Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection (original) (raw)
Abstract
Background
The biological processes associated with postacute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) are unknown.
Methods
We measured soluble markers of inflammation in a SARS-CoV-2 recovery cohort at early (<90 days) and late (>90 days) timepoints. We defined PASC as the presence of 1 or more coronavirus disease 2019 (COVID-19)–attributed symptoms beyond 90 days. We compared fold-changes in marker values between those with and without PASC using mixed-effects models with terms for PASC and early and late recovery time periods.
Results
During early recovery, those who went on to develop PASC generally had higher levels of cytokine biomarkers including tumor necrosis factor–α (1.14-fold higher mean ratio [95% confidence interval {CI}, 1.01–1.28]; P = .028) and interferon-γ–induced protein 10 (1.28-fold higher mean ratio [95% CI, 1.01–1.62]; P = .038). Among those with PASC, there was a trend toward higher interleukin 6 levels during early recovery (1.29-fold higher mean ratio [95% CI, .98–1.70]; P = .07), which became more pronounced in late recovery (1.44-fold higher mean ratio [95% CI, 1.11–1.86]; P < .001). These differences were more pronounced among those with a greater number of PASC symptoms.
Conclusions
Persistent immune activation may be associated with ongoing symptoms following COVID-19. Further characterization of these processes might identify therapeutic targets for those experiencing PASC.
Keywords: SARS-CoV-2, coronavirus, COVID-19, postacute sequelae of SARS-CoV-2 infection, PASC, long COVID, biomarker, inflammation, immune activation, IL-6, TNF-α
Compared to individuals reporting full recovery, individuals with symptoms for >90 days following COVID-19 had subtle elevations in levels of certain markers of immune activation. Further characterization of these processes might identify therapeutic targets for those experiencing postacute sequelae of SARS-CoV-2tion.
The acute phase of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is characterized by inflammation and immune dysregulation [1, 2], but the recovery phase that follows acute infection is poorly understood. A significant proportion of individuals recovering from coronavirus disease 2019 (COVID-19) do not demonstrate a full return to baseline health and experience postacute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC), a condition that is often associated with the persistence or recurrence of symptoms not explained by an alternative medical diagnosis [3]. Multidisciplinary efforts are underway to characterize the epidemiology, natural history, and biology of this condition [4, 5], but there is limited biological information regarding its predictors and correlates.
Inflammation during early infection has been associated with adverse outcomes, particularly in those who were hospitalized with COVID-19 [1, 2, 6–10]. Emerging data suggest that inflammation related to acute SARS-CoV-2 infection can persist for weeks to months [11, 12]. For example, individuals well enough to donate convalescent plasma have elevations in certain markers of inflammation, as compared to prepandemic plasma donors [11]. These markers include interferon gamma (IFN-γ), certain interleukin proteins, and monocyte chemoattractant protein 1 (MCP-1). Furthermore, viral proteins and nucleic acids have been detected months after the acute phase in some small studies [13]. However, there are limited data from COVID-19 recovery cohorts with large numbers of individuals managed in the outpatient setting, which constitute the majority of those infected with SARS-CoV-2, and from cohorts with careful prospective clinical phenotyping of symptoms present during COVID-19 recovery.
The clinical implications of persistent inflammation have been established for chronic infections, including human immunodeficiency virus (HIV) [14–16], but are less well understood for acute infections such as SARS-CoV-2. We therefore implemented in April 2020 a prospective cohort study of individuals who had documented SARS-CoV-2 infection (Long-term Impact of Infection with Novel Coronavirus [LIINC], www.liincstudy.org; NCT04362150). The majority of participants had not been hospitalized, and biologic specimens and detailed clinical data were collected at regular time points. Here, we report on clinical data and markers of systemic immune activation and inflammation that may contribute to the early understanding of PASC pathogenesis. A better understanding of these pathogenic processes is critical for identifying therapies to treat and/or prevent this condition among the millions of individuals who have recovered from acute SARS-CoV-2 infection.
METHODS
Study Participants and Procedures
Volunteers with a documented history of SARS-CoV-2 infection confirmed by nucleic acid amplification testing were enrolled at our clinical research center at San Francisco General Hospital in San Francisco, California [17]. Participants were recruited through a combination of clinician referrals, mailings to consecutive patients testing positive at university-affiliated testing sites, and responses to medical center recruitment postings and websites (including www.liincstudy.org and www.ClinicalTrials.gov). All adults with a positive test were eligible and recruitment was agnostic to the presence of persistent symptoms.
