NGF Controls Dendrite Development in Hippocampal Neurons by Binding to p75NTR and Modulating the Cellular Targets of Notch (original) (raw)
Abstract
Notch and neurotrophins control neuronal shape, but it is not known whether their signaling pathways intersect. Here we report results from hippocampal neuronal cultures that are in support of this possibility. We found that low cell density or blockade of Notch signaling by a soluble Delta-Fc ligand decreased the mRNA levels of the nuclear targets of Notch, the homologues of enhancer-of-split 1 and 5 (Hes1/5). This effect was associated with enhanced sprouting of new dendrites or dendrite branches. In contrast, high cell density or exposure of low-density cultures to NGF increased the Hes1/5 mRNA, reduced the number of primary dendrites and promoted dendrite elongation. The NGF effects on both Hes1/5 expression and dendrite morphology were prevented by p75-antibody (a p75NTR-blocking antibody) or transfection with enhancer-of-split 6 (Hes6), a condition known to suppress Hes activity. Nuclear translocation of NF-kappaB was identified as a link between p75NTR and Hes1/5 because it was required for the up-regulation of these two genes. The convergence of the Notch and p75NTR signaling pathways at the level of Hes1/5 illuminates an unexpected mechanism through which a diffusible factor (NGF) could regulate dendrite growth when cell-cell interaction via Notch is not in action.
INTRODUCTION
Notch activity has a strong impact on the morphology of developing neurons (reviewed in Whitford et al., 2002). It is a type I cell surface protein that functions as a receptor for the membrane-associated ligands Delta 1-3 and Jagged 1-2 (reviewed in Mumm and Kopan, 2000; Redmond and Ghosh, 2001). Activation by its ligands results in the cleavage and nuclear translocation of the intracellular domain of the receptor. In the nucleus, the intracellular domain of Notch binds to the mammalian homologue of Suppressor-of-hairless, thereby modulating the transcription of the homologues of Enhancer-of-split (Hes) and _Hes_-related (Herp) genes, in particular Hes1 and Hes5. In turn, this cascade of events controls the expression of proneural genes that are essential for the differentiation of neurons and glia (reviewed in Mumm and Kopan, 2000; Weinmaster, 2000). In postmitotic neurons, activation of Notch affects the growth pattern of dendrites. For instance, in cultured embryonic cortical neurons up-regulation of Notch activity accounted for the contact-dependent inhibition of dendrite growth (Sestan et al., 1999). A suppression of dendrite outgrowth was observed both in the presence of a membrane-associated Notch ligand or after transfection of a constitutively active form of Notch1 (Franklin et al., 1999; Redmond et al., 2000).
An important open question is whether the signaling pathway of Notch can also be accessed by signals generated upon the release and binding of diffusible molecules. As a rule, the development and maintenance of neuron shape involves both direct cell-cell contact and the release and uptake of growth factors, but the respective signaling pathways rarely use the same sets of molecules. In the present study, we sought to determine whether neurotrophins can use the signaling pathway of Notch to regulate the growth of hippocampal dendrites, especially under conditions when Notch activity is low due to reduced cell-cell contact.
A contribution of neurotrophins to the regulation of dendrite morphology has already been demonstrated in a variety of preparations (McAllister et al., 1995, 1997; Baker et al., 1998; Jin et al., 2003). As neurotrophins are released in an activity-dependent manner, they can play a major role in the structural adjustment of developing and mature neuronal circuits (reviewed in Whitford et al., 2002). However, the signaling pathways underlying neurotrophin effects on neurite outgrowth are not well elucidated. Although most studies in this area emphasize the importance of Trk receptors, the additional possibility exists that neurotrophins regulate neuronal morphology by binding to p75NTR, the common neurotrophin receptor (Rodríguez-Tébar et al., 1990; Chao and Hempstead, 1995).
The present study was undertaken i) to characterize the effects of Notch and NGF/p75NTR on the dendrite shape of hippocampal neurons and ii) to find out whether a point of convergence exists between the signaling pathways of Notch and p75NTR. We found that up-regulation of Hes1/5 genes could either be caused by activation of Notch or, if the cell density is sufficiently low, by activation of p75NTR with NGF. In both cases this was associated with a decrease in the number of primary dendrites and an increase in dendrite length. Thus, in case of Notch inactivation or desensitization, a diffusible factor—NGF—could substitute for Notch, thereby keeping the level of Hes expression under continuous control.
MATERIALS AND METHODS
Antibodies and Other Chemicals
MAP-2 monoclonal mouse antibody was from Sigma (St. Louis, MO). Anti-p65/NF-kappaB polyclonal rabbit antiserum was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and the rabbit anti-EGFP was purchased from Molecular Probes Europe (Leiden, Holland). The anti-p75NTR rabbit polyclonal antiserum (p75-antibody) was kindly provided by Moses Chao (New York University Medical Center). All secondary antibodies, such as anti-rabbit IgG, were from Jackson ImmunoResearch (West Grove, PA). SN50, an inhibitor of NF-kappaB activation, was purchased from Calbiochem (La-Jolla, CA). The Delta recombinant ligand (Delta-Fc) was obtained from the conditioned medium of the 293T-Delta-Fc cell line (Morrison et al., 2000) after purification in protein-G affinity chromatography.
Hippocampal Neuronal Cultures, Neuron Transfection, and Incubation Conditions
The hippocampus was isolated from embryonic day 17 mouse embryos and dissociated after digestion with trypsin (Worthington, Lakewood, NJ) and DNase I (Sigma; Goslin et al., 1998). Neurons were plated on glass coverslips coated with poly-l-lysine (Sigma) and cultured in Neurobasal A supplemented with B-27 and GlutaMAX I (Life Technologies, Crewe, Cheshire, United Kingdom). The plating density was varied between 15,000 and 150,000/cm2 and will be indicated at any experiment described. Low density (LD) refers to 15.000/cm2, medium density to 30,000-50,000/cm2, and high density (HD) to 150,000/cm2. Under the conditions used, cultures were devoid of glia and displayed minimal cell death (<10% apoptotic nuclei). After 7 d in vitro (DIV) the neurons were transfected using the Effectene Transfection Reagent (Qiagen GmbH, Hilden, Germany), following the manufacturer's instructions. Cells were either transfected with an _Hes6_- and EGFP-expressing pIRES vector (Cossins et al., 2002) or an EGFP-expressing vector (pEGFP-N1; Clontech, Palo Alto, CA). Before the application of extracellular ligands, the medium was replaced by fresh medium devoid of B27 supplement and incubated for 2 h. The cultures were then incubated in fresh standard medium devoid of B27 supplement (control) or in medium containing a neurotrophin (NGF, BDNF, NT-3, or NT-4/5, 100 ng/ml each), Delta-Fc (14 nM), p75-antibody (1:100), or SN50 (28 μg/ml). After the indicated incubation time (3.5 h for PCR and analysis of nuclear extracts, 16 h for morphometry) the cells were harvested or prepared for fluorescence microscopy.
