Innate genetic evolution of lung cancers and spatial heterogeneity: analysis of treatment naïve lesions (original) (raw)
. Author manuscript; available in PMC: 2019 Oct 1.
Published in final edited form as: J Thorac Oncol. 2018 Jun 19;13(10):1496–1507. doi: 10.1016/j.jtho.2018.05.039
Abstract
Introduction
Data regarding the pre-treatment inter-tumor heterogeneity of potential biomarkers in advanced-stage lung cancers is limited. A finding of such heterogeneity between primary and metastatic lesions would prove valuable to determine if a metastatic lesion can be a surrogate for the primary tumor, as more biomarkers will likely be used in the future to inform treatment decisions.
Methods
We performed RNA sequencing to analyze inter-tumor heterogeneity in thirty specimens (primary tumors, intra-thoracic, and extra-thoracic metastatic lesions) obtained from five treatment-naïve lung cancer patients.
Results
The global unsupervised clustering analysis showed that the lesions clustered at the individual patient level, rather than on the metastatic sites, suggesting that the characteristics of specific tumor cells have a greater impact on the gene expression signature than the microenvironment in which the metastasis develops. While, the mutational and transcriptional data highlight the presence of inter-tumor heterogeneity, showing that the primary tumors are usually distinct from metastatic lesions. Through a comparison between metastatic lesions and the primary tumors, we observed that pathways related to cell proliferation were upregulated, while immune-related pathways were downregulated in metastatic lesions.
Conclusion
These data not only provide insight into the evolution of lung cancers, but also imply possibilities and limitations of biomarker-based treatment in lung cancers.
Keywords: Biomarkers, Tumor heterogeneity, RNA sequencing, Autopsy, Immune-related markers, RET fusion
Introduction
The recent development of personalized molecular targeted therapies in lung cancer, based on genetic aberrations of tumor cells, has dramatically improved the efficacy of systemic therapies and prolonged patient survival1–3. These personalized therapies include epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) for lung cancers with EGFR activating mutations, anaplastic lymphoma kinase (ALK) TKIs for those with ALK translocations, and ROS1-TKIs for those with ROS1 translocations 1. Such targetable mutations, which are often called “oncogenic driver mutations”, usually distribute homogeneously throughout and between lesions in a single patient 4–6,7. However, in the clinic, individual lesions in a patient sometimes respond to therapy differently, suggesting the potential of tumor heterogeneity at genetic and/or transcriptional levels between lesions. These inter-tumor heterogeneities could eventually inform future diagnostic strategies such that a metastatic lesion could serve as a surrogate for the primary tumor.
The data for tumor heterogeneity is especially important in patients with advanced-stage disease prior to the initiation of front-line systemic therapy. However, the analysis of innate tumor heterogeneity in lung cancer is challenging for various reasons; i) surgical tumor resection is very rare in lung cancer patients with multiple metastases, ii) multiple biopsies are not standard practice for a single lung cancer patient (no perceived clinical benefit on decision making) and iii) systemic treatments, such as chemotherapies and molecular targeted therapies, affect mutational status and/or gene expression status of tumor cells 8–11, altering the level of tumor heterogeneity. To date, three independent studies have compared genetic aberrations between multiple regions in surgically resected early-stage primary lung adenocarcinomas (intra-tumor heterogeneity) 6, between multiple regions in clinically resectable non-small cell lung cancers (NSCLCs; intra-tumor heterogeneity) 7, or between multiple intrathoracic lesions obtained from operable NSCLCs5. However, no data regarding the inter-tumor heterogeneity is available for patients with advanced-stage disease.
To understand innate inter-tumor heterogeneity and investigate tumor evolution between primary and multiple metastatic lesions, we performed comprehensive RNA-sequencing (RNA-seq) analysis on multiple lesions obtained at autopsy from advanced-stage lung cancer patients who did not receive systemic anti-cancer therapy for various reasons.
Materials and Methods
Patients and samples
Our cohort consisted of five autopsied lung cancer patients; two adenocarcinoma patients, two squamous cell carcinoma patients, and one small cell lung cancer (SCLC) patient, who did not receive systemic cancer treatment for various reasons including ages and performance status. Tissue specimens were obtained in accordance with ethical guidelines after obtaining written informed consent from their legal guardians as per the requirements of the Higashi-Hiroshima Medical Center (Higashi-Hiroshima, Japan). Tumor and non-cancerous tissue were obtained at autopsy and were stored at − 80 °C in RNA_later_ RNA stabilization reagent (Qiagen, Hilden, Germany). Total RNA was extracted using the RNeasy Mini Kit (Qiagen) and the quality of RNA was assessed by the RNA Integrity Number (RIN) score.
