PTEN Expression Causes Feedback Upregulation of Insulin Receptor Substrate 2 (original) (raw)
Abstract
PTEN is a tumor suppressor that antagonizes phosphatidylinositol-3 kinase (PI3K) by dephosphorylating the D3 position of phosphatidylinositol (3,4,5)-triphosphate (PtdIns-3,4,5-P3). Given the importance of PTEN in regulating PtdIns-3,4,5-P3 levels, we used Affymetrix GeneChip arrays to identify genes regulated by PTEN. PTEN expression rapidly reduced the activity of Akt, which was followed by a G1 arrest and eventually apoptosis. The gene encoding insulin receptor substrate 2 (IRS-2), a mediator of insulin signaling, was found to be the most induced gene at all time points. A PI3K-specific inhibitor, LY294002, also upregulated IRS-2, providing evidence that it was the suppression of the PI3K pathway that was responsible for the message upregulation. In addition, PTEN, LY294002, and rapamycin, an inhibitor of mammalian target of rapamycin, caused a reduction in the molecular weight of IRS-2 and an increase in the association of IRS-2 with PI3K. Apparently, PTEN inhibits a negative regulator of IRS-2 to upregulate the IRS-2–PI3K interaction. These studies suggest that PtdIns-3,4,5-P3 levels regulate the specific activity and amount of IRS-2 available for insulin signaling.
PTEN is a tumor suppressor gene that is lost or mutated at a high frequency in glioblastomas (20 to 44%) (26, 53) and endometrial carcinomas (50%) (22, 44, 58). Additionally, PTEN mutations have been found to a lesser extent in cancers of the prostate, bladder, ovary, lung, breast, skin, and lymphatic systems (1). Two autosomally dominant hamartoma syndromes, Cowden disease and Bannayan-Zonana syndrome, are associated with germline mutations in PTEN (27, 28; D. J. Marsh, P. L. Dahia, Z. Zheng, D. Liaw, R. Parsons, R. J. Gorlin, and C. Eng, Letter, Nat. Genet. **16:**333–334, 1997). Consistent with the role of PTEN as a tumor suppressor, heterozygous PTEN mice develop tumors in multiple organs (7, 39, 52).
The PTEN gene encodes a 403-amino-acid phosphatase that dephosphorylates phosphoinositides and phosphoamino acids. PTEN has been shown to dephosphorylate the D3 position of phosphatidylinositol (3,4,5)-triphosphate (PtdIns-3,4,5-P3) and phosphatidylinositol (3,4)-bisphosphate (PtdIns-3,4-P2), important second messengers in signal transduction (29). PtdIns-3,4-P2 and PtdIns-3,4,5-P3 activate a variety of signaling proteins by stabilizing their interaction with the membrane via a pleckstrinhomology (PH) domain. These proteins include Akt, PDK1, PKCɛ, Btk, PHISH, insulin receptor substrates 1 to 3 (IRS-1 to -3), Gab1, and many others (40, 42, 43, 45, 64). Akt, a serine/threonine kinase, is one of the best characterized of these and a useful marker of the levels of PtdIns-3,4-P2 and PtdIns-3,4,5-P3 in the cell. Activation of Akt is stimulated by a variety of growth factors, such as insulin, insulin-like growth factor 1 (IGF-1), platelet-derived growth factor, and epidermal growth factor (5). PTEN has been shown to downregulate insulin, IGF-1, and epidermal growth factor-stimulated activation of Akt, confirming its importance as a signaling intermediate that may regulate gene expression (29, 54, 60).
Consistent with the observations that PTEN acts antagonistically to this pathway, several groups have shown that PTEN induces cell cycle arrest and/or apoptosis associated with the downregulation of Akt activity and that dominant active Akt can rescue cells from PTEN inhibition (8, 23, 25, 60). Moreover, tumor lines with mutations in PTEN have increased levels of Akt activity (4, 62). PTEN-null embryonic fibroblasts also exhibit decreased sensitivity to apoptosis and abnormal cell cycle regulation and have increased levels of PtdIns-3,4,5-P3 and Akt activity (52, 54).
PTEN is a close homolog of the Caenorhabditis elegans gene for DAF-18 (48). DAF-18 has been shown to be a negative regulator of the insulin signaling pathway in C. elegans (10, 31, 33). With Drosophila, several groups have examined both the overexpression and loss of PTEN in different cell types (9, 11, 16). They have shown that PTEN affects cell size, cell cycle progression, and apoptosis. As in the C. elegans model, Drosophila PTEN acts as an inhibitor of the insulin signaling pathway (11, 16).
Many signaling pathways in addition to insulin regulate the activity of phosphatidylinositol-3 kinase (PI3K). However, in Drosophila and C. elegans, PI3K and PTEN serve as major mediators of insulin and IGF signaling. Is PTEN's role as a tumor suppressor due to its influence on this pathway, or are other pathways involved? To identify genes that are regulated by PTEN and thereby elucidate more fully the role of PTEN in various signaling pathways, we used a PTEN adenoviral vector to express PTEN in MDA-MB-468, a PTEN-null breast cancer cell line. When PTEN is expressed in this cell line, it induces G1 arrest and apoptosis. Through the use of oligonucleotide chip technology, we identified a number of candidate genes that were regulated by the expression of PTEN. The gene for IRS-2, an insulin signaling family member, was the most highly induced gene at all time points out of more than 40,000 candidates tested. Induction of expression was confirmed at the message and protein levels. In addition to showing increased levels, IRS-2 was tyrosine phosphorylated and associated with p85, proving that PTEN was able to stimulate the ligand-dependent activation of IRS-2 necessary for insulin signaling. A PI3K inhibitor had a similar effect on IRS-2 message and protein, demonstrating that the lipid phosphatase activity of PTEN was likely to be responsible for the upregulation. Furthermore, an inhibitor of mammalian target of rapamycin (mTOR) did not upregulate the level of IRS-2 but did increase the association of IRS-2 and p85. These results indicate that the mTOR/p70S6 kinase pathway is not involved in increasing the level of IRS-2 in the cell but is responsible for the increase in p85-associated IRS-2. Our data suggest that reduced PtdIns-3,4,5-P3 levels elicit a feedback mechanism involving IRS-2 and PI3K that attempts to restore the level of PtdIns-3,4,5-P3 in the cell.
MATERIALS AND METHODS
Cell lines.
The breast cancer cell lines MDA-MB-468, BT-549, and HBL100 and human embryonic kidney 293 cells were obtained from the American Type Culture Collection. All media were supplemented with 10% fetal bovine serum (FBS) as indicated, 100 U of penicillin G/ml, and 100 μg of streptomycin sulfate/ml.
Oligonucleotide arrays.
Oligonucleotide arrays (Affymetrix) containing probes representing 42,000 unigene clusters or full-length genes were used in RNA hybridization experiments. Total RNA was prepared from 107 uninfected, Ad-β-gal-infected, or Ad-PTEN-infected MDA-MB-468 cells at various time points. Total RNA (20 μg) was used to synthesize double-stranded cDNA, which served as a template for the synthesis of antisense RNA. RNA hybridization and data collection were performed as described previously (12).
Northern blot analysis.
Total cellular RNA was prepared using the Qiagen Rneasy kit (Qiagen) according to the manufacturer's instructions. The RNA was resolved on a 1% agarose formaldehyde denaturing gel by electrophoresis and transferred onto nitrocellulose membranes. DNA probes were labeled with [α-32P]dCTP by the random hexamer priming method. Blots were hybridized at 68°C in Express Hyb (Clontech) with the appropriate 32P-labeled DNA probes. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probes were used as loading controls. Membranes were washed in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at 55°C and exposed to BioMax MS films at −80°C using intensifying screens.
Lipid extractions.
To determine the level of PtdIns-3,4,5-P3 in the cell, cells cultured on six-well dishes under various conditions (as indicated in Results) were labeled with 32Pi (100 μCi/ml) for 4 h in Dulbecco modified Eagle phosphate-free and serum-free medium (Gibco). Cells were then washed with cold phosphate-buffered saline (PBS) to remove unincorporated radioisotope, and 0.93 ml of CH3OH–CHCl3–1% HClO4 (50/25/18, vol/vol/vol) was added. Lipids were extracted as described previously (34). The lipids were then separated on a thin-layer chromatography plate and visualized by autoradiography (50).
