The Sorting Nexin 3 Retromer Pathway Regulates the Cell Surface Localization and Activity of a Wnt-Activated Polycystin Channel Complex (original) (raw)
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is caused by inactivating mutations in PKD1 (85%) or PKD2 (15%). The ADPKD proteins encoded by these genes, polycystin-1 (PC1) and polycystin-2 (PC2), form a plasma membrane receptor–ion channel complex. However, the mechanisms controlling the subcellular localization of PC1 and PC2 are poorly understood. Here, we investigated the involvement of the retromer complex, an ancient protein module initially discovered in yeast that regulates the retrieval, sorting, and retrograde transport of membrane receptors. Using yeast two-hybrid, biochemical, and cellular assays, we determined that PC2 binds two isoforms of the retromer-associated protein sorting nexin 3 (SNX3), including a novel isoform that binds PC2 in a direct manner. Knockdown of SNX3 or the core retromer protein VPS35 increased the surface expression of endogenous PC1 and PC2 in vitro and in vivo and increased Wnt-activated PC2-dependent whole-cell currents. These findings indicate that an SNX3-retromer complex regulates the surface expression and function of PC1 and PC2. Molecular targeting of proteins involved in the endosomal sorting of PC1 and PC2 could lead to new therapeutic approaches in ADPKD.
Keywords: ADPKD, polycystic kidney disease, retromer, PKD1, PKD2
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease. It is a major cause of ESRD worldwide and accounts for approximately 10% of all patients on RRT.1 ADPKD is caused by germline mutations in PKD1 or PKD2. The ADPKD proteins, polycystin-1 (PC1 or PKD1) and polycystin-2 (PC2, PKD2 or TRPP2), form a heterodimeric protein complex which is essential for maintaining tubular differentiation, polarity and diameter.2
Despite the demonstration of a PC1-PC2 complex in heterologous or native systems,3–5 the subcellular localization of both proteins only overlaps in a few domains of the plasma membrane or the primary cilium.6 Some studies have reported that PC2 can function independently of PC1 as a cell surface receptor–operated Ca2+-permeable channel complexed with other members of the TRP superfamily of ion channels such as TRPC1,7 TRPV4,8,9 or both.10 We recently reported that the cell surface pool of PC1-PC2 in mouse embryonic fibroblasts and transfected CHO-K1 cells can be activated by Wnt proteins, suggesting that the PC1-PC2 complex can function as a ligand-activated channel complex.11 In addition to the cell surface pool of PC2, the majority of PC2, but not PC1, can be detected in the ER,4,12 where it enhances ER Ca2+ release in response to G protein–coupled receptors linked to the production of IP3 as a second messenger.13,14 Therefore, PC2 is likely to have multiple roles in Ca2+ signaling depending on its subcellular localization, particularly in relation to PC1.3,4,12,15–18
Here, we report for the first time that PC1 and PC2 are retromer cargos and the surface expression of PC2 is regulated by distinct SNX3 isoforms at the stages of clathrin-mediated endocytosis (SNX3–102) and retrograde transport and sorting (in case of the classic SNX3–162 and VPS35) in the endosomal pathway.
Results
SNX3–102, a Novel SNX3 Isoform, Is a New Interacting Partner of PC2
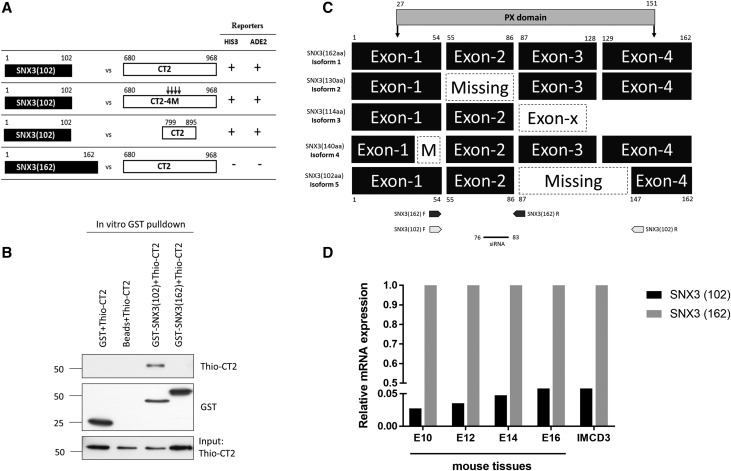
To identify novel regulators of PC2 sorting and trafficking, we performed a yeast two-hybrid (Y2H) screen of a mouse E17 cDNA library using a region (aa799–895) of the PC2 C-terminus (CT2) as bait. This region contains several important functional motifs including several important serine phosphorylation residues (Ser801, Ser812, Ser829), a coiled-coil domain (CC2, aa833–871) essential for CT2 dimerization and interaction with the C-terminus of PC1 (CT1), and a heterodimerization sequence (aa871–895) for PC1 binding.5,17,19 Among several positive interactors (data not shown), we identified SNX3–102, a novel isoform of SNX3. We confirmed its interaction to CT2 (aa 680–968) in directed Y2H assays (Figure 1A). Mutation of CC2 (4M) which disrupts CT2 dimerization and PC1 recognition,5 however, had no effect on binding. Interestingly, CT2 did not bind to the classic SNX3 isoform (SNX3–162, isoform 1) (Figure 1A). To confirm that the interaction between SNX3–102 and CT2 was direct, we carried out in vitro GST pull-down assays using recombinant GST-SNX3 and Thio-CT2 proteins. Consistent with Y2H assays, GST-SNX3–102 but not GST-SNX3–162 showed direct binding to both Thio-CT2 (799–871) (not shown) and Thio-CT2 (680–968) (Figure 1B).
Figure 1.
Identification of a new SNX3 isoform and its interaction with PC2. (A) Y2H screens of an E17 embryonic mouse cDNA library using a portion (aa799–895) of the C-terminus of human PKD2 (CT2) as bait identified a novel isoform of SNX3. Yeast cotransformants were retested on selective media to activate HIS3 and ADE2 selection markers. The new isoform SNX3–102 interacted with CT2 (799–871) and full-length CT2 (680–968) but was unaffected by mutations (4M) disrupting the coiled-coil domain (CC2) which mediates CT2 homodimerization. In contrast, no interaction between CT2 and SNX3–162 was detected. (B) In vitro GST pull-down assays indicate that GST-SNX3–102 but not GST-SNX3–162 bound to recombinant Thio-CT2 directly. Neither GST nor glutathione beads bound to Thio-CT2. (C) Exon map showing the alternative splicing of different human isoforms of SNX3. Compared with the classic isoform SNX3–162, the new isoform SNX3–102 is missing exon 3 and part of exon 4. The PX domain region is marked by the shaded bar. Numbers indicate the amino-acid boundaries for different exons and domains. Dotted boxes represent missing exons. The two isoforms were amplified independently using specific primers indicated by arrows on the figure. The sequence targeted by the SNX3 siRNA is indicated. Swiss-Prot Accession numbers: O60493 (isoform 1); O60493–2 (isoform 2); O60493–3 (isoform 3); O60493–4 (isoform 4). (D) Ratio of SNX3–102 versus SNX3–162 in developing mouse embryos between E10 and E16. The relative mRNA level of SNX3–102 was approximately 3%–5% that of SNX3–162 (set to 100%) at each developmental stage. A similar ratio between both isoforms was found in mouse IMCD cells. Expression of murine SNX3–102 mRNA showed a trend to increase during development. The relative mRNA level was calculated in relation to that of HPRT.
Sequence analysis revealed that compared with SNX3–162, SNX3–102 lacked exon 3 and a part of exon 4 due to alternative splicing at a cryptic splice site (Figure 1C, Supplemental Figure 1). This deletes most of the PX domain (aa27–151). Because four other SNX3 isoforms have previously been reported, we have named this new isoform as isoform 5. Q-PCR analysis of developing mouse embryos (E10-E16) and a number of mouse and human kidney cell lines confirmed that SNX3–102 is widely expressed but at much lower levels (3%–5%) relative to SNX3–162 (Figure 1D) or other kidney cell lines (Supplemental Figure 2C).