Image Acquisition and Morphometric Analysis of Labeled Hippocampal Neurons
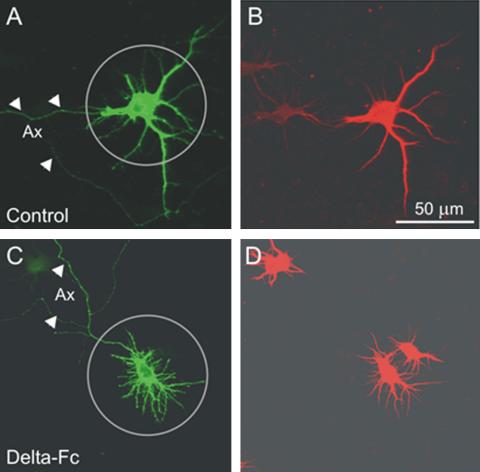
The low efficacy of transfection (∼0.1%) allowed single-labeled neurons to be observed under a Zeiss Axiophot 2 microscope, using a plan-neofluar 40× oil objective with a numerical aperture of 1.3 (Zeiss, Oberkochen, Germany). Parallel experiments demonstrated that, in all conditions used, >90% of nonaxonal processes (see below) were MAP-2 positive, thus indicating that they were true dendrites (see Figure 1). Images of ∼20 neurons per coverslip were digitally acquired (Olympus camera DP-10, Tokyo, Japan) and imported to the final figures by CorelDraw 11 (Ottawa, Ontario, Canada). Dendrite outgrowth was manually evaluated. Only neurons with a defined axon were used for evaluation, but the data from the axon were not included in the analysis. Axons were distinguished from dendrites by the following criteria (Dotti et al., 1988): i) It has a diameter that is much smaller than that of a stem dendrite and that remains constant over the length; ii) Axons emit collaterals at an angle close to 90°, whereas dendrites typically bifurcate at an angle >45°; iii) An axon can be traced far beyond the limits of the dendrite field (usually over hundreds of μm); iv) As expected, the axon was also negative for immunostaining with an antibody against microtubule-associated protein 2 (MAP-2; Figure 1, A and B, arrowheads). Frequently, the axon emerged not from the soma but from a stem dendrite that was MAP-2 positive.
Figure 1.
Identification of axons and dendrites. Shown are confocal micrographs of two neurons, one grown under control conditions (A and B) and another neuron treated with Delta-Fc, a condition that increases the number of dendrites (C and D; see Figure 3C). (A and C) Immunostaining of EGFP; (B and D) immunostaining of MAP2a/b. Note the absence of MAP-2 immunoreactivity from the longest neuron process, the putative axon (arrowheads in A and C) and the presence of MAP-2 staining on thin first- and second-order nonaxonal branches. Circles, circular region of interest (ROI), as used for morphometric evaluation of dendrites.
At DIV7 hippocampal neurons showed, depending on cell density, between 5 and 15 first-order neurites and many second- and third-order branches. To determine whether these presumably still immature neurites already displayed dendrite characteristics, we performed double immunostaining with antibodies against EGFP and MAP-2a/b. Figure 1 illustrates an experiment with control cultures and sister cultures after a 16-h treatment with Delta-Fc ligand, a condition known to increase the number of neurites (see Results). MAP-2 immunoreactivity was found along the entire length of the nonaxonal processes. We therefore classified these processes as dendrites. A region of interest (ROI) with a radius of 50 μm was projected onto the EGFP-labeled neurons with its center roughly coinciding with the center of the soma. The dendrite length was expressed as the fraction of dendrite trees that exceeded the limit of the ROI (fraction dendrites >50 μm). The branching pattern was characterized by the number of primary dendrites, i.e., the number of dendrites associated with the soma and the number of dendrite branch points per ROI. A branch point was defined as the point of origin of at least two daughter branches with a length >7 μm.
Quantitative Real-time PCR
Total RNA was extracted from DIV7 cultures with Absolutely RNA kit (Stratagene, La Jolla, Ca). First-strand cDNA was prepared from RNA using the First Strand Synthesis kit from Fermentas GMBH (St. Leon-Rot, Germany) with a random hexamer primer according to the manufacturer's instructions.
Quantitative PCR was performed using the ABI Prism 7000 Sequence Detector (Applied Biosystems, Weiterstadt, Germany). TaqMan probes and primers for Hes1, Hes5, and for the control housekeeping gene, Gapdh, were Assay-on-Demand gene expression products (Applied Biosystems). All TaqMan probes were labeled with 6-carboxy fluorescein (FAM). Real-time PCRs were performed following the supplier's instructions (Applied Biosystems) in a 20-μl volume reaction using the TaqMan Universal PCR Master Mix. All reactions were done in triplicates and the experiments were repeated at least three times. The standard curves for Hes1, Hes5, and Gapdh were generated using serial dilutions of cDNAs from DIV7 hippocampal cultures. Hes1 and Hes5 expression was normalized for Gapdh expression by using the standard curve method described by the manufacturer. Data were analyzed with an unpaired Student's t test (Graph Pad Prism 4.0, Sorrento, CA) and are expressed as mean ± SEM.
Preparation of Nuclear Extracts
Nuclear extracts from cultured hippocampal neurons were prepared as described previously with some modifications (Coffey et al., 2000). Briefly, cells were washed once with cold PBS containing 1 mM MgCl2 and then lysed for 10 min on ice in 500 μl of the following lysis buffer: 20 mM HEPES, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 2.5 mM EGTA, 0.1 mM DTT, 50 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 0.05% NP-40 (Igepal CA-630, Sigma) and an antiprotease cocktail (Roche Diagnostics GmbH, Mannheim, Germany). The cells were scraped from the culture dish and the suspension was centrifuged at 300 × g for 10 min at 4°C. The pellet was washed once in lysis buffer devoid of NP-40 and finally dissolved in a 60 μl Laemmli denaturation buffer. The protein content was estimated by the DC&RC method (Bio-Rad Laboratories, Munich, Germany) and equal amounts of protein were subjected to SDS/PAGE.
Western Blot Analysis
After electrophoresis, samples were transferred to nitrocellulose membranes, which were incubated in blocking buffer (TBS with 0.3% Tween 20, Sigma, and 5% nonfat dry milk). Membranes were subsequently probed with anti-p65/NF-kappaB, 1:500, for 16 h at 4°C. The blots were washed and then incubated with peroxidase-conjugated anti-rabbit IgG (1:5000). Immunoreactivity was developed by enhancer chemoluminescence (Amersham Biosciences Europe GmbH, Freiburg, Germany).