At each study visit, participants were systematically queried regarding the presence of 32 individual symptoms derived from the Centers for Disease Control and Prevention list of COVID-19 symptoms [18] and from the Patient Health Questionnaire Somatic Symptom Scale [19]. A symptom was considered to be present if it was new in onset since the time of SARS-CoV-2 infection or, for preexisting symptoms, if it had worsened since the diagnosis of SARS-CoV-2 infection. Symptoms that had been present prior to SARS-CoV-2 infection and did not worsen were not considered to represent PASC. At each visit, blood was collected by venipuncture. Plasma was isolated via centrifugation of heparinized blood and stored at –80°C. Due to the known associations between HIV infection and chronic inflammation [14–16], we excluded people living with HIV infection from the current analyses.
Clinical Measurements
We assessed the presence or absence of symptoms at a visit occurring >90 days from initial COVID-19 symptom onset. The primary outcome (PASC) was defined as the presence of 1 or more symptoms at this timepoint. A subset of participants (92/121 [76%]) had an additional blood sample that had been collected at an earlier (ie, before 90 days) timepoint. For further sensitivity and exploratory analyses, we defined the top 25% most symptomatic individuals (based on the number of symptoms reported) as having severe PASC.
Biomarker and Antibody Assays
The fully automated HD-X Simoa platform was used to measure biomarkers in blood plasma including MCP-1, Cytokine 3-PlexA (interleukin 6 [IL-6], interleukin 10 [IL-10], tumor necrosis factor alpha [TNF-α]), IFN-γ–induced protein 10 (IP-10), IFN-γ, and SARS-CoV-2 receptor-binding domain (RBD) immunoglobulin G (IgG) according to the manufacturer’s instructions. These markers were selected based upon their biological importance during acute SARS-CoV-2 infection [1, 2]. Samples were assayed blinded with respect to associated patient and clinical information. Assay performance was consistent with the manufacturer’s specifications.
Statistical Analysis
We used descriptive statistics to characterize the cohort and nonparametric analyses to compare the groups with and without PASC. We log10-transformed all biomarkers as they were not normally distributed. We then compared the ratio of the mean transformed values for each biomarker between those with and without persistent symptoms using mixed-effects models with terms for PASC and time period (early vs late recovery). This approach permits comparison of the values at early and late time points as well as assessment of whether trajectories in marker values differ between those with and without persistent symptoms. We calculated fold-changes and 95% confidence intervals (CIs) by exponentiating the coefficients to give the ratio between the untransformed biomarker values. We used Spearman correlations to evaluate relationships between levels of binding antibodies and immune markers. All P values are 2 sided. We used Stata version 16.1 (StataCorp, College Station, Texas) and Prism version 9.1.2 (GraphPad Software, San Diego, California) software.
Ethics Approval
All participants provided written informed consent. The study was approved by the Institutional Review Board at the University of California, San Francisco.
RESULTS
Study Participants
Our analysis cohort included 121 participants and was diverse in terms of sex, gender, race, ethnicity, and level of education (Table 1). Medical comorbidities present in >10% of the cohort included lung problems, diabetes, and obesity. Nine participants had a history of an autoimmune disorder, which included autoimmune thyroid disease (n = 4), gastrointestinal disease (n = 2), multiple sclerosis (n = 1), rheumatoid arthritis (n = 1), and an unspecified autoimmune condition (n = 1). A majority of participants (78%) had been managed as outpatients during their COVID-19 illness. Of those who had been hospitalized, 9 (33%) were managed in an intensive care setting and 3 (11%) required mechanical ventilation. Few participants received SARS-CoV-2 targeted therapy, including remdesivir (n = 3) and corticosteroids (n = 2). No participants received convalescent plasma or monoclonal antibodies. No participants received a SARS-CoV-2 vaccine prior to sample collection for this study.
Table 1.