The Disrupted PX Domain in SNX3–102 Cannot Mediate Phospholipid Binding
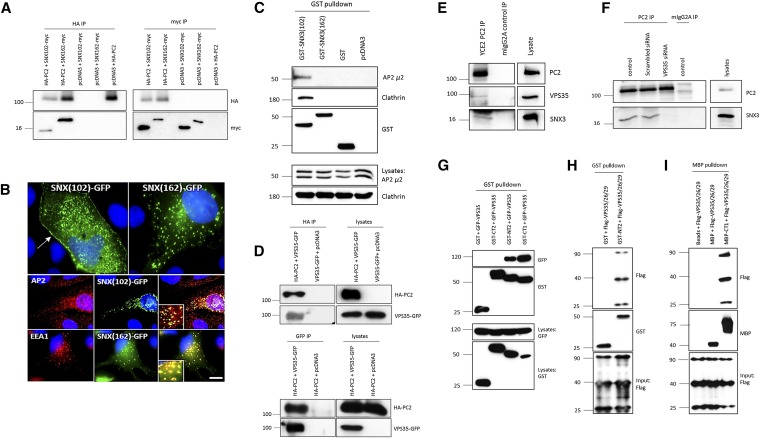
We next performed coimmunoprecipitation experiments to confirm the interactions between full-length SNX3–102 and PC2. Using HEK293 cells, SNX3–102 coimmunoprecipitated with PC2 (Figure 2A). Unexpectedly, binding was also observed with SNX3–162 (Figure 2A). This raised the possibility of an indirect interaction or binding to a different domain of PC2. To better understand this discrepancy, we decided to better characterize SNX3–102. SNX3–162 has been shown to be recruited to endosomes in a PtdIns(3)P-dependent manner.20 Binding has been demonstrated to be mediated by a PX domain although evidence of a second noncanonical PtdIns(3)P binding site in the C-terminus (aa147–162) has been reported.21 To determine whether the missing exons in SNX3–102 were functionally important for phospholipid binding, protein-lipid overlay assays were carried out using commercial lipid strips. As predicted, GST-SNX3–162 bound strongly to PtdIns(3)P and weakly to PtdIns(5)P (Supplemental Figure 2A). In contrast, GST-SNX3–102 showed no lipid binding under the same conditions. These results are consistent with the hypothesis that an intact PX domain is essential for phospholipid binding (and endosomal recruitment). Thus, SNX3–102 is likely to function in a different compartment from that of SNX3–162.
Figure 2.
SNX3–162 binds to the N-terminus of PC2 via the core retromer protein VPS35. (A) Coimmunoprecipitation assays between full-length HA-PC2 and myc-SNX3–102 or myc-SNX3–162 in transfected HEK293 cells. Both isoforms bound to full-length PC2 in both directions. (B) A small plasma membrane pool of GFP-SNX3–102 (70% transfected cells show membrane expression) could be visualized in MDCK II cells (arrow) whereas GFP-SNX3–162 did not show clear surface expression under the same conditions (top panel). GFP-SNX3–102 colocalized with the endocytosis-associated protein AP2 (middle panel). In contrast, GFP-SNX3–162 colocalized with the early endosome marker, EEA1 (bottom panel). Similar findings were observed in HeLA, MDCK, and IMCD3 cells (not shown). Scale bars, 10 _μ_m. (C) GST pull-down assays from cell lysates showing that GST-SNX3–102 but not GST-SNX3–162 binds to endogenous clathrin and the AP2_μ_2 subunit. Loading controls and binding of GST fusion proteins to beads is shown in the lower panels. The doublet bands observed for AP2_μ_2 could represent different splice forms or phosphorylation status. (D) Coimmunoprecipitation assays between full-length HA-PC2 and full-length GFP-VPS35 in transfected HEK293 cells. VPS35 bound to full-length PC2 in both directions. (E) Immunoprecipitation of endogenous PC2 by the PC2 mAb YCE2 (Santa Cruz) from LLCPK1 cells coimmunoprecipitated endogenous VPS35 and SNX3 in the same complex. An irrelevant mIgG2A antibody was used as a negative control. (F) siRNA knockdown of VPS35 abolished the binding of SNX3 to endogenous PC2 in co-IP assays from LLCPK1 cells. A scrambled siRNA control had no effect on this interaction. An irrelevant mIgG2A antibody was used as a negative control for the IP. (G) GST pull-down assays from HEK293 cell lysates showing that a recombinant GST protein encoding the N-terminus of PC2 (GST-NT2, aa1–223) but not the C-terminus of PC2 (GST-CT2, aa 680–968) bound to coexpressed GFP-VPS35. Binding of the C-terminus of PC1 (GST-CT1, aa 4107–4303) to GFP-VPS35 was also detected. (H) In vitro GST binding assays using purified GST-NT2 and recombinant FLAG-VPS35/26/29-His proteins confirmed a direct interaction between both proteins. GST protein was used as a negative control. (I) In vitro binding between recombinant MBP-CT1 and recombinant FLAG-VPS35/26/29-His proteins indicates a direct interaction between the two proteins. MBP protein was used as a negative control.
The Subcellular Localization of SNX3–102 and SNX3–162 Are Distinct
Expression of GFP-tagged SNX3 isoforms in several cell lines revealed a striking difference in their localization. GFP-SNX3–162 showed a strong overlapping distribution to EEA1-positive early endosomes whereas GFP-SNX3–102 colocalized with adaptor protein-2 (AP2) which is enriched in clathrin-coated endocytic vesicles (Figure 2B). Consistent with this, we observed GFP-SNX3–102 expression at or close to the plasma membrane in the majority (70% or more) of transiently transfected cell lines including HeLA, MDCK, LLC-PK1, and IMCD3 (Figure 2B and data not shown). Taken together, these results suggest that SNX3–102 could be involved in AP2 or clathrin-mediated endocytosis of PC2 whereas SNX3–162 is involved in its retrograde transport and sorting in early endosomes. GST pull-down assays from HEK293 using coexpressed GST-SNX3–102 revealed specific interactions with endogenous clathrin heavy chain (CHC) and the AP2 _μ_2 (AP2M1) subunit (Figure 2C). In contrast, GST-SNX3–162 did not interact with either protein consistent with its localization to early endosomes.
The Retromer Complex Protein VPS35 Binds Directly to the N-Terminus of PC2
Because SNX3–162 was localized to early endosomes and could associate with full-length PC2, we investigated the possibility that it could bind to either the N- or C-terminus of PC2 (NT2 or CT2) indirectly via the retromer complex. The retromer complex originally identified in yeast comprises two subcomplexes: a trimer (Vps26–29–35) which functions as a cargo-selective adaptor and a membrane deforming subcomplex consisting of an SNX-BAR heterodimer. We found that the full-length HA-PC2 and GFP-VPS35 proteins coimmunoprecipitated when expressed in HEK293 cells (Figure 2D) and confirmed that endogenous PC2, VPS35, and SNX3–162 were present in the same complex in LLC-PK1 cells (Figure 2E). Significantly, knockdown of endogenous VPS35 abolished the binding between PC2 and SNX-162 in LLC-PK1 (Figure 2F, Supplemental Figure 2E). To define the binding domain in PC2 mediating the interaction with VPS35, GST pull-down assays from whole-cell extracts were next performed. This revealed a clear interaction between GFP-VPS35 and GST-NT2 (aa1–223) but not with GST-CT2 (aa680–968) (Figure 2G). Finally, we confirmed that the interaction between NT2 and VPS35 was direct using a recombinant protein containing the entire VPS35, VPS26, and VPS29 trimer (Figure 2H).
To investigate whether the involvement of the retromer complex in PC2 trafficking could be extended to PC1, we investigated the interaction between VPS35 and the C-terminus of PC1 (CT1). In HEK293 cells, binding between GFP-VPS35 and GST-CT1 (aa4107–4303) was detected (Figure 2G). Similar to NT2, we confirmed a direct interaction between recombinant MBP-CT1 and the FLAG-VPS35/26/29 proteins in vitro (Figure 2I). Interestingly, coimmunoprecipitation could not be confirmed between GFP-VPS35 and full-length PC1 (not shown), suggesting that the interaction between the full-length proteins could be conformationally restricted or depend on the presence of PC2 in vivo. Our results, however, are consistent with PC2 being a direct retromer cargo.
