Lymphocytosis after treatment with dasatinib in chronic myeloid leukemia: Effects on response and toxicity (original) (raw)
Abstract
BACKGROUND
The proliferation of clonal cytotoxic T‐cells or natural killer cells has been observed after dasatinib treatment in small studies of patients with chronic myeloid leukemia (CML).
METHODS
The incidence of lymphocytosis and its association with response, survival, and side effects were assessed in patients from 3 large clinical trials. Overall, 1402 dasatinib‐treated patients with newly diagnosed CML in chronic phase (CML‐CP), CML‐CP refractory/intolerant to imatinib, or with CML in accelerated or myeloid‐blast phase were analyzed.
RESULTS
Lymphocytosis developed in 32% to 35% of patients and persisted for >12 months. This was not observed in the patients who received treatment with imatinib. Dasatinib‐treated patients in all stages of CML who developed lymphocytosis were more likely to achieve a complete cytogenetic response, and patients who had CML‐CP with lymphocytosis were more likely to achieve major and deep molecular responses. Progression‐free and overall survival rates were significantly longer in patients with CML‐CP who were refractory to or intolerant of imatinib and had lymphocytosis. Pleural effusions developed more commonly in patients with lymphocytosis.
CONCLUSIONS
Overall, lymphocytosis occurred and persisted in many dasatinib‐treated patients in all phases of CML. Its presence was associated with higher response rates, significantly longer response durations, and increased overall survival, suggesting an immunomodulatory effect. Prospective studies are warranted to characterize the functional activity of these cells and to assess whether an immunologic effect against CML is detectable. Cancer 2016;122:1398–1407. © 2016 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: dasatinib, leukemia, chronic myeloid, killer cells, natural, lymphocytosis, T‐lymphocytes
Short abstract
Lymphocytosis develops frequently after treatment of chronic myeloid leukemia with dasatinib and is associated with higher response rates, significantly longer response durations, and increased overall survival. Prospective studies are warranted to assess whether dasatinib produces an immunomodulatory effect against chronic myeloid leukemia.
INTRODUCTION
Dasatinib is a multitargeted tyrosine kinase inhibitor (TKI) approved for the treatment of adults with newly diagnosed, Philadelphia chromosome (Ph)‐positive chronic myeloid leukemia (CML) in chronic phase (CML‐CP); adults with CML‐CP, CML in accelerated phase (CML‐AP), CML in myeloid‐blast phase (CML‐MBP), or CML in lymphoid‐blast phase (CML‐LBP) who have resistance/intolerance to prior therapy, including imatinib; and adults with Ph‐positive, acute lymphoblastic leukemia (ALL) who have resistance/intolerance to prior therapy.1 Although dasatinib primarily inhibits the constitutively active tyrosine kinase breakpoint cluster region/Abelson 1 proto‐oncogene (BCR‐ABL1),2 it was initially developed as an SRC family kinase inhibitor.3 It also acts on other kinases, including KIT, platelet‐derived growth factor receptor, the ephrin‐A receptor, and TEC family kinases.2, 3, 4, 5, 6
The immunosuppressive effects of dasatinib were initially observed in preclinical studies.3 The known effects of dasatinib on normal hematopoiesis and lymphopoiesis are limited, but in vitro and animal models suggest that dasatinib may inhibit lymphocyte proliferation and function without inducing apoptosis.7, 8, 9, 10, 11 ABL1 and SRC family kinase signaling pathways, both of which are inhibited by dasatinib, are essential for T‐cell proliferation, function, and survival.12, 13, 14
Therefore, it was somewhat surprising that clonal expansion of cytotoxic T‐cells or natural killer (NK) cells (T/NK) was detected (median time to detection, 1‐4 months) after initiation of dasatinib therapy in small studies of CML or patients with Ph‐positive ALL.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Few studies have evaluated whether this population was present before treatment, although Kreutzman et al25 demonstrated expansion of a BCR‐ABL1–negative lymphocyte population with T‐cell receptor γ/δ rearrangements (present at the time of CML diagnosis) after dasatinib treatment. Some analyses have suggested that T/NK lymphoproliferation was associated with higher than expected rates of cytogenetic and molecular response, particularly in patients with advanced disease, suggesting an immunomodulatory effect.16, 17, 18, 19, 20, 21 Also, an increase in certain side effects, such as pleural effusions, has been reported.20, 21, 22, 23, 24 Resolution of pleural effusion and T/NK lymphocytosis has been described after switching from dasatinib to another TKI.26 T/NK cell expansion has not been described after CML treatment with other TKIs. To our knowledge, no prior study has systematically evaluated the effects of these findings on response to treatment, progression‐free survival (PFS), or overall survival (OS).
