Catechol O-Methyltransferase val158met Genotype Influences Frontoparietal Activity during Planning in Patients with Parkinson's Disease (original) (raw)
Abstract
Cognitive dysfunction commonly occurs even in the early stages of Parkinson's disease (PD). Impairment on frontostriatally based executive tasks is particularly well described but affects only a proportion of early PD patients. Our previous work suggests that a common functional polymorphism (val158met) within the catechol _O_-methyltransferase (COMT) gene underlies some of this executive heterogeneity. In particular, an increasing number of methionine alleles, resulting in lower enzyme activity, is associated with impaired performance on the “Tower of London” planning task. The main objective of this study was to investigate the underlying neural basis of this genotype–phenotype effect in PD using functional magnetic resonance imaging. We scanned 31 patients with early PD who were homozygous for either valine (val) (n = 16) or methionine (met) (n = 15) at the COMT val158met polymorphism during performance of an executive task comprising both Tower of London (planning) and simple subtracting (“control”) problems. A cross-group comparison between genetic subgroups revealed that response times for planning problems were significantly longer in met compared with val homozygotes, whereas response times for control problems did not differ. Furthermore, imaging data revealed a significant reduction in blood oxygen level-dependent signal across the frontoparietal network involved in planning in met/met compared with val/val patients. Hence, we have demonstrated that COMT genotype impacts on executive function in PD through directly influencing frontoparietal activation. Furthermore, the directionality of the genotype–phenotype effect observed in this study, when interpreted in the context of the existing literature, adds weight to the hypothesis that the relationship between prefrontal function and dopamine levels follows as an inverted U-shaped curve.
Keywords: Parkinson's disease, cognition, planning, COMT, functional MRI, prefrontal cortex
Introduction
Parkinson's disease (PD) is typically defined in terms of its motor symptomatology as a syndrome of tremor, rigidity, bradykinesia, and postural instability (Gibb and Lees, 1988). However, cognitive dysfunction is common in PD (Aarsland et al., 2003) and has important implications in terms of quality of life (Schrag et al., 2000) and care requirements (Aarsland et al., 2000). Several types of cognitive impairment have been identified, but the most well described deficits are “executive” in nature, demonstrable on frontostriatally mediated tasks of working memory, planning, and attentional set shifting (Owen et al., 1992, 1995), on which performance is dopamine dependent (Lange et al., 1992; Owen et al., 1995). These executive deficits occur in some patients from the earliest stages of the disease but are by no means universal (Lewis et al., 2003b; Foltynie et al., 2004a). Although functional magnetic resonance imaging (fMRI) studies have confirmed that the underlying neural locus of executive heterogeneity in PD lies within frontostriatal networks (Lewis et al., 2003a), its determinants remain unclear. One highly plausible hypothesis is that this heterogeneity has a genetic basis, and indeed our own previous work has demonstrated that a common polymorphism in the catechol _O_-methyltransferase gene (COMT) significantly influences performance on a test of working memory and planning in PD (Foltynie et al., 2004b).
COMT is an important regulator of CNS dopamine levels, and its activity is in turn modulated by a polymorphism of valine (val) for methionine (met) at codon 158 in the peptide sequence (val158met). The met variant has a lower thermostability, resulting in a threefold reduction in brain COMT activity in met homozygotes compared with val homozygotes (Chen et al., 2004). This has a particularly important effect on dopamine levels in the prefrontal cortex (PFC) (Karoum et al., 1994; Gogos et al., 1998; Mazei et al., 2002) in which there are few dopamine transporters (Lewis et al., 2001), in keeping with the observed influence of the polymorphism on prefrontally mediated behavior. In both healthy subjects (Malhotra et al., 2002) and schizophrenics (Egan et al., 2001), for example, low-activity COMT genotypes are associated with improved performance on the Wisconsin Card Sorting Test (WCST), presumably as a consequence of higher prefrontal dopamine levels. fMRI studies in these subject groups have also confirmed that prefrontal activation during working memory (Egan et al., 2001) and attentional control tasks (Blasi et al., 2005) is significantly altered by COMT genotype.