RNA sequencing and Bioinformatic analysis
Extracted RNA was made into a cDNA library by the University of Colorado Cancer Center Genomics Shared Resource, and samples were sequenced on the Illumina HiSeq 4000 sequencer for 1×150 cycles. Sequencing data were obtained and converted to FASTQ files. We mapped the sequencing reads to the hg19 reference genome using the TopHat/Cufflinks workflow as previously described 12–15. Transcripts were quantified using fragments per kilobase per million mapped reads (FPKM), and samples were quartile normalization prior to data analysis.
We used the Gene Set Enrichment Analysis (GSEA 16; gsea-3.0.jar version) for this analysis. We used the MSigDB C2 curated KEGG gene sets (c2.cp.kegg.v6.1.symbols.gmt) as the gene sets. For each patient, we compared the individual lesions against the normal sample using genotype permutation (n = 1000). We considered pathways that have a nominal P-value < 0.05 as significant and selected them for further analyses.
For the gene fusion detection, we used TopHat-Fusion 17 for detecting gene fusions in the RNA-seq data. We only considered a gene fusion to be positive when there are > 20 supporting reads detected in each sample. For detecting mutations in the RNA-seq data, we adopted the IMPACT (Integrating Molecular Profiles with Actionable Therapeutics) whole-exome sequencing pipeline that we previously developed 18. Briefly, IMPACT takes sequence data as an input and outputs a VCF file containing predicted deleterious mutations. The sequencing reads were mapped to the human hg19 reference exome using the Burrows-Wheeler Aligner 19, 20. SAMTools 21 and BCFtools (v1.1) were utilized to detect variants from the BAM file and create a VCF file output. In the IMPACT pipeline, we used ANNOVAR (v2014-11-12) 22 to annotate the variants. Synonymous and intronic variants were removed. Non-synonymous variants were further analyzed by deleterious prediction tools such as SIFT 23 and PolyPhen2 24. To call a variant in RNA-seq, in this study, we used the following criteria and only called high-confidence somatic variants if the mutations were detected as somatic (after comparing with the paired normal sample), non-synonymous and deleterious mutations with total read counts ≥ 20 and variant read counts ≥ 5 and ≥ 2%.
To understand the development of tumor evolution, we identified acquired somatic and deleterious mutations on reliably expressed genes in different legions. We assumed that mutations occurred independently by chance. After generating the binary mutation matrices for each patient, we constructed phylogenetic trees for the multiple lesions of each patient using Neighbor joining method implemented in the R phangorn package 25.
Immunohistochemistry (IHC) analysis
Formalin-fixed, paraffin-embedded tumor tissue were sectioned, and then IHC staining was performed using a PD-L1 antibody (E1L3N, Cell Signaling Technology, Danvers, MA) as described previously 26. A trained pathologist (S.S.) examined the proportions of the PD-L1 positive tumor cells.
Results
Samples and general RNA-Seq data
Thirty lesions from five autopsied patients (range: 4–9 lesions per patient), as well as non-cancerous tissue from each patient, were analyzed in this study (Table 1). Histologically, this cohort consists of four NSCLC patients (two adenocarcinomas and two squamous cell carcinomas) and one SCLC patient. One adenocarcinoma patient was a never-smoker, whereas one of squamous cell carcinoma patients and a SCLC patient were heavy smokers (50 or more pack-years). Total RNA was extracted from the lesions, and libraries of RNAs were constructed for RNA-seq profiling by next-generation sequencing. On average, we obtained about 61 million (range: 45–97 million) single-end 150 bp sequencing reads per sample, and the average mapping of 83.2% (53.9–90.4%) to the hg19 reference genome using the TopHat/Cufflinks workflow was performed as previously described (ref. 12–15, see Methods for details).
Table 1.
