Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury (original) (raw)
Abstract
Fas-mediated apoptosis has been suggested to contribute to tubular cell death after renal ischemia-reperfusion injury. Here we investigate whether small interfering RNA (siRNA) duplexes targeting Fas protect mice from acute renal failure after clamping of the renal artery. Renal ischemia-reperfusion injury was induced by clamping the renal vein and artery for 15 or 35 min. Mice were treated before or after ischemia with siRNA targeting Fas or a control gene, administered by hydrodynamic injection, low-volume renal vein injection, or both. Treated mice were evaluated for renal Fas protein and mRNA expression, tissue histopathology, and apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining. Blood urea nitrogen and survival were monitored in mice in which the contralateral kidney had been removed. A single hydrodynamic injection of Fas siRNA reduced Fas mRNA and protein expression in the kidney 4-fold. Kidneys from mice that received Fas siRNA two days earlier had substantially less renal tubular apoptosis by TUNEL staining and less tubular atrophy and hyaline damage. Whereas 90% of mice pretreated with saline or GFP siRNA died, only 20% of _Fas_-siRNA-pretreated animals died. The same survival advantage was provided by a single low-volume Fas siRNA injection into the renal vein. Moreover, postischemic injection through the renal vein protected 38% of mice from death. This study confirms the importance of Fas-mediated apoptosis in renal ischemia-reperfusion injury. Silencing Fas by systemic or local catheterization holds therapeutic promise to limit ischemia-reperfusion injury.
Acute renal failure from ischemic damage to the kidney is an important cause of morbidity and mortality in hospitalized intensive care unit patients. Most commonly, tubular epithelial cell death is the ultimate cause of kidney failure. Increasing evidence implicates Fas-mediated apoptosis in extending infarct size during reperfusion of ischemic tissue in multiple tissues, including the brain, heart, kidney, and gut (1-4). Renal tubular epithelial cells abundantly express Fas, and this expression increases in proportion to the duration of ischemia, especially in the distal tubules, the renal site most vulnerable to ischemic damage. lpr mice, lacking Fas expression, have less kidney tissue damage after ischemia-reperfusion than wild-type mice (5, 6). During reperfusion, lymphocytes expressing Fas ligand accumulate and may contribute to Fas-mediated tissue damage (7). We previously showed that silencing Fas expression in the liver by hydrodynamic injection of duplex small interfering RNAs (siRNAs) protects mice from autoimmune hepatitis (8). Hydrodynamic injection involves the rapid injection of a large-volume bolus to cause transient high venous pressures, which are believed to facilitate delivery of siRNAs into cells. Hydrodynamic injection of siRNAs also efficiently silences reporter gene expression in other organs with high blood flow, such as the lung and kidneys (9, 10). We therefore investigated whether siRNAs targeting Fas could inhibit Fas expression in the murine kidney in vivo, and whether it could protect mice from postischemic acute renal failure.
Methods
Animals. Ten-week-old male NMRI (Naval Medical Research Institute) mice weighing 27-32 g (Toxi-Coop, Budapest, Hungary) were maintained under specific pathogen-free conditions. All procedures were performed sterilely in accordance with guidelines set by the National Institutes of Health, the Institutional Animal Care and Use Committee of Semmelweis University, and the Hungarian law on animal care and protection.
siRNAs. Deprotected and annealed siRNAs, synthesized by using 2′-O-ACE-RNA phosphoramidites (Dharmacon Research, Lafayette, CO), were dissolved in RNase-free PBS. The sense and antisense strands of siRNA were as before (8): Fas sequence, 5′-pGUGCAAGUGCAAACCAGACdTdT-3′ (sense) and 5′-pGUCUGGUUUGCACUUGCACdTdT-3′ (antisense); GFP sequence, 5′-pGGCUACGUCCAGGAGCGCACC-3′ (sense) and 5′-pUGCGCUCCUGGACGUAGCCUU-3′ (antisense).