RESULTS
Effect of Cell Density on Hes1/5 mRNA Levels and Dendrite Morphology of Hippocampal Neurons
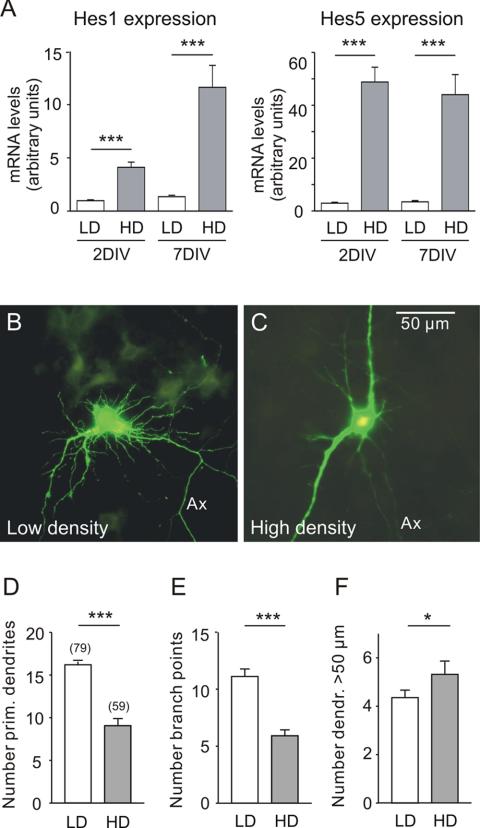
Neurons from E17 mouse hippocampi were plated at low (15,000/cm2) or high (150,000/cm2) cell density and maintained for up to 7 DIV. Based on previous data, it was expected that high density will stimulate the activity of Notch, thereby increasing the levels of Hes1 and Hes5 mRNA (Sestan et al., 1999). Quantitative PCR analysis of hippocampal neuronal cultures showed that this was the case. The effects were seen after 2 and 7 DIV (Figure 2A). To characterize the cellular responses associated with low or high cell density, 7 DIV hippocampal cultures were transfected with an EGFP-expressing vector. The dendrites were subjected to morphometric analysis. Figure 2, B and C, illustrates that neurons grown in cultures at different density exhibited obvious differences in dendrite morphology. Quantitative evaluation showed that low density promoted the initiation of new dendrites (Figure 2D) or dendrite branches (Figure 2E) at the same time stimulating dendrite elongation (Figure 2F).
Figure 2.
The effect of cell density on Hes1/5 mRNA levels and on dendrite morphology of EGFP-labeled hippocampal neurons in culture. (A) Neurons from E17 mouse hippocampi were plated at an initial cell density of 15,000/cm2 (LD) or 150,000/cm2 (HD) and maintained for either 2 or 7 DIV. The mRNA levels of Hes1 and Hes5 were determined by reverse transcription and quantitative PCR as described in the experimental section. Shown is that at high cell density the mRNA levels of both Hes1 and Hes5 were higher. (B and C) Digital fluorescence images of EGFP-expressing hippocampal neurons after 7 DIV. Ax, axon. Note that low cell density favored the appearance of additional first-order (primary) dendrites and second-order (secondary) dendrite branches, whereas high cell density favored dendrite elongation. (D-F) Results of morphometric evaluation. The graphs show the mean, SE, and number of observations (in brackets) for each treatment. Here and in the following, significance levels (asterisks) were determined for the data sets connected by horizontal lines using an unpaired t test. *p < 0.05, **p < 0.01, ***p < 0.001. (D) Number of primary dendrites, i.e., dendrites emerging from the soma. (E) Number of branch points. This parameter represents the total number of bifurcations of the labeled neuron within a radius of 50 μm. (F) Number of dendrite arbors that exceeded the limits of the ROI, i.e., a length >50 μm.
Blockade of Notch Activity Reduces the Level of Hes1/5 Expression and Stimulates Dendrite Initiation
To verify whether, in our preparation, changes in dendrite growth were indeed dependent on the activity of Notch, we applied the blocking Notch ligand Delta-Fc to cultures of intermediate cell density (50,000/cm2). Indeed, this manipulation caused both a decrease of Hes1/5 mRNA levels (Figure 3A) and a dendrite pattern resembling that observed at low cell density, i.e., the initiation of new primary dendrites and dendrite branches was clearly enhanced (Figure 3, B-E), whereas the fraction of dendrites >50 μm decreased (Figure 3F).
Figure 3.
The effect of Notch blockade on Hes1/5 mRNA levels and on dendrite morphology of EGFP-labeled hippocampal neurons in culture. (A) Neurons from E17 mouse hippocampi were plated at an initial cell density of 50,000/cm2. After 7 DIV the recombinant Notch ligand Delta-Fc (14 nM) was added for 3.5 h. The mRNA levels of Hes1 and Hes5 were determined by RT and quantitative PCR. Observe that under the given experimental conditions Delta-Fc behaved as an antagonist of Notch activity, inducing a decrease in the mRNA levels of both Hes1 and Hes5. (B and C) Digital fluorescence images of EGFP-labeled hippocampal neurons. Same cell density as in A. Note Delta-Fc induced the appearance of additional primary dendrites and second-order dendrite branches and decreased the fraction of dendrites >50 μm. (D-F) Summary of morphometric evaluation. The difference in absolute number of dendrites >50 μm was not significantly different in this experiment. Symbols and parameters as in Figure 2.
