Lipotoxicity and β Cell Maintenance in Obesity and Type 2 Diabetes (original) (raw)
Abstract
Obesity and diabetes are often associated with lipotoxic conditions in multiple tissues. The insulin-producing β cells are susceptible to elevated lipid levels and the ensuing lipotoxicity. The preservation of β cell mass and function is one of the main goals of diabetes management under these metabolically stressful conditions. However, the adverse effects from the adaptive signaling pathways that β cells use to counteract lipotoxic stress have secondary negative effects in their own right. Antilipotoxic signaling cascades in β cells can contribute to their eventual failure. Such dual roles are seen for many other biological adaptive processes as well.
Keywords: β cell, lipotoxicity, diabetes, adaptive signaling, insulin-sparing effect
Lipotoxicity refers the detrimental effects of lipid metabolites on nonadipose tissues, such as liver, skeletal muscle, heart, kidney, and pancreatic β cells [1]. It is a pathologic process seen in many metabolic disorders, most notably obesity and type 2 diabetes [2]. Excessive caloric intake or defective lipid metabolism leads to an oversupply of triglycerides, cholesterol, and free fatty acids (FFAs), which eventually exceed the storage capacity of adipose tissue and are enriched in plasma. This process leads systemic pressure on many tissues and cells to increase their lipid uptake. Interstitial or intracellular accumulation of lipids and their toxic metabolic products, such ceramides, diacylglycerol, and fatty acyl-CoA, can impair tissue function and cellular metabolism [3]. The resultant complications include hepatic steatosis, cardiovascular disease, renal failure, and peripheral insulin resistance [4].
Pancreatic β cells are professional secretory cells releasing insulin, an essential hormone regulating glucose and lipid metabolism. With minimal regenerative capacity during adulthood, β cells are susceptible to cellular stresses caused by reactive oxidative species (ROS), protein misfolding, and lipotoxicity [5]. The failure of β cells paves the path to end-stage type 2 diabetes. Here, we discuss recent insights obtained from studies on the impact of lipotoxicity on β cell function and survival, the cellular processes and molecular signaling that β cells use to counteract lipotoxic effects, and the adverse consequences of inducing these counterregulatory mechanisms. These insights derived from studying the antilipotoxic responses in β cells may provide the basis for more effective clinical approaches geared toward β cell preservation in obesity and type 2 diabetes.
1. β Cell Deterioration in Lipotoxic Environments
Hyperlipidemia is a key pathological feature shared by obesity, diabetes mellitus, and metabolic syndromes, and it imposes chronic insults on β cells via generation of intracellular cytotoxic metabolites and activation of detrimental signaling pathways, eventually leading to β cell dysfunction and death [6]. The critical role of environmental lipids in β cell pathology was first proven in rodent models. For instance, Unger [7] showed that in Zucker diabetic fatty rats, a genetic model of obesity and type 2 diabetes, mitigation of plasma FFAs can prevent β cell dysfunction. Interestingly, even in insulinopenic diabetes, high circulating lipids contribute to further β cell loss, driving a vicious positive feedback toward a collapse of systemic lipid homeostasis [8]. Studies in humans have generated mixed results. A 3-year follow-up study in Europe found associations between plasma nonesterified fatty acid levels and insulin resistance but not glucose-stimulated insulin secretion (GSIS) in β cells. In contrast, a recently reported 6-year follow-up study in Canada showed a strong negative correlation between serum nonesterified fatty acids and β cell function, as indicated by the insulinogenic index over homeostatic model assessment of insulin resistance (IR) and the insulin secretion-sensitivity index-2 [9]. More studies will be needed to explain the discrepancies and generalize the conclusions.
As for mechanistic studies, Jacqueminet et al. [10] investigated the effects of long-term saturated FFA exposure (i.e., palmitate over the course of days and weeks) on the transcription of the insulin genes. With isolated rat islets in culture, they discovered that excessive FFA suppresses insulin expression at high glucose levels but not with basal glucose. This effect is also observed in vivo in rat models [11] and can be explained by the high glucose-driven FFA esterification into triglycerides [12]. Other groups reported impairments in proinsulin synthesis and insulin secretion induced by high FFAs and high glucose [13]. The synergistic effects of glucose and saturated FFAs also apply to β cell apoptosis, as seen in rat and human β cell cultures [14], which can be reversed by monounsaturated fats [15]. The underlying reasons for the increased rate of apoptosis are attributed at least in part to increased _β_-oxidative activity [12, 14] that is associated with higher levels of DNA breaks [16].
Multiple additional factors and signaling pathways have been reported to mediate the lipotoxic effects in β cells. Ceramides are a class of sphingolipids that can induce apoptosis in a variety of tissues [17]. In human β cells exposed to FFAs, this effect is mediated by caspase activation [18]. Serine palmitoyltransferase catalyzes a key step of FFA conversion into ceramides [19]. Inhibitors of ceramide synthases can block the palmitate-induced β cell death [15]. The ceramidase activity of adiponectin receptors protects β cells against lipotoxic apoptosis [20]. Kelpe et al. [21] also linked ceramides to the transcriptional suppression of insulin in FFA-treated β cells.
Accumulation of ROS can result in oxidative stress and irreversible β cell injury [22]. Considering the low antioxidant capacity of human β cells, they are highly susceptible to the ROS generated by increased mitochondrial activity under excess nutrient supply [23]. Long-term FFA exposure inhibits the transcription of KIF12, a microtubule motor protein in β cells with potent antioxidative capacity. KIF12 promotes the function of peroxisomes by stabilizing the newly synthesized transcription factor Sp1, which in turn induces the expression of Hsc70 [24]. ROS accumulation can also result from the upregulation of the proinflammatory cytokine IL-1_β_–induced divalent metal transporter 1, which triggers increased intracellular iron deposition [25]. In diabetic mouse islets, the cytokines IL-23, IL-24, and IL-33 function as pro-oxidants, and IL-22 has antioxidant capabilities [26]. A recent study has identified the redox adaptor enzyme p66SHC as a link between saturated FFA and oxidative stress in β cells [27]. Oxidative stress can activate the nuclear factor κB pathway, leading to increased inflammation [28]. In line with that, the inhibition of the proinflammatory kinase IKK_β_ ameliorates FFA-induced β cell dysfunction in mice and rats [29].
Saturated FFAs can induce islet inflammation and β cell dysfunction via the TLR4-MyD88 pathway in β cells [30]. Chemokines and cytokines secreted from β cells lead to the recruitment of M1 macrophages and resulting inflammatory damage. A recent report identified IL-1R signaling as another culprit for impairments in β cell GSIS and proliferation upon inflammation [31].
