Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons (original) (raw)
Abstract
Exposure to unpredictable environmental stress is widely recognized as a major determinant for risk and severity in neuropsychiatric disorders such as major depressive disorder, anxiety, schizophrenia, and PTSD. The ability of ostensibly unrelated disorders to give rise to seemingly similar psychiatric phenotypes highlights a need to identify circuit-level concepts that could unify diverse factors under a common pathophysiology. Although difficult to disentangle a causative effect of stress from other factors on medial prefrontal cortex (PFC) dysfunction, a wealth of data from humans and rodents demonstrates that the PFC is a key target of stress. The present study sought to identify a model of chronic unpredictable stress (CUS) which induces affective behaviors in C57BL6J mice and once established, measure stress-related alterations in intrinsic excitability and synaptic regulation of mPFC layer 5/6 pyramidal neurons. Adult male mice received 2 weeks of ‘less intense’ stress or 2 or 4 weeks of ‘more intense’ CUS followed by sucrose preference for assessment of anhedonia, elevated plus maze for assessment of anxiety and forced swim test for assessment of depressive-like behaviors. Our findings indicate that more intense CUS exposure results in increased anhedonia, anxiety, and depressive behaviors, while the less intense stress results in no measured behavioral phenotypes. Once a behavioral model was established, mice were euthanized approximately 21 days post-stress for whole-cell patch clamp recordings from layer 5/6 pyramidal neurons in the prelimbic (PrL) and infralimbic (IL) cortices. No significant differences were initially observed in intrinsic cell excitability in either region. However, post-hoc analysis and subsequent confirmation using transgenic mice expressing tdtomato or eGFP under control of dopamine D1-or D2-type receptor showed that D1-expressing pyramidal neurons (D1-PYR) in the PrL exhibit reduced thresholds to fire an action potential (increased excitability) but impaired firing capacity at more depolarized potentials, whereas D2-expressing pyramidal neurons (D2-PYR) showed an overall reduction in excitability and spike firing frequency. Examination of synaptic transmission showed that D1-and D2-PYR exhibit differences in basal excitatory and inhibitory signaling under naïve conditions. In CUS mice, D1-PYR showed increased frequency of both miniature excitatory and inhibitory postsynaptic currents, whereas D2-PYR only showed a reduction in excitatory currents. These findings demonstrate that D1-and D2-PYR subpopulations differentially undergo stress-induced intrinsic and synaptic plasticity that may have functional implications for stress-related pathology, and that these adaptations may reflect unique differences in basal properties regulating output of these cells.
Keywords: Prefrontal cortex, Stress, Intrinsic excitability, Excitatory and inhibitory synaptic transmission, Affective behavior, Dopamine receptor
1. Introduction
Exposure to unpredictable environmental stress is widely recognized as a major determinant of risk and severity in neuropsychiatric disorders such as major depressive disorder (MDD), anxiety, and post-traumatic stress disorder (Bale, 2005; Kendler et al., 1998, 1999; Moghaddam and Javitt, 2012). The medial prefrontal cortex (mPFC) is intricately involved in cognitive performance, as well as top-down regulation of affect and stress responsivity (Anisman and Matheson, 2005; Clark et al., 2009; Fossati et al., 1999; Herman et al., 2005; Keedwell et al., 2005; Krishnan and Nestler, 2008; Miller and Lewis, 1977; Murphy et al., 1999; Murrough et al., 2011; Radley et al., 2006a, 2006b; Sullivan, 2004; Treadway and Zald, 2011).
Functional integrity of mPFC information processing and downstream communication relies on a dynamic balance of intrinsic and synaptic excitatory and inhibitory signaling, with disruptions in this balance implicated in stress-related pathologies including flattened affect (anhedonia), anxiety-like behavior, and impaired cognition (Gandal et al., 2012; Holmes and Wellman, 2009; Matsuo et al., 2007; Sohal et al., 2009; Yizhar et al., 2011). Structural modifications in pyramidal neurons (PYR) – the principle output neurons in the mPFC – have long been thought to play a critical role in stress-induced cortical dysfunction, however to date only a handful of studies have examined the impact of this reorganization on neurotransmission and cellular physiology, with even fewer examining the cell-specific locus of these adaptations (McEwen and Morrison, 2013; McKlveen et al., 2016; Radley et al., 2005; Radley et al., 2006a, 2006b; Shansky and Morrison, 2009; Urban and Valentino, 2017).
Growing evidence indicates that distinctions in molecular (e.g., ion channels, receptors), neurophysiology, and anatomical connectivity endow specific subpopulations of PYR with unique properties to integrate input and communicate information downstream (Brown and Hestrin, 2009; Degenetais et al., 2002; Dembrow et al., 2010; Gee et al., 2012; Kim et al., 2016; Seong and Carter, 2012; Sohal et al., 2009; Yang et al., 1996). For example, recent evidence indicates that PYR neurons expressing either the dopamine D1 (D1-PYR) or D2 (D2-PYR) receptor exhibit distinctions in spike firing, ion channel expression and conductance, inhibitory synaptic innervation, and subcortical projection targets, that likely define how they contribute to behavior and undergo experience-induced plasticity (e.g., stress) (Anastasiades et al., 2018; Benes et al., 1993; Gee et al., 2012; Santana et al., 2009; Seong and Carter, 2012; Xu and Yao, 2010). As these cortical networks likely provide a neuroanatomical framework for complex regulation of behavior (Brumback et al., 2018; Gaspar et al., 1995; Gee et al., 2012; Jenni et al., 2017; Santana et al., 2009; Seong and Carter, 2012; Vincent et al., 1993), a critical step towards understanding how stress influences behavior include identifying the selectivity of stress-induced plasticity and associated mechanisms (Jenni et al., 2017).
The current study set out to establish a model of chronic unpredictable stress (CUS) in C57BL/6J mice - a common mouse strain notorious for stress resilience -- that induces consistent affective behaviors as well as determine how CUS differentially impacts mPFC D1-and D2-expressing PYR neuron intrinsic physiology and synaptic regulation. Findings from this study have implications to increase our understanding of how mPFC subcircuits are differentially regulated under naive conditions and how stress-induced adaptations in these circuits may uniquely contribute to stress pathology.
2. Materials and methods
2.1. Animals
Adult male mice (PN51-74) were a combination of wild-type (C57BL/6 J) bred in house or purchased from Jackson Laboratories, heterozygous bacterial artificial chromosome (BAC) transgenic mice (Jackson Laboratories) expressing tdtomato or eGFP expression, or double transgenics expressing tdtomato and eGFP driven by either DR1 (drd1a-tdtomato) or DR2 (drd2-eGFP) dopamine receptors. Recordings performed from single transgenics expressing only tdtomato driven by DR1 were used as the tdtomato signaling is greater in cortical neurons compared to eGFP and also exhibits decreased photobleaching compared to eGFP, therefore cells were identified as D1+ or D1-. Mice were maintained in a temperature and humidity-controlled room with all procedures approved by the Institutional Animal Care and Use Committee at Marquette University.
2.2. Chronic unpredictable stress
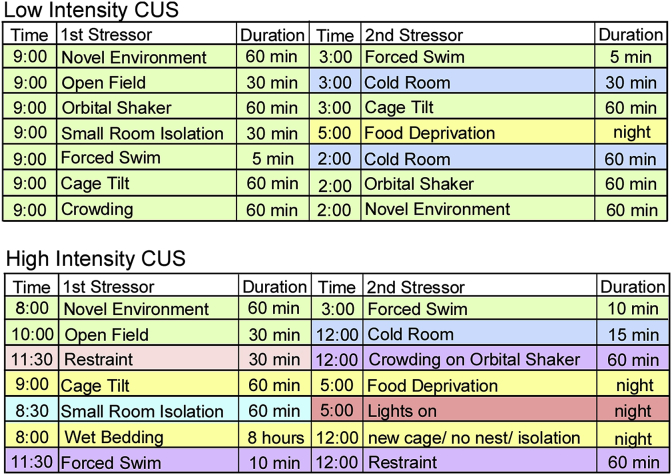
Mice were exposed to two weeks of less intense (LI) stress or exposed to two or four weeks of more intense (MI) stress (Fig. 1). To increase stress intensity, the level of unpredictability was increased by further varying the times, durations, and locations, as well as combining stressors (e.g. cage tilt in cold room) and using MI stressors with increased frequency.
Fig. 1.
One week sample of unpredictable stressors of various durations, intensities, and in various locations (green = stress room A, red = stress room B, purple = stress room C, blue = cold room, yellow = colony). Mice received two weeks of less intense stress (top) or two or four weeks of more intense stress (bottom).
