Src-Mediated Phosphorylation of Focal Adhesion Kinase Couples Actin and Adhesion Dynamics to Survival Signaling (original) (raw)
Abstract
Integrin-associated focal adhesions not only provide adhesive links between cellular actin and extracellular matrix but also are sites of signal transmission into the cell interior. Many cell responses signal through focal adhesion kinase (FAK), often by integrin-induced autophosphorylation of FAK or phosphorylation by Src family kinases. Here, we used an interfering FAK mutant (4-9F-FAK) to show that Src-dependent FAK phosphorylation is required for focal adhesion turnover and cell migration, by controlling assembly of a calpain 2/FAK/Src/p42ERK complex, calpain activation, and proteolysis of FAK. Expression of 4-9F-FAK in FAK-deficient fibroblasts also disrupts F-actin assembly associated with normal adhesion and spreading. In addition, we found that FAK's ability to regulate both assembly and disassembly of the actin and adhesion networks may be linked to regulation of the protease calpain. Surprisingly, we also found that the same interfering 4-9F-FAK mutant protein causes apoptosis of serum-deprived, transformed cells and suppresses anchorage-independent growth. These data show that Src-mediated phosphorylation of FAK acts as a pivotal regulator of both actin and adhesion dynamics and survival signaling, which, in turn, control apparently distinct processes such as cell migration and anchorage-independent growth. This also highlights that dynamic regulation of actin and adhesions (which include the integrin matrix receptors) is critical to signaling output and biological responses.
Elevated expression of the nonreceptor tyrosine kinases Src and focal adhesion kinase (FAK) correlates with malignancy potential and poor clinical prognosis in colon and breast tumors (2, 19, 20, 71, 90, 91). Recent studies monitoring focal adhesion dynamics in cells deficient for FAK and Src implicate Src and FAK as critical mediators of integrin adhesion turnover that promote cell migration (97). Cells devoid of FAK exhibit impaired migration and have large peripheral focal adhesion structures (58), while cells lacking the three ubiquitous Src family members Src, Fyn, and Yes also demonstrate altered distribution of focal adhesions and impaired cell migration (64, 94). Src kinase activity is clearly necessary for focal adhesion turnover and cell motility, presumably by tyrosine phosphorylation of key focal adhesion substrates, such as FAK (22, 34). The extracellular regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway is also important in regulating focal adhesion dynamics during cell motility (39, 63, 69, 99, 100), and it is likely that ERK/MAPK contributes to Src-induced focal adhesion turnover. We have recently reported that ERK/MAPK, which is recruited to focal adhesions following v-Src activation, is required for maximal activity of the protease calpain 2 promoting focal adhesion turnover and migration of v-Src-transformed cells (17, 35). ERK/MAPK-induced activation of calpain 2 is also required for epidermal growth factor-induced substrate deadhesion and cell motility (40, 41).
The calpains are a highly conserved family of intracellular calcium-dependent proteases (for a review, see reference 42). Calpain was first implicated in cell migration by studies utilizing pharmacological inhibition of calpain activity, which impaired retraction at the rear of the cell and decreased cell movement (55, 72). Further studies on calpain-null cells confirmed a role for calpain in regulating focal adhesion turnover, which is necessary for cell migration (29). Several components of the focal adhesion complex, including FAK (15, 16, 18, 25, 89, 95, 105), paxillin (103), talin (104), α-actinin (85), and β3 integrin (30), are known substrates for calpain-mediated proteolysis, suggesting that calpain cleavage of one or more of these components contributes to focal adhesion disassembly.
The relationship between Src and FAK activity and the precise mechanisms by which FAK promotes cell migration remain elusive. Several studies suggest that FAK promotes cell migration by activating multiple signaling pathways involving Src family kinases and phosphatidylinositol (PI) 3-kinase or by phosphorylation of p130CAS, paxillin, or other focal adhesion substrates (21, 45, 79, 86, 88). FAK-dependent cell motility may also require its adaptor protein function, as it is reported that FAK expression, but not its kinase activity, is required for both platelet-derived growth factor- and epidermal growth factor-stimulated cell motility (86). A recent study demonstrated that FAK can also act upstream of calpain, suggesting a novel role by which FAK promotes focal adhesion turnover and cell motility (28). In this regard, we have also shown that FAK can act as an adaptor protein that permits the assembly of a complex consisting of calpain 2 and its upstream activator p42ERK, promoting calpain activity at the membrane (17).
FAK also plays a role in suppressing cell apoptosis, particularly in response to cell detachment (anoikis) (5, 31, 38, 54, 73, 101). Indeed, a previous study suggested that FAK may transmit extracellular matrix (ECM)-derived survival signals that suppress p53-mediated apoptosis (57). However, the upstream mechanisms that regulate FAK-mediated survival signals are not understood.
The question of how tyrosine phosphorylation of FAK contributes to its biological functions has not been addressed directly. Six major tyrosine phospho-acceptor sites have been identified on FAK, at positions 397, 407, 576, 577, 861, and 925 (13, 14, 83, 84). Tyr 397 becomes phosphorylated (presumed by autophosphorylation) upon integrin engagement (82). This leads to the formation of a consensus binding site for the Src SH2 domain (32), promoting association between Src and FAK (82). Phosphorylation of the remaining tyrosine residues on FAK is considered to be Src-dependent (13, 14, 83, 84).
In the present study we have generated a FAK mutant (4-9F-FAK) in which each of the putative Src-induced tyrosine phosphorylation sites (Tyr 407, 576, 577, 861, and 925) has been mutated to a phenylalanine (Phe). We found that v-Src-induced phosphorylation of FAK on tyrosine residues is necessary to enhance the adaptor function of FAK with regard to assembly of the calpain 2/FAK/p42ERK complex. Src-induced phosphorylation of FAK is also required for FAK to undergo proteolytic cleavage by calpain in v-Src-transformed cells and is necessary for calpain-mediated focal adhesion turnover during transformation and cell migration. In addition, we show for the first time that Src-induced phosphorylation of FAK also regulates F-actin assembly and cell spreading. We further demonstrate a role for Src-induced tyrosine phosphorylation of FAK in survival and anchorage-independent growth of transformed cells. These studies demonstrate that Src-induced tyrosine phosphorylation of FAK is a key event that simultaneously regulates actin and adhesion dynamics and cell survival.
MATERIALS AND METHODS
Cell culture.
Primary chicken embryo fibroblasts (CEFs) were cultured as previously described (36). Low-density cultures were transfected with replication-competent avian retroviral constructs, SFCV-v-src encoding the temperature-sensitive (ts) _LA_29 v-Src mutant or the kinase-defective ts LA29KD1 mutant. Transfected CEFs were cultured at the permissive temperature of 35°C until cells were uniformly infected and expressing v-Src protein. For analysis of v-Src-induced transformation, cells were cultured at the restrictive temperature (41°C) and then examined following a shift to the permissive temperature (35°C). FAK-null (FAK−/−) mouse embryo fibroblasts (MEFs) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and nonessential amino acid supplements and cultured at 37°C. Wild-type FAK (wt-FAK)- and 4-9-F-FAK-reconstituted FAK−/− cells were cultured in the same medium containing 1 mg of hygromycin per ml.
Construct design and expression.
Myc-tagged wt-FAK and the 4-9F-FAK construct containing selected tyrosine residues mutated to phenylalanine were constructed as previously described (3), using the Stratagene Quick-Change site-directed mutagenesis kit. After sequencing, the constructs were subcloned into the RCAS avian retroviral vector. CEFs were cotransfected with SFCV-v-Src and either RCAS-wt-FAK or RCAS-4-9F-FAK constructs. For expression in FAK−/− cells, wt-FAK and 4-9F-FAK constructs were subcloned into the pWZL retroviral expression vector. Phoenix ecotropic cells were transfected with pWZL-wt-FAK or -4-9F-FAK. The virus supernatant was then collected and used to infect FAK−/−cells. FAK−/− cells stably expressing either wt-FAK or 4-9F-FAK were selected by hygromycin resistance. Virtually 100% of cells in selected cultures expressed each of the FAK mutants as determined by immunocytochemistry with an anti-Myc antibody.
Antibodies and reagents.
Antibodies for Western blot detection and immunocytochemistry included 354-534N-pp125FAK (Transduction Laboratories), N-pp125FAK (Santa Cruz), paxillin (Transduction Laboratories), calpain 2 (Research Diagnostics, Inc.), anti-Myc 9E10 (Sigma), anti-avian Src EC10 (Upstate Ltd.), anti-phospho-ERK (Cell Signaling), anti-α-tubulin (Sigma), anti-p130cas (Transduction Laboratories), anti-α-actinin (Sigma), antiphosphotyrosine (Transduction Laboratories), and anti-phosphotyrosine397-FAK (BioSource UK). Anti-mouse and -rabbit peroxidase-conjugated secondary antibodies were purchased from New England Biolabs, Inc.
Protein immunoblotting.
Cells were washed twice with phosphate-buffered saline (PBS) and lysed in lysis buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 0.5% NP-40, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM NaF, 10 mM β-glycerophosphate, 10 mM Na4P2O7, 100 μM NaVO4, and protease inhibitors [1 mM phenylmethylsulfonyl fluoride {PMSF}, 10 μg of leupeptin per ml, and 10 μg of aprotinin per ml]). Lysates were clarified by high-speed centrifugation at 4°C, supplemented with sodium dodecyl sulfate (SDS) sample buffer, separated by SDS-10% polyacrylamide gel electrophoresis (PAGE), and immunoblotted with specific antibodies.
Protein IP.
Cells were washed twice with PBS and lysed in modified radioimmunoprecipitation assay buffer (0.5% NP-40 with inhibitors [1 mM PMSF, 100 μM NaVO4, 10 μg of benzamidine per ml, 10 μg of leupeptin per ml, and 10 μg of aprotinin per ml]). The lysates were clarified by high-speed centrifugation at 4°C and incubated overnight with 8 μg of anti-Myc, 3 μg of anti-calpain 2 (Calbiochem), and 2.5 μg of antiphosphotyrosine, antipaxillin, and anti-p130cas at 4°C. Either anti-mouse immunoglobulin G (IgG) conjugated to agarose beads or protein A-Sepharose (for anti-calpain 2 immunoprecipitations [IPs]) was added to the reaction mixtures and left for 1 h at 4°C with constant mixing prior to washing three times with radioimmunoprecipitation assay buffer. IP reaction products were resuspended in SDS sample buffer, and separated by SDS-PAGE, transferred to a membrane, and immunoblotted with specific antibodies.
Immunocytochemistry.
Cells were cultured on Permanox plastic chamber slides (Nalge Nunc International). Cells were fixed in 3.7% formaldehyde for 10 min at room temperature, permeabilized in 0.5% NP-40 in PBS for 10 min at room temperature, and washed serially in PBS, 0.15 M glycine-PBS plus 0.02% NaN3, and PBS. Cells were blocked in 10% fetal calf serum-PBS prior to 1 h of incubation at room temperature with primary antibodies, affinity-purified monoclonal antipaxillin (Transduction Laboratories), antivinculin (Sigma), and anti-Myc epitope clone 9E10 (Sigma). Primary antibody incubation was followed by several washes in PBS and subsequent incubation with fluorescein isothiocyanate-labeled secondary antibodies (Jackson Immunoresearch Laboratories). Actin stress fibers were monitored following staining with FITC-labeled phalloidin (Sigma). Immunostaining and phalloidin staining of cells were analyzed by confocal microscopy.
Wound-healing migration assay.
CEFs (2 × 105)expressing _ts LA_29 v-Src in combination with Myc-tagged wt-FAK or 4-9F-FAK mutants were cultured at 41°C in 60-mm-diameter dishes until 70 to 80% confluent. The cell monolayers were wounded by scoring with a sterile micropipette tip, and the cultures were incubated at 35°C for a further 12 h. FAK−/− cells and wt-FAK- and 4-9F-FAK-reconstituted FAK−/− cells (2 × 105) were also cultured until 70 to 80% confluence prior to generation of a wound in the monolayer and subsequent culture for 12 h at 37°C. For each sample three defined areas along the wound were monitored, the area of wound remaining after 12 h was calculated, and results were expressed as mean percentage of wound closure.
FAK assay.