Endoplasmic reticulum (ER) stress is also closely associated with β cell failure in diabetes, because of the heavy secretory load that β cells have to carry with proper processing of large amounts of proinsulin [32, 33]. As the major organelle for protein folding and posttranslational modifications, the ER is under stress when the unfolded or misfolded protein load exceeds its capacity. In an effort to adapt to these unfavorable conditions, the unfolded protein response (UPR) signaling pathways are activated to reduce protein synthesis, increase ER-associated protein degradation, and promote the expression of protein chaperones [34]. However, if the ER stress is prolonged and beyond the adaptive capacity of the UPR, downstream proapoptotic signaling cascades are activated, leading to a paradoxical upregulation of protein synthesis, depletion of ATP, oxidative stress, and cell death [35]. ER stress in human β cells can be induced by amyloid aggregates [36] or under other conditions characteristic of type 2 diabetes [37]. UPR signaling molecules, such as eIF-2_α_ phosphorylation, ATF4, CHOP, BiP, ATF6, and spliced XBP-1, are induced in β cells by FFA exposure [38, 39]. Thioredoxin-interacting protein was suggested to link ER stress to inflammation and β cell death [40], and its upregulation was detected in islets from diabetic patients [41]. Upstream of ER stress, saturated FFAs can overload ER by disrupting protein trafficking from ER to Golgi [42], an effect that can be ameliorated by high-density lipoproteins [43]. The loss of sphingomyelin seen under these conditions may disrupt the lipid rafts of ER membrane, thereby reducing the efficacy of vesicular trafficking [44].
In summary, lipotoxicity, usually combined with glucotoxicity in diabetes [6], damages the insulin secretory function and survival of β cells. Ceramides, ROS, inflammation, and ER stress signaling are intracellular mediators of β cell failure in this context. Moreover, lipotoxicity may also impair the very limited proliferation and regeneration capacity of β cells [45], leading to an insulinopenic state.
2. Adaptive Signaling Pathways in β Cells Responding to Metabolic Distresses
In response to lipotoxicity and other metabolic challenges during obesity and diabetes, multiple molecular signaling pathways are modulated in β cells to support their function, survival, and growth. In this section, we discuss the signaling cascades mediated by insulin, mammalian target of rapamycin complex (mTORC) 1, protein kinase C (PKC) ζ, autophagy, glucagon-like peptide-1 receptor (GLP1R), peroxisome proliferator-activated receptors (PPARs), and G-protein–coupled receptor (GPR) 40, their crosstalk, and their effects on β cells.
Insulin signaling is intensively studied in β cells, especially for its prominent roles in promoting compensatory growth and functional enhancement for obesity and systemic insulin resistance [46]. The autocrine and paracrine effects of insulin on its own producing cell are interesting in light of its extremely high local concentration in islets that leaves limited room for modulation of signal intensity [47]. Loss of insulin receptor (IR) in β cells triggers diminished insulin secretion and causes type 2 diabetes [48]. It also abolishes the compensatory hyperplasia of β cells when insulin resistance is present [49]. Double knockouts of both the IR and the insulin-like growth factor 1 receptor ablate postnatal β cell mass by suppressing Akt phosphorylation and MafA expression and induction of apoptosis [50]. Immediately downstream of IR, insulin receptor substrate (IRS) 1 and IRS-2 play different roles in β cell function and survival. IRS-1 supports the transcription of insulin genes [51]. However, ablation of IRS-1 reduces the apoptosis of β cells by compensatory upregulation of IRS-2 [52], underscoring the prosurvival role of the latter. In fact, IRS-2 has been intensively studied and established as a pivotal player for β cell compensation in response to insulin resistance [53–58]. As a major downstream kinase effector of insulin signaling, Akt/protein kinase B induces key transcription factors that drive the core β cell transcriptional profile, such as Pdx-1, and promotes β cell viability [59–61]. Between the two isoforms, Akt2 is more critical for β cell survival than Akt1 [62, 63]. On the other hand, overexpression of Akt1 results in β cell hypertrophy and hyperplasia, improved glucose tolerance, and resistance to diabetes [64, 65]. Akt1 activates the cyclin-dependent kinase 4 and the promitotic cyclins D1 and D2 to reignite β cell proliferation in adulthood. Cyclin D2 is critical for maintenance of adult β cell mass by marginal proliferation, and cyclin D1 supports the postnatal rapid growth of β cells together with cyclin D2 [66].
Cyclin induction by Akt is mediated through inhibition of tuberous sclerosis complex 2 and subsequent activation of the mTORC1 signaling pathway [67, 68]. Recent reports have advanced our understandings of the downstream effectors of mTORC1. S6 kinase 1, an immediate target of mTORC1, is necessary for fetal β cell growth [69]. A β cell–specific knockout of mTOR, one of the three proteins that make up mTORC1, impairs GSIS and survival of β cells, which also involves the induction of carbohydrate-responsive element-binding protein, thioredoxin-interacting protein, and oxidative stress [41]. Conditional knockout of raptor, another mTORC1 protein, also damages β cell function and viability [70]. Here, the prosurvival effects of S6 kinase 1 associate with inhibition of autophagosome formation. Eukaryotic translation initiation factor 4E (eIF4E) binding protein 2 is identified as another effector of mTORC1. Inactivation of eIF4E binding protein 2 removes the inhibition of eIF4E and supports β cell proliferation. eIF4E also increases the cap-dependent translation of carboxypeptidase E, the key enzyme for insulin processing.
PKC_ζ_ is another inducer of mTORC1 signaling in the presence of insulin resistance. Activated by extracellular glucose and insulin, PKC_ζ_ is necessary for the adaptive β cell proliferation mediated by mTOR and cyclin D2 but not Akt, as revealed by overexpression of a kinase dead mutant [71]. Consistent with these observations, overexpression of functional PKC_ζ_ does not change Akt phosphorylation but upregulates mTOR phosphorylation and cyclins A, D1, D2, and D3 [72].
Autophagy is a widespread cellular process adapting to nutrient starvation or other stresses by self-degradation [73]. Similar to the UPR, the prosurvival and proapoptotic roles of autophagy can depend on the stress level and the adaptive capacity of the cell, and both prevent further tissue damage [74]. Autophagy is initiated in rodent and human islets by FFA exposure [75] and protects against death [76–78]. However, FFAs also enforce cell apoptosis by blocking the autolysosome formation step of autophagy [79, 80]. Proinflammatory cytokines, such as IL-1_β_ and IFN-γ, induce lysosomal dysfunction and prevent the fusion of lysosomes and autophagosomes in β cells [81], whereas IL-6 [82] and IL-22 [83] augment autophagy.
Restoration of lysosomal function and increases in autophagic flux are part of the GLP1R signaling leading to β cell survival [84]. The other prosurvival mechanisms initiated by GLP1R include crosstalk with insulin signaling via cAMP response element binding protein-induced IRS-2 upregulation [85], reversal of ER stress-induced translational attenuation [86], and nuclear translocation of PKC_ζ_ [87]. Liraglutide, a US Food and Drug Administration–approved GLP1R agonist, has in vitro protective effects on β cells against FFAs or cytokine-induced apoptosis [88]. Randomized clinical trials were carried out and established the GLP1R axis as an important approach to diabetes management [89–91].
