Catechol-O-Methyltransferase val158met Genotype Affects Processing of Emotional Stimuli in the Amygdala and Prefrontal Cortex (original) (raw)
Abstract
Catechol-_O_-methyltransferase (COMT) degrades the catecholamine neurotransmitters dopamine, epinephrine, and norepinephrine. A functional polymorphism in the COMT gene (val158met) accounts for a fourfold variation in enzyme activity. The low-activity met158 allele has been associated with improved working memory but with higher risk for anxiety-related behaviors. Using functional magnetic resonance imaging, we assessed the effects of COMT genotype on brain activation by standardized affective visual stimuli (unpleasant, pleasant, and neutral) in 35 healthy subjects. The analysis of genotype effects was restricted to brain areas with robust activation by the task. To determine genedose effects, the number of met158 alleles (0, 1, or 2) was correlated with the blood oxygen level-dependent (BOLD) response elicited by pleasant or unpleasant stimuli compared with neutral stimuli. COMT genotype had no significant impact on brain activation by pleasant stimuli but was related to the neural response to unpleasant stimuli: reactivity to unpleasant stimuli was significantly positively correlated with the number of met158 alleles in the limbic system (left hippocampus, right amygdala, right thalamus), connected prefrontal areas (bilateral ventrolateral prefrontal cortex, right dorsolateral prefrontal cortex), and the visuospatial attention system (bilateral fusiform gyrus, left inferior parietal lobule). Genotype explained up to 38% of interindividual variance in BOLD response elicited by unpleasant stimuli. We conclude that (1) genetic variations can account for a substantial part of interindividual variance in task-related brain activation and that (2) increased limbic and prefrontal activation elicited by unpleasant stimuli in subjects with more met158 alleles might contribute to the observed lower emotional resilience against negative mood states.
Keywords: gene, catecholamine, emotion, amygdala, prefrontal, fMRI, COMT
Introduction
Catechol-_O_-methyltransferase (COMT) degrades the catecholamine neurotransmitters dopamine (DA), epinephrine (EPI), and norepinephrine (NE). The COMT gene contains a G to A missense variant (Lachman et al., 1996) that translates into a substitution of methionine for valine at codon 158 (val158met). The enzyme containing met158 is unstable at 37°C and has one-third to one-fourth of the activity of the val158 enzyme (Spielman and Weinshilboum, 1981; Lotta et al., 1995). The alleles are codominant, because heterozygous individuals have enzyme activity that is midway between homozygous individuals (Weinshilboum et al., 1999). Despite this rather strong effect of COMT genotype on neurotransmitter metabolism, effects on behavior are moderate: the val158 allele was associated with impaired working memory (Goldberg et al., 2003) and increased perseverative errors in an executive function task (Egan et al., 2001), but only 4% of variance was attributable to COMT genotype. Intermediate phenotypes such as task-dependent brain activation may more sensitively measure gene effects on the brain, and, indeed, functional magnetic resonance imaging (fMRI) revealed a greater efficiency in the prefrontal cortex (PFC) of carriers of the met158 allele (Egan et al., 2001). Egan et al. (2001) suggested that the COMT val158 allele is associated with faster prefrontal DA metabolism, lower DA concentrations, and a reduced PFC neuronal signal/noise ratio.
Given its disadvantages for PFC function, it is curious that the val158 allele is highly prevalent (∼50%) in various human populations (Palmatier et al., 1999). Recent findings indicate that the compensating advantage of the val158 allele may be an increase in emotional resilience against anxiety and dysphoric mood (Enoch et al., 2003a). Zubieta et al. (2003) demonstrated that individuals homozygous for the met158 allele of COMT displayed higher sensory and affective ratings of pain and a more negative affective response to sustained pain. The homozygous met158 genotype was associated with higher levels of dimensionally measured anxiety among women in two populations (Enoch et al., 2003b). Moreover, the met158 allele has been associated with several mental disorders characterized by negative mood states such as panic disorder (Woo et al., 2004), alcoholism (Tiihonen et al., 1999; Wang et al., 2001) as well as increased alcohol intake in social drinkers (Kauhanen et al., 2000), major depression (Ohara et al., 1998a), bipolar affective disorder (Mynett-Johnson et al., 1998; Papolos et al., 1998), and obsessive-compulsive disorder in males (Karayiorgou et al., 1999). However, Ohara et al. (1998b) found no significant association of COMT genotype and anxiety disorder, and Domschke et al. (2004) reported an association of panic disorder with the val158 allele in women.
If genotype effects on brain activation are stronger than on overt behavior, functional brain imaging should be an excellent tool to assess genotype effects on central processing of emotional stimuli (Hariri and Weinberger, 2003). Therefore, we examined the effects of the COMT val158met genotype on the brain response to standardized affective visual stimuli (Lang et al., 1988) in healthy volunteers using fMRI. We hypothesized that COMT activity determined by the val158met genotype would be correlated with brain activity elicited by emotional stimuli.
