Cell cycle proteins as promising targets in cancer therapy (original) (raw)
. Author manuscript; available in PMC: 2017 Jul 27.
Published in final edited form as: Nat Rev Cancer. 2017 Jan 27;17(2):93–115. doi: 10.1038/nrc.2016.138
Preface
Cancer is characterized by uncontrolled proliferation resulting from aberrant activity of various cell cycle proteins; therefore, cell cycle regulators are considered attractive targets in cancer therapy. Intriguingly, animal models demonstrated that some of these proteins are not essential for proliferation of non-transformed cells and development of most tissues. In contrast, many cancers are uniquely dependent on these proteins and are hence selectively sensitive to their inhibition. After decades of research on the physiological functions of cell cycle proteins and their relevance for cancer, this knowledge recently translated into the first approved cancer therapeutic targeting of a direct regulator of the cell cycle. Here, we review the role of cell cycle proteins in cancer, the rationale for targeting them in cancer treatment and results of clinical trials, as well as future therapeutic potential of various inhibitors. We focus only on proteins that directly regulate cell cycle progression. Cyclin-dependent kinases with transcriptional functions, as well as PARP inhibitors, which are highly successful in targeting BRCA1/BRCA2-mutant tumours, are not covered by this review.
Introduction
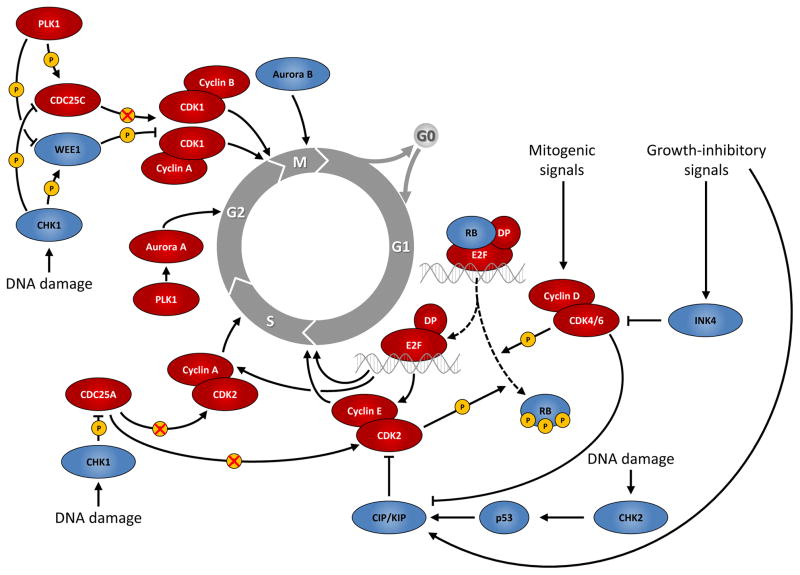
The mammalian cell cycle is a highly organized and regulated process that ensures duplication of genetic material and cell division. This regulation involves growth-regulatory signals as well as signals by proteins monitoring the genetic integrity to ascertain the absence of any genetic damage. Proliferation depends on progression through four distinct phases of the cell cycle (G0/G1, S, G2 and M), which is regulated by several cyclin-dependent kinases (CDKs) that act in complex with their cyclin partners. The activity of CDKs involved in cell cycle regulation is tightly controlled; it is induced by mitogenic signals and can be inhibited by activation of cell cycle checkpoints in response to DNA damage (FIG. 1).
Figure 1. Cell cycle progression and major regulatory proteins.
Mitogenic signals activate complexes of cyclins and cyclin-dependent kinases (CDKs) that promote progression from the G1 phase into S phase mainly by phosphorylating the retinoblastoma protein (RB) and subsequent activation of transcription by the E2F family of transcription factors. Growth-inhibitory signals antagonize G1-S progression by upregulating CDK inhibitors of the INK4 and CIP/KIP families. Progression through S phase and from G2 phase into mitosis (M phase) is also controlled by cyclin-CDK complexes, together with a variety of other proteins, such as Polo-like kinase 1 (PLK1) and Aurora kinases (Aurora A/B). Cells can also exit the cell cycle and enter a reversible or permanent cell cycle arrest (G0 phase). In addition, DNA damage is sensed by several specialized proteins and triggers cell cycle arrest via checkpoint kinase 2 (CHK2) and p53 in G1 phase or via checkpoint kinase 1 (CHK1) in S or G2 phase. Red and blue ovals denote positive and negative regulators of cell cycle progression, respectively.
CDC25, cell division cycle 25; CIP, CDK-interacting protein; G1, gap 1; G2, gap 2; INK4, inhibitor of CDK4; KIP, kinase-inhibitory protein.
Cancer is characterized by aberrant cell cycle activity. This occurs either as result of mutations in upstream signalling pathways or by genetic lesions within genes encoding cell cycle proteins. Aberrant activation of CDKs, which is frequently seen in human cancers, provided a rationale for designing synthetic inhibitors of CDKs as anticancer drugs.
Cell cycle proteins and their role in physiology and cancer
The biology of the CDK4/CDK6-RB pathway
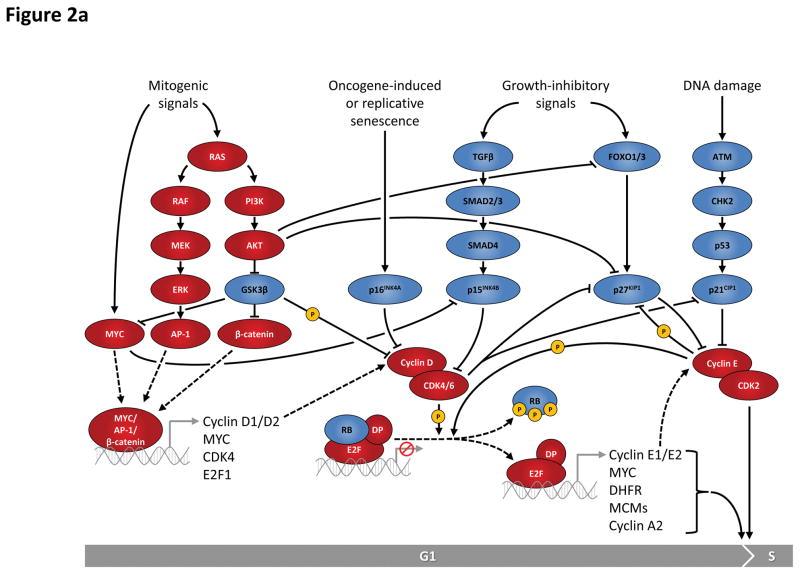
In most adult tissues, cells are residing in a cell cycle arrested state termed G0 phase, which can be either transient (quiescence) or permanent (upon terminal differentiation or senescence). Quiescent cells can be triggered to reenter the cell cycle through stimulation with mitogenic factors. Most of these factors activate cascades of intracellular signalling networks and impinge on CDK4 and CDK6 to drive cell cycle progression from G0/G1 into S phase, in which DNA replication occurs (FIG. 2a). CDK4 and CDK6 are highly homologous serine/threonine kinases that are expressed in a tissue-specific manner. CDK4 and CDK6 phosphorylate a largely overlapping set of target proteins1. Indeed, gene knockout experiments supported a significant redundancy between CDK4 and CDK6 in most tissues2. Apart from that, CDK6 was shown to possess some unique, cyclin-independent transcriptional roles in haematopoietic cells3. The activity of CDK4 and CDK6 is controlled by several mechanisms: positively by association with D-type cyclins (D1, D2 and D3) and negatively by binding to CDK inhibitors of the INK4 family (p16INK4A, p15INKB, p18INK4C and p19INK4D)4.
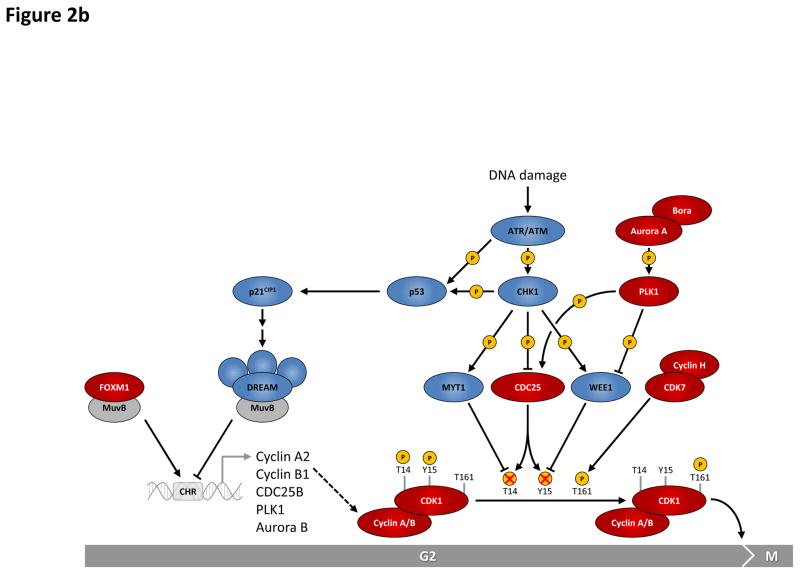
Figure 2. Regulation of G1-S and G2-M cell cycle transitions is controlled by multiple proteins and pathways.
a: Entry into the cell cycle is typically induced in response to mitogenic signals that activate signalling pathways such as the RAS pathway. These pathways eventually impinge on transcriptions factors such as MYC, AP-1 or β-catenin and lead to induction of a number of cell cycle proteins including D-type cyclins. Formation of active complexes of D-type cyclins and cyclin-dependent kinases (CDKs) 4 and 6 drives phosphorylation of the RB (retinoblastoma) protein and is antagonized by the INK4 family (p16INK4A and p15INK4B) in response to senescence-inducing or growth-inhibitory signals, such as the transforming growth factor β (TGFβ). Upon RB phosphorylation, E2F transcription factors are able to activate transcription of a plethora of S phase-promoting genes, including cyclins E1 and E2. Cyclin E-CDK2 complexes are kept inactive by interaction with inhibitors p27KIP1 and p21CIP1 that are regulated by growth-inhibitory signals and the p53-dependent G1 DNA damage checkpoint. Activation of cyclin E-CDK2 involves several mechanisms including the sequestration of p27KIP1 and p21CIP1 by cyclin D-CDK4/6 complexes, and phosphorylation of p27KIP1 by cyclin E-CDK2 kinase. Active cyclin E-CDK2 complexes further phosphorylate RB, as well as many other targets culminating in S phase entry.
b: During G2 phase, the MuvB complex associates with the transcription factor FOXM1 and binds promoters containing cell cycle genes homology region (CHR) elements, thereby inducing transcription of genes required for entry into and progression through mitosis (M phase), including B-type cyclins. Activation of cyclin B-CDK1 kinase requires phosphorylation of CDK1 at Thr-161 by the cyclin H-CDK7 complex (CAK, CDK-activating kinase) as well as dephosphorylation of Thr-14 and Tyr-15 on CDK1 by cell division cycle 25 (CDC25) family phosphatases, the latter process being antagonized by protein kinases MYT1 and WEE1. Activation of CDK1 is prevented in response to activation of the CHK1-dependent G2 DNA damage checkpoint. Upon recovery from DNA damage, Polo-like kinase 1 (PLK1) is essential to re-activate CDK1. Activation of cyclin A/B-CDK1 complexes is required and sufficient for entry into mitosis. Red and blue ovals denote positive and negative regulators of cell cycle transitions, respectively.
AKT, v-akt murine thymoma viral oncogene homolog (kinase); AP-1, activator protein 1; ATM, ataxia telangiectasia mutated (kinase); ATR, ataxia telangiectasia and Rad3 related (kinase); CHK, checkpoint kinase; DHFR, dihydrofolate reductase; DREAM, multiprotein complex consisting of p107/p130, E2F4/E2F5, DP1 and MuvB; ERK, extracellular signal-regulated kinase; FOXM1, forkhead box M1; FOXO, forkhead box O; GSK3β, glycogen synthase kinase 3 beta; MEK, mitogen-activated protein kinase kinase; MCMs, minichromosome maintenance complex component proteins (DNA helicases); MuvB, synthetic multivulva class B complex; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; SMAD, SMAD family of transcription factors.
Cyclin D-CDK4/6 complexes promote cell cycle progression by two major mechanisms5. First, they sequester p21CIP1 and p27KIP1, two CDK inhibitors that bind and prevent activation of cyclin E-CDK2 kinase (BOX 1). Second, active cyclin D-CDK4/6 complexes phosphorylate a variety of cellular targets, most importantly the retinoblastoma tumour suppressor protein (RB) and its closely related proteins p107 and p130, thereby enabling E2F transcription factors to activate transcription of a plethora of genes involved in cell cycle progression from G1 into S phase, DNA replication, chromatin structure, chromosome segregation and mitotic spindle assembly checkpoint. Among the E2F transcriptional targets are cyclins E1 and E2, which bind and activate CDK2. Cyclin E-CDK2 complexes further phosphorylate RB, thereby initiating a positive feedback loop. In addition to these canonical cell cycle functions, D-type cyclins, CDK4 and CDK6 were shown or postulated to perform a number of non-canonical functions, some of which may be relevant for regulation of proliferation6.
Box 1. Cyclin-dependent kinase inhibitor proteins and their role in cancer.
The activity of cyclin-dependent kinases (CDKs) is also regulated by their association with cyclin-dependent kinase inhibitor proteins (CKIs). These include members of the INK4 family (p16INK4A, p15INK4B, p18INK4C and p19INK4D), which bind to CDK4 and CDK6 and block their association with D-type cyclins, thereby extinguishing the kinase activity of CDK4 and CDK6. In contrast, CKIs from the CIP/KIP family (p21CIP1, p27KIP1 and p57KIP2) bind to all CDK complexes and inhibit the kinase activity of CDK2 and CDK1.
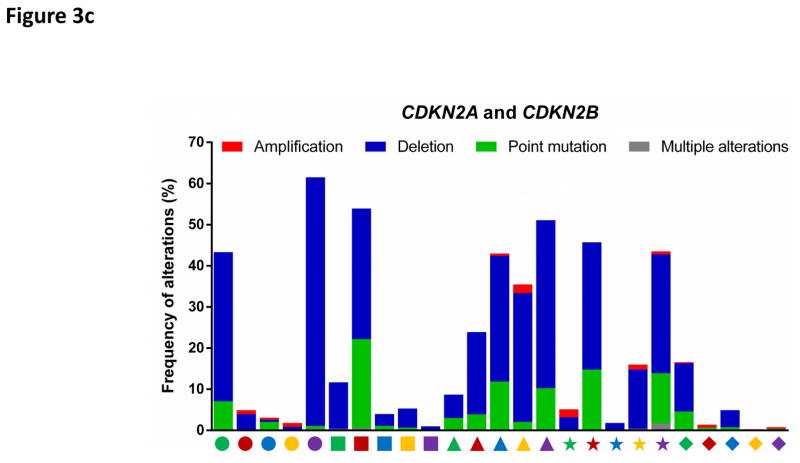
As expected from their role as negative regulators of the cell cycle, CKIs display certain tumour-suppressive properties. Expression of INK4 proteins, in particular p16INK4A and p15INK4B (encoded by CDKN2A and CDKN2B), is silenced in human tumours by genomic deletions, loss-of-function point mutations or promoter methylation (FIG. 3c). Furthermore, p27KIP1 is frequently downregulated as a result of enhanced protein degradation in human tumours, an event associated with poor survival246–248, although deletion of its genomic locus (CDKN1B) is only rarely observed249.
Several mouse models were generated to address the role of CKIs in tumorigenesis. For example, mice deficient for p16INK4A spontaneously developed tumours and exhibited increased susceptibility to carcinogen-induced neoplasia250. Similarly, mice deficient for p21CIP1 exhibited an increased frequency of spontaneous tumour formation in a variety of tissues47. Interestingly, mice heterozygous for Cdkn1b (encoding p27KIP1) displayed increased susceptibility to tumorigenesis following exposure of animals to gamma radiation or to chemical carcinogens, but did not exhibit the loss of the remaining wild-type allele, indicating a haplo-insufficient tumour suppressor role of this CKI46. These findings illustrate that CKIs generally function as tumour-suppressors, presumably by restricting uncontrolled CDK activity and thereby serving as an additional barrier to malignant transformation.
The role of the CDK4/CDK6-RB pathway in cancer
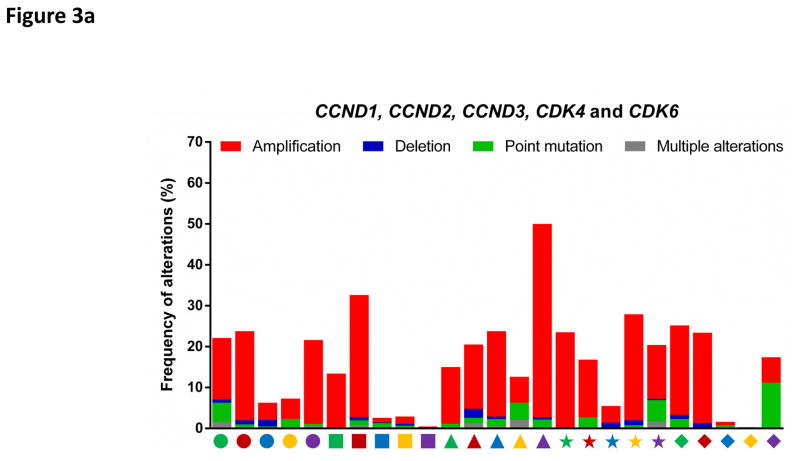
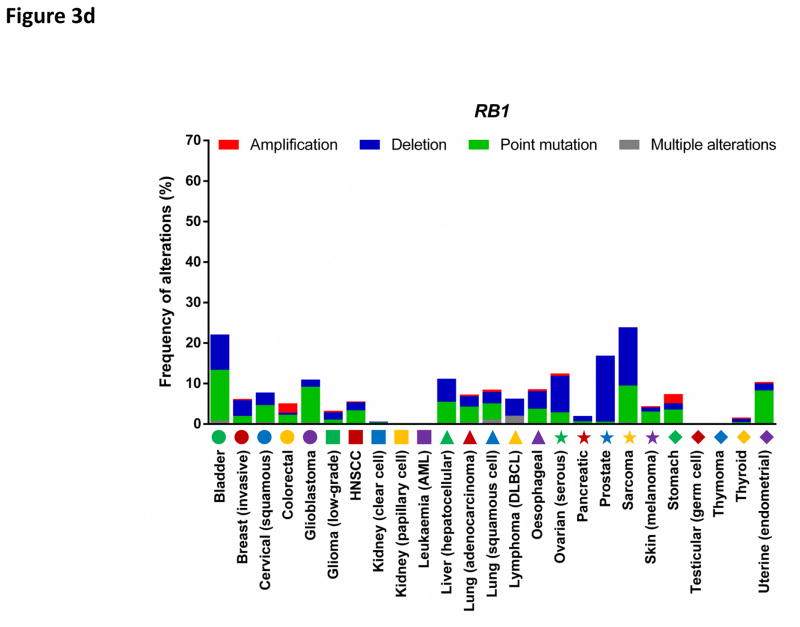
Components of the CDK4/6-RB pathway are commonly mutated in human cancers (FIG. 3a, 3c, 3d). For example, the cyclin D1 gene (CCND1) represents the second most frequently amplified locus among all human cancer types7. CDK4 is amplified in 50% of glioblastomas8 and constitutively activated by a point mutation (R24C, which renders CDK4 insensitive to inhibition by INK4 family members) in melanomas9. Similarly, CDK6 is activated by genomic translocations in splenic marginal zone lymphomas10. Furthermore, the CDKN2A gene (which encodes the tumour suppressors p16INK4A and p14ARF) represents the most frequently deleted locus in human cancers and its expression is also commonly silenced by promoter methylation7. Finally, deletion of the retinoblastoma gene (RB1) occurs frequently in many tumour types and allows proliferation independently of cyclin D-CDK4/6 activity7.