Shared Cellular Targets of p75NTR and Notch
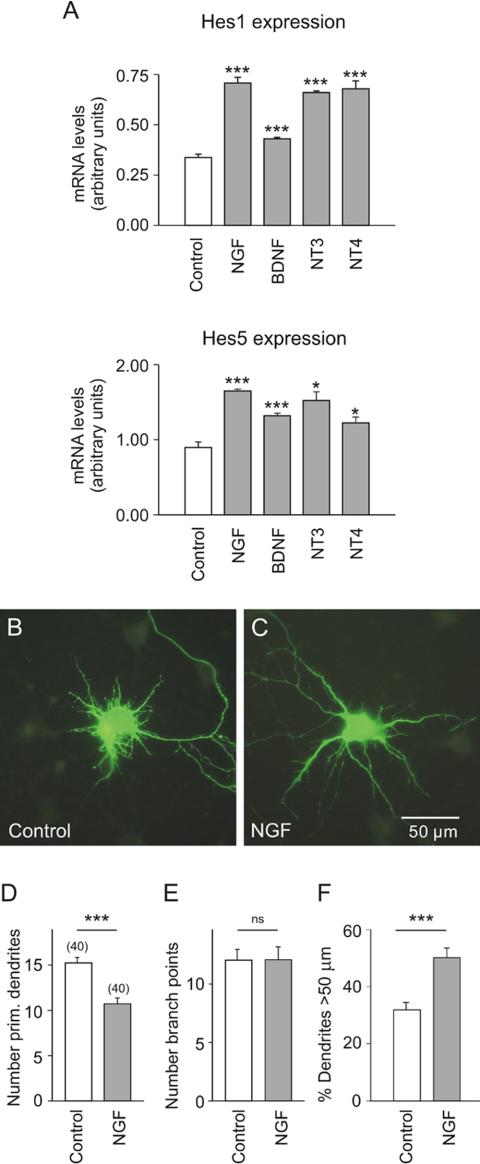
Dendritic morphology does not solely depend on cell-cell contact, but is also controlled by a variety of diffusible factors, including neurotrophins. This implies that both regulatory systems must at some point converge. The question arouse whether or not neurotrophins have access to the regulation of Hes1/5, the nuclear target of Notch. To answer this question, hippocampal neurons were cultured at intermediate cell density (30,000/cm2) and on DIV7 for 3.5 h exposed to NGF, BDNF, NT3, or NT4/5. All four neurotrophins significantly increased the levels of Hes1 and Hes5 mRNAs (Figure 4A), although there was some variability in the efficacy of individual neurotrophins. The similarity of the action observed with the different neurotrophins was a first hint that the increase of Hes1 and Hes5 expression could be mediated by the common neurotrophin receptor p75NTR (see below).
Figure 4.
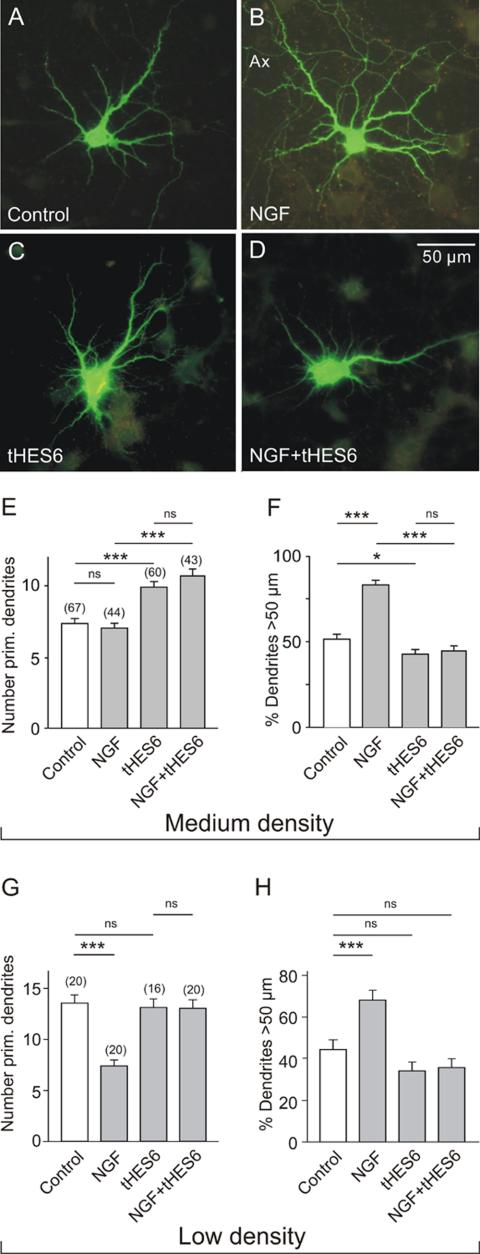
The effect of neurotrophins/NGF on Hes1/5 mRNA levels and on dendrite morphology of EGFP-labeled hippocampal neurons in culture. (A) Results of quantitative RT-PCR experiments on hippocampal neurons that were plated at an initial cell density of 30,000/cm2 and after 7 DIV for 3.5 h exposed to the corresponding neurotrophin at a final concentration of 100 ng/ml. Shown is that all neurotrophins induced an increase of Hes1 and Hes5 mRNA. (B-F) Data from low-density cultures (15,000/cm2). Transfection on DIV 7 and exposure to NFG (100 ng/ml) for 16 h. Observe that in the controls the number of primary dendrites was higher (B and D). The presence of NGF decreased the number of primary dendrites and promoted dendrite elongation (C and F). Symbols and parameters as in Figure 2.
NGF Regulates the Morphology of Hippocampal Dendrites
There is still little information available on the role of NGF in the regulation of dendrite growth (but see Aibel et al., 1998). Here we show that in cultures grown at low cell density (15,000/cm2) NGF caused a significant reduction in the number of primary dendrites (Figure 4, B-D) and increased the fraction of dendrites >50 μm (Figure 4F). An effect of NGF on the number of branch points was not observed (Figure 4E). These experiments suggest that under conditions of low cell density NGF may simulate the action of Notch (compare with Figure 2, D and F).
The Effects of NGF on Hes1/5 Expression and Dendrite Morphology are Mediated by p75NTR
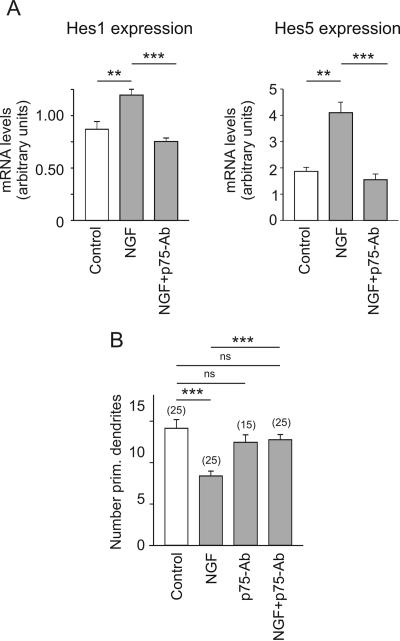
In situ hybridization and PCR demonstrated the presence TrkA and p75NTR in the embryonic and mature hippocampus (unpublished data). Taking into account that all neurotrophins increased the expression of Hes1/5, we pursued the hypothesis that the NGF effects on Hes1/5 and dendrite morphology were mediated by the common low-affinity receptor, p75NTR. Indeed, addition of p75-antibody, an antibody with blocking properties (Huber and Chao, 1995), prevented the NGF-induced increase of Hes1/5 mRNA levels (Figure 5A). In these low-density cultures (15,000/cm2) p75-antibody also blocked the effect of NGF on the number of primary dendrites (Figure 5B). By itself, it had no discernable effects (Figure 5B). Together, these results render strong support to the idea that NGF regulates Hes1/5 expression and dendrite initiation via its low-affinity receptor p75NTR.