Materials and Methods
Subjects. Thirty-five right-handed healthy volunteers [9 women and 26 men; age, 40.6 ± 7.6 (mean ± SD) years] participated in the study after providing informed, written consent according to the Declaration of Helsinki. The Ethics Committee of the University of Heidelberg approved the study. All subjects were Caucasians of central European descent. Standardized clinical assessment with the Structured Clinical Interview I (First et al., 2001) was performed to exclude axes I and II psychiatric disorders according to Diagnostic and Statistical Manual of Mental Disorders, Revision IV and International Classification of Diseases of the World Health Organization, Revision 10. Pregnancy and present drug abuse were excluded with urine tests. Only subjects free of any medication were included. Additionally, levels of anxiety [Symptom Checklist 90 -Revised (SCL-90-R) (Derogatis, 1983); State-Trait-Anxiety Inventory (STAI) (Spielberger et al., 1970)] and depression [Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1997); Hamilton Rating Scale for Depression (HAMD) (Hamilton, 1986)] as well as personality [Temperament and Character Inventory (TCI) (Cloninger et al., 1994)] and education were assessed (Table 1).
Table 1.
Sociodemographic and clinical data
| | met/met | val/met | val/val | p | | | ------------------------------ | ------------ | ------------- | ------------ | ----- | | Sex (females/males) | 3/6 | 4/12 | 2/8 | 0.799 | | Age | 43.0 ± 6.0 | 37.7 ± 8.0 | 43.2 ± 7.1 | 0.106 | | Education years | 12.33 ± 4.53 | 13.31 ± 3.61 | 11.70 ± 4.19 | 0.486 | | STAI state | 31.67 ± 6.00 | 32.75 ± 6.23 | 33.60 ± 5.74 | 0.818 | | STAI trait (t value) | 43.22 ± 8.51 | 46.13 ± 10.51 | 46.80 ± 4.69 | 0.547 | | SCL-90-R anxiety (t value) | 42.00 ± 6.41 | 39.07 ± 4.23 | 40.40 ± 4.84 | 0.407 | | TCI harm avoidance (t value) | 44.22 ± 7.55 | 43.56 ± 9.61 | 44.50 ± 8.71 | 0.967 | | CES-D (t value) | 38.67 ± 7.62 | 40.69 ± 7.68 | 40.20 ± 8.44 | 0.837 | | HAMD | 0.44 ± 1.01 | 0.19 ± 0.40 | 0.10 ± 0.32 | 0.356 |
Imaging study. For emotion induction, we used affectively unpleasant, pleasant, and neutral pictures. Each category was represented by 18 pictures. Pleasant and unpleasant cues were taken from the International Affective Picture System (IAPS) (Lang et al., 1988), in which images are standardized across the dimensions of emotion, arousal, and valence (Lang, 1995). Participants were instructed to passively view the stimuli, because even simple rating tasks can alter brain activation pattern (Taylor et al., 2003).
The stimuli were presented for 750 ms using an event-related design and were arranged in an individually randomized order for each subject. To reconstruct the blood oxygen level-dependent (BOLD) event-related time course, it is necessary to sample data points at different peristimulus time points. This was achieved by a random jitter between intertrial interval and acquisition time, resulting in an equal distribution of data points after each single stimulus. The intertrial interval was randomized between three and six acquisition times (i.e., 9.9-19.8 s). During the intertrial interval, a fixation cross was presented.
Scanning was performed with a 1.5 T whole-body tomograph (Magnetom VISION; Siemens, Erlangen, Germany) equipped with a standard quadrature head coil. For fMRI, 24 slices were acquired every 3.3 s (4 mm thickness, 1 mm gap) using an EPI-Sequence [repetition time (TR), 1.8 ms; echo time (TE), 66 ms; α = 90°] with in-plane resolution of 64 × 64 pixels [field of view (FOV), 220 mm]. fMRI slices were oriented axially parallel to the anterior commissure-posterior commisure. A morphological three-dimensional T1-weighted magnetization prepared rapid gradient echo image data set (1 × 1 × 1 mm3 voxel size; FOV, 256 mm; 162 slices; TR, 11.4 ms; TE, 4.4 ms; α = 12°) covering the whole head was acquired for anatomical reference.
After the MR scan, a subset of the stimuli (8 of 18 per category) was again presented on a computer monitor for 6 s and assessed for arousal and valence according to the standardized procedure described by Lang et al. (1988).
Data analysis. Data were analyzed with Statistical Parametric Mapping (SPM2) (Welcome Department of Neurology, University College London, London, UK). The structural three-dimensional data set was coregistered to the first T2* image. The structural image was spatially normalized to a standard template using a 12-parameter affine transformation with additional nonlinear components. A nonlinear transformation was subsequently applied to the T2* data, and voxels were resampled at a resolution of 3 × 3 × 3 mm3. The functional data were smoothed using an isotropic Gaussian kernel for group analysis (12 mm full-width at half-maximum).
Statistical analysis was performed by modeling the different conditions (pleasant, unpleasant, and neutral pictures; δ functions convolved with a synthetic hemodynamic response function and its time derivative) as explanatory variables within the context of the general linear model on a voxel-by-voxel basis with SPM2. To detect association between COMT genotype and fMRI activation on a voxel-by-voxel basis, the contrast images of all subjects (signal change of pleasant vs neutral and unpleasant vs neutral pictures) were included in a second level regression analysis with SPM2. To model the assumed genedose effect, COMT genotype was coded as a covariate by the number of met158 alleles (0, 1, or 2). To control for potential confounding of genotype effects by gender, we performed an additional SPM analysis using a multiple regression with genotype and sex as independent variables.