PPARs are transcriptional activators of many lipid metabolism genes [92]. PPAR_γ_ is induced in various tissues by obesity and insulin [93] and is meant to overcome lipotoxicity [94]. Mouse β cell hyperplasia is stimulated by a conditional knockout of PPAR_γ_ in β cells on a regular diet, but hyperplasia is suppressed on a high-fat diet [95]. The positive effects of PPAR_γ_ on β cell function and survival are supported by many studies [96]. However, recent reports have provided in vivo evidence that local overexpression of PPAR_γ_ isoforms can impair β cell mass and function in diet-induced obese (DIO) mice [97, 98], suggesting a dose-dependent effect of PPAR_γ_ action. On the other hand, PPAR_α_ expression in β cells is induced by fasting, leading to increased β_-oxidation of FFAs and decreased insulin production [99]. Conversely, high glucose reduces PPAR_α transcription and the associated PPAR_α_ lipid metabolism [100]. In mouse β cells and human islets subjected to long-term FFA-induced lipotoxicity, overexpression of PPAR_α_ promotes β_-oxidation and esterification of FFA, as well as GSIS [101]. The in vivo roles of PPAR_α in β cells under physiological and pathological conditions remain unclear and await further investigation.
Last but not least, short-term (minutes to hours) exposure to FFAs can paradoxically stimulate insulin secretion, which is mediated by GPR40 and intracellular Ca2+ signaling [102]. In the 1960s, a rapid increase in dog plasma insulin was observed after direct infusion of FFA but not triglycerides [103]. In 2003, GPR40 was first identified as a transmembrane receptor for FFAs. It potentiates β cell GSIS by facilitating the influx of extracellular Ca2+ [104]. In vitro experiments with RNA interference identified G_α_q, phospholipase C, sarcoendoplasmic reticulum calcium transport ATPase, l-type calcium channels, and the KATP channel as targets, linking GPR40 to intracellular Ca2+ increase [105]. GPR40 is not necessary for systemic glucose tolerance under physiological conditions and not involved in long-term FFA-impaired insulin secretion [106]. GPR40 can promote the second phase of GSIS via protein kinase D1 and remodeling of F-actin [107]. These findings support GPR40 as a promising target for diabetes drugs.
In summary, β cells use a variety of signaling pathways to handle the metabolic stresses imposed by obesity and diabetes. These short-term adaptive responses may eventually lead to favorable or unfavorable outcomes regarding β cell function and viability over the long term.
3. β Cell Adaptive Signaling Pathways in Obesity and Diabetes Interfere With Each Other
Although the adaptive signaling pathways are meant to neutralize specific detrimental effectors derived from lipotoxicity, they do not always act additively or synergistically on β cells, unfortunately. The antagonism between adaptive signaling pathways is a universal phenomenon, underlying unfavorable drug-drug interactions in many clinical therapies. Although insulin signaling in β cells is one of the most important pathways adapting cells to the increased metabolic demand in obesity and diabetes, insulin signal transduction is vulnerable to the inhibitory effects of many other signaling pathways.
The UPR is meant to alleviate ER stress, but it also impairs insulin signaling and causes insulin resistance in peripheral tissues [108, 109]. Subsequent studies have provided evidence that this also happens in β cells. Cultured β cells treated with palmitate show a concomitant increase in active c-Jun N_-terminal kinase, a downstream effector of the UPR, but also a decrease in phosphorylated Akt [110]. This also happens in the β cells of diabetic db/db mice [111]. Pharmacological induction of ER stress and the UPR in β cells suppresses insulin signaling [111]. Interestingly, insulin signaling exerts dual effects on ER stress in β cells. Inhibition of upstream insulin signaling molecules (e.g., IR and phosphoinositide 3-kinase) diminishes the sequential phosphorylation of Akt and GSK-3_β and exacerbates ER stress in tissue culture [109, 111]. In contrast, in vivo ablation of the phosphoinositide 3-kinase regulatory subunit p85_α_ in β cells reduces the nuclear translocation of XBP-1 and ameliorates ER stress in the Akita mouse model [112]. The different roles of IRS-1 and IRS-2 in β cell function and survival may be mediated by their differential actions on UPR signaling. A β cell-specific knockout of IRS-1 increases the proteasomal degradation of XBP-1 and protects against ER stress-induced apoptosis. It also facilitates the prosurvival role of the UPR by strengthening phosphorylated eIF-2_α_-directed global translational attenuation. In contrast, an IRS-2 knockout in β cells increases nuclear XBP-1 and also ER stress-induced apoptosis [113]. The apparently contradictive interactions between ER stress and insulin signaling may reflect the double-edged nature of the UPR, with early prosurvival and late proapoptotic roles.
PPAR_γ_, the master regulator of lipogenesis, improves insulin signaling in peripheral tissues [114]. However, the crosstalk between PPAR_γ_ and insulin signaling in β cells remains unclear. The upregulation of β cell PPAR_γ_ by obesity and diabetes is also under control of the low-density lipoprotein receptor-related protein 1 (LRP1) [98], a transmembrane receptor and integration hub of multiple signaling pathways [115, 116]. The lack of LRP1 prompts excessive PPAR_γ_ levels that mitigate not only lipotoxic conditions but also insulin signaling components in β cells, which eventually leads to a reduction in GSIS. Transgenic overexpression of PPAR_γ_ in β cells on top of DIO recapitulates the unopposed PPAR_γ_ levels and shows impaired insulin signaling, decreased GSIS, and systemic glucose intolerance in mice with β cell specific LRP1 knockout [98]. Therefore, insulin signaling mediates the dose-dependent effect of PPAR_γ_ on β cell function.
Collectively, the antagonistic interactions between insulin signaling and other adaptive pathways disrupt a “unified” front against lipotoxicity and other associated metabolic stresses and contribute to the eventual failure of β cells at later stages of diabetes.
4. The Potential Insulin-Sparing Effects of Diabetes Therapies
“Insulin-sparing” refers to the reduction in insulin production by antidiabetic treatment regimens. This reduction can reflect a direct action on β cell function or a secondary response to improved systemic insulin sensitivity and hence a decreased demand for insulin. Here, we focus on mechanisms supporting direct action on the β cell.