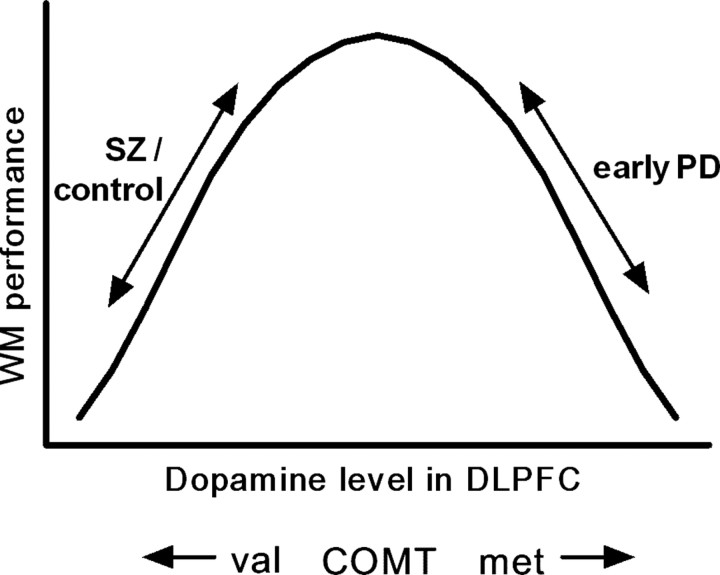
Interestingly, in PD, the direction of this genotype–phenotype relationship appears to differ. In a behavioral study of 288 early PD patients, we have previously shown that an increasing number of met alleles (i.e., lower COMT activity) is associated with impairment on the “Tower of London” (TOL) test of working memory and planning, with the effect being greatest in patients on dopaminergic medications (Foltynie et al., 2004b). Coupled with the observation that there is a hyperdopaminergic state in the PFC in early PD relative to controls (Rakshi et al., 1999; Kaasinen et al., 2001), these findings suggest that excessively high prefrontal dopamine levels are actually detrimental to performance on the TOL. If it is assumed that performance on the TOL is dependent on dopaminergic mechanisms similar to the WCST, there is an apparent reversal of the genotype–phenotype effect in PD. This might be explained by a hypothetical inverted U-shaped curve relating working memory performance to prefrontal dopaminergic activity (Goldman-Rakic et al., 2000) (Fig. 1).
Figure 1.
Hypothesized inverted U-shaped relationship between working memory (WM) performance and dopamine level in the DLPFC (Goldman-Rakic et al., 2000). The COMT met/met genotype is expected to confer a higher baseline dopamine level than the val/val genotype. This has opposing behavioral consequences in schizophrenics (SZ)/controls and those with early PD, suggesting that their relative positions on the curve differ (see Introduction).
The main purpose of this study was to explore further the relationship between COMT genotype and executive function in PD through investigating its underlying neural basis using fMRI. This work has important implications for better understanding the basis of cognitive heterogeneity in this disorder.
Materials and Methods
We examined blood oxygen-level dependent (BOLD) responses using fMRI during the TOL task in two subgroups of patients with early PD who were homozygous for either val or met at the COMT val158met polymorphism. We then performed cross-group comparisons to test the hypothesis that this polymorphism exerts its behavioral effects in PD through modulating activation in the prefrontal cortex.
Subjects.
Thirty-two patients with early Parkinson's disease were recruited to the study from the Cambridge Centre for Brain Repair PD research clinic. Inclusion criteria were a disease duration of <6 years from diagnosis, homozygosity for the COMT val158met polymorphism, mild to moderate disease stage [Hoehn and Yahr stage ≤2.5 (Hoehn and Yahr, 1967)], no significant cognitive deficit [Mini-Mental State Examination score ≥26 (Folstein et al., 1975)], and no major depression [Beck depression score ≤18 (Beck et al., 1988)]. All patients met the United Kingdom Parkinson's Disease Society Brain Bank diagnostic criteria for PD (Gibb and Lees, 1988). Patients were also assessed using the Unified Parkinson's disease rating scale (UPDRS) (Fahn and Elton, 1987) and completed the National Adult Reading Test, a measure of verbal intelligence quotient (IQ) (Nelson and O'Connell, 1978) before scanning.
Each subject's current dopaminergic drug regimen was recorded and converted to an equivalent levodopa dose to facilitate comparison between patients. The following formula was used, which is based on those previously developed and reported in the literature (Brodsky et al., 2003). Equivalent levodopa dose = [levodopa (× 1.2 if COMT inhibitor)(× 1.2 if 10 mg of selegiline or × 1.1 if 5 mg of selegiline)] + [pramipexole × 400] + [ropinirole × 40] + [cabergoline × 160] + [pergolide × 200] + [bromocriptine × 10] + [lisuride × 160]; all doses are in milligrams. No patients were taking acetylcholinesterase inhibitors. All testing was performed with patients taking their usual medications.
Genotyping.
DNA was extracted from peripheral blood samples using standard phenol/chloroform methods. COMT val158met genotypes were determined using an allelic discrimination TaqMan assay. This uses the 5′ exonuclease activity of Taq polymerase to generate an allele-specific fluorescent reporter signal, which is measured after PCRs using an HT7900 detection system (Applied Biosystems, Foster City, CA).
Experimental design.