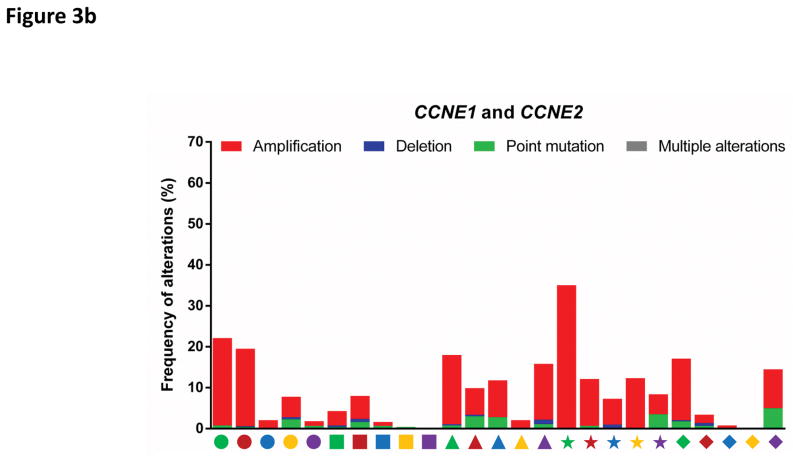
Figure 3. Deregulation of cell cycle proteins in human cancers.
The frequencies of genetic alterations within genes encoding major cell cycle regulators across 25 types of human cancers. Genetic alterations include amplifications (red bars), deletions (blue bars), point mutations (green bars) and multiple alterations (grey bars). Each cancer type is denoted by a symbol with unique shape and colour below the graph (for symbol legend, see FIG. 3d). Data were obtained from The Cancer Genome Atlas (TCGA) and accessed through the cBioPortal for Cancer Genomics (http://www.cbioportal.org/) (26th August 2016). For each cancer type, the TCGA data set with the highest number of tumours was selected. The figures summarize genetic alterations from 48 (for lymphoma) to 1105 (for breast cancer) individual tumours (median of 479 individual tumours per cancer type). More detailed information for each cancer type is available via the cBioPortal for Cancer Genomics.
a: Alterations of cyclin D1 (CCND1), D2 (CCND2), D3 (CCND3) and cyclin-dependent kinase 4 (CDK4) and 6 (CDK6) genes.
b: Alterations of cyclin E1 (CCNE1) and cyclin E2 (CCNE2) genes.
c: Alterations of genes encoding CDK inhibitors p15INK4B (CDKN2B) and p16INK4A (CDKN2A); the latter locus also encodes p14ARF (alternate reading frame protein), which inhibits ubiquitin ligase MDM2 and stabilizes p53.
d: Alterations of the retinoblastoma gene (RB1).
AML, acute myeloid leukaemia; DLBCL, diffuse large B-cell lymphoma; HNSCC; head and neck squamous cell carcinoma.
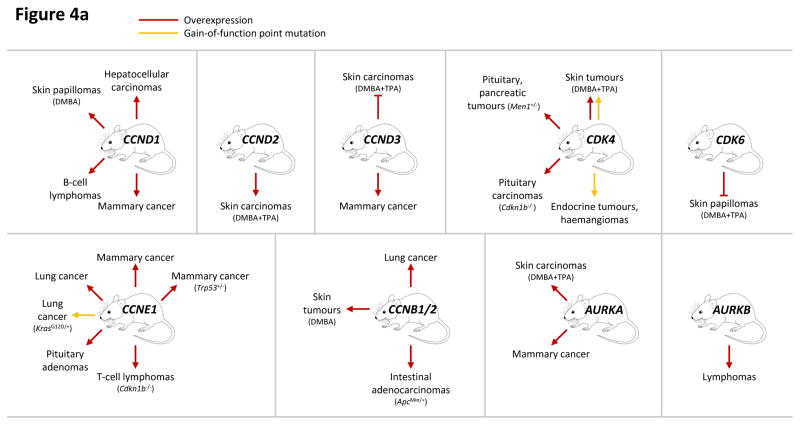
To test the role of D-type cyclins and their catalytic partners CDK4 and CDK6 in tumorigenesis and tumour maintenance, a variety of genetically engineered mouse models were developed (FIG. 4). For instance, introduction of the CDK4 point mutation found in human melanoma (R24C) into the mouse Cdk4 locus caused tumorigenesis in various tissues11 and increased susceptibility to carcinogen-induced melanoma formation12. Furthermore, transgenic mice engineered to overexpress cyclin D1 in mammary glands developed mammary hyperplasia and mammary carcinomas13. These results highlighted the oncogenic properties of D-type cyclins, CDK4 and CDK6. Surprisingly, however, carcinogen-induced skin tumorigenesis was compromised by transgenic overexpression of cyclin D3 or CDK6 in mice14, 15, whereas cyclin D1, D2 or CDK4 overexpression enhanced skin tumorigenesis as expected14, 16, 17.
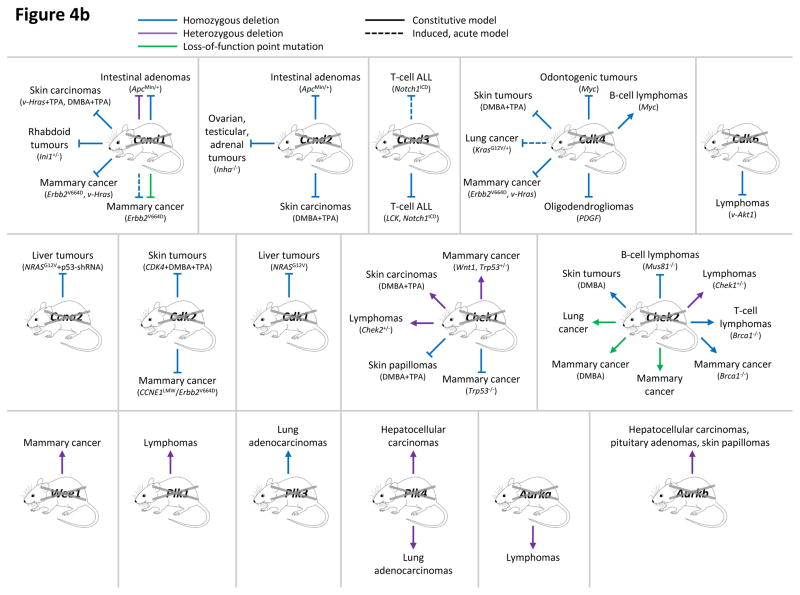
Figure 4. Analyses of cell cycle proteins in cancer using genetically engineered mouse models.
This figure summarizes genetic mouse models used to investigate the role of cell cycle proteins in tumorigenesis. In case of transgenic overexpression, enhanced cancer formation is depicted by red arrows, inhibition of tumorigenesis by red inhibition symbols. Orange arrows indicate cancer formation induced by gain-of-function point mutations. In case of loss-of-function mutations (depicted by crossed out gene symbols), blue inhibition symbols indicate that homozygous ablation of a given gene prevented tumorigenesis, thereby revealing the requirement for this cell cycle protein in cancer formation. Blue dashed inhibition symbols depict an inducible, acute shutdown of Ccnd1, Ccnd3 or Cdk4, used to demonstrate a critical role for these proteins in tumour progression. Arrows indicate that homozygous (blue) or heterozygous (violet) deletion of a cell cycle gene accelerated tumorigenesis. In case of loss-of-function point mutations (as opposed to gene inactivation by deletion described above), enhanced cancer formation is depicted by green arrows, suppressed cancer formation by green inhibition symbols. For tumours induced by a cooperating event (i.e. overexpression or mutation of oncogenes, loss of tumour suppressors or carcinogen treatment), this cooperating event is indicated in parentheses.
a: Genetic mouse models with increased activity of cell cycle proteins, i.e. cyclin D1 (CCND1), D2 (CCND2), D3 (CCND3), CDK4, CDK6, cyclin E1 (CCNE1), cyclin B1 or B2 (CCNB1/2), Aurora A (AURKA) and Aurora B (AURKB).
b: Genetic mouse models with reduced or abolished activity of cell cycle proteins, i.e. cyclin D1 (Ccnd1), D2 (Ccnd2), D3 (Ccnd3), CDK4, CDK6, cyclin A2 (Ccna2), CDK2, CDK1, checkpoint kinase 1 (Chek1) and 2 (Chek2), WEE1, Polo-like kinases 1 (Plk1), 3 (Plk3) and 4 (Plk4), Aurora A (Aurka) and Aurora B (Aurka).
AKT1, thymoma viral proto-oncogene 1; ALL, acute lymphoblastic leukaemia; APC, adenomatosis polyposis coli; BRCA1, breast cancer 1; CDKN1B, CDK inhibitor 1b (p27KIP1); DMBA, 7,12-Dimethylbenz[a]anthracene (a carcinogen); ERBB2 (HER2), erb-b2 receptor tyrosine kinase 2; HRAS, Harvey rat sarcoma virus oncogene; INHA, Inhibin alpha; INI1 (SMARCB1), SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1; KRAS, Kirsten rat sarcoma viral oncogene homolog; LCK, lymphocyte protein tyrosine kinase; MEN1, multiple endocrine neoplasia 1; MUS81, MUS81 endonuclease homolog; MYC, myelocytomatosis oncogene; NRAS, neuroblastoma ras oncogene; p53-shRNA, short hairpin RNA targeting p53; PDGFB, platelet derived growth factor, B polypeptide; TPA, 12-O-Tetradecanoylphorbol-13-acetate (a tumour promoter); TRP53, transformation related protein 53 (p53); WNT1, wingless-type MMTV integration site family, member 1.
| CCND1 gain: | Hepatocellular carcinomas251Skin papillomas (DMBA)16B-cell lymphomas252Mammary cancer13 |
|---|---|
| CCND2 gain: | Skin carcinomas (DMBA+TPA)14 |
| CCND3 gain: | Skin carcinomas (DMBA+TPA)14Mammary cancer253 |
| CDK4 gain: | Skin tumours (DMBA+TPA)12, 17Pituitary, pancreatic tumours (Men1+/−)54Pituitary carcinomas (_Cdkn1b_−/−)254Endocrine tumours, haemangiomas11 |
| CDK6 gain: | Skin papillomas (DMBA+TPA)15 |
| CCNE1 gain: | Mammary cancer (Trp53+/−)255Mammary cancer45Lung cancer256Lung cancer (_Kras_G12D/+)257Pituitary adenomas258T-cell lymphomas (_Cdkn1b_−/−)259 |
| CCNB1/2 gain: | Lung cancer64Skin tumours (DMBA)64Intestinal adenocarcinomas (_Apc_Min/+)64 |
| AURKA gain: | Skin carcinomas (DMBA+TPA)260Mammary cancer107 |
| AURKB gain: | Lymphomas102 |
| Ccnd1 loss: | Intestinal adenomas (_Apc_Min/+)261Skin carcinomas (v-Hras+TPA, DMBA+TPA)262Rhabdoid tumours (Ini1+/−)263Mammary cancer (_Erbb2_V664D, v-Hras)18, 19Mammary cancer (_Erbb2_V664D)22, 26 |
| Ccnd2 loss: | Intestinal adenomas (_Apc_Min/+)264Ovarian, testicular, adrenal tumours (_Inha_−/−)265Skin carcinomas (DMBA+TPA)14 |
| Ccnd3 loss: | T-cell ALL (_Notch1_ICD)26T-cell ALL (LCK, _Notch1_ICD)23 |
| Cdk4 loss: | Odontogenic tumours (Myc)266Skin tumours (DMBA+TPA)267Lung cancer (_Kras_G12V/+)25Mammary cancer (_Erbb2_V664D, v-Hras)20, 21, 268Oligodendrogliomas (PDGF)269B-cell lymphomas (Myc)270 |
| Cdk6 loss: | Lymphomas (v-Akt1)24 |
| Ccna2 loss: | Liver tumours (_NRAS_G12V+53-shRNA)271 |
| Cdk2 loss: | Skin tumours (CDK4+DMBA+TPA)53Mammary cancer (_CCNE1_LMW, _Erbb2_V664D)37, 58 |
| Cdk1 loss: | Liver tumours (_NRAS_G12V)65 |
| Chek1 loss: | Mammary cancer (Wnt1, Trp53+/−)77, 78Skin carcinomas (DMBA+TPA)79Lymphomas (Chek2+/−)272Skin papillomas (DMBA+TPA)79Mammary cancer (Trp53+/−)78 |
| Chek2 loss: | B-cell lymphomas (_Mus81_−/−)273Skin tumours (DMBA)274Lung cancer275Mammary cancer (DMBA)275Mammary cancer276Mammary cancer (_Brca1_−/−)277T-cell lymphomas (_Brca1_−/−)277Lymphomas (Chek1+/−)272 |
| Wee1 loss: | Mammary cancer87 |
| Plk1 loss: | Lymphomas95 |
| Plk3 loss: | Lung adenocarcinomas278 |
| Plk4 loss: | Hepatocellular carcinomas279Lung adenocarcinomas279 |
| Aurka loss: | Lymphomas108 |
| Aurkb loss: | Hepatocellular carcinomas, pituitary adenomas, skin papillomas109 |
In contrast, mice lacking cyclin D1 were resistant to mammary cancer formation induced by specific oncogenes (such as v-Hras or Erbb2V664E)18, 19; at least for Erbb2, this critically depended on the kinase activity of CDK420–22. _Ccnd3_-null mice were resistant to Notch1ICD-driven T-cell acute lymphoblastic leukaemia23, whereas Cdk6 knockout mice were resistant to lymphoma formation induced by constitutively active AKT24. Intriguingly, lung cancer driven by oncogenic KrasG12V exhibited selective sensitivity to CDK4 inhibition, since acute deletion (i.e. conditional deletion after tumour formation) of Cdk4, but not of Cdk6 or Cdk2, induced senescence and prevented tumour progression25. Similarly, an acute and global ablation of Ccnd1 or pharmacological inhibition of CDK4 and CDK6 kinase activity in mice bearing Erbb2V664E-driven mammary tumours blocked cancer progression and triggered tumour cell-specific senescence without having any obvious effect on normal tissues26. Surprisingly, an acute and ubiquitous deletion of Ccnd3 or inhibition of CDK4 and CDK6 in mice with Notch1ICD-induced T-cell acute lymphoblastic leukaemia provoked tumour cell-specific apoptosis, rather than senescence, although the mechanism for this response has not yet been elucidated26, 27. Collectively, these analyses revealed that individual D-type cyclins, CDK4 and CDK6 are required for tumour initiation, and that their continued expression is critical for tumour maintenance. This is in stark contrast to normal non-transformed tissues, in which shutdown of individual D-type cyclins or inhibition of CDK4 and CDK6 catalytic activity had no major effects26. Collectively, these studies illustrate that tumours are frequently dependent on individual cyclins and CDKs and hence susceptible to their targeted inhibition, which is in noticeable contrast to the redundancy observed in most normal, non-transformed tissues5.
CDK2
This CDK is activated through its association with E-type or A-type cyclins. In the absence of mitogens CDK2 complexes are inhibited by association with the CDK inhibitors p27KIP1 or p21CIP1. During the late G1 phase, CDK2 activity increases as a result of E2F-mediated transcription of cyclin E genes, cyclin D-CDK4- and cyclin D-CDK6-mediated sequestration of p27KIP1 and p21CIP1, as well as ubiquitin-mediated proteolysis of p27KIP1 and p21CIP1 following their phosphorylation by CDK2. In addition, CDK2 activity is inhibited by WEE1-mediated phosphorylation at Tyr-15, and this inhibitory phosphorylation is removed by the CDC25 family of phosphatases such as CDC25A and CDC25B28. Cyclin E-CDK2 complexes phosphorylate a variety of proteins required for cell cycle progression, DNA replication and centrosome duplication29, 30. During S phase, cyclin E is rapidly degraded following FBXW7-mediated ubiquitination31, 32 and CDK2 associates with newly synthesized cyclin A2 to form active cyclin A-CDK2 complexes.
CDK2 mutations are rarely found in human cancers; however the catalytic activity of CDK2-containing complexes is hyperactivated via several mechanisms. The CCNE1 locus is frequently amplified, for example in ovarian and breast cancers (FIG. 3b)33, 34. In some tumour types cyclin E overexpression occurs as a result of loss-of-function mutations within the gene encoding FBW7 (FBWX7), a ubiquitin ligase component involved in cyclin E degradation35, 36. Alternatively, certain tumours express a hyperactive, truncated form of cyclin E137. Similarly, cyclin A is frequently overexpressed, sometimes as a result of genomic amplification, for example in hepatocellular carcinomas38, colorectal39 and breast cancers40. In some tumours CDK2 activity is enhanced following reduced expression of the CDK inhibitor p27Kip1, e.g. due to increased SKP2-mediated degradation41. In addition, CDC25A and CDC25B are overexpressed in various tumours42–44.
These different mechanisms of CDK2 activation have been validated using mouse cancer models. Thus, transgenic overexpression of cyclin E1 in mammary glands led to mammary cancer formation45. Increased activity of cyclin E-CDK2 resulting from deletion of genes encoding CDK inhibitor proteins p27KIP1 or p21CIP1 also increased the susceptibility to tumour formation (BOX 1)46, 47. Transgenic overexpression of CDC25A or CDC25B enhanced v-HRAS-induced, ERBB2V664E-induced and carcinogen-induced mammary cancer formation48, 49. Conversely, heterozygous deletion of Cdc25a delayed v-HRAS-induced and ERBB2V664E-induced mammary tumorigenesis50.
It is not clear whether CDK2 activity is required for tumour initiation and maintenance. Several human cancer cell lines were shown to proliferate despite inhibition of CDK2 activity51. Likewise, mice lacking CDK2 displayed unperturbed tumorigenesis in several tissues25, 52–54. However, MYC-overexpressing tumours were shown to require CDK2-mediated phosphorylation of MYC to suppress senescence55, 56. Indeed, deletion of Cdk2 delayed tumour formation in a mouse model of MYC-overexpressing B-cell lymphoma (Eμ-Myc)55. Moreover, CDK2 depletion suppressed cell cycle progression in melanoma cells57. Also, mouse cancer models showed that CDK2 is critically required for mammary cancer formation induced by overexpression of Erbb2V664E or a cancer-associated truncated cyclin E1 isoform37, 58. Hence, CDK2 function may be required in specific cancer types.
CDK1
CDK1 represents the only CDK that is essential for cell cycle progression59. During G2 phase CDK1 binds and becomes activated by cyclins A2 and B. Upon entry into mitosis, cyclin A2 is degraded and CDK1 activity is maintained in complexes with B-type cyclins; CDK1 kinase activity is required for mitotic entry and several mitotic events. B-type cyclins are degraded by the anaphase-promoting complex (APCCDC20 and APCCDH1) in late mitosis60. This attenuates CDK1 activity and allows chromosome separation and completion of mitosis and cytokinesis. In addition to regulation by its cyclin partners, CDK1 activity is inhibited by phosphorylation at Thr-14 and Tyr-15, mediated by kinases MYT161 and WEE162, respectively; this phosphorylation is relieved by CDC25 phosphatases (FIG. 2b)62.
Interestingly, CDK1 activity is not commonly deregulated in cancer; one of the few examples being CCNB3 gene amplifications in neuroendocrine prostate cancers63. Transgenic overexpression of cyclins B1 or B2 increased susceptibility to carcinogen-induced skin and lung tumours, revealing a potential role for elevated CDK1 activity in tumorigenesis64. CDK1 was shown to be required for tumour formation and progression. For example, liver-specific ablation of Cdk1 conferred resistance to NRASG12V-induced liver tumorigenesis65, while CDK1 inhibition blocked the growth of _KRAS_-mutant (G12V, G12D or G12S) colorectal cancer xenografts66. However, CDK1 activity is essential for proliferation also in normal, non-transformed cells59, arguing against inhibition of CDK1 as a viable therapeutic strategy. Intriguingly, inhibition of CDK1 triggered apoptosis of MYC-overexpressing mouse lymphomas and liver tumours67, as well as human basal-like triple-negative breast cancer cells68. These findings raise a possibility that CDK1 inhibition might specifically kill tumour cells, while causing only a transient cell cycle arrest in normal tissues, a notion that requires further investigation using genetic mouse models.
DNA damage checkpoint kinases and WEE1
Cells have checkpoints to halt cell cycle progression in response to DNA damage, thereby allowing time for DNA repair. Several DNA damage checkpoints exist and they impinge on the activity of specific CDK complexes (FIG. 1). Depending on the type of DNA damage, ATR or ATM protein kinases phosphorylate and activate checkpoint kinase 1 (CHK1, encoded by the CHEK1 gene)69. Similarly, ATM can also activate CHK2 (encoded by CHEK2), which in turn participates in the activation of p5370. Activation of p53 transcriptionally induces expression of the CDK inhibitor p21CIP1, leading to inhibition of cyclin E-CDK2 complexes and a G1 arrest (FIG. 2a)71. Activated CHK1 mediates a temporary S phase arrest by phosphorylating and inactivating CDC25A and a G2 checkpoint arrest by phosphorylating CDC25A, CDC25B and CDC25C72, 73. These events prevent dephosphorylation of Tyr-15 on CDK2 and CDK1, thereby rendering these CDKs inactive. CHK1 also activates WEE1 via direct phosphorylation, leading to enhanced inhibitory Tyr-15 phosphorylation of CDK2 and CDK1 and subsequent cell cycle arrest in G2 phase (FIG. 2b)74. In summary, CHK1 is an essential mediator of DNA damage-induced cell cycle arrest in S and G2 phases, particularly in cancer cells with inactivated p53, which depend on G2 checkpoint to halt cell proliferation.
