Expression of Programmed Cell Death 1 Ligand 2 (PD-L2) is a Distinguishing Feature of Primary Mediastinal (Thymic) Large B-cell Lymphoma and Associated with PDCD1LG2 Copy Gain (original) (raw)
. Author manuscript; available in PMC: 2016 Aug 23.
Published in final edited form as: Am J Surg Pathol. 2014 Dec;38(12):1715–1723. doi: 10.1097/PAS.0000000000000297
Abstract
Primary mediastinal (thymic) large B-cell lymphoma (PMBL) and diffuse large B-cell lymphoma (DLBCL) are tumors with distinct clinical and molecular characteristics that are difficult to distinguish by histopathological and phenotypic analyses alone. Programmed cell death 1 ligand 2 (PD-L2) is a cell surface protein expressed by activated macrophages and dendritic cells that binds PD-1 on T-cells to inhibit immune responses. Amplification and/or translocations involving chromosome 9p24.1, a region that includes PDCD1LG2 encoding PD-L2, is a common event in PMBL but not DLBCL and suggests that PD-L2 expression might be a distinguishing feature of PMBL. We developed an assay for the immunohistochemical detection of PD-L2 protein in fixed biopsy specimens (PD-L2 IHC) which we applied to a cohort of PMBLs and DLBCLs. For a subset of cases, we correlated the results of PD-L2 IHC with PDCD1LG2 copy number as determined by qPCR. Twenty-three of 32 (72%) PMBLs but only 1 of 37 (3%) DLBCLs were positive by PD-L2 IHC. Among PMBLs with PDCD1LG2 copy number gain, all were positive by PD-L2 IHC. One PMBL without copy number gain was positive by PD-L2 IHC. When expressed in PMBL, PD-L2 was restricted to tumor cells and not detected on intra-tumoral macrophages. We conclude that PD-L2 protein is robustly expressed by the majority of PMBLs but only rare DLBCLs and often associated with PDCD1LG2 copy gain. PD-L2 IHC may serve as a useful ancillary test for distinguishing PMBL from DLBCL and for the rational selection of patients for therapeutic antibodies that inhibit PD-1 signaling.
Keywords: PD-1, PD-L2, Primary Mediastinal Large B-cell Lymphoma, Diffuse Large B-cell Lymphoma, PDCD1LG2
INTRODUCTION
Primary mediastinal (thymic) large B-cell lymphoma (PMBL) and diffuse large B-cell lymphoma, not otherwise specified (DLBCL) are aggressive tumors of mature B-cells with distinct clinical and genetic characteristics that can be difficult to distinguish in routine surgical pathology practice.(1–5) PMBL and DLBCL have extensive histomorphologic and phenotypic similarities including a sheet-like growth pattern of large lymphoid cells and the expression of pan-B lineage markers such as CD19, CD20, CD79a, and PAX5.(6, 7) Other phenotypic markers useful for the subclassification of B-cell lymphomas, including BCL6, IRF4/MUM1, and CD30, can be expressed by PMBL and DLBCL. MAL, TRAF1, nuclear cRel, CD23, and CD200 are preferentially expressed by PMBL, and immunohistochemical analysis for one or more can facilitate final tumor classification, but no single marker is entirely sensitive and specific.(8–12)
Programmed cell death-1 (PD-1) and the programmed death ligands (PDLs), PD-L1 (CD274, B7-H1) and PD-L2 (CD273, B7-DC), form a signaling network that limits T-cell immunity during chronic viral infection and during pregnancy.(13, 14) A subset of malignant tumors co-opts the PD-1 signaling axis to evade immune surveillance.(15) Targeting PD-1 signaling with inhibitory antibodies can relieve the block in anti-tumor immunity and achieve durable clinical responses in a subset of patients with solid tumors.(16, 17) We, and others, have found that 70% of PMBLs but <5% of DLBCL, have amplification of chromosome 9p24.1- a region that includes CD274 (encoding PD-L1) and PDCD1LG2 (encoding PD-L2).(5, 18, 19, 20) Consistent with these results, we have found that PMBL cell lines express PDLs whereas DLBCL cell lines do not.(18) These results indicate that CD274 and PDCD1LG2 amplification can result in increased PD-L1 and PD-L2 protein production.
Recently, we surveyed the protein expression of PD-L1 on aggressive lymphomas and found tumor-specific expression of PD-L1 to be common in PMBL but not DLBCL.(21) We also found that PD-L1 is highly expressed by tumor-associated macrophages (TAMs) in most PMBL and in a subset of DLBCL- a result that complicates the interpretation of PD-L1 IHC.(21) A detailed examination of PD-L2 protein expression in PMBL and DLBCL has not been performed due to the lack of quality antibodies that are amenable to immunohistochemistry (IHC) on formalin-fixed paraffin-embedded (FFPE) tissue sections. Herein, we establish an immunohistochemical staining protocol for the detection of PD-L2 protein in FFPE samples that we apply to a series of fixed, primary PMBL and DLBCL tissue biopsies. For a subset of cases with matched frozen tissue, we compared the results of PD-L2 IHC with the results of PDCD1LG2 copy number analysis.