The analysis of the genotype × task interaction was restricted to areas showing robust activation related to the task (emotional vs neutral stimuli) as defined by an F-contrast of p < 0.01 and a cluster size of at least 10 voxels.
A statistical threshold of p < 0.05 corrected for the volume of the entire mask was used for the regression analyses. Correction for multiple testing was performed with the False Discovery Rate (FDR) procedure. The FDR is the proportion of suprathreshold voxels that are false positives. In comparison, a traditional multiple comparisons procedure (e.g., Bonferroni or random field correction) controls Family-Wise Error Rate (FWER) at or below α. FWER is the chance of one or more false positives anywhere (not just among suprathreshold voxels). If there is truly no signal in the image anywhere, then an FDR procedure controls FWER, just as Bonferroni and random field methods do. If there is some signal in the image, an FDR method will be more powerful than the traditional methods (Genovese et al., 2002).
Genotyping. The genotype information and flanking sequences of the COMT polymorphism were collected using public single-nucleotide polymorphism (SNP) databases and analyzed using the following procedure: DNA was isolated from venous blood with the QIAamp DNA Mini kit (Qiagen, Hilden, Germany). PCR was performed with HotStarTaq-DNA Polymerase (Qiagen), 4 ng of template DNA in a total volume of 25 μl of PCR, and the primers COMTfor (5′-CACCTGTGCTCACCTCTCCT-3′) and COMTrev (5′-GGGTTTTCAGTGAACGTGGT-3′). PCR conditions were as follows: 95°C for 15 min initial heating, then 35 cycles of 94°C for 60 s, 58°C for 60 s, 72°C for 60 s, and final extension for 10 min at 72°C. After purification of the amplified products (QIAquick PCR Purification kit; Qiagen), the oligonucleotide primer 5′-CGGATGGTGGATTTCGCTGGC-3′ was extended according to the manufacturer's instructions (AcycloPrime-FP SNP detection kit; Perkin-Elmer Life Sciences, Emeryville, CA), and fluorescence polarization was measured using a Wallac Victor2 1420 Multilabel Counter (Perkin-Elmer). We assessed the genotype of the promoter of the serotonin transporter gene (5-HTT) with PCR using oligonucleotide primers (stpr5, 5′-GGCGTTGCCGCTCTGAATGC; int1, 5′-CAGGGGAGATCCTGGGAGGA). PCR amplification was performed in a final volume of 30 μl consisting of 50 ng of genomic DNA, 2.5 mm deoxyribonucleotides (dGTP/7-deaza-2′-dGTP; 1:1), 0.1 μg of sense and antisense primers, 10 mm tris-HCl, pH 8.3, 50 mm KCl, 1.5 mm MgCl2, and 1 U of _Taq_DNA polymerase. Annealing was performed at 61°C for 30 s, extension was performed at 72°C for 1 min, and denaturation was performed at 95°C for 30 s for 35 cycles. The resulting PCR product was electrophoretically separated on a 1.5% agarose gel.
Results
Genotyping
Of the 35 healthy subjects, 9 (3 females and 6 males) were homozygous for the met158 allele and 10 (2 females and 8 males) were homozygous for the val158 allele of the COMT gene; 16 subjects (4 females and 12 males) were heterozygous. Genotype and sex were not associated (χ2 = 0.499; df = 2; p = 0.799). We also assessed the promoter region of the serotonin transporter gene (5-HTTLPR), which has recently been associated with amygdala activation elicited by angry or fearful faces (Hariri et al., 2002a). No association between the two genotypes analyzed in this study was found (χ2 = 2.3; df = 4; p = 0.68) (for details, see supplemental Table S1, available at www.jneurosci.org as supplemental material).
Behavioral data
Data concerning anxiety, depression, and education are shown in Table 1. All scores of the rating scales were in the normal range and not significantly associated with COMT genotype. Subjective ratings of valence and arousal induced by the visual stimuli (unpleasant, neutral, and pleasant) showed that subjects experienced the positive pictures as arousing and affectively pleasant, whereas they rated the negative pictures as equally arousing (p = 0.19) but unpleasant (Table 2). Valence and arousal were not significantly correlated with COMT genotype (p > 0.18).
Table 2.