Knockdown of SNX3 or VPS35 Increases PC2 Surface Expression
Because our data suggested a potential role for SNX3 in the recycling of plasma membrane PC2, we hypothesized that knockdown of endogenous SNX3 would affect the surface expression of endogenous PC2. In previous studies, we and others had shown that in kidney epithelial cells the vast majority of PC2 is localized in the ER, but a small percentage of PC2 (<10%) can be detected at the plasma membrane and ciliary membrane.4,7,18,22 This cell surface pool of PC2 is functional, because it has been shown to contribute to basal conductance and can be further activated by EGF, G protein–coupled receptor agonists,23 and Wnt ligands11 in diverse cell types.
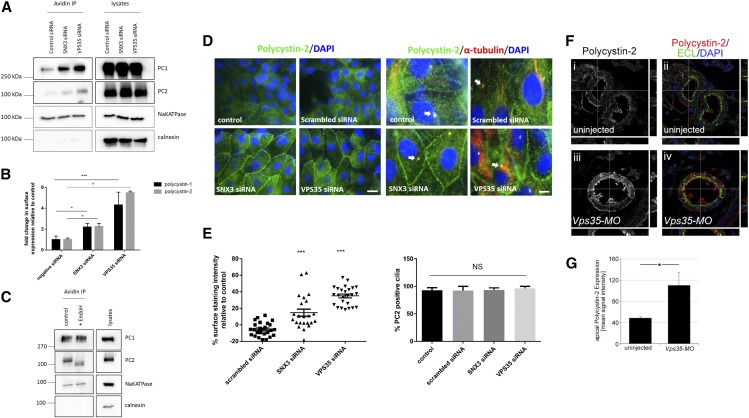
Efficient knockdown of both SNX3 isoforms was achieved using sequence-specific siRNA in LLC-PK1 cells (Supplemental Figure 2, B and D). Because the endogenous expression of SNX3–102 was not easily detectable by immunoblotting, we confirmed knock-down of this isoform at the mRNA level by semiquantitative RT-PCR using isoform-specific primers (Supplemental Figure 2B). Significantly, we observed an increase in surface PC2 and PC1 after SNX3 knockdown by both surface biotinylation (Figure, 3 A and B) and of PC2 by immunofluorescence labeling (Figure 3, D and E). Unfortunately, increased cell surface expression of endogenous PC1 by indirect immunofluorescence could not be achieved, because several available PC1 antibodies did not work in this assay. However, deglycosylation analysis of surface labeled PC1 and PC2 complex confirmed that surface labeled PC1 was EndoH-resistant whereas labeled PC2 remained fully EndoH-sensitive (Figure 3C). As expected, the membrane protein Na+ K+ ATPase was EndoH-resistant in the same assay (Figure 3C).
Figure 3.
Knockdown of SNX3 or VPS35 leads to increased surface expression of endogenous PC1 and PC2 in LLC-PK1 cells and in Xenopus pronephric tubules. (A) Surface expression of endogenous PC1 and PC2 in LLC-PK1 cells was increased after selective knockdown of SNX3 or VPS35 siRNA (20 nM) compared with a scrambled control siRNA. Surface biotinylated PC1 and PC2 was compared with total PC1/PC2. Na+-K+-ATPase and calnexin were used as positive and negative controls, respectively, for surface biotinylation. (B) The ratio of biotinylated/total PC1 calculated from four independent experiments showed a significant increase after SNX3 (P<0.05) and VPS35 (P<0.05) knockdown: Scrambled siRNA = 0.139±0.047; SNX3 siRNA = 0.306±0.048; VPS35 siRNA = 0.600±0.171. Similarly, a significant increase in biotinylated PC2 was observed after SNX3 (P<0.05) and VPS35 (P<0.01) knockdown in triplicate experiments: Scrambled siRNA = 0.235±0.035; SNX3 siRNA = 0.527±0.076; VPS35 siRNA = 1.289±0.038. The increase in biotinylated PC1 and PC2 was quantitatively greater after VPS35 knockdown compared with SNX3 despite a more complete inhibition of the latter. (C) EndoH digestion of surface biotinylated proteins in LLCPK1 cells. Whereas PC1 and Na+ K+ ATPase were EndoH resistant, surface labeled PC2 was EndoH sensitive. Calnexin, an ER resident protein, acts as a negative control for the biotinylation assay. Representative experiment of two shown. (D) Endogenous PC2 expression in LLC-PK1 cells detected by widefield immunofluorescence microscopy using a specific PC2 antibody (g20, Santa Cruz, CA) as previously described.18 A proportion of PC2 was detectable in the lateral plasma membrane as well as in primary cilia colabeled with an antibody to acetylated tubulin (arrows). Left: A clear increase in plasma membrane PC2 staining was observed after knockdown of SNX3 or VPS35 relative to untransfected controls or cells transfected with a scrambled siRNA. Right: No change in cilia PC2 expression was detectable. Images are representative of three separate experiments. Scale bars, 10 _μ_m. (E) Surface staining intensity was calculated as a percentage relative to control untransfected cells and expressed as bar diagrams. Left: A significant increase in surface expression of PC2 was seen after SNX3 and VPS35 knockdown (P<0.01): Scrambled siRNA = −5.54%±1.68, _n_=30; SNX3 siRNA = 14.74%±4.24, _n_=30; VPS35 siRNA = 35.01%±2.28, _n_=30. Right: there was no significant difference in cilia localization of PC2: control siRNA = 92.88±4.52, _n_=50; scrambled siRNA = 92.42%±7.58, _n_=32; SNX3 siRNA = 93.3%±3.88, _n_=31; VPS35 siRNA = 96.43%±3.57, _n_=39. (F) Uninjected Xenopus controls (i, ii) and embryos injected unilaterally with a total of 1.6 pmol Vps34-MO (iii, iv) were analyzed at stage 40 by PC2 immunofluorescence (red). ECL was used to visualize the apical side of the proximal tubules (green) and nuclei were counterstained with DAPI (blue). Images were analyzed by confocal microscopy and all three z planes are shown. The white lines indicate the position used for x,z and y,z planes, respectively. (G) Bar diagram to quantify the apical pixel intensity of PC2 staining. Error bars represent SD, and statistical significance was determined using paired t test (* P<0.05). Note that the pronephric tubules of Vps35 morphants are dilated, which is likely due to the effect of the retromer complex on the secretion of Wnt ligands and their role in kidney tubulogenesis.11,26 *, P<0.05; ***, P<0.001.
To substantiate our findings, we next depleted endogenous VPS35 by siRNA. Similar to SNX3, this resulted in a marked effect on surface PC1 and PC2 expression by surface biotinylation (Figure 3, A and B) and on PC2 by immunofluorescence labeling (Figure 3, D and E). This effect was even more pronounced than the knockdown of SNX3 (both isoforms). Finally, we did not observe detectable changes in ciliary expression of PC2 after knockdown of SNX3 or VPS35 (Figure 3D). Taken together, these results suggest that PC2 and PC1 recycling to the plasma membrane is likely to be mediated to a large extent by the SNX3–162-VPS35 interaction at the level of early endosomes.
