Cell Type- and Input-Specific Differences in the Number and Subtypes of Synaptic GABAA Receptors in the Hippocampus (original) (raw)
Abstract
Networks of parvalbumin (PV)-expressing basket cells are implicated in synchronizing cortical neurons at various frequencies, through GABAA receptor-mediated synaptic action. These cells are interconnected by GABAergic synapses and gap junctions, and converge with a different class of cholecystokinin-expressing, PV-negative basket cells onto pyramidal cells. To define the molecular specializations in the synapses of the two basket cell populations, we used quantitative electron microscopic immunogold localization of GABAA receptors. Synapses formed by PV-positive basket cells on the somata of pyramidal cells had several-fold higher density of α1 subunit-containing receptors than synapses made by PV-negative basket cells, most of which were immunonegative. The density of the β2/3 subunits was similar in the two populations of synapse, indicating similar overall receptor density. Synapses interconnecting parvalbumin-expressing basket cells contained a 3.6 times higher overall density of GABAA receptor (β2/3 subunits) and 3.2 times higher density of α1 subunit labeling compared with synapses formed by boutons of PV-positive basket cells on pyramidal cells. Thus, PV-positive basket cells mainly act through α1subunit-containing GABAA receptors, but the receptor density depends on the postsynaptic cell type. These observations, together with previously reported enrichment of the α2subunit-containing receptors in synapses made by PV-negative basket cells, indicate that the number and subtypes of GABAAreceptors present in different synapse populations are regulated by both presynaptic and postsynaptic influences. The high number of GABAA receptors in synapses on basket cells might contribute to the precisely timed phasing of basket cell activity.
Keywords: basket cell, pyramidal cell, IPSP, interneuron, inhibition, receptor targeting
The heterooligomeric GABAA receptors are anion channels opened by GABA and modulated by a variety of pharmacologically and clinically important drugs. (Macdonald and Olsen, 1994; Sieghart, 1995). They are composed of five subunits, and, so far, 19 different subunits have been identified in the mammalian brain (Barnard et al., 1998; Sieghart et al., 1999). Most receptors consist of two α, two β, and one γ subunit, but the subunit composition is highly variable, suggesting the existence of receptors with different functional and pharmacological properties (McKernan and Whiting, 1996; Rudolph et al., 2001).
Hippocampal pyramidal cells receive GABAergic innervation from several distinct interneurons (Freund and Buzsaki, 1996). For example, axo-axonic cells innervate only axon initial segments, basket cells innervate mainly somata and the proximal dendrites, and other interneurons innervate only dendrites. The same postsynaptic domain of pyramidal cells may be targeted by more than one class of interneuron. Thus, pyramidal cell somata are innervated by two distinct basket cells expressing either parvalbumin (PV) or cholecystokinin (CCK). These distinct basket cells differ in their soma position, local and subcortical innervation, and in the presynaptic control of transmitter release (Hajos et al., 1998; Katona et al., 1999), predicting distinct roles in the hippocampal network.
The multiple sources of GABA, released by distinct interneurons, and the large variety of distinct GABAA receptors raise the possibility that the segregation of inputs is supported by molecular specializations in postsynaptic receptors. The α1, β2/3, and γ2 subunits have been demonstrated in many GABAergic synapses on pyramidal cells (Nusser et al., 1996; Somogyi et al., 1996). However, the α2 subunit was found more frequently in synapses on axon-initial segments than on somata (Nusser et al., 1996), and, on the latter, α2subunit immunoreactivity was present at much higher levels in synapses formed by PV-negative (presumably CCK-positive) basket cells than in synapses formed by PV-positive cells (Nyiri et al., 2001). The input-specific enrichment of α2subunit-containing receptors raises the question whether the synapses formed by PV-positive basket cells contain receptors formed by other subunits expressed by pyramidal cells, such as the α1 subunit (Wisden et al., 1992; Fritschy and Mohler, 1995). This may be important, because the α1 and α2subunit-containing receptors are responsible for different behavioral and pharmacological responsiveness in mice (McKernan et al., 2000;Rudolph et al., 2001).
In the present study, we tested the relative abundance of α1 subunit-containing GABAA receptors in synapses made by PV-positive or PV-negative boutons on pyramidal cell somata. The subunit composition of synaptic receptors may be influenced by both the source of the input and the identity of the target cell, but, for GABAergic connections, this has not been tested. Because basket cells innervate both pyramidal cells and other basket cells (Fukuda et al., 1996; Cobb et al., 1997), the subunit composition and abundance of receptors at the synapses made by basket cells on other PV-positive basket cells was compared with those made on pyramidal cells.
MATERIALS AND METHODS
Preparation of animals and tissues. Three adult male Wistar rats (∼150 gm) obtained from Charles River (Kent, UK) were anesthetized with Sagatal (pentobarbitone sodium, 220 mg/kg, i.p.) and perfused through the heart with 0.9% NaCl, followed by a fixative containing 4% paraformaldehyde, 0.05% glutaraldehyde, and 0.2% picric acid in 0.1 m phosphate buffer, pH 7.4 (PB), for 20–25 min. After perfusion, the brains were left in situ for 10–15 min, and then they were removed from the skull. Blocks from the dorsal hippocampi were dissected and washed in 0.1m PB, followed by sectioning on a vibratome at 500 μm thickness. They were post-fixed for 15–20 min and washed in 0.1 m PB overnight.
Freeze substitution and low-temperature embedding in Lowicryl resin. The same procedure was used as described previously (Baude et al., 1993; Nusser et al., 1995; Nyiri et al., 2001). Briefly, after washing in PB overnight, the sections were placed into increasing concentration of sucrose solutions (0.5, 1, and 2m sucrose for 0.5, 1, and 2 hr, respectively) for cryoprotection. After slamming onto copper blocks cooled in liquid N2, and after low-temperature dehydration and freeze substitution, the sections were embedded in Lowicryl HM 20 resin (Chemische Werke Lowi, Waldkraiburg, Germany).