For an illustrative example, a woman who progressed to CML‐MBP despite prior imatinib treatment was treated with dasatinib alone in June 2005 and, as of her last visit in late 2015, remains in remission with undetectable BCR‐ABL1 transcripts (Fig. 1). Her treatment was complicated by the development of a chronic pleural effusion. Lymphocytosis, reaching > 7000/mm3, had been present for approximately 9 years, with cells characterized as T/NK cells by flow cytometry. Although elevated on most occasions, several lymphocyte counts were in the normal range, which may reflect the documented pharmacodynamic effect of the plasma level of dasatinib and the lymphocyte count.27
Figure 1.
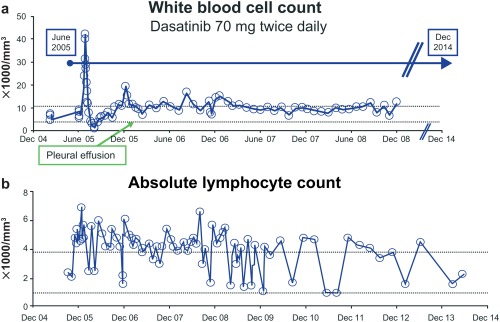
(a,b) The treatment of a woman who had imatinib‐resistant, blast‐phase chronic myeloid leukemia with splenomegaly, fever, and bone pain was complicated by the development of a chronic pleural effusion.
To expand on these observational studies, we analyzed the incidence of lymphocytosis in a large group of patients (n = 1402) with CML‐CP, CML‐AP, and CML‐MBP who received treatment with dasatinib and were enrolled in prospective clinical trials with long‐term follow‐up. Here, we report our results describing the association between the development of lymphocytosis and initial response, long‐term outcome, and the development of pleural effusions.
MATERIALS AND METHODS
Study Design and Patient Eligibility
We previously presented data in abstract form from patients with CML‐CP in the “Src/ABL Tyrosine Kinase Inhibition Activity Research Trials of Dasatinib” (START) Program's phase 2, second‐line START‐A, START‐B, START‐C, and START‐R trials and the phase 3 Dasatinib Versus Imatinib Study in Treatment‐Naive CML Patients (DASISION) trial (CA180‐056; clinicaltrials.gov NCT00481247).20, 28 This report focuses on randomized trials because of their closely monitored, long‐term follow‐up. Retrospective analyses were conducted using 5‐year, long‐term data from dasatinib‐treated patients with newly diagnosed CML‐CP in the DASISION trial29; 7‐year, long‐term data from patients with CML‐CP resistant/intolerant to imatinib in the phase 3 dose‐optimization trial (CA180‐034; NCT00123474)30; and 5‐year, long‐term data from patients with CML‐AP or CML‐MBP who were resistant to/intolerant of imatinib in the phase 3 dose‐optimization trial for patients with advanced‐phase CML (CA180‐035; NCT00123487).31
Entry criteria for these studies have been published elsewhere.29, 30, 31 Briefly, eligible patients in the DASISION trial included adults with cytogenetically confirmed, Ph‐positive CML‐CP within 3 months from diagnosis who had adequate hepatic and renal function and no serious medical conditions. No prior CML therapy was permitted except for anagrelide or hydroxyurea. Patients received treatment with either dasatinib 100 mg once daily or imatinib 400 mg once daily. Patients in CA180‐034 were required to be aged ≥18 years and to have Ph‐positive CML‐CP with primary or acquired resistance/intolerance to imatinib. Patients in CA180‐034 received dasatinib at doses of 100 mg once daily, 140 mg once daily, 50 mg twice daily, or 70 mg twice daily. In CA180‐035, patients with CML‐AP, CML‐MBP/LBP, or Ph‐positive ALL aged ≥ 18 years and who had developed primary or acquired resistance/intolerance to imatinib were enrolled. Patients in CA180‐035 received treatment with dasatinib either 140 mg once daily or 70 mg twice daily. Each of the 3 trials was conducted in accordance with the Declaration of Helsinki and was approved by local ethics committees or institutional review boards. All patients gave written informed consent.
Lymphocytosis
Lymphocytosis was defined as lymphocyte counts >3.6 × 109/L on at least 2 consecutive occasions after 28 days of treatment. Complete blood counts, typically with automated differential counts, were performed at least weekly for the initial 1 to 2 months of treatment, then monthly, and every 3 months thereafter during long‐term follow‐up. In general, total white blood cell counts were normal in dasatinib‐treated patients after 1 to 2 months (within accuracy range for differential counts). The timing of dasatinib dosing relative to lymphocyte assessments was not known. Flow cytometry was not performed, because the observation of lymphocytosis was not considered until after these trials were under way. We did not study patients in lymphoid blastic phase because serial slides were unavailable. The sole use of an automated lymphocyte count may have caused potential confusion between lymphoblasts and other lymphoid elements.
