Differential Roles of the Dorsolateral and Midlateral Striatum in Punished Cocaine Seeking (original) (raw)
Abstract
Continued instrumental drug seeking despite contingent punishment is a core phenotype of drug addiction. Although the neuroanatomical basis of punished drug seeking is unclear, we hypothesize that the sensorimotor striatum, a structure that mediates habitual drug seeking, also mediates punished cocaine seeking. Forelimb sensorimotor projections into the striatum of the rat extend from the dorsolateral to midlateral striatum. Here, we selectively inactivated the dorsolateral and midlateral striatum in rats responding for cocaine in a seeking–taking task. We inactivated both regions after the acquisition of cocaine seeking, after extended cocaine self-administration and finally after the introduction of intermittent, seeking-contingent foot shock. The results show that inactivation of the dorsolateral striatum selectively disrupted punished drug seeking but did not affect unpunished drug seeking, even after extended training. Inactivation of the midlateral striatum, on the other hand, disrupted drug seeking at all stages of training. The effect of inactivating the dorsolateral striatum under punishment conditions was present before delivery of the first shock in the session, and responding reverted to baseline the next day. Thus, inactivation of the dorsolateral striatum seems to enhance the influence of recalled threat of negative consequences of cocaine seeking. The proportional reduction in responding after inactivation of the dorsolateral striatum did not vary with the individual level of compulsivity. Together, these results suggest a novel differentiation of function in the sensorimotor striatum, where the dorsolateral striatum selectively mediates the rigidity of responding after overtraining, while the midlateral striatum mediates responding itself at all stages of training.
Introduction
Habitual instrumental responding for rewards is characterized by insensitivity to reward devaluation when tested under extinction conditions, but normal sensitivity to devaluation when the devalued outcome is actually delivered after the response (Adams, 1982; Miles et al., 2003). On the other hand, compulsive responding is characterized by insensitivity to reward devaluation even when negative outcomes are actually delivered after the instrumental response (Deroche-Gamonet et al., 2004; Pelloux et al., 2007). Chronic cocaine self-administration in rats leads to habitual responding at the group level (Zapata et al., 2010), and compulsive cocaine seeking in a subgroup of animals (Pelloux et al., 2007).
Habitual responding for food (Yin et al., 2004, 2006) and cocaine (Zapata et al., 2010) is mediated by the dorsolateral striatum (DLS). There are several indications that the DLS may also regulate compulsive cocaine seeking (Everitt and Robbins, 2005). Studies using PET imaging of [11C]raclopride binding in human cocaine addicts have shown a cocaine cue-elicited dopamine response in the putamen that correlated significantly with addiction severity (Volkow et al., 2006). Second, it has been shown that dorsal striatal overexpression of BDNF in rats leads to an extreme form of escalation of cocaine taking that strongly suggests a loss of sensitivity to overdosing (Im et al., 2010).
Rats press levers with their forepaws, and rodent cortical forelimb motor areas project to the lateral striatum in an elongated zone (see Fig. 1B) from the dorsolateral to midlateral striatum (Ebrahimi et al., 1992). Electrophysiological correlates of forepaw lever pressing have been observed in this entire striatal region (West et al., 1990; Carelli et al., 1997). Unfortunately, investigation of the function of this region in compulsive responding after overtraining is complicated by the fact that it has also been associated with forelimb lever pressing per se (Whishaw et al., 1986; Fricker et al., 1996; Yin et al., 2006).
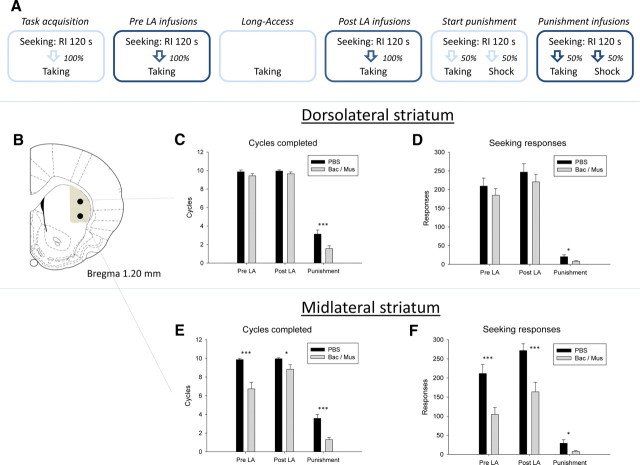
Figure 1.