Antibodies. Rabbit polyclonal antibody (code number P16) was raised to a synthetic peptide corresponding to amino acids 1–9 of the mature rat α1 subunit and was affinity purified. Antibody specificity was described previously (Zezula et al., 1991). This antibody has been used in other postembedding immunocytochemical studies (Nusser et al., 1996, 1997, 1998a,b). Immunoreactions were performed at a final protein concentration of 32 μg/ml. The mouse monoclonal antibody bd-17 (Haring et al., 1985) was kindly provided by Dr. J.-M. Fritschy (Institute of Pharmacology, Zurich, Switzerland) and has been shown to react with both the β2 and β3 subunits of GABAA receptors (Ewert et al., 1990). The antibody was diluted to 20 μg/ml protein. A rabbit polyclonal antiserum (code R302; 1:500 dilution) was raised to rat muscle PV (Calbiochem, Nottingham, UK) and was a gift from Dr. K. G. Baimbridge (University of British Columbia, Vancouver, Canada). It has been described to be specific to PV (Mithani et al., 1987) and was used previously for postembedding immunoreaction (Nyiri et al., 2001).
Postembedding immunocytochemistry. Postembedding immunocytochemistry was performed on ∼70-nm-thick serial sections of slam-frozen, freeze-substituted, Lowicryl-embedded hippocampi. The sections were picked up on pioloform-coated nickel grids. Then they were incubated on drops of blocking solution for 1 hr, followed by incubation on drops of primary antibodies overnight. The blocking solution, which was also used for diluting the primary and secondary antibodies, consisted of 0.05 m Tris-HCl, pH 7.4, containing 0.9% NaCl (TBS) and 2% human serum albumin (Sigma, Poole, UK). After incubation overnight in primary antibodies, sections were washed in TBS and incubated for 4 hr on drops of goat anti-rabbit or goat anti-mouse IgG coupled to 10 or 5 nm (British BioCell International, Cardiff, UK) or ultra small gold particles (<0.8 nm; Aurion, Wageningen, Netherlands) to increase sensitivity. After several washes, sections were fixed in a 2% glutaraldehyde solution for 2 min. Sections labeled with ultra small gold particles were subjected to silver enhancement (Aurion) for 30 min. After washing in ultra pure water, all sections were contrasted with saturated aqueous uranyl acetate, followed by lead citrate.
Because antibodies to the α1 subunit and to PV were both raised in rabbit, no double labeling could be performed on the same section. To identify PV-positive and PV-negative boutons and to differentiate between pyramidal cells and PV-positive interneurons, one to three sections were labeled with rabbit anti-PV serum and were detected with 10 nm gold particle-conjugated secondary antibodies. Other serial sections placed on different grids, both before and after the PV-reacted section(s), were incubated with rabbit anti-α1 subunit antibodies and labeled with ultra small gold particle-conjugated secondary antibodies, followed by silver intensification. For detection of the β2/3 subunits, serial sections were incubated with rabbit anti-PV and mouse anti-β2/3antibodies mixed together. Antibodies to PV were labeled with 5 nm gold particle-conjugated secondary antibodies, and antibodies to β2/3 subunits were colabeled with 10 nm gold particle-conjugated secondary antibodies.
Measurement of immunoreactivity. Overall, 104 synapses in 247 sections for the α1 subunit labeling and 71 synapses in 169 sections for the β2/3 subunit labeling were recorded and analyzed. In each section, several synapses were monitored. Measurements were taken from well preserved strips of Lowicryl-embedded ultrathin sections. One block was used from each of the three rats. Because PV-positive interneurons were relatively infrequent, blocks were sectioned repeatedly and systematically searched until sections with a PV-positive interneuron receiving type I synapses on the soma were found. All type II synapses (Gray, 1959) encountered on the somata of pyramidal cell and PV-positive interneurons were recorded in the hippocampal CA1 region. On interneurons, type II synapses could not always be unequivocally distinguished from type I synapses; therefore, here only the synapses made by PV-positive boutons were collected, which only form type II synapses. Synapses were defined on the basis of the rigid appositions of the plasma membranes, the widening of the extracellular space, and, when present, the postsynaptic membrane thickening. They were photographed or digitally recorded and followed through serial sections. The number of gold particles was counted in a band of 35 nm from either side of the postsynaptic plasma membrane (Nyiri et al., 2001). The α1 subunit labeling of synapses could not be fully reconstructed because of the use of some sections on different grids to detect PV immunoreactivity. Therefore, the density of immunogold particles, calculated as the number of gold particles per length of synaptic junction summed from one to five serial sections, was used to measure immunoreactivity for each individual synapse.
For publication, photographs were scanned, and contrast and brightness of the electronic picture were adjusted. All corrections were subjected to the whole picture; parts of the picture, e.g., gold particles or synapses, were not selectively enhanced.
As a control for the specificity of the method, primary antibodies were either omitted or replaced by 5% normal rabbit or mouse sera. Selective labeling, resembling that obtained with the specific antibodies, could not be detected under these conditions. The concentrations of primary antibodies were chosen such that they resulted in a very low background labeling. To estimate the contribution of background labeling to the labeling of synapses, the density of particles was measured over synaptic vesicle-containing presynaptic terminals, including areas occupied by mitochondria, randomly in the neuropil. These particles are assumed to represent background labeling. Using the calculation described previously (Nyiri et al., 2001), background labeling was estimated to make a small potential contribution of 0.1 ± 0.04 particles/μm synapse length for the α1 antibodies and 0.3 ± 0.1 particles/μm synapse length for the β2/3antibodies. Therefore, correction for background labeling was not performed. Because the center of immunoparticles may be up to 30 nm from the epitope and the synapses are cut at various angles, a contribution of the presynaptic membrane to the labeling cannot be excluded with this method. In addition, the absence of immunolabeling cannot be taken as necessarily representing the absence of receptors.