siRNA Injections. For hydrodynamic injection, synthetic siRNAs (50 μg in 1 ml of PBS) or 1 ml of PBS was rapidly injected (within 10 sec) into one of the tail side veins or the penis vein. To dilate tail veins, the tail was immersed in warm water (50-55°C), under ether narcosis for 5 ± 1 sec. For renal vein injection, from a median laparotomy the left renal pedicle was visualized and the retroperitoneum was left intact to serve as tamponade after removal of the injection needle. Minimal preparation above the renal vessels was performed on the left side of the aorta to insert an occlusion clip (BH31, Aesculap, Center Valley, PA). The aorta and the vena cava were clipped, and the renal vein was punctured with a 26-gauge needle, to inject 0.1 ml of PBS containing siRNA or PBS alone. The needle was kept in place for 5 sec and than removed slowly, while applying compression to the renal vein for 30 sec with a piece of Gelaspon (Chauvin Ankerpharm, Rudolstadt, Germany) held with forceps. The Gelaspon was left in place thereafter. The aorta and vena cava clamp was removed immediately after the injection, having been maintained for a total of at most 10 sec for each injection.
Kidney Ischemia-Reperfusion. Kidney ischemia reperfusion was performed under standardized conditions at 24 ± 0.5°C with average intraabdominal temperature maintained at 35 ± 2°C during the operating period by using a heating pad, controlled by rectal temperature. The left renal pedicle was clamped for 15 or 35 min, and the right kidney was either left intact for control purposes or removed for the survival experiments. For survival experiments, animals were observed for several days after all surviving animals were free of signs of illness.
Blood Urea Nitrogen (BUN). BUN was measured from 32 μl of whole blood on a Reflotron IV automated analyzer (Boehringer Mannheim) with a Fast-test-strip.
Real-Time PCR. Total RNA was isolated from whole kidneys by using TRIzol (Life Technologies, Gaithersburg, MD). Primers for Fas and GAPDH were according to ref. 11. One-step real-time RT-PCR, using Sybr green reagent (Applied Bio-systems) for detection, was performed by using a Bio-Rad iCycler. All reactions were done in a 50-μl reaction volume in triplicate, following the manufacturer's instructions. PCR parameters consisted of 30 min of reverse transcription at 48°C and 10 min of Taq activation at 95°C, followed by 40 cycles of PCR at 95°C for 20 sec, 55°C for 30 sec, and 72°C for 30 sec. Standard curves were generated for both Fas and GAPDH. Relative amounts of Fas mRNA were normalized to GAPDH mRNA. Specificity was verified by melting curve analysis and agarose gel electrophoresis.
Fas Immunohistochemistry. After deparaffinization and rehydration, paraffin sections of the kidneys were incubated with 3% hydrogen peroxide for 15 min to quench endogenous peroxidase activity. After microwaving for 20 min, sections were blocked for 30 min in wash buffer containing 5% normal mouse serum. Sections were incubated for 1 h at room temperature with hamster anti-mouse Fas mAb (BD Pharmingen) diluted 1:100 in PBS. After washing with PBS, sections were incubated with biotinylated mouse anti-hamster Ig and then with streptavidin conjugated with horseradish peroxidase (LSAB detection kit, DAKO). After further washes in PBS, staining was developed with diaminobenzidine (DAB), and slides were lightly counter-stained with hematoxylin. Control slides were stained with hamster IgG replacing primary antibody. Fas immunostaining appears in all or none of the epithelial cells in individual renal tubules. The percentage of positive tubules in five consecutive fields of view (magnification, ×200) was assessed in a blinded manner.
Terminal Deoxynucleotidyltransferase (TdT)-Mediated dUTP Nick End Labeling (TUNEL) Staining. Apoptosis of tubular epithelial cells was detected by in situ TUNEL assay (Roche Diagnostics) according to the supplier's instructions. Paraffin sections were deparaffinized in xylene and rehydrated before analysis. After endogenous peroxidase activity was quenched in 3% hydrogen peroxide for 20 min, sections were treated with proteinase K (20 mg/ml in 10 mM Tris·HCl, pH 7.6) at 37°C for 30 min. before labeling with TdT and biotinylated dUTP in 100 mM potassium cacodylate/2 mM cobalt chloride/0.2 mM DTT, pH 7.2, at 37°C for 60 min in a humidified chamber. TdT was omitted from control slides. Washed sections were incubated with peroxidase-labeled streptavidin for 30 min and then stained with diaminobenzidine, followed by counterstaining with hematoxylin. Approximately 1,000 tubular epithelial cells were counted by a blinded observer at high power (×400) to determine the percentage of TUNEL+ cells with apoptotic morphology.