MATERIALS AND METHODS
Cell lines
Cell lines (300.19, SU-DHL-4, Karpas 1106P, HDLM-2) were maintained as previously described.(18, 22) FFPE cell pellets were generated by harvesting 107 cells, washing, and fixing in 2ml 10% formalin at room temperature for 20 minutes. Cells were then washed, suspended in matrigel, processed, embedded in paraffin as a cell pellet tissue microarray, and cut onto glass slides as per standard histological procedures.
Case Selection
All FFPE biopsy samples (PMBL, n=32; DLBCL, n=37) were obtained from the files of the Department of Pathology at Brigham and Women’s Hospital, Boston, MA, with institutional internal review board approval. Diagnoses were established according to the criteria of the WHO classification.(1) Cases classified as PMBL were confirmed, as part of this study, to satisfy the combination of clinical, radiologic, morphologic and phenotypic criteria for this entity. These include radiologic identification of an isolated mediastinal mass in a young or middle aged individual with or without local extension into adjacent tissues and organs, a morphologic pattern showing sheets of large atypical lymphoid cells without or without a sclerotic background and scattered thymic remnants, and positive staining of tumor cells for mature B lymphoid markers, TRAF1 and/or cRel, and/or CD23 and/or CD30, and an absence of CD15 expression as previously described.(23) For a subset of cases (PMBL, n=12; DLBCL, n=9), matched frozen tumor samples were obtained at the time of biopsy.
Immunohistochemistry
Immunohistochemistry (IHC) was performed using 4-μm-thick, formalin or B+-fixed, paraffin-embedded (FFPE) tissue sections. Slides were baked, deparaffinized in xylene and passed through graded alcohols; antigen was then retrieved with 10mM citrate buffer, pH6.0 (Invitrogen/ Life Technologies, Grand Island, NY), in a steamer for 30 minutes. All further steps were carried out at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (Dako, Carpinteria, CA) for 5 minutes to quench endogenous peroxidase activity, and then washed in 50mM Tris-Cl, pH7.4. Slides were blocked using Protein Block (Dako) as per manufacturer’s instruction, and subsequently incubated with anti-PDL2 (clone 366C.9E5, 0.14 micrograms/ ml final concentration, generated in the laboratory of G. Freeman, gordon_freeman@dfci.harvard.edu) in Da VinCi green diluent (BioCare Medical, Concord, CA) for 1 hour or anti-CD68 (clone PG-M1, DAKO, 1:150 dilution) in antibody diluent (Dako) for 1 hour. Slides were then washed in Tris buffer and, for anti-PD-L2, treated with anti-mouse horseradish peroxidase-conjugated antibody (PowerVision; Leica Biosystems, Buffalo Grove, IL) for 30 minutes or, for anti-CD68, treated with mouse envision secondary antibody (Dako) for 30 minutes. After further washing, immunoperoxidase staining was developed using a 3,3’diaminobenzidine (DAB) chromogen (Dako) for 5 minutes. Slides were counterstained with hematoxylin, dehydrated in graded alcohol and xylene, and mounted and coverslipped.
Double staining for PD-L2 and CD68 was performed using an automated staining system (Bond III, Leica Biosystems). Heat induced antigen retrieval was performed using ER1 solution (pH6) (Leica Biosystems) for 30 minutes. Anti-PD-L2 antibody was incubated for a total of 2 hours with two separate applications. Post-primary AP blocking reagents were incubated for 20 minutes, followed by 15 minutes of AP-labeled polymer. Red substrate was incubated for 15 minutes. All reagents were components of the Bond Polymer AP Red detection system (Leica Biosystems). Anti-CD68 (PG-M1) immunostaining was performed subsequently with a 1:150 dilution using Bond Primary Antibody Diluent. CD68 primary antibody was incubated for one hour, follow by 8 minutes of post-primary blocking reagent, 12 minutes of horseradish peroxidase-labeled polymer and 5 minutes of peroxidase block. All reagents were components of the Bond Polymer Refine detection system (Leica Biosystems). Slides were then taken off the autostainer and HighDef Blue IHC chromogen (HRP) (Enzo Life Sciences, Farmingdale, NY) was applied manually and incubated for 10 minutes. Slides were coverslipped with Dako Faramount Aqueous Mounting medium (Dako).
Evaluation and Scoring of Immunohistochemical Staining
Reactivity for PD-L2 was determined and scored by two hematopathologists (MS and SJR). Discrepant results in staining interpretation (<5% of cases) were resolved in a consensus conference. For each stained slide, the percentage of tumor cells showing positive staining for PD-L2 was recorded in 10% increments (0–100%). In addition, the intensity of positive staining was recorded: (−) = no staining detected, (1+) = weak staining, (2+) = moderate staining, (3+) = strong staining. A case was scored as positive if at least 20% of the tumor cells stained positive for PD-L2 with an intensity of 1+, 2+, or 3+.