Cocaine seeking and taking task and effect of inactivating the dorsolateral and midlateral striatum on compulsive cocaine seeking. A, Experimental procedures and instrumental contingencies. Each infusion block involved PBS and baclofen/muscimol (0.3/0.03 nmol per side) infusion into the DLS and MLS. RI, Random Interval schedule. B, Neuroanatomical representation of cortical forelimb projection area in the lateral striatum (gray shaded area) and definition of the DLS and MLS (black dots). C, Mean (±SEM) seeking cycles completed after inactivation of the DLS at the three time points. Inactivation of the DLS affected responding under punishment but not baseline responding. D, Mean (±SEM) seeking responses after inactivation of the DLS at the three time points. E, Mean (±SEM) seeking cycles completed after inactivation of the MLS at the three time points. Inactivation of the MLS affected responding at all stages. F, Mean (±SEM) seeking responses after inactivation of the MLS at the three time points. ***p < 0.001, *p < 0.05. For all comparisons, N = 23.
However, careful reading of the literature suggests a potential regional differentiation within the forelimb area of the striatum. Inactivation of the dorsal part of the forelimb projection field (DLS) has no effect on baseline responding for food (Corbit and Janak, 2007) or cocaine (Kantak et al., 2009; Zapata et al., 2010), whereas inactivation or lesion studies that have targeted the more ventral part of the forelimb projection field (midlateral striatum, MLS) do show a decrement in baseline responding for food (Fricker et al., 1996) or cocaine (Fuchs et al., 2006).
These findings, together with those from our pilot experiments, led us to investigate the differential contribution of these two regions to cocaine seeking in the context of an extended self-administration study with contingent punishment. We hypothesized that the MLS would mediate drug seeking at all stages of training (early in training, after overtraining, and after introduction of contingent punishment), while the DLS would mediate drug seeking in animals under the threat of punishment, perhaps selectively in animals that become insensitive to punishment after extended cocaine self-administration (Pelloux et al., 2007).
Materials and Methods
Subjects
Male outbred Lister hooded rats (Charles River), weighing 275–325 g at the start of the experiments, were housed in pairs in polycarbonate cages (length, 40 cm; width, 25 cm; height, 18 cm) and maintained under a reversed 12 h light/dark cycle (lights on at 7:00 P.M.) at a constant temperature (21 ± 1°C) with ad libitum access to water. Each subject was given 18 g of laboratory chow (SDS Ltd) per day, which was sufficient to maintain body weight at no less than 85% of free feeding weight. The experimental procedures were conducted in accordance with the United Kingdom's 1986 Animals (scientific procedures) Act (project license PPL 80/2234).
Apparatus
Instrumental training and testing took place in 12 operant conditioning chambers (29.5 × 32.5 × 23.5 cm; Med Associates) equipped with two 4-cm-wide retractable levers that were mounted in the intelligence panel 12 cm apart and 8 cm from the grid floor. Above each lever was a cue light (2.5 W, 24 V), and a white house light (2.5 W, 24 V) was located on the opposite wall. The floor of the chamber was covered with a metal grid with bars separated by 1 cm and connected to a shock generator and scrambler (Campden Instruments), which delivered 0.50 mA foot shocks. SILASTIC tubing shielded with a metal spring extended from each animal's intravenous catheter to a liquid swivel (Stoelting) mounted on an arm fixed outside the operant conditioning chamber. Tygon tubing extended from the swivel to a Razel infusion pump (Semat Technical) located adjacent to the housing. The testing chamber was placed within a sound- and light-attenuating housing equipped with a ventilation fan that also screened external noise. The operant conditioning chambers were controlled by software written in C++ using the Whisker control system (Cardinal, 2001).
Surgery
Subjects were anesthetized with ketamine (Ketalar, 90 mg/kg, i.p.) and xylazine (Rompun, 6.7 mg/kg, i.p.) and implanted with an intravenous catheter and intracranial cannulae. For intravenous surgery, rats were implanted with a single catheter in the right jugular vein. Catheters were made from 22 gauge steel cannulae with elongated ends. SILASTIC tubing (0.012 inner diameter) was secured to one end of the cannula, and the top was fixed to nylon mesh. The mesh end of the catheter was sutured subcutaneously between the scapulae. To prevent infection, rats were treated from the day before to 7 d after the surgery with 10 mg/kg Baytril (Bayer) subcutaneously. For intracranial cannulae implantation, rats were placed in a stereotaxic frame (David Kopf Instruments) with the incisor bar set at −3.3 mm below the interaural line (Paxinos and Watson, 1998). Bilateral stainless steel guide cannulae (26 gauge; Plastics One), targeting the lateral striatum (+1.0 mm anterior from bregma, ±3.0 mm lateral from the midline, 3.0 mm ventral from the skull surface) were implanted stereotaxically and secured to the skull using dental cement anchored to stainless steel screws tapped into the skull. Obturators (Plastics One) were placed into the cannulae to prevent occlusion. The rats were allowed to recover for at least 7 d after surgery before behavioral testing.