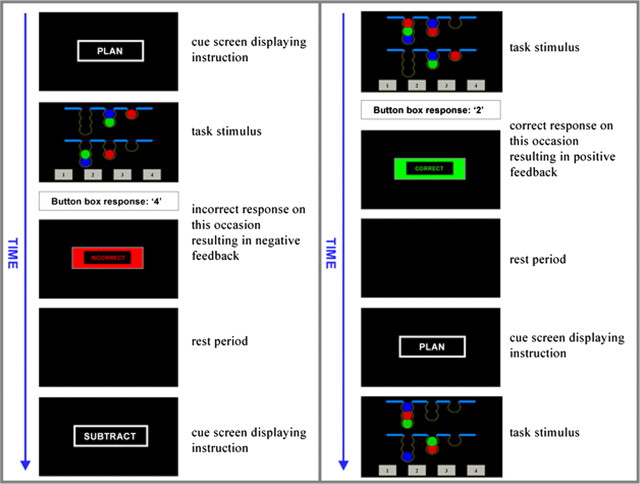
A modified version of the TOL (Shallice, 1982) was chosen as the experimental task for this study because it is known to reliably activate prefrontal association cortex in both positron emission tomography (Baker et al., 1996; Owen et al., 1996; Dagher et al., 1999; Schall et al., 2003) and functional MRI studies (Lazeron et al., 2000; Newman et al., 2003; Schall et al., 2003; van den Heuvel et al., 2003), and the task has been well validated as a measure of executive dysfunction in PD (Morris et al., 1988; Owen et al., 1992, 1995). Furthermore, both performance (Lange et al., 1992) and task-related blood flow in the dorsolateral prefrontal cortex (DLPFC) (Cools et al., 2002) have been shown to be sensitive to manipulations of dopamine level, and we also clearly demonstrated a behavioral effect of COMT genotype in PD using this task (Foltynie et al., 2004b). The TOL requires subjects to rearrange three colored balls, which are distributed between three pockets displayed in the lower half of a computer screen to match a template array displayed in the upper half of the screen (Owen et al., 1990). In this “one-touch” version of the test, subjects were asked to indicate the correct number of moves required to complete the problem by selecting from one of four numbers (1–4) presented at the bottom of the screen [adapted from Owen et al. (1995) and Baker et al. (1996)] (Fig. 2).
Figure 2.
A typical series of trials during the experimental task are illustrated. Subjects were prompted with a cue screen displaying the word PLAN or SUBTRACT before each new problem was presented. Event duration was measured from the time of appearance of the trial stimulus until the time of a response on the button box. Once a response was made, a feedback screen displaying the word CORRECT or INCORRECT appeared. A 5–15 s rest period followed, during which the screen remained blank, before presentation of the next cue. Planning and subtracting problems were presented alternately for a total of 10 min.
The experimental paradigm also incorporated a control task, which used similar visual stimuli and required an identical motor response to the TOL, but the executive demands of the task were lower. Subjects were simply instructed to count the number of colored balls in each of the two arrays displayed on the screen and then to subtract the number in the lower array from the number in the upper array (Fig. 2). Responses were made using a button box in the subject's right hand, with the first, second, third, and fourth fingers being used to indicate numbers 1–4. For both tasks, problems with correct responses of 2, 3, or 4 only were used in the scanner.
The experimental paradigm lasted for 10 min, during which TOL and “subtracting” problems were displayed alternately with an intervening rest interval whose duration was jittered between 5 and 15 s. Subjects were prompted with a cue screen displaying the word “plan” or “subtract” before each new problem was presented and received feedback consisting of the word “correct” or “incorrect” after their response (Fig. 2). The difficulty level of the problems displayed varied according to a randomized sequence. The duration of problem-solving events was response-driven; hence, the number of problems completed by each subject varied. All subjects underwent a training session before scanning to ensure that they understood the rules of the task and were able to perform it adequately.
Data acquisition.
Patients were scanned at the Medical Research Council Cognition and Brain Sciences Unit (Cambridge, UK) using a 3 Tesla Siemens (Munich, Germany) TIM Trio MRI scanner. Three-hundred and thirty T2-weighted echo-planar images depicting BOLD signal were acquired in total, the first 10 of which were discarded to avoid T1-equilibrium effects. Each image consisted of 32 slices of 3 mm thickness with a 1 mm interslice gap, with an in-plane resolution of 3 × 3 mm. The repetition time was 2 s. Slices were angled away from the orbits to avoid signal dropout attributable to magnetic susceptibility inhomogeneity. Stimuli were presented on a computer screen with a resolution of 1024 pixels, which was visualized using a mirror positioned within the scanner at a viewing distance of 90 mm, such that 37 pixels subtended a visual angle of 1°.
Data analysis.
Two variables were used to measure behavioral performance on the tasks, namely percentage of problems attempted that were answered correctly, and mean response time. Repeated-measures ANOVAs were used to compare performance between genetic subgroups across task difficulty level.