2.3. Behavioral testing
Sucrose preference. In a subset of mice, sucrose preference was assessed as a measure of anhedonia (Forbes et al., 1996; Willner et al., 1987). The evening of the last stress exposure (Fig. 2A), mice were provided two separate bottles that were weighed, one containing 1% sucrose solution and the other containing tap water. The mouse had ad libitum access to food and both bottles overnight. The following morning, the bottles were removed and reweighed. Percent sucrose consumed was calculated as the amount of sucrose water consumed divided by the amount of sucrose water consumed plus the amount of tap water consumed.
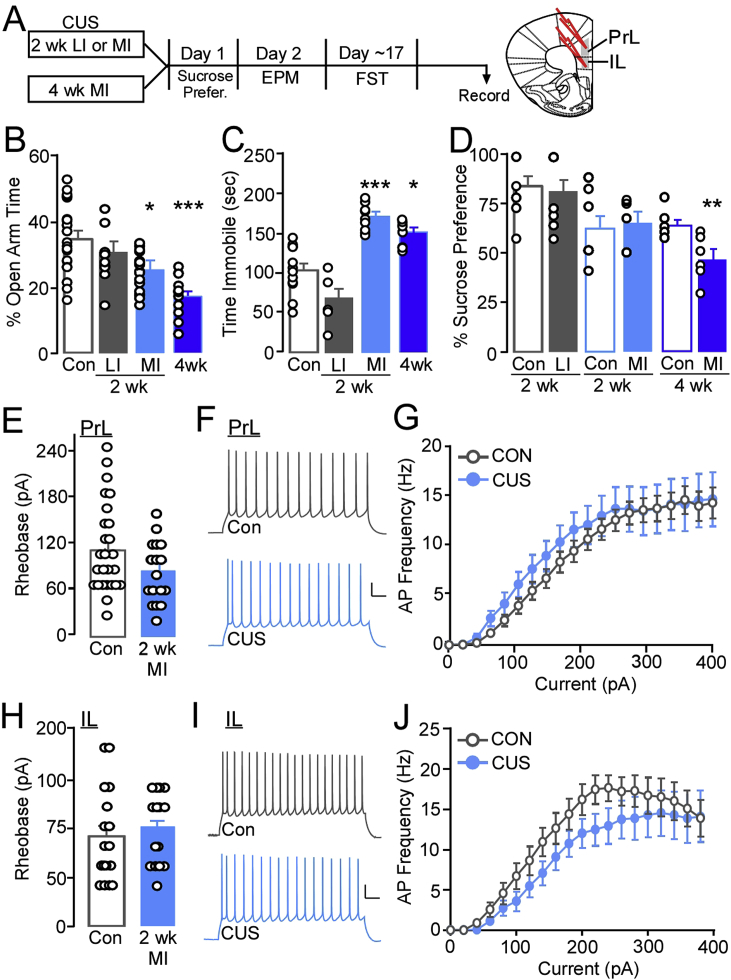
Fig. 2.
(A) Experimental timeline showing mice received either two weeks of less intense (LI) or more intense (MI) stress or four weeks of MI stress followed by behavioral testing and slice electrophysiology in layer 5/6 pyramidal neurons (L5/6 PYR) in the PrL or IL cortices. (B) Percent time in the open arm of the elevated plus maze. Mice exposed to two or four weeks of more intense stress had significant reductions in percent open arm time (N = 9–29/group). (C) Time immobile during a forced swim test (FST). Mice exposed to two or four weeks of more intense stress had significant increases in time immobile during the forced swim test (N = 6–14/group). (D) Percent sucrose consumed during an overnight preference test .Only mice exposed to four weeks of more intense stress showed a significant decrease in sucrose preference compared to respective controls (N = 6–18/group). (E) There were no differences in the current required to evoke an action potential (rheobase) in L5/6 PrL PYR neurons from control mice and mice exposed to two week more intense stress (n = 17–23/group, N = 9–11/group). (F) Action potential (AP) firing frequency elicited during a 1 s 260 pA current injection in L5/6 PrL PYR from control (top) and 2 week MI CUS (bottom) mice. (G) There were no differences in the current-spike plots for control and CUS L5/6 PrL PYR neurons. (H) No differences in rheobase in L5/6 IL PYR neurons from control and 2 week MI CUS mice (n = 16–17/group, N = 8–9/group). (I) Spiking elicited during a 1 s 260 pA current injection in L5/6 IL PYR from control (top) and 2 week MI CUS (bottom) mice. (J) No differences in current-spike plots for control and 2 week MI CUS L5/6 IL PYR neurons. (scale bar, 20pA/500 msec). *p ≤ 0.05 versus Con, ***p < 0.001 versus Con.
Elevated plus maze. On the day following the last stress exposure (Fig. 2A), a subset of mice were tested for anxiety-like behaviors using an elevated plus maze (EPM; San Diego Instruments). The EPM consisted of two opposite open arms (H: 15.25″ W: 2.0″ L: 26.0”) with lights (∼50 lux) and camera mounted above to monitor and record behavior. Individual trials lasted 5 min beginning with the mouse being placed in the center of the maze facing an open arm. Following testing, the maze was cleaned with 70% ethanol and allowed to dry completely between each trial. Behavior was recorded using AnyMaze (Stoelting Co.) tracking software. Percent time in the open arms was calculated as total time in the open arms divided by total time in the maze.
Forced swim test. The forced swim test (FST) can be used to assess depression-like behavior or active (i.e. escape behavior) versus passive (i.e. immobility) coping strategies. In the current study, the apparatus was a transparent glass cylinder (7” diameter). Cylinders were filled with 25 ± 2 °C water to a 10–15 cm depth to prevent the mouse from touching the bottom. Each mouse was individually habituated for 2- min, with behavioral monitoring occurring during a subsequent 4-min test during which the time immobile (sensitivity: 85%, minimum immobility period: 250 ms) using a side mounted Firewire camera directly facing the cylinder and AnyMaze tracking software. Following testing, the mouse was immediately dried and kept in a warming holding cage.
2.4. Slice electrophysiology
Acute slice electrophysiology was performed 20–26 days after the final stress exposure (Fig. 2, Fig. 3A). Mice were anesthetized with an overdose of isoflurane (Henry Schein), decapitated, and the brain removed and put in ice-cold solution containing 229 mM sucrose, 1.9 mM KCl, 1.2 mM NaH2PO4, 33 mM NaHCO3, 10 mM glucose, 0.4 mM ascorbic acid, 6 mM MgCl2, and 0.5 mM CaCl2 oxygenated using 95% O2 5% CO2. Coronal slices (300 μm) containing the mPFC were sliced in the ice-cold sucrose solution using a vibratome (Leica VT1000S) and then incubated at 31 °C for 10 min in a solution containing 119 mM NaCl, 2.5 mM KCl, 1 mM NaH2PO4, 26.2 mM NaHCO3, 11 mM glucose, 0.4 mM ascorbic acid, 4 mM MgCl2, and 1 mM CaCl2 and further incubated a minimum of 35 min at room temperature.
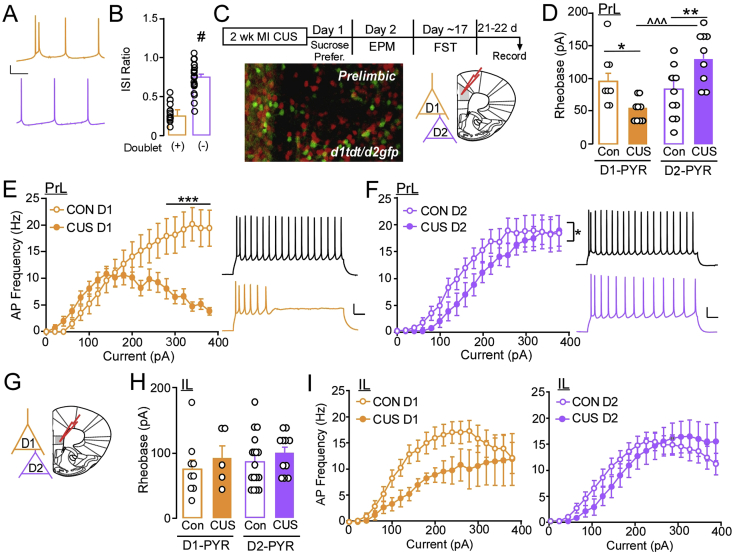
Fig. 3.