Immunoblotting and immunoprecipitation.
For immunoblots, cells were lysed and collected in Laemmli sample buffer. Protein lysates (40 to 80 μg) were resolved by denaturing polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. For immunoprecipitations, cells were lysed in a buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 1% triton, 2 mM EDTA protease inhibitor cocktail set I (CalBiochem), 1 mM Na3VO4, and 40 mM NaF. Cell lysates were then incubated with the appropriate antibody, and the immunocomplex was captured on protein A-agarose beads (CalBiochem). The beads were precipitated, resuspended in Laemmli sample buffer, and subjected to immunoblot analysis as described above. Antibodies to phospho-Akt, Akt (New England Biolabs), IRS-2, p85 (Upstate Biotechnology), PTEN (Cascade BioScience), phosphotyrosine (Signal Transduction Laboratories), and β-tubulin (Covance) were used according to the manufacturer's instructions. Blots were developed with horseradish peroxidase-conjugated secondary antibody using the enhanced chemiluminescence system (Amersham Pharmacia).
PTEN and β-galactosidase delivery systems.
Full-length human PTEN was subcloned from pCEP4-PTEN into the _Not_I site of pShuttle-CMV. The PTEN-containing pShuttle-CMV was homologously recombined with pAdeasy to create an adenoviral vector containing PTEN (15). Ad-β-gal was provided by Bert Vogelstein, Johns Hopkins University. Generation of recombinant virus and amplification in 293 cells were performed as described previously (15). The viruses were purified on a cesium chloride gradient and subjected to dialysis. The titers were determined by using antiadenovirus antibody (provided by Hamish Young, Columbia University), and positive foci were visualized using a fluorescence microscope. PTEN was sequenced from the virus stock used in the present experiments, and its wild-type status was confirmed. Adenoviral infections were performed by inoculating cells with a small volume of growth medium supplemented with 2 or 10% FBS containing the virus and the appropriate viral dilution at 37°C with occasional rocking. After 1 h, additional growth medium with 10% FBS was added.
Flow-cytometric analysis.
Cell suspensions of 2 × 106 cells/ml were made in PBS–3% FBS. Cells were then centrifuged at 400 × g for 5 min. The cells were fixed by resuspending the pellet in PBS–3% FBS with the addition of cold ethanol. The cells were fixed at 4°C for 30 min and pelleted by centrifugation. The cells were resuspended in 0.1 mg of propidium iodide/ml and 0.6% NP-40. RNase A was added to the suspension, and cells were incubated in the dark at room temperature for 30 min. The cells were then filtered through an 85-μm-pore-size Nitrex mesh and analyzed by cytometry (FACScalibur, Becton Dickinson).
RESULTS
Expression of PTEN in a PTEN-null breast cell line induces cell cycle arrest and apoptosis.
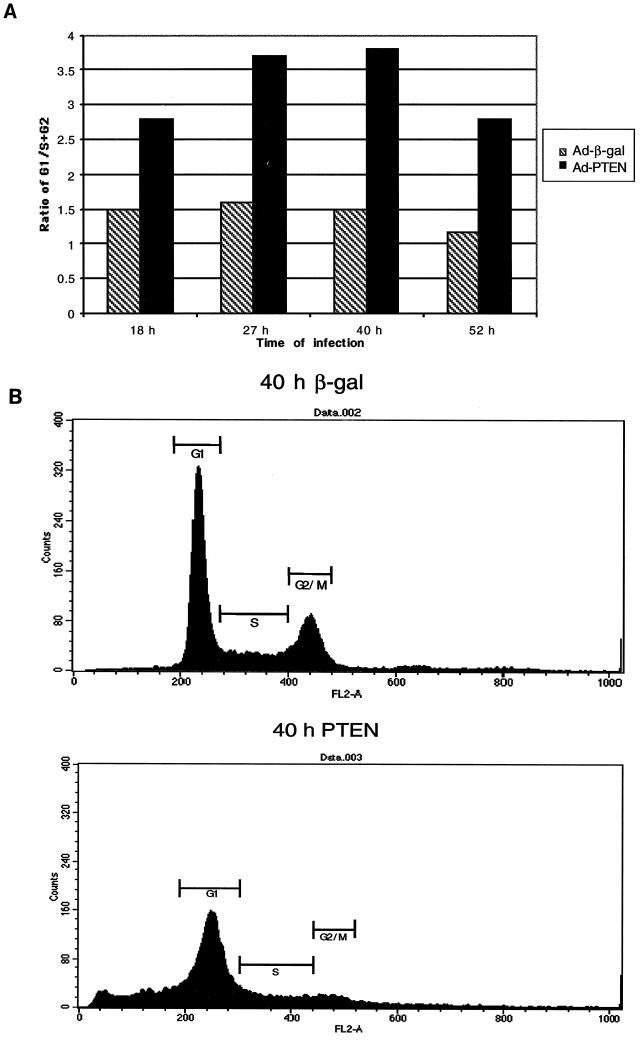
In prior experiments, we identified several PTEN−/− breast cancer cell lines that were growth inhibited by PTEN. Expression of PTEN induced apoptosis in these cell lines as measured by DNA fragmentation and caspase activation (25). To identify genes that are induced by PTEN, we chose to employ a method that would allow for the rapid expression of PTEN in one of these PTEN−/− breast cancer cell lines, MDA-MB-468. For this task, we developed a recombinant adenovirus that employs the cytomegalovirus promoter to express wild-type PTEN (Ad-PTEN). When we examined the cell cycle, we found that the expression of PTEN caused a G1 block which was not seen in the cells infected with the control virus expressing β-galactosidase (Ad-β-gal) (Fig. 1A). During the later time points, there was an induction of apoptosis of Ad-PTEN-infected cells as assessed by the accumulation of a sub-G1 population (Fig. 1B). These results are consistent with recent work by Weng et al., who have shown that PTEN is able to induce a G1 arrest in the breast cancer cell line MCF-7 prior to apoptosis (60).
FIG. 1.
PTEN induces a G1 cell cycle arrest followed by apoptosis. MDA-MB-468 cells were infected with either Ad-PTEN or Ad-β-gal (control) to examine the effects of PTEN on cell cycle and survival. (A) Cells infected with either Ad-PTEN or Ad-β-gal were collected at the indicated time points postinfection and subjected to flow-cytometric analysis as described in Materials and Methods. The graph shows the ratio of cells in G1 to cells in S and G2 phase of the cell cycle versus time points postinfection. (B) Cells infected with either Ad-PTEN or Ad-β-gal were collected at 40 h postinfection and subjected to flow-cytometric analysis to measure DNA content.
PTEN expression reduces the level of PtdIns-3,4,5-P3 and Akt phosphorylation in the cell.
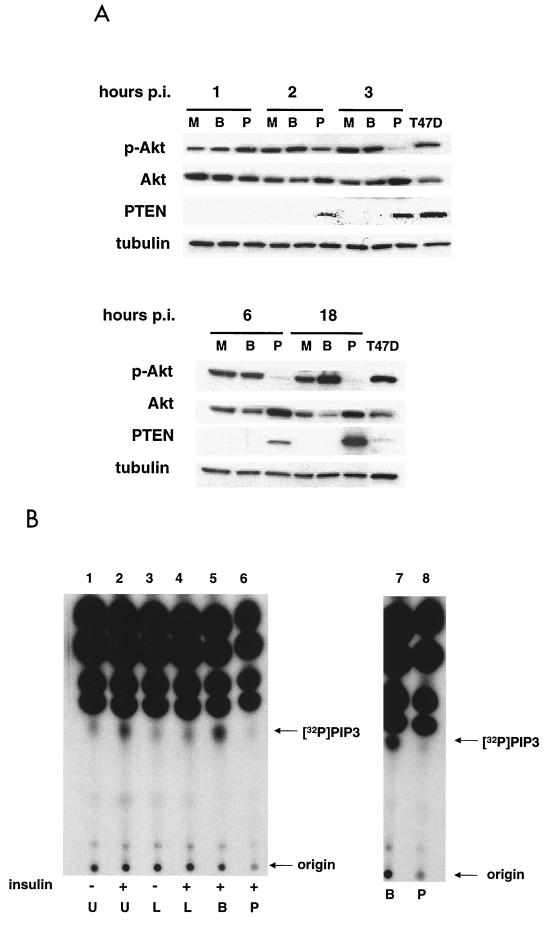
It has been shown that PTEN acts as an antagonist to PI3K and its expression leads to the downregulation of PtdIns-3,4,5-P3 levels and Akt activity (6, 25, 29, 52, 54, 62). Conversely, cell lines that lack PTEN have increased levels of PtdIns-3,4,5-P3 and Akt activity (4, 62). Therefore, we wanted to confirm in our system that the expression of PTEN affected the levels of PtdIns-3,4,5-P3 and Akt activity in the cell. MDA-MB-468 cells were infected with either Ad-PTEN or Ad-β-gal, and the levels of PTEN and phospho-Akt were assessed during various time points postinfection (Fig. 2A). At 2 h postinfection, PTEN could be detected and the level of phospho-Akt was reduced compared to results with both the mock- and Ad-β-gal-infected controls. By 3 h postinfection, the level of PTEN had increased, and Akt activity, as evidenced by phospho-Akt levels, was almost completely abolished. During this same time period, the induction of total Akt was evident, suggesting that the inhibition of Akt activity was sufficient to induce Akt protein in a feedback loop. Interestingly, equivalent amounts of PTEN have different effects on the level of Akt phosphorylation in the MDA-MB-468 and T47D cell lines. This may be due to the ability of cells which continuously express PTEN, such as T47D, to regulate the level of phosphatase activity to ensure cell survival.
