Helicobacter pylori Induces Gastric Epithelial Cell Apoptosis in Association with Increased Fas Receptor Expression (original) (raw)
Abstract
The mechanisms involved in mediating the enhanced gastric epithelial cell apoptosis observed during infection with Helicobacter pylori in vivo are unknown. To determine whether H. pylori directly induces apoptosis of gastric epithelial cells in vitro and to define the role of the Fas-Fas ligand signal transduction cascade, human gastric epithelial cells were infected with H. pylori for up to 72 h under microaerophilic conditions. As assessed by both transmission electron microscopy and fluorescence microscopy, incubation with a _cagA_-positive, _cagE_-positive, VacA-positive clinical H. pylori isolate stimulated an increase in apoptosis compared to the apoptosis of untreated AGS cells (16.0% ± 2.8% versus 5.9% ± 1.4%, P < 0.05) after 72 h. In contrast, apoptosis was not detected following infection with _cagA_-negative, _cagE_-negative, VacA-negative clinical isolates or a Campylobacter jejuni strain. In addition to stimulating apoptosis, infection with H. pylori enhanced Fas receptor expression in AGS cells to a degree comparable to that of treatment with a positive control, gamma interferon (12.5 ng/ml) (148% ± 24% and 167% ± 24% of control, respectively). The enhanced Fas receptor expression was associated with increased sensitivity to Fas-mediated cell death. Ligation of the Fas receptor with an agonistic monoclonal antibody resulted in an increase in apoptosis compared to the apoptosis of cells infected with the bacterium alone (38.5% ± 7.1% versus 16.0% ± 2.8%, P < 0.05). Incubation with neutralizing anti-Fas antibody did not prevent apoptosis of _H. pylori_-infected cells. Taken together, these findings demonstrate that the gastric pathogen H. pylori stimulates apoptosis of gastric epithelial cells in vitro in association with the enhanced expression of the Fas receptor. These data indicate a role for Fas-mediated signaling in the programmed cell death that occurs in response to H. pylori infection.
Infection with the gastric pathogen Helicobacter pylori causes chronic active gastritis and peptic ulcer disease (35). In addition, H. pylori infection has been associated epidemiologically with the development of gastric cancers, including adenocarcinoma (14) and lymphoma (31). The mechanisms by which H. pylori mediates these host responses, however, remain unknown.
Apoptosis is a genetically programmed form of cell death characterized by distinct morphologic and molecular features (32). Programmed cell death plays an important role in the regulation of epithelial cell numbers in the gastrointestinal tract (15). In addition, deregulation of the apoptotic pathway is implicated in a number of disease processes in the intestine, including carcinogenesis (26). Microbes have developed mechanisms to stimulate the apoptotic signal transduction cascade which likely play a role in pathogenesis (37). Microbial pathogens, or their products, can directly activate the cell death signaling cascade. For example, invasive enteric pathogens such as Salmonella can directly induce apoptosis of intestinal epithelial cells (21). Alternatively, immune responses, including infiltrating inflammatory cells and production of inflammatory mediators directed against the microbe, can activate the pathway to cell death. For example, induction of the proinflammatory cytokine tumor necrosis factor alpha mediates in part the cell death of epithelial cells which occurs during Salmonella infection in vitro. Cytotoxic lymphocytes can induce apoptosis of hepatitis C virus-infected cells (1). One mechanism by which immune cells trigger apoptosis of target cells occurs through binding of the Fas receptor to the Fas ligand (32).
Fas, or CD95, is a member of the tumor necrosis factor receptor family which, when bound by its natural ligand, stimulates an apoptotic signal through activation of the caspase cascade (28). Under physiologic conditions, the Fas system is involved in regulating the immune response by eliminating activated lymphocytes (23). Virus-infected cells are also eliminated through Fas-Fas ligand interactions (1). In addition, present evidence links excessive activity of the Fas system with the pathogenic effects associated with infection by certain microbes. For example, the lymphocyte depletion observed in patients infected with the human immunodeficiency virus appears to be Fas mediated (3, 19).