Knockdown of VPS35 in Xenopus Embryos Increases PC2 Surface Expression in Pronephric Kidney Tubules
To examine whether inhibition of the retromer complex had a similar effect on PC2 localization in vivo, we turned to Xenopus as a model for PKD.24,25 In Xenopus, PC2 is expressed along the entire length of the pronephric kidney and the protein can be detected by immunofluorescence confocal microscopy both at the cell membrane and the apical cilia (Figure 3F, Supplemental Figure 3A).24 To inhibit the retromer complex, embryos were injected at the two-cell stage with an antisense morpholino oligomer targeting VPS35 (VPS35-MO), which has been characterized previously.26 At late tail bud stage, knockdown of VPS35 confirmed at the protein level (Supplemental Figure 3C) resulted in increased staining for PC2 protein in kidney tubules at the cell membrane both apically and basolaterally (Figure 3F). This was confirmed by quantification of the apical PC2 signal in the proximal tubules using counterstaining with Erythrina Cristagalli Lectin (ECL, Figure 3G). Finally, as expected from the ubiquitous expression of Vps35, the upregulation of PC2 protein was not limited to the pronephric kidney only, but was also to be observed in other tissues expressing PC2 (e.g., notochord, data not shown).
Knockdown of VPS35 Increases Wnt-Activated PC2 Whole-Cell Current Density
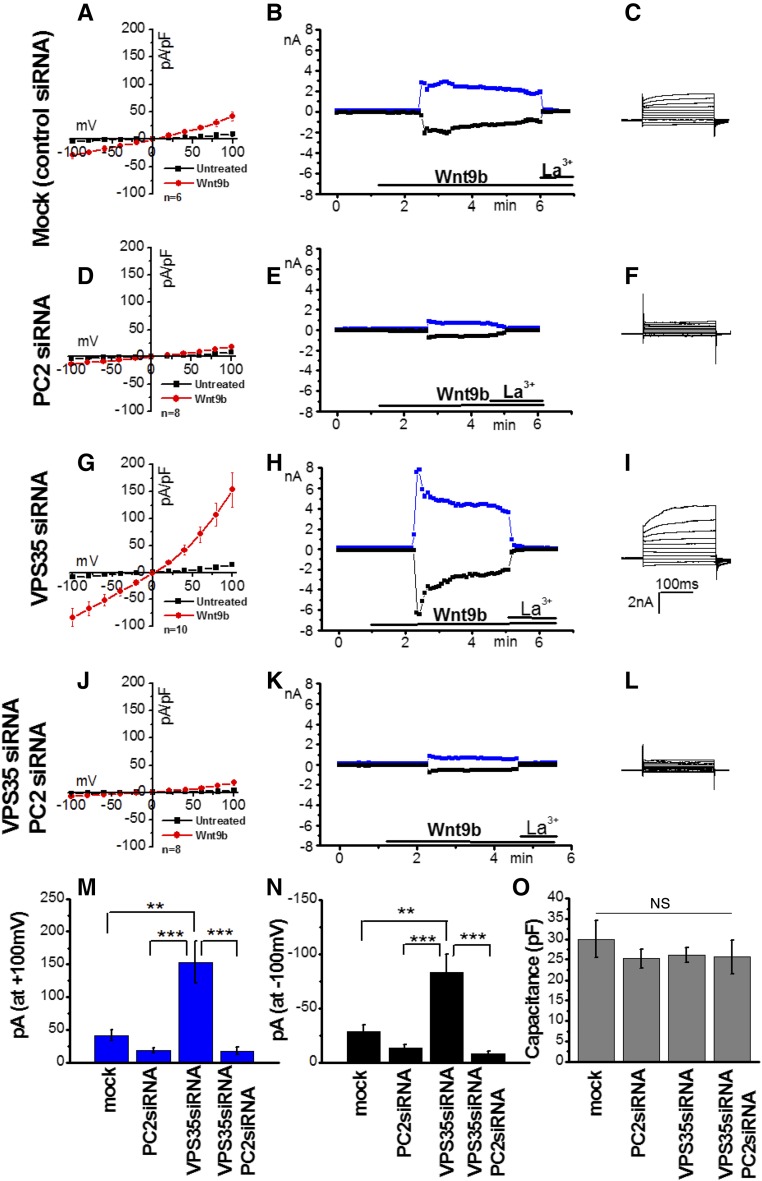
Next, we tested whether retromer function could regulate Wnt-activated PC2-dependent Ca2+ signaling. In a recent paper, we showed that purified Wnt9b induced large PC2-dependent whole-cell currents in mouse embryonic fibroblasts (MEFs) and transfected CHO-K1 cells.11 Here, we tested whether Wnt9b could also induce endogenous PC2-dependent whole-cell currents in LLC-PK1 cells. We confirmed that LLC-PK1 expressed endogenous PC1 (Supplemental Figure 4A) and responded to Wnt9b in a dose-dependent manner. Time course experiments using purified Wnt9b (12.5 nM for a 40 kD protein) induced time-dependent and La3+-inhibited whole-cell currents (Figure 4, A–C). Dose-response experiments indicated that Wnt9b or Wnt5a activated whole-cell inward (−100 mV) and outward (+100 mV) currents with values ranging from 45 to 76 ng/ml (Supplemental Figure 4B). This effect was dependent on endogenous PC2 because knockdown of PC2 completely suppressed the response to Wnt9b27 (Figure 4, D–F).
Figure 4.
Depletion of VPS35 increases functional expression of PC2. (A–L) Current-voltage (I-V [A,D,G, and J]), time course (B,E,H, and K), and representative step whole-cell currents (C,F,I, and L) in cells transfected with a scrambled control siRNA (mock, _n_=6 [A]), PC2-specific siRNA (_n_=8 [B]), VPS35-specific siRNA (_n_=10 [C]), or VPS35- and PC2-specific siRNAs (_n_=8 [D]) in the presence (red IV-curves) or absence (black I-V curves) of 500 ng/ml Wnt9b in the extracellular solution. Outward currents are shown in blue and inward currents are shown in black in time course experiments. Currents were blocked by the addition of 100 _μ_M La3+ added in the extracellular solution at the indicated time points. I-V curves were taken at 4–5 minutes after the addition of Wnt9b, when currents were stable and toward the end of each pulse (195 ms). (M–N) Summary data of outward ([M] at +100mV, blue) and inward current densities ([N] −100mV, black) in all four groups. **P<0.01, ***_P_<0.001. (O) Total cell capacitance in pF in all four groups. ns, _P_>0.01.
Upon depletion of VPS35, Wnt9b (500 ng/ml)–induced whole-cell current density was approximately two-fold larger (Figure 4, G–I) than current density induced by Wnt9b in mock-transfected cells (Figure 4, A–C). To test whether PC2 contributed to Wnt9b-induced currents in VPS35-depleted cells, PC2 was downregulated together with VPS35. Doubly transfected cells showed diminished response to Wnt9b (Figure 4, J–L), indicating that the increased magnitude in Wnt9b-induced currents in VPS35-depleted cells was due to a specific upregulation of plasma membrane PC2. Wnt5a showed a similar effect to Wnt9b (Supplemental Figure 4B). Summary data for inward and outward current densities are shown in Figure 4, M and N. Because VPS35 knockdown could potentially affect the trafficking of other channels to and from the plasma membrane, we compared cell capacitance in mock and VPS35-depleted cells. However, we could not record a measurable difference in total cell capacitance between the two cells types (Figure 4O), indicating that depletion of VPS35 did not have a substantial effect on the trafficking of other channels to and from the plasma membrane. We were unable to test the effect of SNX3 knockdown on PC1-PC2 channel activity because whole-cell currents were unstable for electrophysiology experiments. Overall, these studies showed that depletion of VPS35 did not only increase the cell surface expression of PC2 protein, but also PC2-dependent whole-cell currents.