Metformin is a well-established first-line drug for management of insulin resistance and type 2 diabetes, and its insulin-sparing effect has raised concerns since the 1990s [117, 118]. As an inhibitor of the mitochondrial enzyme glycerol-3-phosphate dehydrogenase, metformin blocks gluconeogenesis from liver [119] and kidney [120]. Through ATP depletion, metformin also activates AMP-activated protein kinase (AMPK), a key homeostat of intracellular energy, in multiple tissues [121, 122]. AMPK promotes _β_-oxidation of FFAs by facilitating their translocation from cytosol into mitochondria [123]. However, the AMPK solution to lipotoxicity is at the price of β cell dysfunction. Adenoviral overexpression of active AMPK in rodent islets results in decreased GSIS and increased apoptosis, which can be reversed by overexpression of a dominant negative mutant of AMPK [124]. AMPK inhibition by elimination of its activating kinase liver kinase B1, on the other hand, supports β cell growth and insulin secretion [125]. Overexpression of an active liver isoform of carnitine palmitoyl-transferase I, the downstream effector of AMPK, increases _β_-oxidation of FFAs but impairs GSIS in β cells [126]. It is possible that the insulin-sparing effects of metformin are masked by the improved systemic insulin sensitivity and reduced demand on insulin production. But this does not preclude the combination of insulin and metformin in treating diabetes [127–129].
Insulin-sparing effects were also observed with thiazolidinediones, a class of PPAR_γ_ agonists decreasing insulin transcription and secretion in rat islets [130]. Although clinical trials showed PPAR_γ_ agonists can increase β cell function in patients with type 2 diabetes [131, 132], such an increase cannot be pinpointed as a primary effect on β cells, because it could be secondary to the improved systemic lipid metabolism. In the setting of diabetes, thiazolidinediones may induce PPAR_γ_ activity to a supraphysiological level in β cells, which in turn may diminish insulin signaling and GSIS [97, 98]. Although the physiological roles of PPAR_α_ in β cells are largely unknown, fenofibrate, a PPAR_α_ agonist, protects β cells from lipotoxicity in obese rats [133] but reduces basal and stimulated insulin secretion in primary human islets [134]. This again reflects the dichotomous results of PPAR_α_ action, similar to what is seen for PPAR_γ_.
Agonists for adiponectin receptors are a class of promising drugs for diabetes and the metabolic syndrome [135]. As a well-known metabolically beneficial adipokine, adiponectin exerts pleiotropic actions via the widely expressed adiponectin receptor 1 (AdipoR1) and AdipoR 2 [136, 137]. AdipoR1 and AdipoR2 activate AMPK and PPAR_α_ signaling, respectively, in peripheral tissues [138]. Meanwhile, they both degrade intracellular lipotoxic sphingolipids by their intrinsic ceramidase activity [20], which is supported by their crystal structures [139]. AdipoR1 and AdipoR2 are expressed in β cells of human and rodents [140], and the antilipotoxic effects of adiponectin preserve the β cell mass in insulinopenic diabetes [8, 141]. However, during the progression of insulin resistance and type 2 diabetes, downstream AMPK and PPAR_α_ activation may raise concerns about the potential insulin-sparing effects of AdipoR agonists. AdipoRon, a recently discovered small molecule, activates AdipoRs and improves insulin sensitivity of muscle and liver in DIO mice, mimicking the function of adiponectin [142]. It stimulates AMPK activity in cardiomyocytes [143] and protects liver, adipose, and kidney via the ceramidase activity of AdipoRs [144, 145]. As for β cells in obesity and diabetes, the functions of AdipoR signaling need a delicate assessment. A selective agonist to specifically increase the ceramidase activity of AdipoRs may circumvent the potential insulin-sparing effects of AMPK and PPAR_α_ signaling, but it is not clear whether such a selective activity with regard to the downstream activation of signaling components can be achieved.
5. Conclusions
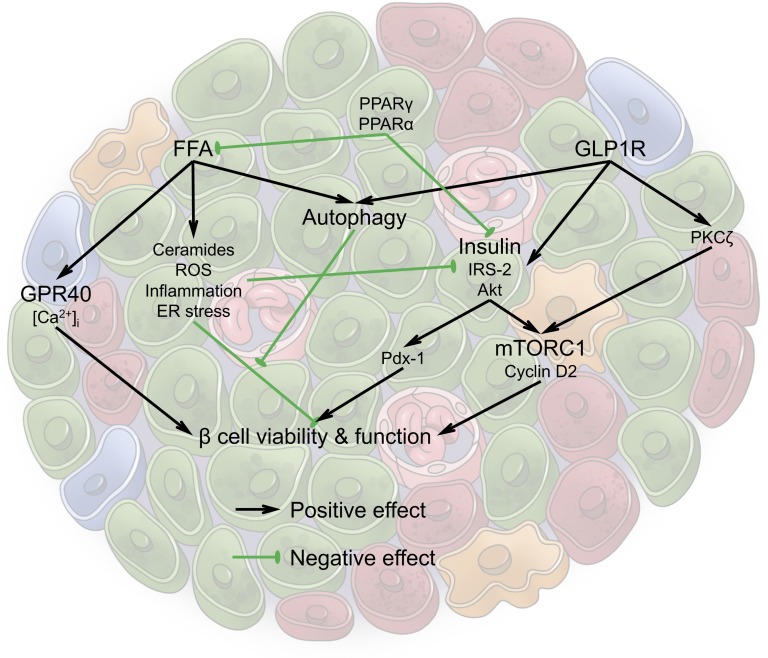
Lipotoxicity associated with obesity and type 2 diabetes provides insults to β cells via multiple causative agents, including ceramides, ROS, inflammation, and ER stress. β cells are able to use multiple adaptive pathways to protect against lipotoxicity and other metabolic stresses (Fig. 1). However, some of these pathways are not always synergistic and can eventually lead to the demise of the function and viability of β cells. An analogy could be drawn to a scenario in which a fire is extinguished and the collateral damage is the flooding of the premises caused by excessive water use. We could therefore refer to the phenomenon as the “lipo-fighter’s dilemma.” As a concept, it can explain the insulin-sparing effects of some diabetic drugs, but it may also be generalized to many other adaptive processes in the body.
Figure 1.
Multiple pathways interact as part of an antilipotoxic response, with some pathways exerting a positive effect (in black) and other pathways leading to a repressive effect (indicated in green) in the β cells in the islet. See text for details on the individual pathways mentioned and how they interact.
6. Search Strategies
Combinations of the following terms were searched on PubMed and Google: lipotoxicity, lipid metabolism, obesity, diabetes, metabolic syndromes, muscle, heart, liver, kidney, beta cells, islets, insulin secretion, insulin expression, proliferation, hyperplasia, growth, hypertrophy, dysfunction, apoptosis, death, human, mouse, nutrient, lipid, glucose, fatty acids, beta oxidation, lipogenesis, glucolipotoxicity, hyperlipidemia, ceramides, lipotoxic sphingolipids, oxidative stress, ROS, inflammation, NF_κ_B, ER stress, UPR, pro-survival, pro-apoptotic, insulin signaling, knockout, overexpression, insulin receptor, IRS, Akt, mTOR, mTORC1, S6K, cyclin, PKC zeta, autophagy, lysosome, GLP-1, extendin, CREB, Pdx-1, MAPK, Erk, liraglutide, clinical trial, PPAR gamma, TZD, PPAR alpha, fibrate, GPR40, Ca2+, calcium, JNK, PI3K, insulin sparing effect, metformin, AMPK, CPT, ACC, malonyl-CoA, acetyl-CoA, mitochondria, Lkb, adiponectin, adiponectin receptor, AdipoRon, ceramidase.