Patients characteristics and details of the specimens
| Patient | Histology | Age/Sex | Smoking (PY) | Metastatic sites* | |
|---|---|---|---|---|---|
| Intra-thoracic | Extra-thoracic | ||||
| Case 1 | AC | 96/F | 0 | Pleura (visceral & mediastinal), Lung | Liver |
| Case 2 | AC | 85/M | 22 | Lung (RML & LUL), LNs (hilar) | n.a. |
| Case 3 | SQ | 82/M | 65 | Pleura (visceral & parietal), Lung, LNs (hilar)Pleura (visceral & parietal & diaphragm), | Retroperitoneum 1 & 2, Peritoneum, Colon |
| Case 4 | SQ | 77/F | 20 | LNs (hilar, peribronchial, mediastinal, & supraclavicular) | n.a. |
| Case 5 | SCLC | 86/M | 50 | LNs (supraclavicular) | Liver, LNs (hepatic hilar) |
Individual tumors have unique gene expression signatures
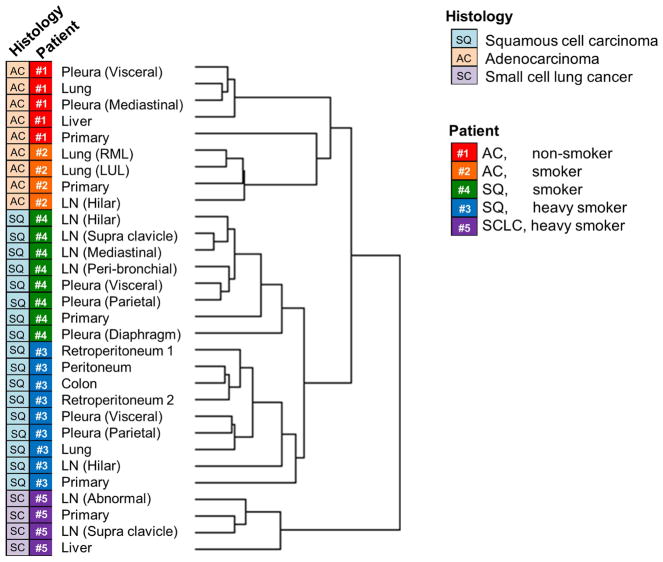
Initially we compared global gene expression data throughout lesions to determine which of the specific tumor characteristics or the microenvironment/metastasis site had a greater impact on gene expression status. As shown in Fig. 1, a global unsupervised clustering analysis of the expression data revealed that lesions from each patient clustered together, indicating that the global gene expression signature is affected more by the tumor cells themselves than the tumor microenvironment (i.e. specific metastatic sites). This suggests that, if the global gene expression pattern is used as a biomarker, a metastatic lesion can be a surrogate for the primary lesion. We also observed that lesions from four NSCLC patients clustered together in contrast to lesions from the single SCLC patient. In addition, among NSCLC lesions, the two adenocarcinoma patients clustered together as did the two squamous cell carcinoma patients.
Figure 1.
The global unsupervised clustering analysis of expression data of all lesions analyzed. The left and right rows indicate the histology and the individual patient, respectively. LN, lymph node metastases; RML, right middle lobe of the lung; LUL, left upper lobe of the lung; AC, adenocarcinoma; SQ, squamous cell carcinoma; SCLC, small-cell lung cancer.
Inter-tumor heterogeneity based on dysregulated pathways
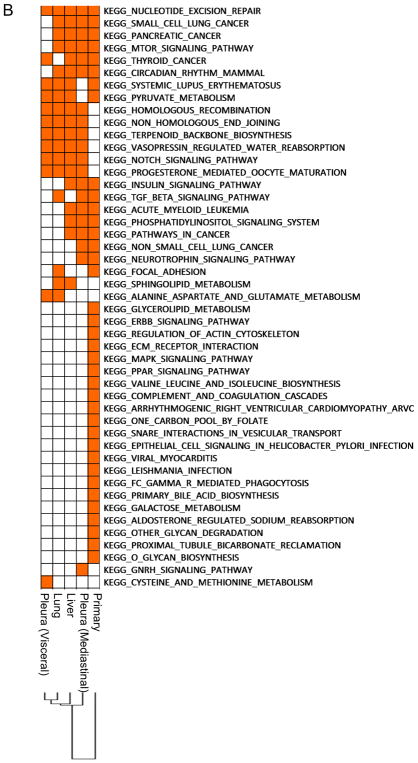
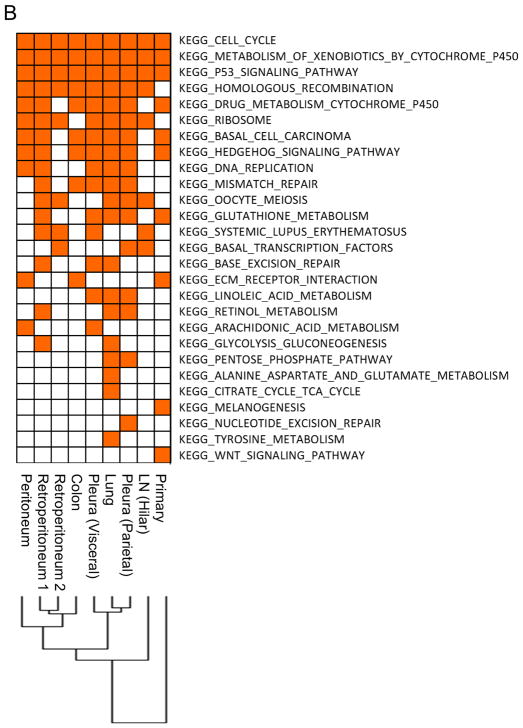
Next, we performed gene set enrichment analysis (GSEA16) comparing lesions within a single patient to identify dysregulated pathways and compared dysregulated pathways between lesions (inter-tumor heterogeneity). A heatmap of KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways with nominal P value < 0.05 revealed 27 – 98 dysregulated pathways in each patient (Figs. 2A & B, 3A & B, Supplementary Figs. S1A & B, S2A & B, and S3A & B). We found that 52 pathways were dysregulated in all lesions obtained from a never-smoker (Case 1; Supplementary Table 1), followed by 15 and 35 pathways in smokers (Case 2 & 4; Supplementary Tables 2 & 3, respectively), and in heavy smokers, only one (Case 3) and 5 (Case 5) dysregulated pathway(s) were shared in all lesions.