Imaging data were analyzed using SPM 5 (Wellcome Department of Imaging Neuroscience, University College London, London, UK). Preprocessing was undertaken with the aa version 1 batch system using aarecipe_general_ver02.m (http://imaging.mrc-cbu.cam.ac.uk/imaging/AutomaticAnalysisManualReference). Images were subject motion corrected, slice time acquisition corrected, coregistered to the structural magnetization-prepared rapid-acquisition gradient echo, normalized to the standard Montreal Neurological Institute echo-planar imaging template using the SPM 5 normalization/segment routine, and smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
The BOLD response was modeled to the onset times and durations of two events: planning (TOL) and subtracting. Onsets were the time of appearance of the stimuli on the screen and durations were measured to the time of the button box response. Contrast images were extracted for each individual for three contrasts of interest: planning minus baseline (rest), subtracting minus baseline, and planning minus subtracting. These contrasts were further explored using group level random effects analyses.
It was anticipated that any effects of a single genetic polymorphism on BOLD response would be small; hence, we attempted to optimize sensitivity for detecting such changes using the following strategy. First, in the whole patient group, we identified those regions specifically involved in planning by performing a group level analysis of planning minus subtracting events using a threshold of p = 0.05 after false discovery rate (FDR) correction for whole brain mass. Second, regions of interest (ROIs) were defined on the basis of this analysis as 5 mm radius spheres at peak height coordinates within each cluster of signal change. Third, we selected the contrast of planning minus baseline, which was expected to generate maximal signal change, and modeled our planning-specific ROIs for each individual subject using the Marseille Bôıte A Region d'Intérét (Marsbar) toolbox (Brett et al., 2002). Finally, these ROI data were extracted for a cross-group comparison between COMT val/val and met/met subgroups. This analysis involved a two-way repeated-measures ANOVA with BOLD response as the dependent variable, genotype as the between-subject factor, and ROI as the within-subject factor. Potential confounding factors including age, gender, disease duration, UPDRS motor score, and equivalent levodopa dose were corrected for. To further explore the possibility of an interacting effect between dopaminergic medication and COMT genotype, which has been raised in our previous work (Foltynie et al., 2004b), we performed subgroup analyses comparing the relationships between medication dose and BOLD activation within each genotypic group.
To explore the relationship between cortical activation and behavioral performance, we performed nonparametric tests for correlations between BOLD response in each ROI and two measures of planning performance, namely (1) overall accuracy (percentage correct) and (2) mean response time (across all difficulty levels). All analyses were performed using SPSS version 11.5 (SPSS, Chicago, IL).
Results
One patient was excluded from the study because of a technical problem with fMRI data acquisition. Of the remaining 31 patients included, 16 were homozygous for valine, and 15 were homozygous for methionine. The two genetic subgroups were well matched in terms of demographic and clinical characteristics (Table 1).
Table 1.
Demographic and clinical characteristics of genotypic subgroups
| Variable | COMT genotype | p | |
|---|---|---|---|
| val/val (n = 16) | met/met (n = 15) | ||
| Age | 64.3 (9.8) | 65.5 (9.6) | 0.75 |
| Gender (male:female) | 11:5 | 9:6 | 0.72 |
| Disease duration (years) | 3.9 (2.0) | 3.4 (1.5) | 0.41 |
| UPDRS motor score | 22.2 (10.5) | 26.1 (9.4) | 0.29 |
| MMSE | 28.9 (1.2) | 28.7 (0.8) | 0.59 |
| Beck depression score | 6.8 (4.3) | 6.7 (4.5) | 0.99 |
| NART (verbal IQ) | 113.1 (7.3) | 114.7 (6.2) | 0.50 |
| Equivalent levodopa dose (mg)a | 618.8 (479.6) | 621.7 (430.6) | 0.99 |
| Actual levodopa dose (mg) | 218.8 (152.6) | 313.3 (253.2) | 0.22 |
Behavioral data
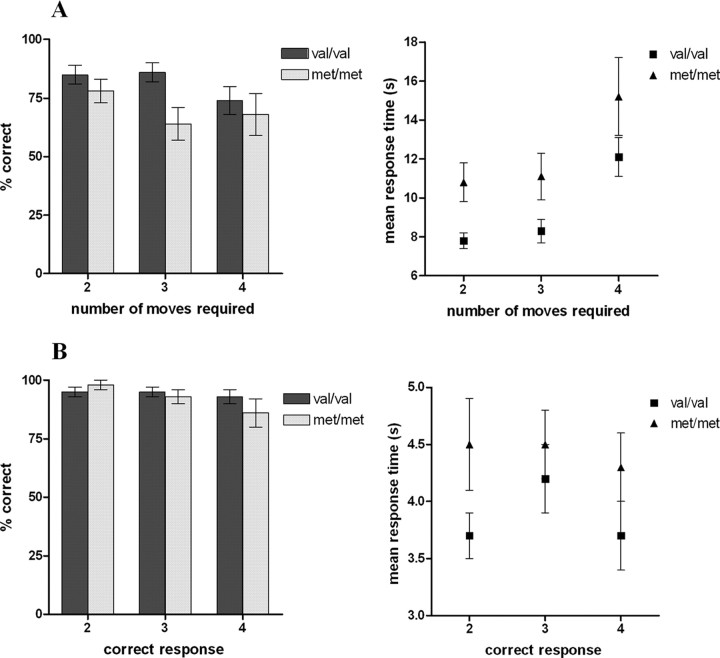
Behavioral performance on both planning and subtracting tasks in the two genetic subgroups is illustrated in Figure 3. For the TOL task, repeated-measures ANOVA confirmed a main effect of COMT genotype on response time (F = 4.68; p = 0.039), with met homozygotes being significantly slower to respond than val homozygotes. There was no significant interaction between number of moves required and genotype, indicating that this effect was not restricted to the more difficult problems. There was also a nonsignificant trend toward impaired accuracy in met homozygotes with no interaction between difficulty and genotype. For the subtracting task, neither accuracy nor response time varied significantly between COMT subgroups; hence, the slower performance of the met group on the TOL task was likely to reflect a specific impairment of planning performance rather than a generalized motor or cognitive slowing.