Acknowledgments
Graphics created by R. Howdy (Visually Medical, Allen, TX).
Financial Support: Supported by National Institutes of Health grants R01-DK55758, R01-DK099110, and P01-DK088761-01; JDRF grant 2-SRA-2016-149-Q-R, an unrestricted grant from the Novo Nordisk Research Foundation (to P.E.S.); a research fellowship from the Naomi Berrie Diabetes Center, Columbia University Medical Center (to R.Y.); and grants from the MSD Life Science Foundation and Mochida Memorial Foundation for Medical and Pharmaceutical Research, Japan (to T.O.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
AdipoR
adiponectin receptor
AMPK
AMP-activated protein kinase
DIO
diet-induced obese
eIF4E
eukaryotic translation initiation factor 4E
ER
endoplasmic reticulum
FFA
free fatty acid
GLP1R
glucagon-like peptide-1 receptor
GPR
G-protein–coupled receptor
GSIS
glucose-stimulated insulin secretion
IR
insulin receptor
IRS
insulin receptor substrate
LRP1
lipoprotein receptor-related protein 1
mTORC
mammalian target of rapamycin complex
PI3K
phosphoinositide 3-kinase
PKC
protein kinase C
PPAR
peroxisome proliferator-activated receptor
ROS
reactive oxidative species
UPR
unfolded protein response
References and Notes
- 1.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14(3):281–287. [DOI] [PubMed] [Google Scholar]
- 2.Yazıcı D, Sezer H. Insulin resistance, obesity and lipotoxicity In: Engin AB, Engin A, eds. Obesity and Lipotoxicity. Cham, Germany: Springer International Publishing; 2017:277–304. [Google Scholar]
- 3.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–1346. [DOI] [PubMed] [Google Scholar]
- 5.Porte D Jr, Kahn SE. beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. 2001;50(suppl 1):S160–S163. [DOI] [PubMed] [Google Scholar]
- 6.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;1801(3):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44(8):863–870. [DOI] [PubMed] [Google Scholar]
- 8.Ye R, Holland WL, Gordillo R, Wang M, Wang QA, Shao M, Morley TS, Gupta RK, Stahl A, Scherer PE. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife. 2014;3:e03851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston LW, Harris SB, Retnakaran R, Giacca A, Liu Z, Bazinet RP, Hanley AJ. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia. 2018;61(4):821–830. [DOI] [PubMed] [Google Scholar]
- 10.Jacqueminet S, Briaud I, Rouault C, Reach G, Poitout V. Inhibition of insulin gene expression by long-term exposure of pancreatic β cells to palmitate is dependent on the presence of a stimulatory glucose concentration. Metabolism. 2000;49(4):532–536. [DOI] [PubMed] [Google Scholar]
- 11.Briaud I, Kelpe CL, Johnson LM, Tran POT, Poitout V. Differential effects of hyperlipidemia on insulin secretion in islets of Langerhans from hyperglycemic versus normoglycemic rats. Diabetes. 2002;51(3):662–668. [DOI] [PubMed] [Google Scholar]
- 12.Briaud I, Harmon JS, Kelpe CL, Segu VBG, Poitout V. Lipotoxicity of the pancreatic β-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50(2):315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994;93(2):870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Assaad W, Buteau J, Peyot M-L, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic β-cell death. Endocrinology. 2003;144(9):4154–4163. [DOI] [PubMed] [Google Scholar]
- 15.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes. 2003;52(3):726–733. [DOI] [PubMed] [Google Scholar]
- 16.Shimabukuro M, Zhou Y-T, Levi M, Unger RH. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95(5):2498–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72. [DOI] [PubMed] [Google Scholar]
- 18.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patané G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51(5):1437–1442. [DOI] [PubMed] [Google Scholar]
- 19.Shimabukuro M, Higa M, Zhou Y-T, Wang M-Y, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273(49):32487–32490. [DOI] [PubMed] [Google Scholar]
- 20.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo M-S, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278(32):30015–30021. [DOI] [PubMed] [Google Scholar]
- 22.Drews G, Krippeit-Drews P, Düfer M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010;460(4):703–718. [DOI] [PubMed] [Google Scholar]
- 23.Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal. 2017;26(10):501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Tanaka Y, Bundo M, Hirokawa N. Antioxidant signaling involving the microtubule motor KIF12 is an intracellular target of nutrition excess in beta cells. Dev Cell. 2014;31(2):202–214. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JB, Tonnesen MF, Madsen AN, Hagedorn PH, Friberg J, Grunnet LG, Heller RS, Nielsen AØ, Størling J, Baeyens L, Anker-Kitai L, Qvortrup K, Bouwens L, Efrat S, Aalund M, Andrews NC, Billestrup N, Karlsen AE, Holst B, Pociot F, Mandrup-Poulsen T. Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic β cell fate in response to cytokines. Cell Metab. 2012;16(4):449–461. [DOI] [PubMed] [Google Scholar]
- 26.Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, Das I, Wang R, Chen ACH, Loudovaris T, Kay TW, Thomas HE, Whitehead JP, Forbes JM, Prins JB, McGuckin MA. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med. 2014;20(12):1417–1426. [DOI] [PubMed] [Google Scholar]
- 27.Natalicchio A, Tortosa F, Labarbuta R, Biondi G, Marrano N, Carchia E, Leonardini A, Cignarelli A, Bugliani M, Marchetti P, Fadini GP, Giorgio M, Avogaro A, Perrini S, Laviola L, Giorgino F. The p66(Shc) redox adaptor protein is induced by saturated fatty acids and mediates lipotoxicity-induced apoptosis in pancreatic beta cells [published correction appears in Diabetologia. 2015;58:2682] Diabetologia. 2015;58(6):1260–1271. [DOI] [PubMed] [Google Scholar]
- 28.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivovic A, Oprescu AI, Koulajian K, Mori Y, Eversley JA, Zhang L, Nino-Fong R, Lewis GF, Donath MY, Karin M, Wheeler MB, Ehses J, Volchuk A, Chan CB, Giacca A. IKKβ inhibition prevents fat-induced beta cell dysfunction in vitro and in vivo in rodents. Diabetologia. 2017;60(10):2021–2032. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15(4):518–533. [DOI] [PubMed] [Google Scholar]
- 31.Böni-Schnetzler M, Häuselmann SP, Dalmas E, Meier DT, Thienel C, Traub S, Schulze F, Steiger L, Dror E, Martin P, Herrera PL, Gabay C, Donath MY. β Cell-specific deletion of the IL-1 receptor antagonist impairs β cell proliferation and insulin secretion. Cell Reports. 2018;22(7):1774–1786. [DOI] [PubMed] [Google Scholar]
- 32.Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in β-cells and development of diabetes. Curr Opin Pharmacol. 2009;9(6):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papa FR. Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb Perspect Med. 2012;2(9):a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. [DOI] [PubMed] [Google Scholar]