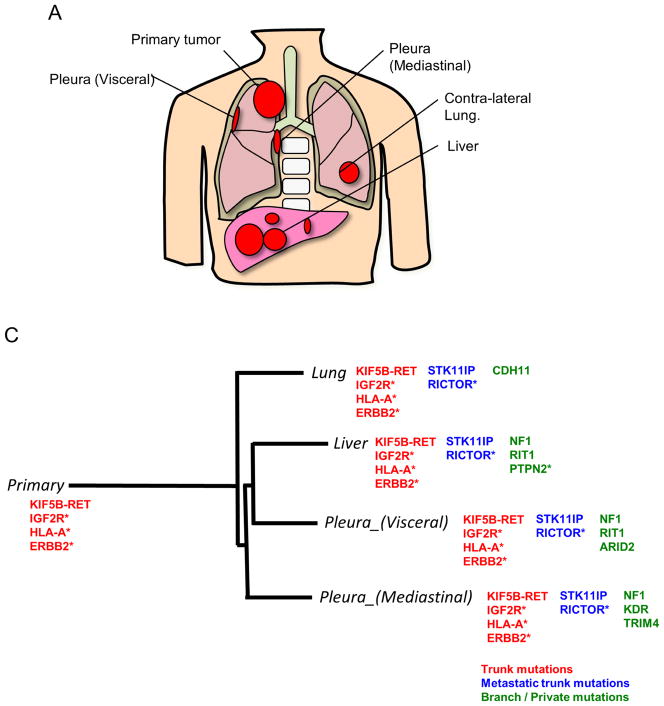
Figure 2.
Mutational and pathway analyses of lesions obtained from a never-smoking lung adenocarcinoma patient (Case 1). A, Geographic locations of analyzed lesions are shown. B, Pathway analysis was performed with Gene Set Enrichment Analysis based on MSigDB C2 curated KEGG gene sets. Dysregulated pathways in all lesions are summarized in Supplementary Table 1, and are excluded from the figure. C, Representative mutations identified in Case 1. KIF5B-RET fusion was also identified in all lesions, but not in non-cancerous tissue, using TopHat-Fusion on the RNA-seq. IGF2R, HLA-A, and ERBB2 mutations were also identified as trunk mutations, while NF1 mutation was present only in some metastatic lesions. The phylogenetic tree was constructed using a Neighbor joining method implemented in the R phangom package, based on somatic and deleterious mutations in reliably expressed genes. The genes with asterisk indicate that somatic/deleterious mutations in those genes were identified in lesions obtained from the other lung adenocarcinoma patient (Case 2).
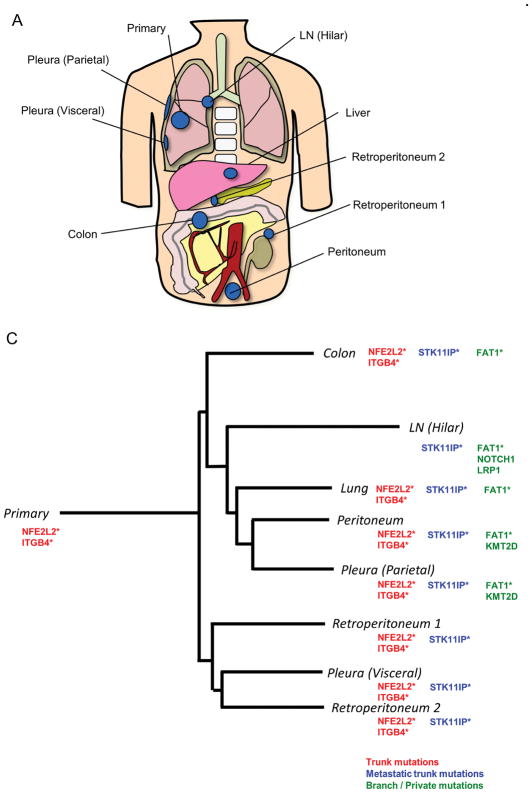
Figure 3.