Discussion
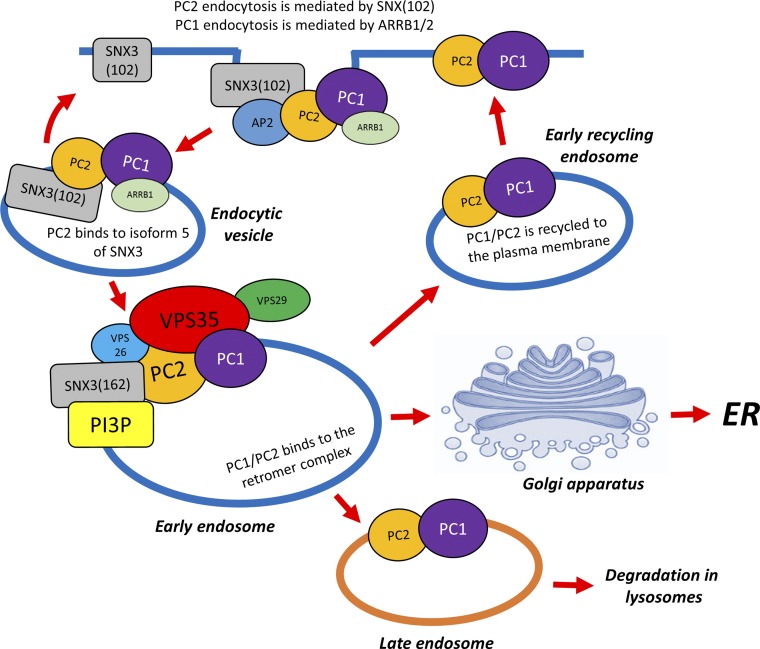
The SNXs are a family of proteins first identified in yeast which share a Phox (PX) domain with affinity for PtdIns(3)P. In mammals, at least 33 SNXs have been identified.28 In yeast, the best characterized function of the retromer complex is to regulate retrograde transport of cytosolic proteins from late endosomes to the _trans_-Golgi Network (TGN). In mammalian cells, however, the functions of the retromer complex are now thought to be broader because components of the retromer complex are found throughout the endosomal network (including clathrin-coated vesicles),29 early endosomes (EGF receptor retrieval)20,30 with enrichment in sorting endosomes where decisions on the fate of endocytosed cargo toward rapid recycling (to the plasma membrane), degradation (in lysosomes), or recycling (via the TGN) are made. Recently, a nonclassic retromer complex consisting of the VPS26–29–35 trimer associated with monomeric SNX3 (rather than with a SNX-BAR heterodimer) was reported.31,32 This SNX3 retromer was shown to be essential for Wnt secretion in C. elegans and D. melanogaster through regulating the retrograde transport of Wntless and was preferentially localized in early endosomes rather than sorting endosomes where SNX-BAR retromer complexes are enriched.32 This spatial segregation could favor sequential cargo handling between both retromer complexes. Our discovery of a novel SNX3 isoform (SNX3–102) refines the role for SNX3 in this pathway by linking clathrin-mediated endocytosis to early endosomes (Figure 5).
Figure 5.
Model of SNX3 and retromer regulated PC1-PC2 trafficking and recycling to the PM. A sequential model of PC2 sorting by the two SNX3 isoforms is displayed. SNX3–102 mediates surface PC2 retrieval and clustering in clathrin-coated vesicles by binding both to surface PC2 and AP2/clathrin. A direct interaction between PC1 and AP2/ARRB1 mediated through the PLAT domain has also been reported.41 PC1-PC2 are then delivered to early endosomes where they can bind to SNX3–162 indirectly via VPS35 to the retromer complex. Sorting of different pools of endosomal PC1-PC2 for recycling to the plasma membrane, retrograde transport to the TGN (retromer), or to lysosomes for degradation takes place here.
Here, we report for the first time that PC1 and PC2 are hitherto unrecognized cargos of an SNX3-retromer complex and demonstrate that VPS35 regulates the surface expression of PC1 and PC2 in vitro and in vivo. An initial Y2H screen using the C-terminus of PC2 as bait identified a novel isoform of SNX3 as a direct PC2 binding partner. We have named this isoform 5. The new isoform (SNX3–102) is likely to be generated by alternative splicing because it excludes exon 3 and part of exon 4 and consequently lacks most of the predicted PX domain found in the classic isoform 1 (SNX3–162). Quantitative gene expression analysis has shown that it is expressed at much lower levels (5%) compared with isoform 1. Strikingly, SNX3–102 was localized predominantly to clathrin-coated vesicles, where it colocalized with AP2. This is in contrast to SNX3–162, which was localized predominantly in EEA-1–positive early endosomes. On the basis of our interaction studies, these findings suggest that PC2 is endocytosed by clathrin-AP2–mediated binding. This observation was confirmed by the fact that SNX3–102, but not SNX3–162, bound to the endogenous clathrin heavy chain (CHC) and the AP2 _μ_2 (AP2M1) subunit in HEK293 cells. However, our results do not exclude possible roles for the other SNX3 isoforms (isoforms 3 and 4 but not 2) which would also have been targeted by the SNX3 siRNA used (Figure 1C).
Importantly, we showed that LLC-PK1 cells depleted of VPS35 showed an increase in Wnt9b-induced current density directly proportional to the increase in the cell surface expression of endogenous PC2 in these cells. Given the fact that PC2 cannot form a Wnt-activated channel without PC1, our data indicate that the limiting factor in PC1-PC2 channel function is PC2 in LLC-PK1 cells. Despite significant efforts, we could not obtain stable currents in cells depleted of SNX3, suggesting that structural changes in the plasma membrane could have affected the quality of the plasma membrane required for reliable current measurements.
An important observation was that the SNX3-retromer complex regulated surface expression and function of PC1 and PC2 at the plasma membrane but did not regulate cilia localization of PC2, and by inference the function of the cilia PC1-PC2 complex. This is not unexpected because distinct mechanisms regulating the forward trafficking of PC2 to either cilia or plasma membranes have also been described.33 Although there is agreement that PC2 is present in several subcellular compartments such as the ER, endosomes, basolateral plasma membrane, and ciliary membrane, there is currently inconclusive evidence that an exclusive function in any one compartment (including primary cilia) is sufficient to prevent cystogenesis.2,5,34 Indeed, reduced cilia PC2 due to impaired Golgi sorting (GMAP210 null mice)35,36 or absent cilia PC1 due to defective PC1 cleavage (_Pkd1_v/v mutant mice)37,38 did not result in an early-onset PKD phenotype in vivo, suggesting that different functional pools of the PC1-PC2 complex are important during kidney development, in different tubular segments37 and, by inference, in the mature organ.34 In addition, because our experiments were not conducted under conditions that promoted optimal cilia formation, it is probable that the Wnt-activated Ca2+ currents we measured can be attributed to plasma membrane PC1-PC2 and not the cilia PC1-PC2 complex. This is in agreement with recent work suggesting that the PC1-PC2 cilia signal is unlikely to be either activated by flow or mediated by Ca2+.39 Also, because we were unable to identify an EndoH-resistant PC2 cilia fraction reported by one group,38 our observations are most likely relevant to the plasma membrane PC1-PC2 complex.4,40
In summary, our results support a sequential model whereby SNX3–102 first regulates PC2 endocytosis by directly binding PC2 and AP2/clathrin to cluster PC2 in clathrin-coated vesicles before relaying it to early endosomes where SNX3–162 and VPS35 regulate its sorting and fate, together with that of PC1 (Figure 5). In a recent paper, we have reported an alternative pathway for PC1-PC2 endocytosis via an AP2/ARBB1 mechanism mediated by direct binding to the PC1-PLAT domain; here, however, endocytosis from plasma membrane and cilia membranes was similarly affected and dependent on PKA-phosphorylation of PLAT at a specific residue (Ser3164).41 The precise relationship between these two retrieval pathways is unclear but likely represents both constitutive (VPS35-PC2) and regulated (ARBB1-PC1) mechanisms. In any case, we suggest that a disruption in retrograde transport to the TGN and possibly clathrin-mediated endocytosis leads to increased surface delivery of PC1 and PC2 by promoting rapid recycling from early endosomes. Finally, our study could have potential therapeutic applications. It is possible that compounds that downregulate SNX3 or VPS35 expression, or which inhibit their recruitment or function,21,30 could have therapeutic benefit in patients with ADPKD with hypomorphic mutations by enhancing PC1-PC2 surface expression.