- 35.Han J, Back SH, Hur J, Lin Y-H, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced β-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab. 2007;293(6):E1656–E1662. [DOI] [PubMed] [Google Scholar]
- 37.Laybutt DR, Preston AM, Åkerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50(4):752–763. [DOI] [PubMed] [Google Scholar]
- 38.Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145(11):5087–5096. [DOI] [PubMed] [Google Scholar]
- 39.Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. Selective inhibition of eukaryotic translation initiation factor 2 α dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic β-cell dysfunction and apoptosis. J Biol Chem. 2007;282(6):3989–3997. [DOI] [PubMed] [Google Scholar]
- 40.Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16(2):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chau GC, Im DU, Kang TM, Bae JM, Kim W, Pyo S, Moon EY, Um SH. mTOR controls ChREBP transcriptional activity and pancreatic β cell survival under diabetic stress. J Cell Biol. 2017;216(7):2091–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia. 2009;52(11):2369–2373. [DOI] [PubMed] [Google Scholar]
- 43.Pétremand J, Puyal J, Chatton J-Y, Duprez J, Allagnat F, Frias M, James RW, Waeber G, Jonas J-C, Widmann C. HDLs protect pancreatic β-cells against ER stress by restoring protein folding and trafficking. Diabetes. 2012;61(5):1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boslem E, Weir JM, MacIntosh G, Sue N, Cantley J, Meikle PJ, Biden TJ. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J Biol Chem. 2013;288(37):26569–26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma RB, Alonso LC. Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr Diab Rep. 2014;14(6):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leibiger IB, Leibiger B, Berggren P-O. Insulin signaling in the pancreatic β-cell. Annu Rev Nutr. 2008;28(1):233–251. [DOI] [PubMed] [Google Scholar]
- 47.Wang M, Li J, Lim GE, Johnson JD. Is dynamic autocrine insulin signaling possible? A mathematical model predicts picomolar concentrations of extracellular monomeric insulin within human pancreatic islets. PLoS One. 2013;8(6):e64860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96(3):329–339. [DOI] [PubMed] [Google Scholar]
- 49.Okada T, Liew CW, Hu J, Hinault C, Michael MD, Kr̈tzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA. 2007;104(21):8977–8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN. Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat Genet. 2006;38(5):583–588. [DOI] [PubMed] [Google Scholar]
- 51.Kulkarni RN, Winnay JN, Daniels M, Brüning JC, Flier SN, Hanahan D, Kahn CR. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured β-cell lines. J Clin Invest. 1999;104(12):R69–R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennige AM, Ozcan U, Okada T, Jhala US, Schubert M, White MF, Kulkarni RN. Alterations in growth and apoptosis of insulin receptor substrate-1-deficient β-cells. Am J Physiol Endocrinol Metab. 2005;289(2):E337–E346. [DOI] [PubMed] [Google Scholar]
- 53.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet. 1999;23(1):32–40. [DOI] [PubMed] [Google Scholar]
- 54.Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49(11):1880–1889. [DOI] [PubMed] [Google Scholar]
- 55.Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, Moroi M, Okada-Iwabu M, Ezaki O, Nagai R, Ueta Y, Kadowaki T, Noda T. Insulin receptor substrate 2 plays a crucial role in β cells and the hypothalamus. J Clin Invest. 2004;114(7):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford MLJ, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and β cell function. J Clin Invest. 2005;115(4):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol. 2009;29(11):3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamateris RE, Sharma RB, Kong Y, Ebrahimpour P, Panday D, Ranganath P, Zou B, Levitt H, Parambil NA, O’Donnell CP, García-Ocaña A, Alonso LC. Glucose induces mouse β-cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes. 2016;65(4):981–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, Larock K, Everill B, Leahy JL, Jetton TL. Regulation of pancreatic β-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes. 2006;55(12):3289–3298. [DOI] [PubMed] [Google Scholar]
- 60.Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, Beith JL, Gross J, Warnock GL, Townsend RR, Permutt MA, Polonsky KS. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA. 2006;103(51):19575–19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Humphrey RK, Yu S-M, Flores LE, Jhala US. Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J Biol Chem. 2010;285(5):3406–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276(42):38349–38352. [DOI] [PubMed] [Google Scholar]
- 63.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB β. J Clin Invest. 2003;112(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuttle RL, Gill NS, Pugh W, Lee J-P, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7(10):1133–1137. [DOI] [PubMed] [Google Scholar]
- 65.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108(11):1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol. 2005;25(9):3752–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–162. [DOI] [PubMed] [Google Scholar]
- 68.Balcazar N, Sathyamurthy A, Elghazi L, Gould A, Weiss A, Shiojima I, Walsh K, Bernal-Mizrachi E. mTORC1 activation regulates β-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem. 2009;284(12):7832–7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Um SH, Sticker-Jantscheff M, Chau GC, Vintersten K, Mueller M, Gangloff Y-G, Adams RH, Spetz J-F, Elghazi L, Pfluger PT, Pende M, Bernal-Mizrachi E, Tauler A, Tschöp MH, Thomas G, Kozma SC. S6K1 controls pancreatic β cell size independently of intrauterine growth restriction. J Clin Invest. 2015;125(7):2736–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blandino-Rosano M, Barbaresso R, Jimenez-Palomares M, Bozadjieva N, Werneck-de-Castro JP, Hatanaka M, Mirmira RG, Sonenberg N, Liu M, Rüegg MA, Hall MN, Bernal-Mizrachi E. Loss of mTORC1 signalling impairs β-cell homeostasis and insulin processing. Nat Commun. 2017;8:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lakshmipathi J, Alvarez-Perez JC, Rosselot C, Casinelli GP, Stamateris RE, Rausell-Palamos F, O’Donnell CP, Vasavada RC, Scott DK, Alonso LC, Garcia-Ocaña A. PKCζ is essential for pancreatic β-cell replication during insulin resistance by regulating mTOR and cyclin-D2. Diabetes. 2016;65(5):1283–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Velazquez-Garcia S, Valle S, Rosa TC, Takane KK, Demirci C, Alvarez-Perez JC, Mellado-Gil JM, Ernst S, Scott DK, Vasavada RC, Alonso LC, Garcia-Ocaña A. Activation of protein kinase C-ζ in pancreatic β-cells in vivo improves glucose tolerance and induces β-cell expansion via mTOR activation. Diabetes. 2011;60(10):2546–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. [DOI] [PubMed] [Google Scholar]