Mutational and pathway analyses of lesions obtained from a lung squamous cell carcinoma patient with heavy smoking history (Case 3). A, Geographic locations of analyzed lesions are shown. B, Pathway analysis was performed with Gene Set Enrichment Analysis based on MSigDB C2 curated KEGG gene sets. C, Representative mutations identified in Case 3. NFE2L2 and ITGB4 mutations were identified in all lesions except for the hilar lymph node metastasis. The phylogenetic tree was constructed using a Neighbor joining method implemented in the R phangom package, based on somatic and deleterious mutations in reliably expressed genes. The genes with asterisks indicate that somatic/deleterious mutations in those genes were identified in lesions obtained from the other lung squamous cell carcinoma patient (Case 4).
Clustering analysis of these dysregulated pathways found that the primary lesions were always distinct from their respective metastatic lesions (Figs. 2B, 3B, Supplementary Figs. S1B, S2B, and S3B). In addition, we observed that data from metastatic lesions often clustered based on their metastatic sites (i.e. lymph node metastases, pleural or pulmonary metastases, and distant extra-thoracic metastases). For example, in Case 3, four distant metastases to abdominal organs (colon, retroperitoneum, and peritoneum) clustered together, a pulmonary metastasis and two pleural metastases (visceral and parietal) clustered together, and a hilar lymph node metastasis was distinct from other metastases (Fig. 3B). We also found that pleural disseminations and pulmonary metastases often clustered together.
Dysregulated pathways in metastatic lesions in NSCLC lesions
Next, we investigated dysregulated pathways in metastatic lesions (compared to primary lesions) in NSCLCs to identify pathways that are upregulated or downregulated based on a metastatic pattern. Metastatic lesions were grouped into pleural disseminations, pulmonary metastases, distant metastases, and lymph node metastases. As shown in Supplementary Fig. S4, pathways related to cell proliferation (such as cell cycle, DNA replication and repair, RNA polymerase, and spliceosome) were upregulated in all metastatic patterns. On the other hand, immune-related pathways (such as Allograft rejection, antigen processing and presentation, or NK cell mediated cytotoxicity) were downregulated in metastatic lesions compared to the primary lesions. In particular, we observed that multiple immune-related pathways were downregulated in pleural disseminations (Supplementary Fig. S5). These results suggest that the metastatic sites have diverse sets of dysregulated pathways, compared with the primary lesions, for promoting the metastatic potential and adapting to the foreign microenvironment.
Mutation analyses in NSCLC lesions
We also compared mutated genes between lesions in each patient. Due to the limitation of RNA-seq, we could not detect all mutations but only those in highly expressed genes (as defined below). Here we used the following criteria to extract high-confidence somatic variants; somatic/non-synonymous/and deleterious mutations with total read counts ≥ 20 and variant read counts ≥ 5 and ≥ 2%. We identified a total of 272 – 442 mutations per each NSCLC patient.
We divided these mutations into three groups: trunk mutations (those present in all lesions), metastatic trunk mutations (those present in all metastases but not in primary lesions) and branch/private mutations. We observed that the numbers of trunk/metastatic trunk mutations were smallest in the squamous cell carcinoma patient with heavy smoking status, followed by the two smokers (one with adenocarcinoma and one with squamous cell carcinoma), and the non-smoking adenocarcinoma patient harbored the highest number of trunk/metastatic trunk mutations (Table 2). We next focused on the mutational analysis by evaluating a list of gene mutations that have been previously reported in NSCLCs by next-generation sequencing5, 6, 27–32. We also performed fusion gene detection using TopHat-Fusion on the RNA-seq results. We found that Case 1 (a never-smoking adenocarcinoma patient) harbored a KIF5B-RET fusion as a trunk mutation (Fig. 2C), while the other adenocarcinoma patient had trunk RICTOR and DUSP5 mutations, both of which may have the potential to be driver mutations (Supplementary Fig. 1C). On the other hand, both of the squamous cell carcinoma patients harbored a trunk NFE2L2 mutation, which was reported as one of the hallmark mutations in squamous cell carcinomas related to oxidative stress response28. We also detected a trunk ITGB4 mutation in both of the squamous cell carcinoma patients. Based on all of the detected mutations, we evaluated the evolution of lesions in each patient using a phylogenetic tree analysis. The results were similar to those obtained from the analysis of dysregulated pathways in Cases 2 and 4, but were quite different from those in Cases 1 and 3. However, similar to the results of the pathway analysis, the mutational status of primary lesions was distinct from that of metastatic lesions in all NSCLC cases.