DNA Isolation and PDCD1LG2 Copy Number Analysis
Genomic DNA was extracted from cryostat sections of frozen biopsy tissues as previously described.(24,18) Copy number (CN) of PDCD1LG2 (locus of PD-L2) at chromosome 9p24.1 was assessed with the Taqman CN assay Hs00797077_cn. RNase P was used as reference control (#4403326). Quantitative Polymerase Chain Reaction (PCR) was performed with Taqman Universal Genotyping Master Mix (Applied Biosystems/ Life Technologies) on an ABI 7300/7500 real-time PCR machine according to the manufacturer’s protocol. The CN of the loci of PD-L2 was inferred by the 2-ΔΔCT method,(25) normalized to the mean of 9 normal samples (2 reactive tonsils and 7 reactive lymph nodes). A CN above the threshold of 2.2 was assigned as CN gain, consistent with previous analyses.(18). Hodgkin lymphoma cell lines with well-defined CN status of this region were used as positive controls.(18)
Imaging and Statistics
Cases were photographed with an Olympus BX41 microscope with 100x/0.75 Olympus UPlanFL (Olympus, Melville, NY) objective at 1000x final magnification. All pictures were taken using Olympus QColor3 camera and analyzed with acquisition software QCapture v2.60 (QImaging, Burnaby, BC, Canada) and Adobe Photoshop 6.0 (Adobe, San Jose, CA).
RESULTS
Validation of anti-PD-L2 antibody for IHC
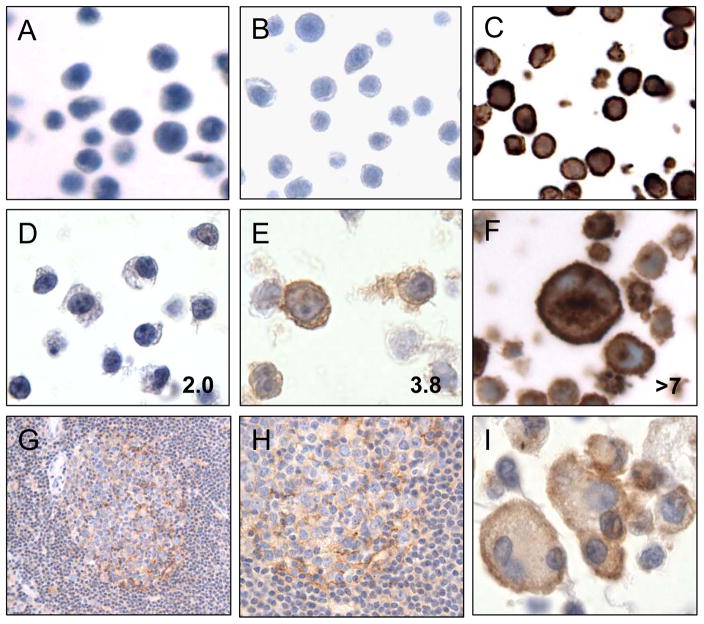
We optimized IHC staining using a mouse monoclonal antibody targeting human PD-L2 (PD-L2 IHC) on a series of genetically defined, FFPE cell lines. We observed no staining of an untransfected, mouse pre-B cell line 300.19 (Figure 1A) and no staining of line 300.19 stably transfected with full-length, human PD-L1 (Figure 1B). In contrast, we observed intense staining of line 300.19 stably transfected with full-length, human PD-L2 in a cytoplasmic and cell membrane pattern (Figure 1C). The DLBCL cell line SU-DHL-4, which has the normal two copies of 9p24.1 and no detectable PD-L2 protein by flow immunophenotyping or western blot analysis,(18, 21) showed no staining for PD-L2 protein by IHC (Figure 1D). In contrast, the PMBL cell line Karpas 1106P, which has intermediate-level amplification of PDCD1LG2 at 9p24.1 (average 3.8 copies) and expresses PD-L2 protein,(18) showed weak but positive staining for PD-L2 by IHC (Figure 1E). The Hodgkin lymphoma cell line, HDLM2, which has high-level amplification of PDCD1LG2 at 9p24.1 (>7 copies) and expresses PDL2 protein,(18, 21) showed intense cytoplasmic and membrane staining for PD-L2 by IHC (Figure 1F). These results verify that PD-L2 IHC is sensitive and specific and demonstrate a correlation between the intensity of staining and PDCD1LG2 copy number.
Figure 1.
Immunohistochemical staining of formalin-fixed, paraffin-embedded (FFPE) cell lines and select tissues with anti-PD-L2 antibody. Staining of A) Untransfected murine pre-B cell line, 300.19, B) Pre-B line stably expressing human PD-L1, C) Pre-B line stably expressing human PD-L2, D) Diffuse large B-cell lymphoma cell line SU-DHL-4, E) Mediastinal large B-cell lymphoma cell line Karpas 1106P, F) Hodgkin lymphoma line HDLM-2, G) Lymph node showing a reactive germinal center (400x), H) High power image of a reactive germinal center (1000x), I) Macrophages, including a multi-nucleated giant cell, associated with necrotic tissue. The average PDCD1LG2 copy number for each human cell line is indicated in the bottom right corner of each image. All images 1000x original magnification unless indicated.