- 74.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115(10):2679–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, Masiello P, Marchetti P, De Tata V. Palmitate activates autophagy in INS-1E β-cells and in isolated rat and human pancreatic islets [published correction appears in PLoS ONE. 10(3):e0122235] PLoS One. 2012;7(5):e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi S-E, Lee S-M, Lee Y-J, Li L-J, Lee S-J, Lee J-H, Kim Y, Jun H-S, Lee K-W, Kang Y. Protective role of autophagy in palmitate-induced INS-1 β-cell death. Endocrinology. 2009;150(1):126–134. [DOI] [PubMed] [Google Scholar]
- 77.Sheng Q, Xiao X, Prasadan K, Chen C, Ming Y, Fusco J, Gangopadhyay NN, Ricks D, Gittes GK. Autophagy protects pancreatic beta cell mass and function in the setting of a high-fat and high-glucose diet. Sci Rep. 2017;7(1):16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernández ÁF, Bárcena C, Martínez-García GG, Tamargo-Gómez I, Suárez MF, Pietrocola F, Castoldi F, Esteban L, Sierra-Filardi E, Boya P, López-Otín C, Kroemer G, Mariño G. Autophagy counteracts weight gain, lipotoxicity and pancreatic β-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017;8(8):e2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in β-cells. J Biol Chem. 2011;286(49):42534–42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trudeau KM, Colby AH, Zeng J, Las G, Feng JH, Grinstaff MW, Shirihai OS. Lysosome acidification by photoactivated nanoparticles restores autophagy under lipotoxicity. J Cell Biol. 2016;214(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lambelet M, Terra LF, Fukaya M, Meyerovich K, Labriola L, Cardozo AK, Allagnat F. Dysfunctional autophagy following exposure to pro-inflammatory cytokines contributes to pancreatic β-cell apoptosis. Cell Death Dis. 2018;9(2):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Linnemann AK, Blumer J, Marasco MR, Battiola TJ, Umhoefer HM, Han JY, Lamming DW, Davis DB. Interleukin 6 protects pancreatic β cells from apoptosis by stimulation of autophagy. FASEB J. 2017;31(9):4140–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu M, Yang S, Yang L, Cheng Y, Zhang H. Interleukin-22 alleviated palmitate-induced endoplasmic reticulum stress in INS-1 cells through activation of autophagy. PLoS One. 2016;11(1):e0146818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JAM, Lovat PE, Arden C. Glucagon-like peptide 1 protects pancreatic β-cells from death by increasing autophagic flux and restoring lysosomal function. Diabetes. 2017;66(5):1272–1285. [DOI] [PubMed] [Google Scholar]
- 85.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17(13):1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves β cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4(5):391–406. [DOI] [PubMed] [Google Scholar]
- 87.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes. 2001;50(10):2237–2243. [DOI] [PubMed] [Google Scholar]
- 88.Bregenholt S, Møldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits β-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330(2):577–584. [DOI] [PubMed] [Google Scholar]
- 89.Gough SCL. Liraglutide: from clinical trials to clinical practice. Diabetes Obes Metab. 2012;14(suppl 2):33–40. [DOI] [PubMed] [Google Scholar]
- 90.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, All in combination with metformin, in type 2 diabetes. The LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Retnakaran R, Kramer CK, Choi H, Swaminathan B, Zinman B. Liraglutide and the preservation of pancreatic β-cell function in early type 2 diabetes: the LIBRA trial. Diabetes Care. 2014;37(12):3270–3278. [DOI] [PubMed] [Google Scholar]
- 92.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97(11):2553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GSH, Lopez M, Seppänen-Laakso T, Ashcroft FM, Orešič M, Vidal-Puig A. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3(4):e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu C-H, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM. Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. 2003;23(20):7222–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta D, Kono T, Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12(12):1036–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hogh KLN, Craig MN, Uy CE, Nygren H, Asadi A, Speck M, Fraser JD, Rudecki AP, Baker RK, Orešič M, Gray SL. Overexpression of PPARγ specifically in pancreatic β-cells exacerbates obesity-induced glucose intolerance, reduces β-cell mass, and alters islet lipid metabolism in male mice. Endocrinology. 2014;155(10):3843–3852. [DOI] [PubMed] [Google Scholar]
- 98.Ye R, Gordillo R, Shao M, Onodera T, Chen Z, Chen S, Lin X, SoRelle JA, Li X, Tang M, Keller MP, Kuliawat R, Attie AD, Gupta RK, Holland WL, Beutler B, Herz J, Scherer PE. Intracellular lipid metabolism impairs β cell compensation during diet-induced obesity. J Clin Invest. 2018;128(3):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gremlich S, Nolan C, Roduit R, Burcelin R, Peyot ML, Delghingaro-Augusto V, Desvergne B, Michalik L, Prentki M, Wahli W. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor α transcriptional up-regulation of fatty acid oxidation. Endocrinology. 2005;146(1):375–382. [DOI] [PubMed] [Google Scholar]
- 100.Roduit R, Morin J, Massé F, Segall L, Roche E, Newgard CB, Assimacopoulos-Jeannet F, Prentki M. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. J Biol Chem. 2000;275(46):35799–35806. [DOI] [PubMed] [Google Scholar]
- 101.Frigerio F, Brun T, Bartley C, Usardi A, Bosco D, Ravnskjaer K, Mandrup S, Maechler P. Peroxisome proliferator-activated receptor α (PPARalpha) protects against oleate-induced INS-1E beta cell dysfunction by preserving carbohydrate metabolism. Diabetologia. 2010;53(2):331–340. [DOI] [PubMed] [Google Scholar]
- 102.Mancini AD, Poitout V. The fatty acid receptor FFA1/GPR40 a decade later: how much do we know? Trends Endocrinol Metab. 2013;24(8):398–407. [DOI] [PubMed] [Google Scholar]
- 103.Crespin SR, Greenough WB III, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969;48(10):1934–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature. 2003;422(6928):173–176. [DOI] [PubMed] [Google Scholar]
- 105.Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD. Role of GPR40 in fatty acid action on the β cell line INS-1E. Biochem Biophys Res Commun. 2005;335(1):97–104. [DOI] [PubMed] [Google Scholar]
- 106.Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DCH, Poitout V. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56(4):1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferdaoussi M, Bergeron V, Zarrouki B, Kolic J, Cantley J, Fielitz J, Olson EN, Prentki M, Biden T, MacDonald PE, Poitout V. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia. 2012;55(10):2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. [DOI] [PubMed] [Google Scholar]