Characteristics of the Study Cohort
| Characteristic | All (N = 121) | No PASC (n = 48) | PASC (n = 73) |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y, median (IQR) | 44 (37–57) | 44.5 (37–58.5) | 44 (36–56) |
| Sex assigned at birth | |||
| Female | 66 (54.5) | 21 (43.8) | 45 (61.6) |
| Male | 55 (45.5) | 27 (56.3) | 28 (38.4) |
| Gender | |||
| Cisgender female | 65 (53.7) | 21 (43.8) | 44 (60.3) |
| Cisgender male | 54 (44.6) | 27 (56.3) | 27 (37.0) |
| Transgender male | 2 (1.7) | 0 (0.0) | 2 (2.7) |
| Race and ethnicity | |||
| Hispanic/Latino | 30 (24.8) | 8 (16.7) | 22 (30.1) |
| White | 69 (57.0) | 28 (58.3) | 41 (56.2) |
| Black/African American | 3 (2.5) | 1 (2.1) | 2 (2.7) |
| Asian | 14 (11.6) | 8 (16.7) | 6 (8.2) |
| Pacific Islander/Native Hawaiian | 1 (0.8) | 1 (2.1) | 0 (0.0) |
| Not provided | 4 (3.3) | 2 (4.2) | 2 (2.7) |
| Highest level of education completed | |||
| At least some high school | 21 (17.4) | 9 (18.8) | 12 (16.4) |
| At least some college | 46 (38.0) | 15 (31.3) | 31 (42.5) |
| At least some graduate school | 54 (44.6) | 24 (50.0) | 30 (41.1) |
| Tobacco use history | 27 (22.3) | 9 (18.8) | 18 (24.7) |
| Clinical characteristics | |||
| Preexisting medical conditions | |||
| Autoimmune disease | 9 (7.4) | 1 (2.1) | 8 (11.0) |
| Cancer (with treatment received within 2 y prior to COVID-19 diagnosis) | 3 (2.5) | 1 (2.1) | 2 (2.7) |
| Diabetes | 14 (11.6) | 6 (12.5) | 8 (11.0) |
| Lung problems | 23 (19.0) | 10 (20.8) | 13 (17.8) |
| BMI category, kg/m2 | |||
| ≤24.9 | 46 (38.0) | 20 (41.7) | 26 (35.6) |
| 25–29.9 | 34 (28.1) | 16 (33.3) | 18 (24.7) |
| ≥30 | 39 (32.2) | 11 (22.9) | 28 (38.4) |
| Hospitalized during acute COVID-19 | 27 (22.3) | 8 (16.7) | 19 (26.0) |
Although we did not identify significant demographic differences between groups, those reporting PASC tended to be more likely to have been assigned female sex at birth (61.6% vs 43.8%; P = .053) and to report a history of autoimmune disease preceding their COVID-19 diagnosis (11% vs 2.1%; P = .087). The presence of PASC did not differ according to hospitalization status during acute COVID-19.
The early recovery measurement occurred at a median of 52 (interquartile range [IQR], 38–64) days from symptom onset and the late recovery measurement occurred at a median of 124 (IQR, 116–136) days from symptom onset. The timing of the early follow-up sample among those with PASC was slightly later than those without PASC (median, 57 vs 50 days after symptom onset; P = .04); the timing of the late sample did not differ (median, 123 vs 124 days after symptom onset; P = .26).
Persistent Symptoms
Seventy-three individuals reported 1 or more symptoms at the late recovery timepoint. The median number of symptoms reported by individuals with PASC was 5 (IQR, 2–8; absolute range, 1–18). Common symptoms (Table 2) included problems with memory or concentration (57%), fatigue (56%), shortness of breath (38%), and anosmia/dysgeusia (37%).
Table 2.
Symptoms Reported at Late Follow-up Among Participants With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection
| Symptoms Reported at Late Follow-up | No. (%) (n = 73) |
|---|---|