Analyses
The incidence, timing, and duration of lymphocytosis were determined. The duration of lymphocytosis was defined as the interval between the date of original detection and the date of last available follow‐up sample that was positive for lymphocytosis. Rates of complete cytogenetic response (CCyR) (based on analysis of ≥20 metaphases), major molecular response (MMR) (BCR‐ABL1 transcripts ≤ 0.1% on the International Scale [IS]), molecular response4.5 (MR4.5) (BCR‐ABL1 transcripts ≤ 0.0032% on the IS), PFS, OS, and incidence of pleural effusion were compared between patients with and without lymphocytosis. Landmark analyses of PFS and OS for patients with or without lymphocytosis at 3 months were also performed. Progression in patients with CML‐CP was defined as an increasing white blood cell count; loss of a complete hematologic response or a major cytogenetic response; an increase ≥ 30% in Ph‐positive metaphases; or transformation to CML‐AP, CML‐MBP, or CML‐LBP.
The log‐rank test was used to calculate P values for PFS and OS (Fig. 2), the Fisher exact test was used to calculate P values for incidences of responses and pleural effusion, and logistic regression was used to examine the relation between the binary response variable lymphocytosis (yes/no) and a set of predictive variables. Statistical comparisons were not prespecified, and comparisons were not adjusted for multiplicity; therefore, P values are provided solely for informative purposes.
Figure 2.
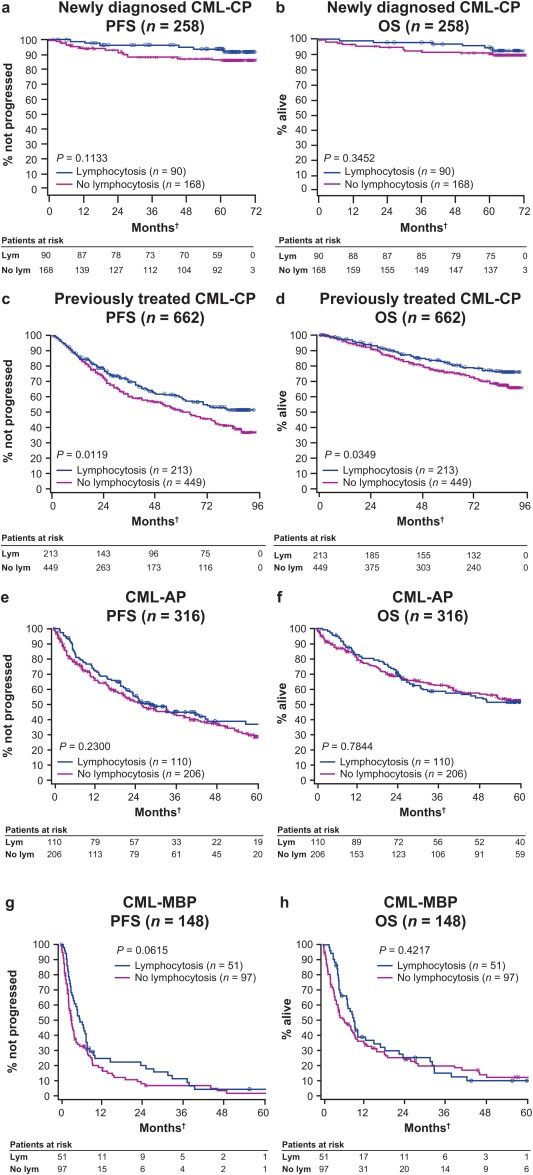
Progression‐free survival (PFS) and overall survival (OS) are illustrated according to the presence of lymphocytosis. (a) PFS and (b) OS are illustrated in patients who had chronic myeloid leukemia in chronic phase (CML‐CP) in the dasatinib treatment arm from the Dasatinib Versus Imatinib Study in Treatment‐Naive CML Patients (DASISION) trial (median follow‐up, 60.5 months). (c) PFS and (d) OS are illustrated in patients who had previously treated CML‐CP from the CA180‐034 trial (median follow‐up, 29.8 months). (e) PFS and (f) OS are illustrated in patients who had CML in accelerated phase (CML‐AP), and (g) PFS and (h) OS are illustrated in patients who had CML in myeloid‐blast phase (CML‐MBP) from the CA180‐035 trial (median follow‐up, 6.1 months). †PFS, OS, and lymphocytosis were considered from start of dasatinib treatment.
RESULTS
Patients and Incidence of Lymphocytosis
Patients' disease and demographic characteristics at baseline in the different studies are provided in Table 1. The median age ranged from 46 to 56 years, and there were slightly more men than women. For patients in the second‐line dasatinib studies, the median duration of prior imatinib therapy was from 16 to 40 months, and up to 46% of patients received ≥ 800 mg daily. Overall, 74% to 81% of patients were resistant to imatinib, whereas 16% to 26% of patients were intolerant. The baseline median lymphocyte counts in treated patients ranged from 1.7 × 109/L to 3.0 × 109/L in the 3 studies. A minority of patients (range, 14%‐41%) had a single lymphocyte count >3.6 × 109/L at enrollment. However, most such patients in chronic and blast phases had elevated white blood cells at study entry, and automated differential counts likely are less accurate in such circumstances. In contrast, our definition of lymphocytosis demanded that the count be elevated on at least 2 consecutive occasions, and this almost always occurred at a time when the total white blood cell count was relatively normal after the cytoreductive effects from the dasatinib.