Statistics. We used nonparametric statistics for the analysis of the distribution of gold particles and for the comparison of populations of synapses because, in many cases, their distribution was not normal, as shown by the Kolmogorov–Smirnov test. The cut lengths of synaptic junctions were also described with nonparametrical statistics, although their distribution was found normal with the Kolmogorov–Smirnov test but not normal by the χ2 test. The Kruskal–Wallis test was used for comparing data from three different groups, followed by_post hoc_ comparisons using the Dunn test (Zar, 1999). Two groups were compared using the Mann–Whitney U test. Statistical analysis was performed using the software package Statistica (StatSoft, Tulsa, OK), except for the Dunn test.
RESULTS
Quantitative comparison of immunolabeling for the α1subunit of the GABAA receptor in different synapses
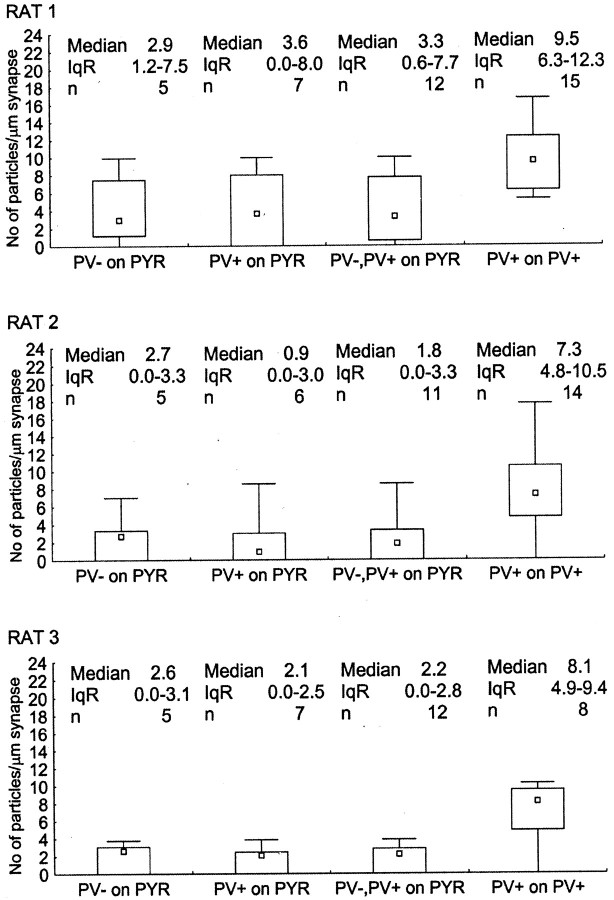
To test a possible differential distribution of the α1 subunit-containing GABAA receptors in synapses formed by PV-negative and PV-positive boutons on CA1 pyramidal cell somata, we performed postembedding immunoreactions using α1 subunit- and PV-specific antibodies. Because both antibodies were raised in rabbit, double labeling could not be performed on the same section. Instead, one to three sections were labeled with antibodies to PV (Fig.1A), and other serial sections of the same synapse were labeled for α1 subunits (Fig. 1B–D). The immunoreaction indicated a higher amount of α1subunit in synapses formed by PV-positive than in synapses formed by PV-negative boutons (Fig. 1).
Fig. 1.
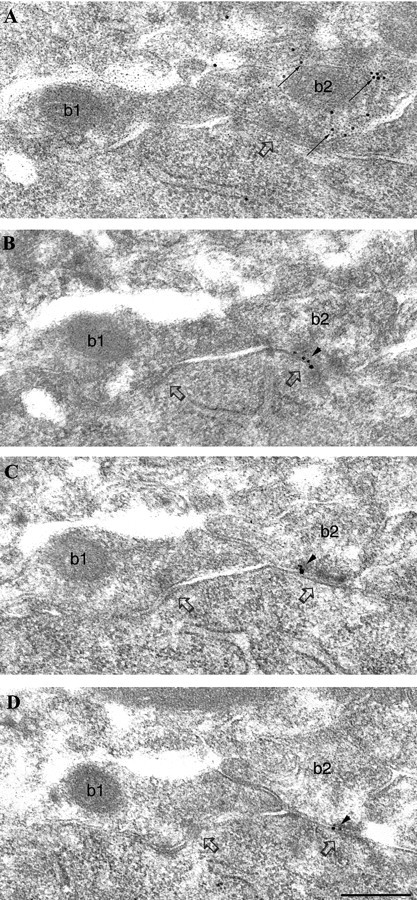
Differential immunolabeling for the α1 subunit of the GABAA receptor in synapses (open arrows) formed by PV-negative (b1) or PV-positive (b2) boutons on a pyramidal cell soma.A, Electron micrograph of a section immunolabeled for PV (10 nm gold particles; small arrows) showing an immunopositive (b2, small arrows) and an immunonegative (b1) bouton converging on the same pyramidal cell body. B–D, Three sections serial to A are immunolabeled for the α1subunit (silver-intensified ultra small gold particles;arrowheads). The synapse made by the PV-positive, but not the one made by the PV-negative, bouton is consistently labeled (arrowheads) for the α1 subunit. Scale bar: A–D, 0.2 μm.
To compare the degree of immunolabeling of GABAergic synapses on pyramidal cells and interneurons, synapses formed by PV-positive boutons on PV-positive cells in the pyramidal cell layer were also investigated within the same experiment (Fig.2). These synapses were uniformly strongly labeled for α1 subunits. In the pyramidal cell layer of the hippocampus, basket cells and axo-axonic cells have been described to express PV (Katsumaru et al., 1988). The cells investigated in this study received a high density of type II synapses on their soma (Fig. 2) and, therefore, presumably represent basket cells, because they have been described to be encrusted with numerous GABAergic boutons (Fukuda et al., 1996). The somata of axo-axonic cells receive a much lower density of synapses (P. Somogyi, unpublished observation). The hippocampus receives a GABAergic innervation from PV-positive cells of the septum, which terminate on interneurons, including PV-positive ones, but not on pyramidal cells (Freund and Antal, 1988; Gulyas et al., 1990). Nevertheless, most, if not all, of the PV-positive boutons terminating on PV-positive cells are of hippocampal origin, as shown by transection of the fimbria/fornix (Fukuda et al., 1996). This might be attributable to a very low level of PV immunoreactivity of the septo-hippocampal boutons.