The role of CHK1 and WEE1 in cancer development is controversial. CHK1 was initially regarded as a tumour suppressor. Indeed, heterozygous loss-of-function mutations of the CHEK1 locus were detected in breast75 and gastric cancer76; however, no homozygous loss-of-function mutations have been identified so far. Consistent with these findings, heterozygous deletion of Chek1 in mice enhanced mammary tumorigenesis induced by the Wnt oncogene or by heterozygous deletion of Trp53 (which encodes p53 in mice)77, 78. In contrast, tissue-specific homozygous deletion of Chek1 in mice inhibited mammary tumorigenesis induced by p53 loss78 and prevented carcinogen-induced skin tumour formation79. Consistent with an oncogenic role for CHK1, this protein is overexpressed in many cancers, such as triple-negative breast cancers, hepatocellular and cervical cancers80–82. Furthermore, an extra allele of Chek1 protected mouse fibroblasts from replicative stress and enhanced HRASG12V-induced transformation by reducing DNA damage-associated apoptosis in vitro83. Collectively, these observations suggest that although reduced CHK1 levels (resulting from heterozygous deletion) may enhance tumorigenesis, CHK1 is required for tumour cell growth and survival by allowing DNA damage repair. In contrast to CHK1, CHK2 is thought to play mostly a tumour-suppressive role, since several loss-of-function mouse models exhibited enhanced tumorigenesis. Hence, CHK2 does not seem to represent a suitable target for cancer therapy (FIG. 4b).
WEE1 kinase is overexpressed in several cancer types, for instance in hepatocellular carcinoma84, glioblastoma85 and melanoma86. In contrast, heterozygous deletion of Wee1 in the mammary gland induced spontaneous development of mammary cancers in a small percentage of older mice, while no tumours were observed upon homozygous deletion87. Hence, tumorigenesis may be incompatible with complete loss of WEE1 activity, similar to CHK1. Despite these contradictory results, WEE1 is generally considered to be an oncogene and a potential target in cancer therapy.
Polo-like kinases
The family of Polo-like kinases consists of five members, of which PLK1 has been studied in most detail. During G2 phase PLK1 participates in the maturation of centrosomes by regulating the centrosomal localization of Aurora A88. Moreover, PLK1 plays an important role in activation of cyclin B-CDK1 complexes by at least two mechanisms. First, it activates CDC25C phosphatase, which in turn removes the inhibitory Tyr-15 phosphorylation of CDK189. Second, PLK1 induces phosphorylation-dependent degradation of WEE1, thereby preventing further phosphorylation of CDK1 at Tyr-15 (FIG. 2b)90. Subsequently, PLK1 is involved in triggering chromosome segregation during the metaphase-anaphase transition and plays important roles in cytokinesis. PLK1 is also crucial for mitotic entry following recovery from DNA damage-induced G2 phase arrest, providing a rationale for its exploitation as a target in cancer therapy.
The role of PLK1 in cancer is not clear. PLK1 expression is frequently elevated in tumours, correlates with poor prognosis and is thought to contribute to tumorigenesis by compromising cell cycle checkpoints and inducing genetic instability91–93. In contrast, a few cancer cell lines exhibited mutations that reduce PLK1 stability94. Furthermore, heterozygous deletion of Plk1 in mice increased the incidence of spontaneous tumours, suggesting a potential tumour-suppressive role for PLK195. Despite these conflicting results, PLK1 is generally considered oncogenic and a potential target in cancer therapy.
Aurora kinases
Aurora kinases are serine/threonine kinases that play major roles in mitosis and cytokinesis. Aurora A localizes to the centrosomes starting in S phase and is essential for centrosome maturation, spindle assembly and spindle orientation. Furthermore, Aurora A phosphorylates and activates PLK1, thereby promoting CDK1 activation and mitotic entry, especially after DNA damage checkpoint-dependent G2 phase arrest96, 97. Aurora A also stabilizes the transcription factor N-MYC (encoded by MYCN) by preventing its proteasomal degradation, thereby promoting G1-S progression98. Aurora B is found at chromosomes and at the mitotic spindle during mitosis where it constitutes a part of the chromosomal passenger complex. Aurora B controls chromosome condensation and orientation as well as proper execution of cytokinesis.
Ectopic overexpression of Aurora A caused inactivation of DNA damage checkpoint during the G2 phase99 and inactivation of the spindle assembly checkpoint during mitosis100, leading to tetraploidy and centrosome amplification, especially in cells with defective p53-dependent DNA damage checkpoint101. Also, overexpression of Aurora B caused defective chromosome separation leading to aneuploidy102. Analyses of human tumours support oncogenic roles for Aurora A and Aurora B. The gene encoding Aurora A is frequently amplified in prostate103 and breast cancers104, while several other cancer types express elevated levels of Aurora A protein105. Aurora B is also found overexpressed in several cancer types, although its genomic locus is rarely amplified, e.g. in only 5% of myelodysplastic syndromes106. Importantly, transgenic overexpression of Aurora A in mouse mammary epithelium induced tetraploidy and centrosome amplification leading to mammary cancer formation107. Likewise, mice ubiquitously overexpressing Aurora B spontaneously developed lymphomas102. Surprisingly, despite their role as oncogenes, heterozygous deletion of the genes encoding Aurora A or Aurora B in mice also increased tumour incidence in various organs suggesting some tumour-suppressive roles108, 109.
Rationale for targeting specific cell cycle proteins
Cell cycle proteins are frequently overactive in cancer cells leading to uncontrolled proliferation. As we described earlier, genetic ablation of individual cyclins or CDKs, or inhibition of cyclin-CDK kinase activity in tumour-bearing mice selectively blocked tumour initiation and progression of specific cancer types driven by particular oncogenic insults, without having major effects on normal tissues. This suggests that tumour cells are dependent on (or “addicted” to) specific CDKs, depending on genetic lesions they carry, and hence CDK inhibition may selectively target cancer cells while sparing normal tissues. In some instances, inhibition of CDK activity in mouse cancer models not only led to cell cycle arrest but also provoked tumour cell senescence or apoptosis. This indicates that tumours carrying particular genetic lesions critically depend on specific cell cycle proteins to inhibit tumour-suppressive programs such as senescence and apoptosis, thereby selectively sensitizing cancer cells to inhibition of these proteins.
In contrast, inhibition of cell cycle proteins critical for checkpoint function, such as CHK1 and WEE1, follows an opposite strategy. Cell cycle checkpoints are essential to halt cell cycle progression in response to DNA damage, thereby allowing time for DNA repair. Inhibition of CHK1 or WEE1 in cancer cells prevents cell cycle arrest during S or G2 phase and allows cell proliferation despite accumulation of DNA damage. This can lead to cell death during mitosis by a process sometimes referred to as “mitotic catastrophe”. This strategy particularly applies to cancer cells with compromised G1 checkpoint due to loss of p53 function; these cancer cells critically depend on the G2 checkpoint, especially in the presence of DNA damage-inducing drugs. For this reason, inactivation of p53 selectively renders cancer cells sensitive to inhibition of CHK1 or WEE1, an example of the so-called “synthetic lethality”.
Targeting CDKs in cancer therapy
Development of pan-CDK inhibitors
Most of the early compounds exhibited little specificity towards individual CDKs and are therefore commonly referred to as pan-CDK inhibitors. The first generation of these inhibitors include flavopiridol, (R)-roscovitine and olomoucine.
Flavopiridol is a semisynthetic flavone targeting many CDKs and represents the most extensively studied CDK inhibitor with over 60 clinical trials initiated since 1997 (TABLE 1). It causes cell cycle arrest in G1 and G2 phases110. Administration of flavopiridol induced apoptosis in several mouse tissues leading to organ atrophy111, an effect attributed to inhibition of cyclin T1-CDK9 (P-TEFb) kinase112. Although flavopiridol exhibited significant anti-tumour activity in preclinical studies111, clinical phase II studies reported insufficient efficacy for solid cancers. However, some evidence for clinical activity was observed in haematological malignancies (TABLE 2)113, 114.
Table 1.
Inhibitors of cell cycle proteins in clinical development
| Inhibitor (synonym) [company] | Major targets (IC50) | Preclinical studies (in vitro, mouse models) | Clinical trials (open/active/completed) |
|---|---|---|---|
| Pan-CDK inhibitors | |||
| Flavopiridol‡ (alvocidib) [Tolero Pharmaceuticals] | CDK9 (6 nM), CDK1 (30–50 nM), CDK2 (70–170 nM), CDK4 (100 nM)112, 280 | Caused G1 arrest, G2 arrest and apoptosis in vitro110, 111 Induced tumour regression in leukaemia and lymphoma xenografts111 | Phase II: AML, lymphoma, AML, multiple myeloma and many others |
| (R)-Roscovitine* (seliciclib) [Cyclacel Pharmaceuticals] | CDK2 (100–710 nM), CDK7 (490 nM), CDK1 (650–2690 nM), ERK2 (1.2–14 μM)281, 282 | Induced G2/M arrest and cell death in vitro281, 282 Slightly inhibited growth of colorectal cancer and uterine cancer xenografts281 | Phase I: advanced solid tumours |
| Dinaciclib‡ (SCH 727965/ MK-7965) [Merck & Co.] | CDK2 (1 nM), CDK5 (1 nM), CDK1 (3 nM), CDK9 (4 nM), CDK7 (NA), CDK6 (NA)115 | Induced G1 arrest, G2/M arrest and apoptosis in vitro115 Reduced migration in vitro116 Exhibited anti-tumour activity in ovarian115 and pancreatic cancer116, ALL117 and _NRAS_Q61L-mutant melanoma118 | Phase III: CLL Phase II: melanoma, CLL, lung, breast, multiple myeloma |
| AT7519‡ (AT7519M) [Astex Therapeutics] | CDK9 (<10 nM), CDK5 (13 nM), CDK2 (47 nM), GSK3β (89 nM), CDK4 (100 nM), CDK6 (170 nM), CDK1 (210 nM)283 | Induced mainly G2/M arrest in vitro283 Showed promising anti-tumour activity in ovarian284 and colon cancer283 and AML xenografts285 Achieved tumour regression and improved survival in MYCN transgenic neuroblastoma model286 | Phase II: CLL, mantle cell lymphoma, multiple myeloma Phase I: non-Hodgkin’s lymphoma |
| Milciclib* (PHA-848125/PHA-848125AC) [Tiziana Life Sciences] | CDK2 (45–363 nM), TRKA (53 nM), CDK7 (150 nM), CDK4 (160 nM), CDK5 (265 nM), CDK1 (398 nM)287 | Induced G1 arrest and cell death via autophagy in vitro287, 288 Inhibited tumour growth of ovarian cancer287 and glioma xenografts288, KRASG12D-induced lung cancer289 and DMBA-induced mammary cancer290; extended survival of mice bearing leukaemia290 and intracranial glioma xenografts288 | Phase II: thymoma, thymic carcinoma Phase I: advanced solid tumours |
| TG02* [Tragara Pharmaceuticals] | CDK9 (3 nM), CDK5 (4 nM), CDK2 (5 nM), CDK3 (8 nM), CDK1 (9 nM), LCK (11 nM), TYK2 (14 nM), FYN (15 nM), JAK2 (19 nM), FLT3 (19 nM)291 | Induced G1 arrest and apoptosis in vitro291 Caused tumour regression and extended survival of mice with AML xenografts291 | Phase I: CLL, AML, ALL, MDS, multiple myeloma |
| CYC065* [Cyclacel Pharmaceuticals] | CDK2 (5 nM), CDK5 (21 nM), CDK9 (26 nM), CDK3 (29 nM), CDK7 (193 nM), CDK4 (232 nM)292 | Induced apoptosis in trastuzumab-resistant breast cancer cells33 Inhibited growth of trastuzumab-resistant breast cancer xenografts33 | Phase I: advanced solid tumours and lymphomas |
| RGB-286638‡ [Agennix] | CDK9 (1 nM), FMS (1 nM), CDK1 (2 nM), CDK2 (3 nM), GSK3β (3 nM), CDK4 (4 nM), CDK3 (5 nM), CDK5 (5 nM), TAK1 (5 nM)293 | Induced cell cycle arrest and apoptosis and inhibited transcription in vitro293 Inhibited tumour growth and extended survival of mice bearing multiple myeloma xenografts293 | Phase I: advanced solid tumours294 |
| CDK4 and CDK6-selective inhibitors | |||
| Palbociclib* (PD0332991) [Pfizer] | CDK4 (9–11 nM), CDK6 (15 nM)131 | Inhibited cell proliferation and induced G1 arrest in RB-positive cancer cells131 Inhibited growth of rhabdomyosarcoma133, multiple myeloma134, AML135, ALL136 and dermatofibrosarcoma137 xenografts Induced tumour regression in glioblastoma and colorectal cancer xenografts131 Showed synergistic anti-tumour activity with PI3K inhibition in _PI3KCA_-mutant triple-negative breast cancer xenografts142 | Phase III: breast, lung Phase II: breast, lung, head and neck, multiple myeloma, AML, ALL, gastrointestinal, ovarian, hepatocellular, prostate, melanoma, liposarcoma, urothelial, lymphoma, endometrial, oligoastrocytoma, oligodendroglioma |
| Ribociclib* (LEE011) [Novartis] | CDK4 (10 nM), CDK6 (39 nM)160 | Induced G1 arrest and senescence in vitro161 Inhibited tumour growth in neuroblastoma161, rhabdomyosarcoma145 and Ewing sarcoma xenografts162 Caused tumour regression in liposarcoma xenografts143 | Phase III: breast Phase II: breast, melanoma, liposarcoma, prostate, lung, uterine, gastrointestinal, ovarian, paediatric glioma, hepatocellular, teratoma, pancreatic, colorectal |
| Abemaciclib* (LY2835219) [Eli Lilly] | CDK4 (2 nM), CDK6 (10 nM), HIPK2 (31 nM), PIM1 (50 nM), CDK9 (57 nM), DYRK2 (61 nM), CK2 (117 nM), GSK3β (192 nM)165 | Induced G1 arrest in vitro165 Showed anti-tumour activity in colorectal cancer165, AML165, glioblastoma (orthotopic)167 and vemurafenib-resistant melanoma xenografts166 | Phase III: breast, lung Phase II: breast, lung, melanoma, mantle cell lymphoma |
| CHK1 and WEE1 inhibitors | |||
| MK-8776‡ (SCH 900776) [Merck & Co.] | CHK1 (3 nM), CDK2 (160 nM), PIM1 (NA)182 | Induced DNA double-strand breaks, G2/M arrest and apoptosis in vitro182; sensitized cancer cells to various chemotherapeutics183 and to histone deacetylase inhibition295 Combination with gemcitabine inhibited tumour growth in pancreatic cancer and induced tumour regression in ovarian cancer xenografts182 | Phase II: AML Phase I: non-Hodgkin’s lymphoma |
| LY2606368‡ (prexasertib) [Eli Lilly] | CHK1 (<1 nM), CHK2 (8 nM), RSK1 (9 nM), MELK (38 nM), SIK (42 nM), BRSK2 (48 nM), ARK5 (64 nM)188 | Caused DNA double-strand breaks during S phase (“replication catastrophe”), leading to fragmented chromosomes and mitotic cell death in vitro188 Inhibited tumour growth in lung cancer xenografts188 | Phase II: breast, ovarian, prostate, lung Phase I: head and neck, AML, MDS |
| AZD1775* (MK-1775) [AstraZeneca] | WEE1 (5.2 nM), YES (14 nM)190 | Sensitized p53-deficient tumour cells to apoptosis induction by DNA damaging agents and radiation190–192 Induced tumour regression in lung cancer194 and (combined with gemcitabine) in pancreatic cancer xenografts193 Extended survival of mice with AML195 and high-grade glioma (intracerebral) xenografts196 Synergized with targeted inhibition of CHK1201, 202, 296, histone deacetylases198, 199, mTOR200 and PARP197 | Phase II: lung, ovarian, pancreatic, stomach, AML, MDS, head and neck Phase I: head and neck, glioma, pancreatic, cervical, CML, AML, bladder |
| GDC-0575* (Arry-575) [Genentech] | CHK1 (NA)297 | NA | Phase I: solid tumours and lymphoma |
| PLK inhibitors | |||
| Rigosertib‡ (ON 01910.Na) [SymBio Pharmaceuticals] | PLK1 (9 nM), PDGFR (18 nM), BCR-ABL (32 nM), FLT1 (42 nM), SRC (155 nM), FYN (182 nM), PLK2 (260 nM), CDK1 (260 nM)205 | Induced spindle abnormalities, mitotic arrest and apoptosis in vitro205 Caused tumour regression in orthotopic head and neck squamous cell carcinoma xenografts206 Combination with chemotherapy led to tumour regression in hepatocellular and breast carcinoma xenografts205 Combination with radiotherapy achieved long-lasting tumour regression in cervical cancer xenografts207 | Phase III: MDS, pancreatic Phase II: MDS, AML, ALL, CMML, ovarian, squamous cell |
| Volasertib‡ (BI 6727) [Boehringer Ingelheim] | PLK1 (0.9 nM), PLK2 (5 nM), PLK3 (56 nM)211 | Induced G2/M arrest and apoptosis in vitro211 Caused tumour regression in colorectal cancer211, neuroblastoma213 and paediatric ALL xenografts213 Caused tumour regression in combination with cytarabine or FLT3 inhibitor quizartinib in AML214 and with vincristine in rhabdomyosarcoma xenografts216 | Phase III: AML Phase II: AML, lung, ovarian, urothelial, MDS |
| TKM-080301‡ (TKM-PLK1) [Arbutus Biopharma] | PLK1 (targeted by a lipid nanoparticle formulation of an siRNA)298 | NA | Phase II: liver, adrenocortical, neuroendocrine Phase I: liver |
| CFI-400945* [University Health Network, Toronto] | PLK4 (2.8 nM), ABL-T315I (5 nM), TRKA (6 nM), TRKB (9 nM), BMX (17 nM), TIE2 (22 nM)299 | Induced defects in centriole duplication and mitosis leading to apoptosis299 Caused tumour regression in carboplatin-resistant, PTEN−/− ER+ breast cancer xenografts299 | Phase I: advanced cancer |
| Aurora inhibitors | |||
| Alisertib* (MLN8237) [Millennium Pharmaceuticals] | Aurora A (1.2 nM), EPHA2 (NA)223 | Induced mitotic arrest, spindle abnormalities, polyploidy, followed by senescence or apoptosis in vitro224, 225 Caused tumour regression in neuroblastoma226, paediatric ALL226 and lymphoma xenografts223 Induced tumour regression and prolonged survival in _MYCN_-driven mouse model of neuroblastoma227 Combination with chemotherapy induced tumour regression in AML228, oesophageal229 and gastric cancer xenografts230 Synergized with a DR5 agonist236 and inhibitors for BCR-ABL231, CD20232, MEK234 and BCL-2 in various cancer xenografts235 | Phase III: peripheral T-cell lymphoma Phase II: lymphoma, lung, breast, ovarian, prostate, AML, gastroesophageal, melanoma, multiple myeloma, uterine, head and neck, mesothelioma, neuroblastoma, MDS, rhabdoid, urothelial |
| ENMD-2076* [Miikana Therapeutics] | FLT3 (3 nM), Aurora A (14 nM), SRC (23 nM), VEGFR2 (40 nM), FGFR1 (93 nM), KIT (120 nM)300 | Induced G2/M arrest and apoptosis in vitro301 Reduced tumour vascularity, vascular permeability and perfusion in vivo300 Induced tumour regression in breast and colorectal cancer, melanoma, AML and multiple myeloma xenografts302 | Phase II: ovarian, breast, hepatocellular, sarcoma Phase I: multiple myeloma |
| AMG 900* [Amgen] | Aurora C (1 nM), Aurora B (4 nM), Aurora A (5 nM), p38α (53 nM)303 | Induced mitotic arrest, polyploidy and apoptosis in vitro303, 304 Caused tumour regression in combination with ixabepilone in triple-negative breast cancer xenografts304 | Phase I: AML, advanced solid tumours |
Table 2.