| Constitutional | |
| Fatigue | 41 (56.2) |
| Subjective fever | 2 (2.7) |
| Chills | 2 (2.7) |
| Objective fever | 2 (2.7) |
| Upper respiratory | |
| Rhinorrhea | 14 (19.2) |
| Sore throat | 7 (9.6) |
| Cardiopulmonary | |
| Cough | 13 (17.8) |
| Shortness of breath | 28 (38.4) |
| Chest pain | 18 (24.7) |
| Palpitations | 15 (20.5) |
| Fainting | 0 (0.0) |
| Gastrointestinal | |
| Diarrhea | 9 (12.3) |
| Nausea | 17 (23.3) |
| Loss of appetite | 12 (16.4) |
| Abdominal pain | 6 (8.2) |
| Vomiting | 1 (1.4) |
| Constipation | 3 (4.1) |
| Genitourinary | |
| Menstrual cramps | 2 (2.7) |
| Dyspareunia | 0 (0.0) |
| Rash | 10 (13.7) |
| Musculoskeletal | |
| Myalgia | 20 (27.4) |
| Back pain | 7 (9.6) |
| Joint pain | 9 (12.3) |
| Neurologic | |
| Anosmia/dysgeusia | 27 (37.0) |
| Headache | 18 (24.7) |
| Concentration problems | 42 (57.5) |
| Dizziness | 18 (24.7) |
| Balance problems | 13 (17.8) |
| Neuropathy | 17 (23.3) |
| Vision problems | 13 (17.8) |
| Parosmia | 8 (11.0) |
| Trouble sleeping | 32 (43.8) |
Levels of Plasma Biomarkers Among Those With and Without PASC
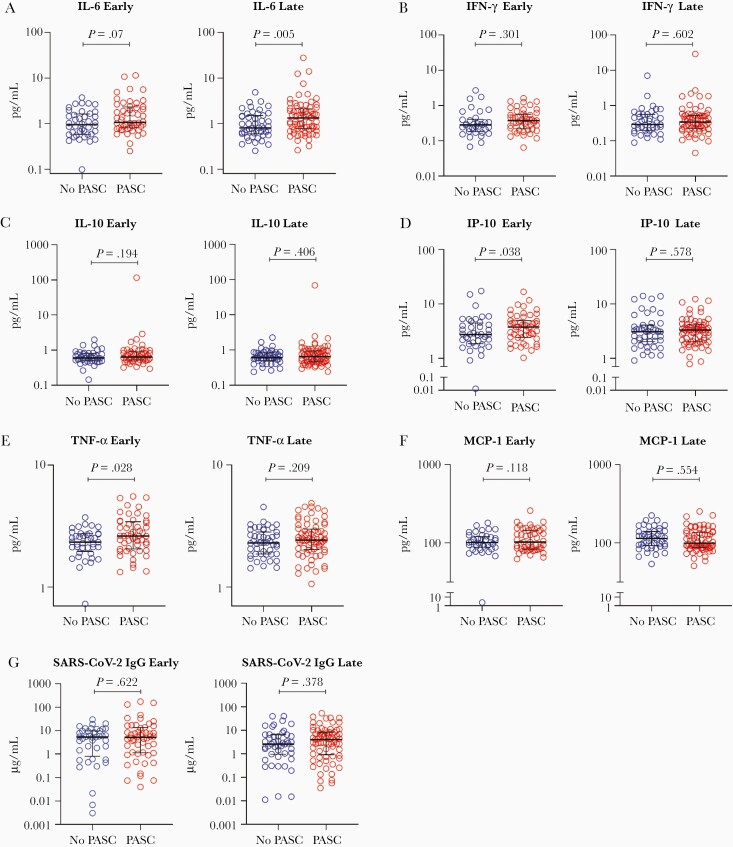
In our cross-sectional analyses, we first compared levels of each plasma marker measured during early recovery between those with and without PASC (Figure 1, Supplementary Tables 1–2). Those who went on to develop PASC demonstrated significantly higher levels of TNF-α (1.14-fold higher mean ratio [95% CI, 1.01–1.28]; P = .028) and IP-10 (1.28-fold higher mean ratio [95% CI, 1.01–1.62]; P = .038). The mean IL-6 level during early recovery was on average 29% higher among those with PASC, although the difference did not achieve statistical significance (95% CI, .98–1.70; P = .07). Several other markers showed higher levels among those with PASC even when the comparisons were not significant.
Figure 1.
Cross-sectional levels of biomarkers among those with and without PASC during late recovery. Abbreviations: IFN-γ, interferon gamma; IgG, immunoglobulin G; IL-6, interleukin 6; IL-10, interleukin 10; IP-10, interferon-γ–induced protein 10; MCP-1, monocyte chemoattractant protein 1; PASC, postacute sequelae of severe acute respiratory syndrome coronavirus 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF-α, tumor necrosis factor alpha.
We next compared levels of each biomarker measured during late recovery between those with and without PASC (Figure 1). During the late recovery period, the mean IL-6 level was on average 44% higher among those with PASC (95% CI, 1.11–1.86; P = .0005). No other markers differed between the groups.
Levels of SARS-CoV-2 RBD IgG did not differ between groups at either the early or late timepoints. Among those with PASC, levels of binding antibodies correlated with TNF-α (r = 0.33, P = .018), IFN-γ (r = 0.30, P = .038), and MCP-1 (r = 0.39, P = .005) at the early timepoint; at the later timepoint, levels of binding antibodies correlated with IL-6 (r = 0.29, P = .015), TNF-α (r = 0.28, P = .016), and MCP-1 (r = 0.40, P = .0006). In the group without PASC, these antibodies correlated only with IFN-γ at the early timepoint (r = 0.38, P = .024) and IP-10 at the late timepoint (r = 0.33, P = .025).