Table 1.
Baseline Demographic and Disease Characteristics of Randomized Patients
| DASISIONa | CA180‐034b | CA180‐035b | ||
|---|---|---|---|---|
| Characteristic | CML‐CP, n = 259 | CML‐CP, n = 670 | CML‐AP, n = 320 | CML‐MBP, n = 153 |
| Median age, y | 46 | 55 | 56 | 50 |
| Sex, % | ||||
| Men | 56 | 47 | 57 | 54 |
| Women | 44 | 53 | 43 | 46 |
| Prior treatment, % | ||||
| Stem cell transplantation | NA | 4 | 6 | 12 |
| Interferon‐α | NA | 52 | 54 | 38 |
| Chemotherapy | NA | 26 | 45 | 57 |
| Prior imatinib therapy | ||||
| Median duration, mo | NA | 27 | 40 | 16 |
| Highest dose ≥ 800 mg/d, % | NA | 33 | 43 | 46 |
| Best response to prior imatinib, % | ||||
| CHR | NA | 84 | 76 | 64 |
| MCyR | NA | 41 | 29 | 32 |
| Imatinib‐resistant, % | NA | 74 | 77 | 81 |
| Imatinib‐intolerant, % | NA | 26 | 23 | 16 |
The cumulative incidence of lymphocytosis was comparable across all phases of CML (range, 32%‐35%) (Table 2) and was similar in patients who had CML‐CP, irrespective of prior imatinib treatment. The maximal lymphocyte counts and the counts at the time of initial detection of lymphocytosis are provided in Table 2. There was no difference in the occurrence of lymphocytosis among the different dasatinib doses and schedules evaluated in study CA180‐034. The distribution of Hasford risk scores was similar for patients who did and did not develop lymphocytosis in the DASISION trial (data not shown), and there was no effect of age or sex. Lymphocytosis developed rapidly after dasatinib initiation, with a median time to detection of 3 to 5 months. A first incidence of lymphocytosis after 5 to 6 months of treatment was very uncommon; however, many patients, particularly those with CML‐AP and CML‐MBP, were no longer receiving dasatinib at that time (median on‐treatment follow‐up, 6.1 months). In the DASISION study, lymphocytosis was observed in 35% and 8% of dasatinib‐ and imatinib‐treated patients, respectively, and was almost invariably transient in the imatinib‐treated patients (data not shown).
Table 2.
Incidence, Timing, and Duration of Lymphocytosis During Treatment With Dasatinib
| DASISIONa | CA180‐034b | CA180‐035b | ||
|---|---|---|---|---|
| Variable | CML‐CP, n = 258 | CML‐CP, n = 662 | CML‐AP, n = 316 | CML‐MBP, n = 148 |
| Incidence, % | 35 | 32 | 35 | 34 |
| Time to onset: Median (range), mo | 5.2 (1.0‐35.9) | 3.2 (1.0‐72.0) | 3.5 (1.0‐25.1) | 2.6 (1.0‐16.3) |
| On‐treatment follow‐up: Median (range), mo | 60.5 (0‐72.7) | 29.8 (0.1‐92.9) | 6.1 (0‐67.0) | |
| Lymphocyte counts in those with lymphocytosis at the time of initial detection and maximal lymphocyte count: Median (range), × 103/μL | ||||
| At initial detection | 4.2 (3.6‐8.7) | 4.2 (2.8‐1350.0)e | 4.5 (3.6‐32.9)e | 5.1 (3.6‐25.3)e |
| Maximal | 5.4 (3.7‐29.1)e | 5.7 (3.7‐5226.0)e | 6.9 (3.9‐215.6)e | 9.5 (3.9‐66.2)e |
| Duration of lymphocytosis: Median (range), moc | 33.8 (28.3‐37.2+)d | 12.9 (11.7‐15.1+)d | 8.4 (4.9‐12.2+)d | 1.4 (0.6‐2.9+)d |
| Lymphocytosis duration > 12 mo, %d | 77 | 52 | 42 | 18 |
The duration of lymphocytosis varied across the different phases of CML (range, 1.4‐33.8 months) (Table 2), possibly influenced by the differing rates of dasatinib discontinuation, specifically in patients with advanced disease. In addition, lymphocytosis may have been missed in some patients because only single samples were obtained approximately every 3 months during long‐term follow‐up. Thus, the median durations of lymphocytosis of 33.8 and 12.9 months in patients with CML‐CP in the DASISION and CA180‐034 trials, respectively, may have been underestimated. Nonetheless, at minimum, from 52% to 77% of patients with CML‐CP who developed lymphocytosis had it persist for ≥12 months. Furthermore, it is likely that some patients in whom lymphocytosis was no longer detected had discontinued dasatinib treatment.