(A) Putative D1-PYR were characterized by a spike ‘doublet’ (orange; top) whereas D2-PYR were characterized by lack of the doublet (purple; bottom). (B) The presence of a spike doublet was positively correlated with the interspike interval (ISI) ratio. (C) Mice received no stress or two weeks of more intense stress with a portion of mice receiving behavioral testing. Image of D1 (red) and D2 (green) fluorescent cells in PrL of double heterozygous BAC transgenic mice (image was modified and enhanced for contrast). (D) Mean current required to evoke an action potential (Rheobase) in L5/6 D1-PYR PrL neurons was not significantly different in D1- versus D2-PYR in control mice. Rheobase was signfiicantly lower in PrL D1-PYR from CUS mice compared to control mice, while D2-PYR required significantly more current in CUS mice compared to control (n = 8–11/group, N = 4–6/group). (E) Mean current-spike plots for control and CUS L5/6 PrL D1-PYR neurons show lower firing at more depolarized potentials in CUS mice. (F) CUS L5/6 PrL D2-PYR neurons had overall lower spike firing during current injections compared to D2-PYR neurons from control mice. (G) Representative coronal section showing IL region of recordings. (H) Mean current required to evoke an action potential in L5/6 D1 and D2-PYR neurons in the IL was similar for control and CUS mice. (I) There were no differences in current-spike plots for control versus CUS L5/6 IL D1-or D2-PYR neurons. #p < 0.001 versus presence of doublet, *p ≤ 0.05, **p < 0.01. ***p < 0.001 versus Con vs CUS; ˆˆˆp < 0.001 CUS vs CUS.
During whole-cell recordings, slices were continuously perfused with oxygenated aCSF (125 mM NaCl, 2.5 mM KCl, 25 mM NaHCO3, 10 mM glucose, 0.4 mM ascorbic acid, 1.3 mM MgCl2, 2 mM CaCl2) at a temperature of 29°C–33 °C using a gravity-fed perfusion system with a flow rate of ∼2-2.5 ml/min. All recordings were performed with adequate whole-cell access (Ra<40 MΩ). Data was filtered at 2 kHz and sampled at 5 kHz for current-clamp recordings and 20 kHz for miniature postsynaptic current recordings using a Sutter Integrated Patch Amplifier (IPA) with Igor Pro (Wave Metrics, Inc.) data acquisition software.
Deep L5/6 PYR neurons were identified based on morphology and/or the presence of fluorescence, as well as physiologically by capacitance (PrL >100pf; IL > 75 pF) and minimum resting membrane potential (−55 mV). For rheobase and action potential firing, borosilicate glass pipettes were filled with internal solution containing 140mM K-Gluconate, 5.0 mM HEPES, 1.1 mM EGTA, 2.0 mM MgCl2, 2.0 mM Na2-ATP, 0.3 mM Na-GTP, and 5.0 mM phosphocreatine (pH 7.3, 290 mOsm). Miniature excitatory (mEPSCs) and inhibitory (mIPSCs) postsynaptic currents were recorded using borosilicate glass pipettes (Sutter Instruments; 2.5–4.5 MΩ) filled with a cesium-based internal solution (120 mM CsMeSO4, 15 mM CsCl, 10 mM TEA-Cl, 8 mM NaCl, 10 mM HEPES, 5 mM EGTA, 0.1 mM spermine, 5 mM QX-314, 4 mM ATP-Mg, and 0.3 mM GTP-Na). mEPSCs and mIPSCs were recorded in the presence of 0.7 mM lidocaine to block Na + -dependent at −72 and 0 mV, respectively.
Data analysis. Statistical significance was determined using independent-samples t-test, analysis of variance (ANOVA), two-way ANOVA, or two-way RM ANOVA where appropriate/indicated. Bonferroni post-hoc comparisons were conducted when necessary. Data points ±2 standard deviations from the mean were excluded which included a total of two control and one stress mouse from EPM analysis. A total of three cells from assessment of unidentified PrL PYR and one D2 putative cell recording were excluded, but none were from mice excluded based on behavior. Data was analyzed using SPSS 24 (IBM Statistics) or SigmaPlot, and graphed using GraphPad Prism. Experimental sample size is presented as n for the number of cells and N for the number of mice.
3. Results
3.1. Influence of CUS intensity and duration on affective behavior
The influence of chronic stress exposure on affective behaviors related to anxiety, depression, and anhedonia have been well established in rats, however prior research has indicated that the most widely used mouse strain (i.e. C57BL/6) exhibit attenuated stress-induced neuroendocrine responsivity and behavioral deficits compared to other strains (e.g. Balb/c; DBA/2 J) (Anisman et al., 2001; Anisman et al., 1998; Razzoli et al., 2011a, 2011b; Savignac et al., 2011). Although recent work established a chronic unpredictable stress (CUS) protocol in mice that results in behavioral phenotypes, these protocols required either 4 or 8 weeks of exposure (Monteiro et al., 2015). In an attempt to identify a more efficient protocol that will increase throughput and produce reliable deficits in commonly examined affect-related behavior in mice, initial studies examined three CUS protocols that varied in intensity/predictability as well as duration.
3.1.1. Elevated plus maze
To identify effects of variable stress intensity on anxiety-like behavior, mice underwent testing in an elevated plus maze (EPM). There were no significant differences between the three control groups, thus data were combined (F(2, 26) = 0.67, p = 0.52). There were significant differences comparing the four conditions (F(3, 68) = 11.53, p < 0.001), an effect that was not due to differences in locomotion as assessed by combining the number of open, closed, and center arm entries (F(3, 68) = 2.52, p = 0.07). Bonferroni post-hoc comparisons indicate that less intense stress was similar to control, but mice undergoing both two weeks and four weeks of MI stress had significantly less percent open arm time compared to non-stressed controls [_Con:_ 34.12 ± 2.04%; _LI:_ 28.60 ± 2.85%, p = 0.67; _two week:_ 25.95 ± 1.78%, p = 0.02; _four week:_ 17.16 ± 1.74%, p < 0.001; Fig. 2B].
3.1.2. Forced swim
Mice were tested in a forced swim test to determine if chronic stress intensity alters depression-like behavior - a measure previously shown to respond to anti-depressant treatment (Castagne et al., 2010; Kara et al., 2018) and induce immobility, a passive coping strategy (Commons et al., 2017; Molendijk and de Kloet, 2015). No differences were observed between control groups, thus they were combined (F(2, 14) = 2.58, p = 0.12). Similar to measures of anxiety-like behavior, there was a significant difference comparing the four conditions (F(3, 41) = 17.81, p < 0.001). Exposure to two weeks of LI stress did not alter time spent immobile , however both lengths of the MI stressors significantly increased immobility time (Con: 113.16 ± 10.56s, LI: 75.38 ± 17.96s, p = 0.16; two week: 181.30 ± 6.43s, p < 0.001; f_our week_: 154.56 ± 9.99s, p = 0.05; Fig. 2C).
3.1.3. Sucrose preference
To assess for the potential influence of chronic stress exposure on anhedonia, the percent of sucrose water to tap water consumed over a 24 h period was compared (Fig. 2D). There were significant differences in sucrose consumption across the three control groups and therefore data were not combined (F(2,24) = 6.38, p < 0.01). Mice that were exposed to two weeks of CUS, whether LI or MI, showed similar preference for sucrose compared to control mice [LI Con: 83.57 ± 4.16%; LI: 81.48 ± 5.23%, t(18) = 0.31, p = 0.76; two week Con: 62.54 ± 6.00%, two week MI: 65.28 ± 5.01%, t(13) = −0.32, p = 0.75]. Conversely, mice that were exposed to four weeks of MI CUS showed a significant reduction in the percent of sucrose compared to water that was consumed, indicating increased anhedonia [Con 63.40 ± 3.08%, four week: 46.43 ± 4.78%, t(10) = 2.99, p = 0.01].
3.2. Region specific effects of CUS on randomly selected L5/6 mPFC pyramidal neurons
Subregions of the mPFC demonstrate distinct patterns of connectivity and make dissociable contributions to behavior, including those related to affect (Dalley et al., 2004; Marquis et al., 2007; Vertes, 2004). Previous findings have shown that intrinsic properties (e.g., excitability) are altered during an acute post-stress period (24 h) following repeated resident-intruder social stress in mid-adolescent, but not adult male mice (Urban and Valentino, 2017), however it is unclear whether a CUS model of exposure alters PYR physiology and/or if these effects persist in adult males. Using whole-cell current clamp recordings, we assessed the threshold of current needed to reach depolarization threshold to fire an initial action potential (rheobase) in L5/6 PYR of the PrL and IL regions of the mPFC 20–26 days post stress. The pattern (frequency) of action potential firing in response to increasing current amplitude injections was also assessed to determine whether intrinsic firing properties of these neurons was altered following 2 week MI CUS (Fig. 2A). As LI stress did not alter measures of affective behavior, and increased intensity for both lengths of exposure prompted similar deficits - particularly performance in the FST that aligned temporally with recordings - electrophysiology measures were focused on mice undergoing two weeks of increased CUS intensity for all subsequent studies.