FIG. 2.
PTEN expression blocks insulin-induced production of PtdIns-3,4,5-P3 and inhibits Akt activity. (A) MDA-MB-468 cells were either mock (M), Ad-β-gal (B), or Ad-PTEN (P) infected, and total cell lysates were collected at various time points postinfection (hours p.i.) as described in Materials and Methods. T47D, total cell lysate of a breast cancer cell line expressing endogenous PTEN. Cell lysates were subjected to immunoblot analysis with phospho-Akt, Akt, PTEN, and tubulin antibodies. Tubulin antibody was used as a loading control. (B) MDA-MB-468 cells were either left uninfected or infected with Ad-β-gal or Ad-PTEN overnight. Cells were serum starved for 4 h in phosphate-free medium containing 32Pi (100 μCi/ml) before 30 min of treatment with 20 μM LY294002. Insulin (100 μ/ml) or FBS (10%) was added as indicated for 10 min. Lipids were subsequently extracted and separated on a thin-layer chromatography plate as described in Materials and Methods and visualized by autoradiography. U, untreated/uninfected; L, treated with 20 μM LY294002; B, Ad-β-gal infected; P, Ad-PTEN infected; [32P], [32P]PtdIns-3,4,5-P3.
Next, we wished to examine the ability of PTEN to reduce PtdIns-3,4,5-P3 levels. MDA-MB-468 cells serum starved for 4 h have low levels of PtdIns-3,4,5-P3 compared to cells starved for 4 h and then stimulated with insulin (Fig. 2B, lanes 1 and 2). Treatment of cells with LY294002, a specific inhibitor of PI3K, prior to insulin stimulation blocked the insulin-induced increase in PtdIns-3,4,5-P3 as expected (Fig. 2B, lane 4). To determine if PTEN expression would also suppress insulin-induced PtdIns-3,4,5-P3 production, cells were infected for 15 h with either Ad-β-gal or Ad-PTEN, starved for 4 h, and then stimulated with insulin. As seen in Fig. 2B, infection with Ad-β-gal had no effect on the production of PtdIns-3,4,5-P3 (lane 5) whereas infection with Ad-PTEN completely blocked the increase of PtdIns-3,4,5-P3 levels upon insulin stimulation (lane 6). Additionally, cells infected with Ad-PTEN had reduced levels of PtdIns-3,4,5-P3 (Fig. 2B, lane 8) upon stimulation with serum compared to those infected with Ad-β-gal (Fig. 2B, lane 7).
These data suggest that PTEN exerts its signaling effects soon after its initial expression. The rapid expression of PTEN by infection with the adenoviral vector and its effect on the PI3K/Akt signaling pathway in MDA-MB-468 cells made this an excellent system in which to identify genes induced by PTEN.
Identification of candidate genes that are regulated by PTEN.
For the initial identification of genes regulated by PTEN, RNA was harvested from MDA-MB-468 cells that were infected for 12 h with either Ad-PTEN or Ad-β-gal. The artisense RNA was hybridized to a series of 5 DNA chips representing 42,000 unigene clusters or full-length genes. Hybridization signals generated from Ad-PTEN and Ad-β-gal RNA sets were compared. Of the 42,000 genes, only 4 genes were induced eightfold or more. Interestingly, 2 of these 4 corresponded to IRS-2. In addition, the IRS-2 gene was the most induced gene in this experiment. However, given that PTEN protein levels at 12 h of infection were above that of the endogenous wild-type protein of the breast cancer cell line (T47D), we were concerned that the induction was not physiologic. Moreover, PTEN had been present in the cell for more than 10 h. Therefore, signals responding directly to PTEN may have been missed.
As mentioned previously, PTEN controls the levels of PtdIns-3,4,5-P3 in the cell. The levels of PtdIns-3,4,5-P3 in turn regulate a variety of signaling kinases that affect gene expression. Since the half-life of PtdIns-3,4,5-P3 is relatively short, a matter of minutes, signals transduced by PtdIns-3,4,5-P3 would regulate gene expression rapidly (40). Therefore, induction of PTEN would be expected to elicit changes in gene expression swiftly. To address the issues of the dose and timing of PTEN expression, we analyzed the kinetic profile of genes induced in response to PTEN. RNA was collected at 0, 3, 6, 9, 15, and 18 h after infection with Ad-PTEN and hybridized to the same series of chips representing 42,000 transcripts. At 3 h, the IRS-2 gene was the most induced gene, and this induction persisted throughout the infection. There were 32 genes that responded at least threefold to the expression of PTEN by 3 h. Of these 32, induction of 21 remained elevated throughout the infection, but none of these inductions were within an order of magnitude of that of IRS-2 (Table 1). Surprisingly, no genes were suppressed by PTEN.
TABLE 1.
Genes induced more than threefold, confirmed by Northern analysis
| Gene product | Level of expressiona with treatment | Accession no. | Result by Northern analysisb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ad-BG (12 h) | None | PTEN (3 h) | PTEN (6 h) | PTEN (9 h) | PTEN (12 h) | PTEN (15 h) | PTEN (18 h) | |||
| IRS-2 | 101 | 2 | 2,462 | 2,356 | 1,265 | 1,208 | 1,957 | 1,910 | AA347674 | +++ |
| HEF | −3 | 44 | 204 | 275 | 234 | 199 | 177 | 96 | L43821 | +++ |
| DAF | 69 | 30 | 1,789 | 834 | 474 | 357 | 525 | 320 | AA424741 | ++ |
| Zf9/Bcd | 212 | 490 | 2,303 | 782 | 544 | 506 | 1,002 | 1,128 | AA018922 | ++ |
| Keratin 6B | 1,617 | 786 | 5,409 | 7,786 | 7,447 | 7,376 | 3,041 | 1,470 | AA026418 | + |
| IR | 2 | 12 | 77 | 86 | 68 | 98 | 161 | 75 | X02160 | + |
| H98071c | 262 | 262 | 2,551 | 1,386 | 400 | 479 | 878 | 1,305 | H98071 | + |
| AA609795c | −2 | 3 | 357 | 204 | 266 | 312 | 350 | 345 | AA609795 | + |
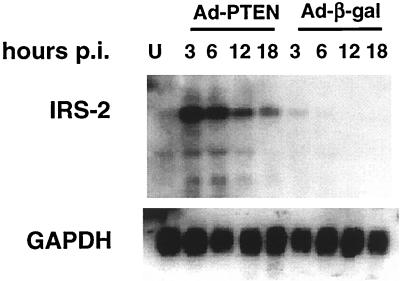
To validate the chip data on the 32 genes that were induced within 3 h of Ad-PTEN infection of MDA-MB-468 cells, infections with either Ad-PTEN or Ad-β-gal were repeated and RNA samples were collected at 0, 3, 6, 12, and 18 h postinfection and then subjected to Northern blot analysis. We were able to confirm a PTEN-specific induction for eight of the genes. Table 1 shows the genes that were induced at least threefold, with results later confirmed by Northern blot analysis. Clearly, IRS-2 was rapidly and specifically induced by the expression of PTEN (Fig. 3). IRS-2 message levels peaked at 3 h in the Ad-PTEN-infected cells and slowly declined over the subsequent time points. In the Ad-β-gal-infected cells, there was very little IRS-2 message over the various time points. Therefore, the peak of IRS-2 message at 3 h correlated with the first time point in which phospho-Akt was inhibited (Fig. 2A) and not the total amount of PTEN expressed.