Response to Treatment
Response to treatment was assessed at approximately 3‐month intervals. Lymphocytosis was associated with statistically significantly higher rates of CCyR in all phases of CML (newly diagnosed CML‐CP, P = .0166; resistant/intolerant CML‐CP, P = .0011; CML‐AP, P = .0004; CML‐MBP, P = .0030) (Table 3). Furthermore, the rates of MMR and MR4.5 were statistically significantly increased in patients who had CML‐CP with lymphocytosis in both the DASISION trial (MMR, P = .0438; MR4.5, P = .0253), which evaluated newly diagnosed patients, and in the CA180‐034 trial, which evaluated imatinib‐resistant/intolerant patients (MMR, P = .0010; MR4.5, P = .0068).
Table 3.
Cumulative Incidence of Response According to the Presence or Absence of Lymphocytosis
| DASISIONa | CA180‐034b | CA180‐035b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response | CML‐CP | CML‐CP | CML‐AP | CML‐MBP | ||||||||
| Presence of lymphocytosis | Yes, n = 90 | No, n = 168 | P | Yes, n = 213 | No, n = 449 | P | Yes, n = 110 | No, n = 206 | P | Yes, n = 51 | No, n = 97 | P |
| CCyR, % | 94 | 84 | .0166 | 61 | 47 | .0011 | 45 | 25 | .0004 | 27 | 8 | .0030 |
| MMR, % | 84 | 73 | .0438 | 52 | 38 | .0010 | — | — | — | — | — | — |
| MR4.5, % | 54 | 39 | .0253 | 20 | 12 | .0068 | — | — | — | — | — | — |
PFS (P = .0119) and OS (P = .0349) were significantly longer in patients with lymphocytosis from the CA180‐034 trial (Fig. 2c,d), which had the longest follow‐up of any of the studies analyzed (7 years). The separation of the curves began at approximately 2 years after the start of treatment. Although patients with newly diagnosed CML‐CP who had lymphocytosis had more prolonged PFS and OS than patients without lymphocytosis, these results were not significantly different with 5 years of follow‐up (PFS, P = .1133; OS, P = .3452) (Fig. 2a,b). There were no significant differences in PFS (CML‐AP, P = .23; CML‐MBP, P = .0615) or OS (CML‐AP, P = .7844; CML‐MBP, P = .4217) in patients with more advanced phases of CML, irrespective of lymphocytosis (Fig. 2e‐h). Landmark analyses evaluating patients with or without lymphocytosis at 3 months (ie, the median time until the detection of lymphocytosis) did not demonstrate statistically significant differences in PFS or OS for patients with or without lymphocytosis, regardless of disease phase (data not shown).
Side Effects: Pleural Effusion
Pleural effusion is arguably the most unique, common nonhematologic toxicity after treatment with dasatinib, and patients have been described with elevated T/NK lymphocytes in their pleural fluid after such treatment.22 The incidence of pleural effusion was numerically greater in patients with lymphocytosis in all phases of CML than in patients without lymphocytosis (Table 4). These differences were statistically significant in patients with CML‐AP (P = .0003) and of borderline significance (P = .0655) in patients with presumably more advanced CML‐CP who received dasatinib as second‐line or third‐line treatment. In an earlier analysis of dasatinib‐treated patients who had lymphocytosis from the DASISION trial, except for a slight increase in the incidence of rash (all incidences were grade 1 or 2), there were no differences in the frequency of other side effects, including diarrhea, fatigue, constitutional symptoms, or myalgias.20
Table 4.
Incidence of Pleural Effusion According to Lymphocytosis
| DASISIONa | CA180‐034b | CA180‐035b | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | CML‐CP | CML‐CP | CML‐AP | CML‐MBP | ||||
| Presence of lymphocytosis | Yes, n = 90 | No, n = 168 | Yes, n = 213 | No, n = 449 | Yes, n = 110 | No, n = 206 | Yes, n = 51 | No, n = 97 |
| Pleural effusion, % | 33 | 26 | 39 | 32 | 53 | 31 | 27 | 27 |
| P | .2493 | .0655 | .0003 | 1.0000 |
DISCUSSION
We demonstrate here for the first time in large studies that lymphocytosis commonly occurs after treatment with dasatinib, but not imatinib. Analyses of 3 large clinical trial populations (n = 1402) in different stages of CML who received treatment with a variety of different doses of dasatinib and were followed for as long as 7 years, demonstrate that lymphocytosis develops in a minimum of 32% of patients and persists for ≥1 year in at least 50% of such patients. Although immunophenotyping was not done, it is very likely that this represents expansion of a T/NK‐cell population, given the findings from smaller studies that have characterized this proliferation in dasatinib‐treated patients. Lymphocytosis was demonstrated in a similar proportion of patients in all clinical phases and occurred relatively rapidly, with most cases identified within 3 to 5 months after the initiation of dasatinib treatment.