Histologic Score. The mean was calculated from the blinded analysis of 50 cortical tubules with visible basement membrane on cross section by using a score of 0, no damage; 1, mild damage with rounding of epithelial cells and dilated tubular lumen; 2, severe damage with flattened epithelial cells, loss of nuclear staining, and dilated lumen; and 3, destroyed tubules with flat epithelial cells lacking nuclear staining.
Statistical Analysis. Statistical comparison was by two-sided Student's t test. Survival was analyzed by Kaplan-Meier test.
Results
We first delivered synthetic siRNA duplexes (50 μg, 2.0-2.5 mg/kg) by a single hydrodynamic injection into the tail vein, using a Fas sequence that silenced effectively and specifically in the liver (8, 12). Twenty-four hours later, Fas mRNA in the kidney was reduced by 74 ± 8% as determined by reverse transcriptase (RT)-PCR of whole kidney homogenates (Fig. 1_a_). Reduction in Fas mRNA was comparable to Fas silencing in the liver after three 50-μg hydrodynamic injections of the same siRNA (86% by RNase protection assay) (8).
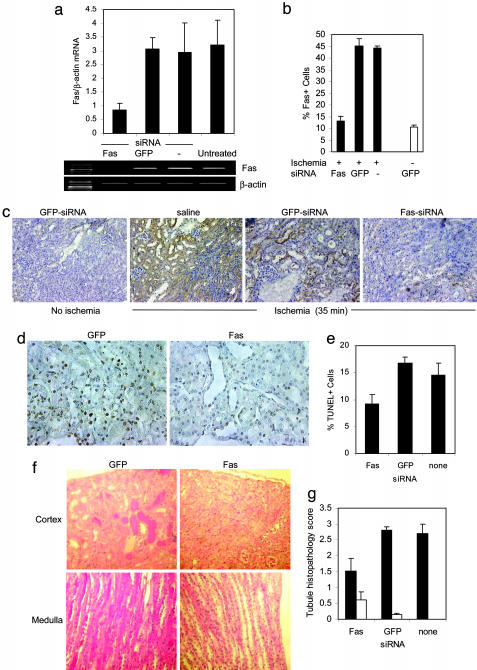
Fig. 1.
A single hydrodynamic injection of Fas siRNA silences Fas expression in kidneys subjected to 35 min of ischemia. (a) Silencing of Fas mRNA analyzed by RT-PCR of Fas and β-actin expression in kidney tissue homogenates from untreated mice or mice receiving a single hydrodynamic injection of PBS, Fas siRNA, or GFP siRNA. A representative sample is shown. These mice were not subjected to renal ischemia. However, similar results were obtained by real-time RT-PCR in injured mice (data not shown). (b and c) Silencing of Fas protein shown by immunohistochemistry (c). Mice injected 2 days earlier with PBS, Fas siRNA, or GFP siRNA were subjected to 35 min of renal ischemia and killed 1 day later. Control mice did not undergo ischemic insult. Graph (b) depicts percent Fas+ cells (mean and SD of all animals, three to five per group). (d) Reduced apoptosis in response to ischemia assessed by TUNEL staining. (Magnification, ×400.) (e) Graph of results from d. (f and g) Less cortical hyaline necrosis in mice pretreated with Fas siRNA compared with mice given GFP siRNA. (f) Representative hematoxylinand eosin-stained sections are shown. (Magnification, ×200.) (g) The histology score (mean, SD) was assessed in a blinded manner in groups of three to five mice subjected to ischemia (filled bars) or not (open bars).