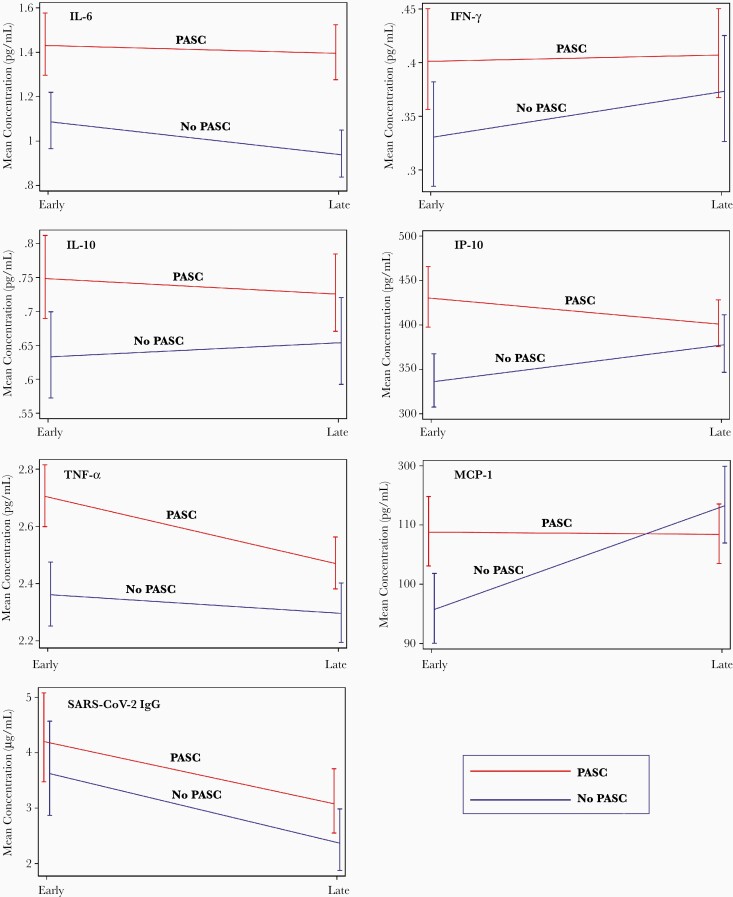
Changes in Levels of Plasma Biomarkers Over Time
In our longitudinal analyses, we used mixed models to indicate changes in levels of biomarkers among those with and without PASC between the early and late recovery period time points (Figure 2). Overall, there were no statistically significant differences in the trends of biomarkers over time between the PASC and non-PASC groups. As would be predicted from cross-sectional analyses, higher levels of IL-6 were observed across time points in those with PASC compared to those without persistent symptoms.
Figure 2.
Longitudinal changes in levels of biomarkers among individuals with and without PASC during late recovery. Abbreviations: IFN-γ, interferon gamma; IgG, immunoglobulin G; IL-6, interleukin 6; IL-10, interleukin 10; IP-10, interferon gamma–induced protein 10; MCP-1, monocyte chemoattractant protein 1; PASC, postacute sequelae of severe acute respiratory syndrome coronavirus 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF-α, tumor necrosis factor alpha.
Sensitivity Analyses
To further assess the effects of certain demographic factors potentially important in PASC, we performed adjustment for age, sex, and hospitalization status during acute SARS-CoV-2 infection. These adjustments did not fundamentally change the interpretation of the earlier analyses, although the IP-10 elevation during early recovery was no longer statistically significant (mean ratio, 1.24 [95% CI, .98–1.57]; P = .071) (Supplementary Table 3). Further adjustment for autoimmune disease and body mass index did not alter interpretation of the results (Supplementary Table 4).
To determine whether the relationships observed in the primary analysis would be more pronounced among those with the most severe manifestations of PASC, we examined the subset of individuals reporting the greatest number of symptoms (upper 25% of symptom count) in comparison to those without any persistent symptoms (Supplementary Table 5). During early recovery, severe PASC was associated with the presence of elevated levels of IL-6 (mean ratio, 1.47 [95% CI, 1.10–1.97]; P = .009), IFN-γ (mean ratio, 1.35 [95% CI, 1.00–1.81]; P = .049), IL-10 (mean ratio, 1.25 [95% CI, 1.00–1.57]; P = .05), and TNF-α (mean ratio, 1.16 [95% CI, 1.03–1.31]; P = .017). IL-6 levels were persistently elevated during late follow-up, although the relationship did not remain statistically significant (mean ratio, 1.47 [95% CI, 1.10–1.97]; P = .089). Further analysis demonstrated that more symptomatic individuals (≥3 symptoms, compared to 1–2 symptoms or 0 symptoms) tended to have higher levels of certain markers (Supplementary Table 6).