Figure 3.
Behavioral performance stratified according to COMT genotype for planning problems (A) and subtracting problems (B).
Regions activated during planning
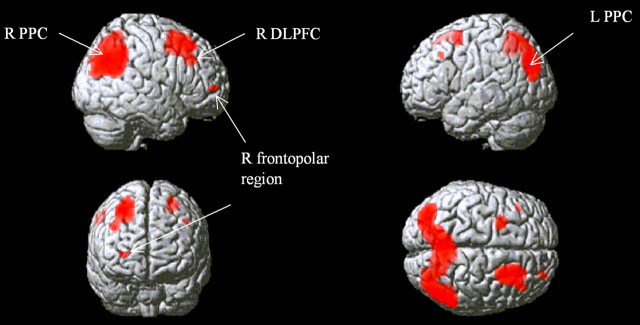
To determine brain regions activated specifically by planning, the contrast of all planning events minus all subtracting events was used. The predominant areas in which significant BOLD signal change was observed were the right DLPFC, the right frontopolar cortex, and the posterior parietal cortices (PPCs) bilaterally (Fig. 4). ROIs were defined in these three areas, centered on coordinates of peak activation (right DLPFC, x = 44, y = 24, z = 38; right frontopolar cortex, x = 28, y = 52, z = 4; left PPC, x = −36, y = −78, z = 34; right PPC, x = 36, y = −78, z = 34, mirrored from left hemisphere). An additional “cortical control” ROI was defined in the right temporal lobe (x = 56, y = 10, z = −28), an area in which there was no significant signal change during planning versus subtracting. Finally, we defined anatomical ROIs in the caudate nuclei using the Marsbar ROI toolbox (Tzourio-Mazoyer et al., 2002). Although we did not observe significant striatal activation in association with the planning component of the TOL, this area is a central site of dopaminergic pathology, and the caudate in particular may be relevant in terms of mediating executive performance in PD (Lewis et al., 2003a) via its connections with the prefrontal cortex. Furthermore, previous studies have demonstrated caudate activation during the TOL in association with increasing task difficulty (Owen et al., 1996; Dagher et al., 1999).
Figure 4.
Activity during planning relative to subtracting rendered onto a canonical brain image. Figure shows areas of signal change above a threshold of p = 0.05 after FDR correction for whole brain volume. The approximate positions of peak height signal change used to define ROIs for subsequent analyses are indicated. R, Right; L, left.
ROI analyses
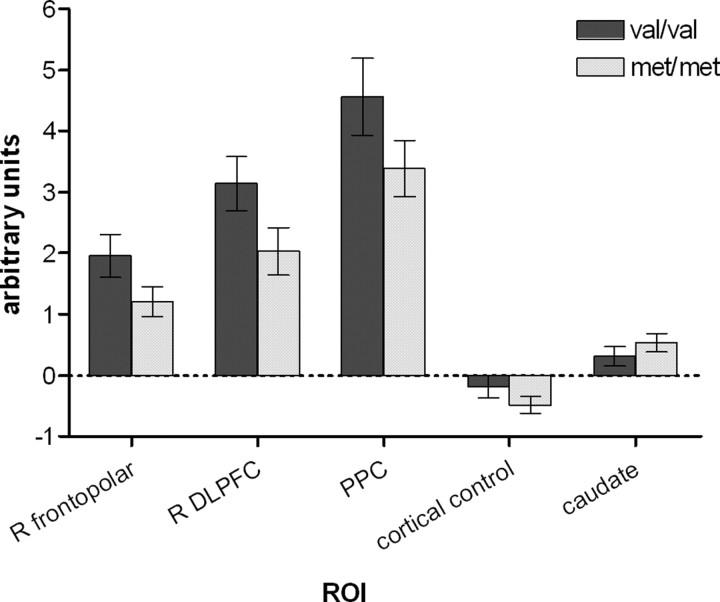
ROI analyses focusing on those areas known to be significantly and specifically activated during planning revealed a consistent overactivation in val homozygotes compared with met homozygotes throughout the frontoparietal planning network during planning compared with baseline (Fig. 5). Repeated-measures ANOVA confirmed a significant main effect of COMT genotype on signal change (p = 0.05) in the right frontopolar cortex, right DLPFC, and PPC with no interaction between ROI and genotype. This effect remained significant even after the inclusion of potential confounding variables including age, gender, disease duration, UPDRS motor score, and either equivalent or actual levodopa dose as covariates in the repeated-measures ANOVA model. In contrast, there was no significant difference in signal change between genotypic groups in the right temporal control ROI (Student's t test, p = 0.20) (Fig. 5), indicating that the COMT effect was specific to particular cortical regions. Furthermore, there was no effect of genotype on BOLD signal in the caudate (Student's t test, p = 0.31) (Fig. 5).