It is also likely that the 32% to 35% incidence of lymphocytosis represents an underestimate, because 2 consecutive blood samples with an elevated count were required. The case study in Figure 1 indicates that occasional lymphocyte counts were in the normal range, suggesting that lymphocytosis in some patients may have been missed because of the use of a somewhat conservative definition. Some of these fluctuations may be related to the temporal relation between the ingestion of dasatinib and the acquisition of the blood sample, as reported in a recent study demonstrating that increased numbers of T/NK cells can be detected within 1 to 2 hours after dasatinib administration, even in normal individuals, with a correlation between peak blood levels and T/NK counts.27
It is noteworthy that higher rates of CCyR were observed in dasatinib‐treated patients who had lymphocytosis in all phases of CML, as were the rates of MMR and deeper MRs (MR4.5) in patients with CML‐CP. For the first time, we demonstrate that PFS and OS are significantly longer in patients with imatinib‐resistant/intolerant CML‐CP who have longer follow‐up, which might be expected if an “immunologic” effect were operative. This is consistent with other immune‐mediated therapies in which positive effects on longer term outcomes are often the most prominent findings. Given the substantial impact of subsequent therapies and the somewhat shorter follow‐up, it is perhaps not unexpected that there was no significant impact on OS in patients with newly diagnosed CML‐CP. It is somewhat disappointing that there was no difference in either PFS or OS in the overall population with more advanced stages of CML, given the previous suggestions regarding unexpectedly long responses from the many cases and anecdotal observations (Fig. 1). However, it may be difficult for any putative immunomodulatory effect to overcome the proliferative thrust of active disease in patients with CML‐MBP, although longer term benefits may occur in patients who can maintain dasatinib treatment. More detailed studies of the immunologic activity of cells from “exceptional” responders would be of interest.
Interpretation of these retrospective data may have been affected by other considerations. For example, the lymphocyte cutoff we used would not detect lower levels of T/NK proliferation, which may also influence clinical outcome. Nonetheless, given the clear correlation between the occurrence of lymphocytosis and the development of pleural effusions, the effects on response rate for all stages of CML, and the significant increase in PFS and OS in patients with previously treated CML‐CP, it appears that our definition enabled us to capture clinically relevant associations. Certainly, lymphocytosis after dasatinib treatment is a consistent and potentially clinically important biologic phenomenon, and it is hoped that several of these questions can be addressed with prospective evaluations of lymphocyte subpopulations in future studies.
The mechanism by which dasatinib induces these changes is unclear. Lymphocytosis has not been noted after CML treatment with other TKIs,32, 33, 34 and we convincingly confirm this observation here through analysis of patients with CML‐CP who were treated on a randomized study of dasatinib versus imatinib. A few trials evaluating dasatinib in patients with solid tumors have been conducted in which lymphocytosis has not been reported,35 although it could have been missed, because it is not a typical focus of toxicity assessments. Although primarily targeted against BCR‐ABL1 in CML, dasatinib has effects on multiple kinases and signaling pathways.2, 4, 5, 6 Indeed, there had been some initial concern about lymphopenia and immunosuppression because of the effect of dasatinib on SRC signaling. Clinical reports have documented mutations in signal transducer and activator of transcription 3 (STAT3) and STAT5B among patients with large granular lymphocytic leukemia,36, 37 a pathway that may be of interest for patients with CML who have dasatinib‐induced lymphocytosis. Finally, a recent publication evaluating dasatinib‐treated patients with CML documented increases in the numbers of functionally active memory CD4‐positive and CD8‐positive Th1‐type T‐helper cells capable of producing interferon‐γ, suggesting potential for an immunomodulatory effect that is undetected in patients who are taking imatinib or nilotinib.38
In summary, lymphocytosis occurs frequently with dasatinib treatment, persists for ≥1 year and is associated with higher response rates, increased PFS and OS in patients with CML‐CP who are refractory to or intolerant of imatinib, and the development of pleural effusions. Prospective studies can better characterize the functional activity of these lymphocytes and assess whether an immunologic effect against CML cells is detectable, perhaps focusing on patients with CML‐AP or CML in blast crisis who have unexpectedly long responses to treatment and those with deep molecular responses who are considering treatment discontinuation. Given the frequency of the effect on lymphocytosis in CML, trials using dasatinib to treat other diseases, possibly in combination with other immunomodulatory agents, would be of interest. Two trials are currently evaluating dasatinib postautologous and postallogeneic transplantation, with the aim of augmenting the NK proliferation that frequently accompanies bone marrow reconstitution (NCT01609816 and NCT01643603).
FUNDING SUPPORT
This analysis was funded by Bristol‐Myers Squibb. Professional medical writing and editorial assistance was provided by Samantha L. Dwyer, PhD, of StemScientific, an Ashfield Company, part of UDG Healthcare plc, funded by Bristol‐Myers Squibb.