- 109.Srinivasan S, Ohsugi M, Liu Z, Fatrai S, Bernal-Mizrachi E, Permutt MA. Endoplasmic reticulum stress-induced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3β in mouse insulinoma cells. Diabetes. 2005;54(4):968–975. [DOI] [PubMed] [Google Scholar]
- 110.Chen YY, Sun LQ, Wang BA, Zou XM, Mu YM, Lu JM. Palmitate induces autophagy in pancreatic β-cells via endoplasmic reticulum stress and its downstream JNK pathway. Int J Mol Med. 2013;32(6):1401–1406. [DOI] [PubMed] [Google Scholar]
- 111.Matsuda T, Kido Y, Uchida T, Kasuga M. Reduced insulin signaling and endoplasmic reticulum stress act synergistically to deteriorate pancreatic beta cell function. Kobe J Med Sci. 2008;54(2):E114–E121. [PubMed] [Google Scholar]
- 112.Winnay JN, Dirice E, Liew CW, Kulkarni RN, Kahn CR. p85α deficiency protects β-cells from endoplasmic reticulum stress-induced apoptosis. Proc Natl Acad Sci USA. 2014;111(3):1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takatani T, Shirakawa J, Roe MW, Leech CA, Maier BF, Mirmira RG, Kulkarni RN. IRS1 deficiency protects β-cells against ER stress-induced apoptosis by modulating sXBP-1 stability and protein translation [published correction appears in Sci Rep. 2016;6:34969] Sci Rep. 2016;6(1):28177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F. Cross-talk between PPARgamma and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009;2009:818945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88(3):887–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boucher P, Herz J. Signaling through LRP1: protection from atherosclerosis and beyond. Biochem Pharmacol. 2011;81(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Slama G. The insulin sparing effect of metformin in insulin-treated diabetic patients. Diabete Metab. 1991;17(1 Pt 2):241–243. [PubMed] [Google Scholar]
- 118.Golay A, Guillet-Dauphiné N, Fendel A, Juge C, Assal JP. The insulin-sparing effect of metformin in insulin-treated diabetic patients. Diabetes Metab Rev. 1995;11(suppl 1):S63–S67. [DOI] [PubMed] [Google Scholar]
- 119.Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez J-P, Lee H-Y, Cline GW, Samuel VT, Kibbey RG, Shulman GI. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kiersztan A, Modzelewska A, Jarzyna R, Jagielska E, Bryła J. Inhibition of gluconeogenesis by vanadium and metformin in kidney-cortex tubules isolated from control and diabetic rabbits. Biochem Pharmacol. 2002;63(7):1371–1382. [DOI] [PubMed] [Google Scholar]
- 121.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54(12):3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(suppl):S138–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic beta-cell function in vivo. J Endocrinol. 2005;187(2):225–235. [DOI] [PubMed] [Google Scholar]
- 125.Fu A, Ng AC-H, Depatie C, Wijesekara N, He Y, Wang G-S, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, Screaton RA. Loss of Lkb1 in adult β cells increases β cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10(4):285–295. [DOI] [PubMed] [Google Scholar]
- 126.Herrero L, Rubí B, Sebastián D, Serra D, Asins G, Maechler P, Prentki M, Hegardt FG. Alteration of the malonyl-CoA/carnitine palmitoyltransferase I interaction in the β-cell impairs glucose-induced insulin secretion. Diabetes. 2005;54(2):462–471. [DOI] [PubMed] [Google Scholar]
- 127.Pagano G, Tagliaferro V, Carta Q, Caselle MT, Bozzo C, Vitelli F, Trovati M, Cocuzza E. Metformin reduces insulin requirement in type 1 (insulin-dependent) diabetes. Diabetologia. 1983;24(5):351–354. [DOI] [PubMed] [Google Scholar]
- 128.Wulffelé MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, Donker AJM, Stehouwer CDA. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care. 2002;25(12):2133–2140. [DOI] [PubMed] [Google Scholar]
- 129.Beysel S, Unsal IO, Kizilgul M, Caliskan M, Ucan B, Cakal E. The effects of metformin in type 1 diabetes mellitus. BMC Endocr Disord. 2018;18(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bollheimer LC, Troll S, Landauer H, Wrede CE, Schölmerich J, Buettner R. Insulin-sparing effects of troglitazone in rat pancreatic islets. J Mol Endocrinol. 2003;31(1):61–69. [DOI] [PubMed] [Google Scholar]
- 131.Juhl CB, Hollingdal M, Pørksen N, Prange A, Lönnqvist F, Schmitz O. Influence of rosiglitazone treatment on β-cell function in type 2 diabetes: evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab. 2003;88(8):3794–3800. [DOI] [PubMed] [Google Scholar]
- 132.Wallace TM, Levy JC, Matthews DR. An increase in insulin sensitivity and basal beta-cell function in diabetic subjects treated with pioglitazone in a placebo-controlled randomized study. Diabet Med. 2004;21(6):568–576. [DOI] [PubMed] [Google Scholar]
- 133.Liu SN, Liu Q, Li LY, Huan Y, Sun SJ, Shen ZF. Long-term fenofibrate treatment impaired glucose-stimulated insulin secretion and up-regulated pancreatic NF-kappa B and iNOS expression in monosodium glutamate-induced obese rats: is that a latent disadvantage? J Transl Med. 2011;9(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Burns SM, Vetere A, Walpita D, Dančík V, Khodier C, Perez J, Clemons PA, Wagner BK, Altshuler D. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic beta-cell function. Cell Metab. 2015;21(1):126–137. [DOI] [PubMed] [Google Scholar]
- 135.Greenhill C. Obesity: adiponectin receptor agonists--possible therapeutic approach? Nat Rev Endocrinol. 2014;10(1):4. [DOI] [PubMed] [Google Scholar]
- 136.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–339. [DOI] [PubMed] [Google Scholar]
- 139.Vasiliauskaité-Brooks I, Sounier R, Rochaix P, Bellot G, Fortier M, Hoh F, De Colibus L, Bechara C, Saied EM, Arenz C, Leyrat C, Granier S. Structural insights into adiponectin receptors suggest ceramidase activity. Nature. 2017;544(7648):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic β cells. Biochem Biophys Res Commun. 2003;312(4):1118–1122. [DOI] [PubMed] [Google Scholar]
- 141.Ye R, Wang M, Wang QA, Scherer PE. Adiponectin-mediated antilipotoxic effects in regenerating pancreatic islets. Endocrinology. 2015;156(6):2019–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503(7477):493–499. [DOI] [PubMed] [Google Scholar]
- 143.Wang Y, Liang B, Lau WB, Du Y, Guo R, Yan Z, Gan L, Yan W, Zhao J, Gao E, Koch W, Ma X-L. Restoring diabetes-induced autophagic flux arrest in ischemic/reperfused heart by ADIPOR (adiponectin receptor) activation involves both AMPK-dependent and AMPK-independent signaling. Autophagy. 2017;13(11):1855–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holland WL, Xia JY, Johnson JA, Sun K, Pearson MJ, Sharma AX, Quittner-Strom E, Tippetts TS, Gordillo R, Scherer PE. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab. 2017;6(3):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Choi SR, Lim JH, Kim MY, Kim EN, Kim Y, Choi BS, Kim Y-S, Kim HW, Lim K-M, Kim MJ, Park CW. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism. 2018;85:348–360. [DOI] [PubMed] [Google Scholar]