FIG. 3.
PTEN upregulates IRS-2 message. MDA-MB-468 cells were infected with either Ad-PTEN or Ad-β-gal, and RNA was collected at various time points postinfection (hours p.i.) as described in Materials and Methods. RNA was subjected to Northern blot analysis. Blots were hybridized with 32P-labeled IRS-2 probe and then stripped and hybridized with GAPDH probe as a control. U, uninfected cell lysate.
The IRS-2 protein is upregulated by PTEN.
We chose to focus on the relationship between PTEN and IRS-2 for two reasons. First, IRS-2 was the most induced message by all criteria examined. Second, the C. elegans and Drosophila models place PTEN primarily on the insulin signaling pathway (10, 11, 16, 33). In the Drosophila model, researchers found that mutations in DPTEN completely suppressed the reduction in tissue growth caused by mutations in chico, which codes for a homolog of mammalian insulin receptor substrates, IRS-1 to -4 (11). This placed DPTEN downstream of the IRS homolog as a negative regulator of this pathway. In mammals, IRS-2 is a key regulator of insulin signaling. IRS-2−/− mice develop severe type 2 diabetes due to impaired peripheral insulin signaling and pancreatic β-cell function (61).
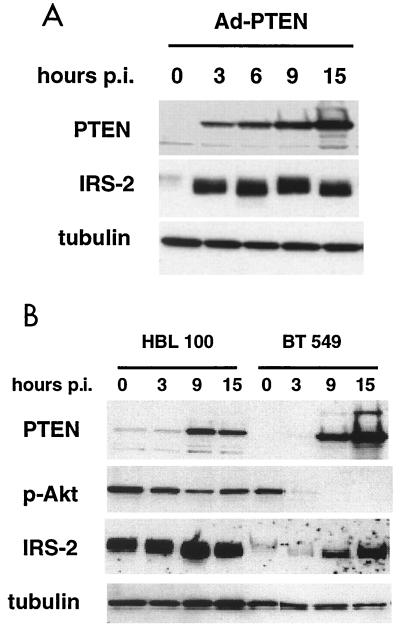
Since IRS-2 was specifically upregulated by PTEN at the RNA level, we wanted to determine whether IRS-2 was upregulated at the protein level. Protein lysates were collected from Ad-PTEN-infected cells at various time points, and immunoblotting was performed with IRS-2 antibody (Fig. 4A). PTEN substantially increased the level of IRS-2 protein at the 3-h time point, and the level of IRS-2 remained elevated for the remainder of the time points. The level of IRS-1 was also assessed to determine if the expression of PTEN specifically upregulated IRS-2 or perhaps other IRS proteins were altered as well. There was only a slight increase in the level of IRS-1 upon PTEN expression and this did not occur until approximately 6 h postinfection (data not shown). From this data, we concluded that PTEN's main target for early upregulation is IRS-2.
FIG. 4.
IRS-2 protein is upregulated by PTEN. MDA-MB-468, BT-549, and HBL100 cell lines were infected with Ad-PTEN, and cell lysates were collected at various time points postinfection (hours p.i.). (A) Immunoblot analysis of MDA-MB-468 cell lysates with antibodies to PTEN, IRS-2, and tubulin. (B) Immunoblot analysis of BT-549 and HBL100 cell lines with antibodies to PTEN, phospho-Akt, IRS-2, and tubulin.
In addition to an observed increase in the level of IRS-2 protein induced by PTEN, there was also an obvious shift in the mobility of IRS-2 which may correspond to a change in the phosphorylation status. Treatment of cells with PI3K inhibitors has been shown to increase the mobility of IRS-2 in IGF-1-treated cells, with a concomitant increase in the tyrosine phosphorylation of IRS-2 (19). These results demonstrate that PTEN expression could potentially lead to an upregulation in the protein levels and tyrosine phosphorylation of IRS-2. However, the increase in the electrophoretic mobility may correspond to a decrease in serine/threonine phosphorylation. Indeed, studies with IRS-1 show that treatment of cells with okadaic acid, a serine/threonine phosphatase inhibitor, leads to the reduction of IRS-1 tyrosine phosphorylation, which was linked to a decrease in its electrophoretic mobility due to phosphorylation on serine/threonine residues (57).
PTEN upregulates IRS-2 in another PTEN-null breast cancer cell line but not in a wild-type PTEN breast cancer cell line.
We were interested in whether the induction of IRS-2 as seen in the MDA-MB-468 cells was unique to this cell line or PTEN expression would induce IRS-2 in other breast cancer cell lines. To investigate this possibility, we infected BT-549 (PTEN-null) and HBL100 (PTEN-wild-type) breast cancer cell lines with Ad-PTEN and examined the levels of IRS-2 by Western blotting. IRS-2 was induced in the BT-549 cell line at 9 h when expression of PTEN was evident (Fig. 4B). There was also a sharp decrease in the level of phospho-Akt at the 3-h time point when the expression of PTEN was very low. Infection of HBL100 with Ad-PTEN did not lead to increased levels of IRS-2 (Fig. 4B). In addition, the expression of PTEN did not significantly decrease the levels of phospho-Akt. It may be necessary to substantially reduce Akt activity to upregulate IRS-2 expression. This was not an altogether surprising result, given that HBL100 cells were previously seen to be resistant to PTEN-induced growth arrest in a colony suppression assay (25). These data suggest that the upregulation of IRS-2 expression by PTEN is not an isolated phenomenon of MDA-MB-468 cells. However, there may be circumstances in certain cell lines when expression of PTEN does not lead to downregulation of Akt activity and IRS-2 is not induced.
Inhibition of PI3K leads to the upregulation of IRS-2.
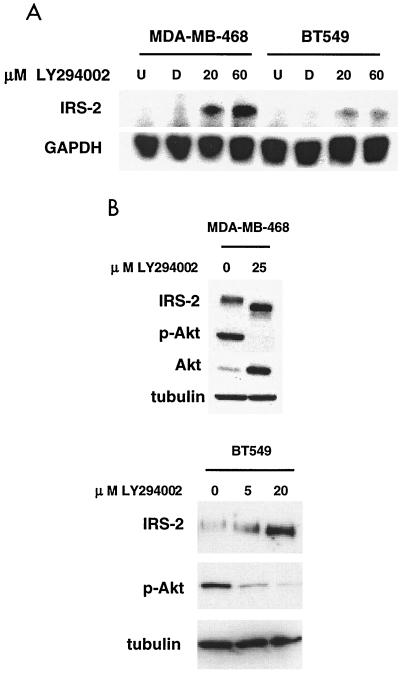
Since PTEN antagonizes the action of PI3K by dephosphorylating the D3 position on PtdIns-3,4,5-P3, we wanted to determine if the upregulation of IRS-2 was due to this antagonistic function or occurred through another PTEN-dependent mechanism. To investigate these possibilities, LY294002 was added to both MDA-MB-468 and BT-549 cells. RNA was collected from untreated cells and dimethylsulfoxide (DMSO)-, 20 μM LY294002-, and 60 μM LY294002-treated cells 3 h after the addition of the drug. Northern blot analysis (Fig. 5A) showed that IRS-2 message was upregulated in the drug-treated MDA-MB-468 and BT-549 cell lines, although at lower levels in the BT-549 cell line. In both cell lines, the addition of LY294002 also upregulated IRS-2 protein levels as detected by immunoblot analysis (Fig. 5B). As with PTEN, LY294002 caused a shift in the molecular weight of IRS-2 and downregulation of Akt activity and induced total Akt expression (Fig. 5B). These data indicate that the inhibition of PI3K is sufficient to upregulate IRS-2 levels and has an effect similar to that of PTEN on gel mobility.