Discussion
Postacute sequelae of SARS-CoV-2 infection is now recognized as a public health priority [3]. In a variety of cohorts and electronic medical record–based studies [4, 5, 12, 20], approximately 10%–40% of individuals report new symptoms that persist for weeks to months. Risk factors for PASC may include more severe acute infection, advanced age, female sex, and socioeconomic factors [5, 20, 21]. However, the study of the pathogenesis of PASC is only just beginning. Leveraging a prospective cohort designed to study how SARS-CoV-2 infection affects long-term health, we measured a variety of potential biomarkers in individuals recovering from COVID-19, a substantial proportion of whom reported symptoms beyond 3 months following initial infection. Importantly, we did not prospectively seek to enroll individuals with PASC. We identified several biomarkers—particularly IL-6 and TNF-α—that differ during early and late recovery among those who continue to experience symptoms at a median of 124 days following infection. These elevations are part of a consistent pattern suggesting persistent immune activation among those who go on to develop PASC. These observations may inform on biological pathways contributing to PASC and aid in the identification of potential therapeutic strategies.
IL-6 and TNF-α are both proinflammatory cytokines that contribute to leukocyte recruitment, activation, and differentiation, as well as B-cell maturation and the expansion of T-helper cell subsets [22, 23]. Both have been identified as key factors in the immune response during acute COVID-19 [2, 9, 10, 24, 25], although in general IL-6 levels appear to be more predictive of poor outcomes such as respiratory failure and the need for mechanical ventilation [2, 8, 10, 26]. Results from interventions to reduce the inflammatory milieu during acute infection by targeting cytokines, such as IL-6, however, have been mixed [27–32]. While various immune activating cytokines can have protective or homeostatic effects [33–35], their dysregulation may lead to detrimental clinical conditions. For example, IL-6 has been implicated in models of chronic inflammatory diseases [33], and overexpression in mouse models is associated with tissue-specific manifestations such as lung or neurologic disease [36–38]. Similarly, TNF-α induces tissue inflammation and endothelial activation and uncontrolled activity of this cytokine underlies various inflammatory diseases that can involve multiple organ systems [39].
Among those recovering from COVID-19, there are limited data on immunologic trends over time and in association with ongoing clinical symptoms. Although our findings need to be supported with larger studies, we found that individuals with persistent symptoms were likely to demonstrate higher levels of markers of inflammation and immune activation during the first 90 days of COVID-19 recovery. While not all markers achieved statistical significance, there was a consistent trend across all markers of interest during this period. Furthermore, we found that elevations in IL-6 persisted into the late recovery period during which we defined the primary clinical outcome (PASC). This observation builds upon prior work identifying persistent elevations in levels of cytokines in COVID-19 convalescent plasma donors [11] and individuals who were previously hospitalized with COVID-19 [12]. In the latter study, previously hospitalized individuals who reported persistent symptoms had higher levels of MCP-1 and platelet-derived growth factor during early convalescence, but these differences were no longer detected at 6 months and the study included a lower proportion of participants with persistent symptoms. Our findings suggest that differences in cytokines and chemokines may also be relevant among individuals who were not hospitalized during the acute phase, and that persistent immune activation might be associated with the processes that drive some persistent symptoms. While the magnitude of the elevations we detected was not dramatic, the direction was consistent across markers and suggests that these subtle immunologic differences warrant further investigation.
The factors driving persistent inflammation during COVID-19 recovery have yet to be determined. One possibility is that residual inflammation from the acute phase of infection persists among certain individuals, either those with the most severe illness or in relation to other unknown factors. This could be supported by the fact that most differences in the levels of these markers appeared to resolve by the late recovery timepoint. Although antibody levels, which consistently correlate with disease severity [40–46] and have been shown to be predictive of persistent symptoms at 6 months in other cohorts [4], did not differ between the groups, among those with PASC antibody levels did correlate with levels of several inflammatory markers. In addition, IL-6 levels remained elevated throughout the observation period. Many factors could contribute to persistent IL-6 elevations including antigen persistence in tissues, compromised mucosal barrier integrity with increased microbial translocation, and/or autoreactive immunity, or an intrinsic failure of the host immune system to return to baseline homeostasis. Further work will be needed to determine whether our findings represent a persistent inflammatory response that is slower to resolve among those with PASC, or whether there is ongoing pathology that would benefit from intervention during either early or later recovery.