Initial examination of rheobase in unidentified subpopulations of PYR showed no significant difference in PrL PYR rheobase in stress naïve and 2 week MI CUS exposed mice [t(42) = 1.26, p = 0.22; Con: 102.22 ± 10.03 pA, CUS: 83.53 ± 9.74 pA; Fig. 2F]. Examination of current-spike relationship curves showed that CUS did not significantly alter the number of action potentials produced by increasing (20 pA) current steps (two-way repeated-measures ANOVA; condition: Con, CUS; F(1, 42) = 0.42, p = 0.52); interaction: F(19, 798) = 0.45, p = 0.98; Fig. 2E and G). Similarly, L5/6 PYR in the IL did not show effects of 2 week MI CUS on rheobase [t(29) = −0.48, p = 0.64; Con: 91.25 ± 12.11 pA, CUS: 98.67 ± 9.45 pA; Fig. 2I] or action potential firing frequency (condition: F(1, 29) = 2.174, p = 0.15; interaction: F(19, 551) = 0.55, p = 0.94; Fig. 2H and J). Taken together, these findings suggested that 2 week MI CUS does not produce a global effect on PrL or IL PYR intrinsic excitability or that these adaptations do not persist three weeks following conclusion of stress.
3.3. Effects of CUS on mPFC D1-and D2-expressing pyramidal neuron intrinsic excitability
PYR express either the dopamine D1- (D1-PYR) or D2 (D2-PYR) receptor, with little overlap (Gaspar et al., 1995; Gee et al., 2012; Santana et al., 2009; Vincent et al., 1993), and may define how they undergo experience-induced plasticity and/or their contribution to behavior (Gee et al., 2012; Jenni et al., 2017; Santana et al., 2009; Seong and Carter, 2012). To determine whether the lack of effect on excitability in randomly selected PYR following stress reflected cell-specific adaptations, we initially reanalyzed rheobase and spike-firing data in PrL PYR based on previously identified physiological characteristics shown to align with D1-and D2-PYR populations (Lee et al., 2014; Seong and Carter, 2012). Briefly, neurons were classified by the presence of a spike “doublet” (putative D1+) or not (putative D1-; Fig. 3A). In agreement with previous work, the presence of a doublet was positively correlated (r = 0.76, p < 0.001) with the inter-spike interval (ISI) ratio of the first and second action potential and the fourth and fifth action potential in a train of at least five action potentials during current-step injections ((AP2-AP1)/(AP5-AP4) = ISI Ratio; Fig. 3B). In stress naïve mice, rheobase values of PrL putative D1-PYR did not differ compared to values observed in putative D2-PYR (D1-PYR: 93.33 ± 13.78 pA; D2-PYR: 110.00 ± 15.51 pA; t(24) = −0.79, p = 0.44; data not shown), indicating that baseline excitability of PYR is not defined by the presence of D1-or D2-receptors. Conversely, putative D1-PYR neurons from stress-exposed mice, albeit statistically underpowered, exhibited lower threshold to fire an action potential compared to putative D2-PYR (D1-PYR: 53.33 ± 6.67 pA; D2-PYR: 90.00 ± 11.04 pA; t(13) = −2.84, p = 0.01; data not shown).
To confirm our initial findings, we used bacterial artificial chromosome (BAC) transgenic mice expressing tdTomato and/or enhanced green fluorescent protein (eGFP) in D1R- and D2R- PYR, respectively (Fig. 3C). These mice were also ran through behavioral assessments and did not differ compared to wild-type mice, thus this behavioral data was included in Fig. 1. A main effect of treatment but not cell-type on resting membrane potential (RMP) was observed (Treatment: F(1, 34) = 4.30, p = 0.046). Post-hoc analysis showed that D1-PYR in CUS mice exhibited a significantly more depolarized RMP (−69.68 ± 1.75 mV) vs Con mice (−65.60 ± 1.42 mV) (t(16) = −2.70, p = 0.016), but no difference in D2-PYR (D2-PYR: Con -69.00 ± 2.78 mV; CUS -67.00 ± 1.30; t(15) = −0.624, p = 0.54; data not shown). Previous research findings in L5 showing D1-PYR neurons are more hyperpolarized than D2-PYR (Seong and Carter, 2012), an inconsistency with our findings that may be due to the heterogeneity of PYR within L5/6 of the mPFC (Kawaguchi, 1993; Yang et al., 1996). Examination of rheobase (Fig. 3D) showed a significant interaction between experience and cell-type (F(1,36) = 12.38, p = 0.001). Post-hoc analysis showed that similar to findings in putative neurons, rheobase in fluorescently identified D1- versus D2-PYR was not significantly different in control mice, however CUS significantly reduced rheobase of D1-PYR compared to Con mice (Con: 95.00 ± 15.00 pA, CUS: 55.56 ± 5.56 pA; p = 0.04). Alternatively, D2-PYR in CUS exposed mice exhibited a significantly higher rheobase compared to Con D2-PYR (Con: 83.64 ± 13.43 pA, CUS: 131.11 ± 12.52 pA, p = 0.008). These changes indicate that the lack of effect on rheobase following CUS in randomly selected populations of cells likely reflects a bidirectional change in firing threshold among two distinct cell populations.
Examination of action potential frequency showed that although D1-PYR in CUS mice had a reduced firing threshold, frequency of firing was significantly reduced at higher current amplitude (interaction: F(19,285) = 14.56, p < 0.001; Fig. 3E). Conversely, comparison of firing in D2-PYR found only a main effect of condition, whereby CUS significantly reduced the overall firing frequency compared to cells from control mice (condition: F(1,18) = 4.36, p = 0.05; interaction: F(19,342) = 1.00, p = 0.46; Fig. 3F). These findings indicate that stress increases the likelihood of D1-PYR to fire, but reduces their firing capacity at more depolarized potentials, whereas CUS reduces overall activation and firing of D2-PYR.
As no significant effects on firing were also observed in randomly selected IL PYR, we next examined whether stress produced a cell-specific change in firing properties based on D1-and D2R expression (Fig. 3G). Here a combination of putative and fluorescently identified neurons was used, with no significant differences in the threshold to fire an action potential observed based on cell-type, condition, or a condition by cell-type interaction (condition: F(1,38) = 1.38, p = 0.25; cell-type: F(1,38) = 0.54, p = 0.47; cell x condition: F(1,38) = 0.03, p = 0.87; Fig. 3H). Additionally, no differences were observed in action potential frequency in either D1-PYR (condition: F(1,12) = 4.04, p = 0.07; condition x current: F(19,228) = 0.79, p = 0.71; Fig. 3I) or D2-PYR (condition: F(1, 24) = 0.10, p = 0.75; condition x current: (F(19, 456) = 1.11, p = 0.33; Fig. 3I).
3.4. Cell-specific effects of CUS on PrL D1-and D2-PYR synaptic transmission
Functional integrity of mPFC information processing relies on a dynamic balance of excitatory and inhibitory transmission (Isaacson and Scanziani, 2011; Kim et al., 2016; Sohal et al., 2009; Yizhar et al., 2011). Recent evidence indicates that sub-types of mPFC PYR receive distinct inhibitory synaptic regulation and responsivity to excitatory drive (Lee et al., 2014). To further explore CUS dependent cell-specific plasticity, we performed ex vivo recordings to examine changes in miniature excitatory (mEPSC) and inhibitory (mIPSC) currents in the PrL – a direct and selective measure of synaptic AMPA and GABAA receptor function, respectively (Fig. 4). In the current study, we found no significant differences in the amplitude of mEPSCs (Fig. 4A–C; D1-PYR: 10.73 ± 0.83 pA, D2-PYR: 10.97 ± 0.50 pA) or mIPSCs (Fig. 4E–H; D1-PYR: 14.13 ± 0.84 pA, D2-PYR: 13.84 ± 1.14 pA) in controls. No sigificant effect of CUS on the amplitude of mEPSCs (Fig. 4C) or mIPSCs (Fig. 4G) was observed based on a lack of condition, cell-type, or cell-type x condition interaction (condition: F(1,37) = 0.008, p = 0.93; cell-type: F(1,37) = 1.02, p = 0.32; cell x condition: F(1,37) = 1.67, p = 0.21).