Fig. 2.
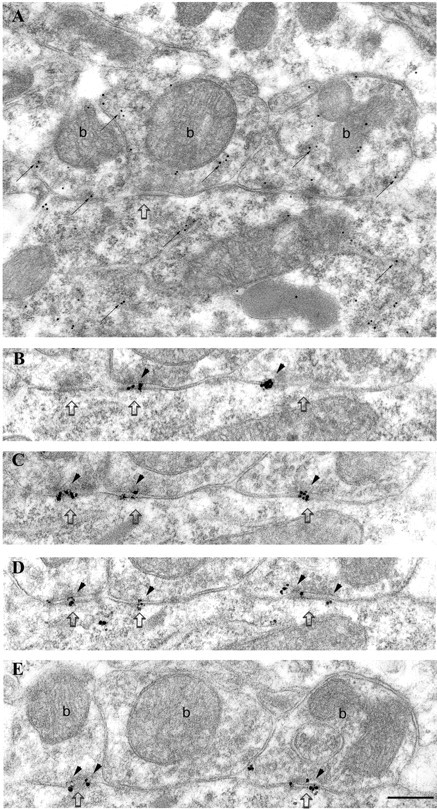
Strong immunolabeling for the α1 subunit of the GABAA receptor in synapses made by PV-positive boutons on a PV-positive interneuron soma in the pyramidal cell layer. A, Electron micrograph of a section immunolabeled for PV (10 nm gold particles; small arrows). Three synaptic boutons (b), as well as the soma of the interneuron, are PV positive. The rightmost bouton is connected to the cell body via three punctae adherentiae. B–E, Four sections serial to A are immunolabeled for the α1 subunit (silver-intensified ultra small gold particles; arrowheads), demonstrating consistent and strong immunolabeling in the synapses made by all three boutons. Scale bar: A–E, 0.2 μm.
Quantitative analysis of the α1subunit immunoreactivity from three animals revealed differences in three populations of synapse (Kruskal–Wallis test,p < 0.001; post hoc Dunn test,p < 0.05): synapses from PV-negative boutons on pyramidal cell somata, synapses from PV-positive boutons on pyramidal cell somata, and synapses from PV-positive boutons on PV-positive interneurons (Fig. 3). The median level of immunoreactivity was different in the three animals, possibly attributable to different antigen preservation of the blocks. However, the differences between the synapse populations were comparable in the three animals tested (Fig. 3). The average median particle densities in PV-positive synapses on PV-positive interneurons were 3.2 ± 1.5 (mean ± SD; 2.1, 2.7, and 4.9, respectively) times higher than the median particle density of PV-positive synapses on pyramidal cells. Differences between PV-positive and PV-negative synapses on pyramidal cells could be demonstrated for all three rats but could be calculated only for rat 3, because the median particle density of PV-negative synapses on pyramidal cells was 0 for rats 1 and 2 (Fig. 3). In rat 3, the median particle density was 3.0 times higher in synapses of PV-positive boutons than in those of PV-negative boutons on pyramidal cells. In addition, in rat 3, the median particle density in synapses of PV-positive boutons on PV-positive interneurons was 6.2 times higher than that in PV-negative boutons on pyramidal cells.
Fig. 3.
Differences in immunoreactivity for the α1 subunit of the GABAA receptor in synapses made by PV-negative or PV-positive boutons on pyramidal cell somata (PYR) and PV-positive boutons on PV-positive interneuron somata in three adult rats. Immunoreactivity is measured as density values (number of gold particles per length of synaptic junction) obtained from one to five serial sections of each synaptic membrane. Small squares, rectangles, and_bars_ indicate median, interquartile range (IqR), and minimum–maximum values, respectively. The three synapse populations were different from each other in all combinations and in all three rats (Kruskal–Wallis test,p < 0.001; post hoc Dunn test,p < 0.05). Note that the overall level of immunoreactivity was different in the three rats, but differences among synapse populations were comparable.
Quantitative comparison of immunolabeling for the β2/3 subunits of the GABAA receptor in different synapses
The different densities of α1 subunits in the three types of synapses investigated could have been attributable to an overall different density of GABAAreceptors or to an α subunit-dependent differential distribution of GABAA receptor subtypes in these synapses. The latter has been shown to be true for the α2subunit-containing GABAA receptors in synapses of PV-negative and PV-positive boutons on CA1 pyramidal cell somata (Nyiri et al., 2001). To test whether the same holds true for synapses on PV-positive interneurons and pyramidal cells, synaptic immunoreactivity for the β2/3 subunits was investigated. The great majority of GABAA receptors in the hippocampus are thought to contain β2 and/or β3 subunits, because a β subunit is required for a functional receptor and the β1 subunit is expressed at a relatively low level in hippocampal CA1 pyramidal cells (Persohn et al., 1992; Sperk et al., 1997).
Serial sections were coimmunolabeled with mouse antibodies to β2/3 subunits (10 nm gold particles) and rabbit antibodies to PV (5 nm gold particles). Synapses made by PV-negative and PV-positive boutons on pyramidal cells seemed to express the similar amount of β2/3 subunits (Fig.4). In the same experiment, immunolabeling for β2/3 subunits was also investigated in synapses made by PV-positive boutons on PV-positive interneurons in the pyramidal cell layer. These synapses were strongly immunolabeled (Fig. 5).
Fig. 4.