Concise Methods
All chemicals were purchased from Sigma (Poole, UK) unless otherwise stated. Antibodies were purchased from the following suppliers: mouse anti-Thio (Invitrogen), mouse anti-GST (Serotec), mouse anti–Clathrin HC (BD Biosciences), rabbit anti-SNX3 (Abcam, Inc.), mouse anti-EEA1 (BD Biosciences), goat anti-PC2 (G20) (Santa Cruz Biotechnology), mouse anti–AP2 _μ_2 subunit (AP50; BD Biosciences), rat anti-HA (Roche), rabbit anti-HA (Santa Cruz Biotechnology), rabbit anti–c-Myc (Santa Cruz Biotechnology), rat anti–c-Myc (Serotec), mouse anti-Pk/V5 (Serotec), mouse anti-Flag (Sigma-Aldrich), and mouse anti-MBP (NEB). The AP2 mAb (AP6) was the kind gift of E. Smythe (University of Sheffield). Purified Wnt9b and Wnt5a were obtained from R&D in the carrier-free formulation.
pET23d-3xFLAG retromer (FLAG-VPS35–26–29-His) and pIRES-neo-GFP-VPS35 plasmids were kind gifts of F. Kishi (Kawasaki Medical School, Okayama, Japan). cDNA of SNX3–162 and a C-terminal EGFP vector (pDG0) were gifts of E. Kiss-Toth (University of Sheffield) and K. Ross (Liverpool John Moores University, Liverpool, UK).
DNA Constructs and Site-Directed Mutagenesis
Unless otherwise stated, the PKD2 and PKD1 plasmids used in this paper have been previously reported.5 N-terminal c-Myc tagged SNX3–102 and SNX3–162 constructs were generated by PCR and ligation into pcDNA3. GFP-tagged SNX3–102 and SNX3–162 were prepared by TOPO cloning into the Gateway vector pD-G0. SNX3–102 and SNX3–162 were subcloned into pGEX-6P-1 or pEBG vectors to express N-terminal bacterial or mammalian GST-fusion proteins, respectively. Similarly, NT2 (PKD2 aa1–223), CT2 (PKD2 aa680–968), and CT1 (PKD1 aa4107–4303) were subcloned into pEBG to express GST fusion proteins in mammalian cells. Site-specific mutations were introduced using the Quick-change site-directed mutagenesis protocol (Stratagene) as previously described and verified by sequencing.
Y2H Assays
A region of the PC2 C-terminus (CT2 799–895) was cloned into the bait vector pGBKT7 (Clontech) as previously reported.5 This was transformed into the yeast strain AH109 (containing ADE2, HIS3, and LacZ reporter genes under the control of the GAL4 UAS) and used to screen an E17 mouse cDNA library pretransformed in the yeast strain Y187 (Clontech). Screening was performed by a yeast-mating method using the yeast strains AH109 (MATa) and Y187 (MAT_α_). Reporter genes HIS3 and ADE2 and LacZ were employed for triple selection of positive transformants. Directed Y2H assays were carried out by cotransforming bait and prey plasmids into AH109 as previously described.42 The plasmids pGBKT7–53 (p53) and pGBADT7-T (SV40 T-antigen) were used as a pair of positive controls. The PC1 and PC2 truncated constructs pGBAD-B-CT1 (4107–4227) and pACT2-B-CT2 (680–742) were used as a pair of negative controls as previously reported.42
Recombinant Protein Preparation
Plasmids were transformed into the E. coli strain BL21-RIPL and recombinant protein expression was induced at 37°C for 3 hours with 0.5 mM IPTG. Expression and purification of the FLAG-tagged retromer complex was performed as previously described.43 GST fusion proteins and His-tagged proteins were purified with Nickel or Glutathione-Sepharose columns, respectively, as previously described.5
GST Pull-Down Assays
One to two micrograms of the bacterial GST fusion protein and the His-Thio tagged potential partner protein (1–2 _μ_g) were incubated in 300 _μ_l binding buffer (1×TBST with 0.2% Tween20) for 1 hour at RT with gentle rotation. Forty microliters of 50% Glutathione Sepharose 4B beads (GE Healthcare) were then added and the mixture and incubated with rotation for an additional hour. The beads were sedimented by centrifugation at 6000 rpm for 2 minutes and washed up to six times with 1 ml volumes of ice-cold PBS. Bound proteins were eluted either using 25 _μ_l of elution buffer or by boiling for 5–10 minutes in reducing sample buffer. The same procedure was followed for pull-down of mammalian GST-fusion proteins from cell lysates. Mammalian GST proteins bound to glutathione beads were similarly prepared for in vitro binding assays except that the beads were washed up to six times with 1 ml of ice-cold PBS, and resuspended in 1×TBST (0.2% Tween20) before the addition of recombinant protein. Coomassie staining showed that the predominant bands eluted from the beads after incubation with cell lysates corresponded to the respective GST fusion proteins.
In Vitro MBP Pull-Down Assays
Two micrograms of MBP or MBP-CT1 and 2 _µ_g of recombinant FLAG-tagged retromer complex (3×FLAG- VPS35, VPS26, VPS29-His6) were added to 300 _µ_l of binding buffer (5 mM HEPES pH 7.9, 150 mM NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 0.1% NP-40) and incubated at RT with gentle rotation for 4 hours. Sixty microlitres of 50% amylose resin was then added and the mixture incubated for a further 2 hours. The beads were then sedimented by centrifugation at 4°C, and washed six times with 1.5 ml amylose column washing buffer to remove unbound proteins. Bound proteins were then eluted from the washed beads into loading buffer and clarified supernatants analyzed by SDS-PAGE and western blotting.
Protein-Lipid Overlay Assays
Commercial lipid strips (Echelon PIP strips P-6001) were blocked with 1% nonfat dry milk in PBS (pH 7.4) for one hour at RT, followed by incubation with recombinant protein in 1% nonfat dry milk in PBS (pH 7.4) at 4°C overnight with gentle agitation. The membrane was washed in 1×PBST buffer (0.1% v/v Tween-20) for 3×10 minutes and bound protein detected by immunodetection.
Quantitative Real-Time PCR
Normal mouse embryos (kind gift of A. Furley, University of Sheffield) at different developmental stages (E10, E12, E14, E16) were pooled (_n_=3) and homogenized for RNA extraction. Total RNA was isolated from the embryos or cultured cells using TRIzol (Invitrogen). Each sample was then treated with Ambion DNA-free DNase kit to remove contaminating DNA, and 0.8 _μ_g of DNase-digested total RNA was reverse transcribed into cDNA using a cDNA reverse transcription kit (AB Applied Biosystems) according to the manufacturer’s instructions. Specific primers were designed to amplify SNX3–102 and SNX3–162 independently from human and mouse samples: primer details are provided below: human SNX3(162), forward, 5′-CACTTACGAAATCAGGGTCAAG-3′; human SNX3(162), reverse, 5′- GGAACTACGACCTTGCTCT-3′; human SNX3(102), forward, 5′- CACTTACGAAATCAGGGTCAAG-3′; human SNX3(102), reverse, 5′- CTATTATTTCATCCTTGCTCTCTC-3′; mouse SNX3(162), forward, 5′- CACCTACGAGATCAGGGTCAAG-3′; mouse SNX3(162), reverse, 5′- GGAACTACAACCTTGCTCTCTC-3′; mouse SNX3(102), forward, 5′- CACCTACGAGATCAGGGTCAA-3′; mouse SNX3(102), reverse, 5′- CTATAATTTCATCCTTGCTCTCTC-3′.
Real-time PCR was performed using the IQ Cybr Green system (BioRad). Hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as housekeeping gene and the data of CT value was calculated in relation to HPRT. To analyze the data, a _∆∆_Ct method was used to calculate the relative mRNA level.