Alterations in the gastric epithelial cell cycle, including both enhanced proliferation and increased apoptosis of gastric cells, are identified during infection with H. pylori (4, 27). These changes in cell turnover are present in both _H. pylori_-infected children (16) and adults (25). Investigations of the molecular determinants mediating apoptosis have identified both enhanced expression of the tumor suppressor p53 (16) and increased expression of the proapoptotic protein Bak in response to H. pylori infection (5). Among _H. pylori_-infected children, gastric epithelial cell apoptosis returns to baseline levels only following both eradication of the bacterium and resolution of the accompanying gastritis (16). These findings suggest a role for immune-mediated apoptosis of gastric epithelial cells during H. pylori infection. Therefore, the aims of this study were to determine if H. pylori can directly stimulate programmed cell death of gastric epithelial cells and to characterize the role of Fas-Fas ligand signaling in this cell death cascade.
MATERIALS AND METHODS
Bacteria and growth conditions.
H. pylori LC 11, a _cagA_-positive, _cagE_-positive, vacuolating cytotoxin (VacA)-producing H. pylori strain originally isolated from a child with duodenal ulcer disease, and two _cagA_-negative, _cagE_-negative, VacA-negative strains, LC 3 and LC 20, were employed for these studies (13). The presence of cagA and cagE was determined by isolation of genomic DNA and PCR (24) with the primer pair GCCTACTGGTGGGGATTG and GCCTGTAGTTGGTCTTC for cagA and both primer pair AGACATGCAAAAAGGTAT and CAATCTAGTGGGGTGGTA and primer pair TGCTGATACGATTAGAGA and TAGTCCCTTAGTGATGAT (kindly provided by Robin Beech, McGill University, Montreal, Quebec, Canada) for cagE. The presence of the vacuolating cytotoxin was determined by the method of Cover et al. (7). Concentrated broth supernatants were incubated with HEp-2 cells for 24 h at 37°C, and vacuolation was assessed by bright-field microscopy (24).
H. pylori strains were grown under microaerophilic conditions on Columbia blood agar plates for 72 h at 37°C, harvested, and resuspended in brucella broth (Difco) supplemented with 10% heat-inactivated fetal calf serum, vancomycin, and trimethoprim. Bacterial cells were grown overnight at 37°C in an Erlenmeyer flask with shaking at 120 rpm, as described previously (17). Cells were then pelleted and resuspended in phosphate-buffered saline at a concentration of 109 CFU/ml. Campylobacter jejuni TGH 9011 was kindly provided by V. L. Chan (University of Toronto).
Cell culture.
The human gastric adenocarcinoma cell line AGS was grown as a monolayer in tissue culture flasks at 37°C in 5% CO2. The tissue culture medium was Ham’s F-12 medium (Life Technologies, GIBCO BRL, Grand Island, N.Y.) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (Cansera International, Rexdale, Ontario, Canada) and 0.1% sodium bicarbonate. The human gastric adenocarcinoma cell line KATO III was grown under similar conditions in RPMI 1640 media supplemented with 10% fetal calf serum. Cells were incubated for up to 72 h with H. pylori (5 × 108 CFU/ml) under microaerophilic conditions. Control cells were incubated under the same conditions in the absence of bacteria.
Assessment of apoptosis. (i) Transmission electron microscopy.
For transmission electron microscopy, cells were grown to confluence in tissue culture flasks and incubated with H. pylori as described above. Cells incubated in the absence of H. pylori served as controls. Both cells in suspension and trypsinized cells were pelleted, fixed with 2% glutaraldehyde (vol/vol) in 0.1 M phosphate buffer, postfixed in 2% osmium tetroxide, and dehydrated through a series of graded acetone washes (9). Samples were embedded in epoxy resin, and ultrathin sections were placed onto 300-mesh copper grids. The grids were then stained with uranyl acetate and lead salts, as described previously (9). Grids were examined for the presence of apoptotic cells with a transmission electron microscope at an accelerating voltage of 60 kV (20).
(ii) Fluorescent dye staining.