Drug infusions
Before the start of experimental infusions, the rats were habituated to the infusion procedure with a surrogate infusion, which consisted of the removal and replacement of the obturator during gentle restraint within a time course identical to that of drug infusion. During infusions, the rats were gently restrained while the obturators were removed and 33-gauge bilateral injectors (Plastics One) that protruded either 1.5 mm (dorsolateral striatum) or 2.5 mm (midlateral striatum) beyond the guide cannulae were inserted, and 0.5 μl of solution was then infused over a 1 min period into each injection site. The injector was left in place for 1 min to allow the drug to diffuse in the local vicinity of the injector tip. The injector was then carefully removed and the obturator was replaced. All infusions were given in the behavioral testing room. The animals were infused with either PBS or a mixture of the GABA-B and GABA-A receptor agonists baclofen (bac) and muscimol (mus) at the doses of 0.3 nmol and 0.03 nmol per side, respectively, in PBS to inactivate neurons in the region of the infusion (McFarland and Kalivas, 2001).
Procedure
Task acquisition.
The procedures are illustrated in Figure 1A. Each session began with the insertion of the taking lever, and the assignment of the left or right lever as the taking lever was counterbalanced across rats. Responding on the taking lever was reinforced under a fixed ratio (FR) 1 schedule and each lever press produced a 0.25 mg/kg infusion of cocaine at the rate of 0.1 ml/5 s, accompanied by the withdrawal of the taking lever for 20 s, the extinction of the house light, and the illumination of the stimulus light above the lever for 5 s. The sessions terminated after 2 h, or before if 30 infusions had been earned. After 5 sessions with just the taking lever, the seeking lever was introduced. The seeking and taking levers were never present simultaneously, because meeting the response requirement on the seeking lever led to retraction of the seeking lever and introduction of the taking lever. The schedule on the seeking lever was gradually increased from Random Interval 2 s (RI 2), to RI 15 s, RI 30 s, RI 60 s, and RI 120 s over 5 d, while the schedule on the taking lever remained at FR 1. Completion of the seeking and taking components together constituted one seeking–taking cycle. Thereafter, the postinfusions timeout was increased from 20 s to 2 min, 4 min, and 10 min over 3 more days. The 10 min postinfusion timeout was chosen to minimize psychostimulant effects of cocaine infusion on the next seeking component (Olmstead et al., 2000).
Pre-long-access infusions.
Once the animals had acquired the task, the first set of four infusions was conducted over 8 d; two infusions (PBS or bac/mus) into the dorsolateral striatum, and two more infusions (PBS or bac/mus) into the midlateral striatum. Infusions were always preceded by an infusion-free baseline day to allow for recovery. The order of the experimental and control infusions, and of the DLS and MLS infusions, was counterbalanced at all stages, but the two infusions for one structure were always conducted sequentially to minimize potential effects of baseline drift in relevant comparisons.
Long-access cocaine self-administration.
Subsequently, the animals were given 12 daily sessions of ad libitum cocaine self-administration in which only the taking lever was presented, and pressing that lever resulted in a cocaine infusion and the standard 20 s timeout. The sessions terminated after 6 h, or earlier if 150 infusions had been earned.
Post-long-access infusions.
After one re-baseline session under the seeking (RI 120 s)/taking (FR 1) with 10 min timeout schedule, the second set of four infusions was conducted over another 8 d, again with two infusions (PBS or bac/mus) into the dorsolateral striatum, and two infusions (PBS or bac/mus) into the midlateral striatum. The procedures were identical to the pre-long-access infusions, except that the order of infusions was newly counterbalanced.
Introduction of punishment.
Next, intermittent and unpredictable contingent punishment of the seeking response was introduced, which resulted in the emergence of compulsive drug seeking (Pelloux et al., 2007). During each punishment session, half of the cycles contained no punishment and terminated with access to the taking lever and cocaine availability. In the remaining half of the cycles, the seeking response was punished: the first response that met the RI requirement in the seeking link delivered the foot shock (0.5 s, 0.5 mA) and led to a direct transition to the timeout period without the taking link. Reinforced and punished cycles were presented randomly within the daily sessions, except that no more than two sequential cycles were punished. Three punishment sessions were run before the last infusions phase (see Infusions during punished responding), as previous experiments have shown that responding of both punishment-sensitive and -resistant (compulsive) subgroups stabilized after three punishment sessions (Pelloux et al., 2007).
Infusions during punished responding.
After stabilization of responding under punishment, the third set of four infusions was conducted over another 8 d, again with two infusions (PBS or bac/mus) into the dorsolateral striatum, and two infusions (PBS or bac/mus) into the midlateral striatum. The procedures were identical to the earlier infusions, except that an additional postinfusion session was conducted the day after the last infusion to obtain complete postinfusion data.