Staining of human lymphoid tissues revealed staining of expected structures. Specifically, reactive lymph nodes showed weak staining in a pattern consistent with follicular dendritic cells within germinal centers (Figure 1G, H). Lymphocytes within the B-cell rich follicles and T-cell rich inter-follicular regions were negative for staining. Similarly macrophages within germinal centers, inter-follicular regions and sub-capsular sinuses were negative for PD-L2 (Figure 1H, and data not shown). Upon staining additional tissue specimens, we observed positive staining of activated macrophages and multi-nucleated giant cells associated with marked, acute inflammation in a distinct, membranous pattern (Figure 1I). These data suggest that PD-L2 is minimally expressed by immune cells within reactive lymphoid tissue but can be induced to high levels on tissue macrophages and giant cells associated with acute inflammation.
Expression of PD-L2 in PMBL and DLBCL
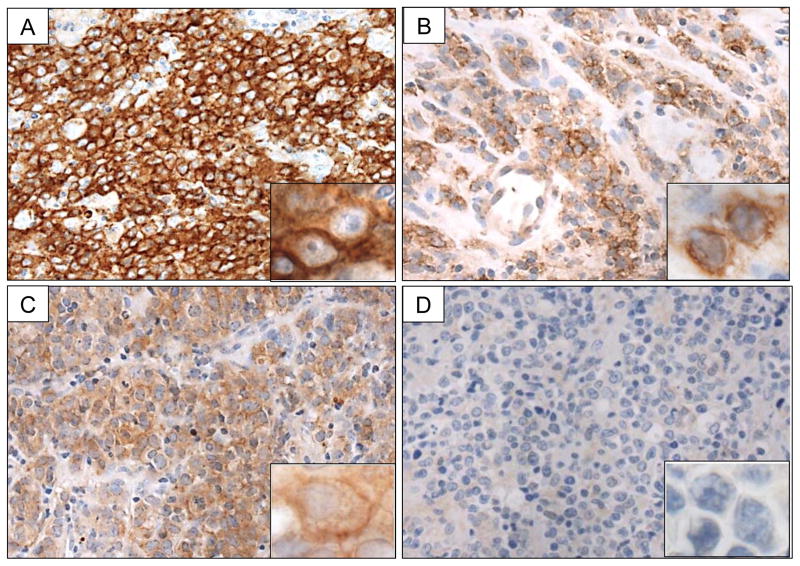
Next, we investigated PD-L2 expression by IHC in a collection of 32 PMBLs and 37 DLBCLs. There was a spectrum of staining patterns across tumor samples. Cases scored as positive for PD-L2 showed distinct membranous staining in ≥20% tumor cells at 3+ (Figure 2A), 2+ (Figure 2B), or 1+ (Figure 2C) intensity. Cases not fulfilling these criteria were scored as negative for PD-L2 (Figure 2D). Twenty-three of 32 (72%) PMBLs were scored as positive for PD-L2 by this method (Table 1). In contrast, only 1 of 37 (3%) DLBCLs were scored as positive (p < 0.001, Fisher's exact test). Among the positive PMBLs, 19 cases (83%) had membranous PD-L2 expression in ≥60% of the malignant cells and 4 cases (17%) had similar staining in 20–59% of the malignant cells (Table 2). The majority of positive PMBLs expressed PD-L2 in a membranous pattern and at 3+ intensity (18/23, 78%) (Table 2). Four PMBLs (17%) showed positive staining at 2+ intensity and one PMBL (4%) showed positive staining 1+ intensity (Table 2). The single DLBCL case scored as positive for PD-L2 showed positive staining of 20% of the malignant cells at 2+ intensity. Re-review of the clinical, histomorphological, and phenotypic features of this case did not suggest misclassification of a PMBL (data not shown). Taken together these findings indicate that PD-L2 IHC is a highly specific (97%) and moderately sensitive (72%) method for distinguishing PMBL from DLBCL.
Figure 2.
PD-L2 IHC Staining Patterns in PMBL. Cases showing A) intense membranous staining scored as 3+, B) moderate membranous staining scored as 2+, C) light membranous staining scored as 1+, D) no membranous staining scored as 0. All images 1000x original magnification.
TABLE 1.
PD-L2 Expression in PMBL and DLBCL
| Diagnosis | No. Cases | Positive * | Negative |
|---|---|---|---|
| PMBL | 32 | 23 (72%) | 9 (28%) |
| DLBCL | 37 | 1 (3%) | 36 (97%) |
TABLE 2.