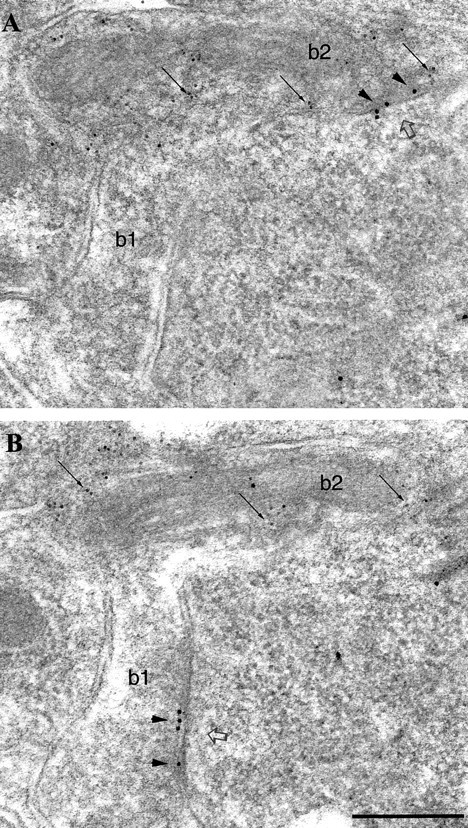
Similar immunolabeling for the β2/3subunits of the GABAA receptor in synapses (open arrows) formed by PV-negative (b1) or PV-positive (b2) boutons on a pyramidal cell soma.A, B, Electron micrographs showing two serial sections coimmunolabeled for β2/3 subunits (10 nm gold particles; arrowheads) and for PV (5 nm gold particles; small arrows). The synaptic junction of each bouton comes into the section plane in different sections. Scale bar:A, B, 0.2 μm.
Fig. 5.
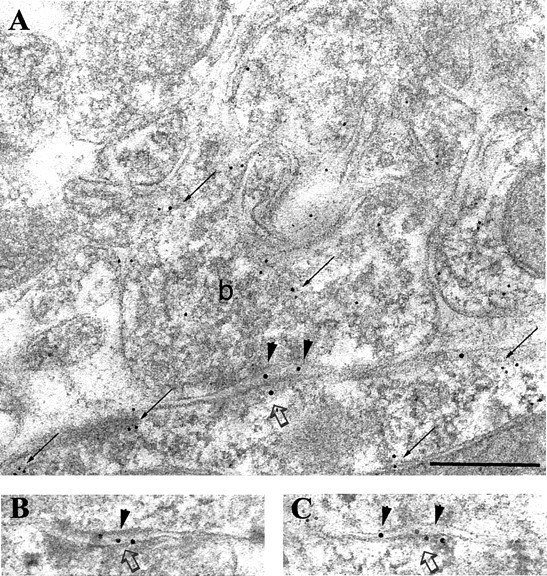
Immunolabeling for the β2/3 subunits of the GABAA receptor in a synapse (open arrows) on the soma of a PV-positive interneuron in the pyramidal cell layer. A–C, Electron micrographs showing three serial sections coimmunolabeled for the β2/3subunits (10 nm gold particles; arrowheads) and for PV (5 nm gold particles; small arrows). Note that the bouton (b), as well as the soma of the interneuron, is PV positive (small arrows). Scale bar:A–C, 0.2 μm.
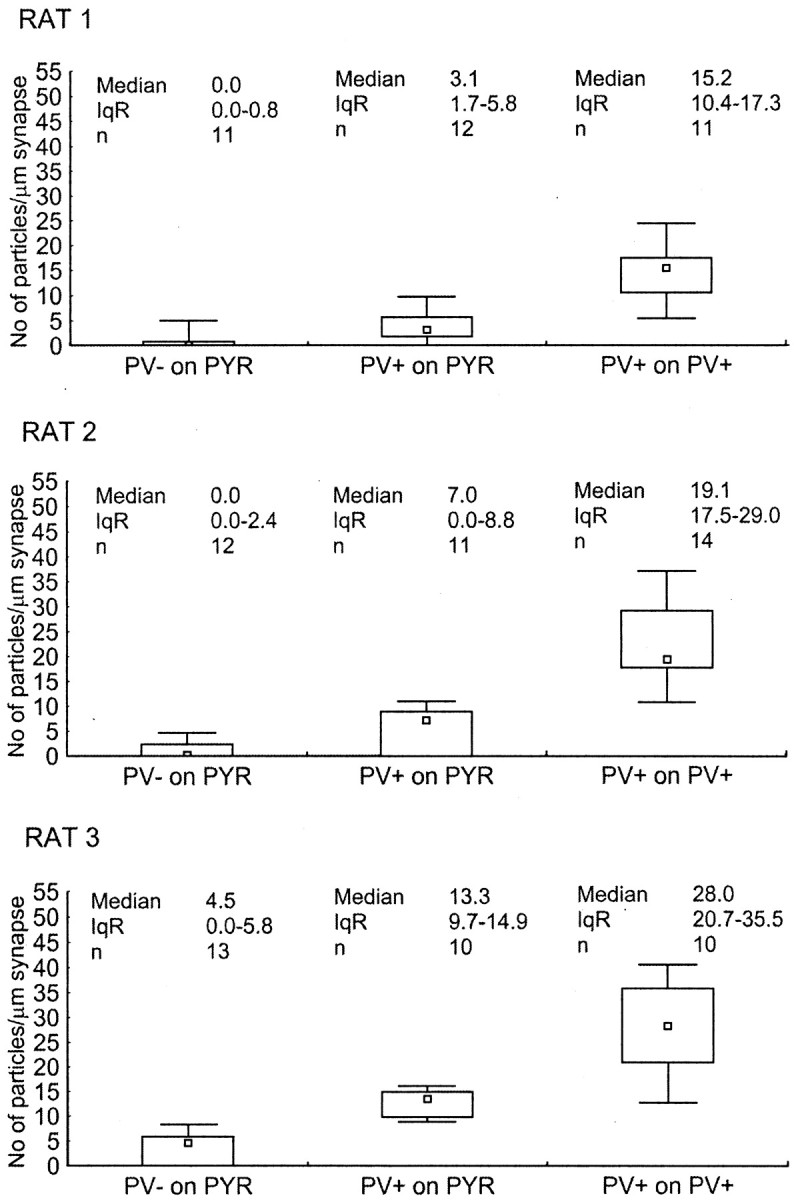
Quantitative analysis of β2/3 subunit immunoreactivity showed that synapses made by PV-negative and PV-positive boutons on pyramidal somata were not different in any of the three animals (Mann–Whitney U test, _p_> 0.5), confirming a previous study (Nyiri et al., 2001). Therefore, these synapses were pooled and compared with the immunoreactivity of synapses on interneurons (Fig. 6). Interestingly, the immunolabeling for β2/3subunits was significantly lower in synapses on pyramidal cell somata than in synapses on PV-positive interneurons in all three rats (Mann–Whitney U test, p < 0.005). The average median particle density in synapses made by PV-positive boutons on PV-positive interneurons was 3.6 ± 0.6 (mean ± SD; range of 2.9, 3.7, and 4.1, respectively) times higher than the median particle density of synapses on pyramidal cells. These results indicate that synapses made by PV-positive boutons on basket cell somata have a higher overall GABAA receptor density than synapses on pyramidal cell somata.