Histology
Following the completion of the experiment, subjects were anesthetized with a lethal dose of sodium pentobarbitone (Euthatal, 200 mg/ml; Genus Express) and perfused transcardially with 0.01 m PBS followed by 4% paraformaldehyde. The brains were removed and postfixed in paraformaldehyde. Before being sectioned, the brains were transferred to 20% sucrose in 0.2 m phosphate buffer and left overnight. Coronal sections were cut at 60 μm on a freezing microtome and stained with cresyl violet. Cannulae locations were mapped onto standardized sections of the rat brain (Paxinos and Watson, 1998), and investigated for any signs of tissue damage.
Statistics
Statistical analysis was based on analysis of the number of cycles completed and seeking responses per session for each infusion. The data were analyzed using ANOVAs with appropriate between- and within-subject factors. All statistical tests used an α level of p < 0.05 for the rejection of the null hypothesis.
Results
Twenty-three rats completed the entire experiment, and the cannulae locations were mapped onto standardized sections of the rat brain (Paxinos and Watson, 1998). The cannulae placements of all 23 rats fell within the dorsolateral and midlateral striatum (Fig. 2) and there was no evidence for gross damage to the striatum beyond the location of the guide and infusion cannulae. The data were analyzed at the group level, including all subjects (N = 23) and including all sessions with a preceding intracranial infusion. Drug seeking was quantified by two measures: the number of seeking cycles completed and the number of seeking responses per session.
Figure 2.
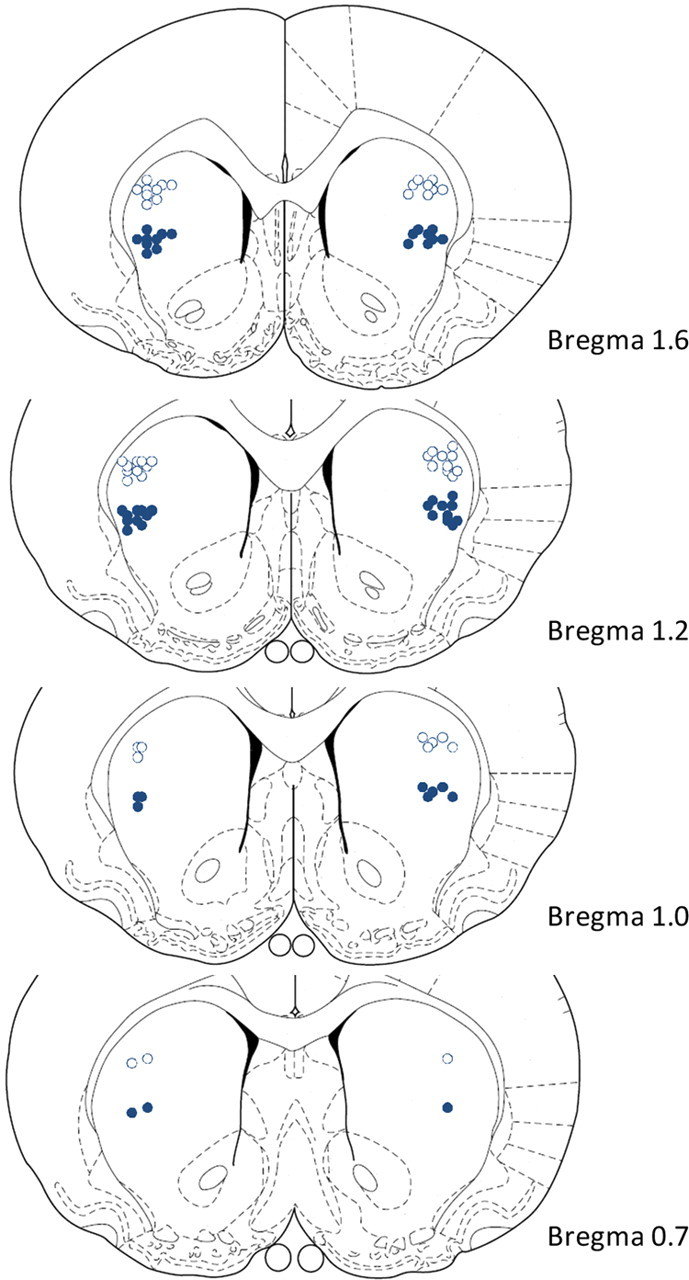
Neuroanatomical representation of cannulae placements. Open circles represent the dorsolateral striatum and closed circles represent the midlateral striatum (N = 23 for both regions). Sections at various distances from bregma in mm.