Table 2.
Numbers of detected mutations in each patient
| # of mutations detected | |||
|---|---|---|---|
| Total | Trunk | Metastatic Trunk | |
| Case 1 | 351 | 51 (KIF5B-RET) | 77 |
| Case 2 | 362 | 81 (RICTOR/DUSP5) | 18 |
| Case 3 | 272 | 7 (NFE2L2) | 5 |
| Case 4 | 442 | 53 (NFE2L2/CDKN2A) | 11 |
| Case 5 | 316 | 63 (TP53/PARP2) | 69 |
Dysregulated pathways and mutational analysis in a SCLC patient
Our cohort had only one patient with SCLC who had a heavy-smoking history (Case 5). We observed that only five dysregulated pathways were shared in all lesions analyzed (Supplementary Fig. S3B), however we found 63 trunk and 69 metastatic trunk mutations in this patient (Table 2). A TP53 mutation, as well as a PARP2 mutation, was identified as trunk mutation (Supplementary Fig. S3C), while an ASPM mutation, which was reported by TCGA analysis 28, was identified in only one lymph node metastatic lesion. The result of a phylogenetic tree analysis was the same as pathway analysis, revealing that the primary lesion was distinct from the metastatic lesions.
Inter-tumor heterogeneity in immune-related markers
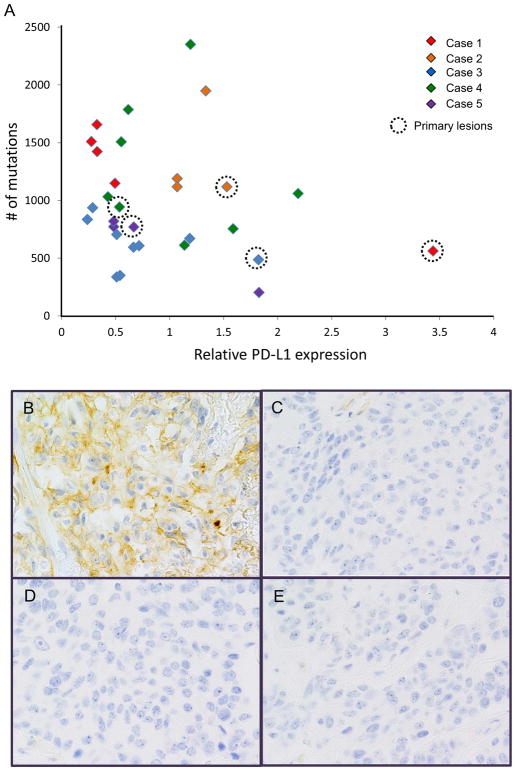
Finally, we evaluated inter-tumor heterogeneity of immune markers that may predict the efficacy of current immunotherapy in lung cancers that target the PD-1/PD-L1 pathway. As shown in Fig. 4, the expression level of PD-L1 (CD274) and total numbers of somatic non-synonymous mutations (not restricted to deleterious) varied significantly between lesions in each patient, suggesting an uncertainty of these markers as predictive biomarkers for immunotherapies. In addition, we did not observe any correlation between the PD-L1 expression and the numbers of mutations. PD-L1 protein expression was evaluated in lesions obtained from Case 1. Sixty-percent of tumor cells were positive for PD-L1 in the primary lesion, while all metastatic lesions were negative (< 1%) for PD-L1 staining. These results were compatible to the results of RNA expression data (Fig. 4).
Figure 4.
The inter-tumor heterogeneity of potential “predictive” biomarkers for PD-1/PD-L1 targeted immunotherapy. A, The relative expression of CD274 (PD-L1) and tumor mutation burden (the numbers of somatic/non-synonymous mutations detected) are shown. Primary lesion from each patient was marked by a dotted circle. B–D, Comparison of PD-L1 IHC staining in the primary tumor (B), visceral pleura (C), contra-lateral lung metastasis (D), and liver metastasis (E) in Case 1 is shown.
Discussion
Completion of The Cancer Genome Atlas (TCGA) project and other efforts using the next generation sequencing (NGS) technique have provided enormous information about genetic aberrations in cancers, including lung cancers 27–33. These studies have revealed the complexity of genomic alterations in lung cancers, and in addition, have identified potentially targetable genetic aberrations. However, these studies were not able to evaluate tumor heterogeneity, which would be clinically important when we try to target these genetic aberrations using molecular targeted agents or when we consider tumor tissue biopsies from metastatic lesions as surrogates for primary tumors. Recent development of liquid biopsy enables us to detect mutations from different sites, however the result of a liquid biopsy is affected by various factors (e.g. if tumors shed mutant DNA or not 34) and it is not possible to evaluate if the identified mutation is shared between lesions or not.