FIG. 5.
LY294002 upregulates IRS-2 RNA and protein levels in two breast cancer cell lines. MDA-MB-468 and BT-549 cells were treated with DMSO (control) or LY294002 or were left untreated for 3 h. (A) RNA was collected from treated cells as described previously and subjected to Northern blot analysis. Blots were hybridized with 32P-labeled IRS-2 probe, stripped, and hybridized with GAPDH probe as a control. U, untreated; D, DMSO. (B) Total cell lysates were collected and subjected to immunoblot analysis using antibodies to PTEN, phospho-Akt, and tubulin.
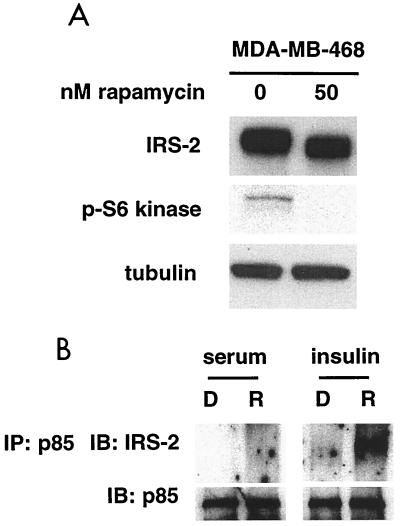
Inhibition of PI3K leads to the downregulation of Akt activity due to the reduction of PtdIns-3,4,5-P3 concentrations. It is possible that the suppression of PtdIns-3,4,5-P3 levels allows for the activation of a signaling pathway responsible for the upregulation of IRS-2 message. PtdIns-3,4,5-P3 regulates the activity of a number of different proteins involved in cell growth and survival, such as NF-κB, FKHR, BAD, p70S6 kinase, and 4E-BP1 (17). We used rapamycin, an inhibitor of mTOR, to block the p70S6 kinase arm of the PI3K pathway to determine if this inhibition would lead to upregulation of IRS-2 message. As with LY294002, RNA was collected from untreated cells and DMSO-, 50 nM rapamycin-, and 100 nM rapamycin-treated cells 3 h after the addition of the drug. Northern blot analysis showed no upregulation of IRS-2 message with rapamycin (data not shown). These data reveal that upregulation of IRS-2 is dependent on the inhibition of PI3K but not on the inhibition of its downstream effector, p70S6 kinase.
Insulin stimulates IRS-2 association with p85 in MDA-MB-468 cells.
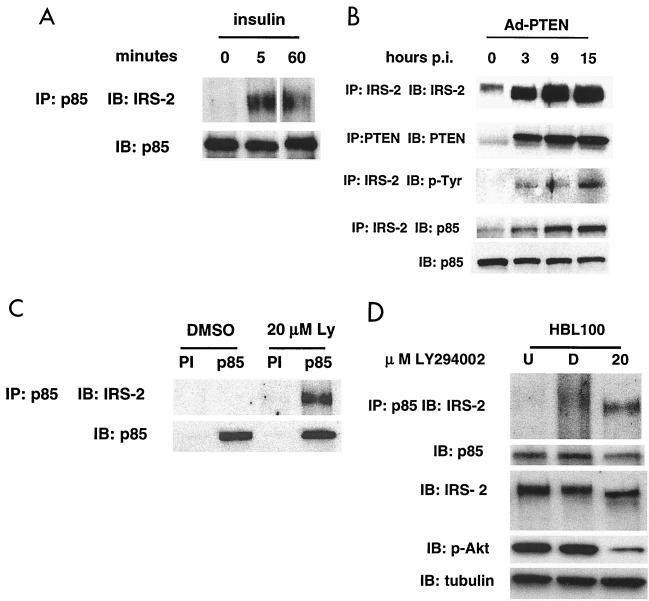
We have shown that both PTEN and a PI3K inhibitor can upregulate IRS-2 message in MDA-MB-468 cells, but we wanted to know whether IRS-2 participates in insulin signal transduction in this cell line. Stimulation of MDA-MB-468 cells by insulin leads to tyrosine phosphorylation of the insulin receptor and to a corresponding increase in PI3K activity (Fig. 2B) (49). To determine if IRS-2 is important in mediating insulin signaling through the PI3K pathway in this cell line, cells were serum starved for 24 h and subsequently pulsed with insulin. Cell lysates were collected, and immunoprecipitations were performed with an antibody that recognizes the p85 subunit of PI3K. The resulting immunocomplexes contained IRS-2 as evidenced by immunoblotting (Fig. 6A). This result indicates that IRS-2 has the ability to transduce insulin signals to the PI3K pathway in MDA-MB-468 cells.
FIG. 6.
Inhibition of the PI3K pathway leads to increased association of IRS-2 and p85. (A) MDA-MB-468 cells were serum starved for 24 h and pulsed with 100 ng of insulin/ml for the indicated time periods. Cell lysates were collected and immunoprecipitations with p85 antibody were performed as described previously. The resulting immmunocomplexes were subjected to immunoblot analysis with IRS-2 and p85 antibody. IP, immunoprecipitation; IB, immunoblot. (B) MDA-MB-468 cells were infected with Ad-PTEN, and cell lysates were collected at various time points postinfection (hours p.i.). Cell lysates were immunoprecipitated with IRS-2, and the resulting immunocomplexes were subjected to immunoblot analysis with antibodies to IRS-2, p85, and p-Tyr. Also, cell lysates were immunoprecipitated with PTEN and immunoblot analysis was performed on the immunocomplexes using PTEN antibody to determine the level of PTEN expression. Total cell lysates were subjected to immunoblot analysis with p85 antibody as a control. IP, immunoprecipitation; IB, immunoblot. (C) MDA-MB-468 cells growing in medium with 10% FBS were treated with DMSO or 20 μM LY294002. Cell lysates were collected after 3 h of treatment and immunoprecipitated with p85 antibody. Immunocomplexes were subjected to immunoblot analysis with IRS-2 and p85 antibody (control). PI, preimmune serum; IP, immunoprecipitation; IB, immunoblot. (D) HBL100 cells growing in medium with 10% bovine serum were left untreated or treated with DMSO or 20 μM LY294002. Cell lysates were collected after 3 h of treatment. A portion of the cell lysates was immunoprecipitated with p85 antibody. Immunocomplexes were subjected to immunoblot analysis with IRS-2 and p85 antibody (control). Total cell lysates were subjected to immunoblot analysis with IRS-2, phospho-Akt, and tubulin (loading control) to analyze protein levels. U, untreated; D, DMSO.
IRS-2 produced by the expression of PTEN is tyrosine phosphorylated and binds the p85 subunit of PI3K.
Tyrosine phosphorylation of the IRS proteins is a necessary step for the binding of the SH2-containing p85 subunit of PI3K and transduction of the insulin signal (3, 46). Since the expression of PTEN caused an increase in the mobility of IRS-2, we wanted to know if this change in the mobility corresponded to functionally active IRS-2. Therefore, we examined its tyrosine phosphorylation status and ability to bind p85. MDA-MB-468 cells were infected with Ad-PTEN, and IRS-2 was immunoprecipitated from the cell lysates at various time points. Immunoblotting of these lysates with a phosphotyrosine and a p85 antibody showed that the IRS-2 immunoprecipitated from the Ad-PTEN-infected cells was tyrosine phosphorylated and associated with p85 (Fig. 6B). There was no increase in the level of p85 as evidenced by immunoblotting of the total cell lysate. Control immunoprecipitations with a PTEN antibody demonstrated that PTEN expression correlated with the induced association of p85 with IRS-2. Additionally, we immunoprecipitated IRS-2 from MDA-MB-468 cells expressing PTEN to determine if Grb2 and/or IR (insulin receptor) binds IRS-2 in the IRS-2–p85 complex. Neither Grb2 nor IR appears to bind IRS-2 in this complex (data not shown). These data indicate that not only does PTEN upregulate the level of IRS-2 in the cell, it also stimulates the association of IRS-2 with the p85 subunit of PI3K. The absence of IR and Grb2 in this complex suggests that the PI3K pathway is specifically upregulated and this upregulation may not occur at the receptor complex.
Inhibitors of PI3K and mTOR lead to upregulated association of IRS-2 and p85.