Figure 5.
Activity in selected ROIs during planning relative to baseline in subgroups defined according to COMT genotype. Bars represent means ± SEM. For the PPC and the caudate nuclei, values were averaged across symmetrical ROIs in the right and left hemispheres. The cortical control ROI is in the right temporal cortex, a randomly chosen area not specifically activated during planning.
Subgroup analysis within each genotypic group revealed no significant correlations between either equivalent or actual levodopa dose and BOLD response in any of the 3 ROIs involved in planning, providing no evidence to support an interactive effect of COMT genotype and dopaminergic medication.
There was a significant positive correlation between accuracy during planning problems and BOLD response in the right DLPFC (Spearman's r = 0.44, p = 0.01). Although correlations between performance and BOLD response did not reach significance in the right frontopolar cortex and the PPC, there was a consistent trend toward positive correlations between accuracy and activation and toward negative correlations between response time and activation (Table 2).
Table 2.
Correlations between BOLD response and behavioral performance during planning problems (Spearman's rho)
| ROI | Behavioral measure | |
|---|---|---|
| % correct | Mean response time | |
| R frontopolar cortex | 0.18 | −0.20 |
| R DLPFC | 0.44* | −0.12 |
| PPC | 0.20 | −0.13 |
Discussion
We investigated the influence of the COMT val158met polymorphism on brain activation in PD patients performing a planning task and demonstrated a significant reduction in BOLD response in met compared with val homozygotes across the frontoparietal executive network. We also observed subtle impairment in behavioral performance in the met/met group, which is likely to reflect this difference in activation, although the relationship between behavioral performance and BOLD response remains speculative.
The brain regions activated specifically by planning included areas of frontal association cortex (DLPFC and frontopolar cortex) and posterior parietal cortex, in keeping with previous imaging studies using the TOL in healthy subjects (Baker et al., 1996; Owen et al., 1996; Dagher et al., 1999; Lazeron et al., 2000; Newman et al., 2003; Schall et al., 2003; van den Heuvel et al., 2003) and PD patients (Owen et al., 1998; Dagher et al., 2001). In our study, prefrontal activation was lateralized to the right hemisphere, although previous findings regarding laterality of function during the TOL have been inconsistent. Newman et al. (2003) specifically investigated this issue and concluded that the right PFC has a strategic role in constructing the plan for solving the TOL problem, whereas the left DLPFC has a supervisory role in plan execution. Such an explanation might account for the predominantly right-sided DLPFC activation observed during studies using a one-touch version of the TOL such as this one (Baker et al., 1996; Lazeron et al., 2000; van den Heuvel et al., 2003), in which the execution stage is minimized. Furthermore, interstudy differences between control tasks are likely to account for disparities in activation patterns: the demands placed on the left PFC by our control task may have been comparable with those of the TOL.
The COMT-dependent changes in cortical activity observed here were region specific, implying a localized rather than generalized effect on neuronal activation. An effect in the PFC was anticipated given the well established finding that COMT predominantly influences dopamine levels in this region. We also observed a significant effect of genotype on posterior parietal activation, which is not altogether surprising, given that prefrontal and posterior parietal regions are consistently coactivated during planning tasks. Newman et al. (2003) have demonstrated functional connectivity between the right DLPFC and bilateral superior parietal cortices, which is modulated by task difficulty, thus COMT-dependent alteration in activity in the right PFC would be expected to produce a yoked change in parietal activity. Furthermore, in the human brain, unlike the rodent brain, the parietal cortex receives significant dopaminergic innervation (Berger et al., 1991; Hurd et al., 2001), hence COMT may well have a direct influence on parietal activation, although such an effect is not apparent in animal models. Finally, while considering the anatomical basis of the COMT effect, we should point out that although a direct effect of genotype on cortical activation seems likely, it is possible that the polymorphism exerts some of its effects subcortically through modulating midbrain dopamine synthesis (Akil et al., 2003; Meyer-Lindenberg et al., 2005). Our data do not suggest a genotypic effect on striatal function, however, which would argue against this theory.