Clinical trial results of selected CDK inhibitors
| Tumour type | Study characteristics, ClinicalTrials.gov Identifier | Drug dosage and combination | Efficacy | Major grade 3/4 adverse effects (≥10%) |
|---|---|---|---|---|
| Flavopiridol (alvocidib) | ||||
| AML (poor prognosis)305 | Phase II N=62 NCT00407966 | 50 mg/m2 IV OD (days 1–3) Combination with cytarabine (2 g/m2 IV over 72 hours, days 6–8) and mitoxantrone (40 mg/m2 IV on day 9) (“FLAM”) | CR: 52% (32/62) PR: 5% (3/62) CR (newly diagnosed secondary AML): 75% (12/15) Median OS: 8 months | Tumour lysis syndrome (53%) |
| AML (newly diagnosed)306 | Phase II N=165 “FLAM” (N=112) vs “7+3” (N=56) NCT01349972 | “FLAM” (see study above) “7+3”: cytarabine (100 mg/m2 IV daily, days 1–7) with daunorubicin (90 mg/m2 IV daily, days 1–3), for residual leukaemia after 14 days: cytarabine (100 mg/m2 IV daily, days 1–5) with daunorubicin (45 mg/m2 IV daily, days 1–2) | Median EFS: 9.7 months vs 3.4 months (HR=0.74, p=0.15) Median OS: 17.5 months vs 22.2 months (HR=1.2, p=0.39) CR: 70% (73/109) vs 57% (32/56) (p=0.08) | Febrile neutropaenia (48% vs 45%), infection (35% vs 38%), hepatic dysfunction (21% vs 23%), gastrointestinal dysfunction (11% vs 9%) |
| CLL (relapsed)113 | Phase II N=64 NCT00098371 | 60–80 mg/m2 IV over 4 hours (days 1, 8, 15 of 28-day cycle) Monotherapy | CR: 2% (1/64) PR: 52% (33/64) Median PFS: 8.6 months | Neutropaenia (88%), diarrhoea (64%), tumour lysis syndrome (42%), elevated transaminases (34%), infection (31%), thrombocytopenia (27%) |
| CLL (fludarabine-refractory)114 | Phase II N=159 NCT00464633 | 60–80 mg/m2 IV over 4 hours (days 1, 8, 15 of 28-day cycle) Monotherapy | CR: 2% (3/159) PR: 24% (38/159) SD: 33% (53/159) Median PFS: 7.6 months Median OS: 14.6 months | Neutropaenia (34%), infections (30%), gastrointestinal (25%), tumour lysis syndrome (21%) and other |
| Dinaciclib (SCH 727965, MK-7965) | ||||
| CLL (relapsed or refractory)124 | Phase I N=52 NCT00871663 | 5–17 mg/m2 IV weekly (3 weeks on, 1 week off), RP2D: 14 mg/m2 Monotherapy | PR: 54% (28/52) SD: NA Median PFS: 15.8 months | Neutropaenia (75%), thrombocytopaenia (40%), increased AST (29%), anaemia (29%), hyperglycaemia (21%) and other |
| Multiple myeloma (relapsed after prior therapy)123 | Phase I/II N=27 NCT01096342 | 30–50 mg/m2 IV every 3 weeks, MTD: 50 mg/m2 Monotherapy | PR: 11% (3/27) SD: 56% (15/27) | Neutropaenia (12%), diarrhoea (12%), blurred vision (12%) |
| Palbociclib (PD0332991) | ||||
| Breast cancer (advanced, ER+ HER2-, first-line treatment, post-menopausal)154 | Phase II N=165 Palbociclib + letrozole (N=84) vs letrozole alone (N=81) NCT00721409 (PALOMA-1) | 125 mg PO OD (3 weeks on, 1 week off) Combination with letrozole (2.5 mg PO OD, continuous) | Median PFS: 20.2 months vs 10.2 months (HR=0.488, p=0.0008) Median OS: 37.5 months vs 33.3 months (HR=0.813, p=0.42) CR: 1% vs 1% PR: 42% vs 32% SD: 44% vs 37% SD ≥ 24 weeks: 38% vs 25% | Neutropaenia (54% vs 1%) |
| Breast cancer (advanced, ER+ HER2-, relapsed or progressed during prior hormone therapy)156, 157 | Phase III N=521 Palbociclib + fulvestrant (N=347) vs placebo + fulvestrant (N=174) NCT01942135 (PALOMA-3) | 125 mg PO OD (3 weeks on, 1 weeks off) Combination with fulvestrant (500 mg IM every 2–4 weeks) Additional goserelin for pre/peri-menopausal women | Median PFS: 9.5 months vs 4.6 months (HR=0.46, p<0.001) Effect on OS yet unknown | Neutropaenia (65% vs 1%) |
| Breast cancer (metastatic, RB+)159 | Phase II N=37 (84% ER+ HER2-) NCT01037790 | 125 mg PO OD (3 weeks on, 1 week off) Monotherapy | PR: 5% (2/37) SD ≥ 6 months: 14% (5/37) Median PFS: 3.7 months | Neutropaenia (54%), lymphopaenia (30%), thrombocytopaenia (19%) |
| Breast cancer (metastatic, RB+)173 | Phase I N=15 NCT01320592 | 50–125 mg PO OD (on days 2–6, 9–14, 16–20 of 28-day cycle) Combination with paclitaxel (80 mg/m2 IV weekly) | PR: 40% (6/15) SD: 33% (5/15) | Neutropaenia (67%) |
| Non-small cell lung cancer (previously-treated, recurrent or metastatic, RB+, with p16INK4A loss)152 | Phase II N=19 NCT01291017 | 125 mg PO OD (3 weeks on, 1 weeks off) Monotherapy | RR: 0% (0/16) SD: 50% (8/16) | Neutropaenia (16%) |
| Head and neck squamous cell carcinoma (incurable)307 | Phase I N=9 NCT02101034 | 100–125 mg PO OD (3 weeks on, 1 week off), RP2D: 125 mg Combination with cetuximab (250–400 mg/m2 IV weekly) | PR: 22% (2/9) SD: 56% (5/9) | None reported |
| Mantle cell lymphoma (relapsed, with cyclin D1 overexpression)149 | Phase Ib N=17 NCT00420056 | 125 mg PO OD (3 weeks on, 1 week off) Monotherapy | CR: 6% (1/17) PR: 12% (2/17) SD: 41% (7/17) PFS > 1 year: 29% (5/17) | Neutropaenia (35%), thrombocytopaenia (24%) |
| Liposarcoma (advanced, well-differentiated or dedifferentiated, RB+, with CDK4 amplification)150 | Phase II N=30 NCT01209598 | 200 mg PO OD (2 weeks on, 1 week off) Monotherapy | PR: 3% (1/29) SD: NA PFS at 12 weeks: 66% (19/29) Median PFS: 18 weeks | Neutropaenia (50%), thrombocytopaenia (30%), anaemia (17%) |
| Germ cell tumours (incurable, refractory, RB+)151 | Phase II N=29 (arm 4) NCT01037790 | 125 mg PO OD (3 weeks on, 1 week off) Monotherapy | PFS at 24 weeks: 28% (8/29) PFS at 24 weeks among patients with teratomas: 45% (5/11) | Neutropaenia (43%), thrombocytopaenia (17%) |
| Ribociclib (LEE011) | ||||
| Breast cancer (advanced, ER+ HER2-, post-menopausal)164 | Phase Ib N=10 (arm 1) NCT01872260 | 600 mg PO OD (3 weeks on, 1 week off) Combination with letrozole (2.5 mg PO OD, continuous) | PR: 17% (1/6) SD: 33% (2/6) | Neutropaenia (50%) |
| Melanoma (NRAS mutant)175 | Phase Ib/II N=14 NCT01781572 | 200–300 mg PO OD (3 weeks on, 1 week off) Combination with binimetinib (25 mg PO BD, continuous) | PR: 43% (6/14), pending confirmation SD: 43% (6/14) | Various DLTs (21%) |
| Abemaciclib (LY2835219) | ||||
| Breast cancer (metastatic)170 | Phase I N=47 (arm 1) NCT01394016 | 200 mg PO BD (continuous) Monotherapy | PR: 23% (11/47), pending confirmation PR among ER+: 31% (11/36) | NA |
| Breast cancer (metastatic, ER+ HER2-)172 | Phase Ib N=36 (parts A+B) N=16 (part C) NCT02057133 | 120–200 mg PO BD (continuous) Combination with letrozole (2.5 mg PO OD) (part A) or anastrozole (1 mg PO OD) (part B) Combination with tamoxifen (20 mg PO OD) (part C) | PR: 6% (2/36) (parts A+B) SD: 61% (22/36) (parts A+B) PR: 0% (0/16) (part C) SD: 75% (12/16) (part C) | Diarrhoea (NA) |
| Non-small cell lung cancer (advanced, relapsed/ progressed) | Phase I N=49 NCT01394016 | 150–200 mg PO BD (continuous) Monotherapy | PR: 2% (1/49) SD: 49% (24/49) Median PFS: 2.1 months | Rare |
In contrast, pan-CDK inhibitors (R)-roscovitine and olomoucine did not show promising anti-tumour activities in preclinical and clinical studies; nevertheless, (R)-roscovitine is still under clinical investigation. In general, first-generation pan-CDK inhibitors suffered from a low therapeutic index leading to toxicities at concentrations necessary to inhibit their targets. To circumvent these limitations, second-generation pan-CDK inhibitors were developed; these include dinaciclib, AT7519, milciclib, TG02, CYC065 and RGB-286638 (TABLE 1).
Dinaciclib is a CDK inhibitor with over 100-fold higher potency in inhibiting RB phosphorylation and a more than ten-fold higher therapeutic index than flavopiridol (TABLE 1)115. It was shown to block proliferation of tumour cells in xenograft models of ovarian and pancreatic cancers, paediatric ALL and _NRAS_-mutant melanoma115–118. Unfortunately, the first clinical trials in several cancer types demonstrated little clinical activity119–122. However, recent results from a phase I/II clinical trial showed promising results with partial responses in 11% of patients with relapsed multiple myeloma (TABLE 2)123. Moreover, a recent phase I clinical trial reported partial responses in 54% of patients with relapsed or refractory CLL124. Interestingly, dinaciclib synergized with the AKT inhibitor MK-2206 and caused strong tumour growth inhibition in xenografts of pancreatic cancer125, a treatment strategy currently investigated in a phase I clinical trial126. Finally, dinaciclib treatment may be efficacious in MYC-overexpressing triple-negative breast cancers and MYC-driven B-cell lymphomas since it caused tumour regression and enhanced survival in preclinical mouse models68, 127. Currently, an ongoing phase I study investigates the utility of dinaciclib in treating patients with MYC-overexpressing solid cancers128.
Development and clinical success of CDK4/CDK6-selective inhibitors
Following promising results from genetic and preclinical studies, the first group of CDK-selective compounds that entered the clinics were CDK4/6 inhibitors palbociclib, ribociclib and abemaciclib129.
Palbociclib was originally developed by David Fry and Peter Toogood in 2001, although it took many years until its potential therapeutic value became appreciated, and phase II clinical trials eventually started in 2009130. Palbociclib potently inhibits both CDK4 and CDK6 kinase activity whereas other kinases are barely affected (TABLE 1)131. As expected, palbociclib prevents RB phosphorylation by CDK4/6 and causes cell cycle arrest in G1 phase131. Consistent with the notion that RB represents the major rate-limiting target of CDK4/6 during cell cycle progression, cells that lost RB did not respond to palbociclib132. Analyses of human cancer xenografts demonstrated a strong anti-tumour activity against glioblastomas131, colorectal cancers131, rhabdomyosarcomas133, multiple myelomas134, AML135, ALL136 and dermatofibrosarcomas137. Noteworthy, systemically administered palbociclib crossed the blood-brain barrier and blocked tumour progression in an orthotopic xenograft model of glioblastoma138. In search for cancer types particularly sensitive to palbociclib, Finn and colleagues demonstrated that luminal-type breast cancer cells expressing oestrogen receptor (called ER+), including luminal-type cells with amplification of the HER2 (ERBB2) receptor (referred to as HER2+), were significantly more sensitive to palbociclib than ER-negative (ER-) breast cancer cells with basal-like histology139. Moreover, palbociclib sensitivity was increased upon loss of p16INK4A, p15INK4B or E2F1, low cyclin E1 expression or high androgen receptor levels; on the other hand, loss of RB abolished and amplification of the CCNE1 gene or overexpression of E2F2 decreased the sensitivity132, 140–142. The effect of CDK4 amplification on CDK4/6 inhibitor sensitivity remains controversial; although it enhanced sensitivity in liposarcoma143, it caused resistance in glioblastoma144 and rhabdomyosarcoma145. Importantly, palbociclib also induced senescence in glioblastoma 138, melanoma1, breast cancer146 and liposarcoma cells147. This is of clinical relevance since senescence induction could trigger an immune response leading to tumour clearance in patients148. However, it is not clear what determines whether palbociclib induces transient quiescence or permanent senescence. Recently, it was suggested that in _CDK4_-amplified liposarcoma, proteolytic degradation of the ubiquitin ligase MDM2 upon palbociclib treatment is required for senescence induction in vitro and represents a predictor of good clinical response in patients147. It remains to be seen how generally applicable this is, whether MDM2 degradation causes accumulation of a senescence mediator, and whether any of the involved factors can be exploited as biomarkers of clinical outcome.
The first promising clinical study of palbociclib focussed on mantle cell lymphoma (TABLE 2), a tumour type often harbouring a CCND1-IGH translocation that juxtaposes the CCND1 gene to the immunoglobulin heavy chain enhancer, thereby driving cyclin D1 overexpression149. The study demonstrated complete or partial responses in 18% and stable disease in 41% of patients. Further studies with preliminary signs of efficacy included patients with _CDK4_-amplified liposarcoma150, germ cell tumours151 and non-small-cell lung carcinoma (NSCLC)152. In all these studies, adverse effects of palbociclib included neutropaenia (reduced number of neutrophil granulocytes) and thrombocytopaenia. The former likely represents an on-target effect, as genetic ablation of Ccnd3, the major D-type cyclin in haematopoietic cells, resulted in severe neutropaenia in mice153.
The randomized phase II clinical trial PALOMA-1 compared treatment with palbociclib and letrozole (a standard-of-care inhibitor of aromatase, an enzyme responsible for a key step in oestrogen biosynthesis) versus treatment with letrozole alone for postmenopausal women with previously untreated ER+ HER2 non-amplified (HER2-) advanced breast cancer. Addition of palbociclib strongly increased the median progression-free survival (PFS) from 10.2 months to 20.2 months154. The median overall survival also showed an improvement in the combination treatment, although a larger study needs to evaluate whether this is statistically significant. Based on these results, palbociclib received accelerated (i.e. provisional) approval by the U.S. Food and Drug Administration (FDA) in February 2015155. A phase III study (PALOMA-2) was initiated to validate the clinical benefit of this treatment (see Supplementary Information S1 (table)). Furthermore, a randomized, double-blind, placebo-controlled phase III trial PALOMA-3 compared treatment with palbociclib and fulvestrant (an ER antagonist) to treatment with placebo and fulvestrant for women with ER+ HER2- metastatic breast cancer that have relapsed or progressed during prior hormone therapy, including a substantial portion of patients (33%) with prior chemotherapy for metastatic disease. The interim analysis of this study demonstrated a significantly improved median PFS (9.5 months versus 4.6 months, respectively)156, 157. Although an analysis of overall survival is not yet possible, this second-line treatment for metastatic breast cancer received approval by the FDA in February 2016158. Palbociclib is currently studied in over 50 clinical trials involving a wide variety of cancer types (TABLE 1). Improvement of the clinical outcome will depend on identification of predictive biomarkers. So far, only ER (in breast cancer) and RB expression have shown some value to predict positive outcome and are used in clinical trials, whereas CCND1 amplification154, 159, CDKN2A loss154, 159, PIK3CA mutation157 and RB localization159 were not informative.
Ribociclib also selectively inhibits CDK4 and CDK6 with high potency (TABLE 1)160. Similar to palbociclib, it blocks RB phosphorylation and causes cell cycle arrest of RB-positive tumour cells161. Furthermore, it showed anti-tumour activity in neuroblastoma (including senescence induction)161, liposarcoma143, rhabdomyosarcoma145 and Ewing sarcoma xenografts162. The first clinical phase I trial involving various advanced RB-positive cancers reported partial responses in a patient with _CCND1_-amplified, _PIK3CA_-mutated breast cancer and a patient with _CCND1_-amplified melanoma; the major dose-limiting toxicities were neutropaenia and thrombocytopaenia163. Ribociclib was then studied in combination with hormonal therapy for postmenopausal women with ER+ HER2- advanced breast cancer and exhibited preliminary signs of clinical activity (TABLE 2)164. These phase Ib results await validation in a large phase III study (MONALEESA-2) (see Supplementary Information S1 (table)). Ribociclib is currently investigated in over 30 clinical trials involving several tumour types (TABLE 1).
Abemaciclib inhibits not only CDK4 and CDK6 but also a number of other kinases with lower potency, including CDK9 and PIM1 (TABLE 1)165. Similar to palbociclib and ribociclib, it inhibits RB phosphorylation and causes cell cycle arrest in the G1 phase. It demonstrated anti-tumour activity in xenograft models of colorectal cancer165, AML165 and melanoma166. Furthermore, systemically administered abemaciclib crossed the blood-brain barrier more efficiently than palbociclib and blocked tumour progression in an orthotopic glioblastoma xenograft model167. The first phase I trial for patients with various advanced cancer types reported responses in three patients (ovarian cancer, _KRAS_-mutant NSCLC and _CDKN2A_-negative, _NRAS_-mutant melanoma)168. The major adverse effects were fatigue, neutropaenia and diarrhoea. Further studies evaluated abemaciclib as monotherapy for patients with advanced NSCLC that have relapsed or progressed during previous treatment (TABLE 2). Partial responses were observed in only 2% of patients but an additional 49% achieved stable disease169. Whether this improves progression-free and overall survival is currently under investigation in a large phase III trial (JUNIPER, see Supplementary Information S1 (table)). In another study, 23% of metastatic breast cancer patients showed partial responses to abemaciclib monotherapy170, leading to “breakthrough therapy” designation for abemaciclib by the FDA in October 2015171. As expected from previous preclinical studies, all responses occurred in patients with ER+ breast cancers whereas no tumour regression was observed in patients with HER2+ or triple-negative breast cancers. Moreover, combination of abemaciclib and aromatase inhibitors (letrozole or anastrozole) demonstrated partial responses in 6% and stable disease in 61% of patients with ER+ HER2- metastatic breast cancers172. The clinical benefit of this combination treatment is currently being validated in a large phase III trial (MONARCH 3).
Several studies investigated the value of combining CDK4/6 inhibitors with additional compounds. A phase I study investigated the utility of combining palbociclib with paclitaxel, a microtubule stabilizer, and showed partial responses in 40% of patients with RB-positive metastatic breast cancers173. The combined inhibition of MEK and CDK4 had a synergistic effect and led to tumour regression in several preclinical mouse models of _NRAS_-mutant melanoma 174. The potential value of this combination was then evaluated in a small clinical phase Ib/II trial175. Indeed, combination of ribociclib and the MEK inhibitor binimetinib resulted in partial responses in 43% of patients with _NRAS_-mutant melanoma175. Furthermore, resistance of _PIK3CA_-mutant breast cancer to PI3Kα inhibition was attributed to increased CDK4/6 activity176. Indeed, combination of PI3Kα and CDK4/6 inhibition caused synergistic tumour regression in several _PIK3CA_-mutant breast cancer xenograft models176. The efficacy of combining ribociclib with a PI3Kα inhibitor (BYL719) and an aromatase inhibitor (letrozole) is currently being investigated in a phase Ib study for advanced ER+ breast cancer164. Finally, following an increase in efficacy revealed in preclinical studies, combination of ribociclib with an mTOR inhibitor (everolimus) and an aromatase inhibitor (exemestane) is also currently under clinical investigation in ER+ breast cancer177.