In our present study, we observed an increase in the mobility of IRS-2 that corresponded to tyrosine phosphorylation and its association with p85 upon the expression of PTEN. In addition, the treatment of cells with LY294002 led to a similar increase in the mobility of IRS-2. This is in agreement with one study that showed that the treatment of cells with PI3K inhibitors (wortmannin and LY294002) increased the mobility of IRS-2 with a corresponding increase in tyrosine phosphorylation of IRS-2 (19). Given this evidence, we hypothesized that PTEN's ability to antagonize PI3K was responsible not only for the upregulation of IRS-2 but also for the induced association of IRS-2 with p85. To test this hypothesis, MDA-MB-468 cells grown in 10% serum were treated with DMSO (control) or 20 μM LY294002 or left untreated. Cell lysates were collected after 3 h, and immunoprecipitations were performed with p85 antibody. The immunoprecipitates were subjected to immunoblot analysis with IRS-2 antibody. As seen in Fig. 6C, there was no IRS-2 associated with p85 in the untreated and DMSO controls, but IRS-2 was found to be associated with p85 in the LY294002-treated cells. These data show that it is the inhibition of the PI3K pathway and not another PTEN-dependent mechanism that is responsible for stimulating the association of IRS-2 and p85.
In our system, reduction of PtdIns-3,4,5-P3 and PtdIns-3,4-P2 by either PTEN or LY294002 led to an increased amount of IRS-2 that could associate with p85. Does this increased association of IRS-2 and p85 result simply from increased levels of IRS-2 or is another mechanism involved? To address this question, we examined the association of IRS-2 and p85 in LY294002-treated HBL100 cells. The HBL100 cell line showed no increase in IRS-2 upon PTEN expression or when treated with LY294002, although there was a clear reduction in phospho-Akt levels when the drug was used (Fig. 4B and 6D). Untreated and DMSO- and LY294002-treated cell lysates were collected and immunoprecipitated with anti-p85 antibody. As seen in Fig. 6D, there is an increased association of p85 and IRS-2 without a concomitant increase in IRS-2 levels. The increased association is, therefore, not dependent on an increase in the levels of IRS-2.
To further investigate the mechanism for the increased association of IRS-2 and p85, we treated MDA-MB-468 cells with rapamycin. Although we had seen no increase in the level of IRS-2 message upon treatment with rapamycin, we did see a shift in the mobility of IRS-2 which coincided with reduction of p70S6 kinase activity (Fig. 7A). Since a shift in the mobility of IRS-2 upon expression of PTEN or treatment with LY294002 corresponded to increased association of IRS-2 and p85, we wanted to know if rapamycin would lead to an increased association of IRS-2 and p85 in MDA-MB-468 cells. Cells cultured in media with 10% serum were treated with DMSO (control) or 50 nM rapamycin or left untreated. Cell lysates were collected, and immunoprecipitations were performed with p85 antibody. Immunoblot analysis was performed on the immunocomplexes, and there was no increase found in the amount of IRS-2 associated with p85 in the rapamycin-treated cells over the control level (Fig. 7B). Previous reports have shown that long-term treatment of cells with insulin leads to increased serine/threonine phosphorylation of IRS-1 and IRS-2, indicating the existence of a negative regulator of this pathway (37, 55). Other reports have provided evidence that this negative regulator is rapamycin sensitive, indicating that it lies downstream of mTOR (13, 24). Believing this might be true with IRS-2 in our case as well, cells were cultured for 24 h in the presence of 100 ng of insulin/ml in serum-free medium to activate this inhibitor. Cells were subsequently treated with DMSO or 50 nM rapamycin or left untreated. Again, cell lysates were immunoprecipitated with p85 antibody, and the resulting immunocomplexes were subjected to immunoblot analysis with IRS-2 antibody. Figure 7B shows that there is an increase in the amount of IRS-2 associated with p85 in the rapamycin-treated cells over the control level, with the same level of p85 immunoprecipitated. Similar results were obtained from insulin-treated cells in the presence of LY294002 (data not shown). These results suggest that there is a negative regulator of insulin signaling downstream of PtdIns-3,4,5-P3 on the p70S6 kinase pathway that PTEN inhibits in MDA-MB-468 cells, allowing for the increased association of p85 and IRS-2. This negative regulation is likely to occur via a serine/threonine kinase that phosphorylates IRS-2.
FIG. 7.
Inhibition of the p70S6 kinase pathway leads to increased association of IRS-2 and p85. (A) MDA-MB-468 cells were treated with either DMSO or 50 nM rapamycin for 3 h, and cell lysates were collected. Total cell lysates were subjected to immunoblot analysis with antibodies to IRS-2, phospho-p70S6 kinase, and tubulin. (B) MDA-MB-468 cells growing in medium with 10% FBS or in serum-free medium supplemented with 100 ng of insulin/ml (24 h) were treated with DMSO (D) or 50 nM rapamycin (R). Cell lysates were collected after 3 h of treatment and immunoprecipitated with p85 antibody. Immunocomplexes were subjected to immunoblot analysis with IRS-2 and p85 antibodies. IP, immunoprecipitation; IB, immunoblot.
DISCUSSION
To investigate which genes are regulated by PTEN, we employed an adenoviral vector expressing PTEN to infect MDA-MB-468, a PTEN-null breast cancer cell line. Expression of PTEN is rapid, causing a G1 cell cycle arrest followed by apoptosis (Fig. 1). Subsequent analysis showed that PTEN blocks insulin-induced production of PtdIns-3,4,5-P3 by PI3K and greatly inhibits Akt activity (Fig. 2). Thus, PTEN is a potent inhibitor of the PI3K pathway in this cell line. To study the effects of PTEN on gene expression, we chose to use oligonucleotide arrays. This is a powerful, unbiased approach which allowed us to screen more than 40,000 genes and expressed sequence tags, which would have been virtually impossible using the candidate approach. IRS-2 was identified as the gene most induced by PTEN expression (Table 1). Northern blot analysis confirmed that the induction of IRS-2 was rapid and specific, with the peak expression occuring at 3 h postinfection (Fig. 3). The amount of IRS-2 protein was increased and in an active state (Fig. 4A and 6B). The level of IRS-1 protein was only slightly increased at 6 h after infection with Ad-PTEN (data not shown). Therefore, PTEN acts to specifically upregulate IRS-2 levels and activity.
In studies examining insulin signaling in Drosophila, homozygous clones mutant for both chico, whose product is the homolog of IRS-1 to -4, and DPTEN display an overgrowth phenotype which completely masks the reduced-growth phenotype normally seen in chico mutants (11). This places DPTEN downstream of chico as a regulator of the insulin signaling pathway. IRS-2 also plays an important role in mediating the actions of insulin in mammals. IRS-2-deficient mice develop diabetes as a result of insulin resistance and impaired insulin production (61). In hepatocytes, IRS-2 has been identified as the main effector of both metabolic and growth-promoting effects of insulin through the PI3K pathway (18, 36, 47, 63). Recently, the effect of PTEN on insulin signaling in 3T3-L1 adipocytes was examined. Overexpression of PTEN inhibited insulin-induced PI3K-dependent processes, such as GLUT4 translocation, Akt phosphorylation, and p70S6 kinase phosphorylation, whereas the tyrosine phosphorylation of the insulin receptor and IRS-1 were unaffected. PTEN, therefore, negatively regulates PI3K-dependent insulin signaling in adipocytes. Our results showing that PTEN regulates IRS-2 in a mammalian cell line provide additional evidence that the relationship between PTEN and insulin signaling is evolutionarily conserved.
During the insulin signaling cascade, insulin binds to its receptor, activating the receptor's intrinsic kinase, leading to autophosphorylation of the receptor. IRS-2 is then recruited to the membrane and binds to the tyrosine-phosphorylated receptor through its phosphotyrosine-binding domain. The p85 subunit of PI3K subsequently binds to the phosphorylated IRS-2 protein, resulting in the activation of the PI3K pathway (64). We have shown that stimulation of MDA-MB-468 cells with insulin results in increased PtdIns-3,4,5-P3 levels and the increased association of IRS-2 and the p85 subunit of PI3K (Fig. 2B and 6A). From these data, we concluded that IRS-2 serves as a mediator of insulin signaling in this cell line. MDA-MB-468 cells growing normally in culture have low levels of IRS-2 associated with p85. Upon expression of PTEN or addition of a PI3K inhibitor, the levels of p85-associated IRS-2 increased, indicating that PTEN is upregulating IRS-2 activity in the absence of added insulin (Fig. 6B and C). In addition, a PI3K inhibitor increased the association of IRS-2 and p85 in cells stimulated with insulin (data not shown). This finding is in agreement with the results of studies that showed that PI3K inhibitors prolonged the tyrosine phosphorylation of IRS-1 and IRS-2, thereby enabling them to remain in an active state (19, 38).