Figure 5.
The effect of p75NTR blockade on Hes1/5 mRNA levels and on dendrite morphology of EGFP-labeled hippocampal neurons in culture. (A) Results of quantitative RT-PCR experiments on hippocampal neurons that were plated at an initial density of 15,000/cm2 and after 7 DIV for 3.5 h exposed to standard medium (control), NGF (100 ng/ml) or NGF plus blocking p75-antibody (1:100). Observe that the presence of the antibody prohibited the NGF-induced increase in the mRNA levels of Hes1 and Hes5. (B) Results of morphometric evaluation in cultures of 15,000 cells/cm2. Observe that the presence of the p75NTR-blocking antibody prevented the NGF-induced decrease in the number of primary dendrites. Symbols and parameters as in Figure 2.
Dendrite Initiation Is Facilitated by Low Levels of HES1/5 Activity, and This Limits the Ability of NGF to Decrease the Number of Primary Dendrites and to Stimulate Dendrite Elongation
To further elucidate the signaling mechanisms leading to either dendrite initiation or elongation, cultures were transfected with a pIRES vector expressing both EGFP and Hes6. HES6 was shown to bind to HES1 and Xhairy 2A and to inhibit the transcriptional repressor activity of HES1 (Bae et al., 2000; Koyano-Nakagawa et al., 2000), probably by forming heteromultimers with HES proteins (Cossins et al., 2002). In cultures of intermediate cell density (50,0000/cm2), where the number of primary dendrites is already low in the controls, NGF failed to decrease the number of primary dendrites (Figure 6, A, B, and E), but it still promoted dendrite elongation (Figure 6, A, B, and F). Expression of Hes6 initiated the outgrowth of additional primary (Figure 6E) and secondary (unpublished data) dendrites, an effect unaltered by the presence of NGF (Figure 6, D and E). Furthermore, the expression of Hes6 prevented the NGF-induced dendrite elongation (Figure 6, G and F). In contrast, when neurons were grown at low cell density, therefore exhibiting a large number of primary dendrites, NGF was able to reduce dendrite number and to increase dendrite length (Figure 6, G and H; also see also Figure 5B). However, both effects were totally abolished by transfection with Hes6. This dominant negative action of Hes6 is consistent with the proposal that the transcription factors Hes1/5 are intercalated in the signaling pathway through which NGF/p75NTR control the growth of dendrites.
Figure 6.
Effect of Hes6 expression on the dendrite morphology of hippocampal neurons in medium- and low-density cultures. (A-D) Digital fluorescence images of EGFP-labeled neurons in medium-density cultures after 16 h in control medium (A and C) or in medium with NGF (100 ng/ml; B and D). E17 hippocampal neurons were cultured for 7 DIV and then transfected with pIRES vectors that either induced expression of EGFP alone (A and B) or the expression of EGFP and Hes6 (C and D). Note the larger dendritic field of the NGF-treated neuron (B) and the lack of response to NGF in the cell expressing Hes6 (D). (E and F) Results of morphometric evaluation of medium density cultures (50,000 cells/cm2). Note that NGF was unable to prevent the _Hes6_-stimulated outgrowth of new primary dendrites. _Hes6_-transfection also effectively blocked the NGF-induced dendrite elongation. (G and H) Results of morphometric evaluation of low cell density cultures (15,000 cells/cm2). Note that NGF decreased the number of primary dendrites and increased the relative length of dendrites, but these effects were prevented by transfection with Hes6. Symbols and parameters as in Figure 2.
The NGF-induced Increase of Hes1/5 mRNA Levels Implies NF-kappaB Activity
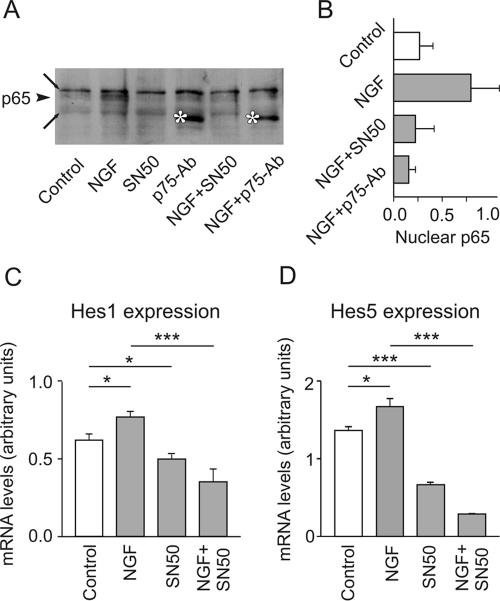
In the light of these results, the question arose as to how NGF might control the levels of Hes1/5 transcripts. Because activation of p75NTR by neurotrophins induced the translocation of NF-kappaB to the cell nucleus (Carter et al., 1996; Ladiwala et al., 1998), we tested the possibility that NF-kappaB mediates the NGF-induced increase of Hes1/5 mRNA levels. To this end, hippocampal cultures were exposed to NGF in the absence or presence of SN50, a specific inhibitor of NF-kappaB (Lin et al., 1995). The application of NGF facilitated the appearance of the NF-kappaB subunit p65/RelA in the nucleus, and this effect was prevented by SN50 (Figure 7, A and B). As expected, a block of p75NTR signaling with p75-antibody also precluded the nuclear translocation of p65/RelA. Figure 7, C and D, shows that the presence of the NF-kappaB inhibitor alone decreased the levels of Hes1/5 mRNAs. Furthermore, the inhibitor SN50 prevented the NGF-induced up-regulation of both Hes1 and Hes5 mRNA. Taken together, these data support the hypothesis that NF-kappaB is an essential link between p75NTR-mediated signaling and Hes1/5 expression.
Figure 7.
NGF promotes the migration of p65/RelA to the nucleus, a process necessary for the increase of Hes1 and Hes5 mRNA. (A) Hippocampal neurons were plated at an initial density of 30,000/cm2. After 7 DIV they were for 3.5 h incubated in NGF, SN50 (an inhibitor of NF-kappaB migration into the nucleus, used here at 28 μg/ml), p75-antibody, NGF plus SN50, or NGF plus p75-antibody (control, no treatment) and then processed to obtain nuclear extracts. The same amount of nuclear protein was separated by SDS/PAGE and transferred to nitrocellulose membranes, which were subsequently probed with an anti-p65 antibody. Note the presence of p65 in the nucleus after treatment with NGF (arrowhead). Both SN50 and p75-antibody prevented the NGF-induced appearance of p65 in the nucleus. Long arrows point to nonspecific bands, asterisks to the heavy chain of the p75-antibody. (B) The intensity of the p65 band was determined by densitometry. Shown is the quantitative analysis from three different experiments. (C) Cultured hippocampal neurons were treated with NGF, with NGF plus SN50, or with SN50 alone. The presence of the inhibitor of the nuclear translocation of NF-kappaB prevented the NGF-induced increase of both Hes1 and Hes5 mRNA.