Fig. 4.
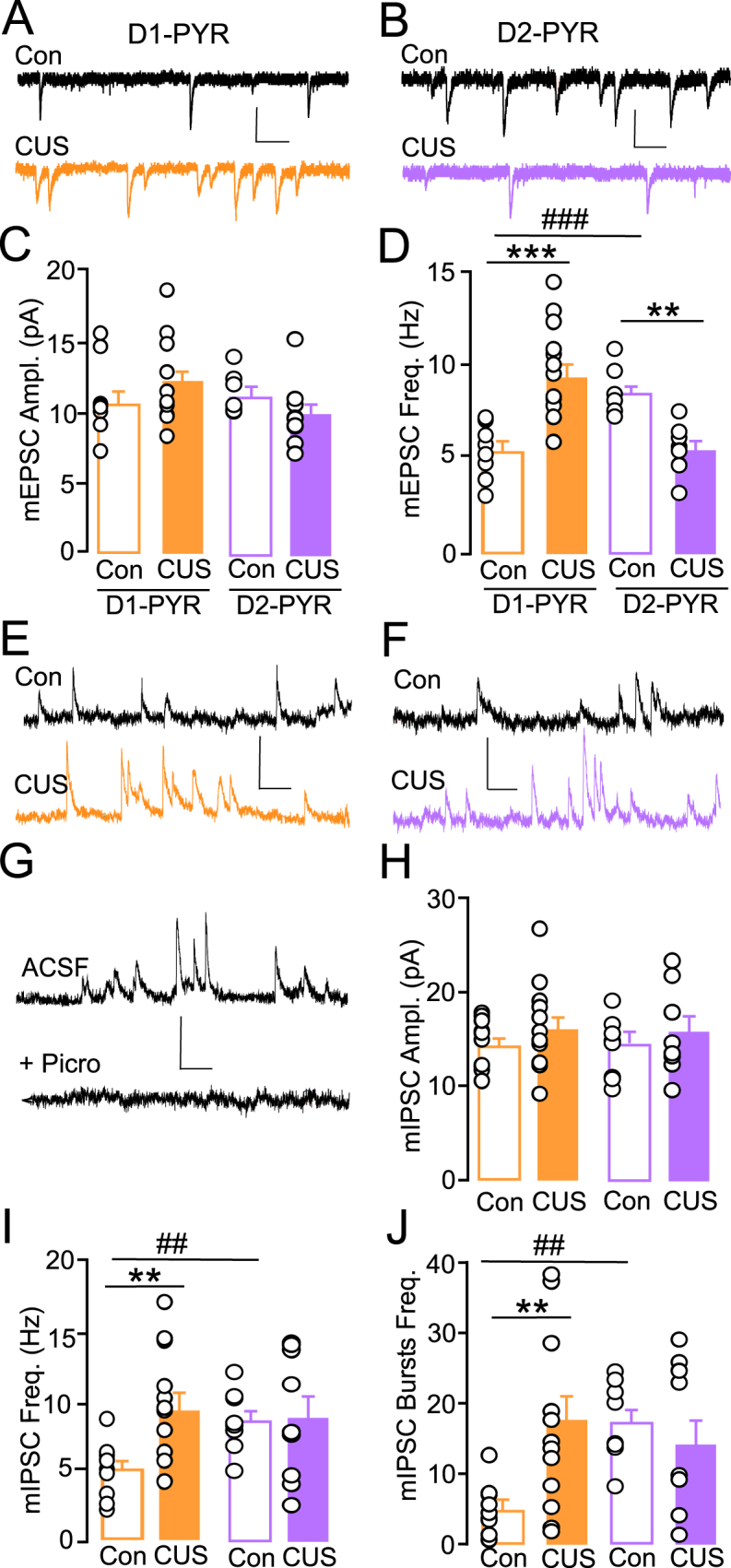
(A, B) Representative traces of mEPSC in L5/6 PYR PrL from Con (black) and CUS (D1-PYR, orange; D2-PYR, purple) mice. (C) There were no differences in mean mEPSC amplitude across cell-type or condition. (D) In Con mice, mean frequency of mEPSCs was significantly higher in D2-PYR compared to D1-PYR. CUS increased mean mEPSC frequency in D1-PYR and reduced it in D2-PYR compared to respective controls (n = 10–12/group, N = 6–7/group). (E, F) Representative traces of mIPSC in L5/6 PYR PrL from Con and CUS mice. (G) Representative mIPSC traces under ACSF alone versus lack of events following subsequent application of the selective GABAA antagonist, picrotoxin (Picro), shows a lack of mEPSCs at 0 mV and that outward inhibitory currents (upward deflection) are mediated by GABAARs. (H) No significant differences in mean mIPSC amplitude were observed across cell-type or condition. (I) Mean mIPSC frequency and (J) mean bursts of mIPSCs (4 + events/150 ms) per sweep was significantly higher in D2-PYR compared to D1-PYR in Con mice. Mean mIPSC event and burst frequency was significantly greater in D1-PYR of CUS vs Con mice, while no difference was observed in D2-PYR (n = 10–12/group, N = 7/group). mEPSC scale bar, 20pA/100 msec; mIPSC scale bar, 30 pA/100. **p < 0.01, ***p < 0.001 CUS versus Con; ##p < 0.01, ###p < 0.001 D1-PYR Con versus D2-PYR Con.
Alternatively, we found a significant interaction of cell-type and condition on mEPSC frequency (F(1,37) = 32.31, p < 0.001). D2-PYR from control mice showed significantly higher mEPSC frequency compared to D1-PYR (Con D1-PYR: 5.26 ± 0.47 Hz, Con D2-PYR: 8.28 ± 0.43 Hz, p < 0.001; Fig. 4D). Compared to controls, CUS significantly increased mEPSC frequency at D1-PYR (CUS 9.14 ± 0.72 Hz, p < 0.001), while reduced frequency at D2-PYR (CUS 5.44 ± 0.59 Hz; p = 0.003; Fig. 4D). A similar interaction of condition and cell-type was observed for mIPSC frequency (F(1,38) = 5.70, p = 0.023) and frequency of IPSC bursts per sweep (F(1,38) = 8.51, p = 0.006). Post hoc analysis showed that under control conditions, D2-PYR exhibit higher mIPSC frequency (Con D1-PYR: 4.78 ± 0.61 Hz, Con D2-PYR: 9.39 ± 0.77 Hz; p < 0.01) and burst frequency (Con D1-PYR: 4.24 ± 1.17, Con D2-PYR: 17.48 ± 2.01; p < 0.004) compared to D1-PYR (Fig. 4I and J). Compared to controls, CUS significantly increased mIPSC frequency and burst frequency in D1-PYR (frequency: 9.14 ± 0.72 Hz; burst frequency: 16.83 ± 2.01 bursts; Fig. 4J). CUS did not alter frequency or burst frequency in D2-PYR (frequency: 8.08 ± 1.21; burst frequency: 12.31 ± 3.32 bursts). Taken together, these data show that D1-and D2-PYR maintain distinctly different excitatory and inhibitory synaptic regulation under naïve conditions, and that CUS promotes opposing effects on excitatory drive and cell-specific changes in inhibitory drive at D1-and D2-PYR that may contribute to divergent effects on intrinsic excitability, and that these effects may result in secondary adaptations in inhibitory signaling at D1-PYR.
4. Discussion
The current study demonstrated that using a chronic unpredictable model of stress with increased variability and enhanced intensity of stressors produced anhedonia- and anxiety-like behavior and increased passive coping strategy in a significantly shorter time frame (2–4 weeks) than previous reports using a single daily exposure to CUS in C57BL/6 mice (Monteiro et al., 2015). We find that under control conditions, PrL D1-and D2-PYR did not exhibit differences in firing threshold, but showed distinctions in excitatory:inhibitory synaptic drive, and that our model of CUS exposure produced enduring and opposing adaptations in both intrinsic physiology and synaptic regulation of these pyramidal subpopulations. D1-PYR from CUS mice exhibited a reduction in firing threshold (increased excitability) but impaired maintenance of firing capacity at more depolarized potentials that was paralleled by enhanced frequency of excitatory AMPAR-mEPSCs and inhibitory GABAA-mIPSCs. Alternatively, CUS promoted an increase in D2-PYR firing threshold (reduced excitation) that was paralleled by reductions in excitatory drive. Taken together, these results build upon previously identified intrinsic differences in D1-and D2-PYR and demonstrate for the first time, that prolonged stress may produce abnormalities in PFC-dependent behavior by uniquely modifying mPFC circuits comprised of D1-and D2-PYR, and that these modifications reflect overlapping and distinct forms of plasticity.