Percentage and Intensity of PD-L2 Expression in PMBL and DLBCL
| Diagnosis | PD-L2 + | Percentage | Intensity | |||
|---|---|---|---|---|---|---|
| ≥60% | 20–59% | +++ | ++ | + | ||
| PMBL | 23 | 19(83%) | 4(17%) | 18(78%) | 4(17%) | 1(4%) |
| DLBCL | 1 | 0(0%) | 1(100%) | 0(0%) | 1(100%) | 0(0%) |
9p24.1/ PDCD1LG2 copy number and PD-L2 Expression in PMBL and DLBCL
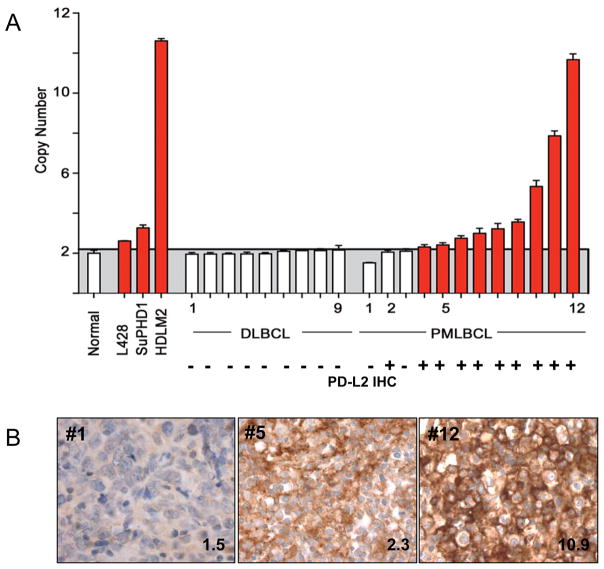
Up to 70% of PMBLs have amplification of chromosome 9p24.1, a region that includes the genes encoding both PD-1 ligands, PDCD1LG2 and CD274.(18, 26) In primary PMBL, there is a close association between PDCD1LG2 copy gain and increased PD-L2 transcript abundance.(18) We therefore evaluated the relationship between PDCD1LG2 copy number and PD-L2 protein expression in 12 PMBLs and 9 DLBCLs for which we had matched frozen and paraffin tissue samples using a quantitative PCR assay for PDCD1LG2 copy number and PD-L2 IHC (details see Materials and Methods). Previously characterized Hodgkin lymphoma cell lines with known low (L428, 2.6 copies), intermediate (SUP-HD1, 3.3 copies), and high (HDLM2, >7 copies) PDCD1LG2 copy number served as positive controls (Figure 3).(18) None of the DLBCLs showed amplification of PDCD1LG2 consistent with the lack of staining for PD-L2 by IHC (Figure 3). In contrast, 9 of 12 (75%) PMBLs had PDCD1LG2 amplification and all 9 were positive for PD-L2 by IHC (Figures 3A and B). Interestingly, one PMBL without PDCD1LG2 copy gain (PMBL case #2,) was positive for PD-L2 by IHC. Whereas two of three PMBLs lacking PDCD1LG2 copy number gain (PMBL cases #1; #3) showed no PD-L2 staining, cases with even low PDCD1LG2 copy number gain (i.e. PMBL case #5, 2.3 average copies) exhibited distinctly positive, membranous PD-L2 staining (Figure 3B). As expected, cases with high copy number gain (i.e. PMBL case #12, 10.9 average copies) were associated with very intense, membranous staining for PD-L2 (Figure 3B). These data indicate that PD-L2 IHC effectively captures PD-L2 protein expression in all cases of PMBL with PDCD1LG2 copy number gain, including those with low-level gain, and identifies additional cases in which PD-L2 may be dysregulated by alternative mechanisms.
Figure 3.
Correlation between PDCD1LG2 copy number and PD-L2 IHC for select DLBCLs and PMBLs. A) PDCD1LG2 copy number as determined by quantitative PCR for 9 normal controls, Hodgkin lymphoma cell lines L428, SUP-HD-1, and HDLM-2, 9 DLBCLs, and 12 PMBLs. The corresponding PD-L2 IHC result is indicated below the PDCD1LG2 copy number for each case. Cases without copy number gain (white bars) and with copy number gain (red bars) are indicated. B) PD-L2 IHC corresponding to PMBL case #1, #5, and #12, respectively, in (A) with the PDCD1LG2 copy number indicated in the bottom right corner of each image.