Do NGF/p75NTR and Notch Activities Collaborate to Induce Hes1/5 Expression?
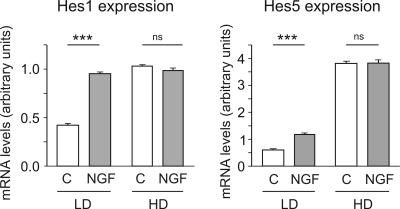
The involvement of NF-kappaB in the NGF-induced increase of Hes1/5 mRNA levels suggests that until the level of Hes1/5 the signaling pathway of NGF might be different from that of Notch. To determine how, with respect of Hes1/5 expression, Notch and NGF/p75NTR cooperate, we measured the effects of NGF on both Hes1 and Hes5 mRNA levels in low (15,000/cm2) and high (150,000/cm2) cell density cultures. Figure 8 shows that NGF increased Hes1/5 mRNA levels only when cell density was low. In contrast, in high cell density cultures, with high levels of Hes1/5 mRNA, NGF had no effect. These results indicate that the Hes1/5 mRNA levels are under the control of two different mechanisms that are additive only as long as one of them does not produce saturation.
Figure 8.
Quantitative PCR experiments to show the cell-density-dependent effect of NGF on Hes1/5 mRNA levels. Hippocampal neurons were for 7 DIV cultured at low (LD, 15,000 cells/cm2) or high (HD, 150,000 cells/cm2) cell density and for 3.5 h incubated in the absence or presence of NGF (100 ng/ml). Observe that NGF increased Hes1/5 mRNA levels only in low cell density cultures.
DISCUSSION
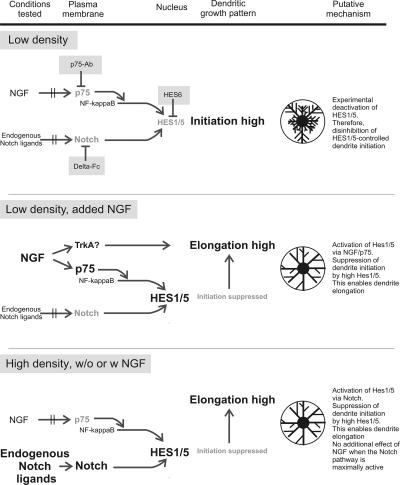
Our principal findings and the proposed signal flow are summarized in Figure 9. We have shown that not only high cell density and Notch activation, but also exposure to NGF, BDNF, NT-3, and NT-4/5 can increase the levels of Hes1 and Hes5 mRNA. This effect of NGF on Hes1/5 was prevented by the p75NTR-blocking antibody p75-antibody and mediated by nuclear translocation of NF-kappaB. Conditions leading to activation of HES1/5, including the exposure to NGF, resulted in a decrease in the number of primary dendrites and an increase in dendrite length. Accordingly, conditions resulting in HES1/5 deactivation, such as low cell density, incubation in Delta-Fc or transfection with Hes6 produced an increase in the number of primary dendrites and an inhibition of dendrite elongation. Thus, low Hes1/5 mRNA levels are concomitant with short and bushy dendrites. Together, these observations support the hypothesis that by binding to p75NTR NGF can partially substitute for the activation of Notch.
Figure 9.
Summarizing scheme to illustrate experimental manipulations (shaded fields), molecular interactions and resultant effects on dendrite growth.
With regard to the direction of effects, our results from hippocampal neurons are at variance with the data obtained from cultured cortical neurons, where high levels of Hes1/5 expression were associated with enhanced branching and reduced elongation of neuronal dendrites (Sestan et al., 1999; Redmond et al., 2000). We cannot, at present, explain the contrasting behavior of hippocampal and cortical neurons. However, it is known that in response to the same stimulus neurons may exhibit variable growth patterns. For example, neurons from different cortical layers were reported to display differential morphological changes under the influence of BDNF or NT3 (McAllister et al., 1997; reviewed in McAllister et al., 1999). Phenotype-specific responses to environmental signals may represent a requirement for optimal adjustment of neuron shape in a mixed population of neurons.
HES as a Common Target of p75NTR and Notch Signaling
Until now the only known physiological source of Hes activation was Notch, and the only known effectors of Notch signaling were the Hes and the Herp genes (see Iso et al., 2003 for review). However, according to previous reports, tissue distributions of Notch and Hes do not always overlap, thereby already suggesting that other membrane receptors could participate in the modulation of HES activity. Now we found that Hes1/5 activation may also be derived from activation of p75NTR by NGF. Although this result is somewhat surprising, it was clear that the Notch and the p75NTR pathway must converge if both are able to regulate the morphology of dendrites. However, until now, it was not known that NGF can have so drastic effects on dendrite morphology of hippocampal neurons. Our present findings also illuminate the conditions under which NGF can contribute to the structural plasticity of hippocampal dendrites.
Our experiments addressed the question of whether or not the actions of Notch and NGF/p75NTR were additive. We found that NGF only increased the levels of Hes1/5 mRNA when the latter were submaximal. Likewise, NGF had clear effects on the number of primary dendrites only in low-density cultures, i.e., when Notch and Hes1/5 activity were not saturated. Already at medium cell density added NGF failed to induce a clear effect on the number of primary dendrites. It therefore seems very likely that NGF-dependent stimulation of Hes1/5 expression represents a regulatory mechanism that complements the regulation by Notch. Thus, in the case of low Notch activity, i.e., under condition of reduced cell-cell contact or Notch desensitization, an appropriate level of Hes1/5 expression and dendrite elongation could be maintained because of the longer-distance action of NGF on p75NTR.
To determine how the signaling pathways of p75NTR proceeds toward Hes1/5, we studied the role of NF-kappaB. It had already been suggested that the activation of p75NTR by NGF can promote the activation of NF-kappaB (Carter et al., 1996). Here we showed that NGF-induced activation of NF-kappaB is a mechanism also shared by hippocampal neurons. The export of p65/NF-kappaB to the nucleus was concomitant with the up-regulation of the Hes1/5 genes, and the pharmacological inhibition of the former prevented the NGF-induced increase of Hes1/5 mRNA levels. Under basal conditions, the inhibition of NF-kappaB activity decreased Hes1/5 mRNA levels, which represents further evidence for the involvement of NF-kappaB in the up-regulation of Hes1/5 expression.