The overall analysis of the cycles completed showed that inactivation of the MLS produced a greater reduction in responding than inactivation of the DLS, and that inactivation of the DLS reduced the number of seeking cycles completed under punishment, but not in the baseline task either before or after long-access cocaine self-administration (Fig. 1C,E). Thus, the three-way ANOVA of the number of cycles completed revealed significant main effects of inactivation (F(3,20) = 39.5, p < 0.001), test (_F_(3,20) = 244.3, _p_ < 0.001), and striatal site (_F_(3,20) = 7.6, _p_ = 0.012), significant two-way interactions between inactivation and test (_F_(3,20) = 5.5, _p_ = 0.012), and inactivation and striatal site (_F_(3,20) = 16.3, _p_ = 0.001), and a three-way interaction between inactivation, test, and striatal site (_F_(3,20) = 3.514, _p_ = 0.048). Separate one-way ANOVAs revealed a significant effect of inactivating the DLS on punished responding (_F_(1,22) = 29.8, _p_ < 0.001), but not unpunished responding pre-long-access (pre-LA; _F_(1,22) = 2.6, _p_ > 0.05) or post-long-access (post-LA; F(1,22) = 1.9, p > 0.05). The same analyses for the MLS revealed significant effects of inactivation at the pre-LA (F(1,22) = 16.7, p < 0.001), post-LA (F(1,22) = 5.3, p = 0.032), and punishment (F(1,22) = 25.8, p < 0.001) stages.
The overall analysis of the seeking responses gave nearly identical results (Fig. 1D,F). Thus, three-way ANOVA of the number of seeking responses per session revealed significant main effects of inactivation (F(3,20) = 22.7, p < 0.001) and test (_F_(3,20) = 93.9, _p_ < 0.001), and significant two-way interactions between inactivation and test (_F_(3,20) = 4.7, _p_ = 0.021) and inactivation and striatal site (_F_(3,20) = 15.0, _p_ = 0.001), and a three-way interaction between inactivation, test, and striatal site (_F_(3,20) = 5.5, _p_ = 0.012). Separate one-way ANOVAs revealed a significant effect of inactivating the DLS on punished responding (_F_(1,22) = 7.1, _p_ = 0.014), but not pre-LA (_F_(1,22) = 1.3, _p_ > 0.05) or post-LA (F(1,22) = 1.0, p > 0.05) responding. The same analyses for the MLS revealed significant effects of inactivation at the pre-LA (F(1,22) = 22.2, p < 0.001), post-LA (F(1,22) = 22.1, p < 0.01), and punishment (F(1,22) = 5.8, p = 0.025) stages.
Subsequently, it was investigated whether the effect of striatal inactivation in the punishment phase also carried over to the day after the infusion. To this end, the number of seeking cycles completed per session on the day before infusion, the day of infusion, and the day after infusion was investigated. The overall analysis showed that for inactivation of both the DLS and MLS, responding under punishment was lower immediately after infusion, but not on the days before or after infusion (Fig. 3A,B). Thus, in the two-way ANOVA of the DLS inactivation, there was a significant interaction between day and inactivation (F(2,21) = 6.2, p = 0.004), with a significant effect on the day of infusion (F(1,22) = 29.8, p < 0.001), but not the day before (_F_(1,22) = 0.4, _p_ > 0.05) or after (F(1,22) = 0.0, p > 0.05). In the two-way ANOVA of the MLS inactivation, there was a significant interaction between day and inactivation (F(2,21) = 9.2, p < 0.001), with a significant effect on the day of infusion (_F_(1,22) = 25.8, _p_ < 0.001), but not the day before (_F_(1,22) = 0.6, _p_ > 0.05) or after (F(1,22) = 1.0, p > 0.05).
Figure 3.