We next determined whether Fas siRNA injection could silence up-regulated Fas expression after ischemic damage. In pilot experiments in which the contralateral kidney was removed or clamped, 15 min of ischemia led to fatality in 16% (1 of 6) of mice and 30 min of ischemia killed 40-60% of mice, whereas 35-45 min of ischemia killed 80-100% of mice. The solitary fatal event after 15 min of ischemia may have been due to a cause other than acute tubular necrosis, because the typical increase in BUN after 15 min of ischemia was small and transient (see below and Fig. 2_a_). These survival data suggest that the strain of mice used in these experiments is more sensitive to ischemic renal injury than are some inbred laboratory strains. In subsequent experiments we clamped the renal pedicle for either 15 or 35 min. Two days after a single hydrodynamic injection of 50 μg of Fas or GFP siRNA or saline, the left renal pedicle (artery and vein) was clamped for 35 min (leaving the right kidney intact), and mice were killed 1 day later for analysis of renal Fas expression and apoptosis. Fas silencing was also effective in the setting of ischemia. The ratio of Fas to GAPDH mRNA assayed by real-time PCR was 4 and 5 times lower in mice that received Fas siRNA compared with mice that received GFP siRNA (P < 0.001) or hydrodynamic injection of saline (P < 0.01), respectively (data not shown). The reduction in Fas mRNA up-regulation after ischemia in mice that received Fas siRNA was similar to the mRNA reduction in the nonischemic setting (Fig. 1_a_). Histological sections prepared from both kidneys were stained for Fas protein expression and for TUNEL to detect apoptotic cells and were evaluated in a blinded manner for histological evidence of kidney damage (Fig. 1 b-e). In the absence of ischemia after hydrodynamic injection of GFP siRNA, 10 ± 1% of tubule epithelial cells stained for Fas. In clamped kidneys from _GFP_-siRNA- or saline-treated control mice, almost half of the tubule epithelial cells (45 ± 3% and 44 ± 1%, respectively) had detectable Fas protein. However, in _Fas_-siRNA-treated mice, only 13 ± 2% of tubule cells stained for Fas. This percentage was statistically indistinguishable from Fas staining in control mice in which the renal pedicle was not clamped (P = 0.13). The clamped kidneys from _Fas_-siRNA-treated mice also had significantly fewer apoptotic tubular epithelial cells (Fig. 1 d and e). Whereas 9 ± 2% of renal tubule epithelial cells from _Fas_-siRNA-treated mice were TUNEL+, 17 ± 1% of cells from mice that received GFP siRNA (P < 0.001) and 14 ± 2% of cells from saline-treated (P < 0.01) were TUNEL+. Fas siRNA also protected the kidneys from ischemic damage, assessed by a blinded histopathology score that emphasized cortical tubular epithelial cell damage (Fig. 1 f and g). All control saline and _GFP_-siRNA-treated mice had extensive cortical tubular damage with massive tubular atrophy and cell loss with tubulointerstitial inflammatory cell infiltrates. Most surviving tubular epithelial cells had evidence of cytoplasmic swelling, and there was frequent nuclear chromatin condensation, indicative of apoptosis. In the medulla, tubular lumens were filled with hyaline material indicative of intense tubular cell loss in upper segments. In contrast, pretreatment with Fas siRNA prevented tubular epithelial cell loss and lessened inflammatory infiltration. The tubule histology score in the absence of ischemic insult in control siRNA-injected mice was <1 (on a scale that ranged from 0 to 3); it increased to 2.7 ± 0.3 in the ischemic kidney of saline control mice and to 2.8 ± 0.1 in _GFP_-siRNA-injected mice, but there was half as much damage (score 1.5 ± 0.4) in _Fas_-siRNA-treated mouse kidneys (P < 0.01 vs. saline, P < 0.003 vs. GFP siRNA).
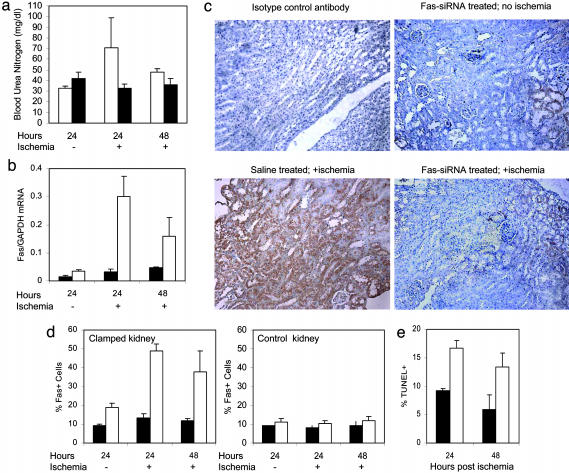
Fig. 2.