The molecular mechanism linking NF-kappaB activation and Hes1/5 up-regulation in postmitotic neurons has remained unresolved. Our data are in contrast with that of Espinosa et al. (2003), who found that the sequestration of NF-kappaB in the cytoplasm of NIH-3T3 and 293T cells freed the Hes1 promoter from the repression by SMRT/N-CoR. A synergistic action of Notch and NF-kappaB has, however, been described by Bash et al. (1999). In their experiments Jagged1 transcripts were up-regulated by activated NF-kappaB. At higher concentration Jagged1 should activate Notch receptors and then increase the Hes1/5 transcription levels in neighboring cells. We tested this possibility, but found that the exposure of cultured hippocampal neurons to NGF had no effect on the expression of Jagged1 (our unpublished results). Therefore, we consider the observed increase of Hes1/5 expression to be a direct consequence of NGF-induced NF-kappaB activation.
Multiple Functions of Hes Genes
Although the role of HES1/5 has been extensively studied in neural stem cells (see Solecki et al., 2001; Cossins et al., 2002 for more details), little is known about the functions of HES1/5 in the development of postmitotic neurons. Taking into account i) the existence of antagonistic relationships between different members of the Hes family, ii) the differences in the functions of the proneural bHLH genes under the control of Hes1/5 (our unpublished results), and iii) the multitude of possible Hes effectors downstream of the proneural genes, one may expect the consequences of Hes1/5 activation to be rather varied. Nonetheless, in the experimental model used here, the cellular responses to the modulators of Hes1/5 expression were surprisingly reproducible. Conditions resulting in a decrease of Hes1/5 mRNA inevitably led to the outgrowth of additional dendrites/branches. However, because our present results are at variance with the previously reported suppressive effect of high cell density on dendrite elongation (Sestan et al., 1999; Redmond et al., 2000), we assume that the mechanisms controling dendrite morphology downstream of Hes1/5 exhibit phenotype-specific differences.
A New Role for p75NTR in the Regulation of Dendrite Growth
It is already well established that neurotrophins can play a critical role in the morphogenesis of neurons by controlling the outgrowth and ramification of axons and dendrites (see McAllister et al., 1999; Huang and Reichardt, 2001 for review). Most of the previously described actions were mediated by Trk receptors. In particular it was shown that NGF promoted neurite outgrowth by binding to TrkA, (see Kaplan, 1998; Yamashita et al., 1999; Nakamura et al., 2002; Katoh and Negishi, 2003). These studies identified molecular links between TrkA and Rho GTPases as well as downstream targets of the latter. Interestingly, it has been shown that in cultured hippocampal neurons TrkA mutations interfered with neurite elongation (Aibel et al., 1998). In peripheral neurons, p75NTR proved to be essential for axon growth. Hence, p75NTR-knockout mice presented reduced axon elongation (Bentley and Lee, 2000; McQuillen et al., 2002). p75NTR may control axonal outgrowth by promoting a signal through the small GTPases (Wong et al., 2002; Yamashita et al., 2002; Yamashita and Tohyama, 2003) or the ceramide pathways (Brann et al., 1999). Contrasting with these studies on axon growth, the effects of p75NTR on dendrite morphology have so far remained elusive. The present experiments made clear that p75NTR activity can indeed be a potent factor in the regulation of dendrite structure.
As the most important outcome of our current work we consider the finding that NGF/p75NTR can substitute for the signals normally derived from Notch (Figure 8). In hippocampal neurons, the efficacy of the NGF/p75NTR pathway was inversely related to the efficacy of the Notch pathway. In other words, an NGF/p75-mediated decrease in dendrite number and an increase in dendrite length can only been expected when Hes1/5 activity is released from the control by Notch. This was the case in low-density cultures, and it may happen after massive neuron death or any other condition leading to deactivation or desensitization of Notch.
Clarifying the mechanisms by which neurotrophins regulate dendrite morphology is crucial for reaching a complete understanding of the cellular interactions that enable a developing network to adjust to the gain or loss of neurons. Identification of p75NTR activity as another starting point for Hes-dependent signaling should help to direct further studies on the development and structural plasticity of dendrites.
Acknowledgments
We thank Vanessa Blanca and Elisa Baides (Madrid) and David Betances and Karin Przezdzecki (Berlin) for excellent technical assistance. We also thank Phil Jones and Moses Chao for providing a pIRES vector expressing HES6 and the anti-p75 antiserum. This work was financed by grants PB08477 from Ministerio deCiencia y Tecnología, PX-0124 from Comunidad deMadrid, and 00/003-00 from Fundació La Caixa, Barcelona. Further support was received from the Deutsche Forschungsgemeinschaft (GRK 238 travel grant to P.S.-C. and project grant Ro925/2 to R.G.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-05-0438. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0438.
References
- Aibel, L., Martin-Zanca, D., Perez, P., and Chao, M. V. (1998). Functional expression of TrkA receptors in hippocampal neurons. J. Neurosci. Res. 54, 424-431. [DOI] [PubMed] [Google Scholar]
- Bae, S., Bessho, Y., Hojo, M., and Kageyama, R. (2000). The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development 127, 2933-2943. [DOI] [PubMed] [Google Scholar]
- Baker, R. E., Dijkhuizen, P. A., Van Pelt, J., and Verhaagen, J. (1998). Growth of pyramidal, but not non-pyramidal, dendrites in long-term organotypic explants of neonatal rat neocortex chronically exposed to neurotrophin-3. Eur. J. Neurosci. 10, 1037-1044. [DOI] [PubMed] [Google Scholar]
- Bash, J., Zong, W. X., Banga, S., Rivera, A., Ballard, D. W., Ron, Y., and Gelinas, C. (1999). Rel/NF-kappaB can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. EMBO J. 18, 2803-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley, C. A., and Lee, K. F. (2000). p75 is important for axon growth and Schwann cell migration during development. J. Neurosci. 20, 7706-7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann, A. B., Scott, R., Neuberger, Y., Abulafia, D., Boldin, S., Fainzilber, M., and Futerman, A. H. (1999). Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J. Neurosci. 19, 8199-8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, B. D., Kaltschmidt, C., Kaltschmidt, B., Offenhauser, N., Bohm-Matthaei, R., Baeuerle, P. A., and Barde, Y. A. (1996). Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science 272, 542-545. [DOI] [PubMed] [Google Scholar]
- Chao, M. V., and Hempstead, B. L. (1995). p75 and Trk: a two-receptor system. Trends Neurosci. 18, 321-326. [PubMed] [Google Scholar]
- Coffey, E. T., Hongisto, V., Dickens, M., Davis, R. J., and Courtney, M. J. (2000). Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J. Neurosci. 20, 7602-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins, J., Vernon, A. E., Zhang, Y., Philpott, A., and Jones, P. H. (2002). Hes6 regulates myogenic differentiation. Development 129, 2195-2207. [DOI] [PubMed] [Google Scholar]
- Dotti, C. G., Sullivan, C. A., and Banker, G. A. (1988). The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa, L., Ingles-Esteve, J., Aguilera, C., and Bigas, A. (2003). Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J. Biol. Chem. 278, 32227-32235. [DOI] [PubMed] [Google Scholar]
- Franklin, J. L., Berechid, B. E., Cutting, F. B., Presente, A., Chambers, C. B., Foltz, D. R., Ferreira, A., and Nye, J. S. (1999). Autonomous and non-autonomous regulation of mammalian neurite development by Notch1 and Delta1. Curr. Biol. 9, 1448-1457. [DOI] [PubMed] [Google Scholar]
- Goslin, K., Asmussen, H., and Banker, G. (1998). Rat hippocampal neurons in low density cultures. In: Culturing Nerve Cells, ed. G. Banker and K. Goslin, Cambridge, MA: Massachusetts Institute of Technology, 339-370.