Since the addition of PTEN or LY294002 leads to increased levels of IRS-2 in MDA-MB-468 cells, we wanted to know if the increased association of p85 and IRS-2 resulted simply from increased levels of IRS-2 or if there was another mechanism responsible. To resolve this issue, we examined the association of IRS-2 and p85 in LY294002-treated HBL100 cells. The HBL100 cell line showed no increase in IRS-2 upon PTEN expression or when treated with LY294002 (Fig. 4B and 6D), so if an increased association between p85 and IRS-2 was observed upon the addition of LY294002, it would not be due merely to increased levels of IRS-2. As seen in Fig. 6D, there is an increased association of p85 and IRS-2 without an increase in IRS-2 levels. The increased association is therefore not dependent on an increase in the levels of IRS-2. To further examine the mechanism involved in the increased association of IRS-2 and p85, MDA-MB-468 cells growing in the presence of insulin were treated with rapamycin. This treatment led to the increased association of IRS-2 and p85 (Fig. 7B). This finding suggests that PTEN upregulated IRS-2 activity by inhibiting a negative regulator which lies on the mTOR/p70S6 kinase pathway. Similarly, studies have shown that negative regulation of IRS-1 occurs though a rapamycin-sensitive pathway (13, 24).
From these data, it is possible to conclude that the increased association of IRS-2 and p85 is the result of a feedback mechanism due to a reduction in PtdIns-3,4,5-P3 levels in the cell in an attempt to restore signaling through the PI3K pathway. Interestingly, we did not see an increase in the association of IRS-2 and IR upon PTEN expression (data not shown), suggesting that IRS-2 is not a part of the receptor complex throughout the activation of the feedback loop. A recent report showed that the IRS-2 PH domain preferentially binds PtdIns-3,4-P2, and stimulation with insulin caused a translocation of IRS-2 from the cytoplasm to the plasma membrane (43). This may partially explain why we do not see the association of IRS-2 and IR given the paucity of PtdIns-3,4,5-P3 and potentially PtdIns-3,4-P2. Another potential feedback mechanism may involve the direct interaction of PtdIns-3,4,5-P3 with the SH2 domain of p85. Rameh et al. have shown that PtdIns-3,4,5-P3 competes with the IR and IRS-1 for binding to the SH2 domain of p85 (41). Such competition may also be occurring between PtdIns-3,4,5-P3 and tyrosine-phosphorylated IRS-2 for binding to p85. This may lead to the increased association of IRS-2 and p85 when PtdIns-3,4,5-P3 levels are low due to PTEN expression.
PTEN not only upregulates the IRS-2 and p85 interaction, it also upregulates the level of IRS-2 in the cell. Through the use of a PI3K inhibitor, we were able to show that it was PTEN's ability to act antagonistically to PI3K that led to this induction (Fig. 5A). The addition of rapamycin, an inhibitor of mTOR, did not upregulate IRS-2 message in the cell, which provided evidence that the p70S6 kinase arm of the PI3K pathway was not involved (data not shown). While this report was in preparation, two reports were published showing that the levels of IRS-2 in the liver can be affected by constitutive changes in either insulin or its receptor (30, 51). Chronic hyperinsulinema leads to reduced IRS-2 mRNA and protein, while liver-specific loss of the IR leads to upregulation of IRS-2 levels. Based upon our work, it is likely that changes in IRS-2 expression are due to altered levels of PtdIns-3,4,5-P3 in these livers.
Regarding the mechanism of induction of the level of IRS-2, it is possible that the inhibition of Akt may play a role. Several groups have shown that Akt phosphorylates forkhead transcription factors, FKHR, FKHRL1, and AFX, preventing their translocation to the nucleus (2, 20, 56). Indeed, a recent study showed that in PTEN-null cells, FKHRL1 and exogenously expressed FKHR are retained in the cytoplasm and exogenous FKHR fails to activate transcription. When PTEN is expressed, the ability of FKHR to activate transcription is restored (59). Indeed, we find that PTEN inhibits FKHRL1 phosphorylation by 3 h postinfection (I. Hennessy and R. Parsons, unpublished observations). Although the forkhead transcription factors seem to be attractive candidates in mediating PTEN's ability to upregulate IRS-2, it is quite possible that other transcription factors or a combination of factors that are dependent on PtdIns-3,4,5-P3 could be responsible for this upregulation. Also, PTEN is a more potent inducer of IRS-2 than LY294002, suggesting that there is a component of this upregulation that is independent of PTEN's phosphatase activity.
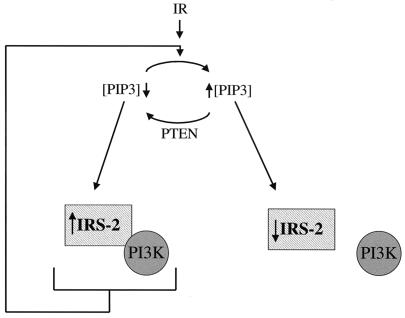
From our present data, we believe that PTEN activates a feedback loop which upregulates the level and activity of IRS-2 (Fig. 8). It is possible that IRS-2 in this state is maximally prepared to activate PI3K in response to insulin in an effort to upregulate PtdIns-3,4,5-P3 levels. Consistent with the existence of a feedback loop upstream of PTEN, total Akt levels were upregulated in the presence of either PTEN or a PI3K inhibitor when the levels of phospho-Akt were low (Fig. 2A and 5B). The ability of a tumor suppressor to upregulate its antagonist in a signaling pathway is not unprecedented. After irradiation, wild-type p53 upregulates the mdm2 gene (14, 21, 32, 35). In this feedback loop, MDM2 blocks the transcriptional activity of p53 and targets p53 for degradation. Therefore, we propose that IRS-2 may be oncogenic, as is Akt.
FIG. 8.
Model outlining PTEN's involvement in signal transduction. PIP3 represents PtdIns-3,4,5-P3. Feedback modulation of insulin signaling by PTEN is shown. Expression of PTEN reduces the level of PtdIns-3,4,5-P3 in the cell, which leads to increased amounts of IRS-2 and an increased association with PI3K. Increased levels of PtdIns-3,4,5-P3 are associated with increased posttranslational modification of IRS-2 and disassociation with PI3K.
In conclusion, our study presents evidence that the tumor suppressor PTEN antagonizes PI3K, causing the activation of a feedback loop involving IRS-2 in a futile attempt by the cell to circumvent apoptosis and growth inhibition by upregulating signaling through PI3K.
ACKNOWLEDGMENTS
We thank L. C. Cantley, T. Franke, and J. Luo for their advice on phospholipid extraction and analysis. L. Simpson and J. Li contributed equally to this work.
This work was supported by National Cancer Institute grants CA75553 and CA82783.