Paradoxically, combination of palbociclib with conventional chemotherapeutics decreased their anti-tumour activity178, 179. These results were observed only in RB+ tumours and can be explained by the fact that palbociclib induces G1 arrest of cancer cells, thereby protecting them from the cytotoxic action of chemotherapeutics. These results caution against combining CDK4/CDK6 inhibitors with chemotherapy for RB+ tumours. Importantly, administration of CDK4/CDK6 inhibitors was shown to protect normal bone marrow cells from the effects of cytotoxic drugs or radiation, by reducing the proliferation of haematopoietic progenitor cells178, 180. Hence, this “chemo-protective” effect of CDK4/CDK6 inhibition might be valuable in reducing haematological toxicities of chemotherapy or radiotherapy in patients bearing CDK4/CDK6-independent (e.g. RB-negative) tumours and is currently investigated in a clinical trial181.
It is expected that tumour cells will eventually develop resistance to CDK4/CDK6 inhibition. While the molecular basis is currently unknown, possible mechanisms include the loss RB, overexpression of cyclin D1, CDK4 or E2F, hyperactivation of cyclin E-CDK2 kinase via cyclin E overexpression or the loss of CDK inhibitors p21CIP1 or p27KIP2, as well as overexpression of certain ABC transporters.
Targeting of other cell cycle proteins
Inhibitors of CHK1 and WEE1
During the last decade, a number of CHK1 and WEE1 inhibitors have been developed and tested in preclinical and clinical studies. Currently, three of them seem promising: the CHK1 inhibitors MK-8776 and LY2606368 and the WEE1 inhibitor AZD1775 (TABLE 1).
MK-8776 exhibits high potency and selectivity for CHK1182. Treatment of cancer cells with MK-8776 caused accumulation of DNA double-strand breaks leading to apoptotic cell death in vitro. Furthermore, it synergized with gemcitabine, hydroxyurea and cytarabine in causing apoptosis of AML and breast cancer cells in vitro, as well as with gemcitabine in ovarian and pancreatic cancer xenografts182–184. Based on these studies, the first clinical phase I trial with MK-8776 in combination with gemcitabine was initiated for patients with advanced solid tumours. The trial showed preliminary activity and little toxicity185. Another phase I clinical trial investigated the sequential administration of cytarabine and MK-8776 in patients with relapsed or refractory acute leukaemias. This combination achieved complete remission in 33% of patients (TABLE 3)186 and is currently being evaluated in a phase II trial for patients with relapsed AML187.
Table 3.
Clinical trial results of selected inhibitors of other cell cycle proteins
| Tumour type | Study characteristics, ClinicalTrials.gov Identifier | Drug dosage and combination | Efficacy | Major grade 3/4 adverse effects (≥10%)? |
|---|---|---|---|---|
| MK-8776 (SCH 900776) | ||||
| Acute leukaemias (relapsed or refractory)186 | Phase I N=24 (AML: N=21) NCT00907517 | 10–80 mg/m2 IV OD (days 2, 3, 11 and 12), RP2D: 56 mg/m2 Combination with cytarabine (2 g/m2 IV over 72 hours, days 1–3 and 10–12) | CR/CRi: 33% (8/24) | Hepatic dysfunction (17%) |
| LY2606368 (prexasertib) | ||||
| Anal squamous cell carcinoma (metastatic)189 | Phase I N=26 (subgroup expansion) NCT01115790 | 105 mg/m2 IV every 14 days Monotherapy | CR: 4% (1/26) PR: 12% (3/26) SD: 42% (11/26) | Neutropaenia (grade 4: 77%) |
| AZD1775 (MK-1775) | ||||
| Ovarian cancer (refractory or resistant, p53 mutant)203 | Phase II N=24 NCT01164995 | 225 mg PO BD (for 2.5 days in a 21-day cycle) Combination with carboplatin (IV, day 1) | PR: 27% (6/22) SD: 41% (9/22) | NA |
| Ovarian cancer (platinum-sensitive, p53-mutant)204 | Phase II N=121 AZD1775 + “P/C” (N=59) vs placebo + “P/C” (N=62) NCT01357161 | 225 mg PO BD (for 2.5 days in a 21-day cycle) Combination with “P/C”: paclitaxel (175 mg/m2 IV, day 1) and carboplatin (IV, day 1) | Median PFS: 42 weeks vs 35 weeks (HR=0.55, p=0.03) RR: 81% vs 76% (p=0.459) | Various (overall: 78% vs 65%) |
| Rigosertib (ON 01910.Na) | ||||
| MDS (primary HMA failure)209 | Phase III N=169 (subgroup) Rigosertib (N=117) vs best supportive care (N=52) NCT01241500 (ONTIME) | 1800 mg IV OD (for 3 days; every 2 weeks for 16 weeks, then every 4 weeks) | Median OS: 8.6 months vs 4.5 months (HR=0.63, p=0.011) | Anaemia (16% vs 10%), thrombocytopaenia (15% vs 6%), neutropaenia (15% vs 8%), febrile neutropaenia (13% vs 10%), pneumonia (12% vs 12%) |
| MDS (very high risk)210 | Phase III N=134 (subgroup) Rigosertib (N=93) vs best supportive care (N=41) NCT01241500 (ONTIME) | 1800 mg IV OD (for 3 days; every 2 weeks for 16 weeks, then every 4 weeks) | Median OS: 7.6 months vs 3.2 months (HR=0.56, p=0.005) | Anaemia (24% vs 11%), thrombocytopaenia (21% vs 11%), febrile neutropaenia (17% vs 11%), neutropaenia (15% vs 13%), pneumonia (12% vs 13%) |
| Pancreatic cancer (metastatic, first-line treatment)308 | Phase II/III N=160 (subgroup) Rigosertib + gemcitabine (N=106) vs gemcitabine alone (N=54) NCT01360853 (ONTRAC) | 1800 mg/m2 IV twice per week (3 weeks on, 1 week off) Combination with gemcitabine (1000 mg/m2 weekly, 3 weeks on, 1 week off) | Median PFS: 3.4 months vs 3.4 months (HR=0.96) Median OS: 6.1 months vs 6.4 months (HR=1.24) PR: 19% vs 13% SD: 50% vs 56% | Hyponatremia (17% vs 4%) |
| Volasertib (BI 6727) | ||||
| AML (patients ineligible for intensive treatment)217 | Phase II N=87 Volasertib + cytarabine (N=42) versus cytarabine alone (N=45) NCT00804856 | 350 mg IV OD (on days 1 and 15 of 28-day cycle) Combination with cytarabine (20 mg s.c. BD, days 1–10) | Median EFS: 5.6 months vs 2.3 months (HR=0.57, p=0.021) Median OS: 8.0 months vs 5.2 months (HR=0.63, p=0.047) CR/CRi: 31% vs 13% | Febrile neutropaenia (55% vs 16%), infections (48% vs 22%), gastrointestinal (24% vs 7%) |
| Alisertib (MLN8237) | ||||
| Peripheral T-cell lymphoma and transformed | Phase II N=37 NCT01466881 | 50 mg PO BD (1 week on, 2 weeks off) Monotherapy | Peripheral T-cell lymphoma: CR: 7% (2/30) PR: 23% (9/30) SD: 17% (5/30) Transformed Mycosis Fungoides: RR: 0% (0/7) SD: 28% (0/7) | Neutropaenia (32%), anaemia (30%), thrombocytopaenia (24%), lymphopaenia (22%), febrile neutropaenia (14%) |
| B-cell and T-cell non-Hodgkin lymphoma (relapsed or refractory)243 | Phase II N=48 NCT00807495 | 50 mg PO BD (1 week on, 2 weeks off) Monotherapy | CR: 10% (5/48) PR: 17% (8/48) SD: 33% (16/48) | Neutropaenia (63%), anaemia (35%), thrombocytopaenia (33%), stomatitis (15%), febrile neutropaenia (13%) |
| Ovarian, fallopian tube, primary peritoneal and breast cancer (recurrent)239, 309 | Phase I N=28 (ovarian: N=20) NCT01091428 | 10–50 mg PO BD (days 1–3, 8–10, 15–17 in 28-day cycle), RP2D: 40 mg Combination with paclitaxel (60–80 mg/m2 IV BD, days 1+8+15, RP2D: 60 mg/m2) | PR: 29% (8/28) SD: 11% (3/28) | Neutropaenia (54%) |
| Breast, small-cell lung, non-small-cell lung, head and neck squamous cell, gastro-oesophageal cancer (advanced, relapsed or refractory)240 | Phase II N=249 NCT01045421 | 50 mg PO BD (1 week on, 2 weeks off) Monotherapy | Breast cancer: PR: 18% (9/49) SD: 51% (25/49) Small-cell lung cancer: PR: 21% (10/48) SD: 33% (16/48) | Breast cancer:Neutropaenia (57%), stomatitis (15%), fatigue (11%)Small-cell lung cancer:Neutropaenia (37%), anaemia (17%), thrombocytopaenia (10%) |
| Solid tumours (advanced) including prostate cancer (castration-resistant)241 | Phase I N=35 NCT01094288 | 10–50 mg PO BD (1 week on, 2 weeks off); RP2D: 20 mg Combination with docetaxel (60–75 mg/m2 IV OD, on day 1, RP2D: 75 mg/m2) | For castration-resistant prostate cancer: PR: 35% (6/17) SD: 35% (6/17) | Neutropaenia (86%), febrile neutropaenia (23%), stomatitis (14%) |
| Multiple myeloma242 | Phase Ib N=26 NCT01034553 | 20–50 mg PO BD (1 week on, 3 weeks off) Combination with bortezomib (1.5 mg/m2 IV weekly) | CR: 4% (1/26) PR: 23% (6/26) SD: 38% (10/26) Median PFS: 5.9 months Median OS: 23.6 months | Neutropaenia (38%), thrombocytopaenia (31%), lymphopaenia (19%), infection (15%), muscle weakness (12%) |
| Neuroblastoma (relapsed or refractory)310 | Phase I N=22 NCT01601535 | 45–80 mg/m2 PO OD (days 1–7 in 21-day cycle) Combination with irinotecan (50 mg/m2 IV OD, on days 1–5) and temozolimide (100 mg/m2 PO OD, on days 1–5) | CR: 23% (5/22) PR: 9% (2/22) SD: 50% (11/22) | NA |
| ENMD-2076 | ||||
| Ovarian cancer (recurrent, platinum-resistant)311 | Phase II N=64 NCT01104675 | 250–325 mg PO OD (continuous) Monotherapy | PR: 8% (5/64) SD: 50% (32/64) PFS at 6 months: 22% Median OS: ≈12 months | Hypertension (27%), fatigue (19%) |
| Soft tissue sarcoma (advanced)312 | Phase II N=10 NCT01719744 | 275 mg PO OD (continuous) Monotherapy | PR: 20% (2/10) SD ≥ 6 months: 10% (1/10) Median PFS: 1.8 months | Hypertension (20%), elevated transaminases (10%), leukopaenia (10%), diarrhoea (10%) |
LY2606368 is a recently developed inhibitor with higher selectivity for CHK1 than CHK2 (TABLE 1)188. As expected, it causes activation of CDC25A in cancer cells, leading to increased CDK2 activity. The inappropriate activation of the CDC25A-CDK2 axis promotes S phase progression with increased number of replication forks, resulting in DNA double-strand breaks at stalled replication forks (termed “replication catastrophe”), chromosome fragmentation and eventually mitotic cell death188. LY2606368 reduced tumour growth in a xenograft model of lung cancer188. The first clinical trial demonstrated anti-tumour activity in the subgroup of patients with metastatic squamous cell carcinoma of the anus with responses in 15% of patients (TABLE 3)189. This agent will be further investigated in several clinical studies that are currently recruiting participants.
AZD1775 specifically targets WEE1 and (less potently) YES kinase (TABLE 1)190. Inhibition of WEE1 blocks DNA-damage induced inhibitory phosphorylation of CDK1 and CDK2 at Tyr-15. This causes cells with damaged DNA to prematurely enter mitosis, triggering mitotic arrest and apoptosis190. AZD1775 synergized with a variety of chemotherapeutic compounds as well as radiation and was particularly active against tumour cells with a defective DNA damage checkpoint in G1 phase due to loss of p53 function, an example of synthetic lethality190–192. Treatment with AZD1775 (either alone or together with chemotherapeutics or gamma-radiation) achieved promising anti-tumour activity in xenograft models of pancreatic cancer, NSCLC, AML and glioma193–196. Moreover, WEE1 and PARP inhibition synergistically increased radiosensitivity in a xenograft model of pancreatic cancer197. AZD1775 also acted synergistically with inhibitors of histone deacetylases (HDACs) both in vitro and in xenograft models of AML and pancreatic cancer198, 199. Furthermore, combined WEE1 and mTOR inhibition achieved tumour regression in a mouse model of _KRAS_G12D-induced lung cancer as well as in _KRAS_A18D-mutant AML xenografts200. Finally, combined WEE1 and CHK1 inhibition induced DNA damage and apoptosis in the absence of chemotherapeutics and inhibited tumour growth in neuroblastoma xenografts201, 202. A phase II trial investigated AZD1775 in combination with carboplatin for treatment of p53-mutant ovarian cancer and showed partial responses in 27% of patients (TABLE 3)203. Subsequently, a randomized phase II trial compared the combination of AZD1775 with chemotherapeutics (carboplatin and paclitaxel) versus chemotherapeutics alone for patients with recurrent, platinum-sensitive, p53-mutant ovarian cancer, and reported an improved median PFS of 43 weeks versus 35 weeks204. Currently, AZD1775 is being studied in over 20 clinical trials involving a variety of cancer types, including combinations with chemotherapeutics, HDAC and PARP inhibitors.
Inhibitors of Polo-like kinases
Development of Polo-like kinase inhibitors has mainly focussed on PLK1. Currently, two promising PLK1 inhibitors, rigosertib and volasertib, are under clinical investigation (TABLE 1).
Rigosertib is a multi-kinase inhibitor with highest affinity for PLK1205. Rigosertib caused tumour regression in a xenograft model of head and neck squamous cell carcinoma206 and (in combination with gamma-radiation) in xenografts of cervical cancer207. Clinical trials mainly focussed on pancreatic cancer and myelodysplastic syndromes (MDS). Whereas treatment with rigosertib and gemcitabine did not improve survival of pancreatic cancer patients (TABLE 3), the outcomes for MDS patients were more promising. In patients with higher-risk MDS, an analysis of four clinical phase I/II trials reported bone marrow responses in 40% and cytogenetic responses in 6% of patients208. Rigosertib was then compared to best supportive care for patients with higher-risk MDS, who have relapsed after, failed to respond to, or progressed during treatment with hypomethylating agents (HMAs), in a randomized phase III trial (ONTIME). Prolonged median overall survival was observed for patients with primary HMA failure (8.6 months versus 4.5 months)209, as well as for patients with very poor prognosis (7.6 months versus 3.2 months)210. The clinical benefit of this treatment is currently being validated in another phase III trial (INSPIRE, see Supplementary Information S1 (table)).
Volasertib is a highly selective Polo-like kinase family inhibitor with highest potency against PLK1 (TABLE 1)211. Similar to rigosertib, it causes cell arrest and apoptosis. In contrast to rigosertib, volasertib induces oncogenic AKT and ERK signalling and synergizes with AKT or mTOR inhibition in vitro212. Volasertib demonstrated impressive anti-tumour activity in xenograft models of neuroblastoma (as monotherapy)213, ALL (as monotherapy and in combination with cytarabine or quizartinib)213, 214, breast cancer (in combination with fulvestrant)215 and rhabdomyosarcoma (with vincristine)216. A clinical phase II trial compared volasertib in combination with low-dose cytarabine versus cytarabine alone for older patients with AML (TABLE 3). This combination achieved an improved complete response rate (31% versus 13%), improved median event-free survival (5.6 months versus 2.3 months) and improved median overall survival (8.0 months versus 5.2 months)217. The clinical benefit is currently being validated in a phase III trial (POLO-AML-2, see Supplementary Information S1 (table)). In contrast, several phase II clinical trials for patients with solid tumours showed disappointing results with lack of sufficient clinical activity218–220. Future clinical application will depend on identification of biomarkers that can predict clinical response. Based on in vitro data it was suggested that p53-negative cancers would be particularly sensitive to PLK1 inhibition221. Importantly, overexpression of the ABC transporter ABCB1 (P-glycoprotein) conferred resistance to volasertib treatment in vitro, supporting future co-administration of ABCB1 inhibitors to improve clinical responses222.
Inhibitors of Aurora kinases
A number of inhibitors targeting the major family members Aurora A or Aurora B, such as alisertib, ENMD-2076, danusertib and AMG-900, have been developed and are under clinical investigation (TABLE 1). In contrast, a selective Aurora B inhibitor, barasertib (AZD1152), was discontinued after a number of clinical phase II trials showed no substantial clinical benefit.
Alisertib exhibits high selectivity for Aurora A223. Treatment of cancer cells with alisertib was shown to induce mitotic arrest and polyploidy, and resulted in senescence or apoptosis224, 225. Monotherapy with alisertib showed tumour regression in preclinical mouse models of neuroblastoma226, ALL226 and lymphoma223. Interestingly, Aurora A inhibition using alisertib triggered degradation of N-MYC and hence caused tumour regression in a MYCN-driven mouse model of neuroblastoma227. Furthermore, combination of alisertib with chemotherapeutics induced tumour regression in mouse models of AML228, oesophageal229 and gastric cancer230. Alisertib also synergized with inhibitors of BCR-ABL (in CML)231, CD20 (in mantle cell lymphoma232 and diffuse large B-cell lymphoma233), MEK (in colorectal cancer)234 and BCL2 (in neuroblastoma)235, as well as with an agonist of the cell death receptor DR5 (in melanoma)236. The first clinical phase I trial involving alisertib monotherapy started in 2007 and reported a partial response in a patient with refractory ovarian cancer237. Although alisertib monotherapy did not achieve sufficient clinical activity in a subsequent phase II trial for patients with platinum-resistant/refractory ovarian cancer238, an ongoing phase I/II study reported an encouraging response rate of 29% in combination with paclitaxel for patients with recurrent ovarian cancer (TABLE 3)239. Furthermore, another phase II study for solid tumours showed promising activity for patients with small cell lung carcinoma (response rate 21%) and breast cancer (response rate 18%)240. Combination with docetaxel demonstrated good preliminary activity in castration-resistant prostate cancer (response rate approx. 50%)241 and in combination with bortezomib (a proteasome inhibitor) in multiple myeloma (response rate 27%)242. Moreover, two clinical phase II studies reported promising anti-tumour activity in relapsed/refractory B-cell and T-cell non-Hodgkin lymphoma (response rate 27%)243 and in relapsed/refractory peripheral T-cell lymphoma (response rate 30%)244. The potential clinical benefit for patients with peripheral T-cell lymphoma is currently being investigated in a phase III trial (see Supplementary Information S1 (table)). Alisertib is currently studied in over 30 clinical trials involving a wide variety of cancers (TABLE 1).
Conclusions
While basic cell cycle regulators were discovered over 30 years ago, the last decade saw a dramatic increase in our understanding of their role in cancer and their potential as targets for cancer therapy. The development of novel compounds using structure-based drug design and efficient high-throughput screening platforms allowed bringing cell cycle studies from bench to bedside. Indeed, the provisional approval of a CDK4/CDK6-selective inhibitor (palbociclib) for breast cancer treatment by the FDA represents the first successful clinical translation in this field. Other CDK4/CDK6-selective inhibitors demonstrated very encouraging results and their approval is expected within the next years. Inhibitors that selectively inhibit CDK4 but not CDK6 (and vice versa) may also be developed, and they could possibly reduce the adverse effects without compromising the therapeutic benefit. Furthermore, CDK2- and CDK1-selective inhibitors will likely be developed, as they may have clinical utility for specific cancer subtypes55, 66–68
The success of future cell cycle-targeted therapies will depend on development of selective and potent compounds and on identification of specific vulnerabilities of cancer cells. Cell culture-based screening approaches, patient-derived xenografts and genetically engineered mouse cancer models will likely remain essential to uncover synthetic lethal interactions between genomic lesions and selective inhibition of individual cell cycle proteins. Novel treatment modalities capable of targeting multiple components of the same pathway, such as microRNAs may also provide therapeutic benefits. Indeed, MRX34, a miR-34 mimic targeting multiple cell cycle gene transcripts, recently entered clinical phase I evaluation245. Moreover, combination of several selective inhibitors may substantially improve clinical activity; however, in several cases combination therapies have shown a significant increase of adverse effects and may thus not be tolerated by many patients. Furthermore, current targeted therapies suffer from the relatively low percentage of patients showing satisfactory, long-term response. Hence, genomic technologies will become an invaluable diagnostic tool to identify predictive biomarkers for responsive patient subpopulations. Finally, since many preclinical studies as well as clinical experience indicate the occurrence of resistance (i.e. relapse after an initial response), research on resistance mechanisms against current compounds will help to identify treatment options for relapsed/refractory patients, or suggest combinatorial therapies that might prevent acquisition of resistance.