Fig. 6.
Difference in immunoreactivity for the β2/3 subunits of the GABAA receptor on PV-negative, PV-positive, or pooled populations of synapses on pyramidal cell somata (PYR), and PV-positive synapses on PV-positive interneuron somata in three adult rats. Immunoreactivity is measured as density values (number of gold particles per length of synaptic junction) obtained from one to five serial sections of each synaptic membrane. Small squares,rectangles, and bars indicate median, interquartile range (IqR), and minimum–maximum values, respectively. Immunoreactivity for the β2/3 subunits in synapses made by PV-negative and PV-positive boutons on pyramidal cell somata was not different in any of the rats (Mann–Whitney_U_ test, p > 0.5) (Nyiri et al., 2001); therefore, the data were pooled. Synapses on pyramidal cell somata and PV-positive synapses on interneurons were different in all three rats (Mann–Whitney U test, p< 0.005).
Comparison of the size of GABAergic synapse populations
To test whether the three synapse populations differ in the size of the synaptic specialization, in addition to the density of α1 and β2/3subunit-containing receptors, the lengths of synapses presented in Figures 3 and 6 were compared. Synapses from the same population with respect to PV labeling, but from three different animals, were not different in size; therefore, they were pooled (Kruskal–Wallis test,p > 0.05). The median length of synaptic membrane per section was 0.21 μm (interquartile range of 0.17–0.26;n = 122) for synapses made by PV-negative boutons on pyramidal cell somata, 0.20 μm (interquartile range of 0.17–0.27;n = 126) for synapses made by PV-positive on pyramidal cell somata, and 0.21 μm (interquartile range of 0.16–0.25;n = 166) for synapses made by PV-positive boutons on PV-positive interneuron somata. The three synapse populations do not differ in their average synaptic length per section (Kruskal–Wallis test, p > 0.3). These results indicate that the higher overall density of GABAA receptors in synapses on basket cell somata compared with synapses on pyramidal cell somata is not a compensation for a smaller synaptic junctional area.
DISCUSSION
We showed that at least three synapse populations have different densities of α1 subunit-containing GABAA receptors in the hippocampal pyramidal cell layer. Synapses made by PV-negative boutons on pyramidal cell somata contain very low density of α1 subunit; those made by PV-positive boutons on pyramidal cell somata contain an intermediate level, and synapses on PV-positive basket cell somata contain the highest density of α1 subunits. The high density of α1 subunits in synapses on basket cells corresponds to a higher overall density of GABAA receptors compared with pyramidal cell synapses, as shown by the correspondingly higher density of immunoreactivity for the β2/3 subunits. In contrast, the different densities of α1 subunit labeling in synapses made by PV-negative and PV-positive boutons on pyramidal cells are attributable to an input-specific difference in the α subunit composition of GABAA receptors.
Different amounts of α1 subunit-containing GABAA receptor in synapses on pyramidal cells
A relatively even frequency of synapses with α1 subunit-containing receptors were reported on distinct postsynaptic domains of pyramidal cells (Nusser et al., 1996). However, within one domain, the soma, the present study revealed differences between two synapse populations. The results are consistent with a higher amount of α2 subunit-containing GABAA receptor in synapses made by PV-negative boutons compared with those made by PV-positive boutons (Nyiri et al., 2001). The evidence provided here shows that the enrichment of α2 subunits in one of the synapse populations is accompanied by a lower amount of α1 subunits and vice versa. The immunocytochemical findings are in agreement with the differential effect of an α1subunit-selective benzodiazepine agonist on synaptic responses (Thomson et al., 2000). The results together clearly indicate that different GABAA receptor subtypes are selectively distributed in an input-dependent manner. For such a distribution of receptors, it is necessary that the presynaptic bouton signals the postsynaptic cell its identity and the postsynaptic cell converts this information to a selective targeting and/or maintenance of different GABAA receptor subtypes (Connolly et al., 1996;Moss and Smart, 2001). Whether other α subunits, such as α4 and α5 subunits (Sperk et al., 1997; Fritschy et al., 1998), are also selectively distributed will be investigated in future studies, but pharmacological evidence indicates further receptor specializations (Pawelzik et al., 1999). In addition, different GABAA receptor subtypes may be targeted also selectively to different cell domains, such as the axon (MacDermott et al., 1999), or the extrasynaptic plasma membrane (Nusser et al., 1998b).