In this study, via a clustering analysis using gene expression data, we found that lesions obtained from each patient clustered together irrespective of the lesion location. This may indicate that, when we use the gene expression signature as a biomarker, e.g. as a predictive biomarker for immunotherapies35, metastatic lesions can be a surrogate for primary tumors.
However, at the same time, we observed inter-tumor heterogeneity between a primary tumor and the metastatic lesions in terms of dysregulated pathways and somatic mutations. Our results suggest that the levels of tumor heterogeneity determined by either dysregulated pathways or somatic mutations were correlated with smoking status in NSCLCs; i.e. higher levels of inter-tumor heterogeneity was observed in the heavy smoker, followed by smokers, and finally, by a non-smoker. Although further studies are necessary to confirm these findings, we consider that our results are reasonable since cigarette smoking is related to higher mutation burden and genomic instability in cancers29, 36.
Through the comparison of dysregulated pathways in our cohort, we identified that the primary lesions were always distinct from their metastatic counterparts. These data cannot be obtained from previous publications, including a large prospective TRACERx study, that analyzed intra-tumor heterogeneity via multi-region sequencing within the primary lesions 7. Our results indicate that a caution is necessary when we use a metastatic lesion as a surrogate for the primary tumor for some types of biomarker analysis (e.g. pathway-based biomarkers or a somatic mutation with no evidence to be a trunk mutation).
We identified a KIF5B-RET fusion as a trunk mutation in all metastatic and primary lesions in an adenocarcinoma patient with non-smoking history. Although the KIF5B-RET fusion is considered to drive lung cancers 37–40, recent clinical trials found that RET-TKIs for lung cancer patients with identified RET fusions were not as effective as EGFR-TKIs for those with EGFR mutations 1, 41, 42. The results from this study may indicate that this lower efficacy is due to reasons other than the inter-tumor heterogeneity of RET fusion, since our result showed a homogeneous distribution of this fusion gene. Currently, unlike the EGFR mutation or ALK/ROS1 fusion, RET fusion analysis is not routinely performed. Therefore, she did not have an opportunity to be treated with a specific TKI. For the other adenocarcinoma patient, we identified the RICTOR mutation 43 as one of the trunk mutations. In the analysis of squamous cell carcinoma patients, we identified the NFE2L2 mutation as a trunk mutation in both cases, suggesting its importance in squamous cell carcinomas. Both patients also harbored a trunk ITGB4 mutation, however the collaborative role of these two mutated genes in squamous cell carcinomas is currently unclear. Nevertheless, our results indicate that these trunk mutations occurred at the early phase of tumorigenesis and may serve as driver mutations in lung cancers,
Our comparison of metastatic lesions vs. primary tumors in NSCLCs identified that pathways related to cell proliferation are upregulated while immune-related pathways are downregulated in metastatic sites. Among these metastatic patterns, pleural dissemination was the most immunosuppressive metastatic pattern. These differences may have a direct impact on the efficacy of treatment, especially for immunotherapeutic agents.
In summary, these data demonstrate both similarity and the heterogeneity between primary and metastatic lesions in lung cancer patients. Our results support the use of metastatic lesions as surrogates for primary lesions when we analyze the gene expression signature or trunk mutations, although we have to be aware that metastatic lesions are usually distinct from the primary tumors in terms of dysregulated pathways and mutational patterns.
Supplementary Material
supplement
Acknowledgments
The authors thank the patients and their legal guardians. We also appreciate Prof. Paul A. Bunn, Jr. (Division of Medical Oncology, University of Colorado Anschutz Medical Campus) for his valuable comments for this study.
Footnotes
Conflicts of Interest and Source of Funding
T. Mitsudomi has received honoraria from AstraZeneca, Chugai, Boehringer-Ingelheim, Pfizer and Roche; has received compensation from AstraZeneca, Chugai, Boehringer-Ingelheim, Pfizer, Roche and Clovis Oncology for participating in advisory boards; and has received research funding (through Kindai University Faculty of Medicine) from AstraZeneca and Chugai. F. R. Hirsch has received compensation from Genentech/Roche, Pfizer, BMS, Lilly, Merck, Ventana/Roche, Novartis and Abbvie for participating in advisory boards; and has received research funding (through the University of Colorado) from Genetech/Roche, BMS, Lilly, Bayer, Amgen and Ventana/Roche. All other authors declare that they have no conflict of interest related to this study.