The apparent underactivation of the frontoparietal network in met compared with val homozygotes is in keeping with the directionality of the behavioral differences between genetic subgroups observed here and in our previous behavioral study. The latter demonstrated that response accuracy on a similar one-touch version of the TOL was impaired with an increasing number of met alleles in a cohort of 288 patients (Foltynie et al., 2004b). The behavioral differences reported here are more subtle, with a clear disparity in terms of response times (p = 0.039) but only a trend toward reduced accuracy in met compared with val homozygotes, which did not reach significance. This is probably a consequence of relatively small subgroup sizes and subgroup matching in terms of global cognitive ability and IQ to minimize confounding influences. Furthermore, functional imaging might provide a more powerful method of detecting genotypic differences in brain function than behavioral testing (Goldberg and Weinberger, 2004), with important implications for future explorations of genetic influences on cognitive processing.
Our results suggest that the met allele confers an impairment of prefrontal activation during planning in the early stages of PD, and it seems likely that this underlies the observed behavioral deficit given the significant correlation between performance and BOLD response in the DLPFC, and the direction of the trend in the remaining ROIs. However, the nature of the relationship between BOLD response and behavioral performance is far from straightforward. In studies using the _n_-back working memory task in schizophrenics (Manoach et al., 1999; Callicott et al., 2000) and PD patients (Mattay et al., 2002), authors have reported an inverse correlation between prefrontal activation and performance and have interpreted their findings by suggesting that increased fMRI activation in the PFC represents cortical inefficiency of processing. A recent study using an alternative working memory task in PD patients reported findings similar to our own, however, with cognitive deficits being associated with reductions in activity in prefrontal and striatal ROIs (Lewis et al., 2003a). The reason for such discrepancies in the literature is unclear, but one possibility is that the relationship between BOLD response and performance might be task dependent. Certainly, comparisons between fMRI studies using different tasks must be made with caution.
Several previous studies have investigated the COMT val158met effect on frontal activation. Egan et al. (2001) used the _n_-back working memory task in healthy controls and schizophrenic subjects and observed reduced activation in the DLPFC and anterior cingulate cortex with an increasing number of met alleles in the context of stable behavioral performance. They suggested that this reflected increased cortical efficiency, in keeping with behavioral data revealing improved performance in the Wisconsin Card Sorting Test with an increasing met allele load (Egan et al., 2001). Similarly, an increasing number of met alleles was associated with improved performance but reduced activity in the anterior cingulate in an attentional control task (Blasi et al., 2005). Additional evidence supporting a role for this polymorphism in determining prefrontal function comes from a study in which amphetamine was used to manipulate the dopaminergic system: in val homozygotes, with presumed low baseline prefrontal dopamine levels, this monoaminergic stimulant drug enhanced prefrontal efficiency during the _n_-back task, whereas in met homozygotes, in whom baseline prefrontal dopamine levels are higher, a decrease in prefrontal efficiency was observed at high working memory load (Mattay et al., 2003). This supports the concept of an inverted U-shaped relationship between prefrontal function and dopamine level (Goldman-Rakic et al., 2000), which has been suggested by experimental work involving D1 receptor-mediated modulation of dopaminergic transmission in animals (Williams and Goldman-Rakic, 1995; Zahrt et al., 1997; Lidow et al., 2003). Our study adds to this existing literature in two respects. First, we have demonstrated for the first time that the COMT polymorphism has a significant impact on prefrontal activation in PD. Second, using a task, which appears to provide a direct measure of cortical function, our imaging data support our previous suggestion that the relationship between prefrontal function and the COMT polymorphism is reversed in early PD in contrast to healthy controls and schizophrenic patients, as predicted by the “inverted U” hypothesis (Fig. 1) (Foltynie et al., 2004b).
Based on this hypothesis, we might also expect an interaction between dopaminergic medication and genotype, such that medication has differing effects according to an individual's genetically determined position on the inverted U-shaped curve. Indeed, the impairment of performance on the TOL associated with met allele load in our previous behavioral study was greater in the subgroup on dopaminergic medication (Foltynie et al., 2004b). Here, we found no effect of medication on prefrontal function in either genetic subgroup, but the study was designed primarily to examine cross-group differences between two genetic subgroups and may have been underpowered to detect the influence of a continuous variable such as medication dose on BOLD response. One way to address this question in the future would be to perform a similar study in a larger patient group scanned both on and off levodopa. Furthermore, given that the majority of our subjects were on levodopa-based medications (n = 23), we cannot exclude the possibility that the observed genotype effect was mediated at least in part through an alteration in the metabolism of exogenous levodopa rather than an effect on intrinsic cortical dopamine levels. However, regardless of the underlying mechanism, we have demonstrated a clear genotypic effect relevant to the majority of PD patients in clinical practice who are on dopaminergic therapy.