It is notable that 8 of 9 participants with a history of previously diagnosed autoimmune disorders experienced PASC. It has been theorized that elevated autoantibodies may be present during acute hospitalization with COVID-19 and may persist during convalescence [47, 48], although the association with persistent symptoms has yet to be established. Others have observed new diagnoses or exacerbations of clinical entities with autoimmune mechanisms, such as diabetes and thyroiditis, following SARS-CoV-2 infection [49, 50]. The relationship between SARS-CoV-2 infection, autoreactive immunity, and the inflammatory milieu that may persist following COVID-19 warrants further investigation, particularly if it can be tied to specific PASC phenotypes.
Strengths of this analysis include the inclusion of a large number of individuals with deeply characterized persistent symptoms and the large proportion who had not been hospitalized during acute infection, which is reflective of the majority of people recovering from COVID-19. Limitations of this study include the use of a convenience sample that may not be representative of the SARS-CoV-2 epidemic and lack of specimens from the acute infection period. There is currently no widely agreed upon case definition for PASC, and we adopted a broad case definition that might be overly sensitive. We also used a rough temporal cutoff to classify PASC, which may have led to misclassification bias in our results. Still, our sensitivity analyses showed similar findings, suggesting the presence of a true effect. We measured a relatively small number of biomarkers based on hypotheses derived from published signatures during acute infection, but more in-depth biomarker characterization may be more revealing. While we performed multiple statistical analyses, our findings were consistent with our prespecified hypotheses. Finally, because specimens preceding SARS-CoV-2 infection were not available, it is not possible to discern whether individuals with PASC had elevated levels of these markers preinfection, which could possibly alter their responses during infection and recovery and/or predispose them to PASC. Nonetheless, our observation will help inform on mechanistic pathways that could contribute to PASC and direct future research leading to the identification of potential therapeutic targets.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
jiab490_suppl_Supplementary_Materials
Notes
Author contributions. M. J. P., H. H., J. D. K., J. N. M., S. G. D., and T. J. H. designed the study. M. J. P., S. E. M., R. H., and V. T. collected clinical data and biospecimens. A. C., B. C. Y., J. W. W., and C. J. P. analyzed the specimens. M. J. P., S. L., A. F. T., M. S. D., J. D. K., and D. V. G. performed and/or interpreted the statistical analyses. M. J. P., S. L., A. F. T., H. H., C. F., J. D. K., S. G. D., and T. J. H. drafted the initial manuscript with input from A. C., J. W. W., P. W. H., P. S. Y., and J. N. M. All authors edited, reviewed, and approved the final manuscript.
Acknowledgments. We are grateful to the Long-term Impact of Infection with Novel Coronavirus (LIINC) study participants and to the clinical staff who provided care to these individuals during their acute illness period and during their recovery. We thank Dr Isabel Rodriguez-Barraquer and Dr Rachel Rutishauser for their contributions to the LIINC leadership team. We acknowledge current and former LIINC clinical study team members Tamara Abualhsan, Andrea Alvarez, Mireya Arreguin, Emily Fehrman, Monika Deswal, Heather Hartig, Yanel Hernandez, Marian Kerbleski, Lynn Ngo, Dylan Ryder, Ruth Diaz Sanchez, Cassandra Thanh, Leonel Torres, Fatima Ticas, and Meghann Williams; and LIINC laboratory team members Joanna Donatelli, Jill Hakim, Nikita Iyer, Owen Janson, Christopher Nixon, and Keirstinne Turcios. We thank Elnaz Eilkhani for coordination with the Institutional Review Board. We acknowledge the contributions of the University of California, San Francisco (UCSF) Clinical and Translational Science Unit, Core Immunology Laboratory, and AIDS Specimen Bank. We thank Jeremy Lambert from Quanterix for his advice and assistance with the severe acute respiratory syndrome coronavirus 2 immunoglobulin G antibody assay kits.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number 3R01AI141003–03S1 to T. J. H.) and by the Zuckerberg San Francisco General Hospital Department of Medicine and Division of HIV, Infectious Diseases, and Global Medicine. M. J. P. is supported by the National Institutes of Health (T32 AI60530–12) and by the UCSF Resource Allocation Program.