Ratings of valence and arousal of the visual stimuli
| | Valence (mean ± SD) | Arousal (mean ± SD) | | | | | | | ---------------------- | ------------------- | ----------- | ----------- | ----------- | ----------- | ----------- | | | Unpleasant | Neutral | Pleasant | Unpleasant | Neutral | Pleasant | | | All genotypes | 1.78 ± 0.79 | 6.02 ± 1.08 | 7.67 ± 0.76 | 5.35 ± 2.48 | 2.31 ± 1.52 | 4.65 ± 1.43 | | met/met | 1.61 ± 0.68 | 6.20 ± 0.79 | 7.54 ± 0.59 | 6.50 ± 2.47 | 2.22 ± 1.45 | 4.31 ± 1.31 | | val/met | 1.80 ± 0.93 | 6.01 ± 1.26 | 7.68 ± 0.90 | 4.46 ± 2.47 | 2.02 ± 1.43 | 4.51 ± 1.45 | | val/val | 1.98 ± 0.62 | 5.80 ± 1.09 | 7.83 ± 0.64 | 6.03 ± 1.98 | 3.18 ± 1.84 | 5.50 ± 1.50 |
Main effect of the task regardless of genotype
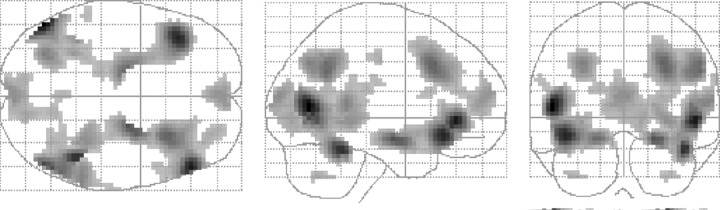
Significant differences in brain activity elicited by emotional stimuli (pleasant and unpleasant) compared with neutral stimuli were found in a total of 4247 voxels (3 × 3 × 3 mm). The main effect of emotional stimuli comprised a distributed neuronal network of secondary and tertiary visual regions in the occipital and parietal lobe, bilateral amygdala, hippocampus and parahippocampus, dorsal cingulate, and regions in the left and right medial, ventrolateral, and dorsolateral PFC (compare Fig. 1). The following analyses of the genotype × task interactions were restricted to these brain regions [volume of interest (VOI)].
Figure 1.
Brain areas activated by pleasant and unpleasant emotional stimuli compared with neutral stimuli (p < 0.01; uncorrected; cluster size ≥10 voxels) and used as VOI in the task × genotype analyses.
COMT val158met genotype and processing of pleasant visual stimuli
No significant positive (p > 0.2; corrected) or negative (p = 1; corrected) correlations between COMT genotype and brain activity elicited by pleasant stimuli were found inside the predefined VOI. Also, when gender was controlled for, no significant association between COMT genotype and brain activity was found.
COMT val158met genotype and processing of unpleasant visual stimuli
For unpleasant visual stimuli, regression analyses revealed a significant (p < 0.05 corrected for entire VOI) positive correlation between the number of met_158_ alleles and the BOLD fMRI response in limbic and prefrontal brain areas as well as in brain areas related to visuospatial attention (Table 3, Fig. 2). Brain activation in 573 of 4247 voxels comprised by the VOI (13%) was significantly affected by genotype. The highest correlations were found in the ventrolateral PFC [Brodmann area (BA) 47]: 38% of the interindividual variance of the BOLD response in the left ventrolateral PFC and 31% in the right ventrolateral PFC were explained by genotype. Besides being strong, the effect of genotype in the ventrolateral PFC was rather widespread. The clusters comprised 258/141 (left/right) voxels. Moreover, a significant correlation was found in the right dorsolateral PFC. The number of met_158_ alleles was also positively correlated with brain activation elicited by unpleasant cues in the left hippocampus, the right amygdala, and in the right thalamus. In these areas, 25-33% of interindividual variance was explained by genotype. Above all, brain activation elicited by unpleasant stimuli in the left and right fusiform gyrus and the inferior parietal lobule, which are secondary and tertiary visual areas, was also positively correlated with the dosage of met_158_ alleles. When gender was controlled for, the relationship of COMT genotype and the processing of unpleasant stimuli remained statistically significant in all brain regions described above (for details, see supplemental Table S2, available at www.jneurosci.org as supplemental material), indicating that the observed effects of COMT on brain activation are not considerably modulated by gender.
Table 3.
Effect of COMT val 158 met genotype on the activation of prefrontal, limbic, and sensory brain areas by unpleasant stimuli
| | | | | MNI coordinates | | | | | | | | | ------------------------ | ----------------- | ---- | --------------- | ---- | ----- | ----- | ----- | ------ | ---- | ---- | | System | Region | Side | BA | x | y | z | t | _p_FDR | r | _R_2 | | Prefrontal | Ventrolateral PFC | L | 47 | -42 | 33 | -9 | 4.49 | 0.021 | 0.62 | 38% | | R | 47 | 51 | 33 | -3 | 3.84 | 0.021 | 0.56 | 31% | | | | Middle frontal gyrus | L | n.s. | | | | | | | | | | R | 6/8 | 27 | 9 | 57 | 3.32 | 0.026 | 0.50 | 25% | | | | Limbic | Hippocampus | L | -24 | -39 | -3 | 4.03 | 0.021 | 0.57 | 33% | | | R | n.s. | | | | | | | | | | | Amygdala | L | n.s. | | | | | | | | | | R | 21 | -9 | -9 | 3.29 | 0.027 | 0.50 | 25% | | | | | Thalamus | L | n.s. | | | | | | | | | | R | 21 | -33 | 3 | 3.75 | 0.021 | 0.55 | 30% | | | | | Sensory | Fusiform gyrus | L | 37 | -36 | -42 | -21 | 3.50 | 0.023 | 0.52 | 27% | | R | 37 | 42 | -42 | -18 | 3.57 | 0.021 | 0.53 | 28% | | | | Inferior parietal lobule | L | 40 | -60 | -39 | 30 | 3.08 | 0.032 | 0.47 | 22% | | | | | R | | | | | | n.s. | | | |
Figure 2.