Cell Culture and Transfection
HEK-293, IMCD3, MDCKII, Hela, and LLCPK cells were cultured in DMEM supplemented with 10% FBS. Transient transfection was carried out on cells cultured to 60%–80% confluency using Genejuice transfection reagent (Novagen) or Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Protein Biochemistry
Cells were lysed by extraction at 4°C using the IP lysis Buffer (25 mM NaCl, 150 mM EDTA, 1 mM 0.5% NP40, 1% Triton X-100, pH 7.0) supplemented with a protease inhibitor cocktail (Roche). Immunoblotting and immunoprecipitation were performed as previously described.4
Immunofluorescence Staining
Cells were grown on coverslips and fixed with 7:3 methanol/acetone or 4% paraformaldehyde at RT for 10 minutes followed by air drying for 10 minutes. Blocking was carried out for 20 minutes with 5% BSA/PBS, and incubated with primary antibody overnight at 4°C in 5% BSA/PBS. Controls included cells stained with primary antibody omitted. Antibody binding was visualized using fluorescein isothiocyanate–conjugated donkey anti–goat IgG secondary antibody (Santa Cruz Biotechnology). Slides were viewed using an Imaging Systems inverted IX71 microscope (Olympus, Tokyo, Japan) configured for multifluorescence image capture. Images were acquired using SimplePCI imaging software (Compix; Hamamatsu, PA). For PC2 surface expression, images were quantified by identifying regions of surface staining on individual cells (_n_=30 for each transfection) and determining surface staining intensity using ImageJ analysis software (National Institutes of Health [NIH]).
siRNA Knockdown
Isoform-specific siRNA to pig SNX3 was chemically synthesized by Ambion (Austin, TX) according to the following sequence: (sense: 5′-GCUUCGAAGUGAAUUAGAATT-3′, antisense: 5′-UUCUAAUUCACUUCGAAGCCA-3′). This targets a sequence common to isoforms 1, 3, 4, and 5 (Figure 1C). A scrambled negative control siRNA (Silencer) was purchased from Ambion. For knockdown of endogenous VPS35 in LLC-PK1 cells, the following sequences were synthesized: VPS35 siRNA 1: 5′-UAAAGAUACAACAUCCUCUGAAGGC-3′, and VPS35 siRNA 2: 5′-GCCUUCAGAGGAUGUUGUAUCUUUA-3′. Transfection of 20 nM siRNA into cells was achieved using RNAimax reagent (Invitrogen). The efficiency of knockdown was assessed by immunoblotting or RT-PCR 72 hours post-transfection.
Cell Surface Biotinylation
Cells were cultured to 90% confluence in six-well dishes, washed three times with PBS, and incubated for 40 minutes with 1 mg/ml sulpho-NHS-SC-Biotin in PBS pH 8.0 at 4°C. Cells were rinsed twice with PBS containing 100 mM glycine and excess biotin quenched by further incubation for 20 minutes with PBS containing 100 mM glycine and 0.1% BSA. After washing twice with PBS, they were lysed in 500 _µ_l buffer (50 mM Tris, 500 mM NaCl, 5 mM EDTA, 1% TX-100, pH 7.5) for 1 hour at 4°C. Equal amounts of cell lysate were incubated with 100 _µ_l streptavidin beads overnight at 4°C. Beads were washed three times each with lysis buffer, high salt buffer (50 mM Tris, 500 mM NaCl, 0.1% TX-100), and nonsalt buffer (10 mM Tris, pH 7.5) before being resuspended in 40 _µ_l 2×SDS sample buffer. Samples were analyzed by SDS-PAGE and western blotting using the antibodies described and bands quantified using a Biorad Gel Doc XR+ Imager.
EndoH Deglycosylation
After cell surface biotinylation, labeled proteins were incubated with 500 U EndoH (New England Biolabs, UK) as previously described.4
Xenopus Embryo Manipulations
Xenopus experiments were approved by the Institutional Animal Care and Use Committee and adhered to the NIH Guide for the Care and Use of Laboratory Animals. Embryos obtained by in vitro fertilization were maintained in 0.1×modified Barth medium44 and staged following.45 Knockdown of Vps35 was performed using an antisense morpholino oligomer (Vps35-MO, 5′-GGA CTG CTG GGT CGT GGG CAT CAT C’-3′; GeneTools) previously reported in Coudreuse et al.26 and confirmed by immunoblotting using a validated Vps35 antibody (ab154647, Abcam, Inc.). Injections were performed at the two-cell stage targeting a single blastomere with the contralateral side serving as uninjected control. Embryos were analyzed when sibling uninjected control embryos reached stage 40.
For immunofluorescence analysis, embryos were fixed in Dent’s fixative (methanol/DMSO=4:1). Xenopus embryos were then embedded in paraplast and sectioned at 25 _μ_m. Sections were probed with an antibody recognizing Xenopus PC2 (#AB9088; EMD Millipore, see Supplemental Figure 2 for antibody validation) and visualized by staining with anti–rabbit Alexa-647. Sections were counterstained with DAPI and ECL (Vector Laboratories) to visualize nuclei and the apical domain of the proximal tubules as described previously.46 Slides were analyzed by confocal microscopy and apical PC2 staining was quantified using ImageJ determining the number of pixels within the ECL staining domain.
Electrophysiology
LLC-PK1 cells were cotransfected with a porcine VPS35-specific siRNA 1 (see sequence above) or porcine PC2-specific (sense: 5′-UGUAGUAGUACACUGGAGC-3′) siRNA and CD8_α_ using Lipofectamine 2000 (Invitrogen). Transfected cells were identified using anti-CD8_α_–coated beads (DYNAL). CD8_α_ plus a scrambled siRNA (sense: 5′-UUCUCCGAACGUGUCACGU-3′; Qiagen) were used in mock-transfected cells. Transfection reactions were performed in 35-mm dishes using 1_μ_g DNA (CD8_α_ cDNA) and 40 pmol of one or two siRNAs (VPS35 siRNA 1 or PC2 siRNA) in a total of 500 _μ_l media containing 6 _μ_l Lipofectamine2000.
Immediately before each experiment, a coverslip bearing LLC-PK1 cells was removed from the culture plate and put into a recording chamber, which was mounted on the stage of a Nikon Diaphote inverted microscope. The extracellular solution was normal Tyrode solution containing (in mM): 135 NaCl, 5.4 KCl, 0.33 NaH2PO4, 1 MgCl2, 1.8 CaCl2, 5 HEPES, 5.5 glucose (pH 7.4). Recording pipettes were made from capillary glass (plain; Fisher Scientific, Pittsburgh, PA) using a micropipette puller and polisher (PP-830 and MF-830, respectively; Narishige, Tokyo, Japan). Pipettes were back-filled with internal solution composed of (in mM): 100 K-aspartate, 30 KCl, 0.3 Mg-ATP, 10 HEPES, 10 EGTA, and 0.03 GTP (pH 7.2). Pipette resistance varied from 3 to 5 M_Ω_ when filled with this internal solution. Offset potential was corrected just before a gigaohm seal formation. Series resistance and capacitance transients were compensated with a Warner PC-505B amplifier (Warner Instrument Corp., Hamden, CT) and pClamp 10.0 software (Axon Instrument, Foster City, CA). Currents were digitized with a Digidata 1440A converter (Molecular Devices), filtered through an internal four-pole Bessel filter at 1 kHz, and sampled at 2 kHz. Inward and outward whole-cell currents were elicited by employing a step-pulse protocol from −100 to +100 mV every 3 seconds for 200 milliseconds’ duration from a holding potential of −60 mV. Current-voltage (I-V) curves were derived using a voltage step protocol ranging from −100 to +100 mV in 20-mV increments for 200 milliseconds’ duration from a holding potential of −60 mV. Steady-state currents were averaged between 50 and 100 milliseconds. Cells with a similar diameter were selected for recordings, and only those cells with stable baseline currents were included in the results. Purified Wnt proteins were directly applied to the bath solution from a stock solution of 100 ng/_μ_l.
Statistical Analyses
Data are presented as mean values±SEM. Paired t test was used for statistical analyses with a P<0.05 indicating statistical significance. All analyses were carried out using Prism (Graphpad). Current traces were analyzed off-line with pClamp 10.0. All results are presented as mean±SEM. One-way analysis of mean and variance computation was used to analyze data with unequal variance between each group. A probability level of 0.05 was considered significant. The Boltzmann equation was used to plot _I_normalized as a function of concentration and calculate EC50 as follows: y=A1−A2/1+e(x−x0)/dx+A2, where y is the _I_normalized at a given concentration (x), A1 and A2 are the _I_normalizedmax and _I_normalizedmin, respectively, x is the given concentration, xo is the EC50, and dx is the slope factor. Microcal Origin 6.0 software was used to fit the data.