4.1. Impact of stress intensity and predictability on affective behavior
The influence of chronic stress exposure on affective behaviors has been well-established in rats, with a variety of mild, unpredictable, and social stress paradigms able to reduce sucrose preference, as well as increase anxiety- and depression-like behavior (Vasconcelos et al., 2015; Willner, 2017). Alternatively, inherent strain differences in stress susceptibility have presented a challenge towards the use of mice – an approach that would greatly expand genetic manipulations and cell-specific identification through the use of transgenic animals. Recent work by Monteiro et al. (2015) laid the groundwork for establishing mouse-specific CUS protocols that produce reliable deficits in affective behavior, however these protocols involved up to eight weeks of once daily stress exposure - a length of time that not only reduces throughput, but negates advantages related to per diem costs associated with housing mice versus rats. As unpredictable stressors often more negatively impact humans compared to predictable ones (Anisman and Matheson, 2005; Bale, 2005; Kendler et al., 1998, 1999; Moghaddam and Javitt, 2012; Willner and Mitchell, 2002), it was plausible that manipulations of intensity and predictability would allow for the reduction in length of stress exposure, while resulting in similar behavioral phenotypes. Similar to CUS models in rats, the current protocol utilized two daily stressors, but sought to increase overall stress exposure intensity by combining stressors, using stressors with greater intensity (e.g. forced swim and restraint) more frequently, and decreasing predictability by using multiple distinctly different contexts and varying the time of day in which stressors were given. These changes reduced time spent in the open arm of an EPM, reduced preference for sucrose, and increased passive coping strategies – the latter of which persisted around 17 days following stress exposure – as indicative of increased anxiety-, anhedonia- and depression-like behavior. Notably, our unpublished data indicate that reductions in open arm time following 2 weeks of more intense stress were no longer present at 17–21 days post-stress, suggesting that our CUS protocol produces enduring deficits in depression- but not anxiety-like behaviors. Our data support the ability to use C57BL/6 mouse models to study plasticity associated with CUS without drastically prolonging stress exposure, however, as previous reports have shown a delayed emergence of affect behavior following chronic stress exposure in rats (Matuszewich et al., 2007), it will be important for future studies to characterize the timeline of the behavioral and physiological changes produced by this CUS regimen in order to identify causal relationships between the two.
4.2. Bidirectional changes in prelimbic D1-and D2-PYR intrinsic excitability
Neuroanatomical studies indicate that similar to medium spiny neurons in the striatum, mPFC pyramidal neurons can be canonically divided based on the expression of D1 or D2 receptors (Gaspar et al., 1995; Santana et al., 2009; Vincent et al., 1993). Pharmacological evidence indicates that mPFC dopamine D1 and D2 receptors modulate dissociable (often opposing) aspects of cognitive and affective behavior (Bai et al., 2017; Jenni et al., 2017; Sawaguchi and Goldman-Rakic, 1994; Seamans et al., 1998; Shinohara et al., 2018), through distinct PFC circuits (Durstewitz et al., 2000; Durstewitz et al., 2010; Jenni et al., 2017; Seamans and Yang, 2004). Evidence utilizing optogenetics, chemogenetics, and D1-or D2-Cre transgenic mouse lines supports this notion, with numerous studies demonstrating that manipulating activity of mPFC D1 and D2R-expressing cell-bodies produces distinct modifications in behavior related to feeding, social interaction, and depression-associated behavior, that may not be reproducible with general population manipulations (Brumback et al., 2018; Hare et al., 2019; Land et al., 2014; Narayanan et al., 2012; Shinohara et al., 2018). A number of these studies have directly demonstrated that D1-PYR networks are specifically involved in regulating this behavior through downstream terminal stimulation approaches (Hare et al., 2019; Land et al., 2014). However, as D1-and D2R have been found pre- and postsynaptically, as well as on principle and interneuron populations (Anastasiades et al., 2018; Benes et al., 1993; Santana et al., 2009; Vincent et al., 1993), and recent findings indicate that pharmacological stimulation of D1-and D2R on PYR exert an excitatory effect (Gee et al., 2012; Robinson and Sohal, 2017; Seong and Carter, 2012), approaches involving intra-cranial pharmacological manipulations as well as intra-PFC manipulations in Cre-mice should be interpreted cautiously.
Studies of patients and animal models suggest that a functional imbalance in the ratio of PFC cellular excitation:inhibition causally underlies impaired working memory, social withdrawal, and anxiety-like behavior in stress-related psychiatric disorders (Fuchs et al., 2016; Gandal et al., 2012; Gonzalez-Burgos and Lewis, 2012; Holmes and Wellman, 2009; Javitt et al., 2011; Matsuo et al., 2007; Moghaddam and Javitt, 2012; Sohal et al., 2009; Yizhar et al., 2011). Intrinsic membrane properties play a critical part in determining this balance, as they directly shape neuronal output by influencing the probability of a neuron firing an action potential in response to excitatory synaptic inputs and modulate firing capacity. The lack of significant effect of stress on rheobase and action potential firing frequency in randomly selected populations in the current study closely resembles findings in adult male rats 24 h following conclusion of social defeat stress (Urban and Valentino, 2017). Thus, it is possible that null effects following social stress also reflect an opposing reduction and increase in firing thresholds (rheobase) in D1-and D2-PYR, respectively.
The underlying intrinsic mechanisms contributing to alterations in firing threshold remain unclear. Our previous work has shown that G protein-gated inwardly rectifying K+ channels (Girks) mediate ~70% of the GABABR-dependent inhibition of Layer 5/6 PYR in the mPFC, essentially acting as a neuronal off switch (Hearing et al., 2013; Hearing et al., 2012). Given that knockout of these channels or experience-dependent suppression of this signaling reduces firing thresholds akin to that observed in D1-PYR here, it is possible that CUS promotes a downregulation of GABABR-Girk signaling in D1-PYR and perhaps an upregulation in D2-PYR. In addition to reduced firing threshold in D1-PYR, CUS promotes an apparent depolarization-induced blockade at higher current injections. As activity-dependent Girk channel plasticity has recently been implicated in the transition between tonic and burst firing modes in midbrain dopamine neurons, it is also possible that alterations in Girk channels contribute to the stress-related reduction in firing capacity (Lalive et al., 2014). Alternatively, this may reflect a shift in feedforward inhibition or impaired hyperpolarization-activated ionic conductances responsible for maintaining a constant firing rate or increased inhibitory transmission, as elevations in the frequency and bursting of mIPSCs were observed in D1-PYR (Winograd et al., 2008).As persistent activity in PFC networks is thought to be important for memory formation, conditioned associations, and working memory (Gilmartin and Helmstetter, 2010; Gilmartin et al., 2012; Gilmartin and McEchron, 2005; Gilmartin et al., 2013; Kwapis et al., 2014; Runyan et al., 2004; Seamans et al., 2003), it is possible that deficits in these facets of cognition may be due in part to reduced firing capacity in D1- or D2-PYR.
4.3. Effects of CUS on infralimbic pyramidal neuron excitability
Although the present study identified adaptations in D1-and D2-PYR within the PrL that were not initially observed when examining a general population of cells, data obtained from putative and fluorescently identified D1-and D2-PYR in the IL did not show significant differences in intrinsic excitability. The lack of effect on IL intrinsic excitability is particularly surprising given previous work demonstrating that CUS increases inhibitory synaptic transmission that is paralleled by increased inhibitory appositions on glutamatergic neurons when sampling from unidentified populations of IL PYR (McKlveen et al., 2016).
Although outside the scope of the current study, it is possible that stress promotes modifications in the IL that are not only cell-specific but also pathway specific, as stress is known to alter basolateral amygdala (BLA) -to-PFC input without influencing BLA inputs projecting to the bed nucleus of the stria terminalis (BNST) (Lowery-Gionta et al., 2018). Circuit specific structural modifications have also been implicated in apical dendrite retraction following chronic restraint stress in IL circuits, as a population of Layer 2/3 PYR projecting to the BLA appear to be spared from stress plasticity (Shansky and Morrison, 2009). These findings indicate that stress almost assuredly promotes enduring plasticity in IL neurons, however these modifications may be more complex and/or specific based on anatomical connectivity - a possibility we are currently exploring.