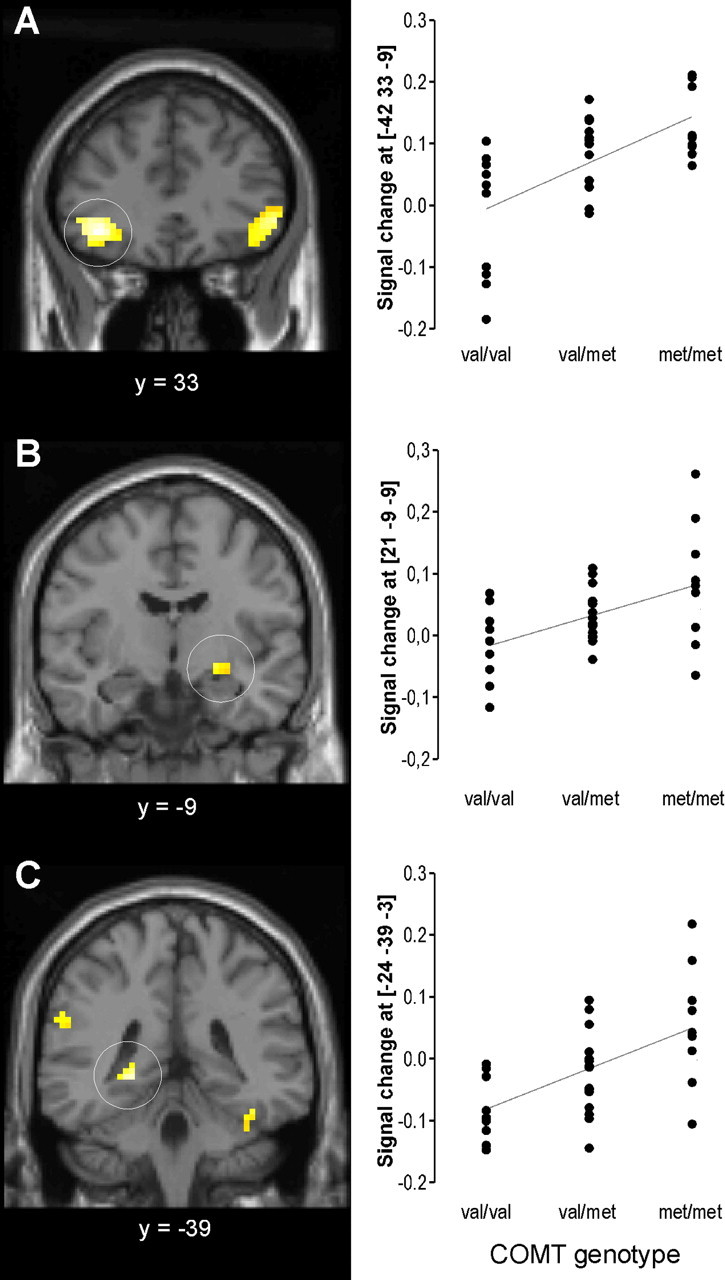
Correlation between COMT met158 allele dosage (0 = val/val, 1 = val/met, or 2 = met/met) and activation by unpleasant stimuli of the ventrolateral PFC (A), right amygdala (B), and left dorsal hippocampus (C). Left, Statistical maps are shown (p < 0.05 corrected for volume of mask; cluster size ≥10 voxels). Right, Neural responses of individuals are displayed related to COMT genotype. Individual BOLD responses were extracted from the voxel with the highest t value inside the cluster encircled at the left.
Notably, in none of the investigated brain areas, a significant negative correlation between the number of met_158_ alleles and BOLD response was found. This was also true when gender was controlled for (p = 1; corrected).
Discussion
Val158met, a functional polymorphism in the COMT gene, interacted substantially with the neural processing of unpleasant stimuli in the limbic system, connected PFC, and visuospatial attention system: up to 38% of the interindividual variance of the fMRI BOLD response to unpleasant emotional stimuli was explained by a gene-dose effect of the COMT val158met genotype. The magnitude of the BOLD response to unpleasant stimuli always increased with the number of met158 alleles; in none of these brain areas, a negative correlation was detected.
The COMT variant containing met158 has 25-70% of the activity of the val158 enzyme (Spielman and Weinshilboum, 1981; Chen et al., 2004). COMT is expressed in all regions of the human brain, including the limbic and prefrontal areas (Hong et al., 1998). Therefore, the positive correlation between the number of met158 alleles and the BOLD response suggests that the amplified activation of limbic and prefrontal areas elicited by unpleasant stimuli may be related to increased levels of DA and/or NE in these brain regions.