Cells in suspension and trypsinized cells were pelleted and resuspended in 1 ml of phosphate-buffered saline. Acridine orange-ethidium bromide in phosphate-buffered saline (100 μg/ml) was added to the suspension (11). A drop of the suspension was applied to a microscope slide, and apoptotic cells were assessed by fluorescence microscopy, as previously described (11). Apoptotic cells were enumerated by counting 500 cells at multiple randomly selected fields. The apoptotic index was expressed as the percent of apoptotic cells per 500 cells enumerated.
Determination of Fas receptor expression. (i) Fluorescence microscopy.
AGS cells were grown on Lab-Tek chamber slides (Nunc, Naperville, Ill.), as described above, until they were semiconfluent. The cells were then incubated in the presence or absence of gamma interferon (IFN-γ) (12.5 ng/ml) for 24 h at 37°C (12). Cells were washed with phosphate-buffered saline and then fixed with 100% acetone for 5 min at room temperature. Cells were next incubated with an anti-Fas receptor monoclonal antibody (clone DX2; Oncogene Research Products, Cambridge, Mass.) at a concentration of 2.5 μg/ml for 1 h at 37°C. Following the washings, cells were incubated with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (1:100) for 1 h at 37°C. After incubation and further washings, slides of the cells were mounted with coverslips and viewed under fluorescence microscopy.
(ii) Immunoassay.
A commercially available enzyme-linked immunosorbent assay (Oncogene Research Products) was employed to measure Fas receptor expression in tissue culture cells that had been incubated in the presence or absence of H. pylori LC 11 for 24 h. Cells treated with IFN-γ (12.5 ng/ml) were used as a positive control (12). Fas receptor expression was calculated according to the manufacturer’s instructions per 106 cells enumerated. Briefly, cell extracts were obtained by incubating harvested cells in the supplied buffer with an antigen extraction agent and then centrifuged to obtain a clear lysate. The lysates were diluted and added to the supplied microtiter plates. Following incubation with the detector antibody and streptavidin conjugate, absorbances of wells in the plates were read spectophotometrically at dual wavelengths of 450 to 490 nm.
Functional assessment of Fas receptor.
Untreated and _H. pylori_-infected AGS cells were exposed for 24 h to an activating anti-Fas monoclonal antibody (Upstate Biotechnology, Lake Placid, N.Y.) at a concentration which had previously been shown to induce apoptosis in Fas-sensitive cells (100 ng/ml) (12). Apoptotic cells were enumerated by fluorescence microscopy following staining with acridine orange and ethidium bromide, as described above.
_H. pylori_-infected cells were incubated with a neutralizing anti-Fas antibody (Upstate Biotechnology) at 1,000 ng/ml. Dose-response studies (concentrations ranging from 100 to 2,000 ng/ml) determined that the maximal inhibition of apoptosis (50%) in AGS cells stimulated with the activating Fas antibody (100 ng/ml) was achieved at a dosage of 1,000 ng/ml. Apoptotic cells were enumerated as described above. Results are expressed as the means of the data obtained from two independent experiments.
Statistical analysis.
Results are expressed as means ± standard errors (SE). To test the statistical significance between multiple groups, a one-way analysis of variance (ANOVA) was used, followed by post hoc comparisons with the Newman-Keuls test.
RESULTS
Evaluation of apoptosis in _H. pylori_-infected gastric epithelial cells.
To determine if infection with H. pylori alone could stimulate apoptosis of gastric epithelial cells in vitro, AGS and KATO III cells were incubated with the bacteria for up to 72 h. AGS cells infected with a _cagA_-positive, _cagE_-positive, VacA-positive isolate, strain LC 11, underwent apoptosis as assessed by fluorescence microscopy. As shown in Fig. 1, apoptotic cells displayed the characteristic features of reduced size, cytoplasmic vacuolation, and enhanced fluorescence of condensed and marginated nuclear chromatin. These results were confirmed by transmission electron microscopy. Unlike untreated cells, AGS cells infected with strain LC 11 demonstrated the ultrastructural features which characterize the process of programmed cell death, including cytoplasmic vacuolation, condensed nuclear chromatin, and formation of apoptotic bodies (Fig. 2).
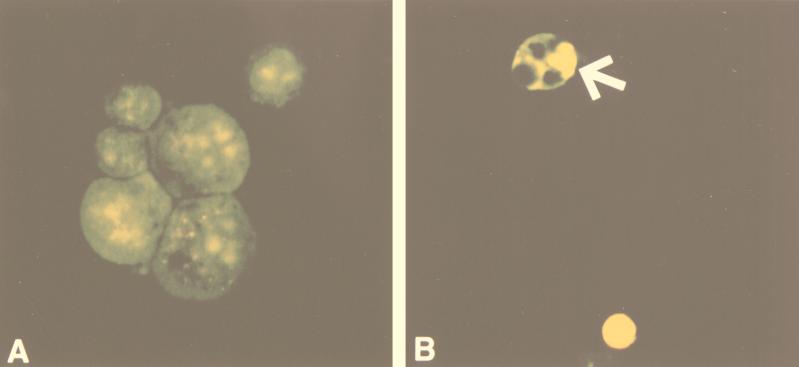
FIG. 1.
Identification of apoptotic cells in untreated AGS cells (A) and _H. pylori_-infected cells (B) after 72 h by acridine orange-ethidium bromide staining and fluorescence microscopy. (A) AGS cells demonstrate normal morphology. (B) _H. pylori_-infected AGS cells show morphologic features of apoptosis (arrow), including condensed and marginated chromatin with apoptotic body formation. Approximate magnifications, ×1,000.
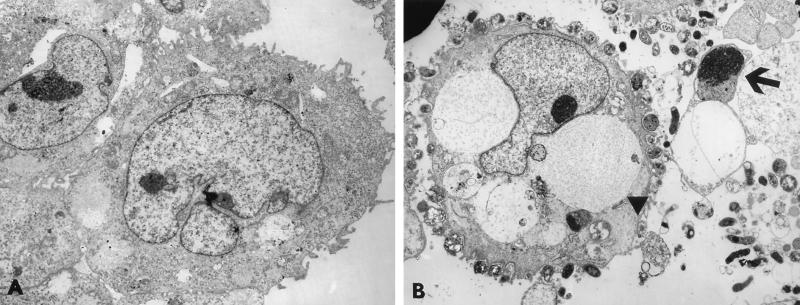
FIG. 2.
Transmission electron photomicrographs of uninfected (A) and _H. pylori_-infected (B) AGS cells. (A) Control cells show normal cellular morphology. (B) _H. pylori_-infected AGS cells demonstrate the characteristic features of programmed cell death, including cytoplasmic vacuolation (arrowhead) and apoptotic body formation (arrow). Approximate magnifications, ×7,800.
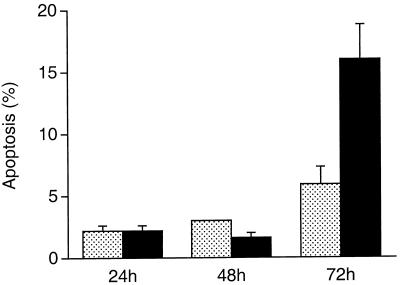
Quantitation of apoptotic AGS cells by fluorescence microscopy demonstrated that H. pylori LC 11-mediated cell death was time dependent. An increase in the death of gastric epithelial cells was observed following 72 h of infection with the bacterium (16.0% ± 2.8% versus 5.9% ± 1.4%, P < 0.05) (Fig. 3). When AGS cells were infected for 72 h with two clinical isolates which lack the putative virulence genes cagE and cagA and vacuolating cytotoxin activity, apoptosis of gastric epithelial cells was not detected (6.5% ± 1.3% versus 7% ± 2.1%). Similarly, infection with the related enteric pathogen C. jejuni did not induce apoptosis of gastric cells (5.9% ± 2.7% versus 6.4% ± 2.3%). In contrast to AGS cells, KATO III cells more readily underwent necrosis in response to infection with H. pylori strain LC 11 for 72 h; therefore, the remaining studies were performed with AGS cells.
FIG. 3.
Quantitation of apoptotic AGS cells infected with H. pylori for various lengths of time. Incubation with the bacterium resulted in an increase in apoptosis compared to the apoptosis of untreated cells at 72 h (P < 0.05 [ANOVA]). Results are expressed as the mean percentages of apoptotic cells per 500 cells enumerated. Variations are represented as the SE.
Expression of the Fas receptor during H. pylori infection.
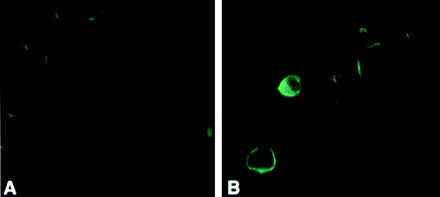
To determine if AGS cells had a basal expression of Fas, which could be enhanced by IFN-γ, which is known to upregulate expression of the receptor in other cell lines (12), fluorescence microscopy with a monoclonal antibody to the Fas receptor was employed. As shown in Fig. 4, AGS cells had a low level of Fas expression which was enhanced following stimulation by IFN-γ (12.5 ng/ml).
FIG. 4.
Fluorescence micrograph demonstrating Fas expression. (A) A low level of Fas expression is detected in untreated AGS cells. (B) Fas expression is enhanced following incubation of AGS with IFN-γ (12.5 ng/ml).
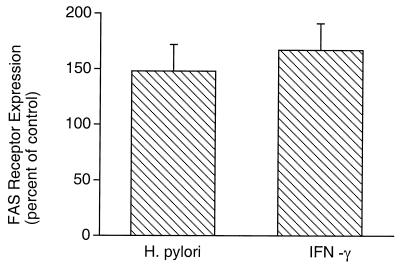
Compared to uninfected (control) cells, infection with H. pylori LC 11 also enhanced expression of Fas as determined by enzyme-linked immunosorbent assay (Fig. 5). _H. pylori_-stimulated Fas receptor expression was comparable to that mediated by IFN-γ (148% ± 24% and 167% ± 24% of the control, respectively; n = 3). The _H. pylori_-mediated Fas expression was not a result of cross-reactivity with a bacterial product, since assessment of bacterial extracts alone showed no detectable Fas expression.
FIG. 5.
Effect of H. pylori on Fas receptor expression in AGS cells. Incubation with H. pylori enhanced Fas receptor expression in AGS cells to a degree comparable to that obtained by treatment with IFN-γ (12.5 ng/ml). Results are expressed as the percent increase in Fas receptor expression compared to the expression in untreated (control) cells (+ SE).
Sensitivity of _H. pylori_-infected cells to Fas-stimulated cell death.
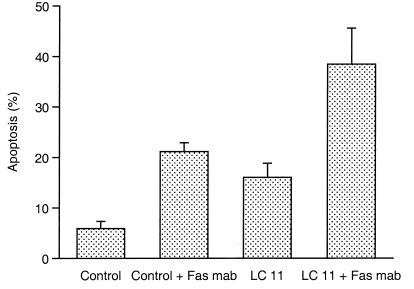
An agonistic monoclonal antibody to the Fas receptor, which stimulates Fas-sensitive cells to undergo apoptosis (12), was then employed to determine if the enhanced Fas expression was functional. As shown in Fig. 6, incubation of AGS cells with the anti-Fas antibody mediated an increase in apoptosis compared to the apoptosis of untreated cells (21.1% ± 1.8% versus 5.9% ± 1.4%). Furthermore, incubation of LC 11-infected gastric cells with the anti-Fas antibody resulted in a marked increase in programmed cell death compared to the death of cells infected with the bacterium alone (38.5% ± 7.1% versus 16.0% ± 5.5%, P < 0.05 [ANOVA]). Incubation of LC 11-infected AGS cells with a neutralizing anti-Fas receptor antibody did not prevent cell death (7.7% versus 9.9%), indicating that the induction of apoptosis observed following infection with the bacterium alone was not mediated by the enhanced Fas receptor expression.
FIG. 6.
Effect of H. pylori infection on Fas-stimulated apoptosis. Untreated (control) and _H. pylori_-infected (strain LC 11) AGS cells were incubated in the presence or absence of an agonistic monoclonal antibody (mab) to the Fas receptor, and apoptosis was assessed. Incubation with the Fas agonist enhanced programmed cell death in control cells (21.1% ± 1.8% versus 5.9% ± 1.4%, P < 0.05 [ANOVA]). The apoptotic index of _H. pylori_-infected cells incubated with an anti-Fas antibody increased compared to that of AGS cells incubated with the bacterium alone (38.5% ± 7.1% versus 16.0% ± 2.8%, P < 0.05 [ANOVA]). Results are expressed as the percentages of apoptotic cells per 500 cells enumerated (+ SE).
DISCUSSION
In vivo studies demonstrate that infection with H. pylori triggers apoptosis of gastric epithelial cells (34). However, in this setting it is unclear whether immune factors or bacterial factors contribute to cell death. This study supports and extends recent evidence indicating that several mechanisms are involved in stimulating apoptosis of gastric epithelial cells during H. pylori infection (2, 33, 36).
These data demonstrate that H. pylori is capable of directly inducing the death of gastric epithelial cells in vitro in the absence of immune cells. The mechanism of cell death differed between the two gastric cell lines. KATO III cells underwent necrosis in response to prolonged infection with the bacterium, while AGS cells underwent apoptosis. The response of AGS cells to infection with the bacterium mimics the in vivo setting, indicating that the AGS cell line serves as a better model system for investigating these apoptotic pathways than the KATO III cell line.
The exact bacterial factors which directly mediate the death signal are not known. Fan et al. (10) recently provided evidence that binding of H. pylori to the class II major histocompatibility complex expressed on gastric epithelial cells can transduce the cell death signal in vitro. In this study, the presence of factors considered to be associated with virulence, including cagE and cagA, two genes found on the pathogenicity island, as well as vacuolating cytotoxin activity, was associated with apoptosis. In contrast, apoptosis was not detected following infection with clinical isolates lacking these virulence factors. These results are in agreement with the recent findings of Rudi et al. (33), who detected apoptosis of gastric epithelial cells following incubation with culture supernatants from a _cagA_-positive H. pylori isolate with cytotoxic activity but not with supernatants from a _cagA_-negative, noncytotoxic strain. In contrast, another study detected apoptosis during infection with both _cagA_-positive, VacA-producing strains and _cagA_-negative, VacA-negative H. pylori strains (36). However, the cagE status of the strains utilized in both of these studies was not determined. Taken together, these findings suggest that the induction of programmed cell death could play a role in mediating disease outcome. Of interest, a preliminary study demonstrated that infection with _cagE_-positive strains is associated with peptic ulcer disease in children (8).
In addition to cell death triggered directly by infection with H. pylori, upregulation of the Fas receptor is observed in association with increased sensitivity to apoptosis upon ligation of the receptor. Wagner and colleagues (36) also identified enhanced apoptosis in the gastric epithelial cell line HM02 following infection with H. pylori and Fas ligation. However, the mechanism for the enhanced sensitivity to Fas signaling was not determined. This study suggests that H. pylori infection enhances expression of the Fas receptor in gastric epithelial cells, thereby resulting in an increased sensitivity to Fas-triggered cell death. _H. pylori_-mediated enhanced Fas expression does not directly stimulate apoptosis since a neutralizing antibody did not prevent cell death. These findings indicate that immune system-mediated cell death through the Fas-Fas ligand pathway likely also contributes to the apoptosis that is observed during infection in vivo.
The factors mediating enhanced Fas receptor expression and Fas-mediated cell death during H. pylori infection are not known. A recent study demonstrated that IFN-γ, which is increased in the gastric mucosa during H. pylori infection (18), augments apoptosis. IFN-γ also upregulates expression of the Fas death receptor (30). Cytokines produced by inflammatory cells in the lamina propria in response to H. pylori infection could also modulate cell death. Further studies are required to determine the factors which increase the susceptibility of gastric cells to the Fas death cascade.
Our findings indicate that Fas-stimulated cell death could play a role in _H. pylori_-mediated pathogenesis in vivo. During infection with the bacterium, gastric epithelial cells exhibiting enhanced Fas receptor expression could be eliminated by infiltrating lymphocytes that express the Fas ligand. In support of this contention, a recent study identified an increase in Fas ligand mRNA expression in lymphocytes within the lamina propria and enhanced Fas receptor expression in both gastric epithelial cells and cells within the superficial lamina propria of _H. pylori_-infected gastric biopsy tissue (33). Furthermore, the region of the gastric mucosa with enhanced Fas ligand mRNA corresponded to areas of enhanced apoptosis.
Also, animal models suggest that Fas signaling plays a role in gastric injury. In a murine model of autoimmune gastritis generated by either thymectomy or adoptive transfer of a Th1 cell clone recognizing an epitope of the H+, K+-ATPase, enhanced Fas expression was detected on gastric parietal cells (29). In contrast, gastric tissue from normal controls lacked detectable Fas. The topographic expression of this death receptor in parietal cells correlated with the induction of apoptosis as assessed by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling method (29). This indicates that one mechanism by which autoimmune-mediated target cell destruction may be effected is Fas-Fas ligand interactions. This is of particular interest since autoantibodies directed against gastric parietal H+, K+-ATPase are detected in sera from _H. pylori_-infected subjects and correlate with the presence of gastric atrophy (6).
In summary, the present study shows that H. pylori infection is capable of activating the apoptotic cell death cascade in gastric epithelial cells by more than one mechanism. The bacterium can directly stimulate programmed cell death and also enhances both expression of the cell death receptor Fas and sensitivity to Fas-mediated apoptosis. In vivo studies, including those with animal models of human disease (22), should now be undertaken to further delineate the role of Fas signaling in the pathogenesis of _H. pylori_-mediated disease.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council of Canada. N. L. Jones is the recipient of a Research Initiative Award from the Canadian Association of Gastroenterology/Astra Pharmaceuticals/Medical Research Council of Canada. A. S. Day is a recipient of a Research Fellowship Award from the Canadian Association of Gastroenterology/Solvay Pharma/Medical Research Council of Canada. P. M. Sherman is a recipient of an AC Finkelstein Award from the Medical Research Council of Canada.
REFERENCES
- 1.Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283–5291. [PubMed] [Google Scholar]
- 2.Bennett M W, O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. Fas-mediated apoptosis in autoimmune and Helicobacter pylori-associated gastritis. Gastroenterology. 1998;114:A930. [Google Scholar]
- 3.Bohler T, Nedel S, Debatin K M. CD95-induced apoptosis contributes to loss of primed/memory but not resting/naive T cells in children infected with human immunodeficiency virus type 1. Pediatr Res. 1997;41:878–885. doi: 10.1203/00006450-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Brenes F, Ruiz B, Correa P, Hunter F, Rhamakrishnan T, Fontham E, Shi T Y. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre- and post-eradication indices of proliferating cell nuclear antigen. Am J Gastroenterol. 1993;88:1870–1875. [PubMed] [Google Scholar]
- 5.Chen G, Sordillo E M, Ramey W G, Reidy J, Holt P R, Krajewski S, Reed J C, Blaser M J, Moss S F. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of Bak. Biochem Biophys Res Commun. 1997;239:626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- 6.Claeys D, Faller G, Appelmelk B J, Negrini R, Kirchner T. The gastric H+, K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 7.Cover T L, Halter S A, Blaser M J. Characterization of HeLa cell vacuoles induced by Helicobacter pylori broth culture supernatant. Hum Pathol. 1992;23:1004–1010. doi: 10.1016/0046-8177(92)90261-z. [DOI] [PubMed] [Google Scholar]
- 8.Day, A. S., H. A. Jennings, J. T. Lynett, N. L. Jones, C. A. Fallone, R. Beech, and P. M. Sherman.cagE is a virulence factor associated with _Helicobacter pylori_-induced duodenal ulceration in children. In Abstracts of the 99th General Meeting of the American Society for Microbiology 1999, abstr. D1B-139, p. 236. American Society for Microbiology, Washington, D.C. [DOI] [PubMed]
- 9.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P M. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan X J, Crowe S E, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley W K, Ernst P B, Reyes V E. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossmann J, Mohr S, Lapetina E G, Fiocchi C, Levine A D. Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am J Physiol. 1998;274:G1117–G1124. doi: 10.1152/ajpgi.1998.274.6.G1117. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Tatebe S, Osaki M, Goto A, Suzuki Y, Ito H. Expression of Fas antigen and its mediation of apoptosis in human gastric cancer cell lines. Jpn J Cancer Res. 1997;88:49–55. doi: 10.1111/j.1349-7006.1997.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemalatha S G, Drumm B, Sherman P. Adherence of Helicobacter pylori to human gastric epithelial cells in vitro. J Med Microbiol. 1991;35:197–202. doi: 10.1099/00222615-35-4-197. [DOI] [PubMed] [Google Scholar]
- 14.Huang J-Q, Hunt R H. An overview of Helicobacter pylori epidemiology studies. In: Hunt R H, Tytgat G N J, editors. Helicobacter pylori: basic mechanisms to clinical cure 1998. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 295–307. [Google Scholar]
- 15.Jones B A, Gores G J. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;237:G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- 16.Jones N L, Shannon P T, Cutz E, Yeger H, Sherman P M. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 17.Jones N L, Shabib S, Sherman P M. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiol Lett. 1997;146:223–227. doi: 10.1111/j.1574-6968.1997.tb10197.x. [DOI] [PubMed] [Google Scholar]
- 18.Karttunen R, Karttunen T, Ekre H P, MacDonald T T. Interferon gamma and interleukin-4 secreting cells in the gastric antrum of Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L A, Herzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerr J F R, Gobe G C, Winterford C M, Harmon B V. Anatomical methods in cell death. Methods Cell Biol. 1995;46:1–26. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- 21.Kim J M, Eckmann L, Savidge T C, Lowe D C, Witthoft T, Kagnoff M F. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Investig. 1998;102:1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A, O’Rourke J, Corazon de Ungria M, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 23.Lenardo M J. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeb M, Jayaratne P, Jones N, Sihoe A, Sherman P. Lack of correlation between vacuolating cytotoxin activity, cagA gene in Helicobacter pylori, and peptic ulcer disease in children. Eur J Clin Microbiol Infect Dis. 1998;17:653–656. doi: 10.1007/BF01708350. [DOI] [PubMed] [Google Scholar]
- 25.Mannick E E, Bravo L E, Zarama G, Realpe J L, Zhang X J, Ruiz B, Fontham E T H, Mera R, Miller M J S, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 26.Merritt A J, Potten C S, Watson A J M, Loh D Y, Nakayama K, Nakayama K, Hickman J A. Differential expression of bcl-2 in intestinal epithelium: correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995;108:2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- 27.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 29.Nishio A, Katakai T, Oshima C, Kasakura S, Sakai M, Yonehara S, Suda T, Nagata S, Masuda T. A possible involvement of Fas-Fas ligand signaling in the pathogenesis of murine autoimmune gastritis. Gastroenterology. 1996;111:959–967. doi: 10.1016/s0016-5085(96)70063-x. [DOI] [PubMed] [Google Scholar]
- 30.Ossina N K, Cannas A, Powers V C, Fitzpatrick P A, Knight J D, Gilbert J R, Shekhtman E M, Tomei L D, Umansky S R, Keifer M C. Interferon-γ modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem. 1997;272:16351–16357. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- 31.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 32.Raff M. Cell suicide for beginners. Nature. 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- 33.Rudi J, Kuck D, Strand S, von Herbay A, Mariani S M, Krammer P H, Galle P R, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial cell apoptosis. J Clin Investig. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirin H, Moss S F. Helicobacter pylori induced apoptosis. Gut. 1998;43:592–594. doi: 10.1136/gut.43.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 36.Wagner S, Beil W, Westermann J, Logan R P H, Bock C T, Trautwein C, Bleck J S, Manns M P. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 37.Zychlinsky A, Sansonetti P J. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]