4.4. Cell-specific effects of stress on synaptic transmission
Although stress-induced structural plasticity in the mPFC has been recognized for more than a decade as a prominent factor in PFC dysfunction, few studies have examined the pathway- and cell-specific locus of these adaptations (Lowery-Gionta et al., 2018; McEwen and Morrison, 2013; McKlveen et al., 2016; Shansky and Morrison, 2009; Yuen et al., 2012). Previous work has shown that in early adolescent male mice, CUS transiently reduces PrL PYR glutamate receptor expression and excitatory synaptic transmission which returned to control levels by day 5 post stress, although the exact population of pyramidal neurons (layer 2/3 or layer 5/6) examined was not apparent (Yuen et al., 2012). It is possible that the ostensible lack of enduring change in excitatory plasticity reflects examination of unidentified populations, as we observed divergent effects on mEPSC frequency in D1 vs D2-PYR. Alternatively, early reductions in glutamate signaling may represent a generalized permissive functional plasticity that precedes divergent adaptations (Kourrich et al., 2015). It is also possible that early reductions in excitatory signaling is specific for adolescents, as PrL PYR from mid-adolescent but not adult male rats, exhibit reductions in excitatory transmission 24 h following prolonged social defeat stress (Urban and Valentino, 2017). The current findings of increased mEPSC frequency but not amplitude in D1-PYR tangentially aligns with other findings of increased presynaptic glutamate release in BLA to PFC synapses, with no differences in AMPA/NMDA ratio noted (Lowery-Gionta et al., 2018). Given the opposing effects of CUS on the frequency of mEPSCs in D1-PYR but not D2-PYR, it is possible that social defeat and restraint did in fact alter excitatory transmission in adults, albeit in an opposing fashion.
Recent work has shown that type A and B PYR in the PrL exhibit properties akin to D2-and D1-PYR, respectively, and that these populations receive distinct forms of input that may subserve divergent functions (Gee et al., 2012; Lee et al., 2014; Seong and Carter, 2012). For example, type A PYR (i.e., D2-PYR) are preferentially inhibited by fast-spiking parvalbumin interneurons but not somatostatin interneurons (Lee et al., 2014). Our findings build on these observations, showing elevated excitatory and inhibitory signaling in D2-vs D1-PYR - highlighting a need to understand how these intrinsic differences contribute to CUS-dependent plasticity. Interestingly, we find that increases in excitatory transmission in D1-PYR were paralleled by enhanced inhibitory drive. Although the source of inhibitory change is unclear, local neocortical circuitry includes collateral connections between pyramidal neurons as well as reciprocal connections between pyramidal and local interneurons (Isaacson and Scanziani, 2011), thus it is possible that increased excitation of D1-PYR increases activation of and transmitter release from local GABA neurons – a possibility that aligns with the selective increase in mIPSC frequency and bursting rather than amplitude (Sohal, 2012). As all of our observed synaptic adaptations were selective for changes in the frequency of PSCs, it will also be important to determine whether these reflect presynaptic or postsynaptic structural modifications.
5. Functional implications
Emerging evidence indicates that PFC-dependent regulation of cognition and affective behavior are governed through a complex array of cortical networks comprised of neuronal subpopulations that express innate physiological and synaptic properties that likely determine how they influence behavior and undergo plasticity (McEwen and Morrison, 2013). It is tempting to speculate that the divergent effects on D1-and D2-PYR in the current study underlie specific stress-related pathologies. For example, reductions in the output of D2-PYR may reflect reduced top-down control over affect related behavior, and thus permit the emergence of increased anxiety- and depression-like behavior. On the other hand, recent findings indicate that cognitive deficits in a number of disorders may reflect a shift towards cortical excitation and increased (disorganized) firing of mPFC PYR that disrupts cortical information flow and cognitive performance (Fuchs et al., 2016; Gonzalez-Burgos and Lewis, 2012; Javitt et al., 2011; Matsuo et al., 2007; Moghaddam and Javitt, 2012).
6. Conclusion
The ability of ostensibly unrelated disorders to give rise to seemingly similar psychiatric phenotypes highlights a need to identify circuit-level concepts that could unify diverse factors under a common pathophysiology. The current work indicates that stress-related pathophysiology likely manifests through dynamic alterations in communication within specific cortical networks. Our findings highlight the need to gain a better understanding of which neural pathways are responsible for regulating behavior under naïve and pathological states to identify more targeted approaches to effectively treat neuropsychiatric disorders that encompass varied, co-occurring symptoms, and divergent responses to treatment.
Conflicts of interest
No conflicts of interest to disclose.
Acknowledgements
These studies were supported by funding from the Brain and Behavior Research Foundation (#26299), Marquette University Regular Research Grant, and the Charles E Kubly Mental Health Research Foundation at Marquette University.
Footnotes
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- Anastasiades P.G., Boada C., Carter A.G. Cell-type-specific D1 dopamine receptor modulation of projection neurons and interneurons in the prefrontal cortex. Cerebr. Cortex. 2018 doi: 10.1093/cercor/bhy299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H., Hayley S., Kelly O., Borowski T., Merali Z. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav. Neurosci. 2001;115(2):443–454. [PubMed] [Google Scholar]
- Anisman H., Lacosta S., Kent P., McIntyre D.C., Merali Z. Stressor-induced corticotropin-releasing hormone, bombesin, ACTH and corticosterone variations in strains of mice differentially responsive to stressors. Stress. 1998;2(3):209–220. doi: 10.3109/10253899809167284. [DOI] [PubMed] [Google Scholar]
- Anisman H., Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29(4–5):525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Bai M., Zhu X., Zhang L., Zhang Y., Xue L., Wang Y., Zhang X. Divergent anomaly in mesocorticolimbic dopaminergic circuits might be associated with different depressive behaviors, an animal study. Brain Behav. 2017;7(10) doi: 10.1002/brb3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm. Behav. 2005;48(1):1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Benes F.M., Vincent S.L., Molloy R. Dopamine-immunoreactive axon varicosities form nonrandom contacts with GABA-immunoreactive neurons of rat medial prefrontal cortex. Synapse. 1993;15(4):285–295. doi: 10.1002/syn.890150405. [DOI] [PubMed] [Google Scholar]
- Brown S.P., Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457(7233):1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback A.C., Ellwood I.T., Kjaerby C., Iafrati J., Robinson S., Lee A.T., Sohal V.S. Identifying specific prefrontal neurons that contribute to autism-associated abnormalities in physiology and social behavior. Mol. Psychiatr. 2018;23(10):2078–2089. doi: 10.1038/mp.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V., Moser P., Roux S., Porsolt R.D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Pharmacol. 2010 doi: 10.1002/0471141755.ph0508s49. Chapter 5, Unit 5.8. [DOI] [PubMed] [Google Scholar]
- Clark L., Chamberlain S.R., Sahakian B.J. Neurocognitive mechanisms in depression: implications for treatment. Annu. Rev. Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Commons K.G., Cholanians A.B., Babb J.A., Ehlinger D.G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Cardinal R.N., Robbins T.W. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Degenetais E., Thierry A.M., Glowinski J., Gioanni Y. Electrophysiological properties of pyramidal neurons in the rat prefrontal cortex: an in vivo intracellular recording study. Cerebr. Cortex. 2002;12(1):1–16. doi: 10.1093/cercor/12.1.1. [DOI] [PubMed] [Google Scholar]
- Dembrow N.C., Chitwood R.A., Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J. Neurosci. 2010;30(50):16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D., Seamans J.K., Sejnowski T.J. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J. Neurophysiol. 2000;83(3):1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Durstewitz D., Vittoz N.M., Floresco S.B., Seamans J.K. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66(3):438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Forbes N.F., Stewart C.A., Matthews K., Reid I.C. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol. Behav. 1996;60(6):1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- Fossati P., Amar G., Raoux N., Ergis A.M., Allilaire J.F. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatr. Res. 1999;89(3):171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Fuchs T., Jefferson S.J., Hooper A., Yee P.H., Maguire J., Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol. Psychiatr. 2016 doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal M.J., Sisti J., Klook K., Ortinski P.I., Leitman V., Liang Y.…Siegel S.J. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl. Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P., Bloch B., Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur. J. Neurosci. 1995;7(5):1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Gee S., Ellwood I., Patel T., Luongo F., Deisseroth K., Sohal V.S. Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. J. Neurosci. 2012;32(14):4959–4971. doi: 10.1523/JNEUROSCI.5835-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin M.R., Helmstetter F.J. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn. Mem. 2010;17(6):289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin M.R., Kwapis J.L., Helmstetter F.J. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiol. Learn. Mem. 2012;97(4):452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin M.R., McEchron M.D. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav. Neurosci. 2005;119(6):1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Gilmartin M.R., Miyawaki H., Helmstetter F.J., Diba K. Prefrontal activity links nonoverlapping events in memory. J. Neurosci. 2013;33(26):10910–10914. doi: 10.1523/JNEUROSCI.0144-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G., Lewis D.A. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr. Bull. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B.D., Shinohara R., Liu R.J., Pothula S., DiLeone R.J., Duman R.S. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat. Commun. 2019;10(1):223. doi: 10.1038/s41467-018-08168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M., Kotecki L., Marron Fernandez de Velasco E., Fajardo-Serrano A., Chung H.J., Lujan R., Wickman K. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80(1):159–170. doi: 10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M.C., Zink A.N., Wickman K. Cocaine-induced adaptations in metabotropic inhibitory signaling in the mesocorticolimbic system. Rev. Neurosci. 2012;23(4):325–351. doi: 10.1515/revneuro-2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Ostrander M.M., Mueller N.K., Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Holmes A., Wellman C.L. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33(6):773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson J.S., Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72(2):231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D.C., Schoepp D., Kalivas P.W., Volkow N.D., Zarate C., Merchant K., Lee C.M. Translating glutamate: from pathophysiology to treatment. Sci. Transl. Med. 2011;3(102):102mr102. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni N.L., Larkin J.D., Floresco S.B. Prefrontal dopamine D1 and D2 receptors regulate dissociable aspects of decision making via distinct ventral striatal and amygdalar circuits. J. Neurosci. 2017;37(26):6200–6213. doi: 10.1523/JNEUROSCI.0030-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara N.Z., Stukalin Y., Einat H. Revisiting the validity of the mouse forced swim test: systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci. Biobehav. Rev. 2018;84:1–11. doi: 10.1016/j.neubiorev.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J. Neurophysiol. 1993;69(2):416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Keedwell P.A., Andrew C., Williams S.C., Brammer M.J., Phillips M.L. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol. Psychiatry. 2005;58(6):495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski L.M., Prescott C.A. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J. Nerv. Ment. Dis. 1998;186(11):661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski L.M., Prescott C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kim H., Ahrlund-Richter S., Wang X., Deisseroth K., Carlen M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164(1–2):208–218. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S., Calu D.J., Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat. Rev. Neurosci. 2015;16(3):173–184. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis J.L., Jarome T.J., Helmstetter F.J. The role of the medial prefrontal cortex in trace fear extinction. Learn. Mem. 2014;22(1):39–46. doi: 10.1101/lm.036517.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive A.L., Munoz M.B., Bellone C., Slesinger P.A., Luscher C., Tan K.R. Firing modes of dopamine neurons drive bidirectional GIRK channel plasticity. J. Neurosci. 2014;34(15):5107–5114. doi: 10.1523/JNEUROSCI.5203-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B.B., Narayanan N.S., Liu R.J., Gianessi C.A., Brayton C.E., Grimaldi D.M., DiLeone R.J. Medial prefrontal D1 dopamine neurons control food intake. Nat. Neurosci. 2014;17(2):248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.T., Gee S.M., Vogt D., Patel T., Rubenstein J.L., Sohal V.S. Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron. 2014;81(1):61–68. doi: 10.1016/j.neuron.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta E.G., Crowley N.A., Bukalo O., Silverstein S., Holmes A., Kash T.L. Chronic stress dysregulates amygdalar output to the prefrontal cortex. Neuropharmacology. 2018;139:68–75. doi: 10.1016/j.neuropharm.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis J.P., Killcross S., Haddon J.E. Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats. Eur. J. Neurosci. 2007;25(2):559–566. doi: 10.1111/j.1460-9568.2006.05295.x. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Glahn D.C., Peluso M.A., Hatch J.P., Monkul E.S., Najt P., Soares J.C. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol. Psychiatr. 2007;12(2):158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- Matuszewich L., Karney J.J., Carter S.R., Janasik S.P., O'Brien J.L., Friedman R.D. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol. Behav. 2007;90(4):674–681. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen J.M., Morano R.L., Fitzgerald M., Zoubovsky S., Cassella S.N., Scheimann J.R., Herman J.P. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry. 2016;80(10):754–764. doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E., Lewis P. Recognition memory in elderly patients with depression and dementia: a signal detection analysis. J. Abnorm. Psychol. 1977;86(1):84–86. doi: 10.1037//0021-843x.86.1.84. [DOI] [PubMed] [Google Scholar]
- Moghaddam B., Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Monteiro S., Roque S., de Sa-Calcada D., Sousa N., Correia-Neves M., Cerqueira J.J. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatry. 2015;6:6. doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.C., Sahakian B.J., Rubinsztein J.S., Michael A., Rogers R.D., Robbins T.W., Paykel E.S. Emotional bias and inhibitory control processes in mania and depression. Psychol. Med. 1999;29(6):1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Murrough J.W., Iacoviello B., Neumeister A., Charney D.S., Iosifescu D.V. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol. Learn. Mem. 2011;96(4):553–563. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Narayanan N.S., Land B.B., Solder J.E., Deisseroth K., DiLeone R.J. Prefrontal D1 dopamine signaling is required for temporal control. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Arias C.M., Sawchenko P.E. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J. Neurosci. 2006;26(50):12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Janssen W.G., Hof P.R., McEwen B.S., Morrison J.H. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196(1):199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Rocher A.B., Miller M., Janssen W.G., Liston C., Hof P.R., Morrison J.H. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebr. Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Carboni L., Andreoli M., Ballottari A., Arban R. Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav. Brain Res. 2011;216(1):100–108. doi: 10.1016/j.bbr.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Razzoli M., Carboni L., Andreoli M., Michielin F., Ballottari A., Arban R. Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol. Biochem. Behav. 2011;97(3):566–576. doi: 10.1016/j.pbb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Robinson S.E., Sohal V.S. Dopamine D2 receptors modulate pyramidal neurons in mouse medial prefrontal cortex through a stimulatory G-protein pathway. J. Neurosci. 2017;37(42):10063–10073. doi: 10.1523/JNEUROSCI.1893-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan J.D., Moore A.N., Dash P.K. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci. 2004;24(6):1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N., Mengod G., Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebr. Cortex. 2009;19(4):849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Savignac H.M., Finger B.C., Pizzo R.C., O'Leary O.F., Dinan T.G., Cryan J.F. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011;192:524–536. doi: 10.1016/j.neuroscience.2011.04.054. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P.S. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994;71(2):515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Seamans J.K., Floresco S.B., Phillips A.G. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Nogueira L., Lavin A. Synaptic basis of persistent activity in prefrontal cortex in vivo and in organotypic cultures. Cerebr. Cortex. 2003;13(11):1242–1250. doi: 10.1093/cercor/bhg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Yang C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seong H.J., Carter A.G. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J. Neurosci. 2012;32(31):10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky R.M., Morrison J.H. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara R., Taniguchi M., Ehrlich A.T., Yokogawa K., Deguchi Y., Cherasse Y., Furuyashiki T. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol. Psychiatr. 2018;23(8):1717–1730. doi: 10.1038/mp.2017.177. [DOI] [PubMed] [Google Scholar]
- Sohal V.S. Insights into cortical oscillations arising from optogenetic studies. Biol. Psychiatry. 2012;71(12):1039–1045. doi: 10.1016/j.biopsych.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal V.S., Zhang F., Yizhar O., Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7(2):131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Treadway M.T., Zald D.H. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban K.R., Valentino R.J. Age- and sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cerebr. Cortex. 2017;27(1):244–253. doi: 10.1093/cercor/bhw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos M., Stein D.J., de Almeida R.M. Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: a systematic review of the last decade. Trends Psychiatry Psychother. 2015;37(2):51–66. doi: 10.1590/2237-6089-2014-0034. [DOI] [PubMed] [Google Scholar]
- Vertes R.P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vincent S.L., Khan Y., Benes F.M. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J. Neurosci. 1993;13(6):2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P., Mitchell P.J. The validity of animal models of predisposition to depression. Behav. Pharmacol. 2002;13(3):169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Willner P., Towell A., Sampson D., Sophokleous S., Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Winograd M., Destexhe A., Sanchez-Vives M.V. Hyperpolarization-activated graded persistent activity in the prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2008;105(20):7298–7303. doi: 10.1073/pnas.0800360105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T.X., Yao W.D. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107(37):16366–16371. doi: 10.1073/pnas.1004108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.R., Seamans J.K., Gorelova N. Electrophysiological and morphological properties of layers V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J. Neurosci. 1996;16(5):1904–1921. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O., Fenno L.E., Prigge M., Schneider F., Davidson T.J., O'Shea D.J.…Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Wei J., Liu W., Zhong P., Li X., Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73(5):962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.