The impact of COMT genotype on the processing of unpleasant stimuli was most pronounced in the ventrolateral PFC. Studies in COMT-deficient mice and studies in rats with the COMT inhibitor tolcapone showed increased DA but unaltered NE levels in the PFC (Gogos et al., 1998; Tunbridge et al., 2004). Therefore, the modulating effect of COMT genotype on PFC function might primarily be attributable to differences in DA levels. Activation of the ventrolateral PFC has been reported during induction of sadness (Levesque et al., 2003a,b) and recall of sad autobiographical memories (Markowitsch et al., 2003; Pelletier et al., 2003). Increased activation in the ventrolateral PFC was also found in patients suffering from major depression (Drevets, 2000; Brody et al., 2001). In patients with obsessive-compulsive disorder, response to treatment was associated with metabolic decreases in the ventrolateral PFC (Saxena et al., 2002), and provocation of obsessive and compulsive symptoms in healthy controls activated the ventrolateral PFC (Mataix-Cols et al., 2003). These findings indicate that the ventrolateral PFC participates in the processing of negative emotions. This prefrontal region receives extensive sensory inputs as well as limbic afferences from the amygdala. Therefore, the observed activation of the ventrolateral PFC may be associated with the integration of sensory inputs and information regarding changes in the subjects' emotional state.
In this study, activation of the right amygdala by unpleasant stimuli also increased with the number of met158 alleles and may indicate identification of stimuli with emotional significance (Phan et al., 2002; Phillips et al., 2003). In rats, DA potentiates the response of the amygdala by attenuating the effect of inhibitory input from the PFC and augmenting the effect of excitatory input from sensory cortices (Rosenkranz and Grace, 1999). Consistent with this, two fMRI studies in humans showed that DA modulates the responsivity of the amygdala to emotional stimuli (Hariri et al., 2002b; Tessitore et al., 2002). The potentiated amygdala response we report may thus reflect DA gating of amygdala inputs. Moreover, COMT genotype interacted with activation in the right thalamus and left dorsal hippocampus elicited by unpleasant stimuli. The thalamus is closely linked to limbic areas and is involved in controlling attentional processes. The level of its activation is modified by NE (Coull, 1998). The hippocampus has been implicated in the inhibition of stress responses (Lopez et al., 1999) and is crucial in the regulation of arousal and affective states (McNaughton and Gray, 2000). COMT genotype also seems to modulate thalamic endorphin release and associated pain perception (Zubieta et al., 2003). Together with our findings, these data indicate that in carriers of the met158 allele, limbic networks may be more sensitive to unpleasant stimuli and signaling of emotional salience might be enhanced.
Activation in the fusiform gyrus and parietal lobe elicited by unpleasant stimuli was also linked to a higher dosage of the met158 allele. Activation of these secondary and tertiary visual association areas may reflect increased attentional processing of emotionally salient visual stimuli and has been observed in a series of brain imaging studies using the IAPS (Lang et al., 1998). Positive feedback from the amygdala to the fusiform gyrus might contribute to the observed effect in this brain area (Morris et al., 1998; Vuilleumier et al., 2002).
Interestingly, in our study, the COMT val158met genotype was only associated with processing of unpleasant but not affectively pleasant stimuli. Because stimuli of both categories were rated as equally arousing, this specific effect cannot be explained on the basis of arousal differences. Another explanation might be that only stressful but not pleasant events activate the locus ceruleus, which results in increased NE release in its projection sites, including the amygdala, hippocampus, and the PFC (Tanaka et al., 2000; Charney, 2004). Therefore, in our study, presentation of aversive stimuli may have induced NE efflux in limbic structures and the connected PFC, and the resulting extracellular NE concentrations may then be affected by differences in NE metabolism attributable to COMT genotype.
Because COMT val158met genotype interacted with brain activity elicited by unpleasant but not pleasant stimuli, it seems to be primarily relevant for the processing and regulation of negative affective states. It can be speculated that in carriers of the met158 allele, heightened signaling of emotional salience and increased allocation of attentional resources leads to an increased demand on prefrontal capacities for integration and regulation of emotional states. Interestingly, ratings of emotional valence and arousal did not differ between genotypes. Thus, genetically determined differences in emotional processing detected with fMRI may be compensated at the behavioral level. Viewing unpleasant, aversive, or disgusting pictures inside the scanner may only be a minor challenge for emotional brain circuits, and highly demanding situations in real life should have a much stronger impact. In subjects with one met158 allele, and progressively more in carriers of two copies, the limits for integration and regulation of emotional states might be reached earlier, resulting in a reduced resilience against negative mood states (Enoch et al., 2003b; Zubieta et al., 2003).
In the study by Egan et al. (2001), the met158 allele was associated with a more focused dorsolateral prefrontal response during performance of a test of executive function. In our study, the met158 allele was associated with increased activation of the ventrolateral PFC during passive viewing of unpleasant stimuli, indicating that this allele is not generally associated with a low BOLD response in the PFC and that the associated changes in DA or NE concentrations may have differential effects on the performance of executive functions and the passive perception of affective stimuli in different parts of the PFC.