A final consideration which limits the interpretability of the functional imaging literature to date is that there is some evidence that not only the directionality of the BOLD response, but also the COMT effect itself, may be task dependent. Thus, Nolan et al. (2004) have reported that, in healthy subjects, met alleles improve performance on tasks requiring cognitive stability (e.g., holding information within working memory) but impair performance on tasks dependent on cognitive flexibility (e.g., switching behavior). They hypothesize that these opposing effects reflect differential involvement of tonic and phasic dopamine signaling in the two types of task. However, TOL and working memory tasks appear to be closely correlated in terms of both performance (Owen et al., 1995; Robbins, 1996) and task-related prefrontal blood flow changes (Cools et al., 2002). Furthermore, fMRI studies investigating the COMT effect in similar patient groups but using different tasks have produced comparable findings (Egan et al., 2001; Blasi et al., 2005). Nonetheless, future studies should attempt to use a single task across different subject groups to confirm that the apparent reversal of the relationship between COMT genotype and prefrontal function observed in early PD is indeed a consequence of a dysfunctional dopaminergic system rather than a consequence of task demand.
In conclusion, this study demonstrates for the first time that COMT genotype directly influences cognitive phenotype in PD through altering activation in a frontoparietal executive neural network. Our findings also support existing evidence suggesting that COMT genotype has differing effects on prefrontal function according to underlying dopaminergic state, as predicted by an inverted U-shaped relationship. Thus, this work adds to our understanding of the complex influence of the COMT val158met polymorphism on cognitive performance but may also have clinical implications for PD patients in terms of optimizing their dopaminergic medication as a function of disease stage and COMT genotype to minimize cognitive dysfunction.
Footnotes
This work was supported by the Medical Research Council. C.H.W.-G. is a Patrick Berthoud Clinical Research Fellow and holds a Raymond and Beverley Sackler studentship.
This paper is dedicated to the memory of Imogen Rose Barker, who inspired us so much, and was tragically killed on February 24, 2007, aged 15 years.
References
- Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based-prospective study. J Am Geriatr Soc. 2000;48:938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin GM. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Paper presented at 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, June. 2002 [Google Scholar]
- Brodsky MA, Godbold J, Roth T, Olanow CW. Sleepiness in Parkinson's disease: a controlled study. Mov Disord. 2003;18:668–672. doi: 10.1002/mds.10429. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain. 1999;122:1973–1987. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. The role of the striatum and hippocampus in planning: a PET activation study in Parkinson's disease. Brain. 2001;124:1020–1032. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson's disease. Florham Park, NJ: MacMillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004a;127:550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Goldberg TE, Lewis SG, Blackwell AD, Kolachana BS, Weinberger DR, Robbins TW, Barker RA. Planning ability in Parkinson's disease is influenced by the COMT val158met polymorphism. Mov Disord. 2004b;19:885–891. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, III, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hurd Y, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat. 2001;22:127–137. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, Rinne JO. Increased frontal [(18)F] fluorodopa uptake in early Parkinson's disease: sex differences in the prefrontal cortex. Brain. 2001;124:1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-dopa withdrawal in Parkinson's disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology (Berl) 1992;107:394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- Lazeron RH, Rombouts SA, Machielsen WC, Scheltens P, Witter MP, Uylings HB, Barkhof F. Visualizing brain activation during planning: the tower of London test adapted for functional MR imaging. AJNR Am J Neuroradiol. 2000;21:1407–1414. [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003a;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson's disease. Neuropsychologia. 2003b;41:645–654. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, Hyde TM, Weinberger DR. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 2002;936:58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Morris RG, Downes JJ, Sahakian BJ, Evenden JL, Heald A, Robbins TW. Planning and spatial working memory in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:757–766. doi: 10.1136/jnnp.51.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Hodges JR, Summers BA, Polkey CE, Robbins TW. Dopamine-dependent frontostriatal planning deficits in early Parkinson's disease. Neuropsychology. 1995;9:126–140. [Google Scholar]
- Owen AM, Doyon J, Petrides M, Evans AC. Planning and spatial working memory: a positron emission tomography study in humans. Eur J Neurosci. 1996;8:353–364. doi: 10.1111/j.1460-9568.1996.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson's disease identified with PET. Implications for higher cortical functions. Brain. 1998;121:949–965. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease A 3D [(18)F]dopa-PET study. Brain. 1999;122:1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Schall U, Johnston P, Lagopoulos J, Juptner M, Jentzen W, Thienel R, Dittmann-Balcar A, Bender S, Ward PB. Functional brain maps of Tower of London performance: a positron emission tomography and functional magnetic resonance imaging study. Neuroimage. 2003;20:1154–1161. doi: 10.1016/S1053-8119(03)00338-0. [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15:273–283. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van Dyck R, Veltman DJ. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18:367–374. doi: 10.1016/s1053-8119(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]