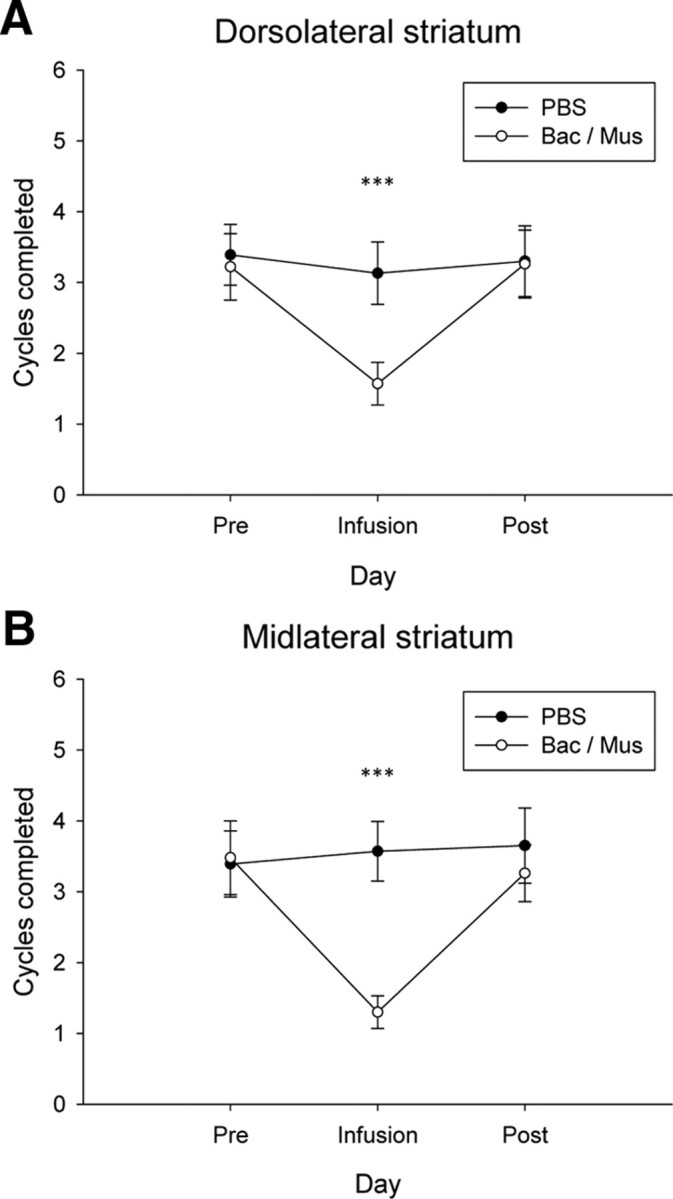
Effect of inactivating lateral striatal subregions on cocaine seeking under punishment the day after baclofen/muscimol infusion. A, Mean (±SEM) seeking cycles completed after inactivation of the DLS the day before infusion, the day of infusion, and the day after infusion. Inactivation of the DLS only affected responding on the day of infusion. B, Mean (±SEM) seeking cycles completed after inactivation of the MLS the day before infusion, the day of infusion, and the day after infusion. Inactivation of the MLS only affected responding on the day of infusion. ***p < 0.001. For all comparisons, N = 23.
Next, the relationship between baseline compulsivity and the effect of inactivating the DLS was investigated. Based on the distribution of the average number of seeking–taking cycles completed under punishment after PBS infusion into the DLS (Fig. 4A), the relative level of compulsive responding was compared with the proportional suppression of responding after DLS inactivation (cycles completed after bac/mus infusion divided by cycles completed after PBS infusion into the DLS; Fig. 4B), excluding the single animal that completed zero cycles after PBS infusion (N = 22). The statistical analysis showed that there was no significant correlation between these two variables (Spearman's ρ = 0.06, p > 0.05). Thus, the effect of inactivation of the DLS to reduce seeking responses was not specific to either high- or low-compulsive animals.
Figure 4.
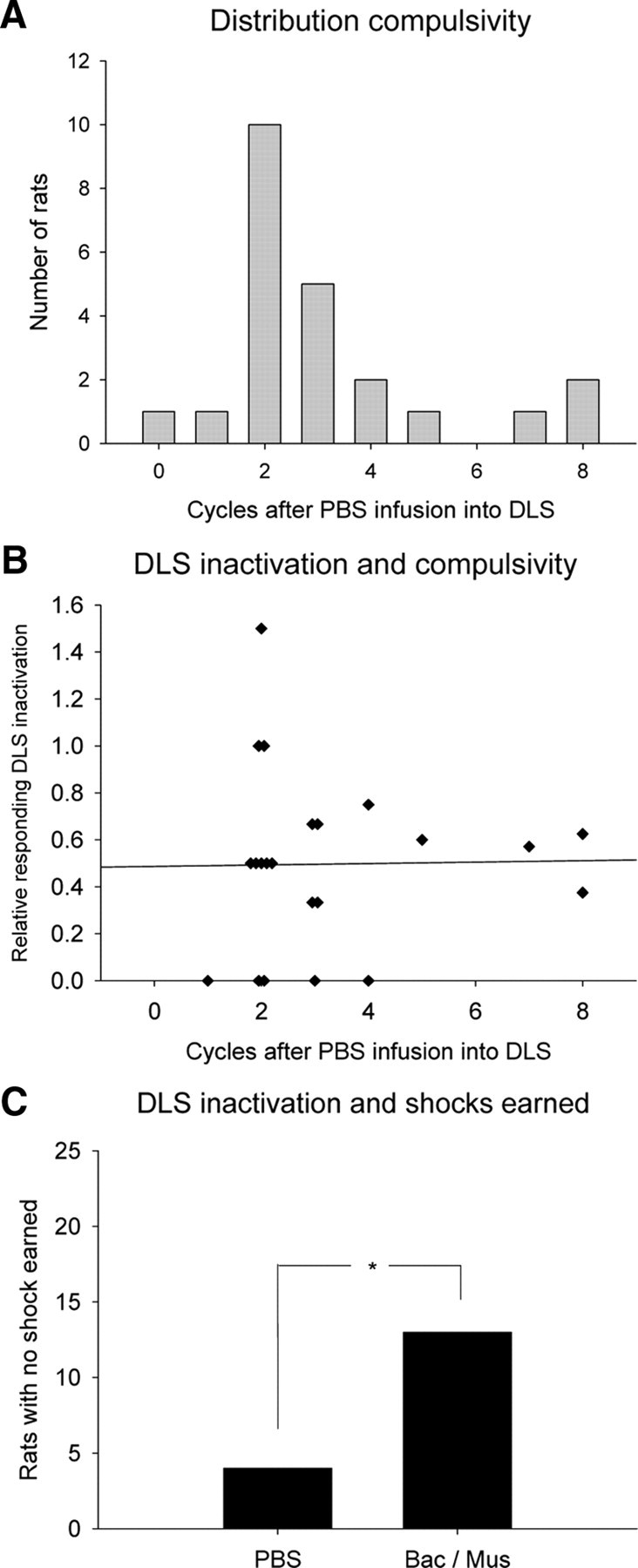
Relation between compulsivity and effect of DLS inactivation. A, Distribution of compulsivity by seeking cycles completed in the punished sessions after PBS infusion into the DLS (N = 23). B, Relation between compulsivity and relative suppression of responding by DLS inactivation (N = 22). Regression line represents correlation coefficient of 0.06, which was not significant. C, Number of animals (of 23) that did not earn shock in session after infusion into the DLS. Inactivation of the DLS significantly increased the number of animals that did not earn shock in the session, demonstrating that the effect of DLS inactivation was present before shock administration. *p < 0.05.
Finally, we investigated whether the reduced responding under punishment after DLS inactivation was due to altered sensitivity to shocks within the test session. To this end, we compared the number of animals that did not receive a single shock in the test session (due to low response rates) after control infusion (4 of 23) and inactivation of the DLS (13 of 23). A χ2 test with Yates correction showed that this difference was significant (p = 0.0134). This result demonstrates that inactivation of the DLS reduced responding before delivery of foot shock, ruling out altered sensitivity to shock delivery within the test session as an explanation for the selective effect of DLS inactivation under punishment conditions (Fig. 4C).