Tumor infiltrating macrophages do not express detectable PD-L2 in PMBL and DLBCL
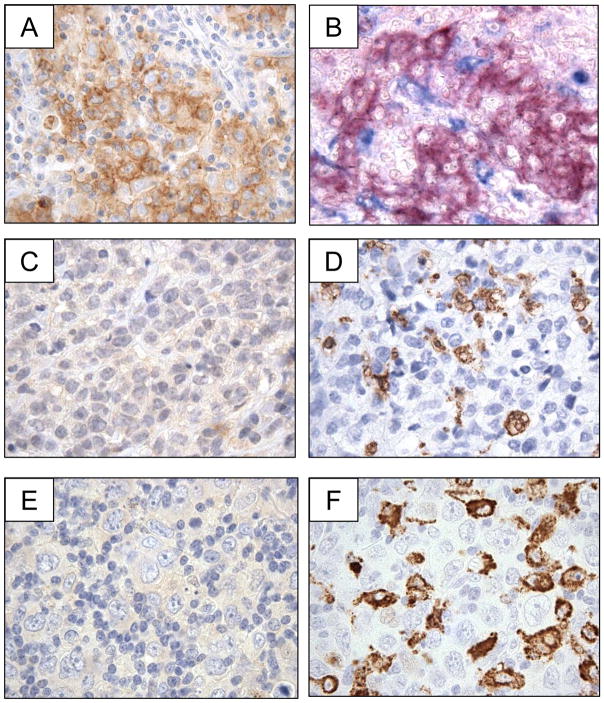
Our data revealed low to undetectable PD-L2 expression on the resident macrophage populations colonizing reactive lymphoid tissue, but high expression on tissue macrophages at sites of acute inflammation. We therefore wished to determine whether PD-L2 protein is detected on tumor-associated macrophages (TAMs) in PMBL and DLBCL. Review of PD-L2 IHC revealed that distinct, membranous staining for PD-L2 was restricted to the large, neoplastic tumor cells despite the presence of small lymphocytes and macrophages within the tumor microenvironment (Figure 4A). A case of PMBL which was double-stained for PD-L2 and the macrophage marker CD68 revealed distinct cellular populations expressed the two proteins (Figure 4B). In addition, cases of PMBL and DLBCL without any positive staining for PD-L2 contained frequent CD68+ TAMs (Figure 4C, D; Figure 4E, F, respectively).
Figure 4.
Expression of PD-L2 within the tumor microenvironment of PMBL and DLBCL. (A) Case of PMBL stained with anti-PD-L2 antibody and showing positive staining of tumor cells (positive staining = brown). (B) The case of PMBL shown in (A) double-stained with anti-PD-L2 and anti-CD68 antibodies and showing positive staining in distinct cellular populations (positive staining for anti-PD-L2= red, positive staining for anti-CD68= blue). (C) A second case of PMBL stained with anti-PD-L2 antibody and showing no positive staining of tumor cells (positive staining = brown). (D) The case of PMBL shown in (C) stained with anti-CD68 antibody and showing positive staining intratumoral macrophages (positive staining = brown). (E) Case of DLBCL stained with anti-PD-L2 antibody and showing no positive staining of tumor cells (positive staining = brown). (F)The case of DLBCL shown in (E) stained with anti-CD68 antibody and showing positive staining intratumoral macrophages (positive staining = brown).
DISCUSSION
PMBL is an aggressive large B-cell lymphoma with a distinct clinical presentation and course.(3, 7) The major differential diagnosis for PMBL is classical Hodgkin lymphoma (cHL) and DLBCL.(1) For the practicing pathologist, the distinction between PMBL and cHL is usually straightforward, although occasional cases can exhibit features intermediate between PMBL and cHL.(27) In contrast, the distinction between PMBL and DLBCL on histopathologic and phenotypic findings alone is often very difficult in the absence of ancillary clinical and radiographic data.(6) This distinction is important, as treatment regimens are increasingly tailored to patients with PMBL,(28, 29) and, in an era of molecularly targeted therapies, critical to ensure that the appropriate patient population is selected for clinical trials.(30)
Several proteins, detectable by IHC in formalin-fixed paraffin embedded (FFPE) biopsy specimens, have been proposed to distinguish PMBL from DLBCL. These include MAL, activated (phosphorylated) STAT6 (p-STAT6), p63, TRAF1, activated (nuclear) cRel, TNFAIP2, CD23, and CD200.(8, 10, 11, 12, 31, 32). The most robust of these markers show sensitivities ranging from 69% (CD23) to 94% (CD200) and specificities ranging from 77% (TRAF1) to 97% (MAL).(12) In this study we found that the expression of PD-L2 is 72% sensitive and 97% specific for the diagnosis of PMBL relative to DLBCL, and therefore comparable in sensitivity to most, and superior in specificity to almost all IHC markers described to date.
PD-L2 and PD-L1 are unique among the diagnostic markers of PMBL because these proteins are often over-expressed as a consequence of specific, genetic lesions. Abnormalities involving chromosome 9p24.1 are common in PMBL and in classical Hodgkin lymphoma (cHL), occurring approximately 70% and 30% of cases, respectively; in contrast, these lesions are rare in DLBCL.(18, 24, 33) The frequency of 9p24.1 abnormalities in PMBL suggests critical biological roles for four major genes that reside at this locus, PDCD1LG2, CD274, JAK2, and JMJ2DC, and amplification of 9p24.1 is often associated with increased transcription of all four genes.(18, 20, 26, 30, 33, 34) However gene expression profiling studies indicate that the relative over-expression of PD-L2 typically exceeds that of PD-L1 in PMBL.(18, 20) Indeed, PD-L2 was the best overall gene transcript detected on a tissue microarray for distinguishing PMBL from DLBCL with 5.6-fold more expression in PMBL compared to DLBCL.(20) This may explain the exceptionally high specificity for PMBL that we observe with PD-L2 IHC.