CONFLICT OF INTEREST DISCLOSURES
Charles A. Schiffer has acted as a consultant for Bristol‐Myers Squibb, Celgene, MEI Pharma, Novartis, Onconova, Pfizer, Pharmacyclics, Takeda, and Teva; and his institution has received research funding from Ambit, Ariad, Bristol‐Myers Squibb, Celegene, Cephied, Micromedix, Novartis, Pharmacyclics, and Takeda. Jorge E. Cortes has received research support from and has acted as a consultant for Ariad, Bristol‐Myers Squibb, Novartis, and Pfizer; has received research support from Teva; and has been paid for travel expenses by Bristol‐Myers Squibb, Novartis, and Pfizer. Andreas Hochhaus has received research funding and honoraria from Ariad, Bristol‐Myers Squibb, MSD, Novartis, and Pfizer. Giuseppe Saglio has acted as a consultant for and has received honoraria from Ariad, Bristol‐Myers Squibb, Novartis, and Pfizer. Philipp le Coutre has received honoraria from Ariad, Bristol‐Myers Squibb, Novartis, and Pfizer and has received research funding from Novartis. Kimmo Porkka has received honoraria from and has acted as a consultant for Bristol‐Myers Squibb, Novartis, and Pfizer and has received research funding from Bristol‐Myers Squibb and Pfizer. Satu Mustjoki has received research funding from Bristol‐Myers Squibb, Novartis, and Pfizer and has received honoraria from and has acted as a consultant for Bristol‐Myers Squibb and Novartis. Hesham Mohamed is an employee of Bristol‐Myers Squibb, has stock or other ownership interest in Bristol‐Myers Squibb, and has been paid for travel expenses by Bristol‐Myers Squibb. Neil P. Shah has received research funding from Ariad, Bristol‐Myers Squibb, Daiichi Sankyo, Pfizer, and Plexxikon. The authors did not receive financial compensation from Bristol‐Myers Squibb for writing this article.
AUTHOR CONTRIBUTIONS
Charles A. Schiffer: Conceptualization, methodology, validation, formal analysis, investigation, resources, writing‐original draft, writing‐review and editing, visualization, supervision, and project administration. Jorge E. Cortes: Conceptualization, methodology, formal analysis, investigation, resources, writing‐review and editing, and visualization. Andreas Hochhaus: Conceptualization, methodology, formal analysis, investigation, data curation, and writing‐review and editing. Giuseppe Saglio: Conceptualization, and writing‐review and editing. Philipp le Coutre: Conceptualization, validation, investigation, data curation, and writing‐review and editing. Kimmo Porkka: Conceptualization, and writing‐review and editing. Satu Mustjoki: Conceptualization, validation, and writing‐review and editing. Hesham Mohamed: Conceptualization, methodology, and writing‐review and editing. Neil P. Shah: Conceptualization, methodology, and writing‐review and editing.
Presented as a poster at the 55th American Society of Hematology Annual Meeting; December 7‐10, 2013; New Orleans, LA.
We thank all study sites that participated in this Bristol‐Myers Squibb‐sponsored analysis.
REFERENCES
- 1.Bristol‐Myers Squibb Company . Sprycel [package insert]. Princeton, NJ: Bristol‐Myers Squibb Company; 2015. [Google Scholar]
- 2.Quintas‐Cardama A, Cortes J. Chronic myeloid leukemia in the tyrosine kinase inhibitor era: what is the best therapy? Curr Oncol Rep. 2009;11:337‐345. [DOI] [PubMed] [Google Scholar]
- 3.Lindauer M, Hochhaus A. Dasatinib. Recent Results Cancer Res. 2014;201:27‐65. [DOI] [PubMed] [Google Scholar]
- 4.Hantschel O, Rix U, Schmidt U, et al. The Btk tyrosine kinase is a major target of the Bcr‐Abl inhibitor dasatinib. Proc Natl Acad Sci U S A. 2007;104:13283‐13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rix U, Hantschel O, Durnberger G, et al. Chemical proteomic profiles of the BCR‐ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055‐4063. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez P, DiPersio JF. Therapy options in imatinib failures. Oncologist. 2008;13:424‐434. [DOI] [PubMed] [Google Scholar]
- 7.Blake S, Hughes TP, Mayrhofer G, Lyons AB. The Src/ABL kinase inhibitor dasatinib (BMS‐354825) inhibits function of normal human T‐lymphocytes in vitro. Clin Immunol. 2008;127:330‐339. [DOI] [PubMed] [Google Scholar]
- 8.Fei F, Yu Y, Schmitt A, et al. Dasatinib exerts an immunosuppressive effect on CD8+ T cells specific for viral and leukemia antigens. Exp Hematol. 2008;36:1297‐1308. [DOI] [PubMed] [Google Scholar]
- 9.Fei F, Yu Y, Schmitt A, et al. Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol. 2009;144:195‐205. [DOI] [PubMed] [Google Scholar]
- 10.Fraser CK, Lousberg EL, Kumar R, Hughes TP, Diener KR, Hayball JD. Dasatinib inhibits the secretion of TNF‐alpha following TLR stimulation in vitro and in vivo. Exp Hematol. 2009;37:1435‐1444. [DOI] [PubMed] [Google Scholar]