- Huber, L. J., and Chao, M. V. (1995). Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev. Biol. 167, 227-238. [DOI] [PubMed] [Google Scholar]
- Huang, E. J. and Reichardt, L. F. (2001). Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso, T.., Kedes, L., and Hamamori, Y. (2003). HES and HERP families: multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 194, 237-255. [DOI] [PubMed] [Google Scholar]
- Jin, X., Hu, H., Mathers, P. H., and Agmon, A. (2003). Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J. Neurosci. 23, 5662-5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D. R. (1998). Studying signal transduction in neuronal cells: the Trk/NGF system. Prog. Brain Res. 117, 35-46. [DOI] [PubMed] [Google Scholar]
- Katoh, H., and Negishi, M. (2003). RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 424, 461-464. [DOI] [PubMed] [Google Scholar]
- Koyano-Nakagawa, N., Kim, J., Anderson, D., and Kintner, C. (2000). Hes6 acts in a positive feedback loop with the neurogenins to promote neuronal differentiation. Development 127, 4203-4216. [DOI] [PubMed] [Google Scholar]
- Ladiwala, U., Lachance, C., Simoneau, S. J., Bhakar, A., Barker, P. A., and Antel, J. P. (1998). p75 neurotrophin receptor expression on adult human oligodendrocytes: signaling without cell death in response to NGF. J. Neurosci. 18, 1297-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. Z., Yao, S. Y., Veach, R. A., Torgerson, T. R., and Hawiger, J. (1995). Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 270, 14255-14258. [DOI] [PubMed] [Google Scholar]
- McAllister, A.K., Lo, D. C., and Katz, L. C. (1995). Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791-803. [DOI] [PubMed] [Google Scholar]
- McAllister, A. K., Katz, L. C., and Lo, D. C. (1997). Opposing roles for endogenous BDNF and NT-3 in regulation cortical dendritic growth. Neuron 18, 767-778. [DOI] [PubMed] [Google Scholar]
- McAllister, A. K., Katz, L. C., and Lo, D. C. (1999). Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295-318. [DOI] [PubMed] [Google Scholar]
- McQuillen, P. S., DeFreitas, M. F., Zada, G., and Shatz, C. J. (2002). A novel role for p75NTR in subplate growth cone complexity and visual thalamocortical innervation. J. Neurosci. 22, 3580-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, S. J., Perez, S. E., Qiao, Z., Verdi, J. M., Hicks, C., Weinmaster, G., and Anderson, D. J. (2000). Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499-510. [DOI] [PubMed] [Google Scholar]
- Mumm, J. S., and Kopan, R. (2000). Notch signaling: from the outside in. Dev. Biol. 228, 151-165. [DOI] [PubMed] [Google Scholar]
- Nakamura, T., Komiya, M., Sone, K., Hirose, E., Gotoh, N., Morii, H., Ohta, Y., and Mori, N. (2002). Grit, a GTPase-activating protein for the Rho family, regulates neurite extension through association with the TrkA receptor and N-Shc and CrkL/Crk adapter molecules. Mol. Cell. Biol. 22, 8721-8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond, L., and Ghosh, A. (2001). The role of Notch and Rho GTPase signaling in the control of dendritic development. Curr. Opin. Neurobiol. 11, 111-117. [DOI] [PubMed] [Google Scholar]
- Redmond, L., Oh, S. R., Hicks, C., Weinmaster, G., and Ghosh, A. (2000). Nuclear Notch1 signaling and the regulation of dendritic development. Nat. Neurosci. 3, 30-40. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tébar, A., Dechant, G., and Barde, Y.-A. (1990). Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron 4, 487-492. [DOI] [PubMed] [Google Scholar]
- Sestan, N., Artavanis-Tsakonas, S., and Rakic, P. (1999). Contact-dependent inhibition of cortical neurite growth mediated by Notch signaling. Science 286, 741-746. [DOI] [PubMed] [Google Scholar]
- Solecki, D. J., Liu, X. L., Tomoda, T., Fang, Y., and Hatten, M. E. (2001). Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron 31, 557-568. [DOI] [PubMed] [Google Scholar]
- Weinmaster, G. (2000). Notch signal transduction: a real rip and more. Curr. Opin. Genet. Dev. 10, 363-369. [DOI] [PubMed] [Google Scholar]
- Whitford, K. L., Dijkhuizen, P., Polleux, F., and Ghosh, A. (2002). Molecular control of cortical dendrite development. Annu. Rev. Neurosci. 25, 127-149. [DOI] [PubMed] [Google Scholar]
- Wong, S. T., Henley, J. R., Kanning, K. C., Huang, K. H., Bothwell, M., and Poo, M. M. (2002). A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 5, 1302-1308. [DOI] [PubMed] [Google Scholar]
- Yamashita, H., Avraham, S., Jiang, S., Dikic, I., and Avraham, H. (1999). The Csk homologous kinase associates with TrkA receptors and is involved in neurite outgrowth of PC12 cells. J. Biol. Chem. 274, 15059-15065. [DOI] [PubMed] [Google Scholar]
- Yamashita, T., Higuchi, H., and Tohyama, M. (2002). The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 157, 565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, T., and Tohyama, M. (2003). The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 6, 461-467. [DOI] [PubMed] [Google Scholar]