Gene copy number gain is not the only mechanism for deregulation of PD-1 ligands but is the most common one in B-cell malignancies.(18, 33) Translocations involving PDCD1LG2 or CD274 can occur and result in marked upregulation of the respective target genes.(33, 34) Based on integrative genetic and expression data, other mechanisms of deregulation also likely exist.(33) In this study, we focused on the detection of PDCD1LG2 amplification, which showed excellent correlation with PD-L2 IHC. However, we also found a case of PMBL that was positive for PD-L2 by IHC but lacked PDCD1LG2 amplification. The origin of PD-L2 deregulation in this case is unknown but, based upon prior published analyses of PMBL, a translocation involving PDCD1LG2 is possible.
The interpretation of PD-L2 IHC is facilitated by the specificity of the stain for malignant cells. We have found that tumor-associated macrophages express little PD-L2 in PMBL and DLBCL. In contrast, PD-L1 is highly expressed by TAMs in a wide variety of aggressive lymphomas, including PMBL and a subset of DLBCL.(21) TAMs expressing high PD-L1 protein are also characteristic of other tumor types and thought to have clinical significance.(35, 36) The differential expression of PD-L2 and PD-L1 on TAMs in PMBL may be attributable to the differential sensitivity of the PDCD1LG2 and CD274 promoters to cytokine-mediated JAK-STAT signaling. The human PDCD1LG2 promoter contains a partially conserved interferon (IFN)-stimulated regulatory element/IFN-regulatory factor 1 (ISRE/IRF1) motif that is weakly responsive to JAK-STAT signaling.(18) In contrast, human CD274 promoter contains a highly conserved ISRE/IRF1 motif that is highly responsive to JAK-STAT signaling. (18, 37) Thus the multitude of cytokines within the tumor microenvironment of PMBL and DLBCL may be less effective in inducing PD-L2 than PD-L1.
Our results have clinical implications for patients with PMBL. Recent trials of anti-PD-1 and anti-PD-L1 antibodies have shown dramatic and long-lasting clinical responses in subset of patients with solid tumors including melanoma, non-small cell lung cancer and renal cell carcinoma.(16, 17) These findings emphasize the importance of the PD-1/PD-L1 axis in regulating anti-tumor immunity and the benefits of targeting this signaling pathway in clinical practice. In addition, clinical responsiveness to PD-1 blockade correlated with tumor-specific expression of PD-L1 as detected by IHC in a small series of cases for which biopsy tissue was available for analysis.(16) Our data indicate that up to 70% of patients with PMBL are rational candidates for immunotherapy that targets PD-1 signaling. Given that PMBLs typically express both PD-L2 and PD-L1,(18, 21) PD-1 receptor blockade should be considered a better option than selective PD-L1 blockade.
In summary, we have validated a protocol for the detection of PD-L2 in formalin-fixed paraffin-embedded tissue sections by standard IHC. We find that PD-L2 IHC is sensitive and specific, with robust positive staining of cell lines that harbor even low-level PDCD1LG2 copy number gain. The majority of PMBLs (72%) exhibit distinct, membranous PD-L2 expression, whereas the vast majority of DLBCLs (97%) do not. In most, but not all cases of PMBL, positive staining for PD-L2 is associated with PDCD1LG2 copy number gain. Finally, we find that PD-L2 expression is restricted to neoplastic cells within the tumor microenvironment. PD-1 blockade, which is currently being evaluated in clinical trials, may be a possible new treatment option for patients with PMBL.
Acknowledgments
This work was financially supported, in part, by the following: DFCI Center for Immune Oncology (SJR), R01 CA161026 (MAS), a Specialized Center of Research (SCOR) grant from the Leukemia and Lymphoma Society (MAS; SJR), and NIH grants AI56299, U19AI082630, HHSN27220110001, and U54CA163125 (GJF).