Fas silencing after hydrodynamic and renal vein injection of Fas siRNA. Mice received a single hydrodynamic injection of Fas siRNA in PBS (filled bars) or just PBS (open bars) 4 days before and low-volume renal vein injection 2 days before the renal pedicle was clamped for 15 min (subcritical ischemia) or not in sham-operated animals. Samples were harvested 1 or 2 days after clamping, as indicated. (a) BUN increased when the right kidney was removed in control animals but not in _Fas_-siRNA-treated mice. (b) Ischemia-induced up-regulation of Fas mRNA, compared with GAPDH mRNA by real-time RT-PCR, was largely silenced in animals receiving a single hydrodynamic injection of Fas siRNA. (c and d) Fas protein up-regulation was also blunted in _Fas_-siRNA-treated mice. (c) Representative sections. (Magnification, ×200.) (d) Fas staining of the ischemic left kidney and the unclamped right kidney (mean and SD). (e) The proportion of TUNEL+ tubules was also reduced by Fas siRNA treatment.
We next treated mice with a single hydrodynamic injection of Fas siRNA, as above, followed by a low-volume injection (50 μg in 0.1 ml) into the left renal vein 2 days later, and we induced subcritical ischemia 2 days after that by clamping the left renal pedicle for 15 min. If the right kidney was removed at the time of the renal injection, transient renal insufficiency developed in control mice (Fig. 2_a_). The BUN rose to 71 ± 4 mg/dl the next day, compared with a normal value of 33 ± 1 mg/dl without ischemia. Two days later the BUN had normalized. In mice treated with hydrodynamic and renal vein injections of Fas siRNA, the BUN remained normal (33 ± 4 mg/dl 1 day and 36 ± 6 mg/dl 2 days after clamping). We next looked at how effectively Fas was silenced in the setting of 15 min of subcritical ischemia. In mice sham-treated with saline injections without clamping the renal pedicle, the Fas/GAPDH mRNA ratio by real-time PCR was 0.03 ± 0.01, which was reduced to 0.015 ± 0.01 in mice that received Fas siRNA (Fig. 2_b_). After subcritical ischemia, the ratio increased 10-fold to peak 1 day later at 0.30 ± 0.07 and remained elevated at 0.16 ± 0.07 2 days later. However, in the mice that received Fas siRNA, the Fas/GAPDH mRNA ratio in the ischemic kidney hardly rose above that of the control mice not subjected to ischemia. The Fas/GAPDH ratio was 0.032 ± 0.01 and 0.046 ± 0.003 on days 1 and 2 after clamping (P < 0.001 compared with control on day 1). Moreover, Fas protein expression, assayed by counting the numbers of Fas-staining tubule cells by immunohistochemistry, was also substantially reduced in the challenged kidney (Fig. 2 c and d). In the _Fas_-siRNA-treated mice, 13 ± 2% of tubule cells in the ischemic kidney became Fas+ at the peak response on day 1, whereas 49 ± 4% of tubule cells stained for Fas in the ischemic control mouse kidney. Fas staining in the ischemic kidney of mice that received Fas siRNA was not significantly different from Fas staining in the nonischemic right kidney of control mice that received only saline injections. When the numbers of TUNEL+ apoptotic cells in the ischemic kidney were counted 1 and 2 days after clamping, there were about half as many TUNEL+ cells in the _Fas_-siRNA-treated mice as in the controls (Fig. 2_e_, P < 0.002 day 1, P < 0.05 day 2). Renal pathology determined by hematoxylin staining was also significantly reduced in the ischemic kidney with silenced Fas expression (tubule histopathology score, 1.4 ± 0.5 in _Fas_-siRNA-treated animals vs. 2.2 ± 0.3 in control mice, P < 0.05).
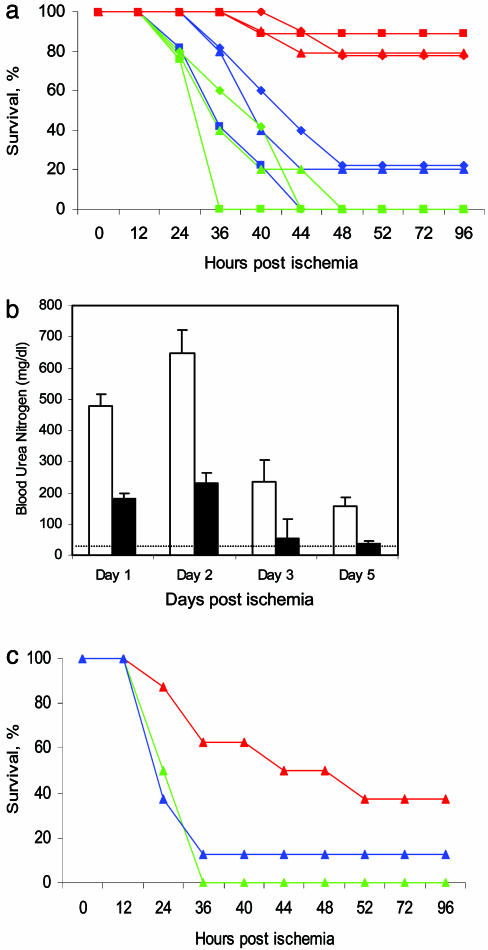
Because Fas expression in the kidney and tubular apoptosis and ischemic damage were suppressed, we next determined whether Fas siRNA could provide protection from critical ischemia in mice in which the left renal pedicle was clamped for 35 min and the contralateral kidney was removed. The right kidney was removed by median laparotomy 2 days before a single hydrodynamic injection of saline, GFP siRNA, or Fas siRNA. Two days later the renal pedicle of the remaining kidney was clamped for 35 min and the mice were observed (Fig. 3_a_). Four of five mice that received saline and all five mice that received GFP siRNA by hydrodynamic tail vein injection died of acute renal failure within 2 days. However, 8 of 10 mice injected with Fas siRNA survived (P < 0.0001 vs. GFP siRNA, P < 0.005 vs. saline). Kidney function, assessed by following BUN in surviving mice, was less perturbed in mice that received Fas siRNA than in those that received a hydrodynamic injection of saline (Fig. 3_b_). Whereas the peak BUN in surviving control mice was 646 ± 77 mg/dl, it was a third of that (232 ± 33 mg/dl, P < 0.0001) in _Fas_-siRNA-treated mice, compared with a normal value of 33 ± 1 mg/dl.
Fig. 3.
Hydrodynamic or renal vein injection of Fas siRNA protects mice from lethal kidney ischemia: survival and BUN levels of surviving mice after 35 min of kidney ischemia and reperfusion. (a) Mice were treated with saline (blue) (n = 5), GFP siRNA (green) (n = 5), or Fas siRNA (red) (n = 10) by hydrodynamic injection (♦), renal vein injection (▴), or both (▪). (b) BUN is reduced in surviving _Fas_-siRNA-treated mice (filled bars) compared with saline-treated controls (open bars). (c) Intraoperative (postischemia) treatment via the renal vein also offered some protection (color code as in a).
We also tested whether low-volume injection of siRNAs (100 μl) into the left renal vein (performed at the same time the right kidney was removed), which was well tolerated in the previous experiments, could enhance or substitute for hydrodynamic injection. The survival curves of mice treated with just renal vein infusion or with both hydrodynamic and renal vein injection were indistinguishable from those of mice that received a single hydrodynamic injection (Fig. 3_a_). Renal vein injection provided a significant survival advantage from critical ischemia-reperfusion injury (P < 0.001 vs. GFP siRNA, P < 0.01 vs. saline). Although hydrodynamic injection is unlikely to be possible in humans, catheterization of the renal vein is a feasible therapeutic option.
Although in some situations, such as preoperatively, it may be possible to anticipate ischemia-reperfusion injury, in most clinical situations ischemic damage arises without forewarning. We therefore evaluated survival when Fas siRNA was administered after the ischemic insult. Fas siRNA was injected in 100 μl into the renal vein during reperfusion 5 min after releasing the renal vessel clamp, when the kidney had recovered its red color. Postischemic renal vein injection protected three of eight mice from 35-min ischemia, whereas all eight _GFP_-siRNA-treated control mice and seven of eight saline-treated controls did not survive (P < 0.01 vs. GFP siRNA, P = 0.07 vs. saline). (Fig. 3_c_) In Fig. 3 a and c, all of the _GFP_-siRNA-treated mice died, whereas in each saline-treated group, one mouse survived. Although the differences between saline and GFP siRNA treatment in the experiments shown in Fig. 3 and elsewhere are not statistically significant, we cannot exclude subtle off-target effects induced by the GFP siRNA.
Discussion
This study confirms the importance of Fas-mediated apoptosis in renal ischemia-reperfusion injury, because silencing Fas protected mice from lethal acute ischemic renal failure. The tissues of other organs, such as the heart or brain, might also be protected from ischemia-reperfusion injury by silencing Fas. Protection was provided not only by hydrodynamic injection but also by a single low-volume injection into the renal vein. How hydrodynamic injection works is not well understood. It is hypothesized that parenchymal cells are transduced when they are subjected to increased hydrostatic pressure induced by a sudden increase in intravascular volume. It is unlikely that this approach can be scaled up to human therapy. However, renal vein catheterization is feasible in humans and is likely to target the part of the kidney most vulnerable to ischemic damage, the tubulointerstitium. However, this study did not directly measure which kidney cells take up siRNAs after either hydrodynamic or renal vein injection. Future studies to detect the distribution of fluorescently labeled siRNAs into different renal cells will be useful to guide the development of therapeutic strategies in the kidney. In this study we chose an injection volume that roughly corresponds to the volume of a single mouse kidney (13). These injections may have created a transient localized increase in intravascular pressure within the kidney resulting in retrograde flow with siRNA transduction of tubule cells by a similar mechanism as for hydrodynamic injection, but without the risk of right-sided heart failure. Further studies to measure local and systemic intravascular pressures are necessary.
In a previous study we found that this Fas siRNA sequence specifically silenced Fas, but not other genes in the apoptotic pathway (8). However, recent in vitro studies have suggested that some duplex siRNA sequences have off-target effects and can induce an IFN response, particularly at high concentrations (14-16). Further studies are required to investigate whether these problems are of concern in vivo and for this sequence. However, preliminary in vitro studies in which cells were transfected with the Fas siRNA used in this study did not indicate any induction of IFN-inducible genes (data not shown). Our data suggest that silencing Fas expression in renal tubular epithelial cells is the mechanism for protection by Fas siRNA injection. However, we cannot exclude the possibility that indirect antiinflammatory effects of silencing Fas expression elsewhere contribute to the protective outcome.
Local injection of Fas siRNAs after ischemia provided some, but less complete, protection. Survival of _Fas_-siRNA-treated mice was significant when compared with _GFP_-siRNA-treated mice or with _GFP_-siRNA- and saline-treated control mice considered together (P < 0.02), but not when compared with the saline-treated mice, although there was a trend toward protection. If the half-life and delivery efficiency of duplex siRNAs in vivo can be improved by chemical modification (17, 18), protection might be more effective. Postischemic protection is possible because Fas is up-regulated after ischemia, allowing a window of opportunity for therapeutic intervention.
Acknowledgments
We thank P. Lipták (University of Szeged, Szeged, Hungary) for establishing the histologic score system, M. Godó (Semmelweis University) for in vitro measurements, and P. Shankar and M. Swamy for helpful comments. This work was supported by Egészségügyi Tudományos Tanács/Medical Scientific Board 225/2000 and 432/2003, and Országos Tudományos Kutatási Alap/National Scientific Research Fund F034498, (P.H.), and National Institutes of Health Grant AI-056900 (to J.L.). P.H. is a recipient of a Békésy scholarship from the Hungarian Ministry of Education.
Author contributions: P.H., E.S., and J.L. designed research; P.H., E.S., G.K., A.C., and N.O. performed research; P.H. contributed new reagents/analytical tools; P.H., E.S., and J.L. analyzed data; and P.H. and J.L. wrote the paper.
Abbreviations: siRNA, small interfering RNA; BUN, blood urea nitrogen; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Nogae, S., Miyazaki, M., Kobayashi, N., Saito, T., Abe, K., Saito, H., Nakane, P. K., Nakanishi, Y. & Koji, T. (1998) J. Am. Soc. Nephrol. 9**,** 620-631. [DOI] [PubMed] [Google Scholar]
- 2.Paschen, W. (2003) J. Cereb. Blood Flow Metab. 23**,** 773-779. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Villalba, A., Hahne, M., Kleber, S., Vogel, J., Falk, W., Schenkel, J. & Krammer, P. H. (2001) Cell Death Differ. 8**,** 679-686. [DOI] [PubMed] [Google Scholar]
- 4.Castaneda, M. P., Swiatecka-Urban, A., Mitsnefes, M. M., Feuerstein, D., Kaskel, F. J., Tellis, V. & Devarajan, P. (2003) Transplantation 76**,** 50-54. [DOI] [PubMed] [Google Scholar]
- 5.Lee, P., Sata, M., Lefer, D. J., Factor, S. M., Walsh, K. & Kitsis, R. N. (2003) Am. J. Physiol. 284**,** H456-H463. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz, A., Justo, P., Catalan, M. P., Sanz, A. B., Lorz, C. & Egido, J. (2002) Curr. Drug Targets Immune Endocr. Metabol. Disord. 2**,** 181-192. [PubMed] [Google Scholar]
- 7.Miyazawa, S., Watanabe, H., Miyaji, C., Hotta, O. & Abo, T. (2002) J. Lab. Clin. Med. 139**,** 269-278. [DOI] [PubMed] [Google Scholar]
- 8.Song, E., Lee, S. K., Wang, J., Ince, N., Ouyang, N., Min, J., Chen, J., Shankar, P. & Lieberman, J. (2003) Nat. Med. 9**,** 347-351. [DOI] [PubMed] [Google Scholar]
- 9.McCaffrey, A. P., Meuse, L., Pham, T. T., Conklin, D. S., Hannon, G. J. & Kay, M. A. (2002) Nature 418**,** 38-39. [DOI] [PubMed] [Google Scholar]
- 10.Lewis, D. L., Hagstrom, J. E., Loomis, A. G., Wolff, J. A. & Herweijer, H. (2002) Nat. Genet. 32**,** 107-108. [DOI] [PubMed] [Google Scholar]
- 11.Terrazzino, S., Bauleo, A., Baldan, A. & Leon, A. (2002) J. Neuroimmunol. 124**,** 45-53. [DOI] [PubMed] [Google Scholar]
- 12.Zhang, G., Budker, V. & Wolff, J. A. (1999) Hum. Gene Ther. 10**,** 1735-1737. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama, H., Higuchi, N., Nishikawa, Y., Hirahara, H., Iino, N., Kameda, S., Kawachi, H., Yaoita, E., Gejyo, F. & Miyazaki, J. (2002) Hum. Gene Ther. 13**,** 455-468. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, A. L., Bartz, S. R., Schelter, J., Kobayashi, S. V., Burchard, J., Mao, M., Li, B., Cavet, G. & Linsley, P. S. (2003) Nat. Biotechnol. 21**,** 635-637. [DOI] [PubMed] [Google Scholar]
- 15.Sledz, C. A., Holko, M., de Veer, M. J., Silverman, R. H. & Williams, B. R. (2003) Nat. Cell Biol. 5**,** 834-839. [DOI] [PubMed] [Google Scholar]
- 16.Scacheri, P. C., Rozenblatt-Rosen, O., Caplen, N. J., Wolfsberg, T. G., Umayam, L., Lee, J. C., Hughes, C. M., Shanmugam, K. S., Bhattacharjee, A., Meyerson, M. & Collins, F. S. (2004) Proc. Natl. Acad. Sci. USA 101**,** 1892-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamada, M., Ohtsuka, T., Kawaida, R., Koizumi, M., Morita, K., Furukawa, H., Imanishi, T., Miyagishi, M. & Taira, K. (2002) Antisense Nucleic Acid Drug Dev. 12**,** 301-309. [DOI] [PubMed] [Google Scholar]
- 18.Chiu, Y. L. & Rana, T. M. (2003) RNA 9**,** 1034-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]