Myc-tagged 4-9F-FAK, wt-FAK, and kinase-defective (kd)-FAK were immunoprecipitated from cells as described above. After washing in lysis buffer, FAK immunoprecipitates were washed twice in kinase buffer (20 mM Tris [pH 7.2], 10 mM magnesium chloride, 100 μM sodium orthovanadate). The FAK immunoprecipitates were resuspended in kinase buffer containing 20 μM ATP and incubated with a FAK peptide encompassing amino acids 361 to 463. This peptide fragment contains the autophosphorylation site of FAK (tyrosine 397) and also tyrosine 407, which was mutated to phenylalanine so that the ability of FAK to phosphorylate tyrosine 397 alone could be measured. The reaction was started by addition of 5 μCi of [γ-32P]ATP and, after 10 min at 30°C, was stopped by addition of 2× Laemmli buffer. The phosphorylated peptide was then detected by autoradiography following separation by SDS-15% PAGE. Levels of immunoprecipitated FAK in each reaction were monitored by immunoblotting with anti-FAK antibody.
Calpain activity assays.
Analysis of calpain activity in total cell lysates extracted from FAK−/− cells and wt- and 4-9F-FAK-reconstituted FAK−/− cells was performed with a calpain activity assay kit from (BioVision Inc.) according to the manufacturer's instructions. The calpain activity assay kit consists of a fluorogenic peptide calpain substrate (Ac-LLY-AFC). Briefly, clarified cell lysates were incubated with fluorogenic peptide calpain substrate for 1 h at 37°C in the dark. Upon cleavage by calpain, the fluorogenic portion (AFC) of the peptide is released, emitting UV light at a wavelength of 505 nm, which was measured by using a FLEXstation fluorescent plate reader (Molecular Devices). Results are expressed as relative fluorescence units.
Sensitivity to trypsin-induced cell-substrate detachment.
FAK−/− cells reexpressing wt-FAK or 4-9F-FAK were cultured on uncoated tissue culture-treated plastic dishes for several days prior to washing twice with PBS and incubation with trypsin (0.25-mg/ml solution). The number of cells detached from the substrate cells at sequential time points following trypsin incubation was quantified by counting suspended cells with a CASY 1 cell counter.
Isolation of G-actin and F-actin pools.
Triton-soluble and -insoluble pools of G-actin and F-actin, respectively, were prepared as previously described (27). Briefly, 2 × 106 cells were cultured on 90-mm-diameter uncoated tissue culture plastic dishes for 8 h prior to being rinsed twice in CSK buffer [10 mM piperazine-N,_N_′-bis(2-ethanesulfonic acid) (PIPES; pH 6.8), 50 mM NaCl, 3 mM MgCl2, 300 mM sucrose] and then treated as follows.
For G-actin isolation, cells were then incubated with 0.5 ml of lysis buffer 1 (CSK buffer with 1% Triton X-100, 1 μg of phalloidin per ml, 10 μg of leupeptin per ml, 10 μg of pepstatin A per ml, and 1 mM PMSF). The cells were gently mixed with lysis buffer on a rotatory shaker for 5 min at room temperature. Supernatants containing the G-actin pool were collected, and an SDS solution was added to give a final concentration of 2%.
For F-actin isolation, cellular protein remaining on the dishes was washed three times with CSK buffer and then incubated with 0.5 ml of lysis buffer 2 (lysis buffer 1 containing 2% SDS) and mixed on a rotatory shaker for 5 min at room temperature. Lysates were then scraped from dishes and collected. The lysates were sheared by being passed through a 1-ml syringe with a 25-gauge needle three times. Lysates were supplemented with SDS sample buffer prior to separation by SDS-PAGE.
G-actin and F-actin pools were also isolated from cells following 12 h of preincubation with the calpain inhibitor _N_-acetyl-leucine-leucine-norleucinyl-CHO (ALLN; 50 μM) prior to plating on plastic for 8 h in the presence of ALLN (50 μM).
Cell adhesion assay.
The adhesion of FAK−/− cells expressing wt-FAK and 4-9F-FAK to fibronectin was assayed as previously described (12). Briefly, FAK−/− cells expressing either wt-FAK, 4-9F-FAK, or empty vector were radiolabeled with chromium 51. Labeled cells (104) were then plated on to non-tissue culture-treated wells of a flexible plastic 96-well assay plate coated with either bovine serum albumin (BSA) as control or 1 μg of fibronectin per cm2. At 30 and 60 min following cell plating, cells were washed twice with PBS and briefly air dried. The wells were removed from the plate, and counts per minute corresponding to adherent chromium 51-labeled cells were measured with a gamma counter. The percentage of cells adherent to fibronectin was calculated by relating counts per minute of cells attached to wells to 104 cells in suspension. The percentage of cells adherent to BSA-coated plates was subtracted from the percentage of cells adherent to fibronectin-coated plates to monitor specifically adhesion to fibronectin.
Analysis of sub-G1 DNA content and annexin V-FITC staining to detect apoptotic cells.
Flow cytometric analysis was used to obtain cell cycle profiles as previously described (62). Briefly CEFs coexpressing either wt-FAK or 4-9F-FAK with ts v-Src were cultured under serum-free conditions for 24 h at restrictive (41°C, v-src off) and permissive (35°C, v-src on) temperatures for v-Src activity. Cells were harvested, fixed in 70% ethanol, and resuspended in PBS containing RNase A (250 μg/ml) and propidium iodide (10 μg/ml). The cells were incubated for 30 min in the dark prior to analysis by flow cytometry. The percentage of cells containing sub-G1 DNA was recorded as the proportion of apoptotic cells.
Cell apoptosis was also monitored by using the annexin V-FITC apoptosis detection kit I (BD PharMingen) according to the manufacturer's instructions. Briefly, CEFs coexpressing either wt-FAK or 4-9F-FAK with ts v-Src were cultured under serum-free conditions for 24 h at restrictive and permissive temperatures for v-Src activity. Cells were washed twice with cold PBS and resuspended in 1× binding buffer. The cells were then incubated with FITC-conjugated annexin V and propidium iodide prior to analysis by flow cytometry.
Anchorage-independent growth assay.
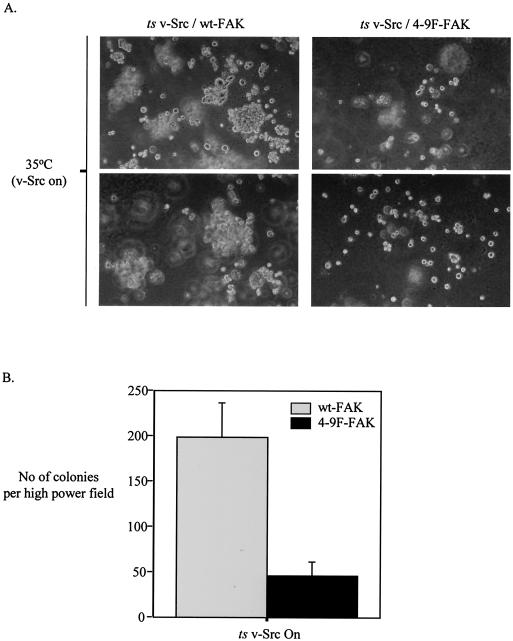
Anchorage-independent growth assays were performed as previously described (46). Briefly, 60-mm-diameter bacterial culture dishes were coated with 0.5% base agar supplemented with normal CEF culture medium as described above. CEFs (2 × 105) coexpressing ts v-Src with either wt-FAK or 4-9F-FAK were suspended in double-concentrated CEF growth medium and added to an equal volume of 0.6% molten top layer agar. Cell-agar preparations were added to base agar dishes and cultured at permissive culture temperatures for v-Src activity. Following several days in culture, top layer agar containing cells was overlaid with base agar supplemented with culture medium. Fourteen days after seeding, the formation of cell colonies was quantified as the number of colonies (defined as four or more cells) per high-power field (magnification, ×25).
RESULTS
A major obstacle in the study of focal adhesion dynamics is that in migrating cells, focal adhesion structures are in a constant equilibrium between assembly and disassembly. To examine the mechanisms that regulate focal adhesion disassembly specifically, we have used a ts mutant of v-Src (_ts LA_29) (98) expressed in CEFs, a well-established model for studying Src-dependent focal adhesion turnover (34, 37). Activation of v-Src by culture at the permissive temperature (35°C) shifts the balance of focal adhesion assembly and disassembly to almost complete focal adhesion disassembly, resulting in an extreme transformed cell phenotype (34, 36). We have recently used this model system to demonstrate that Src-induced disassembly of focal adhesions and morphological transformation is accompanied by calpain-mediated cleavage of FAK (15, 18).
v-Src kinase activity is required for FAK proteolysis and focal adhesion disassembly during v-Src transformation.
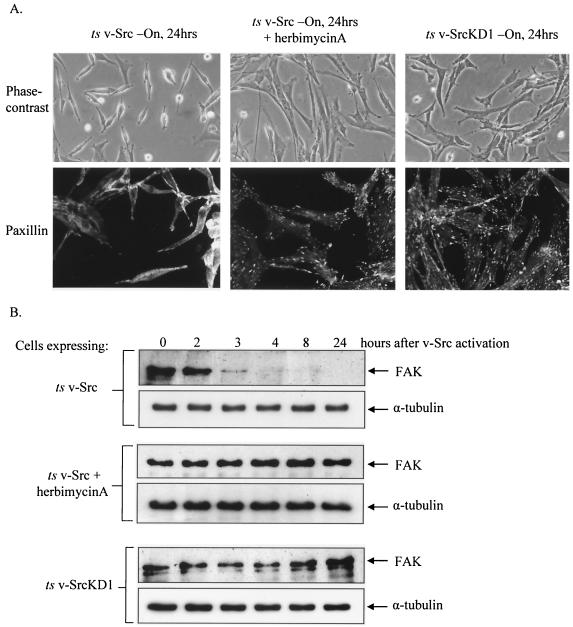
Src is a nonreceptor tyrosine kinase that also contains SH2 and SH3 domains, thus functioning not only as a kinase but also as an adaptor molecule to exert its biological effects (37). To determine whether FAK proteolysis and focal adhesion disassembly associated with Src are consequences of v-Src's kinase activity, we examined focal adhesion disassembly and cell morphology, first following activation of ts v-Src in the absence or presence of a general inhibitor of tyrosine kinases, herbimycin A, and second by activation of a kinase-defective mutant ts v-Src (_ts LA_29KD1) (Fig. 1). As expected, treatment with herbimycin A (1 μM) prevented focal adhesion disassembly, morphological transformation, and substrate detachment (Fig. 1A, middle panels [compare with left panels]). In contrast to the case for _ts LA_29 v-Src, shift of the kinase-defective _ts LA_29KD1 v-Src-expressing cells to the permissive temperature did not result in focal adhesion disassembly or in morphological transformation and detachment (Fig. 1A, right panels). Treatment with herbimycin A (1 μM) also prevented v-Src-induced proteolytic cleavage of FAK (Fig. 1B, middle panels [compare with upper panels]). Shift of the kinase-defective _ts LA_29KD1 v-Src cells to the permissive temperature also did not result in FAK proteolysis (Fig. 1B, bottom panels). These results clearly demonstrate that the tyrosine kinase activity of v-Src is required for initiating the proteolytic cleavage of FAK that accompanies disassembly of focal adhesions during transformation.
FIG. 1.
v-Src kinase activity is required for FAK proteolysis and focal adhesion disassembly during v-Src transformation. (A) CEFs expressing _ts LA_29 v-Src with and without herbimycin A treatment and CEFs expressing kinase-defective _ts LA_29KD1 v-Src were cultured at the permissive temperature for v-Src activity for 24 h. Cell morphology and distribution of focal adhesions were evaluated by phase-contrast microscopy (magnification, ×200) and confocal microscopy (magnification, ×400) following immunocytochemistry with an antipaxillin antibody. (B) Cell lysates were prepared at sequential time points following v-Src activation (by a shift to the permissive temperature of 35°C) from CEFs expressing _ts LA_29 v-Src (upper panels), CEFs expressing ts LA29 v-Src following pretreatment with 1 μM herbimycin A (middle panels), and CEFs expressing _ts LA_29KD1 v-Src (lower panels). FAK protein levels were detected by SDS-PAGE and immunoblotting with anti-FAK antibody. Equal protein loading was determined by immunoblotting with anti-α-tubulin antibody.
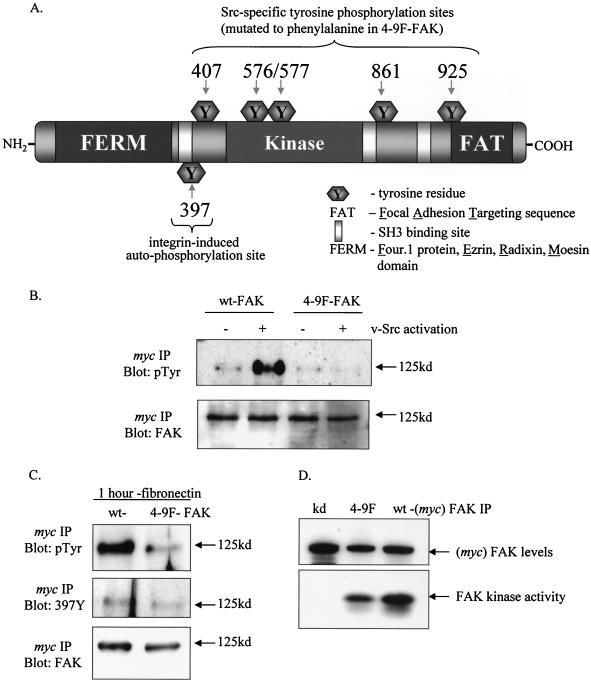
Mutation of tyrosines Y407, Y576, Y577, Y861, and Y925 of FAK inhibit v-Src-induced tyrosine phosphorylation of FAK.