Supplementary Material
Supplementary Information
ONLINE SUMMARY.
- Many cell cycle proteins are overexpressed or overactive in human cancers, in particular D-type and E-type cyclins, cyclin-dependent kinases (CDK4, CDK6 and CDK2), Polo-like kinase 1 (PLK1) and Aurora kinases (Aurora A and B). In transgenic mice, overexpression of several of these cell cycle proteins induces or contributes to tumorigenesis, revealing their prominent oncogenic roles.
- Some of these cell cycle proteins are also required for tumorigenesis and their ablation in mice impairs tumour formation induced by specific genetic lesions or by carcinogen treatment, as demonstrated for several cyclins (D1, D2, D3 and A2) and CDKs (CDK4, CDK6, CDK2 and CDK1), as well as for checkpoint kinase 1 (CHK1). Importantly, in some cases the continued presence of a cell cycle protein has been shown to be also required for tumour maintenance and progression, e.g. for cyclin D1, D3 and CDK4, thereby providing a clear rationale for targeting these proteins in cancer treatment.
- Kinases involved in cell cycle checkpoint function such as CHK1 and WEE1 also constitute potential therapeutic targets. Their inhibition compromises checkpoint function, causes excessive DNA damage and eventually leads to apoptosis, particularly in cells with compromised p53 function.
- CDK4/6-selective inhibitors, such as palbociclib, ribociclib and abemaciclib, have shown significant benefits in clinical studies, particularly in breast cancer, but also in non-small cell lung cancer, melanoma and head and neck squamous cell carcinoma. Importantly, following demonstration of a substantial improvement in progression-free survival, combination of palbociclib and letrozole received accelerated approval for first-line treatment of patients with advanced ER+ HER2- breast cancer.
- Inhibitors of PLK1, such as rigosertib and volasertib, have also shown encouraging results in clinical phase II/III studies for patients with myelodysplastic syndromes and acute myelogenous leukaemia, respectively, and several phase III trials are currently ongoing.
- Compounds targeting Aurora A, particularly alisertib, have been extensively studied in preclinical models and demonstrated synergy with many other targeted therapies, leading to tumour regression in a variety of cancer models. Moreover, clinical studies revealed encouraging activity of alisertib in peripheral T-cell lymphoma, non-Hodgkin lymphoma, non-small cell lung cancer and breast cancer.
GLOSSARY
Clinical breast cancer subgroups
- ER+ or HR+ (hormone receptor-positive) breast cancer: cancer with expression of ER (oestrogen receptor) and/or PR (progesterone receptor) and with normal HER2 (human epidermal growth factor receptor 2, ERBB2) expression;
- HER2+ breast cancer: with HER2 amplification or overexpression;
- triple-negative breast cancer (TNBC): with low/absent expression of ER/PR without HER2 overexpression.
Cell cycle checkpoints
A number of surveillance pathways that monitor occurrence of DNA damage (DNA damage checkpoints), as well as proper assembly of the mitotic spindle (spindle assembly checkpoint), and are capable of transiently arresting the cell cycle to allow time for repair or proper assembly.
Clinical responses
Complete response (CR, complete disappearance of all tumours in a given patient), partial response (PR, tumour shrinkage by ≥30%), stable disease (SD, <30% tumour shrinkage or <20% tumour growth), progressive disease (PD, ≥20% growth).
Clinical trial phases
New agents with promising preclinical results (animal models) are first tested for safety (adverse effects), optimal dosage and preliminary signs of efficacy (phase I), then for their efficacy using the optimal dosage in a defined, small group of patients (phase II), and finally in large, randomized, double-blind study in comparison to a placebo or the current “gold standard” of treatment (phase III).
Mitotic catastrophe
A particular form of apoptosis occurring during mitosis as a result of aberrant chromosome segregation or DNA damage, typically related to inactivation of cell cycle checkpoints.
Pan-CDK inhibitor
An inhibitor of cyclin-dependent kinases (CDKs) with a broad specificity (i.e. not selective for individual CDKs).
Replication catastrophe
A form of DNA damage involving DNA double-strand breaks and chromosome fragmentation. Replication catastrophe occurs during S phase as a result of unscheduled firing of DNA replication origins that causes breakage of stalled replication forks. It is typically related to an impaired ATR- and CHK1-dependent DNA damage checkpoint.
Therapeutic index
The ratio between the drug dose causing the desired pharmacological effect and the dose causing toxicity (e.g. toxicity or lethality in 50% of patients or animals, respectively).
Xenograft
Transplantation of human cancer cells (either cancer cell lines or patient-derived primary cancer specimens) into immunocompromised mice – either under the skin (subcutaneous) or into the location of the original tumour (orthotopic).
Biographies
Tobias Otto
Tobias Otto received his Ph.D. degree from the University of Marburg in Marburg, Germany. During his graduate studies in the laboratory of Dr. Martin Eilers, he studied the role of the MYC family of transcription factors in neuroblastoma and T-cell lymphoma. He then joined the laboratory of Dr. Piotr Sicinski in Dana-Farber Cancer Institute as a postdoctoral research fellow. He investigates the role of cell cycle-targeting microRNAs during development and cancer formation using genetically engineered mouse models.
Piotr Sicinski
Piotr (Peter) Sicinski received his M.D. and Ph.D. degrees from the Warsaw Medical School in Warsaw, Poland. He was a visiting scientist at the Medical Research Council in Cambridge, UK, before joining the laboratory of Dr. Robert A. Weinberg at the Whitehead Institute for his postdoctoral training. Peter joined the faculty of the Harvard Medical School in 1997, where he is now a Professor of Genetics. His laboratory, located in the Dana-Farber Cancer Institute, studies the roles of cell cycle proteins in physiology and in cancer.
References
- 1.Anders L, et al. A Systematic Screen for CDK4/6 Substrates Links FOXM1 Phosphorylation to Senescence Suppression in Cancer Cells. Cancer Cell. 2011;20:620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 3.Kollmann K, et al. A new kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013;24 doi: 10.1016/j.ccr.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 6.Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat Rev Mol Cell Biol. 2016;17:280–92. doi: 10.1038/nrm.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt EE, Ichimura K, Reifenberger G, Collins VP. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994;54:6321–6324. [PubMed] [Google Scholar]
- 9.Wölfel T, et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 10.Corcoran MM, et al. Dysregulation of cyclin dependent kinase 6 expression in splenic marginal zone lymphoma through chromosome 7q translocations. Oncogene. 1999;18:6271–7. doi: 10.1038/sj.onc.1203033. [DOI] [PubMed] [Google Scholar]
- 11.Sotillo R, et al. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 2001;20:6637–47. doi: 10.1093/emboj/20.23.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotillo R, et al. Invasive melanoma in Cdk4-targeted mice. Proc Natl Acad Sci US A. 2001;98:13312–13317. doi: 10.1073/pnas.241338598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TC, et al. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–71. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 14.Rojas P, et al. Cyclin D2 and cyclin D3 play opposite roles in mouse skin carcinogenesis. Oncogene. 2007;26:1723–30. doi: 10.1038/sj.onc.1209970. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Sistrunk C, Rodriguez-Puebla ML. Unexpected reduction of skin tumorigenesis on expression of cyclin-dependent kinase 6 in mouse epidermis. Am J Pathol. 2011;178:345–54. doi: 10.1016/j.ajpath.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto H, et al. Enhanced skin carcinogenesis in cyclin D1-conditional transgenic mice: cyclin D1 alters keratinocyte response to calcium-induced terminal differentiation. Cancer Res. 2002;62:1641–7. [PubMed] [Google Scholar]
- 17.Miliani de Marval PL, Macias E, Conti CJ, Rodriguez-Puebla ML. Enhanced malignant tumorigenesis in Cdk4 transgenic mice. Oncogene. 2004;23:1863–73. doi: 10.1038/sj.onc.1207309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–21. doi: 10.1038/35082500. The first study demonstrating the oncogene-dependent requirement for cyclin D1 in mammary tumorigenesis in vivo using mouse cancer models. [DOI] [PubMed] [Google Scholar]
- 19.Bowe DB, Kenney NJ, Adereth Y, Maroulakou IG. Suppression of Neu-induced mammary tumor growth in cyclin D1 deficient mice is compensated for by cyclin E. Oncogene. 2002;21:291–8. doi: 10.1038/sj.onc.1205025. [DOI] [PubMed] [Google Scholar]
- 20.Yu Q, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. These studies demonstrated the requirement for CDK4 kinase activity in mammary tumorigenesis using mouse cancer models, indicating a potential value of inhibiting CDK4 in breast cancer therapy. [DOI] [PubMed] [Google Scholar]
- 21.Reddy HK, et al. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 2005;65:10174–8. doi: 10.1158/0008-5472.CAN-05-2639. These studies demonstrated the requirement for CDK4 kinase activity in mammary tumorigenesis using mouse cancer models, indicating a potential value of inhibiting CDK4 in breast cancer therapy. [DOI] [PubMed] [Google Scholar]
- 22.Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22. doi: 10.1016/j.ccr.2005.12.019. These studies demonstrated the requirement for CDK4 kinase activity in mammary tumorigenesis using mouse cancer models, indicating a potential value of inhibiting CDK4 in breast cancer therapy. [DOI] [PubMed] [Google Scholar]
- 23.Sicinska E, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 24.Hu MG, et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 2009;69:810–818. doi: 10.1158/0008-5472.CAN-08-2473. This work showed that mice lacking CDK6 are resistant to v-AKT-induced lymphoma formation and therefore suggested that CDK6-selective inhibitors may be used for treatment of lymphoid malignancies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puyol M, et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18:63–73. doi: 10.1016/j.ccr.2010.05.025. This seminal work provided evidence for the dependence of KRASG12V-induced mouse lung cancers on the activity of CDK4 not only for tumour initiation but also during tumour progression, using an acute genetic shutdown of CDK4 or inhibition of CDK4/6 kinase activity in tumour-bearing mice. [DOI] [PubMed] [Google Scholar]
- 26.Choi YJ, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. This study validated cyclins D1 and D3 and their associated kinases as suitable therapeutic targets in mammary cancer and T-cell acute lymphoblastic leukaemia, respectively, by using an acute and ubiquitous genetic ablation as well as CDK4/6-selective inhibitors in tumour-bearing mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawai CM, et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell. 2012;22:452–465. doi: 10.1016/j.ccr.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–91. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma T, et al. Cell cycle-regulated phosphorylation of p220(NPAT) by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 2000;14:2298–313. doi: 10.1101/gad.829500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okuda M, et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell. 2000;103:127–40. doi: 10.1016/s0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 31.Strohmaier H, et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413:316–322. doi: 10.1038/35095076. [DOI] [PubMed] [Google Scholar]
- 32.Koepp DM, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–177. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 33.Scaltriti M, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A. 2011;108:3761–6. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etemadmoghadam D, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci U S A. 2013;110:19489–94. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spruck CH, et al. hCDC4 gene mutations in endometrial cancer. Cancer Res. 2002;62:4535–9. [PubMed] [Google Scholar]
- 36.Kemp Z, et al. CDC4 mutations occur in a subset of colorectal cancers but are not predicted to cause loss of function and are not associated with chromosomal instability. Cancer Res. 2005;65:11361–6. doi: 10.1158/0008-5472.CAN-05-2565. [DOI] [PubMed] [Google Scholar]
- 37.Akli S, Van Pelt CS, Bui T, Meijer L, Keyomarsi K. Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res. 2011;71:3377–86. doi: 10.1158/0008-5472.CAN-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao Y, et al. Overexpression of cyclin A but not Skp 2 correlates with the tumor relapse of human hepatocellular carcinoma. Cancer Res. 1998;58:985–90. [PubMed] [Google Scholar]
- 39.Handa K, Yamakawa M, Takeda H, Kimura S, Takahashi T. Expression of cell cycle markers in colorectal carcinoma: superiority of cyclin A as an indicator of poor prognosis. Int J Cancer. 1999;84:225–33. doi: 10.1002/(sici)1097-0215(19990621)84:3<225::aid-ijc5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 40.Michalides R, et al. Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br J Cancer. 2002;86:402–8. doi: 10.1038/sj.bjc.6600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gstaiger M, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci U S A. 2001;98:5043–8. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galaktionov K, et al. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575–7. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 43.Takemasa I, et al. Overexpression of CDC25B phosphatase as a novel marker of poor prognosis of human colorectal carcinoma. Cancer Res. 2000;60:3043–50. [PubMed] [Google Scholar]
- 44.Broggini M, et al. Cell cycle-related phosphatases CDC25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res. 2000;20:4835–40. [PubMed] [Google Scholar]
- 45.Bortner DM, Rosenberg MP. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol. 1997;17:453–9. doi: 10.1128/mcb.17.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin-Caballero J, Flores JM, Garcia-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–8. [PubMed] [Google Scholar]
- 48.Yao Y, et al. Increased susceptibility to carcinogen-induced mammary tumors in MMTV-Cdc25B transgenic mice. Oncogene. 1999;18:5159–66. doi: 10.1038/sj.onc.1202908. [DOI] [PubMed] [Google Scholar]
- 49.Ray D, et al. Deregulated CDC25A expression promotes mammary tumorigenesis with genomic instability. Cancer Res. 2007;67:984–91. doi: 10.1158/0008-5472.CAN-06-3927. [DOI] [PubMed] [Google Scholar]
- 50.Ray D, et al. Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res. 2007;67:6605–11. doi: 10.1158/0008-5472.CAN-06-4815. [DOI] [PubMed] [Google Scholar]
- 51.Tetsu O, McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–45. doi: 10.1016/s1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 52.Padmakumar VC, Aleem E, Berthet C, Hilton MB, Kaldis P. Cdk2 and Cdk4 activities are dispensable for tumorigenesis caused by the loss of p53. Mol Cell Biol. 2009;29:2582–93. doi: 10.1128/MCB.00952-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macias E, Kim Y, Miliani de Marval PL, Klein-Szanto A, Rodriguez-Puebla ML. Cdk2 deficiency decreases ras/CDK4-dependent malignant progression, but not myc-induced tumorigenesis. Cancer Res. 2007;67:9713–20. doi: 10.1158/0008-5472.CAN-07-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gillam MP, et al. MEN1 tumorigenesis in the pituitary and pancreatic islet requires Cdk4 but not Cdk2. Oncogene. 2015;34:932–8. doi: 10.1038/onc.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campaner S, et al. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. NatCell Biol. 2010;12:54–59. doi: 10.1038/ncb2004. [DOI] [PubMed] [Google Scholar]
- 56.Hydbring P, et al. Phosphorylation by Cdk2 is required for Myc to repress Ras-induced senescence in cotransformation. Proc Natl Acad Sci U S A. 2010;107:58–63. doi: 10.1073/pnas.0900121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du J, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565–76. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Ray D, Terao Y, Christov K, Kaldis P, Kiyokawa H. Cdk2-null mice are resistant to ErbB-2-induced mammary tumorigenesis. Neoplasia. 2011;13:439–44. doi: 10.1593/neo.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santamaria D, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–5. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 60.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–43. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 62.Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–7. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 63.Beltran H, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016 doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nam HJ, van Deursen JM. Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol. 2014;16:538–49. doi: 10.1038/ncb2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diril MK, et al. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci U S A. 2012;109:3826–31. doi: 10.1073/pnas.1115201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costa-Cabral S, et al. CDK1 Is a Synthetic Lethal Target for KRAS Mutant Tumours. PLoS One. 2016;11:e0149099. doi: 10.1371/journal.pone.0149099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goga A, Yang D, Tward AD, Morgan DO, Bishop JM. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med. 2007;13:820–7. doi: 10.1038/nm1606. These two reports introduced the potential therapeutic value of CDK1-selective inhibitors for targeting MYC-overexpressing tumours such as triple-negative breast cancers. [DOI] [PubMed] [Google Scholar]
- 68.Horiuchi D, et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J Exp Med. 2012;209:679–96. doi: 10.1084/jem.20111512. These two reports introduced the potential therapeutic value of CDK1-selective inhibitors for targeting MYC-overexpressing tumours such as triple-negative breast cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsuoka S, et al. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97:10389–94. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El-Deiry WS, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez Y, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 73.Peng CY, et al. Mitotic and G2 checkpoint control: regulation of 14–3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–5. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 74.O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–54. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mu K, et al. Detection of CHK1 and CCND1 gene copy number changes in breast cancer with dual-colour fluorescence in-situ hybridization. Histopathology. 2011;58:601–7. doi: 10.1111/j.1365-2559.2011.03780.x. [DOI] [PubMed] [Google Scholar]
- 76.Menoyo A, et al. Somatic mutations in the DNA damage-response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res. 2001;61:7727–30. [PubMed] [Google Scholar]
- 77.Liu Q, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 78.Fishler T, et al. Genetic instability and mammary tumor formation in mice carrying mammary-specific disruption of Chk1 and p53. Oncogene. 2010;29:4007–17. doi: 10.1038/onc.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tho LM, Libertini S, Rampling R, Sansom O, Gillespie DA. Chk1 is essential for chemical carcinogen-induced mouse skin tumorigenesis. Oncogene. 2012;31:1366–75. doi: 10.1038/onc.2011.326. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, et al. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32:976–87. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 81.Verlinden L, et al. The E2F-regulated gene Chk1 is highly expressed in triple-negative estrogen receptor /progesterone receptor /HER-2 breast carcinomas. Cancer Res. 2007;67:6574–81. doi: 10.1158/0008-5472.CAN-06-3545. [DOI] [PubMed] [Google Scholar]
- 82.Xie Y, et al. Checkpoint kinase 1 is negatively regulated by miR-497 in hepatocellular carcinoma. Med Oncol. 2014;31:844. doi: 10.1007/s12032-014-0844-4. [DOI] [PubMed] [Google Scholar]
- 83.Lopez-Contreras AJ, Gutierrez-Martinez P, Specks J, Rodrigo-Perez S, Fernandez-Capetillo O. An extra allele of Chk1 limits oncogene-induced replicative stress and promotes transformation. J Exp Med. 2012;209:455–61. doi: 10.1084/jem.20112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Masaki T, et al. Cyclins and cyclin-dependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology. 2003;37:534–43. doi: 10.1053/jhep.2003.50112. [DOI] [PubMed] [Google Scholar]
- 85.Mir SE, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell. 2010;18:244–57. doi: 10.1016/j.ccr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magnussen GI, et al. High expression of Wee1 is associated with poor disease-free survival in malignant melanoma: potential for targeted therapy. PLoS One. 2012;7:e38254. doi: 10.1371/journal.pone.0038254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vassilopoulos A, et al. WEE1 murine deficiency induces hyper-activation of APC/C and results in genomic instability and carcinogenesis. Oncogene. 2015;34:3023–35. doi: 10.1038/onc.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Luca M, Lavia P, Guarguaglini G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle. 2006;5:296–303. doi: 10.4161/cc.5.3.2392. [DOI] [PubMed] [Google Scholar]
- 89.Roshak AK, et al. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000;12:405–11. doi: 10.1016/s0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 90.van Vugt MA, Bras A, Medema RH. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 91.Wolf G, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543–9. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- 92.Knecht R, et al. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794–7. [PubMed] [Google Scholar]
- 93.Kanaji S, et al. Expression of polo-like kinase 1 (PLK1) protein predicts the survival of patients with gastric carcinoma. Oncology. 2006;70:126–33. doi: 10.1159/000093003. [DOI] [PubMed] [Google Scholar]
- 94.Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat Cell Biol. 2000;2:852–4. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- 95.Lu LY, et al. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870–6. doi: 10.1128/MCB.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Macurek L, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 98.Otto T, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 99.Marumoto T, et al. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 2002;7:1173–1182. doi: 10.1046/j.1365-2443.2002.00592.x. [DOI] [PubMed] [Google Scholar]
- 100.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 101.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gonzalez-Loyola A, et al. Aurora B Overexpression Causes Aneuploidy and p21Cip1 Repression during Tumor Development. Mol Cell Biol. 2015;35:3566–78. doi: 10.1128/MCB.01286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mosquera JM, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15:1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Staff S, Isola J, Jumppanen M, Tanner M. Aurora-A gene is frequently amplified in basal-like breast cancer. Oncol Rep. 2010;23:307–12. [PubMed] [Google Scholar]
- 105.Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004;10:2065–2071. doi: 10.1158/1078-0432.ccr-1057-03. [DOI] [PubMed] [Google Scholar]
- 106.Heredia FF, et al. Proteins related to the spindle and checkpoint mitotic emphasize the different pathogenesis of hypoplastic MDS. Leuk Res. 2014;38:218–24. doi: 10.1016/j.leukres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, et al. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25:7148–7158. doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- 108.Lu LY, et al. Aurora a is essential for early embryonic development and tumor suppression. J Biol Chem. 2008;283:31785–31790. doi: 10.1074/jbc.M805880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fernandez-Miranda G, et al. Genetic disruption of aurora B uncovers an essential role for aurora C during early mammalian development. Development. 2011;138:2661–72. doi: 10.1242/dev.066381. [DOI] [PubMed] [Google Scholar]
- 110.Kaur G, et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86–8275. J Natl Cancer Inst. 1992;84:1736–40. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- 111.Arguello F, et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity In vivo against human leukemia and lymphoma xenografts. Blood. 1998;91:2482–90. [PubMed] [Google Scholar]
- 112.Chao SH, et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem. 2000;275:28345–8. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- 113.Lin TS, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–8. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lanasa MC, et al. Final results of EFC6663: a multicenter, international, phase 2 study of alvocidib for patients with fludarabine-refractory chronic lymphocytic leukemia. Leuk Res. 2015;39:495–500. doi: 10.1016/j.leukres.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parry D, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344–53. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 116.Feldmann G, et al. Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol Ther. 2011;12:598–609. doi: 10.4161/cbt.12.7.16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gorlick R, et al. Initial testing (stage 1) of the cyclin dependent kinase inhibitor SCH 727965 (dinaciclib) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;59:1266–74. doi: 10.1002/pbc.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Desai BM, et al. The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling. PLoS One. 2013;8:e59588. doi: 10.1371/journal.pone.0059588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lao CD, et al. SWOG S0826: A phase II trial of SCH 727965 (NSC 747135) in patients with stage IV melanoma. J Clin Oncol. 2012;30(suppl) abstr 8521. [Google Scholar]
- 120.Gojo I, et al. Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother Pharmacol. 2013;72:897–908. doi: 10.1007/s00280-013-2249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stephenson JJ, et al. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer. 2014;83:219–23. doi: 10.1016/j.lungcan.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 122.Mita MM, et al. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer. 2014;14:169–76. doi: 10.1016/j.clbc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 123.Kumar SK, et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood. 2015;125:443–8. doi: 10.1182/blood-2014-05-573741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Flynn J, et al. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia. 2015;29:1524–9. doi: 10.1038/leu.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu C, et al. Combined Inhibition of Cyclin-Dependent Kinases (Dinaciclib) and AKT (MK-2206) Blocks Pancreatic Tumor Growth and Metastases in Patient-Derived Xenograft Models. Mol Cancer Ther. 2015;14:1532–9. doi: 10.1158/1535-7163.MCT-15-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.US National Library of Medicine. ClinicalTrials.gov. 2013 https://www.clinicaltrials.gov/ct2/show/NCT01783171.