REFERENCES
- 1.Ali I U, Schriml L M, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 2.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter C L, Cantley L C. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim Biophys Acta. 1996;1288:M11–M16. doi: 10.1016/0304-419x(96)00018-2. [DOI] [PubMed] [Google Scholar]
- 4.Dahia P L, Aguiar R C, Alberta J, Kum J B, Caron S, Sill H, Marsh D J, Ritz J, Freedman A, Stiles C, Eng C. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum Mol Genet. 1999;8:185–193. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- 5.Datta S R, Brunet A, Greenberg M E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 6.Davies M A, Lu Y, Sano T, Fang X, Tang P, LaPushin R, Koul D, Bookstein R, Stokoe D, Yung W K, Mills G B, Steck P A. Adenoviral transgene expression of MMAC/PTEN in human glioma cells inhibits Akt activation and induces anoikis. Cancer Res. 1998;58:5285–5290. [PubMed] [Google Scholar]
- 7.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi P P. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 8.Furnari F B, Huang H J, Cavenee W K. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–5008. [PubMed] [Google Scholar]
- 9.Gao X, Neufeld T P, Pan D. Drosophila PTEN regulates cell growth and proliferation through P13K-dependent and -independent pathways. Dev Biol. 2000;221:404–418. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- 10.Gil E B, Malone Link E, Liu L X, Johnson C D, Lees J A. Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumor suppressor gene. Proc Natl Acad Sci USA. 1999;96:2925–2930. doi: 10.1073/pnas.96.6.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goberdhan D C, Paricio N, Goodman E C, Mlodzik M, Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harkin D P, Bean J M, Miklos D, Song Y H, Truong V B, Englert C, Christians F C, Ellisen L W, Maheswaran S, Oliner J D, Haber D A. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 13.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma P M, Olefsky J M, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 14.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 15.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Potter C J, Tao W, Li D M, Brogiolo W, Hafen E, Sun H, Xu T. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development. 1999;126:5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- 17.Kandel E S, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 18.Kido Y, Burks D J, Withers D, Bruning J C, Kahn C R, White M F, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Investig. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim B, Leventhal P S, White M F, Feldman E L. Differential regulation of insulin receptor substrate-2 and mitogen-activated protein kinase tyrosine phosphorylation by phosphatidylinositol 3-kinase inhibitors in SH-SY5Y human neuroblastoma cells. Endocrinology. 1998;139:4881–4889. doi: 10.1210/endo.139.12.6348. [DOI] [PubMed] [Google Scholar]
- 20.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 21.Kubbutat M H, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 22.Levine R L, Cargile C B, Blazes M S, van Rees B, Kurman R J, Ellenson L H. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58:3254–3258. [PubMed] [Google Scholar]
- 23.Li D M, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA. 1998;95:15406–15411. doi: 10.1073/pnas.95.26.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, DeFea K, Roth R A. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J Biol Chem. 1999;274:9351–9356. doi: 10.1074/jbc.274.14.9351. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Simpson L, Takahashi M, Miliaresis C, Myers M P, Tonks N, Parsons R. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998;58:5667–5672. [PubMed] [Google Scholar]
- 26.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 27.Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, Eng C, Parsons R. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 28.Lynch E D, Ostermeyer E A, Lee M K, Arena J F, Ji H, Dann J, Swisshelm K, Suchard D, MacLeod P M, Kvinnsland S, Gjertsen B T, Heimdal K, Lubs H, Moller P, King M C. Inherited mutations in PTEN that are associated with breast cancer, cowden disease, and juvenile polyposis. Am J Hum Genet. 1997;61:1254–1260. doi: 10.1086/301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maehama T, Dixon J E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 30.Michael M D, Kulkarni R N, Postic C, Previs S F, Shulman G I, Magnuson M A, Kahn C R. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 31.Mihaylova V T, Borland C Z, Manjarrez L, Stern M J, Sun H. The PTEN tumor suppressor homolog in Caenorhabditis elegans regulates longevity and dauer formation in an insulin receptor-like signaling pathway. Proc Natl Acad Sci USA. 1999;96:7427–7432. doi: 10.1073/pnas.96.13.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 33.Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- 34.Okada T, Hazeki O, Ui M, Katada T. Synergistic activation of PtdIns 3-kinase by tyrosine-phosphorylated peptide and beta gamma-subunits of GTP-binding proteins. Biochem J. 1996;317:475–480. doi: 10.1042/bj3170475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliner J D, Pietenpol J A, Thiagalingam S, Gyuris J, Kinzler K W, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 36.Patti M E, Sun X J, Bruening J C, Araki E, Lipes M A, White M F, Kahn C R. 4PS/insulin receptor substrate (IRS)-2 is the alternative substrate of the insulin receptor in IRS-1-deficient mice. J Biol Chem. 1995;270:24670–24673. doi: 10.1074/jbc.270.42.24670. [DOI] [PubMed] [Google Scholar]
- 37.Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H, Zick Y. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem. 1997;272:29911–29918. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- 38.Paz K, Liu Y F, Shorer H, Hemi R, LeRoith D, Quan M, Kanety H, Seger R, Zick Y. Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J Biol Chem. 1999;274:28816–28822. doi: 10.1074/jbc.274.40.28816. [DOI] [PubMed] [Google Scholar]
- 39.Podsypanina K, Ellenson L H, Nemes A, Gu J, Tamura M, Yamada K M, Cordon-Cardo C, Catoretti G, Fisher P E, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 41.Rameh L E, Chen C S, Cantley L C. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 42.Rao V R, Corradetti M N, Chen J, Peng J, Yuan J, Prestwich G D, Brugge J S. Expression cloning of protein targets for 3-phosphorylated phosphoinositides. J Biol Chem. 1999;274:37893–37900. doi: 10.1074/jbc.274.53.37893. [DOI] [PubMed] [Google Scholar]
- 43.Razzini G, Ingrosso A, Brancaccio A, Sciacchitano S, Esposito D L, Falasca M. Different subcellular localization and phosphoinositides binding of insulin receptor substrate protein pleckstrin homology domains. Mol Endocrinol. 2000;14:823–836. doi: 10.1210/mend.14.6.0486. [DOI] [PubMed] [Google Scholar]
- 44.Risinger J I, Hayes A K, Berchuck A, Barrett J C. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–4738. [PubMed] [Google Scholar]
- 45.Rodrigues G A, Falasca M, Zhang Z, Ong S H, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rordorf-Nikolic T, Van Horn D J, Chen D, White M F, Backer J M. Regulation of phosphatidylinositol 3′-kinase by tyrosyl phosphoproteins. Full activation requires occupancy of both SH2 domains in the 85-kDa regulatory subunit. J Biol Chem. 1995;270:3662–3666. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- 47.Rother K I, Imai Y, Caruso M, Beguinot F, Formisano P, Accili D. Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J Biol Chem. 1998;273:17491–17497. doi: 10.1074/jbc.273.28.17491. [DOI] [PubMed] [Google Scholar]
- 48.Ruvkun G, Hobert O. The taxonomy of developmental control in Caenorhabditis elegans. Science. 1998;282:2033–2041. doi: 10.1126/science.282.5396.2033. [DOI] [PubMed] [Google Scholar]
- 49.Sepp-Lorenzino L, Rosen N, Lebwohl D E. Insulin and insulin-like growth factor signaling are defective in the MDA MB-468 human breast cancer cell line. Cell Growth Differ. 1994;5:1077–1083. [PubMed] [Google Scholar]
- 50.Serunian L A, Auger K R, Cantley L C. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 1991;198:78–87. doi: 10.1016/0076-6879(91)98010-4. [DOI] [PubMed] [Google Scholar]
- 51.Shimomura I, Matsuda M, Hammer R E, Bashmakov Y, Brown M S, Goldstein J L. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 52.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 53.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng D H, Tavtigian S V. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 54.Sun H, Lesche R, Li D M, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3, 4, 5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X J, Miralpeix M, Myers M G, Glasheen E M, Backer J M, Kahn C R, White M F. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992;267:22662–22672. [PubMed] [Google Scholar]
- 56.Tang E D, Nunez G, Barr F G, Guan K L. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 57.Tanti J F, Gremeaux T, van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 58.Tashiro H, Blazes M S, Wu R, Cho K R, Bose S, Wang S I, Li J, Parsons R, Ellenson L H. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 59.Vazquez F, Ramaswamy S, Nakamura N, Sellers W R. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weng L P, Smith W M, Dahia P L, Ziebold U, Gil E, Lees J A, Eng C. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res. 1999;59:5808–5814. [PubMed] [Google Scholar]
- 61.Withers D J, Gutierrez J S, Towery H, Burks D J, Ren J M, Previs S, Zhang Y, Bernal D, Pons S, Shulman G I, Bonner-Weir S, White M F. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 62.Wu X, Senechal K, Neshat M S, Whang Y E, Sawyers C L. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, Takahashi Y, Yoshizawa F, Aizawa S, Akanuma Y, Sonenberg N, Yazaki Y, Kadowaki T. Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol. 1996;16:3074–3084. doi: 10.1128/mcb.16.6.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yenush L, White M F. The IRS-signalling system during insulin and cytokine action. Bioessays. 1997;19:491–500. doi: 10.1002/bies.950190608. [DOI] [PubMed] [Google Scholar]