Our study was supported by an IASLC Young investigator Award (2015 – 2017) to K. Suda, JSPS KAKENHI Grant Number 18K07336 to K. Suda, the Pia and Fred Hirsch Chair in Lung Cancer at the University of Colorado Anschutz Medical Campus, the National Institutes of Health P50CA058187, P30CA046934, Cancer League of Colorado, and the David F. and Margaret T. Grohne Family Foundation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirsch FR, Suda K, Wiens J, Bunn PA., Jr New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388(10048):1012–24. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 2.Takano T, Fukui T, Ohe Y, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol. 2008;26(34):5589–95. doi: 10.1200/JCO.2008.16.7254. [DOI] [PubMed] [Google Scholar]
- 3.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol. 2011;29(22):2972–7. doi: 10.1200/JCO.2010.33.3906. [DOI] [PubMed] [Google Scholar]
- 5.de Bruin EC, McGranahan N, Mitter R, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 2014;346(6206):251–6. doi: 10.1126/science.1253462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346(6206):256–9. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 8.Suda K, Murakami I, Katayama T, et al. Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res. 2010;16(22):5489–5498. doi: 10.1158/1078-0432.CCR-10-1371. [DOI] [PubMed] [Google Scholar]
- 9.Suda K, Tomizawa K, Fujii M, et al. Epithelial to Mesenchymal Transition in an Epidermal Growth Factor Receptor-Mutant Lung Cancer Cell Line with Acquired Resistance to Erlotinib. J Thorac Oncol. 2011;6(7):1152–61. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 10.Suda K, Murakami I, Sakai K, et al. Heterogeneity in resistance mechanisms causes shorter duration of epidermal growth factor receptor kinase inhibitor treatment in lung cancer. Lung Cancer. 2016;91:36–40. doi: 10.1016/j.lungcan.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Mizuuchi H, Suda K, Murakami I, et al. Oncogene swap as a novel mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor in lung cancer. Cancer Sci. 2016;107(4):461–8. doi: 10.1111/cas.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson-Taylor SK, Le AT, Yoo M, et al. Resistance to RET-Inhibition in RET-Rearranged NSCLC Is Mediated By Reactivation of RAS/MAPK Signaling. Mol Cancer Ther. 2017;16(8):1623–1633. doi: 10.1158/1535-7163.MCT-17-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keysar SB, Le PN, Miller B, et al. Regulation of Head and Neck Squamous Cancer Stem Cells by PI3K and SOX2. J Natl Cancer Inst. 2017;109(1) doi: 10.1093/jnci/djw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keysar SB, Astling DP, Anderson RT, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7(4):776–90. doi: 10.1016/j.molonc.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware KE, Hinz TK, Kleczko E, et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis. 2013;2:e39. doi: 10.1038/oncsis.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D, Salzberg SL. TopHat-Fusion: an algorithm for discovery of novel fusion transcripts. Genome Biol. 2011;12(8):R72. doi: 10.1186/gb-2011-12-8-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hintzsche J, Kim J, Yadav V, et al. IMPACT: a whole-exome sequencing analysis pipeline for integrating molecular profiles with actionable therapeutics in clinical samples. J Am Med Inform Assoc. 2016;23(4):721–30. doi: 10.1093/jamia/ocw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–95. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Handsaker B, Wysoker A, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 24.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7(Unit7 20) doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schliep KP. phangorn: phylogenetic analysis in R. Bioinformatics. 2011;27(4):592–3. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimoji M, Shimizu S, Sato K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1) Lung Cancer. 2016;98:69–75. doi: 10.1016/j.lungcan.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–34. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y, Hammerman PS, Kim J, et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. J Clin Oncol. 2014;32(2):121–8. doi: 10.1200/JCO.2013.50.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paik PK, Shen R, Won H, et al. Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discov. 2015;5(6):610–21. doi: 10.1158/2159-8290.CD-14-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell JD, Alexandrov A, Kim J, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48(6):607–16. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suda K. DNA shedding in non-small-cell lung cancer: useful to assess? Lancet Respir Med. 2018;6(2):77–78. doi: 10.1016/S2213-2600(17)30479-4. [DOI] [PubMed] [Google Scholar]
- 35.Jamieson NB, Maker AV. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther. 2017;24(3):134–140. doi: 10.1038/cgt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22(3):436–45. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18(3):375–7. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18(3):382–4. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378–81. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 41.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5(1):42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- 43.Cheng H, Zou Y, Ross JS, et al. RICTOR Amplification Defines a Novel Subset of Patients with Lung Cancer Who May Benefit from Treatment with mTORC1/2 Inhibitors. Cancer Discov. 2015;5(12):1262–70. doi: 10.1158/2159-8290.CD-14-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplement