Inflammation-induced STING-dependent autophagy restricts Zika virus infection in the Drosophila brain (original) (raw)
. Author manuscript; available in PMC: 2019 Jul 11.
Published in final edited form as: Cell Host Microbe. 2018 Jun 19;24(1):57–68.e3. doi: 10.1016/j.chom.2018.05.022
Summary
The emerging arthropod-borne flavivirus Zika virus (ZIKV) is associated with neurological complications. Innate immunity is essential for the control of virus infection, but the innate immune mechanisms that impact viral infection of neurons remain poorly defined. Using the genetically tractable Drosophila system we show that ZIKV infection of the adult fly brain leads to NF-kB-dependent inflammatory signaling, which serves to limit infection. ZIKV-dependent NF-kB activation induces the expression of Drosophila stimulator of interferon genes (STING) in the brain. dSTING protects against ZIKV by inducing autophagy in the brain. Loss of autophagy led to increased ZIKV infection of the brain and death of the infected fly, while pharmacological activation of autophagy was protective. These data suggest an essential role for an inflammation-dependent STING pathway in the control of neuronal infection and a conserved role for STING in antimicrobial autophagy, which may represent an ancestral function for this essential innate immune sensor.
In Brief
Innate immune mechanisms that protect neurons against Zika virus infection remain unclear. In a Drosophila model of infection, Liu et al. show that ZIKV is largely restricted to the brain, where it is controlled by the inflammatory, NF-kB-dependent, expression of Drosophila STING, which is required to induce antiviral autophagy.
Graphical Abstract
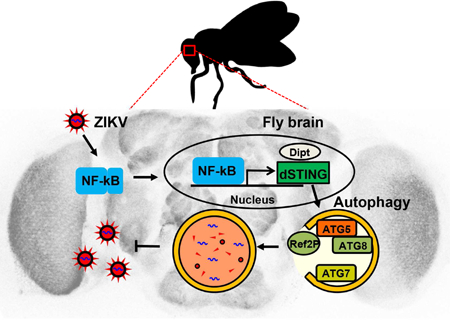
eTOC blurb
Introduction
Zika virus (ZIKV) is a member of flavivirus genus of RNA viruses that includes dengue, West Nile and yellow fever viruses (Fields et al., 2013; Lazear and Diamond, 2016). ZIKV is arthropod-borne and largely transmitted by Aedes mosquitoes, but can also be transmitted by other routes (D’Ortenzio et al., 2016; Yockey et al., 2016). The recent global emergence of ZIKV has produced considerable concern because of its potential to cause serious neurological complications during fetal development (Franca et al., 2016; Rodrigues, 2016). While most infections of humans are self-limiting, ZIKV can infect the fetal brain during pregnancy, resulting in significant brain abnormalities (Mlakar et al., 2016; Rubin et al., 2016). This has led to extensive research into cellular tropism of ZIKV (Dang et al., 2016; Lazear et al., 2016; Li et al., 2016b; Li et al., 2016c; Miner and Diamond, 2017; Oh et al., 2017; Tang et al., 2016). ZIKV most prominently infects neural stem cells and progenitors of the developing central nervous system (Dang et al., 2016; Li et al., 2016b; Tang et al., 2016). Neural precursors infected with ZIKV are severely affected explaining why the fetal brain, which has abundant neuroprogenitors, supports widespread infection accompanied by brain damage (Dang et al., 2016; Li et al., 2016a; Li et al., 2016b; Tang et al., 2016). In contrast, ZIKV infection of adults has fewer deleterious consequences to the adult brain, with rare cases of encephalitis (da Silva et al., 2017; Munoz et al., 2017; Zhu et al., 2017). The innate mechanisms that control ZIKV infection of mature neurons are poorly understood.
Drosophila melanogaster has been a powerful model for elucidating molecular mechanism involved in innate immunity (Lemaitre and Hoffmann, 2007). As in higher organisms, NF-kB-dependent inflammatory signaling cascades are activated by diverse microbial infections (Buchon et al., 2014; Kleino and Silverman, 2014). This leads to the transcriptional induction of downstream antimicrobial effectors including anti-microbial peptides (AMPs) that can attenuate or clear infection (Buchon et al., 2014; Kleino and Silverman, 2014). While the antibacterial and antifungal effectors downstream of NF-kB signaling are well characterized, the roles of these pathways in antiviral defense remain poorly understood. During systemic infection, NF-kB pathways have been implicated in the control of some viral infections, but the mechanisms are unclear (Avadhanula et al., 2009; Costa et al., 2009). In the gut, NF-kB signaling is required downstream of the microbiota to prime antiviral immunity through the production of an antiviral cytokine, Pvf2 (Sansone et al., 2015). The role of NF-kB signaling in innate immune defense against pathogens in the brain has not been explored.
Autophagy is another conserved antimicrobial effector pathway that can be induced by infections. Autophagy is an ancient degradative pathway that involves the targeted sequestration of cytoplasmic contents in double-membrane vesicles, autophagosomes, which fuse with lysosome to degrade the engulfed cargo (He and Klionsky, 2009; Levine and Kroemer, 2008; Mizushima and Komatsu, 2011). Capture of microbes or microbial components leads to their degradation, protecting cells from infection (Deretic and Levine, 2009; Levine et al., 2011). Activation of antimicrobial autophagy can protect cells from diverse pathogens including bacteria, viruses and eukaryotic parasites from flies to mammals (Campoy and Colombo, 2009; Choi et al., 2014; Gutierrez et al., 2004; Jia et al., 2009; Lee and Iwasaki, 2008; Moy et al., 2014; Nakagawa et al., 2004; Nakamoto et al., 2012; Shelly et al., 2009; Sumpter and Levine, 2010; Yano et al., 2008). Non-lytic autophagic clearance of pathogens has been shown to be critical in mature neurons which cannot self-renew (Nixon and Yang, 2012; Yoshimori, 2010). And defects in autophagy can lead to increases in pathogenesis and/or infection by some neurotropic viruses (Moy et al., 2014; Orvedahl et al., 2010; Yordy et al., 2012).
Diverse mechanisms can activate autophagy during viral infections. In some cases attenuation of nutrient signaling can lead to the activation of antiviral autophagy (Moy et al., 2014; Shelly et al., 2009). In other cases autophagy genes themselves can be induced (Levine et al., 2011). Furthermore, STING is a signaling molecule that can be activated during virus and bacterial infections, and can induce autophagy (Barber, 2015; Ishikawa and Barber, 2008; Ishikawa et al., 2009; Moretti et al., 2017; Watson et al., 2015; Xu et al., 2012). While STING can be activated by both endogenous and bacterially-derived cyclic dinucleotides, increased expression of STING mRNA is also sufficient to induce (Burdette et al., 2011; Burdette and Vance, 2013; Ishikawa and Barber, 2008; Ishikawa et al., 2009). Roles for STING in antiviral autophagy have not been described and while Drosophila encodes a STING ortholog (CG1667) it has not been directly implicated in immune defense.
In this study, we have developed a fly model to study innate immune responses to ZIKV infection. We show that ZIKV infects the fly brain, but is controlled by NF-kB-dependent inflammatory signaling that activates the expression of dSTING to attenuate ZIKV infection of the brain. This pathway is required for antiviral autophagy in neurons which is necessary and sufficient to control infection. Indeed, loss of autophagy leads to increased ZIKV replication and death of the animal while pharmacological activation can block infection of the brain. Altogether, we describe inflammatory and STING mediated autophagy as an antiviral innate defense mechanism against ZIKV infection that plays an essential role in the control of infection of the brain.
Results
ZIKV targets the fly brain
Drosophila is a powerful model system to identify genes important in innate immunity and viral infections (Buchon et al., 2014; Lamiable and Imler, 2014; Xu and Cherry, 2014). Moreover, we have previously used this system to uncover host factors that regulate arbovirus infection including flaviviruses such as West Nile virus and dengue virus (Hackett et al., 2015; Xu and Cherry, 2014; Xu et al., 2012; Yasunaga et al., 2014; Zhang et al., 2016). Therefore, we set out to develop a fly model to study the related flavivirus, ZIKV. To this end, we systemically challenged Drosophila with ZIKV (MR766) and monitored the level of infection by RT-qPCR and found that the levels of ZIKV RNA increased over time (Figure 1A). Next, we infected flies and dissected various tissues 7 days post infection (dpi) and observed that the highest levels of ZIKV RNA were within the brain (Figure 1B). A time course revealed increasing ZIKV RNA in the brain over time (Figure 1C). Furthermore, ZIKV virus load also increased over time in heads and was substantially higher than the bodies (Figure 1D). Confocal microscopy of the fly brain using an anti-ZIKV envelope antibody revealed widespread infection of the brain (Figure 1E). Taken together, these data reveal that ZIKV productively infects and replicates in the fly brain.
Figure 1. Characterization of ZIKV infection in Drosophila.
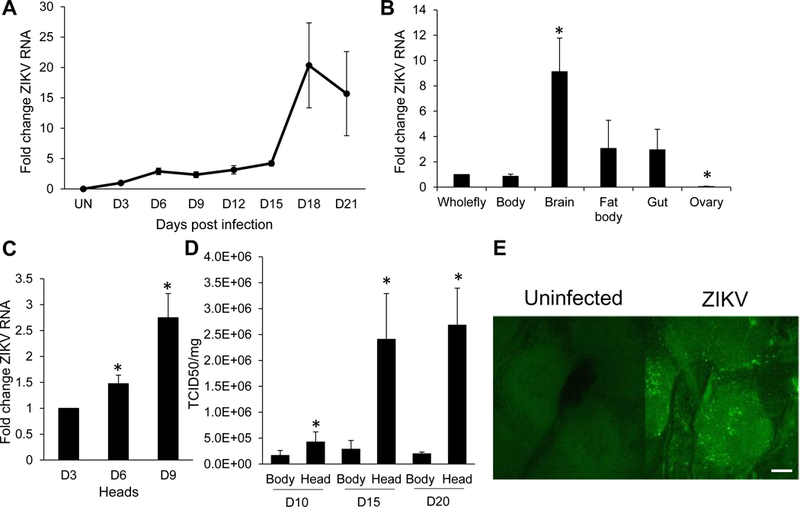
(A) Time course of ZIKV infection in adult flies challenged with ZIKV. On the indicated days post-infection (dpi), whole flies were collected and analyzed by RT-qPCR. Viral RNA normalized to rp49 shown relative to control (3 dpi) with mean ± SEM; n=3. (B) Tissues of ZIKV infected flies were dissected on 7 dpi and levels of ZIKV RNA was analyzed by RT-qPCR. ZIKV RNA normalized to rp49 shown relative to control (whole fly) with mean ± SEM; n=3, *p<0.05. (C) Time course of ZIKV infection in fly heads determined by RT-qPCR. ZIKV RNA normalized to rp49 shown relative to 3 dpi with mean ± SEM; n=3, *p<0.05. (D) ZIKV titers were assayed for bodies or heads at the indicated time points post infection by TCID50 normalized to total protein with mean ± SEM; n=3, *p<0.05. (E) Representative confocal images of brains of wild type flies or ZIKV infected analyzed 7 dpi (40x; anti-flavivirus E-green; bar 25µm).
RNAi pathway is not antiviral against ZIKV
We next set out to determine the innate immune responses active against ZIKV infection in the fly brain. RNA interference (RNAi) is a well-established antiviral pathway that controls many diverse viruses in Drosophila (Gammon and Mello, 2015). We challenged flies mutant for either of two core components of the RNAi pathway, Dcr-2 and Ago2, to test if RNAi plays a role in limiting ZIKV replication either systemically or in the brain. We monitored ZIKV infection both of the whole animal, as well as in the head. Perhaps surprisingly, we found no change in ZIKV RNA levels in either the whole flies or in the heads of mutant flies relative to controls (Figure 2A). As a positive control, we also tested Sindbis virus (SINV) which has been previously shown to be controlled by antiviral RNAi systemically (Galiana-Arnoux et al., 2006). In contrast to our results with ZIKV, Ago2 mutants exhibited 30-fold increases in SINV replication systemically and 8-fold increases in the head (Figure 2B). These results suggest that the RNAi pathway is active in the brain, but that ZIKV may encode a suppressor of the antiviral RNAi pathway and is thus controlled by other antiviral pathways (Gammon and Mello, 2015; Samuel et al., 2016).
Figure 2. The RNAi pathway does not restrict ZIKV infection.
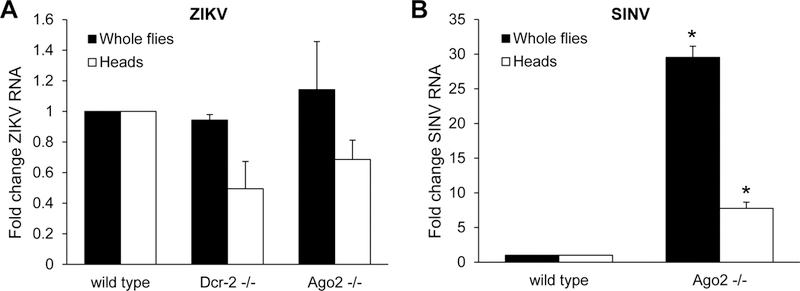
(A) Control or RNAi pathway component Dcr-2 −/− and Ago2 −/− mutant flies were infected with ZIKV and RT-qPCR was performed on whole flies and heads at 7 dpi. ZIKV RNA normalized to rp49 shown relative to wild type with mean ± SEM; n=3. (B) Wild type and RNAi pathway component Ago2 −/− mutant flies were infected with SINV and RT-qPCR was performed on whole flies and heads at 7 dpi. SINV RNA normalized to rp49 shown relative to wild type with mean ± SEM; n=3, *p<0.05.
ZIKV induces antiviral NF-kB signaling in the brain
The inflammatory NF-κB pathways, TOLL and IMD, are involved in diverse immune responses in flies including some viral infections (Avadhanula et al., 2009; Buchon et al., 2014; Costa et al., 2009). Therefore, we next explored whether these pathways are activated upon ZIKV infection of the brain. Compared to uninfected flies, we observed that the IMD pathway target gene Diptericin (Dipt) was significantly induced upon ZIKV challenge in whole flies and heads, but not in bodies (Figure 3A). This was specific to the IMD pathway, since no change in the TOLL pathway target gene Drosomycin (Drs) was observed upon ZIKV infection (Figure 3B). As expected, we found that this activation was dependent on Relish, the NF-kB transcription factor in the IMD pathway. Flies carrying a loss-of-function Relish allele (E38) no longer showed ZIKV-dependent induction of Diptericin (Figure 3C).
Figure 3. ZIKV infection induces NF-kB signaling which restricts infection.
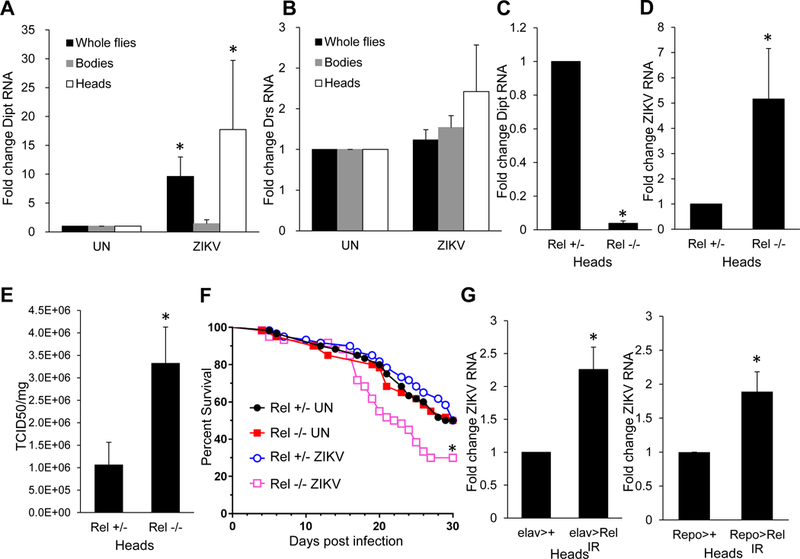
(A–B) Adult flies were uninfected or infected with ZIKV and RT-qPCR analysis of whole flies, bodies and heads was performed monitoring (A) Diptericin (Dipt) or (B) Drosomycin (Drs) normalized to rp49, and shown relative to uninfected control. Mean ± SEM; n=3, *p<0.05. (C-E) Relish_ mutant flies or heterozygous sibling controls were challenged with ZIKV and heads collected 7 dpi. (C-D) RT-qPCR was performed and (C) Diptericin or (D) ZIKV RNA was normalized to rp49, shown relative to control with mean ± SEM; n=3, *p<0.05. (E) ZIKV titers were assayed by TCID50 normalized to protein input with mean ± SEM; n=3, *p<0.05. (F) Percent survival of _Relish_ mutant or heterozygous sibling controls uninfected, or infected with ZIKV, with mean shown for n=3 (*p<0.01, log-rank test). (G) Control (_elav>+ or Repo>+, respectively) or flies depleted for Relish in neurons or glia (elav> Rel IR or Repo> Rel IR, respectively) were challenged with ZIKV and viral RNA was quantified by RT-qPCR normalized to rp49 shown relative to control (elav>+ or Repo>+, respectively) from heads 7 dpi with mean ± SEM; n=3, *p<0.05. See also Figure S1.
Next, we tested whether ZIKV replication was controlled by this pathway. We challenged Relish mutant flies with ZIKV and monitored viral RNA and titers in the brain. Compared to sibling controls, Relish mutant flies exhibited increased viral infection compared to sibling controls by both assays, indicating that the NF-kB pathway controls ZIKV infection (Figure 3D–E). Moreover, we observed increased lethality in Relish mutant flies challenged with ZIKV (Figure 3F). There are two major cell types in the brain, neurons and glia. Therefore, we set out to determine whether IMD signaling was required in neurons or glia or both. We specifically depleted Relish using a validated in vivo RNAi line and a neuron-specific gal4 driver (elav-gal4) or a glia-specific gal4 driver (repo-gal4) (Morris et al., 2016; Sepp et al., 2001). Depletion of Relish in neurons or glia led to increased ZIKV infection (Figure 3G). And loss of Relish in neurons and glia significantly, but modestly decrease in Diptericin expression (Figure S1A). IMD pathway activation is initiated by the pattern recognition receptors, Peptidoglycan Recognition Proteins-LC or -LE (PGRP-LC or –LE). We challenged flies mutant for both PGRP-LC and LE or sibling controls, and found ZIKV RNA was significantly elevated in PGRP-LC/LE mutant whole flies and heads (Figure S1B). Collectively these data show that ZIKV infection induces IMD pathway NF-kB signaling and furthermore that this is required to restrict viral replication in the brain.
dSTING is induced by ZIKV-induced NF-kB signaling and is antiviral
To determine the downstream Relish-dependent genes that control viral infection we performed transcriptional profiling on flies either uninfected or infected with the model virus Drosophila C virus for 9h. We profiled three independent experiments, identifying 145 genes that were induced at least 2-fold with a false discovery <0.05 (Table S1). Gene ontology (GO) enrichment found that ‘defense response to bacterium’ and related categories were highly enriched (Figure 4A). Canonical IMD dependent genes including antimicrobial peptides were some of the highest induced genes (Table S1). 37 of these genes were identified by De gregorio et al. as NF-kB-dependent genes, and 25 of these genes were identified by Dosert et al. as virus-induced (Table S1) (De Gregorio et al., 2001; Dostert et al., 2005). Interestingly, Relish was also transcriptionally induced. Altogether, these data suggest that we identified virus-induced Relish-dependent genes.
Figure 4. ZIKV induced NF-kB signaling induces dSTING which is antiviral.
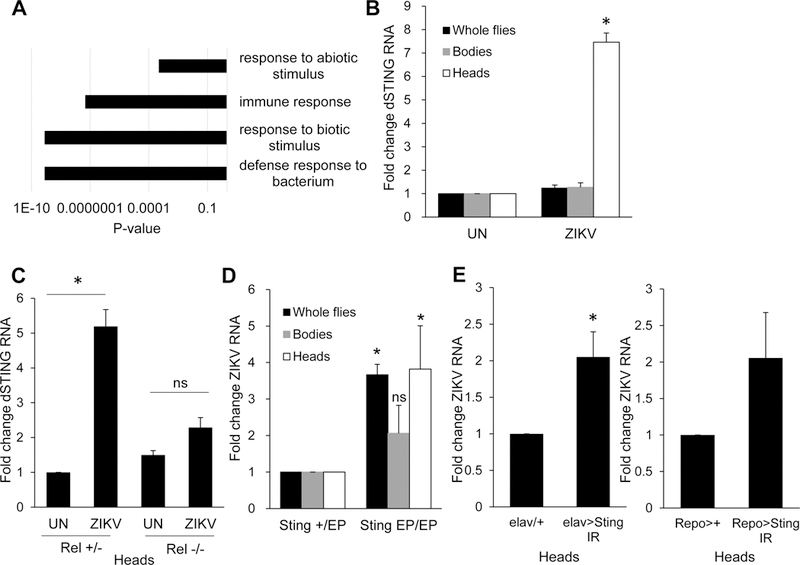
(A) Transcriptional profiling of virus-infected flies show enrichment for innate immune and antibacterial pathways. Analysis of the 145 genes induced by DCV infection in whole flies 9 hpi. The p-values of the significantly enriched Panther slim biological processes are shown. (B) Uninfected (UN) or ZIKV-infected flies whole flies, bodies, and heads were collected 7 dpi and RT-qPCR analysis of dSTING normalized to rp49, and shown relative to uninfected control. Mean ± SEM; n=3, *p<0.05. (C) Relish_ mutant or heterozygous sibling control flies were uninfected (UN) or infected with ZIKV, and dSTING expression in heads was monitored by RT-qPCR on 7 dpi and shown relative to uninfected control (Rel +/−). Mean ± SEM; n=3, *p<0.05, ns, not significant. (D) _Sting_ _EP/EP_ or heterozygous sibling control flies _Sting_ _EP/+_ were challenged with ZIKV and RT-qPCR analysis of whole flies, bodies and heads was performed monitoring ZIKV RNA normalized to _rp49_, shown relative to heterozygous control. Mean ± SEM; n=3, *p<0.05, ns, not significant. (E) Control (_elav>+ or Repo>+, respectively) or flies depleted for dSTING in neurons or glia (elav> Sting IR or Repo> Sting IR, respectively) were challenged with ZIKV and viral RNA was quantified by RT-qPCR normalized to rp49 shown relative to control (elav>+ or Repo>+, respectively) from heads 7 dpi with mean ± SEM; n=3, *p<0.05. See also Figure S2 and Table S1.
When mining this dataset for innate immune regulators that may control viral infections we found that the fly ortholog of STING, encoded by CG1667, was induced 2.8-fold in our study and was also identified by Dosert, et al. as virally induced (Dostert et al., 2005; Kranzusch et al., 2015). To determine if ZIKV infection induces dSTING expression, we challenged flies with ZIKV and monitored the level of dSTING by RT-qPCR. Compared to uninfected flies, we found dSTING expression was significantly induced upon ZIKV challenge in the fly head (Figure 4B). Moreover, we found that Relish expression was also induced upon ZIKV infection in fly heads (Figure S2A). We tested whether induction of dSTING was dependent on Relish by challenging Relish mutants or heterozygous sibling controls with ZIKV. Indeed, we found that ZIKV-induced dSTING expression is _Relish_-dependent (Figure 4C).
We obtained a P-element insertion allele of dSTING (EY06491) and observed decreased expression of dSTING as measured by RT-qPCR (Figure S2B). Compared to sibling controls, Sting EP/EP flies exhibit a significantly elevated level of ZIKV viral load in whole flies and heads but not in bodies (Figure 4D). To determine whether dSTING is required in neurons or glia or both, we use elav-gal4 or repo-gal4 to deplete dSTING in neurons or glia respectively. We validated knockdown by qRT-PCR (Figure S2C). Depletion of dSTING in neurons led to increased replication of ZIKV (Figure 4E). In contrast, depletion of dSTING in glia did not significantly impact ZIKV infection (Figure 4E), which could be due to the poor depletion (Figure S2C). Altogether, these data suggest that NF-kB is upstream, and restricts ZIKV replication through induced dSTING expression.
ZIKV infection activates autophagy in the fly brain
Since we found that dSTING is downstream of inflammatory NF-kB signaling in the brain, we reasoned that it may be inducing antiviral effector mechanisms. Recent studies found that STING can induce autophagy to resist intracellular pathogens (Moretti et al., 2017; Watson et al., 2015). Autophagy plays important roles in protection of neurons from diverse stressors including viral infection (Levine et al., 2011; Moy et al., 2014; Orvedahl and Levine, 2008; Orvedahl et al., 2010; Yordy et al., 2012). Autophagy can capture intracellular pathogens including viruses to clear infection in the absence of cell death which would be beneficial to mature neurons (Orvedahl and Levine, 2008; Sumpter and Levine, 2011). Moreover, autophagy has been shown to play important roles in the restriction of several arboviruses (Moy et al., 2014; Nakamoto et al., 2012; Shelly et al., 2009). Therefore, we monitored autophagy during ZIKV infection. One hallmark of autophagy is the lipidation of LC3 (dAtg8) where the unmodified form, Atg8-I, is conjugated to phosphatidylethanolamine and its lipidated form, Atg8-II, is associated with the growing autophagosomal membrane (Deretic et al., 2013). An elevation in Atg8-II levels indicates autophagy activation. Compared to uninfected flies, we observed an increase in Atg8-II accumulation upon ZIKV challenge. This was more apparent in the head samples, suggesting ZIKV infection activates autophagy in the brain (Figure 5A–B).
Figure 5. ZIKV infection induces autophagy in the fly brain.
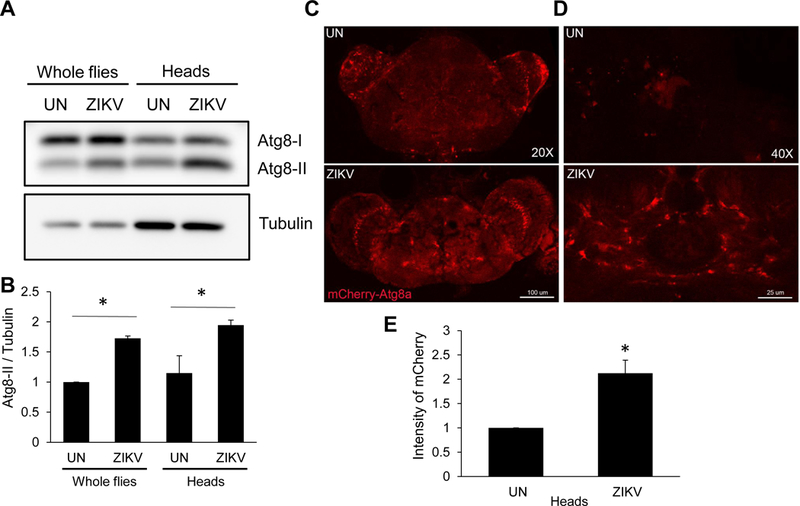
(A–B) Wild type flies were uninfected (UN) or infected with ZIKV collected 7 dpi. (A) A representative immunoblot of Atg8 and tubulin from whole fly and head lysates is shown. (B) Quantification of Atg8a-II/tubulin is shown with mean ± SEM; n=3, *p<0.05. (C-E) Representative confocal images of uninfected (UN) or ZIKV-infected brains expressing Atg8a-mCherry (Act>Atg8a-mCherry) analyzed 7 dpi at (C) 20X (bar 100µm), or (D) 40x (bar 25µm). (E) Quantification of 40X mCherry intensity is shown with mean ± SEM; n=3, *p<0.05.
To further confirm increased autophagy in the infected brain, we challenged flies ubiquitously expressing mCherry tagged Atg8 (Actin-gal4) (Chang and Neufeld, 2009). Basally, mCherry-Atg8 is diffuse and difficult to detect. However, upon autophagy induction, mCherry-Atg8 accumulates in autophagic punctae which can be observed by microscopy (Chang and Neufeld, 2009). As expected, we found diffuse and weak signal in the uninfected brain; however, during ZIKV infection we observed an increase in cells containing mCherry foci in the brain (Figure 5C–E). Taken together, these data suggest that ZIKV infection induces autophagy in the fly brain.
Autophagy restricts ZIKV infection in adult flies
Next, we investigated whether autophagy limits ZIKV infection. We used heat shock inducible gal4 (HS-gal4) to silence core autophagy genes Atg5 and Atg7 using established in vivo RNAi transgenic flies (Scott et al., 2004). Compared to sibling controls, flies inducibly and ubiquitously deficient in core autophagy genes harbored significantly increased levels of ZIKV RNA in whole flies and heads (Figure 6A). In addition, ZIKV titers were increased in Atg5-deficient heads (Figure 6B).
Figure 6. Autophagy is antiviral against ZIKV of the brain.
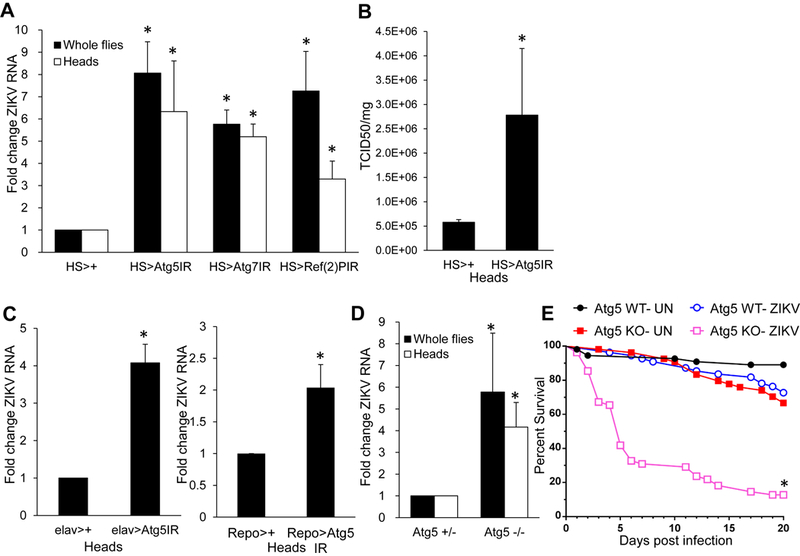
(A) Flies of the indicated genotypes were infected with ZIKV. RT-qPCR analysis of viral RNA normalized to rp49 and shown relative to control (HS>+) from whole flies or heads 7 dpi with mean ± SEM; n=3, *p<0.05. (B) Control (HS>+) or Atg5-depleted (HS> Atg5 IR) flies were challenged with ZIKV and viral titers from heads were quantified 7 dpi with mean ± SEM; n=3, *p<0.05. (C) Control (elav>+ or Repo>+, respectively) or flies depleted for Atg5 in neurons or glia (elav> Atg5 IR or Repo> Atg5 IR, respectively) were challenged with ZIKV and viral RNA was quantified by RT-qPCR normalized to rp49 shown relative to control (elav>+ or Repo>+, respectively) from heads 7 dpi with mean ± SEM; n=3, *p<0.05. (D) Atg5 null or heterozygous sibling controls were infected with ZIKV and viral infection was monitored from whole flies or heads 7dpi. Viral RNA was quantified by RT-qPCR normalized to rp49 shown relative to control with mean ± SEM; n=3, *p<0.05. (E) Percent survival of Atg5 null or sibling controls uninfected, or infected with ZIKV, with mean shown for n=3 (*p<0.0001, log-rank test). See also Figure S3–S4.
Autophagy captures intracellular cargos through the use of cargo receptors (Stolz et al., 2014). Drosophila encodes two well characterized autophagy cargo receptors, the p62 ortholog Ref(2)P and the ALFY ortholog Bchs (Nezis, 2012). We determined whether either cargo receptor is antiviral against ZIKV. While bchs-deficient animals did not show increased levels of infection, heat shock driven depletion of Ref(2)P led to loss of the mRNA accompanied by increased infection of ZIKV in whole flies and heads (Figure 6A, Figure S3).
To determine whether autophagy is required in neurons or glia or both to control ZIKV infection, we specifically depleted Atg5 in neurons using elav-gal4 and glia using repo-gal4. Loss of autophagy in either compartment led to increased replication of ZIKV (Figure 6C).
Since RNAi leads to hypomorphic phenotypes we also challenged Atg5 null mutants (5cc5) (Kim et al., 2016). Atg5 null flies exhibit an increased level of ZIKV RNA in whole flies and heads compared to heterozygous sibling controls (Figure 6D). In addition, whereas sibling controls survived infection, Atg5 null animals succumbed to ZIKV infection (Figure 6E).Therefore, autophagy is essential for control of ZIKV infection, and limits pathogenesis.
NF-kB-dependent dSTING-dependent autophagy controls ZIKV infection
Taken together, these data suggest that ZIKV-induces Relish-dependent dSTING expression which is required for antiviral autophagy. To test this model we first challenged Relish mutant flies with ZIKV and monitored Atg8-II accumulation by immunoblot. ZIKV-induced Atg8-II accumulation was apparent in heterozygous control flies but lost in Relish mutant heads (Figure 7A). Next, we tested whether dSTING is also required for ZIKV-induced autophagy. Again, while heterozygous control flies show increased Atg8-II upon ZIKV infection in the head, dSTING mutants are unable to mount this response (Figure 7B). These data show that Relish and dSTING are required for ZIKV-induced autophagy. Altogether, these data suggest that neurons respond to ZIKV infection by inducing inflammatory signaling which induces STING dependent autophagy to restrict infection.
Figure 7. Antiviral autophagy is regulated by NF-kB and dSTING and is sufficient to block infection.
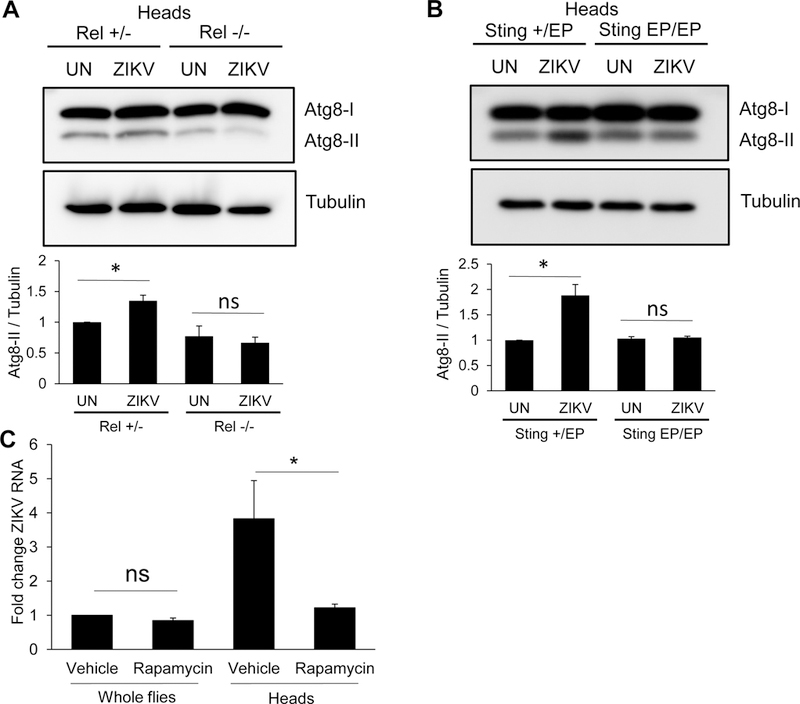
(A–B) Representative immunoblots of fly heads from uninfected (UN) or ZIKV infected flies of the indicated genotypes 7 dpi. Quantification of Atg8a-II/tubulin is shown with mean ± SEM; n=3, *p<0.05. (C) Flies fed vehicle or rapamycin were challenged with ZIKV and viral RNA was quantified 7 dpi by RT-qPCR normalized to rp49 shown relative to control (vehicle fed, whole flies) with mean ± SEM; n=3, *p<0.05, ns, not significant.
Activation of autophagy blocks ZIKV infection of the brain
Given the importance of autophagy in attenuating ZIKV infection of the brain, we reasoned that activation of autophagy prior to infection may limit infection. To accomplish this goal we fed flies rapamycin, a potent activator of autophagy, and subsequently challenged with ZIKV. We found that rapamycin treatment protected the brain from ZIKV infection (Figure 7C). These data demonstrate the essential role that autophagy plays in impacting neuronal infection and pathogenesis.
Discussion
Encephalitic viruses cause severe disease. Our understanding of the innate mechanisms that restrict neuronal infections is limited, thus making it difficult to devise new treatment strategies. We set out to develop a model system to explore innate and intrinsic mechanisms that control ZIKV infection of the adult brain and found that ZIKV specifically infects the adult fly brain, but this infection is limited. We found, in contrast to many other RNA viruses, ZIKV is not restricted by the RNAi pathway in the fly. Instead, we found that ZIKV induces NF-kB-dependent inflammatory signaling in the fly brain. This transcriptional response induces the expression of dSTING, which activates antiviral autophagy in neurons. Loss of autophagy, dependent on the NF-kB transcription factor Relish and dSTING, leads to uncontrolled viral replication and death of the animal. Importantly, pharmacological activation of autophagy was protective, suggesting a path toward neuronal protection.
Inflammatory signaling in flies
Drosophila encodes two parallel inflammatory, NF-kB-dependent, signaling pathways that regulate the expression of a battery of antimicrobial effectors including antimicrobial peptides (AMPS) (Buchon et al., 2014; Lemaitre and Hoffmann, 2007). These inflammatory pathways have been best characterized for their anti-fungal and anti-bacterial activities; however, some studies have shown that NF-kB-dependent genes can be modestly induced by systemic viral infections (Avadhanula et al., 2009; Deddouche et al., 2008; Zambon et al., 2005). Moreover, flies deficient for the NF-kB transcription factor Relish harbor increases in the systemic infection of some viruses (Avadhanula et al., 2009; Costa et al., 2009; Deddouche et al., 2008; Zambon et al., 2005). The mechanism by which Relish impacts these particular infections is unclear, and may be related to differences in tissue tropism of these viruses. Specifically in the gut, we found that gram negative commensals prime the NF-kB transcription factor Relish for the virus-induced expression of an antiviral cytokine (Sansone et al., 2015).
Roles for Relish in antiviral or antimicrobial defense of the brain have not been explored. However, there are clear roles for this signaling pathway in neuronal homeostasis and protection from neurodegeneration (Cantera and Barrio, 2015; Cao et al., 2013; Kounatidis et al., 2017; Petersen et al., 2013). Autophagy is also essential for protection from neurodegeneration in flies and higher organisms suggesting that autophagy regulation is an essential component of neuronal stress responses and maintenance of neuronal homeostasis (Frake et al., 2015; Kim et al., 2017). In the brain, we found that the NF-kB transcription factor was required for the transcriptional induction of dSTING which activated antiviral autophagy.
Conservation of STING
STING was first identified in an ectopic expression screen for activators of type I interferons hence named stimulator of interferon genes (Ishikawa and Barber, 2008). STING is antiviral against both DNA and RNA viruses (Barber, 2015; Ishikawa and Barber, 2008; Ishikawa et al., 2009). While STING activation of IRF3 and type I interferon transcription was the first identified downstream activity of STING, STING can also activate NF-kB signaling and autophagy in mammalian cells (Ishikawa et al., 2009; Moretti et al., 2017; Watson et al., 2015). Bioinformatic analyses revealed the presence of STING orthologs in most animal phyla, including insects (Kranzusch et al., 2015; Margolis et al., 2017). STING can be activated by binding to cyclic dinucleotides (CDNs) and biochemical studies revealed an ancient ability of STING to bind CDNs (Burdette et al., 2011; Wu et al., 2013). However, insect genomes, including Drosophila, encode a clear STING ortholog that did not bind the CDNs tested (Kranzusch et al., 2015). While STING has clear one-to-one orthologs, cGAS, the enzyme that produces CDNs upon DNA recognition in mammals, is less well conserved (Kranzusch et al., 2015; Margolis et al., 2017).
While STING is clearly conserved, the domain required for activation of NF-kB and IRF3 arose during vertebrate evolution (Margolis et al., 2017). IRFs also arose during vertebrate evolution, and Monosiga bevicollis, the most ancestral organism with a clear STING ortholog, lacks NF-kB pathway components. Altogether, these data suggest that the ancestral function likely predates those that emerged later, including IRF3 induction and NF-kB activation. STING stimulates autophagy in mammals (Moretti et al., 2017; Watson et al., 2015) which is an ancient innate immune pathway that targets and kills intracellular bacteria and viruses from flies to humans (Levine et al., 2011; Sumpter and Levine, 2010). The deep conservation of autophagy, and our data together suggests that regulation of autophagy pathway may be the ancestral function of STING.
Since we had also observed dSTING induction during DCV infection, we also tested whether dSTING impacts systemic DCV infection. We challenged Sting EP/EP or sibling controls with DCV and found that mutant flies were more susceptible to infection as measured by RT-qPCR (Figure S4A). However, this is not through NF-kB or autophagy as Relish mutants and Atg5 mutants were not more susceptible to infection (Figure S4B–C). Therefore, there are likely additional cell-type specific mechanisms by which dSTING is antiviral.
ZIKV and autophagy
While autophagy can be used by neurons to both clear viral infection and promote cell survival, at least against some viruses, autophagy is not always antiviral, and can promote some viral infections (Dong and Levine, 2013; Lennemann and Coyne, 2015). ZIKV displays diverse tissue tropism (Miner and Diamond, 2017), and infection induces autophagy in many cell types (Chiramel and Best, 2017). Proviral roles for autophagy during ZIKV infection have been demonstrated in epithelial cells, fibroblasts, and human fetal stem cells, all dividing cells that can potentially self-renew albeit to varying extents (Cao et al., 2017; Hamel et al., 2015; Liang et al., 2016). Studies at the maternal-fetal interface have shown that primary human trophoblasts, which are post-mitotic, use autophagy to restrict infections including ZIKV (Delorme-Axford et al., 2013). Recent studies in murine systems found that mice harboring a hypomorphic allele of ATG16L1, a gene required for autophagy, showed decreased ZIKV infection of placental tissues (Cao et al., 2017). In addition, ZIKV and other flaviviviruses replicate within the ER, and reticulophagy which is selective autophagy of the ER, limits these viral infections (Lennemann and Coyne, 2017). Therefore, depending on the cell type and context, autophagy can interface with ZIKV in diverse ways. The role of autophagy during ZIKV infection of mature mammalian neurons has not been explored, although it is clear that these cells have restrictive mechanisms as infection of these cells is difficult (Zhu et al., 2017). And we suggest that autophagy may play a role in restricting ZIKV infection of mature neurons. Indeed, studies with the related West Nile virus found that in non-neuronal cells autophagy did not limit infection while pharmacological activation of autophagy protected neurons from WNV infection and death (Martin-Acebes et al., 2015; Shoji-Kawata et al., 2013; Vandergaast and Fredericksen, 2012).
Conclusion
Viral infection of neurons is pathogenic, yet the factors that govern innate control in neurons remain incompletely understood. By taking advantage of Drosophila we have defined the essential role that inflammatory dependent STING activation plays in inducing antiviral autophagy in the brain. Our discovery that dSTING is an essential mediator of innate defenses in Drosophila provides further support for an ancient and conserved pathway that may have evolved to target intracellular pathogens for autophagic degradation. While pharmacological activation of autophagy restricted infection of the brain, this may not be a tenable strategy since ZIKV infection of other tissues may be exacerbated. Likewise, blocking autophagy may reduce infection of some tissues, while enhancing infection of the brain. A clearer understanding of tissue specific responses is required for us to define the appropriate therapeutics during infection by viruses, such as ZIKV, which display diverse tissue tropisms.
STAR METHODS
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sara Cherry (cherrys@pennmedicine.upenn.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila melanogaster
All fly stocks used in this study are _Wolbachia_-free and listed in Key Resources Table. Flies were maintained on standard cornmeal medium at room temperature. Four- to seven-day-old adult female flies were used for all molecular experiments and male flies were used for the survival experiments.
Zika Virus
ZIKV (MR766) was obtained from Michael Diamond. ZIKV were grown in C636 cells as described (Rausch et al., 2017).
Sindbis Virus
SINV (hrSp) was obtained from Richard Hardy. SINV were grown in C636 cells as described
Drosophila C Virus
Drosophila C Virus (DCV) was obtained from Peter Christian (Johnson and Christian, 1999).
DCV were grown and purified as described (Cherry and Perrimon, 2004).
METHOD DETAILS
Drosophila genetics and infection
Flies of the stated genotypes were inoculated with ~50nL of ZIKV (MR766), SINV (hrSp) or DCV using an Eppendorf Femtojet as previously described (Cherry and Perrimon, 2004; Rose et al., 2011; Yasunaga et al., 2014). Heat shock was performed by incubation at 37°C for 1 hour every day for 3 days prior to infection. Once infected, flies were incubated at 37°C for 1 hour every other day for the duration of the experiment. Flies were processed at the indicated time point post-infection. For Rapamycin treatment, flies were provided a PBS-only diet overnight and then starved for one hour to synchronize ingestion. Then flies were fed Rapamycin (100uM, with a final concentration of 5% ethanol) or vehicle (5% ethanol) in PBS supplemented with 5% (vol/vol) sucrose and systemically infected the next day. Flies were transferred onto fresh Rapamycin- or vehicle-containing food every 3 days for the duration of the experiment (Moy et al., 2014).
RNA and Quantitative Real-Time PCR
To quantify ZIKV tropism, 30 brains, 6 whole flies, 6 bodies, 5 ovaries, 15 intestines or 6 fat bodies were collected for each experiment. For other experiments, 30 heads, 6 whole flies, 6 bodies were collected for each experiment. Total RNA was extracted using TRIzol (Invitrogen) according to manufacturer’s protocol and as previously described (Xu et al., 2012). cDNA was prepared using M-MLV reverse transcriptase (Invitrogen). cDNA was analyzed using Power SYBR Green PCR Master Mix (Applied Biosystems), along with gene specific primers in triplicate, for at least three independent experiments. Data was analyzed by relative quantification, by normalizing to rp49. Oligonucleotides are listed in Table S2.
Immunofluorescence and Confocal Microscopy
Brains were processed as previously described (Luo and Sehgal, 2012). Briefly, 10 brains per condition for each experiment were dissected in PBS, fixed in 4% formaldehyde solution for 15 minutes on ice, rinsed 3 times in PBS with 0.1% Triton-X (PBST), and blocked with 5% normal donkey serum in PBST (NDST) for 45 minutes. Samples were incubated with primary antibody (ZIKV 4G2; 1:1000) diluted in NDST overnight at 4℃, rinsed 3 times in PBST, and incubated with secondary antibody (1:1000) at room temperature for 2 hr. Samples were rinsed 3 times in PBST and mounted in Vectashield (Vector Laboratories). Tissues were imaged on Leica TCS SPE confocal microscope. Three independent experiments were performed.
Immunoblotting
6 whole flies or 30 heads were pooled per condition per experiment and lysed in 200ul radioimmunoprecipitation (RIPA) buffer supplemented with a protease inhibitor cocktail (Boehringer) and were processed for immunoblot as described previously (Shelly et al., 2009).Three independent experiments were performed.
TCID50 assay
50 heads or 25 bodies were pooled per condition per experiment. Viral titers were measured by TCID50 on Vero cells as described (Rausch et al., 2017). Total protein was measured by BCA assay and used to normalize input. Data is shown as TCID50/mg of protein.
Transcriptional profiling
Flies were uninfected or infected with DCV and three independent experiments were collected 9 hpi. Total RNA was purified and hybridized to Affymetrix Drosophila Genome 2.0. We calculated fold changes relative to uninfected controls and considered significant genes with at least a 2-fold change and a false discovery rate of <0.05.
QUANTIFICATION AND STATISTICAL ANALYSIS
For survival curves, at least 20 flies for each genotype were used for each condition, three independent experiments were performed, and the average is shown. Pairwise comparisons of each experimental group with its control were carried out using a log-rank test. For other experiments, the Student’s two-tailed t test was used to measure the statistical significance and then considered significant if p < 0.05 in three independent experiments. Quantification of western blot and immunofluorescence images were performed using ImageJ software. For brain Atg8a-mCherry immunofluorescence quantification, mean gray value of the 40X images were used. All error bars represent stand error of mean. GraphPad Prism software was used for statistical analysis.
Supplementary Material
Highlights.
- ZIKV is neurotropic in Drosophila but RNAi is not protective
- ZIKV induces NF-kB-dependent inflammatory signaling in the fly brain
- Inflammatory NF-kB signaling activates dSTING expression and is antiviral
- dSTING induces autophagy, which restricts ZIKV infection in the adult fly brain
Acknowledgements
We would like to thank the following for fly stocks: Bloomington, VDRC, NIG, BDGP, DRSC, Thomas Neufeld, Neal Silverman, Jun Hee Lee, Daniel J Klionsky and Margit Burmeister. We would like to thank M. Diamond for antibodies and ZIKV, R. Hardy for SINV and P. Christian for DCV. We would like to thank Julie To for technical help with tittering, and the S.C. lab for helpful discussions and advice. This work was supported by National Institute of Health grants R01AI074951 and 5R01AI122749 to S.C. S.C. is a recipient of the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References:
- Aggarwal K, Rus F, Vriesema-Magnuson C, Erturk-Hasdemir D, Paquette N, and Silverman N (2008). Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway. PLoS pathogens 4, e1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhanula V, Weasner BP, Hardy GG, Kumar JP, and Hardy RW (2009). A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS pathogens 5, e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN (2015). STING: infection, inflammation and cancer. Nat Rev Immunol 15, 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Silverman N, and Cherry S (2014). Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nature reviews Immunology 14, 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, and Vance RE (2011). STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, and Vance RE (2013). STING and the innate immune response to nucleic acids in the cytosol. Nature immunology 14, 19–26. [DOI] [PubMed] [Google Scholar]
- Burnham AJ, Gong L, and Hardy RW (2007). Heterogeneous nuclear ribonuclear protein K interacts with Sindbis virus nonstructural proteins and viral subgenomic mRNA. Virology 367, 212–221. [DOI] [PubMed] [Google Scholar]
- Campoy E, and Colombo MI (2009). Autophagy in intracellular bacterial infection. Biochimica et biophysica acta 1793, 1465–1477. [DOI] [PubMed] [Google Scholar]
- Cantera R, and Barrio R (2015). Do the genes of the innate immune response contribute to neuroprotection in Drosophila? Journal of innate immunity 7, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Parnell LA, Diamond MS, and Mysorekar IU (2017). Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. The Journal of experimental medicine 214, 2303–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Chtarbanova S, Petersen AJ, and Ganetzky B (2013). Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proceedings of the National Academy of Sciences of the United States of America 110, E1752-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YY, and Neufeld TP (2009). An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Molecular biology of the cell 20, 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S, and Perrimon N (2004). Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat Immunol 5, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiramel AI, and Best SM (2017). Role of autophagy in Zika virus infection and pathogenesis. Virus Res [DOI] [PMC free article] [PubMed]
- Choi J, Park S, Biering SB, Selleck E, Liu CY, Zhang X, Fujita N, Saitoh T, Akira S, Yoshimori T, et al. (2014). The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity 40, 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Jan E, Sarnow P, and Schneider D (2009). The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One 4, e7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, and Leparc-Goffart I (2016). Evidence of Sexual Transmission of Zika Virus. The New England journal of medicine 374, 2195–2198. [DOI] [PubMed] [Google Scholar]
- da Silva IRF, Frontera JA, Bispo de Filippis AM, Nascimento O, and Group R-G-ZR (2017). Neurologic Complications Associated With the Zika Virus in Brazilian Adults. JAMA Neurol 74, 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, and Rana TM (2016). Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell stem cell 19, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, and Lemaitre B (2001). Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proceedings of the National Academy of Sciences of the United States of America 98, 12590–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, and Imler JL (2008). The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nature immunology 9, 1425–1432. [DOI] [PubMed] [Google Scholar]
- Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, et al. (2013). Human placental trophoblasts confer viral resistance to recipient cells. Proceedings of the National Academy of Sciences of the United States of America 110, 12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, and Levine B (2009). Autophagy, immunity, and microbial adaptations. Cell host & microbe 5, 527–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, and Akira S (2013). Autophagy in infection, inflammation and immunity. Nature reviews Immunology 13, 722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, and Levine B (2013). Autophagy and viruses: adversaries or allies? J Innate Immun 5, 480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, and Imler JL (2005). The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol 6, 946–953. [DOI] [PubMed] [Google Scholar]
- Fields BN, Knipe DM, and Howley PM (2013). Fields virology, Vol 2 (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins) [Google Scholar]
- Frake RA, Ricketts T, Menzies FM, and Rubinsztein DC (2015). Autophagy and neurodegeneration. J Clin Invest 125, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, Nunes ML, Castro MC, Serruya S, Silveira MF, et al. (2016). Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 388, 891–897. [DOI] [PubMed] [Google Scholar]
- Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, and Imler JL (2006). Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol 7, 590–597. [DOI] [PubMed] [Google Scholar]
- Gammon DB, and Mello CC (2015). RNA interference-mediated antiviral defense in insects. Curr Opin Insect Sci 8, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, and Deretic V (2004). Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766. [DOI] [PubMed] [Google Scholar]
- Hackett BA, Yasunaga A, Panda D, Tartell MA, Hopkins KC, Hensley SE, and Cherry S (2015). RNASEK is required for internalization of diverse acid-dependent viruses. Proc Natl Acad Sci U S A 112, 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, et al. (2015). Biology of Zika Virus Infection in Human Skin Cells. J Virol 89, 8880–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, and Klionsky DJ (2009). Regulation mechanisms and signaling pathways of autophagy. Annual review of genetics 43, 67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, and Barber GN (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, and Barber GN (2009). STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Thomas C, Akbar M, Sun Q, Adams-Huet B, Gilpin C, and Levine B (2009). Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proceedings of the National Academy of Sciences of the United States of America 106, 14564–14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KN, and Christian PD (1999). Molecular Characterization ofDrosophilaC Virus Isolates. Journal of invertebrate pathology 73, 248–254. [DOI] [PubMed] [Google Scholar]
- Kim M, Ho A, and Lee JH (2017). Autophagy and Human Neurodegenerative Diseases-A Fly’s Perspective. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Sandford E, Gatica D, Qiu Y, Liu X, Zheng Y, Schulman BA, Xu J, Semple I, Ro SH, et al. (2016). Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleino A, and Silverman N (2014). The Drosophila IMD pathway in the activation of the humoral immune response. Developmental and comparative immunology 42, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, and Ligoxygakis P (2017). NF-kappaB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep 19, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzusch PJ, Wilson SC, Lee AS, Berger JM, Doudna JA, and Vance RE (2015). Ancient Origin of cGAS-STING Reveals Mechanism of Universal 2’,3’ cGAMP Signaling. Mol Cell 59, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamiable O, and Imler JL (2014). Induced antiviral innate immunity in Drosophila. Current opinion in microbiology 20, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, and Diamond MS (2016). Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. Journal of virology 90, 4864–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, and Diamond MS (2016). A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 19, 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, and Iwasaki A (2008). Autophagy and antiviral immunity. Current opinion in immunology 20, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, and Hoffmann J (2007). The host defense of Drosophila melanogaster. Annual review of immunology 25, 697–743. [DOI] [PubMed] [Google Scholar]
- Lennemann NJ, and Coyne CB (2015). Catch me if you can: the link between autophagy and viruses. PLoS Pathog 11, e1004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennemann NJ, and Coyne CB (2017). Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 13, 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, and Kroemer G (2008). Autophagy in the pathogenesis of disease. Cell 132, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Mizushima N, and Virgin HW (2011). Autophagy in immunity and inflammation. Nature 469, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, and Xu Z (2016a). Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell stem cell 19, 120–126. [DOI] [PubMed] [Google Scholar]
- Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, Terskikh AV, Shresta S, and Gleeson JG (2016b). Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell stem cell 19, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Saucedo-Cuevas L, Shresta S, and Gleeson JG (2016c). The Neurobiology of Zika Virus. Neuron 92, 949–958. [DOI] [PubMed] [Google Scholar]
- Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, Ge J, Wang S, Goldman SA, Zlokovic BV, et al. (2016). Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell stem cell [DOI] [PMC free article] [PubMed]
- Luo W, and Sehgal A (2012). Regulation of circadian behavioral output via a MicroRNA-JAK/STAT circuit. Cell 148, 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SR, Wilson SC, and Vance RE (2017). Evolutionary Origins of cGAS-STING Signaling. Trends Immunol 38, 733–743. [DOI] [PubMed] [Google Scholar]
- Martin-Acebes MA, Blazquez AB, and Saiz JC (2015). Reconciling West Nile virus with the autophagic pathway. Autophagy 11, 861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, and Diamond MS (2017). Zika Virus Pathogenesis and Tissue Tropism. Cell host & microbe 21, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, and Komatsu M (2011). Autophagy: renovation of cells and tissues. Cell 147, 728–741. [DOI] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, et al. (2016). Zika Virus Associated with Microcephaly. The New England journal of medicine 374, 951–958. [DOI] [PubMed] [Google Scholar]
- Moretti J, Roy S, Bozec D, Martinez J, Chapman JR, Ueberheide B, Lamming DW, Chen ZJ, Horng T, Yeretssian G, et al. (2017). STING Senses Microbial Viability to Orchestrate Stress-Mediated Autophagy of the Endoplasmic Reticulum. Cell [DOI] [PMC free article] [PubMed]
- Morris O, Liu X, Domingues C, Runchel C, Chai A, Basith S, Tenev T, Chen H, Choi S, Pennetta G, et al. (2016). Signal Integration by the IκB Protein Pickle Shapes Drosophila Innate Host Defense. Cell host & microbe 20, 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy RH, Gold B, Molleston JM, Schad V, Yanger K, Salzano MV, Yagi Y, Fitzgerald KA, Stanger BZ, Soldan SS, et al. (2014). Antiviral autophagy restricts Rift Valley fever virus infection and is conserved from flies to mammals. Immunity 40, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz LS, Parra B, Pardo CA, and Neuroviruses Emerging in the Americas, S. (2017). Neurological Implications of Zika Virus Infection in Adults. The Journal of infectious diseases 216, S897-S905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, et al. (2004). Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Moy RH, Xu J, Bambina S, Yasunaga A, Shelly SS, Gold B, and Cherry S (2012). Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity 36, 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP (2012). Selective autophagy in Drosophila. Int J Cell Biol 2012, 146767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, and Yang DS (2012). Autophagy and neuronal cell death in neurological disorders. Cold Spring Harbor perspectives in biology 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Zhang F, Wang Y, Lee EM, Choi IY, Lim H, Mirakhori F, Li R, Huang L, Xu T, et al. (2017). Zika virus directly infects peripheral neurons and induces cell death. Nature neuroscience 20, 1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, and Levine B (2008). Autophagy and viral neurovirulence. Cellular microbiology 10, 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, MacPherson S, Sumpter R Jr., Talloczy Z, Zou Z, and Levine B (2010). Autophagy protects against Sindbis virus infection of the central nervous system. Cell host & microbe 7, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AJ, Katzenberger RJ, and Wassarman DA (2013). The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics 194, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch K, Hackett BA, Weinbren NL, Reeder SM, Sadovsky Y, Hunter CA, Schultz DC, Coyne CB, and Cherry S (2017). Screening Bioactives Reveals Nanchangmycin as a Broad Spectrum Antiviral Active against Zika Virus. Cell Rep 18, 804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues LC (2016). Microcephaly and Zika virus infection. Lancet 387, 2070–2072. [DOI] [PubMed] [Google Scholar]
- Rose PP, Hanna SL, Spiridigliozzi A, Wannissorn N, Beiting DP, Ross SR, Hardy RW, Bambina SA, Heise MT, and Cherry S (2011). Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and mammalian hosts. Cell host & microbe 10, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin EJ, Greene MF, and Baden LR (2016). Zika Virus and Microcephaly. The New England journal of medicine 374, 984–985. [DOI] [PubMed] [Google Scholar]
- Samuel GH, Wiley MR, Badawi A, Adelman ZN, and Myles KM (2016). Yellow fever virus capsid protein is a potent suppressor of RNA silencing that binds double-stranded RNA. Proceedings of the National Academy of Sciences of the United States of America 113, 13863–13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone CL, Cohen J, Yasunaga A, Xu J, Osborn G, Subramanian H, Gold B, Buchon N, and Cherry S (2015). Microbiota-Dependent Priming of Antiviral Intestinal Immunity in Drosophila. Cell Host Microbe 18, 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, and Neufeld TP (2004). Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7, 167–178. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, and Auld VJ (2001). Peripheral glia direct axon guidance across the CNS/PNS transition zone. Developmental biology 238, 47–63. [DOI] [PubMed] [Google Scholar]
- Shelly S, Lukinova N, Bambina S, Berman A, and Cherry S (2009). Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30, 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, et al. (2013). Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A, Ernst A, and Dikic I (2014). Cargo recognition and trafficking in selective autophagy. Nature cell biology 16, 495–501. [DOI] [PubMed] [Google Scholar]
- Sumpter R Jr., and Levine B (2010). Autophagy and innate immunity: triggering, targeting and tuning. Seminars in cell & developmental biology 21, 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter R Jr., and Levine B (2011). Selective autophagy and viruses. Autophagy 7, 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, et al. (2016). Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 18, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandergaast R, and Fredericksen BL (2012). West Nile virus (WNV) replication is independent of autophagy in mammalian cells. PloS one 7, e45800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, and Cox JS (2015). The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe 17, 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, and Chen ZJ (2013). Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, and Cherry S (2014). Viruses and antiviral immunity in Drosophila. Dev Comp Immunol 42, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Grant G, Sabin LR, Gordesky-Gold B, Yasunaga A, Tudor M, and Cherry S (2012). Transcriptional pausing controls a rapid antiviral innate immune response in Drosophila. Cell Host Microbe 12, 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, Takada H, Goldman WE, Fukase K, Silverman N, et al. (2008). Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nature immunology 9, 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga A, Hanna SL, Li J, Cho H, Rose PP, Spiridigliozzi A, Gold B, Diamond MS, and Cherry S (2014). Genome-wide RNAi screen identifies broadly-acting host factors that inhibit arbovirus infection. PLoS Pathog 10, e1003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, et al. (2016). Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 166, 1247–1256 e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordy B, Iijima N, Huttner A, Leib D, and Iwasaki A (2012). A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe 12, 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T (2010). How autophagy saves mice: A cell-autonomous defense system against Sindbis virus infection. Cell host & microbe 7, 83–84. [DOI] [PubMed] [Google Scholar]
- Zambon RA, Nandakumar M, Vakharia VN, and Wu LP (2005). The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci U S A 102, 7257–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Miner JJ, Gorman MJ, Rausch K, Ramage H, White JP, Zuiani A, Zhang P, Fernandez E, Zhang Q, et al. (2016). A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Gorman MJ, McKenzie LD, Chai JN, Hubert CG, Prager BC, Fernandez E, Richner JM, Zhang R, Shan C, et al. (2017). Zika virus has oncolytic activity against glioblastoma stem cells. J Exp Med 214, 2843–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.