Discussion
These results establish that the dorsolateral striatum selectively mediates drug seeking in the face of contingent punishment, but is not necessary for responding under unpunished conditions, even after overtraining. The effect of DLS inactivation on punished cocaine seeking was not due to enhanced learning about negative outcomes within the test session, as the reduction in responding was present before delivery of the first shock, and response levels returned to baseline the next day when the DLS was back online. The most parsimonious explanation of this pattern of results is that the influence of recalled threat of negative consequences of lever pressing is enhanced after DLS inactivation. Apparently, the response drive in the DLS that is acquired after overtraining renders drug seeking less sensitive to (more recently acquired) intermittent punishment-driven inhibition of responding. This interpretation is consistent with findings that implicate the DLS in instrumental responding based on foot shock avoidance (Wietzikoski et al., 2012). We speculate that the DLS may play a role in this process because of the lack of direct connectivity between threat related areas such as the amygdala and ventral hippocampus and the DLS (Killcross et al., 1997; Voorn et al., 2004). Thus, the DLS seems to drive a type of rigidity of responding that appears to be related to the DLS-mediated habitual control of responding that renders overtrained food or cocaine seeking insensitive to (later acquired) outcome devaluation when tested under extinction conditions (Yin et al., 2004, 2006; Zapata et al., 2010). The present results extend these findings to a conflict test with actually delivered negative outcomes.
However, in the absence of response conflict, the same response drive in the DLS is not necessary for responding under baseline (unpunished) conditions, even if the response has been overtrained. Inactivation of the midlateral striatum, on the other hand, impaired drug seeking more or less equally at all stages of training, suggesting that this region is important for the initiation of previously rewarded instrumental responses. This consistent response reduction could potentially be explained by a reduction in the rewarding efficacy of cocaine, but this is highly unlikely, as even omission of 50% of cocaine infusions did not affect response rates in this task (Pelloux et al., 2007). Instead, the effect of inactivation of this region is likely to be an instrumental performance effect, consistent with the motor cortical connectivity of this region. The decrease in appetitive instrumental responding after inactivation of this region is consistent with previous reports (Fricker et al., 1996; Fuchs et al., 2006; Yin et al., 2006).
Lateral striatum and reinforced lever pressing
Together, these findings suggest a model whereby activity in the MLS is always necessary for reinforced lever pressing, while the DLS comes to control a selective aspect of responding after overtraining. Apparently, activity in the DLS is not necessary for responding under normal (unpunished) conditions, as activity in the MLS is presumably sufficient to drive normal responding. However, the role of the DLS is unmasked in conditions where an inhibitory response drive that was more recently acquired is superimposed on the excitatory response drive. Although there are only limited data on the differential afferent projections to the MLS and DLS regions, one possible explanation for the selective role of the MLS in reinforced responding could be the considerably denser primary forelimb motor cortex (caudal forelimb area, CFA) projection into the MLS (Rouiller et al., 1993).
Dorsolateral striatum and compulsive drug seeking
Contrary to our expectations, there was no evidence for a selective effect of DLS inactivation on responding under punishment in highly compulsive animals, as the proportional suppression of responding after DLS inactivation was unrelated to the level of compulsivity. Thus, these results do not provide an explanation for the differential levels of compulsivity that were previously observed after an extended history of cocaine self-administration (Deroche-Gamonet et al., 2004; Pelloux et al., 2007). Nevertheless, the present results do demonstrate that the DLS is a neural structure that exerts a strong influence on drug seeking under threat of punishment. Therefore, it remains possible that interindividual variability in the response of the DLS to extended cocaine self-administration is related to the variability in behavioral compulsivity. This hypothesis would be consistent with human (Volkow et al., 2006), macaque monkey (Letchworth et al., 2001; Porrino et al., 2004), and rodent studies (Welch et al., 2007; Im et al., 2010; Peça et al., 2011) implicating the dorsal striatum in compulsive behavior, and deserves further investigation in the future.
In conclusion, these results show that inactivation of the dorsolateral striatum does not affect cocaine seeking, even after overtraining. Instead, it is selectively important for punished drug seeking. Activity in the DLS after extended cocaine self-administration seems to drive punished responding by reducing the influence of threat of intermittent and unpredictable contingent shock on response output. Furthermore, the results show that the midlateral striatum mediates instrumental responding at all stages of training, thereby suggesting a novel differentiation in the functions of the sensorimotor striatum.
Footnotes
This work was supported by United Kingdom Medical Research Council (MRC) Grant 9536855 and was conducted within the MRC/Wellcome Trust Behavioural and Clinical Neuroscience Institute.
References
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol. 1982;34B:77–98. [Google Scholar]
- Cardinal RN. Whisker. 2001. version 1.0, www.whiskercontrol.com.
- Carelli RM, Wolske M, West MO. Loss of lever press-related firing of rat striatal forelimb neurons after repeated sessions in a lever pressing task. J Neurosci. 1997;17:1804–1814. doi: 10.1523/JNEUROSCI.17-05-01804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Inactivation of the lateral but not medial dorsal striatum eliminates the excitatory impact of pavlovian stimuli on instrumental responding. J Neurosci. 2007;27:13977–13981. doi: 10.1523/JNEUROSCI.4097-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Ebrahimi A, Pochet R, Roger M. Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neurosci Res. 1992;14:39–60. doi: 10.1016/s0168-0102(05)80005-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fricker RA, Annett LE, Torres EM, Dunnett SB. The placement of a striatal ibotenic acid lesion affects skilled forelimb use and the direction of drug-induced rotation. Brain Res Bull. 1996;41:409–416. doi: 10.1016/s0361-9230(96)00083-4. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate–putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res. 2009;201:128–136. doi: 10.1016/j.bbr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: action or habit? Behav Neurosci. 2003;117:927–938. doi: 10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A. Cocaine seeking by rats: regulation, reinforcement and activation. Psychopharmacology. 2000;152:123–131. doi: 10.1007/s002130000498. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. New York: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MO, Carelli RM, Pomerantz M, Cohen SM, Gardner JP, Chapin JK, Woodward DJ. A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. J Neurophysiol. 1990;64:1233–1246. doi: 10.1152/jn.1990.64.4.1233. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, O'Connor WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain. 1986;109:805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- Wietzikoski EC, Boschen SL, Miyoshi E, Bortolanza M, Dos Santos LM, Frank M, Brandão ML, Winn P, Da Cunha C. Roles of D1-like dopamine receptors in the nucleus accumbens and dorsolateral striatum in conditioned avoidance responses. Psychopharmacology. 2012;219:159–169. doi: 10.1007/s00213-011-2384-3. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]