Potential conflicts of interest. A. C., B. C. Y., J. W. W., and C. J. P. are employees of Monogram Biosciences, Inc, a division of LabCorp. D. V. G. reports grants and/or personal fees from Merck and Co. and Gilead Biosciences, outside the submitted work. S. G. D. reports grants and/or personal fees from Gilead Sciences, Merck & Co., ViiV, AbbVie, Eli Lilly, ByroLogyx, and Enochian Biosciences, outside the submitted work. T. J. H. reports grants from Merck and Co., Gilead Biosciences, and Bristol-Myers Squibb, outside the submitted work. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lucas C, Wong P, Klein J, et al. ; Yale IMPACT Team. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020; 26:1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27:1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021; 594:259–64. [DOI] [PubMed] [Google Scholar]
- 6.Lavillegrand JR, Garnier M, Spaeth A, et al. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann Intensive Care 2021; 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharifpour M, Rangaraju S, Liu M, et al. ; Emory COVID-19 Quality and Clinical Research Collaborative. C-reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS One 2020; 15:e0242400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold T, Jurinovic V, Arnreich C, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv [Preprint]. Posted online 10 April 2020. doi:10.1101/2020.04.01.20047381. [Google Scholar]
- 9.Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 2020; 26:1623–35. [DOI] [PubMed] [Google Scholar]
- 10.Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 2020; 9:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonny TS, Patel EU, Zhu X, et al. Cytokine and chemokine levels in coronavirus disease 2019 convalescent plasma. Open Forum Infect Dis 2021; 8:ofaa574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong SWX, Fong S-W, Young BE, et al. Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect Dis 2021; 8:ofab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peluso MJ, Kelly JD, Lu S, et al. Rapid implementation of a cohort for the study of post-acute sequelae of SARS-CoV-2 infection/COVID-19. medRxiv [Preprint]. Posted online 13 March 2021. doi: 10.1101/2021.03.11.21252311. [DOI] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Symptoms of COVID-19. 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 7 July 2021.
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002; 64:258–66. [DOI] [PubMed] [Google Scholar]
- 20.Ayoubkhani D. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK—Office for National Statistics. Office for National Statistics.2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021. Accessed 7 April 2021.
- 21.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol 2018; 18:773–89. [DOI] [PubMed] [Google Scholar]
- 23.Fong Y, Tracey KJ, Moldawer LL, et al. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med 1989; 170:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol 2021; 6:eabg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Yu B, Yang Y, et al. Immunological and inflammatory profiles during acute and convalescent phases of severe/critically ill COVID-19 patients. Int Immunopharmacol 2021; 97:107685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol 2021; 147:72–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RECOVERY Collaborative Group; Horby PW, Pessoa-Amorim G, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P; CORIMUNO-19 Collaborative Group. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. ; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383:2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvarani C, Dolci G, Massari M, et al. ; RCT-TCZ-COVID-19 Study Group. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021; 384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16:448–57. [DOI] [PubMed] [Google Scholar]
- 34.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 2001; 4:1116–22. [DOI] [PubMed] [Google Scholar]
- 35.Papathanasiou S, Rickelt S, Soriano ME, et al. Tumor necrosis factor-α confers cardioprotection through ectopic expression of keratins K8 and K18. Nat Med 2015; 21:1076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suematsu S, Matsuda T, Aozasa K, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A 1989; 86:7547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell IL, Abraham CR, Masliah E, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A 1993; 90:10061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104:236–44, 28p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 2016; 12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020; 5:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
- 42.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383:1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Zuiani A, Fischinger S, et al. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell 2020; 183:1496–507.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kowitdamrong E, Puthanakit T, Jantarabenjakul W, et al. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One 2020; 15:e0240502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 2020; 71:2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peluso MJ, Takahashi S, Hakim J, et al. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci Adv. 2021; 7: eabh3409. doi: 10.1126/sciadv.abh3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang SE, Feng A, Meng W, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun 2021; 12:5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhadelia N, Belkina AC, Olson A, et al. Distinct autoimmune antibody signatures between hospitalized acute COVID-19 patients, SARS-CoV-2 convalescent individuals, and unexposed pre-pandemic controls. medRxiv [Preprint]. Posted 25 January 2021. doi: 10.1101/2021.01.21.21249176. [DOI] [Google Scholar]
- 49.Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in Covid-19. N Engl J Med 2020; 383:789–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after SARS-CoV-2 infection. J Clin Endocrinol Metab 2020; 105:dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jiab490_suppl_Supplementary_Materials