- 127.Gregory GP, et al. CDK9 inhibition by dinaciclib potently suppresses Mcl-1 to induce durable apoptotic responses in aggressive MYC-driven B-cell lymphoma in vivo. Leukemia. 2015;29:1437–41. doi: 10.1038/leu.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.US National Library of Medicine. ClinicalTrials.gov. 2012 https://www.clinicaltrials.gov/ct2/show/NCT01676753.
- 129.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2015 doi: 10.1158/2159-8290.CD-15-0894. A recent review specifically focussing on the clinical use of CDK4/6-selective inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garber K. The cancer drug that almost wasn’t. Science. 2014;345:865–7. doi: 10.1126/science.345.6199.865. [DOI] [PubMed] [Google Scholar]
- 131.Fry DW, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 132.Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29:4018–32. doi: 10.1038/onc.2010.154. These two studies revealed that the loss of RB causes complete resistance of tumour cells to the anti-proliferative effect of CDK4/6 inhibitors. This observation has important implications for patient selection in clinical studies. [DOI] [PubMed] [Google Scholar]
- 133.Saab R, et al. Pharmacologic inhibition of cyclin-dependent kinase 4/6 activity arrests proliferation in myoblasts and rhabdomyosarcoma-derived cells. Mol Cancer Ther. 2006;5:1299–308. doi: 10.1158/1535-7163.MCT-05-0383. [DOI] [PubMed] [Google Scholar]
- 134.Baughn LB, et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66:7661–7. doi: 10.1158/0008-5472.CAN-06-1098. [DOI] [PubMed] [Google Scholar]
- 135.Wang L, et al. Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood. 2007;110:2075–83. doi: 10.1182/blood-2007-02-071266. [DOI] [PubMed] [Google Scholar]
- 136.Nemoto A, et al. Specific Antileukemic Activity of PD0332991, a CDK4/6 Inhibitor, against Philadelphia Chromosome-Positive Lymphoid Leukemia. Mol Cancer Ther. 2016;15:94–105. doi: 10.1158/1535-7163.MCT-14-1065. [DOI] [PubMed] [Google Scholar]
- 137.Eilers G, et al. CDKN2A/p16 Loss Implicates CDK4 as a Therapeutic Target in Imatinib-Resistant Dermatofibrosarcoma Protuberans. Mol Cancer Ther. 2015;14:1346–53. doi: 10.1158/1535-7163.MCT-14-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Michaud K, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–38. doi: 10.1158/0008-5472.CAN-09-4559. These two studies revealed that the loss of RB causes complete resistance of tumour cells to the anti-proliferative effect of CDK4/6 inhibitors. This observation has important implications for patient selection in clinical studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Finn RS, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. This very important study indicated that ER+ breast cancer cells are particularly sensitive to growth inhibition by palbociclib, thereby providing the basis for its current clinical use in ER+ breast cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Konecny GE, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17:1591–602. doi: 10.1158/1078-0432.CCR-10-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Logan JE, et al. PD-0332991, a potent and selective inhibitor of cyclin-dependent kinase 4/6, demonstrates inhibition of proliferation in renal cell carcinoma at nanomolar concentrations and molecular markers predict for sensitivity. Anticancer Res. 2013;33:2997–3004. [PubMed] [Google Scholar]
- 142.Asghar U, et al. Identification of subtypes of triple negative breast cancer (TNBC) that are sensitive to CDK4/6 inhibition. J Clin Oncol. 2015;33(suppl) abstr 11098. [Google Scholar]
- 143.Zhang YX, et al. Antiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivo. Mol Cancer Ther. 2014;13:2184–93. doi: 10.1158/1535-7163.MCT-14-0387. [DOI] [PubMed] [Google Scholar]
- 144.Cen L, et al. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol. 2012;14:870–81. doi: 10.1093/neuonc/nos114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Olanich ME, et al. CDK4 Amplification Reduces Sensitivity to CDK4/6 Inhibition in Fusion-Positive Rhabdomyosarcoma. Clin Cancer Res. 2015;21:4947–59. doi: 10.1158/1078-0432.CCR-14-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thangavel C, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–45. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kovatcheva M, et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget. 2015;6:8226–43. doi: 10.18632/oncotarget.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leonard JP, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119:4597–607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 150.Dickson MA, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–8. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vaughn DJ, et al. Phase 2 trial of the cyclin-dependent kinase 4/6 inhibitor palbociclib in patients with retinoblastoma protein-expressing germ cell tumors. Cancer. 2015;121:1463–8. doi: 10.1002/cncr.29213. [DOI] [PubMed] [Google Scholar]
- 152.Gopalan PK, et al. A phase II clinical trial of the CDK 4/6 inhibitor palbociclib (PD 0332991) in previously treated, advanced non-small cell lung cancer (NSCLC) patients with inactivated CDKN2A. J Clin Oncol. 2014;32(suppl) abstr 8077. [Google Scholar]
- 153.Sicinska E, et al. Essential role for cyclin D3 in granulocyte colony-stimulating factor-driven expansion of neutrophil granulocytes. Mol Cell Biol. 2006;26:8052–8060. doi: 10.1128/MCB.00800-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. This clinical phase II trial showed substantial clinical benefit and provided the basis for the accelerated approval of the CDK4/6-selective inhibitor palbociclib in combination with letrozole for first-line treatment of advanced ER+ breast cancer. [DOI] [PubMed] [Google Scholar]
- 155.Beaver JA, et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2015;21:4760–6. doi: 10.1158/1078-0432.CCR-15-1185. [DOI] [PubMed] [Google Scholar]
- 156.Turner NC, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. This interim analysis of an ongoing clinical phase III trial reported a clinical benefit of using the CDK4/6-selective inhibitor palbociclib in combination with fulvestrant for ER+ breast cancer patients that have relapsed after previous hormone therapy. [DOI] [PubMed] [Google Scholar]
- 157.Cristofanilli M, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 158.US Food and Drug Administration. fda.gov. 2016 http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm487080.htm.
- 159.DeMichele A, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 160.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–46. doi: 10.1038/nrd4504. An excellent review focussing on CDK inhibitors in cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rader J, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173–82. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kennedy AL, et al. Functional, chemical genomic, and super-enhancer screening identify sensitivity to cyclin D1/CDK4 pathway inhibition in Ewing sarcoma. Oncotarget. 2015;6:30178–93. doi: 10.18632/oncotarget.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Infante JR, et al. A phase I study of the single-agent CDK4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas. J Clin Oncol. 2014;32(suppl) abstr 2528. [Google Scholar]
- 164.Munster PN, et al. Ph IB study of LEE011 and BYL719 in combination with letrozole in ER+, HER2- breast cancer. J Clin Oncol. 2014;32(suppl 26) abstr 143. [Google Scholar]
- 165.Gelbert LM, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–37. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yadav V, et al. The CDK4/6 inhibitor LY2835219 overcomes vemurafenib resistance resulting from MAPK reactivation and cyclin D1 upregulation. Mol Cancer Ther. 2014;13:2253–63. doi: 10.1158/1535-7163.MCT-14-0257. [DOI] [PubMed] [Google Scholar]
- 167.Raub TJ, et al. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab Dispos. 2015;43:1360–71. doi: 10.1124/dmd.114.062745. [DOI] [PubMed] [Google Scholar]
- 168.Shapiro G, et al. A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. J Clin Oncol. 2013;31(suppl) abstr 2500. [Google Scholar]
- 169.Goldman JW, et al. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with non-small cell lung cancer. J Clin Oncol. 2014;32(suppl) abstr 8026. [Google Scholar]
- 170.Patnaik A, et al. LY2835219, a novel cell cycle inhibitor selective for CDK4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. J Clin Oncol. 2014;32(suppl) abstr 534. [Google Scholar]
- 171.DiGiulio S. Oncology Times. Wolters Kluwer Health, Inc; 2015. http://journals.lww.com/oncology-times/blog/fdaactionsandupdates/pages/post.aspx?PostID=119. [Google Scholar]
- 172.Tolaney SM, et al. A phase Ib study of abemaciclib with therapies for metastatic breast cancer. J Clin Oncol. 2015;33(suppl) abstr 522. [Google Scholar]
- 173.Clark AS, et al. A phase I trial of palbociclib and paclitaxel in metastatic breast cancer. J Clin Oncol. 2014;32(suppl) abstr 527. [Google Scholar]
- 174.Kwong LN, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med. 2012;18:1503–10. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sosman JA, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J Clin Oncol. 2014;32(suppl) abstr 9009. [Google Scholar]
- 176.Vora SR, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–49. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bardia A, et al. Phase Ib/II study of LEE011, everolimus, and exemestane in postmenopausal women with ER+/HER2-metastatic breast cancer. J Clin Oncol. 2014;32(suppl) abstr 535. [Google Scholar]
- 178.Roberts PJ, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–87. doi: 10.1093/jnci/djs002. These two studies cautioned against combining CDK4/6-selective inhibitors with conventional chemotherapeutics for treatment of RB+ CDK4/6-dependent tumours, but also suggested a potential protective value of CDK4/6 inhibitors for normal tissues during chemotherapeutic treatment of CDK4/6-independent tumours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McClendon AK, et al. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle. 2012;11:2747–55. doi: 10.4161/cc.21127. These two studies cautioned against combining CDK4/6-selective inhibitors with conventional chemotherapeutics for treatment of RB+ CDK4/6-dependent tumours, but also suggested a potential protective value of CDK4/6 inhibitors for normal tissues during chemotherapeutic treatment of CDK4/6-independent tumours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Johnson SM, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120:2528–36. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Medicine, U.N.L.o. ClinicalTrials.gov. 2015 https://clinicaltrials.gov/ct2/show/NCT02499770.
- 182.Guzi TJ, et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol Cancer Ther. 2011;10:591–602. doi: 10.1158/1535-7163.MCT-10-0928. [DOI] [PubMed] [Google Scholar]
- 183.Montano R, Chung I, Garner KM, Parry D, Eastman A. Preclinical development of the novel Chk1 inhibitor SCH900776 in combination with DNA-damaging agents and antimetabolites. Mol Cancer Ther. 2012;11:427–38. doi: 10.1158/1535-7163.MCT-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schenk EL, et al. Effects of selective checkpoint kinase 1 inhibition on cytarabine cytotoxicity in acute myelogenous leukemia cells in vitro. Clin Cancer Res. 2012;18:5364–73. doi: 10.1158/1078-0432.CCR-12-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Daud AI, et al. Phase I dose-escalation trial of checkpoint kinase 1 inhibitor MK-8776 as monotherapy and in combination with gemcitabine in patients with advanced solid tumors. J Clin Oncol. 2015;33:1060–6. doi: 10.1200/JCO.2014.57.5027. [DOI] [PubMed] [Google Scholar]
- 186.Karp JE, et al. Phase I and pharmacologic trial of cytosine arabinoside with the selective checkpoint 1 inhibitor Sch 900776 in refractory acute leukemias. Clin Cancer Res. 2012;18:6723–31. doi: 10.1158/1078-0432.CCR-12-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.US National Library of Medicine. ClinicalTrials.gov. 2013 https://www.clinicaltrials.gov/ct2/show/NCT01870596.
- 188.King C, et al. LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms. Mol Cancer Ther. 2015;14:2004–13. doi: 10.1158/1535-7163.MCT-14-1037. This study illustrated how inhibition of CHK1 triggers cell death by inducing DNA damage during S phase (termed replication catastrophe), followed by chromosome fragmentation. [DOI] [PubMed] [Google Scholar]
- 189.Bendell JC, et al. Checkpoint kinase (CHK) 1/2 inhibitor LY2606368 in a phase I, dose-expansion study in patients (pts) with metastatic squamous cell carcinoma (mSCC) of the anus. J Clin Oncol. 2015;33(suppl) abstr 3520. [Google Scholar]
- 190.Hirai H, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8:2992–3000. doi: 10.1158/1535-7163.MCT-09-0463. [DOI] [PubMed] [Google Scholar]
- 191.Hirai H, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther. 2010;9:514–22. doi: 10.4161/cbt.9.7.11115. [DOI] [PubMed] [Google Scholar]
- 192.Bridges KA, et al. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin Cancer Res. 2011;17:5638–48. doi: 10.1158/1078-0432.CCR-11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rajeshkumar NV, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799–806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guertin AD, et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol Cancer Ther. 2013;12:1442–52. doi: 10.1158/1535-7163.MCT-13-0025. [DOI] [PubMed] [Google Scholar]
- 195.Van Linden AA, et al. Inhibition of Wee1 sensitizes cancer cells to antimetabolite chemotherapeutics in vitro and in vivo, independent of p53 functionality. Mol Cancer Ther. 2013;12:2675–84. doi: 10.1158/1535-7163.MCT-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mueller S, et al. Targeting Wee1 for the treatment of pediatric high-grade gliomas. Neuro Oncol. 2014;16:352–60. doi: 10.1093/neuonc/not220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Karnak D, et al. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin Cancer Res. 2014;20:5085–96. doi: 10.1158/1078-0432.CCR-14-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou L, et al. A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations. Leukemia. 2015;29:807–18. doi: 10.1038/leu.2014.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang G, et al. Synergistic antitumor interactions between MK-1775 and panobinostat in preclinical models of pancreatic cancer. Cancer Lett. 2015;356:656–68. doi: 10.1016/j.canlet.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weisberg E, et al. Identification of Wee1 as a novel therapeutic target for mutant RAS-driven acute leukemia and other malignancies. Leukemia. 2015;29:27–37. doi: 10.1038/leu.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Davies KD, et al. Chk1 inhibition and Wee1 inhibition combine synergistically to impede cellular proliferation. Cancer Biol Ther. 2011;12:788–96. doi: 10.4161/cbt.12.9.17673. [DOI] [PubMed] [Google Scholar]
- 202.Russell MR, et al. Combination therapy targeting the Chk1 and Wee1 kinases shows therapeutic efficacy in neuroblastoma. Cancer Res. 2013;73:776–84. doi: 10.1158/0008-5472.CAN-12-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leijen S, et al. Phase II study with Wee1 inhibitor AZD1775 plus carboplatin in patients with p53 mutated ovarian cancer refractory or resistant (<3 months) to standard first line therapy. J Clin Oncol. 2015;33(suppl) doi: 10.1200/JCO.2016.67.5942. abstr 2507. [DOI] [PubMed] [Google Scholar]
- 204.Oza AM, et al. An international, biomarker-directed, randomized, phase II trial of AZD1775 plus paclitaxel and carboplatin (P/C) for the treatment of women with platinum-sensitive, TP53-mutant ovarian cancer. J Clin Oncol. 2015;33(suppl) doi: 10.1158/1078-0432.CCR-20-0219. abstr 5506. [DOI] [PubMed] [Google Scholar]
- 205.Gumireddy K, et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:275–86. doi: 10.1016/j.ccr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 206.Anderson RT, et al. The dual pathway inhibitor rigosertib is effective in direct patient tumor xenografts of head and neck squamous cell carcinomas. Mol Cancer Ther. 2013;12:1994–2005. doi: 10.1158/1535-7163.MCT-13-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Agoni L, et al. Rigosertib is a more effective radiosensitizer than cisplatin in concurrent chemoradiation treatment of cervical carcinoma, in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2014;88:1180–7. doi: 10.1016/j.ijrobp.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Silverman LR, et al. Clinical activity and safety of the dual pathway inhibitor rigosertib for higher risk myelodysplastic syndromes following DNA methyltransferase inhibitor therapy. Hematol Oncol. 2015;33:57–66. doi: 10.1002/hon.2137. [DOI] [PubMed] [Google Scholar]
- 209.Fenaux P, et al. Overall survival (OS) and baseline disease characteristics in MDS patients with primary HMA failure in a randomized, controlled, phase III study of rigosertib. J Clin Oncol. 2015;33(suppl) abstr e18079. [Google Scholar]
- 210.Silverman LR, et al. Prognostic and predictive value of IPSS-R in assessing overall survival (OS) in a phase III study of rigosertib in second-line higher-risk (HR) MDS patients. J Clin Oncol. 2015;33(suppl) abstr 7092. [Google Scholar]
- 211.Rudolph D, et al. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094–102. doi: 10.1158/1078-0432.CCR-08-2445. [DOI] [PubMed] [Google Scholar]
- 212.Sparta AM, et al. Therapeutic targeting of Polo-like kinase-1 and Aurora kinases in T-cell acute lymphoblastic leukemia. Cell Cycle. 2014;13:2237–47. doi: 10.4161/cc.29267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gorlick R, et al. Initial testing (stage 1) of the Polo-like kinase inhibitor volasertib (BI 6727), by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2014;61:158–64. doi: 10.1002/pbc.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rudolph D, et al. Efficacy and mechanism of action of volasertib, a potent and selective inhibitor of Polo-like kinases, in preclinical models of acute myeloid leukemia. J Pharmacol Exp Ther. 2015;352:579–89. doi: 10.1124/jpet.114.221150. [DOI] [PubMed] [Google Scholar]
- 215.Bhola NE, et al. Kinome-wide functional screen identifies role of PLK1 in hormone-independent, ER-positive breast cancer. Cancer Res. 2015;75:405–14. doi: 10.1158/0008-5472.CAN-14-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hugle M, Belz K, Fulda S. Identification of synthetic lethality of PLK1 inhibition and microtubule-destabilizing drugs. Cell Death Differ. 2015;22:1946–56. doi: 10.1038/cdd.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Döhner H, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–33. doi: 10.1182/blood-2014-03-560557. This clinical phase II study reported a clinical benefit of using the PLK1-selective inhibitor volasertib in combination with cytarabine for older patients with acute myelogenous leukaemia, which is now being validated in a phase III trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pujade-Lauraine E, et al. Volasertib Versus Chemotherapy in Platinum-Resistant or -Refractory Ovarian Cancer: A Randomized Phase II Groupe des Investigateurs Nationaux pour l’Etude des Cancers de l’Ovaire Study. J Clin Oncol. 2016;34:706–13. doi: 10.1200/JCO.2015.62.1474. [DOI] [PubMed] [Google Scholar]
- 219.Stadler WM, et al. An open-label, single-arm, phase 2 trial of the Polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer. 2014;120:976–82. doi: 10.1002/cncr.28519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ellis PM, et al. A Randomized, Open-Label Phase II Trial of Volasertib as Monotherapy and in Combination With Standard-Dose Pemetrexed Compared With Pemetrexed Monotherapy in Second-Line Treatment for Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2015;16:457–65. doi: 10.1016/j.cllc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 221.Sur S, et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci U S A. 2009;106:3964–9. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wu CP, et al. Human ATP-Binding Cassette Transporter ABCB1 Confers Resistance to Volasertib (BI 6727), a Selective Inhibitor of Polo-like Kinase 1. Mol Pharm. 2015;12:3885–95. doi: 10.1021/acs.molpharmaceut.5b00312. [DOI] [PubMed] [Google Scholar]