Disclosures
None.
Supplementary Material
Supplemental Data
Acknowledgments
We thank Elizabeth Smythe, Andrew Furley, Endre Kiss-Toth, Fumio Kishi, and Kehinde Ross for generously sharing reagents; Michael Caplan and Andrew Peden for helpful discussions.
This project was funded by the Sheffield Kidney Research Foundation (A.C.M.O.), Research Councils UK (A.J.S.), Oklahoma Center for the Advancement of Science and Technology (L.T.), The John S. Gammill Chair in Polycystic Kidney Disease (L.T.), National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (DK59599, L.T.; DK080745, O.W.), National Science Foundation of China (81670010, H.N.), and a Lerner Research Institute Chair’s Innovative Research Grant (O.W.). S.F. was a Sheffield Kidney Research Foundation Brayshaw Fellow and A.J.S. was a Research Councils UK Academic Fellow.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Harris PC, Torres VE: Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong AC, Harris PC: A polycystin-centric view of cyst formation and disease: The polycystins revisited. Kidney Int 88: 699–710, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G: Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci U S A 94: 6965–6970, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC: Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J Biol Chem 277: 20763–20773, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Giamarchi A, Feng S, Rodat-Despoix L, Xu Y, Bubenshchikova E, Newby LJ, Hao J, Gaudioso C, Crest M, Lupas AN, Honoré E, Williamson MP, Obara T, Ong AC, Delmas P: A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J 29: 1176–1191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong AC, Harris PC: Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P: Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472–479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G: TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang ZR, Chu WF, Song B, Gooz M, Zhang JN, Yu CJ, Jiang S, Baldys A, Gooz P, Steele S, Owsianik G, Nilius B, Komlosi P, Bell PD: TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS One 8: e73424, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X: TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J 28: 4677–4685, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Nie H, Nesin V, Tran U, Outeda P, Bai CX, Keeling J, Maskey D, Watnick T, Wessely O, Tsiokas L: The polycystin complex mediates Wnt/Ca(2+) signalling. Nat Cell Biol 18: 752–764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S: Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem 279: 19987–19995, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Mekahli D, Sammels E, Luyten T, Welkenhuyzen K, van den Heuvel LP, Levtchenko EN, Gijsbers R, Bultynck G, Parys JB, De Smedt H, Missiaen L: Polycystin-1 and polycystin-2 are both required to amplify inositol-trisphosphate-induced Ca2+ release. Cell Calcium 51: 452–458, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Santoso NG, Yu S, Woodward OM, Qian F, Guggino WB: Polycystin-1 interacts with inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling with implications for polycystic kidney disease. J Biol Chem 284: 36431–36441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF: Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köttgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Höpker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G: Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J 24: 705–716, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streets AJ, Wessely O, Peters DJ, Ong AC: Hyperphosphorylation of polycystin-2 at a critical residue in disease reveals an essential role for polycystin-1-regulated dephosphorylation. Hum Mol Genet 22: 1924–1939, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC: Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet 15: 1465–1473, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streets AJ, Needham AJ, Gill SK, Ong AC: Protein kinase D-mediated phosphorylation of polycystin-2 (TRPP2) is essential for its effects on cell growth and calcium channel activity. Mol Biol Cell 21: 3853–3865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Hortsman H, Seet L, Wong SH, Hong W: SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol 3: 658–666, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Mizutani R, Yamauchi J, Kusakawa S, Nakamura K, Sanbe A, Torii T, Miyamoto Y, Tanoue A: Sorting nexin 3, a protein upregulated by lithium, contains a novel phosphatidylinositol-binding sequence and mediates neurite outgrowth in N1E-115 cells. Cell Signal 21: 1586–1594, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J: Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsiokas L: Function and regulation of TRPP2 at the plasma membrane. Am J Physiol Renal Physiol 297: F1–F9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran U, Zakin L, Schweickert A, Agrawal R, Döger R, Blum M, De Robertis EM, Wessely O: The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 137: 1107–1116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran U, Pickney LM, Ozpolat BD, Wessely O: Xenopus Bicaudal-C is required for the differentiation of the amphibian pronephros. Dev Biol 307: 152–164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coudreuse DY, Roël G, Betist MC, Destrée O, Korswagen HC: Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312: 921–924, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Yin H, He J, Ye J, Ding F, Wang S, Hu X, Meng Q, Li N: cDNA cloning of porcine PKD2 gene and RNA interference in LLC-PK1 cells. Gene 476: 38–45, 2011 [DOI] [PubMed] [Google Scholar]
- 28.McGough IJ, Cullen PJ: Recent advances in retromer biology. Traffic 12: 963–971, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS: Comparative proteomics of clathrin-coated vesicles. J Cell Biol 175: 571–578, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiow KH, Tan Y, Chua RY, Huang D, Ng ML, Torta F, Wenk MR, Wong SH: SNX3-dependent regulation of epidermal growth factor receptor (EGFR) trafficking and degradation by aspirin in epidermoid carcinoma (A-431) cells. Cell Mol Life Sci 69: 1505–1521, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strochlic TI, Schmiedekamp BC, Lee J, Katzmann DJ, Burd CG: Opposing activities of the Snx3-retromer complex and ESCRT proteins mediate regulated cargo sorting at a common endosome. Mol Biol Cell 19: 4694–4706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, van Weering JR, van Heesbeen RG, Middelkoop TC, Basler K, Cullen PJ, Korswagen HC: A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol 13: 914–923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmeister H, Babinger K, Gürster S, Cedzich A, Meese C, Schadendorf K, Osten L, de Vries U, Rascle A, Witzgall R: Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J Cell Biol 192: 631–645, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nigro EA, Castelli M, Boletta A: Role of the polycystins in cell migration, polarity, and tissue morphogenesis. Cells 4: 687–705, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ: The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet 4: e1000315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Follit JA, Tuft RA, Fogarty KE, Pazour GJ: The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17: 3781–3792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu S, Hackmann K, Gao J, He X, Piontek K, García-González MA, Menezes LF, Xu H, Germino GG, Zuo J, Qian F: Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc Natl Acad Sci USA 104: 18688–18693, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Xu H, Yao Q, Li W, Huang Q, Outeda P, Cebotaru V, Chiaravalli M, Boletta A, Piontek K, Germino GG, Weinman EJ, Watnick T, Qian F: Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun 5: 5482, 2014. 25405894 [Google Scholar]
- 39.Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE: Primary cilia are not calcium-responsive mechanosensors. Nature 531: 656–660, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gainullin VG, Hopp K, Ward CJ, Hommerding CJ, Harris PC: Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J Clin Invest 125: 607–620, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Streets AJ, Hounslow AM, Tran U, Jean-Alphonse F, Needham AJ, Vilardaga JP, Wessely O, Williamson MP, Ong AC: The polycystin-1, lipoxygenase, and α-toxin domain regulates polycystin-1 trafficking. J Am Soc Nephrol 27: 1159–1173, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng S, Okenka GM, Bai CX, Streets AJ, Newby LJ, DeChant BT, Tsiokas L, Obara T, Ong AC: Identification and functional characterization of an N-terminal oligomerization domain for polycystin-2. J Biol Chem 283: 28471–28479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabuchi M, Yanatori I, Kawai Y, Kishi F: Retromer-mediated direct sorting is required for proper endosomal recycling of the mammalian iron transporter DMT1. J Cell Sci 123: 756–766, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Sive HL, Grainger RM, Harland RM: Early Development of Xenopus leavis: A Laboratory Manual, Cold Spring Harbor, New York, Cold Spring Harbor Laboratory Press, 2000 [Google Scholar]
- 45.Nieuwkoop PD, Faber J: Normal Table of Xenopus Laevis, New York, Garland Publishing, 1994 [Google Scholar]
- 46.Cerqueira DM, Tran U, Romaker D, Abreu JG, Wessely O: Sterol carrier protein 2 regulates proximal tubule size in the Xenopus pronephric kidney by modulating lipid rafts. Dev Biol 394: 54–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data