Distribution of α1 subunit-containing GABAA receptors on pyramidal and basket cells
Immunoreactivity for the α1 subunit is expressed throughout the entire hippocampus (Fritschy and Mohler, 1995;Sperk et al., 1997; Pirker et al., 2000). Consistent with the present study, very strong immunostaining for the α1subunit was reported in hippocampal PV-positive cells (Gao and Fritschy, 1994; Fritschy and Mohler, 1995; Sperk et al., 1997). Much of this is clearly attributable to extrasynaptic receptors (Nusser et al., 1995; Somogyi et al., 1996). The strong α1subunit immunoreactivity of PV-positive cells has sometimes been interpreted as showing that most α1 subunits are on interneurons, and, consequently, drugs acting on α1 subunit-containing receptors might exert their action through these cells. Because of the expression of the α1 subunit by all pyramidal cells (Persohn et al., 1992; Wisden et al., 1992) and the low number of basket cells compared with pyramidal cells, this suggestion is reevaluated in the following calculation.
Synapses made by PV-positive boutons on PV-positive basket cells somata have 3.2 times more immunolabeling for the α1subunit than PV-positive synapses on pyramidal cells and 6.2 times more labeling than PV-negative synapses (see rat 3). The dimensions of synapses were not different. Assuming that the density of immunolabeling is proportional to the abundance of functional receptors containing that subunit (Nusser et al., 1997), neglecting the minority of PV-negative boutons on basket cell somata (Fukuda et al., 1996), and considering that 68% of synapses on pyramidal cell somata are from PV-positive boutons (Nyiri et al., 2001), it can be calculated that a type II synapse on a basket cell soma contains on average of 3.8. times more α1 subunits than one on a pyramidal cell soma. Because PV-positive basket cells receive 1.9 times more type II synapses on the soma than pyramidal cells (Gulyas et al., 1999; Megias et al., 2001) and there are 48 times more pyramidal than PV-positive cells (Aika et al., 1994), it follows that overall there are ∼6.7 times more α1 subunits in somatic synapses on pyramidal cells than in somatic synapses on PV-positive basket cells. Although this rough estimation neglects α1subunits in dendritic synapses, in the extrasynaptic membranes, and on other cell types, it predicts that the majority of synaptic α1 subunits in the CA1 area of the hippocampus are on pyramidal cells and not on basket cells. The presence of the same subunit of the GABAA receptor on different cell types indicates that receptor subtype-specific drugs might not provide selective tools for specific cell types.
Pathway-dependent synaptic enrichment of α1subunit-containing GABAA receptors
Although most receptor subtypes are expressed by several cell types, there seems to be a pathway-dependent distribution of some GABAA receptor subtypes. Basket cells expressing PV are strongly interconnected with each other and also innervate pyramidal cell somata (Katsumaru et al., 1988; Fukuda et al., 1996). In both synapse populations, a high amount of α1subunit-containing GABAA receptor has been found. Interestingly, the preferential use of α1subunits by this pathway is consistent with recent genetic, pharmacological, and behavioral studies, indicating that α1 subunit-containing receptors are important for mnemonic processes. A point mutation in the α1 subunit, rendering α1 subunit-containing receptors insensitive to benzodiazepines, leads to a decrease in the amnesic and sedative effects of diazepam (Rudolph et al., 1999; McKernan et al., 2000). It is possible that diazepam causes memory impairment and sedation via synapses interconnecting PV-positive basket cells and the basket cell to pyramidal cell circuit, which are enriched in α1 subunit-containing receptors. This synaptic organization is present probably also in the isocortex and amygdala. During exploration, when new memories are formed and established ones recalled, network oscillations in the theta and gamma frequency range occur in the hippocampus (Bragin et al., 1995) and other cortical areas (Jefferys et al., 1997). These oscillations are thought to be strongly influenced by GABAergic and electrical interactions between PV-positive basket cells, which are able to regulate the precise timing of principal cell discharge (Buzsaki and Chrobak, 1995; Cobb et al., 1995;Tamas et al., 2000).
In contrast, PV-negative (CCK/vasoactive intestinal poly-peptide-positive) basket cells do not appear to express the α1 subunit (Gao and Fritschy, 1994), and also their synapses made on pyramidal cells contain only a low amount of α1 subunit. The role of this pathway remains to be clarified. However, the pathway-dependent distribution of GABAA receptor subtypes and their specific functions in the brain demonstrate the opportunity for developing receptor subtype-specific drugs for selective functional effects.
Implications of the high number of GABAA receptor in synapses on basket cells
Synapses on PV-positive basket cell somata contain a higher density of GABAA receptors than synapses on pyramidal cells. This might influence the amplitude of miniature IPSCs (Nusser et al., 1997), but we are unaware of electrophysiological data comparing the two populations of synapse in the hippocampal CA1 region. The amplitude of IPSCs in other types of interneuron and pyramidal cells do not differ greatly (Hajos and Mody, 1997; Hajos et al., 2000), which could reflect a delicate balance between receptor number and receptor occupancy (Nusser et al., 1997). However, in the dentate gyrus, the mean decay time constant of IPSCs was approximately twofold faster in interbasket cell synapses than in granule cell synapses (Bartos et al., 2001). This may be explained by the different GABAA receptor subtypes of these cells (Lavoie et al., 1997) or by steric relationships of synapses or modulation of receptors, dependent on the postsynaptic cell (Overstreet et al., 2000;Moss and Smart, 2001). Bartos et al. (2001) also report a larger peak amplitude of IPSCs in basket cells compared with granule cells. This might be caused by a higher number of GABAAreceptors on basket cell synapses, similar to that found in the present study for basket cells in the CA1 area. As a consequence, the synaptic peak conductance change was much higher in basket cell synapses compared with granule cell synapses. This is proposed to have a critical role in the generation of coherent, high-frequency oscillations (Wang and Buzsaki, 1996; Bartos et al., 2001), which are believed to be important for mnemonic processes. Overall, the higher number of synaptic GABAA receptors and the bias for receptor subtypes containing α1 subunits by PV-positive basket cells supports a phasic, precisely timed inhibition of basket cells, leading to a coherent interneuron network oscillation. The lower amount of GABAA receptors in GABAergic synapses on pyramidal cell somata, together with a wide variety of potential synaptic receptors, predicts a large variability in their inhibitory responses.