Hariri et al. (2002a) found that carriers of the short allele of the serotonin transporter gene (5-HTTLPR) showed a significantly enhanced amygdala response to angry and fearful faces, which probably reflects hyperresponsiveness of their amygdala to relevant environmental stimuli. In carriers of the short allele, slightly increased anxiety-related personality traits have been found (Lesch et al., 1996), and stressful life events more often lead to mood disorder and suicidality (Caspi et al., 2003). The genetically driven increase of amygdala reactivity may thus contribute to higher vulnerability to stressful life events. fMRI paradigms like the ones used by Hariri et al. (2002a) and in our study may be useful tools to define intermediate phenotypes that are related to reduced resilience against negative mood states and therefore to vulnerability for anxiety or mood disorders.
Studies investigating associations of a genetic polymorphism with an (intermediate) phenotype are critically dependent on the assumption that the genotype groups are not different in factors other than genotype. To exclude confounding attributable to the effect of an occult substructure, we tested for differences in ethnicity, age, gender, education, anxiety or depression scores, 5-HTTLPR genotype, as well as arousal and valence ratings of the affective stimuli in the COMT genotype groups. None of these potentially confounding variables was associated with COMT genotype. Moreover, in our study, stratification attributable to population differences in clinical ascertainment is extremely unlikely because genotype is correlating with a difference in brain function, not with diagnosis.
The substantial influence of genetic polymorphisms on emotional as well as cognitive processing found by us and others indicates that effects of genetic variants on individual brain function might obscure other differences between subjects or groups of subjects. Therefore, controlling for genetic heterogeneity may reduce noise in data and facilitate detection of differences related to other variables of interest.
In summary, our data show that the processing of unpleasant visual stimuli increased with met158 allele dosage. These COMT-driven differences in emotional processing are possibly attributable to altered DA and NE levels. The increased activation in limbic circuits elicited by unpleasant stimuli was accompanied by increased activity in prefrontal regions that are involved in the regulation of emotions. When confronted with unpleasant cues, the load limit of systems concerned with emotional and cognitive behavior control may be reached earlier in met158 allele carriers, contributing to their lowered resilience against negative mood states.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (He 2597/4-2) and the German Ministry of Education and Research (01GS0117).
Correspondence should be addressed to Dr. Andreas Heinz, Department of Psychiatry and Psychotherapy, Charité Campus Mitte, Charité-University Medicine Berlin, Schumannstrasse 20-21, 10117 Berlin, Germany. E-mail: andreas.heinz@charite.de.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250836-07$15.00/0
References
- Brody AL, Barsom MW, Bota RG, Saxena S (2001) Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry 6: 102-112. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386-389. [DOI] [PubMed] [Google Scholar]
- Charney DS (2004) Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 161: 195-216. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR (2004) Functional analysis of genetic variation in catechol-_O_-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75: 807-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD (1994) The temperament and character inventory (TCI): a guide to its development and use. St. Louis: Center for Psychobiology of Personality, Washington University.
- Coull JT (1998) Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55: 343-361. [DOI] [PubMed] [Google Scholar]
- Derogatis LR (1983) Symptom checklist 90 -revised/SCL-90-R: administration, scoring and procedures manual II. Baltimore: Clinical Psychometric Research.
- Domschke K, Freitag CM, Kuhlenbaumer G, Schirmacher A, Sand P, Nyhuis P, Jacob C, Fritze J, Franke P, Rietschel M, Garritsen HS, Fimmers R, Nothen MM, Lesch KP, Stogbauer F, Deckert J (2004) Association of the functional V158M catechol-_O_-methyl-transferase polymorphism with panic disorder in women. Int J Neuropsychopharmacol 7: 183-188. [DOI] [PubMed] [Google Scholar]
- Drevets WC (2000) Neuroimaging studies of mood disorders. Biol Psychiatry 48: 813-829. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Schuckit MA, Johnson BA, Goldman D (2003a) Genetics of alcoholism using intermediate phenotypes. Alcohol Clin Exp Res 27: 169-176. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D (2003b) Genetic origins of anxiety in women: a role for a functional catechol-_O_-methyltransferase polymorphism. Psychiatry Genet 13: 33-41. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2001) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute.
- Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870-878. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M (1998) Catechol-_O_-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95: 9991-9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR (2003) Executive subprocesses in working memory: relationship to catechol-_O_-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60: 889-896. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1986) The Hamilton Rating Scale for Depression. In: Assessment of depression (Sartorius N, Ban TA, eds), pp 143-152. Berlin: Springer.
- Hariri AR, Weinberger DR (2003) Imaging genomics. Br Med Bull 65: 259-270. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR (2002a) Serotonin transporter genetic variation and the response of the human amygdala. Science 297: 400-403. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR (2002b) Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology 27: 1036-1040. [DOI] [PubMed] [Google Scholar]
- Hong J, Shu-Leong H, Tao X, Lap-Ping Y (1998) Distribution of catechol-_O_-methyltransferase expression in human central nervous system. NeuroReport 9: 2861-2864. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, Ott J, Gogos JA (1999) Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol Psychiatry 45: 1178-1189. [DOI] [PubMed] [Google Scholar]
- Kauhanen J, Hallikainen T, Tuomainen TP, Koulu M, Karvonen MK, Salonen JT, Tiihonen J (2000) Association between the functional polymorphism of catechol-_O_-methyltransferase gene and alcohol consumption among social drinkers. Alcohol Clin Exp Res 24: 135-139. [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996) Human catechol-_O_-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6: 243-250. [DOI] [PubMed] [Google Scholar]
- Lang PJ (1995) The emotion probe. Studies of motivation and attention. Am Psychol 50: 372-385. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Öhman A, Vaitl D (1988) The International Affective Picture System (slides). Gainesville, FL: Center for Research in Psychophysiology, University of Florida.