To address whether FAK was a critical substrate for v-Src, we generated a FAK mutant (4-9F-FAK) that does not undergo further tyrosine phosphorylation upon v-Src activation. Specifically, each of the recognized Src-specific phospho-acceptor sites (Tyr 407, 576, 577, 861, and 925) was mutated to nonphosphorylatable phenylalanine (F). These sites are shown relative to other FAK domains in Fig. 2A. Myc-tagged wt- and 4-9F-FAK proteins were coexpressed with _ts LA_29 v-Src. When wt- and 4-9F-FAK were immunoprecipitated with an anti-Myc antibody at nonpermissive and permissive temperatures and immunoblotted with antiphosphotyrosine antibody, we found that tyrosine phosphorylation of 4-9F-FAK was not enhanced upon v-Src activation compared to that of wt-FAK (Fig. 2B). This indicates that the 4-9F-FAK is refractory to v-Src-induced phosphorylation. The residual phosphotyrosine signal exhibited by 4-9F-FAK is most likely due to tyrosine phosphorylation on Tyr 397. We examined the ability of 4-9F-FAK to undergo phosphorylation in response to cell adhesion to fibronectin. Following adhesion of nontransformed CEFs to fibronectin for 1 h, Myc-tagged wt-FAK or 4-9F-FAK was immunoprecipitated as described above and subsequently immunoblotted with antiphosphotyrosine antibody and a site-specific anti-phosphotyr397-FAK antibody (Fig. 2C). We found that the 4-9F-FAK mutant is phosphorylated on tyrosine 397 following adhesion to fibronectin. However, total tyrosine phosphorylation of 4-9F-FAK following adhesion to fibronectin is significantly reduced compared to that of wt-FAK. These data indicate that in response to adhesion both wt-FAK and 4-9F-FAK become autophosphorylated on Tyr 397. This phosphorylation event is likely to recruit the SH2 domain of Src, resulting in further phosphorylation of wt-FAK on Src-dependent tyrosine residues. In contrast to wt-FAK the 4-9F-FAK mutant cannot undergo further phosphorylation at Src-dependent sites, resulting in a reduced level of total tyrosine phosphorylation.
FIG. 2.
Mutation of tyrosines Y407, Y576, Y577, Y861, and Y925 of FAK inhibits v-Src-induced tyrosine phosphorylation of FAK. (A) Illustration of FAK protein domains, indicating locations of autophosphorylation and Src-regulated phosphotyrosine sites. (B) CEFs coexpressing _ts LA_29 v-Src with either wt- or 4-9F-FAK mutant (Myc tagged) were cultured at restrictive (−) and permissive (+) temperatures for v-Src activation. Total cell lysates were prepared from these cells, followed by IP with anti-Myc antibody. Tyrosine phosphorylation of wt-FAK and 4-9F-FAK (both 125 kDa) was detected following separation of anti-Myc IPs by SDS-PAGE and immunoblotting with antiphosphotyrosine antibody (pTyr). (C) Total tyrosine phosphorylation status and specific phosphorylation of the tyrosine 397 site on wt- and 4-9F-FAK was monitored 1 h following adhesion of nontransformed cells to fibronectin. Anti-Myc IPs were separated by SDS-PAGE and immunoblotting with antiphosphotyrosine antibody and a site-specific anti-phosphotyrosine397-FAK (397Y). (D) The kinase activities of wt- and 4-9F-FAK were directly compared by kinase assay following IP with anti-Myc antibody and incubation with substrate in the presence of [γ-32P]ATP. kd-FAK was also immunoprecipitated and included in the kinase assay as a negative control. Levels of each FAK mutant used in the kinase assay were monitored by immunoblotting of immunoprecipitates with anti-FAK antibody.
We next monitored the kinase activity of the 4-9F-FAK mutant by using a fragment of FAK that contains the autophosphorylation Tyr 397 site as a substrate. The kinase activity of immunoprecipitated 4-9F-FAK was compared with that of wt-FAK and kd-FAK as a negative control (Fig. 2D). This analysis clearly indicates that 4-9F-FAK retains a readily detectable amount of kinase activity; however, kinase activity is reduced in comparison to that of wt-FAK.
Src-induced tyrosine phosphorylation of FAK is necessary for FAK proteolysis during v-Src-induced transformation.
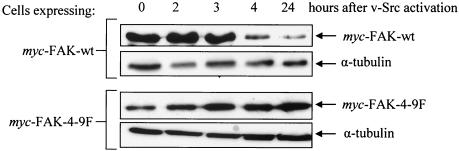
Previous studies have reported that Src-mediated tyrosine phosphorylation of cortactin, spectrin, and the NR2 subunits of _N_-methyl-d-aspartate receptors influences the ability of these proteins to be cleaved by calpain (6, 52, 70). To determine whether Src-induced tyrosine phosphorylation of FAK influences its stability, we monitored total protein levels of exogenous wt-FAK, in comparison with 4-9F-FAK, following activation of _ts LA_29 v-Src. Total cell lysates were prepared at sequential time points following v-Src activation. The lysates were then immunoblotted with anti-Myc antibody to determine protein levels of exogenous FAK proteins, or α-tubulin as a control (Fig. 3). Consistent with the kinetics for endogenous FAK degradation (15, 36), wt-FAK levels decreased at 4 h following v-Src activation and were substantially diminished after 24 h. However, unlike endogenous FAK (Fig. 1A), exogenous wt-FAK protein was not completely degraded following v-Src activation, presumably due to the high level of overexpression. In contrast to those of exogenous wt-FAK, the protein levels of the 4-9F mutant FAK did not decrease following v-Src activation and appeared to accumulate at later time points (Fig. 3). As we have previously shown that proteolytic cleavage of FAK following v-Src activation is dependent on calpain activity (15, 18), these results suggest that Src-induced phosphorylation of tyrosine residues (Tyr 407, 576, 577, 861, and 925) is required for FAK to undergo calpain-mediated proteolysis in v-Src-transformed cells in vivo.
FIG. 3.
Src-induced tyrosine phosphorylation of FAK is necessary for FAK proteolysis during v-Src-induced transformation. Cell lysates were prepared at sequential time points following v-Src activation from CEFs coexpressing, Myc-tagged wt-FAK and 4-9F-FAK with _ts LA_29 v-Src. Protein levels of Myc-tagged wt-FAK and 4-9F-FAK were detected by SDS-PAGE and immunoblotting with anti-Myc antibody. Equal protein loading was analyzed by immunoblotting with anti-α-tubulin antibody.
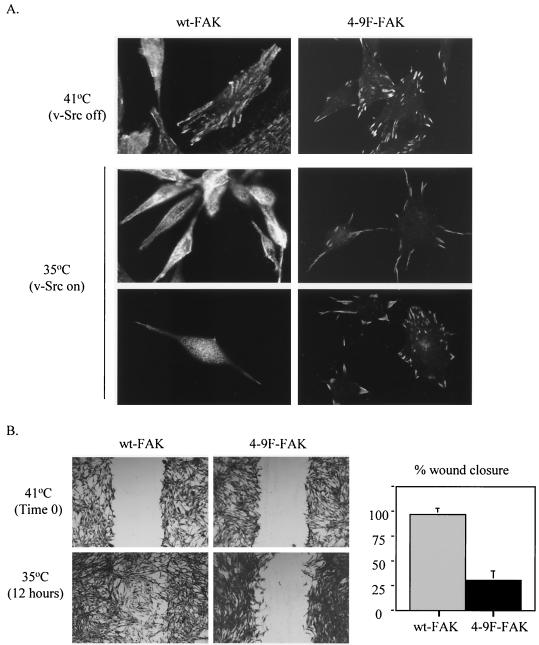
Src-induced tyrosine phosphorylation of FAK is required for focal adhesion turnover and migration of v-Src-transformed cells.
We previously demonstrated that v-Src-induced proteolytic cleavage of FAK occurs in parallel with focal adhesion disassembly and morphological transformation of CEFs (15). We analyzed v-Src's ability to initiate focal adhesion disassembly in cells coexpressing wt- or 4-9F-FAK. We monitored the localization and loss of wt- FAK and 4-9F-FAK proteins from focal adhesion sites prior to and following v-Src activation. CEFs coexpressing the Myc-tagged FAK proteins with ts v-Src were cultured at the restrictive (41°C) or permissive (35°C) temperature for v-Src activation. Cells were analyzed by confocal microscopy with an anti-Myc antibody to visualize exogenous FAK protein (Fig. 4A). At the restrictive temperature, cell morphology and distribution of FAK-containing focal adhesions appeared similar for cells expressing wt-or 4-9F-FAK proteins. The wt- and 4-9F-FAK proteins were both efficiently expressed and targeted to focal adhesion sites (Fig. 4A). Following v-Src activation, cells expressing wt-FAK became morphologically transformed and wt-FAK was redistributed from focal adhesion sites to a diffuse cytoplasmic localization. In contrast, cells expressing 4-9F-FAK were not efficiently transformed at 24 h following v-Src activation. At this time point, the 4-9F-FAK mutant protein was maintained at peripheral focal adhesion sites (Fig. 4A). The persistence of 4-9F-FAK at focal adhesion sites is consistent with increased stability of this mutant protein (Fig. 3). The reduction in v-Src-induced focal adhesion disassembly exhibited by cells expressing 4-9F-FAK mutants was also confirmed by antivinculin staining (results not shown).
FIG. 4.
Src-induced tyrosine phosphorylation of FAK is required for focal adhesion turnover and migration of v-Src-transformed cells. (A) CEFs coexpressing Myc-tagged wt-FAK or 4-9F-FAK with _ts LA_29 v-Src were cultured under restrictive conditions (41°C, v-Src off) or permissive conditions (35°C, v-Src on [24 h; two representative fields are shown]) for v-Src activity. The cell morphology and distribution of Myc-tagged wt-FAK or 4-9F-FAK were evaluated by confocal microscopy (magnification, ×600) following immunocytochemistry with an anti-Myc antibody. (B) CEFs coexpressing _ts LA_29 v-Src with wt-FAK or 4-9F-FAK were initially cultured at the restrictive temperature of 41°C. Once cells were preconfluent, a wound was generated, and wound size was recorded at time zero. Cells were subsequently incubated for 12 h at the permissive temperature (35°C) for v-Src activation, and cell migration into the wound was analyzed by phase-contrast microscopy (magnification, ×50). The percent distance of wound closure at 12 h following wound generation was calculated from three separate areas and expressed as mean plus standard error.
Since we have previously shown that Src-induced focal adhesion disassembly is required for cell migration (15, 34), we next examined whether cells expressing the more stable 4-9F-FAK protein exhibited a reduced capacity to migrate. Wound repair assays were performed on CEFs coexpressing ts v-Src with either wt-FAK or 4-9F-FAK (Fig. 4B). After wounding, CEFs were cultured for 12 h at the permissive temperature. Following activation of v-Src, CEFs expressing 4-9F-FAK mutants demonstrated a visibly reduced capacity to migrate into the wounded area compared to CEFs expressing wt-FAK (Fig. 4B).
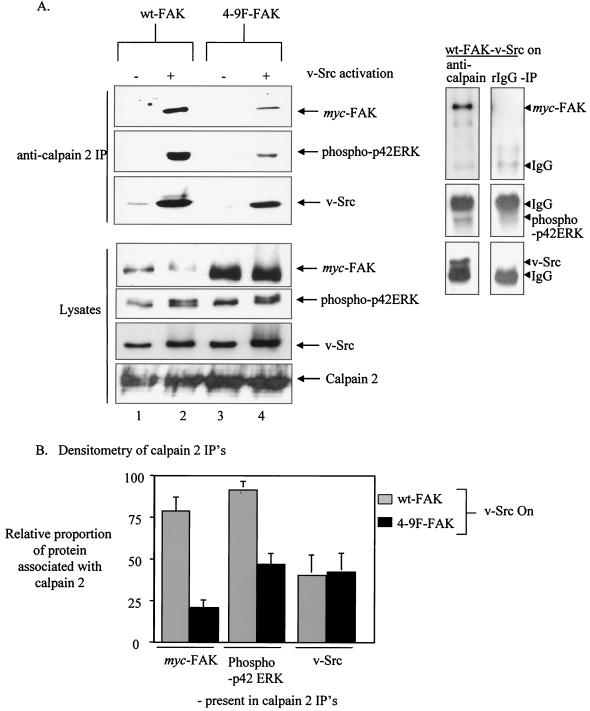
v-Src-induced tyrosine phosphorylation of FAK enhances the association of calpain 2 with FAK, p42ERK, and v-Src.
We recently identified a novel function for FAK as an adaptor molecule that recruits both calpain 2 and p42ERK (an upstream activator of calpain 2) to focal adhesion sites (17). This adaptor function of FAK is required for assembly of a calpain 2/FAK/p42ERK complex, which, in turn, activates calpain 2 at the cell periphery and promotes proteolysis of FAK, focal adhesion turnover, and cell migration and transformation (17). To determine whether v-Src-induced tyrosine phosphorylation of FAK affects complexing with calpain and p42ERK, we immunoprecipitated calpain 2 from CEFs coexpressing ts v-Src with either Myc-tagged wt- or 4-9F-FAK, at either restrictive or permissive temperatures (Fig. 5A). When v-Src is inactive, only a small amount of calpain 2 associates with either wt-FAK or the 4-9F-FAK mutant protein (only visible following long exposures) (results not shown). However, at 24 h following v-Src activation, calpain 2 strongly associates with wt-FAK (Fig. 5A, lane 2). The association between calpain 2 and the 4-9F-FAK mutant is also enhanced following v-Src activation (Fig. 5A, lane 4), but to a lesser extent than when wt-FAK is expressed even though 4-9F-FAK expression is greater (Fig. 5A, lane 2). This suggests that v-Src-induced phosphorylation of FAK on tyrosine is not absolutely required but enhances its ability to associate with calpain 2. The association of calpain 2 with activated p42ERK is also induced following v-Src activation in cells expressing wt-FAK (Fig. 5A, lane 2). However, the v-Src-induced association between calpain 2 and p42ERK is reduced in cells expressing the 4-9F-FAK mutant (Fig. 5A, lane 4). These results suggest that while Src-induced phosphorylation of FAK enhances its ability to function as an adaptor protein linking calpain 2 to its upstream activator p42ERK, it is not absolutely required for complex formation. Although a complex between 4-9F-FAK, calpain 2, and p42ERK forms following v-Src activation, this is apparently not sufficient to promote the cleavage of 4-9F-FAK (Fig. 3), implying that v-Src-induced tyrosine phosphorylation of FAK is also required for it to act as an efficient proteolytic substrate for calpain 2.
FIG. 5.
v-Src-induced tyrosine phosphorylation of FAK enhances the association of calpain 2 with FAK, p42ERK, and v-Src. (A) CEFs coexpressing _ts LA_29 v-Src with either wt-FAK or 4-9F-FAK were cultured at the restrictive (−) or permissive (+) temperature for v-Src activation. Total cell lysates were prepared from these cells, followed by IP with anti-calpain 2 antibody. Calpain 2-associated v-Src, phospho-p42ERK, and Myc-tagged FAK mutants were detected after SDS-PAGE, transfer to membranes and immunoblotting (top). Direct Western blots of the same total cell lysates used for IP experiments are also shown (bottom). Control IPs with normal rabbit IgG are shown on the right. (B) The fractions of wt-FAK, 4-9F-FAK, phospho-p42ERK, and v-Src coimmunoprecipitating with calpain 2 following v-Src activation in relation to total levels present in cell lysates were calculated from three separate experiments. Values were quantified by densitometry using NIH3 image software and represent mean values plus standard errors.
The relative proportions of Myc-tagged wt-FAK, 4-9F-FAK, phospho-p42ERK, and v-Src coprecipitating with calpain 2 relative to total protein levels found in cell lysates were calculated by densitometric analysis from three separate experiments (Fig. 5B). This analysis demonstrates that calpain 2 association with v-Src is not significantly altered following expression of 4-9F-FAK, suggesting that this interaction may be independent of FAK phosphorylation. Control IPs performed with nonspecific normal rabbit IgG antibody did not coprecipitate Myc-tagged FAK proteins, p42ERK, or v-Src (Fig. 5A, right panels). Western blot analysis on lysates performed with anti-Myc antibody demonstrates expression of exogenously expressed wt- and 4-9F-FAK proteins as well as p42ERK, v-Src, and calpain 2 (Fig. 5A). Calpain 2 protein levels were not affected by 4-9F-FAK protein expression.
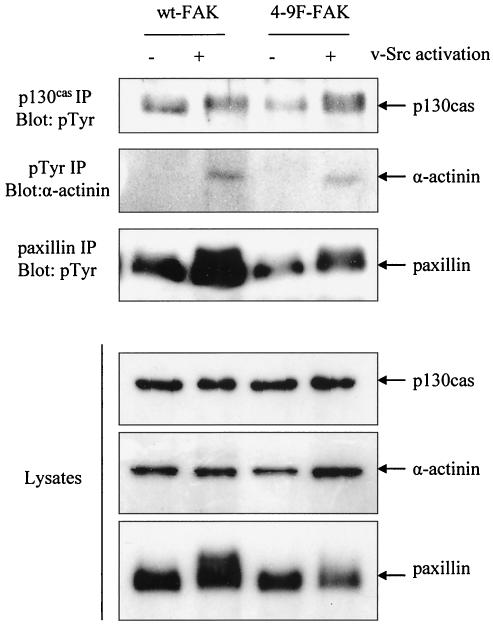
4-9F-FAK impairs v-Src-induced phosphorylation of paxillin and α-actinin but not p130cas.
Previous studies indicate that expression of FAK-related nonkinase, a C-terminally truncated form of FAK that lacks kinase activity, can impair v-Src-induced phosphorylation of p130cas (48). We examined the role that Src-induced phosphorylation of FAK has in phosphorylation of other Src substrates, including p130cas, paxillin, and α-actinin. The tyrosine phosphorylation status of p130cas, paxillin, and α-actinin was monitored following activation of ts v-Src in cells coexpressing either wt- or 4-9F-FAK (Fig. 6). These data show that expression of 4-9F-FAK significantly impairs v-Src-induced phosphorylation of paxillin and may also slightly reduce v-Src-induced phosphorylation of α-actinin. In contrast, v-Src-induced phosphorylation of p130cas was not affected by expression of 4-9F-FAK. These results suggest that Src-induced phosphorylation of FAK is required for mediating maximal phosphorylation of paxillin and also, to a lesser degree, that of α-actinin in v-Src-transformed cells. The reduced levels of paxillin and α-actinin phosphorylation in cells expressing 4-9F-FAK may contribute to the impaired morphological transformation and reduced motility exhibited by these cells.
FIG. 6.
4-9F-FAK impairs v-Src-induced phosphorylation of paxillin and α-actinin but not p130cas. The tyrosine phosphorylation status of paxillin, p130cas, and α-actinin was monitored prior to and 3 h following activation of v-Src in cells coexpressing wt-FAK and 4-9F-FAK. p130cas and paxillin were immunoprecipitated with specific antibodies and then separated by SDS-PAGE and immunoblotted with antiphosphotyrosine (pTyr) antibody. To detect tyrosine phosphorylation of α-actinin, antiphosphotyrosine was used to immunoprecipitate phosphorylated proteins, which were then separated by SDS-PAGE and immunoblotted with anti-α-actinin antibody.
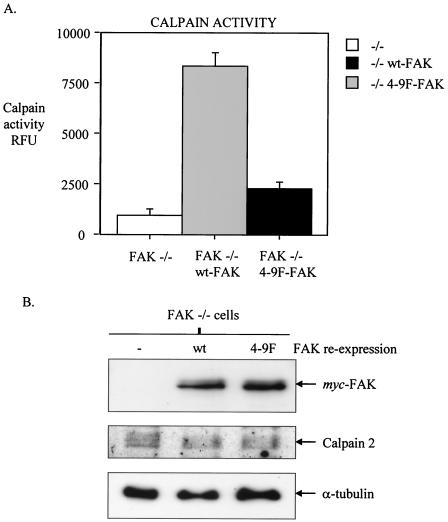
4-9F-FAK does not restore calpain activity in FAK−/− cells.
Recent studies demonstrate that FAK−/− MEFs have reduced levels of calpain proteolytic activity (17, 28). We have reported that FAK may contribute to calpain activity by acting as an adaptor protein linking calpain 2 with its activator p42ERK at the plasma membrane (17). Our data demonstrate that expression of the 4-9F-FAK mutant protein reduced the physical association of calpain 2 with p42ERK in v-Src-transformed cells (Fig. 5). Thus, we addressed whether Src-induced phosphorylation of FAK influenced total cellular calpain activity by measuring calpain activity in FAK−/− MEFs following reexpression of either wt-FAK or 4-9F-FAK. Quantification of total cellular levels of calpain activity in cell extracts showed that reexpression of wt-FAK promotes calpain activity to a substantially greater extent than reexpression of 4-9F-FAK (Fig. 7A). wt- and 4-9F-FAK were expressed at relatively equal levels, and expression of 4-9F FAK did not influence the protein levels of calpain 2 (Fig. 7B). These data demonstrate that the linked activities of Src and FAK are upstream of calpain activity.
FIG. 7.
4-9F-FAK does not restore calpain activity in FAK−/− cells. wt-FAK and the 4-9F-FAK mutant were stably expressed in FAK−/− MEFs. (A) Total cellular levels of calpain activity were measured in cell lysates extracted from FAK−/− MEFs and FAK−/− MEFs expressing either wt-FAK or 4-9F-FAK. Calpain activity in cell extracts was determined by using a calpain activity assay kit (BioVision Inc.) that monitors fluorescence emission induced by cleavage of a specific fluorogenic peptide substrate. Calpain activity was quantified with a fluorescence plate reader, and results are expressed as relative fluorescence units (RFU). Data represent mean values plus standard errors from three separate experiments. (B) Total cell lysates were prepared from FAK−/− cells or wt-FAK- and 4-9F-FAK-reconstituted FAK−/− cells. Lysates were separated by SDS-PAGE and immunoblotted with anti-Myc, anti-calpain 2, and anti-α-tubulin antibodies.
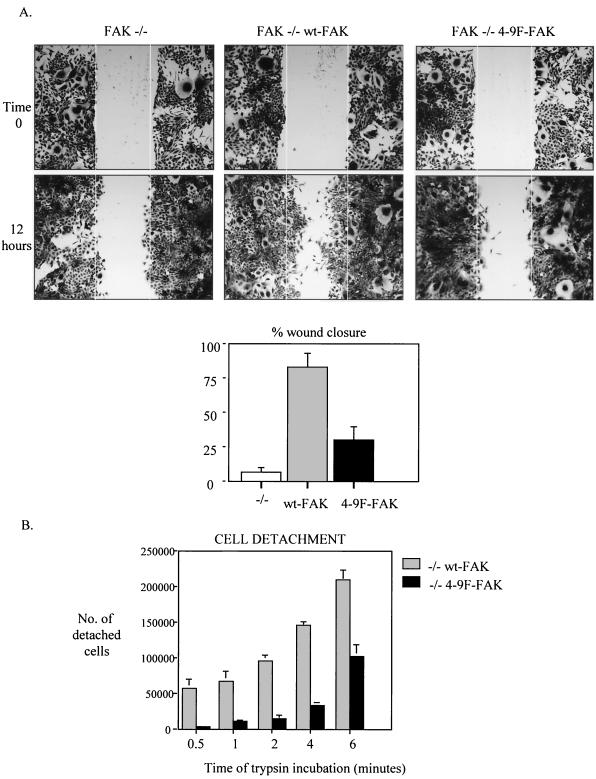
Reexpression of wt-FAK but not 4-9F-FAK rescues motility of FAK−/− cells and facilitates cell deadhesion.
FAK−/− MEFs exhibit a distinctive phenotype with larger focal adhesions and impaired cell motility compared to wild-type MEFs (58). Reexpression of FAK in FAK−/− cells rescues the motility defect (86, 87). These studies and others indicate that FAK plays a role in promoting focal adhesion turnover that facilitates cell-substrate deadhesion and cell motility. To determine whether Src-induced tyrosine phosphorylation of FAK contributes to focal adhesion turnover and cell motility of nontransformed cells, we reexpressed wt-FAK or 4-9F-FAK in FAK−/− cells. As previously reported, reexpression of wt-FAK rescued the migration of FAK−/− cells into a wounded monolayer (Fig. 8A, middle panels). In contrast, reexpression of 4-9F-FAK at similar levels (Fig. 7B) did not promote cell migration to a similar extent (Fig. 8A, right panels). To determine whether Src-induced phosphorylation of FAK facilitates cell-substrate deadhesion, we quantified the number of wt-FAK- and 4-9F-FAK-reconstituted FAK−/− cells that detached from their substrate at sequential time points following incubation with trypsin (Fig. 8B). These results clearly demonstrate that 4-9F-FAK-expressing cells are more resistant to trypsin-induced substrate detachment than wt-FAK-expressing cells.
FIG. 8.
Reexpression of wt-FAK but not 4-9F-FAK rescues motility of FAK−/− cells and facilitates cell deadhesion. (A) A wound was generated in subconfluent monolayers of FAK−/− cells and wt-FAK- and 4-9F-FAK-reconstituted FAK−/− cells. Wound size was recorded immediately following wound generation (time zero), and cell migration into the wound was monitored following incubation at 37°C for 12 h by phase-contrast microscopy (magnification, ×25). The percent distance of wound closure at 12 h following wound generation was calculated from three separate areas and expressed as mean plus standard error. (B) The adhesive properties of FAK−/− cells reexpressing wt- or 4-9F-FAK were assessed by monitoring trypsin-induced substrate detachment. Data represent mean values plus standard errors from three independent experiments.
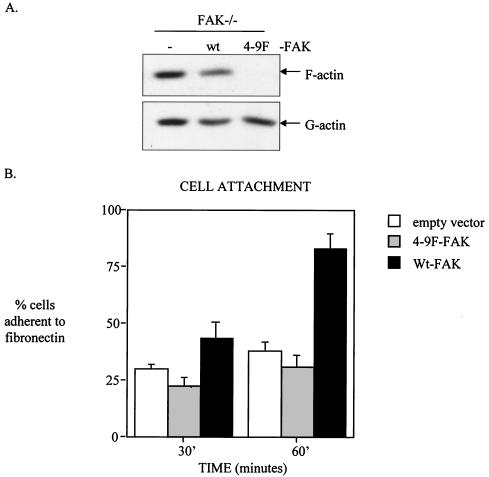
4-9F-FAK disrupts the normal F-actin/G-actin ratio and fails to rescue actin stress fiber assembly, cell attachment, and spreading of FAK−/− cells.
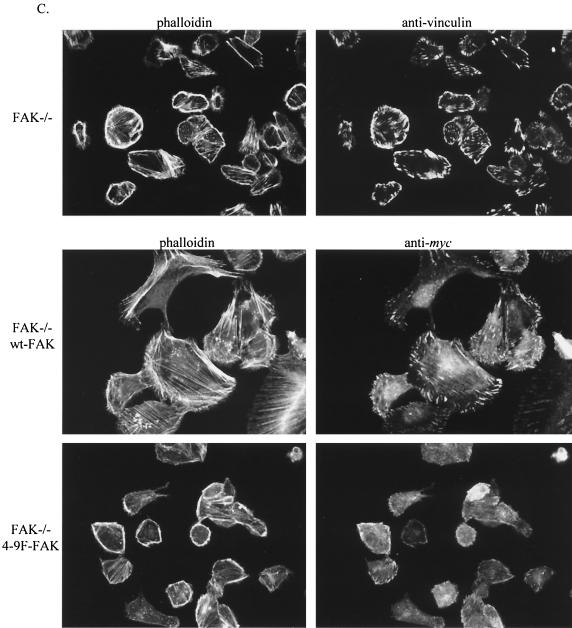
Previous studies suggested an important role for FAK in regulating the actin cytoskeleton, an effect that is mediated in part by downstream signaling to the Rho family of small GTPases (24, 106). To determine whether Src-induced tyrosine phosphorylation of FAK plays a particular role in influencing F-actin assembly and/or maintenance, we isolated pools of G-actin and F-actin from FAK−/− cells reconstituted with wt-FAK or 4-9F-FAK. We found that cells expressing 4-9F-FAK protein possessed substantially less F-actin relative to G-actin compared to cells expressing wt-FAK or control FAK−/− cells (Fig. 9A). This implies that the 4-9F-FAK mutant acts to reduce levels of F-actin, possibly through sequestration of an essential component of actin polymerization. Because assembly of F-actin is required for cell adhesion and spreading, we next addressed whether Src-induced phosphorylation of FAK was associated with integrin-dependent cell adhesion and spreading. Relative to expression of wt-FAK, expression of 4-9F FAK impaired cell attachment to a fibronectin-coated substrate (Fig. 9B). In comparison to FAK-null cells expressing empty vector, reexpression of 4-9F-FAK was unable to promote cell attachment to fibronectin to the same extent as wt-FAK reexpression. The percentage of cells adhering to BSA-coated substrates was subtracted prior to determining the values shown in Fig. 9B to specifically monitor adhesion to fibronectin. Expression of 4-9F-FAK also delayed subsequent cell spreading and formation of distinct focal adhesions and actin stress fibers following adhesion to fibronectin (Fig. 9C). After adhesion to fibronectin for 2 h, F-actin can be visualized in both FAK−/− cells and counterparts expressing wt-FAK; however, the F-actin is restricted to the cortex in FAK−/− cells but is rapidly incorporated into stress fibers in cell expressing wt-FAK. Attached cells expressing 4-9F-FAK eventually formed some focal adhesions and spread on fibronectin by 18 h following adhesion (results not shown). These results indicate that Src-induced phosphorylation of FAK promotes the formation of actin stress fibers and focal adhesions, thus facilitating rapid cell attachment and spreading on fibronectin.
FIG. 9.
4-9F-FAK disrupts the normal F-actin/G-actin ratio and fails to rescue actin stress fiber assembly, cell attachment, and spreading of FAK−/− cells. (A) Pools of G-actin and F-actin were isolated from FAK−/− cells and FAK−/− cells expressing either wt-FAK or the 4-9F-FAK mutant. (B) Attachment of FAK−/− cells expressing either empty vector, wt-FAK, or 4-9F-FAK to fibronectin was quantified 30 and 60 min following plating. Data represent mean values with BSA background subtracted plus standard errors from three separate experiments. (C) The formation of actin stress fibers and focal adhesions in FAK−/− cells expressing wt- and 4-9F-FAK was monitored 2 h following adhesion to fibronectin. Formation of actin stress fibers and organization of the actin cytoskeleton were detected by staining fixed cells with FITC-labeled phalloidin. The distribution of focal adhesions and intracellular localization of exogenously expressed Myc-tagged FAK proteins were analyzed by immunocytochemistry with antivinculin and anti-Myc antibodies, respectively. Cells were analyzed by confocal microscopy (magnification, ×600).
Actin stress fiber assembly and cell spreading mediated by expression of wt-FAK is dependent on calpain activity.
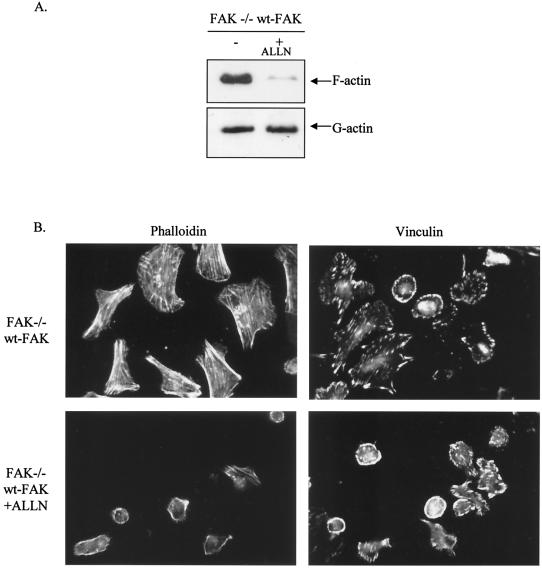
Since calpain has been shown to promote initial cell adhesion and spreading events, presumably by modulating proteolysis of proteins that regulate the actin cytoskeleton (75), we addressed whether the elevated calpain activity which follows reexpression of wt-FAK in FAK−/− cells is likely to contribute to enhanced actin stress fiber assembly and cell spreading. We treated FAK−/− cells expressing wt-FAK with an inhibitor of calpain activity, ALLN (calpain inhibitor 1), and found it to suppress F-actin levels (Fig. 10A). Preincubation with ALLN also suppressed the spreading of wt-FAK cells plated on fibronectin and impaired the formation of actin stress fibers and focal adhesions (Fig. 10B). These studies imply that the ability of the 4-9F-FAK protein to disrupt F-actin assembly and formation of actin stress fibers and focal adhesions during cell attachment and spreading may be due, in part, to reduced calpain activity.
FIG. 10.
Actin stress fiber assembly and cell spreading mediated by expression of wt-FAK are dependent on calpain activity. (A) Pools of F-actin and G-actin were isolated from FAK−/− cells reconstituted with wt-FAK, with or without preincubation with the calpain inhibitor ALLN (50 μM). (B) FAK−/− cells reexpressing wt-FAK were preincubated with the calpain inhibitor ALLN (50 μM) prior to adhesion to fibronectin (for 2 h). Formation of actin stress fibers, organization of the actin cytoskeleton, and formation of focal adhesions were monitored by staining with FITC-labeled phalloidin or immunostaining with antivinculin antibody. Cells were analyzed by confocal microscopy (magnification, ×600).
Src-induced phosphorylation of FAK is required for survival of serum-deprived v-Src-transformed fibroblasts.
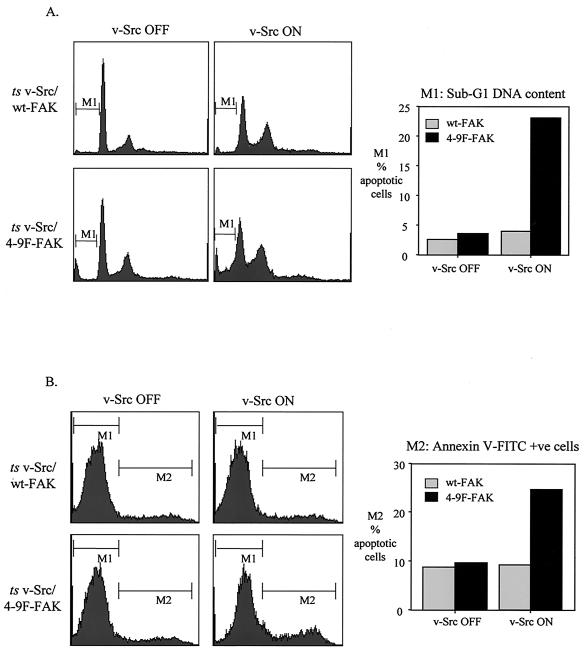
Because FAK transmits survival signals from the ECM and suppresses anoikis (detachment-induced apoptosis) (38, 54, 57, 101), we examined whether survival signaling mediated by v-Src was attenuated in cells expressing the 4-9F-FAK mutant protein. Previously we and others showed that v-Src primes cells for apoptosis but provides survival signals that keeps them viable when serum is withdrawn (61, 97). Thus, when v-Src is switched off by transfer to the nonpermissive temperature, serum-deprived transformed cells undergo rapid programmed cell death (61). However, the immediate substrates that mediate v-Src-dependent survival of serum-deprived transformed cells were not identified. Thus, we addressed whether FAK phosphorylation was involved. We found that under serum-deprived conditions, activation of v-Src in cells expressing wt-FAK promoted the typical transformed phenotype, whereas expression of the interfering 4-9F-FAK mutant protein with active v-Src caused rapid cell rounding and detachment (results not shown). To investigate this further, we analyzed the proportion of cells with sub-G1 DNA content, which is indicative of cell apoptosis. Following v-Src activation under serum-deprived conditions, an increase in the proportion of cells with sub-G1 DNA content was observed in cells coexpressing 4-9F-FAK (Fig. 11A). In contrast, no increase in sub-G1 DNA content was detected following v-Src activation in cells coexpressing wt-FAK (Fig. 11A). Positive staining of exposed membrane phospholipids, an early event during apoptosis, was detected by annexin V-FITC staining as an independent monitor of apoptosis (Fig. 11B). Under serum-deprived conditions, the percentage of annexin V-positive (apoptotic) cells was increased following v-Src activation in cells coexpressing 4-9F-FAK but not in cells coexpressing wt-FAK (Fig. 11B).
FIG. 11.
Src-induced phosphorylation of FAK is required for survival of serum-deprived v-Src-transformed fibroblasts. (A) Flow cytometric analysis of propidium iodide-stained CEFs coexpressing ts v-Src with wt-FAK or 4-9F-FAK was used to determine the proportion of cells with a sub-G1 DNA content (M1, indicative of apoptosis) in response to v-Src activation under serum deprivation conditions. (B) Flow cytometric analysis was also used to determine positive staining of annexin V-FITC (M2) to the surface of apoptotic CEFs coexpressing ts v-Src with wt-FAK or 4-9F-FAK following v-Src activation under serum deprivation conditions.
Therefore, these results imply that Src-induced tyrosine phosphorylation of FAK is required for the transmission of survival signals that prevent v-Src-induced apoptosis of serum-deprived transformed cells.
Src-induced phosphorylation of FAK promotes optimal anchorage-independent growth of v-Src-transformed cells.
A characteristic feature of cell transformation induced by v-Src is the acquisition of anchorage-independent cell proliferation (65). To date the link between actin and adhesion regulation and survival signaling has not been elucidated. To determine whether Src-induced phosphorylation of FAK is required for anchorage-independent growth of v-Src-transformed cells, we performed assays of colony formation in soft agar. Our results indicate that CEFs coexpressing activated v-Src with wt-FAK proliferate in agar, forming significantly more colonies than do CEFs coexpressing activated v-Src with 4-9F-FAK (Fig. 12). Thus, Src-induced phosphorylation of FAK is a key event in promoting optimal anchorage-independent growth of v-Src-transformed cells. This, together with our observation that Src-induced phosphorylation of FAK controls actin and adhesion dynamics as well as survival signaling, implies that the induction of these events by v-Src inside the cell is permissive for anchorage-independent growth.
FIG. 12.
Src-induced phosphorylation of FAK promotes optimal anchorage-independent growth of v-Src-transformed cells. CEFs expressing ts v-Src with either wt-FAK or 4-9F-FAK were cultured in soft agar at the permissive temperature (35°C) for v-Src activity. (A) Phase pictures illustrate colony formation 14 days following cell seeding (two representative fields are shown). (B) Colony formation after 14 days was quantified by counting the number of colonies per high-power field (magnification, ×25). Values represent the mean number of colonies plus standard errors from three separate fields.
DISCUSSION
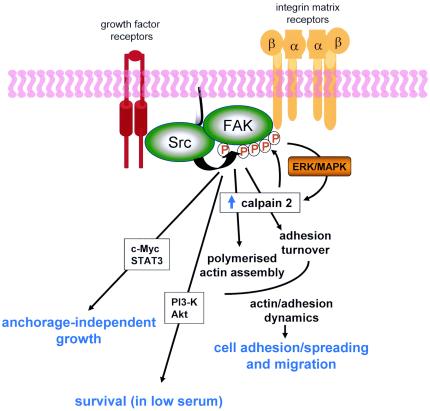
Dynamic regulation of focal adhesion assembly and disassembly is clearly necessary for cell migration (56) and for signaling output that regulates a variety of cellular functions (11). Here, we present a model whereby Src-induced phosphorylation of FAK represents a single critical event in the regulation of actin and adhesion dynamics and coordinates multiple intracellular processes in both normal and transformed cells (Fig. 13). Src-induced phosphorylation of FAK is needed for actin stress fiber formation and focal adhesion assembly associated with cell attachment and spreading on ECM. These events are mediated, at least in part, by regulated activity of calpain proteases. Src-induced phosphorylation of FAK is also required for focal adhesion turnover by calpain, as a result of FAK-mediated recruitment of calpain and its upstream regulator p42ERK to the membrane. Surprisingly, v-Src-induced phosphorylation of FAK is also required for cell survival and anchorage-independent growth of v-Src-transformed cells, apparently by substituting for adhesion-derived growth and survival signals (Fig. 13). Thus, Src-induced phosphorylation of FAK is a key event during v-Src-induced neoplastic transformation and subverts normal processes that are perturbed in cancer cells.
FIG. 13.
Src-induced phosphorylation of FAK coordinates adhesion dynamics, cell migration, survival, and anchorage-independent growth. A model proposing Src-induced tyrosine phosphorylation of FAK as a crucial event at adhesion sites that coordinates multiple cellular processes is shown. Src-induced phosphorylation of FAK is required for rapid actin stress fiber assembly and focal adhesion formation that promote initial cell adhesion and spreading, events that are in part mediated by calpain activity. Src-induced phosphorylation of FAK also promotes focal adhesion turnover and motility of cells via activation of calpain, most likely by linking calpain to upstream regulation by the ERK/MAPK pathway. v-Src-induced phosphorylation of FAK also promotes cell survival and anchorage-independent growth of transformed cells by substituting for integrin-derived growth and survival signals that are normally induced in response to cell-ECM adhesion and associated actin remodeling. These signals are likely to involve activation of PI 3-K/Akt, STAT3, and c-Myc, which have previously been identified as downstream mediators of both Src and FAK and are implicated in cell survival and anchorage-independent growth of transformed cells.
F-actin assembly, cell attachment to fibronectin, and cell spreading.
It is widely accepted that autophosphorylation of FAK on Tyr 397 recruits signaling molecules such as Src, PI 3-kinase, phospholipase C-γ, and Grb7, which promote focal adhesion assembly and cell adhesion and migration (23, 32, 47, 77, 87). We found that the Src-dependent tyrosine phosphorylation sites on FAK were also required for optimal adhesion to fibronectin and initial assembly of focal adhesions and actin stress fibers that facilitate cell spreading. Moreover, expression of 4-9F-FAK, which blocked v-Src-induced phosphorylation of FAK, reduced levels of F-actin, presumably by inhibition of the normal processes that govern F-actin dynamics. The association of FAK with GRAF (a RhoA-specific GTPase-activating protein), ASAP1 (a GTPase-activating protein for Arf1 and Arf6), and p190 RhoGEF (a guanine nucleotide exchange factor for Rho) provides important links between integrin-mediated adhesion events and actin remodeling (106). Such links may be modified by expression of the 4-9F-FAK mutant protein. Interestingly, impaired actin assembly and spreading may also be a consequence of depleted levels of calpain activity measured in cells expressing the 4-9F-FAK mutant. This is in keeping with previous findings that calpain activity is required for actin stress fiber formation and cell spreading following substrate adhesion (67, 75). Calpain is both a positive and negative regulator of actin and adhesion dynamics, by regulating the turnover of a number of proteins that modulate the actin cytoskeleton, such as RhoA and FAK, and also the actin binding proteins, cortactin, spectrin, and ezrin (51, 52, 66, 75). Modulation of either F-actin or calpain activity has also been shown to influence cell adhesion to ECM by regulating integrin activity (7, 8, 104). Although Tyr 397 is still phosphorylated in cells expressing the 4-9F-FAK protein, this is clearly not sufficient for optimal cell attachment and spreading, indicating that Src-induced tyrosine phosphorylation of FAK is also required for the actin and adhesion remodeling events that these processes require.
Focal adhesion turnover and cell migration.
A recent study showed that calpain activity is significantly decreased in both FAK−/− and MEKK1−/− cells, correlating with reduced cell-substrate deadhesion and impaired cell motility (28). In addition, we demonstrated that FAK can act as an adaptor molecule mediating the assembly of a calpain 2/FAK/p42 ERK complex that enhances total cellular levels of calpain activity, focal adhesion turnover, and cell motility (17). Here, we show that Src-induced tyrosine phosphorylation of FAK, while not essential for assembly of the calpain 2/FAK/p42ERK complex, can enhance its formation. Furthermore, reexpression of wt-FAK, but not 4-9F-FAK, in FAK−/− cells restores depleted levels of calpain activity. These results confirm that FAK, particularly Src-mediated tyrosine phosphorylation of FAK, is upstream of calpain activation. Despite the residual association between 4-9F-FAK and calpain 2, the 4-9F-FAK mutant remains resistant to calpain-mediated proteolysis after v-Src activation. This reduction in 4-9F-FAK association with calpain 2 may be sufficient to reduce its degradation, but it is equally likely that v-Src-induced phosphorylation of FAK may be an essential prerequisite for FAK to be cleaved by calpain.
Reexpression of 4-9F-FAK induced only a very slight restoration of cell motility in FAK−/− cells compared to expression of wt-FAK. These data provide the first direct demonstration that phosphorylation of FAK at the Src-specific tyrosine residues is required to integrate signals from the MEK/ERK/MAPK pathway to the calpain proteases that are necessary for actin and adhesion assembly, adhesion turnover, and cell migration. Our results provide a mechanistic explanation for the similar adhesion and motility defects shared by Src-, FAK-, MEKK1-, and calpain-deficient cells (28, 29, 58, 64).
FAK survival signaling.
Previous evidence demonstrated that v-Src activation protects against apoptosis in part via activation of Ras, PI 3-kinase, and STAT3 signaling pathways (1, 10, 61, 74, 93). However, when the Ras and PI 3-kinase pathways are inhibited, v-Src-transformed cells undergo apoptosis to a greater extent than corresponding nontransformed cells (96). These studies indicate that in a manner similar to that for the c-Myc and E1A oncogenes (33, 76), activation of v-Src, in the absence of survival signals, primes cells for apoptosis. We show that coexpression of 4-9F-FAK with activated v-Src is sufficient to induce apoptosis of serum-deprived transformed cells, implying that phosphorylation of FAK is important for v-Src-mediated cell survival. This is consistent with recent studies that suggest that Src and FAK act synergistically to suppress apoptosis (43, 49).
Anchorage-independent growth.
v-Src-induced activation of a number of downstream regulators, including Ras-MAPK, PI 3-kinase-mTOR, STAT3, and c-Myc, has been proposed to promote anchorage-independent growth by both maintaining cell survival and promoting cell cycle progression (74, 92). FAK expression alone is not sufficient to transform cells but substantially potentiates anchorage-independent growth induced by other oncogenes, notably v-Ras (78). FAK also suppresses transformation-associated anoikis of human breast cancer cells and stimulates anchorage-independent growth (102). While it is apparent that v-Src can stimulate anchorage-independent growth by a variety of mechanisms, including some acting independently of FAK (48, 68), our results demonstrate that Src-induced phosphorylation of FAK can contribute significantly to anchorage-independent growth of v-Src-transformed cells.
Expression of FAK mutants in which each of the six tyrosine residues (Tyr 397, 407, 576, 577, 861, and 925) has been mutated to phenylalanine individually had no observable effect on v-Src-induced morphological transformation or survival signaling (results not shown). Mutation of the Tyr 397 site did inhibit cell motility, while individual mutation of the other Src-dependent tyrosine residues had no significant effect on cell migration. Reconstitution of the two C-terminal tyrosine residues Tyr 861 and Tyr 925 can rescue the motility defect exhibited by 4-9F-FAK expressing cells (results not shown). These data suggest that Src-dependent phosphorylation of at least two or more tyrosine residues is required for the transmission of signals that mediate cell survival, morphological transformation, and optimal cell migration of v-Src-transformed cells.
In contrast to our findings, recent studies in which v-Src was introduced into FAK−/− cells suggest that FAK is not required for v-Src-induced morphological transformation or anchorage-independent growth (50, 81). However, characteristics unique to FAK−/− cells may compensate for the role of FAK during v-Src-induced transformation. First, FAK−/− cells have undergone adaptation to the absence of FAK by upregulating levels of the FAK homologue PYK2. PYK2 phosphotyrosine content is elevated following v-Src expression in FAK−/− cells (81), and PYK2 has previously been shown to promote p42ERK activation in FAK−/− cells (88), a signaling pathway that contributes to both morphological transformation and anchorage-independent growth of transformed cells (17, 26). Second, FAK−/− cells are deficient in p53 (58), and FAK can induce cell survival through inhibition of p53-dependent apoptosis (58). Alternatively, it is possible that the primary CEFs used in our study may differ from the MEFs used in the previous studies in their requirements for FAK signaling. In addition, while in mammalian cells FAK phosphorylation appears to be required for signaling by endogenous Src family kinases, as demonstrated by our studies on reexpression of wt-FAK and 4-9F-FAK in FAK−/− cells, FAK phosphorylation may not be required to the same extent for transformation by overexpressed v-Src. Our evidence strongly suggests that in normal primary cells, with endogenous FAK and p53, Src-induced phosphorylation of FAK is required for v-Src-induced morphological transformation, survival, and anchorage-independent growth. It is possible, however, that the dominant negative effect of 4-9F-FAK on these pathways may be due, in part, to sequestration of Src or some property other than resistance to proteolysis.
The proliferation of normal nontransformed cells requires both mitogenic signals and survival signals derived from cell-ECM adhesion (53, 60). Previous studies using cytochalasin D showed that the integrity of the actin cytoskeleton facilitates cell cycle progression and also mediates cell survival after integrin engagement (9, 59, 80). Furthermore, c-Myc expression, which contributes to cell cycle progression and anchorage-independent growth (44), is increased following integrin-mediated adhesion through a mechanism dependent on Src and actin reorganization (4). Here we show for the first time that regulation of actin and adhesion assembly and disassembly, via Src-mediated phosphorylation of FAK, is tightly linked to survival signaling. More specifically, the same phosphorylation events on FAK clearly integrate, and coordinately regulate, actin and adhesion dynamics with survival signaling, consistent with the idea that dynamic regulation of cellular actin and adhesion networks, and structures containing the integrin-matrix receptors themselves, is essential for signal output and multiple downstream biological responses. We have previously shown that calpain activity, an important regulator of actin dynamics, is also required for stimulating anchorage-independent growth of v-Src-transformed cells (18). Thus, v-Src-induced phosphorylation of FAK may functionally substitute for integrin-mediated cell adhesion to reorganize the actin cytoskeleton and induce signals that promote survival and anchorage-independent growth.
Since the status of integrin adhesion complexes and survival signaling from these may jointly contribute to the anchorage-independent growth that is a hallmark of oncogenic transformation, these findings may explain why high levels of Src and FAK are selected for during cancer development.
Acknowledgments
We thank John Wyke for critical reading of the manuscript.
This work was funded by Cancer Research UK.
REFERENCES
- 1.Aftab, D. T., J. Kwan, and G. S. Martin. 1997. Ras-independent transformation by v-Src. Proc. Natl. Acad. Sci. USA 94**:**3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agochiya, M., V. G. Brunton, D. W. Owens, E. K. Parkinson, C. Paraskeva, W. N. Keith, and M. C. Frame. 1999. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene 18**:**5646-5653. [DOI] [PubMed] [Google Scholar]
- 3.Avizienyte, E., A. W. Wyke, R. J. Jones, G. W. McLean, M. A. Westhoff, V. G. Brunton, and M. C. Frame. 2002. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 4**:**632-638. [DOI] [PubMed] [Google Scholar]
- 4.Benaud, C. M., and R. B. Dickson. 2001. Regulation of the expression of c-Myc by beta1 integrins in epithelial cells. Oncogene 20**:**759-768. [DOI] [PubMed] [Google Scholar]
- 5.Beviglia, L., V. Golubovskaya, L. Xu, X. Yang, R. J. Craven, and W. G. Cance. 2003. Focal adhesion kinase N-terminus in breast carcinoma cells induces rounding, detachment and apoptosis. Biochem. J. 373**:**201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi, R., Y. Rong, A. Bernard, M. Khrestchatisky, and M. Baudry. 2000. Src-mediated tyrosine phosphorylation of NR2 subunits of NMDA receptors protects from calpain-mediated truncation of their C-terminal domains. J. Biol. Chem. 275**:**26477-26483. [DOI] [PubMed] [Google Scholar]
- 7.Bialkowska, K., S. Kulkarni, X. Du, D. E. Goll, T. C. Saido, and J. E. Fox. 2000. Evidence that beta3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active. J. Cell Biol. 151**:**685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobak, D., J. Moorman, A. Guanzon, L. Gilmer, and C. Hahn. 1997. Inactivation of the small GTPase Rho disrupts cellular attachment and induces adhesion-dependent and adhesion-independent apoptosis. Oncogene 15**:**2179-2189. [DOI] [PubMed] [Google Scholar]
- 9.Bohmer, R. M., E. Scharf, and R. K. Assoian. 1996. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol. Biol. Cell. 7**:**101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromberg, J. F., C. M. Horvath, D. Besser, W. W. Lathem, and J. E. Darnell, Jr. 1998. Stat3 activation is required for cellular transformation by v-src. Mol. Cell. Biol. 18**:**2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, E., and E. Dejana. 2003. Cell-to-cell contact and extracellular matrix. Cell-cell and cell-matrix interactions—running, jumping, standing still. Curr. Opin. Cell Biol. 15**:**505-508. [Google Scholar]
- 12.Brunton, V. G., V. J. Fincham, G. W. McLean, S. J. Winder, C. Paraskeva, J. F. Marshall, and M. C. Frame. 2001. The protrusive phase and full development of integrin-dependent adhesions in colon epithelial cells require FAK- and ERK-mediated actin spike formation: deregulation in cancer cells. Neoplasia 3**:**215-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calalb, M. B., T. R. Polte, and S. K. Hanks. 1995. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15**:**954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calalb, M. B., X. Zhang, T. R. Polte, and S. K. Hanks. 1996. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem. Biophys. Res. Commun. 228**:**662-668. [DOI] [PubMed] [Google Scholar]
- 15.Carragher, N. O., V. J. Fincham, D. Riley, and M. C. Frame. 2001. Cleavage of focal adhesion kinase by different proteases during SRC-regulated transformation and apoptosis. Distinct roles for calpain and caspases. J. Biol. Chem. 276**:**4270-4275. [DOI] [PubMed] [Google Scholar]
- 16.Carragher, N. O., B. Levkau, R. Ross, and E. W. Raines. 1999. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J. Cell Biol. 7**:**619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carragher, N. O., M. A. Westhoff, V. J. Fincham, M. D. Schaller, and M. C. Frame. 2003. A novel role for FAK as a protease-targeting adaptor protein: regulation by p42 ERK and Src. Curr. Biol. 13**:**1442-1450. [DOI] [PubMed] [Google Scholar]
- 18.Carragher, N. O., M. A. Westhoff, D. Riley, D. A. Potter, P. Dutt, J. S. Elce, P. A. Greer, and M. C. Frame. 2002. v-Src-induced modulation of the calpain-calpastatin proteolytic system regulates transformation. Mol. Cell. Biol. 22**:**257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cartwright, C. A., C. A. Coad, and B. M. Egbert. 1994. Elevated c-Src tyrosine kinase activity in premalignant epithelia of ulcerative colitis. J. Clin. Investig. 93**:**509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartwright, C. A., A. I. Meisler, and W. Eckhart. 1990. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc. Natl. Acad. Sci. USA 87**:**558-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cary, L. A., D. C. Han, T. R. Polte, S. K. Hanks, and J. L. Guan. 1998. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140**:**211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cary, L. A., R. A. Klinghoffer, C. Sachsenmaier, and J. A. Cooper. 2002. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22**:**2427-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, H. C., P. A. Appeddu, H. Isoda, and J. L. Guan. 1996. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 271**:**26329-26334. [DOI] [PubMed] [Google Scholar]
- 24.Chikumi, H., S. Fukuhara, and J. S. Gutkind. 2002. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J. Biol. Chem. 277**:**12463-12473. [DOI] [PubMed] [Google Scholar]
- 25.Cooray, P., Y. Yuan, S. M. Schoenwaelder, C. A. Mitchell, H. H. Salem, and S. P. Jackson. 1996. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 318**:**41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowley, S., H. Paterson, P. Kemp, and C. J. Marshall. 1994. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77**:**841-852. [DOI] [PubMed] [Google Scholar]
- 27.Cramer, L. P., L. J. Briggs, and H. R. Dawe. 2002. Use of fluorescently labelled deoxyribonuclease I to spatially measure G-actin levels in migrating and non-migrating cells. Cell Motil. Cytoskeleton 51**:**27-38. [DOI] [PubMed] [Google Scholar]
- 28.Cuevas, B. D., A. N. Abell, J. A. Witowsky, T. Yujiri, N. L. Johnson, K. Kesavan, M. Ware, P. L. Jones, S. A. Weed, R. L. DeBiasi, Y. Oka, K. L. Tyler, and G. L. Johnson. 2003. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO J. 22**:**3346-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dourdin, N., A. K. Bhatt, P. Dutt, P. A. Greer, J. S. Arthur, J. S. Elce, and A. Huttenlocher. 2001. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J. Biol. Chem. 276**:**48382-48388. [DOI] [PubMed] [Google Scholar]
- 30.Du, X., T. C. Saido, S. Tsubuki, F. E. Indig, M. J. Williams, and M. H. Ginsberg. 1995. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J. Biol. Chem. 270**:**26146-26151. [DOI] [PubMed] [Google Scholar]
- 31.Duxbury, M. S., H. Ito, E. Benoit, M. J. Zinner, S. W. Ashley, and E. E. Whang. 2003. RNA interference targeting focal adhesion kinase enhances pancreatic adenocarcinoma gemcitabine chemosensitivity. Biochem. Biophys. Res. Commun. 311**:**786-792. [DOI] [PubMed] [Google Scholar]
- 32.Eide, B. L., C. W. Turck, and J. A. Escobedo. 1995. Identification of Tyr-397 as the primary site of tyrosine phosphorylation and pp60src association in the focal adhesion kinase, pp125FAK. Mol. Cell. Biol. 15**:**2819-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69**:**119-128. [DOI] [PubMed] [Google Scholar]
- 34.Fincham, V. J., and M. C. Frame. 1998. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 17**:**81-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fincham, V. J., M. James, M. C. Frame, and S. J. Winder. 2000. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 19**:**2911-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fincham, V. J., J. A. Wyke, and M. C. Frame. 1995. v-Src-induced degradation of focal adhesion kinase during morphological transformation of chicken embryo fibroblasts. Oncogene 10**:**2247-2252. (Erratum, **11:**2185, 1995.) [PubMed] [Google Scholar]
- 37.Frame, M. C., V. J. Fincham, N. O. Carragher, and J. A. Wyke. 2002. v-Src's hold over actin and cell adhesions. Nat. Rev. Mol. Cell. Biol. 3**:**233-245. [DOI] [PubMed] [Google Scholar]
- 38.Frisch, S. M., K. Vuori, E. Ruoslahti, and P. Y. Chan-Hui. 1996. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 134**:**793-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujio, Y., F. Yamada, K. Takahashi, and N. Shibata. 1993. Altered fibronectin-dependent cell adhesion by PDGF accompanies phenotypic modulation of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 196**:**997-1002. [DOI] [PubMed] [Google Scholar]
- 40.Glading, A., R. J. Bodnar, I. J. Reynolds, H. Shiraha, L. Satish, D. A. Potter, H. C. Blair, and A. Wells. 2004. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell. Biol. 24**:**2499-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glading, A., P. Chang, D. A. Lauffenburger, and A. Wells. 2000. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J. Biol. Chem. 275**:**2390-2398. [DOI] [PubMed] [Google Scholar]
- 42.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83**:**731-801. [DOI] [PubMed] [Google Scholar]
- 43.Golubovskaya, V. M., S. Gross, A. S. Kaur, R. I. Wilson, L. H. Xu, X. H. Yang, and W. G. Cance. 2003. Simultaneous inhibition of focal adhesion kinase and SRC enhances detachment and apoptosis in colon cancer cell lines. Mol. Cancer Res. 1**:**755-764. [PubMed] [Google Scholar]
- 44.Grandori, C., S. M. Cowley, L. P. James, and R. N. Eisenman. 2000. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16**:**653-699. [DOI] [PubMed] [Google Scholar]
- 45.Gu, J., M. Tamura, R. Pankov, E. H. Danen, T. Takino, K. Matsumoto, and K. M. Yamada. 1999. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J. Cell Biol. 146**:**389-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guadagno, T. M., M. Ohtsubo, J. M. Roberts, and R. K. Assoian. 1993. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science 262**:**1572-1575. [DOI] [PubMed] [Google Scholar]
- 47.Han, D. C., and J. L. Guan. 1999. Association of focal adhesion kinase with Grb7 and its role in cell migration. J. Biol. Chem. 274**:**24425-24430. [DOI] [PubMed] [Google Scholar]
- 48.Hauck, C. R., D. A. Hsia, X. S. Puente, D. A. Cheresh, and D. D. Schlaepfer. 2002. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 21**:**6289-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hisano, C., R. Tanaka, H. Fujishima, H. Ariyama, T. Tsuchiya, T. Tatsumoto, K. Mitsugi, M. Nakamura, and S. Nakano. 2003. Suppression of anoikis by v-Src but not by activated c-H-ras in human gallbladder epithelial cells. Cell Biol. Int. 27**:**415-421. [DOI] [PubMed] [Google Scholar]
- 50.Hsia, D. A., S. K. Mitra, C. R. Hauck, D. N. Streblow, J. A. Nelson, D. Ilic, S. Huang, E. Li, G. R. Nemerow, J. Leng, K. S. Spencer, D. A. Cheresh, and D. D. Schlaepfer. 2003. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 160**:**753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu, R. J., and V. Bennett. 1991. In vitro proteolysis of brain spectrin by calpain I inhibits association of spectrin with ankyrin-independent membrane binding site(s). J. Biol. Chem. 266**:**18200-18205. [PubMed] [Google Scholar]
- 52.Huang, C., N. N. Tandon, N. J. Greco, Y. Ni, T. Wang, and X. Zhan. 1997. Proteolysis of platelet cortactin by calpain. J. Biol. Chem. 272**:**19248-19252. [DOI] [PubMed] [Google Scholar]
- 53.Hueber, A. O., and G. I. Evan. 1998. Traps to catch unwary oncogenes. Trends Genet. 14**:**364-367. [DOI] [PubMed] [Google Scholar]
- 54.Hungerford, J. E., M. T. Compton, M. L. Matter, B. G. Hoffstrom, and C. A. Otey. 1996. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J. Cell Biol. 135**:**1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huttenlocher, A., S. P. Palecek, Q. Lu, W. Zhang, R. L. Mellgren, D. A. Lauffenburger, M. H. Ginsberg, and A. F. Horwitz. 1997. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272**:**32719-32722. [DOI] [PubMed] [Google Scholar]
- 56.Huttenlocher, A., R. R. Sandborg, and A. F. Horwitz. 1995. Adhesion in cell migration. Curr. Opin. Cell Biol. 7**:**697-706. [DOI] [PubMed] [Google Scholar]
- 57.Ilic, D., E. A. Almeida, D. D. Schlaepfer, P. Dazin, S. Aizawa, and C. H. Damsky. 1998. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 143**:**547-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377**:**539-544. [DOI] [PubMed] [Google Scholar]
- 59.Ingber, D. E., D. Prusty, Z. Sun, H. Betensky, and N. Wang. 1995. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J. Biomech. 28**:**1471-1484. [DOI] [PubMed] [Google Scholar]
- 60.Ishizaki, Y., L. Cheng, A. W. Mudge, and M. C. Raff. 1995. Programmed cell death by default in embryonic cells, fibroblasts, and cancer cells. Mol. Biol. Cell 6**:**1443-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson, D., M. Agochiya, K. Samejima, W. Earnshaw, M. Frame, and J. Wyke. 2000. Regulation of both apoptosis and cell survival by the v-Src oncoprotein. Cell Death Differ. 7**:**685-696. [DOI] [PubMed] [Google Scholar]
- 62.Johnson, D., M. C. Frame, and J. A. Wyke. 1998. Expression of the v-Src oncoprotein in fibroblasts disrupts normal regulation of the CDK inhibitor p27 and inhibits quiescence. Oncogene 16**:**2017-2028. [DOI] [PubMed] [Google Scholar]
- 63.Klemke, R. L., S. Cai, A. L. Giannini, P. J. Gallagher, P. de Lanerolle, and D. A. Cheresh. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137**:**481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18**:**2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kryceve-Martinerie, C., D. A. Lawrence, J. Crochet, P. Jullien, and P. Vigier. 1982. Cells transformed by Rous sarcoma virus release transforming growth factors. J. Cell Physiol. 113**:**365-372. [DOI] [PubMed] [Google Scholar]
- 66.Kulkarni, S., D. E. Goll, and J. E. Fox. 2002. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 277**:**24435-24441. [DOI] [PubMed] [Google Scholar]
- 67.Kulkarni, S., T. C. Saido, K. Suzuki, and J. E. Fox. 1999. Calpain mediates integrin-induced signaling at a point upstream of Rho family members. J. Biol. Chem. 274**:**21265-21275. [DOI] [PubMed] [Google Scholar]
- 68.Moissoglu, K., and I. H. Gelman. 2003. v-Src rescues actin-based cytoskeletal architecture and cell motility and induces enhanced anchorage independence during oncogenic transformation of focal adhesion kinase-null fibroblasts. J. Biol. Chem. 278**:**47946-47959. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen, D. H., A. D. Catling, D. J. Webb, M. Sankovic, L. A. Walker, A. V. Somlyo, M. J. Weber, and S. L. Gonias. 1999. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J. Cell Biol. 146**:**149-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicolas, G., C. M. Fournier, C. Galand, L. Malbert-Colas, O. Bournier, Y. Kroviarski, M. Bourgeois, J. H. Camonis, D. Dhermy, B. Grandchamp, and M. C. Lecomte. 2002. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol. Cell. Biol. 22**:**3527-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Owens, L. V., L. Xu, R. J. Craven, G. A. Dent, T. M. Weiner, L. Kornberg, E. T. Liu, and W. G. Cance. 1995. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 55**:**2752-2755. [PubMed] [Google Scholar]
- 72.Palecek, S. P., A. Huttenlocher, A. F. Horwitz, and D. A. Lauffenburger. 1998. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J. Cell Sci. 111**:**929-940. [DOI] [PubMed] [Google Scholar]
- 73.Park, H. B., V. Golubovskaya, L. Xu, X. Yang, J. W. Lee, S. Scully II, R. J. Craven, and W. G. Cance. 2004. Activated Src elevates adhesion, survival and alpha2-integrin expression in human breast cancer cells. Biochem. J. 378**:**559-567. [DOI] [PMC free article] [PubMed]
- 74.Penuel, E., and G. S. Martin. 1999. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol. Biol. Cell. 10**:**1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Potter, D. A., J. S. Tirnauer, R. Janssen, D. E. Croall, C. N. Hughes, K. A. Fiacco, J. W. Mier, M. Maki, and I. M. Herman. 1998. Calpain regulates actin remodeling during cell spreading. J. Cell Biol. 141**:**647-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao, L., M. Debbas, P. Sabbatini, D. Hockenbery, S. Korsmeyer, and E. White. 1992. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc. Natl. Acad. Sci. USA 89**:**7742-7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reiske, H. R., S. C. Kao, L. A. Cary, J. L. Guan, J. F. Lai, and H. C. Chen. 1999. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J. Biol. Chem. 274**:**12361-12366. [DOI] [PubMed] [Google Scholar]
- 78.Renshaw, M. W., L. S. Price, and M. A. Schwartz. 1999. Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J. Cell Biol. 147**:**611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richardson, A., R. K. Malik, J. D. Hildebrand, and J. T. Parsons. 1997. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol. Cell. Biol. 17**:**6906-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosen, K., J. Rak, T. Leung, N. M. Dean, R. S. Kerbel, and J. Filmus. 2000. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J. Cell Biol. 149**:**447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roy, S., P. J. Ruest, and S. K. Hanks. 2002. FAK regulates tyrosine phosphorylation of CAS, paxillin, and PYK2 in cells expressing v-Src, but is not a critical determinant of v-Src transformation. J. Cell Biochem. 84**:**377-388. [DOI] [PubMed] [Google Scholar]
- 82.Schaller, M. D., J. D. Hildebrand, J. D. Shannon, J. W. Fox, R. R. Vines, and J. T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14**:**1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schlaepfer, D. D., S. K. Hanks, T. Hunter, and P. van der Geer. 1994. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372**:**786-791. [DOI] [PubMed] [Google Scholar]
- 84.Schlaepfer, D. D., and T. Hunter. 1996. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell. Biol. 16**:**5623-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Selliah, N., W. H. Brooks, and T. L. Roszman. 1996. Proteolytic cleavage of alpha-actinin by calpain in T cells stimulated with anti-CD3 monoclonal antibody. J. Immunol. 156**:**3215-3221. [PubMed] [Google Scholar]
- 86.Sieg, D. J., C. R. Hauck, D. Ilic, C. K. Klingbeil, E. Schaefer, C. H. Damsky, and D. D. Schlaepfer. 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2**:**249-256. [DOI] [PubMed] [Google Scholar]
- 87.Sieg, D. J., C. R. Hauck, and D. D. Schlaepfer. 1999. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112**:**2677-2691. [DOI] [PubMed] [Google Scholar]
- 88.Sieg, D. J., D. Ilic, K. C. Jones, C. H. Damsky, T. Hunter, and D. D. Schlaepfer. 1998. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO J. 17**:**5933-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Small, G. W., T. Y. Chou, C. V. Dang, and R. Z. Orlowski. 2002. Evidence for involvement of calpain in c-Myc proteolysis in vivo. Arch. Biochem. Biophys. 400**:**151-161. [DOI] [PubMed] [Google Scholar]
- 90.Talamonti, M. S., C. R. Shumate, G. W. Carlson, and S. A. Curley. 1993. Locally advanced carcinoma of the colon and rectum involving the urinary bladder. Surg. Gynecol. Obstet. 177**:**481-487. [PubMed] [Google Scholar]
- 91.Termuhlen, P. M., S. A. Curley, M. S. Talamonti, M. H. Saboorian, and G. E. Gallick. 1993. Site-specific differences in pp60c-src activity in human colorectal metastases. J. Surg. Res. 54**:**293-298. [DOI] [PubMed] [Google Scholar]
- 92.Tokumitsu, Y., S. Nakano, H. Ueno, and Y. Niho. 2000. Suppression of malignant growth potentials of v-Src-transformed human gallbladder epithelial cells by adenovirus-mediated dominant negative H-Ras. J. Cell Physiol. 183**:**221-227. [DOI] [PubMed] [Google Scholar]
- 93.Turkson, J., T. Bowman, R. Garcia, E. Caldenhoven, R. P. De Groot, and R. Jove. 1998. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 18**:**2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Volberg, T., L. Romer, E. Zamir, and B. Geiger. 2001. pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J. Cell Sci. 114**:**2279-2289. [DOI] [PubMed] [Google Scholar]
- 95.Wang, Y. K., Y. H. Wang, C. Z. Wang, J. M. Sung, W. T. Chiu, S. H. Lin, Y. H. Chang, and M. J. Tang. 2003. Rigidity of collagen fibrils controls collagen gel-induced down-regulation of focal adhesion complex proteins mediated by alpha2beta1 integrin. J. Biol. Chem. 278**:**21886-21892. [DOI] [PubMed] [Google Scholar]
- 96.Webb, B. L., E. Jimenez, and G. S. Martin. 2000. v-Src generates a p53-independent apoptotic signal. Mol. Cell. Biol. 20**:**9271-9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Webb, D. J., K. Donais, L. A. Whitmore, S. M. Thomas, C. E. Turner, J. T. Parsons, and A. F. Horwitz. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6**:**154-161. [DOI] [PubMed] [Google Scholar]
- 98.Welham, M. J., and J. A. Wyke. 1988. A single point mutation has pleiotropic effects on pp60v-src function. J. Virol. 62**:**1898-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Welsh, J. B., G. N. Gill, M. G. Rosenfeld, and A. Wells. 1991. A negative feedback loop attenuates EGF-induced morphological changes. J. Cell Biol. 114**:**533-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie, H., M. A. Pallero, K. Gupta, P. Chang, M. F. Ware, W. Witke, D. J. Kwiatkowski, D. A. Lauffenburger, J. E. Murphy-Ullrich, and A. Wells. 1998. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCgamma signaling pathway. J. Cell Sci. 111**:**615-624. [DOI] [PubMed] [Google Scholar]
- 101.Xu, L. H., L. V. Owens, G. C. Sturge, X. Yang, E. T. Liu, R. J. Craven, and W. G. Cance. 1996. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ. 7**:**413-418. [PubMed] [Google Scholar]
- 102.Xu, L. H., X. Yang, C. A. Bradham, D. A. Brenner, A. S. Baldwin, Jr., R. J. Craven, and W. G. Cance. 2000. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J. Biol. Chem. 275**:**30597-30604. [DOI] [PubMed] [Google Scholar]
- 103.Yamaguchi, R., M. Maki, M. Hatanaka, and H. Sabe. 1994. Unphosphorylated and tyrosine-phosphorylated forms of a focal adhesion protein, paxillin, are substrates for calpain II in vitro: implications for the possible involvement of calpain II in mitosis-specific degradation of paxillin. FEBS Lett. 356**:**114-116. [DOI] [PubMed] [Google Scholar]
- 104.Yan, B., D. A. Calderwood, B. Yaspan, and M. H. Ginsberg. 2001. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J. Biol. Chem. 276**:**28164-28170. [DOI] [PubMed] [Google Scholar]
- 105.Yuan, Y., S. M. Dopheide, C. Ivanidis, H. H. Salem, and S. P. Jackson. 1997. Calpain regulation of cytoskeletal signaling complexes in von Willebrand factor-stimulated platelets. Distinct roles for glycoprotein Ib-V-IX and glycoprotein IIb-IIIa (integrin alphaIIbbeta3) in von Willebrand factor-induced signal transduction. J. Biol. Chem. 272**:**21847-21854. [DOI] [PubMed] [Google Scholar]
- 106.Zhai, J., H. Lin, Z. Nie, J. Wu, R. Canete-Soler, W. W. Schlaepfer, and D. D. Schlaepfer. 2003. Direct interaction of focal adhesion kinase with p190RhoGEF. J. Biol. Chem. 278**:**24865-24873. [DOI] [PubMed] [Google Scholar]