Footnotes
T.K. was supported by an Erwin Schroedinger Fellowship of the Austrian Science Fund. We thank Dr. Werner Sieghart (Brain Research Institute, Vienna, Austria) for providing the antibody to the α1subunit, Dr. J.-M. Fritschy (Institute of Pharmacology, Zurich, Switzerland) for antibody bd17, and Dr. K. G. Baimbridge, (Department of Physiology, University of British Columbia, Vancouver, Canada) for the rabbit antibodies to parvalbumin. We also thank Dr. Yannis Dalezios for help with statistics, and Dr. Zoltan Nusser and Gabor Nyiri for helpful comments on an earlier version of this manuscript. Philip Cobden, Paul Jays, and Dr. Laszlo Marton provided excellent technical assistance.
Correspondence should be addressed to Dr. Thomas Klausberger, Medical Research Council, Anatomical Neuropharmacology Unit, Oxford University, Department of Pharmacology, Mansfield Road, Oxford OX1 3TH, UK. E-mail:thomas.klausberger@pharm.ox.ac.uk.
REFERENCES
- 1.Aika Y, Ren JQ, Kosaka K, Kosaka T. Quantitative analysis of GABA-like-immunoreactive and parvalbumin-containing neurons in the CA1 region of the rat hippocampus using a stereological method, the disector. Exp Brain Res. 1994;99:267–276. doi: 10.1007/BF00239593. [DOI] [PubMed] [Google Scholar]
- 2.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 3.Bartos M, Vida I, Frotscher M, Geiger JRP, Jonas P. Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci. 2001;21:2687–2698. doi: 10.1523/JNEUROSCI.21-08-02687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 5.Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 7.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 8.Cobb SR, Halasy K, Vida I, Nyiri G, Tamas G, Buhl EH, Somogyi P. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience. 1997;79:629–648. doi: 10.1016/s0306-4522(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 9.Connolly CN, Wooltorton JRA, Smart TG, Moss SJ. Subcellular localization of γ-aminobutyric acid type A receptors is determined by receptor β subunits. Proc Natl Acad Sci USA. 1996;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewert M, Shivers BD, Luddens H, Mohler H, Seeburg PH. Subunit selectivity and epitope characterization of mAbs directed against the GABAA/benzodiazepine receptor. J Cell Biol. 1990;110:2043–2048. doi: 10.1083/jcb.110.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;366:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 12.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Fritschy J-M, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 14.Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- 15.Fukuda T, Aika Y, Heizmann CW, Kosaka T. Dense GABAergic input on somata of parvalbumin-immunoreactive GABAergic neurons in the hippocampus of the mouse. Neurosci Res. 1996;26:181–194. doi: 10.1016/s0168-0102(96)01102-9. [DOI] [PubMed] [Google Scholar]
- 16.Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the α1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 17.Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- 18.Gulyas AI, Gorcs TJ, Freund TF. Innervation of different peptide-containing neurons in the hippocampus by GABAergic septal afferents. Neuroscience. 1990;37:31–44. doi: 10.1016/0306-4522(90)90189-b. [DOI] [PubMed] [Google Scholar]
- 19.Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajos N, Mody I. Synaptic communication among hippocampal interneurons: properties of spontaneous IPSCs in morphologically identified cells. J Neurosci. 1997;17:8427–8442. doi: 10.1523/JNEUROSCI.17-21-08427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express M2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- 22.Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci. 2000;12:810–818. doi: 10.1046/j.1460-9568.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- 23.Haring P, Stahli C, Schoch P, Takacs B, Staehelin T, Mohler H. Monoclonal antibodies reveal structural homogeneity of γ-aminobutyric acid/benzodiazepine receptors in different brain areas. Proc Natl Acad Sci USA. 1985;82:4837–4841. doi: 10.1073/pnas.82.14.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jefferys JGR, Traub RD, Whittington MA. Neuronal networks for induced 40 Hz rhythms. Trends Neurosci. 1997;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- 25.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsumaru H, Kosaka T, Heizmann CW, Hama K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res. 1988;72:347–362. doi: 10.1007/BF00250256. [DOI] [PubMed] [Google Scholar]
- 27.Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 30.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 31.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 32.Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 33.Mithani S, Atmadja S, Baimbridge KG, Fibiger HC. Neuroleptic-induced oral dyskinesias: effects of progabide and lack of correlation with regional changes in glutamic acid decarboxylase and choline acetyltransferase activities. Psychopharmacology. 1987;93:94–100. doi: 10.1007/BF02439593. [DOI] [PubMed] [Google Scholar]
- 34.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 35.Nusser Z, Roberts JDB, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- 38.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998a;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998b;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 40.Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of γ2-subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- 41.Overstreet LS, Jones MV, Westbrook GL. Slow desensitization regulates the availability of synaptic GABAA receptors. J Neurosci. 2000;20:7914–7921. doi: 10.1523/JNEUROSCI.20-21-07914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawelzik H, Bannister AP, Deuchars J, Ilia M, Thomson AM. Modulation of bistratified cell IPSPs and basket cell IPSPs by pentobarbitone sodium, diazepam and Zn2+: dual recordings in slices of adult rat hippocampus. Eur J Neurosci. 1999;11:3552–3564. doi: 10.1046/j.1460-9568.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- 43.Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- 44.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 45.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 46.Rudolph U, Crestani F, Mohler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 47.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 48.Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABAA receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 49.Somogyi P, Fritschy JM, Benke D, Roberts JDB, Sieghart W. The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 50.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus. I. Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 51.Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 52.Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci. 2000;12:425–436. doi: 10.1046/j.1460-9568.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang X-J, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zar JH. Biostatistical analysis, pp 224–225. Prentice Hall; Upper Saddle River, NJ: 1999. [Google Scholar]
- 56.Zezula J, Fuchs K, Sieghart W. Separation of α1, α2 and α3 subunits of the GABAA-benzodiazepine receptor complex by immunoaffinity chromatography. Brain Res. 1991;563:325–328. doi: 10.1016/0006-8993(91)91556-g. [DOI] [PubMed] [Google Scholar]