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. Tumours of haematopoietic and lymphoid tissues. 4. Lyon: IARC; 2008. [Google Scholar]
- 2.Johnson PW, Davies AJ. Primary mediastinal B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2008:349–58. doi: 10.1182/asheducation-2008.1.349. [DOI] [PubMed] [Google Scholar]
- 3.Cazals-Hatem D, Lepage E, Brice P, et al. Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA (“groupe d'etude des lymphomes de l'adulte”) study. Am J Surg Pathol. 1996;20(7):877–88. doi: 10.1097/00000478-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Joos S, Otano-Joos MI, Ziegler S, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood. 1996;87(4):1571–8. [PubMed] [Google Scholar]
- 5.Bentz M, Barth TF, Bruderlein S, et al. Gain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): Comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell line. Genes Chromosomes Cancer. 2001;30(4):393–401. doi: 10.1002/1098-2264(2001)9999:9999<::aid-gcc1105>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Paulli M, Strater J, Gianelli U, et al. Mediastinal B-cell lymphoma: A study of its histomorphologic spectrum based on 109 cases. Hum Pathol. 1999;30(2):178–87. doi: 10.1016/s0046-8177(99)90273-3. [DOI] [PubMed] [Google Scholar]
- 7.Barth TF, Leithauser F, Joos S, et al. Mediastinal (thymic) large B-cell lymphoma: Where do we stand? Lancet Oncol. 2002;3(4):229–34. doi: 10.1016/s1470-2045(02)00714-3. [DOI] [PubMed] [Google Scholar]
- 8.Copie-Bergman C, Plonquet A, Alonso MA, et al. MAL expression in lymphoid cells: Further evidence for MAL as a distinct molecular marker of primary mediastinal large B-cell lymphomas. Mod Pathol. 2002;15(11):1172–80. doi: 10.1097/01.MP.0000032534.81894.B3. [DOI] [PubMed] [Google Scholar]
- 9.Copie-Bergman C, Gaulard P, Maouche-Chretien L, et al. The MAL gene is expressed in primary mediastinal large B-cell lymphoma. Blood. 1999;94(10):3567–75. [PubMed] [Google Scholar]
- 10.Rodig SJ, Savage KJ, LaCasce AS, et al. Expression of TRAF1 and nuclear c-rel distinguishes primary mediastinal large cell lymphoma from other types of diffuse large B-cell lymphoma. Am J Surg Pathol. 2007;31(1):106–12. doi: 10.1097/01.pas.0000213334.40358.0e. [DOI] [PubMed] [Google Scholar]
- 11.Salama ME, Rajan Mariappan M, Inamdar K, et al. The value of CD23 expression as an additional marker in distinguishing mediastinal (thymic) large B-cell lymphoma from hodgkin lymphoma. Int J Surg Pathol. 18(2):121–8. doi: 10.1177/1066896909331994. [DOI] [PubMed] [Google Scholar]
- 12.Dorfman DM, Shahsafaei A, Alonso MA. Utility of CD200 immunostaining in the diagnosis of primary mediastinal large B cell lymphoma: Comparison with MAL, CD23, and other markers. Mod Pathol. 2012;25(12):1637–43. doi: 10.1038/modpathol.2012.129. [DOI] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 15.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD- 1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24. 1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–77. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steidl C, Telenius A, Shah SP, et al. Genome-wide copy number analysis of hodgkin reed-sternberg cells identifies recurrent imbalances with correlations to treatment outcome. Blood. 2010;116(3):418–27. doi: 10.1182/blood-2009-12-257345. [DOI] [PubMed] [Google Scholar]
- 20.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to hodgkin lymphoma. J Exp Med. 2003;198(6):851–62. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–73. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai G, Anumanthan A, Brown JA, et al. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9(2):176–85. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 23.Kondratiev S, Duraisamy S, Unitt CL, et al. Aberrant expression of the dendritic cell marker TNFAIP2 by the malignant cells of hodgkin lymphoma and primary mediastinal large B-cell lymphoma distinguishes these tumor types from morphologically and phenotypically similar lymphomas. Am J Surg Pathol. 2011;35(10):1531–9. doi: 10.1097/PAS.0b013e31822bd476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monti S, Chapuy B, Takeyama K, et al. Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell. 2012;22(3):359–72. doi: 10.1016/j.ccr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Rui L, Emre NC, Kruhlak MJ, et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. 2010;18(6):590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant C, Dunleavy K, Eberle FC, et al. Primary mediastinal large B-cell lymphoma, classic hodgkin lymphoma presenting in the mediastinum, and mediastinal gray zone lymphoma: What is the oncologist to do? Curr Hematol Malig Rep. 2011;6(3):157–63. doi: 10.1007/s11899-011-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–16. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinzani PL, Martelli M, Bertini M, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: A retrospective multinational study on 426 previously untreated patients. Haematologica. 2002;87(12):1258–64. [PubMed] [Google Scholar]
- 30.Hao Y, Chapuy B, Monti S, et al. Selective JAK2 inhibition specifically decreases hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guiter C, Dusanter-Fourt I, Copie-Bergman C, et al. Constitutive STAT6 activation in primary mediastinal large B-cell lymphoma. Blood. 2004;104(2):543–9. doi: 10.1182/blood-2003-10-3545. [DOI] [PubMed] [Google Scholar]
- 32.Zamo A, Malpeli G, Scarpa A, et al. Expression of TP73L is a helpful diagnostic marker of primary mediastinal large B-cell lymphomas. Mod Pathol. 2005;18(11):1448–53. doi: 10.1038/modpathol.3800440. [DOI] [PubMed] [Google Scholar]
- 33.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123(13):2062–5. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 34.Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–81. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]