- 223.Manfredi MG, et al. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–24. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 224.Görgün G, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115:5202–13. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Qi W, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011;81:881–90. doi: 10.1016/j.bcp.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Maris JM, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. These three studies showed an impressive and long-lasting tumour regression upon selective inhibition of Aurora A using alisertib in various preclinical neuroblastoma models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brockmann M, et al. Small Molecule Inhibitors of Aurora-A Induce Proteasomal Degradation of N-Myc in Childhood Neuroblastoma. Cancer Cell. 2013 doi: 10.1016/j.ccr.2013.05.005. These three studies showed an impressive and long-lasting tumour regression upon selective inhibition of Aurora A using alisertib in various preclinical neuroblastoma models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kelly KR, et al. Targeting Aurora A kinase activity with the investigational agent alisertib increases the efficacy of cytarabine through a FOXO-dependent mechanism. Int J Cancer. 2012;131:2693–703. doi: 10.1002/ijc.27579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sehdev V, et al. The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Mol Cancer Ther. 2012;11:763–74. doi: 10.1158/1535-7163.MCT-11-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sehdev V, et al. The combination of alisertib, an investigational Aurora kinase A inhibitor, and docetaxel promotes cell death and reduces tumor growth in preclinical cell models of upper gastrointestinal adenocarcinomas. Cancer. 2013;119:904–14. doi: 10.1002/cncr.27801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kelly KR, et al. The novel Aurora A kinase inhibitor MLN8237 is active in resistant chronic myeloid leukaemia and significantly increases the efficacy of nilotinib. J Cell Mol Med. 2011;15:2057–70. doi: 10.1111/j.1582-4934.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mahadevan D, et al. Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18:2210–9. doi: 10.1158/1078-0432.CCR-11-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mahadevan D, et al. Alisertib added to rituximab and vincristine is synthetic lethal and potentially curative in mice with aggressive DLBCL co-overexpressing MYC and BCL2. PLoS One. 2014;9:e95184. doi: 10.1371/journal.pone.0095184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Davis SL, et al. Combined inhibition of MEK and Aurora A kinase in KRAS/PIK3CA double-mutant colorectal cancer models. Front Pharmacol. 2015;6:120. doi: 10.3389/fphar.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ham J, et al. Exploitation of the Apoptosis-Primed State of MYCN-Amplified Neuroblastoma to Develop a Potent and Specific Targeted Therapy Combination. Cancer Cell. 2016;29:159–72. doi: 10.1016/j.ccell.2016.01.002. These three studies showed an impressive and long-lasting tumour regression upon selective inhibition of Aurora A using alisertib in various preclinical neuroblastoma models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu Y, et al. Combining an Aurora Kinase Inhibitor and a Death Receptor Ligand/Agonist Antibody Triggers Apoptosis in Melanoma Cells and Prevents Tumor Growth in Preclinical Mouse Models. Clin Cancer Res. 2015;21:5338–48. doi: 10.1158/1078-0432.CCR-15-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dees EC, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–84. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 238.Matulonis UA, et al. Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol. 2012;127:63–9. doi: 10.1016/j.ygyno.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 239.Falchook GS, et al. Phase I/II study of weekly paclitaxel with or without MLN8237 (alisertib), an investigational aurora A kinase inhibitor, in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer (OC), or breast cancer (BrC): Phase I results. J Clin Oncol. 2012;30(suppl) abstr 5021. [Google Scholar]
- 240.Melichar B, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. doi: 10.1016/S1470-2045(15)70051-3. [DOI] [PubMed] [Google Scholar]
- 241.Sarantopoulos J, et al. Phase I trial of the investigational aurora A kinase (AAK) inhibitor MLN8237 (alisertib) in combination with docetaxel (DTX) in patients (pts) with advanced solid tumors, including castration-resistant prostate cancer (CRPC) J Clin Oncol. 2014;32(suppl 4) abstr 217. [Google Scholar]
- 242.Rosenthal A, et al. A Phase Ib Study of the combination of the Aurora Kinase Inhibitor Alisertib (MLN8237) and Bortezomib in Relapsed Multiple Myeloma. Br J Haematol. 2015 doi: 10.1111/bjh.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Friedberg JW, et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014;32:44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Barr PM, et al. Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J Clin Oncol. 2015;33:2399–404. doi: 10.1200/JCO.2014.60.6327. This clinical phase II trial indicated potential clinical benefit of using the Aurora A-selective inhibitor alisertib for patients with peripheral T-cell lymphoma and prompted further clinical investigation in a currently ongoing phase III trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.US National Library of Medicine. ClinicalTrials.gov. 2013 https://www.clinicaltrials.gov/ct2/show/NCT01829971.
- 246.Loda M, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–4. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 247.Catzavelos C, et al. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227–30. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 248.Porter PL, et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–5. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 249.Spirin KS, et al. p27/Kip1 mutation found in breast cancer. Cancer Res. 1996;56:2400–4. [PubMed] [Google Scholar]
- 250.Sharpless NE, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 251.Deane NG, et al. Hepatocellular carcinoma results from chronic cyclin D1 overexpression in transgenic mice. Cancer Res. 2001;61:5389–95. [PubMed] [Google Scholar]
- 252.Gladden AB, Woolery R, Aggarwal P, Wasik MA, Diehl JA. Expression of constitutively nuclear cyclin D1 in murine lymphocytes induces B-cell lymphoma. Oncogene. 2006;25:998–1007. doi: 10.1038/sj.onc.1209147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pirkmaier A, et al. Alternative mammary oncogenic pathways are induced by D-type cyclins; MMTV-cyclin D3 transgenic mice develop squamous cell carcinoma. Oncogene. 2003;22:4425–33. doi: 10.1038/sj.onc.1206488. [DOI] [PubMed] [Google Scholar]
- 254.Macias E, Miliani de Marval PL, Senderowicz A, Cullen J, Rodriguez-Puebla ML. Expression of CDK4 or CDK2 in mouse oral cavity is retained in adult pituitary with distinct effects on tumorigenesis. Cancer Res. 2008;68:162–71. doi: 10.1158/0008-5472.CAN-07-2461. [DOI] [PubMed] [Google Scholar]
- 255.Smith AP, et al. Deregulated cyclin E promotes p53 loss of heterozygosity and tumorigenesis in the mouse mammary gland. Oncogene. 2006;25:7245–59. doi: 10.1038/sj.onc.1209713. [DOI] [PubMed] [Google Scholar]
- 256.Ma Y, et al. Transgenic cyclin E triggers dysplasia and multiple pulmonary adenocarcinomas. Proc Natl Acad Sci U S A. 2007;104:4089–94. doi: 10.1073/pnas.0606537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Loeb KR, et al. A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell. 2005;8:35–47. doi: 10.1016/j.ccr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 258.Roussel-Gervais A, et al. Cooperation between cyclin E and p27(Kip1) in pituitary tumorigenesis. Mol Endocrinol. 2010;24:1835–45. doi: 10.1210/me.2010-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Geisen C, Karsunky H, Yucel R, Moroy T. Loss of p27(Kip1) cooperates with cyclin E in T-cell lymphomagenesis. Oncogene. 2003;22:1724–9. doi: 10.1038/sj.onc.1206340. [DOI] [PubMed] [Google Scholar]
- 260.Torchia EC, et al. A genetic variant of Aurora kinase A promotes genomic instability leading to highly malignant skin tumors. Cancer Res. 2009;69:7207–15. doi: 10.1158/0008-5472.CAN-09-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hulit J, et al. Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice. Mol Cell Biol. 2004;24:7598–7611. doi: 10.1128/MCB.24.17.7598-7611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Robles AI, et al. Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev. 1998;12:2469–74. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tsikitis M, Zhang Z, Edelman W, Zagzag D, Kalpana GV. Genetic ablation of Cyclin D1 abrogates genesis of rhabdoid tumors resulting from Ini1 loss. Proc Natl Acad Sci U S A. 2005;102:12129–34. doi: 10.1073/pnas.0505300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cole AM, et al. Cyclin D2-cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res. 2010;70:8149–58. doi: 10.1158/0008-5472.CAN-10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Burns KH, Agno JE, Sicinski P, Matzuk MM. Cyclin D2 and p27 are tissue-specific regulators of tumorigenesis in inhibin alpha knockout mice. Mol Endocrinol. 2003;17:2053–69. doi: 10.1210/me.2003-0038. [DOI] [PubMed] [Google Scholar]
- 266.Miliani de Marval PL, et al. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol Cell Biol. 2004;24:7538–7547. doi: 10.1128/MCB.24.17.7538-7547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rodriguez-Puebla ML, et al. Cdk4 deficiency inhibits skin tumor development but does not affect normal keratinocyte proliferation. Am J Pathol. 2002;161:405–11. doi: 10.1016/S0002-9440(10)64196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Reddy HK, Grana X, Dhanasekaran DN, Litvin J, Reddy EP. Requirement of Cdk4 for v-Ha-ras-Induced Breast Tumorigenesis and Activation of the v-ras-Induced Senescence Program by the R24C Mutation. Genes Cancer. 2010;1:69–80. doi: 10.1177/1947601909358105. These studies demonstrated the requirement for CDK4 kinase activity in mammary tumorigenesis using mouse cancer models, indicating a potential value of inhibiting CDK4 in breast cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ciznadija D, Liu Y, Pyonteck SM, Holland EC, Koff A. Cyclin D1 and cdk4 mediate development of neurologically destructive oligodendroglioma. Cancer Res. 2011;71:6174–83. doi: 10.1158/0008-5472.CAN-11-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lu Y, et al. CDK4 deficiency promotes genomic instability and enhances Myc-driven lymphomagenesis. J Clin Invest. 2014;124:1672–84. doi: 10.1172/JCI63139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gopinathan L, et al. Loss of Cdk2 and cyclin A2 impairs cell proliferation and tumorigenesis. Cancer Res. 2014;74:3870–9. doi: 10.1158/0008-5472.CAN-13-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Niida H, et al. Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo. EMBO J. 2010;29:3558–70. doi: 10.1038/emboj.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.El Ghamrasni S, et al. Inactivation of chk2 and mus81 leads to impaired lymphocytes development, reduced genomic instability, and suppression of cancer. PLoS Genet. 2011;7:e1001385. doi: 10.1371/journal.pgen.1001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hirao A, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–32. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bahassi el M, et al. Mice with the CHEK2*1100delC SNP are predisposed to cancer with a strong gender bias. Proc Natl Acad Sci U S A. 2009;106:17111–6. doi: 10.1073/pnas.0909237106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kwak EL, et al. Mammary tumorigenesis following transgenic expression of a dominant negative CHK2 mutant. Cancer Res. 2006;66:1923–8. doi: 10.1158/0008-5472.CAN-05-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.McPherson JP, et al. Collaboration of Brca1 and Chk2 in tumorigenesis. Genes Dev. 2004;18:1144–53. doi: 10.1101/gad.1192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yang Y, et al. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008;68:4077–85. doi: 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ko MA, et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet. 2005;37:883–8. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- 280.Kim KS, et al. Thio- and oxoflavopiridols, cyclin-dependent kinase 1-selective inhibitors: synthesis and biological effects. J Med Chem. 2000;43:4126–34. doi: 10.1021/jm000231g. [DOI] [PubMed] [Google Scholar]
- 281.McClue SJ, et al. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine) Int J Cancer. 2002;102:463–8. doi: 10.1002/ijc.10738. [DOI] [PubMed] [Google Scholar]
- 282.Meijer L, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–36. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 283.Squires MS, et al. Biological characterization of AT7519, a small-molecule inhibitor of cyclin-dependent kinases, in human tumor cell lines. Mol Cancer Ther. 2009;8:324–32. doi: 10.1158/1535-7163.MCT-08-0890. [DOI] [PubMed] [Google Scholar]
- 284.Wyatt PG, et al. Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. J Med Chem. 2008;51:4986–99. doi: 10.1021/jm800382h. [DOI] [PubMed] [Google Scholar]
- 285.Squires MS, et al. AT7519, a cyclin-dependent kinase inhibitor, exerts its effects by transcriptional inhibition in leukemia cell lines and patient samples. Mol Cancer Ther. 2010;9:920–8. doi: 10.1158/1535-7163.MCT-09-1071. [DOI] [PubMed] [Google Scholar]
- 286.Dolman ME, et al. Cyclin-Dependent Kinase Inhibitor AT7519 as a Potential Drug for MYCN-Dependent Neuroblastoma. Clin Cancer Res. 2015;21:5100–9. doi: 10.1158/1078-0432.CCR-15-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Brasca MG, et al. Identification of N,1,4,4-tetramethyl-8-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-4,5-dihydro-1H-py razolo[4,3-h]quinazoline-3-carboxamide (PHA-848125), a potent, orally available cyclin dependent kinase inhibitor. J Med Chem. 2009;52:5152–63. doi: 10.1021/jm9006559. [DOI] [PubMed] [Google Scholar]
- 288.Albanese C, et al. Anti-tumour efficacy on glioma models of PHA-848125, a multi-kinase inhibitor able to cross the blood-brain barrier. Br J Pharmacol. 2013;169:156–66. doi: 10.1111/bph.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Degrassi A, et al. Efficacy of PHA-848125, a cyclin-dependent kinase inhibitor, on the K-Ras(G12D)LA2 lung adenocarcinoma transgenic mouse model: evaluation by multimodality imaging. Mol Cancer Ther. 2010;9:673–81. doi: 10.1158/1535-7163.MCT-09-0726. [DOI] [PubMed] [Google Scholar]
- 290.Albanese C, et al. Dual targeting of CDK and tropomyosin receptor kinase families by the oral inhibitor PHA-848125, an agent with broad-spectrum antitumor efficacy. Mol Cancer Ther. 2010;9:2243–54. doi: 10.1158/1535-7163.MCT-10-0190. [DOI] [PubMed] [Google Scholar]
- 291.Goh KC, et al. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia. 2012;26:236–43. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- 292.Cyclacel Pharmaceuticals. 2015 http://www.cyclacel.com/pdf/cyclacel_CYC065_program_summary.pdf.
- 293.Cirstea D, et al. Small-molecule multi-targeted kinase inhibitor RGB-286638 triggers P53-dependent and -independent anti-multiple myeloma activity through inhibition of transcriptional CDKs. Leukemia. 2013;27:2366–75. doi: 10.1038/leu.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.van der Biessen DA, et al. Phase I study of RGB-286638, a novel, multitargeted cyclin-dependent kinase inhibitor in patients with solid tumors. Clin Cancer Res. 2014;20:4776–83. doi: 10.1158/1078-0432.CCR-14-0325. [DOI] [PubMed] [Google Scholar]
- 295.Dai Y, et al. The novel Chk1 inhibitor MK-8776 sensitizes human leukemia cells to HDAC inhibitors by targeting the intra-S checkpoint and DNA replication and repair. Mol Cancer Ther. 2013;12:878–89. doi: 10.1158/1535-7163.MCT-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chila R, et al. Combined inhibition of Chk1 and Wee1 as a new therapeutic strategy for mantle cell lymphoma. Oncotarget. 2015;6:3394–408. doi: 10.18632/oncotarget.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Duan W, et al. Fanconi anemia repair pathway dysfunction, a potential therapeutic target in lung cancer. Front Oncol. 2014;4:368. doi: 10.3389/fonc.2014.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Northfelt DW, et al. A phase I dose-escalation study of TKM-080301, a RNAi therapeutic directed against polo-like kinase 1 (PLK1), in patients with advanced solid tumors: Expansion cohort evaluation of biopsy samples for evidence of pharmacodynamic effects of PLK1 inhibition. J Clin Oncol. 2013;31(suppl) abstr TPS2621. [Google Scholar]
- 299.Mason JM, et al. Functional characterization of CFI-400945, a Polo-like kinase 4 inhibitor, as a potential anticancer agent. Cancer Cell. 2014;26:163–76. doi: 10.1016/j.ccr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 300.Tentler JJ, et al. Assessment of the in vivo antitumor effects of ENMD-2076, a novel multitargeted kinase inhibitor, against primary and cell line-derived human colorectal cancer xenograft models. Clin Cancer Res. 2010;16:2989–98. doi: 10.1158/1078-0432.CCR-10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wang X, et al. Preclinical activity of a novel multiple tyrosine kinase and aurora kinase inhibitor, ENMD-2076, against multiple myeloma. Br J Haematol. 2010;150:313–25. doi: 10.1111/j.1365-2141.2010.08248.x. [DOI] [PubMed] [Google Scholar]
- 302.Fletcher GC, et al. ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol Cancer Ther. 2011;10:126–37. doi: 10.1158/1535-7163.MCT-10-0574. [DOI] [PubMed] [Google Scholar]
- 303.Payton M, et al. Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res. 2010;70:9846–54. doi: 10.1158/0008-5472.CAN-10-3001. [DOI] [PubMed] [Google Scholar]
- 304.Bush TL, et al. AMG 900, a small-molecule inhibitor of aurora kinases, potentiates the activity of microtubule-targeting agents in human metastatic breast cancer models. Mol Cancer Ther. 2013;12:2356–66. doi: 10.1158/1535-7163.MCT-12-1178. [DOI] [PubMed] [Google Scholar]
- 305.Karp JE, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13:4467–73. doi: 10.1158/1078-0432.CCR-07-0381. [DOI] [PubMed] [Google Scholar]
- 306.Zeidner JF, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100:1172–9. doi: 10.3324/haematol.2015.125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Michel LS, Ley JC, Wildes TM, Trinkaus K, Adkins D. A phase I trial of the addition of the CDK 4/6 inhibitor palbociclib to cetuximab in patients with incurable head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2015;33(suppl) abstr 6043. [Google Scholar]
- 308.O’Neil BH, et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann Oncol. 2015;26:1923–9. doi: 10.1093/annonc/mdv264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Venkatakrishnan K, et al. Recommended phase (Ph) II dose (RP2D) selection for investigational Aurora A kinase (AAK) inhibitor MLN8237 (Alisertib; A) combined with paclitaxel (P): Clinical pharmacokinetics (PK), drug-drug interaction (DDI) assessment, and translational exposure-efficacy modeling. J Clin Oncol. 2013;31(suppl) abstr 2598. [Google Scholar]
- 310.DuBois SG, et al. Phase I Study of the Aurora A Kinase Inhibitor Alisertib in Combination With Irinotecan and Temozolomide for Patients With Relapsed or Refractory Neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) Trial. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Matulonis UA, et al. ENMD-2076, an oral inhibitor of angiogenic and proliferation kinases, has activity in recurrent, platinum resistant ovarian cancer. Eur J Cancer. 2013;49:121–31. doi: 10.1016/j.ejca.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 312.Loong HH, et al. A phase II study of oral ENMD-2076 administered to patients (pts) with advanced soft tissue sarcoma (STS) J Clin Oncol. 2013;31(suppl) abstr TPS10593. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information