- 11.Schade AE, Schieven GL, Townsend R, et al. Dasatinib, a small‐molecule protein tyrosine kinase inhibitor, inhibits T‐cell activation and proliferation. Blood. 2008;111:1366‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906‐7909. [DOI] [PubMed] [Google Scholar]
- 13.Seddon B, Zamoyska R. TCR signals mediated by Src family kinases are essential for the survival of naive T cells. J Immunol. 2002;169:2997‐3005. [DOI] [PubMed] [Google Scholar]
- 14.Gu JJ, Ryu JR, Pendergast AM. Abl tyrosine kinases in T‐cell signaling. Immunol Rev. 2009;228:170‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powers JJ, Painter JS, Dubovsky JA, Epling‐Burnette PK, Pinilla J. A comprehensive lymphocyte analysis of dasatinib treated chronic myelogenous leukemia patients reveals T‐cell oligoclonality [abstract]. Blood. 2008;112:1114. [Google Scholar]
- 16.Lee SJ, Jung CW, Kim DY, et al. Retrospective multicenter study on the development of peripheral lymphocytosis following second‐line dasatinib therapy for chronic myeloid leukemia. Am J Hematol. 2011;86:346‐350. [DOI] [PubMed] [Google Scholar]
- 17.Valent JN, Schiffer CA. Prevalence of large granular lymphocytosis in patients with chronic myelogenous leukemia (CML) treated with dasatinib. Leuk Res. 2011;35:e1‐e3. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Kamel‐Reid S, Chang H, et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica. 2009;94:135‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo Rossi A, Ricco A, Carluccio P, et al. T/NK cell lymphocytosis in CML Ph + patients during dasatinib therapy [abstract]. Blood. 2009;114:3279. [Google Scholar]
- 20.Schiffer CA, Cortes JE, Saglio G, et al. Lymphocytosis following first‐line treatment for CML in chronic phase with dasatinib is associated with improved responses: a comparison with imatinib [abstract]. Blood. 2010;116:358. [Google Scholar]
- 21.Nagata Y, Ohashi K, Fukuda S, Kamata N, Akiyama H, Sakamaki H. Clinical features of dasatinib‐induced large granular lymphocytosis and pleural effusion. Int J Hematol. 2010;91:799‐807. [DOI] [PubMed] [Google Scholar]
- 22.Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK‐cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23:1398‐1405. [DOI] [PubMed] [Google Scholar]
- 23.Porkka K, Khoury HJ, Paquette RL, Matloub Y, Sinha R, Cortes JE. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer. 2010;116:377‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka H, Nakashima S, Usuda M. Rapid and sustained increase of large granular lymphocytes and rare cytomegalovirus reactivation during dasatinib treatment in chronic myelogenous leukemia patients. Int J Hematol. 2012;96:308‐319. [DOI] [PubMed] [Google Scholar]
- 25.Kreutzman A, Juvonen V, Kairisto V, et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood. 2010;116:772‐782. [DOI] [PubMed] [Google Scholar]
- 26.Nagata Y, Fukuda S, Kobayashi T, et al. Safe switching from dasatinib to nilotinib after a 1‐month off‐drug period for persistent pleural effusion in patients with chronic myelogenous leukemia in chronic phase. Int J Hematol. 2010;91:539‐541. [DOI] [PubMed] [Google Scholar]
- 27.Mustjoki S, Auvinen K, Kreutzman A, et al. Rapid mobilization of cytotoxic lymphocytes induced by dasatinib therapy. Leukemia. 2013;27:914‐924. [DOI] [PubMed] [Google Scholar]
- 28.Schiffer CA, Cortes JE, Saglio G, et al. Association of lymphocytosis following treatment with dasatinib with response and outcome [abstract]. J Clin Oncol. 2010;28S: Abstract 6553. [Google Scholar]
- 29.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic‐phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260‐2270. [DOI] [PubMed] [Google Scholar]
- 30.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib‐resistant and ‐intolerant chronic‐phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204‐3212. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian H, Cortes J, Kim DW, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15‐month median follow‐up. Blood. 2009;113:6322‐6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novartis Pharmaceuticals Corp . Tasigna [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2015. [Google Scholar]
- 33.Pfizer Inc . Bosulif [package insert]. New York, NY: Pfizer Inc; 2015. [Google Scholar]
- 34.Ariad Pharmaceuticals Inc . Iclusig [package insert]. Cambridge, MA: Ariad Pharmaceuticals Inc; 2015. [Google Scholar]
- 35.Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36:492‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajala HL, Eldfors S, Kuusanmaki H, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121:4541‐4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreutzman A, Ilander M, Porkka K, Vakkila J, Mustjoki S. Dasatinib promotes Th1‐type responses in granzyme B expressing T‐cells [serial online]. Oncoimmunology. 2014;3:e28925. [DOI] [PMC free article] [PubMed] [Google Scholar]