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V (1998) Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology 35: 199-210. [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527-1531. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaun G, Leroux JM, Bourgouin P, Beauregard M (2003a) Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 53: 502-510. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaun G, Leroux JM, Bourgouin P, Beauregard M (2003b) Neural correlates of sad feelings in healthy girls. Neuroscience 121: 545-551. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Akil H, Watson SJ (1999) Neural circuits mediating stress. Biol Psychiatry 46: 1461-1471. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J (1995) Kinetics of human soluble and membrane-bound catechol _O_-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34: 4202-4210. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhovel MM, Lanfermann H, Russ MO (2003) Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex 39: 643-665. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, Brammer MJ, Williams SC, Speckens A, Phillips ML (2003) Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry 53: 482-493. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA (2000) Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord 61: 161-176. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ (1998) A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121: 47-57. [DOI] [PubMed] [Google Scholar]
- Mynett-Johnson LA, Murphy VE, Claffey E, Shields DC, McKeon P (1998) Preliminary evidence of an association between bipolar disorder in females and the catechol-_O_-methyltransferase gene. Psychiatry Genet 8: 221-225. [DOI] [PubMed] [Google Scholar]
- Ohara K, Nagai M, Suzuki Y, Ohara K (1998a) Low activity allele of catechol-_O_-methyltransferase gene and Japanese unipolar depression. NeuroReport 9: 1305-1308. [DOI] [PubMed] [Google Scholar]
- Ohara K, Nagai M, Suzuki Y, Ochiai M, Ohara K (1998b) No association between anxiety disorders and catechol-_O_-methyltransferase polymorphism. Psychiatry Res 80: 145-148. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK (1999) Global variation in the frequencies of functionally different catechol-_O_-methyltransferase alleles. Biol Psychiatry 46: 557-567. [DOI] [PubMed] [Google Scholar]
- Papolos DF, Veit S, Faedda GL, Saito T, Lachman HM (1998) Ultra-ultra rapid cycling bipolar disorder is associated with the low activity catecholamine-_O_-methyltransferase allele. Mol Psychiatry 3: 346-349. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Bouthillier A, Levesque J, Carrier S, Breault C, Paquette V, Mensour B, Leroux JM, Beaun G, Bourgouin P, Beauregard M (2003) Separate neural circuits for primary emotions? Brain activity during self-induced sadness and happiness in professional actors. NeuroReport 14: 1111-1116. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 16: 331-348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R (2003) Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 54: 504-514. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1997) The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1: 385-401. [Google Scholar]
- Rosenkranz JA, Grace AA (1999) Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation _in vivo_J Neurosci 19: 11027-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N, Ho MK, Huang SC, Wu HM, Baxter Jr LR (2002) Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry 59: 250-261. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE (1970) Manual for the State-Trait-Anxiety Inventory. Palo Alto, CA: Consulting Psychologists.
- Spielman RS, Weinshilboum RM (1981) Genetics of red cell COMT activity: analysis of thermal stability and family data. Am J Med Genet 10: 279-290. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H (2000) Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 405: 397-406. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I (2003) Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage 18: 650-659. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, Hyde TM, Weinberger DR, Mattay VS (2002) Dopamine modulates the response of the human amygdala: a study in Parkinson's disease. J Neurosci 22: 9099-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, Salonen JT, Ryynanen OP, Koulu M, Karvonen MK, Pohjalainen T, Syvalahti E, Hietala J (1999) Association between the functional variant of the catechol-_O_-methyltransferase (COMT) gene and type 1 alcoholism. Mol Psychiatry 4: 286-289. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ (2004) Catechol-_O_-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24: 5331-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ (2002) Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia 40: 2156-2166. [DOI] [PubMed] [Google Scholar]
- Wang T, Franke P, Neidt H, Cichon S, Knapp M, Lichtermann D, Maier W, Propping P, Nothen MM (2001) Association study of the low-activity allele of catechol-_O_-methyltransferase and alcoholism using a family-based approach. Mol Psychiatry 6: 109-111. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL (1999) Methylation pharmacogenetics: catechol _O_-methyltransferase, thiopurine methyltransferase, and histamine _N_-methyltransferase. Annu Rev Pharmacol Toxicol 39: 19-52. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Choi YH, Oh KS, Lee YS, Yu BH (2004) The association between panic disorder and the L/L genotype of catechol-_O_-methyltransferase. J Psychiatry Res 38: 365-370. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D (2003) COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 299: 1240-1243. [DOI] [PubMed] [Google Scholar]