1l blockade
Acknowledgments
We graciously thank Drs. Y. Xiong and K. Humphries for providing constructs, M. Kamps and M. Minden for leukemia lines, M. Torres for Meis1 antibody, D. Bauer and F. Zhang for CRISPR/Cas9 systems, M. Rehli for a CpG-free reporter, and J. Bear for an shRNA vector used in this study. Thanks to Drs. D. Allison and L. Cai and other members of the Wang laboratory for helpful discussion and technical support. We thank UNC’s Genomics Core, Animal Studies Core, Flow Core, and HTSF core for their supports of this work. This work is supported by NCI K99/R00 grant CA151683 to G.G.W., a DoD grant CA130247 to G.G.W., and grants of Gabrielle’s Angel Foundation to O.A. and G.G.W. G.G.W. is an American Society of Hematology (ASH) Scholar and a Kimmel Scholar. R.L. is a Lymphoma Research Foundation Postdoc Fellow. UNC Cores including flow cytometry facility are supported in part by the North Carolina Biotech Center Institutional Support Grant (2012-IDG-1006) and UNC Cancer Center Core Support Grant (P30 CA016086).
Footnotes
ACCESSION NUMBERS
The microarray, eRRBS-seq, and ChIP-seq data reported in this paper have been deposited to Gene Expression Omnibus (GEO) with an accession number GSE71475.
AUTHOR CONTRIBUTIONS
R.L. designed the research, performed experiments and computational analysis, interpreted data, and wrote the manuscript. P.W., S.R. and D.Z. performed computational analysis. T.P., Y.Z. and W.C. helped on animal modeling, viral transduction, and immunostaining studies, respectively. K.C. and P.A.W. participated in DNA methylome studies. O.A. helped on human AML cell studies. D.Z. supervised the computational studies. G.G.W. supervised the work, designed the research, interpreted data, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramovich C, Pineault N, Ohta H, Humphries RK. Hox genes: from leukemia to hematopoietic stem cell expansion. Annals of the New York Academy of Sciences. 2005;1044:109–116. doi: 10.1196/annals.1349.014. [DOI] [PubMed] [Google Scholar]
- Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26:6766–6776. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- Baubec T, Schubeler D. Genomic patterns and context specific interpretation of DNA methylation. Current opinion in genetics & development. 2014;25:85–92. doi: 10.1016/j.gde.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik H, Mallaney C, Kothari A, Ostrander EL, Eultgen E, Martens A, Miller CA, Hundal J, Klco JM, Challen GA. Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood. 2015;125:619–628. doi: 10.1182/blood-2014-08-594564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nature genetics. 2012;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YI, You X, Kong G, Ranheim EA, Wang J, Du J, Liu Y, Zhou Y, Ryu MJ, Zhang J. Loss of Dnmt3a and endogenous Kras(G12D/+) cooperate to regulate hematopoietic stem and progenitor cell functions in leukemogenesis. Leukemia. 2015;29:1847–1856. doi: 10.1038/leu.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Koche RP, Sinha AU, Deshpande AJ, Zhu N, Eng R, Doench JG, Xu H, Chu SH, Qi J, et al. DOT1L inhibits SIRT1-mediated epigenetic silencing to maintain leukemic gene expression in MLL-rearranged leukemia. Nature medicine. 2015;21:335–343. doi: 10.1038/nm.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10:457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nature medicine. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The New England journal of medicine. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang L, Li J, Ding Z, Xiao J, Yin X, He S, Shi P, Dong L, Li G, et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature. 2015;517:640–644. doi: 10.1038/nature13899. [DOI] [PubMed] [Google Scholar]
- Heuser M, Yun H, Berg T, Yung E, Argiropoulos B, Kuchenbauer F, Park G, Hamwi I, Palmqvist L, Lai CK, et al. Cell of origin in AML: susceptibility to MN1-induced transformation is regulated by the MEIS1/AbdB-like HOX protein complex. Cancer cell. 2011;20:39–52. doi: 10.1016/j.ccr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz-Schietinger C, Matje DM, Reich NO. Mutations in DNA methyltransferase (DNMT3A) observed in acute myeloid leukemia patients disrupt processive methylation. The Journal of biological chemistry. 2012;287:30941–30951. doi: 10.1074/jbc.M112.366625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Sitwala K, Bronstein J, Sanders D, Dandekar M, Collins C, Robertson G, MacDonald J, Cezard T, Bilenky M, et al. Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood. 2012;119:388–398. doi: 10.1182/blood-2011-03-341081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. Age-related clonal hematopoiesis associated with adverse outcomes. The New England journal of medicine. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews Genetics. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Zhao H, Hardikar S, Singh AK, Goodell MA, Chen T. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood. 2013;122:4086–4089. doi: 10.1182/blood-2013-02-483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, Zaretsky I, Jaitin DA, David E, Keren-Shaul H, Mildner A, Winter D, Jung S, et al. Immunogenetics. Chromatin state dynamics during blood formation. Science (New York, NY. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, et al. DNMT3A mutations in acute myeloid leukemia. The New England journal of medicine. 2010;363:2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wen H, Xi Y, Tanaka K, Wang H, Peng D, Ren Y, Jin Q, Dent SY, Li W, et al. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell. 2014;159:558–571. doi: 10.1016/j.cell.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV, Challen GA, Li W, Wheeler D, Rebel VI, Goodell MA. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood. 2015;125:629–638. doi: 10.1182/blood-2014-08-594648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh KM, Wang H, Kim HR, Wenderski W, Fang F, Li CH, Dewell S, Hughes SH, Melnick AM, Patel DJ, et al. Engineering of a Histone-Recognition Domain in Dnmt3a Alters the Epigenetic Landscape and Phenotypic Features of Mouse ESCs. Molecular cell. 2015;59:89–103. doi: 10.1016/j.molcel.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. The New England journal of medicine. 2012;366:1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russler-Germain DA, Spencer DH, Young MA, Lamprecht TL, Miller CA, Fulton R, Meyer MR, Erdmann-Gilmore P, Townsend RR, Wilson RK, Ley TJ. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer cell. 2014;25:442–454. doi: 10.1016/j.ccr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidl C, Klug M, Boeld TJ, Andreesen R, Hoffmann P, Edinger M, Rehli M. Lineage-specific DNA methylation in T cells correlates with histone methylation and enhancer activity. Genome research. 2009;19:1165–1174. doi: 10.1101/gr.091470.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- Shih AH, Jiang Y, Meydan C, Shank K, Pandey S, Barreyro L, Antony-Debre I, Viale A, Socci N, Sun Y, et al. Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer cell. 2015;27:502–515. doi: 10.1016/j.ccell.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GG, Cai L, Pasillas MP, Kamps MP. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nature cell biology. 2007;9:804–812. doi: 10.1038/ncb1608. [DOI] [PubMed] [Google Scholar]
- Wang GG, Pasillas MP, Kamps MP. Meis1 programs transcription of FLT3 and cancer stem cell character, using a mechanism that requires interaction with Pbx and a novel function of the Meis1 C-terminus. Blood. 2005;106:254–264. doi: 10.1182/blood-2004-12-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GG, Pasillas MP, Kamps MP. Persistent transactivation by meis1 replaces hox function in myeloid leukemogenesis models: evidence for co-occupancy of meis1-pbx and hox-pbx complexes on promoters of leukemia-associated genes. Molecular and cellular biology. 2006;26:3902–3916. doi: 10.1128/MCB.26.10.3902-3916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GG, Song J, Wang Z, Dormann HL, Casadio F, Li H, Luo JL, Patel DJ, Allis CD. Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature. 2009;459:847–851. doi: 10.1038/nature08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Iwasaki M, Somervaille TC, So CW, Cleary ML. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes & development. 2007;21:2762–2774. doi: 10.1101/gad.1602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang P, Wei W, Lo C, Rosten P, Hou J, Hoodless PA, Bilenky M, Bonifer C, Cockerill PN, Kirkpatrick A, et al. Delineating MEIS1 cis-regulatory elements active in hematopoietic cells. Leukemia. 2014;28:433–436. doi: 10.1038/leu.2013.287. [DOI] [PubMed] [Google Scholar]
- Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature medicine. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang YY, Dai YJ, Zhang W, Zhang WN, Xiong SM, Gu ZH, Wang KK, Zeng R, Chen Z, Chen SJ. DNMT3A Arg882 mutation drives chronic myelomonocytic leukemia through disturbing gene expression/DNA methylation in hematopoietic cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2620–2625. doi: 10.1073/pnas.1400150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, Shi JY, Zhu YM, Tang L, Zhang XW, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nature genetics. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- Yang L, Rau R, Goodell MA. DNMT3A in haematological malignancies. Nat Rev Cancer. 2015;15:152–165. doi: 10.1038/nrc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang J, Liu Y, Sidik H, Young KH, Lodish HF, Fleming MD. Oncogenic Kras-induced leukemogeneis: hematopoietic stem cells as the initial target and lineage-specific progenitors as the potential targets for final leukemic transformation. Blood. 2009;113:1304–1314. doi: 10.1182/blood-2008-01-134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4